Personalized Care Management for Persons with Parkinson’s Disease
Abstract
There is a growing awareness that delivery of integrated and personalized care is necessary to meet the needs of persons living with Parkinson’s disease. In other chronic diseases than Parkinson’s disease, care management models have been deployed to deliver integrated and personalized care, yielding positive effects on patients’ health outcomes, quality of life and health care utilization. However, care management models have been highly heterogeneous, as there is currently no clear operationalization of its core elements. In addition, most care management models are disease-specific and not tailored to the individual needs and preferences of a patient. In this viewpoint we present an integrated and personalized care management model for persons with Parkinson’s disease costing of five core elements: (1) care coordination, (2) patient navigation, (3) information provision, (4) early detection of signs and symptoms through proactive monitoring and (5) process monitoring. Following the description of each core element, implications for implementing the model into practice are discussed. Finally, we provide clinical and methodological considerations on the evaluation of care management models.
INTRODUCTION
Parkinson’s disease (PD) is the fastest growing neurodegenerative disorder, affecting more than eight million people worldwide [1]. It is characterized by a variable combination of motor and non-motor symptoms that jointly affect peoples’ quality of life and everyday functioning. Unfortunately, our current health care systems are not well equipped to deal with the complex and highly individualized needs of persons living with PD. First, given the multifaceted nature of PD, care for affected individuals ideally involves a seamless interdisciplinary collaboration between health care providers from a wide range of different professional disciplines, all of whom can potentially add value to the care management team [2]. In reality, however, care is often monodisciplinary (usually just a medical specialist or family physician). And even when multiple providers are involved, interdisciplinary collaboration and even communication is typically insufficient. Second, there is often a lack of continuity of care. For example, recommendations made by hospital-based teams are followed up poorly in the community. Third, many health-related problems are managed reactively, instead of taking a proactive approach, aiming to address health issues early on before going awry – causing avoidable disability for patients and unnecessary costs for society. Finally, care remains largely physician-centered, patients and their families not being involved adequately in the decision making process [2, 3]. Taken together, these limitations emphasize the need for an improved care model for persons with PD.
The chronic care model (CCM), which was originally developed to improve quality of care for persons with various other chronic health conditions [4], could serve as the basis for a novel care model that is tailored to the personal needs of persons with PD. The CCM emphasizes the necessity of supportive and productive interactions between patients, their families, and all members of the patient’s health care team [5]. Important elements of the CCM are self-management support, integrated decision support, proactive care delivery, mobilization and use of available community resources, and use of patient registries and other supportive information systems [4, 6]. Bolstered by these complementary elements, the CCM embodies a model of care known as case or care management, which is a collaborative and proactive approach to interdisciplinary care that ascertains links between specialists and generalists for, and in close collaboration with, persons living with a disease and their carers [7]. In previous publications, the terms ‘care’ and ‘case’ management have been used more or less interchangeably. We strongly prefer to use the term care management, for several reasons. First, someone living with a chronic disease is much more than a ‘case’; he or she is an individual with a unique profile and specific personal needs and wishes, surrounded by a complex environment. Second, the term ‘case management’ inadvertently places the patient in a more passive role as a care receiver. In contrast, the term care management refers to the management of the entire care process, in which the patient acts as an active partner in disease management through shared decision making, and with recognition of each patient’s ability and desire for self-management.
Previous research on care management in chronic health populations other than PD revealed its potential in improving patient outcomes [8, 9], quality of life [10], patient satisfaction [11], and also in preventing complications that also common occur in PD patients, including reduction in feelings of anxiety [12, 13] and depressive symptoms [13, 14]. Research has demonstrated that implementation of care management models is successful in guiding quality improvement and in providing effective and high quality of chronic care [8– 16]. Until now, only two studies examined the effect of care management on patient outcomes in a PD population [17, 18]. The study results of a nurse-led chronic care management intervention among veterans with PD showed an improved adherence to PD care quality indicators [17]. This finding may have meaningful impact for clinical practice: the increased adherence to PD quality care indicators suggest that the care management intervention might improve the quality of the care process. In addition, of the eight secondary outcomes measures, the screen for depressive symptoms was better in the intervention group compared to usual care group. Considering the fact that mood, including depression, impact quality of life of persons with PD, this can indeed be considered as an important outcome. However, the generalizability of these study findings is limited as recruited population is restricted to male veterans located in specific geographical areas. A recent randomized controlled trial (RCT) [18] evaluated an intervention that consisted of individualized therapeutic plans and care adaptations as needed, delivered during home visits by a specialized Parkinson nurse who essentially acted as an individual care manager. The intervention patients also received care as usual, which essentially consisted of hospital-based consultations with a neurologist. The control group only received care as usual. The results showed that the people with PD allocated to the intervention arm experienced an improvement in quality of live scores as well as of motor and non-motor symptoms. However, it remains unclear which specific components of this patient-centered integrated healthcare approach were responsible for the observed impact on individual patient outcomes. Another challenge was the fact that many otherwise eligible patients could not receive the home visits because of long travel distances, emphasizing the importance of alternatives via telemedicine approaches [19, 20].
Although these results are promising, the huge heterogeneity in care management models make it difficult to establish which elements– and in which frequency and intensity– are responsible for the success of care management. Although there is consensus on which core elements constitute care management [21], most research lacked a concrete operationalization of these core elements [22, 23]. To achieve this, a clear description of the content, form and intensity of each element is necessary. Furthermore, all current care management models were tailored around a disease, instead of around the person living with the disease. Such a personalized approach is especially important for elderly patients who typically have often more than one chronic health condition [24]– emphasizing the need to look after the person, instead of a single disease. Moreover, the heterogeneity of motor and non-motor symptoms across different persons with PD highlights the need for a highly personalized care approach [17].
A previous study revealed that persons with PD desire integrated care management, including better interdisciplinary collaboration between health care providers and, at the same time, to be involved as active agents in managing their own health [25]. It follows that a balance is needed between guiding and directing persons with PD through their journey, while leaving enough room for self-management. Furthermore, persons with PD indicated that one of the top priorities to improve PD care was to have a single point of access, i.e., a single health care provider acting as a personal care manager, who could either answer simple questions directly, or else navigate the patient towards other professionals who are better suited to address the specific issue at hand, thus ascertaining integration as well as continuity of care across disciplines and across different work places [25]. It is questionable, however, whether a single person can achieve this much desired integration, realizing that PD care is– at least in a desired optimal scenario– delivered by multiple health care providers who operate in very different settings. In that regard, it is presumably more desirable that the entire health care team assumes a responsibility in delivering integrated and personalized care management.
TOWARDS PERSONALIZED CARE MANAGEMENT
To optimize care for persons with PD, we envisage a personalized care management model that is tailored to each individual’s needs and preferences, with the following core elements: (1) care coordination, (2) patient navigation, (3) information provision, (4) early detection of signs and symptoms through proactive monitoring, and (5) process monitoring (Fig. 1). In this paper, we present our view on the design of a personalized care management model for people with PD. We first describe each of these five core elements, followed by a discussion on the practical as well as clinical and methodological considerations of the personalized care management model. Table 1 provides a summary, including the definition, key points, brief examples and quality improvement indicators for each of the five components of the personalized care management model.
Fig.1
The five core elements of personalized care management for persons with PD.
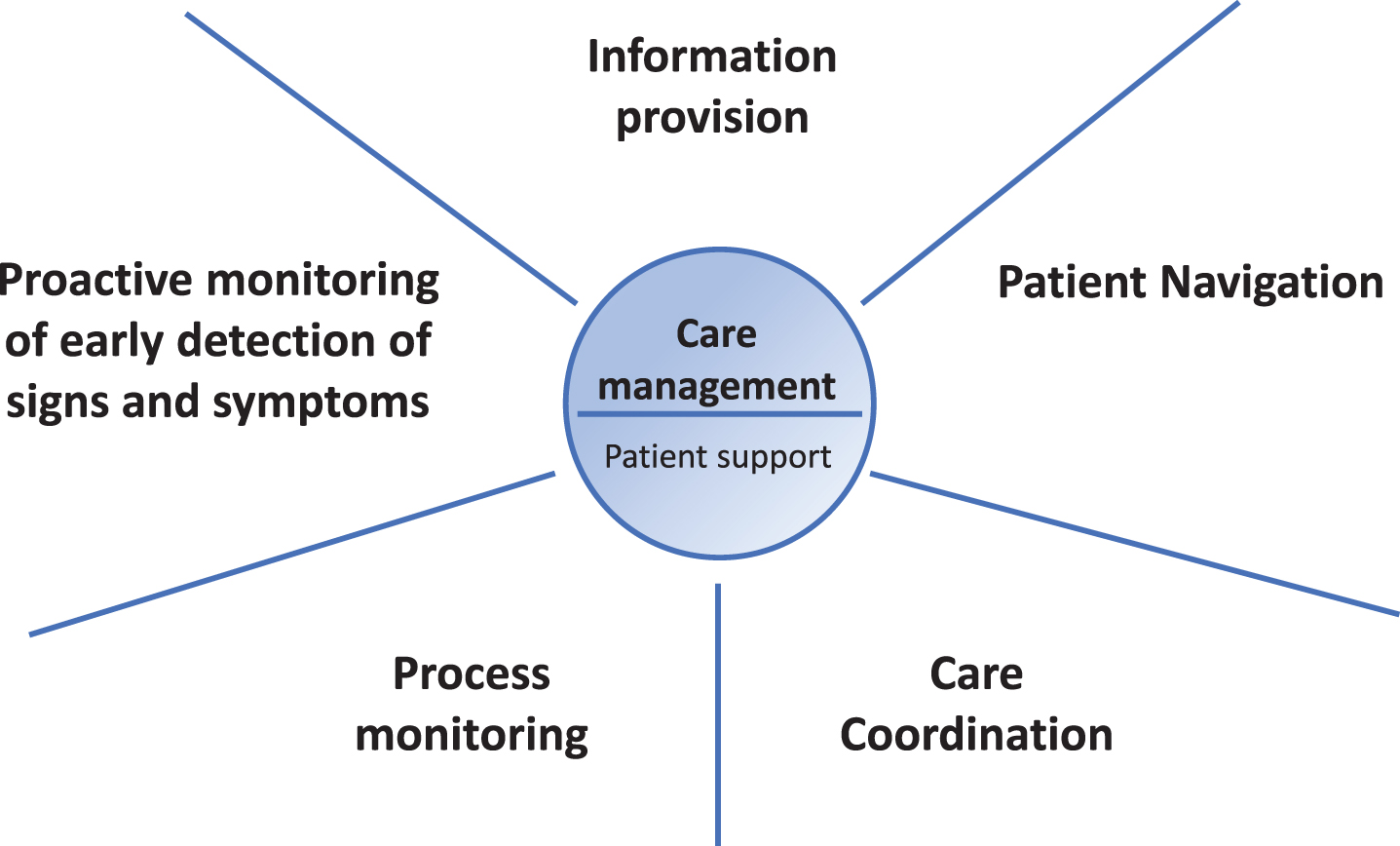
Table 1
Components of a personalized care management model for people with Parkinson’s disease
| COMPONENT | DEFINITION | KEY POINTS | EXAMPLE | QUALITY IMPROVEMENT INDICATORS INDINDICATORS |
| Care coordination | Team-based activity of involved health care providers to ensure sharing of relevant health information across all healthcare layers, creation of a common understanding of care needs of each patient, alignment of treatment plans to prevent contradictory disease management, and assurance of certainty about responsibilities of each discipline in the management process. | •Development of individualized care plans covering individuals’ unique needs and preferences •Sharing of the individualized care plans among all involved health care providers | Use of individualized care plans in people with gastrointestinal cancer led to significant improvement in patient reported quality of life outcomes, a significant decrease in feelings of anxiety, fewer depressive symptoms, and a reported higher satisfaction compared to the usual care group [29]. | •Improved patients and carers care experience and satisfaction with received care •Lower caregiver burden |
| Patient navigation | Proactively guidance and support for patients to find their way through the complex health care system, referring them timely to the appropriate health care provider. | •Mapping of care team network of each patient •Building relationships between primary, secondary and tertiary care, and community resources | Patient navigation programs in cancer care have revealed improvement in continuity of care through timely receipt of disease treatment and follow-up care [52] | •People with PD and their carers report to receive the right care at the right place and time. |
| Information provision | Providing PD-related information in oral, written or other form. | •Establishment of an information delivery system •Availability of a single point of access | Education programme in combination with home visits and tele-consulting showed that cancer patients benefit from websites offering information on disease management [12]. The results supported earlier findings that education increases acceptance of disease, symptom control and also improves the quality of life. | •Level of shared decision making in disease treatment and care •Improved self-management skills•Patients are more ‘in control. |
| Proactive monitoring for early detection of signs and symptoms | The timely detection of the first changes in signs or symptoms, allowing for preemptive interventions to prevent further worsening of problems and to avoid complications that might lead to emergency department visits, hospital admission and use of unnecessary resources. | •Monitoring adherence to treatment plans •Supported by home-based monitoring, i.e., wearable motion sensors | Proactive monitoring of falls with wearable sensors in people with PD allow identification of patients with a high risk of falling, which In turn, allows for timely referral to fall prevention programs, which impacts activity of daily living [53]. | •Less emergency department admissions •Less unneeded hospital admissions •Lower prevalence of preventable motor and non-motor complications •Improved functional status •Improved Work status •Treatment according to pre-defined care goals |
| Process monitoring | Routine review and evaluation of the care management process regarding adherence to care plans. | •Recognizing facilitators and barriers in the care management process •Evaluation that all involved health care providers work on the same pre-defined care goals. | Telemedicine based disease management programs including monitoring of adherence to care plans, improved health outcomes in veterans with poor diabetes by improving diabetes self-care [54]. |
Care coordination
The first element is care coordination, which we operationally define as a team-based activity of involved health care providers to ensure sharing of relevant health information across all healthcare layers, creation of a common understanding of care needs of each patient, alignment of treatment plans to prevent contradictory disease management, and assurance of certainty about responsibilities of each discipline in the management process. Care coordination is key to ensure continuity of care and to deliver integrated high-quality care. Poor care coordination is associated with lower quality of care, inadequate treatment of symptoms, unnecessary use of resources, worse health outcomes and higher healthcare costs [26, 27]. It often results in a duplication of care services and lack of delivery of needed health care services, including preventive care. A study on the effect of a cancer care coordination approach found that patients in the intervention group had fewer clinical visits, unexpected hospitalizations and bed days of care for preventable hospital admission than the control group [28]. Research revealed that sharing multidisciplinary expertise is key for delivering integrated care [18]. The aim of team-based care coordination is thus to ensure that persons with PD receive an integrated care package.
In addition, care coordination also involves collaboration with the patient, aiming to design an individualized multidisciplinary care plan that covers the unique needs, preferences and goals of each patient. Such care plans are already frequently integrated in the care for persons with other chronic diseases, including cancer [29, 30] and diabetes [31]. This care plan considers the individual circumstances and specific patient characteristics. For example, around 5% of patients are diagnosed before the age of 50 [32, 33], and the primary concerns of these young individuals might well relate to working capacity and fear of job loss. Their care plan should then primarily aim to support work abilities for as long as possible, considering each patient’s self-management skills and support needs to reach this goal. So rather than focusing on suppressing symptoms or signs (e.g., reducing tremor ratings during clinic-based assessments), the goals shift towards to functionally relevant targets (e.g., how does tremor interfere with this individual’s ability to perform his or her job?).
Developing such an individualized care plan should follow a cyclic process, starting with the formulation of a personal mission statements that entails the unique needs and preferences from the patient perspective, followed by formulating a multidisciplinary therapy advice which is based on the personal mission statement. The implementation of the individual care plan forms the second step and finally, the care management team monitors the implementation and evaluates if the goals have been achieved and if the individual care plan needs to be adjusted.
Patient navigation
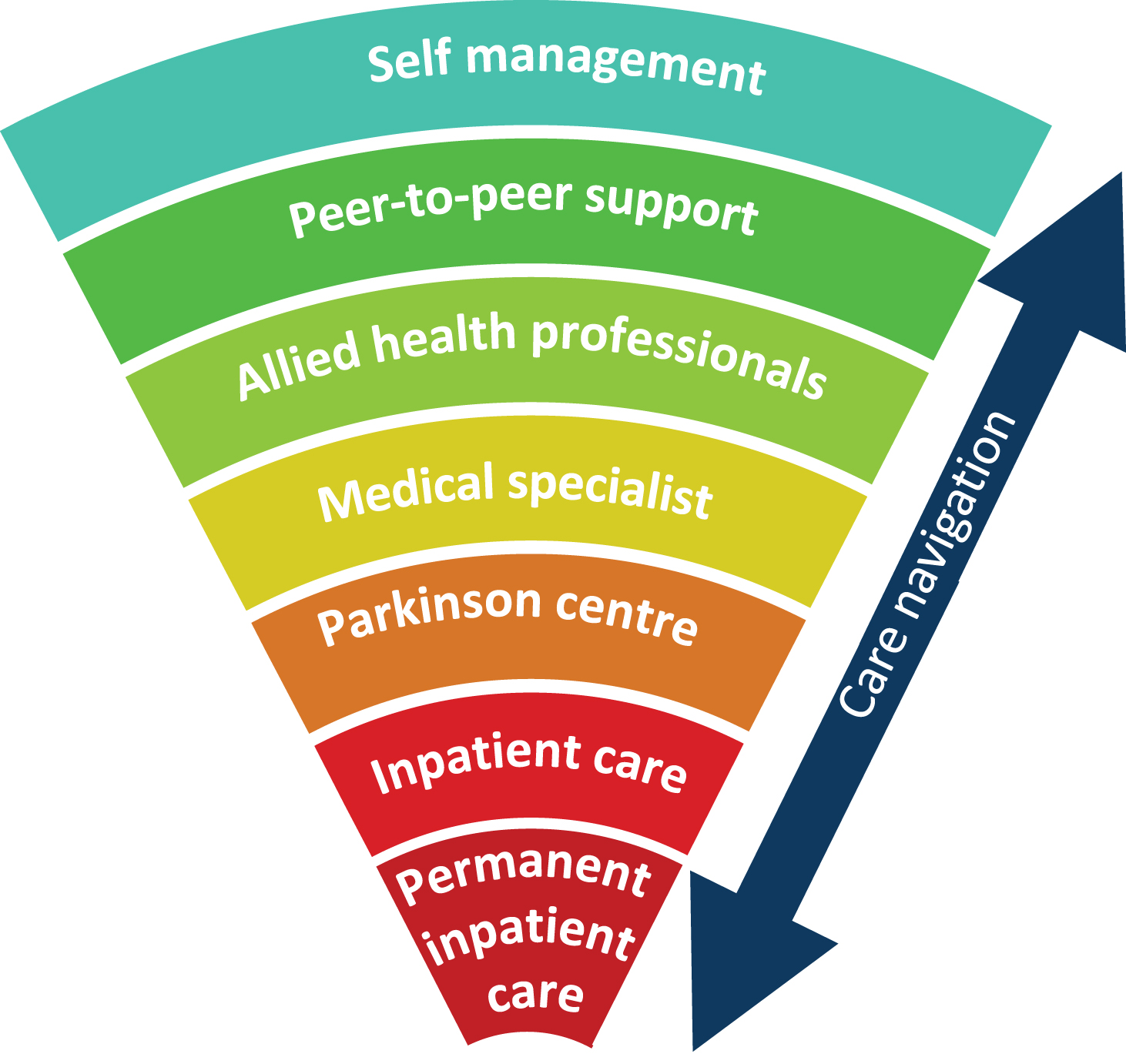
The second element is patient navigation which we define as an integrated service delivery to proactively guide and support patients through the complex health care system, referring them timely to the appropriate health care provider [34]. Fragmentation of care is a key factor limiting the efficacy and quality of healthcare delivery [35]. Research with claims data of commercial insurance company revealed that fragmentation is indeed associated with poorer quality of care, higher healthcare costs and more preventable hospitalizations among chronically ill people [36]. Examples are abundant: the hospital-based neurologist identifies a need for occupational therapy, but this is never picked up by the community team. Or a physiotherapist who witnesses frequent off-state periods at home, but this not followed up by the neurologist with tailored medication adjustments. In addition, care fragmentation puts a great burden on patients and carers, leaving them with the responsibility to navigate through the healthcare system. Consequently, treatment plans are poorly implemented. The main aim of patient navigation is to offer patients seamless care by integrating primary, secondary and tertiary care, as well as community resources (Fig. 2). Important within this context is the cooperation between patient navigators, the primary care physician or nursing home specialists, and community workers. It follows that building productive relationships with patients and their carers, health care providers and community service providers forms the basis for patient navigation. Mapping the care team and network of the patient is a key tool for effective patient navigation. Next to the care management team of a patient, patient navigation might also entail information on networks of allied health care providers specifically trained in PD according to evidence-based guidelines, such as those provided by the Dutch ParkinsonNet approach [37].
Fig.2
Connecting all different layers of health care to ensure patient navigation.
Information provision
Information provision is the third element of our personalized care management model and relates to all PD-related information in oral, written or other forms. Receiving information and education about treatments and disease management is a top priority among persons with PD [25]. Being adequately informed is essential to empower patient in terms of self-management and active involvement in decision-making. By contrast, being underinformed is equated with lower quality of care, greater safety risks and poorer health outcomes. Furthermore, scattered, incomplete or conflicting information keeps patients from understanding their disease and the accompanying treatment choices, thereby increasing uncertainty. An information delivery system containing reliable and validated information is therefore necessary. In doing so, it is essential to examine the actual information needs of each patient; some might want to know everything, whereas others feel overwhelmed or even anxious when receiving too much information [38, 39]. Similarly, newly diagnosed patients will likely not be interested in advanced care planning, whereas wheelchair-bound nursing home residents may not want to hear about the importance of daily aerobic exercise.
The need for information can be addressed in various ways. When available, relevant information can be extended directly by a personal care manager who acts as the patient’s single point of access (for example a Parkinson’s nurse [18]). The advantage here is that this personal care manager also knows the specific context of the patient, such as current medication use or prior history. A more scalable alternative is offered by online central helplines, such as the ones provided by various patient associations, e.g., in the US or the UK. A disadvantage here is that the online support employees have no insight in recent medical files and have no formal treatment relation with the patient, so only generic advice can be given. Development of standardized protocols for common medical issues can help those answering the call, and act as a guide in the triage process, as some enquiries may necessitate a subsequent referral to an appropriate health care provider. A website or printed brochure with information is even more scalable, but this is also the least personally customized solution. It follows that general questions and requests can be answered by an online helpline team, a website or a brochure, whereas personal issues requiring medical action must be directed to the appropriate health care professional.
All information streams depicted above will benefit from the availability of a rich knowledge base system that contains reliable information about PD and management options, as well as information and advice from different professional disciplines. This knowledge base should also include information about self-management skills and social and community resources for support.
Early detection of signs and symptoms through proactive monitoring
The fourth element concerns the early detection of signs and symptoms through proactive monitoring. We define proactive monitoring as the timely detection of the first changes in signs or symptoms, allowing for preemptive interventions to prevent further worsening of problems and to avoid complications that might lead to emergency department visits, hospital admission and use of unnecessary resources. Estimates of the prevalence of disease-related and treatment-related complications [40] reveal that falls due to postural instability and other axial features were the most common reported complications among people with PD (64%), having a significant impact on daily functioning and quality of life.
Theoretically, the early detection process would be enabled by continuous home-based monitoring by patients themselves, either passively in the background (e.g., using wearable motion sensors to detect falls or changes in physical activity) or actively (e.g., using digital diaries that patients or carers should complete periodically). However, such an approach requires a careful individual assessment of each patient’s wishes and needs, because self-monitoring can be time-consuming (particularly self-report) and might increase feelings of anxiety or uncertainty among those desiring little information. In addition, changes in mental state such as feelings of anxiety and depressive symptoms affect approximately one fifth of all patients with PD [40]. Also, proactive monitoring for signs and symptoms of anxiety and depression will help to prevent increase in severity and timely referral to a psychologist.
Process monitoring
The final element is process monitoring which we define as the routine review and evaluation of the care management process regarding adherence to care plans. Monitoring the personalized care management process is important to ascertain the delivery of high quality, effective and efficient care. Monitoring also helps to identify possible barriers and facilitators in the management process. This process involves observing the patient’s adherence to treatment plans and evaluating whether professionals are working on the appropriate pre-specified care goals.
PRACTICAL CONSIDERATIONS
Personalized care management is a true team effort, where every involved health professional delivers care in line with the presented framework of personalized care management. However, we also emphasize that certain providers might assume a more active role than others. A currently formulated set of recommendations for the organization of multidisciplinary care in PD suggests that every patient should have a core care management team, which can consist of a dietician, movement disorder neurologist, Parkinson’s nurse, occupational therapist, physiotherapist, psychiatrist or (neuro)psychologist, speech and language therapist, and social worker [41]. All members of this core team are important for the treatment of PD, but we acknowledge that their contribution can vary based on disease stage and patients’ current needs and preferences. Next to these core members, the primary care physician plays a crucial role in making referrals to medical specialists [41] and managing health issues that are not directly related to PD, such as hypertension, diabetes or high cholesterol. In practice, there will be considerable overlap between PD-specific treatments and the care provided by the primary care physician; it is therefore important to integrate specialist expertise with primary care to ensure the delivery of continuous and integrated care management.
Another consideration relates to the possibilities offered by technology. For example, telemedicine using video conferencing allows care to be delivered in the patient’s home environment, offering a perspective of how PD affects the patient’s actual functioning [20, 42, 43]. Moving care back into the patients’ homes is a widely recognized need for improving care [43]. Remarkably, studies consistently found that care delivered through telecommunication systems had outcomes comparable to those of face-to-face evaluations [44, 45]. Telenursing, as part of telemedicine, has the potential to deliver many of the integrated care services discussed here: to pragmatically answer simple questions remotely, to share information and to initiate timely referrals. Although telemedicine cannot replace in-person contact completely, it may strengthen the relationship between clinicians and patients, and improve patients daily functioning, health outcomes and quality of life, without increasing social and healthcare costs [46]. The present outbreak of the corona disease 2019 (COVID-19) has forced us to rethink the way we deliver care and has accelerated the use of telemedicine for remote monitoring of patients [20, 47, 48]. Telemedicine has indeed become a key tool in the current time of crisis for ensuring continuity of care, especially among people who are at a high risk of complications, including people with a chronic disease such as PD. Remote monitoring technologies using wearable sensors, for example, allow clinicians to monitor the natural activities of people with mobility impairments, including those living with PD, in their own living environment, which is now more important than ever before because the risk of contagion has markedly limited the access to in-person assessments. It is well known that increased levels of stress as well as a decrease in physical activity— both consequences of the present COVID-19 crisis— can worsen various motor symptoms, including dyskinesias, freezing of gait and tremor, and also non-motor symptoms such as insomnia [49]. Telemedicine can play a crucial role here (using either web- or telephone-based solutions) by allowing people with PD to continue with remote physical exercise classes or to join mindfulness-based interventions through telemedicine, which can reduce feelings of anxiety and depressive symptoms [49].
Also interestingly, thanks to technological advances, it is foreseeable that a virtual care manager system, based on standardized protocols and enhanced by artificial intelligence, can soon replace some tasks and responsibilities of human personnel, e.g., by answering common questions and by providing information tailored to the issues raised.
Another important point refers to the implementation of such a personalized care management model. In a first step, the locally relevant unmet needs and preference of people with PD, their carers and health care providers should be analyzed, resulting in a shared understanding of what needs to be improved. In a next step, a prioritization of the desired changes takes place, together with an identification of the population that would benefit most from certain interventions. Accordingly, a vision statement is shared in which the goals, instruments for measuring (changes in) outcomes over time and the time frame for implementation of the interventions are listed. To ensure sustainability, evaluation of both the process and its health outcomes are assessed on a regular basis, to see if the desired goals are met, and to analyze what is needed to reach the aims.
CLINICAL AND METHODOLOGICAL CONSIDERATIONS WHEN EVALUATING CARE MANAGEMENT MODELS
Implementing the model described here should be an iterative process, capitalizing on new insights from regular evaluations.
For the evaluation of such care management models several considerations should be taken into account. First, the focus of previous studies on population health outcomes (e.g., quality of life) and cost savings as the only criteria for measuring the effectiveness of care management models was too limited. It is necessary to also evaluate the two other domains that jointly constitute the quadruple aim [50]: patient (and carer) experience of care; and healthcare professional experience. Only studies which consider all four domains, both qualitatively and quantitatively, allow for a thorough evaluation of the effectiveness of a care management model.
Second, evaluating what is done and how it is done (i.e., process evaluation through a ‘quality improvement approach’) is essential to understand what drives the efficacy of any care management model. Furthermore, a quality improvement approach can be used to adapt care management interventions on the basis of new insights. Future studies should thereby focus on barriers and facilitators for implementing care management in the long-term.
Third, patient and public involvement has become a cornerstone for improving quality of care. As acknowledged earlier in this viewpoint, patients are not passive receivers of care, but are integral to the design and implementation process of the care management model. Incorporating patients’ first-hand experiences throughout the implementation cycle and evaluation processes is essential in this regard. Before implementation, it is vital to assess which patients’ needs and preferences are likely to benefit from the intervention. During implementation, patients play a key role in identifying and prioritizing the potential problems related to the nature of the intervention and in searching for possible solutions. When analyzing the results of an intervention, patients provide key insight on the implications for their (previously) unmet needs and preferences.
Fourth, the design of a study which evaluates the effects of a care management approach is affected by the nature of the intervention. Ideally, each care management intervention should be evaluated in an RCT to minimize potential influences of confounding and selection bias. Recently, methodological adaptations to RCTs have been developed which account for quality improvement throughout an intervention period, such as a stepped wedge cluster RCT, which involves random and sequential crossover of clusters from control to intervention until all clusters are exposed [51]. If a care management intervention strongly relies on regional collaboration, a clustered RCT can only be performed if all healthcare providers of patients work within a single, well-defined region. Since there will be overlap across regions in most settings, prospective observational studies with meticulous assessment of potential sources of confounding and selection bias are a valid alternative design to evaluate the effects of care management interventions. In such studies, a specific method to quantify possible selection bias that may arise because of differences in recruitment between regions is to perform baseline assessments of patients’ expectations of the quality of care in their region throughout the intervention period.
CONFLICT OF INTEREST
Prof. Bastiaan Bloem currently serves as Editor in Chief for the Journal of Parkinson’s disease, serves on the editorial board of Practical Neurology and Digital Biomarkers, has received honoraria from serving on the scientific advisory board for Abbvie, Biogen and UCB, has received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare and Bial, and has received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, UCB, Abbvie, the Stichting Parkinson Fonds, the Hersenstichting Nederland, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, the Gatsby Foundation and the Parkinson Vereniging.
Dr. Sirwan Darweesh was supported in part by a Parkinson’s Foundation- Postdoctoral Fellowship (PF-FBS-2026).
ACKNOWLEDGMENTS
We appreciate Marlies van Bemmel, Jacqueline Deenen, Martha Huvenaars, Dr. Agnes Smink and Fred Wolters for their support in providing insights and expertise that were very valuable and greatly contributed to this manuscript. In addition, we thank Gijs Haerkens for reviewing the manuscript which clearly improved its quality.
The Centre of Expertise for Parkinson & Movement Disorders was supported by a centre of excellence grant by the Parkinson Foundation. This research is part of the PRIME Parkinson project. The collaboration project is financed by the Gatsby Foundation and co-funded by the PPP Allowance made available by Health∼Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships.
REFERENCES
[1] | Dorsey ER , Sherer T , Okun MS , Bloem BR ((2018) ) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8: , S3–S8. |
[2] | van der Eijk M , Faber MJ , Al Shamma S , Munneke M , Bloem BR ((2011) ) Moving towards patient-centered healthcare for patients with Parkinson’s disease. Parkinsonism Relat Disord 17: , 360–364. |
[3] | Tension E , Smink A , Redwood S , Darweesh SKL , Cottle H , van Halteren AD , van den Haak P , Hamlin R , Ypinga J , Bloem BR , Ben-Shlomo Y , Munneke M , Henderson EJ ((2020) ) Proactive and integrated management and empowerment in Parkinson’s disease: Designing a new model of care. Parkinsons Dis 2020: , 8673087. |
[4] | Wagner EH , Austin BT , Davis C , Hindmarsh M , Schaefer J , Bonomi A ((2001) ) Improving chronic illness care: Translating evidence into action. Health Affairs 20: , 64–78. |
[5] | Bodenheimer T , Wagner EH , Grumbach K ((2002) ) Improving primary care for patients with chronic illness: The chronic care model, Part 2. JAMA 288: , 1909–1914. |
[6] | Coleman K , Austin BT , Brach C , Wagner EH ((2009) ) Evidence on the chronic care model in the new millennium. Health Aff (Millwood) 28: , 75–85. |
[7] | ((2007) ) Care Management Definition and Framework. https://www.chcs.org/resource/care-management-definition-and-framework/ |
[8] | Davy C , Bleasel J , Liu H , Tchan M , Ponniah S , Brown A ((2015) ) Effectiveness of chronic care models: Opportunities for improving healthcare practice and health outcomes: A systematic review. BMC Health Serv Res 15: , 194. |
[9] | Joo JY , Liu MF ((2019) ) Case management effectiveness for managing chronic illnesses in Korea: A systematic review. Int Nurs Rev 66: , 30–42. |
[10] | Joo JY , Liu MF ((2019) ) Effectiveness of nurse-led case management in cancer care: Systematic review. Clin Nurs Res 28: , 968–991. |
[11] | Levine S , Steinman BA , Attaway K , Jung T , Enguidanos S ((2012) ) Home care program for patients at high risk of hospitalization.e. Am J Manag Care 18: , 269–276. |
[12] | Avci IA , Altay B , Cavusoglu F , Cal A , Mumcu N , Eren DC , Oz O , Altin A , Karaoglanoglu O , Buberci A ((2020) ) Evaluation of the efficacy of the three-component health care management program HEWCOT in colorectal cancer patients receiving chemotherapy. J Cancer Educ 35: , 274–283. |
[13] | Mertz BG , Dunn-Henriksen AK , Kroman N , Johansen C , Andersen KG , Andersson M , Mathiesen UB , Vibe-Petersen J , Dalton SO , Envold Bidstrup P ((2017) ) The effects of individually tailored nurse navigation for patients with newly diagnosed breast cancer: A randomized pilot study. Acta Oncol 56: , 1682–1689. |
[14] | Chen Y , Funk M , Wen J , Tang X , He G , Liu H ((2018) ) Effectiveness of a multidisciplinary disease management program on outcomes in patients with heart failure in China: A randomized controlled single center study. Heart Lung 47: , 24–31. |
[15] | Minkman M , Ahaus K , Huijsman R ((2007) ) Performance improvement based on integrated quality management models: What evidence do we have? A systematic literature review. Int J Qual Health Care 19: , 90–104. |
[16] | Nutting PA , Dickinson WP , Dickinson LM , Nelson CC , King DK , Crabtree BF , Glasgow RE ((2007) ) Use of chronic care model elements is associated with higher-quality care for diabetes. Ann Fam Med 5: , 14–20. |
[17] | Connor KI , Cheng EM , Barry F , Siebens HC , Lee ML , Ganz DA , Mittman BS , Connor MK , Edwards LK , McGowan MG , Vickrey BG ((2019) ) Randomized trial of care management to improve Parkinson disease care quality. Neurology 92: , e1831–e1842. |
[18] | Eggers C , Dano R , Schill J , Fink GR , Hellmich M , Timmermann L ((2018) ) Patient-centered integrated healthcare improves quality of life in Parkinson’s disease patients: A randomized controlled trial. J Neurol 265: , 764–773. |
[19] | Beck CA , Beran DB , Biglan KM , Boyd CM , Dorsey ER , Schmidt PN , Simone R , Willis AW , Galifianakis NB , Katz M , Tanner CM , Dodenhoff K , Aldred J , Carter J , Fraser A , Jimenez-Shahed J , Hunter C , Spindler M , Reichwein S , Mari Z , Dunlop B , Morgan JC , McLane D , Hickey P , Gauger L , Richard IH , Mejia NI , Bwala G , Nance M , Shih LC , Singer C , Vargas-Parra S , Zadikoff C , Okon N , Feigin A , Ayan J , Vaughan C , Pahwa R , Dhall R , Hassan A , DeMello S , Riggare SS , Wicks P , Achey MA , Elson MJ , Goldenthal S , Keenan HT , Korn R , Schwarz H , Sharma S , Stevenson EA , Zhu W ((2017) ) National randomized controlled trial of virtual house calls for Parkinson disease. Neurology 89: , 1152–1161. |
[20] | Bloem BR , Dorsey ER , Okun MS ((2020) ) The coronavirus disease 2019 crisis as catalyst for telemedicine for chronic neurological disorders. JAMA Neurol, doi: 10.1001/jamaneurol.2020.1452. |
[21] | Drennan V , Goodman C ((2004) ) Nurse-led case management for older people with long-term conditions. Br J Community Nurs 9: , 527–533. |
[22] | Lukersmith S , Millington M , Salvador-Carulla L ((2016) ) What is case management? A scoping and mapping review. Int J Integr Care 16: , 2. |
[23] | Schaefer J , Davis C ((2004) ) Case management and the chronic care model: A multidisciplinary role. Lippincotts Case Manag 9: , 96–103. |
[24] | Fortin M , Bravo G , Hudon C , Vanasse A , Lapointe L ((2005) ) Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med 3: , 223–228. |
[25] | Vlaanderen FP , Rompen L , Munneke M , Stoffer M , Bloem BR , Faber MJ ((2019) ) The voice of the Parkinson customer. J Parkinsons Dis 9: , 197–201. |
[26] | O’Malley AS ((2011) ) Tapping the unmet potential of health information technology. N Engl J Med 364: , 1090–1091. |
[27] | Moore JMC , Dolansky M , Hudak C , Kenneley I ((2015) ) Care coordination between convenient care clinics and healthcare homes. J Am Assoc Nurse Pract 27: , 262–269. |
[28] | Chumbler NR , Kobb R , Harris L , Richardson LC , Darkins A , Sberna M , Dixit N , Ryan P , Donaldson M , Kreps GL ((2007) ) Healthcare utilization among veterans undergoing chemotherapy: The impact of a cancer care coordination/home-telehealth program. J Ambul Care Manage 30: , 308–317. |
[29] | Hird AE , Lemke M , Turovsky M , Malecki V , Kumar K , DeAngelis C , Chow E , Ko YJ ((2015) ) Doctor, what are my options? A prospective cohort study of an individualized care plan for patients with gastrointestinal cancer. Curr Oncol 22: , e171–177. |
[30] | Ell K , Xie B , Quon B , Quinn DI , Dwight-Johnson M , Lee PJ ((2008) ) Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol 26: , 4488–4496. |
[31] | Lion KC , Mangione-Smith R , Britto MT ((2014) ) Individualized plans of care to improve outcomes among children and adults with chronic illness: A systematic review. Care Manag J 15: , 11–25. |
[32] | Koerts J , Konig M , Tucha L , Tucha O ((2016) ) Working capacity of patients with Parkinson’s disease - A systematic review. Parkinsonism Relat Disord 27: , 9–24. |
[33] | Wickremaratchi MM , Perera D , O’Loghlen C , Sastry D , Morgan E , Jones A , Edwards P , Robertson NP , Butler C , Morris HR , Ben-Shlomo Y ((2009) ) Prevalence and age of onset of Parkinson’s disease in Cardiff: A community based cross sectional study and meta-analysis. J Neurol Neurosurg Psychiatry 80: , 805–807. |
[34] | Freeman HP , Rodriguez RL ((2011) ) History and principles of patient navigation. Cancer 117: , 3539–3542. |
[35] | van der Eijk M , Nijhuis FA , Faber MJ , Bloem BR ((2013) ) Moving from physician-centered care towards patient-centered care for Parkinson’s disease patients. Parkinsonism Relat Disord 19: , 923–927. |
[36] | Frandsen BR , Joynt KE , Rebitzer JB , Jha AK ((2015) ) Care fragmentation, quality, and costs among chronically ill patients. Am J Manag Care 21: , 355–362. |
[37] | Nijkrake MJ , Keus SHJ , Overeem S , Oostendorp RAB , Vlieland TPMV , Mulleners W , Hoogerwaard EM , Bloem BR , Munneke M ((2010) ) The ParkinsonNet Concept: Development, implementation and initial experience. Mov Disord 25: , 823–829. |
[38] | Leydon GM , Boulton M , Moynihan C , Jones A , Mossman J , Boudioni M , McPherson K ((2000) ) Cancer patients’ information needs and information seeking behaviour: In depth interview study. BMJ 320: , 909–913. |
[39] | Bawden D , Robinson L ((2009) ) The dark side of information: Overload, anxiety and other paradoxes and pathologies. J Inf Sci 35: , 180–191. |
[40] | Schrag A , Ben-Shlomo Y , Quinn N ((2002) ) How common are complications of Parkinson’s disease? J Neurol 249: , 419–423. |
[41] | Radder DLM , Nonnekes J , van Nijmegen M , Eggers C , Abbruzzese G , Alves G , Browner N , Chaudhuri KR , Ebersbach G , Ferreira JJ , Fleisher JE , Fletcher P , Frazzitta G , Giladi N , Guttman M , Iansek R , Khandhar S , Klucken J , Lafontaine A , Marras C , Nutt J , Okun MS , Parashos SA , Munneke M , Bloem BR ((2020) ) Recommendations for the organisation of multidisciplinarz clinical care teams in Parkinson’s disease. J Parkinsons Dis 10: , 1087–1098. |
[42] | Bloem BR , Henderson EJ , Dorsey ER , Okun MS , Okubadejo N , Chan P , Adrejack J , Darweesh SKL , Munneke M ((2020) ) Integrated and patient-centred management of Parkinson’s disease: A network model for reshaping chronic neurological care. Lancet Neurol 19: , 623–634. |
[43] | Dorsey ER , Vlaanderen FP , Engelen LJ , Kieburtz K , Zhu W , Biglan KM , Faber MJ , Bloem BR ((2016) ) Moving Parkinson care to the home. Mov Disord 31: , 1258–1262. |
[44] | Wilkinson JR , Spindler M , Wood SM , Marcus SC , Weintraub D , Morley JF , Stineman MG , Duda JE ((2016) ) High patient satisfaction with telehealth in Parkinson disease: A randomized controlled study. Neurol Clin Pract 6: , 241–251. |
[45] | Sekimoto S , Oyama G , Hatano T , Sasaki F , Nakamura R , Jo T , Shimo Y , Hattori N ((2019) ) A randomized crossover pilot study of telemedicine delivered via iPads in Parkinson’s disease. Parkinsons Dis 2019: , 9403295. |
[46] | Kotsani K , Antonopoulou V , Kountouri A , Grammatiki M , Rapti E , Karras S , Trakatelli C , Tsaklis P , Kazakos K , Kotsa K ((2018) ) The role of telenursing in the management of Diabetes Type 1: A randomized controlled trial. Int J Nurs Stud 80: , 29–35. |
[47] | Guidon AC , Amato AA ((2020) ) COVID-19 and neuromuscular disorders. Neurology 94: , 959–969. |
[48] | Cilia R , Mancini F , Bloem BR , Eleopra R ((2020) ) Telemedicine for parkinsonism: A two-step model based on the COVID-19 experience in Milan, Italy. Parkinsonism Relat Disord 75: , 130–132. |
[49] | Helmich RC , Bloem BR ((2020) ) The impact of the COVID-19 pandemic on Parkinson’s disease: Hidden sorrows and emerging opportunities. J Parkinsons Dis 10: , 351–354. |
[50] | Bodenheimer T , Sinsky C ((2014) ) From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med 12: , 573–576. |
[51] | Hemming K , Haines TP , Chilton PJ , Girling AJ , Lilford RJ ((2015) ) The stepped wedge cluster randomised trial: Rationale, design, analysis, and reporting. BMJ 350: , h391. |
[52] | Hopkins J , Mumber MP ((2009) ) Patient navigation through the cancer care continuum: An overview. J Oncol Pract 5: , 150–152. |
[53] | Silva de Lima AL , Smits T , Darweesh SKL , Valenti G , Milosevic M , Pijl M , Baldus H , de Vries NM , Meinders MJ , Bloem BR ((2020) ) Home-based monitoring of falls using wearable sensors in Parkinson’s disease. Mov Disord 35: , 109–115. |
[54] | Crowley MJ , Edelman D , McAndrew AT , Kistler S , Danus S , Webb JA , Zanga J , Sanders LL , Coffman CJ , Jackson GL , Bosworth HB ((2016) ) Practical telemedicine for veterans with persistently poor diabetes control: A randomized pilot trial. Telemed E-Health 22: , 376–384. |




