Gastrointestinal Immunity and Alpha-Synuclein
Abstract
The gastrointestinal (GI) tract is equipped with robust immune defenses which protect the organism from infection. Enteric nerves are front and center in this defensive network, even in the most primitive organisms. Neuropeptides exhibit potent antimicrobial activity in the vicinity of the nerve and attract the innate and adaptive immune systems to help confine the invading agent. Alpha-synuclein (αS) has many biophysical characteristics of antimicrobial peptides and binds small vesicles such as those carrying endocytosed viruses. It is induced in nerve cells in response to viral and bacterial infections. It renders the nerve cell resistant to viral infection and propagation. It signals the immune system by attracting neutrophils and macrophages, and by activating dendritic cells. Most remarkably αS is trafficked to the central nervous system (CNS) conferring immunity in advance of an infection. Chronic GI infection or breakdown of the epithelial barrier can cause αS to accumulate and form neurotoxic aggregates. Overproduction of αS in the enteric nervous system (ENS) and its chronic trafficking to the CNS may damage nerves and lead to Parkinson’s disease. Targeting the formation of αS aggregates in the ENS may therefore slow the progression of the disease.
THE IMMUNE DEFENSE OF THE ENTERIC NERVOUS SYSTEM IS NOT WELL UNDERSTOOD BUT MUST BE ROBUST
Understandably, most neurologists give little attention to structures “below the neck” and the gastrointestinal (GI) tract is no exception. The principal diseases of the GI tract, including constipation, have traditionally fallen into the clinical and research domains of the gastroenterologist. Other than serving as a portal for the entry of drugs, toxins and infectious agents, how does the GI tract influence neurologic disease [1]?
The enteric nervous system (ENS) consists of the myenteric plexus that controls peristaltic activity, the submucosal plexus that sits within the lamina propria and helps orchestrate secretion, absorption, and vascular flow, and the intrinsic primary afferent neurons that capture sensory data. Spinal and vagal nerves connect the ENS with the central nervous system (CNS) [2]. The ENS is comprised of about the same number of neurons as the spinal cord. Although the ENS serves as the neural substrate coordinating the digestion of nutrients and the elimination of wastes, it also represents the largest sensory organ of the brain [3, 4]. The ENS extends the 5 meter length of the GI tract, and is said to cover the surface area the size of a tennis court [5]. If blindness and atrophy of visual pathways in the brain occur as a consequence of the destruction of the retina, what might be the consequences to the brain of reduced sensory input from this vast ENS?
Neuronal processes, such as the neurites of the submucosal plexus, lie beneath the single epithelial layer, where they form a dense network that mirrors the surface contours of the luminal surface of the intestine (Fig. 1). Recently, sensory neurons have been shown to form direct contact with enteroendocrine cells (EEC), neurotropic viruses that infected the EEC, or made their way across the epithelial barrier, could then traffic to the brain following invasion of the sensory nerve ending [6–8]. A mystery that remains by and large unanswered is how the ENS defends itself from the pathogens that infect the GI tract, or from the luminal microbes that enter the lamina propria in inflammatory conditions, such as inflammatory bowel disease. What protects the ENS of an infant during the first few years of life as it suffers through episodes of viral gastroenteritis? What prevents GI viruses from infecting nerve endings within the GI tract and trafficking to the brain? Since childhood GI infections that functionally damage the ENS or cause encephalitis are exceedingly rare, the immune defenses that protect the ENS must be extraordinarily robust.
Fig.1
Subepithelial enteric neurons visualized with PGP9.5 immunostaining from human small intestine. The epithelial layer was detached from the underlying lamina propria by EDTA treatment [51] and visualized as described [25].
![Subepithelial enteric neurons visualized with PGP9.5 immunostaining from human small intestine. The epithelial layer was detached from the underlying lamina propria by EDTA treatment [51] and visualized as described [25].](https://content.iospress.com:443/media/jpd/2019/9-s2/jpd-9-s2-jad191702/jpd-9-jpd191702-g001.jpg)
Antimicrobial peptides and proteins play a major role in the immune defense of the GI tract [9]. They are produced in high concentration in Paneth cells at the base of the crypts, and from the apical surface of secretory enterocytes. Antimicrobial proteins (AMPs) reach microbicidal concentrations in the fluid layer in direct contact with the apical surface of the epithelium. Microbes that penetrate the mucous barrier in contact with the lumen will enter the AMP-rich layer and face rapid microbicidal action before they can attach to the epithelial cell and invade.
Epithelial surfaces in contact with the outside world, like the lining of the GI tract undergo continual renewal every several days, ensuring the integrity of the physical barrier. The intestinal stem cells, located within the crypts, are protected from infectious agents by their proximity to AMP-secreting Paneth cells [10].
The mammalian GI tract is home to about 70% of the lymphoid tissue of the adaptive immune system, positioned to defend the individual from ingested pathogens. In a healthy human, the adaptive immune system is held under tight reins permitting the education of the immune system of ingested antigens and commensal bacteria without provoking clinically apparent inflammation. Precisely how this immune balance is achieved is not fully understood. The shield created by antimicrobial peptides and proteins clearly limits the exposure of the immune cells within the lamina propria to microbes that would induce an inflammatory response. In Crohn’s disease, for example, inadequate expression of Paneth cell AMPs results in the failure of the epithelium to restrain commensal bacteria from attaching and invading, resulting in an unremitting defensive tissue destructive inflammatory response mounted against luminal microbes [11].
A BRIEF LESSON ABOUT IMMUNE-DEFENSIVE NEUROPEPTIDES FROM A SIMPLE METAZOAN
Hydra is the simplest metazoan, composed of an epithelial outer layer (ectoderm), an epithelial layer that surrounds its inner cavity (endoderm), and a network of neurons that lie between the epithelial layers. The ectoderm is populated by a stable species-specific microbiome. The composition of the microbiome is determined, in part, by the nervous system. The neuropeptide NDA-1 is secreted from nerve ends onto the surface of the ectoderm, inhibiting Gram positive bacteria and suppressing the growth of the primary colonizer, the Gram-negative bacterium Curvibacter sp. In addition, several of the many well characterized neuropeptides from Hydra exhibit antimicrobial activity in vitro. These observations support the hypothesis that the nervous system of this simple animal plays a major role in immunity [12].
MANY NEUROPEPTIDES EXHIBIT ANTIMICROBIAL ACTIVITY
Antimicrobial peptides are widely distributed across all animal and plant species and constitute an essential arm of innate immunity [13]. In general, AMPs are short (between 15–80 amino acids), processed from ribosomal synthesized precursors and expressed on epithelial surfaces exposed to microbes or within vesicles of phagocytes. They are amphipathic, with hydrophilic and hydrophobic amino acid segregated on different faces of the molecule, a property that permits them to exist in aqueous solution and yet bury into a lipid membrane. Almost universally they have a net cationic charge. This feature permits them to target the plasma membranes of bacteria. The outer leaflet of the plasma membranes that surround the cells of our tissues are composed of zwitterionic phospholipids. In contrast, the plasma membranes surrounding bacteria are composed of phospholipids with anionic headgroups. Positively charged AMPs, usually as a cocktail of different peptides, are attracted to the negatively charged surfaces of the outer bacterial membranes, organize within the membranes and disrupt membrane function. As a consequence of this simple mechanism the likelihood of bacterial resistance is low. In addition to their direct antimicrobial activity, AMPs exhibit chemoattractant activity towards immune cells and in some cases, growth stimulatory activity.
Like AMPs, neuropeptides are generally amphipathic since they too must partition from an aqueous solution onto a membrane based receptor [14]. In addition, many also exhibit a net cationic charge. Several of these latter group of neuropeptides have been shown to exhibit antimicrobial activity in vitro and play an immune role in vivo in the GI tract [15]. Substance P (SP) is the best studied and best understood with respect to its role in immunity. SP is synthesized in sensory neurons and released from a nerve ending following noxious stimulation, whereupon it binds to NK1 and NK2 receptors signaling pain. SP exhibits bactericidal activity against both Gram-positive and Gram-negative bacteria but at concentrations that could only be obtained in vivo in the immediate microenvironment of the nerve ending. In addition, NK1 and NK2 receptors are present on endothelial cells, macrophages and neutrophils and as a consequence SP acts as a potent immune cell chemoattractant. Thus, SP supports several defensive functions that unfold at progressively later times after a physical insult: The rapid induction of localized pain, followed by the rapid disinfection within the micro-environment of the nerve ending, followed by the more gradual migration of neutrophils and macrophages. Since SP receptors are present on keratinocytes, this neuropeptide might also play a role in directly promoting tissue repair of the initial injury. In the oral cavity SP released from sensory nerves that penetrate the junction of the tooth surface and the epithelium of the gum (junctional epithelium) maintain a standing army of neutrophils poised to defend the periodontal tissues. In the colon, sensory nerve endings are the target of Clostridium difficile toxin A. The toxin binds to the nerve ending releasing SP which in turn stimulates the firing of enteric neurons within the lamina propria resulting in diarrhea and severe local inflammation. Pretreatment with an SP antagonist inhibits toxin A mediated enterocolitis [16].
αS EXHIBITS MANY BIOPHYSICAL CHARACTERISTICS OF AN AMP AND IT BINDS TO VESICLES OF THE SIZE THAT PACKAGE NEUROTRANSMITTERS OR VIRUSES
In 2003, Braak proposed that Parkinson’s disease (PD) might begin in the ENS through the gradual accumulation of neurotoxic aggregates within neurons, followed by their transport via nerves connecting the ENS to the CNS [17]. This hypothesis was based on pathological analyses of the nervous systems of patients with PD suffering from mild to severe disease and is supported by the observation that truncal vagotomy reduces the risk of developing PD [18, 19]. Furthermore, a majority of patients with PD have constipation and in many, the onset of constipation precedes the movement disorder by years or decades [20]. Precisely why αS should accumulate in the GI tract was unclear, nor was its functional role.
αS is described as an “intrinsically disordered” protein comprised of 140 amino acids. This means that in an aqueous solvent αS assumes no specific structure. However, if the molecule is placed in the presence of lipid membranes that contain a high proportion of phospholipids with negative charged headgroups (like phosphatidyl serine), αS will be pulled toward the membrane electrostatically by the N-terminal ∼60 residues, which exhibit a net cationic charge [21]. Surprisingly, however, once in the vicinity of the negatively charged membrane, docking of the N-terminus requires that the membrane surface exhibit a curvature similar to that of a secretory vesicle, between 35–200 nm in diameter [22]. The cationic motifs in the N-terminus are arranged in five blocks of six amino acids each, separated by linkers of between 5 and 9 residues. Presumably the energetics of binding of this chain link structure favors binding to a curved surface. In our hands, monomeric αS does not exhibit direct antimicrobial activity, a property to be expected since micron-sized bacteria present a relatively flat surface in comparison to 35–200 nm vesicle. Because of this topological constraint monomeric αS would also not be expected to damage the bacterial sized mitochondria, with which they share the cytoplasm. Classical AMPs, by contrast, rapidly depolarize mitochondrial membranes [23]. On the other hand, many viruses enter the cell through receptor mediated endocytosis, enveloped by an endosomal membrane of both phospholipid composition and size that accommodates decoration by αS. Intracellular αS would be expected to coat endocytosed virions, perhaps explaining the antiviral activity of αS demonstrated in vivo (see below).
Upon binding to its membrane target, the N-terminal portion of αS organizes into an alpha-helix, with the hydrophobic faces buried within the lipid phase, the hydrophilic surface in contact with the polar environment, and the carboxyl terminus remaining relatively untethered [24]. As the surface concentration of membrane bound αS increases, and the bound molecules become closer to one another they begin to aggregate. Because of the regular periodicity of the alpha helix, adjacent regions can assemble into stable structures, analogous to crystallization. This property of concentration dependent aggregation has been well described for many AMPs.
As the aggregates grow in size, at some stage they damage the membranes on which they are growing. A poorly understood mechanism exists that disposes of these aggregates. In PD the formation of neurotoxic αS aggregates exceeds clearance, resulting in neuronal injury and its downstream consequences.
αS IS INDUCED IN THE SETTING OF GI INFECTION IN CHILDREN
The similarity with respect to biophysical properties between AMPs and αS suggested to us that αS might have an immune function. To address this hypothesis we examined biopsies that received a pathological diagnosis of acute or chronic inflammation conducted in a single hospital over a 9 year period taken from 42 children who experienced upper GI symptoms of a severity that warranted endoscopy [25]. A pediatric population was selected to avoid bias from an adult population with ‘pre-clinical’ PD. The sections were immune stained separately for αS, PGP 9.5 (to identify neurons), and CD68 (to identify macrophages). Greater than 90% of the duodenal and jejunal biopsies stained positively for neuronal αS, with the intensity of the αS abundance proportional to the degree of inflammation, as reflected in the numbers of neutrophils and mononuclear cells in the tissue specimen. To more explicitly explore the relationship between αS expression and infection we examined endoscopic biopsies taken from 14 children who had received an intestinal transplant and subsequently developed a Norovirus enteritis, a common infection in this immunosuppressed population. Since endoscopic biopsies are routinely taken prospectively following an intestinal transplant to monitor for early pathological signs of rejection, we were able to examine tissue specimens from children before, during, and after Norovirus enteritis. The biopsies from all of these patients taken during the infection exhibited intense immunoreactive αS and remained positive for several months post infection, possibly due to persistent viral carriage. αS, however, was not seen in tissue specimens taken from several patients within weeks prior to the Norovirus infection. These observations support the hypothesis that αS expression is induced in the human upper GI tract during a viral infection and can persist for some time following the clinical resolution of the illness.
NEURONAL αS CHEMOATTRACTS IMMUNE CELLS TO CONFINE INFECTION
The localization of CD68 macrophages around αS positive neurons suggested that macrophages were attracted to the αS expressing neurons (Fig. 2) [25]. The chemoattractant properties of αS were measured directly using Boyden chamber methodology. Human αS exhibited chemoattractant activity towards both mouse and human macrophages and neutrophils, comparable in potency to the classical chemokine, interleukin 8 (IL-8). Monomeric and oligomeric forms of human αS exhibited comparable chemoattractant activity. Surprisingly the N-acetylated N-terminal 21 amino acids retained the chemoattractant activity of the full-length molecule, while an unacetylated peptide corresponding to the first 25 amino acids of the N-terminus did not. Since N-acetylation promotes stabilization of the alpha helical secondary structure of the N-terminus [26], these data provide insight into the structural motif of αS involved in chemoattraction.
Fig.2
Macrophages cluster around neurons expressing αS. Human duodenal biopsy from a pediatric patient presenting with upper GI distress. Left, immune-stained for αS; Right, immune-stained for CD68 antigen (macrophage) [25].
![Macrophages cluster around neurons expressing αS. Human duodenal biopsy from a pediatric patient presenting with upper GI distress. Left, immune-stained for αS; Right, immune-stained for CD68 antigen (macrophage) [25].](https://content.iospress.com:443/media/jpd/2019/9-s2/jpd-9-s2-jad191702/jpd-9-jpd191702-g002.jpg)
Based on the report by Wang et al. that aggregates of αS chemoattract mouse brain microglia via interaction with the integrin CD11b [27], expressed on these cells, we examined the importance of CD11b for the chemoattractant activity of αS towards macrophages and neutrophils. Indeed, neutrophils isolated from CD11b–/– mice were unresponsive to both monomeric and aggregated αS, or the N-acetylated 1–21 residue peptide. Similarly, the chemoattractant activity of all three molecules was inhibited when human neutrophils were presented in the presence of an antibody directed against CD11b. CD11b on immune cells is known to interact with many well recognized antimicrobial peptides presumably for the purpose of directing these defensive cells towards sites of injury where AMPs are normally released [28].
Dendritic cells lie at the interface between the innate and adaptive immune systems. Exposure of naïve human monocyte dendritic cells to either monomeric or aggregated αS stimulated phenotypic maturation. Thus, the presence of αS poised the dendritic cell to respond to antigen and orchestrate a subsequent immune response.
Our observations provide compelling support for the hypothesis that αS serves an immune function in the GI tract. Induction of αS in the setting of an infectious insult would initially attract protective immune cells, followed by the engagement of the adaptive immune system. As yet we do not know the mechanisms that are involved in the induction of neuronal αS by infection, such as the role of TLRs (which are present on neurons) [29, 30], or the release of pro-inflammatory mediators, such as IL-17 and IL-22 (which potently stimulate induction of epithelial AMPs) [31].
αS IN RED BLOOD CELLS COULD SERVE AN IMMUNE FUNCTION
Human red blood cells contain αS [32, 33]. In fact, studies indicate that most of the αS found in human blood is of red cell origin [34]. In this cell, αS cannot be playing a role in secretory vesicle dynamics since the red blood cell has no such capacity. However, it is well known that microbleeds (best studied in the brain) rapidly induce a local inflammatory response consisting of an initial wave of neutrophils [35]. By this mechanism any physical breach of the vascular bed that resulted in extravasation of red blood cells, carrying a payload of αS, would signal the immune system.
αS CAN PROTECT THE BRAIN FROM DANGERS DETECTED IN THE GI TRACT
αS synthesized within the ENS can traffic to the CNS, at the very least, via the vagus nerves, as demonstrated by Holmqvist et al. [36]. Human αS, whether as a monomer or aggregate, could be followed travelling up the vagus over the course of hours after injection into the submucosa of the stomach wall of a rat. Thus, αS induced in the GI tract in response to infection would be expected to make its way to the brain, delivering its immune protection. Since DNA viruses, such as Herpes simplex [37] and RNA viruses, such as influenza [38] and polio [39], can traffic via peripheral nerves to the brain, the trafficking of αS from the ENS could provide the brain with an immune defense in advance of a potential infection.
αS KNOCKOUT MICE SUFFER GREATER MORBIDITY THAN WILD TYPE MICE AFTER VIRAL AND BACTERIAL INFECTIONS
αS knockout mice are surprisingly healthy and do not exhibit a strong neurological phenotype, even when all three synuclein genes are removed [40]. Since αS is known to associate with secretory vesicles, much of the research in these models has focused on neurotransmitter vesicle dynamics [41]. Indeed, subtle differences in vesicle behavior dependent on the presence or absence of αS have been detected [42]. However, the fact that complete absence of the mouse synuclein family has no impact on the development of the central or peripheral nervous system does make one question the role of αS in neurotransmission.
Recently, however, two reports demonstrated that αS knockout mice exhibit immune incompetence in the setting of systemic infections, compared with wild type mice [43–45]. In the studies from Beckham’s laboratory αS knockout mice were exposed to West Nile virus survival was drastically reduced compared with wild type animals, correlating with viral titers in the CNS. About 24 hours following infection, expression of αS was significantly induced within specific neurons in the brain of the wild type animals, with the induced αS colocalizing within the ER with viral capsid proteins. The expression of several pro-inflammatory cytokines that could have potentially impacted the anti-viral response, did not differ between knockout and wild type animals. This study supports the hypothesis that neuronal αS can protect the neuron from viral infection by direct action.
A report by Tomlinson et al. [43] examined the role of αS within the olfactory system of the mouse following nasal inoculation of reovirus. Uninfected wild type mice robustly express αS within the olfactory epithelium, olfactory glomeruli, and olfactory cortex, reflecting either constitutive expression or expression induced by continuous exposure to commensal microbes. The authors demonstrated that αS knockout mice were more susceptible to reovirus, administered via an intranasal route, than were wild type controls. In addition to viral infection, αS appears to play a role in immune defense against bacterial infection, since αS knockout mice were also more susceptible to intravenously administered Salmonella typhimurium than the wild type.
A SIMPLIFIED MODEL OF THE MANNER BY WHICH αS COULD DEFEND THE NERVOUS SYSTEM
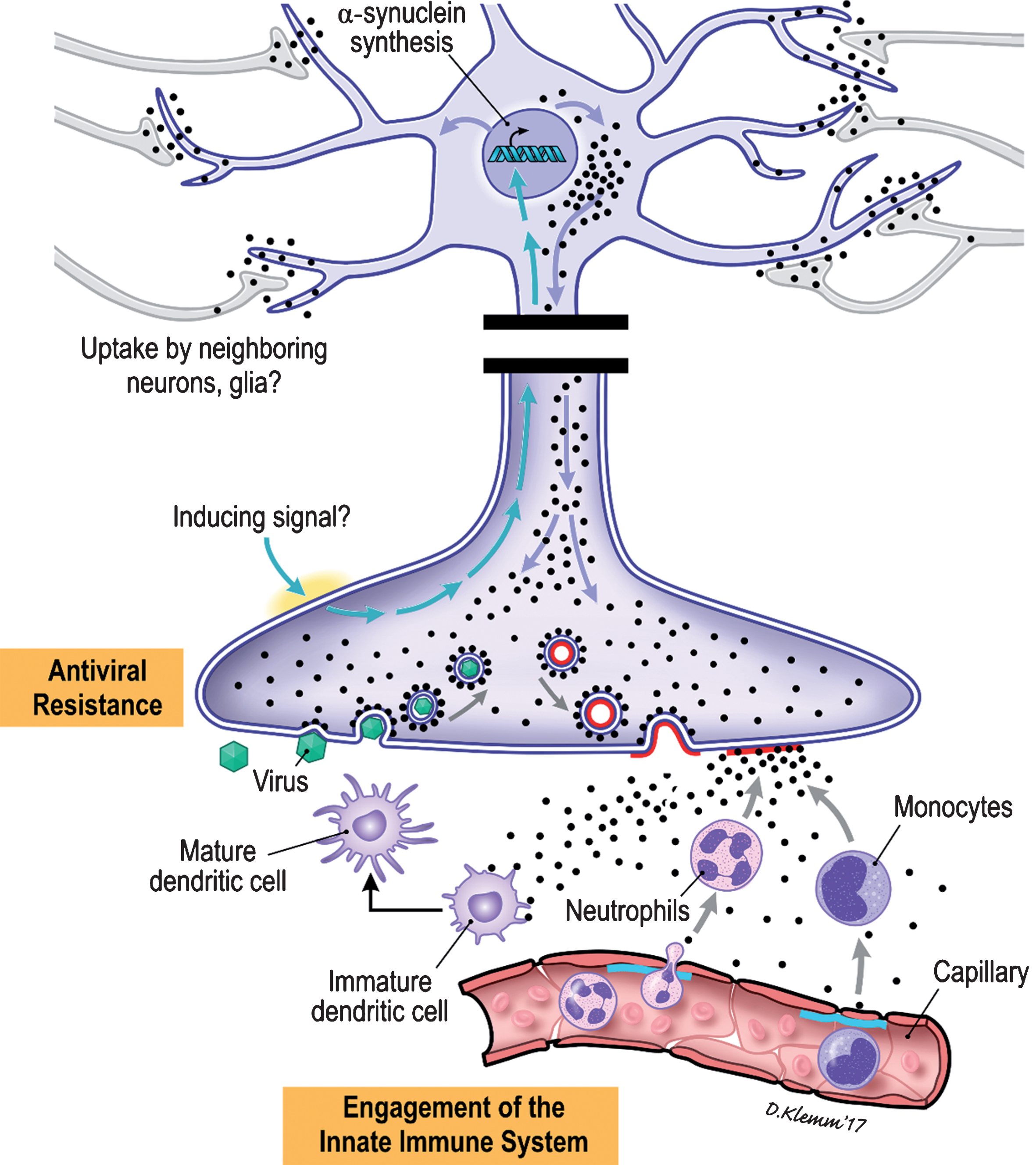
Neuronal αS is induced in the setting of viral and bacterial infections by mechanisms still unknown (Fig. 3). Cytoplasmic αS binds to the outer leaflet of intracellular vesicles, such as those in which neurotransmitters are packaged, by virtue of its affinity for membrane surfaces of appropriate phospholipid composition and topology. Excitation of the neuron results in fusion of the αS coated vesicle with the nerve ending [46]. This fusion event results in the mixing of the lipids within the bilayers of the vesicle and nerve ending. αS bound to phospholipids that partition to the “flat” outer leaflet of the membrane of the nerve ending would detach into the surrounding tissues. The presence of αS recruits immune cells to the vicinity of the nerve terminal and local dendritic cells are armed to direct a specific immune response. As a consequence of the presence of intracellular αS the normal infective life cycle of a virus might be disrupted. Normally the endocytosed virions would be trafficked to sites within the cell for uncoating; in a cell in which αS is being expressed these virion-containing vesicles would likely be coated with αS, possibly altering the normal intracellular pathways anticipated by the virus and creating a state of viral resistance.
Fig.3
Cartoon illustration of the proposed immune roles of αS within the ENS.
TARGETING αS WITHIN THE ENTERIC NERVOUS SYSTEM TO TREAT PARKINSON’S DISEASE
Together, these studies support the hypothesis that αS within the ENS of the GI tract accumulates in response to an infectious insult. The induction of αS is a normal immune response directed by the nervous system. From this perspective, PD appears to be a neurodegenerative disease caused by excessive expression of an inflammatory mediator. Excessive expression leads to the formation of aggregates at a rate that overwhelms clearance mechanisms. Excessive expression of αS within the ENS could be caused by chronic GI infections, or by impaired epithelial barrier functions resulting in exposure of the submucosa to commensal microbes [47]. The robust expression of αS in the appendix likely is an inflammatory response to the presence of the commensal microbes that sequester in the blind pouch [48, 49].
Since αS can accumulate in the ENS, and aggregates formed within the ENS can traffic to the brainstem and beyond, targeting αS dynamics within ENS might have therapeutic benefit in PD.
In mid-2018 we completed a 50-patient open label Phase 2a clinical study (RASMET) evaluating ENT-01 for the treatment of constipation associated with PD [50]. ENT-01 is an orally administered synthetic salt of squalamine, a cationic aminosterol originally isolated from the liver and gall bladder of the dogfish shark. In preclinical studies squalamine was shown to compete with αS for membrane binding sites, restore normal peristalsis in mouse models of PD, and prevent αS aggregation and paralysis in a C. elegans model engineered to express αS in its muscle cells [24]. These studies support the hypothesis that by competing with αS for membrane binding sites, the squalamine ion restores normal electrical activity of enteric neurons, reduces the rate of formation of surface promoted αS aggregation and the subsequent morbidity that results from the accumulation of αS aggregates. The RASMET study demonstrated that ENT-01 could safely and effectively correct constipation in over 80% of patients with PD, with each patient titrated up to a dose of ENT-01 that stimulated a prokinetic response. Surprisingly, we also observed benefits in both motor and non-motor symptoms. The RASMET study demonstrated that it is possible to correct long standing dysfunction of the ENS which might have been assumed to be irreversibly damaged. A 110-patient double-blind, placebo-controlled Phase 2b trial evaluating the effect of oral ENT-01 tablets on constipation and neurologic symptoms is currently in progress (KARMET).
CONCLUSION
The hypothesis that neurotoxic aggregates of αS arise within the ENS and subsequently traffic to the CNS where they ultimately cause inflammatory destruction of the substantia nigra, imposes a paradigm shift on our understanding of the etiology of PD. Recent data from our laboratory and others demonstrate that αS is induced in the setting of viral and bacterial infection and serves an immune function, by protecting the ENS, by alerting the adaptive immune system, and through pre-emptive defense of the CNS in advance of the infectious agent. In the setting of chronic GI infections or impaired intestinal barrier function, when the expression of αS exceeds its clearance, neurotoxic aggregates of αS form damaging the ENS and trafficking to the CNS. Based on this perspective, we are testing the hypothesis that by targeting the ENS with ENT-01, a compound that can inhibit the intracellular aggregation of αS, we can restore ENS functioning in the short term, and possibly slow the progressive deterioration of the CNS in the long term.
CONFLICTS OF INTEREST
Drs. Barbut and Zasloff are co-founders of Enterin, Inc. and hold equity. Dr. Stolzenberg has no conflict of interest.
REFERENCES
[1] | Rao M , Gershon MD ((2016) ) The bowel and beyond: The enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 13: , 517–528. |
[2] | Kunze WA , Furness JB ((1999) ) The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol 61: , 117–142. |
[3] | Furness JB , Kunze WA , Clerc N ((1999) ) Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: Neural, endocrine, and immune responses. Am J Physiol 277: , G922–G928. |
[4] | Furness JB , Rivera LR , Cho HJ , Bravo DM , Callaghan B ((2013) ) The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10: , 729–740. |
[5] | Helander HF , Fandriks L ((2014) ) Surface area of the digestive tract - revisited. Scand J Gastroenterol 49: , 681–689. |
[6] | Bohorquez DV , Shahid RA , Erdmann A , Kreger AM , Wang Y , Calakos N , Wang F , Liddle RA ((2015) ) Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125: , 782–786. |
[7] | Bellono NW , Bayrer JR , Leitch DB , Castro J , Zhang C , O’Donnell TA , Brierley SM , Ingraham HA , Julius D ((2017) ) Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170: , 185–198 e116. |
[8] | Brun P , Giron MC , Zoppellaro C , Bin A , Porzionato A , De Caro R , Barbara G , Stanghellini V , Corinaldesi R , Zaninotto G , Palu G , Gaion RM , Tonini M , De Giorgio R , Castagliuolo I ((2010) ) Herpes simplex virus type 1 infection of the rat enteric nervous system evokes small-bowel neuromuscular abnormalities. Gastroenterology 138: , 1790–1801. |
[9] | Bevins CL , Salzman NH ((2011) ) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9: , 356–368. |
[10] | Clevers HC , Bevins CL ((2013) ) Paneth cells: Maestros of the small intestinal crypts. Annu Rev Physiol 75: , 289–311. |
[11] | Wehkamp J , Wang G , Kubler I , Nuding S , Gregorieff A , Schnabel A , Kays RJ , Fellermann K , Burk O , Schwab M , Clevers H , Bevins CL , Stange EF ((2007) ) The Paneth cell alpha-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J Immunol 179: , 3109–3118. |
[12] | Augustin R , Schroder K , Murillo Rincon AP , Fraune S , Anton-Erxleben F , Herbst EM , Wittlieb J , Schwentner M , Grotzinger J , Wassenaar TM , Bosch TCG ((2017) ) A secreted antibacterial neuropeptide shapes the microbiome of Hydra. Nat Commun 8: , 698. |
[13] | Zasloff M ((2002) ) Antimicrobial peptides of multicellular organisms. Nature 415: , 389–395. |
[14] | Brogden KA , Guthmiller JM , Salzet M , Zasloff M ((2005) ) The nervous system and innate immunity: The neuropeptide connection. Nat Immunol 6: , 558–564. |
[15] | Aresti Sanz J , El Aidy S ((2019) ) Microbiota and gut neuropeptides: A dual action of antimicrobial activity and neuroimmune response. Psychopharmacology (Berl) 236: , 1597–1609. |
[16] | Pothoulakis C , Castagliuolo I , LaMont JT , Jaffer A , O’Keane JC , Snider RM , Leeman SE ((1994) ) CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A 91: , 947–951. |
[17] | Braak H , Del Tredici K , Rub U , de Vos RA , Jansen Steur EN , Braak E ((2003) ) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: , 197–211. |
[18] | Svensson E , Horvath-Puho E , Thomsen RW , Djurhuus JC , Pedersen L , Borghammer P , Sorensen HT ((2015) ) Does vagotomy reduce the risk of Parkinson’s disease: The authors reply. Ann Neurol 78: , 1012–1013. |
[19] | Liu B , Fang F , Pedersen NL , Tillander A , Ludvigsson JF , Ekbom A , Svenningsson P , Chen H , Wirdefeldt K ((2017) ) Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 88: , 1996–2002. |
[20] | Fasano A , Visanji NP , Liu LW , Lang AE , Pfeiffer RF ((2015) ) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14: , 625–639. |
[21] | Davidson WS , Jonas A , Clayton DF , George JM ((1998) ) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273: , 9443–9449. |
[22] | Middleton ER , Rhoades E ((2010) ) Effects of curvature and composition on alpha-synuclein binding to lipid vesicles. Biophys J 99: , 2279–2288. |
[23] | Westerhoff HV , Juretic D , Hendler RW , Zasloff M ((1989) ) Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci U S A 86: , 6597–6601. |
[24] | Perni M , Galvagnion C , Maltsev A , Meisl G , Muller MB , Challa PK , Kirkegaard JB , Flagmeier P , Cohen SI , Cascella R , Chen SW , Limbocker R , Sormanni P , Heller GT , Aprile FA , Cremades N , Cecchi C , Chiti F , Nollen EA , Knowles TP , Vendruscolo M , Bax A , Zasloff M , Dobson CM ((2017) ) A natural product inhibits the initiation of alpha-synuclein aggregation and suppresses its toxicity. Proc Natl Acad Sci U S A 114: , E1009–E1017. |
[25] | Stolzenberg E , Berry D , Yang , Lee EY , Kroemer A , Kaufman S , Wong GCL , Oppenheim JJ , Sen S , Fishbein T , Bax A , Harris B , Barbut D , Zasloff MA ((2017) ) A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun 9: , 456–463. |
[26] | Bartels T , Ahlstrom LS , Leftin A , Kamp F , Haass C , Brown MF , Beyer K ((2010) ) The N-terminus of the intrinsically disordered protein alpha-synuclein triggers membrane binding and helix folding. Biophys J 99: , 2116–2124. |
[27] | Wang S , Chu CH , Stewart T , Ginghina C , Wang Y , Nie H , Guo M , Wilson B , Hong JS , Zhang J ((2015) ) alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc Natl Acad Sci U S A 112: , E1926–E1935. |
[28] | Podolnikova NP , Podolnikov AV , Haas TA , Lishko VK , Ugarova TP ((2015) ) Ligand recognition specificity of leukocyte integrin alphaMbeta2 (Mac-1, CD11b/CD18) and its functional consequences. Biochemistry 54: , 1408–1420. |
[29] | Caputi V , Giron MC ((2018) ) Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int J Mol Sci 19: , E1689. |
[30] | Perez-Pardo P , Dodiya HB , Engen PA , Forsyth CB , Huschens AM , Shaikh M , Voigt RM , Naqib A , Green SJ , Kordower JH , Shannon KM , Garssen J , Kraneveld AD , Keshavarzian A ((2019) ) Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut 68: , 829–843. |
[31] | Valeri M , Raffatellu M ((2016) ) Cytokines IL-17 and IL-22 in the host response to infection. Pathog Dis 74: , ftw111. |
[32] | Bartels T , Choi JG , Selkoe DJ ((2011) ) alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477: , 107–110. |
[33] | Fauvet B , Mbefo MK , Fares MB , Desobry C , Michael S , Ardah MT , Tsika E , Coune P , Prudent M , Lion N , Eliezer D , Moore DJ , Schneider B , Aebischer P , El-Agnaf OM , Masliah E , Lashuel HA ((2012) ) alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem 287: , 15345–15364. |
[34] | Barbour R , Kling K , Anderson JP , Banducci K , Cole T , Diep L , Fox M , Goldstein JM , Soriano F , Seubert P , Chilcote TJ ((2008) ) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5: , 55–59. |
[35] | Ahn SJ , Anrather J , Nishimura N , Schaffer CB ((2018) ) Diverse inflammatory response after cerebral microbleeds includes coordinated microglial migration and proliferation. Stroke 49: , 1719–1726. |
[36] | Holmqvist S , Chutna O , Bousset L , Aldrin-Kirk P , Li W , Bjorklund T , Wang ZY , Roybon L , Melki R , Li JY ((2014) ) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128: , 805–820. |
[37] | Gesser RM , Koo SC ((1996) ) Oral inoculation with herpes simplex virus type 1 infects enteric neuron and mucosal nerve fibers within the gastrointestinal tract in mice. J Virol 70: , 4097–4102. |
[38] | Jang H , Boltz D , Sturm-Ramirez K , Shepherd KR , Jiao Y , Webster R , Smeyne RJ ((2009) ) Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A 106: , 14063–14068. |
[39] | Flexner S ((1936) ) Respiratory versus gastro-intestinal infection in poliomyelitis. J Exp Med 63: , 209–226. |
[40] | Greten-Harrison B , Polydoro M , Morimoto-Tomita M , Diao L , Williams AM , Nie EH , Makani S , Tian N , Castillo PE , Buchman VL , Chandra SS ((2010) ) αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci U S A 107: , 19573–19578. |
[41] | Pineda A , Burre J ((2017) ) Modulating membrane binding of alpha-synuclein as a therapeutic strategy. Proc Natl Acad Sci U S A 114: , 1223–1225. |
[42] | Logan T , Bendor J , Toupin C , Thorn K , Edwards RH ((2017) ) alpha-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci 20: , 681–689. |
[43] | Tomlinson JJ , Shutinoski B , Dong L , Meng F , Elleithy D , Lengacher NA , Nguyen AP , Cron GO , Jiang Q , Roberson ED , Nussbaum RL , Majbour NK , El-Agnaf OM , Bennett SA , Lagace DC , Woulfe JM , Sad S , Brown EG , Schlossmacher MG ((2017) ) Holocranohistochemistry enables the visualization of alpha-synuclein expression in the murine olfactory system and discovery of its systemic anti-microbial effects. J Neural Transm (Vienna) 124: , 721–738. |
[44] | Massey AR , Beckham JD ((2016) ) Alpha-synuclein, a novel viral restriction factor hiding in plain sight. DNA Cell Biol 35: , 643–645. |
[45] | Beatman EL , Massey A , Shives KD , Burrack KS , Chamanian M , Morrison TE , Beckham JD ((2015) ) Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol 90: , 2767–2782. |
[46] | Paillusson S , Clairembault T , Biraud M , Neunlist M , Derkinderen P ((2013) ) Activity-dependent secretion of alpha-synuclein by enteric neurons. J Neurochem 125: , 512–517. |
[47] | Kelly LP , Carvey PM , Keshavarzian A , Shannon KM , Shaikh M , Bakay RA , Kordower JH ((2014) ) Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29: , 999–1009. |
[48] | Gray MT , Munoz DG , Gray DA , Schlossmacher MG , Woulfe JM ((2014) ) Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord 29: , 991–998. |
[49] | Killinger BA , Madaj Z , Sikora JW , Rey N , Haas AJ , Vepa Y , Lindqvist D , Chen H , Thomas PM , Brundin P , Brundin L , Labrie V ((2018) ) The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med 10: , eaar5280. |
[50] | Hauser RA SD , Madrid JA , Rol MA , Frucht S , Isacson S , Pagan F , Maddux BN , Li G , Tse W , Walter BL , Kumar R , Kremens D , Lew MF , Ellenbogen A , Oguh O , Vasquez A , Kinney W , Lowery M , Resnick M , Huff N , Posner J , Ballman KV , Camilleri M , Zasloff M , Barbut D ((2019) ) Targeting neurons in the gatrointestinal tract to treat Parkinson’s disease. Clin Parkinsonism Relat Disord 1: , 2–7. |
[51] | Fishbein T , Novitskiy G , Mishra L , Matsumoto C , Kaufman S , Goyal S , Shetty K , Johnson L , Lu A , Wang A , Hu F , Kallakury B , Lough D , Zasloff M ((2008) ) NOD2-expressing bone marrow-derived cells appear to regulate epithelial innate immunity of the transplanted human small intestine. Gut 57: , 323–330. |




