Plasma IL-6 and IL-17A Correlate with Severity of Motor and Non-Motor Symptoms in Parkinson’s Disease
Abstract
The nature of the inflammatory response in Parkinson’s disease (PD) remains to be better understood. Here, we used highly sensitive Single Molecule Array (SIMOA) technology to measure the levels of the inflammatory mediators Interleukin 6 (IL-6), Interleukin 17A (IL-17A), Tumour Necrosis Factor α (TNFα) and Transforming Growth Factor β (TGFβ) in plasma from PD patients and age- and gender-matched healthy controls. We report that IL-17A correlates with non-motor symptoms (NMS) scores, while IL-6 positively correlates with motor scores. We found no correlations between cytokines and disease duration suggesting that IL-6 and IL-17A are associated with disease severity rather than disease duration in this cohort, furthermore IL-17A may be involved in the underlying pathophysiology of NMS in PD.
INTRODUCTION
Increasing recognition is being given to non-motor symptoms (NMS) associated with Parkinson’s Disease (PD). Depressive symptoms are present in 35% of PD patients and up to 60% develop some form of anxiety, with prevalences varying in the literature for both disorders [1, 2]. Mood disorders in PD are often under-recognised due to the overlap with other PD-related somatic and mental symptoms [1, 2]. In addition to this, the majority of late-stage PD patients experience cognitive decline over time [3], which ranges from mild cognitive impairment (MCI) to PD dementia (PDD) where patients develop a multi-domain debilitating cognitive deficit.
The negative effects of excessive neuroinflammation have been well documented in PD and evidence now suggests that activated microglia in PD may be involved in dopaminergic neuronal cell death, not just a response to PD pathology [4]. With considerable cross talk between the periphery and the central nervous system (CNS), assessing peripheral inflammatory profiles may be an easily accessible proxy to assess CNS changes. Indeed, altered levels of peripheral cytokines have been reported in PD, cognitive decline, depression and anxiety [5], however, data on the inflammatory profile of PD patients remain inconsistent and vary widely [6]. Despite the lack of consensus on the peripheral changes occurring, it is nevertheless apparent that changes do occur and may play a role in the underlying disease aetiology. Further research using highly-sensitive methodologies, is required to elucidate how these peripheral changes relate to or indeed reflect CNS changes in PD.
We measured IL-6, IL-17A, TNFα and TGFβ in a cohort of well-phenotyped PD patients at different disease stages using the highly sensitive single molecule array (SIMOA) platform. IL-6 and TNFα have previously been implicated in PD [6] and growing evidence suggests a role for the proinflammatory IL-17A-expressing T cells in PD-associated neurodegeneration [7]. We also investigated (TGF)-β, an anti-inflammatory mediator that has been shown to have protective effects in rodent models of PD [8] and is increased in ventricular CSF from PD patients. PD is more prevalent in men than women and the clinical phenotypes, including NMS, show gender differences [9]. Moreover, studies have shown gender different immune system activation and, for example, women with autoimmune diseases have stronger immune responses than men [10]. We have therefore investigated if there are gender differences in the studied inflammatory mediators in our cohort of PD patients.
MATERIALS AND METHODS
Experiments were carried out in accordance with the Declaration of Helsinki, and were approved by the Regional Review Board and the local ethical committee of the Karolinska Institute, Stockholm. Participants were recruited by a MD at the Neurology clinic at the Karolinska University Hospital, Sweden and all subjects gave written informed consent.
PD patients (n = 66) were defined by standard criteria. Disease progression and stage were scored at the Karolinska University Hospital using the Unified Parkinson’s Disease Rating Scale (UPDRS) and the modified Hoehn & Yahr (H&Y) scale respectively, and the Montreal Cognitive Assessment (MoCA) was used for cognitive impairment. MoCA is a widely used psychometric test which tests eight domains of cognition and is validated for the diagnosis of MCI [11]. Anxiety and dementia were self-assessed by the patients using the Hospital Anxiety and Depression Scale (HADS), which is commonly used in an outpatient setting [12]. Patients’ levodopa daily equivalent dose (LEDD) was calculated for the day before blood draw. Healthy controls (HCs, n = 45) were recruited through family members of patients and hospital/research personnel and were included based on the absence of PD. Blood samples were collected by venipuncture and plasma was isolated during the preparation of leukocytes from whole blood.
For SIMOA experiments plasma samples were prepared according to the kit specific manufacturers instruction. Cytokine levels were measured using a Quanterix cytokine 3-Plex B kit and a TGFβ-discovery kit on the SIMOA HD-1 platform. Standards and samples were run in duplicate utilizing the manufactures assay instructions. The lower limits of quantification (LLOQ) for IL-6, IL-17A, TNFα and TGFβ were 0.023 pg/mL, 0.026 pg/mL, 0.0068 pg/mL and 0.514 pg/ml respectively, and values below these are not recomemended to be used by the company. Accordingly, in the present study, IL-17A measures from two patients were excluded. Samples were coded by a third party and each run contained patients and age and gender matched HCs.
Data were analyzed using IBM SPSS statistics v25. Duplicates were averaged to give one value per patient per cytokine or excluded where the concentration coefficient of variation >20%. Outliers were identified as values outside 3x the interquartile range. Outliers and concentrations below the LLOQ were excluded. Data were tested for normality using Shapiro-Wilk test, and histograms and Q-Q plots were visualized. To compare groups, Student’s t-test, Chi-squared test, Mann Whitney test, Fisher’s Exact test or Kruskal Wallis test with Dunn’s post hoc test were used as appropriate and are indicated in the text where used. Spearman partial correlations were carried out between cytokine levels and demographic or clinical data, correcting for age, gender and LEDD scores as apropriate. Statistical significance assumed at p < 0.05.
RESULTS
There was no significant difference in the age or gender ratios of participants or in cytokine levels across the groups (Student’s t-test and Chi-squared test, Table 1, Supplementary Figure 1). To investigate gender differences, data were divided based on disease group and gender. Female PD patients had significantly higher IL-17A levels than male PD patients (Kruskal-Wallis test, Dunn’s multiple comparisons test p < 0.01, Fig. 1A).
Table 1
Demographics and clinical characteristics of patients with Parkinson’s disease (PD) and healthy controls (HC)
| PD (n = 66) | HC (n = 45) | P value | |
| Age | 69.9±8.1 | 68.2±7.1 | 0.25 |
| Gender (male/female) | 34/32 | 24/21 | 1.00 |
| Age of onset | 64.3±8.9 | ||
| Disease duration | 5 (0–26) | ||
| LEDD | 505 (0–1730) | ||
| Hoehn and Yahr (n = 61) | 2 (1–5) | ||
| UPDRS-III (n = 60) | 28.9±13.3 | ||
| MoCA (n = 66) | 23.1±5.6 | ||
| MADRS-S (n = 47) | 11.7±10.1 | ||
| HADS-Anxiety (n = 46) | 6.4±4.9 | ||
| HADS-Depression (n = 46) | 4.9±4.6 | ||
| Patients on Parkinson’s medication | 56 | ||
| Other Medication | |||
| Psychiatric/cognitive | 25 | 7 | 0.011 |
| Neurological | 7 | 1 | 0.14 |
| Cardiovascular | 31 | 15 | 0.17 |
| Other internal medicine | 20 | 12 | 0.83 |
| Others | 19 | 14 | 0.83 |
| Comorbidities | |||
| Psychiatric/cognitive | 20 | 3 | 0.0035 |
| Neurological | 13 | 5 | 0.30 |
| Cardiovascular | 23 | 13 | 0.54 |
| Other internal medicine | 27 | 12 | 0.16 |
| Others | 41 | 15 | 0.0037 |
| Cytokine | |||
| IL-6 (pg/ml, mean±SEM) | 1.43±0.17 (n = 57) | 1.17±0.12 (n = 43) | 0.32 |
| TNFα (pg/ml, mean±SEM) | 1.72±0.08 (n = 63) | 1.76±0.07 (n = 43) | 0.72 |
| IL-17 (pg/ml mean±SEM) | 0.19±0.02 (n = 39) | 0.14±0.03 (n = 18) | 0.17 |
| TGFβ (pg/ml, mean±SEM) | 3482±262 (n = 55) | 3871±379 (n = 36) | 0.65 |
Data presented as number, mean±SEM, or median (range). LEDD, levodopa equivalent daily dose; UPDRS-III, unified Parkinson’s disease rating scale part 3; MoCA, Montreal cognitive assessment; MADRS-S, self-reported Montgomery-Åsberg depression rating scale; HADS, Hospital anxiety and depression scale; IL-interleukin; TNF, tumor necrosis factor; TGF, transforming growth factor. Mann Whitney test used for age and cytokines, and Fisher’s exact test was used for gender, medication and comorbidities.
Fig.1
Plasma cytokines in PD patients correlate with motor and non-motor scores. Plasma IL-17A levels in healthy controls and PD patients divided by gender (A). IL-17A levels are significantly higher in female PD patients as compared to male PD patients (Kruskal-Wallis test, Dunn’s multiple comparisons test p < 0.01). Data expressed as mean±SD. (B) IL-6 and TNFα plasma levels positively correlate with age in PD patients. (C and D) IL-6 levels in PD patients positively correlate with H&Y and UPDRSIII scores. (E and F) IL-17A levels positively correlate with anxiety subscale of HADS and negatively correlate with MoCA scores in PD patients. (G) IL-17A levels positively correlate with depression subscale of HADS in female PD patients only. *p < 0.05, Spearman partial correlation, corrected for gender and LEDD score (B) or corrected for age, gender and LEDD score (C-F) or corrected for age and LEDD score (G). Other gender specific significant partial Spearman correlations indicated in text.
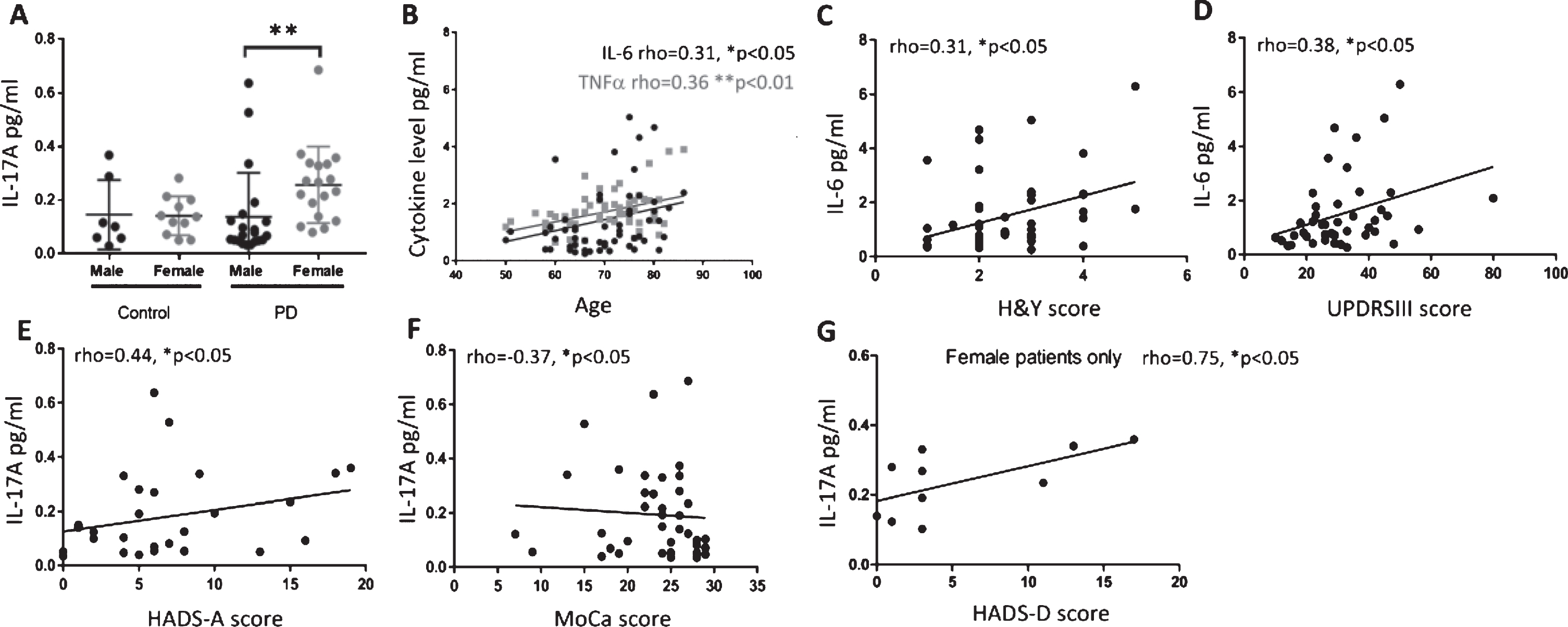
Cytokines (IL-6, TNFα, IL-17A and TGFβ), were correlated with clinical scores (UPDRSIII, H&Y, MoCA, MADRS and HADS) and correlations were corrected for age, gender and LEDD unless specified otherwise. Only statistically significant correlations are reported. Both IL-6 and TNFα were positively correlated with age, when correcting for gender and LEDD score (p < 0.05, rho = 0.31 and p < 0.01, rho = 0.36, respectively Fig. 1B). IL-6 positively correlated with H&Y and UPDRSIII score in PD patients (p < 0.05, rho = 0.31 and 0.38 respectively, Fig. 1C and 1D). IL-17A was positively correlated with the anxiety subscale of HADS (HADS-A, p < 0.05, rho = 0.44, Fig. 1E) and negatively correlated with MoCA score (p < 0.05, rho = –0.37, Fig. 1F). TGFβ was not correlated with any motor symptom or NMS investigated.
Gender specific correlations were corrected for age and LEDD only unless specified otherwise. In male patients, IL-6 levels positively correlated with H&Y and UPDRSIII (p < 0.05, rho = 0.4 and 0.44 respectively, male only data not shown, full data Fig. 1C and D) but negatively correlated with MoCA score (p < 0.05, rho = –0.45, data not shown). TNFα positively correlated with age (p < 0.05, rho = 0.44, male only data not shown, full data Fig. 1B) when corrected for LEDD score. In female patients IL-17A levels positively correlated with H&Y as well as with both the anxiety (HADS-A, female only data not shown, full data Fig. 1E) and depression (HADS-D) subsets of HADS (p < 0.05, rho = 0.52, 0.77 and 0.75 respectively, Fig. 1E and G). In addition to this IL-17A in female PD patients was negatively correlated with MoCA score (p < 0.05, rho = –0.54, female patients only data not shown, data for all patients in Fig. 1F).
DISCUSSION
We have shown here that plasma inflammatory mediators correlate with motor symptoms and NMS in a cytokine specific manner in PD patients. Specifically, we demonstrate that the pro-inflammatory cytokine IL-6 positively correlates with motor scores while IL-17A correlates with NMS, specifically mood and cognition scores. Furthermore, we show that it may be important to investigate sex specific alterations in peripheral cytokines in PD patients.
While IL-6 and TNFα have been previously investigated in the context of PD, the role of Th cells in the pathophysiology of PD is gaining momentum, and there is a lack of studies investigating peripheral levels of IL-17A in PD patients. IL-17A is a proinflammatory cytokine, mainly produced by T-helper 17 cells when stimulated by cytokines such as IL-6 or IL-1β and stimulates the production of cytokines and chemokines. Increased levels of Th17 cells have been reported in PD patients and IL-17A levels have been found to be increased in supernatant from human induced pluripotent stem cell-induced neurons co-cultured with autologous T cells from PD patients as compared to healthy controls [7, 13]. Previously, IL-17A plasma levels were difficult to detect, however, the SIMOA platform has now overcome this sensitivity issue. Our data suggests that IL-17A does not correlate with motor symptoms but correlates with NMS, including anxiety and depression in PD patients. HADS focuses on questions specific for depression and anxiety excluding questions overlapping with somatic disorders. Although the scale has been criticized for its inability to consistently differentiate between depression and anxiety and accurately assess the levels of the two, it was designed to be cost-effective and has been thoroughly validated and is frequently used by clinicians [12, 14]. We show here that higher IL-17A levels are associated with higher anxiety and depression scores in PD patients. In support of this, IL-17A has previously been shown to positively correlate with anxiety in patients with rheumatoid arthritis [15]. Moreover, in our cohort IL-17A in female PD patients correlated negatively with MoCA scores. There was also a trend for an increase in IL-17A in female PD patients as compared to healthy females, however this did not reach significance when group comparisons were made.
IL-6 and TNFα have well established roles in neuroinflammation, and have been widely investigated in PD. However, results are inconsistent, which, may be due to the sensitivity of methods used. A general increase in IL-6 and TNFα in PD plasma has been reported when compiling data from multiple studies with different level of clinical information [6]. However, a high heterogeneiety among the results is noted with some studies even showing a decrease in proinflammatory cytokines in PD plasma [6]. In the present study, we used highly sensitive SIMOA, with detection limits below that of commercially available ELISA kits. SIMOA is an immunoassay that uses paramagnetic beads coupled with antibodies for a specific target, that in theory can bind and detect single molecules down to femtomolar sample concentrations, and uses a digital readout method of beads that are either bound or not to the antigen. IL-6 has previously been found to be elevated in plasma of PD patients [6] and moreover studies have demonstrated its association with motor scores. Our data provides further evidence that IL-6 correlates with motors symptom severity. Although we did not find any change in IL-6 levels between PD patients compared to controls (Supplementary Figure 1), this may be due to the fact that we used a mixed cohort of patients in our study. The patients ranged from de novo patients to those with severe motor symptoms. Thus discrete changes in a cytokines due to disease duration or severtity may be masked due to the heterogenetiy present when all samples are grouped as PD patients. Interestingly, when we investigated IL-6 levels in female and male patients alone, we found that IL-6 in male patients positively correlates with motor score, while higher IL-6 levels in female patients was associated with worse cognitive performance. These kind of gender differences in cytokines is supported in a PD mouse model, where different pro-inflammatory cytokines increased in male and female mice, and even increased at different time points [16].
The lack of correlations with disease duration, suggests that IL-6 and IL-17A are associated with disease severity rather than disease duration in this cohort. Our data shows for the first time that plasma IL-17A in PD patients correlates with NMS in PD patients, and thus may be involved in the underlying pathophysiology of NMS in PD.
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to declare.
ACKNOWLEDGMENTS
This work was supported by John and Lucille van Geest Foundation at Kings College London, the Swedish Foundation of Strategic Research and the ALF program of Stockholm County Council. Per Svenningsson is a Wallenberg Clinical Scholar.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-191699.
REFERENCES
[1] | Chen JJ , Marsh L ((2014) ) Anxiety in Parkinson’s disease: Identification and management. Ther Adv Neurol Disord 7: , 52–59. |
[2] | Aarsland D , Pahlhagen S , Ballard CG , Ehrt U , Svenningsson P ((2011) ) Depression in Parkinson disease–epidemiology, mechanisms and management. Nat Rev Neurol 8: , 35–47. |
[3] | Svenningsson P , Westman E , Ballard C , Aarsland D ((2012) ) Cognitive impairment in patients with Parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol 11: , 697–707. |
[4] | Halliday GM , Stevens CH ((2011) ) Glia: Initiators and progressors of pathology in Parkinson’s disease. Mov Disord 26: , 6–17. |
[5] | Miller AH , Haroon E , Raison CL , Felger JC ((2013) ) Cytokine targets in the brain: Imact on neurotransmitters and neurocircuits. Depress Anxiety 30: , 297–306. |
[6] | Qin XY , Zhang SP , Cao C , Loh YP , Cheng Y ((2016) ) Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol 73: , 1316–1324. |
[7] | Storelli E , Cassina N , Rasini E , Marino F , Cosentino M ((2019) ) Do Th17 Lymphocytes and IL-17 Contribute to Parkinson’s Disease? A Systematic Review of Available Evidence. Front Neurol 10: , 13. |
[8] | Tesseur I , Nguyen A , Chang B , Li L , Woodling NS , Wyss-Coray T , Luo J ((2017) ) Deficiency in Neuronal TGF-beta Signaling Leads to Nigrostriatal Degeneration and Activation of TGF-beta Signaling Protects against MPTP Neurotoxicity in Mice. J Neurosci 37: , 4584–4592. |
[9] | Picillo M , Nicoletti A , Fetoni V , Garavaglia B , Barone P , Pellecchia MT ((2017) ) The relevance of gender in Parkinson’s disease: A review. J Neurol 264: , 1583–1607. |
[10] | Klein SL , Flanagan KL ((2016) ) Sex differences in immune responses. Nature Reviews Immunology 16: , 626. |
[11] | Nasreddine ZS , Phillips NA , Bedirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[12] | Bjelland I , Dahl AA , Haug TT , Neckelmann D ((2002) ) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: , 69–77. |
[13] | Sommer A , Marxreiter F , Krach F , Fadler T , Grosch J , Maroni M , Graef D , Eberhardt E , Riemenschneider MJ , Yeo GW , Kohl Z , Xiang W , Gage FH , Winkler J , Prots I , Winner B ((2018) ) Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell 23: , 123–131 e126. |
[14] | Cosco TD , Doyle F , Ward M , McGee H ((2012) ) Latent structure of the Hospital Anxiety And Depression Scale: A 10-year systematic review. J Psychosom Res 72: , 180–184. |
[15] | Liu Y , Ho RC , Mak A ((2012) ) The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis 15: , 183–187. |
[16] | Ciesielska A , Joniec I , Kurkowska-Jastrzebska I , Przybylkowski A , Gromadzka G , Czlonkowska A , Czlonkowski A ((2007) ) Influence of age and gender on cytokine expression in a murine model of Parkinson’s disease. Neuroimmunomodulation 14: , 255–265. |




