Parkinson’s Disease in Women and Men: What’s the Difference?
Abstract
Increasing evidence points to biological sex as an important factor in the development and phenotypical expression of Parkinson’s disease (PD). Risk of developing PD is twice as high in men than women, but women have a higher mortality rate and faster progression of the disease. Moreover, motor and nonmotor symptoms, response to treatments and disease risk factors differ between women and men. Altogether, sex-related differences in PD support the idea that disease development might involve distinct pathogenic mechanisms (or the same mechanism but in a different way) in male and female patients. This review summarizes the most recent knowledge concerning differences between women and men in PD clinical features, risk factors, response to treatments and mechanisms underlying the disease pathophysiology. Unraveling how the pathology differently affect the two sexes might allow the development of tailored interventions and the design of innovative programs that meet the distinct needs of men and women, improving patient care.
INTRODUCTION
Parkinson’s disease (PD) is the second most common, age-related neurodegenerative disorder, affecting about 3% of the population by the age of 65 and up to 5% of the people over 85 years [1]. The main pathological feature of PD is the progressive loss of midbrain dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) and the presence of alpha-synuclein positive cytoplasmic inclusions, termed Lewy bodies, in surviving neurons [2]. Degeneration of the nigrostriatal DA pathway leads to the primary motor symptoms of PD, which include bradykinesia, rigidity, resting tremor and gait disturbances. The most common non-motor symptoms associated with the disorder include autonomic dysfunctions, cognitive abnormalities, psychiatric symptoms such as anxiety, depression and apathy, and sleep disorders with high prevalence of insomnia and REM behavior disorder [3]. The vast majority of PD cases occur sporadically, only 10% of patients carrying disease-causing genetic mutations.
Together with aging, genetics, environment and immune status, the role of biological sex as an important factor in the development of PD has been widely discussed in the past decade. There are clear sex-related differences in epidemiological and clinical features of the disease: PD affects men twice more often than women [4, 5], but women have a higher mortality rate and faster progression of the disease [6]. Moreover, women show distinctive symptoms as well as differences in the response to pharmacological therapies and deep brain stimulation procedure, and in the personal evaluation of the quality of life compared with men [7].
Although women diagnosed with PD are a sizable portion of the PD population, their specific needs are still partially overlooked. A retrospective observational study looking at Medicare data— a government-financed health insurance program for the elderly and disabled in the United States— highlighted that women are less likely to have specialist (neurologist) care, together with nonwhites [8]. Accordingly, an a posteriori analysis of multiprofessional treatment approach PD-MCT (i.e., “Parkinson’s disease multimodal complex treatment”) conducted in Germany in the years 2010– 2016 and involving pharmacological and non-pharmacological treatment options such a physical therapy, occupational therapy, and speech therapy, revealed that more male than female patients were treated under this program [9]. A recent study covering 7209 patients at 21 centers in the United States, Canada, the Netherlands, and Israel, disclosed that women are also less likely than men to have informal caregiver support (i.e., support from spouse, family or friends). As a result, more women use paid caregiver services than men. The reasons for this discrepancy may be linked to the longer average lifespan of women and their natural inclination toward being caregivers rather than receivers of care, even when their spouse or caregiver is still present in their lives [6].
Studies considering female sex as a crucial variable to consider are highly under-represented in PD research. In line with this, a notice disseminated by the National Institutes of Health in 2015 exhorts scientists to factor sex into the design, analysis and reporting of vertebrate animal and human studies (NOT-OD-15-102). Together with the NIH initiative, the World Health Organization (WHO) also stated the necessity of the “adequate demographic (including gender) characterization, analysis and assessment of the patient population together with separate ICH (International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use) guideline on women as a special population in clinical trials” as the priority for European and world medicine [10].
This review summarizes the most recent knowledge concerning differences between women and men in PD clinical features, PD risk factors, response to treatments and mechanisms underlying the disease pathophysiology.
CLINICAL DIFFERENCES
According to a recent meta-analysis, an age-related rising incidence of PD is observed in both sexes, but with a steeper increase in males in the 60– 69 and 70– 79 decades of life [11]. The increasing prevalence of PD in men is reported both for disease with and without dementia [12]. Sex-based differences have been also found for factors that influence life prognosis in PD. A recent study, using relative survival methods, showed that a diagnosis of PD with dementia has a larger impact on life expectancy in females than in males [13]. Park and colleagues demonstrated that a low body mass index (<18.5) is strongly associated with reduced survival time, but this reduction is significant only in males [14].
In addition to the differences between women and men in PD prevalence and prognosis, many studies have reported sex-related differences in the clinical phenotype (Fig. 1).
Motor symptoms
Fig.1
Differences in PD symptomatology and risk factors between women and men. PD patients show a different clinical phenotype according to the gender. Moreover, distinct factors seem to contribute to PD risk in women and men. GLA, galactosidase alpha.
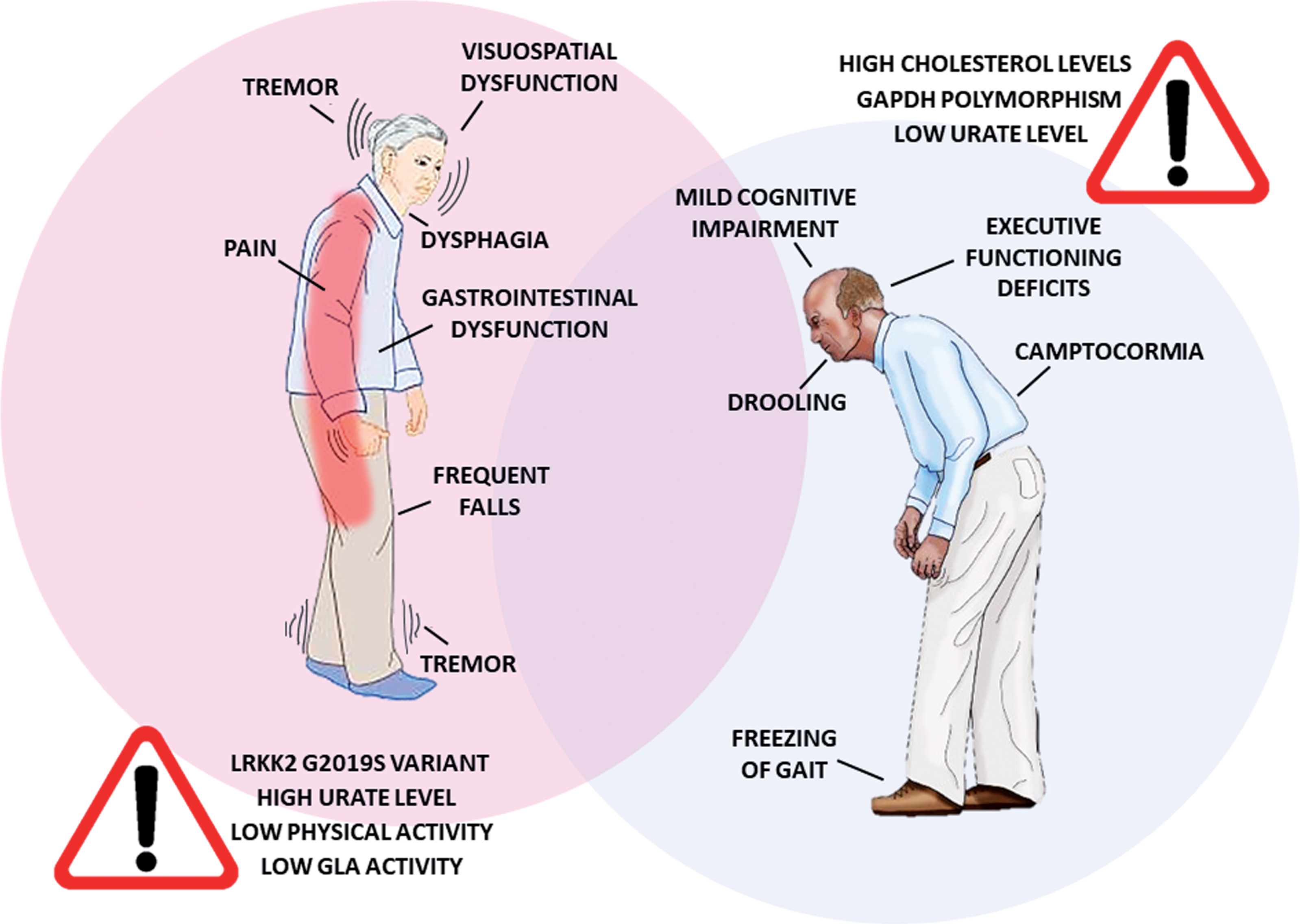
PD diagnosis is essentially based on the presence of motor symptoms. The characterization of possible sex-related differences in motor symptom patterns may play a crucial role in terms of diagnostic accuracy and therapeutic strategies. Over the past decades, the effect of biological sex on the expression and severity of PD motor symptoms has been broadly discussed in the literature. Motor symptoms emerge later in women, with specific characteristics such as reduced rigidity [15], tremor as a more common first presenting symptom [16], higher propensity to develop postural instability and elevated risk for levodopa-related motor complications [17]. Male sex, on the other hand, has been recently associated with later development of freezing of gait— the most disabling motor complication of PD [18]— whereas female sex was mentioned in the list of predictors of progression to falling in PD [19]. Another specific motor disturbance of PD is camptocormia, which refers to abnormal severe forward flexion of the trunk that occurs while standing or walking and abates or disappears in a supine position. It has been recently reported that PD male patients have higher risk of developing this symptom along the disease progression [20]. An ongoing clinical trial is evaluating the prevalence in PD and the biological sex impact on other postural abnormalities, such as Pisa syndrome, antecollis, scoliosis and striatal deformities related to hand and/or toes (ClinicalTrials.gov Identifier: NCT03573232).
Non-motor symptoms
Martinez-Martin and colleagues performed a complex study in a cohort of 951 PD patients to assess the prevalence and severity of non-motor symptoms according to biological sex. They concluded that symptoms such as fatigue, depression, restless legs, constipation, pain, loss of taste or smell, weight change and excessive sweating are more severe and common in women [21].
The relationship between female sex and pain has been recently confirmed in a large clinical study demonstrating that, together with affective and autonomic symptoms, motor complications and younger age, female sex predicts overall pain severity [22]. A clinical trial designed to study the pathophysiology underlying spontaneous and evoked pain in PD patients with motor fluctuations is currently ongoing (NCT03648671); among other factors, the trial aims to evaluate the impact of biological sex on the quality and distribution of different pain syndromes associated with PD.
Within the autonomic disturbances occurring in PD, gastrointestinal dysfunctions play a major role, also because of their profound impact on the quality of life. Based on the evidence that intestinal inflammation is consistent with intestinal symptoms and may act as a driver of disease pathology, Housers and colleagues conducted an extensive analysis of immune and angiogenetic factors in stool of PD patients and healthy controls. They found disease-associated increase in numerous immune and angiogenesis mediators, but only in the stool of female PD patients while the stool of male patients did not differ significantly from controls [23]. Female PD patients have also shown higher predisposition to develop critical dysphagia [24], whereas male patients are more prone to severe drooling [25].
Different studies showed that male PD patients have worse general cognitive abilities and male sex is the primary predictive factor for mild cognitive impairment and its more rapid progression in the severe stage of the disease [26– 28]. Accordingly, female PD patients perform better at the Symbol Digit Modalities Test, verbal fluency tests and overall cognition measured by the Montreal Cognitive Assessment scale, but present with worse visuospatial function [29]. Significant sex differences are also observed for frontal executive abilities (attention and working memory), with male PD patients showing greater deficits than female non-demented patients.
Regarding PD-associated complications, female sex is associated with more severe, persistent and episodic anxiety and profound depression [30, 31], whereas impulse control disorders, such as pathological gambling and hypersexuality, are more common in male PD patients [32– 34]. Nevertheless, men show greater sexual dysfunction and impairment of their sexual relationship than women [35]. Biological sex also differently impact on emotion processing in PD with men showing worse recognition performance of the emotion anger accompanied by reduced neural response [36].
REM sleep behavior disorder (RBD), the strongest known prodromal symptom of neurodegenerative synucleinopathies, is classically associated with male sex [37]. However, a recent large population-based study reverted the traditional RBD clinical profile showing no difference between men and women [38]. Women generally show less aggressive and injurious RBD than men, thus they are less prone to present for medical attention. According to the authors, this factor might have introduced a bias in the previous studies.
Quality of life
Gender also affects how clinical symptoms affect quality of life of PD patients and their ability to perform activities of daily living, to participate in social activities and to access medical care. The health-related quality of life (HrQoL) is a multi-dimensional scale used to evaluate the impact of disease and treatments on the lives of patients. A recent study exploring the relationship between three HrQoL domains (physical-functioning, cognition, socioemotional) and sociodemographic variables did not reveal a global gender effect [39]. However, female gender proved to be a negative predictor for physical-functioning and socioemotional HrQoL, whereas male gender mainly affected the HrQoL cognition domain. Conversely, in a prospective study on a cohort of patients with idiopathic PD in Germany, female patients reported more problems in all dimensions, except selfcare [40]. At the same time, a longitudinal study designed to analyze the effect of PD onset on life satisfaction showed marked reduction of life satisfaction among individuals in the second half of life in men, but not in women [41].
DISEASE PREDICTORS AND RISK FACTORS
The etiology of PD is not well understood. Despite the presence of familial cases, PD is substantially an idiopathic, multi-factorial disease caused by the interplay between genetic and environmental factors. Genetic studies have identified increasing numbers of risk polymorphisms, whereas little is still known about environmental risk factors and how these affect PD risk.
Heinzel and coll. recently highlighted the sex-related differences in prodromal PD. They concluded that women and men show distinctive prodromal markers of PD (subthreshold parkinsonism, constipation, olfactory loss, depression, probable RBD, nonsmoking, nonuse of caffeine, nigral hyperechogenicity), suggesting that these differences should be taken into account to guarantee the diagnostic accuracy of prodromal PD [42].
Genetic risk factors
Urate, an endogenous purine metabolite with antioxidant and neuroprotectant properties, is a genetically and environmentally determined modifiable factor and a potential PD biomarker, as high urate levels are associated with reduced risk and slower progression of idiopathic PD [43, 44]. However, the contribution of urate levels to PD risk according to biological sex is still controversial. Higher urate levels were associated with reduced prevalence and slower progression of PD in men, while the opposite trend was observed in women [45, 46]. More recently, a large population-based study conducted in Norway demonstrated that the treatment with urate-lowering drugs correlates with lower PD risk especially in men, even if no statistically significant difference by sex was detected. In addition, the association varied significantly by age among women, showing a protective effect only in women above 70 years, when urate levels are comparable to those in men [47]. The neuroprotective effect of urate in men, especially in terms of cognitive functions, seems to be related to its ability to influence resting-state networks (RSN), which reflect the spontaneous neural activities and provide indirect information regarding brain functional status [48]. Interestingly, a recent study focused on the potential of urate plasma levels in subject carrying mutations in the gene encoding leucine-rich repeat kinase 2 (LRRK2) [49], the most common genetic cause of PD [50]. The study demonstrated that subjects with a LRRK2 mutation who had not developed PD had higher urate levels than LRRK2-PD subjects; moreover, a significant difference in urate levels between control subjects and PD patients was observed among women with LRRK2 mutations [49]. The strength of the urate association observed in both women and men with LRRK2 mutations may help to explain the comparable PD penetrance of LRRK2 mutations between sexes, in contrast to the well-established lower risk of idiopathic PD in women. Although this seems to be valid for the most part of variants in LRRK2, a recent meta-analysis highlighted that carriers of LRRK2 G2019S variant are predominantly females [51] and show sex-dependent phenotypic differences, with cognitive impairment and depression being less common in G2019S male carriers compared with females.
The association between mutations in GBA1 gene, which encodes the lysosomal enzyme glucocerebrosidase (GCase), and PD development has highlighted the potential role of mutations in lysosome-related genes as risk factors for PD. Since lysosomes are involved in the degradation of alpha-synuclein, lysosomal dysfunction could trigger alpha-synuclein accumulation, thus contributing to PD pathogenesis [52]. Alcalay and coll. showed a link between PD status and reduced activity of galactosidase alpha (GLA), a lysosomal enzyme encoded by a gene on the X chromosome whose mutations cause Fabry’s disease. Differently from GCase, this association should be sex-dependent, reaching significance among women only [53].
Lastly GAPDH gene, encoding a highly conserved protein involved in various cellular processes (e.g., glycolysis metabolism, mitochondria damage, autophagy) was identified as another gene with a potential role in PD [54]. Ping and coll. showed that rs1136666 polymorphism of GAPDH strongly correlates with sporadic PD and increases PD risk in older male [55].
Environmental risk factors
One of the most prominent environmental factors connected to elevated PD risk is chronic stress [56]. Adverse psychosocial work conditions are a potential source of stress relevant for public health, which can be described by the “job demand-control” model. This model consists of two dimensions: the “demand component” measuring time pressure and psychological/cognitive demands and the “control component” including decision making authority and skill discretion abilities. A recent study on the association between occupational stress according to this model and the risk for PD showed that high job demands appear to increase PD risk in men, especially in men with high education, whereas high job control increases PD risk more strongly in low educated women [57].
Occupational exposure to environmental factors with neurotoxic potential has received considerable attention in the PD field. Increased risk of PD has been classically reported in specific occupational groups, including farmers, wood workers, painters, metallurgy and medical workers exposed to pesticides, solvents and metals [58]. A recent nationwide study conducted in France, based on a comprehensive analysis of industry sectors, showed a significant association between PD incidence and specific sectors (e.g., agriculture, metallurgy, textile). However, the difference in the distribution of occupations and exposure patterns between men and women complicate the realistic assessment of gender-related impact of this risk factor [59].
A recent population-based, longitudinal large-scale cohort study conducted in statin-free individuals examined the association between cholesterol levels and PD risk. The authors found that total cholesterol levels greater than 180 mg/dL and low-density lipoprotein cholesterol levels greater than 110 mg/dL are associated with a decreased risk of PD in middle-aged men and elderly women [60]. The age-pooled analyses also showed a significantly reduced PD risk for men, but not for women. Given the absence of the potentially confounding effect of statin use, this study could provide a relevant contribution in clarifying the controversial relationship between cholesterol and PD. Although the study was not designed to provide mechanistic information, the authors suggested a modulatory effect of sex hormones on lipoprotein metabolism and apolipoprotein E phenotype.
Physical activity is another important lifestyle factor that may affect PD onset, severity and progression. High levels of exercise in midlife are associated with lower PD risk, better disease prognosis and lower rates of serious complications [61]. A large international multicenter cohort study on early PD patients showed that higher self-reported activity scores were associated with younger age and male gender. Older patients, especially women, may be particularly vulnerable to inactivity and its complications [62].
BIOLOGICAL SEX AND PD THERAPY
Pharmacological therapy of motor symptoms
In the absence of a disease-modifying therapy, PD treatment is currently based on the control of motor symptoms by levodopa supplementation. However, long-term therapy with levodopa is associated with the development of motor complications, such as levodopa-induced-dyskinesia, wearing off and on-off phenomena. It is generally assumed that dyskinesia is associated with sustained levodopa plasma levels [63]. Commonly, women present greater levodopa bioavailability, which is further supported by lower levodopa clearance levels [64, 65]. Dopamine bioavailability in the central nervous system is dependent on the activity of two catabolic enzymes: catechol-O-methyltransferase (COMT) and monoamine oxidase-B (MAO-B), whose encoding genes are located on the chromosome 22 and X chromosome, respectively [66]. A study that explored the relationship between MAO-B or COMT functional SNPs and levodopa therapy reported that male (but not female) PD patients carrying the MAO-B G allele had a 2.84-fold increased risk of developing motor complications when treated with high doses of levodopa [67].
The main genetic variant of the DRD2 gene, encoding for the D2 dopamine receptor, is TaqIA (SNP rs1800497), which has been associated with higher frequency of motor fluctuations and dyskinesia in response to PD treatment [68]. The SLC6A3 gene encodes the dopamine transporter and the most studied polymorphism of this locus (SNP rs28363170) has been associated with an increased susceptibility to PD in selected different populations, although not unanimously [69]. In a very recent study conducted in a Brazilian cohort of PD patients, an association between rs1800497, rs28363170 SNPs and susceptibility to levodopa-induced dyskinesia was described, with a significant protective effect played by age and male sex [70].
Pharmacological therapy of non-motor symptoms
Antipsychotic are an important drug class for the treatment of patients with PD or dementia with Lewy bodies in cases where hallucinations and psychosis can be disabling. However, these drugs have been associated with increased mortality and morbidity in this population, especially in older PD patients. Two independent studies on Canadian cohorts of PD patients under treatment with antipsychotic drugs showed that older age and male sex were significantly associated with an increased rate of antipsychotic prescriptions during follow-up [71]. As reported in another study, male PD patients more often receive a prescription of antipsychotic drugs in the absence of a clear psychosis diagnosis, with respect to female patients. This, as suggested by the authors, may be related to the fact that male patients are more prone to become aggressive and difficult to assist than women, when the disease is complicated by psychosis [72].
Surgical procedures
Recently, subthalamic nucleus deep brain stimulation (STN DBS) has been proposed as a promising therapeutic tool for the management of abnormal trunk and neck postures commonly affecting patients in an advanced stage of the disease. Male sex was identified as a predictor of STN DBS-induced improvement in upper camptocormia, with a trend towards greater improvement also in lower camptocormia and global postural angle [73]. Curiously, despite the higher motor improvement in males, quality of life measures improved more in women than in men [74].
PD and steroids
The epidemiological evidence of sex differences in PD suggests a possible beneficial activity of female gonadal hormones on the dopaminergic system, particularly of circulating estradiol, which may act as a neuroprotective agent. Therefore, several observational studies have investigated the relationship between estrogen exposure and PD risk. Women with higher cumulative estrogen exposure over their lifetime have a significantly reduced PD risk [75]. Estrogens have also proved effective in improving PD symptoms and levodopa-induced dyskinesia [76, 77]. One study reported beneficial effects of 17b-estradiol also in a male PD patient with severe motor fluctuations and dyskinesias [78].
Due to their peripheral action on reproductive organs, which can promote the risk of cancer in both sexes [79] and the feminizing effect in males, estrogens cannot be recommended as a treatment for PD. A different line of reasoning may apply to selective estrogen receptor modulators (SERMs), a promising group of drugs that exert estrogen antagonist activity in the mammary tissue while mimicking the effects of estrogen in other tissues, such as bone and uterus. SERMs are used in the clinical practice to treat and prevent osteoporosis, and to reduce risk of breast cancer in postmenopausal women [80]. Whereas the effect of SERMs in patients with PD has not been documented, there are indications that they may display beneficial effects on the of elderly brain, such a reduced risk of mild cognitive impairment and improvement of verbal memory in women [81, 82] and enhancement of attention, memory and executive function in men without inducing feminizing effects [83].
There is some evidence that also progesterone metabolism is impaired in PD, since the concentration of the enzyme 5a-reductase - responsible for the conversion of progesterone to its metabolites - has been found reduced in the SN of PD patients [84]. The 5a-reductase inhibitors have received attention for their role in DA neurotransmission, with potential therapeutic effects in several disorders associated with dopaminergic hyperactivity [85]. In animal models, these agents were shown to reduce the development and expression of levodopa-induced dyskinesia in both female and male rats [86], thus making this class of drugs potential candidates for the reduction of the side effects related to dopaminergic medications.
Fig.2
Impact of biological sex on PD pathophysiology. The figure summarizes the main sex-related differences in the key players of PD pathogenesis, focusing attention on the vulnerability of dopaminergic system (upper part), neuroinflammatory cells (central part) and oxidative stress (lower part). IP10, interferon-inducible protein 10.
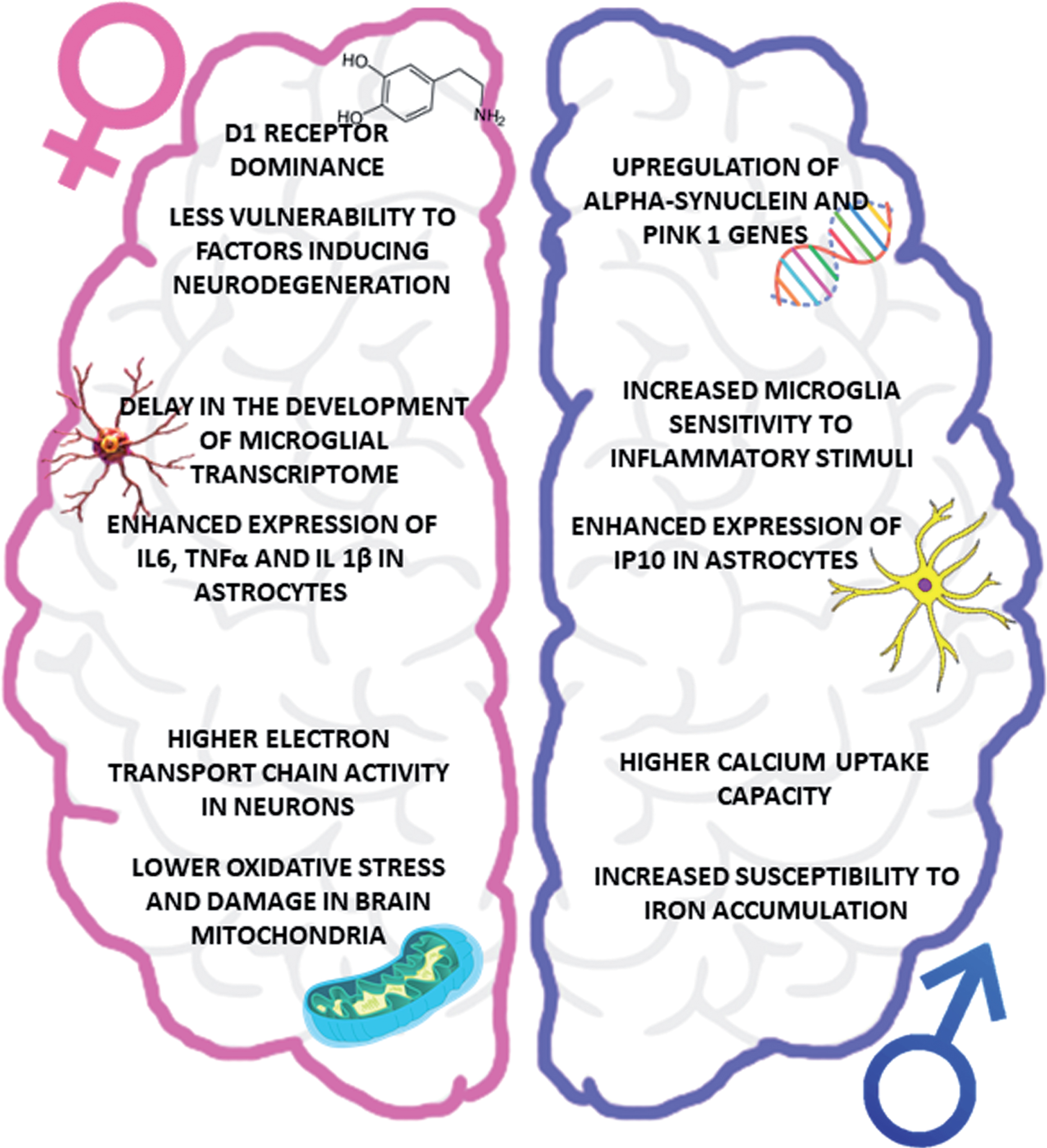
IMPACT OF BIOLOGICAL SEX ON PD PATHOPHYSIOLOGY
The distinctive clinical features as well as the contribution of different risk factors support the idea that PD development might involve distinct pathogenetic mechanisms (or the same mechanism but in a different way) in male and female patients. It is clear that estrogens play a preponderant role in the sex differences in PD, providing disease protection as demonstrated by the similar incidence of the disease in men and post-menopausal women. Moreover, it is noteworthy that sex hormones act throughout the entire brain of both males and females and sex differences are now highlighted in brain regions and functions not previously considered as subjected to such differences, opening the way to a better understanding of gender-related behavior and functions.
This section presents an overview of the most recent evidence corroborating the hypothesis of a sex-related PD pathophysiology, with a special focus on the role of estrogens (Fig. 2).
Dopaminergic neurodegeneration
Dopaminergic neurons in the substantia nigra are highly vulnerable to stress conditions, compared to other neuronal types. This vulnerability is ascribable to different factors, such as high oxidative burden during dopamine metabolism, excitotoxicity, high iron content and low mitochondrial mass. Consequently, sex differences in these factors may account for the different propensity of dopaminergic neurons to degenerate.
Gene expression studies in human SNc dopamine neurons of control and PD subjects revealed a sex-specific genomic signature: genes that are upregulated in females are mainly involved in signal transduction and neuronal maturation, while in males upregulated genes encode proteins involved in PD pathogenesis, such as alpha-synuclein and PINK1 [87, 88]. Together with differential gene expression, a stereological analysis of dopamine receptor 1 (D1) and 2 (D2) distribution during development highlighted maturational changes in the expression of these receptors between the sexes. In particular, females had a higher D1:D2 ratio compared to males in all regions analyzed (including dorsal and ventral striatum) except for the insular cortex, suggesting that D1 dominance may make one resilient to some disorders (e.g., addiction) but vulnerable to others (e.g., anxiety) [89].
Female dopaminergic cells generally show lesser vulnerability to factors inducing degeneration than male neurons. Accordingly, reserpine-treated female rats did not show any reduction of tyrosine hydroxylase immunoreactivity in the SNc and dorsal striatum compared to males [90]. The lack of Ras homolog enriched in striatum (Rhes), a protein that exert pleiotropic effects on cell function, in KO mice induced a decrease in tyrosine hydroxylase expression in males only, while KO females showed resistance to DA neuron degeneration, which tended to decrease only with aging [91, 92]. Interestingly, neuronal loss in male SNc is accompanied by a significant reduction in total ganglioside content, especially neuronal- or synaptic-enriched gangliosides GD1a and GT1b. No significant abnormalities were found in female PD subjects [93]. Accordingly, it has been demonstrated that estradiol acts as homeostatic modulator of lipid rafts, thus preserving lipid balance in neuronal membrane microdomains [94].
The specific impact of estradiol on dopamine metabolism mainly accounts for the reduced vulnerability observed in female brain. It is well known that estradiol increases the synthesis, release, reuptake and turnover of DA. Moreover, this hormone potentiates amphetamine-stimulated DA release in the dorsolateral striatum in ovariectomized rats by interaction with estradiol and mGlu5 receptors [95]. Nevertheless, Conway and coll. demonstrated that dopamine production and release in female rats can also be independent by estrogens and provide protection from kappa opioid receptors-mediated negative effects on brain stimulation reward [96].
Neuroinflammation
Neuroinflammation is an important piece of the pathogenic puzzle of PD. Current evidence suggests that the physiological role exerted by microglial and astrocytic cells could become compromised during aging, thus contributing to PD onset and progression [97– 100]. Since estrogens have anti-inflammatory properties, their actions throughout the lifespan could partially account for sex-related risk and manifestation of PD.
Transcriptomic analysis of male and female microglia isolated from adult healthy mice revealed that microglia is sexually differentiated and that its sensitivity and ability to respond to specific hormonal and environmental stimuli is affected by the neonatal estrogen priming which permanently induces a sexual phenotype that is maintained in the adult animals [101]. In particular, estrogen priming of male microglia changes their immune capacity by increasing the ability to react to inflammatory stimuli. Accordingly, Hanamsagar and coll. have demonstrated that the mechanisms driving development and immune reactivity in microglia are dissociable in males vs. females and that male microglial transcriptome is more developmentally mature than female microglia [102]. As mentioned before, postnatal sexual dimorphism persists in the adult brain, as demonstrated by the differences in the number and morphology of microglia in different anatomical regions (i.e., hippocampus, cortex and amygdala) between males and females [103, 104]. This heterogeneity may lead to development of distinct, sex-dependent microglia inflammatory responses in the brain. Several studies in animal models of PD showed that the neuroprotective and symptomatic effect of estrogens is due to their ability to attenuate microglia activation and to modulate microglia polarization toward a cytoprotective phenotype [105– 107].
Similarly to microglia, also astrocytes show sex differences under physiological and pathological conditions. Male and female cortical astrocytes respond differentially to an inflammatory challenge, such as a treatment with lipopolysaccharide [108], leading to increased levels of distinct inflammatory factors. In detail, male astrocytes showed enhanced expression of IL6, TNFα and IL1β after LPS treatment whereas the levels of interferon-inducible protein 10 were higher in astrocytes derived from females. Further, a recent study demonstrated sex-dependent mitochondrial bioenergetic properties of rat cortical astrocytes, with male astrocytes having a higher maximal respiration than female astrocytes at low physiologically relevant oxygen tension [109].
Astrocytes participate in the protective actions of estrogenic compounds [110]. Recent findings point to the role of these inflammatory cells in mediating the effect of sex hormones on cognition. The proven role of estrogens in hippocampal plasticity and the evidence of estrogen effects on memory, suggested a positive relationship between estrogens and cognition. Yun and coll. demonstrated that estrogens might protect against memory impairment through the regulation of neurogenic inflammation by inhibiting NF-κB activity [111]. Analogously, the reduction of estradiol level and the expression of its receptors in hippocampus of aged female rat has been shown to contribute to the deficit of spatial memory performance in the Morris water maze test [112]. Nevertheless, a recent paper recommended to carefully consider the positive effect of estrogens on cognition, suggesting that these hormones can enhance and impair learning depending on the memory system [113]. In this context, astrocytes would play the role of providers of metabolic substrates - especially lactate - during cognition, thus mediating the opposing effects of estrogens on learning. Most interestingly, a study on adult zebra finches indicates that astrocytes can increase estradiol synthesis through cytokine-dependent up-regulation of aromatase and release of cytokines; this, in turn, could alter metabolic and neural plasticity phenomena that are essential for learning and memory [114].
Oxidative stress
Different factors seem to contribute to oxidative stress in PD, including dopamine metabolism, mitochondrial dysfunction, iron overload, neuroinflammation, calcium dysregulation and aging.
In particular, given the vital functions exerted by mitochondria and the high metabolic rate and increased sensitivity to oxidative damages of the brain, the maintenance of mitochondrial homeostasis is central to neuronal viability and function. Because of their exclusive maternal transmission, mitochondria exhibit a strong sex-specific behavior and exert differential effects in males and females. Gender indiscriminately impacts on all mitochondria functions. Studies conducted in animals and postmortem human samples showed that female neurons have higher electron transport chain activity and greater functional capacities compared with male neurons [115– 117]. Generally, lower oxidative stress and damage have been observed in brain mitochondria from female rats compared with males, independently of age and estrus cycle [115, 118]. Contrary to positive influence of female gender on cell respiration and generation of redox oxygen species, calcium uptake capacity is lower in female than male brain mitochondria [119], which can negatively affect mitochondrial calcium buffering and consequently cell homeostasis.
Mitochondria are both the site of steroidogenesis and target of sex-steroids in stimulating mitochondrial functions, especially biogenesis. Estradiol counteracted the loss of mitochondria in aged female hippocampus, restoring mitochondrial number to the levels observed in young animals [120]. G-1, an agonist of G protein-coupled estrogen receptor (GPER), reversed the reduction of estrogen receptors in the hippocampus of 16-month-old female rats, increased mitochondrial membrane potential and activity of anti-oxidant enzymes [121]. These effects are accompanied by a relief of anxiety and depression-like behavior suggesting GPER as a potential therapeutic target for estrogen deficiency-related affective disorders. Analogously, estradiol and the selective estrogen receptor modulators tamoxifen and raloxifene have been shown to reduce oxidative stress, apoptosis, mitochondrial membrane depolarization and Ca2+ influx through the inhibition of thermosensitive transient receptor potential (TRP) channels [122]. Nakano and coll. showed that esculetin, a small chemical with agonist action on all three estrogen-related receptors, enhances glycolysis and mitochondrial respiration when added to culture media of neuronally differentiated PC12 cells, leading to elevated cellular ATP levels [123]. Interestingly, the effects of estrogens are not restricted to neuronal cells as demonstrated by the protective outcomes of the treatment with tibolone— a synthetic steroid with estrogenic, progestogenic and androgenic actions— on astrocytic mitochondria [124].
Although the sexual dimorphism in brain mitochondria has been proven, few recent studies have dealt with sex-related differences of oxidative stress in the context of PD. Sex variance in prooxidant-antioxidant balance and malondialdehyde levels, a product of lipid peroxidation, has been observed in PD patients [125]. Interestingly, a recent neuromelanin (NM) imaging study highlighted a bigger normalized NM-rich volume in the women SN compared with men older than 47 years, suggesting that this difference may underpin the high male-to-female ratio of the PD prevalence [126]. Neuromelanin, a pigment with paramagnetic properties, acts as a scavenger removing potentially toxic substances through the autooxidation of catecholamines and/or binding redox-active metal ions such as iron. The relationship between estrogens and iron in PD has been known for decades. Experimental and epidemiological evidence suggest that estrogens play a regulatory role in iron metabolism. Striatum of male mice has been shown more susceptible to iron accumulation than female [127]. Analogously, a study conducted on humans showed that at the same plasmatic concentrations of iron, women had a lower probability of having PD [128]. Recently, it has been clarified that estradiol-related effects on iron metabolism exploit different pathways between the sexes, with GPER1 mediating the suppressive effects of estradiol on iron overload-induced autophagy in males while estrogen receptor α on induced lipid peroxidation in females [129]. Moreover, Xu et al., discovered a cell-dependent regulation of iron metabolism by estrogens. According to these findings, estrogen would increase the expression of iron exporter ferroportin 1 (FPN1) and iron importer divalent metal transporter 1 (DMT1) in astrocytes, whereas the down-regulation of iron regulatory protein may account for the decreased DMT1 and increased FPN1 expression in neurons [130].
CONCLUSION
Increasing experimental and clinical evidence supports the idea that PD differs between women and men. Not only do men and women experience the disease differently, but different mechanisms seem to be involved in the pathogenesis of the disease. Nevertheless, we are still far away from the actual understanding of what underlies such differences. Studies in this area are under-represented, both from the clinical and research perspective, especially for females. In line with this, governmental and private initiatives are strongly encouraging scientists and clinicians to have special consideration for gender characterization and sex-specific issues in PD. Recently, the Parkinson’s Foundation, a US-based national organization, created a national agenda to identify research and care practices that better capture the needs of women. The overall goal is to develop tailored interventions and design innovative programs that meet the distinct requirements of men and women with PD. It is a line that is worth pursuing and will deserve further attention by the scientific community and policy makers.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Dexter DT , Jenner P ((2013) ) Parkinson disease: From pathology to molecular disease mechanisms. Free Radic Biol Med 62: , 132–144. |
[2] | Heller J , Dogan I , Schulz JB , Reetz K ((2014) ) Evidence for gender differences in cognition, emotion and quality of life in Parkinson’s disease? Aging Dis 5: , 63–75. |
[3] | Picillo M , Erro R , Amboni M , Vitale C , Iavarone A , Moccia M , Allocca R , Orefice G , Barone P ((2014) ) Gender differences in non-motor symptoms in early Parkinson’s disease: A 2-years follow-up study on previously untreated patients. Parkinsonism Relat Disord 8: , 850–854. |
[4] | Baldereschi M , Di Carlo A , Rocca WA , Vanni P , Maggi S , Perissinotto E , Grigoletto F , Amaducci L , Inzitari D ((2000) ) Parkinson’s disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 9: , 1358–1363. |
[5] | Solla P , Cannas A , Ibba FC , Loi F , Corona M , Orofino G , Marrosu MG , Marrosu F ((2012) ) Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson’s disease. J Neurol Sci 323: , 33–39. |
[6] | Dahodwala N , Shah K , He Y , Wu SS , Schmidt P , Cubillos F , Willis AW ((2018) ) Sex disparities in access to caregiving in Parkinson disease.48-e. Neurology 90: , 54. |
[7] | Georgiev D , Hamberg K , Hariz M , Forsgren L , Hariz G-M ((2017) ) Gender differences in Parkinson’s disease: A clinical perspective. Acta Neurol Scand 136: , 570–584. |
[8] | Willis AW , Schootman M , Evanoff BA , Perlmutter JS , Racette BA ((2011) ) Neurologist care in Parkinson disease: A utilization, outcomes, and survival study. Neurology 77: , 851–857. |
[9] | Richter D , Bartig D , Muhlack S , Hartelt E , Scherbaum R , Katsanos AH , Müller T , Jost W , Ebersbach G , Gold R , Krogias C , Tönges L ((2019) ) Dynamics of Parkinson’s disease multimodal complex treatment in Germany from 2010-2016: Patient characteristics, access to treatment, and formation of regional centers. Cells 8: , E151. |
[10] | Kaplan W , Wirtz VJ , Mantel-Teeuwisse A , Stolk P , Duthey B , Laing R ((2013) ) Priority Medicines for Europe and the World 2013 Update. World Health Organization in collaboration with Utrecht University and Boston University, Geneva. |
[11] | Hirsch L , Jette N , Frolkis A , Steeves T , Pringsheim T ((2016) ) The incidence of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 46: , 292–300. |
[12] | Savica R , Grossardt BR , Rocca WA , Bower JH ((2018) ) Parkinson disease with and without dementia: A prevalence study and future projections. Mov Disord 33: , 537–543. |
[13] | Larsson V , Torisson G , Londos E ((2018) ) Relative survival in patients with dementia with Lewy bodies and Parkinson’s disease dementia. PLoS One 8: , e0202044. |
[14] | Park K , Oeda T , Kohsaka M , Tomita S , Umemura A , Sawada H ((2018) ) Low body mass index and life prognosis in Parkinson’s disease. Parkinsonism Relat Disord 55: , 81–85. |
[15] | Baba Y , Putzke JD , Whaley NR , Wszolek ZK , Uitti RJ ((2005) ) Gender and the Parkinson’s disease phenotype. J Neurol 252: , 1201–1205. |
[16] | Haaxma CA , Bloem BR , Borm GF , Oyen WJ , Leenders KL , Eshuis S , Booij J , Dluzen DE , Horstink MW ((2007) ) Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78: , 819–824. |
[17] | Colombo D , Abbruzzese G , Antonini A , Barone P , Bellia G , Franconi F , Simoni L , Attar M , Zagni E , Haggiag S , Stocchi F ((2015) ) The “gender factor” in wearing-off among patients with Parkinson’s disease: Aanalysis of DEEP study. Scientific World Journal 2015: , 787451. |
[18] | Kim R , Lee J , Kim Y , Kim A , Jang M , Kim HJ , Jeon B , Kang UJ , Fahn S ((2018) ) Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson’s disease: An analysis of the PPMI cohort. Parkinsonism Relat Disord 51: , 49–54. |
[19] | Parashos SA , Bloem BR , Browner NM , Giladi N , Gurevich T , Hausdorff JM , He Y , Lyons KE , Mari Z , Morgan JC , Post B , Schmidt PN , Wielinski CL ((2018) ) What predicts falls in Parkinson disease? Observations from the Parkinson’s Foundation registry. Neurol Clin Pract 8: , 214–222. |
[20] | Ou R , Liu H , Hou Y , Song W , Cao B , Wei Q , Yuan X , Chen Y , Zhao B , Shang H ((2018) ) Predictors of camptocormia in patients with Parkinson’s disease: A prospective study from southwest China. Parkinsonism Relat Disord 52: , 69–75. |
[21] | Martinez-Martin P , Falup Pecurariu C , Odin P , van Hilten JJ , Antonini A , Rojo-Abuin JM , Borges V , Trenkwalder C , Aarsland D , Brooks DJ , Ray Chaudhuri K ((2012) ) Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J Neurol 259: , 1639–1647. |
[22] | Silverdale MA , Kobylecki C , Kass-Iliyya L , Martinez-Martin P , Lawton M , Cotterill S , Chaudhuri KR , Morris H , Baig F , Williams N , Hubbard L , Hu MT , Grosset DG ; UK Parkinson’s Pain Study Collaboration ((2018) ) A detailed clinical study ofpain in 1957 participants with early/moderate Parkinson’s disease. Parkinsonism Relat Disord 56: , 27–32. |
[23] | Houser MC , Chang J , Factor SA , Molho ES , Zabetian CP , Hill-Burns EM , Payami H , Hertzberg VS , Tansey MG ((2018) ) Stool immune profiles evince gastrointestinal inflammation in Parkinson’s disease. Mov Disord 33: , 793–804. |
[24] | Nienstedt JC , Bihler M , Niessen A , Plaetke R , Pötter-Nerger M , Gerloff C , Buhmann C , Pflug C ((2019) ) Predictive clinical factors for penetration and aspiration in Parkinson’s disease. Neurogastroenterol Motil 3: , e13524. |
[25] | Mao CJ , Xiong YT , Wang F , Yang YP , Yuan W , Zhu C , Chen J , Liu CF ((2018) ) Motor subtypes and other risk factors associated with drooling in Parkinson’s disease patients. Acta Neurol Scand 137: , 509–514. |
[26] | Cholerton B , Johnson CO , Fish B , Quinn JF , Chung KA , Peterson-Hiller AL , Rosenthal LS , Dawson TM , Albert MS , Hu SC , Mata IF , Leverenz JB , Poston KL , Montine TJ , Zabetian CP , Edwards KL ((2018) ) Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord 50: , 29–36. |
[27] | Szewczyk-Krolikowski K , Tomlinson P , Nithi K , Wade-Martins R , Talbot K , Ben-Shlomo Y , Hu MT ((2014) ) The influence of age and gender on motor and non-motor features of early Parkinson’s disease: Initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord 20: , 99–105. |
[28] | Liu R , Umbach DM , Peddada SD , Xu Z , Tröster AI , Huang X , Chen H ((2015) ) Potential sex differences in non-motor symptoms in early drug-naïve Parkinson disease. Neurology 84: , 2107–2115. |
[29] | Lin SJ , Baumeister TR , Garg S , McKeown MJ ((2018) ) Cognitive profiles and hub vulnerability in Parkinson’s disease. Front Neurol 9: , 482. |
[30] | Broen MPG , Leentjens AFG , Hinkle JT , Moonen AJH , Kuijf ML , Fischer NM , Perepezko K , Bakker A , Pontone GM ((2018) ) Clinical markers of anxiety subtypes in Parkinson disease. J Geriatr Psychiatry Neurol 31: , 55–62. |
[31] | Wissel BD , Dwivedi AK , Merola A , Chin D , Jacob C , Duker AP , Vaughan JE , Lovera L , LaFaver K , Levy A , Lang AE , Morgante F , Nirenberg MJ , Stephen C , Sharma N , Romagnolo A , Lopiano L , Balint B , Yu XX , Bhatia KP , Espay AJ ((2018) ) Functional neurological disorders in Parkinson disease. J Neurol Neurosurg Psychiatry 6: , 566–571. |
[32] | Bhattacharjee S ((2018) ) Impulse control disorders in Parkinson’s disease: Review of pathophysiology, epidemiology, clinical features, management, and future challenges. Neurol India 66: , 967–975. |
[33] | Weintraub D , Koester J , Potenza MN , Siderowf AD , Stacy M , Voon V , Whetteckey J , Wunderlich GR , Lang AE ((2010) ) Impulse control disorders in Parkinson disease: A cross-sectional study of 3090 patients. Neurol 67: , 589–595. |
[34] | Kon T , Ueno T , Haga R , Tomiyama M ((2018) ) The factors associated with impulse control behaviors in Parkinson’s disease: A 2-year longitudinal retrospective cohort study. Brain Behav 8: , 01036. |
[35] | Buhmann C , Dogac S , Vettorazzi E , Hidding U , Gerloff C , Jurgens TP ((2017) ) The impact of Parkinson’s disease on patients’ sexuality and relationship. J Neural Transm (Vienna) 128: , 983–996. |
[36] | Heller J , Mirzazade S , Romanzetti S , Habel U , Derntl B , Freitag NM , Schulz JB , Dogan I , Reetz K ((2018) ) Impact of gender and genetics on emotion processing in Parkinson’s disease - a multimodal study. Neuroimage Clin 18: , 305–314. |
[37] | Zhou J , Zhang J , Li Y , Du L , Li Z , Lei F , et al ((2015) ) Gender differences in REM sleep behavior disorder: A clinical and polysomnographic study in China. Sleep Med 16: , 414–418. |
[38] | Haba-Rubio J , Frauscher B , Marques-Vidal P , Toriel J , Tobback N , Andries D , Preisig M , Vollenweider P , Postuma R , Heinzer R ((2018) ) Prevalence and determinants of REM sleep behavior disorder in the general population. Sleep 41: , zsx197. |
[39] | Ophey A , Eggers C , Dano R , Timmermann L , Kalbe E ((2018) ) Health-related quality of life subdomains in patients with Parkinson’s Disease: The role of gender. Parkinsons Dis 1: , 6532320. |
[40] | Balzer-Geldsetzer M , Klotsche J ; LANDSCAPE Consortium, Dodel R , Riedel O ((2018) ) Quality of life in a German cohort of Parkinson’s patients assessed with three different measures. J Neurol 265: , 2713–2722. |
[41] | Buczak-Stec EW , König HH , Hajek A ((2018) ) Impact of incident Parkinson’s Disease on satisfaction with life. Front Neurol 9: , 589. |
[42] | Heinzel S , Kasten M , Behnke S , Vollstedt EJ , Klein C , Hagenah J , Pausch C , Heilmann R , Brockmann K , Suenkel U , Yilmaz R , Liepelt-Scarfone I , Walter U , Berg D ((2018) ) Age- and sex-related heterogeneity in prodromal Parkinson’s disease. Mov Disord 33: , 1025–1027. |
[43] | Savica R , Rocca WA , Ahlskog JE ((2010) ) When does Parkinson disease start? Arch Neurol 67: , 798–801. |
[44] | De Lau LM , Koudstaal PJ , Hofman A , Breteler MM ((2005) ) Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 58: , 797e80. |
[45] | O’Reilly EJ , Gao X , Weisskopf MG , Chen H , Schwarzschild MA , Spiegelman D , Ascherio A ((2010) ) Plasma urate and Parkinson’s disease in women. Am J Epidemiol 172: , 666–670. |
[46] | Ascherio A , LeWitt PA , Xu K , Eberly S , Watts A , Matson WR , Marras C , Kieburtz K , Rudolph A , Bogdanov MB , Schwid SR , Tennis M , Tanner CM , Beal MF , Lang AE , Oakes D , Fahn S , Shoulson I , Schwarzschild MA ; Parkinson Study Group DATATOP Investigators ((2009) ) Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 66: , 1460–1468. |
[47] | Cortese M , Riise T , Engeland A , Ascherio A , Bjørnevik K ((2018) ) Urate and the risk of Parkinson’s disease in men and women. Parkinsonism Relat Disord 52: , 76–82. |
[48] | Lee Y , Park YH , Lee JJ , Sohn YH , Lee JM , Lee PH ((2018) ) Gender-specific effect of uric acid on resting-state functional networks in de novo Parkinson’s disease. Parkinsonism Relat Disord 52: , 49–54. |
[49] | Bakshi R , Macklin EA , Logan R , Zorlu MM , Xia N , Crotty GF , Zhang E , Chen X , Ascherio A , Schwarzschild MA ((2019) ) Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann Neurol 85: , 593–599. |
[50] | Martin I , Kim JW , Dawson VL , Dawson TM ((2014) ) LRRK2 pathobiology in Parkinson’s disease. J Neurochem 131: , 554–565. |
[51] | Shu L , Zhang Y , Pan H , Xu Q , Guo J , Tang B , Sun Q ((2018) ) Clinical heterogeneity among LRRK2 variants in Parkinson’s disease: A meta-analysis. Front Aging Neurosci 10: , 283. |
[52] | Migdalska-Richards A , Schapira AH ((2016) ) The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem 139: , 77–90. |
[53] | Alcalay RN , Wolf P , Levy OA , Kang UJ , Waters C , Fahn S , Ford B , Kuo SH , Vanegas N , Shah H , Liong C , Narayan S , Pauciulo MW , Nichols WC , Gan-Or Z , Rouleau GA , Chung WK , Oliva P , Keutzer J , Marder K , Zhang XK ((2018) ) Alpha galactosidase A activity in Parkinson’s disease. Neurobiol Dis 112: , 85–90. |
[54] | Liu L , Xiong N , Zhang P , Chen C , Huang J , Zhang G , Xu X , Shen Y , Lin Z , Wang T ((2015) ) Genetic variants in GAPDH confirm susceptibility to sporadic Parkinson’s disease in a Chinese Han population. PLoS One 10: , e0135425. |
[55] | Ping Z , Xiaomu W , Xufang X , Wenfeng C , Liang S , Tao W ((2018) ) GAPDH rs1136666 SNP indicates a high risk of Parkinson’s disease. Neurosci Lett 685: , 55–62. |
[56] | Hemmerle AM , Herman JP , Seroogy KB ((2012) ) Stress, depression and Parkinson’s disease. Exp Neurol 233: , 79–86. |
[57] | Sieurin J , Andel R , Tillander A , Valdes EG , Pedersen NL , Wirdefeldt K ((2018) ) Occupational stress and risk for Parkinson’s disease: A nationwide cohort study. Mov Disord 33: , 1456–1464. |
[58] | Teschke K , Marion SA , Tsui JK , Shen H , Rugbjerg K , Harris MA ((2014) ) Parkinson’s disease and occupation: Differences in associations by case identification method suggest referral bias. Am J Ind Med 57: , 163–171. |
[59] | Vlaar T , Kab S , Schwaab Y , Fréry N , Elbaz A , Moisan F ((2018) ) Association of Parkinson’s disease with industry s: A French nationwide incidence study. Eur J Epidemiol 33: , 1101–1111 sector. |
[60] | Rozani V , Gurevich T , Giladi N , El-Ad B , Tsamir J , Hemo B , Peretz C ((2018) ) Higher serum cholesterol and decreased Parkinson’s disease risk: A statin-free cohort study. Mov Disord 33: , 1298–1305. |
[61] | Rafferty MR , Schmidt PN , Luo ST , Li K , Marras C , Davis TL , Guttman M , Cubillos F , Simuni T , all NPF-QII Investigators ((2017) ) Regular exercise, quality of life, and mobility in Parkinson’s disease: A longitudinal analysis of National Parkinson Foundation Quality Improvement Initiative data. J Parkinsons Dis 7: , 193–202. |
[62] | Mantri S , Fullard ME , Duda JE , Morley JF ((2018) ) Physical activity in early Parkinson disease. J Parkinsons Dis 8: , 107–111. |
[63] | Fabbrini G , Brotchie JM , Grandas F , Nomoto M , Goetz CG ((2007) ) Levodopa-induced dyskinesias. Mov Disord 22: , 1379–1389. |
[64] | Kumagai T , Nagayama H , Ota T , Nishiyama Y , Mishina M , Ueda M ((2014) ) Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin Neuropharmacol 37: , 173–176. |
[65] | Martinelli P1 , Contin M , Scaglione C , Riva R , Albani F , Baruzzi A ((2003) ) Levodopa pharmacokinetics and dyskinesias: Are there sex-related differences? Neurol Sci 24: , 192–193. |
[66] | Goudreau JL , Maraganore DM , Farrer MJ , Lesnick TG , Singleton AB , Bower JH , Hardy JA , Rocca WA ((2002) ) Case-control study of dopamine transporter-1, monoamine oxidase-B, and catechol-O-methyl transferase polymorphisms in Parkinson’s disease. Mov Disord 17: , 1305–1311. |
[67] | Sampaio TF , Dos Santos EUD , de Lima GDC , Dos Anjos RSG , da Silva RC , Asano AGC , Asano NMJ , Crovella S , de Souza PRE ((2018) ) MAO-B and COMT genetic variations associated with levodopa treatment response in patients with Parkinson’s disease. J Clin Pharmacol 58: , 920–926. |
[68] | Wang J , Liu ZL , Chen B ((2001) ) Association study of dopamine D2, D3 receptor gene polymorphisms with motor fluctuations in PD. Neurology 56: , 1757–1759. |
[69] | Kaiser R , Hofer A , Grapengiesser A , Gasser T , Kupsch A , Roots I , Brockmöller J ((2003) ) L-Dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology 60: , 1750–1755. |
[70] | Dos Santos EUD , Sampaio TF , Tenório Dos Santos AD , Bezerra Leite FC , da Silva RC , Crovella S , Asano AGC , Asano NMJ , de Souza PRE ((2019) ) The influence of SLC6A3 and DRD2 polymorphisms on levodopa-therapy in patients with sporadic Parkinson’s disease. J Pharm Pharmacol 71: , 206–212. |
[71] | Marras C , Austin PC , Bronskill SE , Diong C , Rochon PA ((2018) ) Antipsychotic drug dispensing in older adults with Parkinsonism. Am J Geriatr Psychiatry 12: , 1244–1257. |
[72] | Drummond N , McCleary L , Freiheit E , Molnar F , Dalziel W , Cohen C , Turner D , Miyagishima R , Silvius J ((2018) ) Antidepressant and antipsychotic prescribing in primary care for people with dementia. Can Fam Physician 64: , e488–e497. |
[73] | Roediger J , Artusi CA , Romagnolo A , Boyne P , Zibetti M , Lopiano L , Espay AJ , Fasano A , Merola A ((2019) ) Effect of subthalamic deep brain stimulation on posture in Parkinson’s disease: A blind computerized analysis. Parkinsonism Relat Disord. 10.1016/j.parkreldis.2019.01.003. |
[74] | Hariz GM , Limousin P , Zrinzo L , Tripoliti E , Aviles-Olmos I , Jahanshahi M , Hamberg K , Foltynie T ((2013) ) Gender differences in quality of life following subthalamic stimulation for Parkinson’s disease. Acta Neurol Scand 128: , 281–285. |
[75] | Gatto NM , Deapen D , Stoyanoff S , Pinder R , Narayan S , Bordelon Y , Ritz B ((2014) ) Lifetime exposure to estrogens and Parkinson’s disease in California teachers. Parkinsonism Relat Disord 20: , 1149e1156. |
[76] | Tsang KL , Ho SL , Lo SK ((2000) ) Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology 54: , 2292–2298. |
[77] | Nicoletti A , Arabia G , Pugliese P , Nicoletti G , Torchia G , Condino F , Morgante L , Quattrone A , Zappia M ((2007) ) Hormonal replacement therapy in women with Parkinson disease and levodopa-induced dyskinesia: A crossover trial. Clin Neuropharmacol 30: , 276–280. |
[78] | Adams C , Kumar R ((2013) ) The effect of estrogen in a man with Parkinson’s disease and a review of its therapeutic potential. Int J Neurosci 123: , 741–742. |
[79] | Lobo RA ((2017) ) Hormone-replacement therapy: Current thinking. Nat Rev Endocrinol 13: , 220–231. |
[80] | Martinkovich S , Shah D , Planey SL , Arnott JA ((2014) ) Selective estrogen receptor modulators: Tissue specificity and clinical utility. Clin Interv Aging 9: , 1437–1452. |
[81] | Yaffe K , Krueger K , Cummings SR , Blackwell T , Henderson VW , Sarkar S , Ensrud K , Grady D ((2005) ) Effect of raloxifene on prevention of dementia and cognitive impairment in older women: The Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry 162: , 683–690. |
[82] | Jacobsen DE , Melis RJ , Verhaar HJ , Olde Rikkert MG ((2012) ) Raloxifene and tibolone in elderly women: A randomized, double-blind, double-dummy, placebo-controlled trial. J Am Med Dir Assoc 13: , 181–187. |
[83] | Goekoop R , Barkhof F , Duschek EJ , Netelenbos C , Knol DL , Scheltens P , Rombouts SA ((2006) ) Raloxifene treatment enhances brain activation during recognition of familiar items: A pharmacological fMRI study in healthy elderly males. Neuropsychopharmacology 31: , 1508–1518. |
[84] | Luchetti S , Bossers K , Frajese GV , Swaab DF ((2010) ) Neurosteroid biosynthetic pathway changes in substantia nigra and caudate nucleus in Parkinson’s disease. Brain Pathol 20: , 945–951. |
[85] | Paba S , Frau R , Godar SC , Devoto P , Marrosu F , Bortolato M ((2011) ) Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des 17: , 151–167. |
[86] | Frau R , Savoia P , Fanni S , Fiorentini C , Fidalgo C , Tronci E , Stancampiano R , Meloni M , Cannas A , Marrosu F , Bortolato M , Devoto P , Missale C , Carta M ((2017) ) The 5-alpha reductase inhibitor finasteride reduces dyskinesia in a rat model of Parkinson’s disease. Exp Neurol 291: , 1–7. |
[87] | Cantuti-Castelvetri I , Keller-McGandy C , Bouzou B , Asteris G , Clark TW , Frosch MP , Standaert DG ((2007) ) Effects of gender on nigral gene expression and Parkinson disease. Neurobiol Dis 26: , 606–614. |
[88] | Simunovic F , Yi M , Wang Y , Stephens R , Sonntag KC ((2010) ) Evidence for gender-specific transcriptional profiles of nigral dopamine neurons in Parkinson disease. PLoS One 5: , e8856. |
[89] | Cullity ER , Madsen HB , Perry CJ , Kim JH ((2019) ) Postnatal developmental trajectory of dopamine receptor 1 and 2 expression in cortical and striatal brain regions. J Comp Neurol 527: , 1039–1055. |
[90] | Bispo JMM , Melo JEC , Gois AM , Leal PC , Lins LCRF , Souza MF , Medeiros KAAL , Ribeiro AM , Silva RH , Marchioro M , Santos JR ((2019) ) Se differences in the progressive model of parkinsonism induced by reserpine in rats. Behav Brain Res 363: , 23–29. |
[91] | Costa G , Pinna A , Porceddu PF , Casu MA , Di Maio A , Napolitano F , Pinna A , Usiello A , Morelli M ((2018) ) Rhes counteracts dopamine neuron degeneration and neuroinflammation depending on gender and age. Front Aging Neurosci 10: , 163. |
[92] | Costa G , Porceddu PF , Serra M , Casu MA , Schiano V , Napolitano F , Pinna A , Usiello A , Morelli M ((2019) ) Lack of Rhes increases MDMA-induced neuro-inflammation and dopamine neuron degeneration: Role of gender and age. Int J Mol Sci 20: , E1556. |
[93] | Seyfried TN , Choi H , Chevalier A , Hogan D , Akgoc Z , Schneider JS ((2018) ) Sex-related abnormalities in substantia nigra lipids in Parkinson’s disease. ASN Neuro 10: , 1759091418781889. |
[94] | Marin R , Diaz M ((2018) ) Estrogen interactions with lipid rafts related to neuroprotection, impact of brain ageing and menopause. Front Neurosci 12: , 128. |
[95] | Song Z , Yang H , Peckham EM , Becker JB ((2019) ) Estradiol-induced potentiation of dopamine release in dorsal striatum following amphetamine administration requires estradiol receptors and mGlu5. eNeuro 6: , ENEURO.0446-18.2019. |
[96] | Conway SM , Puttick D , Russell S , Potter D , Roitman MF , Chartoff EH ((2019) ) Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology 146: , 231–241. |
[97] | Mukherjee S , Klaus C , Pricop-Jeckstadt M , Miller JA , Struebing FL ((2019) ) Microglial signature directing human aging and neurodegeneration-related gene networks. Front Neurosci 13: , 2. |
[98] | Olmedillas Del Moral M , Asavapanumas N , Uzcátegui NL , Garaschuk O ((2019) ) Healthy brain aging modifies microglial calcium signaling in vivo. Int J Mol Sci 20: , E589. |
[99] | Yao K , Zhao YF ((2018) ) Aging modulates microglia phenotypes in neuroinflammation of MPTP-PD mice. Exp Gerontol 111: , 86–93. |
[100] | Palmer AL , Ousman SS ((2018) ) Astrocytes and aging. Front Aging Neurosci 10: , 337. |
[101] | Villa A , Gelosa P , Castiglioni L , Cimino M , Rizzi N , Pepe G , Lolli F , Marcello E , Sironi L , Vegeto E , Maggi A ((2018) ) Sex-specific features of microglia from adult mice. Cell Rep 23: , 3501–3511. |
[102] | Hanamsagar R , Alter MD , Block CS , Sullivan H , Bolton JL , Bilbo SD ((2017) ) Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65: , 1504–1520. |
[103] | Schwarz JM , Sholar PW , Bilbo SD ((2012) ) Sex differences in microglial colonization of the developing rat brain. J Neurochem 120: , 948–963. |
[104] | Lenz KM , Nugent BM , Haliyur R , McCarthy MM ((2013) ) Microglia are essential to masculinization of brain and behavior. J Neurosci 33: , 2761–2772. |
[105] | Siani F , Greco R , Levandis G , Ghezzi C , Daviddi F , Demartini C , Vegeto E , Fuzzati-Armentero MT , Blandini F ((2017) ) Influence of estrogen modulation on glia activation in a murine model of Parkinson’s disease. Front Neurosci 11: , 306. |
[106] | Mendes-Oliveira J , Lopes Campos F , Videira RA , Baltazar G ((2017) ) GPER activation is effective in protecting against inflammation-induced nigral dopaminergic loss and motor function impairment. Brain Behav Immun 64: , 296–307. |
[107] | Guan J , Yang B , Fan Y , Zhang J ((2017) ) GPER agonist G1 attenuates neuroinflammation and dopaminergic neurodegeneration in Parkinson disease. Neuroimmunomodulation 24: , 60–66. |
[108] | Santos-Galindo M , Acaz-Fonseca E , Bellini MJ , Garcia-Segura LM ((2011) ) Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ 2: , 7. |
[109] | Jaber SM , Bordt EA , Bhatt NM , Lewis DM , Gerecht S , Fiskum G , Polster BM ((2018) ) Sex differences in the mitochondrial bioenergetics of astrocytes but not microglia at a physiologically relevant brain oxygen tension. Neurochem Int 117: , 82–90. |
[110] | Martin-Jiménez C , Gaitán-Vaca DM , Areiza N , Echeverria V , Ashraf GM , González J , Sahebkar A , Garcia-Segura LM , Barreto GE ((2019) ) Astrocytes mediate protective actions of estrogenic compounds after traumatic brain injury. Neuroendocrinology 108: , 142–160. |
[111] | Yun J , Yeo IJ , Hwang CJ , Choi DY , Im HS , Kim JY , Choi WR , Jung MH , Han SB , Hong JT ((2018) ) Estrogen deficiency exacerbates Aβ-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-κB activation in ovariectomized mice. Brain Behav Immun 73: , 282–293. |
[112] | Chamniansawat S , Sawatdiyaphanon C ((2018) ) Age-related memory impairment associated with decreased endogenous estradiol in the hippocampus of female rats. Int J Toxicol 37: , 207–215. |
[113] | Korol DL , Wang W ((2018) ) Using a memory systems lens to view the effects of estrogens on cognition: Implications for human health. Physiol Behav 187: , 67–78. |
[114] | Pedersen AL , Nelson LH , Saldanha CJ ((2016) ) Centrally synthesized estradiol is a potent anti-inflammatory in the injured zebra finch brain. Endocrinology 157: , 2041–2051. |
[115] | Gaignard P , Savouroux S , Liere P , Pianos A , Thérond P , Schumacher M , Slama A , Guennoun R ((2015) ) Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology 156: , 2893–2904. |
[116] | Escames G , Díaz-Casado ME , Doerrier C , Luna-Sánchez M , López LC , Acuña-Castroviejo D ((2013) ) Early gender differences in the redox status of the brain mitochondria with age: Effects of melatonin therapy. Horm Mol Biol Clin Investig 16: , 91–100. |
[117] | Harish G , Venkateshappa C , Mahadevan A , Pruthi N , Bharath MM , Shankar SK ((2013) ) Mitochondrial function in human brains is affected by pre- and post mortem factors. Neuropathol Appl Neurobiol 39: , 298–315. |
[118] | Guevara R , Gianotti M , Oliver J , Roca P ((2011) ) Age and sex-related changes in rat brain mitochondrial oxidative status. Exp Gerontol 46: , 923–928. |
[119] | Kim HJ , Magranè J , Starkov AA , Manfredi G ((2012) ) The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis. Brain 135: , 2865–2874. |
[120] | Waters EM , Mazid S , Dodos M , Puri R , Janssen WG , Morrison JH , McEwen BS , Milner TA ((2019) ) Effects of estrogen and aging on synaptic morphology and distribution of phosphorylated Tyr1472 NR2B in the female rat hippocampus. Neurobiol Aging 73: , 200–210. |
[121] | Wang J , Yu R , Han QQ , Huang HJ , Wang YL , Li HY , Wang HM , Chen XR , Ma SL , Yu J ((2019) ) G-1 exhibit antidepressant effect, increase of hippocampal ERs expression and improve hippocampal redox status in aged female rats. Behav Brain Res 359: , 845–852. |
[122] | Yazğan Y , Naziroğlu M ((2017) ) Ovariectomy-induced mitochondrial oxidative stress, apoptosis, and calcium ion influx through TRPA1, TRPM2, and TRPV1 are prevented by 17β-estradiol, tamoxifen, and raloxifene in the hippocampus and dorsal root ganglion of rats. Mol Neurobiol 54: , 7620–7638. |
[123] | Nakano M , Imamura H , Sasaoka N , Yamamoto M , Uemura N , Shudo T , Fuchigami T , Takahashi R , Kakizuka A ((2017) ) ATP maintenance via two types of ATP regulators mitigates pathological phenotypes in mouse models of Parkinson’s disease. EBioMedicine 22: , 225–241. |
[124] | González-Giraldo Y , Forero DA , Echeverria V , Garcia-Segura LM , Barreto GE ((2019) ) Tibolone attenuates inflammatory response by palmitic acid and preserves mitochondrial membrane potential in astrocytic cells through estrogen receptor beta. Mol Cell Endocrinol 486: , 65–78. |
[125] | Miletić J , Drakulić D , Pejić S , Petković M , Ilić TV , Miljković M , Stefanović A , Prostran M , Stojanov M ((2018) ) Prooxidant-antioxidant balance, advanced oxidation protein products and lipid peroxidation in Serbian patients with Parkinson’s disease. Int J Neurosci 128: , 600–607. |
[126] | Xing Y , Sapuan A , Dineen RA , Auer DP ((2018) ) Life span pigmentation changes of the substantia nigra detected by neuromelanin-sensitive MRI. Mov Disord 33: , 1792–1799. |
[127] | Wang LF , Yokoyama KK , Chen TY , Hsiao HW , Chiang PC , Hsieh YC , Lo S , Hsu C ((2015) ) Male-specific alleviation of iron-induced striatal injury by inhibition of autophagy. PLoS One 10: , e0131224. |
[128] | Mariani S , Ventriglia M , Simonelli I , Bucossi S , Siotto M , Donno S , Vernieri F , Squitti R ((2016) ) Association between sex, systemic iron variation and probability of Parkinson’s disease. Int J Neurosci 126: , 354–360. |
[129] | Chen TY , Lin CL , Wang LF , Tsai KL , Lin JY , Hsu C ((2019) ) Targeting GPER1 to suppress autophagy as a male-specific therapeutic strategy for iron-induced striatal injury. Sci Rep 9: , 6661. |
[130] | Xu M , Tan X , Li N , Wu H , Wang Y , Xie J , Wang J ((2019) ) Differential regulation of estrogen in iron metabolism in astrocytes and neurons. J Cell Physiol 234: , 4232–4242. |




