A New Prospective, Home-Based Monitoring of Motor Symptoms in Parkinson’s Disease
Abstract
Background:
Subjective symptoms, which are retrospectively assessed during clinical interviews in the office, may be influenced by patient recall in Parkinson’s disease (PD). Prospective collection of subjective data might be an effective tool to overcome this bias.
Objective:
We investigated the correspondence between prospectively and retrospectively assessed motor symptoms in PD.
Methods:
Forty-two consecutive patients (9 females, 67±9.8 years old) with mild to moderate PD reported their symptoms four times a day for two weeks, using the “SleepFit” application (app) for tablets. This app incorporates a new Visual Analogue Scale assessing global mobility (m-VAS), and the Scales for Outcome in Parkinson Assessment Diary Card (SCOPA-DC). At day 14, the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) parts II and IV questionnaires were completed at the hospital. Agreement (root mean square difference) and the tendency to under- or overestimate their symptoms by patients (relative difference after normalization) were calculated to compare prospectively vs. retrospectively collected information.
Results:
Although agreement was good for overall scores (m-VAS: 10.0%; SCOPA-DC: 18.3%), and for single motor symptoms (involuntary movements, hand dexterity, walking, changing position; each <20%), some individuals with more advanced disease, higher fatigue or worse sleep quality showed poor symptom recall in retrospect. Moreover, a subgroup of patients (16.7%) either over- or underestimated symptom severity.
Conclusions:
Regular, prospective monitoring of motor symptoms is suitable in PD patients. SleepFit might be a useful tool in routine practice to identify patients tending to under- or overestimate their symptoms, and for their follow-up.
INTRODUCTION
The fine-tuning of antiparkinsonian treatment in Parkinson’s disease (PD) is often a challenging task for clinicians. In fact, these medications need to be prescribed at their minimal efficient doses to optimize mobility while minimizing undesirable side effects [1]. In routine practice, the adjustment of antiparkinsonian therapy relies on a combination of objective data derived from clinical examination, and subjective data from the patient’s interview. The Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) is a helpful, validated tool for a structured patient interview. It focuses on the week prior to the visit [2]. Motor symptoms are assessed by parts II (motor experiences of daily living) and IV (motor complications). However, retrospectively-assessed subjective information might be biased by the subjects’ recall capability [3].
This issue can be critical in patients with PD, as they often show subclinical cognitive dysfunction, even at early stages of the disease [4]. To circumvent these problems, Ecological Momentary Assessment (EMA) can be employed. This technique involves repeated prospective sampling of subjects’ current behaviors or experiences in real time. It makes it possible to study variable phenomena in real-world contexts, minimizing recall bias and maximizing ecological validity [5]. To the best of our knowledge, this approach has been applied to PD in only two preliminary studies [6, 7], while other prospective studies employed pen-and-paper questionnaires [8, 9]. However, there is no guarantee that self-administered questionnaires are actually completed by the patients at the exact time point at which they are supposed to be filled in. Moreover, to our knowledge, no study to date has investigated the agreement between subjective data collected both prospectively and retrospectively in the same patients, and referring to the same period of observation. Our group has developed an application (app) named SleepFit for Android tablets. This app was especially designed for patients with PD, ensuring good compliance and usability (Mascheroni A. et al., under review). SleepFit enables repeated measures of subjective motor symptoms in the patient’s home, proposing questions and tests to the patients at given times of the day, and placing an exact timestamp on the data identifying the time they are collected. Among its various functions, SleepFit combines a validated motor diary and a new scale for overall mobility, providing subjective data unbiased by patient recall. This approach could critically inform clinical decision-making. To evaluate the potential usefulness of SleepFit in a clinical setting, we investigate the agreement between subjective data collected prospectively using SleepFit, and the retrospective assessment by structured interview during in-hospital office consultations.
PATIENTS AND METHODS
Patients
The study consists in a preliminary analysis on the first 46 consecutive participants in the Sleep & Move study. Inclusion criteria were: mild to moderate idiopathic PD (no atypical parkinsonism; Hohen & Yahr stage >1 and <3), stable antiparkinsonian medications for at least 4 weeks, no cognitive impairment (Mini-Mental State Examination ≥26/30, Clinical Dementia Rating Scale ≤0.5), no active depression (Beck Depression Inventory <14/63), no deep brain stimulation. Details of the demographic and clinical characteristics of the study population are given in Table 1.
Table 1
Demographic and clinical characteristics of the patient population
| Demographic and clinical characteristics | N = 42 |
| Age | 67.5±9.8 (43–86) |
| Sex (n, %) | 9 f: 33 m, 21% f: 79% m |
| PD phenotype | |
| tremor-dominant (n, %) | 24 (57.1%) |
| postural instability/gait difficulty (n, %) | 12 (28.6%) |
| undetermined (n, %) | 6 (14.3%) |
| Age at PD onset | 61.1±10.6 (37–82) |
| PD duration | 3.2±0.7 (2–4) |
| Fluctuations | 12 (28.7%) |
| motor (n, %) | 11 (26.2%) |
| non-motor (n, %) | 2 (4.8%) |
| Hoehn &Yahr | 2.0±0.4 (1–3) |
| MDS-UPDRS total score | 49.9±19.9 (15–98) |
| part I | 8.8±4.6 (0–17) |
| part II | 9.5±5.7 (1–24) |
| part III | 30.0±14.0 (4–70) |
| part IV | 1.6±2.3 (0–9) |
| LEDD (mg) | 570±347 (37.5–1637) |
| Medications | |
| levodopa (n, %) | 32 (76.2%) |
| levodopa ER (n, %) | 6 (14.3%) |
| dopamine agonists (n, %) | 12 (28.6%) |
| dopamine agonists ER (n, %) | 14 (33.3%) |
| COMT inhibitors (n, %) | 8 (19.0%) |
| anti-MAO-B (n, %) | 20 (47.6%) |
| safinamide (n, %) | 1 (2.4%) |
| benzodiazepines (n, %) | 11 (26.2% 9 |
| other hypnotics (n, %) | 2 (4.8%) |
| antidepressants (n, %) | 14 (33.3%) |
| antipsychotics (n, %) | 1 (2.4%) |
| Mini-Mental State Examination | 29.2±0.9 (26–30) |
| Active worker | 10 (24%) |
| Cumulative Illness Rating Scale -R | 6.9±3.6 (0–14) |
| Pittsburg Sleep Quality Index | 6.0±2.9 (1–13) |
| Epworth Sleepiness Scale (ESS) | 8.8±4.7 (2–20) |
| Fatigue Severity Scale (FSS) | 4.3±1.5 (1–7) |
SD, standard deviation; PD, Parkinson’s disease; LEDD, levodopa equivalent daily dose; ER, extended release; COMT, catechol-O-methyl transferase; MAO-B, mono-amino-oxidase-B. All numerical values are expressed as mean±standard deviation. Ranges are expressed in brackets for some variables. Categorical values are given as number (percentage).
Assessment of motor symptoms
The SleepFit app
SleepFit is an application for Android tablets that was specifically developed to provide PD patients with an efficient and practical home-based system. It is a handy tool for recording repetitive, objective and subjective metrics of mobility as well as subjective data on sleep, sleepiness and emotional state. It also provides physicians and researchers with a remote portal to access the information, monitor patient compliance in real-time, apply different custom filters and download any data in *.csv format.
SleepFit integrates the motor items of the Scales for Outcome in Parkinson Assessment Diary Card (m-SCOPA-DC) [8] and a Visual Analogue Scale [10] assessing global mobility (m-VAS). The latter consists of a horizontal line, 100 mm in length, anchored by the following word descriptors at each end: “No motor difficulties” and “Very severe motor difficulties”. The subjects are asked to move a slider on the line to the point best representing their perception of current global mobility.
Prospective, home assessment of motor symptoms
Subjective motor symptoms were prospectively collected at four different times during the day, for 14 days in the patient’s home, using SleepFit, namely: in the morning, 30 minutes after waking up (M30) and 1 hour after intake of dopaminergic medication (MDOPA1); in the afternoon, before taking dopaminergic medication, where applicable (A); and in the evening, just before bedtime (E). These four time-points were fixed and decided at the inclusion visit, to adapt to each patient’s routine, so that they could perform the test without being bothered by these tasks. Each patient could decide whether to carry the tablet around all day long or keep it at home, depending on their daily routine, provided they were sure they would be able to perform the test at the predefined time-points of the day. In each session, patients were asked to estimate their perceived momentary motor capability regarding the presence of involuntary movements, hand dexterity, walking, and changing position, using the m-SCOPA-DC; and to estimate their momentary global mobility using the m-VAS. Each patient had to answer 5 questions 4 times per day (starting from E of day 1 to the MDOPA1 of day 14), for a total of 275 questions during his/her participation to the study. Participants were asked to keep their daily routines unchanged during the whole duration of the study, including the dose and timing of antiparkinsonian and psychotropic medications. The only exception to this was that patients were asked to wait at least 30 minutes after waking up before taking their first antiparkinsonian medication. To minimize patient burden, only the time of the first morning antiparkinsonian medication intake was recorded in SleepFit, i.e. the one before the MDOPA1 assessment.
Retrospective, in-office assessment of motor symptoms
To retrospectively assess subjective motor symptoms during the previous period, the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) parts II and IV [2] were administered during an office consultation at 14 days after the inclusion visit. It was permitted for the patient to refer to the previous two weeks, instead of the previous one week, in order to match the home-based observation period. To achieve consistent observations, the MDS-UPDRS was administered by a neurologist specialized in movement disorders (PLR) or one of his five trained co-workers, in his presence.
Statistical analysis
Multidimensional categorization of motor symptoms
To better evaluate the agreement between prospective vs. retrospective assessment of subjective motor symptoms, we considered five mobility items: walking, changing position, hand dexterity, dyskinesia and global mobility. We matched the corresponding questions of m-SCOPA-DC or m-VAS with those of the MDS-UPDRS parts II and IV as follows: a) “walking”: SCOPA-DC question 1 matched with the average of MDS-UPDRS questions 2.12 (walking and balance) and 2.13 (freezing of gait); b) “changing position”: SCOPA-DC question 2 matched with MDS-UPDRS question 2.11 (getting out of bed); c) “hand dexterity”: SCOPA-DC question 3 matched with the average of MDS-UPDRS questions 2.4 (eating tasks), 2.5 (dressing), 2.6 (hygiene) and 2.7 (handwriting); d) “dyskinesias”: SCOPA-DC question 4 matched with the average of MDS-UPDRS questions 4.1 (time spent with dyskinesias) and 4.2 (functional aspects of dyskinesias); e) “Global mobility” assessed by m-VAS matched with the total score of MDS-UPDRS parts II+IV.
A global view of the agreement between m-SCOPA-DC and MDS-UPDRS parts II+IV was also derived from the agreement of the combined mobility items “walking”, “changing position”, “hand dexterity”, and “dyskinesias”.
Agreement between prospective and retrospective subjective evaluation
Overall agreement between prospective information collected at home for two weeks, and retrospective information collected at the office consultation, was calculated per patient and per category, as the Root Mean Square Difference (RMSD), after normalization [11]. Since the MDS-UPDRS parts II and IV retrospectively ask for the recurrence of symptoms over time, we used the mode of prospectively collected data. Perfect agreement between retrospective and prospective scores is defined by a difference of 0%. Since a change of at least 20% is needed to move from one ordinal response category on the MDS-UPDRS to the next (scores ranging from 0 to 4), we defined an RMSD of 20% as the cutoff for good agreement.
To better evaluate agreement variation, we also grouped patients, considering only those above 20% for each item. We also checked agreement for single items in single patients of the overall population, to estimate whether single patients tend to have poor agreement on all motor symptoms or just on single items.
Furthermore, to check for agreement tendencies and to explore a potential recency effect of patient recall [12], we calculated the agreement in different timeframes, namely: the last three days, the first week, and the second week.
Positive vs. negative thinking
In order to discriminate between patients who tend to minimize their symptoms (“positive thinkers”) at office evaluation and patients who tend to enhance them (“negative thinkers”), we calculated the relative difference between subjective motor symptoms assessed prospectively vs. retrospectively, overall and for each category. We applied cutoffs of +20% and – 20% to define positive or negative thinkers, respectively. Similarly to the assessment of agreement, we grouped patients into positive and negative thinkers to better evaluate the range of variation, and we checked whether the patient displayed positive/negative thinking on all items or whether it depended on each specific item.
Correlations
The influence of demographic and clinical parameters on agreement and positive/negative thinking were also investigated by correlation analysis. We considered the following variables as categorical parameters: gender, PD phenotype (defined as tremor dominant, postural instability/gait difficulty, or undetermined, based on the predominant motor symptoms assessed by the MDS-UPDRS-III) [13], presence of motor or non-motor fluctuations, being in active employment, treatment with standard/extended release levodopa, standard/extended release dopamine agonists, mono-amino-oxydase-B inhibitors, psychotropic drugs and the presence of a caregiver during office consultation. The following quantitative parameters were also evaluated: age, PD duration, Levodopa Equivalent Daily Dose (LEDD) [14], Cumulative Illness Rating Scale – Geriatric (CIRS-G) total score, Mini-Mental State Examination score, Pittsburgh Sleep Quality Index (PSQI) score, Epworth Sleepiness Scale (ESS) score, Fatigue Severity Scale (FSS) score, Parkinson’s Disease Sleep Scale-revised score, MDS-UPDRS part III total score, MDS-UPDRS total score.
The following parameters, assessed by MDS-UPDRS part I, were considered as categorical variables, using a cutoff > =1 (i.e. “slight” to “severe” problem): PD phenotype, presence of hallucinations and psychosis, depressed mood, anxious mood, apathy and features of dopamine dysregulation syndrome.
The correlation between quantitative demographic parameters, and both agreement and positive/negative thinking were calculated using Kendall’s (τ) correlation coefficient (using the “cor.test” correlation of the R statistics package [15]). Correlations were considered significant if the p-value was <0.05 and relative achieved power >80%. This coefficient is suitable for the measurement of a monotonic association of non-normally distributed data. Correlation between categorical demographic variables, and both agreement and positive/negative thinking was assessed with the classical Wilcoxon tests (“wilcox.test f” function in the R statistics package [R]), equivalent to a paired t-test for non-parametric distributions. In case of more than two subgroups (e.g. for PD phenotype), ANOVA or (if the assumptions for ANOVA were not met), the Kruskal-Wallis rank sum test were applied. Correlations were considered significant if the p-value was <0.05 and relative achieved power >80%.
RESULTS
Forty-six subjects with mild to moderate PD were included, of whom 42 completed the study procedures and were included in further analyses. Four patients prematurely discontinued their participation because of the excessive burden of following study procedures (n = 3) or due to an inability to use the tablet (n = 1). The demographic and clinical characteristics of the study population are shown in the table.
Overall, answers were obtained on 250.7±23.1 (range 165–275) over 275 questions. The average response rate to the subjective questionnaires was 91.2%.
Agreement per category (measured by the RMSD) was 16.5% (range 0–41.7%) for walking, 18.5% (range 0–75%) for changing position, 15.0% (range 0–43.8%) for hand dexterity, and 7.8% (range 0–37.5%) for involuntary movements.
Overall, agreement was 18.3% (range 0–44.5%) for the four SCOPA-DC categories together (Fig. 1A).
Fig.1
Comparison between prospective and retrospective patient self-reports of motor symptoms over a period of two weeks. Comparison between m-SCOPA-DC and MDS-UPDRS parts II and IV. A) RMSD Agreement. The dashed line indicates the 20% cutoff for good agreement; B) Percentage Difference. Dashed lines indicate the 20% cutoff for positive and negative thinking, respectively.
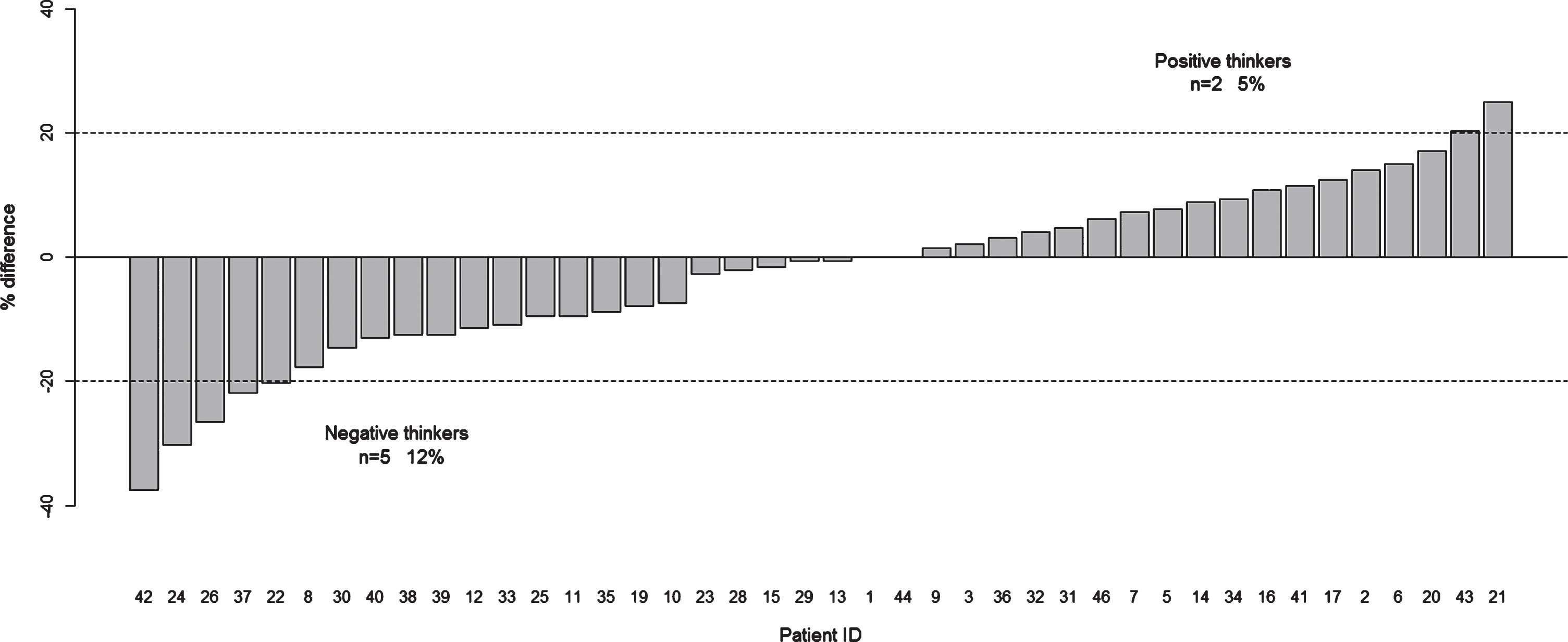
When grouping only the patients who had an RMSD >20% on each item, the following agreement rates were observed: 26.1% (range 20.8–41.7%) for walking, 34.3% (range 25–75%) for changing position, 27.8% (range 20.8–43.8%) for hand dexterity, and 29.6% (range 20.8–37.5%) for involuntary movements.
When we considered single items in single patients in the overall population, we found that 20 patients had poor agreement (RMSD >20%) on only 1 item, 6 patients on 2 items, 9 patients on 3 items and 1 patient on all 4 items. Only 6 patients showed good agreement on all categories.
Global mobility estimated by the m-VAS scale showed better agreement with MDS-UPDRS parts II+IV: 10.0%, range 0.3–40.8%.
Agreement was of similar magnitude when prospective information was evaluated for time frames of different duration (the last three days, the first week, the second week).
Considering the m-SCOPA-DC, we found 4.8% positive thinkers at retrospective, in-hospital evaluation and 11.9% negative thinkers (Fig. 1B). However, the differences were more pronounced for single categories: there were 9.5% / 42.9% positive/negative thinkers for walking, 26.2% / 14.3% for changing position, 7.1% / 21.4% for hand dexterity, 2.4% / 19.0% for dyskinesias, and 0% / 4.8% for global mobility.
In the overall population, we found that 13 patients were positive thinkers on only 1 item, 2 on 2 items, and there were no positive thinkers on 3 or 4 items. Fourteen patients were negative thinkers on one item, 5 on 2 and 5 on 3 items; only one patient was a negative thinker on all 4 items. Five patients were neither negative nor positive thinkers on all four items, whereas 4 patients were sometimes negative, sometimes positive thinkers.
We highlighted the significant correlations between agreement (RMSD) and the following demographic and clinical parameters: a) walking item: with PD duration (τ 0.27, p = 0.04) and FSS score (τ 0.32, p = 0.00); b) hand dexterity item: PSQI (τ 0.30, p = 0.01); c) m-SCOPA-DC: PSQI (τ 0.29, p = 0.01), and LEDD (τ 0.11, p = 0.29); d) m-VAS: MDS-UPDRS total score (τ 0.29, p = 0.01). No correlation was found for changing position and involuntary movements. We did not find any significant correlation between agreement and positive/negative thinking and categorical parameters.
DISCUSSION
The agreement between prospective, home-based (m-SCOPA-DC and m-VAS) and retrospective, in-hospital office assessments (MDS-UPDRS parts II and IV) of subjective motor symptoms over a period of two weeks was good overall (18.3%). Moreover, agreement also proved to be good (<20%) when we considered each single mobility item (involuntary movements, hand dexterity, walking, and changing position).
We did not find any evidence of a recency effect when we grouped the data into shorter time frames.
It is noteworthy that a subgroup of PD patients seemed not to report their motor symptoms accurately at the office consultation. In fact, we noticed a discrepancy between the global mobility estimate on the m-SCOPA-DC, and the corresponding items evaluated by questions in the MDS-UPDRS parts II + IV, in 18 out of 42 patients (42.9%). This disagreement was observed in 2 out of 42 patients (4.8%) when measured by the m-VAS. Correlation analyses suggest that patients with higher disagreement are those who tend to have more advanced disease, higher fatigue or worse sleep quality.
We did not find tendency towards positive or negative thinking in symptoms reported by the patients in the overall population. However, quite a sizeable number of patients tended to retrospectively over- or underestimate their symptoms (12% negative thinkers vs. 5% positive thinkers). This incurs the risk of either under- or overdosing dopaminergic medication in these patients in routine practice based on their retrospective report, possibly leading to serious adverse side effects. Knowing of a given patient’s tendency towards positive or negative thinking could thus critically inform clinical decision-making with regard to dopaminergic medication adjustment. Negative thinking might be interpreted by the patient’s tendency to perform at maximal capacity at the office visit, when their daily capacity is usually lower. This might make them underestimate their performance during the previous period when evaluated retrospectively compared to the momentary capacity at the office visit.
From our analysis, we also observed that agreement, as well as negative/positive thinking, can be item-specific. In other words, some patients can have good recall for specific motor symptoms, but poor recall for others. This finding, together with the small size of our population, might explain the wide range of RMSD within each category.
In conclusion, our preliminary results highlight the benefit of regular, prospective monitoring of mobility of PD patients at home. A sizeable proportion of patients tend to over- or underestimate their symptom severity in retrospect. Consequently, a prospective approach would enable better clinical evaluation of patients’ subjective symptoms, and thus, better clinical management of the patients themselves. Future work should focus on identifying the profiles of selected patients who tend not to report their symptoms accurately retrospectively, and on identifying the symptoms on which individual subjects tend to be less precise. The SleepFit app could be a useful tool in routine clinical practice, especially for this group of patients who warrant closer and specific symptom-oriented follow-up.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ETHICAL COMPLIANCE STATEMENT
The study was approved by the Ethics committee of Canton Ticino (Ref. 2016-00056) and conducted according to the principles of the Declaration of Helsinki of 1975. All the participants gave written informed consent for the study. This study was conducted in the framework of the “Sleep, Awake & Move” project (ClinicalTrials.gov Identifier: NCT02723396).
ACKNOWLEDGMENTS
We are particularly grateful to all the patients and their families who participated in this project. This study was conducted in the framework of the “Sleep, Awake & Move” project, which was financed by Swiss Parkinson’s Association 2015 research award and by a 2015 research grant from the Advisory Board for scientific research of Ente Ospedaliero Cantonale (ABREOC). We also acknowledge the DRCI (Délégation de la Recherche Clinique et de l’Innovation) of University Hospitals of Martinique for contributing to the publication fee of this article.
We thank Prof. Moustapha Dramé for his advice in manuscript revision.
Many thanks to Dr. Stephany Fulda for her support on the first conception of the “Sleep, Awake & Move” research project, to Miss Simona Bonoli, Miss Lucia Guglielmetti and Mr. Francesco Mezzanotte for their help in patient recruitment. We also thank all the colleagues and co-workers who supported this project and shared our enthusiasm: Ing. Michele Marazza, Ing. Marco Bosetti, Ing. Luigi Fiorillo, Dr. Claudio Staedler, Dr. Filippo Scacchi, Mr. Gregor Ruggel, Mr. Pierluigi Lurà, Mrs. Nicole Vago-Caputo, Mr. Matteo Pereno, Mrs. Yasmin Belloni, Mrs. Simona Perrotta, Mr. Giuliano Filippini.
REFERENCES
[1] | Olanow CW , Stern MB , Sethi K ((2009) ) The scientific and clinical basis for the treatment of Parkinson disease. Neurology 72: , S1–S136. |
[2] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , LaPelle N ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[3] | Shiffman S , Stone AA , Hufford MR ((2008) ) Ecological momentary assessment. Annu Rev Clin Psychol 4: , 1–32. |
[4] | Aarsland D , Bronnick K , Larsen JP , Tysnes OB , Alves G , Norwegian ParkWest Study Group ((2009) ) Cognitive impairment in incident, untreated Parkinson disease: The Norwegian ParkWest study. Neurology 72: , 1121–1126. |
[5] | Moskowitz DS , Young SN ((2006) ) Ecological momentary assessment: What it is and why it is a method of the future in clinical psychopharmacology. J Psychiatry Neurosci 31: , 13–20. |
[6] | van Gilst MM , van Mierlo P , Bloem BR , Overeem S ((2015) ) Quantitative motor performance and sleep benefit in Parkinson disease. Sleep 38: , 1567–1573. |
[7] | Bot BM , Suver C , Neto EC , Kellen M , Klein A , Bare C , Doerr M , Pratap A , Wilbanks J , Dorsey ER , Friend SH , Trister AD ((2016) ) The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci Data 3: , 160011. |
[8] | Marinus J , Visser M , Stiggelbout AM , Rabey JM , Bonuccelli U , Kraus PH , van Hilten JB ((2002) ) Activity-based diary for Parkinson’s disease. Clin Neuropharmacol 25: , 43–50. |
[9] | van Gilst MM , Bloem BR , Overeem S ((2015) ) Prospective assessment of subjective sleep benefit in Parkinson disease. BMC Neurol 15: , 2. |
[10] | Wewers ME , Lowe NK ((1990) ) A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 13: , 227–236. |
[11] | Mould RF ((1998) ) Introductory medical statistics, 3rd edition, Institute of Physics Publishing, London, United Kingdom. |
[12] | Talmi D , Goshen-Gottstein Y ((2006) ) The long-term recency effect in recognition memory. Memory 14: , 424–436. |
[13] | Stebbins GT , Goetz CG , Burn DJ , Jankovic J , Khoo TK , Tilley BC ((2013) ) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov Disord 28: , 668–670. |
[14] | Tomlinson CL , Stowe R , Patel S , Rick C , Gray R , Clarke CE ((2010) ) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25: , 2649–2653. |
[15] | R Core Team ((2013) ) R Foundation for Statistical Computing, Vienna, Austria. |




