Using Medical Claims Analyses to Understand Interventions for Parkinson Patients
Abstract
The scientific evidence to support the value of a range of non-pharmacological interventions for people with Parkinson’s disease (PD) is increasing. However, showing unequivocally that specific interventions are better than usual care is not straightforward because of generic drawbacks of clinical trials. Here, we address these challenges, specifically related to the context of evaluating complex non-pharmacological interventions for people with PD. Moreover, we discuss the potential merits of undertaking “real world” analyses using medical claims data. We illustrate this approach by discussing an interesting recent publication in The Lancet Neurology, which used such an approach to demonstrate the value of specialized physiotherapy for PD patients, over and above usual care physiotherapy.
BACKGROUND
It is increasingly being recognized that Parkinson’s disease (PD), given its range of problems, is managed best using a multidisciplinary approach [1, 2]. Such a team approach should include not only conventional medical interventions (pharmacotherapy and deep brain stimulation), but also a range of non-pharmacological approaches including, but not limited to, physiotherapy, occupational therapy and speech-language therapy. The perception that such non-pharmacological interventions have added value for people with PD was, at least initially, based largely on theoretical rationale and anecdotal evidence. An exponential increase in randomized controlled trials and systematic reviews now provides high-quality scientific evidence to support the merits of a range of non-pharmacological interventions for people with PD.
However, to unequivocally demonstrate that specific non-pharmacological interventions are better than usual care is not an easy task. This is related in part to the generic drawbacks of clinical trials, which become even more apparent when evaluating complex healthcare interventions such as physiotherapy or multidisciplinary interventions. Here, we briefly address these generic challenges, and discuss them in the context of evaluating non-pharmacological interventions for people with PD. We then consider a complementary approach, which is based on an analysis of medical claims data, with particular reference to a recent publication in The Lancet Neurology [3]. We conclude by highlighting the broader implications of this approach, and how this and other methodological approaches might help to shape the future of care for neurological patients.
THE CHALLENGES OF CLINICAL TRIALS
The randomized controlled trial (RCT) remains the gold standard for evaluating new healthcare interventions and is a powerful approach to evaluate effectiveness [4]. Clear advantages include the rigorous measures that are taken to avoid bias and to control for influences of confounding factors by means of a concealed randomization procedure. However, the design of RCTs is also subject to a number of limitations [5].
First, the population included in a RCT is typically selected using strict inclusion/exclusion criteria, which by definition means that the study results cannot be generalized to a real-life population. Recent pragmatic RCTs are to be commended for having used broader in- and exclusion criteria, [6, 7] but some populations are still structurally over- or underrepresented in other studies on PD. For example, most patients with PD are older adults with multiple co-morbid conditions, but RCTs usually exclude such co-morbid patients, and consist largely of younger patients. Therefore, we do not know whether evidence-based treatments are also effective in a real-life and more vulnerable population of PD patients [8]. Patients with cognitive decline are also often excluded from trials; this exclusion can be problematic when the outcome is related in part to cognitive functional capacity. For example, an analysis in this journal showed that fall intervention studies typically do not include patients with cognitive decline, yet falls are clearly related to the presence of frontal executive deficits [9]. Patients at the extremes of the age spectrum are often also excluded from trials, even though both treatment effects and adverse events may well be different for very young or very old patients. Underrepresentation of older adults with PD, who represent the majority of the PD population, from clinical trials is particularly evident. The effect of these commonly applied inclusion/exclusion criteria is that patients with mild disease are preferentially included in trials, so the results cannot be generalized to those with more advanced disease. Finally, we recently showed that men are significantly overrepresented in RCTs (even accounting for the slighter higher incidence of PD in men compared to women), with the proportion of men being extra high in more complex or technically oriented studies (Tosserams et al., submitted). Studies of non-pharmacological interventions are certainly no exception, and in fact seem particularly plagued by these recruitment challenges [10, 11]. For example, a recent RCT showed that treadmill walking combined with virtual reality immersion helped to reduce falls in PD patients [12]. However, our own center (which participated in this study) faced serious recruitment issues because the intervention was hospital-based, and many patients refused participation because the time and energy needed for multiple weekly visits to the hospital was perceived as too cumbersome.
Second, since RCTs are very costly and time-consuming, most studies include only a relatively small sample size, and follow their participants for only a limited period. A recent review showed that this issue also applies to the field of non-pharmacological interventions, where the majority of RCTs included only between 20 and 100 patients (mean = 80) [13]. The largest study included 586 patients, but many others appeared to be underpowered. Moreover, these studies followed their patients for only short periods of time, with a median of only 16 weeks [13]. In exceptional cases, a two-year follow-up period was reached, so the long-term effects remain unknown for virtually all non-pharmacological interventions.
Third, many studies of non-pharmacological interventions are troubled by rather high numbers of patients who are lost to follow-up. For example, a recent review showed a great variability in attrition rates, between 10% up to 80% in studies on vocal rehabilitation [14]. These high attrition rates introduce bias to the results of any study. We should point out that loss to follow-up is a problem for any study design, and RCTs actually perform reasonably well here by comparison to other clinical studies.
Finally, after successfully completing an RCT, it often proves difficult to implement new interventions into everyday clinical practice. In fact, also replication of study results is difficult, where initial stronger results as well as contradictions are reported [15]. This issue is potentially particularly vexing for non-pharmacological interventions, which in the clinical trial setting are administered typically by well-trained and highly motivated personnel (‘selection bias of therapists’). It is possible that the positive experiences with, e.g., dance therapy are explained at least in part by the enthusiasm and dedication of the dance trainers in those studies [16]. It is not necessarily feasible to engage similarly motivated and equally skilled personnel to now deliver these interventions in daily practice outside the original study centers, and this may have a detrimental effect on the therapy being administered.
WHAT ANALYSES OF MEDICAL CLAIMS CAN OFFER
Mining medical claims databases, on the other hand, offers a potential solution for at least some of these drawbacks. This includes the opportunity to include very large numbers of patients that can potentially be followed for long periods of time without attrition. Moreover, medical claims data are more likely to reflect healthcare in real-life clinical practice, including access to a much more representative population across all ages, disease severities and co-morbid conditions. By definition, medical claims data contain information about medical expenses, enabling evaluations of cost-effectiveness after a real-life implementation of an intervention, especially when being linked to other databases, for example clinical outcome registries. Studying a medical claims database is also much cheaper than performing a clinical trial, because data collection has already taken place as part of regular healthcare, so only the personnel for data analyses has to be funded [17]. Research has shown that PD patients can be identified reliably in medical claims data using a combination of PD claim factors [17]. Table 1 offers an overview of studies that used medical claims in the field of PD.
Table 1
Studies that have used medical claims analyses in the field of PD
| Study | Database | Population | Objective | Results |
| Ypinga et al. [3] | Medical claims, CZ groep, 2013–2015 | n = 2129 patients with PD receiving specialized physiotherapy | To study the long-term benefits and costs of specialized physiotherapy using the ParkinsonNet approach | Significantly fewer patients that received specialized physiotherapy had a PD related complication. Patients treated by a ParkinsonNet physiotherapist had significantly less physiotherapy costs ($456 per patient per year) and total medical expenses ($612 per patient per year. Specialized physiotherapists used less physiotherapy sessions per patients, had a higher case-load of PD patients and a higher percentage of patients received care from the same physiotherapist |
| n = 2252 patients with PD receiving usual care physiotherapy | ||||
| Sierles-Nielsen et al. [51] | Medicare, 2004–2009, USA | 66–90 years | To predict PD using demographic and medical claims data | It is possible to identify people with a high probability of PD using diagnosis and procedure codes in the 5 years prior to PD diagnosis. This data is readily available in medical claims |
| n = 89,790 incident PD cases | ||||
| n = 118,095 controls | ||||
| Fullard et al. [52] | Medicare, 2007–2009, USA | n = 174,643, patients with PD | To study rehabilitation service utilization in patients with PD | Outpatient rehabilitation utilization was low. In 2007:14.2% physiotherapy or occupational therapy, 14.6% speech therapy. |
| Chou et al. [53] | National Health Insurance Research Database, 1997–2005, Taiwan | n = 1,944 patients diagnosed with sleep apnea | To study the risk of PD following a diagnosis with sleep apnea using a 3-year follow-up period | 17 (0.9%) patients with sleep apnea and 38 (0.4%) controls were diagnosed with PD during follow-up. Patients with sleep apnea had a 1.85-fold higher risk of PD than controls |
| n = 9,720 matched controls without sleep apnea | ||||
| Yang et al. [54] | National Health Insurance Research Database, 2000–2011, Taiwan | n = 36,294 patients newly diagnosed with diabetes mellitus in 2000–2006 | To study the risk of PD following a diagnosis of diabetes mellitus (follow-up until 2011). | The risk of PD was 1.36-fold higher in the patients with diabetes mellitus compared to the healthy controls. |
| n = 108,882 healthy controls | ||||
| Huang et al. [55] | National Health Insurance Research Database, 2008–2012, Taiwan | n = 6455 patients with PD receiving major surgery in 2008–2012. | To study the risk of post-operative complications and mortality after non-neurological surgery in PD patients | Patients with PD had an increased risk of postoperative pulmonary embolism, stroke, pneumonia, urinary tract infection, septicemia, acute renal failure, and mortality |
| n = 12,910 controls | ||||
| Crispo et al. [56] | Cerner Health Facts® database | n = 16,302 PD hospitalized patients hospitalized | To study anticholinergic medication use, diagnoses, and hospital readmission in a PD inpatient population | More than half of the hospitalized PD patients were prescribed medications with moderate to very strong anticholinergic potential. Anticholinergic medication use was associated with increased odds of ED visits and 30-day readmission |
| Kwak, [57] | National Health Insurance Research Database, 2013, Taiwan | n = 4,137 patients with PD | To study the social demographic characteristics and health services use of patients with PD in Korea | The prevalence of PD was 3.54 in 1,000 in 2013. On average 9.83 outpatient visit days and 25.3 inpatient hospitalization days were found. Annual direct medical costs were USD 487 for an outpatient and USD 10,429 for an inpatient. |
| Chen et al. [58] | National Health Insurance Research Database, 2000–2009, Taiwan | n = 5864 patients with newly diagnosed obstructive sleep apnea | To study the risk of PD in patients with obstructive sleep apnea | The incidence of PD was approximately two times higher for patients with sleep apnea with an adjusted hazard ratio of 1.84. |
| n = 23,269 controls | ||||
| Huang et al. [59] | National Health Insurance Research Database, 2000–2003, Taiwan | n = 1,423 patients with PD | To study the risk of fractures and post-fracture outcomes in patients with PD. | Patients with PD had a higher risk of fractures and complications following fractures than controls |
| n = 5,692 controls | ||||
| Followed-up until 2008 | ||||
| Benzinger et al. [60] | Database from a German health insurance company, 2004–2008. | n = 23,469 patients with ‘possible PD’ | To study the risk of femoral fracture in patients with PD | Patients with PD had a more than doubled risk of a femoral fracture |
| n = 12,391 patients with ‘probably PD’ | ||||
| n = 860,388 reference group | ||||
| Wang et al. [61] | National Health Insurance Research Database, 1997–2010, Taiwan | n = 8,325 patients with end-stage renal disease | To study the risk of PD in patients with end stage renal disease | The risk of PD was 1.55-fold higher in patients with end stage renal disease compared to controls |
| n = 33,382 controls | ||||
| Harris-Hayes et al. [45] | Medicare, 2000–2005, USA | n = 1,980,401 people with a hip or pelvic fracture, of which n = 131,215 with PD | To study mortality associated with demographic factors after hip or pelvic fracture in patients with PD. | The adjusted mortality rate after hip/pelvic fracture in individuals with PD was higher than in those without PD |
| Lai et al. [62] | National Health Insurance Research Database, 2000–2010, Taiwan | n = 4,976 patients with hearing loss | To study the risk of PD in patients with hearing loss | The risk of PD was higher in patients with hearing loss than in controls |
| n = 19,904 controls | ||||
| Suh et al. [63] | Medstat MarketScan® Claims and Encounters research database, 2004–2008, USA | n = 1,312 patients with PD and levodopa-induced dyskinesias | To study the treatment patterns, direct healthcare costs and predictors of treatment costs associated with levodopa-induced dyskinesia in PD | Total treatment costs increased from $18,645 to $26,439 (from 12 months preceding levodopa-induced dyskinesia to 12 months after onset). PD-related costs increased from $3917 to $8110. |
| n = 1,312 patients with a prescription for levodopa and a primary or secondary diagnosis of PD, without levodopa-induced dyskinesias | ||||
| Hobson et al. [64] | Administrative data from Manitoba, Canada | n = 1,469 patients with PD | To study healthcare utilization and the factors associated with healthcare utilization and prescription drug use for patients with PD (6-year follow-up) | Patients with PD had greater healthcare utilization than controls (except for visits to non-neurological specialists and hospital use for non-mental disorder diagnoses). |
| n = 2,938 controls | ||||
| Ooba et al. [65] | Vendor, medical claims database, 2005–2008, Japan | n = 574 patients with PD | To study the impact of regulatory actions (e.g., requiring physicians to perform periodic ultrasonic cardiography in patients who take cabergoline or pergolide) on prescribing dopamine receptor agonists | No decrease in the proportion of patients prescribed cabergoline or pergolide was found. Prescription tended to increase |
| Davis et al. [66] | Insurance claims from 30 health plans, 1997–2004, USA | n = 3,119 patients with PD | To study the prevalence of medication nonadherence and its association with healthcare costs | 61% of the PD patients were non-adherent. Higher healthcare costs were found for non-adherent patients compared to adherent patients. |
| Safarpour D et al. [67] | Insurance claims from Medicare program, 2002–2006,USA | n = 469,055 individuals with PD | To study LTCF and hospice use in PD patients | 25% of PD patients resided in a long-term care facility. Hip fracture and dementia were associated with LTCF use. LTCF PD patients infrequently received neurologist care. |
| Willis et al. [68] | Insurance claims from Medicare program, 2002–2005, USA | n = 657,000 Medicare beneficiaries with PD | To study sociodemographic, clinical, and physician/practice factors associated with DBS. | DBS was infrequently used. Non-white and female PD patients were less likely to receive DBS. Beneficiaries treated in diverse physician practices were less likely to receive DBS, regardless of individual race |
| Willis et al. [69] | Insurance claims from Medicare program, 2005, USA | n = 14,354 PD patients aged 30–64 who receive Medicare benefits by virtue of being disabled | To study the characteristics of the Young disabled PD population in the US and to quantify the burden of neuropsychiatric disease manifestations in this group. | The race and sex distribution of Young disabled PD patients was similar to that seen in the general population: White >nonwhite, male >female. Young PD patients more often were diagnosed with depression, dementia, substance abuse, psychosis, and impulse control disorders |
| Willis et al. [70] | Insurance claims from Medicare program, 2002–2006, USA | n = 24,929 incident PD cases | To investigate the impact of neurologist care on PD-related hospitalizations in the US | Neurologist PD care was associated with lower adjusted odds of both initial and repeat hospitalization for psychosis, urinary tract infection and traumatic injury. Odds of general illness hospitalization or hospitalization did not differ by neurologist involvement. |
| Willis et al. [71] | Insurance claims from Medicare program, 2002–2008, USA | n = >138 000 Medicare beneficiaries ages 65 + with incident PD | To determine survival for older patients with PD in the US | Thirty-five percent of patients with PD lived more than 6 years. Female, Hispanic, Asian patients had a lower adjusted risk of death than white men. |
| Willis et al. [72] | Insurance claims from Medicare program, 2002–2005, USA | n = >138 000 Medicare beneficiaries ages 65 + with incident PD | To study the utilization of neurologist providers in the treatment of patients with PD in the US and determine whether neurologist treatment is associated with improved clinical outcomes. | Only 58% of patients with PD received neurologist care between 2002 and 2005. Women and minorities were less likely to be treated by a neurologist |
| Weintraub et al. [73] | Veterans Health Administration data, 1999–2010 | n = 6,679 matched PD pairs | To determine if AP use in PD patients is associated with increased physical morbidity | AP is associated with significantly increased ED, inpatient, and outpatient visits and mortality in PD patients |
| Makaroff et al. [74] | PharMetrics Patient-Centric Database, 2000–2008, USA | n = 5,579 patients with a prescription for levodopa and/ or dopamine agonist | To study the incidence of gastrointestinal disorders in PD patients and to examine subsequent PD-related outcomes | Incidence of gastrointestinal disorders increased over time to 65% at four years after PD diagnosis. Patients with gastrointestinal disorders had higher rates of psychosexual dysfunction, anxiety, depression, ataxia, pain, movement disorders, urinary incontinence and falls. |
| Guttman et al. [75] | Health insurance data, 1993–1998, Canada | n = 15,304 patients with parkinsonism | To study mortality rate in patients receiving treatment for parkinsonism | Cases with parkinsonism had a higher risk of mortality with an overall mortality odds ratio of 2.5 (95% CI: 2.4, 2.6) compared with the control group |
| n = 30,608 controls | ||||
| Chan et al. [76] | The Nationwide Inpatient Sample and Area Resource File, 2002–2009, USA | n = 2,408,302 PD discharges | To study the use of deep brain stimulation in PD and to determine the factors that drive DBS use in the US | Predictors for DBS use included younger age, male sex, increasing income quartile of patient zip code, large hospitals, teaching hospitals, urban setting, hospitals with higher number of annual discharges for PD, and increased countywide density of neurologists Predictors of nonuse included African American race, Medicaid use, and increasing comorbidity score |
| n = 18,312 PD discharges for DBS | ||||
| Dahodwala et al. [77] | Pennsylvania State Medical Claims, USA | n = 307 incident PD cases | To identify racial disparities in the treatment of PD | African-Americans were four times less likely than whites to receive any PD treatment, especially indicated medications. |
| Noyes et al. [78] | Medicare Current Beneficiary Survey, 1992–2000, USA | n = 35,217 Medicare beneficiaries≥65 years old | To evaluate medical utilization and economic burden of self-reported PD on patients and society | PD patients used significantly more health care services and paid significantly more out of pocket for their medical services than controls. PD patients also had higher annual health care expenses and were more likely to use medical care, in particular for long-term care and home health care |
| n = 717 PD patients | ||||
| Lapane et al. [79] | Medicare, 1992–1996, USA | n = 470.000 residents of long term care facilities | To stud the epidemiology of PD in long term care facilities | The prevalence of PD in nursing homes was 5.2%, with peak age-specific prevalence between ages 75 and 84 years. 70% had cognitive impairments, and over 80% had functional disability. Less than 10% had verbal and physical signs of grief and anxiety, and 80% exhibited poor psychosocial well-being. Only 44% received antiparkinsonian drugs. |
| n = 24,402 nursing home residents with a diagnosis of PD |
Search terms: Parkinson’s disease AND medical claims OR administrative data, November 21th 2017, 101 hits. PD, Parkinson’s disease; LTCF, long term care facility; DBS, deep brain stimulation; AP, antipsychotic; ED, emergency department.
However, this approach also has challenges of its own. The first drawback relates to the lack of direct information on disease-specific patient characteristics. Simple demographics such as age and gender are available, but detailed information about disease status is normally lacking (e.g., MDS-UPDRS scores). Therefore, comparability between groups who either did or did not receive a particular intervention on disease-specific measures cannot be guaranteed. A second drawback is that group separation is not based on randomization, and therefore potentially subject to all sorts of bias. For example, it is theoretically possible to mine medical claims data to search for differences in outcome between patients who received multidisciplinary care, versus those who did not. However, it is quite possible that patients with, for example, greater disease severity or those with higher education were preferentially referred to such interventions, leading to baseline differences that could explain differences in outcome. Third, it is impossible to be certain which intervention was ultimately responsible for the results, because patients in everyday clinical practice may receive a broad range of treatments, all of which can contribute to a different outcome. Moreover, coupled to this, there may be changes in social circumstances which are not captured in such databases. Without carefully controlling for each of them (which is a strength of RCTs), it is impossible to pinpoint a particular treatment as being the most effective ingredient. Fourth, medical claims databases also have to deal with attrition. In the Netherlands, this attrition rate is limited because of some national regulatory rules (i.e., health insurance is obligatory for every citizen, insurers have nationwide coverage, and changing from one insurer to another is only allowed at the beginning of each new year). The percentage of people switching their health insurance in the Netherlands (mostly young, healthy individuals) was 6.8% in 2016 and 6.3% in 2017, [18, 19] which is comparable to other European countries (i.e., Germany and Switzerland) and higher than for example Belgium and Israel where only 1% switches insurer [20]. However, also in other medical claims databases where loss to follow-up is higher compared to the Dutch situation, attrition is usually not the main concern because these rates in general do not reach 20% (which is often used as the cut-off for introducing bias [21]). Fifth, the quality of the data in medical claims databases (e.g., reliability, validity, sensitivity) is often unknown, and sometimes even known to be insufficient [22]. A particular concern is the presence of measurement error, which refers to the fact that in medical claims, conditions are defined imprecisely and inaccurately (specifically, it is uncertain how well general neurologists follow accepted international criteria to diagnose PD), that measurement may vary in a biased way, and that little is known about how robust the measures are. For example, the number of claims is often not identical to the number of unique visits [23] and some treatments or components of treatments may either not be claimed or wrongly coded [24]. Augmenting medical claims databases with other data sources, for example with prospective, longitudinal registries, may offer a potential solution for this [22]. Finally, just as in RCTs, researchers who analyze medical claims must define a primary outcome at the outset, prior to performing the analyses. The number of possible outcomes in a medical claims database is almost infinite, creating a risk of spurious findings unless a predefined primary outcome is decided upon before embarking upon the analyses.
Importantly, in light of these challenges, we do not see this approach as a substitute for well-designed trials, but rather as a complementary method to gather converging evidence to help in the design or interpretation of findings in a RCT. Indeed, medical claims data are increasingly being recognized and used as a valuable source of scientific information, next to the more traditional research methods in medical science [25].
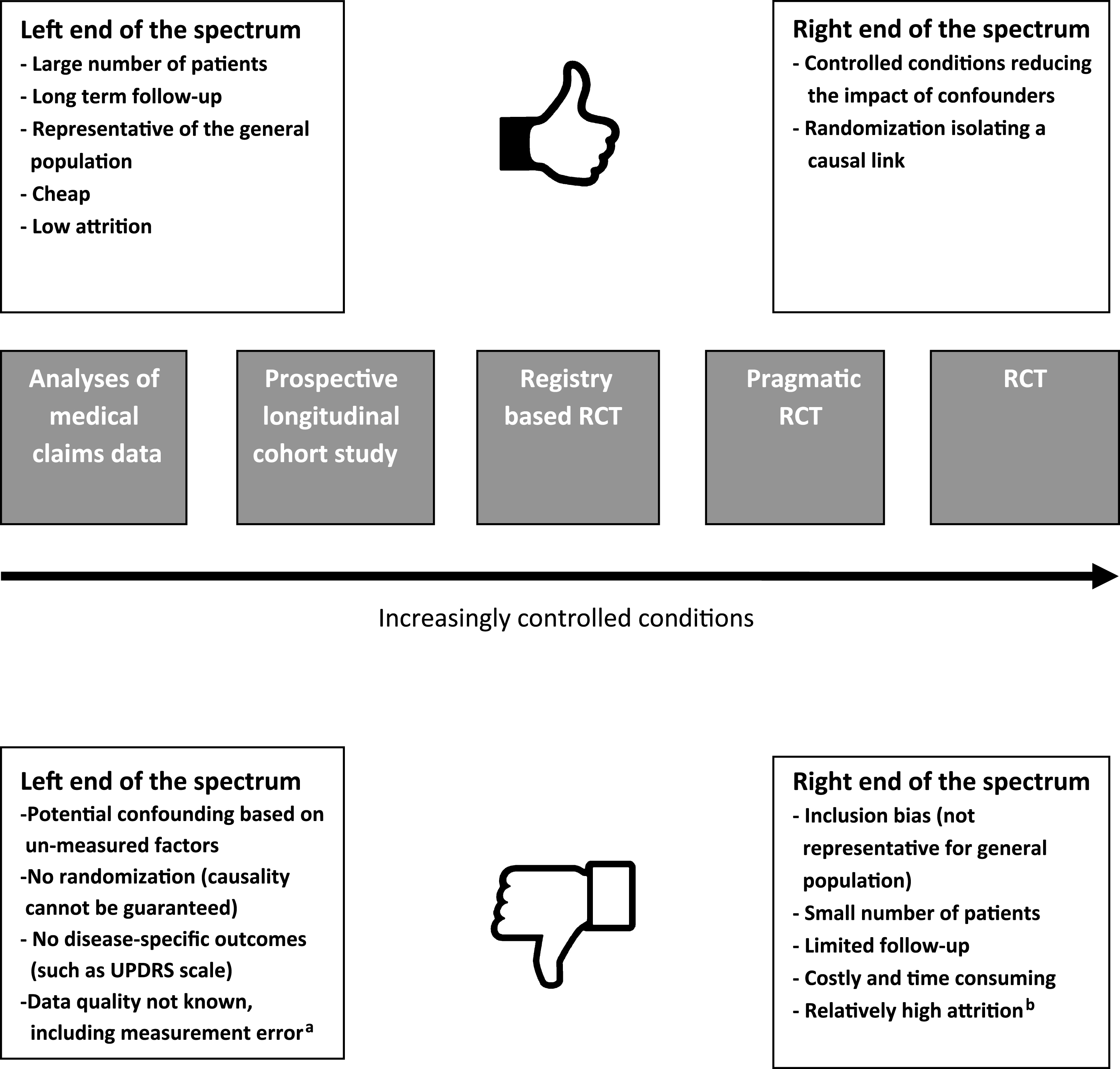
RCTs and analyses of medical claims databases can be seen as two extremes of a spectrum of study methodologies. However, there are also other methodological options along this spectrum that can be used to study the effectiveness of medical treatments, each with their own benefits and challenges (Fig. 1). Examples include the prospective longitudinal cohort study (of which a number of PD specific studies have emerged in the last few years, such as the Parkinson Progression Marker Initiative [26], the National Center of Excellence in Research–PD cohort [27] and the Parkinson Precision Project [28]), prognostic disease modeling [29, 30], the randomized registry trial (i.e., combining a randomization procedure to already existing high-quality observational registries) [4] and the pragmatic RCT (where the basic concept is to combine the strength of randomization with the cost-savings of using administrative data for outcome ascertainment) [31]. Here, we will further focus on the analyses of medical claims data as an example of an alternative research method, complementary to the traditional RCT.
Fig.1
This figure shows the RCT and analyses of medical claims as two extreme ends of the spectrum of study designs, with a low versus a high level of control. In between these two extremes, there are a number of alternative designs, and some of thesehave been included as examples here. aMeasurement error refers to the fact that in medical claims, conditions are defined imprecisely and inaccurately, that measurement may vary in a biased way, and that little is known about how robust the measures are. bAttrition rate is a problem for any study design. RCTs actually perform reasonably well by comparison to other clinical studies.
ANALYZING MEDICAL CLAIMS TO STUDY PHYSIOTHERAPY FOR PARKINSON PATIENTS
A recent study illustrates the value of this whole approach, in this specific case to demonstrate the cost-effectiveness of specialized physiotherapy [3]. There is now class II evidence to support the merits of specific physiotherapy interventions for patients with PD [32]. It is also clear that specific physiotherapy interventions such as external cueing require a great deal of PD-specific expertise to ascertain that these interventions are delivered optimally, i.e., both according to evidence-based guidelines [32] and tailored to each patient’s unique needs and personal profile. For example, inappropriate setting of visual or auditory cues is not only ineffective, but can lead to a worsening of gait disability in PD patients [33, 34]. Similar issues are at play when delivering other non-pharmacological interventions, such as occupational therapy, speech-language therapy or more recently introduced treatments such as cognitive rehabilitation or dance [13].
To address this matter of PD-specific expertise, the ParkinsonNet model of care was introduced in 2004 in the Netherlands as an innovative treatment concept for patients with PD. Specifically, ParkinsonNet consists of regional community-based networks that encompass a number of dedicated allied health therapists who have been trained specifically according to evidence-based guidelines [35]. The core elements of the ParkinsonNet model include: (1) professional empowerment, by concentrating care among specifically trained professionals; (2) patient empowerment, by informing patients and including them as partners in healthcare; and (3) team empowerment, by organizing and supporting care into multidisciplinary, regional networks [36]. The professional training consists of a 4-day baseline course in PD according to evidence-based guidelines (for each discipline separately). Additionally, professionals are required to participate in regional meetings (thrice annually) and national congresses where the latest evidence is presented. ParkinsonNet has reached full national coverage in the Netherlands, and currently includes 70 regional sub-networks with a total of 3,100 specifically trained healthcare professionals, including—among others—physiotherapists, occupational therapists, speech-langue therapists, dieticians, and Parkinson nurses [16].
The cost-effectiveness of this ParkinsonNet model has been studied in several RCTs, which overall revealed a better quality of care, lower healthcare costs and equal, if not better, health outcomes, at least in the short term (the longest follow-up was 6 months) [37–40]. A recent review conservatively estimated the cost-savings to amount to at least 381 euros per patient annually [36], and this would translate into an annual cost saving of around 15,2 million euros for the entire Dutch population (around n = 40,000 patients with PD). However, the aforementioned drawbacks of RCTs also applied here. Most importantly, there has been no information about the cost-effectiveness of specialized ParkinsonNet treatment in the long-term, within a representative real-life population. If the cost-effectiveness found in trials could be extrapolated to daily practice, then health insurers might have reasonable grounds to initiate a process of preferential or even selective reimbursement of ParkinsonNet therapy, thereby promoting better care and contributing to further cost savings.
Stimulated by these considerations, one of the largest Dutch healthcare insurers (the CZ Groep) took the initiative for a new evaluation of the ParkinsonNet model, using their own large medical claims database as their source of information [3]. The specific aim of this study was to compare the health outcomes and healthcare utilization of specialized physiotherapy via ParkinsonNet for a large, real-life population of people with PD, who had been followed for up for at least three years. Compared to RCTs, the inclusion criteria were purposely left broad: merely having a diagnosis of PD and being in receipt of physiotherapy as part of their regular PD care. Importantly, patients with co-morbid conditions were not excluded. Their claims database included a large group of no less than 4,381 patients who fulfilled these broad inclusion criteria.
By chance, about half of these patients had received care by a specialized community-based ParkinsonNet physiotherapist (n = 2,129 patients, with 4,649 observations over the 3-year period), while the other half had received usual care physiotherapy (n = 2,252 patients, with 5,353 observations). Importantly, this group separation did not result from careful randomization, but was created by the fact that Dutch patients (and their referring physicians) are free to choose their physiotherapy providers. Theoretically, all sorts of bias could have affected this choice, but this is what happens in “real life”. Correcting for potential baseline differences was challenging because, as we pointed out earlier, a claims database does not contain any PD-specific information about disease duration or severity. The researchers tackled this issue by comparing the two groups for a number of reasonable proxies for PD-related health status: number of neurology outpatient visits, number of drugs for PD, presence of depression, use of mental healthcare, number of different consulted professionals, and number of co-morbid conditions. Their analyses identified only small and clinically irrelevant group differences, suggesting that at the least, both groups did not differ substantially with respect to their underlying PD. A final concern was socio-economic status, as patients with higher education might be more prone to seek treatment from a well-trained expert. Therefore, socio-economic status was also corrected for in the statistical models and in addition showed to be very comparable between both groups. The results can neither be explained by differences in treatment intensity. In fact, the specialized ParkinsonNet therapists required significantly fewer treatment sessions than regular therapists, yet reached better outcomes. Finally, the investigators also took care to define a primary outcome prior to performing the analyses, and opted for the percentage of patients having one of the following common and major PD-related complications: hospital visits or admissions for sustaining a fracture, other orthopedic injuries, pneumonia, or a combination thereof. This outcome seems reasonable, because ParkinsonNet physiotherapists are specifically trained to promote safer mobility, by offering gait and balance training, reducing freezing of gait events, and thereby preventing falls and fall-related injuries [35].
The results were striking, showing that patients who had been treated by a specialized physiotherapist experienced significantly fewer PD-related complications (17.3%) compared to patients treated by a usual care physiotherapist (21.3%). Post-hoc analyses showed that this reduced risk of sustaining a complication was also significant for each of the three components of the primary outcome. It is likely that these outcomes can be ascribed to the specialized ParkinsonNet physiotherapy, as this was the only variable that consistently separated the two groups. An alternative option, that cannot be totally excluded, is that patients receiving specialized physiotherapy were also receiving specialized care by one or more of the other professional disciplines trained by ParkinsonNet [16]. For example, specialized speech-language therapists might have helped to reduce the risk of sustaining aspiration pneumonia, while home visits by specialized occupational therapists might have helped to remove domestic hazards for falls. Even though this was partially corrected for in the statistical model by including the number of healthcare professionals, the quality of these health professionals was not taken into account. To address this issue, further detailed analyses are now ongoing to identify which professional ParkinsonNet disciplines typically co-treat patients in daily practice. Such analyses would also help to further reinforce the quality of the multidisciplinary team approach for individual patients with PD, which is the ultimate goal of the ParkinsonNet approach. Nevertheless, if patients receiving specialized physiotherapy also incurred additional costs associated with provision of other specialist allied health services via ParkinsonNet, the positive cost-effectiveness outcomes of this study may be considered all the more impressive.
The investigators also analyzed whether the improved outcomes could be related to measures of better quality of care, and this was indeed the case. Specifically, the specialized physiotherapists treated a much higher case load (this helps to gain more expertise i.e. ‘practice makes perfect’) [41] and offered greater continuity of care than regular care therapists (which is greatly valued by patients). Moreover, specialized therapists required significantly fewer treatment sessions, which was expected since this is a part of their ParkinsonNet training (they are trained to support patients in self-management, and to utilize home-based and community-based exercise for maintaining gains made with supervised therapy), [35] whereas usual care therapists tend to offer chronic less intense maintenance treatments [42].
The better outcomes and greater quality of care also translated into tangible cost savings: compared to usual care physiotherapy, direct physiotherapy costs were almost € 400 less annually for specialized therapy (reflecting their greater efficiency), while total healthcare costs (including hospital expenses) were on average € 530 less annually (reflecting the prevented hospital visits). Finally, the investigators performed a very exploratory mortality analysis, motivated by the fact that survival in PD can be affected by specific complications such hip fractures or other fall-related injuries [43–46]. The uncorrected data showed a lower mortality risk for patients in the specialized physiotherapy group as compared to usual care (2.9% risk reduction), but this difference was no longer significant in a Cox’s proportional hazard model for survival time.
SIGNIFICANCE AND FUTURE PERSPECTIVES
The importance of this study lies, first of all, in the fact that this medical claims analysis offered supporting evidence to confirm and extend the findings of prior RCTs, but now within a large and real-life population, and—importantly—over a much longer follow-up period (3 years). In contrast to the cluster-controlled trial performed in 2010, [37] the new claims analysis found better health outcomes (reduced percentage of patients having a complication) for patients treated by specialized physiotherapists. Several factors can explain this difference with the earlier 2010 study. First, the regional ParkinsonNet networks included in the new claims study were quite “mature” (i.e., they had been operational for several years, allowing the participating therapists to accrue additional PD-specific expertise from treating a high case load), while the networks in the 2010 study were newly founded and then immediately analyzed (i.e., the networks included freshly trained physiotherapists, without the additional expertise from years of daily clinical practice). Mature or seasoned therapists are likely to achieve better outcomes than freshly trained therapists [16, 37]. Second, the 2010 study evaluated monodisciplinary networks that consisted of strictly physiotherapists. The new study evaluated regional networks that had meanwhile become multidisciplinary in nature [16]. As we mentioned earlier, we cannot fully exclude that patients who received specialized physiotherapy may also have received treatment by other specialized disciplines, and this may potentially have contributed to the positive outcomes, even though a correction for the number of other health professionals was applied. Finally, the follow-up duration of the RCT in 2010 was only 6 months [37], which is hardly enough to measure clinically relevant changes in health status, and in particular for rare events such as fractures. In contrast, the new study had a much longer follow-up of 3 years.
Both RCTs and claims analyses have their own strengths and weaknesses, and as such, the two approaches can really complement each other. Taking these two evidence sources together, it is now clear that, compared to generically trained professionals who treat PD patients only occasionally, specialized PD therapists offer better care, prevent major complications and hospital admissions, and help to reduce their treatment costs for society. These findings should stir a debate about promotion of evidence-based practice and specialization in the field of allied health, and indeed for many other health professionals delivering non-pharmacological interventions. In that regard, ParkinsonNet is just one example of how physiotherapy specialization in PD management can be achieved. Some other countries provide opportunities for specialization, for example, in Australia, specialist neurological physiotherapy training [47] includes PD, but is not limited to PD. Whatever the process of specialization, a key fundamental is the need for high-level skills in evidence-based practice, so that emerging evidence (not available in current guidelines and, where feasible, including evidence of cost-effectiveness) can be evaluated and implemented appropriately without delay. Recent examples include the PD-WEBB program (progressive strength and progressive balance exercises plus cueing strategies for freezing of gait) for falls prevention, [48] and intensive amplitude training for improving writing [49].
Additionally, the recent study in The Lancet Neurology offers an interesting “proof of concept” for using medical claims (in countries where such systems of health care exist) as a new way of assessing healthcare interventions, and in particular complex treatments like specialized physiotherapy. The main lesson brought forward by this new paper is that medical claims databases contain a surprising wealth of information about relevant care issues in the field of PD, and that mining such databases can offer an inexpensive and attractive approach to learn more about the functioning of clinical interventions in a real-life setting. Linking medical claims databases to a clinical outcomes registry would also be in interesting option, as it would allow entry of more disease-specific outcomes into the analyses, and might help to address the issue of misclassification (by introducing a greater standardisation according to established criteria, for example using the recently published criteria for health outcomes) [50]. Bearing in mind the drawbacks associated with claims analyses, the same approach could now be adopted with other healthcare disciplines, and even for a multidisciplinary team approach. In addition, this approach offers other unique opportunities, such as examining whether specialized interventions can help postpone admission to long-term care facilities such as nursing homes. Finally, other more invasive therapies for PD could also be tracked in similar ways, especially as we are entering an era of cell, gene and immune therapies. For example, do such therapies really impact on the problems of real life PD, and how do they compare with standards of care and non-pharmacological therapies? The time is now fast approaching when we have to radically rethink how we assess what we do for patients, not in clinic, but in the real world.
CONFLICTS OF INTEREST
Several of the authors of the present publication were part of the writing group that published the recent paper in The Lancet Neurology (BRB, JY, MM, NV).
ACKNOWLEDGMENTS
Professor Bastiaan R. Bloem is supported by a research grant of the Parkinson’s Foundation. Dr. Nienke M. de Vries is supported by a research grant from The Netherlands Organization for Health Research and Development. Dr. Allison Willis is supported by the Parkinson’s Foundation, the National Institutes of Health (R01-NS-099129-01A1), the Patient Centred Outcomes Research Institute, and the University of Pennsylvania.
REFERENCES
[1] | Post B , van der EM , Munneke M , Bloem BR ((2011) ) Multidisciplinary care for Parkinson’s disease: Not if, but how! Pract Neurol 11: , 58–61. |
[2] | van der Marck MA , Bloem BR ((2014) ) How to organize multispecialty care for patients with Parkinson’s disease. Parkinsonism Relat Disord 20: (Suppl 1), S167–S173. |
[3] | Ypinga JHL , de Vries NM , Boonen LHHM , Koolman X , Munneke M , Zwinderman AH , Bloem BR ((2017) ) Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: A retrospective analysis of medical claims data. Lancet Neurol, in press. |
[4] | Lauer MS , D’Agostino RB ((2013) ) The randomized registry trial–the next disruptive technology in clinical research? N Engl J Med 369: , 1579–1581. |
[5] | Chalmers I , Glasziou P ((2009) ) Avoidable waste in the production and reporting of research evidence. Lancet 374: , 86–89. |
[6] | Fransen GA , van Marrewijk CJ , Mujakovic S , Muris JW , Laheij RJ , Numans ME , de Wit NJ , Samsom M , Jansen JB , Knottnerus JA ((2007) ) Pragmatic trials in primary care. Methodological challenges and solutions demonstrated by the DIAMOND-study. BMC Med Res Methodol 7: , 16. |
[7] | Oude Rengerink K , Kalkman S , Collier S , Ciaglia A , Worsley SD , Lightbourne A , Eckert L , Groenwold RHH , Grobbee DE , Irving EA ((2017) ) Series: Pragmatic trials and real world evidence: Paper 3. Patient selection challenges and consequences. J Clin Epidemiol 89: , 173–180. |
[8] | Lugtenberg M , Burgers JS , Clancy C , Westert GP , Schneider EC ((2011) ) Current guidelines have limited applicability to patients with comorbid conditions: A systematic analysis of evidence-based guidelines. PLoS One 6: , e25987. |
[9] | Domingos JM , Godinho C , Dean J , Coelho M , Pinto A , Bloem BR , Ferreira JJ ((2015) ) Cognitive impairment in fall-related studies in Parkinson’s disease. J Parkinsons Dis 5: , 453–469. |
[10] | Keus SH , Bloem BR , Hendriks EJ , Bredero-Cohen AB , Munneke M ((2007) ) Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord 22: , 451–460; quiz 600. |
[11] | Ashburn A , Pickering RM , Fazakarley L , Ballinger C , McLellan DL , Fitton C ((2007) ) Recruitment to a clinical trial from the databases of specialists in Parkinson’s disease. Parkinsonism Relat Disord 13: , 35–39. |
[12] | Mirelman A , Rochester L , Maidan I , Del Din S , Alcock L , Nieuwhof F , Rikkert MO , Bloem BR , Pelosin E , Avanzino L , Abbruzzese G , Dockx K , Bekkers E , Giladi N , Nieuwboer A , Hausdorff JM ((2016) ) Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 388: , 1170–1182. |
[13] | Bloem BR , de Vries NM , Ebersbach G ((2015) ) Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 30: , 1504–1520. |
[14] | de Campos Moreira T , Gadenz CD , Capobianco DM , Figueiro LR , Ferigolo M , Vissoci JR , Barros HM , Cassol M , Pietrobon R ((2017) ) Factors associated with attrition in randomized controlled trials of vocal rehabilitation: Systematic review and meta-analysis. J Voice 31: , 259.e229–259.e240. |
[15] | Ioannidis JP ((2005) ) Contradicted and initially stronger effects in highly cited clinical research. JAMA 294: , 218–228. |
[16] | Bloem BR , Munneke M ((2014) ) Revolutionising management of chronic disease: The ParkinsonNet approach. BMJ 348: , g1838. |
[17] | Baldacci F , Policardo L , Rossi S , Ulivelli M , Ramat S , Grassi E , Palumbo P , Giovannelli F , Cincotta M , Ceravolo R , Sorbi S , Francesconi P , Bonuccelli U ((2015) ) Reliability of administrative data for the identification of Parkinson’s disease cohorts. Neurol Sci 36: , 783–786. |
[18] | https://www.vektis.nl/actueel/definitief-overstappercentage-zorgverzekering-2017-is-6-4, Accessed November 29th (2017) . |
[19] | https://www.vektis.nl/uploads/Publicaties/Zorgthermometer/Verzekerden%20in%20beeld%202016.pdf, Latest access November 29th (2017) . |
[20] | Hendriks M , de Jong JD , van den Brink-Muinen A , Groenewegen PP ((2010) ) The intention to switch health insurer and actual switching behaviour: Are there differences between groups of people? Health Expect 13: , 195–207. |
[21] | Dumville JC , Torgerson DJ , Hewitt CE ((2006) ) Reporting attrition in randomised controlled trials. BMJ 332: , 969–971. |
[22] | Noone AM , Lund JL , Mariotto A , Cronin K , McNeel T , Deapen D , Warren JL ((2016) ) Comparison of SEER treatment data with Medicare Claims. Med Care 54: , e55–e64. |
[23] | Tyree PT , Lind BK , Lafferty WE ((2006) ) Challenges of using medical insurance claims data for utilization analysis. Am J Med Qual 21: , 269–275. |
[24] | Mahmoudi E , Kotsis SV , Chung KC ((2015) ) A review of the use of Medicare claims data in plastic surgery outcomes research. Plast Reconstr Surg Glob Open 3: , e530. |
[25] | Brennan N , Oelschlaeger A , Cox C , Tavenner M ((2014) ) Leveraging the big-data revolution: CMS is expanding capabilities to spur health system transformation. Health Aff (Millwood) 33: , 1195–1202. |
[26] | ((2011) ) The Parkinson Progression Marker Initiative. (PPMI). Prog Neurobiol 95: , 629–635. |
[27] | http://www.parkinson.lu/index.php/en/, Latest access 28th November (2017) |
[28] | https://www.parkinsonopmaat.nl/studie, Latest access 28th November (2017) |
[29] | Liu G , Locascio JJ , Corvol JC , Boot B , Liao Z , Page K , Franco D , Burke K , Jansen IE , Trisini-Lipsanopoulos A , Winder-Rhodes S , Tanner CM , Lang AE , Eberly S , Elbaz A , Brice A , Mangone G , Ravina B , Shoulson I , Cormier-Dequaire F , Heutink P , van Hilten JJ , Barker RA , Williams-Gray CH , Marinus J , Scherzer CR ((2017) ) Prediction of cognition in Parkinson’s disease with a clinical-genetic score: A longitudinal analysis of nine cohorts. Lancet Neurol 16: , 620–629. |
[30] | Velseboer DC , de Bie RM , Wieske L , Evans JR , Mason SL , Foltynie T , Schmand B , de Haan RJ , Post B , Barker RA , Williams-Gray CH ((2016) ) Development and external validation of a prognostic model in newly diagnosed Parkinson disease. Neurology 86: , 986–993. |
[31] | Zuidgeest MGP , Goetz I , Groenwold RHH , Irving E , van Thiel G , Grobbee DE ((2017) ) Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J Clin Epidemiol 88: , 7–13. |
[32] | Keus SH , Munneke M , Graziano M , Paltamaa J , Pelosin I , Domingos J , Brühlmann S , Ramaswamy B , Prins J , Struiksma C , Rochester L , Nieuwboer A , Bloem B ((2014) ) European Physiotherapy Guideline for Parkinson’s Disease. KNGF/ ParkinsonNet, The Netherlands. |
[33] | Ginis P , Nackaerts E , Nieuwboer A , Heremans E ((2017) ) Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann Phys Rehabil Med, doi: 10.1016/j.rehab.2017.08.002 |
[34] | Beaulne-Seguin Z , Nantel J ((2016) ) Conflicting and non-conflicting visual cues lead to error in gait initiation and gait inhibition in individuals with freezing of gait. Gait Posture 49: , 443–447. |
[35] | Nijkrake MJ , Keus SH , Overeem S , Oostendorp RA , Vlieland TP , Mulleners W , Hoogerwaard EM , Bloem BR , Munneke M ((2010) ) The ParkinsonNet concept: Development, implementation and initial experience. Mov Disord 25: , 823–829. |
[36] | Bloem BR , Rompen L , de Vries NM , Klink A , Munneke M , Jeurissen P ((2017) ) ParkinsonNet: A low cost health care innovation with a systems approach from the Netherlands. Health Aff (Millwood) 36: , 1987–1996. |
[37] | Munneke M , Nijkrake MJ , Keus SH , Kwakkel G , Berendse HW , Roos RA , Borm GF , Adang EM , Overeem S , Bloem BR , ParkinsonNet Trial Study Group ((2010) ) Efficacy of community-based physiotherapy networks for patients with Parkinson’s disease: A cluster-randomised trial. Lancet Neurol 9: , 46–54. |
[38] | Sturkenboom IH , Graff MJ , Hendriks JC , Veenhuizen Y , Munneke M , Bloem BR , Nijhuis-van der Sanden MW , OTiP study group ((2014) ) Efficacy of occupational therapy for patients with Parkinson’s disease: A randomised controlled trial. Lancet Neurol 13: , 557–566. |
[39] | Sturkenboom IH , Hendriks JC , Graff MJ , Adang EM , Munneke M , Nijhuis-van der Sanden MW , Bloem BR ((2015) ) Economic evaluation of occupational therapy in Parkinson’s disease: A randomized controlled trial. Mov Disord 30: , 1059–1067. |
[40] | van der Marck MA , Munneke M , Mulleners W , Hoogerwaard EM , Borm GF , Overeem S , Bloem BR ((2013) ) Integrated multidisciplinary care in Parkinson’s disease: A non-randomised, controlled trial (IMPACT). Lancet Neurol 12: , 947–956. |
[41] | Luft HS , Hunt SS , Maerki SC ((1987) ) The volume-outcome relationship: Practice-makes-perfect or selective-referral patterns? Health Serv Res 22: , 157–182. |
[42] | Keus SH , Bloem BR , Verbaan D , de Jonge PA , Hofman M , van Hilten BJ , Munneke M ((2004) ) Physiotherapy in Parkinson’s disease: Utilisation and patient satisfaction. J Neurol 251: , 680–687. |
[43] | Pouwels S , Bazelier MT , de Boer A , Weber WE , Neef C , Cooper C , de Vries F ((2013) ) Risk of fracture in patients with Parkinson’s disease. Osteoporos Int 24: , 2283–2290. |
[44] | Pinter B , Diem-Zangerl A , Wenning GK , Scherfler C , Oberaigner W , Seppi K , Poewe W ((2015) ) Mortality in Parkinson’s disease: A 38-year follow-up study. Mov Disord 30: , 266–269. |
[45] | Harris-Hayes M , Willis AW , Klein SE , Czuppon S , Crowner B , Racette BA ((2014) ) Relative mortality in U.S. Medicare beneficiaries with Parkinson disease and hip and pelvic fractures. J Bone Joint Surg Am 96: , e27. |
[46] | Akbar U , Dham B , He Y , Hack N , Wu S , Troche M , Tighe P , Nelson E , Friedman JH , Okun MS ((2015) ) Incidence and mortality trends of aspiration pneumonia in Parkinson’s disease in the United States, 1979-2010. Parkinsonism Relat Disord 21: , 1082–1086. |
[47] | https://www.physiotherapy.asn.au/APAWCM/Careers/Career_Paths/Specialisation_Pathway/APAWCM/Careers/Career_Paths/Specialisation.aspx, Latest access November 20th (2017) . |
[48] | Canning CG , Sherrington C , Lord SR , Close JC , Heritier S , Heller GZ , Howard K , Allen NE , Latt MD , Murray SM , O’Rourke SD , Paul SS , Song J , Fung VS ((2015) ) Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology 84: , 304–312. |
[49] | Nackaerts E , Heremans E , Vervoort G , Smits-Engelsman BC , Swinnen SP , Vandenberghe W , Bergmans B , Nieuwboer A ((2016) ) Relearning of writing skills in Parkinson’s disease after intensive amplitude training. Mov Disord 31: , 1209–1216. |
[50] | de Roos P , Bloem BR , Kelley TA , Antonini A , Dodel R , Hagell P , Marras C , Martinez-Martin P , Mehta SH , Odin P , Chaudhuri KR , Weintraub D , Wilson B , Uitti RJ ((2017) ) A consensus set of outcomes for Parkinson’s disease from the International Consortium for Health Outcomes Measurement. J Parkinsons Dis 7: , 533–543. |
[51] | Searles Nielsen S , Warden MN , Camacho-Soto A , Willis AW , Wright BA , Racette BA ((2017) ) A predictive model to identify Parkinson disease from administrative claims data. Neurology 89: , 1448–1456. |
[52] | Fullard ME , Thibault DP , Hill A , Fox J , Bhatti DE , Burack MA , Dahodwala N , Haberfeld E , Kern DS , Klepitskava OS , Urrea-Mendoza E , Myers P , Nutt J , Rafferty MR , Schwalb JM , Shulman LM , Willis AW , Parkinson Study Group Healthcare Outcomes and Disparities Working Group ((2017) ) Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 89: , 1162–1169. |
[53] | Chou PS , Lai CL , Chou YH , Chang WP ((2017) ) Sleep apnea and the subsequent risk of Parkinson’s disease: A 3-year nationwide population-based study. Neuropsychiatr Dis Treat 13: , 959–965. |
[54] | Yang YW , Hsieh TF , Li CI , Liu CS , Lin WY , Chiang JH , Li TC , Lin CC ((2017) ) Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 96: , e5921. |
[55] | Huang YF , Chou YC , Yeh CC , Hu CJ , Cherng YG , Chen TL , Liao CC ((2016) ) Outcomes after non-neurological surgery in patients with Parkinson’s disease: A nationwide matched cohort study. Medicine (Baltimore) 95: , e3196. |
[56] | Crispo JA , Willis AW , Thibault DP , Fortin Y , Hays HD , McNair DS , Bjerre LM , Kohen DE , Perez-Lloret S , Mattison DR , Krewski D ((2016) ) Associations between anticholinergic burden and adverse health outcomes in Parkinson disease. PLoS One 11: , e0150621. |
[57] | Kwak M ((2015) ) Social demographic characteristics and direct medical costs for patients with Parkinson’s disease In Korea: Big data analysis from the National Health Insurance Claims Dataset. Value Health 18: , A751. |
[58] | Chen JC , Tsai TY , Li CY , Hwang JH ((2015) ) Obstructive sleep apnea and risk of Parkinson’s disease: A population-based cohort study. J Sleep Res 24: , 432–437. |
[59] | Huang YF , Cherng YG , Hsu SP , Yeh CC , Chou YC , Wu CH , Chen TL , Liao CC ((2015) ) Risk and adverse outcomes of fractures in patients with Parkinson’s disease: Two nationwide studies. Osteoporos Int 26: , 1723–1732. |
[60] | Benzinger P , Rapp K , Maetzler W , Konig HH , Jaensch A , Klenk J , Buchele G ((2014) ) Risk for femoral fractures in Parkinson’s disease patients with and without severe functional impairment. PLoS One 9: , e97073. |
[61] | Wang IK , Lin CL , Wu YY , Chou CY , Lin SY , Liu JH , Yen TH , Huang CC , Sung FC ((2014) ) Increased risk of Parkinson’s disease in patients with end-stage renal disease: A retrospective cohort study. Neuroepidemiology 42: , 204–210. |
[62] | Lai SW , Liao KF , Lin CL , Lin CC , Sung FC ((2014) ) Hearing loss may be a non-motor feature of Parkinson’s disease in older people in Taiwan. Eur J Neurol 21: , 752–757. |
[63] | Suh DC , Pahwa R , Mallya U ((2012) ) Treatment patterns and associated costs with Parkinson’s disease levodopa induced dyskinesia. J Neurol Sci 319: , 24–31. |
[64] | Hobson DE , Lix LM , Azimaee M , Leslie WD , Burchill C , Hobson S ((2012) ) Healthcare utilization in patients with Parkinson’s disease: A population-based analysis. Parkinsonism Relat Disord 18: , 930–935. |
[65] | Ooba N , Yamaguchi T , Kubota K ((2011) ) The impact in Japan of regulatory action on prescribing of dopamine receptor agonists: Analysis of a claims database between 2005 and 2008. Drug Saf 34: , 329–338. |
[66] | Davis KL , Edin HM , Allen JK ((2010) ) Prevalence and cost of medication nonadherence in Parkinson’s disease: Evidence from administrative claims data. Mov Disord 25: , 474–480. |
[67] | Safarpour D , Thibault DP , DeSanto CL , Boyd CM , Dorsey ER , Racette BA , Willis AW ((2015) ) Nursing home and end-of-life care in Parkinson disease. Neurology 85: , 413–419. |
[68] | Willis AW , Schootman M , Kung N , Wang XY , Perlmutter JS , Racette BA ((2014) ) Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 82: , 163–171. |
[69] | Willis AW , Schootman M , Kung N , Racette BA ((2013) ) Epidemiology and neuropsychiatric manifestations of young onset Parkinson’s disease in the United States. Parkinsonism Relat Disord 19: , 202–206. |
[70] | Willis AW , Schootman M , Tran R , Kung N , Evanoff BA , Perlmutter JS , Racette BA ((2012) ) Neurologist-associated reduction in PD-related hospitalizations and health care expenditures. Neurology 79: , 1774–1780. |
[71] | Willis AW , Schootman M , Kung N , Evanoff BA , Perlmutter JS , Racette BA ((2012) ) Predictors of survival in patients with Parkinson disease. Arch Neurol 69: , 601–607. |
[72] | Willis AW , Schootman M , Evanoff BA , Perlmutter JS , Racette BA ((2011) ) Neurologist care in Parkinson disease: A utilization, outcomes, and survival study. Neurology 77: , 851–857. |
[73] | Weintraub D , Chiang C , Kim HM , Wilkinson J , Marras C , Stanislawski B , Mamikonyan E , Kales HC ((2017) ) Antipsychotic use and physical morbidity in Parkinson disease. Am J Geriatr Psychiatry 25: , 697–705. |
[74] | Makaroff L , Gunn A , Gervasoni C , Richy F ((2011) ) Gastrointestinal disorders in Parkinson’s disease: Prevalence and health outcomes in a US claims database. J Parkinsons Dis 1: , 65–74. |
[75] | Guttman M , Slaughter PM , Theriault ME , DeBoer DP , Naylor CD ((2001) ) Parkinsonism in Ontario: Increased mortality compared with controls in a large cohort study. Neurology 57: , 2278–2282. |
[76] | Chan AK , McGovern RA , Brown LT , Sheehy JP , Zacharia BE , Mikell CB , Bruce SS , Ford B , McKhann GM 2nd ((2014) ) Disparities in access to deep brain stimulation surgery for Parkinson disease: Interaction between African American race and Medicaid use. JAMA Neurol 71: , 291–299. |
[77] | Dahodwala N , Xie M , Noll E , Siderowf A , Mandell DS ((2009) ) Treatment disparities in Parkinson’s disease. Ann Neurol 66: , 142–145. |
[78] | Noyes K , Liu H , Li Y , Holloway R , Dick AW ((2006) ) Economic burden associated with Parkinson’s disease on elderly Medicare beneficiaries. Mov Disord 21: , 362–372. |
[79] | Lapane KL , Fernandez HH , Friedman JH ((1999) ) Prevalence, clinical characteristics, and pharmacologic treatment of Parkinson’s disease in residents in long-term care facilities. SAGE Study Group. Pharmacotherapy 19: , 1321–1327. |




