Impulsive and Compulsive Behaviors in Parkinson’s Disease: The Norwegian ParkWest Study
Abstract
Background: Impulsive and compulsive behaviors (ICBs) are frequent in Parkinson’s disease (PD), but data from population-based cohorts is lacking.
Objectives: To determine the frequency and associated demographic, clinical, neuropsychiatric and cognitive features of ICBs in a population-based PD cohort.
Methods: This cross-sectional study included 125 patients with PD and 159 age- and gender-matched normal controls recruited from the Norwegian ParkWest study. Participants underwent comprehensive neurological, neuropsychiatric and neuropsychological assessments. ICBs were assessed using the Questionnaire for Impulsive-Compulsive Disorders in PD short form. Multiple logistic regression analyses were performed to compare the odds of ICBs between groups and to identify independent correlates of ICBs in PD.
Results: 30.4% of patients reported at least one ICB, with an odds ratio (OR) of 3.2 (95% confidence interval [CI] 1.8–5.9) compared with controls. Multiple ICBs were experienced by 8.8% of patients vs 1.3% of controls (OR 7.6, 95% CI 1.7–34.8). Compared to controls, the ORs of having an ICB were 7.4 (95% CI 2.6–20.9) in patients taking DA without levodopa, 4.6 (95% CI 2.3–9.3) in those treated with both DA and levodopa, and 1.2 (95% CI 0.5–3.2) in patients using levodopa but not DA. In multivariate models, ICB status in patients was independently associated with DA treatment and depressive symptoms, but not with other dopaminergic medications, motor function, or cognitive performance.
Conclusions: Patients with PD treated with DA, but not other dopaminergic medications, have increased odds of having ICBs compared with age- and gender-matched controls. This has implications for individualized patient management and follow-up.
INTRODUCTION
Impulsive-compulsive behaviors (ICBs) are recognized as serious neuropsychiatric complications in Parkinson disease (PD), with potentially devastating personal, social and financial consequences [1, 2]. These abnormal behaviors include the four major impulse control disorders (ICD) pathological gambling, compulsive shopping, binge eating and hypersexuality [1]. These behaviors are ego-syntonic and impulsive in nature, characterized by an effort to obtain arousal and gratification and cognitive biases [3–5]. In addition, a range of related behaviors have been described in PD, including punding, hobbyism, walkabout and compulsive dopaminergic medication overuse [6]. The related ICBs are ego-dystonic and compulsive in nature, associated with a calming or anxiolytic effect on the patient [5, 6].
Reported prevalence estimates of ICBs in PD vary considerably, ranging from 6% to almost 35% [1, 7]. Potential explanations include differences in the definition and assessment of ICBs, dopaminergic treatment, and patient selection, with most studies performed at highly-specialized movement disorders centers. In addition, since only few studies included normal control subjects, little is known about the risk of ICBs in PD relative to the general population [8–10]. Such information would, however, be important given that social, cultural and economic factors are likely to influence the prevalence of ICBs.
ICBs in PD have been associated most consistently with dopaminergic medication, and dopamine agonist (DA) treatment in particular [9, 11]. Other proposed determinants include premorbid personality traits, younger age, male gender, and depression and anxiety [12]. However, evidence in this respect is not unequivocal and even less clear for a range of other features within the spectrum of motor and non-motor symptoms associated with PD.
Against this background, we investigated the risk and determinants of ICDs and related impulsive-compulsive behaviors in a population-based PD cohort and normal controls (NCs) using comprehensive and standardized assessments of ICBs, as well as neurological, neuropsychiatric and cognitive functioning.
MATERIALS AND METHODS
Study design and participants
All participants were derived from the Norwegian ParkWest project, a population-based longitudinal study of the incidence, neurobiology and prognosis of PD. Details of the case ascertainment and diagnostic procedures to recruit a population-representative PD cohort have been published elsewhere [13]. Briefly, patients with newly diagnosed PD and NC subjects were recruited from four counties in Western and Southern Norway between 2004 and 2006, and followed prospectively by movement disorders neurologists with standardized clinical examinations. Assessment of ICBs was introduced at the 5 year re-examination, in which 155 patients with PD and 159 NCs participated. Of these, we excluded 28 patients and 1 control subject due to dementia [14, 15]. Thus, 125 non-demented PD patients and 159 NC subjects were eligible for this cross-sectional study of ICBs in PD. All PD patients met the National Institute of Neurological Disorders and Stroke and the United Kingdom PD Society Brain Bank criteria for PD [16, 17]. All participants were Caucasian.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Regional Committee for Medical and Health Research Ethics, Western Norway. Signed written informed consent was obtained from all participants.
Assessments
A standardized examination program was administered by trained members of the ParkWest study group. Information regarding demographic variables, lifestyle factors, clinical history, and medication was obtained during semistructured interviews. Motor severity and disease stage were assessed by the Unified PD Rating Scale (UPDRS) and Hoehn and Yahr scale. Levodopa equivalent doses (LEDs) were calculated according to published recommendations [18].
For assessment of ICBs, the self-report short form version of the Questionnaire for Impulsive-Compulsive Disorders in PD (QUIP) was completed by all participants [19]. The QUIP is designed to detect clinically significant impulse control disorders (compulsive gambling, sexual behavior, shopping and eating) and related impulsive-compulsive behaviors (punding, hobbyism, walkabout, and compulsive use of dopaminergic medication), and has been demonstrated to be a valid self-assessment screening instrument for ICBs in patients with PD [20]. Participants with positive response to one or more screening questions of the QUIP were classified to have ICB [20].
Cognitive function was assessed using the Mini-Mental State Examination (MMSE) as a measure of global cognition [21]. In addition, a comprehensive neuropsychological test battery [Stroop test [22], Semantic verbal fluency test [23], California Verbal Learning Test II (CLVT-II) [24], and Silhouettes and Cube subtests of the Visual Object and Space Perception Battery (VOSP) [25]] was administered by trained study nurses to assess a wide range of cognitive domains: attention (Stroop word reading and color naming), executive functioning (Semantic verbal fluency, Stroop interference condition), verbal memory (CVLT-II), and visuospatial skills (VOSP). A diagnosis of PD dementia (PDD) was determined according to published criteria [14], as described previously [26].
Neuropsychiatric symptoms were assessed using the 12-item version of the Neuropsychiatric Inventory (NPI) [27]. A composite score (product of frequency and severity; range 0–12) was calculated for each neuropsychiatric symptom. The validity of the NPI has been established [27], and high reliability in PD has been reported [28]. In addition, more comprehensive assessments of depressive symptoms, daytime sleepiness, and night-time sleep problems were performed using the Montgomery and Aasberg Depression Rating Scale (MADRS) [29], the Epworth Sleepiness Scale [30], and the PD Sleep Scale [31]. To identify possible subcomponents of depressive symptoms, we applied a three-factor model of MADRS as suggested by Suzuki et al. [32].
Statistical methods
All statistical procedures were performed using IBM SPSS Statistics version 22. Group differences were analysed using t tests, Mann–Whitney tests, χ2 tests and Fisher exact tests as appropriate. Logistic regression analyses (enter method) without and with adjustment for potential confounders (age, gender, MADRS and MMSE-scores) were used to compare the risk of ICBs in patients with PD vs controls, expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Since the unadjusted and adjusted analyses yielded similar results, unadjusted OR and CI are reported in the manuscript. Multivariate logistic regression analysis (enter method) was also applied to assess independent correlates of ICBs in patients with PD. For this purpose, variables attaining a significance level of p < 0.10 in univariate analyses were considered for inclusion as independent variables in multivariate models, with the presence or absence of ICBs as the dependent variable. Two-tailed p values < 0.05 were considered statistically significant.
RESULTS
Participant characteristics
Patients with PD had slightly lower MMSE and higher MADRS scores than age- and gender-matched NCs, but there were no between-group differences regarding lifestyle factors, daytime sleepiness, or night-time sleep problems (Table 1).
Frequency of ICBs
The frequencies of ICDs and related behaviors in patients and controls are illustrated in Fig. 1. Overall, 30.4% (38/125) of patients and 11.9% (19/159) of controls reported at least one ICB, yielding an OR of 3.2 (95% CI 1.8–5.9; p < 0.001). Multiple ICBs were reported by 8.8% of patients (28.9% of those with ICBs) compared with 1.3% of controls (10.5% of controls with ICBs). The corresponding OR for multiple ICBs was 7.6 (95% CI 1.7–34.8; p = 0.009).
ICDs were reported by 20.8% (26/125) of patients and 5.7% (9/159) of controls (OR 4.4, 95% CI 2.0–9.7; p < 0.001). The frequencies of ICD subtypes in patients vs controls were as follows: compulsive gambling 1.6% vs. 0.6%, hypersexuality 5.6% vs. 0.6%, compulsive shopping 4.8% vs. 2.5%, and compulsive eating 11.2% vs. 2.5%.
Related impulsive-compulsive behaviors were reported by 16.8% (21/125) of patients and 7.5% (12/159) of controls (OR 2.5, 95% CI 1.3–5.3; p = 0.018). The frequencies of related behavior subtypes in patients vs. controls were as follows: punding 9.6% vs. 5.0%, hobbyism 10.4% vs. 4.4%, walkabout 4.0% vs. 0.6%, and compulsive dopaminergic medication use 2.4% vs. 0%. We did not identify a gender difference between patients with the different ICB types.
Demographic and clinical correlates
Patients with ICBs were younger than patients without ICBs (p = 0.054), but there were no between-group differences in gender distribution, lifestyle factors, disease duration, motor severity, disease stage, daytime sleepiness or night-time sleep problems (Table 2). No patients had a history of deep brain stimulation.
Neuropsychiatric and neuropsychological correlates
Compared to patients without ICBs, those with ICBs had higher MADRS total scores and MADRS subscores related to dysphoria and retardation (Table 2). In addition, NPI items regarding depression, agitation, apathy, and irritability were more common in patients with than without ICBs (Table 3). In contrast, there were no significant between-group differences in global cognition or neuropsychological measures of attention, executive functioning, verbal memory, or visuospatial abilities (Supplemental Table). Supplemental analyses including only patients on DA treatment (n = 78) yielded similar results (data not shown).
Medication effects
There were no differences in monoaminooxidase-B inhibitor (MAO-B) use, levodopa use or dose, DA LED, or total LED between patients with or without ICBs (Table 2). However, patients with ICBs were more likely to use DA than those withoutICBs.
The distribution of ICBs stratified by treatment is summarized in Table 4. The highest frequency was observed among patients using DA only (50%), followed by those on both DAs and levodopa (38.3%), and patients taking levodopa but not DAs (13.9%). Compared to controls, the corresponding ORs were 7.4 (95% CI 2.6–20.9; p < 0.001) for those on DAs only and 4.6 (95% CI 2.3–9.3; p < 0.001) for combination users. Patients using levodopa only had no increased odds of ICBs compared to controls (OR = 1.2; 95% CI 0.5–3.2; p = 0.723). Compared to patients with a single ICB, patients with multiple ICBs did not use higher dosage of dopamine agonist (t = 1.20, P = 0.240).
Combined analysis
A multivariate model with ICB status as dependent variable and age, MADRS score, and DA treatment as independent variables, showed significant effects for higher MADRS score (OR 1.2, 95% CI 1.1–1.3; p = 0.001) and DA treatment (OR 6.4, 95% CI 2.0–20.4; p = 0.001), but not age (OR 1.0, 95% CI 0.9–1.0; p = 0.429). A second model that included age, DA treatment and positive NPI scores (score ≥1) regarding agitation, depression, apathy and irritability as independent variables, showed significant effects for NPI depression (OR 4.0, 95% CI 1.2–13.4; p = 0.022) and DA treatment (OR 5.7, 95% CI 1.6–19.7; p = 0.006), but not age (OR 1.0, 95% CI 0.9–1.0; p = 0.269) or other NPI symptoms (data not shown).
DISCUSSION
The main finding of this population-based study was that patients with PD have a 3-fold increased odds of ICBs compared with age- and gender-matched controls. About 30% of our PD cohort screened positive on the QUIP for at least one ICB and almost 10% for multiple ICBs. Presence of ICBs in PD was strongly and independently associated with DA treatment and depressive symptoms, but not with other clinical or demographic variables, levodopa treatment or neuropsychological measures of attention, executive function, memory or visuospatial skills. These findings have implications for individualized patient management and follow-up.
All ICB subtypes were more common in patients than in NCs, particularly hypersexuality, compulsive eating and walkabout. These findings clearly underline the importance of screening for related behaviors beyond the major ICDs in patients with PD. Compared to our population-based estimate of 30.4% ICBs in PD, previous studies from Mexico, Finland and Denmark reported both lower and higher ICBs rates, ranging from 14.9% to 34.8% [10, 33, 34]. However, these studies comprised convenient PD samples and no control group, making comparisons difficult. Indeed, we are aware of only one other controlled study reporting comparative data on the broad range of ICBs in PD. However, that study investigated drug-naïve patients and found similar ICBs rates compared to healthy controls, affecting about 20% in each group [8]. The frequency of ICBs in our control group was substantially lower, probably reflecting differences in sample recruitment and characteristics, as well as social, cultural and economic factors. These are important to consider when comparing the occurrence of ICBs between continents and countries.
ICBs in patients with PD have consistently been associated with dopaminergic medication, and DA use in particular. Our population-based data support and extend this observation, showing a more than 7-fold increased odds of ICBs among patients using DA but not levodopa, compared with NCs. In contrast, the association of ICBs with levodopa treatment has been less clear and a matter of debate. While some authors reported that levodopa treatment is associated with ICDs in PD [12], others argue that this finding may be an artefact of including patients with comorbid dopamine dysregulation syndrome who are taking high-dose levodopa [35]. Therefore, it has been claimed that levodopa remains a first-line choice in patients at high risk of ICDs and is essential to maintain antiparkinson efficacy in patients who need to reduce or stop DA treatment. Our findings seem to support this view, as the highest odds of ICBs was observed among DA only users, whereas the frequency of ICBs among patients not taking DAs was similar to that observed in NCs.
While ICBs were strongly associated with DA treatment, they were not related to DA dose in our cohort, suggesting a drug class rather than drug dose effect. A caveat of this conclusion is that our study did not differentiate between severe and less severe ICBs, making identification of a potential drug dose effect difficult. Although similar observations have been made previously [10], others report ICBs in PD to be associated with higher DA dosages [35–37]. This is also in line with the common clinical observation that down titration of DA dosage may alleviate ICB symptoms in patients with PD.
Despite conflicting findings, some studies argue that other antiparkinson drugs, such as monoaminoxidase-B (MAO-B) inhibitors and amantadine, may be associated with increased risk of ICBs [38–41]. While amantadine was not used in our cohort, we found no association between treatment with MAO-B inhibitors and frequency of ICBs.
We found strong associations between ICBs and depressive symptoms in our cohort, specifically symptoms of dysphoria and retardation. Although depressive symptoms were mild or even subclinical (i.e. under the cut-off for clinical significant depression in PD [42]) in most patients, the association with ICBs was consistent across several measures (NPI depression item and MADRS) and independent in multivariate analysis. Despite consistent evidence in multiple studies [33, 34, 43, 44], the relationship between ICBs and depressive symptoms in PD is not fully understood. However, it has been hypothesized that denervation of afferent dopaminergic neurons may result in sensitization of the subcortical motivational reward pathways. When exposed to exogenous DAs, affected patients may experience fluctuating symptoms of both depression and ICBs [44].
Presence of ICBs in our PD cohort was not related to other clinical or demographic measures. For example, we were unable to identify significant difference in gender between patients with and without ICBs. In line with previous studies [12, 34, 43], patients with ICBs in our cohort were younger than those without. However, age was not independently associated with ICBs in multivariate analysis that also took into consideration the effects of DAs, which often are the preferred dopaminergic treatment in younger patients with PD. Furthermore, we were not able to demonstrate any association between ICBs and motor disability or disease duration, nor cognitive impairment. Although altered reward and stimulus valuation have been reported in patients with PD and ICBs [3, 4, 9], global cognitive functioning has usually been demonstrated to be preserved [43, 45], in line with our findings. Indeed, a recent longitudinal study in PD even reported lower cognitive decline in patients with than without ICBs [45].
These observations argue against ICBs in PD being a consequence of more widespread brain pathology and rather suggest that the vulnerability to DAs in a substantial subset of patients reflects genetic susceptibility. In support of this, a recent study in PD found that 57% of the variance in ICB incidence was explained by common genetic variants, and that differentiation between patients at risk and patients without risk of ICB development may be possible using a broad candidate genetic panel [46]. These results are promising and highlight the involvement of premorbid genetic factors of multiple neurotransmitter systems in the pathogenesis of ICBs in PD. Continued research into potential genetic markers of ICBs might translate into clinical practice and make identification of at-risk patients possible.
Our study has both strengths and limitations. Major strengths include the population-based design, the well-characterized PD cohort, the comprehensive clinical, neuropsychiatric and cognitive assessments, and the age- and gender-matched control group from the same geographical area. We consider the use of the QUIP, a validated screening instrument covering a broad range of ICBs [20], another strength of our study, although we recognize that the short form version applied in this study does not allow to determine the severity of ICB symptoms, which is a relative limitation. We also recognize the assessment of ICBs by self-report as a potential limitation of this study, as affected individuals not always recognize ICBs as problematic [20]. Using QUIP, only moderate interrater reliability has been found between patients and their caregivers, arguing for a risk of false negatives in the absence of informant-based information on ICBs in PD [47, 48]. On the other hand, we are aware that the QUIP may overestimate the frequency of ICBs. However, this is most likely true for both patients and controls and should therefore not impact the odds ratios between these two groups. Another study limitation is the cross-sectional data presented here. However, these data extend previous evidence by demonstrating that ICBs are very common in the general PD population, with a more than 3-fold increased risk compared to matched control subjects. Importantly, this increased risk of ICBs in PD was driven by patients on treatment with DA, whereas levodopa per se was not associated with ICBs in our population-based cohort. Clinicians are therefore advised to demonstrate caution when administering DAs and to routinely screen for ICB symptoms in PD, as patients not always spontaneously report ICBs in daily clinical practice. Given the very limited long-term data in this field, collection of longitudinal data in our population-based study is ongoing and will hopefully provide further valuable insights into important aspects related to the causes, evolution and consequences of ICBs.
CONFLICT OF INTERESTS
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
The authors are grateful to all patients for their willingness to participate in this study and thank all personnel involved in planning and conducting the Norwegian ParkWest study. This study was supported by the Research Council of Norway (grant# 177966), the Western Norway Regional Health Authority (grant# 911218 and grant# 912014), and the Norwegian Parkinson’s Disease Association.
Appendices
The supplementary information is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-160977.
REFERENCES
[1] | Weintraub D , David AS , Evans AH , Grant JE & Stacy M ((2015) ) Clinical spectrum of impulse control disorder in Parkinson’s disease. Mov Disord, 30: , 121–127. |
[2] | Phu AL , Xu Z , Brakoulias V , Mahant N , Fung VS , Moore GD , Martin A , Starcevic V & Krause M ((2014) ) Effect of impulse control disorders on disability and quality of life in Parkinson’s disease patients. J Clin Neurosci, 21: , 63–66. |
[3] | Housden CR , O’Sullivan SS , Joyce EM , Lees AJ , & Roiser JP ((2010) ) Intact reward learning but elevated delay discounting in Parkinson’s disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology, 35: , 2155–2164. |
[4] | Piray P , Zeighami Y , Bahrami F , Eissa AM , Hewedi DH & Moustafa AA ((2014) ) Impulse control disorders in Parkinson’s disease are associated with dysfunction in stimulus valuation but not action valuation. J Neurosci, 34: , 7814–7824. |
[5] | Hollander E & Stein DJ ((2006) ) Clinical Manual of Impulse Control Disorders, American Psychiatric Publishing, Arlington, VA, USA. |
[6] | Evans AH , Katzenschlager R , Paviour D , O’Sullivan JD , Appel S , Lawrence AD & Lees AJ ((2004) ) Punding in Parkinson’s disease: Its relation to the dopamine dysregulation syndrome. Mov Disord, 19: , 397–405. |
[7] | Ceravolo R , Frosini D , Rossi C & Bonuccelli U ((2009) ) Impulse control disorders in Parkinson’s disease: Definition, epidemiology, risk factors, neurobiology and management. Parkinsonism Relat Disord, 155: , 111–115. |
[8] | Weintraub D , Papay K & Siderowf AD ((2013) ) Screening for impulse control symtpoms in patients with de novo Parkinson disease - A case-control study. Neurology, 80: , 176–180. |
[9] | Voon V , Sohr M , Lang AE , Potenza MN , Siderowf AD , Whetteckey J , Weintraub D , Wunderlich GR & Stacy M ((2011) ) Impulse control disorders in Parkinson disease: A multicenter case–control study. Ann Neurol, 69: , 986–996. |
[10] | Rodriguez-Violante M , Gonzalez-Latapi P , Cervantes-Arriaga A , Camacho-Ordonez A & Weintraub D ((2014) ) Impulse control and related disorders in Mexican Parkinson’s disease patients. Parkinsonism Relat Disord, 20: , 907–910. |
[11] | Garcia-Ruiz PJ , Martinez Castrillo JC , Alonso-Canovas A , Herranz Barcenas A , Vela L , Sanchez Alonso P , Mata M , Olmedilla Gonzalez N & Mahillo Fernandez I ((2014) ) Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: A multicentre study. J Neurol Neurosurg Psychiatry, 85: , 840–844. |
[12] | Weintraub D , Koester J , Potenza MN , Siderowf AD , Stacy M , Voon V , Whetteckey J , Wunderlich GR & Lang AE ((2010) ) Impulse control disorders in Parkinson disease: A cross-sectional study of 3090 patients. Arch Neurol, 67: , 589–595. |
[13] | Alves G , Muller B , Herlofson K , HogenEsch I , Telstad W , Aarsland D , Tysnes OB , Larsen JP & Norwegian ParkWest study group ((2009) ) Incidence of Parkinson’s disease in Norway: The Norwegian ParkWest study. J Neurol Neurosurg Psychiatry, 80: , 851–857. |
[14] | Emre M , Aarsland D , Brown R , Burn DJ , Duyckaerts C , Mizuno Y , Broe GA , Cummings J , Dickson DW , Gauthier S , Goldman J , Goetz C , Korczyn A , Lees A , Levy R , Litvan I , McKeith I , Olanow W , Poewe W , Quinn N , Sampaio C , Tolosa E & Dubois B ((2007) ) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord, 22: , 1689–1707; quiz 1837. |
[15] | McKhann G , Drachman D , Folstein M , Katzman R , Price D & Stadlan E ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDAWork Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34: , 939–944. |
[16] | Gelb D , Oliver E & Gilman S ((1999) ) Diagnostic criteria for Parkinson’s disease. Arch Neurol, 56: , 33–39. |
[17] | Hughes A , Daniel S , Kilford L & Lees A ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry, 55: , 181–184. |
[18] | Tomlinson C , Stowe R , Patel S , Rick C , Gray R & Clarke C ((2010) ) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord, 25: , 2649–2653. |
[19] | Weintraub D , Mamikonyan E , Papay K , Shea JA , Xie SX & Siderowf A ((2012) ) Questionnaire for impulsive-compulsive disorders in Parkinson’s disease-rating scale. Mov Disord, 27: , 242–247. |
[20] | Weintraub D , Hoops S , Shea JA , Lyons KE , Pahwa R , Driver-Dunckley ED , Adler CH , Potenza MN , Miyasaki J , Siderowf AD , Duda JE , Hurtig HI , Colcher A , Horn SS , Stern MB & Voon V ((2009) ) Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord, 24: , 1461–1467. |
[21] | Folstein MF , Folstein SE & McHugh PR ((1975) ) Mini mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12: , 189–198. |
[22] | Golden CJ & Freshwater SM ((1998) ) The Stroop Color and Word Test, The Stoelting Company, Wood Dale, IL. |
[23] | Benton AL & Hamsher KD ((1989) ) Multilingual Aphasia Examination, AJA Associates, Iowa City, IA. |
[24] | Delis DC KJ & Ober BA ((2000) ) CVLT-II. California Verbal Learning Test, Second Edition. Adult Version. The Psychological Corporation: Harcourt Assessment, Inc,San Antonio, TX. |
[25] | Warrington EK & James M ((1991) ) The Visual Object and Space Perception Battery, Thames Valley Test Company, Bury St Edmunds. |
[26] | Alves G , Lange J , Blennow K , Zetterberg H , Andreasson U , Forland M , Tysnes O , Larsen J & Pedersen KF ((2014) ) CSF A[beta]42 predicts early-onset dementia in Parkinson disease. Neurology, 82: , 1784–1790. |
[27] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology, 48: , S10–S16. |
[28] | Aarsland D , Larsen JP , Lim NG , Janvin C , Karlsen K , Tandberg E & Cummings J ((1999) ) Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry, 67: , 492–496. |
[29] | Montgomery SA & Asberg M ((1979) ) A new depression scale designed to be sensitive to change. Br J Psychiatry, 134: , 382–389. |
[30] | Murray JW ((1991) ) A new method for measuring daytime sleepiness: The Epsworth Sleepiness scale. Sleep, 14: , 540–545. |
[31] | Chaudhuri KR , Pal S , DiMarco A , Whately-Smith C , Bridgman K , Mathew R , Pezzela FR , Forbes A , Hogl B & Trenkwalder C ((2002) ) The Parkinson’s disease sleep scale: A new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 73: , 629–635. |
[32] | Suzuki A , Aoshima T , Fukasawa T , Yoshida K , Higuchi H , Shimizu T & Otani K ((2005) ) A three-factor model of the MADRS in major depressive disorder. Depress Anxiety, 21: , 95–97. |
[33] | Joutsa J , Martikainen K , Vahlberg T , Voon V & Kaasinen V ((2012) ) Impulse control disorders and depression in Finnish patients with Parkinson’s disease. Parkinsonism RelatDisord, 18: , 155–160. |
[34] | Callesen MB , Weintraub D , Damholdt MF & Moller A ((2014) ) Impulsive and compulsive behaviors among Danish patients with Parkinson’s disease: Prevalence, depression, and personality. Parkinsonism Relat Disord, 20: , 22–26. |
[35] | Grosset DG , Cardoso F & Lees A ((2011) ) Dopamine agonists vs levodopa in impulse control disorders. Arch Neurol, 68: , 544–546. |
[36] | Rohde K , Riedel O , Lueken U , Rietzel S , Fauser M , Ossig C , Reichmann H & Storch A ((2013) ) Impulsive-compulsive behaviours in a German Parkinson’s disease outpatient sample. Fortschr Neurol Psychiatr, 81: , 503–510. |
[37] | Weintraub D , Siderowf AD , Potenza MN , Goveas J , Morales KH , Duda JE , Moberg PJ & Stern MB ((2006) ) Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol, 63: , 969–973. |
[38] | Sáez-Francása N , Martí Andrésb G , Ramíreza N , de Fàbreguesb O , Álvarez-Sabínb J , Casasc M & Hernández-Varab J ((2016) ) Clinical and psychopathological factors associated with impulse control disorders in Parkinson’s disease. Neurologia, 31: , 231–238. |
[39] | Perez-Lloret S , Rey MV , Fabre N , Ory F , Spampinato U , Brefel-Courbon C , Montastruc JL & Rascol O ((2012) ) Prevalence and pharmacological factors associated with impulse-control disorder symptoms in patients with Parkinson disease. Clin Neuropharmacol, 35: , 261–265. |
[40] | Weintraub D , Sohr M , Potenza MN , Siderowf AD , Stacy M , Voon V , Whetteckey J , Wunderlich GR & Lang AE ((2010) ) Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol, 68: , 963–968. |
[41] | Thomas A , Bonanni L , Gambi F , Di Iorio A & Onofrj M ((2010) ) Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol, 68: , 400–404. |
[42] | Costa FH , Rosso AL , Maultasch H , Nicaretta DH & Vincent MB ((2012) ) Depression in Parkinson’s disease: Diagnosis and treatment. Arq Neuropsiquiatr, 70: , 617–620. |
[43] | Pontieri FE , Assogna F , Pellicano C , Cacciari C , Pannunzi S , Morrone A , Danese E , Caltagirone C & Spalletta G ((2015) ) Sociodemographic, neuropsychiatric and cognitive characteristics of pathological gambling and impulse control disorders NOS in Parkinson’s disease. Eur Neuropsychopharmacol, 25: , 69–76. |
[44] | Vriend C , Pattij T , van der Werf YD , Voorn P , Booij J , Rutten S , Berendse HW & van den Heuvel OA ((2014) ) Depression and impulse control disorders in Parkinson’s disease: Two sides of the same coin? Neurosci Biobehav Rev, 38: , 60–71. |
[45] | Siri C , Cilia R , De Gaspari D , Canesi M , Meucci N , Zecchinelli AL , Pezzoli G & Antonini A ((2010) ) Cognitive status of patients with Parkinson’s disease and pathological gambling. J Neurol, 257: , 247–252. |
[46] | Kraemmer J , Smith K , Weintraub D , Guillemot V , Nalls MA , Cormier-Dequaire F , Moszer I , Brice A , Singleton AB & Corvol JC ((2016) ) Clinical-genetic model predicts incident impulse control disorders in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 87: , 1106–1111. |
[47] | Papay K , Mamikonyan E , Siderowf AD , Duda JE , Lyons KE , Pahwa R , Driver-Dunckley ED , Adler CH & Weintraub D ((2011) ) Patient versus informant reporting of ICD symptoms in Parkinson’s disease using the QUIP: Validity and variability. Parkinsonism Relat Disord, 17: , 153–155. |
[48] | Lim SY , Tan ZK , Ngam PI , Lor TL , Mohamed H , Schee JP , Tan AK , Goh JY , Ooi E & Soh PC ((2011) ) Impulsive-compulsive behaviors are common in Asian Parkinson’s disease patients: Assessment using the QUIP. Parkinsonism Relat Disord, 17: , 761–764. |
Figures and Tables
Fig.1
Frequencies of ICBs among patients with PD and normal controls. ICB = Impulsive-compulsive behavior; PD = Parkinson disease; ICD = Impulse control disorder. Group differences are indicated by significance levels.
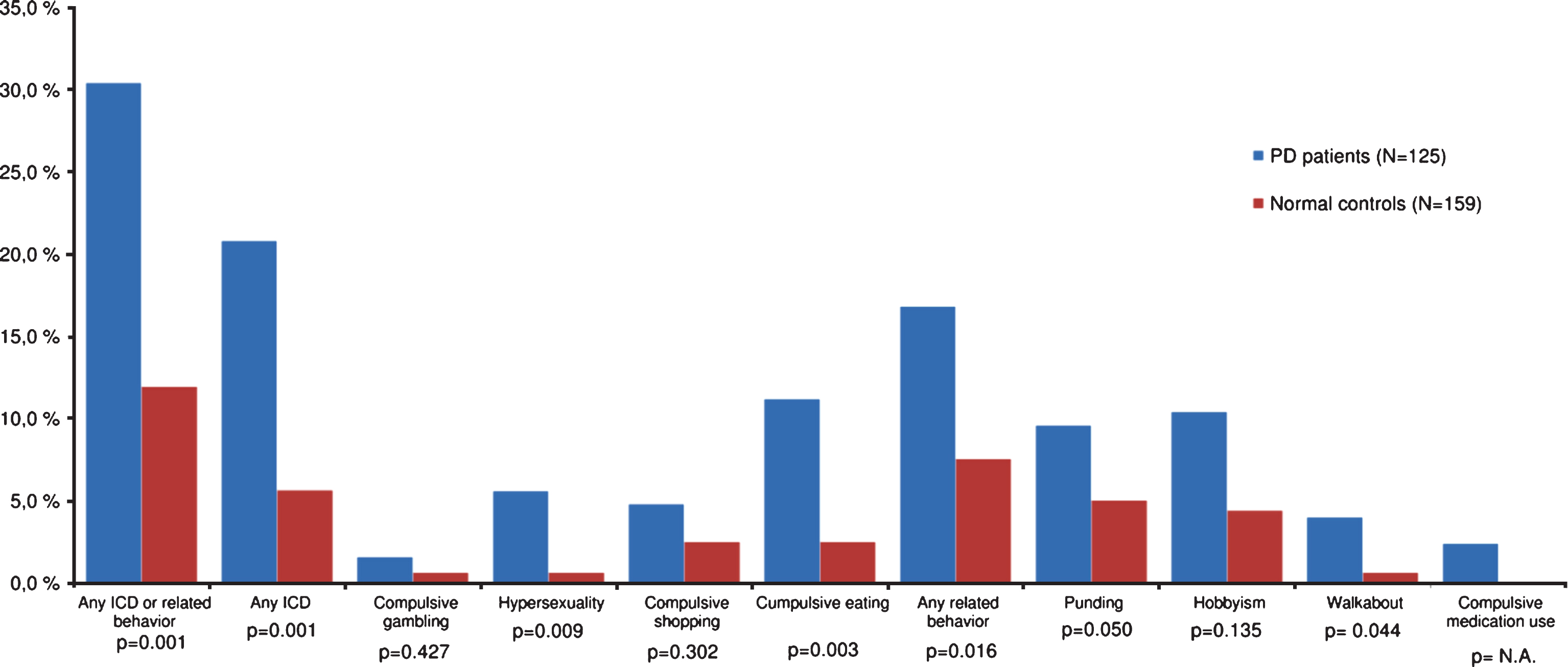
Table 1
Characteristics of patients with PD and normal controls
| Characteristics | PD patients | Normal controls | P value |
| (N = 125) | (N = 159) | ||
| Male, n (%) | 75 (60.0%) | 81 (50.9%) | 0.128 |
| Age, y | 70.3 (9.4) | 70.8 (9.0) | 0.674 |
| Smokinga, n (%) | 16 (12.8) | 17 (10.7) | 0.161 |
| Alcohol usea, n (%) | 86 (68.8) | 119 (74.8) | 0.259 |
| MMSE score | 27.8 (2.5) | 28.7 (1.5) | 0.001 |
| MADRS score | 3.8 (4.4) | 1.5 (2.9) | 0.001 |
| ESS score | 5.8 (4.0) | 6.5 (4.4) | 0.244 |
| PDSS score | 124.7 (17.5) | 123.6 (17.3) | 0.597 |
| Duration of PD, y | 7.4 (1.8) | – | – |
| UPDRS motor score | 22.7 (10.6) | – | – |
| Hoehn and Yahr stage | 2.2 (0.6) | – | – |
MMSE = Mini-Mental Status Examination; MADRS =Montgomery and Aasberg Depression Rating Scale; ESS =Epsworth Sleepiness Scale; PDSS = Parkinson’s Disease Sleep Scale. Data are mean (SD) unless otherwise indicated. aPrevious or current use. Bold values indicate significant p-value.
Table 2
Clinical and demographic characteristics of patients with and without ICBs
| Characteristics | ICB positive (n = 38) | ICB negative (n = 87) | P value |
| Demographic | |||
| Male, n (%) | 26 (68.4) | 49 (56.3) | 0.204 |
| Age, y | 67.9 (7.7) | 71.4 (9.8) | 0.054 |
| Smokinga, n (%) | 7 (18.4) | 12 (13.8) | 0.507 |
| Alcohol usea, n (%) | 26 (68.4) | 64 (73.5) | 0.556 |
| Clinical | |||
| Duration of PD, y | 7.4 (1.6) | 7.4 (1.9) | 0.367 |
| UPDRS motor score | 23.8 (10.5) | 22.2 (10.7) | 0.381 |
| Hoehn and Yahr stage | 2.2 (0.5) | 2.2 (0.6) | 0.598 |
| MMSE score | 28.4 (1.8) | 27.5 (2.8) | 0.108 |
| MADRS score | 5.4 (5.1) | 3.1 (3.9) | 0.009 |
| Dysphoria subscore | 1.0 (1.4) | 0.4 (0.9) | 0.003 |
| Retardation subscore | 2.6 (2.4) | 1.4 (2.1) | 0.006 |
| Vegetative subscore | 1.8 (1.3) | 1.3 (2.0) | 0.292 |
| ESS Scored | 5.6 (5.1) | 5.9 (3.5) | 0.283 |
| PDSS Scored | 121.8 (22.5) | 126.0 (14.5) | 0.775 |
| Medication | |||
| DA use, n (%) | 32 (84.2) | 46 (52.9) | 0.001 |
| Levodopa use, n (%) | 29 (76.3) | 74 (85.1) | 0.238 |
| Total LEDb | 730.6 (343.3) | 658.4 (275.9) | 0.522 |
| DA LEDb | 293.7 (132.4) | 289.5 (150.0) | 0.896 |
| Levodopa dosec | 505.2 (279.1) | 408.7 (266.7) | 0.107 |
| MAO-B use | 13 (34.2) | 31 (35.6) | 0.878 |
| Antidepressant use, n (%) | 5 (13.2) | 11 (12.6) | 0.937 |
MMSE = Mini-Mental Status Examination; MADRS = Montgomery and Aasberg Depression Rating Scale; ESS = Epsworth Sleepiness Scale; PDSS = Parkinson’s Disease Sleep Scale; DA = Dopamine agonist; LED = Levodopa equivalent dose; MAO-B = Monoaminooxidase-B inhibitor. Data are mean (SD) unless otherwise indicated. aPrevious or current use. bAmong DA users. Patients using only levodopa (n = 43) excluded. cAmong levodopa users. Patients using only DA (n = 18) excluded. dN = 102. Bold values indicate significant p-value.
Table 3
Neuropsychiatric characteristics in patients with and without ICBs
| NPI item | NPI score, mean (SD) | Proportion with positive NPI score, n (%) | P valuea | ||
| ICB positive (n = 34) | ICB negative (n = 71) | ICB positive (n = 34) | ICB negative (n = 71) | ||
| Delusions | 0.1 (0.7) | 0.0 (0.4) | 2 (5.9) | 1 (1.4) | 0.244 |
| Hallucinations | 0.1 (0.3) | 0.1 (0.8) | 1 (2.9) | 3 (4.2) | 0.999 |
| Agitation | 0.7 (1.9) | 0.2 (0.8) | 8 (23.5) | 5 (7.0) | 0.028 |
| Depression | 1.4 (2.3) | 0.4 (1.3) | 16 (47) | 11 (15.5) | 0.001 |
| Anxiety | 0.4 (1.1) | 0.2 (0.9) | 4 (11.8) | 5 (7.0) | 0.467 |
| Euphoria | 0.0 (0.2) | 0.0 (0.0) | 1 (2.9) | 0 (0.0) | 0.324 |
| Apathy | 1.3 (2.2) | 0.6 (1.6) | 11 (32.4) | 10 (14.1) | 0.029 |
| Disinhibition | 0.2 (0.7) | 0.2 (1.1) | 4 (11.8) | 3 (4.2) | 0.210 |
| Irritability | 0.9 (2.0) | 0.1 (0.4) | 9 (26.5) | 7 (9.9) | 0.027 |
| Aberrant motor behavior | 0.3 (1.4) | 0.0 (0.0) | 2 (5.9) | 0 (0.0) | 0.105 |
| Sleep disturbance | 2.8 (3.1) | 1.6 (2.4) | 17 (50.0) | 29 (40.8) | 0.337 |
| Appetite disturbance | 2.0 (3.4) | 1.2 (2.7) | 11 (32.4) | 14 (19.7) | 0.155 |
| NPI total | 10.8 (9.3) | 4.8 (5.8) | 28 (82.4) | 43 (60.6) | 0.021 |
ICBs = Impulsive-compulsive behaviors; NPI = Neuropsychiatric Inventory. NPI data missing in 4 with ICB and 16 without ICB. aχ2 test. Bold values indicate significant p-value.
Table 4
Frequency and odds of ICBs in patients stratified by treatment
| Characteristics | DA only users (n = 18) | DA and levodopa users (n = 60) | Levodopa only users (n = 43) |
| ICB positive, n (%) | 9 (50.0) | 23 (38.3) | 6 (13.9) |
| Multiple ICBs, n (%) | 3 (16.6) | 7 (11.6) | 1 (2.3) |
| OR* for any ICB, (95% CI) | 7.4 (2.6–20.9) | 4.6 (2.3–9.3) | 1.2 (0.5–3.2) |
Bold indicates significance (p < 0.05). ICBs = Impulsive-compulsive behaviors; OR = Odds ratio; DA = Dopamine agonist. *Compared to controls (n = 159).




