Ventilatory Dysfunction in Parkinson’s Disease
Abstract
In contrast to some other neurodegenerative diseases, little is known about ventilatory dysfunction in Parkinson’s disease (PD). To assess the spectrum of ventilation disorders in PD, we searched for and reviewed studies of dyspnea, lung volumes, respiratory muscle function, sleep breathing disorders and the response to hypoxemia in PD. Among the studies, we identified some limitations: (i) small study populations (mainly composed of patients with advanced PD), (ii) the absence of long-term follow-up and (iii) the absence of functional evaluations under “off-drug” conditions. Although there are many reports of abnormal spirometry data in PD (mainly related to impairment of the inspiratory muscles), little is known about hypoventilation in PD. We conclude that ventilatory dysfunction in PD has been poorly studied and little is known about its frequency and clinical relevance. Hence, there is a need to characterize the different phenotypes of ventilation disorders in PD, study their relationships with disease progression and assess their prognostic value.
INTRODUCTION
Ventilatory dysfunction is known to have a role in the pathogenesis and progression of neurodegenerative diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis [1–4]. However, little is known about the association between Parkinson’s disease (PD) and ventilatory dysfunction –despite the fact that James Parkinson noted the presence of respiratory abnormalities in his initial description of the disease (“He fetched his breath rather hard ... ”, [5]). Furthermore, it is well known that aspiration pneumonia and pulmonary embolism are among the main causes of death in PD patients [6, 7]. Ventilatory changes in PD might affect the patient’s quality of life by reducing levels of physical activity. However, ventilatory dysfunction in PD patients has not been well characterized even if, in 2010, a general review about respiratory problems in neurologic disorders was published [8]. Nowadays, there are a number of outstanding questions. What are the frequency and the severity of ventilatory dysfunction in PD? When does it start? How might ventilatory dysfunction influence the course of the disease in terms of the phenotype and prognosis? Is it responsive to dopaminergic treatments? Here, we performed a review of the spectrum of ventilatory disorders in PD. Identified publications were classified into five groups, depending on the topic: (i) dyspnea, (ii) lung volumes, (iii) respiratory muscle function and (iv) sleep breathing disorders, (v) response to hypoxemia. Lastly, we discuss these impairments’ putative involvement in the neurodegenerative process.
LITERATURE SEARCH STRATEGY
We performed a systematic review of available literature in Pubmed with the appropriate search terms. Relevant publications in any language were identified by searching the PubMed bibliographic database (http://www.ncbi.nlm.nih.gov/pubmed/) up 1950 until January 2015 with combinations of the keywords “pulmonary function AND Parkinson”, “lung AND Parkinson”, “breathing disorders AND Parkinson” (excluding nocturnal disorders), “ventilation AND Parkinson” and “hypoxemia AND Parkinson”. When relevant, additional references found in the identified publications were included in the review. Thus, we also considered reports of “breathing sleep disorders” in PD focusing on the articles about the impact of antiparkinsonian drugs.
DYSPNEA
Dyspnea corresponds to the subjective experience of respiratory discomfort, and consists of distinct sensations that vary in intensity [9]. The phenomenon appears to result from the erroneous integration of sensory afferents but also has a marked emotional component [10]. Dyspnea is a marker for poor quality of life and is associated with a loss of autonomy in ambulatory elderly patients [11]. Very few studies assessed dyspnea in PD (Table 1).
Dyspnea and fluctuations
In an observational study including all patients attended in a Movement Disorders department, most of the dyspnea was due to cardio-pulmonary diseases [12]. Using the classification of non-motor fluctuations in PD [13], dyspnea can be considered as an “autonomic disorder” (with cough and stridor) or as a “sensory disturbance”. Among non-motor fluctuations, in a cohort composed of advanced-PD patients, 40% reported dyspnea [14]. Several studies have shown that the perception of dyspnea is impaired in PD patients [15, 16]. This misperception was associated with symptoms of anxiety and non-motor fluctuations [17, 18]. Indeed, PD patients report that they feel dyspnea more frequently in the “off-drug” condition [14, 19].
Impact of treatments on dyspnea
However, dopamine does not seem to be the only neurotransmitter involved in dyspnea. Although there is still a doubt about the role of serotonin [20], anti-inflammatory drugs like steroids may interfere in dyspnea sensation [21]. After the administration of L-DOPA, improvements in lung function were not correlated with the reduction in the symptoms reported by the patients [15]. Paradoxically, antiparkinsonian medications can trigger dyspnea. Thus, L-DOPA-induced dyskinesia has been reported as a possible cause of dysregulated breathing [22], perhaps as a result of the loss of muscle control. Likewise, a longitudinal study has shown that dyspnea can be a side effect of subthalamic nucleus deep brain stimulation [23]. The authors mentioned the following mechanisms underlying this phenomenon: An alteration of dyspnea perception, a bronchoconstriction, a disturbance in upper airway control or a disturbed respiratory muscle control. Surprisingly, a fixed epiglottis has been observed in subthalamic nucleus deep brain stimulation patients [24].
Therefore, the precise mechanisms of dyspnea and the effect of treatments remain unknown.
LUNG VOLUMES
Restrictive patterns
Since the 1960 s, many studies have used spirometry to assess lung capacity in PD patients (Table 2). Even though the pulmonary fibrosis caused by ergotamine derivatives has become very rare, restrictive pulmonary syndrome has been reported in patients with severe PD (i.e. Hoehn & Yahr scores between III and V [25]). Nevertheless, in this paper, the severity of the restrictive patterns is unknown as their definition was based on a qualitative analyze of the low-flow volume loop. The precise prevalence of these patterns in PD remains unclear but a recent study found 56.7% of restrictive pulmonary dysfunction in a 30 patients cohort with a mean disease duration of 4.9 years ± 3.1 [26]. The loss of chest wall compliance and the camptocormia have been suggested as a mechanism [27, 28]. Actually, chest expansion was much lower in PD than in control group (1.8 cm ± 0.8 vs. 4.3 cm ± 1.0). A gender effect has been suggested: Women may present a more severe restrictive syndrome, even after adjustment for the severity of PD [29, 30]. Beyond the severity, restrictive patterns affected more women (more than 50%) than men (about 10% - [29]). On the contrary, in a kinematic and spirometric analysis of PD subjects suffering from a speech deficit, no lung volumes abnormalities was observed [31]. Nevertheless, most of the studies used the forced vital capacity (FVC) to define the restrictive pattern although the guidelines recommend using the total lung capacity (TLC).
Obstructive patterns
An analysis of the flow-volume curve highlighted a severe obstructive pulmonary syndrome in patients with advanced-PD [32], and some researchers have reported abnormalities in the upper airways [33, 34]. Some of the participants in these studies were patients with active tobacco intoxication [35, 36], and so the results may also have depended on the patients’ willingness to perform the test. Furthermore, once more, upper airway obstruction was set from the flow-volume loop tracing with difficulty to assess quantitatively. Therefore, the frequency of upper airway obstruction remains unknown even if some researchers estimated an occurrence between one fifth and two-third of the patients [30, 35, 36]. After testing 58 PD patients, Sabaté et al. suggested that the obstructive pulmonary syndrome was associated with bradykinesia, hypertonia and radiologic signs of cervical and dorsal arthrosis [30]. In contrast, no obstructive pulmonary syndrome was found in a cohort of 12 patients with advanced-PD (even in the “off-drug” condition) [25]. In 1989, a respiratory flutter phenomenon (flow-volume loop oscillation of 4–8 Hz) was observed (see Fig. 1; [32]) and found to be correlated with dyskinesia and tremor. It should be due to a vibration of the vocal cords and the supraglottic structures. However, this feature does not seem to be specific for PD since it was described in other extrapyramidal disorders [33]. At last, two studies have highlighted the occurrence of mixed pulmonary dysfunction (association of obstructive and obstructive ventilatory syndromes) in PD patients [28, 30].
Effect of antiparkinsonian drugs on lungs volumes
Regarding the dopasensitivity, some researchers consider that the effect of L-DOPA in the restrictive syndrome is only partial [29] although others did not highlight any signification variation due to treatment [25].Yet, even on “on drug condition”, FVC remained below the norm [29]. Some researchers suggest that acute and chronic administrationL-DOPA can improve the flow-volume curve [35, 36]. These results must be interpreted with caution, since some of the studies included patients with asthma or obstructive bronchopulmonary disease [35, 36]. Furthermore, it has been suggested that obstructive pulmonary syndrome is due to bronchoconstriction caused by sympathetic hyperactivation [37].
RESPIRATORY MUSCLES
Inspiratory and expiratory muscles weakness
Several studies have evidenced weakness of both inspiratory and expiratory muscles in PD (Table 3; [26, 38–41]). The maximal inspiratory mouth pressures (MIP) seems to be more affected than the maximal expiratory mouth pressure (MEP) according to several researchers [26, 42]. Besides, in this latter paper, inspiratory muscles weakness is very severe [42]. The correlation with respiratory symptoms remains unclear, since some of the studies included patients with severe PD and limitations in their activities of daily living. There are few studies of the pathophysiology of this respiratory muscle weakness. Tremor (mainly action tremor) may be involved [43] or jerky movements of the diaphragm [44]. Accessory muscles seem to be affected in PD, although data on diaphragm function in PD are scarce. Vercueil et al. observed a differential impact of the disease on inspiratory muscles (preservation of diaphragmatic activity and impaired accessory inspiratory muscles, mainly intercostal muscles) [45]. A link between respiratory muscles disturbance and impaired lung volumes has been suggested [46]. Spirometry results would be the consequence of a reduced efficiencyduring repetitive motor tasks. Furthermore, respiratory muscles strength seems to decrease with the course of the disease since a negative correlation was highlighted between MIP, MEP and the motor section of UPDRS [26].
Effect of antiparkinsonian drugs on respiratory muscles
Continuous subcutaneous infusion of Apomorphine has a positive effect on upper airways and chest wall muscle coordination [42]. But the MIP was not normalized. Other researchers did not find any significant effect of L-DOPA on mouth pressure values [41]. Likewise, two other studies did not evidence any respiratory muscle weakness in PD patients [23, 33]. Lastly, it is still not clear whether the onset of muscleweakness occurs early in PD, although Guedes et al. has suggested that this is indeed the case. Yet, the mean disease duration in their cohort was 9.1 ± 0.3 years [41].
SLEEP BREATHING DISORDERS
Occurrence of sleep apnea syndrome in PD
It has been known for decades that PD is associated with sleep disorders such as insomnia, excessive daytime sleepness, REM (rapid-eye movement) and sleep behavioral disorders. However, there is still debate as to the prevalence of sleep apnea syndrome (SAS) in PD patients [47]. According to Shill et al. [48], the presence of restrictive or obstructive patterns in PD could be a predisposition to SAS. Some researchers have reported an abnormally high prevalence of SAS in PD (relative to healthy, age-matched controls), whereas others have reported normal or below-normal values [47, 49–51]. The body mass index was a major source of bias in these studies, and most of the patients included were suffering from late-stage PD. Moreover, no predictive clinical features of SAS have been identified [51]. Some researchers have mentioned peripheral SAS caused by upper airway obstruction [48]. However, an occurrence of 48% of sleep breathing disorders with a predominance of central SAS has been observed [52].
Interestingly, a recent study found that 43.3% of de novo PD patients (with a mean ± SD duration of disease of 9.7 ± 9.5 months) had an apnea-hypopnea index higher than 5 per hour [53]. The researchers observed an average of 15.9 ± 20.9 desaturation episodes per hour. Nevertheless, the study lacked an age-matched control group. Furthermore, no other studies (except those with obese patients) have evidenced significant oxyhemoglobindesaturation [47]. In conclusion, there is some evidence of mild nocturnal desaturation in early-stage PD but the underlying mechanisms have not been characterized. The functional consequences of these sleep breathing disorders are unclear, although vigilance does not seem to be affected [54].
Impact of antiparkinsonian drugs on sleep breath disorders
No effect of antiparkinsonian drugs was reported in a review about treatment of sleep disorders in PD [55]. In a study, dopamine agonist enhanced the risk of central SAS (mainly during REM-sleep) without any difference in terms of Epworth Sleepiness Scale [53].
THE RESPONSE TO HYPOXIA
In 1998, Serebrovskaya et al. showed that PD patients had abnormally low alveolar ventilation during severe hypoxia [56]. This result could not be attributed to a mechanical restriction of lung function. It is suggested that the altered response to minor and major hypoxia resulted from a PD-associated impairment of chemoreception [56]. Other researchers confirmed these results in patients with advanceddisease [16] and patient with early-stage disease [57]. Other studies found a reduced ventilatory response to hypercapnia [58]. The putative mechanism is related to low ventilatory chemosensitivity and autonomic dysfunction.
INVOLVEMENT IN THE NEURODEGENERATIVE PROCESS
Neurodegeneration of the substantia nigra pars compacta (the hallmark of PD) is accompanied by extensive loss of neurons in extranigral sites, including the brainstem nuclei involved in sleep physiology and respiratory control [59, 60]. This localized neurodegeneration in some parts of the brainstem may account for the occurrence of ventilatory disorders in PD. However, a number of questions have yet to be resolved.
(i) Since hypoxemia has already been described as one of the mechanisms of cell death in PD [61], does the alteration in pulmonary function accelerate the disease progression? Do cerebral or brainstem structures morphometric alterations impact on the occurrence of ventilator disorders? In a cerebral MRI study, Gama et al. found an association between excessive daytime sleepiness and middle cerebellar peduncle atrophy in PD [62]. It would be interesting to perform a polysomnography and MRI study in this type of patient population.
(ii) When does hypoxemia occur? Asymptomatic hypoxemic episodes seem to occur even in de novo untreated patients [49], although larger patient vs. control cohort studies are required.
(iii) If hypoxemia does occur, can it influence the disease phenotype? As already observed in non-parkinsonian elderly patients [63], Neikrug et al. also highlighted obstructive SAS as a predictor of cognitive impairment in PD [64]. However, in a cohort of 740 early-stage PD patients, pulmonary dysfunction was not found to be a risk factor for cognitive impairment [65]. The main limit of this study is the absence of precise evaluation of the ventilatory function. Furthermore, motor status before and after the treatment of sleep apnea in PD patients has never assessed. However, some researchers found evidence of an abnormally low sympathetic response to hypoxia in patients with PD [57]. Here again, only a large, prospective cohort follow-up will be able to address this point.
CONCLUSION
Ventilatory dysfunction is an underestimated feature of PD. The heterogeneity of the papers hinders the assessment of the frequency and the severity of lung volumes alterations (restrictive and obstructive patterns), the respiratory muscles weakness and the dyspnea, even in advanced PD. The effect of antiparkinsonian drugs is still controversial. Among the studies included in this paper, we identified some limitations: (i) small study populations (mainly composed of patients with advanced PD), (ii) the absence of long-term follow-up and (iii) the absence of functional evaluations under “off-drug” conditions.
Recent data suggested that ventilator dysfunction could affect even early-stage. However, there are too few literature data to adequately confirm thishypothesis.
In conclusion, future research will have to investigate (i) the prevalence of respiratory dysfunction in early-stage patients, (ii) the pathophysiological mechanisms underlying this dysfunction and (iii) any long-term correlations between respiratory muscle weakness, altered lung volumes, breathing sleep disorders and the onset of motor and non-motor signs of PD.
Our manuscript was copy-edited by David Fraser (Biotech Communication SARL).
REFERENCES
[1] | Troussière A-C , Charley CM , Salleron J , Richard F , Delbeuck X , Derambure P , Pasquier F , & Bombois S ((2014) ) Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry, 12: , 1405–1408. |
[2] | Kim S-M , Kim H , Lee J-S , Park KS , Jeon GS , Shon J , Ahn SW , Kim SH , Lee KM , Sung JJ , & Lee KW ((2013) ) Intermittent hypoxia can aggravate motor neuronal loss and cognitive dysfunction in ALS mice. PLoS One, 11: , e81808. |
[3] | Moreau C , Devos D , Gosset P , Brunaud-Danel V , Tonnel AB , Lassalle P , Defebvre L , & Destée A ((2010) ) Mechanisms of deregulated response to hypoxia in sporadic amyotrophic lateral sclerosis: A clinical study. Rev Neurol (Paris), 3: , 279–283. |
[4] | Burns JM , Cronk BB , Anderson HS , Donnelly JE , Thomas GP , Harsha A , Brooks WM , & Swerdlow RH ((2008) ) Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology, 3: , 210–216. |
[5] | Parkinson J ((2002) ) An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci, 2: , 223–236. |
[6] | Ebihara S , Saito H , Kanda A , Nakajoh M , Takahashi H , Arai H , & Sasaki H ((2003) ) Impaired efficacy of cough in patients with Parkinson disease. Chest, 3: , 1009–1015. |
[7] | Fontana GA , Pantaleo T , Lavorini F , Maluccio NM , Mutolo D , & Pistolesi M ((1998) ) Defective motor control of coughing in Parkinson’s disease. Am J Respir Crit Care Med, 2: , 458–464. |
[8] | Mehanna R , & Jankovic J ((2010) ) Respiratory problems in neurologic movement disorders. Parkinsonism Relat Disord, 10: , 628–638. |
[9] | Parshall MB , Schwartzstein RM , Adams L , Banzett RB , Manning HL , Bourbeau J , Calverley PM , Gift AG , Harver A , Lareau SC , Mahler DA , Meek PM , & O’Donnell DE; American Thoracic Society Committee on Dyspnea ((2012) ) An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med, 4: , 435–452. |
[10] | Chenivesse C , Chan P-Y , Tsai H-W , Wheeler-Hegland K , Silverman E , von Leupoldt A , Similowski T , Davenport P ((2014) ) Negative emotional stimulation decreases respiratory sensory gating in healthy humans. Respir Physiol Neurobiol, 204: , 50–57. |
[11] | Ho SF , O’Mahony MS , Steward JA , Breay P , Buchalter M , & Burr ML ((2001) ) Dyspnoea and quality of life in older people at home. Age Ageing, 2: , 155–159. |
[12] | Yust-Katz S , Shitrit D , Melamed E , & Djaldetti R ((2012) ) Respiratory distress: An unrecognized non-motor phenomenon in patients with parkinsonism. J Neural Transm, 1: , 73–76. |
[13] | Susan HF , & Lang AE ((2007) ) Motor and nonmotor fluctuations. Handbook of Clinical Neurology. Elsevier, Edinburgh–Toronto, pp. 159–184. |
[14] | Witjas T , Kaphan E , Azulay JP , Blin O , Ceccaldi M , Pouget J , Poncet M , & Chérif AA ((2002) ) Nonmotor fluctuations in Parkinson’s disease: Frequent and disabling. Neurology, 3: , 408–413. |
[15] | Weiner P , Inzelberg R , Davidovich A , Nisipeanu P , Magadle R , Berar-Yanay N , & Carasso RL ((2002) ) Respiratory muscle performance and the Perception of dyspnea in Parkinson’s disease. Can J Neurol Sci, 1: , 68–72. |
[16] | Onodera H , Okabe S , Kikuchi Y , Tsuda T , & Itoyama Y ((2000) ) Impaired chemosensitivity and perception of dyspnoea in Parkinson’s disease. Lancet, 9231: , 739–740. |
[17] | Storch A , Schneider CB , Wolz M , Stürwald Y , Nebe A , Odin P , Mahler A , Fuchs G , Jost WH , Chaudhuri KR , Koch R , Reichmann H , & Ebersbach G ((2013) ) Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology, 9: , 800–809. |
[18] | Leentjens AFG , Dujardin K , Marsh L , Martinez-Martin P , Richard IH , & Starkstein SE ((2012) ) Anxiety and motor fluctuations in Parkinson’s disease: A cross-sectional observational study. Parkinsonism Relat Disord, 10: , 1084–1088. |
[19] | Bayulkem K , & Lopez G ((2010) ) Nonmotor fluctuations in Parkinson’s disease: Clinical spectrum and classification. J Neurol Sci, 1-2: , 89–92. |
[20] | Martinez JA , Rocha FS , Sobrani E , Galhardo FP , & Terra Filho J ((2002) ) Effects of ondansetron on respiratory pattern and sensation of experimentally induced dyspnea. Sao Paulo Med J, 5: , 141–145. |
[21] | Kallas de Carvalho F , Filho JT , Vianna EO , Silva GA , Martinez JA ((2002) ) Do steroids interfere in dyspnoea sensation? Respir Med, 7: , 511–514. |
[22] | Rice JE , Antic R , & Thompson PD ((2002) ) Disordered respiration as a levodopa-induced dyskinesia in Parkinson’s disease. Mov Disord, 3: , 524–527. |
[23] | Chalif JI , Sitsapesan HA , Pattinson KTS , Herigstad M , Aziz TZ , & Green AL ((2014) ) Dyspnea as a side effect of subthalamic nucleus deep brain stimulation for Parkinson’s disease. Respir Physiol Neurobiol, 192: , 128–133. |
[24] | Yanase M , Kataoka H , Kawahara M , Hirabayashi H , Yamanaka T , Hirano M , & Ueno S ((2008) ) Fixed epiglottis associated with subthalamic nucleus stimulation inParkinson’s disease. J Neurol Neurosurg Psychiatry, 3: , 332–333. |
[25] | De Pandis MF , Starace A , Stefanelli F , Marruzzo P , Meoli I , De Simone G , Prati R , & Stocchi F ((2002) ) Modification of respiratory function parameters in patients with severe Parkinson’s disease. Neurol Sci Suppl, 2: , 69–70. |
[26] | Wang Y , Shao WB , Gao L , Lu J , Gu H , Sun LH , Tan Y , & Zhang YD ((2014) ) Abnormal pulmonary function and respiratory muscle strength findings in Chinese patients with Parkinson’s disease and multiple system atrophy–comparison with normal elderly. PLoS One, 12: , e116123. |
[27] | Cardoso SRX , & Pereira JS ((2002) ) Analysis of breathing function in Parkinson’s disease. Arq Neuropsiquiatr, 1: , 91–95. |
[28] | Izquierdo-Alonso JL , Jiménez-Jiménez FJ , Cabrera-Valdivia F , & Mansilla-Lesmes M ((1994) ) Airway dysfunction in patients with Parkinson’s disease. Lung, 1: , 47–55. |
[29] | Pal PK , Sathyaprabha TN , Tuhina P , & Thennarasu K ((2007) ) Pattern of subclinical pulmonary dysfunctions inParkinson’s disease and the effect of levodopa. Mov Disord, 3: , 420–424. |
[30] | Sabaté M , González I , Ruperez F , & Rodríguez M ((1996) ) Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. J Neurol Sci, 1-2: , 114–119. |
[31] | Murdoch BE , Chenery HJ , Bowler S , & Ingram JC ((1989) ) Respiratory function in Parkinson’s subjects exhibiting a perceptible speech deficit: A kinematic and spirometric analysis. J Speech Hear Disord, 4: , 610–626. |
[32] | Hovestadt A , Bogaard JM , Meerwaldt JD , van der Meché FG , & Stigt J ((1989) ) Pulmonary function in Parkinson’s disease. J Neurol Neurosurg Psychiatr, 3: , 329–333. |
[33] | Vincken WG , Gauthier SG , Dollfuss RE , Hanson RE , Darauay CM , & Cosio M ((1984) ) Involvement of upper-airway muscles in extrapyramidal disorders. A cause of airflow limitation. N Engl J Med, 7: , 438–442. |
[34] | Nakano KK , Bass H , & Tyler HR ((1972) ) Levodopa in Parkinson’s disease: Effect on pulmonary function. Arch Intern Med, 3: , 346–348. |
[35] | Herer B , Arnulf I , & Housset B ((2001) ) Effects of levodopa on pulmonary function in Parkinson’s disease. Chest, 2: , 387–393. |
[36] | Bateman DN , Cooper RG , Gibson GJ , Peel ET , & Wandless I ((1981) ) Levodopa dosage and ventilatory function in Parkinson’s disease. Br Med J (Clin Res Ed), 6285: , 190–191. |
[37] | Obenour WH , Stevens PM , Cohen AA , & McCutchen JJ ((1972) ) The causes of abnormal pulmonary function in Parkinson’s disease. Am Rev Respir Dis, 3: , 382–387. |
[38] | Sathyaprabha TN , Kapavarapu PK , Pall PK , Thennarasu K , & Raju TR ((2005) ) Pulmonary functions in Parkinson’s disease. Indian J Chest Dis Allied Sci, 4: , 251–257. |
[39] | Haas BM , Trew M , & Castle PC ((2004) ) Effects of respiratory muscle weakness on daily living function, quality of life, activity levels, and exercise capacity in mild to moderate Parkinson’s disease. Am J Phys Med Rehabil, 8: , 601–607. |
[40] | Tzelepis GE , McCool FD , Friedman JH , & Hoppin FG Jr ((1988) ) Respiratory muscle dysfunction in Parkinson’sdisease. Am Rev Respir Dis, 2: , 266–271. |
[41] | Guedes LU , Rodrigues JM , Fernandes AA , Cardoso FE , & Parreira VF ((2012) ) Respiratory changes in Parkinson’s disease may be unrelated to dopaminergic dysfunction. Arq Neuropsiquiatr, 11: , 847–851. |
[42] | De Bruin PF , de Bruin VM , Lees AJ , & Pride NB ((1993) ) Effects of treatment on airway dynamics and respiratory muscle strength in Parkinson’s disease. Am Rev Respir Dis, 6: , 1576–1580. |
[43] | Brown P , Corcos DM , & Rothwell JC ((1997) ) Does parkinsonian action tremor contribute to muscle weakness in Parkinson’s disease? Brain, 3: , 401–408. |
[44] | Estenne M , Hubert M , & De Troyer A ((1984) ) Respiratory-muscle involvement in Parkinson’s disease. N Engl J Med, 23: , 1516–1517. |
[45] | Vercueil L , Linard JP , Wuyam B , Pollak P , & Benchetrit G ((1999) ) Breathing pattern in patients with Parkinson’s disease. Respir Physiol, 2-3: , 163–172. |
[46] | Polatli M , Akyol A , Cildag O , & Bayülkem K ((2001) ) Pulmonary function tests in Parkinson’s disease. Eur J Neurol, 4: , 341–345. |
[47] | Da Silva-Júnior FP , do Prado GF , Barbosa ER , Tufik S , & Togeiro SM ((2014) ) Sleep disordered breathing in Parkinson’s disease: A critical appraisal. Sleep Med Rev, 2: , 173–178. |
[48] | Shill H , & Stacy M ((1998) ) Respiratory function inParkinson’s disease. Clin Neurosci, 2: , 131–135. |
[49] | Maria B , Sophia S , Michalis M , Charalampos L , Andreas P , John ME , & Nikolaos SM ((2003) ) Sleep breathing disorders in patients with idiopathic Parkinson’s disease. Respir Med, 10: , 1151–1157. |
[50] | Diederich NJ , Vaillant M , Leischen M , Mancuso G , Golinval S , Nati R , & Schlesser M ((2005) ) Sleep apnea syndrome in Parkinson’s disease. A case-control study in 49 patients. Mov Disord, 11: , 1413–1418. |
[51] | Trotti LM , & Bliwise DL ((2010) ) No increased risk of obstructive sleep apnea in Parkinson’s disease. Mov Disord, 13: , 2246–2249. |
[52] | Valko P , Hauser S , Sommerauer M , Werth E , & Baumann CR ((2014) ) Observations on sleep-disordered breathing in idiopathic Parkinson’s disease. PLoS One, 6: , e100828. |
[53] | Joy SP , Sinha S , Pal PK , Panda S , Philip M , & Taly AB ((2014) ) Alterations in Polysomnographic (PSG) profile in drug-naïve Parkinson’s disease. Ann Indian Acad Neurol, 3: , 287–291. |
[54] | Monaca C , Duhamel A , Jacquesson JM , Ozsancak C , Destée A , Guieu JD , Defebvre L , & Derambure P ((2006) ) Vigilance troubles in Parkinson’s disease: A subjective and objective polysomnographic study. Sleep Med, 5: , 448–453. |
[55] | Trotti LM , & Bliwise DL ((2014) ) Treatment of the sleep disorders associated with Parkinson’s disease. Neurotherapeutics, 1: , 68–77. |
[56] | Serebrovskaya T , Karaban I , Mankovskaya I , Bernardi L , Passino C , & Appenzeller O ((1998) ) Hypoxic ventilatory responses and gas exchange in patients with Parkinson’s disease. Respiration, 1: , 28–33. |
[57] | Seccombe LM , Rogers PG , Hayes MW , Farah CS , Veitch EM , & MJ ((2013) ) Reduced hypoxic sympathetic response in mild Parkinson’s disease: Further evidence of early autonomic dysfunction. Parkinsonism Relat Disord, 11: , 1066–1068. |
[58] | Seccombe LM , Giddings HL , Rogers PG , Corbett AJ , Hayes MW , Peters MJ , & Veitch EM ((2011) ) Abnormal ventilatory control in Parkinson’s disease–further evidence for non-motor dysfunction. Respir Physiol Neurobiol, 2-3: , 300–304. |
[59] | Diederich NJ , & McIntyre DJ ((2012) ) Sleep disorders in Parkinson’s disease: Many causes, few therapeutic options. J Neurol Sci, 1-2: , 12–19. |
[60] | Braak H , Del Tredici K , Rüb U , Rüb U , de Vos RA , Jansen Steur EN , & Braak E ((2003) ) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging, 2: , 197–211. |
[61] | Olanow CW ((2007) ) The pathogenesis of cell death in Parkinson’s disease. Mov Disord Suppl, 17: , S342–S335. |
[62] | Gama RL , Távora DG , Bomfim RC , Silva CE , de Bruin VM , & de Bruin PF ((2010) ) Sleep disturbances and brain MRI morphometry in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy - a comparative study. Parkinsonism Relat Disord, 4: , 275–279. |
[63] | Yaffe K , Laffan AM , Harrison SL , Redline S , Spira AP , Ensrud KE , Ancoli-Israel S , & Stone KL ((2011) ) Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA, 6: , 613–619. |
[64] | Neikrug AB , Maglione JE , Liu L , Natarajan L , Avanzino JA , Corey-Bloom J , Palmer BW , Loredo JS , & Ancoli-Israel S ((2013) ) Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J Clin Sleep Med, 11: , 1119–1129. |
[65] | Uc EY , McDermott MP , Marder KS , Anderson SW , Litvan I , Como PG , Auinger P , Chou KL , & Growdon JC; Parkinson Study Group DATATOP Investigators ((2009) ) Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology, 18: , 1469–1477. |
Figures and Tables
Fig.1
A flow-volume loop in a PD patient (personal observation). Ordinate: Flow (L/s), Abscissa: Volume (L). Ventilatory flutter is predominant during inspiration (black arrow).
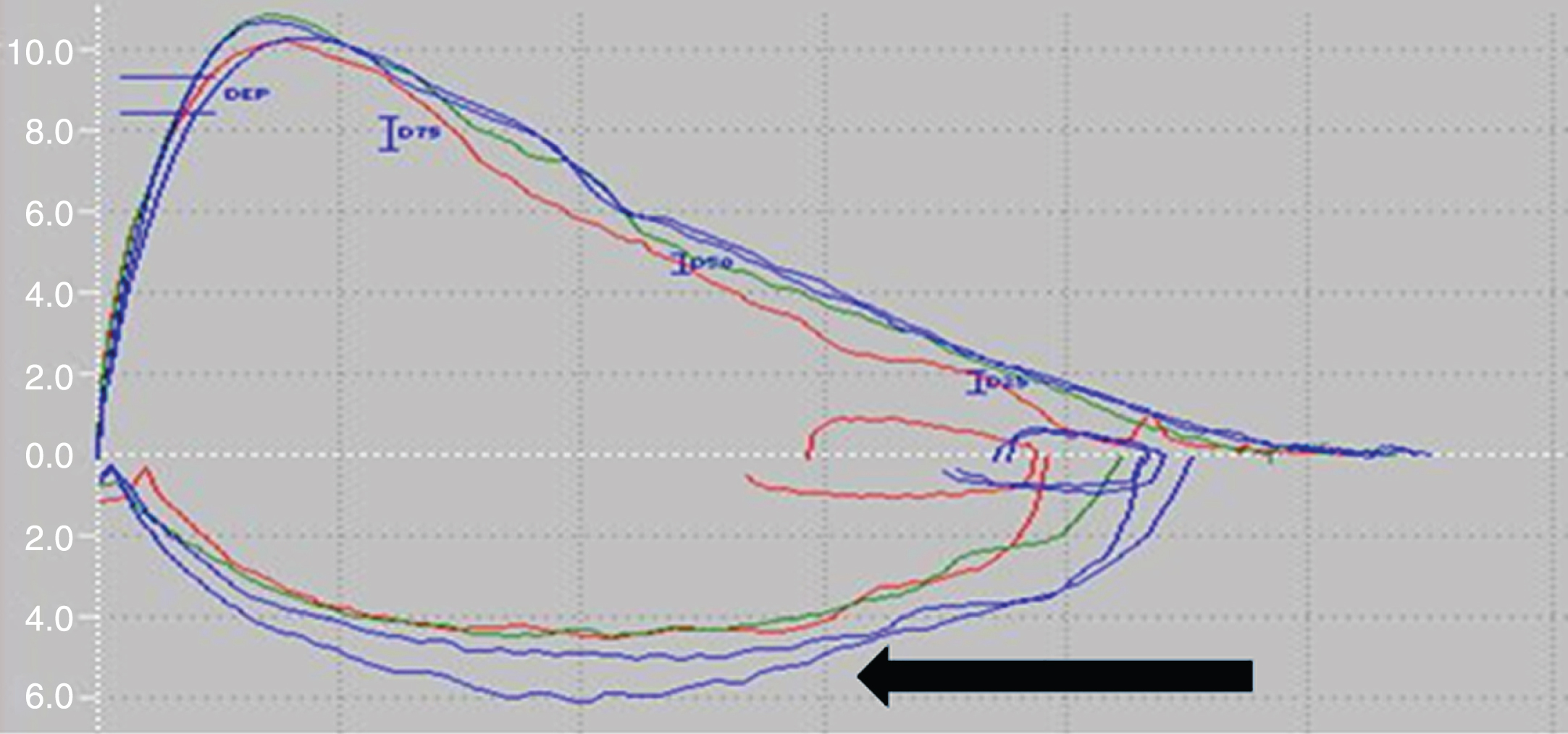
Table 1
Summary of the literature data on dyspnea in PD. HY: Hoehn and Yahr, DBS: Deep brain stimulation. VIM: Ventrale intermediate nucleus of the thalamus, NA: Not available
| Study | Number of patients | Disease duration (year) | Clinical scores | Control group | Misperception of dyspnea | Main results |
| [16] | 25 | HY 2–3 | Yes | Yes | Impaired perception of dyspnea | |
| [14] | 50 | 12.7 ± 5.4 | UPDRS III 44.4 ± 13.4“on drug” | 90% of patients with dyspnea on “off drug” condition | ||
| [15] | 20 | 7.5 ± 1.1 | HY 2–3 | Yes | Yes | No link between reduction in dyspnea and increase in lung volumes |
| [23] | 13 | Patients withDBS | NA | Yes (VIM) | Yes | link between DBS and dyspnea |
Table 2
Summary of the literature data on pulmonary function and PD. HY: Hoehn and Yahr, NS: Not significant, UPDRS: Unified Parkinson’s Disease Rating Scale, MSA: Multiple system atrophy
| Study | Number of patients | Disease duration (year) | Clinical scores | Control group | Spirometry | Dopasensitivity | Main results |
| [37] | 31 | obstruction | No | bronchoconstriction due to hyperactivity of the sympathetic system | |||
| [34] | 23 | proximal obstruction | |||||
| [36] | 6 | obstruction | partial | ||||
| [33] | 27 | proximal obstruction | |||||
| [32] | 31 | 12 HY3, 11 HY4, 8 HY5 | obstruction | link with PD progression | |||
| [32] | 31 | 8 | mixed | description of ventilatory flutter | |||
| [31] | 19 | Yes | no impact of lung volumes on dysarthria | ||||
| [28] | 63 | 5 ± 0.68 | mixed | link with UPDRS III score | |||
| [30] | 58 | mixed | link with clinical aspects of PD (bradykinesia, hypertonia, dorsal and cervical arthrosis) | ||||
| [48] | review | review | restriction associated with hypertonia, obstruction pulmonary syndrome associated with upper airway obstruction | ||||
| [35] | 21 | 5 (mean) | HY 2–4 | obstruction | Yes | lung volumes improved by L-DOPA | |
| [27] | 40 | / | HY 1–3 | Yes | restriction | ||
| [46] | 21 | Yes | obstruction | abnormal agonist-antagonist muscle activity impacts on lungs volumes | |||
| [25] | 12 | HY 3–5 | restriction | NS | no obstructive syndrome, even in the “off-drug” condition | ||
| [29] | 53 | 2.8 in women, 3.2 in men | UPDRS 45 off, 14.6 on | restriction | partial | female patients had worse pulmonary function | |
| [26] | 30 | 4.9 ± 3.1 | UPDRS 32.4“on drug” | Yes (and MSA) | mixed | correlation with motor section of UPDRS |
Table 3
Summary of the literature data on respiratory muscle weakness and PD. HY: Hoehn and Yahr, inspi: Inspiratory muscles, expi: Expiratory muscles, NS: Not significant. UPDRS: Unified Parkinson’s Disease rating Scale
| Study | Number of | Disease | Clinical | Control | Muscles | Dopasensitivity | main results |
| patients | duration (year) | scores | group | ||||
| [36] | 6 | Inspi and expi | Yes | ||||
| [40] | 9 | 2 to 14 | HY 1–3 | Yes | |||
| [42] | 10 | 6 to 20 | HY 2–4 | inspi | Yes(apomorphine) | due to lack of muscle coordination | |
| [27] | 40 | HY 1–3 | Yes | normal | |||
| [39] | 66 | HY 3–5 | Yes | inspi and expi | abnormal response to mild hypoxia | ||
| [29] | 35 | 3 | Yes | inspi and expi | Yes | ||
| [41] | 26 | 9.1 ± 0,3 | UPDRS 43 “on drug” | Yes | inspi and expi | NS | respiratory muscle weakness in early-stage PD |
| [26] | 30 | 4.9 ± 3.1 | UPDRS 32.4 “on drug” | Yes (and MSA) | inspi and expi | correlation with motor section of UPDRS |
Table 4
Summary of the literature data on dyspnea and PD. HY: Hoehn and Yahr
| Study | Number of | Disease | Clinical | Control | Main results |
| patients | duration (year) | scores | group | ||
| [56] (article in Russian) | 7 | abnormal response to hypoxemia | |||
| [57] | 19 | 5.7 ± 0.3 | HY 3–5 | reduced response to hypoxia | |
| [58] | 12 | 9.3 ± 4.6 | HY 1.5 ± 0.7 | Yes | reduced response to hypoxia |




