An Efficient Procedure for Removal and Inactivation of Alpha-Synuclein Assemblies from Laboratory Materials
Abstract
Background: Preformed α-synuclein fibrils seed the aggregation of soluble α-synuclein in cultured cells and in vivo. This, and other findings, has kindled the idea that α-synuclein fibrils possess prion-like properties.
Objective: As α-synuclein fibrils should not be considered as innocuous, there is a need for decontamination and inactivation procedures for laboratory benches and non-disposable laboratory material.
Methods: We assessed the effectiveness of different procedures designed to disassemble α-synuclein fibrils and reduce their infectivity. We examined different commercially available detergents to remove α-synuclein assemblies adsorbed on materials that are not disposable and that are most found in laboratories (e.g. plastic, glass, aluminum or stainless steel surfaces).
Results: We show that methods designed to decrease PrP prion infectivity neither effectively remove α-synuclein assemblies adsorbed to different materials commonly used in the laboratory nor disassemble the fibrillar form of the protein with efficiency. In contrast, both commercial detergents and SDS detached α-synuclein assemblies from contaminated surfaces and disassembled the fibrils.
Conclusions: We describe three cleaning procedures that effectively remove and disassemble α-synuclein seeds. The methods rely on the use of detergents that are compatible with most non-disposable tools in a laboratory. The procedures are easy to implement and significantly decrease any potential risks associated to handling α-synuclein assemblies.
1INTRODUCTION
The protein alpha-synuclein (α-Syn) is the principal component of Lewy bodies and Lewy neurites, which are the neuropathological hallmarks of Parkinson’s disease and other synucleinopathies [1–3]. Monomeric α-Syn assembles in vitro under physiological pH and salt conditions into fibrils of amyloid nature [4–8]. These assemblies, as well as some of their on-assembly pathway precursors, are toxic to cells [9–13]. Fibrils made in vitro amplify by seeding the aggregation of soluble α-Syn following uptake by cells [14–18]. The fibrils are also taken up by cultured primary neurons, transported anterogradely and retrogradely and transmitted to neighboring neurons [19] and/or microglia, oligodendrocytes and astrocytes [20–22]. In addition, mounting evidence suggest that α-Syn made in vitro, when injected within the olfactory bulb, the enteric nervous system, the muscles or the central nervous system of model animals, propagates to connected regions and trigger the formation of protein deposits resembling those present within the central nervous system of Parkinson’s disease patients [23–26]. When injected systemically, the fibrils can also cross the brain-blood barrier, accumulate within the central nervous system and seed endogenous α-Syn aggregation [18]. Altogether, these observations suggest that α-Syn assemblies are toxic to cells, are taken up, amplify and have the potential to propagate pathological protein deposits from one cell to another within the central nervous system. This led to the notion that they possess prion-like properties [27], meaning that they should not be considered as innocuous. Consequently, it is pertinent to develop guidelines for how to best handle pathogenic α-Syn assemblies in the laboratory.
Efficient decontamination and inactivation procedures have been implemented in the prion field for reusable material. These procedures call on the use of sodium hypochlorite (20,000 ppm available free chlorine), sodium hydroxide (1N) and autoclaves (121–134°C for 1 hour) or a combination of hydrogen peroxide and copper ions [28–30]. These procedures may be used to clean material contaminated by α-Syn assemblies, however there are no reports evaluating their efficiency and milder conditions may be sufficient to greatly reduce any potential health hazards related to handling α-Syn assemblies in laboratories or hospitals. Here we assess the cleaning efficiency of established decontamination procedures. We also describe a milder cleaning procedure we implemented that removes α-Syn assemblies adsorbed on plastic, glass, aluminum or stainless steel surfaces yielding efficient decontamination. α-Syn assembly yields several fibrillar polymorphs exhibiting distinct toxicity and seeding propensities [17, 18]. We evaluate here the stability of α-Syn oligomers and two fibrillar polymorphs we named fibrils and ribbons, when they are exposed to the different cleaning solutions and demonstrate efficient depolymerization of the fibrillar polymorphs under cleaning procedures that rely on the use of detergents.
2MATERIALS AND METHODS
2.1Reagents used for decontamination
The following reagent were used in this studies: 10% (W/V) SDS solution (Euromedex # EU0760), (V/V) Hellmanex II (Hellma #500300.11-F10W), (V/V) TFD4 (Franklab), (W/V) NaOH (Euromedex # 21162), MilliQ water (Millipore using a “Milli-Q® Reference“),(W/V) Sodium hypochlorite 9.6% (Lajot, Pintaud Inc., Mansle, France).
2.2Preparation of α-Syn assemblies
The expression and purification of human wild-type α-Syn was performed as previously described [31]. For α-Syn oligomers formation, α-Syn (200μM) was incubated in buffer A (50 mM Tris–HCl, pH 7.5, 150 mM KCl) at 4°C, without shaking, for 7 days. Oligomeric α-Syn was separated from the monomeric form of the protein by size-exclusion chromatography (Superose 6 HR10/300, GE Healthcare) equilibrated in buffer A. For fibril and ribbons formation, α-Syn was incubated in buffer A and buffer A supplemented with 1 mM EDTA, respectively, at 37°C under continuous shaking in an Eppendorf Thermomixer set at 600 r.p.m for 4 days (Fig. S1 A). For labeling, the fibrils and ribbons were centrifuged twice at 15,000 g for 10 min and resuspended twice in PBS while the oligomers were buffer exchanged using NAP10 desalting columns (GE Healthcare) equilibrated in PBS. All preformed α-Syn assemblies were labeled with Atto-550 NHS-ester (Atto-Tec Gmbh # AD 550-35) fluorophore following the manufacturer’s instructions using a protein:dye ratio of 2:1 based on initial monomer concentration (Fig. S1 B). The labeling reactions were arrested by addition of 1 mM Tris pH 7.5. The unreacted fluorophore was removed by NAP10 desalting columns for oligomeric α-Syn and by a final cycle of two centrifugations at 15,000 g for 10 min and resuspensions of the pellets in PBS for α-Syn fibrils and ribbons. The nature of fibrillar α-Syn forms was assessed using a JEOL 1400 transmission electron microscope following adsorption onto carbon-coated 200-mesh grids and negative staining with 1% (W/V) uranyl acetate (Fig. S1 A). The images were recorded with a Gatan Orius CCD camera (Gatan).
2.3Spotting α-Syn assemblies on different surfaces
Plastic slides (Permanox Microscope Slides (Nunc # 160005)), Glass (Glass slides for microscopy, Sigma # S8400-1PAK), Aluminum and stainless steel plates (2×20×60 mm) were pre-treated with sandpaper (grit size P1000) to make the surfaces rougher. 2μl samples of oligomeric or fibrillar α-Syn containing 1μg of protein, were spotted, and allowed to dry over night at 37°C in the dark (Fig. S1 D).
2.4Cleaning procedure
Slides and plates, one per beaker with four spots on each (Fig. S1D), were incubated in the dark, holding on their shortest edge, fully immerged in glass beakers containing 50 ml of washing solution (MilliQ water, SDS (1% , W/V), NaOH 1N, Hellmanex II (1% , V/V), TFD4 (1% , V/V), sodium hypochlorite (20 000 ppm of free available Chlorine, prepared immediately before use form 9.6% (W/V) sodium hypochlorite stock solution), under gentle agitation (50 rpm) with a magnetic stir bar (8×3 mm) at room temperature for 1 hour. Slides and plates were removed, and washed in glass beakers containing 50 ml of MilliQ under the same agitation conditions. All operations were carried out in the dark. Finally slides and plates were removed from the glass beakers and allowed to dry over night at 37°C in the dark.
2.5Quantification of α-Syn assemblies
2.5.1Fluorescence measurements
α-Syn was detected directly on the slides and plates using its Atto-550 fluorescence, by a LAS3000 imager (Fuji) using the excitation Green LED (520 nm) and emission filter at 575 nm (575DF20). Acquisition was performed in duplicate using two sensitivity settings laquo Standard raquo, and laquo Super raquo by exposition increments of 5 seconds. The slides for all replicates on a given surface were imaged at the same time. The 5 s increments were used to increase the signal (additive effect). All comparisons were done on the same acquisition with the same sensitivity of the photomultiplier/camera and exposure time. Images were processed and quantified using Image J, briefly, the fluorescence intensity of each spot was integrated using laquo Oval selection raquo tool with a fixed diameter (fig. S1D blue circles). The background was measured on a neighbouring area and subtracted. The proportion of remaining α-Syn was calculated for each spot as the fluorescence intensity after cleaning divided by the initial fluorescence intensity. The method detection limits assessed from serial dilution experiments are: 0.001μg for plastic and glass, 0.01μg for aluminum and stainless steel surfaces.
2.5.2Inactivation assay
Fibrillar α-Syn (28μg e.g. 20μl at 100μM) was dried in 500μL polycarbonate tubes (Beckman Coulter, cat# 343776). The different cleaning solutions were added (200μl) and the tubes were incubated 1 h at room temperature. The tubes were subjected to ultracentrifugation (100.000 g for 30 min at 25°C in a tabletop TL100 ultra centrifuge using a TLA120.1 rotor) and the absorbance at 550 nm before and after centrifugation were recorded using an HP 8453 UV-Vis spectrophotometer (Hewlett Packard).
3RESULTS
3.1Binding of α-synuclein assemblies
Oligomeric and fibrillar (fibrils and ribbons; Fig. S1 A) α-Syn adhere to various surfaces made of plastic, glass, stainless steel, or aluminum. To better mimic the conditions encountered in laboratories where the surfaces are not necessarily smooth, all surfaces were pre-treated with sandpaper to make the surface rougher, increase the attachment of the material and diminish elimination by gentle wiping. Fluorescently labeled oligomeric and fibrillar α-Syn (1μg in 2μl) were spotted on plastic, glass, stainless steel, or aluminum strips. The droplets were allowed to dry overnight at 37°C. The spots were clearly visible by eye or by fluorescence on the surfaces (Fig. S1 D).
3.2Cleaning procedures and quantitative assessment of the fate of α-Syn assemblies
The washing procedure consisted of two steps where the contaminated surfaces were immersed in washing solutions of different compositions under gentle agitation with a magnetic stirrer (50 rpm) followed by a washing step with MilliQ water. The washing solutions were recovered for further quantification of the amount of fibrillar α-Syn detached from the contaminated surfaces. The surfaces were dried again and the amount of remaining oligomeric (Fig. 1A) and fibrillar (Fig. 1B) α-Syn was quantified by fluorescence measurements. After removal from the contaminated surfaces α-Syn fibrils may either disassemble in the cleaning solutions or remain in the solution, in which case the solutions need further inactivation. We therefore assessed the amount of non-fibrillar α-Syn remaining in the cleaning solutions following centrifugation.
We quantified the amount of oligomeric and fibrillar α-Syn that remained attached to the different surfaces after washing with each one of the following solutions: i) sodium hypochlorite (20,000 ppm); ii) sodium hydroxide (1N), (both efficient in diminishing prion infectivity); iii) SDS (1% , W/V); iv) Hellmanex (1% , V/V); v) TFD4 (1% , V/V). In a control experiment, the surface was washed with just MilliQ water. We quantified the amount of oligomeric and fibrillar α-Syn remaining attached to the surfaces by measuring the amount of fluorescent α-Syn remaining on the contaminated surfaces (Fig. 1A and B). The fluorescence intensity of fibrillar α-Syn initially spotted on the different surfaces was arbitrarily set to 100% . The amount of fibrillar a-Syn remaining on the different surfaces after the washing step, represented by the measured fluorescence intensity, was expressed relative to the initial 100% fluorescence intensity. The washing solutions did not affect the fluorescence of the ATTO-550 dye (Fig. S1C). We found that SDS (1% , W/V, in MilliQ water) removes adsorbed oligomeric and fibrillar α-Syn effectively from all surfaces except for plastic (Fig. 1). Two commercial detergents, TFD4 and Hellmanex (1% , V/V, in MilliQ water), gave slightly better results for all surfaces (Fig. 1). As α-Syn assembles into distinct fibrillar polymorphs, we assessed the cleaning efficacy of SDS and Hellmanex (1% , V/V, in MilliQ water) using the polymorph we named ribbons (Fig. S1A). Fig. 1C shows that ribbons are removed as efficiently as fibrils from plastic, glass, aluminum and stainless steel surfaces.
To determine the fate of desorbed α-Syn fibrils and ribbons and whether they remain fibrillar or disassemble in the washing solutions, α-Syn fibrils and ribbons were incubated with the different cleaning solutions for 1 h and subjected to ultracentrifugation. Based on ATTO-550 absorbance measurements, we determined the amount of non-fibrillar fluorescent α-Syn in the supernatant of the different solutions used to clean plastic, glass, steel, or aluminum and expressed the results as fractions of the initial total fibrillar α-Syn amount before centrifugation (Fig. 2). After being exposed to water alone, 92–94% of the α-Syn that was initially present in fibrillar form remained as fibrils (Fig. 2A) and ribbons (Fig. 2B). α-Syn fibrils (Fig. 2A) detached from all surfaces when exposed to the commercial detergents TFD4 and Hellmanex disassembled almost completely (less than 2±1% remained fibrillar in nature). Similarly, 97±1% of α-Syn fibrils detached by SDS from all surfaces disassembled completely. In short summary, the commercial detergents TFD4 and Hellmanex largely removed α-Syn assemblies from plastic, glass, steel, or aluminum surfaces and also disassembled the fibrils (Fig. 1 and 2). We found that SDS treatment led to disassembly of α-Syn fibrils, with similar efficiency to that we had observed using TFD4 and Hellmanex. The disassembly of α-Syn ribbons did not reach the same extent in the presence of Hellmanex, SDS and TFD4 given that the proportion of fluorescent α-Syn ribbons ranged from 20 to 40% (Fig. 2B) with TFD4 being the less efficient detergent. By contrast, when we cleaned the surfaces using solutions previously shown to effectively diminish prion infectivity, e.g. sodium hypochlorite (20,000 ppm) or sodium hydroxide (1N), we found that they were ineffective at removing fibrillar α-Syn (both fibrils and ribbons) from the contaminated surfaces. Thus, these solutions did not effectively disassemble the fibrils and we found that 58±15% of α-Syn fibrils remained attached to plastic surfaces while only 0 to 25±6% were solublized. We conclude from these observations that the commercial detergents Hellmanex, and SDS (1% , W/V) are the most suitable cleaning reagents for removal and neutralization of α-Syn seeds from contaminated surfaces to a level where they become undetectable by the methods used here.
4DISCUSSION
Findings that fibrillar α-Syn can seed the aggregation of monomeric α-Syn both in cell cultures and in animal models, coupled to observations that injected alpha -Syn fibrils propagate from the olfactory bulb, intestine, muscle and the blood to the central nervous system suggest that α-Syn fibrils should be handled with caution in laboratories and hospital settings.
Akin to what has already been reported for prions [32] and Aβ [33], aggregated α-Syn in homogenates derived from the brains of transgenic mice that progressively develop α-synucleinopathy seeds aggregation of α-Syn when injected into the brains of mice after formaldehyde fixation of the brain tissue homogenate [34]. While this suggests α-synucleinopathies can be transmitted from one mouse to another, in an artificial laboratory setting, it does not provide proof that transmission occurs in any other paradigm or in, e.g., humans. A study performed on recipients of human growth hormone purified from cadavers concluded that there is no evidence for transmission of α-Syn aggregates between humans [35]. This retrospective study, however, does not definitively exclude the possibility that α-synucleinopathy can transmit between humans. The ability of protein seeds to transmit disease depends on several factors. First, the purification procedure of human growth hormone (based on ammonium sulfate precipitation and size exclusion chromatography) [36, 37], while insufficient to fully eliminate infectious prions from the preparation might well destroy other protein assemblies and/or change their seeding propensity. Second, the amount of α-Syn seeds necessary to trigger α-synucleinopathies in human might well be higher than that of the prion protein seeding units required to trigger Creutzfeldt-Jakob disease. Third, difference in the resistance of different types of protein seeds to degradation following injection into a tissue may explain variations in disease transmission/induction efficiencies. Considering that the amounts of α-Syn fibrils that are used in the laboratory exceed by orders of magnitude the amount of prion protein seeds that are needed to transmit disease, we recommend that work safety conditions are based on a worst-case scenario where α-Syn aggregates are considered infectious.
Safety rules have been implemented in facilities where autopsies are performed to confirm Creutzfeldt-Jakob Disease diagnosis and in laboratories where potentially infectious material is handled [38–40]. They are mostly based on avoiding the use of material, such as needles, that can cause penetrating wounds and on the careful collection of potentially infectious biological material and its inactivation by well-defined procedures [28–30]. Reusable surgical instruments decontamination protocols were also implemented to minimize the transmission of spongiform encephalopathies after neurosurgical procedures were suspected to allow such transmission [41]. Similarly, procedures allowing the inactivation of Aβ seeds have been described [42, 43].
We implemented in our laboratories stringent rules to avoid the generation of aerosols when shearing by sonication fibrillar α-Syn into short seeds. As many of the tools we use to handle α-Syn fibrils are not disposable, we first tested methods designed to decrease the infectivity of human and animal prion particles regarding their ability to clean exposed surfaces and to disassemble α-Syn fibrils. Typically, the non-disposable tools we use in the laboratory are made of plastic, glass, stainless steel or other metals. We therefore assessed the ability of prion inactivating procedures to remove α-Syn fibrils, ribbons and oligomers that we had left to dry on the above listedmaterials.
As it is important to determine whether the fibrils have retained the fibrillar structure prior to disposing of the cleaning solutions, we also determined whether they remained fibrillar or had disassembled into their constituting soluble α-Syn in the washing solutions. We found that methods designed to decrease prion infectivity are ineffective at removing fibrillar α-Syn adsorbed to different materials commonly used in the laboratory and do not disassemble α-Syn fibrils (Fig. 1 & 2). The most likely explanation is that these are different assemblies made of distinct proteins. In contrast commercial detergents and SDS not only detach dried fibrillar α-Syn from contaminated surfaces, even when they are scratched, but also disassemble the fibrils. Based on our findings and experience, we have summarized good laboratory practices for fibrillar α-Syn in Table 1. We conclude that cleaning procedures relying on the use of detergents that are compatible with most non-disposable tools in a laboratory are simple to implement and highly recommended when working with fibrillar α-Syn in a laboratory setting. The procedures we describe remove and inactivate α-Syn fibrillar assemblies to a level where they are undetectable using the method we used. Further work is needed to establish the infectious unit of recombinant α-Syn and the biological efficiency of the cleaning methods we describe.
CONFLICT OF INTEREST
The authors have no conflict of interest to report that is relevant to the current paper.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale de la Recherche (ANR-09-MNPS-013-01 and ANR-11-BSV8-021-01), the Centre National de la Recherche Scientifique, a ‘Coup d’Élan à la Recherche Française’ award from Fondation Bettencourt Schueller, Van Andel Research Institute.
Appendices
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-150691.
REFERENCES
[1] | Spillantini MG , Schmidt ML , Lee VM , Trojanowski JQ , Jakes R , & Goedert M ((1997) ) Alpha-synuclein in Lewy bodies. Nature, 388: , 839–840. |
[2] | Gai WP , Power JH , Blumbergs PC , & Blessing WW ((1998) ) Multiple-system atrophy: A new alpha-synuclein disease? Lancet, 352: , 547–548. |
[3] | Newell KL , Boyer P , Gomez-Tortosa E , Hobbs W , Hedley-Whyte ET , Vonsattel JP , & Hyman BT ((1999) ) Alpha-synuclein immunoreactivity is present in axonal swellings in neuroal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol, 58: , 1263–1268. |
[4] | Goldberg MS , & Lansbury PT Jr. ((2000) ) Is there a cause-andeffect relationship between a-synuclein fibrilization and Parkinson’s disease? Nat Cell Biol, 2: , E115–E119. |
[5] | Uversky VN , Li J , Souillac P , Millett IS , Doniach S , Jakes R , Goedert M , & Fink AL ((2002) ) Biophysical properties of the synucleins and their propensities to fibrillate: Inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem, 277: , 11970–11978. |
[6] | Uversky VN , Li J , & Fink AL ((2001) ) Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem, 276: , 10737–10744. |
[7] | Crowther RA , Jakes R , Spillantini MG , & Goedert M ((1998) ) Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett, 436: , 309–312. |
[8] | Hoyer W , Antony T , Cherny D , Heim G , Jovin TM , & Subramaniam V ((2002) ) Dependence of alpha-synuclein aggregate morphology on solution conditions. J Mol Biol, 322: , 383–393. |
[9] | El-Agnaf OM , Jakes R , Curran MD , Middleton D , Ingenito R , Bianchi E , Pessi A , Neill D , & Wallace A ((1998) ) Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett, 440: , 71–75. |
[10] | Caughey B , & Lansbury PT Jr. ((2003) ) Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci, 26: , 267–298. |
[11] | Winner BR , Maji SK , Desplats PA , Boyer L , Aigner S , Hetzer C , Loher T , Vilar M , Campioni S , Tzitzilonis C , Soragni A , Jessberger S , Mira H , Consiglio A , Pham E , Masliah E , Gage FH , & Riek R ((2011) ) demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA, 108: , 4194–4199. |
[12] | Danzer KM , Haasen D , Karow AR , Moussaud S , Habeck M , Giese A , Kretzschmar H , Hengerer B , & Kostka M ((2007) ) Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci, 27: , 9220–9232. |
[13] | Pieri L , Madiona K , Bousset L , & Melki R ((2012) ) Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J, 102: , 2894–2905. |
[14] | Brettschneider J , Del Tredici K , Lee VM , & Trojanowski JQ ((2015) ) Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat Rev Neurosci, 16: , 109–120. |
[15] | Luk KC , Song C , O’Brien P , Stieber A , Branch JR , Brunden KR , Trojanowski JQ , & Lee VM ((2009) ) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A, 106: , 20051–20056. |
[16] | Hansen C , Angot E , Bergström AL , Steiner JA , Pieri L , Paul G , Outeiro TF , Melki R , Kallunki P , Fog K , Li JY , & Brundin P ((2011) ) α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest, 121: , 715–725. |
[17] | Bousset L , Pieri L , Ruiz-Arlandis G , Gath J , Jensen PH , Habenstein B , Madiona K , Olieric V , Böckmann A , Meier BH , & Melki R ((2013) ) Structural and functional characterization of two alpha-synuclein strains. Nat Commun, 4: , 2575. |
[18] | Peelaerts W , Bousset L , Van der Perren A , Moskalyuk A , Pulizzi R , Giugliano G , Van den Haute C , Melki R , & Baekelandt V ((2015) ) alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature, 522: , 340–344. |
[19] | Freundt EC , Maynard N , Clancy EK , Roy S , Bousset L , Sourigues Y , Covert M , Melki R , Kirkegaard K , & Brahic M ((2012) ) Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann Neurol, 72: , 517–424. |
[20] | Reyes JF , Rey NL , Bousset L , Melki R , Brundin P , & Angot E ((2014) ) Alpha-synuclein transfers from neurons to oligodendrocytes. Glia, 62: , 387–398. |
[21] | Lee HJ , Suk JE , Patrick C , Bae EJ , Cho JH , Rho S , Hwang D , Masliah E , & Lee SJ ((2010) ) Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem, 285: , 9262–9272. |
[22] | Kim C , Ho DH , Suk JE , You S , Michael S , Kang J , Joong Lee S , Masliah E , Hwang D , Lee HJ , & Lee SJ ((2013) ) Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun, 4: , 1562. |
[23] | Rey NL , Petit GH , Bousset L , Melki R , & Brundin P ((2013) ) Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol, 126: , 555–573. |
[24] | Holmqvist S , Chutna O , Bousset L , Aldrin-Kirk P , Li W , Björklund T , Wang ZY , Roybon L , Melki R , & Li JY ((2014) ) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol, 128: , 805–820. |
[25] | Sacino AN , Brooks M , Thomas MA , McKinney AB , Lee S , Regenhardt RW , McGarvey NH , Ayers JI , Notterpek L , Borchelt DR , Golde TE , & Giasson BI ((2014) ) Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A, 111: , 10732–10737. |
[26] | Luk KC , Kehm V , Carroll J , Zhang B , O’Brien P , Trojanowski JQ , & Lee VM ((2012) ) Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science, 338: , 949–953. |
[27] | Brundin P , Melki R , & Kopito R ((2010) ) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol, 11: , 301–307. |
[28] | Ernst DR , & Race RE ((1994) ) Comparative analysis of scrapie agent inactivation methods. J Virol Methods, 41: , 193–202. |
[29] | Race RE , & Raymond GJ ((2004) ) Inactivation of transmissible spongiform encephalopathy (prion) agents by Environ LpH. J Virol, 78: , 2164–2165. |
[30] | Solassol J , Pastore M , Crozet C , Perrier V , & Lehmann S ((2006) ) A novel copper-hydrogen peroxide formulation for prion decontamination. J Infect Dis, 194: , 865–869. |
[31] | Ghee M , Melki R , Michot N , & Mallet J ((2005) ) PA700, the regulatory complex of the 26S proteasome, interferes with alpha-synuclein assembly. FEBS J, 272: , 4023–4033. |
[32] | Taylor DM ((1994) ) Survival of mouse-passaged bovine spongiform encephalopathy agent after exposure to paraformaldehyde-lysine-periodate and formic acid. Vet Microbiol, 44: , 111–112. |
[33] | Fritschi SK , Clintron A , Ye L , Mahler J , Buühler A , Baumann F , Neumann M , Nilsson KP , Hammarstroöm P , Walker LC , & Jucker M ((2014) ) Aβ seeds resist inactivation by formaldehyde. Acta Neuropathol, 128: , 477–484. |
[34] | Schweighauser M , Bacioglu M , Fritschi SK , Shimshek DR , Kahle PJ , Eisele YS , & Jucker M ((2015) ) Formaldehyde-fixed brain tissue from spontaneously ill α-synuclein transgenic mice induces fatal α-synucleinopathy in transgenic hosts. Acta Neuropathol, 129: , 157–159. |
[35] | Irwin DJ , Abrams JY , Schonberger LB , Leschek EW , Mills JL , Lee VM , & Trojanowski JQ ((2013) ) Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol, 70: , 462–468. |
[36] | Roos P , Fevold HR , & Gemzell CA ((1963) ) Preparation of human growth hormone by gel filtration. Biochim Biophys Acta, 74: , 525–531. |
[37] | Reisfeld RA , Hallows BG , Williams DE , Brink MG , & Steelman SL ((1963) ) Purification of human growth hormone on “sephadex G-200”. Nature, 197: , 1206–1207. |
[38] | Budka H , Aguzzi A , Brown P , Brucher JM , Bugiani O , Collinge J , Diringer H , Gullotta F , Haltia M , & Hauw JJ ((1995) ) Tissue handling in suspected Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol, 5: , 319–322. |
[39] | Steelman VM ((1994) ) Creutzfeld-Jakob disease: Recommendations for infection control. Am J Infect Control, 22: , 312–318. |
[40] | Taylor DM ((2000) ) Inactivation of transmissible degenerative encephalopathy agents: A review. Vet J, 159: , 10–17. |
[41] | Will RG , & Matthews WB ((1982) ) Evidence for case-to-case transmission of Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry, 45: , 235–238. |
[42] | Eisele YS1 , Bolmont T , Heikenwalder M , Langer F , Jacobson LH , Yan ZX , Roth K , Aguzzi A , Staufenbiel M , Walker LC , & Jucker M ((2009) ) Induction of cerebral beta-amyloidosis: Intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A, 106: , 12926–12931. |
[43] | Meyer-Luehmann M , Coomaraswamy J , Bolmont T , Kaeser S , Schaefer C , Kilger E , Neuenschwander A , Abramowski D , Frey P , Jaton AL , Vigouret J-M , Paganetti P , Walsh DM , Mathews PM , Ghiso J , Staufenbiel M , Walker LC , & Jucker M ((2006) ) Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science, 313: , 1781–1784. |
Figures and Tables
Fig.1
Efficiency of different cleaning solutions to remove α-Syn assemblies from different surfaces. Quantification of the remaining Atto-550 labeled oligomeric, A, α-Syn fibrils, B and α-Syn ribbons, C, spotted and dried on plastic, glass, aluminum and stainless steel surfaces, previously scraped with sandpaper, using a fluorescence imager after cleaning with the different solutions. Error bars represent standard error (SE) (n = 4 independent measurements) performed in quadruplicates. The measured fluorescence data from imaging are presented as averages±standard error in Supplementary Table 1, panels A, B, C and C’.
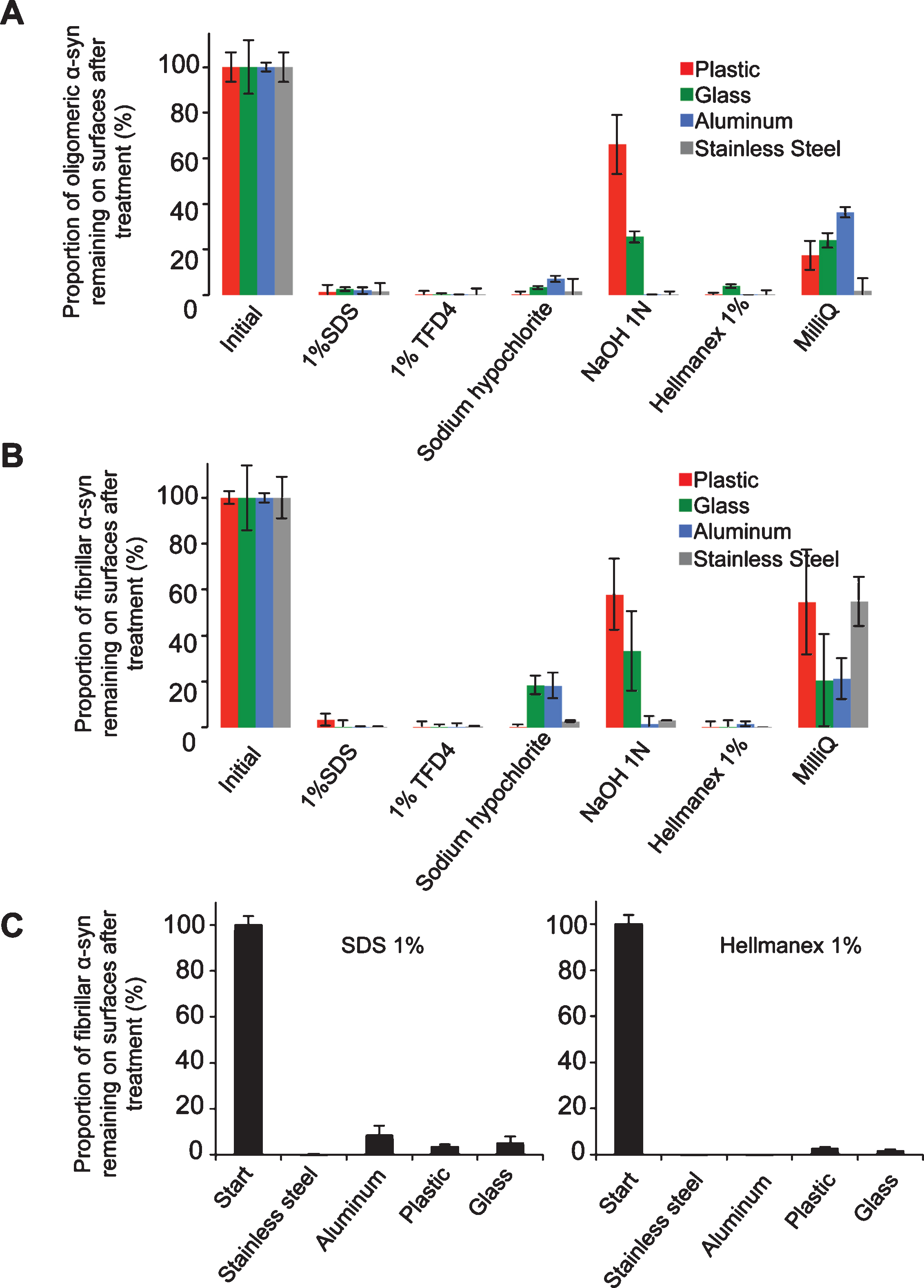
Fig.2
Quantitative assessment of the fraction of fibrillar α-Syn solubilized in the different cleaning solutions. The fraction (%) of α-Syn fibrils, A and ribbons, B, that becomes soluble relative to the initial total amount of fibrillar α-Syn in the cleaning solution was determined following ultracentrifugation by measurement of the absorbance of fluorescently labeled α-Syn in the supernatant fraction as described in the material and methods section. Error bars represent standard error (SE) (n = 4 independent measurements) performed in quadruplicates. The measured absorbance data are presented as averages±standard error in Supplementary Table 1, panels D and E.
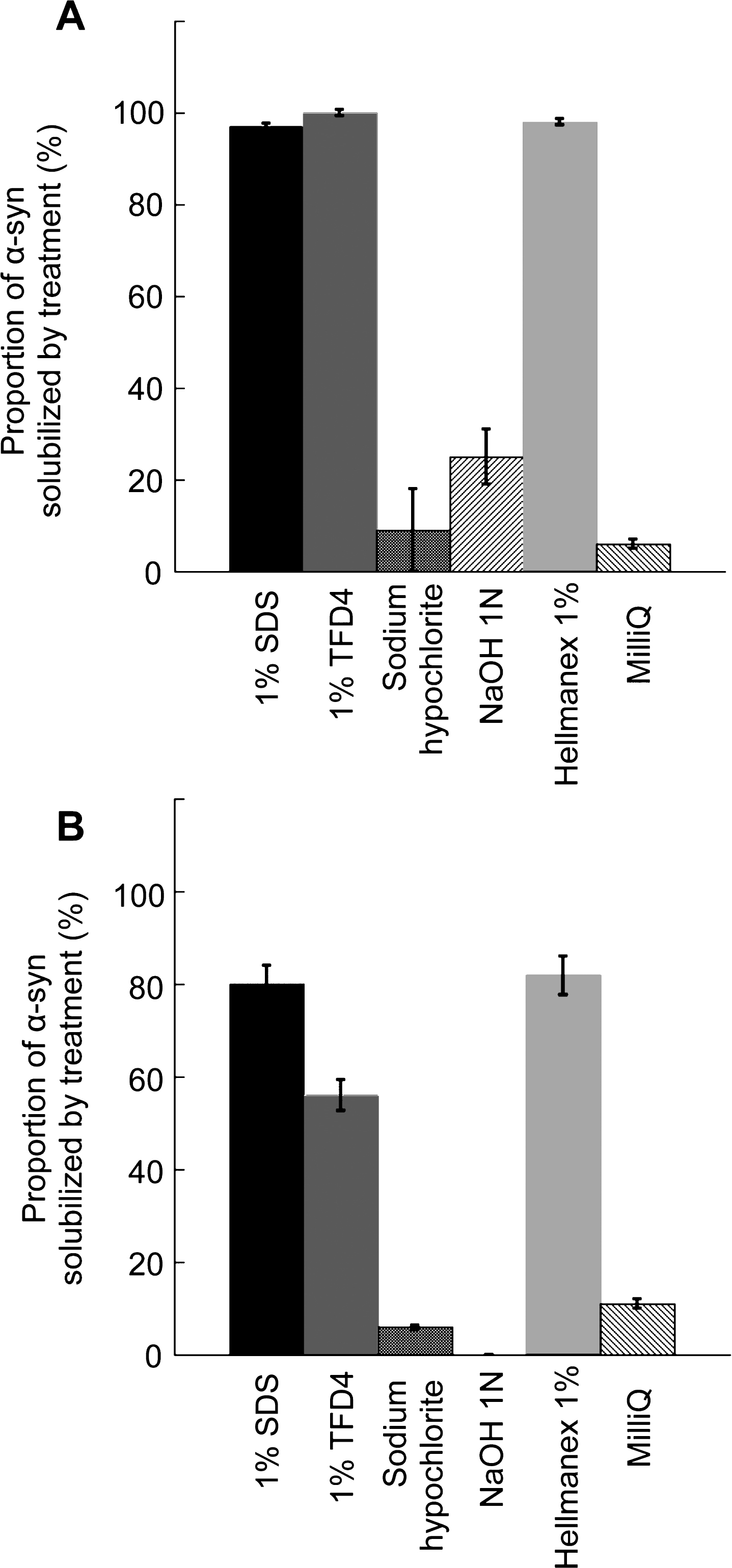
Table 1
Laboratory Standard Operating Procedures for fibrillar α-synuclein
| Recommendations: | ||
| Step | Do | Do NOT |
| General | Use appropriate personal protective equipment: | - Eat or drink in an environment where α-Syn fibrils are used |
| - Laboratory coat | ||
| - Gloves | ||
| - Goggles / safety glasses | ||
| - Mechanical filter respirators such as FFP2 particulate respirator mask, | ||
| brand 3M ref #8822 | ||
| - Prefer disposable supplies | ||
| Purification | - Keep the concentration of α-Syn below 1 mM | - Concentrate α-Syn above 1 mM |
| - Maintain pH > 6.5 to avoid spontaneous assembly | - Sonicate continuously α-Syn solution above 5 min | |
| - Aliquot α-Syn upon purification (1 to 5 mg per fraction) | ||
| Fibrillization | - Use the minimal amount of α-Syn needed for the experiment | - Sonicate in open containers. This generates aerosol containing α-Syn fibrils that conceivably might reach the brain through the olfactory epithelium following inhalation |
| - Assemble in sealed tubes | ||
| - Sonicate in closed/sealed tubes using appropriated apparatus such VialTweeter or cup-horn sonotrode | ||
| - Operate under a PSM2 if working with open tubes/vials | ||
| Storage | - Keep fibrils in closed tubes and discard in biohazard container immediately after use | - Do not dry the fibrils on any surfaces, as this renders them more resistant to detergent solubilization / inactivation |
| - Keep α-Syn fibrils in solution | ||
| Inactivation | - Inactivate samples and contaminated surfaces with 1% SDS (W/V) or commercial inactivation solutions for 1 hours at room temperature | - Do not use NaOH or Sodium Hypochlorite |
| - Discard inactivated samples with limited volume (e.g. less than 100 l) in Biohazard waste container |
| Recommendations: | |||
| α-Syn waste | inactivation | ||
| α-Syn fibril in solution | Dilute the solution 10 folds in inactivation solution | ||
| Incubate 1 h at room temperature | |||
| Discard in biohazard waste | |||
| Contaminated surfaces with α-Syn fibril | Immerse completely in inactivation solution | ||
| Incubate 1 h at room temperature under gentle shaking | |||
| Rinse with water | |||
| Discard inactivation solution in biohazard waste | |||
| If the surface is a bench, wipe with inactivation solution, discard tissues in solid biohazard waste | |||




