Oculo-Visual Dysfunction in Parkinson’s Disease
Abstract
This review describes the oculo-visual problems likely to be encountered in Parkinson’s disease (PD) with special reference to three questions: (1) are there visual symptoms characteristic of the prodromal phase of PD, (2) is PD dementia associated with specific visual changes, and (3) can visual symptoms help in the differential diagnosis of the parkinsonian syndromes, viz. PD, progressive supranuclear palsy (PSP), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and corticobasal degeneration (CBD)? Oculo-visual dysfunction in PD can involve visual acuity, dynamic contrast sensitivity, colour discrimination, pupil reactivity, eye movement, motion perception, and visual processing speeds. In addition, disturbance of visuo-spatial orientation, facial recognition problems, and chronic visual hallucinations may be present. Prodromal features of PD may include autonomic system dysfunction potentially affecting pupil reactivity, abnormal colour vision, abnormal stereopsis associated with postural instability, defects in smooth pursuit eye movements, and deficits in visuo-motor adaptation, especially when accompanied by idiopathic rapid eye movement (REM) sleep behaviour disorder. PD dementia is associated with the exacerbation of many oculo-visual problems but those involving eye movements, visuo-spatial function, and visual hallucinations are most characteristic. Useful diagnostic features in differentiating the parkinsonian symptoms are the presence of visual hallucinations, visuo-spatial problems, and variation in saccadic eye movement dysfunction.
INTRODUCTION
Patients with Parkinson’s disease (PD) can exhibit significant non-motor symptoms including apathy [1], depression, sleep problems, cognitive impairment, dementia, and autonomic, gastrointestinal, and sensory dysfunction [2]. Sensory problems may include oculo-visual dysfunction, loss of smell, auditory problems, and ‘restless legs’ syndrome [3]. A variety of oculo-visual problems have been reported in PD including defects in primary vision such as visual acuity (VA), colour vision, and eye movement, and deficits in more complex visual tasks involving the ability to judge distance or the shape of an object [2, 4]. Visual deficits in PD are important in influencing overall motor function [5], are a risk factor for developing hallucinations [6], and can have a significant influence on general quality of life [2].
There is increasing recognition of a prodromal phase which may precede actual diagnosis of PD by several years and which could involve non-dopamine as well as dopamine-related pathways [7]. In addition, the concept of ‘Parkinson’s disease associated risk syndrome’ has been proposed in which at risk patients may possess genetic factors which predispose to the disease or exhibit early non-motor risk symptoms [8]. Hence, motor features, autonomic system dysfunction, and neuropsychological disturbances may occur early in PD [7, 9, 10]. Some of these changes may reflect degeneration of the central and peripheral dopamine neurotransmitter systems, early in the disease [11]; the delay between onset of dopamine denervation and the appearance of motor symptoms being 5–20 years [7]. It may be possible to identify potential biomarkers that can detect individuals at an early stage of PD thus permitting early intervention and potential neuroprotection [10]. Strictly, prodromal disease defines a condition that exists before PD is definitely diagnosed, and hence studies of early clinical PD are not directly assessing prodromal disease, although such features may ultimately be shown to be present in the prodromal phase [12].
Cognitive disturbance can also occur in PD as the disease develops and can vary from subtle mental dysfunction to overt dementia [13]. Dementia associated with PD (PD dementia) is a particularly debilitating condition, 80% of patients being potentially at risk [14]. The risk of PD dementia increases with patient age, 90% of recorded cases being over 70 years of age [15]. In a longitudinal, community-based study of patients with newly diagnosed PD, 10% of patients developed dementia at a mean of 3.5 years after diagnosis and 57% showed evidence of cognitive impairment, fronto-striatal deficits being the most common [16]. The most important predictors of dementia were tests of semantic fluency and copying geometric figures, tasks which are affected by posterior cortical pathology, and probably associated with non-dopamine dysfunction [16]. Gray matter atrophy in PD varies with the extent of cognitive impairment [17]. Hence, PD patients with little cognitive impairment exhibit no significant differences in atrophy compared with the cognitively unimpaired. By contrast, PD patients with mild cognitive impairment exhibit limited atrophy affecting temporal, parietal, and frontal cortices as well as the caudate nucleus, hippocampus, amygdala, and putamen. In PD dementia, reduced gray matter volume in the parahippocampal gyrus, visual cortex, and cingulate gyrus may be additionally present [17]. Recently, the presence of olfactory deficits in PD has been linked to an increased risk of developing PD dementia [14] although this remains a controversial finding [18].
PD is also a member of a larger group of closely-related motor disorders, viz. the ‘parkinsonian syndromes’. This group includes progressive supranuclear palsy (PSP) [19], dementia with Lewy bodies (DLB) [20], multiple system atrophy (MSA) [21], and corticobasal degeneration (CBD) [22]. Differential diagnosis of PD within this group can be difficult, especially early in the disease, but where visual signs and symptoms are present they may be useful adjuncts to diagnosis [2].
This article reviews the oculo-visual symptomology of PD with special reference to three questions: (1) are there visual symptoms characteristic of the prodromal phase of the disease, (2) is PD dementia associated with an exacerbation of specific visual changes, and (3) can visual symptoms aid the differential diagnosis of the parkinsonian syndromes? The molecular and neurotransmitter deficits which may be responsible for many of these visual changes are also discussed.
MOLECULAR AND NEUROTRANSMITTER DEFICITS IN PD
Although dysfunction of the dopamine neurotransmitter system has long been associated with the pathophysiology of PD [2, 4] accumulating evidence suggests that PD is a multisystem degeneration [23]. Dopamine is an important neurotransmitter in the retina being present in amacrine cells along the inner border of the inner nuclear layer (INL) [24] (Fig. 1), and accumulated by interplexiform cells [25]. Two types of amacrine cells appear to be involved: (1) type 1 cells which send ascending processes to synapse with γ-aminobutyric acid (GABA) interplexiform cells in stratum 1 of the INL and (2) type 2 cells which have dendrites stratifying above those of the type 1 cells of the inner plexiform layer (IPL). Dopamine may be involved in the organisation of the ganglion cell and bipolar cell receptive fields and may modulate the physical activity of the photoreceptors [26]. In addition, dopamine is involved in the coupling of the horizontal and amacrine lateral system [27]. Thinning of the retinal nerve fibre layer (RNFL) has been recorded in PD [28] (Fig. 1). In particular, significant thinning of INL in parafoveal regions has been observed, especially in those patients exhibiting visual hallucinations but without overt signs of dementia [29].
Two main dopamine pathways are present in the brain. First, the striatonigral pathway originates in the substantia nigra (cell group A9) and terminates in the striatum (caudate nucleus, putamen). Second, two pathways originate in the ventral tegmentum (cell groups A8, A10) and project to the nucleus accumbens and limbic system (mesolimbic pathway), and frontal cortex (mesocortical pathway). There are also dopamine pathways within the hypothalamus which transmit dopamine to the pituitary gland and four pathways connecting the ventral tegmentum to the amygdala, hippocampus, cingulate gyrus, and olfactory bulb. Significant dopamine activity is largely limited to frontal and limbic areas of cerebral cortex and there is significantly less activity in visual cortex [30]. Cerebral metabolic rates for glucose are also reduced by up to 23% in primary visual cortex (area V1) of PD patients [31]. Reductions in dopamine levels in basal ganglia and frontal cortex may also deplete levels in the superior colliculus and therefore could be a factor in the production of defective saccades [32]. Dopamine also has a peripheral role in sympathetic ganglia, visceral ganglia, and in artery walls. Hence, reductions in dopamine in some of these regions could be a factor contributing to eye movement problems and defects in pupil reactivity in PD.
There is increasing interest in the clinical effects of the degeneration of cholinergic basal forebrain and tegmental pedunculopontine complex projection in PD [33]. Across the spectrum of PD, whereas dopamine denervation is frequent in individuals with minimal or no cognitive change and increases with more severe cognitive impairment, cortical cholinergic denervation is a major degenerative process associated with cognitive decline [33]. Moreover, extra-nigral pathway and cholinergic deficits are particularly common in PD patients exhibiting ‘freezing of gait’ [34]. Nevertheless, cholinergic deficits in PD are variable and may aid selection of patients for targeted drug therapies [35].
Third, surviving neurons of the substantia nigra in PD often contain Lewy bodies (LB) which differ from other types of neurofibrillary pathology in that they contain abnormal aggregates of the protein α-synuclein [36]. α-Synuclein is a small pre-synaptic protein and the entire molecule undergoes a conformational change in PD resulting in the insoluble protein that forms a major component of the LB. The role of α-synuclein pathology in PD, however, is controversial. In a large post-mortem study of cases with α-synuclein pathology in the dorsal motor vagus, substantia nigra, and basal forebrain, only 32/106 cases had been diagnosed with a neurodegenerative disorder [37]. In addition, neither the distribution nor load of α-synuclein permitted dependable post-mortem diagnosis of extrapyramidal symptoms or cognitive impairment.
OCULO-VISUAL SYMPTOMS IN PD
The oculo-visual problems that may be present in prodromal PD, in PD generally, and most likely to be exacerbated in PD dementia are summarised in columns 2–4 of Table 1.
Colour vision
Colour vision dysfunction maybe a disease specific feature of PD [38–40] and has been suggested as a possible diagnostic sign [41]. Hence, using the Farnsworth-Munsell 100-hue test, PD patients frequently exhibit higher error scores that controls after age adjustment, the frequency of error scores often correlating with the severity of motor symptoms [42]. In addition, vision in PD may be blurred using coloured stimuli [43] with reduced perception of monochromatic contours especially for dark-green, light-blue, and dark-red stimuli [44]. Defective colour vision may also be an early sign of dopamine dysfunction in PD [40]. Hence, in 100 idiopathic PD patients, 27 of whom were expressing PD linked mutations, colour vision was consistently impaired [45]. However, other studied suggest that colour vision may not be consistently impaired in early PD [46]. Although no overall significant differences between PD and controls were present in the study, elevation of error scores exceeding the upper limit of normality occurred in three cases. Colour vision deficits may be more characteristic of disorders characterised by α-synuclein-immunoreactive pathology such as PD/DLB [47]. However the pathophysiology of colour discrimination deficits in PD is likely to be complex. Hence, cognitive impairment can make a major contribution to the degree of colour discrimination deficits in PD [48]. In addition, the effect of coloured lights on gait and freezing of gait have been investigated in PD, with in a pilot experiment, green light improving gait and attenuating freezing of gait better than red or no light [49].
Visual fields
Few studies of visual field defects have been carried out in PD and there is poor evidence for the consistent presence of such deficits [50]. However, there is controversy regarding whether there is increased frequency of glaucomatous visual field defects in PD [51]. In addition, visual fields were also investigated in patients undergoing posterior pallidotomy, a surgical procedure which risks damaging structures such as the optic tract [52]. Of 40 such patients, three had visual field defects which could have been attributable to the surgery, viz., contralateral superior quadrantanopias, associated in two patients with small paracentral scotomas.
Saccadic and smooth pursuit eye movements
Objective assessment of oculomotor function in PD is usually made by electro-oculography. Electro-oculography responses are often normal in PD patients when the eyes are in the primary position or when resting. Abnormal saccadic and smooth pursuit eye movements, however, are well established in PD and reported in about 75% of patients [53]. Both reaction times and the maximum saccadic velocity of horizontal gaze are slower in PD than controls [53]. Saccadic eye movements may exhibit hypometria, i.e., ‘under reaching of task’ [32] while smooth pursuit movements may be interrupted by small additional saccades [53]. In addition, the amplitude of saccadic eye movements is often increased in normal subjects when there is a change from externally cued saccades to self-paced saccades and this effect is often greater in PD [54]. In a study in which the delay of remembered (imagined) saccades was gradually increased in untreated PD, there was a marked hypometria of saccadic gain at all delays suggesting dysfunction of the striato-collicular inhibitory pathway resulting from dopamine deficiency in the basal ganglia [53]. In addition, spatial working memory was studied in relation to eye movements in which a sequence of four targets was memorised by the patient and then the eyes were moved to fixate the targets in their correct order. In PD, several discrete saccadic eye movements of reduced amplitude were necessary before reaching the final eye position and the patients also exhibited an increase in errors while remembering the target sequence.
Electro-oculography recordings have also been made before and after apomorphine treatment in early stage patients suggesting that smooth pursuit movements could be affected during the prodromal phase [57]. In addition, patients with PD often have difficulty in sustaining repetitive actions and hence, smooth pursuit movements exhibit a reduction in response magnitude and a progressive decline of response with stimulus repetition.
Stereopsis
Impairment of stereopsis (‘depth perception’) can be observed in PD associated with impaired VA and colour perception [58, 59], and has been attributable to pathology in extrastriate cortical areas [60]. Stereopsis is mediated by various neural pathways involving the thalamus and posterior parietal lobe, areas likely to be affected in PD and especially in PD dementia, as the pathology spreads to posterior parietal regions.
Nystagmus and convergence
Abnormal optokinetic nystagmus (‘train nystagmus’), an abnormal rhythmical movement of the eyes, induced when a patient looks at a moving object [53], and poor convergence, i.e., the ability of both eyes to fixate a common point [61], have both been reported in PD and are also important signs in PSP. Convergence can be associated with a relatively large outward deviation of the eye (exophoria), and the result is often double vision [62].
Eyelids
Eyelid problems in parkinsonian syndromes can seriously impair vision as a result of difficulties in opening the lids after voluntary closure (‘apraxia of eyelid opening’) [63]. Apraxia of eyelid opening can occur at the onset of disease [64] and the condition may worsen if the patient is given L-dopa as a possible treatment. The problem is likely to be attributable to a loss of the reciprocal relationship between the levator palpebrae and the pretarsal portion of the orbicularis oculi muscles, both of which contract together in PSP rather than exhibiting their normal opponent action. In addition, involuntary eyelid closure can occur after deep brain stimulation of the subthalamic nucleus in PD, implicating this region in pathophysiology [65].
A reduced frequency of blinking leading to a staring appearance is common in PD [60]. Reduced blink rates can also lead to an abnormal tear film, dry eye, and reduced vision. A characteristic ocular sign of PD is the blink reflex, elicited by a light tap above the bridge of the nose, successive taps in normal individuals producing less and less response as the reflex habituates [66]. In PD, the blink reflex may not disappear on repeated tapping. In addition, blink duration may be increased in PD and may be a consequence of the increasing loss of dopamine neurons [67]. The blink reflex is mediated by: (1) the nasociliary branch of the trigeminal nerves which senses the stimulus, (2) the temporal and zygomatic branches of the facial nerve which initiate a motor response, and (3) a number of nuclei in the pons. Hence, the pathophysiology of the blink reflex in PD is likely to be complex [68] with dopamine dysfunction in frontal lobes [69] and a reduction in the inhibitory effect of the nucleus reticularis giganto cellularis as possible contributory factors [70].
Pupil reactivity
Significantly larger pupil diameters, with unequal pupil sizes (anisocoria) after light adaptation, have been reported in PD [71], no such differences being apparent after dark adaptation. In addition, longer light reflex latencies and constriction times have been observed while contraction amplitudes may be reduced [66]. These results suggest autonomic imbalance in PD involving the parasympathetic system. Autonomic dysfunction can be an early manifestation of PD [72] and therefore, changes in pupil reaction could be present in the prodromal phase. In addition, the maximum contraction ability of the iris muscle measured in vitro is greater in PD than in controls suggesting that the muscle may have acquired adaptive sensitivity changes [73].
Contrast sensitivity
Patients with PD and PD dementia frequently complain of poor vision especially as the disease progresses [74, 75]. In addition, contrast sensitivity function (CSF) is affected in a proportion of PD patients [76, 77], performance at high or intermediate frequencies being the most common. In some individuals, a substantial decrease in CSF occurs with disease progression which could explain reports of poor vision in PD. Abnormalities in CSF could be related to dopamine dysfunction in the retina, but are often orientation specific, suggesting cortical involvement [78]. Treatment with L-dopa can improve CSF performance in PD close to that of controls while treatment with apomorphine significantly improves non-colour spatial CSF at all frequencies [79].
Motion detection
Decreased sensitivity to temporally changing stimuli may also occur in PD and is especially well demonstrated by studies of the auditory system. Hence, in psychophysical tests assessing auditory processing, bilateral subthalamic nucleus stimulation caused dysfunction in ability to track rapid fluctuations in sound intensity [80]. In addition, in motor tasks involving finger tapping, PD patients were impaired both in the motor task itself and in assessing its duration implicating pathology in basal ganglia and the thalamocortical connections involved in timing [81]. Subsequently, the substantia nigra was shown to be involved in temporal processing involving motor and perceptual tasks [82]. Hence, there could be deficits in the visual perception of rapidly moving stimuli in PD which could potentially cause problems in tracking fast moving targets.
Electroretinogram
The amplitude of the electroretinogram ‘b’ wave is reduced in PD under a variety of light conditions [83]. The amplitude of the ‘b’ wave may be an indicator of INL function and therefore, its reduction may reflect defects in visual processing involving dopamine neurons. The amplitude of the pattern response electroretinogram (PERG) to a checkerboard stimulus is also decreased in PD [83] and the latency of the P50 component delayed [84]. Subsequent studies have suggested that retinal dopamine depletion may result in attenuated electroretinogram responses to peak stimuli [85]. In addition, steady-state pattern PERG to sinusoidal gratings was studied over a range of spatial frequencies [86]. Aging affected responses at all spatial frequencies but the pattern of age-related loss was different in PD. In PD, a specific deficit at medium spatial frequencies was present accompanied by a distorted PERG spatial frequency response function. PERG is also sensitive to dopamine manipulation in the monkey retina [87]. In an experiment involving the use of a selective D2 antagonist, treatment affected the PERG to a sinusoidal vertical grating presented at four spatial frequencies [87]. Hence, dopamine appears to be involved in retinal processing and the D2 receptor to be necessary for spatial-temporal tuning of pattern vision. Subsequently it was shown that the two dopamine receptors D1 and D2 play different roles in retinal function and therefore affect vision differently in PD [88]. Hence, PERG could be useful both in evaluating retinal dopamine mechanisms and in monitoring dopamine therapies in PD.
Visual evoked potentials (VEP)
Visual evoked potentials (VEP) in response to coloured stimuli are affected in PD [89]. Hence, in some patients, amplitude is decreased and latency increased for all chromatic stimuli and especially for those using blue-yellow horizontal gratings [90]. Increased latency of the VEP P100 component to a checkerboard stimulus has also been reported in PD suggesting a delay in visual processing at one or more stages of the visual system [91–93]. As there is significantly less dopamine activity in visual cortex, these responses are likely to be related to other neurotransomitters such as the cholinergic system.
Complex visual functions
Perceptual abnormalities in PD may result from dopamine dysfunction in the retina (‘bottom-up’) or can be attributable to deficiencies in attention due to dysfunction of the striato-frontal system (‘top-down’) [94]. Hence, mild PD is associated with little perceptual abnormality, moderate PD with top-down abnormalities, and more severe PD with both types of abnormality, data which suggest retinal effects on perception occur later in the disease [94]. In addition, the presence of visuo-perceptual impairment could be an indication of developing PD dementia [13].
There are also prominent deficits in PD involving neuropsychological tests requiring self motivation and a demanding response from the patient [1]. Hence, PD patients may exhibit a variety of deficits in visuo-spatial orientation [95, 96] including difficulty in judging verticals and the position of body parts, and in carrying out a route-walking task. Visuo-spatial working memory also appears to be selectively impaired in early PD which probably reflects degeneration of the basal ganglia, the dorsal visual stream, and the frontal-prefrontal cortex [1]. In a problem solving task involving arranging coloured balls in pockets on a computer screen, PD patients made more errors than controls and also did not show any dissociation in the amount of time fixating the two halves of the display [97]. The results suggested difficulties in encoding and/or maintaining current goals during problem solving in PD.
Studies also suggest deficits in visuo-motor adaptation early in the disease [98]. Hence, in two tasks carried out over three days involving mirror reading and reversed vision using a prism, mirror reading time was increased after one day and there was significantly more slowing on the reversed vision task in early PD, neither effect being correlated with the Wechsler memory score of the patient [98]. In addition, motor adaptation to imposed visual rotation was significantly enhanced when tested days later after a sleep-dependent memory consolidation, PD patients and controls showing similar movements and adaptation to rotation [99]. After a few days, however, PD patients did not show this consolidation.
PD patients also show an impairment of orientation and motion discrimination [100], tasks most likely to involve the visual cortex. In addition, impairments in the ability to perceive and imagine faces have been reported in PD [101]. Medicated and non-medicated patients exhibit facial recognition problems but these deficits are most frequently present in the untreated group [102]. In addition, normal subjects contract their facial muscles while imaging faces, a process which is often impaired in PD.
Visual hallucinations
Visual hallucinations are a chronic complication of PD [103, 104] especially in patients treated with L-dopa and dopamine agonists and those diagnosed with PD dementia. In a large study of PD patients, hallucinations occurred in the previous three months in 40% of patients examined, being visual in 22% and auditory in 10% of patients [103]. Patients with minor hallucinations had higher depression scores than those without. A number of factors were the best predictors of hallucinations, viz., a severe cognitive defect, daytime somnolence, longer duration of disease, poor vision, and reduced activity in primary visual cortex [31]. Hallucinations in PD are often complex with flickering lights, and illusionary misconceptions often preceding the most common manifestation, viz., stereotypical colourful images [104].
IS VISION AFFECTED IN THE PRODROMAL STAGE?
A number of oculo-visual features observed in early PD could be present in the prodromal phase of PD (Table 1). First, autonomic system dysfunction is well documented in early PD [72] and could affect pupil reactivity, and is a feature requiring further investigation. Second, electro-oculographic recordings have been made before and after apomorphine treatment confirming that smooth pursuit movements can be affected during the initial stages [57]. Third, deficits in colour vision may occur early in the disease [40]. Fourth, deficits in visuo-motor adaptation may occur during the early stages [98]. Fifth, in PD cases in which idiopathic rapid eye movement (REM) sleep behaviour disorder is accompanied by abnormal stereopsis, postural instability may be an additional feature [105]. The presence of any of these features in undiagnosed individuals may raise a concern of possible PD. Since early diagnosis may enable intervention and possible neuroprotection [10], further visual studies of the early stage PD are urgently needed.
IS VISION SPECIFICALLY AFFECTED IN PD DEMENTIA?
Many of the oculo-visual features present in early and middle stage PD will become more severe if the patient develops PD dementia. However, some features appear to be particularly exacerbated in PD dementia including deficits in colour vision [47] and changes in pupillary function [73, 106] (Table 1). In addition, there are visual features which may be particularly characteristic of PD dementia. First, prominent visual hallucinations are significantly more frequent in PD dementia than PD [107–110]. Second, severe eye movement problems are more likely to be present in PD dementia and to become more extensive with declining cognitive function [111, 112]. Third, defects in visuospatial orientation are likely to be greater in PD dementia especially when associated with greater cortical atrophy [113]. Many additional visual features, already detected in PD, are likely to be present in a more severe form in PD dementia.
CAN OCULO-VISUAL SYMPTOMS AID DIFFERENTIAL DIAGNOSIS?
Many of the parkinsonian syndromes have overlapping clinical features (Table 1) making differential diagnosis difficult. In patients with unclassifiable or with indeterminate parkinsonian symptoms, however, the presence of visual hallucinations is an important symptom which may indicate underlying LB pathology, i.e., supporting a diagnosis of PD/DLB rather than PSP/CBD or MSA [109].
Dementia with Lewy bodies (DLB)
A recent review of PD carried out by the ‘International Parkinson’s disease and Movement Disorders Societies’ has identified considerable heterogeneity within the disease, has questioned the role of classic α-synuclein pathology in pathophysiology, and considers whether PD and DLB should continue to be regarded as distinct disorders [114]. Separating PD from DLB was thought to be important because patients with visual hallucinations may be treated with antipsychotic drugs, regarded as a hazardous treatment in DLB [115]. In addition, DLB patients were thought to exhibit fewer tremors, more asymmetry of motor symptoms, more falls, and respond less well to dopamine treatment than PD [110]. DLB and PD patients, however, exhibit similarities on a variety of saccadic eye movement tasks. A newly developed portable saccadometer has been used to compare saccadic latency distribution in several parkinsonian syndromes [116] suggesting that a combination of saccadic parameters could discriminate between PD and DLB better than any single parameter. Although there are similarities in general cognitive performance in PD and DLB there may also be subtle differences. For example, deficits in orientation, ‘trail-making’, and reading the names of colours (‘Stroop test’) suggest DLB rather than PD [117]. Visual perception tasks (visual discrimination, space-motion and object-form recognition), however, are usually equally impaired in DLB and PD, especially in patients with visual hallucinations [111]. Cognitive/psychiatric symptoms including overt dementia are generally less frequent in PD than in DLB [118]. However, it is likely that these differences reflect heterogeneity within PD or the presence of distinct disease subtypes of PD.
Progressive supranuclear palsy (PSP) and cortico-basal degeneration (CBD)
PSP and CBD are both four-repeat (4R) tau diseases exhibiting considerable similarities and overlap [19, 22]. Distinguishing PD from PSP can be especially difficult early in the disease. Atypical features of PSP include slowing of upward saccades, moderate slowing of downward saccades, the presence of a full range of voluntary vertical eye movements, a curved trajectory of oblique saccades, and absence of square-wave jerks [119]. Hence, particularly useful in separating PSP from PD is the presence in the former of vertical supranuclear gaze palsy, fixation instability, lid retraction, blepharospasm, and apraxia of eyelid opening and closing [120]. Downgaze palsy is probably the most useful diagnostic clinical symptom of PSP [121]. Deficits in colour vision appear to be more important in PD and directly related to the dopamine system. However, in untreated early PD, no consistent deficits in colour vision were demonstrated making this alone an unreliable indicator of PD [46].
By contrast, typical oculo-visual features of CBD include increased latency of saccadic eye movements [122–124], impaired smooth pursuit movements [122], and visuo-spatial dysfunction especially involving object-based tasks [125]. Less typical features include vertical gaze palsy, visual hallucinations, sleep disturbance, and an impaired ERG. It is also possible that visuospatial deficits are more prominent in CBD, a mixed cortical/subcortical dementia, than in PD dementia.
Multiple system atrophy (MSA)
Anderson et al. [126] suggest several features suggestive of MSA including excessive square-wave jerks, mild to moderate hypometria of saccades, impaired vestibulo-ocular reflex (VOR), and the presence of nystagmus. In addition, visual hallucinations, unrelated to medication, are rare in MSA compared with PD and their presence can often exclude MSA as a possible diagnosis [127]. In addition, there is greater retinal pathology in PD, which results in significant defects in dynamic CSF and in the early components of the VEP, which are not likely to be present in MSA. Moreover, the colour VEP is affected in PD but not in MSA [128]. Nevertheless, where defects in the VEP occur in MSA, they are more likely to involve more complex event-related potentials and components such as the P300 [129, 130]. A feature which may be useful in separating PD and MSA is an ability to fixate an object, which is abnormal in a significant proportion of patients with MSA but less so in PD [131]. In addition, eye movements recorded during sinusoidal tracking by video-oculography show that in MSA, saccades correct for position error (‘catch-up saccades’) while in PD, saccades are directed towards future target positions (‘anticipatory saccades’) [132].
In conclusion, PD can affect visual acuity, CSF, colour discrimination, pupil reactivity, eye movement, motion perception, and visual processing speeds. Disturbances of visuo-spatial orientation, facial recognition problems, and chronic visual hallucinations may also be present. Possible prodromal features of PD include autonomic system dysfunction which could affect pupil reactivity, abnormal colour vision and stereopsis, the latter associated with postural instability, and abnormal smooth pursuit eye movement function. PD dementia is associated with the exacerbation of many of the oculo-visual problems present in PD generally but those involving eye movements, visuo-spatial function and visual hallucinations appear most characteristic. Useful diagnostic features in differentiating the parkinsonian symptoms are the presence/absence of visual hallucinations, visuo-spatial problems, and variation in saccadic eye movementdysfunction.
REFERENCES
1 | Antal A, Bandini P, Keri S, Bodis-Wollner I (1998) Visuo-cognitive dysfunctions in Parkinson’s disease Clin Neurosci 5: 147 152 |
2 | Armstrong RA (2008) Visual signs and symptoms of Parkinson’s disease Clin Exp Optom 91: 129 138 |
3 | Earley CJ (2003) Restless legs syndrome New Eng J Med 348: 2103 2109 |
4 | Armstrong RA (1997) Parkinson’s disease and the eye Ophthalmic Physiol Opt 17: S9 S16 |
5 | Diederich NJ, Raman R, Leurgans S, Goetz CG (2002) Progressive worsening of spatial and chromatic processing deficits in Parkinson’s disease Arch Neurol 59: 1249 1252 |
6 | Matsui H, Udaka F, Tamura A, Oda M, Kubori T, Nishinaka K, Kameyama M (2006) Impaired visual acuity as a risk factor for visual hallucinations in Parkinsons’s disease J Geriatr Psychiatry Neurol 19: 36 40 |
7 | Meissner WG (2012) When does Parkinson’s disease begin? From prodromal disease to motor signs Rev Neurol 168: 809 814 |
8 | Stern MB, Siderowf A (2010) Parkinson’s at risk syndrome: Can Parkinson’s disease be predicted? Mov Disord 25: S89 S93 |
9 | Schrag A, Horsfall L, Walters K, Noyce A, Petersen I (2015) Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study Lancet Neurol 14: 57 64 |
10 | Goldman JG, Postuma R (2014) Premotor and non-motor features of Parkinson’s disease Curr Opin Neurol 27: 434 441 |
11 | Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfier C, Khan NM, Leslie RA, Robertson HA (2011) Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease J Neurol 258: 1254 1260 |
12 | Berg D, Lang AE, Postuma RB, Maetzler W, Deuschi G, Gasser T, Siderowf A, Schapira AH, Oertal W, Obeso OA, Olanow CW, Poewe W, Stern M (2013) Changing the research criteria for the diagnosis of Parkinson’s disease: Obstacles and opportunities Lancet Neurol 12: 514 524 |
13 | Braak H, Rub U, Del Tredici K (2005) Cognitive changes in sporadic Parkinson disease- a cliniconeuropathological correlation Nervenheilkunde 24: 129 136 |
14 | Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, Hasegawa T, Sugeno N, Konno M, Suzuki K, Takahashi S, Fukuda H, Aoki M, Itoyama Y, Mori E, Tajida A (2012) Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: A 3-year longitudinal study Brain 135: 161 169 |
15 | Rana AQ, Yousuf MS, Naz S, Qa’aty (2012) Prevalence and relation to dementia to various factors in Parkinson disease Psychiatry Clin Neurosci 66: 64 68 |
16 | Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA (2007) Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort Brain 130: 1787 1798 |
17 | Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ (2012) Grey matter atrophy in cognitively impaired Parkinson’s disease J Neurol Neurosurg Psychiatry 83: 188 194 |
18 | Anang JBM, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, Montplaisir J, Postuma RB (2014) Predictors of dementia in Parkinson’s disease: A prospective study Neurology 83: 1253 1260 |
19 | Armstrong RA (2011) Visual signs and symptoms of progressive supranuclear palsy Clin Exp Optom 95: 150 160 |
20 | Armstrong RA (2012) Visual signs and symptoms of dementia with Lewy bodies Clin Exp Optom 94: 621 630 |
21 | Armstrong RA (2014) Visual signs and symptoms of multiple system atrophy Clin Exp Optom 97: 483 491 |
22 | Armstrong RA (2015) Corticobasal degeneration Preedy M Diet and Nutrition in Dementia and Cognitive Decline 35 44 London, San Diego, Waltham, Oxford Academic Press |
23 | Bohnen NI, Albin RL, Muller MLTM, Petrou M, Kotagal V, Koeppe R, Scott PJH, Frey KA (2015) Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson’s disease and evidence of interaction effects JAMA Neurol 72: 194 200 |
24 | Dowling JE, Ehinger B, Hedden WL (1976) Interplexiform cell:New type of retinal neuron Invest Ophthal Vis Sci 15: 916 926 |
25 | Frederick JM, Rayborn ME, Laties AM, Lam DMK, Hollyfield JG (1982) Dopaminergic neurones in the human retina J CompNeurol 210: 65 79 |
26 | Masson G, Mestre D, Blin O (1993) Dopaminergic modulation ofvisual sensitivity in man Fundam Clin Pharmacol 7: 449 463 |
27 | Djamgoz MBA, Hankins MW, Hirano J, Archer SN (1997) Neurobiology or retinal dopamine in relation to degenerative states of the tissue Vision Res 37: 3509 3529 |
28 | Moreno-Ramos T, Benito-Leon J, Villarejo A, Bermejo-Pareja F (2013) Retinal nerve fibre layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease J Alzheimers Dis 34: 659 664 |
29 | Lee JY, Kim JM, Ahn J, Kim HJ, Jeon BS, Kim TW (2014) Retinal nerve fibre layer thickness and visual hallucinations in Parkinson’s disease Mov Disord 29: 61 67 |
30 | Nguyen-Legros J, Harnois C, Paolo TD, Simon A (1993) Theretinal dopamine system in Parkinson’s disease Clin VisSci 8: 1 12 |
31 | Eberling JL, Richardson BC, Reed BR, Wolfe N, Jagust WJ (1994) Cortical glucose metabolism in Parkinson’s disease without dementia Neurobiol Aging 15: 329 335 |
32 | Crawford TJ, Goodrich S, Henderson L, Kennard C (1989) Predictive responses in Parkinson’s disease: Manual keypresses and saccadic eye movements to regular stimulus events J Neurol Neurosurg Psychiatry 52: 1033 1042 |
33 | Muller MLTM, Bohnen NI (2013) Cholinergic dysfunction in Parkinson’s disease Curr Neurol Neurosci Rep 13: 377 |
34 | Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Constantine GM, Scott PJH, Albin RL, Muller MLTM (2014) Extra-nigral pathological conditions are common in Parkinson’s disease with freezing of gait: An in vivo positron emission tomographic study Mov Disord 29: 1118 1124 |
35 | Muller MLTM, Bohnen NI, Kotagal V, Scott PJH, Koeppe RA, Frey KA, Albin RL (2015) Clinical markers for identifying cholinergic deficits in Parkinson’s disease Mov Disord 30: 269 273 |
36 | Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M (1998) Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies Neurosci Lett 251: 205 208 |
37 | Parkkinen L, Kauppinen T, Pirthla T, Autere JM, Alafuzoff I (2005) Alpha-synuclein pathology does not predict extra pyramidal symptoms Ann Neurol 57: 82 91 |
38 | Castelo-Branco M, Faria P, Forjaz V, Kosac LR, Azevedo H (2004) Simultaneous comparison of relative damage to chromatic pathways in ocular hypertension and glaucoma: Correlation with clinical measures Invest Ophthalmol Vis Sci 45: 499 505 |
39 | Silva MF, Faria P, Regateiro FS, Forjaz V, Januario C, Freire A, Castelo-Branco M (2005) Independent patterns of damage within magno, parvo, and koniocellular pathways in Parkinson’s disease Brain 128: 2260 2271 |
40 | Piro A, Tagarelli A, Nicoletti G, Fletcher R, Quattrone A (2014) Commentary: Color vision impairment in Parkinson’s disease J Parkinsons Dis 4: 317 319 |
41 | Birch J, Kolle RU, Trenkwalder C, Oertal WR, Paulus W (1995) Acquired color deficiency in patients with Parkinson’s disease Vision Res 38: 3421 3426 |
42 | Oh YS, Kim JS, Chung SW, Song IU, Kim YD, Kim YI, Lee KS (2011) Color vision in Parkinson’s disease and essential tremor Eur J Neurol 18: 577 583 |
43 | Price MJ, Feldman RG, Adelberg D, Kayne H (1992) Abnormalities in colour vision and contrast sensitivity in Parkinson’s disease Neurology 42: 887 890 |
44 | Buttner T, Kuhn W, Klotz P, Steinberg R, Voss L, Bulgaru P, Przuntek H (1993) Disturbance of colour perception in Parkinson’s disease J Neural Transm 6: 11 15 |
45 | Kerteige L, Bruggemann N, Schmidt A, Tadic V, Wisse C, Dankert S, Drude L, van der Vegt J, Sieber H, Pawleck H, Pramstaller PP, Behrens MI, Ramirez A, Reichel D, Buhmann C, Hagenah J, Klein C, Lohmann K, Kasten M (2010) Impaired sense of smell and color discrimination in monogenic and idiopathic Parkinson’s disease Mov Disord 25: 2665 2669 |
46 | Vesela O, Ruzicka E, Jech R, Roth J, Stepenkova K, Mecir P, Solan Z, Preclikova E (2001) Colour discrimination is not a reliable early marker of Parkinson’s disease J Neurol 248: 975 978 |
47 | Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY (2011) Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep disorder Ann Neurol 69: 811 818 |
48 | Bertrand JA, Bedetti C, Postuma RB, Monchi O, Marchand DG, Jubault T, Gagnon JF (2012) Color discrimination deficits in Parkinson’s disease are related to cognitive impairment and white-matter alterations Mov Disord 27: 1781 1788 |
49 | Bryant MS, Rintala DH, Lai EC, Protas EJ (2010) A pilot study: Influence of visual cue color on freezing of gait in persons with Parkinson’s disease Disabil Rehabil Assist Technol 5: 456 461 |
50 | Ture S, Inci I, Gedizlioglu M (2007) Abnormalities of contrast sensitivity, visual fields and visual evoked potentials in Parkinson’s disease and effect of dopaminergic treatment J Neurol 254: 93 |
51 | Bayer AU, Keller ON, Ferrari F, Maag KP (2002) Association of glaucoma with neurodegenerative diseases with apoptotic cell death.: Alzheimer’s disease and Parkinson’s disease Am J Ophthalmol 133: 135 137 |
52 | Biousse V, Newman NJ, Carroll C, Mewes K, Vitek JL, Bakay RAE, Baron MS, DeLong MR (1998) Visual fields in patients with posterior GPi pallidotomy Neurology 50: 258 265 |
53 | Shibasaki H, Tsuji S, Kuroiwa Y (1979) Oculomotor abnormalities in Parkinson’s disease Arch Neurol 36: 360 364 |
54 | Winograd-Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB (2006) Self-paced saccades and saccades to oddball targets in Parkinson’s disease Brain Res 1106: 134 141 |
55 | Shaunak S, O’Sullivan E, Blunt S, Lawden M, Crawford T, Henderson L, Kennard C (1999) Remembered saccades with variable delay in Parkinson’s disease Mov Disord 14: 80 86 |
56 | Hodgson TL, Dittrich WH, Henderson L, Kennard C (1999) Eye movements and spatial working memory in Parkinson’s disease Neuropsychologia 37: 927 938 |
57 | Bares M, Brazdil M, Kanovsky P, Jurak P, Daniel P, Kukleta M, Rector I (2003) The effect of apomorphine administration on smoothpursuit ocular movements in early Parkinson’s disease Parkinsonism Relat Disord 9: 139 144 |
58 | Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH, Banks JH (1991) Visual dysfunction in Alzheimer disease: Relation to normal aging Ann Neurol 29: 41 52 |
59 | Kiyosawa M, Bosley TM, Chawluk J, Jamieson D, Scatz NJ, Savino PJ, Sergott RC, Reivich M, Alavi A (1989) Alzheimer’s disease with prominent visual symptoms; clinical and metabolic evaluation Ophthalmology 96: 1077 1085 |
60 | Sun L, Zhang H, Gu ZQ, Cao M, Li DW, Chan P (2014) Stereopsis impairment is associated with decreased color perception and worse motor performance in Parkinson’s disease Eur J Med Res 19: 29 |
61 | Corin MS, Bender MB, Elizan TS (1971) Oculomotor function in patients with Parkinson’s disease J Neurol Sci 15: 251 265 |
62 | Lepore FE (2006) Parkinson’s disease and diplopia Neuroopthalmology 30: 37 40 |
63 | Lamberti P, De Mari M, Zenzola A, Aniello MS, Defazio G (2002) Frequency of apraxia of eyelid opening in the general population and in patients with extrapyramidal disorders Neurol Sci 23: S81 S82 |
64 | Defazio G, De Mari M, De Salvia R, Lamberti P, Giorelli M, Livrea P (1999) Apraxia of eyelid opening induced by levodopa therapy and apomorphine in atypical parkinsonism (possible progressive supranuclear palsy): A case report Clin Neuropharmacol 22: 292 294 |
65 | Weiss D, Waechter T, Breit S, Jacob SN, Pomper JK, Asmus F, Valls-Sole J, Plewnia C, Gasser T, Gharabaghi A, Kruger R (2010) Involuntary eyelid closure after STN-DBS: Evidence for different pathohphysiological entities J Neurol Neurosurg Psychiatry 81: 1002 1007 |
66 | Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C, Newman NJ (2004) Ophthalmologic features of Parkinson’s disease Neurology 62: 177 180 |
67 | Garland HG (1952) Refresher course for general practitioners: Parkinsonism Brit Med J 1: 153 155 |
68 | Peshori KR, Schicatano EJ, Gopalaswamy R, Sahay E, Evinger C (2001) Aging of the trigeminal blink system Exp Brain Res 136: 351 363 |
69 | Thomas RJ (1994) Blinking and the release reflexes: Are they clinically useful? J Am Geriatr Soc 42: 609 613 |
70 | Lozza A, Pepin JL, Rapisarda G, Moglia A, Delwaide PJ (1997) Functional changes of brainstem reflexes in Parkinson’s disease: Conditioning of the blink reflex R2 component by paired and index finger stimulation J Neural Transm 104: 679 687 |
71 | Micieli G, Tassorelli C, Martignoni E, Pachetti C, Bruggi P, Magri M, Nappi G (1991) Disordered pupil reactivity in Parkinson’s disease Clin Auton Res 1: 55 58 |
72 | Postuma RB, Gagnon JF, Pelletier A, Montplaisir J (2013) Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies Mov Disord 28: 597 604 |
73 | Patil PN, Mauger TF (1992) Cholinergic sensitivity of irides from donors with various pathological conditions and lens implants Naunyn Schmiedebergs Arch Pharmacol 346: 620 628 |
74 | Repka MX, Claro MC, Loupe DN, Reich SG (1996) Ocular motility in Parkinson’s disease J Pediatr Ophthalmol Strabismus 33: 144 147 |
75 | Archibald NK, Clarke MP, Mosimann UP, Burn DJ (2011) Visual symptoms in Parkinsons’ disease and Parkinson’s disease dementia Mov Disord 26: 2387 2395 |
76 | Bulens C, Meerwaldt JD, Van der Wildt GJ, Van Deursen JBP (1987) Effect of levodopa treatment on contrast sensitivity in Parkinson’s disease Ann Neurol 22: 365 369 |
77 | Hutton JT, Morris JL, Elias JW (1993) Levodopa improves spatial contrast sensitivity in Parkinson’s disease Arch Neurol 50: 721 724 |
78 | Rodnitzky RL. Visual dysfunction in Parkinson’s disease. Clin Neurosci 5, 102–106 |
79 | Buttner T, Muller T, Kuhn W (2000) Effects of apomorphine on visual functions in Parkinson’s disease J Neural Transm 107: 87 94 |
80 | Guehl D, Burbaud P, Lorenzi C, Ramos C, Biolac B, Semal C, Demany L (2008) Auditory processing in Parkinson’s disease Neuropsychologia 46: 2326 2335 |
81 | Harrington DL, Haaland KY, Hermanowicz N (1998) Temporal processing in the basal ganglia Neuropsychology 12: 3 12 |
82 | Jones CRG, Jananshahi M (2009) The substantia nigra, the basal ganglia, dopamine and temporal processing J Neural Transm 73: 161 171 |
83 | Gottlob I, Schneider E, Heider W, Skrandies W (1987) Alteration of visual evoked potentials and electroretinograms in Parkinson’s disease Electroencephalogr Clin Neurophysiol 66: 349 357 |
84 | Peppe A, Stanzione P, Pierelli F, De Angelis D, Pierantozzi M, Bernardi G (1995) Visual alterations in de novo Parkinson’s disease: Pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials Neurology 45: 1144 1148 |
85 | Bodis-Wollner I, Tzelepi A (1998) The push-pull action of dopamine on spatial tuning of the monkey retina: The effects of dopaminergic deficiency and selective D-1 and D-2 receptor ligands on the pattern electroretinogram Vision Res 38: 1479 1487 |
86 | Tagliati M, Bodis-Wollner I, Yahr MD (1996) The pattern electroretinogram in Parkinson’s disease reveals lack of retinal spatial tuning. Electroencephalogr Clin Neurophysiol 100: 1 11 |
87 | Tagliati M, Bodis-Wollner I, Kovanecz I, Stanzione P (1994) Spatial-frequency tuning of the monkey pattern ERG depends on D2 receptor-linked action of dopamine Vision Res 34: 2051 2057 |
88 | Stanzione P, Bodis-Wollner I, Pierantozzi M, Semprini R, Tagliati M, Peppe A, Bernardi G (1999) A mixed D1 and D2 antagonist does not replay pattern electroretinogram alterations observed with a selective D2 antagonist in normal humans: Relationship with Parkinson’s disease pattern electroretinogram alterations Clin Neurophysiol 110: 82 85 |
89 | Barbata L, Rinalduzzi S, Laurenti M, Ruggieri S, Accornero N (1994) Color visual evoked potentials in Parkinson’s disease Electroencephalogr Clin Neurophysiol 92: 169 172 |
90 | Sartucci F, Orlandi G, Bonuccelli U, Borghetti D, Murri L, Orsini C, Domenica L, Porciatti V (2006) Chromatic pattern-reversal electroretinogram (ChPERG) are spared in multiple system atrophy compared with Parkinson’s disease Neurol Sci 26: 395 401 |
91 | Bodis-Wollner I, Yahr M (1978) Measurement of visual evoked potentials in Parkinson’s disease Brain 101: 661 671 |
92 | Onofrj M, Bodis-Wollner I (1982) Dopaminergic deficiency causes delayed visual evoked-potentials in rats Ann Neurol 11: 484 490 |
93 | Bodis-Wollner I, Yahr MD, Mylin L, Thornton J (1982) Dopaminergic deficiency and delayed visual evoked-potentials in humans Ann Neurol 11: 478 483 |
94 | Flowers KA, Robertson C (1995) Perceptual abnormalities in Parkinson’s disease: Top-down or bottom-up processes Perception 24: 1201 1221 |
95 | Levin BE, Llabre MM, Reisman S, Weiner WJ, Sanchez-Ramos J, Singer C, Brown MC (1991) Visuospatial impairments in Parkinson’s disease Neurology 41: 365 369 |
96 | Davidsdottir S, Cronin-Golomb A, Lee A (2005) Visual and spatial symptoms in Parkinson’s disease Vision Res 45: 1285 1296 |
97 | Hodgson TL, Tiesman B, Owen AM, Kennard C (2002) Abnormal gaze strategies during problem solving in Parkinson’s disease Neuropsychologia 40: 411 422 |
98 | Sato E, Onitsuka T, Ninomiya H, Nakamura I, Kanba S (2014) Prism adaptation and perceptual skill learning deficits in early-stage Parkinson’s disease Neuropsychobiology 70: 165 172 |
99 | Marinelli L, Crupi D, Di Rocco A, Eidelberg D, Abbruzzese G, Ghilardi MF (2009) Learning and consolidation of visuo-motor adaptation in Parkinson’s disease Parkinsonism Relat Disord 15: 6 11 |
100 | Trick GL, Kaski B, Steinman SB (1994) Visual impairment in Parkinson’s disease: Deficits in orientation and motion discrimination Optom Vis Sci 71: 242 245 |
101 | Lang PJ (1979) A bio-informational theory of emotional imagery Psychophysiology 16: 495 512 |
102 | Sprengelmeyer R, Young AW, Mahn K, Schroeder U, Woitalla D, Buttner T, Kuhn W, Przuntek H (2003) Facial expression recognition in people with medicated and unmedicated Parkinson’s disease Neuropsychology 41: 1047 1057 |
103 | Fenelon G, Mahieux F, Huon R, Ziegler M (2000) Hallucinations in Parkinson’s disease: Prevalence, phenomenology and risk factors 123 733 745 |
104 | Diederich NJ, Goetz CG, Stebbins GT (2005) Repeated visual hallucinations in Parkinsons’s disease as disturbed external/internal perceptions: Focussed review and a new integrative model Mov Disord 20: 130 140 |
105 | Chen TZ, Xu GJ, Zhou GA, Wang JR, Chan P, Du YF (2014) Postural sway in idiopathic eye movement sleep behaviour disorder: A potential marker of prodromal Parkinson’s disease Brain Res 1559: 26 32 |
106 | Granholm E, Morris S, Galasko D, Shults C, Rogers E, Vukov B (2003) Tropicamide effects on pupil size and papillary light reflexes in Alzheimer’s and Parkinson’s disease Int J Psychophysiol 47: 95 115 |
107 | Archibald NK, Clarke MP, Mosimann UP, Burn DJ (2011) Visual symptoms in Parkinsons’ disease and Parkinson’s disease dementia Mov Disord 26: 2387 2395 |
108 | Hely MA, Reid WG, Halliday GM, McRitchie DA, Leicester J, Joffe R, Brooks W, Brae GA, Morris JGL (1996) Diffuse Lewy body disease: Clinical features in nine cases without coexistant Alzheimer’s disease J Neurol Neurosurg Psychiatry 60: 531 538 |
109 | Williams DR, Warren JD, Lees AJ (2008) Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism J Neurol Neurosurg Psychiatry 79: 652 655 |
110 | Ransmayer G (2000) Dementia with Lewy bodies: Prevalence, clinical spectrum and natural history Riederer P, Calne DB, Horowski R, Mizuno Y, Olanow W, Poewe W, Youdim MBH Advances in Research on Neurodegeneration 8: 303 314 Springer |
111 | Mosimann UP, Mather G, Wesnes KA, O’Brien JT, Burn DJ, McKeith IG (2004) Visual perception in Parkinson’s disease dementia and dementia with Lewy bodies Neurology 63: 2091 2096 |
112 | Debruin VM, Lees AJ, Daniel SE (1992) Diffuse Lewy body disease presenting with supranuclear gaze palsy, Parkinsonism and dementia: A case report Mov Disord 7: 355 358 |
113 | Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ (2001) Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer’s disease J Neurol Neurosurg Psychiatry 70: 483 488 |
114 | Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, Goetz CG, Halliday GM, Hardy J, Lang AE, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschi G (2014) Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease Mov Disord 29: 454 462 |
115 | Kaufer DI (2004) Pharmacologic treatment expectations in the management of dementia with Lewy bodies Dement Geriatr Cogn Disord 17: 32 39 |
116 | Antoniades CA, Bak TH, Carpenter RHS, Hodges JR, Barker RA (2007) Diagnostic potential of saccadometry in progressive supranuclear palsy. it Biomark Med 1: 487 490 |
117 | Mondon K, Gochard A, Margue A, Armand A, Beauchamp D, Prunier C, Jacobi D, de Toffol B, Autret A, Camus V, Hommet C (2007) Visual recognition memory differentiates dementia with Lewy bodies and Parkinson’s disease dementia J Neurol Neurosurg Psychiatry 78: 738 741 |
118 | Loins ED, Goldman JE, Powers JM, Fahn S (1995) Parkinsonian features of eight pathologically diagnosed cases of diffuse Lewy body disease Mov Disord 10: 188 194 |
119 | Averbach-Heller L, Paulson GW, Davoff RB, Leigh RJ (1999) Whipple’s disease mimicking progressive supranuclear palsy, the diagnostic value of eye movement recording J Neurol Neurosurg Psychiatry 66: 532 535 |
120 | Friedman DI, Jankovic J, McCrary JA (1992) Neuro-ophthalmic findings in progressive supranuclear palsy J Clin Neuroophthalmol 12: 104 109 |
121 | Grandas F, Esteban A (1994) Eyelid motor abnormalities in progressive supranuclear palsy J Neurol Transm 42: Supp 33 41 |
122 | Revaud-Pechoux S, Vidailhet M, Gallouedec G, Litvan I, Gaymard B, Pierrot-Deseilligny C (2000) Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy Neurology 54: 1029 1032 |
123 | Massman PJ, Kreiter KT, Jankovic J, Doody RS (1996) Neuropsychological functioning in cortical-basal ganglionic degeneration: Differentiation from Alzheimer’s disease Neurology 46: 720 726 |
124 | Pierrot-Deselligny C, Rivauld-Pechoux S (2003) Contribution of oculomotor exploitation for the etiological diagnosis of parkinsonian syndromes Rev Neurol 159: S75 S81 |
125 | Bak TH, Caine D, Nearn VC, Hodges JR (2006) Visuospatial functions in atypical parkinsonian syndromes J Neurol Neurosurg Psychiatr 77: 454 456 |
126 | Anderson T, Luxon L, Quinn N, Daniel S, Marsden CD, Bronstein A (2008) Oculomotor function in multiple system atrophy: Clinical and laboratory features in 30 patients Mov Disord 23: 977 984 |
127 | Kumbler E, Kornhuber M (2002) Delusional parasitosis in multiple system atrophy Nervenarzt 73: 380 383 |
128 | Sartucci F, Orlandi G, Bonuccelli U, Borghetti D, Murri L, Orsini C, Domenica L, Porciatti V (2006) Chromatic pattern-reversal electroretinogram (ChPERG) are spared in multiple system atrophy compared with Parkinson’s disease Neurol Sci 26: 395 401 |
129 | Wang LH, Kuroiwa Y, Kamitani T, Li M, Takahashi T, Suzuki Y, Shimamura M, Hasegawa O (2000) Visual event-related potentials in progressive supranuclear palsy, corticobasal degeneration, striatonigral degeneration and Parkinsons’s disease J Neurol 247: 356 363 |
130 | Koga Y, Nagata K, Hirata K (1997) Hypothesis on the P300 generators based on visual P300 results in neurological disorders Brain Topog Today Int Cong Series 1147: 354 357 |
131 | Rascol O, Sabatini U, Fabre N, Senard JM, Simonett-Amoreau M, Montastruc JL, Clanet M, Rascol A (1995) Abnormal vestibulo-ocular cancellation in multiple system atrophy and progressive supranuclear palsy but not in Parkinson’s disease Mov Disord 10: 163 170 |
132 | Pinkhardt EH, Kassubek J, Sussmuth S, Ludolph AC, Becker W, Jurgens R (2009) Comparison of smooth pursuit eye movement deficits in multiple system atrophy and Parkinson’s disease J Neurol 256: 1438 1446 |
Figures and Tables
Fig.1
Retinal image of a normal control subject using optical coherence tomography (OCT) showing the various functional layers: Fo = Location of fovea, NFL = Nerve fibre layer, GCL = Ganglion cell layer, IPL = Inner plexiform layer, INL = Inner nuclear layer, OPL = Outer plexiform layer, ONL = Outer nuclear layer, RPE = Retinal pigment epithelium, PR = Photoreceptor layer, N = Nasal direction, T = Temporal direction. A specific thinning of INL in the parafoveal region has been recorded in Parkinson’s disease (PD). (Image courtesy Dr R Heitmar, Aston University)
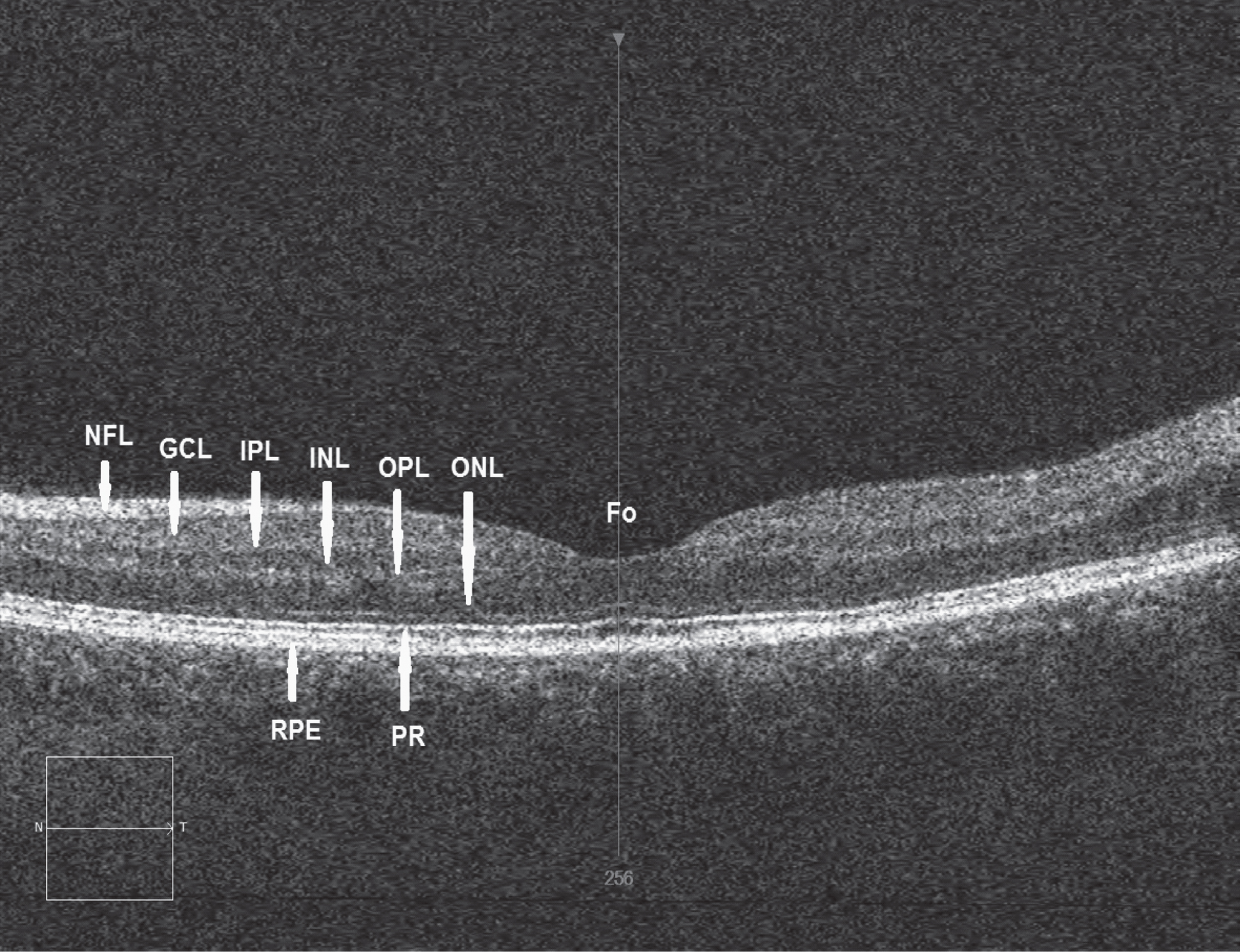
Table 1
Comparison of oculo-visual dysfunction in Parkinson’s disease (PD) and other parkinsonian syndromes
| Disorder | |||||||
| Feature | PR | PD | PD-Dem | DLB | PSP | CBD | MSA |
| Visual acuity | ± | – | – | ||||
| Colour vision | ± | + | + | + | – | ||
| Stereopsis | + | + | + | ||||
| Contrast sensitivity | + | + | – | ||||
| Motion detection | + | ||||||
| Visual fields | + | + | + | ||||
| Eyelid mobility | + | + | + | + | |||
| Blink reflex | + | + | |||||
| Pupil reactivity | ± | + | + | + | + | + | |
| Fixation | + | + | |||||
| Saccadic EM | + | + | + | + | + | + | |
| Smooth pursuit EM | + | + | + | + | + | + | |
| Nystagmus | +on | +on | +on | + | |||
| VOR | + | ± | + | ||||
| ERG | +f | +f | + | ± | |||
| Cortical VEP | +Ch | – | – | ||||
| Visuo-spatial function | + | + | + | + | + | – | |
| Visuo-motor adaptation | + | + | |||||
| Reading ability | + | + | + | ||||
| Object recognition | +fa | + | |||||
| Visual hallucinations | + | + | + | +r | +r | +r | |
Abbreviations: Disorders: PR = Prodromal phase of disease, PD = Parkinson’s disease without dementia, PD-Dem = Parkinson’s disease with dementia DLB = Dementia with Lewy bodies, PSP = Progressive supranuclear palsy, CBD = Corticobasal degeneration; MSA = Multiple system atrophy, Visual features: EM = Eye movements, EP = Evoked potential, ERG = Electroretinogram, VEP = Visual evoked potential; Symbols: Unaffected (–), Affected (+), Controversial (±). Superscripts: Ch = Chromatic VEP, f = Flash VEP, fa = Faces, on = Abnormal optokinetic nystagmus, r = rarely present Blanks indicate where limited available data to assess visual function. PD dementia data include only features where there is evidence suggesting exacerbation with developing cognitivedysfunction.




