Long-Term Effects of Safinamide on Dyskinesia in Mid- to Late-Stage Parkinson’s Disease: A Post-Hoc Analysis
Abstract
Background: Safinamide is a novel α-aminoamide with dopaminergic and non-dopaminergic properties developed as adjunctive therapy for patients with PD. Results from a 24-month double-blind controlled study suggested that as add-on to levodopa (and other PD medications) the benefits of safinamide on dyskinesia may be related to severity of dyskinesia at baseline.
Objective: This post-hoc analysis further characterized the effects of safinamide on dyskinesia in mid- to late-stage PD patients.
Methods: Patients were stratified by the presence or absence of dyskinesia at baseline, and by whether or not the dose of levodopa had been changed during the 24-month treatment period. Differences between safinamide and placebo were evaluated using the Wilcoxon rank-sum test.
Results: For the overall treated population (with or without baseline dyskinesia), safinamide 100 mg/day significantly improved the dyskinesia rating scale score, compared with placebo, in the subgroup of patients with no change in levodopa dose (p = 0.0488). For patients with baseline dyskinesia, improvements over placebo were also significant (p = 0.0153) in patients with or without changes in levodopa dose, and nearly significant (p = 0.0546) in patients with no change in levodopa dose, suggesting that these improvements were not due to levodopa dose reductions.
Conclusions: While no statistically significant difference in mean DRS scores was seen between safinamide and placebo in the original study population, the present post-hoc analysis helps to provide a meaningful interpretation of the long-term effects of safinamide on dyskinesia. These results may be related to safinamide state- and use-dependent inhibition of sodium channels and stimulated glutamate release, and are unlikely due to reduced dopaminergic stimulation.
INTRODUCTION
Levodopa (L-dopa) remains the mainstay for treatment of the motor symptoms of Parkinson’s disease (PD) [1]. However, as the disease progresses, initial therapy becomes less effective and motor complications, including dyskinesia and fluctuations, eventually develop [2]. About 40% of patients will experience motor fluctuations and dyskinesia after 4–6 years of levodopa therapy [3]. Unfortunately, levodopa-induced dyskinesia remains difficult to treat: the efficacy of levodopa decreases with time and disease symptoms recur with subsequent impairment on motor function and quality of life [4]. There are different strategies usually adopted to control motor complications: use of slow-release levodopa formulations, or start with an adjunctive therapy such as a dopamine agonist, a COMT-inhibitor or a MAO-B inhibitor [5, 6]. However, treatment of PD should not be limited to simply targeting the dopaminergic system [7]. In particular, overactive glutamate transmission plays a role in the progression of PD [8–10]. Targeting non-dopaminergic systems is thus a complementary approach to improve and control such motor complications, while maintaining the efficacy of levodopa [7]. A variety of medications may be considered in the attempt to reduce dyskinesia, but only amantadine has a weak evidence-based recommendation for dyskinesia, with an effect that is generally considered to be modest and relatively short-lasting [4].
Safinamide (Xadago™, Zambon S.p.A.) is a novel α-aminoamide that has been developed as adjunctive therapy for PD as add-on to dopamine therapy in early PD [11–13] and as add-on to levodopa in mid- to late-stage PD [14, 15]; safinamide 50 and 100 mg/day has been approved in EU for the treatment of adult patients with idiopathic PD as add-on therapy to a stable dose of levodopa, alone or in combination with other PD medicinal products, in mid-to late-stage fluctuating patients.
Safinamide is a unique compound with a novel dual mechanism of action (dopaminergic and non-dopaminergic) that includes reversible and highly selective monoamine oxidase-B (MAO-B) inhibition, sodium channel blockade and N-type calcium channel modulation. The sodium channel inhibition is both concentration- and state-dependent (different potency at different states of the channel), thus inhibiting the excessive presynaptic release of excitotoxic amino acids like glutamate without influencing the physiological activity [16–20]. Safinamide does not affect L-type calcium channels (no effects on blood pressure or heart rate) [16, 17, 21, 22], is well tolerated with a favorable side effect profile, has no major drug–drug interactions, and no diet restrictions [20, 23, 24].
As add-on to levodopa, safinamide improved motor symptoms and controlled motor complications in the short term, maintaining the benefits in the long term. Results from a long-term (24 months) double-blind controlled study (study 018) suggest that safinamide significantly increased daily ON time without increasing the risk of developing troublesome dyskinesia, and significantly improved the activities of daily living and the quality of life [15]. For the overall population of study 018, the rather low baseline dyskinesia score values allowed little room for improvement, and no statistically significant difference in DRS scores was seen between safinamide and placebo; in fact, a significant improvement in favor of safinamide was seen only in patients with moderate to severe dyskinesia at baseline. A post-hoc analysis of study 018 is presented herein in order to summarize the full set of results in order to provide a clinically-meaningful interpretation about the long-term effects of safinamide on the evolution of dyskinesia in mid- to late-stage PD patients.
MATERIALS AND METHODS
Trial 018 was a multicenter, multinational, double-blind, placebo-controlled, extension study of a pivotal 24-week Phase III trial 016 [14] in 669 mid- to late-stage PD fluctuating patients on a stable levodopa dose. Patients were included in the extension study (study 018) if they had completed the initial study, were treatment compliant and willing to continue, or if they had discontinued but had completed the scheduled efficacy evaluations at weeks 12 and 24. They were excluded if they had experienced clinically significant adverse events or shown clinically significant deterioration in motor symptoms during study 016. Both protocols and patient materials were approved by Independent Ethics Committees and Health Authorities in all three participating countries (India, Romania, Italy). All patients signed an informed consent form and the study was conducted according to the Declaration of Helsinki. Study 018 is registered on Clinicaltrials.gov (NCT01187966). After completing the initial treatment period, patients willing to participate in the extension phase continued on their randomized study medications and were followed-up during an additional period of 18 months [15]. Safinamide (50 or 100 mg/day p.o.) or placebo were given as add-on to levodopa and other PD therapies (dopamine agonists, COMT inhibitors, amantadine, and/or anticholinergics). In case of motor symptom deterioration, dose increases of levodopa or additional PD drugs, except MAO-B inhibitors, were permitted. The levodopa dose could also be decreased based on patient conditions or the occurrence of adverseevents.
The primary variable of study 018 was the mean change from baseline (study 016 start) to study end in the total dyskinesia rating scale (DRS) score during ON time. The DRS is an easy-to-use and practical tool used to rate the severity of dyskinesia and to identify the most disabling dyskinesia [25]. The primary analysis estimated the mean difference between placebo and safinamide in the primary variable.
Study design and statistical analysis
The present study was a post-hoc analysis of study 018 that evaluated the categorical changes in DRS scores at the end of study 018 after stratifying patients based on the presence or absence of dyskinesia (DRS score >0 or DRS score = 0, respectively) at baseline, and by additional subgroups based on whether or not the dose of levodopa had been changed during the entire treatment period of 24 months. Proportions of patients with categorical changes in DRS scores were evaluated, since DRS score distributions were ill-conditioned (because of skewness and the presence of outliers). Changes in DRS scores were classified as “decreased”, “unchanged”, and “increased”, and patients were classified according to changes in DRS scores during the study using a 3-point ordinal scale (1 = improved, DRS score decreased; 2 = stable; 3 = worsened, DRS score increased). The comparisons of active treatments (safinamide 100 mg and safinamide 50 mg) versus placebo were performed using the Wilcoxon rank-sum test for independent samples. Since in the original protocol of study 018 [23] the multiplicity issue (over treatment groups) was handled by using a pre-specified “sequence of comparisons” approach [26], no adjustment of type I error was needed for the multiple comparisons. All statistical analyses were performed using SAS softwareversion 9.4.
RESULTS
In the original 018 trial, safinamide maintained a statistically significant improvement in ON time without dyskinesia over 2 years of treatment, confirming the results obtained in the initial 6-month study 016. After 2 years, there was a decrease from baseline in mean DRS score during ON time of 31% and 27% with safinamide 50 and 100 mg/day, respectively, compared to a decrease of only 3% with placebo. However, the primary endpoint was not met [least-squares mean difference vs. placebo, –0.51 score points (95% CI: –1.32 to 0.29, p = 0.2125) for safinamide 50 mg, and –0.59 (95% CI: –1.40 to 0.21, p = 0.1469) for safinamide100 mg] [15].
The proportions of patients with reduced levodopa dose were higher in both safinamide groups compared to placebo, while the proportions of patients with an increase in levodopa dose had an opposite trend (Fig. 1). Over the study period, the overall mean dose of levodopa increased in the placebo arm and in the safinamide 50 mg/day group, and decreased in the safinamide 100 mg/day group (Table 1). Such dose changes do not appear to be clinically relevant [15].
An already published initial post-hoc analysis of the changes in DRS in patients with moderate to severe dyskinesia at baseline (DRS total score >4, n = 242/594) showed a more consistent decrease in DRS scores from baseline to study end in all treatment groups. Notably, the least-squares (LS) mean change in DRS scores from baseline to study end for the safinamide 100-mg group was significantly different from the placebo group [LS mean difference vs. placebo, –1.22 (95% CI, –2.33 to –0.11, p = 0.0317)] [15].
Post-hoc analysis
Both doses of safinamide (50 and 100 mg/day) were generally well tolerated over 2 years by patients who were receiving polypharmacy. In the subgroup of patients (with or without dyskinesia at baseline, DRS ≥0) with no changes in their levodopa dose during the entire treatment period (n = 431), the proportion of those with an improvement (decrease) in DRS scores was higher in both safinamide groups compared with placebo (Fig. 2). Concurrently, the proportion of patients with a worsening (DRS increase) of dyskinesia was lower in both safinamide groups compared with placebo. The difference between safinamide 100 mg and placebo, considering the 3-point ordinal scale (decreased, unchanged, increased), wasstatistically significant (p = 0.0488). This suggests that the beneficial effect of safinamide 100 mg on DRS scores was not dependent on changes in the levodopa dose, as this analysis excluded patients with a dose reduction.
When the subgroup of patients with dyskinesia at baseline (DRS >0, n = 334) was considered (Fig. 3), more patients treated with 100 mg or 50 mg safinamide vs. placebo had a decrease in DRS scores. This finding was paralleled by lower proportions of patients in both safinamide groups who showed an increase in the DRS score compared with placebo. The difference considering the 3-point ordinal scale (decreased, unchanged, increased) between safinamide 100 mg and placebo was statistically significant(p = 0.0153).
In the subgroup of patients with dyskinesia at baseline (DRS >0) and no change in L-dopa dose (n = 273), the proportion of patients with a decrease in the DRS score was again greater in both safinamide groups compared with placebo, while the proportion of patients with an increase in the DRS score was lower in both safinamide groups vs. placebo (Fig. 4). The distribution difference considering the 3-point ordinal scale (decreased, unchanged, increased) between the safinamide 100 mg and placebo groups showed a trend towards significance (p = 0.0546).
Considering the subgroup of patients without dyskinesia at baseline (DRS = 0, n = 201), DRS scores remained unchanged throughout the 2-year treatment period for the majority (≥75% ) of patients in all treatment groups; the proportions of patients with worsening of dyskinesia were low and similar across treatment groups (Fig. 5). A similar trend was seen in the subgroup of patients with no change in L-dopa dose (n = 158; Fig. 6). None of the observed differences was statistically significant.
DISCUSSION
In study 018, safinamide 100 mg/day was administered as add-on to levodopa and other PD medications in patients with mid- to late-stage PD and motor fluctuations over 2 years of treatment: DRS score mean changes from baseline were not significantly different between safinamide and placebo in the original study 018 analysis, even if scores decreased with safinamide and were almost unchanged with placebo.
In terms of categorical changes of DRS scores (proportions of patients showing worsening, no changes and improvements), the present post-hoc analysis showed that safinamide 100 mg was associated with significant differences compared with placebo in the subgroup of patients with no changes in L-dopa dose, suggesting that these improvements were not due to a reduction in the dose of levodopa, but rather to the effects of safinamide itself. Furthermore, this effect is unlikely to be related to reduced dopaminergic stimulation, as demonstrated by statistically significant effects of safinamide on motor fluctuations (ON and OFF times) over 2 years [14, 15].
Several neurotransmitters, in addition to dopamine, contribute to the appearance of levodopa-induced dyskinesia [27], and overactive glutamate transmission has been shown to play a role in the progression of PD [8, 10]. Moreover, altered neurotransmission is regulated by signal transduction cascades involving glutamatergic receptors belonging to the NMDA and AMPA classes [10, 28]. The results of the present post-hoc analysis may be explained by the blockade of sodium channels and modulation of calcium channels by safinamide, which inhibits altered glutamatergic neurotransmission. At the dose of 100 mg/day, the effective safinamide concentration is expected to be fully active on the glutamate transmission; this may further support the hypothesis that the higher dose is needed to maximize both the dopaminergic and non-dopaminergic effects of safinamide.
Safinamide is a unique treatment for PD that exhibits a combined non-dopaminergic and dopaminergic mode of action. Unlike other drugs that can improve motor functions, safinamide does not worsen dyskinesia: this effect may be related to its dual mechanism, which modulates dopaminergic and glutamatergic pathways. The present post-hoc analysis, in terms of categorical changes of DRS scores (proportions of patients), helps to provide a meaningful interpretation of the long-term effects of safinamide 100 mg/day on dyskinesia.
ACKNOWLEDGMENTS INCLUDING SOURCES OF SUPPORT
The authors have no acknowledgments to disclose.
CONFLICT OF INTEREST
Carlo Cattaneo, Roberto La Ferla, and Marco Sardina are employees at Zambon SpA. Erminio Bonizzoni is a consultant statistician for Zambon SpA.
REFERENCES
1 | LeWitt PA(2009) Levodopa therapeutics for Parkinson’s disease: New developments Parkinsonism Relat Disord15: Suppl 1S31S34 |
2 | Hauser RA(2009) Levodopa: Past, present, and futureEur Neurol62: 18 |
3 | Ahlskog JE, Muenter MD(2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literatureMov Disord16: 448458 |
4 | Pahwa R, Factor SA, Lyons KE, Ondo WG, Gronseth G, Bronte-Stewart H, Hallett M, Miyasaki J, Stevens J, Weiner WJQuality Standards Subcommittee of the American Academy of N(2006) Practice parameter: Treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurologyNeurology66: 983995 |
5 | Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG(2007) Levodopa-induced dyskinesiasMov Disord22: 13791389quiz, 1523. |
6 | Hinson VK(2010) Parkinson’s disease and motor fluctuationsCurr Treat Options Neurol12: 186199 |
7 | Fox SH(2013) Non-dopaminergic treatments for motor control in Parkinson’s diseaseDrugs73: 14051415 |
8 | Blandini F, Porter RH, Greenamyre JT(1996) Glutamate and Parkinson’s diseaseMol Neurobiol12: 7394 |
9 | Borgohain R, Kandadai RM(2013) Safinamide: A novel anti-Parkinsonian drug with multiple actionsNeurodegener Dis Manag3: 231240 |
10 | Chase TN, Bibbiani F, Oh JD(2003) Striatal glutamatergic mechanisms and extrapyramidal movementdisordersNeurotox Res5: 139146 |
11 | Schapira AH, Stocchi F, Borgohain R, Onofrj M, Bhatt M, Lorenzana P, Lucini V, Giuliani R, Anand R, Study I(2013) Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson’s diseaseEur J Neurol20: 271280 |
12 | Stocchi F, Arnold G, Onofrj M, Kwiecinski H, Szczudlik A, Thomas A, Bonuccelli U, Van Dijk A, Cattaneo C, Sala P, Fariello RGSafinamide Parkinson’s Study G(2004) Improvement of motor function in early Parkinson disease by safinamideNeurology63: 746748 |
13 | Stocchi F, Borgohain R, Onofrj M, Schapira AH, Bhatt M, Lucini V, Giuliani R, Anand R, Study I(2012) A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson’s disease patientsMov Disord27: 106112 |
14 | Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt M, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E, Rice P, Anand R, Study I(2014) Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuationsMov Disord29: 229237 |
15 | Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt MH, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E, Rice P, Anand R, Study I(2014) Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s diseaseMov Disord29: 12731280 |
16 | Caccia C, Maj R, Calabresi M, Maestroni S, Faravelli L, Curatolo L, Salvati P, Fariello RG(2006) Safinamide: From molecular targets to a new anti-Parkinson drugNeurology67: S18S23 |
17 | Chazot PL(2007) Safinamide for the treatment of Parkinson’s disease, epilepsy and restless legs syndromeCurr Opin Investig Drugs8: 570579 |
18 | Gregoire L, Jourdain VA, Townsend M, Roach A, Di Paolo T(2013) Safinamide reduces dyskinesias and prolongs L-DOPA antiparkinsonian effect in parkinsonian monkeysParkinsonism Relat Disord19: 508514 |
19 | Kulisevsky J(2014) Emerging role of safinamide inParkinson’s disease therapyEur Neurolog Review9: 37 |
20 | Muller T(2013) Current status of safinamide for the drug portfolio of Parkinson’s disease therapyExpert Rev Neurother13: 969977 |
21 | Caccia C, Salvati P, Rossetti S, Anand R(2008) Safinamide: Modulation of dopaminergic and glutamatergic systemMov Disord2008: S22S23 |
22 | Pevarello P, Bonsignori A, Dostert P, Heidempergher F, Pinciroli V, Colombo M, McArthur RA, Salvati P, PostC , Fariello RG, Varasi M(1998) Synthesis and anticonvulsant activity of a new class of 2-[(arylalky)amino]alkanamide derivativesJ Med Chem41: 579590 |
23 | Cattaneo C, Caccia C, Marzo A, Maj R, Fariello RG(2003) Pressor response to intravenous tyramine in healthysubjects after safinamide, a novel neuroprotectant with selective, reversible monoamine oxidase B inhibitionClin Neuropharmacol26: 213217 |
24 | Onofrj M, Bonanni L, Thomas A(2008) An expert opinion on safinamide in Parkinson’s diseaseExpert Opin Investig Drugs17: 11151125 |
25 | Goetz CG, Stebbins GT, Shale HM, Lang AE, Chernik DA, Chmura TA, Ahlskog JE, Dorflinger EE(1994) Utility of an objective dyskinesia rating scale for Parkinson’s disease: Inter- and intrarater reliability assessmentMov Disord9: 390394 |
26 | Marcus R, Peritz E, Gabriel KR(1976) On closed testing procedures with special reference to ordered analysis of varianceBiometrika63: 665660 |
27 | Brotchie JM(2005) Nondopaminergic mechanisms in levodopa-induced dyskinesiaMov Disord20: 919931 |
28 | Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN(2000) AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeysNeurology54: 15891595 |
Figures and Tables
Fig.2
Proportions of patients with different categorical changes in DRS score (decrease, no change, increase). Subgroups of patients whose dose of L-dopa was not changed throughout the study.
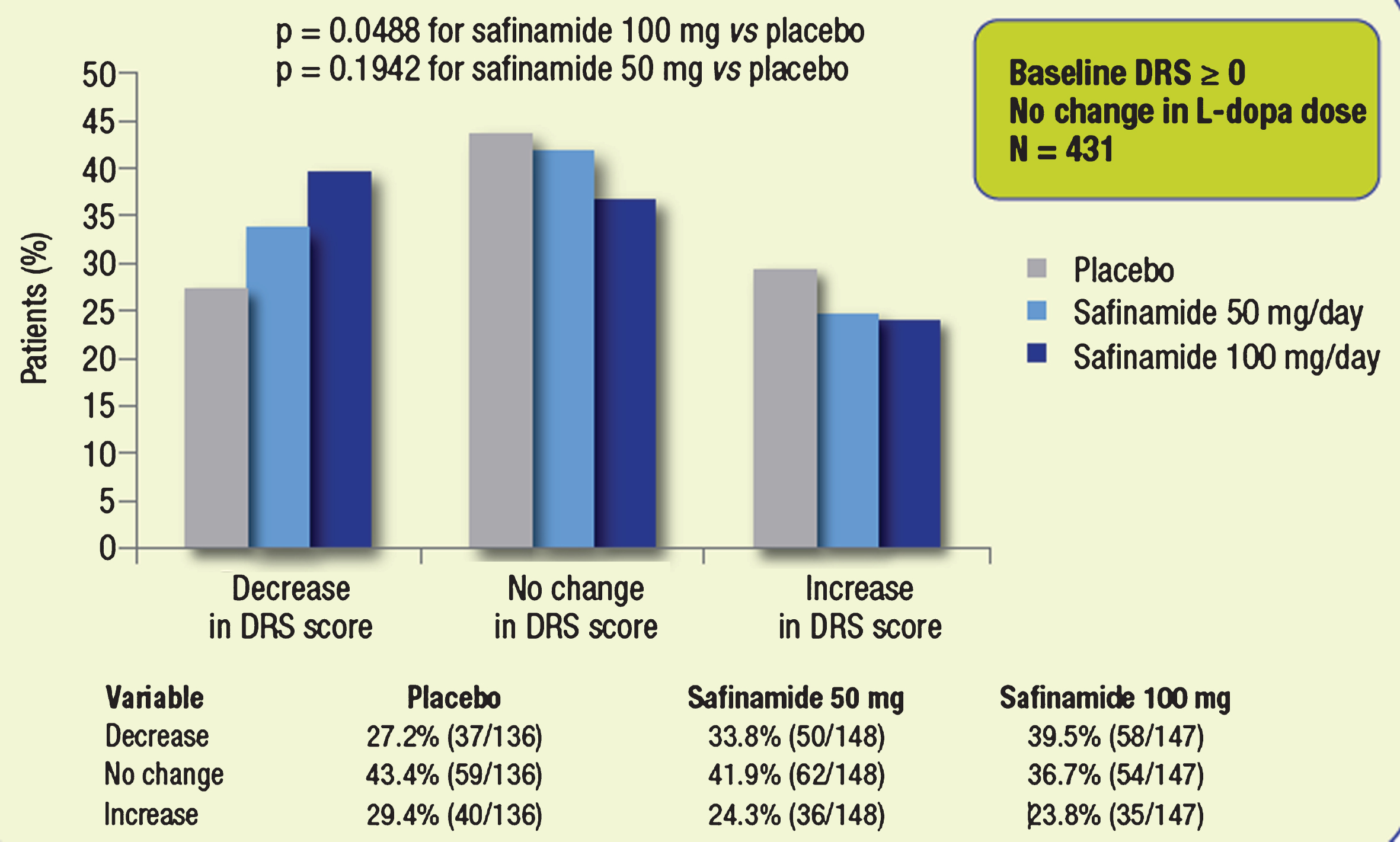
Fig.3
Proportions of patients with different categorical changes in DRS score (decrease, no change, increase). Subgroups of patients with dyskinesia at baseline.
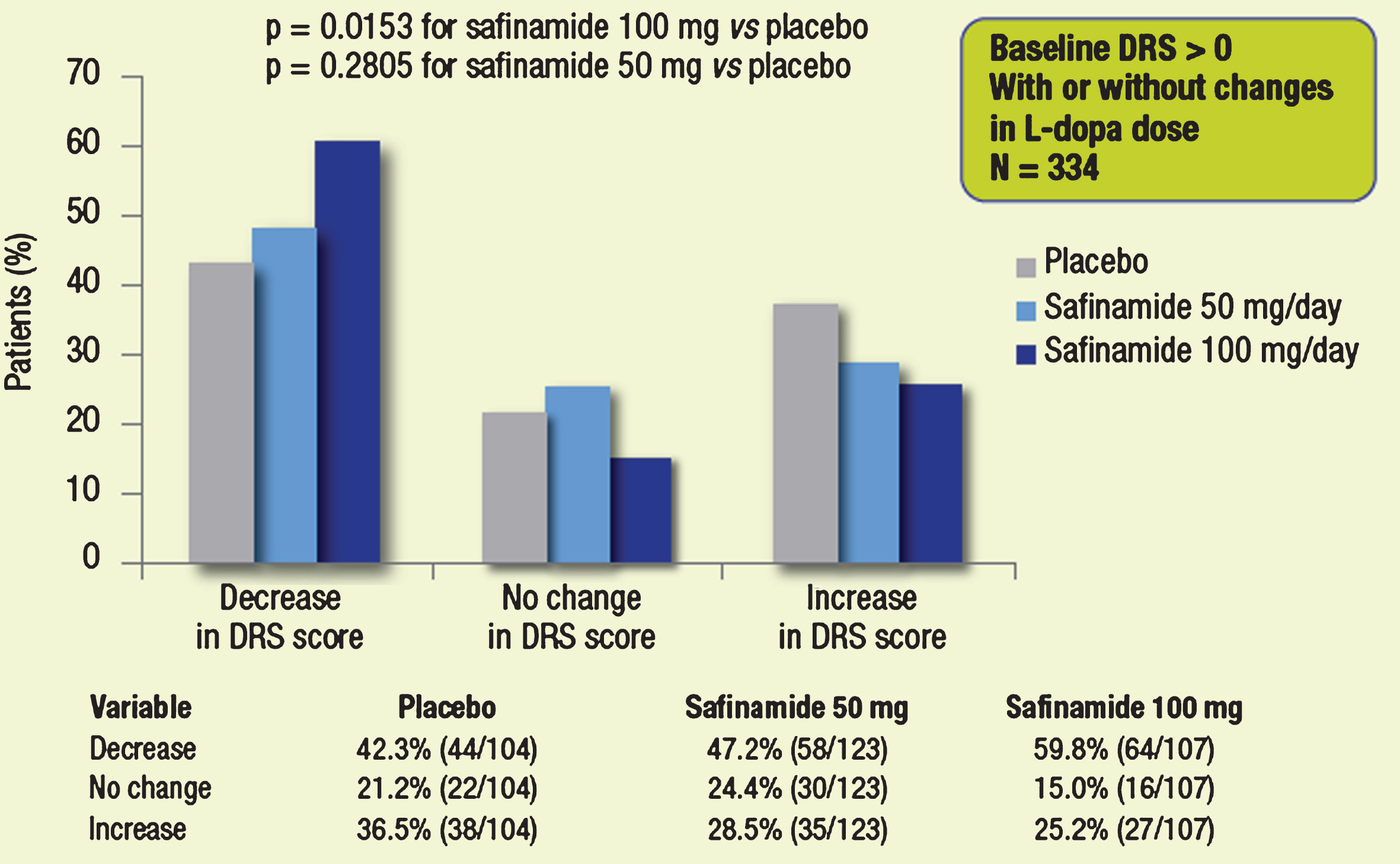
Fig.4
Proportions of patients with different categorical changes in DRS score (decrease, no change, increase). Subgroups of patients with dyskinesia at baseline, whose dose of L-dopa was not changed throughout the study.
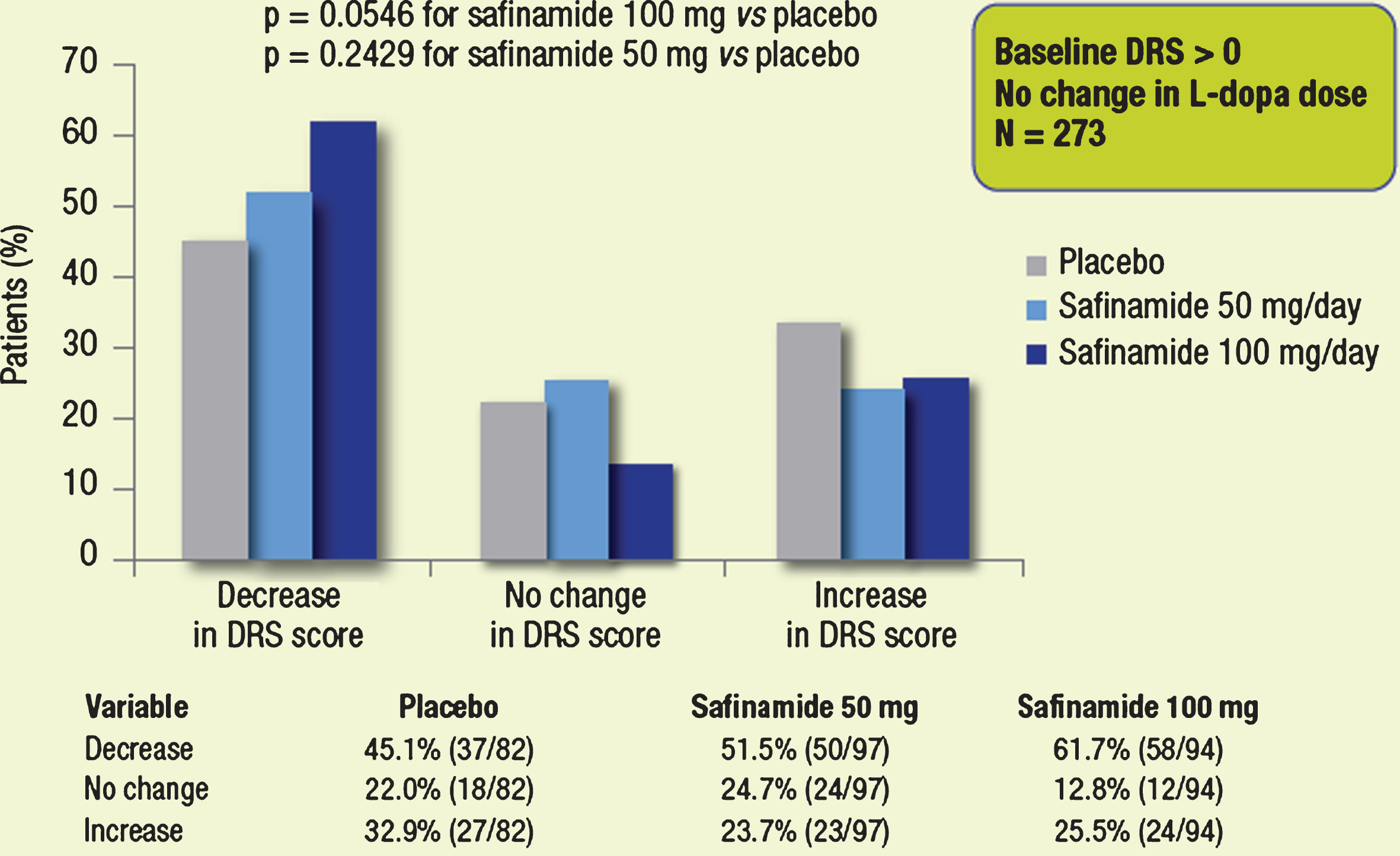
Fig.5
Proportions of patients with different categorical changes in DRS score (decrease, no change, increase). Subgroups of patients without dyskinesia at baseline.
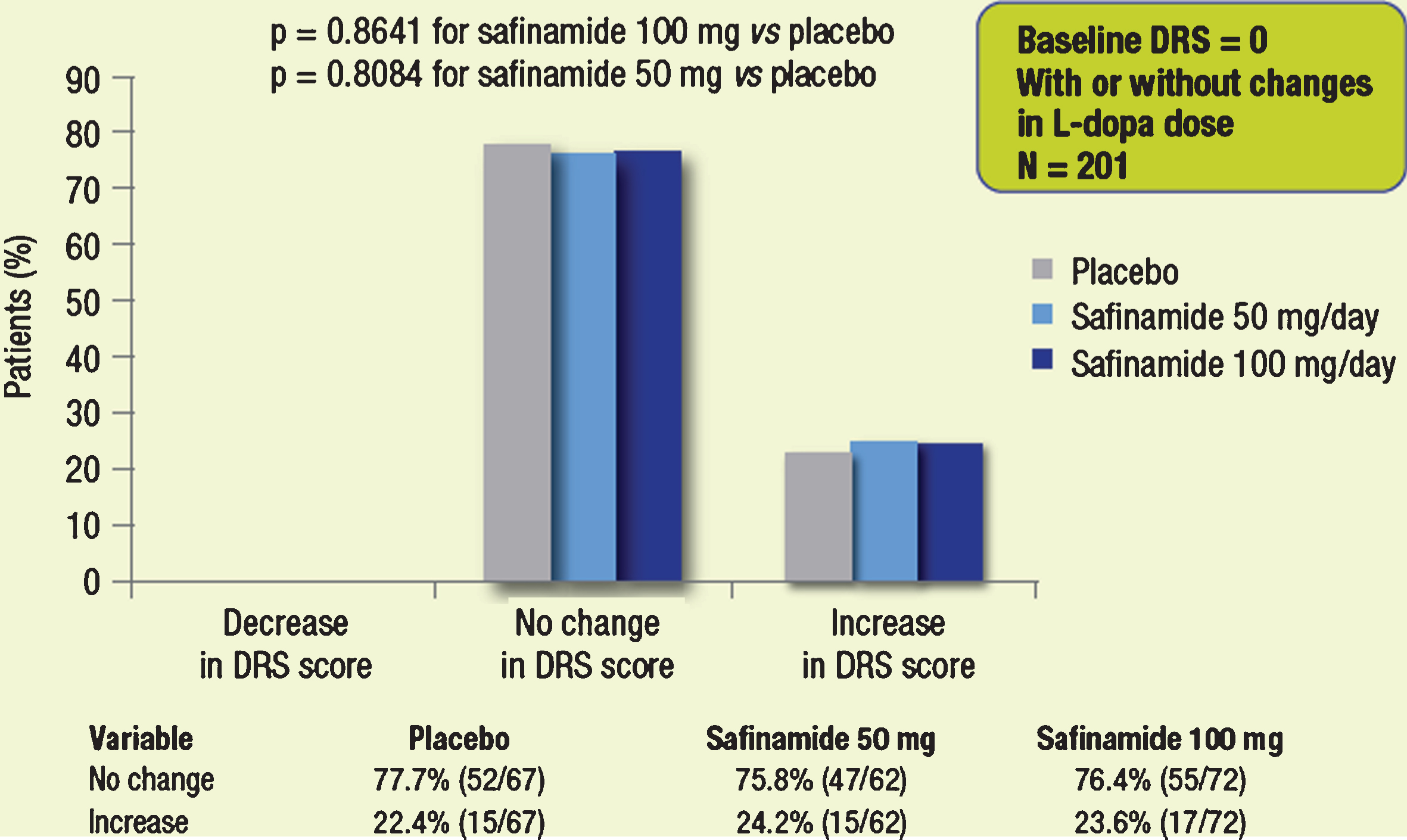
Fig.6
Proportions of patients with different categorical changes in DRS score (decrease, no change, increase). Subgroups of patients without dyskinesia at baseline, whose dose of L-dopa was not changed throughout the study.
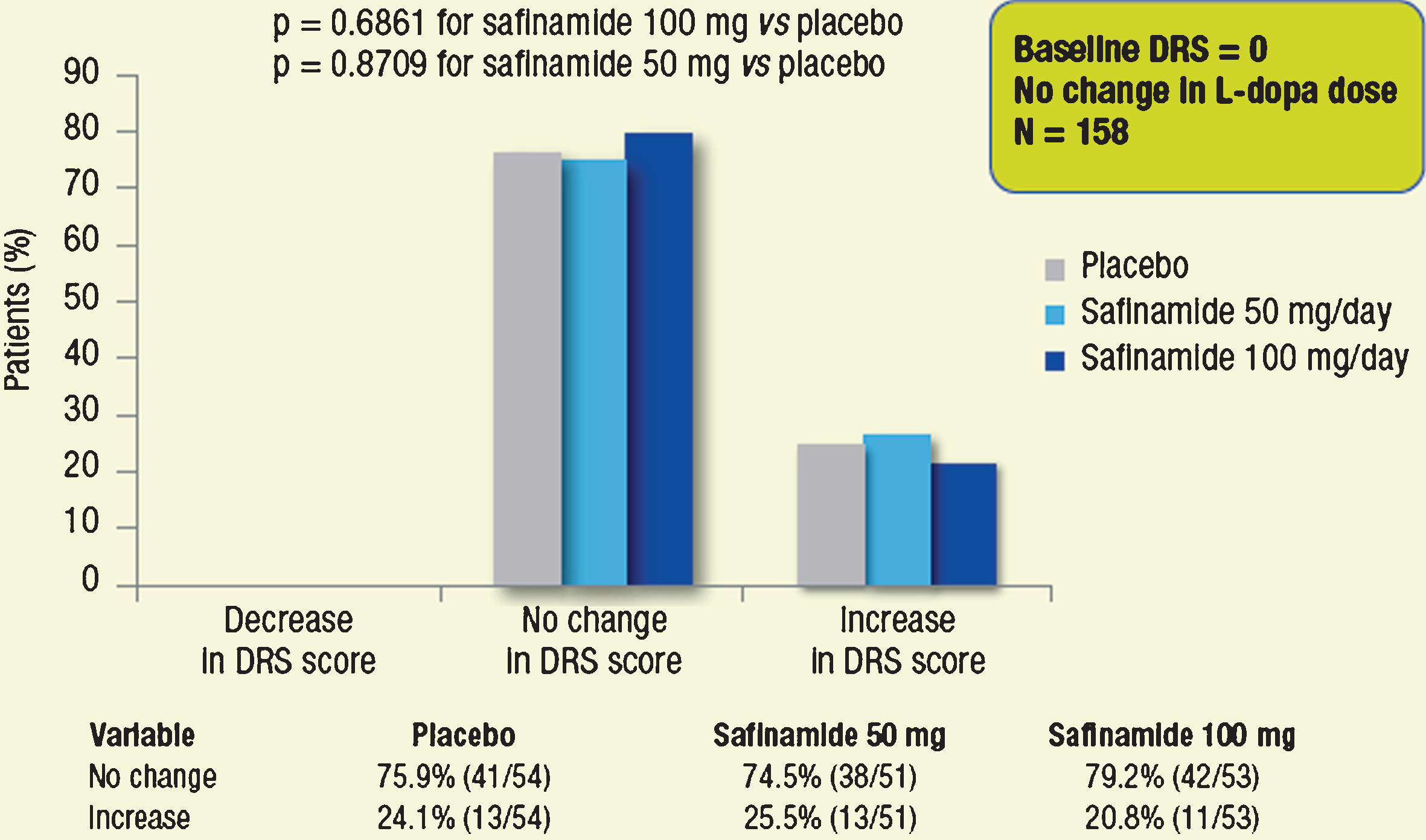
Table 1
Changes in the mean ( ± SD) dose of L-dopa from baseline to 24 months [15]
| Placebo (N = 222) | Safinamide 50 mg/day (N = 223) | Safinamide 100 mg/day (N = 224) | |
| Baseline (mg/day) | 618.5 ± 335.7 | 621.4 ± 329.7 | 579.6 ± 310.0 |
| Month 24 (mg/day) | 650.6 ± 338.0 | 635.2 ± 410.4 | 556.0 ± 381.9 |

![Proportions of patients with changes in L-dopa dose over 2 years [15].](https://content.iospress.com:443/media/jpd/2015/5-3/jpd-5-3-jpd150569/jpd-5-3-jpd150569-g001.jpg)



