E-health Support in People with Parkinson’s Disease with Smart Glasses: A Survey of User Requirements and Expectations in the Netherlands
Abstract
Recent advances in smart glasses, wearable computers in the form of glasses, bring new therapeutic and monitoring possibilities for people with Parkinson’s disease (PD). For example, it can provide visual and auditory cues during activities of daily living that have long been used to improve gait disturbances. Furthermore, smart glasses can personalize therapies based on the state of the user and/or the user environment in real-time using object recognition and motion tracking. To provide guidelines for developers in creating new PD applications for smart glasses, a self-reported questionnaire was designed to survey the requirements, constraints, and attitudes of people with PD with respect to this new technology. The survey was advertised online over an 11 month period on the website of the Parkinson Vereninging. The results were derived from 62 participants (54.8% men and 45.2% women, average age of 65.7 ± 9.1), representing a response rate of 79.5% . The participants were overall very enthusiastic about smart glasses as an assistive technology to facilitate daily living activities, especially its potential to self-manage motor problems and provide navigational guidance, thereby restoring their confidence and independence. The reported level of usage of mobile technologies like tablets and smartphones suggests that smart glasses could be adopted relatively easily, especially by younger people with PD. However, the respondents were concerned about the cost, appearance, efficacy, and potential side effects of smart glasses. To accommodate a wide range of symptoms, personal preferences, and comfort level with technology, smart glasses should be designed to allow simple operation and personalization.
INTRODUCTION
Due to the impaired ability of the basal ganglia to coordinate automatic movement sequences [1], many people with Parkinson’s disease (PD) suffer from gait and balance disturbances [2], characterized by small shuffling steps, bradykinesia, freezing, and falling [3–7]. As these problems can persist in spite of optimal drug treatments and deep brain stimulation, alternative rehabilitation therapies are required [8]. Interestingly, people with PD who typically have difficulty performing simple movements (e.g. walking) may perform complex tasks easily in the presence of an external sensory cue (e.g. cycling) [9]. For this purpose, a cue is defined as an external temporal or spatial stimulus that facilitates movement initiation and continuation [10]. In particular, audiovisual stimuli such as transverse lines on the floor [11, 12] or the beat of a metronome [10, 13–15] can greatly improve walking quality and decrease freezing. In a large-scale multi-site clinical trial of a cueing training program at home, the participants showed improvements immediately after intervention; however, improvements decreased considerably 4–12 weeks after training [16, 17]. To maintain the progress achieved with cueing training and to provide cueing on demand, it is thus essential that we develop cueing devices that are effective, portable and convenient for everyday use outside the laboratory [10, 18].
Recent advances in mobile technology offer promising new ways to provide external cueing and other e-health applications (apps) to people with PD, notably through smart glasses such as the Google Glass, Vuzix M100, and Epson Moverio BT-200 [19]. These wearable computers are worn like conventional spectacles but provide all the conveniences of a smart phone. For example, smart glasses can respond to voice and gestures commands and provide auditory feedback. The embedded camera can capture information about the user environment while GPS, Wi-Fi, and inertial sensors can be used to track the user’s position and movement. Most importantly, smart glasses can augment reality by overlaying pertinent information derived from these sensors on top of the user’s visual field. Given its portability, inconspicuousness, and user friendliness, smart glasses represent an ideal modality to provide personalized feedback and assistance to people with PD in daily living situations. Indeed, according to a short proceedings report by McNaney et al. [20], participants with PD (n = 5) were generally positive about the Google Glass as an everyday assistive device; however usability issues and social stigma still hinder its general acceptance. In developing smart glasses and associated apps for PD, one must therefore account for the specific requirements and constraints of the target group and their environment. Here we report the results of a nationwide survey in the Netherlands on the therapeutic needs and ergonomic preferences of people with PD and their attitudes toward adopting this new technology.
MATERIALS AND METHODS
In accordance with the guidelines of the Declaration of Helsinki and the ethical standards of the local medical ethics committee, we conducted an online survey of people with PD in the Netherlands to uncover public opinions about smart glasses and how such devices may contribute to the quality of life for people living with PD. All research subjects participated voluntary without monetary solicitation and provided written informed consent for inclusion in the study.
Survey design
The initial version of the user survey was developed based on Burgess’ guidelines on the design of surveys [21] and in consultation with two neurologists, two physical therapists, and a human movement scientist who work with people with PD. To optimize its readability and understandability, further revisions were made to the questionnaire based on the evaluations of a physical therapist with a user panel consisting of individuals with PD.
User questionnaire
The questionnaire consisted of multiple choice questions (with the option to fill in an unlisted answer), Likert rating scales (0 to 10), and open ended questions, designed to gather information about the participants (e.g. demographics, PD related difficulties, and current use of mobile technology) and to explore their ideas and opinions about smart glasses and its potential features. In the introduction leading to the online survey, we clearly described the general features of smart glasses (e.g. augmented reality, embedded camera, movement sensors, loudspeaker, microphone, GPS, etc.) and the effects of external cueing (e.g. metronome and stripes on the floor) for people with PD. The specific questions in the survey can be found in Supplement 1 and are addressed individually in the results section. Not all questions were required to be answered.
Data collection
The questionnaire was advertised via the website of the Dutch society for people with PD (Parkinson Vereniging, www.parkinson-vereniging.nl). There were no inclusion or exclusion criteria for participation except that respondents should be diagnosed with PD. The response rate during the data collection period (Jan 22 – Dec 27, 2014) was 79.5% , based on the total number of visitors to the survey invitation webpage (n = 78).
Data analysis
The responses were independently reviewed and categorized by two researchers. Multiple submissions by the same participant were accounted for by comparing the subject data and using the most recent responses. Disagreements about the categorization of answers to open-ended questions were resolved through discussion between the two reviewers. All data was coded and inputted into Microsoft Excel and further data analysis was performed using MATLAB R2012a (Mathworks, Inc., Natick, MA, USA). Tabulated responses were represented as a percentage of the total number of participant responses (n). Numerical ratings were characterized by the mean ± standard deviation (SD), median, and skewness and their distributions compared using a two-sided Kolmogorov-Smirnov (KS) test with Bonferroni correction. Spearman’s rank correlation coefficient (rs) was used to measure statistical dependence between two variables and its effect size was interpreted according to Cohen’s guidelines [22]. A significance level of α= 0.05 was used for all statistical tests.
RESULTS
Demographics
62 persons (54.8% men and 45.2% women) responded to the survey. On average, the respondents were 65.7 ± 9.1 years old (n = 61, range: 39–87) and have been diagnosed with PD for 9.5 ± 6.8 years (n = 60, range: 2–36). 86.9% cohabitate with a partner, while 9.8% live alone and 3.3% reported other living arrangements (n = 61). The participants represented a variety of occupations, educational background, and technical proficiency.
Opinions on smart glasses
We assessed whether people with PD would welcome smart glasses as an everyday assistive device by asking the participants to rate how willing they were to use smart glasses in various situations on a scale of 0 to 10 (0 corresponding to completely uncomfortable and 10 to extremely comfortable). The ratings distributions for use at home (n = 52), outdoors (n = 51), in busy areas (n = 52), and in general (n = 52) were centered around 6–8 and left-skewed (i.e. the distribution is concentrated on the right), indicating that participants were overall receptive towards wearing smart glasses (Fig. 1A-D). No statistically significant differences were found amongst the ratings distributions for the four situations, nor were there significant correlations between the ratings and the participants’ age or the number of years they had PD. Since smart glasses are relatively new to the consumer market, 4 participants explicitly indicated that they could not choose an exact rating while 8 others indicated that they required more hands on experience to make a precise decision. 5 participants expressed concerns about multitasking with smart glasses, namely that “people with PD can only do one thing at a time and might become disoriented ... ” by the extra sensory information provided by smart glasses. This was particularly worrisome in busy environments and may exacerbate their motor and cognitive disabilities. On the other hand, 3 respondents described smart glasses as an ideal therapeutic tool for PD. ‘It was the first thing that came into my mind when I heard about the Google Glass,” recounted one participant. Indeed, 80.7% of the respondents (n = 62) were willing to participate in a clinical trial to further test the functionalities and efficacy of such devices.
We also asked participants to list the features they would like to see in smart glasses and categorized their responses into three subcategories: function, design, and applications (Table 1). First, respondents most frequently requested features for improving the quality of their gait and maintaining balance, thereby allowing them to gain more independence and confidence. Equally important was that side effects (e.g. headaches, dizziness, disorientations, eye strain, etc.) be minimal. Design wise, the control interface must be easy to operate, especially for the elderly, and allow hands-free operation. Furthermore, the device should be comfortable to wear, aesthetically pleasing, and compatible with prescription glasses. Besides the basic smart phone applications (e.g. GPS, calendar, address book, media player, etc.), the participants desired more specialized PD functions such as guide-dog like navigational guidance and object avoidance, external cueing (e.g. metronome, music, and stripes on the floor), real-time detection of motor fluctuations, warnings or instructions on how to improve performance, medication alerts, and logging of daily activities. One participant even suggested using smart glasses to provide virtual realityphysiotherapy.
We further surveyed how much people with PD were willing to pay (out of pocket) for such a device. The percentage of participants who would spend at most €100, €200, €300, €400, €500, or over €500 were respectively 17.7, 27.4, 14.5, 1.6, 17.7, and 8.1% (n = 62). The remaining 12.9% of the participants did not specify a monetary value but provided additional explanations: a few respondents could not judge the monetary value of smart glasses (n = 3) while another indicated that more experience with these devices was needed to make a precise decision (n = 1). Based on the comments gathered from all participants, the monetary value of smart glasses depended on its efficacy in managing PD symptoms (n = 5) and participants were therefore willing to pay more if smart glasses could truly improve their quality of life (n = 6); however, several participants were adamant that medical insurance should reimburse them at least in part (n = 4).
Parkinson’s disease in daily life
To prioritize smart glasses applications in terms of their usefulness in activities of daily living, we asked participants to quantify the extent to which PD impedes them in various setting on a scale of 0 to 10 (0 corresponding to not at all and 10 to extremely disruptive). The ratings distributions for home, work, and in daily life all peaked around 7-8 and were left skewed (Fig. 1E-G), indicating that PD was prohibitive in most aspects of life. No significant differences were found between the distributions of the different settings. In their comments, participants reported that motor problems (n = 6) and tasks requiring multitasking, fine motor skills, and endurance (e.g. cycling, housework, walking outside, etc.) (n = 4) particularly affected their quality of life, with some participants no longer able to work at all (n = 4). Nonetheless, many participants were determined to stay active (n = 6) and independent (n = 2) in spite of the disease. According to one patient: “It is inevitable that [PD is] prevalent in everyday life; however, I will not let this prevent me from pushing boundaries and overcoming limitations by continuing to pursue all that interests me (e.g. driving, traveling, dancing, singing, sports, etc.). ”
We further asked the participants to list the symptoms they experience and specific situations that exacerbate these symptoms. The respondents most frequently cited motor impairments, including gaitdisability (80.6%), balance impairment (72.6%), freezing (37.1%), tremor (43.5%), poor coordination (33.9%), and rigidity (9.7%) (n = 62). 45.2% of the respondents also complained about sleep disturbances and 6.5 reported cognitive impairments. As shown in Table 2, gait or freezing can be triggered by specific movements, spatial obstructions, mental states, and time of the day. For example, participants recounted motor complications after long periods of sitting or lying down, standing up, initiating new movements, and transitioning between movements (e.g. stepping off a bicycle). Notably challenging were tight turns, narrow spaces, doors, and complicated crossings. Mornings were especially problematic and symptoms became more prominent when medication began to wear off. Fatigue, stress, fear, and distractions further exacerbated motor performance. Only 12.9% of all the participants specifically reported little to no problems with gait or freezing on a daily basis.
Treatments for Parkinson’s disease
To assess which existing therapies could be incorporated into smart glasses, we asked participants which therapies they currently use. The majority undergo physiotherapy (76.7%) and take medication (76.7%) while 6.7% use DBS (n = 60). Some participants also follow exercise programs (e.g. boxing, Parkfit program, and Cesar therapy, 10.0%), speech therapy (3.3%) and light-therapy (3.3%). Overall, people living with PD “ ... long for a way to maintain dopamine levels without all the side effects.”
Besides traditional PD therapies, 46.8% of all participants (n = 62) use external cues or cueing devices to improve their gait and balance, including canes (22.6%), auditory metronomes (provided by an external device or self-made, 16.1%), music (listening or singing, 11.3%), stripes on the floor (8.1%), counting (6.5%), and lasers (1.6%). Participants also rated the effectiveness of each cue they used on a scale of 0 to 10 (0 corresponding to severely worsens and 10 to significantly improves), resulting in average ratings of 7.6 ± 1.3 for the cane (n = 13), 7.4 ± 1.1 for the auditory metronome (n = 10), 7.5 ± 0.8 for music (n = 8), 7.0 ± 1.0 for stripes on the floor (n = 7), 6.6 ± 1.1 for counting (n = 5), and 7.0 ± 1.7 for lasers (n = 3). Some participants reported that their symptoms were not severe enough yet to use cueing (16.1%) or that they were, up until now, unfamiliar with cueing strategies (14.5%). Others used walking supports such as rollators (19.4%), Nordic walking poles (4.8%), and scooter mobiles (3.2%). Should smart glasses be able to provide cueing therapy, the participants preferred to control for themselves when the cues are given: on a scale of 0 to 10 (0 corresponding to completely automatic and 10 to completely manual), 66.7% choose a rating of 5 or greater (n = 61). Nevertheless, the respondents suggested that a combination of manual and automatic control might be best, depending on the situation.
Use of mobile technology
To predict how easily people with PD could adapt to smart glasses, we asked the participants to rate how comfortable they were in using mobile technologies (e.g. smart phones and tablets) on a scale of 0 to 10 (0 corresponding to totally uncomfortable and 10 to extremely comfortable). 57.4% of the respondents (n = 61) reported a comfort level of 6 or above (Fig. 2A). These ratings showed a significant inverse correlation with the participants’ age (rs =−0.45, p < 0.001) but not with the number of years they had PD (Fig. 2B-C). 74.6% of the respondents (n = 61) reported using mobile devices in some capacity: 45.9 % on a daily basis, 18.0% 1–5 times a week, and 11.5% less than once per week. The frequency of mobile device usage was also significantly correlated with the age of the patient (rs = 0.43, p < 0.001) but not with how long they lived with PD (Fig. 2D-E). Respondents who never use mobile devices (24.6%) commented that they were more interested in other activities (n = 1), had poor eyesight (n = 1), disliked the sensory experience of using such devices (n = 1), or did not own any mobile devices (n = 1). In the open comments, the respondents particularly singled out the iPad (n = 3) and iPhone (n = 2) (Apple, Cupertino, CA, USA) as useful for researching hobbies, keeping in touch with family and friends, and work related activities (e.g. note taking, reading documents, business communication, administration, organizing events, etc.). Notably, one participant reported: “my iPad has become a part of my brain.”
Other health issues
Preexisting health conditions may preclude some people with PD from using smart glasses. In fact, 57.4% of the respondents (n = 61) reported medical conditions besides PD, including cardiovascular (e.g. high blood pressure, heart attack, and infarcts, n = 12), musculoskeletal (e.g. osteoporosis, arthritis, tendonitis, hip problems, back pains, scoliosis, and cervical stenosis, n = 11), neurodegenerative (e.g. Lewy body dementia and dystonia, n = 6) and pulmonary (e.g. chronic obstructive pulmonary disease, n = 5) diseases and other problems related to chronic pain (n = 3), diabetes (n = 3), the spine (n = 3), and cancer (n = 2). In addition, participants also reported vision and hearing problems that may preclude the use of some audiovisual capabilities of smart glasses. Specifically, 75.4% of the respondents (n = 61) required spectacles (prescription or reading glasses) and 16.4% suffered from some form of visual impairment. On the other hand, 67.2% of the respondents (n = 61) reported no hearing problems while 18.0% suffered from deafness and 13.1% required hearing aids.
DISCUSSION
Recommended applications
As the effects of PD permeate almost every aspect of life, an overarching wish amongst the participants was to regain their independence and self-confidence. In particular, gait and balance impairments afflict most people with PD and have the most impact on their quality of life [8]. With its sensor and feedback capabilities, smart glasses may help by detecting when motor fluctuations occur, alerting the patient to correct this behavior, and providing support via visual or auditory cues, instructional videos, or step by step verbal instructions. The glasses may even be able to predict when the patient might require assistance and take precautionary actions based on situations known to exacerbate PD (Table 2) or a log of daily activities.
Smart glasses might also support existing PD therapies. For example, users could receive reminders to take their medication, meet with their physician, get more light exposure, or exercise. Physiotherapy and exercise apps could further promote and motivate physical activity by guiding users through different movement exercises while tracking their performance and adjusting the therapy accordingly. Verbally guided relaxation exercises, on the other hand, may help relax the patient’s state of mind while gaming apps could stimulate cognitive abilities. Finally, speech therapy apps could alert the user to speak louder, provide external cues to facilitate speech production, or find the appropriate words to finish a sentence. All the aforementioned possibilities for smart glasses apps remain to be developed and thoroughly tested.
Requirements and constraints
Given that most people with PD are above the age of 50 and have visual impairments, smart glasses must accommodate prescription glasses and be easy to operate. Based on the enthusiasm amongst the survey respondents toward the iPad, a simple and natural interface that recognizes broad gestures (versus precise tapping) would help users to navigate the controls. For people with tremor, hands-free operation could be achieved through speech recognition; however, users with speech impediments might require alternative ways to communicate with smart glasses such as eye tracking or winking.
In proposing to use smart glasses as an everyday assistive device, one should also consider its potentially harmful side effects. First, with respect to external cueing, complicated situations that demand increased cognitive processes do indeed aggravate gait impairments in PD [23, 24]; however, external cueing has also been shown to facilitate double task walking [25]. In principle, cueing training could be implemented using smart glass technology and as such facilitate conditions for goal-based exercise training to improve gait and gait-related activities for people with PD [26]. The choice of an appropriate cue as well as when to give the cue is however nontrivial and warrants further exploration [26]. Ideally, users should be able to personalize the apps to accommodate their symptoms, preferences (i.e. automatic versus manual cueing, alerts on or off, etc.), and comfort level with the device.
Second, chronic exposure to blue light (a portion of the visible spectrum ranging from 380 to 500 nm in wavelength), as emitted by light-emitting diode (LED) based video displays, can lead to damage of retinal structures via photochemical mechanisms [27–30]. Moreover, the usage of LED displays such as eReaders in the evening can negatively affect sleep, circadian rhythm, and daytime alertness [31] through the suppression of melatonin release [32–34] and a shift in the circadian clock [32, 35]. To minimize disruptions to the retina and circadian rhythm, manufacturers of smart glasses must carefully tune the spectral power distribution of these devices [36], possibly through blue-light filters or by adapting the spectral distribution throughout the day or according to the frequency of use.
Third, given the prevalence of cardiovascular and musculoskeletal diseases amongst people with PD, physicians and physiotherapists should be involved in assessing the suitability of certain apps (e.g. movement training or exercise apps) for these individuals, perhaps through e-prescriptions (i.e. downloading apps from an app store via prescriptions). The data collected by the embedded sensors and daily activity logs on the smart glasses may also allow clinicians to remotely monitor the progress and symptoms of their patients. The results of this and similar data mining initiatives with other wearable devices (e.g. Michael J. Fox Foundation’s collaboration with Intel) could accelerate PD research and validation of new therapeutics [37].
Lastly, a potential side effect of taking PD medication is a propensity to engage in reward seeking obsessive compulsive behaviors (e.g. hoarding, shopping, eating, and gambling) [38–40]. People with PD must be advised of such risks when using smart glasses and caretakers and healthcare professionals should be vigilant of any addictive activities stemming from its use.
Limitations of the study
Due to the inherent nature of online self-completed questionnaires, there are several issues to consider in the interpretation of the results, namely self-selection bias and trustworthiness of the responses. People already proficient with or interested in technology will be more likely to take part in the online survey; however, these individuals are also more likely to adopt smart glasses in clinical practice. In that regard, self-selection is less critical in the short-term; however, further work is needed to examine the perceptions of other patient groups, including elderly or less educated patients without regular access to the internet. It is furthermore impossible to fully demonstrate the trustworthiness of the responses. For example, the survey could have been completed by or with help from a partner or family member as opposed to the person with PD; nevertheless, this should not detract from the value of responses as caretakers often play an integral role in the care of individuals with PD and their decision-making. To verify the veracity of the responses, we checked the answers for a subset of 10 participants during subsequent face-to-faceinterviews.
Future outlook
Smart glasses possess the potential to empower people with PD by providing everyday assistance in the self-management of their symptoms. Through this study, we uncovered many useful applications of smart glasses for people with PD and established some requirements and constraints for its design. Participants who responded to our survey were generally positive about smart glasses and their usage of current mobile technologies suggests that smart glasses may become as integral to their lives as smart phones and tablets. However, more research is necessary to ensure the safety, efficacy, usability, and social acceptance of smart glasses.
Although unaddressed in our survey, privacy and social stigma will undoubtedly become major points of contention. In the United States and the United Kingdom, where the Google Glass is currently available, lawmakers, citizens, and business owners have already raised concerns about privacy, road safety, violation of intellectual property, and breach of confidentiality. Social stigma may also plague people with PD, who feel especially self-conscious [41], while wearing smart glasses in public. However, as smart glasses become less conspicuous and establish valuable therapeutic benefits for people with PD, its legal and social acceptance should alsoevolve.
ACKNOWLEDGMENTS
This study was funded by the Fonds NutsOhra(1303-071). YZ is also supported by the Whitaker International Scholarship Program, RJAvW and BRB by HealthPAC (Marie-Curie FP7-PEOPLE-2013-ITN), and RJAvW by NWO 314-98-018 and 058-14-001. We thank the Parkinson Vereniging for advertising the survey. The authors have no conflict of interest to report.
REFERENCES
1 | Meara J, Koller WC (2000) Parkinson’s Disease and Parkinsonism in the Elderly Cambridge University Press |
2 | Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V, Fahn S (1992) Motor blocks in Parkinson’s disease Neurology 42: 333 333 |
3 | Knutsson E (1972) An analysis of parkinsonian gait Brain 95: 475 486 |
4 | Murray MP, Sepic SB, Gardner GM, Downs WJ (1978) Walking patterns of men with parkinsonism Am J Phys Med 57: 278 294 |
5 | Stern G, Franklyn S, Imms F, Prestidge S (1982) Quantitative assessments of gait and mobility in Parkinson’s disease J Neural Transm Suppl 19: 201 214 |
6 | Rogers MW (1996) Disorders of posture, balance, and gait in Parkinson’s disease Clin Geriatr Med 12: 825 845 |
7 | Morris ME, Iansek R, Matyas TA, Summers JJ (1994) The pathogenesis of gait hypokinesia in Parkinson’s disease Brain 117: 1169 1181 |
8 | Maetzler W, Nieuwhof F, Hasmann SE, Bloem BR (2013) Emerging therapies for gait disability and balance impairment: Promises and pitfalls Mov Disord 28: 1576 1586 |
9 | Martin JP (1967) Medical The Basal Ganglia and Posture London Pitman |
10 | Lim I, Van Wegen E, De Goede C, Deutekom M, Nieuwboer A, Willems A, Jones D, Rochester L, Kwakkel G (2005) Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: A systematic review Clin Rehabil 19: 695 713 |
11 | Azulay J-P, Mesure S, Amblard B, Blin O, Sangla I, Pouget J (1999) Visual control of locomotion in Parkinson’s disease Brain 122: 111 120 |
12 | Wegen Ev, Lim I, Goede Cd, Nieuwboer A, Willems A, Jones D, Rochester L, Hetherington V, Berendse H, Zijlmans J (2006) The effects of visual rhythms and optic flow on stride patterns of patients with Parkinson’s disease Parkinsonism Relat Disord 12: 21 27 |
13 | McIntosh GC, Brown SH, Rice RR, Thaut MH (1997) Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson’s disease J Neurol Neurosurg Psychiatry 62: 22 26 |
14 | Thaut M, McIntosh G, Rice R, Miller R, Rathbun J, Brault J (1996) Rhythmic auditory stimulation in gait training for Parkinson’s disease patients Mov Disord 11: 193 200 |
15 | Dietz MA, Goetz CG, Stebbins GT (1990) Evaluation of a modified inverted walking stick as a treatment for parkinsonian freezing episodes Mov Disord 5: 243 247 |
16 | Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, Chavret F, Hetherington V, Baker K, Lim I (2007) Cueing training in the home improves gait-related mobility in Parkinson’s disease: The RESCUE trial J Neurol Neurosurg Psychiatry 78: 134 140 |
17 | Lim I, Van Wegen E, Jones D, Rochester L, Nieuwboer A, Willems A-M, Baker K, Hetherington V, Kwakkel G (2010) Does cueing training improve physical activity in patients with Parkinson’s disease? Neurorehabil Neural Repair 24: 469 477 |
18 | Kwakkel G, De Goede C, Van Wegen E (2007) Impact of physical therapy for Parkinson’s disease: A critical review of the literature Parkinsonism Relat Disord 13: S478 S487 |
19 | Wiederhold BK (2013) Time to port augmented reality health apps to smart glasses? Cyberpsychol Behav Soc Netw 16: 157 158 |
20 | McNaney R, Vines J, Roggen D, Balaam M, Zhang P, Poliakov I, Olivier P (2014) Exploring the acceptability of google glass as an everyday assistive device for people with Parkinson’s Proceedings of the 32nd Annual ACM conference on Human factors in computing systems 2551 2554 |
21 | Burgess TF (2001) University of Leeds, Leeds A general introduction to the design of questionnaires for survey research |
22 | Cohen J (2013) Statistical Power Analysis for the Behavioral Sciences Routledge Academic |
23 | Brauer SG, Woollacott MH, Lamont R, Clewett S, O’Sullivan J, Silburn P, Mellick GD, Morris ME (2011) Single and dual task gait training in people with Parkinson’s Disease: A protocol for a randomised controlled trial BMC Neurol 11: 90 |
24 | Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N (2008) Dual-task decrements in gait: Contributing factors among healthy older adults J Gerontol A Biol Sci Med Sci 63: 1335 1343 |
25 | Rochester L, Nieuwboer A, Baker K, Hetherington V, Willems A-M, Chavret F, Kwakkel G, Van Wegen E, Lim I, Jones D (2007) The attentional cost of external rhythmical cues and their impact on gait in Parkinson’s disease: Effect of cue modality and task complexity J Neural Transm 114: 1243 1248 |
26 | van Wegen EE, Hirsch MA, Huiskamp M, Kwakkel G (2014) Harnessing cueing training for neuroplasticity in Parkinson disease Top Geriatr Rehabil 30: 46 57 |
27 | Grimm C, Wenzel A, Williams TP, Rol PO, Hafezi F, Remé CE (2001) Rhodopsin-mediated blue-light damage to the rat retina: Effect of photoreversal of bleaching Invest Ophthalmol Vis Sci 42: 497 505 |
28 | Noell WK (1980) Possible mechanisms of photoreceptor damage by light in mammalian eyes Vis Res 20: 1163 1171 |
29 | Noell WK, Walker VS, Kang BS, Berman S (1966) Retinal damage by light in rats Invest Ophthalmol Vis Sci 5: 450 473 |
30 | Ham WTJr, Mueller HA, Sliney DH (1976) Retinal sensitivity to damage from short wavelength light Nature 260: 153 155 |
31 | Chang AM, Aeschbach D, Duffy JF, Czeisler CA (2015) Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness Proc Natl Acad Sci U S A 112: 1232 1237 |
32 | Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA (2000) Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression J Physiol 526: 695 702 |
33 | Mclntyre IM, Norman TR, Burrows GD, Armstrong SM (1989) Human melatonin suppression by light is intensity dependent J Pineal Res 6: 149 156 |
34 | Brainard GC, Lewy AJ, Menaker M, Fredrickson RH, Miller LS, Weleber RG, Cassone V, Hudson D (1988) Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers Brain Res 454: 212 218 |
35 | Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA (2003) A phase response curve to single bright light pulses in human subjects J Physiol 549: 945 952 |
36 | Wood B, Rea MS, Plitnick B, Figueiro MG (2013) Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression Appl Ergon 44: 237 240 |
37 | Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR (2013) Quantitative wearable sensors for objective assessment of Parkinson’s disease Mov Disord 28: 1628 1637 |
38 | Evans AH, Strafella AP, Weintraub D, Stacy M (2009) Impulsive and compulsive behaviors in Parkinson’s disease Mov Disord 24: 1561 1570 |
39 | Lawrence AD, Evans AH, Lees AJ (2003) Compulsive use of dopamine replacement therapy in Parkinson’s disease: Reward systems gone awry? Lancet Neurol 2: 595 604 |
40 | Wolters EC, Van der Werf YD, van den Heuvel OA (2008) Parkinson’s disease-related disorders in the impulsive-compulsive spectrum J Neurol 255: 48 56 |
41 | MacCarthy B, Brown R (1989) Psychosocial factors in Parkinson’s disease Br J Clin Psychol 28: 41 52 |
Figures and Tables
Fig.1
Smart glasses and Parkinson’s disease in daily living situations. (A-D) Ratings of how comfortable people with PD are in using smart glasses (A) in general (n = 52), (B) at home (n = 52), (C) outdoors (n = 51), and (D) in busy areas (n = 52). (E-G) Ratings of how severely PD hinders people with PD (E) at home (n = 61), (F) at work (n = 37), and (G) in daily life (n = 62). The rating scale ranged from 0 to 10, with 0 corresponding to extremely uncomfortable (A-D) or nondisruptive (E-G) and 10 to extremely comfortable (A-D) or disruptive (E-G). In the histograms, the frequency of each numerical rating is expressed as a percentage of the total number of responses (n). Insets: measures of the mean, median, standard deviation (SD), and skewness (symmetry) to describe the location and variability of the distributions.
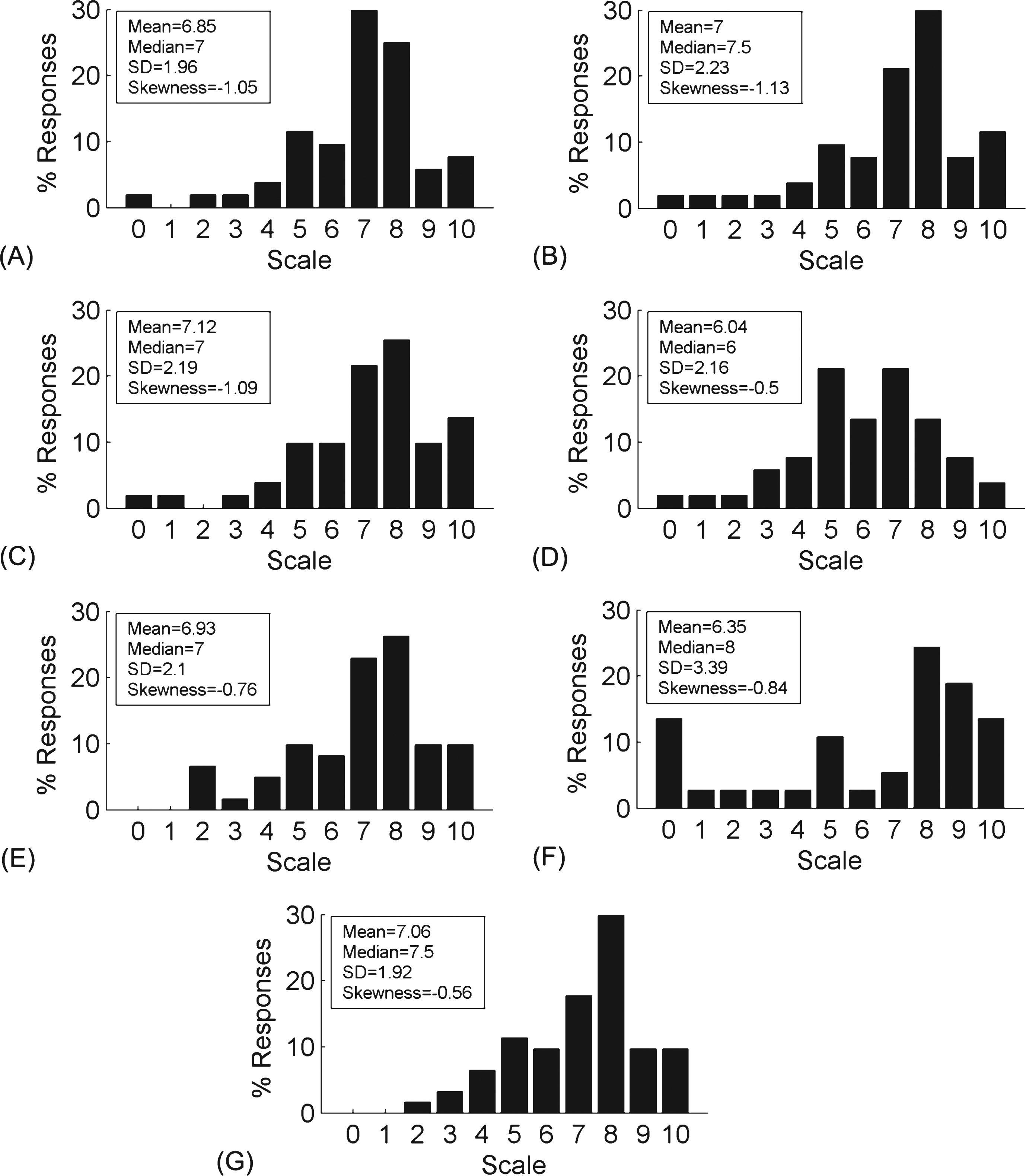
Fig.2
Use of mobile technology. (A-C) Ratings of how comfortable people with PD were in using mobile technologies. The rating scale ranged from 0 to 10, with 0 corresponding to completely uncomfortable and 10 to extremely comfortable. (A) The frequency of each numerical rating is expressed as a percentage of the total number of respondents (n = 61). Inset: measures of the mean, median, standard deviation (SD) and skewness to describe the location and variability of the distribution. (B-C) The comfort ratings as a function of (B) age (n = 60) and (C) disease progression (n = 59). (D-E) The frequency with which people with PD use mobile devices as a function of (D) age (n = 60) and (E) disease progression (n = 59). For each box and whiskers plot, the central mark corresponds to the median and the edges to the 25th and the 75th percentiles. The whiskers encompass the most extreme data points which are not considered to be outliers; outliers are individuallyplotted.
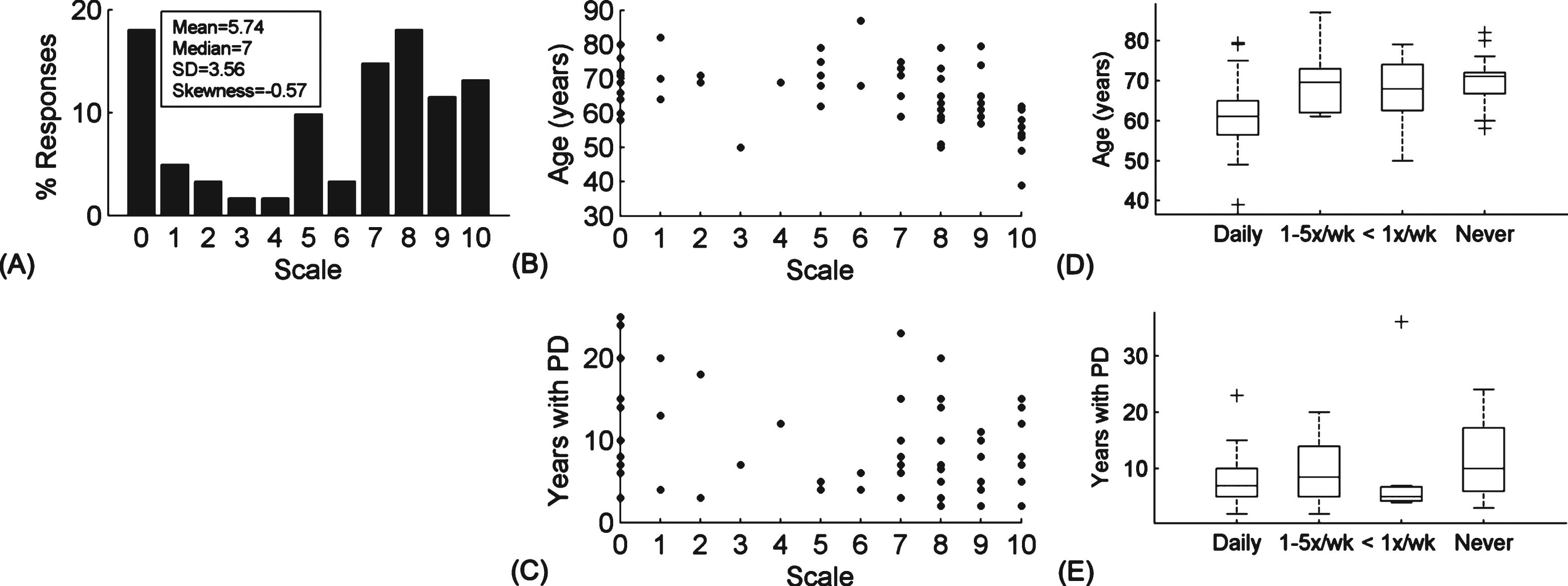
Table 1
Patient requested features for smart glasses
| Features | Number of responses | |
| Function | Improves gait | 10 |
| Maintains balance | 4 | |
| Increase confidence | 3 | |
| No side effects | 3 | |
| Decreases freezing of gait | 2 | |
| Helps standing up | 1 | |
| Facilitates independence | 1 | |
| Design | Easy to operate | 5 |
| Comfortable to wear | 4 | |
| Accommodate prescription lenses | 4 | |
| Aesthetically pleasing | 3 | |
| Durable/Sturdy | 2 | |
| Hands-free operation | 1 | |
| Application | GPS/navigation guidance | 5 |
| Instructions/alerts to improve movement | 5 | |
| Detect gait/balance problems | 5 | |
| Auditory cues | 4 | |
| Visual cues | 2 | |
| Agenda/reminders/to do lists | 2 | |
| (Emergency) calls/contacts | 2 | |
| Object/ QR code recognition | 1 | |
| Log daily activities | 1 | |
| Virtual reality physiotherapy | 1 |
Table 2
Situations that exacerbate the symptoms of Parkinson’s disease
| Exacerbating situations | Number of responses | |
| Movement | Long periods of sitting or lying down | 10 |
| Initiating new movement | 6 | |
| Standing up | 4 | |
| Transitional movements | 3 | |
| Stopping movement | 2 | |
| Walking backwards | 2 | |
| Walking outside | 2 | |
| Multitasking | 2 | |
| Standing still | 1 | |
| Spatial obstruction | Narrow spaces and passages | 6 |
| Turns | 5 | |
| Busy areas | 2 | |
| Complex crossings | 1 | |
| Doors | 1 | |
| Mental state | Fatigue | 4 |
| Stress | 1 | |
| Fear | 1 | |
| Distractions | 1 | |
| Time of the day | Morning | 8 |
| Medication wearing off | 7 | |
| Unpredictable | 3 | |
| Afternoon | 2 | |
| Late afternoon/early evening | 2 | |
| Evening | 2 | |
| Night | 1 | |
| All day | 1 |




