Rising to the Challenges of Clinical Trial Improvement in Parkinson’s Disease
Abstract
Background:
Despite an urgent need for new medications, clinical trials in Parkinson’s have a relatively low rate of success. Although many reasons have been proposed for this, the opinions of patients and scientists, the two principal stakeholders, have not been widely canvassed.
Objective:
The objective of the present study was to establish the main barriers to clinical trials success in Parkinson’s, as perceived by people with Parkinson’s and those engaged in conducting clinical trials in Parkinson’s.
Method:
Three hundred and three people (303) with a connection to Parkinson’s completed an online four-item questionnaire, directed towards discovering the barriers that interfere with the establishment of effective clinical trials.
Results:
87% of respondents were patients and their care partners and 11% were medical professionals involved with clinical research. In the survey, those involved in conducting research cited insufficient financial and administrative support as the biggest obstacles to carrying out effective clinical trials. For responders with Parkinson’s, the principal barrier to their participation in medical research was fear of potential adverse consequences and misconceptions regarding the clinical trial system as a whole, issues rooted in a perceived lack of communication of relevant information between the research and patient communities.
Conclusions:
Areas for future improvement as highlighted by this survey and debated at the Rallying to the Challenge meeting of people with Parkinson’s (PwP) at the Van Andel Research Institute that followed included recommendations in the areas of communication, education, funding, recruitment and compliance.
INTRODUCTION
Clinical trials are necessary to see how effective or safe certain treatments, interventions or diagnostic tests are in humans. They are also important in gaining information about a disease, how it manifests and the clinical course that it takes.
Drug development is not an expeditious process. For a new treatment to get from the idea stage to the pharmacy shelf it takes time, usually 10–15 years [1] as the initial concept journeys through basic research, preclinical testing, clinical trials and finally regulatory approval.
This process is a costly one. A recent projection by the Tufts Center for the Study of Drug Development [2] puts the total cost for developing a drug at $2.9 billion. $1.4 billion of that amount going towards drug discovery and development costs with the remainder attributable to the loss of potential returns on investment and costs incurred following a drug’s approval. Another study review found that estimates of the cost of drug development ranged between $500 million and $2 billion [3]. Ninety percent of drug development costs are incurred in Phase III trials according to the Manhattan Institute for Policy Research [4]. Although there is obvious discrepancy in the estimates, it is indisputable that clinical drug development is extremely costly – both in terms of dollars and time.
Part of the problem, borne out in the survey results below, is the timely and successful recruitment of study subjects. As clinical trials have become more rigorous they have also grown larger and more complex, and the work burden for study staff is increasing. For those who choose to volunteer, the last decade has seen a more selective and more stratified approach to the inclusion and exclusion criteria applied to clinical trials. This narrowing of the goalposts by an estimated 58% is likely to be responsible for volunteer patient enrollment rates dropping by 21% and retention rates falling by 30% [5].
The challenges facing patient recruitment are complex, currently up to 30% of the timeline for the drug development process is spent on suitable subject enrollment [6]. In fact, 45% of clinical studies are delayed because of difficulty enrolling participants [7]. Although a recent US based public opinion poll shows that 72% of Americans would participate in a clinical research study if recommended by their physician, the same poll estimated that only 16% of those surveyed have actually taken part in a study [8]. In an effort to evaluate why participation in Parkinson’s clinical research is so poor The Michael J. Fox Foundation for Parkinson’s Research conducted a poll in 2011. This revealed that even though more than 80% of respondents were at least “somewhat likely” to participate in a clinical trial, only one in ten Parkinson’s patients had actually taken part [9].
It seems there is a discordance between patient perceptions of the likelihood of their involvement and their actual participation in clinical trials. The identification of the cause of this disharmony provides the first step in developing appropriate strategies which could be employed to address these issues.
METHOD
A brief five question online survey (using Survey Monkey) was developed by Parkinson’s Movement (www.parkinsonsmovement.com), an international patient-driven action group created by a UK research charity, The Cure Parkinson’s Trust (www.cureparkinsons.org.uk). The survey was shaped with input from an advisory group of four Parkinson’s advocates, and two Parkinson’s specialist neurologists that run clinical trials. This process highlighted that people with Parkinson’s and those conducting clinical trials have different priorities and requirements and therefore the survey needed to question patients and those involved in clinical trials separately. This meant the responses could not be mapped as direct comparison in the results.
An invitation to participate was sent out to the charity’s database of people with Parkinson’s (consisting of 544 people who receive regular updates and 4,389 members of Parkinson’s Movement HealthUnlocked) along with most of the major UK speaking charitable organizations in the US and UK representing the interests of patients (namely in the US Michael J Fox Foundation, Parkinson’s Disease Foundation, Davis Phinney Foundation, Brian Grant Foundation, Northwest Parkinson’s Association and in the UK, Parkinson’s UK). The survey was also distributed to the Clinical Studies Group of the UK’s Dementias and Neurodegeneration network (DeNDRoN), representatives of the Parkinson Study Group in the US and those attending the Grand Challenges in Parkinson’s conference in Grand Rapids, Michigan, USA organized by the Van Andel Research Institute (VARI). Exact numbers invited are hard to estimate but likely exceed 10,000, placing response rates at 3% or lower.
Questions 1–4 offered responders a choice of answers from a list. Question 1 asked respondents to identify themselves according to their principal role (e.g. patient, care partner, neurologist, clinical researcher, scientist, PD specialist nurse, PD specialist physiotherapist). Question 2 was directed at those directly involved in running clinical trials and asked respondents to identify their top 5 barriers to successful clinical trials. Question 3 asked the same of people with Parkinson’s and their care partners. Question 4 asked respondents to identify the number of Parkinson’s-related clinical trials in which they had participated. Question 5 asked responders in which country they live. This was a free text response.
RESULTS
A total of 303 people connected with Parkinson’s completed the survey. Of the 274 respondents that identified themselves, 197 (72%) were people with Parkinson’s, 41 (15%) were care partners, and 31 (11%) identified themselves as clinical researchers, neurologists or scientists (Fig. 1).
Scientists and other health professionals cited funding as a principal barrier to effective Parkinson’s trials (66%). The next biggest problem reported was the lack of administrative support and time available to manage the trial (46%). The third most common barrier to conducting an effective clinical trial identified was recruitment of people with Parkinson’s to specific studies (43%). Other highly cited issues affecting the clinical trial process were matters such as the lack of practical support from other organizations involved in the trial, and the patient community’s perceptions of the need for the trial and the importance of the subject matter (Table 1).
For patients and care partners, the top five reasons for not participating or engaging in clinical trials were varied. More than 56% cited potential adverse consequences and potential side effects of taking part in clinical studies as the most concerning barrier. 54% worried about the possible disruption to their normal medication regimen. Other principal barriers to patient involvement were the prospect of receiving a placebo instead of the active drug (38%) and the upheaval and inconvenience to life that the trial would cause (37%). The fifth most common concern cited by this group, was fear of not being kept fully informed of both the pro-gress and results of the trial when appropriate (Table 2).
When asked to recall the number of Parkinson’s clinical trials in which the respondents had participated, the vast majority stated between 0 and 5 (83%), followed by 9% having taken part in 6 to 10 studies. 4% of those surveyed participated in 11 to 20 and a small number, 5% , had taken part in more than 20 clinical trials (Fig. 2) – this equates to 13 responders of whom 4 were clinicians, 5 people with Parkinson’s and 5 were unspecified so could have answered the question in error. This question did not ask whether these were drug studies or not. This result needs to be seen in the context of the population sampled. In many respects this is likely to have constituted a highly motivated cohort. Moreover, it is unlikely that all trials are large-scale drug trials. Many may be much simpler trials, often not involving medication.
DISCUSSION
Success or failure of clinical trials is an area in which the patient body and scientific community have significant personal and professional interest. The results of this survey were presented at a meeting called Rallying to the Challenge in September of 2014 which was organized by VARI in association with Parkinson’s Movement. This meeting was attended by 100 people with Parkinson’s predominantly from the US and was organized specifically to discuss how people living with Parkinson’s can be a valuable resource in clinical trials. The highlights of the questionnaire served as a focus of discussion for those advocates, researchers, patients and care partners in attendance in an effort to develop tangible suggestions and calls to action, keeping in mind the overarching objective to improve the effectiveness of clinical trials in Parkinson’s disease.
Of the total number of respondents, 285 people provided details of the country in which they live: 123 were based in the UK; 136 in the USA, 17 in Canada, 2 in Portugal and 1 each from The Netherlands, Germany, Italy, Mexico, Australia, New Zealand and Sweden. The survey did not ask from which source the responders had received the survey. Although the PwP responders are not fully representative of the wider Parkinson’s community, they are representative of a minority that are motivated by research matters, and therefore most likely to take part in clinical trials and this data provides a starting point to understanding the issues and barriers that are preventing PwP taking part in trials. 220 people responded to the survey within one month (3/7/14 to 3/8/14).
The findings identified the most significant obstacles to carrying out effective clinical trials for those involved in conducting research as being lack of funding and support. In contrast for those with Parkinson’s, the principal barriers to their participation in medical research were found to be fear of potential adverse consequences, interruption of their ongoing medical regimen and concern about receiving placebos. This is in keeping with the perceived psychosocial barriers to clinical trial participation in the field of oncology where fear of side effects has been shown to be the most significant barrier to clinical trial participation as well [10].
We feel that many of the principal barriers reported in this survey can be mitigated by the Parkinson’s community, as was discussed during Rallying to the Challenge. This may be achieved by focusing on the areas of communication, patient education, funding, recruitment and compliance throughout the clinical trial process. In fact, the importance of patient engagement in healthcare research has been shown previously to assist with increasing enrollment rates, securing funding for researchers, choosing study outcomes and designing research protocols [11].
However, there is no clear guidance regarding the degree of involvement or method by which to engage patients in clinical research to make a difference. It is also important that perfunctory involvement is avoided. These concerns were addressed in the conclusions which emerged from the Rallying to the Challenge meeting, and were presented to the parallel Grand Challenges in Parkinson’s scientific meeting being run by VARI at the same time.
It seems likely that the gap between the willingness of people living with Parkinson’s to participate in clinical trials and the reality of the shortfall in recruitment numbers could be closed if there was better understanding, information and communication between those conducting the trials and the participants. This was the conclusion of the participants at the Rallying to the Challenge meeting – and as a result, a Charter encapsulating education, understanding and communication concerns is being developed by PwP.
Much of the current divide between the Parkinson’s research and patient communities (and differing priorities as highlighted in the survey) is encapsulated by the problem of the lack of effective communication, an issue illustrated by other studies [12]. The development of a clinical trial training program that addresses misconceptions around the treatment development process, demonstrates best practice in patient engagement and identifies roles where those with Parkinson’s can become involved as partners in the process, could contribute significantly to engaging educated patients. To facilitate this education, there is a need to use existing resources and, where necessary, to develop new tools which together could demonstrate best practices in the global Parkinson’s clinical trial arena. The principles from which these best practices are derived, could then form the framework of a Clinical Trials Charter which could be disseminated to everyone involved in clinical trials.
It is anticipated that establishing a steering committee, composed of scientists and patients, to draft a Clinical Trials Charter and addressing the needs of both parties would help to create successful collaboration. This charter would set best practice guidelines for both patients and scientists before, during and after the trial. The charter would encourage researchers to outline the logistics of milestones and timeframes, bring transparency and clarity to the scope of trial objectives, and the need to reiterate a patient’s rights. For patients, the charter would highlight the key issues to consider when deciding whether or not to participate in a particular trial as well as directing them to further resources for use both during and after their trial experience.
It is also important to demonstrate to the wider Parkinson’s community that those with Parkinson’s can be a useful resource and have a role to play in accelerating clinical trials. The Parkinson’s community has value in identifying which trials are most relevant to them and in constructively engaging and contributing to the conduct of effective clinical trials; to learn from the experiences of current trial participants to help inform the conduct of future studies. Involvement of the patient community from the outset contributes to a culture of partnership and collaboration.
A clinical trial in Plymouth, to evaluate the potential of simvastatin as a treatment for Parkinson’s, jointly funded by The Cure Parkinson’s Trust, Peninsula University and the J P Moulton Trust, is also exploring and evaluating recruitment and retention strategies. The use of the Clinical Trials Charter in this trial will test its effectiveness as a tool for improved education and communication, and, in turn, better understanding, recruitment and retention i.e. employing the recommendations presented at Rallying to the Challenge. This will provide direct practical and experiential input into the process.
The issues of lack of funding and administrative support which continue to plague researchers, along with the delays caused by difficulties in patient recruitment, can also be addressed by involving the patient community. Partnering with patient organizations is, in fact, vital for the scientific community. These organizations can assist with funding, communication and promotion of research opportunities to the Parkinson’s community, and provide recruitment and practical assistance. The voice of Parkinson’s patients may be utilized to instill increased teamwork within the Parkinson’s community and encourage a culture of partnership between patients, patient organizations, scientists and industry.
CONCLUSION
The drug development process is a long and arduous one, with many perceived and real barriers adding to its complexity. For researchers, lack of funding and support are cited as major barriers whereas for patients, the concern over side effects and perceived potential disruption of their ongoing medical management were the most influential determinants. Many of these issues can be mitigated by involving the patient community in all areas of treatment discovery and development. Parkinson’s Movement seeks to develop and deliver stronger partnerships between the research and patient communities, and in so doing, expedite the search for better treatments and ultimately a cure.
ACKNOWLEDGMENTS
The document represents a consensus on the views expressed at the Rallying to the Challenge meeting. We thank the participants at that meeting for their contribution. We also thank Jean Burns (Parkinson’s Plan for Life), Helen Matthews (The Cure Parkinson’s Trust) and Leah Mursaleen (The Cure Parkinson’s Trust) for input, logistical support and critical review.
The authors confirm that no financial or material support was provided in the production of this paper and there are no commercial or other conflicts of interest in connections with its submission.
REFERENCES
1 | Corr P, Williams D (2009) The pathway from idea to regulatory approval: Examples for drug development Conflict of Interest in Medical Research, Education, and Practice National Academies (US) Washington |
2 | DiMassi JA (2014) Cost to develop and win marketing approval for a new drug is $2.6 billion. PR Tufts CSDD 2014 Cost Study. Tufts Center for the Study of Drug Development http://csdd.tufts.edu/news/complete_story/pr_tufts_csdd_2014_cost_study |
3 | Adams CA, Brantner VV (2006) Estimating the cost of new drug development: Is it really $802 million? Health Aff (Millwood) 25: 420 428 |
4 | Roy ASA (2012) Stifling New Cures: The True Cost of Lengthy Clinical Drug Trials. Rep. no. 5. Manhattan Institute for Policy Research, http://www.manhattan-institute.org/html/fda_05.htm |
5 | Getz K (2009) Current investigator landscape poses growing challenges for sponsors, Impact Report Tufts Center for the Study of Drug Development 11: 1 (2009) |
6 | Miseta E (2013) Bring down the cost of clinical trials with improved site selection. Clinical Leader, Dec. 19, 2013 http://www.clinicalleader.com/doc/bring-down-the-cost-of-clinical-trials-with-improved-site-selection-0001 |
7 | Stephenson H, Bhamal M (2010) Applied Clinical Trials Online. 2010 Sep 1. Direct-to-Patient Enrollment Strategies: A Comon of the Yields and Costs of Online Outreach Methods to Other Recruitment Techniques, http://www.appliedclinicaltrialsonline.com/appliedclinicaltrials/CRO%2FSponsor/Direct-to-Patient-Enrollment-Strategies/ArticleStandard/Article/detail/686202 paris |
8 | National Poll: Clinical Research. National Poll. Research America, USA, 2013 |
9 | Meunier CC, Chowdhury S, Cappelletti LW, Sherer TB (2014) Accelerating drug development for the field: Building clinical trial recruitment infrastructure in Parkinson’s Clin Res (Alex) 22 27 |
10 | Meropol NJ (2007) Barriers to clinical trial participation as perceived by oncologists and patients J Nat Comp Cancer Network 5: 655 664 |
11 | Domecq J, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee M, Brito J, Boehmer K, Hasan R, Firwana B, Erwin P, Eton D, Sloan J, Montori V, Asi N, Dabrh AM, Murad M (2014) Patient engagement in research: A systematic review BMC Health Serv Re 14: 89 |
12 | Sood A, Prasad K, Chhatwani L, Shinozaki E, Cha SS, Loehrer LL, Wahner-Roedler DL (2009) Patients’ attitudes and preferences about participation and recruitment strategies in clinical trials Mayo Clinic Proc 8: 243 247 |
Figures and Tables
Fig.1
Breakdown of survey respondents who identified their primary role (274 of 303).
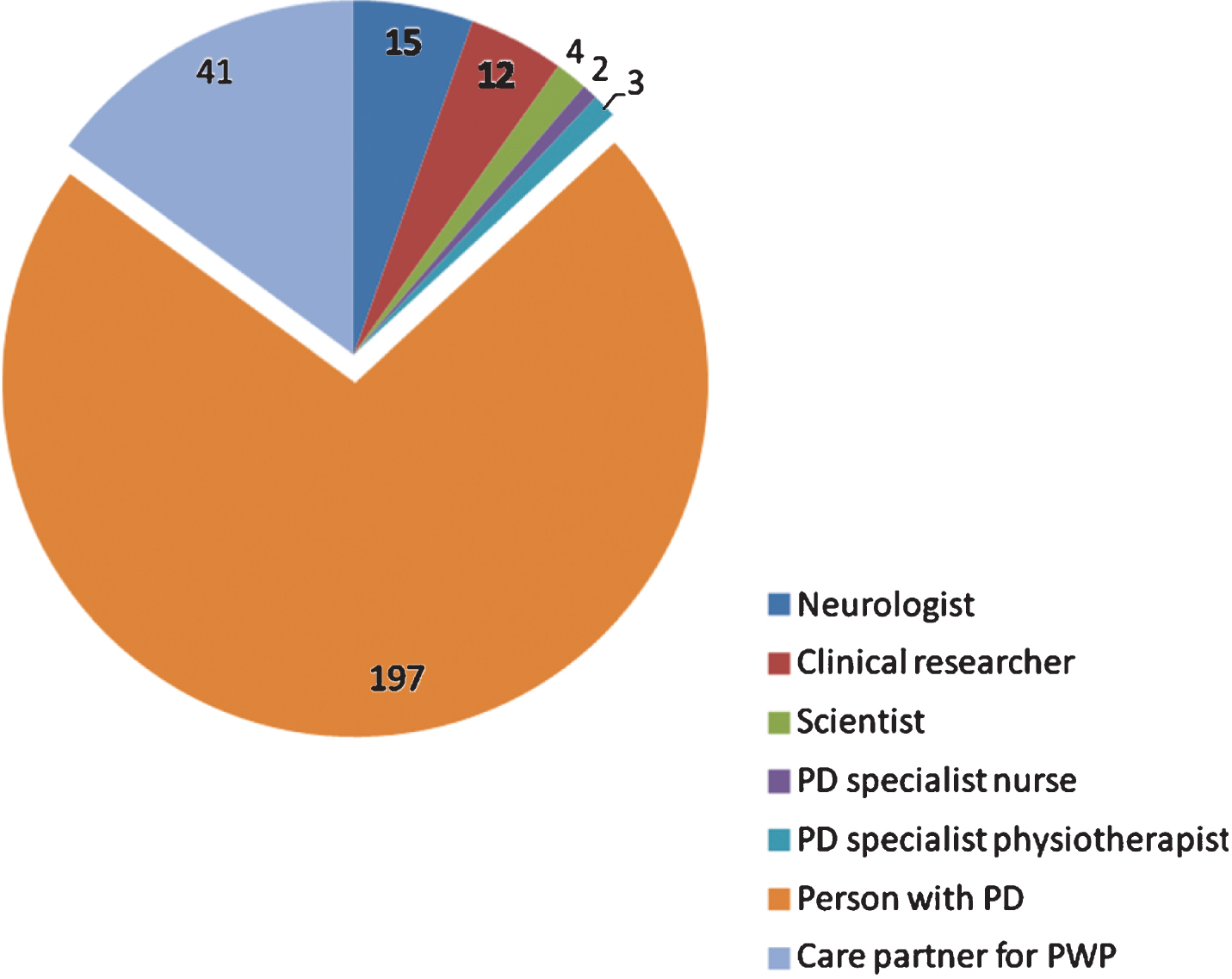
Fig.2
Number of PD clinical trials joined by survey respondents.
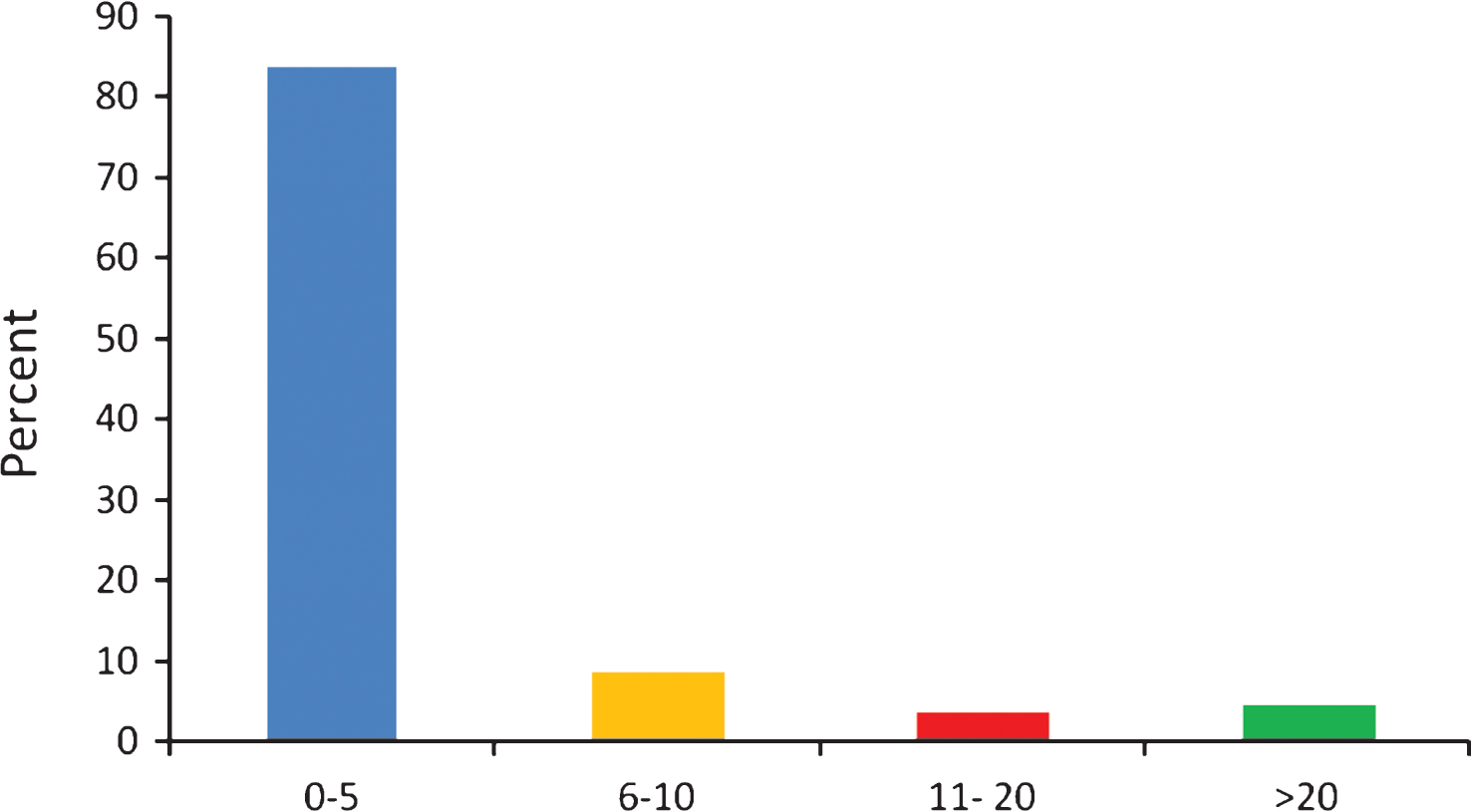
Table 1
Barriers to effective clinical trials *, as perceived by 61 respondents involved in conducting clinical trials
| Barrier | % |
| Funding | 66 |
| Administrative support/time available to manage trial | 46 |
| Recruitment of PwP to trials | 43 |
| Practical support from other organisations involved in the trial e.g. commercial partners, public sector, funders, advisory bodies, patient organisations | 36 |
| Subject matter of the trial and perceptions of need for the trial from patient community | 34 |
| Institutional contracting (time taken) | 33 |
| Problems with heterogeneous nature of Parkinson’s (recruitment/methodology/results of trial) | 28 |
| Patient inertia/ lack of motivation and engagement | 26 |
| Problems associated with outcome measures | 25 |
| Ethics approval (time taken) | 23 |
| Communication issues with involved parties ie. funding bodies, academic institutions, trial participants. | 23 |
| Problems communicating the importance of the trial or general promotion | 16 |
| Placebo effect | 10 |
| Supply of drug/placebo | 10 |
| Gaining consensus on trial design | 8 |
| Inability to publish results | 7 |
| Compliance of PwP to trial protocol | 5 |
| Data collection | 5 |
*61 respondents, self-identified as involved in conducting clinical trials, were asked to pick the 5 most important from the above list of barriers to effective clinical trials.
Table 2
Barriers to effective clinical trials *, as perceived by 240 respondents who were PWPs or care partners
| Barrier | % |
| The potentially adverse consequences/side effects of taking part in clinical trials | 56 |
| The disruption to my current medication | 53 |
| I may be given the placebo and not the real drug | 38 |
| The upheaval to my life that the trial would cause | 37 |
| Being kept fully informed of both the progress and results of the trial when appropriate | 34 |
| Being on one trial may exclude my involvement in other future trials | 32 |
| I may not be able to reclaim all the costs incurred through participating in a trial | 30 |
| Access to understandable information about what a trial involves | 28 |
| I am only interested in trials that seek to delay, stop or reverse the condition permanently | 25 |
| The current system of measuring Parkinson’s does not reflect the overall state of my wellbeing | 24 |
| Clarity on my legal status should something go wrong, eg compensation etc | 23 |
| The effect that participation in a trial will have on my family | 21 |
| The risks of going on a trial outweigh the benefits | 16 |
| The privacy of my medical information | 8 |
| I have had (or have heard of) some bad experiences in previous clinical trials | 6 |
*240 respondents, self-identified as PWPs or care partners, were asked to pick the 5 most important from the above list of barriers to effective clinical trials.




