Clinical Parameters and Tools for Home-Based Assessment of Parkinson’s Disease: Results from a Delphi study
Abstract
Background:
Parkinson’s disease (PD) is a neurodegenerative disorder with fluctuating symptoms. To aid the development of a system to evaluate people with PD (PwP) at home (SENSE-PARK system) there was a need to define parameters and tools to be applied in the assessment of 6 domains: gait, bradykinesia/hypokinesia, tremor, sleep, balance and cognition.
Objective:
To identify relevant parameters and assessment tools of the 6 domains, from the perspective of PwP, caregivers and movement disorders specialists.
Methods:
A 2-round Delphi study was conducted to select a core of parameters and assessment tools to be applied. This process included PwP, caregivers and movement disorders specialists.
Results:
Two hundred and thirty-three PwP, caregivers and physicians completed the first round questionnaire, and 50 the second. Results allowed the identification of parameters and assessment tools to be added to the SENSE-PARK system. The most consensual parameters were: Falls and Near Falls; Capability to Perform Activities of Daily Living; Interference with Activities of Daily Living; Capability to Process Tasks; and Capability to Recall and Retrieve Information. The most cited assessment strategies included Walkers; the Evaluation of Performance Doing Fine Motor Movements; Capability to Eat; Assessment of Sleep Quality; Identification of Circumstances and Triggers for Loose of Balance and Memory Assessment.
Conclusions:
An agreed set of measuring parameters, tests, tools and devices was achieved to be part of a system to evaluate PwP at home. A pattern of different perspectives was identified for each stakeholder.
INTRODUCTION
Clinical visits provide only a brief snapshot of the health condition of Parkinson’s disease (PD) patients. Actually, PD is notorious for its variations in the severity of symptoms, which may occur both within and across days [1]. Moreover, performance during the clinical visit does not always reflect how people with PD (PwP) are at home. Freezing of gait is a classic example of a PD symptom, which is often hard to assess in the examination room as well as the early morning dystonia and nighttime disability [2, 3]. This argues for home-based assessment of PD-associated symptoms, to give both to doctor and PwP an as objective as possible feedback about the real situation of the person suffering from this chronic condition.
Moreover, as medical care models become more patient-centric, patients’ active participation is essential to meet their needs and expectations [4]. It is conceivable that an adequate feedback provided to the user from such a home-based evaluation system may increase self-awareness, and, consequently, self-engagement with positive effects on disease management, and health-related quality of life [5].
In the context of the development of a home-based evaluation system for PwP, which has been created by a consortium (www.sense-park.eu), there was a need to define clinical parameters and (preferably wearable) tools to be applied in the assessment of 6 previously defined clinical domains: gait, bradykinesia, tremor, sleep, balance and cognition. These domains were identified and prioritized by PwP. For this purpose, we conducted a Delphi study to identify relevant parameters and assessment tools, from the perspective of PwPs caregivers and movement disorders specialists [6].
MATERIALS AND METHODS
The e-Delphi was conducted using an online, web-based survey called Survey Monkey ®. This software builds a demographic profile of panelists, present each round of the Delphi and tracks responses. Participants received an e-mail giving them access to the website, a description of the study and a link to the first round of the Delphi. E-mail was also used to alert participants to the second round and to remind them to complete questionnaires. Each round remained open for 3 weeks.
Phase 1
In the first round of the Delphi survey, PwP, caregivers and clinicians with experience in PD were invited to complete a questionnaire (see supplement) aimed to identify parameters of interest and useful tests, tools or devices to evaluate PwP at home. Open questions were asked to identify a list of parameters and tools that are relevant for patients’ assessment, from the perspective of the participant. Questionnaires were designed to obtain the same kind of information between groups but they were adapted according to the group they were intended to.
Participants were invited through e-mail. A total of 3290 members of the contact list of a UK and Portuguese Parkinson’s patient association were invited to the first round of the study. Clinicians were purposively selected among the researcher’s professional networks of practice. PwP and caregivers were recruited through Portuguese and British patients associations’ mailing lists.
Demographic data including gender, age and living country was collected from the panel. Movement disorders specialists were also asked about the number of years of experience with PD.
Responses were therefore analyzed qualitatively. The answers were grouped into categories generating a rank-ordered list of items. Two reviewers (JJF and ATS) performed this categorization process by removing irrelevant, overlapping and repeated contents, looking for common viewpoints, identifying responses that were open to interpretation and making categorization of responses more accurate by discussing the disagreements. Suggestions that did not present an assessment idea or fit better in another domain were excluded. However, those suggestions that could be considered parameters but were mentioned when asked for assessment tools, tests and devices (or the opposite) were retained. Based on this list, closed questions were developed for the second-round questionnaire.
Most frequent responses were carried forward to phase 2. By censoring in this way, we reduced the number of items on the phase 2 questionnaire, without writing off suggestions of potential importance. The criterion for consensus was set after Round 1 so that the attrition rate and the panel heterogeneity could be considered.
All the parameters and tools listed in phase 2 questionnaire were considered important. By asking participants to select their top 3, we identified those of particular relevance.
Phase 2
Only those who participated in phase 1 and were willing to be contacted for the second round were invited to do so. To determine the relative importance of each parameter and tool, participants were asked to rate the relevance of each suggestion using a five point Likert type scale where 1 meant “not at allimportant” and 5 “very important” as answer to the question: “How important, in your opinion, are the following parameters to assess [each of the 6 domains of interest]? Please score on a scale of 1–5 the items below. This list has been created following participants’ suggestions in the first round.” They were also asked to pick the three parameters and tools, devices or tests they felt were the most useful ones to be used. In order to ensure that important points were not missed, participants were asked to suggest any unlisted ones that they would have selected in their top 3. Each suggestion was ranked according to the proportion of participants who selected it in their top 3 and an average of the scores given to each one was alsodetermined.
Reminders were needed to increase response rate.
RESULTS
At the end of the first round, we received 233 completed questionnaires: 12 from clinicians, 67 from British PwP, 14 from British caregivers or relatives and 82 and 58 from Portuguese PwP and caregivers, respectively. From those who participated in the first round, 93 were willing to be contacted again for the second round: 9 clinicians, 28 British and 30 Portuguese patients and 6 British and 20 Portuguese caregivers. Of those, 50 participants completed the second round. Figure 1 gives an overview of this procedure.
More females (N = 28) than males (N = 22) participated (p = 50). Mean age of the participants was 57.8 years. The clinicians included referred an average of 11.5 years of experience with PD.
The parameters considered most important by clinicians, patients and caregivers to monitor are listed in Table 1. Most attractive / useful tools are listed in Table 2.
Gait
Regarding parameters, the most important ones that should be evaluated with a home-based quantitative system were found to be frequency of falls and near falls (67%) and frequency of the freezing of gait episodes (59%). The most preferred tool was U-Step Walker (76%).
Bradykinesia
To monitor bradykinesia, the parameters considered most important for evaluation in a home-based setting were Capability to do activities of Daily Living (91%), Capability to perform fine motor movements (85%) and Easiness to move from sit to stand positions (70%). The most frequently cited tool was To evaluate the performance doing fine motor movements (62%).
Tremor
The most cited parameter was Interference with Activities of Daily Living (77%). The test situationconsidered most useful to assess tremor was Capability to eat (84%).
Sway
Most cited parameters were Frequency of falls and near falls (81%) and Circumstances and triggers that induce falls and near falls. Identify the circumstances and triggers for loose of balance (98%), U-Step walker (86%) and Need of assistive device usage or another person’s assistance support (81%) were considered the best way to assess this domain.
Sleep
Total Sleep Time was the most frequently mentioned parameter that should be assessed with a home-based quantitative device. Tests considered the most useful were Assessment of sleep quality (93%) and Measure the total sleep time (91%).
Cognition
In cognition, both Capacity to process tasks (98%) and Capability to recall and retrieve information (90%) were highly scored as parameters of interest. Memory assessment tools (66%) were judged as the most relevant devices for this domain.
Main findings regarding parameters
At the end of the first round, we noticed some parameters have been mentioned simultaneously as useful to assess two domains. One example was the Frequency of Falls and Near Falls which not only has been mentioned simultaneously for Gait and Sway but also has become, in the second round, the most important parameter for both.
Although the global results reflected the opinion of the 5 interviewed groups, we found some discrepancies between the relevance given to each parameter from each group. For instance, when discussing bradykinesia, both British and Portuguese patients and caregivers scored the Capability to Perform Activities of Daily Living as the most important parameter to be considered unlike the clinician’s opinion who referred the Time Consumed for Doing Specific Tasks as the most useful one. The same occurred in the Gait and Sway domains. British and Portuguese PwP, respectively, expressed different opinions from the othergroups.
In Tremor and Cognition domains, all groups agreed about the most interesting parameter to be assessed by the patients at home.
For the Sleep domain, there was no consensus between groups. Clinicians considered 4 out of the 6 parameters equally important, British caregivers chose the two parameters discarded by the clinicians and the other groups selected different parameters too. This domain reflected the different perspectives of what are the most useful and important parameters.
It was also evident that in Gait, Sleep and Tremor domains, the scores are very homogeneous. For Bradykinesia, Sway and Cognition, some parameters clearly highlighted among the others.
Main findings regarding tools, tests and devices
The determination of the most important tests, tools or devices to be used at home was not as unanimous as the determination of interesting parameters. Only in the Sway domain we had agreement between all groups in selecting Identification the Circumstances and Triggers for Loose of Balance as the most useful test to be used at home.
For Tremor and Sleep domains, patients and caregivers agreed with the globally most chosen test. However, in both domains, clinicians had another point of view. Capability to Eat and Assessment of Sleep Quality were the most rated tests for Tremor and Sleep domains, respectively. Clinicians were unanimous in these different choices.
For the Cognition domain, British patients and all caregivers were in line with the globally most cited test although the clinicians preferred MoCA and SCOPA assessments and 90% of the Portuguese PwP considered Solving Mind Games as the most usefulone.
In Gait and Bradykinesia domains, all caregivers and Portuguese patients agreed with the global opinion. All clinicians preferred the Accelerometer to assess Gait and to monitor Bradykinesia, the Tapping Test and the Cronometration of Doing Specific Tasks. In turn, 89% and 83% of British patients chose Video-Based Observation to assess Gait and Bradykinesia, respectively.
Generally, the scores of each test, tool and device was very conclusive about their importance to assess patients. For each domain, there was always one that stood out from others.
DISCUSSION
In this study, we have conducted a method involving patients, caregivers and clinicians in the process of identifying relevant parameters in PwP and useful tests, tools or devices to be used by the patients to assess themselves at home.
The Delphi Method is a structured communication technique used to obtain the most reliable consensus of opinion about a domain from experts in the field by a series of intensive questionnaires [7]. The process begins with an initial questionnaire (round one), which acts as an idea-generation strategy to uncover the issues pertaining to the topic under study [8]. The response data are summarized and a new questionnaire is designed based solely on the results obtained from the first application. Repeated rounds of this process are carried out to achieve consensus or until the “law of diminishing returns” occurs [7, 9].
One of its key advantages is that participants in a Delphi study do not interact directly with each other, so situations where the group is dominated by the views of certain individuals can be avoided. Invitations were sent by email to each respondent to ensure privacy and freedom to consider responses and modify them without any pressure. Only the person coordinating the exercise knew the identities of respondents, essential since an individual’s previous ratings had to be returned for consideration in a subsequent round.
E-Delphi has additional benefits on cost effectiveness compared with committee meetings or personal interviews and quick feedback of responses to panelists over multiple rounds [10]. We used this instrument to assess preferences of a team of specialists in the field of PD (particularly including PwP, but also caregivers and movement disorders specialists experienced with PD treatment) with regard to parameters to assess, and tools to use within 6 predefined domains (i.e. symptoms) that occur regularly in PD, and should be assessed with the SENSE-PARK device.
The Delphi process has been widely used in health research particularly in indicators development and core sets of outcomes definition. Several studies developed core outcome sets based on patients and clinicians perspectives [11–13].
It is suggested that PwP, relatives and caregivers who take care of PwP and clinicians with long experience in PD have different perspectives of the most important variables to be assessed and used when monitoring patients at home. For this, the Delphi technique revealed to be a reliable collection methodology gathering real-world knowledge. With this exercise, it was possible to bond the vision of top management decision makers who will translate the data obtained with the parameters and tests of the Delphi study, the perspective of those who will use the tests and measure the parameters, and the point of view of professional staff and family members who are close enough to support patients in their home environments
This study has certain limitations and inherent selection bias, including the fact that panel clinicians were selected by the researchers. In addition, we sampled participants from Portugal and UK, so if this study was replicated in other countries, some other parameters and tools would have been considered of special importance.
Despite of good agreement between reviewers, some responses in phase 1 were open to interpretation. One example is related to walkers used in gait. Although some participants, especially PwP and caregivers, specified the U-Step walker, others did not. A pragmatic decision was made to reduce the several detailed walkers described. If our assumption had been false, the number of participants suggesting “walkers” could have been underestimated in phase 1. Ultimately, our results appears to be robust to this decision, because walkers were included in phase 2 questionnaire and were considered the most important tool to be used regarding gait assessment.
Our sample of clinicians only represented 8% of people who answered the two rounds performed. Patients were the largest group representing 68% of our sample, 38% British and 30% Portuguese. Portuguese caregivers totalized 20% but the English ones were even smaller than the clinicians group, only 4% of the global sample.
ACKNOWLEDGMENTS INCLUDING SOURCES OF SUPPORT
This study was performed in the frame of the EU project SENSE-PARK, funded under the Seventh Framework Programme, Cooperation –ICT, Grant Agreement no. 288557.
CONFLICT OF INTERESTS
Joaquim J. Ferreira has held consultancy functions with GlaxoSmithKline, Novartis, TEVA, Lundbeck, Solvay, Abbott, BIAL, Merck-Serono, Merz, Ipsen; has received grants from GlaxoSmithKline, Grunenthal, Fundação MSD (Portugal) and Teva; has been a member of the European Huntington Disease Network and has been employed by Centro Hospitalar Lisboa Norte, Faculdade de Medicina de Lisboa. Helen Matthews has been employed by The Cure Parkinson’s Trust. Walter Maetzler has been employed by the University of Tübingen; and has received speaker honoraria from UCB, GlaxoSmithKline and Rölke Pharma.
Appendices
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-140493.
REFERENCES
1 | Patel S, Lorincz K, Hughes R, Huggins N, Growdon J, Standaert D, Akay M, Dy J, Welsh M, Bonato P (2009) Monitoring motor fluctuations in patients with Parkinson’s disease using wearable sensors IEEE Trans Inf Technol Biomed 13: 864 873 |
2 | Louter M, Munneke M, Bloem BR, Overeem S (2012) Nocturnal hypokinesia and sleep quality in Parkinson’s disease J Am Geriatr Soc 60: 1104 1108 |
3 | Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR (2012) Freezer or non-freezer: Clinical assessment of freezing of gait Parkinsonism Relat Disord 18: 149 154 |
4 | Kleiner-Fisman G, Gryfe P, Naglie G (2013) A patient-based needs assessment for living well with Parkinson disease: Implementation via nominal group technique Parkinsons Di 2013: 6 |
5 | Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR (2013) Quantitative wearable sensors for objective assessment of Parkinson’s disease Mov Disord 28: 1628 1637 |
6 | Maetzler W, Liepelt I, Berg D (2009) Progression of Parkinson’s disease in the clinical phase: Potential markers Lancet Neurol 8: 1158 1171 |
7 | McKenna HP (1994) The Delphi technique: A worthwhile research approach for nursing? J Adv Nurs 19: 1221 1225 |
8 | Keeney S, Hasson F, McKenna HP (2001) A critical review of the Delphi technique as a research methodology for nursing Int J Nurs Stud 38: 195 200 |
9 | Keeney S, Hasson F, McKenna H (2006) Consulting the oracle: Ten lessons from using the Delphi technique in nursing research J Adv Nurs 53: 205 212 |
10 | Gephart SM, Effken JA, McGrath JM, Reed PG (2013) Expert consensus building using e-Delphi for necrotizing enterocolitis risk assessment J Obstet Gynecol Neonatal Nurs 42: 332 347 |
11 | Devane D, Begley CM, Clarke M, Horey D, COB (2007) Evaluating maternity care: A core set of outcome measures Birth 34: 164 172 |
12 | Mease PJ, Arnold LM, Crofford LJ, Williams DA, Russell IJ, Humphrey L, Abetz L, Martin SA (2008) Identifying the clinical domains of fibromyalgia: Contributions from clinician and patient Delphi exercises Arthritis Rheum 59: 952 960 |
13 | Zochling J, Sieper J, van der Heijde D, Braun J (2008) Development of a core set of domains for data collection in cohorts of patients with ankylosing spondylitis receiving anti-tumor necrosis factor-alpha therapy J Rheumatol 35: 1079 1082 |
Figures and Tables
Fig.1
Study flowchart showing participants in each phase of the study.
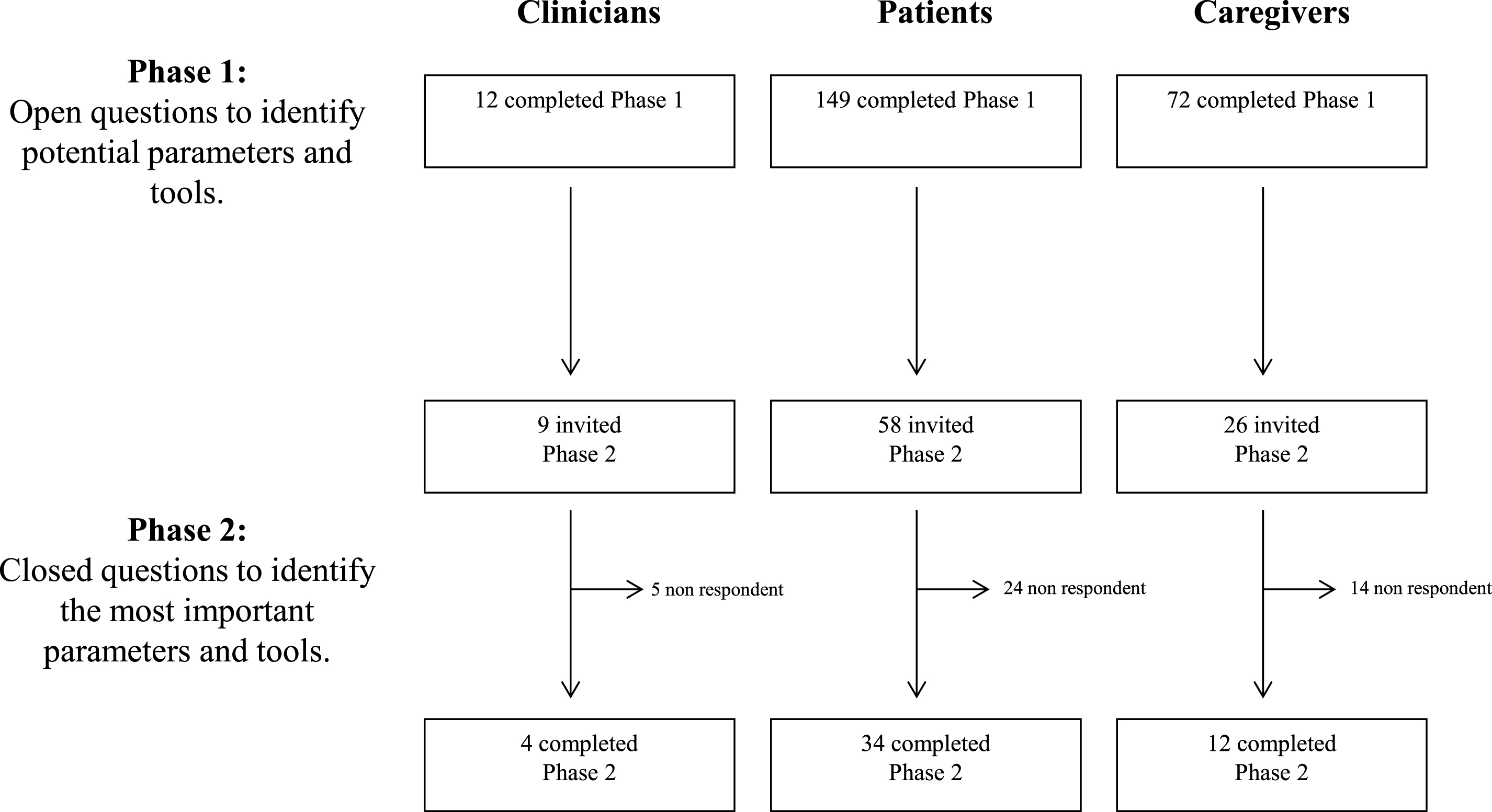
Table 1
The importance of each parameter in phase 1 questionnaire, as scored by people with Parkinson’s Disease, caregivers and clinicians
| Gait | Bradykinesia | multicolumn 2cTremor | Sleep | Sway | Cognition | ||||||
| 9.85% | Frequency of falls and near falls | 22.45% | Capability to do activities of daily living | 12.50% | Interference with Activities of Daily Living | 28.87% | Total sleep time | 26.32% | Frequency of falls and near falls | 39.51% | Capacity to process tasks |
| 21.97% | Frequency of the Freezing of Gait episodes | 12.24% | Capability to perform fine motor movements | 34.09% | Capability to perform fine motor movements | 24.74% | Occurence of midsleep awakenings | 23.68% | Circumstances and triggers (ex. dual task, stress, crowds...) that induce falls and near falls | 12.35% | Capability to recall and retrieve information |
| 11.36% | Need of assistive device usage or another person's assistance support | 8.16% | Easiness to move from sit to stand positions | 9.09% | Activities that aggravate tremor | 43.30% | Frequency of insomnia episodes | 13.16% | Gait abnor malities | 14.81% | Attention |
| 10.61% | Ocurrence of shuffling steps | 6.12% | Time consumed for doing specific tasks | 12.50% | Duration of periods of tremor | 11.34% | Daytime sleepiness | 7.89% | Need of assistive device usage / another person's assistance support | 49.38% | Memory test results |
| 22.73% | Occurrence of difficulties in the first steps of gait initiation | 7.95% | Occurrence of social embarrassment | 11.34% | Occurrence of dream enacting behaviors | 10.53% | Amplitude of sway | ||||
| 8.33% | Patient’s speed of gait | 18.18% | Amplitude of tremor | 12.37% | Time to sleep onset | ||||||
| 23.48% | Patient’s stride length |
Table 2
Demographic, cognitive, and language comparisons
| Gait | Average evaluation | Scoring in top 3 | Bradykinesia | Average evaluation | Scoring in top 3 | Tremor | Average evaluation | Scoring in top 3 | Sleep | Average evaluation | Scoring in top 3 | Sway | Average evaluation | Scoring in top 3 | Cognition | Average evaluation | Scoring in top 3 |
| Frequency of falls and near falls | 4.29 | 67% | Capability to do activities of daily living | 4.66 | 91% | Interference with Activities of Daily Living | 4.61 | 77% | Total sleep time | 4.43 | 66% | Frequency of falls and near falls | 4.62 | 81% | Capacity to process tasks | 4.66 | 98% |
| Frequency of the Freezing of Gait episodes | 4.33 | 59% | Capability to perform fine motor movements | 4.4 | 85% | Capability to perform fine motor movements | 4.52 | 55% | Occurence of midsleep awakenings | 4.34 | 61% | Circumstances and triggers (ex. dual task, stress, crowds...) that induce falls and near falls | 4.45 | 76% | Capability to recall and retrieve information | 4.63 | 90% |
| Need of assistive device usage or another person's assistance support | 4.10 | 43% | Easiness to move from sit to stand positions | 4.23 | 70% | Activities that aggravate tremor | 4.23 | 50% | Frequency of insomnia episodes | 4.23 | 59% | Gait abnorm alities | 4.31 | 60% | Attention | 4.46 | 63% |
| Ocurrence of shuffling steps | 4.00 | 41% | Time consumed for doing specific tasks | 4.32 | 53% | Duration of periods of tremor | 4.20 | 43% | Daytime sleepiness | 4.30 | 55% | Need of assistive device usage / another person's assistance support | 4.26 | 57% | Memory test results | 4.46 | 49% |
| Occurrence of difficulties in the first steps of gait initiation | 4.10 | 37% | Occurrence of social embarrassment | 4.11 | 39% | Occurrence of dream enacting behaviors | 4.23 | 43% | Amplitude of sway | 4.1 | 26% | ||||||
| Patient’s speed of gait | 3.96 | 33% | Amplitude of tremor | 4.09 | 36% | Time to sleep onset | 3.93 | 16% | |||||||||
| Patient’s stride length | 3.73 | 20% |
Table 3
The importance of each test, tool and device in phase 1 questionnaire, as scored by people with Parkinson’s Disease, caregivers and clinicians
| Gait | Bradykinesia | Tremor | Sleep | Sway | Cognition | ||||||
| 8.96% | U-Step Walker | 5.26% | Evaluate performance doing fine motor movements | 7.89% | Capability to eat | 7.89% | Assessment of sleep quality | 11.11% | Identify circumstances and triggers for loose of balance | 18.37% | Memory assessment |
| 4.48% | Vídeo-based observation | 10.53% | Vídeo-based observation | 7.89% | Capability to hold and use tools | 10.53% | Measure the total sleep time | 8.33% | U-Step walker (a Walking Stabilizer designed for Parkinson’s Disease patients) | 14.29% | Solve mind games |
| 10.45% | Mobilaser | 5.26% | Evaluate amplitude of movements | 21.05% | Capability to write | 15.79% | Perform a polyssomnography study | 16.67% | Need of assistive device usage or another person's assistance support | 38.78% | Cognitive scales assessments |
| 5.97% | Accelerometer | 5.26% | Evaluation of writing skills | 7.89% | Measure duration of periods of tremor | 36.84% | Use of a sleep diary | 19.44% | Wii Fit balance board | 10.20% | Reading evaluation |
| 7.46% | Pedometer | 10.53% | Wii Fit balance board | 7.89% | Vídeo-based observation | 2.04% | SCOPA (SCales for Outcomes in Parkinson’s disease) | ||||
| 7.89% | Tapping test | 10.53% | Measure amplitude of tremor | 16.33% | Complete puzzles | ||||||
| 21.05% | Cronometration of doing specific tasks | 32% | 2.04% | MoCA (The Montreal Cognitive Assessment) | |||||||
Table 4
The importance of each test, tool and device in phase 2 questionnaire, as scored by people with Parkinson’s Disease, caregivers and clinicians
| Gait | Average evaluation | Scoring in top 3 | Bradykinesia | Average evaluation | Scoring in top 3 | Tremor | Average evaluation | Scoring in top 3 | Sleep | Average Evaluation | Scoring in top 3 | Sway | Average Evaluation | Scoring in top 3 | Cognition | Average Evaluation | Scoring in top 3 |
| U-Step Walker | 3.65 | 76% | Evaluate performance doing fine motor movements | 4.02 | 62% | Capability to eat | 4.66 | 84% | Assessment of sleep quality | 4.42 | 93% | Identify circumstances and triggers for loose of balance | 4.64 | 98% | Memory assessment | 4.31 | 66% |
| Vídeo-based observation | 3.68 | 68% | Vídeo-based observation | 3.91 | 51% | Capability to hold and use tools | 4.44 | 64% | Measure the total sleep time | 4.16 | 91% | U-Step walker (a Walking Stabilizer designed for Parkinson’s Disease patients) | 4.10 | 86% | Solve mind games | 4.13 | 54% |
| Mobilaser | 3.24 | 58% | Evaluate amplitude of movements | 3.80 | 49% | Capability to write | 4.32 | 50% | Perform a polyssomnography study | 3.95 | 59% | Need of assistive device usage or another person’s assistance support | 4.02 | 81% | Cognitive scales assessments | 3.95 | 44% |
| Accelerometer | 3.24 | 50% | Evaluation of writing skills | 3.91 | 43% | Measure duration of periods of tremor | 3.95 | 39% | Use of a sleep diary | 3.91 | 57% | Wii Fit balance board | 3.78 | 36% | Reading evaluation | 4.10 | 41% |
| Pedometer | 3.10 | 42% | Wii Fit balance board | 3.60 | 32% | Vídeo-based observation | 3.98 | 34% | SCOPA (SCales for Outcomes in Parkinson’s disease) | 3.78 | 39% | ||||||
| Tapping test | 3.55 | 32% | Measure amplitude of tremor | 3.82 | 30% | Complete puzzles | 4.00 | 29% | |||||||||
| Cronometration of doing specific tasks | 3.43 | 32% | MoCA (The Montreal Cognitive Assessment) | 3.78 | 27% |




