Patterns of Tau and α-Synuclein Pathology in the Visual System
Abstract
Background:
Spreading of misfolded proteins has been suggested for neurodegenerative diseases. The hierarchical distribution of protein deposits in Alzheimer‘s (AD) and Parkinson’s disease (PD) supports this concept.
Objectives:
To evaluate α-synuclein and tau-deposition in the optic pathway as an excellent anatomical model, which follows a strict trajectory including a cortico-geniculate feedback connection.
Methods:
We immunostained the optic nerve, lateral geniculate nucleus (LGN), and occipital cortex for AT8 (phosphorylated tau), α-synuclein, and disease-associated prion protein (PrP) in 47 cases with tau pathology (AD type, argyrophilic grain disease, or progressive supranuclear palsy), 16 PD, and 5 Creutzfeldt-Jakob disease (CJD) cases, respectively.
Results:
We detected immunoreactivity for all proteins along the optic pathway. The optic nerve showed immunopositivity only in cases with tau (6/8, 75%) or α-synuclein (5/7, 71%) pathology. The LGN was involved also frequently (tau: 22/47, 46.8% ; α-synuclein: 15/16, 93.7% ; PrP 5/5, 100%). The occipital cortex was variably affected by tau or α-synuclein pathology, but always showed PrP immunoreactivity in the CJD cases. Tau pathology in the LGN correlated with tau immunoreactivity in the occipital cortex and Braak stages of neurofibrillary degeneration. In tauopathies, which do not involve the occipital cortex, like argyrophilic grain disease or progressive supranuclear palsy, tau pathology was more frequently astrocytic in the LGN.
Conclusions:
Our results have implications 1) for the understanding of disease spreading along neural pathways and 2) for the diagnostic evaluation of the visual system in neurodegenerative proteinopathies as a potential biomarker to evaluate disease progression or subgrouping of cases.
INTRODUCTION
Neurodegenerative diseases (NDDs) are characterized by loss of nerve cells and accumulation of misfolded proteins. Due to various causes, the physiological conformation changes from an α-helix to β-sheet structure, leading to protein misfolding and neuronal degeneration [1]. Prion diseases or transmissible spongiform encephalopathies are the model disorders associated with a misfolded protein. Here the autocatalytic conversion of normal cellular prion protein (PrPc) to a pathological conformer (PrPSc) associates with a rapid progressive lethal disease course [2]. The microtubule-associated protein tau is aggregated in an abnormally phosphorylated form in many NDDs, like Alzheimer’s disease (AD), argyrophilic grain disease (AGD), progressive supranuclear palsy (PSP), Pick’s disease, corticobasal degeneration (CBD) and globular glial tauopathies [3]. Tau plays an important role in axonal transport, and its aggregates can be found in nerve cells and neuritic processes, but in some cases also in glia cells [3, 4]. Another key protein involved in NDDs is α-synuclein, which is found as filamentous cytoplasmic glial inclusions in multiple system atrophy (MSA) and in the nerve cell soma and processes in Parkinson’s Disease (PD) and dementia with Lewy bodies (DLB) [5, 6].
According to the prion theory, the disease-associated PrPSc changes the physiological PrPc molecules. Following internalization of PrPSc in the neurons via the endosomal-lysosomal system prions can spread cell-to-cell. Spreading of the disease-associated PrP conformer is thought to follow neural pathways. Recent experimental studies and observations in humans (e.g. grafted neurons in PD) have shown similarities in spreading, including cell-to-cell transmission, and self-perpetuation of other NDD-related proteins [7–17]. The observation of a prion-like internalization process of disease-associated α-synuclein in the human brain supported this notion [18]. Indeed, the hierarchical involvement of different anatomical regions described by Braak et al. for tau in AD-related changes [19] as well as for α-synuclein pathology in PD [20], already suggested that proteins disseminate through anatomical pathways during the disease course. A recent study of Recasens et al. demonstrated that the spreading of α-synuclein pathology to distant brain areas can also be replicated in animals and that it is more prominent in highly interconnected brain regions [21]. On the other hand, contrary findings were reported in an experimental model, where human α-synuclein pathology was induced in transgenic and non-transgenic mice, since it remained restricted to the site of inoculation [22].
To investigate cell-to-cell spreading in the human brain the optic pathway is an excellent model, since it barely has any anatomical connections to other brain regions and can be easily examined. The optic nerve projects to the lateral geniculate nucleus of the thalamus (LGN) and from there fibers project to the primary visual cortex [23]. Moreover, from the sixth layer of the primary visual cortex there is a cortico-geniculate feedback connection [24]. Only 5–10% of the synaptic inputs in the LGN are from retinal ganglion cells, hence the majority of projections stem from the visual cortex and a subset also from the thalamic reticular nucleus, the pulvinar and the brainstem [23–25]. Indeed spreading of prion disease related lesions along the visual pathway has been already demonstrated in mice, which were inoculated with a human brain homogenate of a patient with Creutzfeldt-Jakob disease (CJD) [26]. Based on these observations, in the present study we investigated whether there is a similar distribution pattern along this pathway for tau and α-synuclein in the human brain.
MATERIALS AND METHODS
Selection of cases
Cases were selected from the archives of the Institute of Neurology, Medical University of Vienna. For the evaluation of tau pathology 47 cases were included. From these 28 had only AD-related pathological changes. Further 19 had AGD or PSP-like pathology with or without AD-related changes (9 cases AD+AGD, 5 cases AD+PSP, 2 cases AD+AGD+PSP, 1 case PSP+AGD, 1 case with pure PSP, 1 case with complex tauopathy [27]). In sum, from the 47 cases 3 were Braak stage 0, 3 Braak I, 10 Braak II, 7 Braak III, 8 Braak IV, 6 Braak V, 10 Braak VI) (Table 1). Mean age of death of cases with tau pathology was 81±7 years. α-Synucleinopathy cases comprised samples from 16 individuals with PD (mean age at death: 78±8, 8 Braak stage 4, 3 Braak stage 5, 5 Braak stage 6) (Table 2). Additionally, we also evaluated brain tissue with prion disease (sporadic Creutzfeldt-Jakob disease, CJD, 4 cases MM type 1, 1 case MV type 2, mean age at death 65±5). Neuropathological diagnosis was based on internationally applied diagnostic criteria [19, 20, 28–32]. The presence of the full extent of the LGN on the sections of the posterior hippocampus was a pivotal factor for case selection. Optic nerve was available in eight patients with tau pathology (6 with only AD-related changes, 2 with AD+PSP), in seven patients with α-synucleinopathy and in four patients with sporadic CJD. Samples were collected following local regulations and the study was performed in the frame of a study (“Molecular neuropathologic investigation of neurodegenerative diseases”) approved by the Ethical Committee of the Medical University of Vienna.
Immunohistochemistry and evaluation of tissue sections
Three components of the optic pathway, the optic nerve (either at the optic chiasm or the optic tract), the LGN and the primary visual cortex (layer VI), were examined in formalin-fixed, paraffin-embedded tissue blocks. The blocks were cut into 3μm thick sections. Immunohistochemistry was performed with anti-tau AT8 antibody (1:200; AB223647, Pierce Endogen, USA), α-synuclein antibody (5G4; 1:4000; Roboscreen, Germany) [18], and with a monoclonal antibody against PrP (12F10; 1:2000, AB10080043, Cayman Chemicals, USA). Serial cutting of optic nerve samples was performed in cases that were initially negative. Due to the low amount ofimmunoreactive structures in the optic nerve and LGN we used a dichotomic evaluation (immunoreactivity present or not). For the occipital cortex we applied a three-tiered scoring (−: none; ±; mild degree of immunoreactivity; +: prominent immunoreactivity). In addition, co-immunostaining with anti-tau AT8 and anti-GFAP (rabbit polyclonal, 1:3000; AB10013482, Dako, Denmark) was performed on 10μm thick sections. These antibodies were incubated together for 48 hours at 4°C. After washing, the sections were incubated with anti-rabbit Alexa fluor 488 (Jackson Immunoresearch, USA) and goat anti-mouse Cy3 (Jackson Immunoresearch, USA) for 2 hours at ambient temperature. Sections were examined and photographed with a confocal laser-scanning microscope (Leica TCS SP5 II).
Statistical analysis
We correlated scores for the protein deposits in the LGN, optic nerve and layer VI of the occipital cortex using the Spearman correlation test and also performed a Fisher’s exact test to assess the relation between the frequency of these deposits in the examined cases. Data were regarded as statistically significant at a p-value≤0.05.
RESULTS
Description of immunoreactivity patterns
Tau (AT8) pathology in the optic nerve comprised thread-like and granular structures (Fig. 1a), α-synuclein immunostaining revealed thread-like, granular and dot like positivity (Fig. 1d), while there was no positivity in the sporadic CJD samples (Fig. 1g). In the group of cases with tau pathology 6/8 (75%) of the samples showed immunopositivity for AT8 in the optic nerve, and α-synuclein immunoreactivity was seen in 5/7 (71.1%) in cases with Lewy- related pathology (Tables 1 and 2).
In the LGN tau positive grain-like structures, threads, and astroglial immunoreactivity were seen (Fig. 1b). There were no neuronal cytoplasmic immunoreactivity or neurofibrillary tangles. The rims of occasional corpora amylacea were also immunopositive. The LGN showed threads and grain-like profiles in 22/47 (46.8%) of the cases irrespective of the Braak stage of neurofibrillary degeneration, including cases with additional PSP or AGD (Table 1). Immunostaining for α-synuclein revealed thin and thick neurites as well as dot-like structures (Fig. 1e). Almost in all samples (15/16; 93.7%) of the PD cases some degree of α-synuclein immunoreactivity was present in the LGN, irrespective of the Braak stage (4–6) of Lewy-related pathology (Table 2). Immunostaining for PrP revealed diffuse synaptic and fine somatosynaptic pattern in all five sporadic CJD cases (Fig. 1h).
In layer VI of the primary visual cortex tau immunoreactive neuropil threads, neurofibrillary tangles and dystrophic neurites around amyloid plaques were detectable in cases with Braak stage VI. In Braak stages lower than V few dot- and thin thread-like neuropil immunoreactivity were also seen in a few cases (Fig. 1c) as described already in AD [32]. This was also observed in 4/8 (50%) of the PSP cases with Braak stages ≤ 4 (3 associated with Braak stage II, and 1 pure PSP case). AGD was not associated with tau positivity in the occipital cortex in lower Braak stages (≤ 4). In 7/16 (43.7%) of the PD cases dot- and thread-like, neuronal cytoplasmic and astroglia-related α-synuclein immunoreactivities were seen in the occipital cortex (Fig. 1f). All five (100%) sporadic CJD cases showed diffuse synaptic and fine somatosynaptic PrP deposition in the cortex (Fig. 1i).
Correlation between variables
In cases that showed only neurofibrillary tau pathology related to AD a significant correlation between Braak stages and neuron-related immunopositivity (grain-like profiles and threads) and between Braak stages and total tau-load (neuron-related and glial) in the LGN (for both correlations: n = 28, Spearman R = 0.447, p = 0.017) was observed. Tau pathology in the LGN (grain-like profiles and threads and total tau-load) correlated also with the load of occipital tau pathology (for both correlations: n = 28, R = 0.50, p = 0.007 respectively). Fisher’s exact test also showed a significant association between LGN positivity and occipital tau pathology (n = 28; p = 0.001). After dividing the samples with only AD-type pathology (N = 28) in two groups with regard to their Braak stages (Braak ≤ 4, n = 15, and Braak ≥ 5, n = 13) the correlation between LGN and occipital cortex remained significant in the group with Braak stage ≤ 4 (R = 0.784, p = 0.001) (Table 1).
In addition, a significant correlation between optic nerve and LGN positivity could be detected in cases with only-AD type pathology (n = 6; R = 1.0, p < 0.01) and there was also a trend towards a significant correlation between optic nerve and occipital cortex immunoreactivity (n = 6; R = 0.775, p = 0.07). When pooling all cases with tau pathology, the correlation between tau immunoreactivities in the LGN and occipital cortex remained significant (n = 47, Spearman R = 0.351, p = 0.015). Fisher’s exact showed a significant association between LGN and occipital pathology (n = 47; p = 0.022). Furthermore, a strong, significant correlation was seen between the optic nerve tau immunopositivity and occipital cortex tau pathology (n = 8, R = 0.816, p = 0.013). In summary tau immunopositivity was never observed in the optic nerve without immunoreactivity in the LGN and occipital cortex. Tau immunopositivity in the LGN without corresponding immunoreactivity in the optic nerve or occipital cortex was seen frequently in PSP; however, mostly associated with astrocytes (Fig. 1j-l).
In Lewy body disorders we observed a strong trend for correlation between α-synuclein immunopositivity in the optic nerve and occipital cortex (n = 7, R = 0.730, p = 0.062). In total, 7/16 (43.7%) of cases with positivity in the LGN showed also immunoreactivity in layer VI of the occipital cortex. In addition, in four out of the seven LGN-positive cases optic nerve samples were available and were always positive. Moreover, only one case showed optic nerve positivity without occipital pathology (Table 2). However, we could neither detect a significant correlation between LGN and optic nerve positivity (n = 7; R = 0.645, p = 0.117) nor LGN and occipital positivity (n = 16; R = 0.228, p = 0.396) nor with Braak staging (n = 16; R = 0.244, p = 0.362). There was also no correlation between Braak staging (4–6) and occipital (n = 16; R = 0.179, p = 0.508) or optic nerve immunoreactivity (n = 7; R = 0.669, p = 0.100).
DISCUSSION
Prion-like seeding of misfolded proteins in NDDs is a widely discussed topic. Our aim was to evaluate the optic pathway, whether the distribution of protein deposits support the concept of propagation along neural paths. Indeed, we observed tau,α-synuclein and PrP immunoreactivity in a significant proportion of the examined cases.
We demonstrated a significant correlation of tau pathology in the LGN with Braak staging in patients with only AD-type tau pathology, which is in line with Braak’s theory of hierarchical disease development. However, we observed that in lower Braak stages, cases with tau positivity in the LGN had also some immunoreactivity in the occipital cortex, while cases without LGN tau positivity did not show these immunoreactivities in the occipital cortex. In fact, a relative strong relation between tau inclusions in the LGN and layer VI of the occipital cortex could be shown when we evaluated only the cases with lower stages (Braak stage ≤ 4). Although moderate to severe immunopositive threads in the occipital cortex are only seen in Braak stage V and VI, some positivity has been also reported in lower Braak stages [32]. Altogether, this might suggest that the simultaneous presence of tau pathology in the LGN and occipital cortex is not incidental, but it reflects the spreading between these anatomical structures. In summary, the strong correlation between the presences of tau immunoreactivity in the three examined anatomical structures suggests that, at least in a subset of patients, the visual system is affected during the disease course with a progression along neural pathways. However, we cannot clarify whether tau pathology in the optic nerve is an early event or not, since we cannot exclude the possibility of a retrograde spreading of tau pathology from the LGN to the optic nerve. Visual dysfunction is frequently observed in patients with AD suggesting involvement of the visual system during the disease course. Although tau pathology is known to be present in the primary visual cortex and more severe in the secondary visual cortex, the LGN was thought to be less susceptible for AD related pathology [33, 34]. However, our study used the very sensitive method of detecting disease-associated phosphorylated tau (AT8 antibody) [3], whereas those studies were conducted at a time, where immunohistochemistry was not applied for assessing AD-related pathology.
In addition to evaluating cases with only AD-type tau pathology, we also pooled cases with different concomitant tau pathologies together, since these cases are more representative of ageing in the human brain [35, 36]. We detected a weaker correlation between LGN and occipital pathology. This might suggest that PSP and AGD-related tau pathologies (i.e. strains) do not involve the occipital cortex, thus seeding along neural pathways between LGN and occipital cortex is more characteristic for AD related tau pathology. However, there are limitations of our study due to the fact that immunoreactivities in the optic nerve are small and easily missed even after serial cutting of the samples, furthermore, that the optic nerve samples were available only from a few cases. Due to this limitation in the pure PSP cases we were unable to correlate our findings with the grading of tau pathology as suggested by Williams et al. [37]. In addition, the small amount of tau pathology in these structures (optic nerve and LGN) did not allow the application of a semiquantitative scoring.
Aggregates of intracellular α-synuclein in neurons of the retina of PD patients have been recently reported, linking it to visual impairment seen in these patients [38, 39]. The presence of physiological forms of the synuclein family in human retina and optic nerve has been also reported [40]. Our study complements these observations by demonstrating the presence of disease-associated α-synuclein, using a very sensitive antibody [41], along the visual pathway in PD. As for tau immunoreactivities, α-synuclein deposits in the optic nerve are small. However, most of the patients showed some kind of pathology in the LGN and some cases showed α-synuclein immunoreactivity in the occipital cortex. In addition, a strong correlation between optic nerve and occipital cortex pathology was observed, which, however, showed only a trend towards significance probably due to the small sample size. A study using α-synuclein immunohistochemistry examining the visual pathway of 19 DLB cases described mild cell loss in the LGN; however, in contrast to our study they did not detect Lewy bodies or neurites in the LGN and only single neurites in the primary visual cortex and some more positivity in the secondary visual cortex [42]. Whether, this discrepancy is due to different anti-α-synuclein antibodies used or to a different patient cohort in our and their study is not clear. Furthermore, it must be noted that serial sectioning is recommended in cases where the optic nerve or LGN does not show any immunoreactivity. Thus, our study expands their observations [42] and supports the concept of the involvement of the retino-geniculo-occipital in addition to the retino-colliculo-thalamo-amygdala pathway, which could be one explanation for visual hallucinations and dysfunction in PD patients [42, 43].
Interestingly, immunoreactivity for α-synuclein was more prominent and more frequent in the LGN in PD as compared to tau in AD samples. In addition, the majority of the cases with positivity in the optic nerve also showed positivity in the occipital cortex, suggesting spreading of the protein deposits in the course of disease. Pathologic deposits of α-synuclein are also known in the peripheral, including several cranial nerves [18], and autonomous nervous system as well as in other organs in Lewy body disorders [44]. It must be noted, however, that apart from the retina, the LGN receives input from regions, which are affected in Lewy body disorders (i.e. thalamic nuclei, brainstem); this might explain the immunopositivity in the LGN without deposits in the optic nerve or occipital cortex. Furthermore, optic nerve and occipital positivity might be also dependent of disease progression, as only cases with Braak stage 4 in our cohort show LGN positivity without immunohistochemically detectable involvement of the optic nerve or occipital cortex.
Importantly, all CJD cases showed PrP immunoreactivity both in the LGN and occipital cortex. Although we could not see immunoreactivity in the optic nerves, the optic nerve is considered as highly infectious tissue in patients with suspected CJD [45]. Our cases were mostly MM type 1, which are discussed to have less frequent PrPSc deposits in the retina and optic nerve, due to a shorter disease course in these cases assuming there is a centrifugal spread of disease associated PrP from the brain to the optic nerve and retina [45]. Our findings in AD and PD resemble in some aspects the patterns of protein deposition in human prion disease. However, our study is not sufficient to show the mechanisms underlying this process. Transmission of pathologic tau via synapses has been discussed as a model for spreading along axons to nerve cells in AD [46]. Our observation of thread like and granular deposits could be a morphological support for these experimental observations. A recent study examined human P301S tau transgenic mice infused with human brain extracts containing tau aggregates and showed that spreading of tau pathology is probably dependent on synaptic connectivity [47]. The authors discuss that the lack of tau propagation to the LGN, a nucleus not connected but in close proximity to the hippocampus, discounts the possibility of tau seeds simply diffusing from the infusion site to distal nuclei.
In summary, our study supports 1) the notion that tau and α-synuclein may spread along neural pathways, and that 2) the clinical assessment of visual function and particularly the examination of the retina and the optic nerve, might be a promising diagnostic tool to evaluate disease progression or to stratify the patients with visual system involvement in these neurodegenerative disorders [34].
ACKNOWLEDGMENTS OF FUNDING
Supported by the EU FP7 project DEVELAGE (Grant Agreement N 278486).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
1 | Natale G, Pompili E, Biagioni F, Paparelli S, Lenzi P, Fornai F (2013) Histochemical approaches to assess cell-to-cell transmission of misfolded proteins in neurodegenerative diseases Eur J Histochem 57: e5 |
2 | Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie Science 216: 136 144 |
3 | Kovacs GG (2015) Neuropathology of tauopathies: Principles and practice Neuropathol Appl Neurobiol 41: 3 23 |
4 | Tolnay M, Probst A (1999) REVIEW: Tau protein pathology in Alzheimer’s disease and related disorders Neuropathol Appl Neurobiol 25: 171 187 |
5 | Eisbach SE, Outeiro TF (2013) Alpha-synuclein and intracellular trafficking: Impact on the spreading of Parkinson’s disease pathology J Mol Med (Berl) 91: 693 703 |
6 | Wales P, Pinho R, Lazaro DF, Outeiro TF (2013) Limelight on alpha-synuclein: Pathological and mechanistic implications in neurodegeneration J Parkinsons Dis 3: 415 459 |
7 | Dunning CJ, George S, Brundin P (2013) What’s to like about the prion-like hypothesis for the spreading of aggregated alpha-synuclein in Parkinson disease? Prion 7: 92 97 |
8 | Auli S, Le TT, Moda F, Abounit S, Corvaglia S, Casalis L, Gustincich S, Zurzolo C, Tagliavini F, Legname G (2014) Defined alpha-synuclein prion-like molecular assemblies spreading in cell culture BMC Neurosci 15: 69 |
9 | Betemps D, Verchere J, Brot S, Morignat E, Bousset L, Gaillard D, Lakhdar L, Melki R, Baron T (2014) Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological alpha-synuclein by enhanced ELISA Acta Neuropathol Commun 2: 29 |
10 | Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Ghetti B, Goedert M, Tolnay M (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain Proc Natl Acad Sci U S A 110: 9535 9540 |
11 | Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M (2009) Transmission and spreading of tauopathy in transgenic mouse brain Nat Cell Biol 11: 909 913 |
12 | Guo JL, Lee VM (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases Nat Med 20: 130 138 |
13 | Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease Nat Med 14: 504 506 |
14 | Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation Nat Med 14: 501 503 |
15 | Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, Hasegawa M (2014) Pathological alpha-synuclein propagates through neural networks Acta Neuropathol Commun 2: 88 |
16 | Prusiner SB (2012) Cell biology. A unifying role for prions in neurodegenerative diseases Science 336: 1511 1513 |
17 | Kurowska Z, Englund E, Widner H, Lindvall O, Li JY, Brundin P (2011) Signs of degeneration in 12-22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson’s disease J Parkinsons Dis 1: 83 92 |
18 | Kovacs GG, Breydo L, Green R, Kis V, Puska G, Lorincz P, Perju-Dumbrava L, Giera R, Pirker W, Lutz M, Lachmann I, Budka H, Uversky VN, Molnar K, Laszlo L (2014) Intracellular processing of disease-associated alpha-synuclein in the human brain suggests prion-like cell-to-cell spread Neurobiol Dis 69: 76 92 |
19 | Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes Acta Neuropathol 82: 239 259 |
20 | Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease Neurobiol Aging 24: 197 211 |
21 | Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, Farinas I, Obeso JA, Bezard E, Vila M (2014) Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys Ann Neurol 75: 351 362 |
22 | Sacino AN, Brooks M, Thomas MA, McKinney AB, McGarvey NH, Rutherford NJ, Ceballos-Diaz C, Robertson J, Golde TE, Giasson BI (2014) Amyloidogenic alpha-synuclein seeds do not invariably induce rapid, widespread pathology in mice Acta Neuropathol 127: 645 665 |
23 | De Moraes CG (2013) Anatomy of the visual pathways Journal of glaucoma 22: Suppl 5 S2 S7 |
24 | Briggs F, Usrey WM (2011) Corticogeniculate feedback and visual processing in the primate The Journal of physiology 589: 33 40 |
25 | Erisir A, Van Horn SC, Sherman SM (1997) Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus Proc Natl Acad Sci U S A 94: 1517 1520 |
26 | Liberski PP, Yanagihara R, Gibbs CJJr, Gajdusek DC (1990) Spread of Creutzfeldt-Jakob disease virus along visual pathways after intraocular inoculation Arch Virol 111: 141 147 |
27 | Kovacs GG, Molnar K, Laszlo L, Strobel T, Botond G, Honigschnabl S, Reiner-Concin A, Palkovits M, Fischer P, Budka H (2011) A peculiar constellation of tau pathology defines a subset of dementia in the elderly Acta Neuropathol 122: 205 222 |
28 | Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration J Neurol 246: Suppl 2 II6 I15 |
29 | Kovacs GG, Budka H (2010) Current concepts of neuropathological diagnostics in practice: Neurodegenerative diseases Clin Neuropathol 29: 271 288 |
30 | Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on A, Alzheimer’s A (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach Acta Neuropathol 123: 1 11 |
31 | Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD Neurology 58: 1791 1800 |
32 | Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: A study of the BrainNet Europe Consortium Brain Pathol 18: 484 496 |
33 | Katz B, Rimmer S (1989) Ophthalmologic manifestations of Alzheimer’s disease Surv Ophthalmol 34: 31 43 |
34 | Armstrong RA (2009) Alzheimer’s Disease and the Eye J Optom 2: 103 111 |
35 | Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, Honigschnabl S, Reiner-Concin A, Heinzl H, Jungwirth S, Krampla W, Fischer P, Budka H (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: A community-based autopsy series Acta Neuropathol 126: 365 384 |
36 | Rahimi J, Kovacs GG (2014) Prevalence of mixed pathologies in the aging brain Alzheimers Res Ther 6: 82 |
37 | Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome Brain 130: 1566 1576 |
38 | Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S (2014) Alpha-synuclein in the inner retina in Parkinson’s Disease Ann Neurol 75: 964 966 |
39 | Beach TG, Carew J, Serrano G, Adler CH, Shill HA, Sue LI, Sabbagh MN, Akiyama H, Cuenca N, Arizona Parkinson’s Disease C (2014) Phosphorylated alpha-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects Neurosci Lett 571: 34 38 |
40 | Surguchov A, McMahan B, Masliah E, Surgucheva I (2001) Synucleins in ocular tissues J Neurosci Res 65: 68 77 |
41 | Kovacs GG, Wagner U, Dumont B, Pikkarainen M, Osman AA, Streichenberger N, Leisser I, Verchere J, Baron T, Alafuzoff I, Budka H, Perret-Liaudet A, Lachmann I (2012) An antibody with high reactivity for disease-associated alpha-synuclein reveals extensive brain pathology Acta Neuropathol 124: 37 50 |
42 | Yamamoto R, Iseki E, Murayama N, Minegishi M, Marui W, Togo T, Katsuse O, Kato M, Iwatsubo T, Kosaka K, Arai H (2006) Investigation of Lewy pathology in the visual pathway of brains of dementia with Lewy bodies J Neurol Sci 246: 95 101 |
43 | Diederich NJ, Stebbins G, Schiltz C, Goetz CG (2014) Are patients with Parkinson’s disease blind to blindsight? Brain 137: 1838 1849 |
44 | Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, Marti MJ, Hernandez I, Valldeoriola F, Rene R, Ribalta T (2014) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders Mov Disord 29: 1010 1018 |
45 | Armitage WJ, Tullo AB, Ironside JW (2009) Risk of Creutzfeldt-Jakob disease transmission by ocular surgery and tissue transplantation Eye (Lond) 23: 1926 1930 |
46 | Spires-Jones TL, Hyman BT (2014) The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer’s Disease Neuron 82: 756 771 |
47 | Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S, Bose S, Clavaguera F, Tolnay M, Lavenir I, Goedert M, Hutton ML, O’Neill MJ (2014) A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: The pattern of spread is determined by connectivity, not proximity Acta Neuropathol 127: 667 683 |
Figures and Tables
Fig.1
Patterns of immunoreactivity along the visual pathway. (a-c) Anti-tau (AT8) immunostaining in a case with AD-related pathology Braak stage 4: (a) thread-like and granular immunoreactivity in the optic nerve; (b) neuron-related (threads and grains; indicated by arrows) immunoreactivity in the LGN; (c) very few dots and threads (indicated by arrows) in layer VI of the occipital cortex. (d-f) α-Synuclein staining in a case with PD Braak stage 6: (d) thread-like, granular and dot like positivity in the optic nerve; (e) thin and thick neurites and dot-like immunoreactivity in the LGN; (f) dot-like and thread-like immunoreactivity in layer VI of the occipital cortex. (g-i) Prion protein immunostaining in a case with sporadic CJD (Codon 129 MM type 1): (g) lack of immunoreactivity in the optic nerve; (h) diffuse synaptic and fine somato-synaptic pattern in the LGN and in the occipital cortex (i). (j-l) Double-immunolabeling for AT8 and GFAP in the LGN in a case with PSP: (j) AT8 immunoreactivity (red); (k) Anti-GFAP immunoreactivity showing astrocytes (green); (l) Co-localization of tau and GFAP in astrocytes processes (indicated by arrows). Bar in (a) represent 50μm for all images.
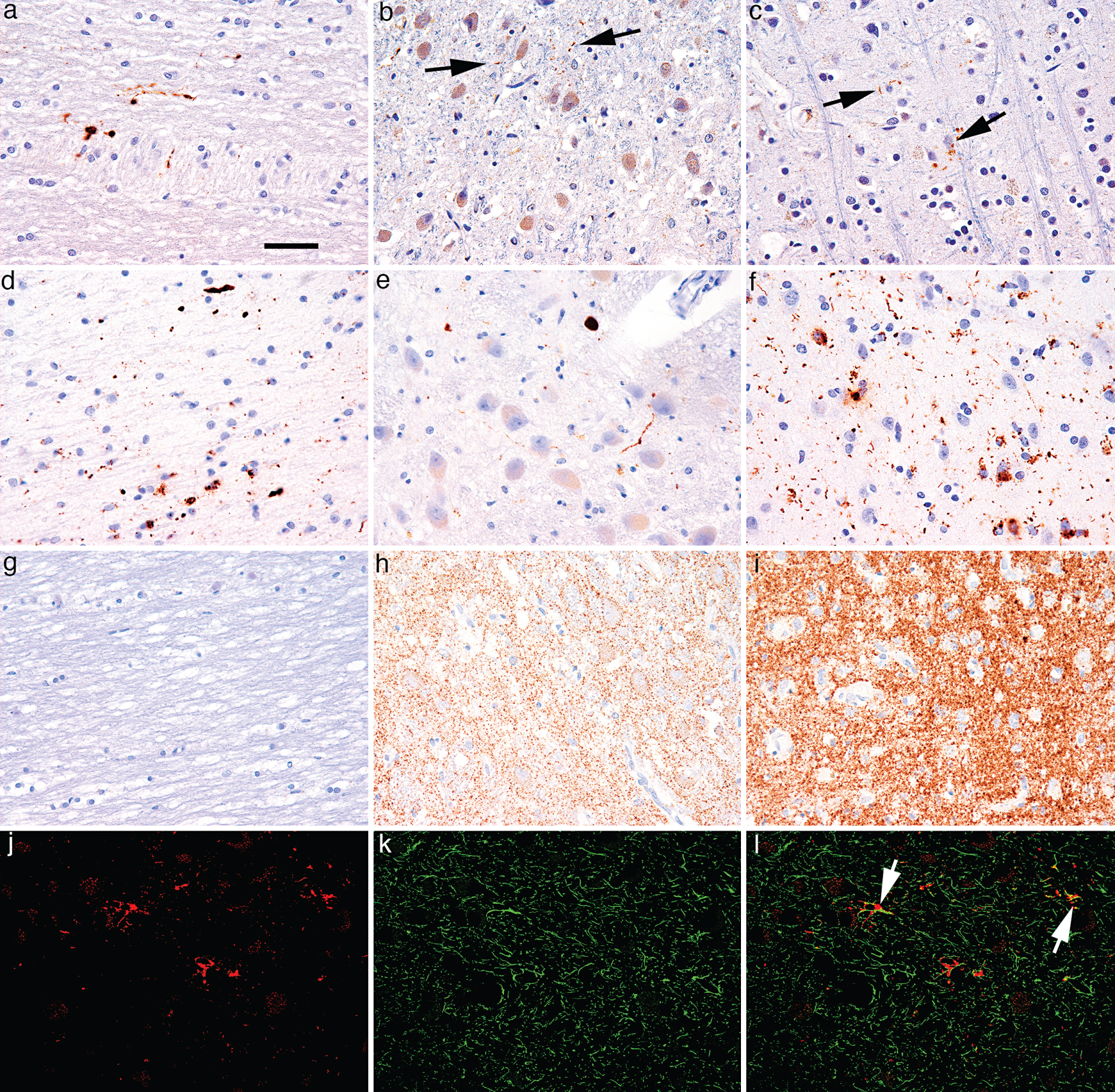
Table 1
Characterization of cases with tau pathologies
| Case | Age | BB stage | Tau-pathologies | optic nerve | LGN | occipital |
| Tau 1 | 79 | 2 | AD+PSP | + | + | + |
| Tau 2 | 83 | 4 | AD | + | + | + |
| Tau 3 | 63 | 6 | AD | + | + | + |
| Tau 4 | 79 | 6 | AD | + | + | + |
| Tau 5 | 89 | 5 | AD | + | + | + |
| Tau 6 | 55 | 6 | AD | + | + | + |
| Tau 7 | 83 | 1 | AD+PSP | – | + | – |
| Tau 8 | 70 | 2 | AD | – | – | – |
| Tau 9 | 76 | 0 | PSP | na | + | + |
| Tau 10 | 77 | 5 | AD+complex tauopathy | na | + | + |
| Tau 11 | 84 | 5 | AD+PSP | na | + | + |
| Tau 12 | 73 | 6 | AD | na | + | + |
| Tau13 | 81 | 6 | AD | na | + | + |
| Tau 14 | 85 | 6 | AD | na | + | + |
| Tau 15 | 87 | 2 | AD+PSP+AGD | na | + | + |
| Tau 16 | 88 | 2 | AD | na | + | + |
| Tau 17 | 82 | 5 | AD | na | + | + |
| Tau 18 | 65 | 0 | PSP+AGD | na | + | – |
| Tau 19 | 79 | 2 | AD+PSP+AGD | na | + | – |
| Tau 20 | 81 | 2 | AD+PSP | na | + | – |
| Tau 21 | 85 | 2 | AD+AGD | na | + | – |
| Tau 22 | 86 | 3 | AD+AGD | na | + | – |
| Tau 23 | 86 | 4 | AD | na | + | – |
| Tau 24 | 83 | 5 | AD | na | – | + |
| Tau 25 | 84 | 5 | AD | na | – | + |
| Tau 26 | 82 | 6 | AD | na | – | + |
| Tau 27 | 84 | 6 | AD | na | – | + |
| Tau 28 | 88 | 6 | AD+AGD | na | – | + |
| Tau 29 | 90 | 6 | AD | na | – | + |
| Tau 30 | 77 | 1 | AD | na | – | – |
| Tau 31 | 88 | 1 | AD | na | – | – |
| Tau 32 | 73 | 2 | AD | na | – | – |
| Tau 33 | 74 | 2 | AD | na | – | – |
| Tau 34 | 80 | 2 | AD+PSP | na | – | – |
| Tau 35 | 86 | 2 | AD+AGD | na | – | – |
| Tau 36 | 81 | 3 | AD | na | – | – |
| Tau 37 | 82 | 3 | AD | na | – | – |
| Tau 38 | 83 | 3 | AD+AGD | na | – | – |
| Tau 39 | 83 | 3 | AD+AGD | na | – | – |
| Tau 40 | 84 | 3 | AD+AGD | na | – | – |
| Tau 41 | 86 | 3 | AD+AGD | na | – | – |
| Tau 42 | 82 | 4 | AD | na | – | – |
| Tau 43 | 82 | 4 | AD | na | – | – |
| Tau 44 | 84 | 4 | AD | na | – | – |
| Tau 45 | 85 | 4 | AD+AGD | na | – | – |
| Tau 46 | 86 | 4 | AD | na | – | – |
| Tau 47 | 88 | 4 | AD | na | – | – |
Abbreviations: AD: Alzheimer disease-related tau pathology; PSP: progressive supranuclear palsy; AGD: argyrophilic grain disease; na: not available; − indicates absence of immunoreactivity; + indicates that immunoreactivity is present.
Table 2
Characterization of cases with α-synucleinopathies
| Case | Age | Braak stage PD | optic nerve | LGN | occipital cortex |
| AS 1 | 74 | 5 | + | + | – |
| AS 2 | 80 | 5 | + | + | + |
| AS 3 | 74 | 6 | + | + | + |
| AS 4 | 78 | 4 | + | + | + |
| AS 5 | 55 | 6 | + | + | + |
| AS 6 | 82 | 4 | – | + | – |
| AS 7 | 79 | 4 | – | + | – |
| AS 8 | 78 | 4 | na | + | + |
| AS 9 | 88 | 4 | na | + | + |
| AS 10 | 70 | 6 | na | + | + |
| AS 11 | 79 | 4 | na | + | – |
| AS 12 | 81 | 4 | na | + | – |
| AS 13 | 83 | 5 | na | + | – |
| AS 14 | 90 | 6 | na | + | – |
| AS 15 | 83 | 6 | na | + | – |
| AS 16 | 86 | 4 | na | – | – |
Abbreviations: AS: α-synucleinopathy; PD: Parkinson disease, na: not available; − indicates absence of immunoreactivity; + indicates that immunoreactivity is present.




