Narrative Discourse Cohesion in Early Stage Parkinson’s Disease
Abstract
Background:
Models of basal ganglia (BG) function suggest that expressive language deficits will likely and consistently present in BG disease. Disparities currently exist between the predictions of models of BG function in expressive language and data from studies of BG disease. Traditional expressive language assessment methodologies that emphasize measures of language form (word and sentence productivity) while not carefully considering how language is used, may only partially account for these disparities.
Objective:
To use measures of cohesion to examine the use of cohesive markers in narrative discourse.
Methods:
Twelve individuals with idiopathic Parkinson’s disease (PD) were compared to 12 matched neurologically intact controls on measures of discourse performance. Three discourse samples (typical day, memorable vacation and family) were analyzed for measures of narrative productivity, number of cohesive ties and cohesive adequacy. Mixed model analyses were completed for group comparisons.
Results:
Group differences were not observed on measures of language form as measured by narrative productivity, communication units, and number of cohesive ties produced. In contrast, group differences were observed in cohesive adequacy as individuals with PD produced a higher percentage of incomplete and erroneous cohesive ties relative the control subjects across narratives.
Conclusions:
These results support the conclusion that the BG in PD may have an executive role in expressive language use that can be disrupted without impacting language form.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative syndrome that is estimated to affect four to six million individuals worldwide in the world’s most populous countries, with 50,000–60,000 new cases beingdiagnosed in the United States annually [1]. While PD affects neuroanatomical regions centering on the basal ganglia (BG), studies by Braak and colleagues have demonstrated that PD related pathology affects multiple neuronal systems as it courses from the brain stem thru the BG and eventually affecting the cerebral cortex [2]. According to Braak et al. [2], the neurodegenerative disease process begins prior to the onset of overt somato-motor dysfunctions. In the earliest stages the disease process begins in the dorsal IX/X motor nucleus and may also extend into the adjoining intermediate reticular zone. The disease then progresses to lower and upper brain stem nuclei followed by an upward course culminating in the cerebral cortex [2]. Because of the extensive disease progression, PD is characterized by progressive reductions in motor as well as cognitive performance.
Models of BG function suggest a critical relationship between the BG and cerebral cortex that should result in predictable expressive language deficits in BG diseases. The BG are connected to the cerebral cortex via a family of cortical-BG-thalamic-cortical circuits that are distinguished by their unique function [3]. Extensive connections exist between most areas of the cerebral cortex, particularly the frontal lobes that are critical to expressive language [4]. Cortical-BG connections provide the anatomical base for the disruption of expressive language which is traditionally linked to the cerebral cortex [5]. Some authors have concluded that the BG and supporting connections contribute to the development of expressive language via word search and generation strategies for verbal expression and therefore expressive language deficits should consistently occur in BG disease [6, 7].
Two recent reviews of language production in PD offer compelling evidence that language disruptions do exist in individuals with PD. Murray’s review [8] designed to examine advances in the literature related to changes in language associated with PD offered evidence that language changes in PD existed even in the earliest disease stages. Deficits included morphosyntactic, lexical semantic and language production breakdowns as linguistic complexity increased. A review by Altmann and Troche [9] emphasized the effects of PD on the brain and cognition, and on language production and the stages of language production impairment found as PD progresses. Importantly they described evidence of language production issues following PD that cannot be solely explained by traditional hypotheses of language impairment due primarily to reductions in working memory and/or executive function.
Studies of expressive language associated with BG disease have traditionally emphasized the examination of “language form” (i.e., word and sentence productivity, syntax, grammaticality, etc.) [10–13]. Previous studies have primarily emphasized production at the word and sentence level. Less emphasis has been placed on how language is being used (language use), particularly at the discourse or conversational level. These issues are important because successful generation of expressive language involves the development of language form and the correct use of that form. As a result, studies designed to examine language use in those with BG disease are needed to establish the hypothesized vulnerability of expressive language use in BG disease. However, approaches designed to study language use and language form have yet to emerge. One method for making this comparison may be to analyze discourse for attributes of language form and use during discourse production. The assessment of discourse can be used to evaluate the complex integration of sentences into coherent communication that represents the speaker’s skills in language use and relating language use to the grammatical form [14]. In summary, discourse contains attributes of language form and language use that can be measured in one sample.
Narrative discourse is used to describe an experience as a sequence of events or episodes [15]. Successful narrative discourse contains the linking of meaning across sentences or cohesion. Cohesion occurs via the use of cohesive markers that direct the listener to information found outside individual sentences [16]. A cohesive marker creates a tie with information found outside individual sentences, thus establishing a meaningful relationship across sentences [17]. Cohesion has been measured in disorders that are known for language use deficits; Alzheimer’s disease [18], traumatic brain injury [19] and stroke [20]. Because sentence production involves a complex sequence of choosing lexical items and appropriate sentence structures, disease of the BG may disrupt the language planning process required to produce fluent and cohesive output [10].
Therefore, the purpose of this study is to use measures of cohesion in narrative discourse to examine language use in individuals with BG disease. We selected individuals with idiopathic PD because they have a disease that begins in the BG and disrupts the cortical-BG circuits that govern various aspects of higher cortical functioning [21]. We hypothesize that individuals with PD would exhibit differences in the cohesiveness of their narratives relative to neurologically intact controls as a result of BG disease and disruptions of cortical-BG functions. We elected to test this hypothesis in individuals with early stage PD because disease related pathology exists even during asymptomatic preclinical phases before the overt signs of the disease emerge [22].
MATERIALS AND METHODS
Description of the subjects
Participants consisted of 12 individuals diagnosed with idiopathic PD (hereafter referred to as experimental subjects) by a movement disorders neurologist using the strict criteria of the UK Brain Bank [23] and 12 individuals who were age, education, ethnicity and gender matched and neurologically intact (hereafter referred to as control subjects). The study was IRB approved and all participants gave written informed consent. All participants were male, right handed, and had no history of prior stroke, dementia, brain tumor, or head trauma. All had at least a seventh grade education, functional hearing for normal conversation, functional vision for reading tasks, spoke English as their primary language, and demonstrated expressive language skills within intact range for normal conversation. All subjects (experimental and control) exhibited scores of 26 or better on the Mini Mental Status Exam (MMSE) [24].
Each experimental subject presented with a minimum of 3 of 4 cardinal features of PD (resting tremor, rigidity, bradykinesea, postural instability) and had no history of deep brain stimulation or brain lesion therapy. The parkinsonism of each experimental subject was rated with the Hoehn & Yahr Staging Scale for PD and classified by predominate feature (tremor vs. rigidity).
Procedure
Standardized assessments
The Boston Naming Test (BNT) [25] and Wechsler Memory Scale –Logical Memory I (WMS-LMI) [26] were administered to examine potential group differences relative to language form (BNT) and the influence of memory on language form and use (WMS-LMI).
Narrative discourse data collection
Narratives were collected from experimental subjects in the “off” state of their parkinsonian medications. Experimental subjects were seen in their homes prior to their first daily dose of anti-parkinsonian medication and at least 12 hours since their last dosage to maximize dopamine depletion. Five of the 12 experimental subjects were newly diagnosed with PD and had no history of parkinsonian medication use at the time of the study. Control subjects were seen primarily in their homes.
Each participant was instructed to discuss 3 topics, which included the following: (1) a typical day, (2) a memorable vacation, and (3) his family for a minimum of 3-minutes. In the event that any subject stopped before 3-minutes, a standardized verbal cue was provided to continue the narrative until the 3-minute minimum was achieved. The order of presentation of the 3 topics was counterbalanced across subjects. All responses were audio recorded for off line transcription and scoring.
Analysis of narrative data
The first 3 minutes of all language samples were transcribed verbatim. Each sample was divided into communication units (CU); defined as the shortest allowable independent clause and related dependent clauses. Individual CU’s were defined primarily by syntax, however prosodic and semantic features were used at times when the unit could not be determined entirely by syntax. All words unintelligible to the investigator were excluded from the analysis. In instances where the location of coordinating conjunctions such as “and”, “but” and “or” was unclear, their prosodic feature determined their final location at the beginning or ending of the communication unit. One-word responses were not considered in the communication unit calculation. Scoring guidelines for communication units were based on Hunt’s procedure [27].
The total number of CU’s and words in each transcribed narrative were calculated for comparisons. Following segmentation of CU’s, the total number of communication units and words for the 3-minute sample was calculated for each subject. Each communication unit was then evaluated for use of cohesive ties. Cohesive ties within three categories (Reference, Conjunction, and Lexical) were identified. Although five types of cohesive ties exist, studies of discourse cohesion report that typically only three ties are used frequently enough for statistical analysis [28] (see supplemental material for definitions and discourse examples).
Each cohesive tie was judged for the adequacy of its tie (complete, incomplete or erroneous). Cohesive ties were judged “complete” when the referent could be easily found in the preceding discourse. Ties were defined “incomplete” cohesive markers when the referent could not be identified in the preceding discourse or was not evident. Ties were defined as “erroneous” when multiple referents could be identified in the discourse therefore making the marker ambiguous.
The percentage of incomplete and erroneous ties were calculated as defined below.
Three trained raters participated in the project to establish reliability for identification of CU’s, words, number of cohesive ties and percent incorrect and erroneous use of cohesive ties. Raters were blinded to the neurological status subjects that generated the samples used for the analyses. One trained rater analyzed 100% of the samples that were used for the analysis. Two additional trained raters independently analyzed 15% of the total sample. Intra-class correlation coefficients were completed to analyze agreement among the 3 raters.
Analysis of motor speech performance
An independent judge blinded to the neurological status of all subjects rated motor speech performance of all audio recorded samples. Each sample was rated on a 5-point scale of speech intelligibility. Ratings ranged from 1 (No detectable disorder) to 5 (No functional speech). Speech ratings were correlated with all language variables.
RESULTS
Scoring reliability
Intra-class correlation coefficients (ICC) were calculated by using a two-way mixed model with repeated measures to evaluate scoring agreement among the raters for CU’s, words, cohesive ties and cohesive adequacy. ICC scores of (0.99) were achieved for CUs and words, (0.91) for cohesive ties and (0.83) for percent correct use of ties.
Statistical analyses
Mixed model analyses (2 group×3 narrative topics; (<0.05) were completed for all analyses unless otherwise stated. The examination of CU’s, total words, cohesive ties, and cohesive adequacy compared only the first 3-minutes of each narrative sample.
Demographic comparisons
A detailed profile of the PD subjects is provided in Table 1. Two-tailed t-test, alpha level = 0.05, revealed no significant group differences between the experimental and control subjects on age, education, and standardized cognitive and language comparisons (see Table 2).
Language form
Communication units and words
Results of a 2 (group)×3 (narrative) comparison indicated non-significant effect for group on total number of CU’s [F(1, 23) = 0.99, p = 0.33] and total number of words [F(1, 23) = 0.00, p = 0.98). The experimental subjects produced similar numbers of CU’s and words relative to the controls across the 3 narratives (typical day, memorable vacation, and family). A group × narrative interaction was significant for CU’s [F(2, 192) = 3.90, p = 0.022]. Pairwise comparisons using independent sample t-tests indicated that controls subjects exhibited a significant reduction in CU’s during their discussion of family relative to vacation (t = 2.865; p = 0.015).
Language use
Cohesive ties
Results of a 2 (group)×3 (narrative) comparison indicated a non-significant effect for group [F(1, 24) = 0.010, p = 0.919]. The experimental subjects produced similar numbers of total cohesive ties relative to the controls across the 3 narratives (typical day, memorable vacation, and family). A group × narrative interaction was significant [F(2, 192) = 10.48, p = 0.000]. Pairwise comparisons indicated that control subjects produced significantly fewer cohesive ties during their discussion of typical day relative to memorable vacation (t =−2.314; p = 0.04). The mean number of cohesive ties produced by the 2 groups is reported in Fig. 1. A percentage distribution of each cohesive tie type (Reference, Lexical, and Conjunction) was calculated and is displayed in Fig. 2.
Cohesive adequacy
Results of a 2 (group)×3 (narrative) comparison indicated a significant main effect for group [F(1, 81) = 5.891, p = 0.017]. The experimental subjects produced a higher percentage of incomplete and erroneous cohesive ties relative to the control subjects across narratives. There were no significant group × narrative interactions. The mean percentage use of incorrect and erroneous of cohesive ties produced by the 2 groups is displayed in Fig. 3.
Further inspection of the cohesive ties judged as incomplete or erroneous indicated that the experimental subjects produced 136 ties identified as incomplete or in error compared to 63 by the controls subjects. Sixty-nine percent of all ties were judged incomplete or erroneous were of reference type for both groups. The total number of ties judged incomplete or erroneous is depicted in Fig. 4.
Motor speech performance
A two-tailed t-test (p < 0.05) revealed a significant group difference between the experimental subjects (M = 2.2, SD 0.72) and control subjects (M = 1.3, SD 0.62) on intelligibility ratings, t (22), p = 0.003. Correlations with language variables indicated non-significant correlations between ratings of motor speech performance and CUs (r = 0.19, p = 0.38), words (r =−0.07, p = 0.76), total ties (r = 0.05, p = 0.81), and percent incomplete and erroneous cohesive ties (r =−0.21, p = 0.33).
DISCUSSION
The results of this study confirmed a disorder of language use (cohesive adequacy) in those with early PD even though significant differences were not observed on measures of language “form” or by motor speech issues. Relative to the controls, the experimental subjects produced similar language form as measured by number of: CU’s, words and cohesive ties. In contrast, individuals with PD produced a greater percentage of incomplete and erroneous ties in their narratives. Thus, while aspects of overall “form” of narrative discourse output (CU’s, total words, total cohesive ties) may be relatively preserved in the earliest stage of PD, the cohesiveness of narrative discourse may be impaired, heralding the emergence of language use disruption.
These findings also support the notion that traditional measures of language “form” and measures designed to capture language production issues at the sentence level may be insensitive to language disruption in the earliest stages of PD. It is presumed that to adequately understand language production among individuals with diseases of the BG such as PD, studies should be designed to emphasize “language use” at a more complex level than word production tasks, i.e., discourse. This presumption is supported by studies which have shown impairments in language pragmatics or the use of verbal and non-verbal social communication in individuals with PD [29].
In this study, a review of all incomplete or erroneous ties revealed that experimental subjects produced double the number of incomplete or erroneous ties compared to the control subjects. Sixty nine-percent of all ties judged as incomplete or in error were of the reference type for both groups. Reference is reportedly susceptible to disruption in neurological disease due to the complexity of the reference system. The use of reference is important to narrative discourse as it directs the listener to the identity of the thing to which the references refers [16].
Our observed greater number of incomplete and erroneous cohesive ties among individuals with PD compared to controls agree with previous studies of cohesion in neurological disease [18, 30]. Ripich and Terrell [31] observed significantly greater use of incomplete and erroneous cohesive ties among individuals with dementia compared to elderly controls. They hypothesized that reference ties serve to structure or organize narrative discourse production and may be more sensitive to neurological disease. The lack of equivalent reductions in cohesive adequacy amonglexical and conjunction ties suggests that general nouns and conjunctions create less opportunity for ambiguity relative to pronouns. Consequently, use of reference ties may be more subject to increased inaccuracy of use relative to lexical and conjunction ties in PD. It is important to note that we only saw the participants in the “off” medication state. Completing the same measures during their “on” state may have offered additional information regarding the impact of PD on cohesiveness during discourse production. Similarly, a more comprehensive measure of cognitive functioning with a particular emphasis on executive function would ensure that the observed language errors were independent of other cognitive issues.
Cohesion is a microlinguistic feature of narrative discourse that has the potential to be masked in PD by more commonly observed and reported motor speech deficits. Van Leer & Turskstra [28] noted that ambiguous intersentential meaning can occur when the cohesive tie is not readily apparent. Reductions in cohesion are believed to result in increased vagueness thereby confusing the intended listener [18]. Glosser and Deser [31] also reported that disrupted cohesion can reflect impaired lexical retrieval during discourse construction. Since cohesion serves to facilitate the continuity of meaning in narrative discourse, it is believed to provide an indirect index of the ability to maintain a topic during discourse production. Therefore, disruptions in cohesive adequacy can indicate a more global disruption of language use in PD.
There is current evidence related to the neurobiological underpinnings of a potential increase in incomplete/erroneous cohesive ties in PD. For example Ford et al. [32] demonstrated that the machinery for basal ganglia participation in language function exists by demonstrating connections between the basal ganglia and Broca’s area and as previously hypothesized by Ullman [33]. However, simple connectivity does not explain the role of the basal ganglia in discourse processing. But, more comprehensive neurocognitive models designed to address linguistic functions of the basal ganglia for word or sentence processing have application for the interpretation of the current discourse data. Evidence is offered from three sources. First, Crosson et al. [34] addressed the role of a left pre-SMA-basal ganglia loop in word production. The authors hypothesized that the basal ganglia increases the signal-to-noise ratio during word selection by enhancing activation of the best lexical candidate while suppressing other competing candidates. More specifically, the best candidate selection in discourse is determined by the surrounding context as well as by semantic considerations. According to this theory, incomplete or erroneous cohesive markers would be selected due a failure to enhance an appropriate choice over less appropriate ones. For example, a pronoun (reference tie) with multiple equally possible prior referents could be selected instead of a word making the reference to the prior concept more specific because of a failure to enhance activation of the specific word more than that of the non-specific pronoun (which was common in our PD discourse samples). The biggest weakness in this explanation is that one would anticipate other word selection difficulties, which typically are not considered to be prominentin PD.
A second potential explanation for more incomplete/erroneous cohesion ties involves automatic processes, in particular procedural memory. Studies by Copland et al. have suggested that lexical-semantic processing difficulties in PD occur in automatic as opposed top-down processing [35, 36]. Support for that explanation emerged from Ullman who suggested the basal ganglia plays a prominent role in the procedural memory system underpinning grammatical functions [33]. In keeping with a vast literature on procedural memory, Ullman defines procedural memory as subserving “the learning of new, and control of established sensory-motor and cognitive ‘habits’, ‘skills’, and other procedures” [33, p. 237]. Ullman’s concept is consistent with the observations of Copland in that procedural memory is capable of operating outside of top-down control mechanisms. This explanation assumes that discourse cohesion is controlled, at least in part, by procedural memory systems affected by PD. However, our data indicate that the overall “form” of narrative discourse (CU’s, total words, total cohesive ties), the mostly likely aspect of discourse to be influenced by procedural memory, was relativelyintact.
The third explanation for greater use of incomplete/erroneous cohesion ties during discourse production in PD involves the role or working memory in cohesion for discourse. Working memory is the ability to hold information in one’s short-term memory for use or manipulation during ongoing cognitive operations. In that regard, Grossman et al. concluded that working memory limitations played a role in the difficulties that PD patients have with complex syntax and that these working memory limitations were due to frontal-basal ganglia dysfunction in PD [37, 38]. In this explanation, incomplete or erroneous cohesive ties would be related to PD patients losing track of all of the prior information in their narrative to whichcohesive ties might be made. Consequently, a PD patient might select a pronoun with multiple potential referents because s/he has lost the ability to track all of the potential referents. Future studies must be designed to test the working memory scenario against the aforementioned scenarios by including more detailed measures of working memory, procedural memory, and word retrieval in a study of discourse beyond the measures used in this study to determine which function(s) are most strongly associated with increases in incomplete/erroneouscohesive ties.
Though many questions persist regarding the influences of BG disease on expressive language performance, our findings indicate that language use deficits do in fact occur. While individuals with BG disease may exhibit fluent verbal output, the cohesiveness of their expressive language may be deceased. It is important that we acknowledge the potential contributions to discourse production from the diversity of cortical and subcortical structures identified by Braak and colleagues that are involved in the PD disease progression [2]. Novel methodologies will be required to test the sensitivity of BG disease to expressive language performance that address potential language use deficits such as correct pragmatic language use and the relative contributions from extra-BG anatomical structures to language use issues. Cohesion analyses with special emphasis on cohesive adequacy can provide a useful means to identify sensitive expressive language impairments that will not be otherwise identified. Similarly, clearly understanding the exact nature of cohesion issues may lead to interventions that parallel motor speech treatments that are designed to reduce the impact on communication and minimize the progressive impact of the disease. Finally, the observation of group by narrative interactions on measures of CU’s and total cohesive ties suggests that multiple samples are required to adequately consider the variability of performance during discourse production when examining language form and language use variables.
CONCLUSIONS
Studies of individuals with disease of the BG have generally emphasized motor speech and language form changes while omitting expressive language use issues. Models of BG functioning suggesting that BG diseases should have a greater influence on expressive language use were supported by the findings of thisstudy.
DISCLOSURES
Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, Quantia, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
ACKNOWLEDGMENTS
The first author was funded by a Pre-Doctoral Fellowship from the VA Office of Academic Affairs awarded to the first author while a predoctoral fellow in the Brain Rehabilitation Research Center, VAMC, Gainesville, FL. We would like to acknowledge the support of the National Parkinson Foundation Center of Excellence at the University of Florida. BC and LGR were funded by Research Career Scientist awards from the VA Rehabilitation Research: Development Service.
Appendices
The supplementary materials is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-140476.
REFERENCES
1 | National Parkinson, Foundation (2014) http://www.parkinson.org/parkinson-s-disease.aspx Accessed on September 3, 2014. |
2 | Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease Neurobiol Aging 24: 197 211 |
3 | Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: Motor and cognitive circuits Brain Res Rev 31: 236 250 |
4 | Alexander MP (2002) Disorders of language after frontal lobe injury: Evidence for the neural mechanisms of assembling language In Principles of Frontal Lobe Function Knight Stuss DT Oxford University Press Oxford 159 167 |
5 | Salmon DP, Heindel WC, Hamilton JM (2001) Cognitive abilities mediated by frontal-subcortical circuits Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders Litcher DG, Cummings JL 114 150 New York Guilford Press |
6 | Copland DA, Chenery HJ, Murdoch BE (2000) Persistant deficits in complex language function following dominant nonthalamic subcortical lesions J Med Speech Lang Pathol 8: 1 14 |
7 | Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, Gopinath K, Soltysik D, Bauer RM, Auerbach EJ, Gokcay D, Leonard CM, Briggs RW (2003) Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes J Int Neuropsychol Soc 9: 1061 1077 |
8 | Murray L (2008) Language and Parkinson’s disease ARAL 28: 113 127 |
9 | Altmann LJ, Troche MS (2011) High-level language production in Parkinson’s disease: A review Parkinsons Dis 2011: 238956 |
10 | Troche MS, Altmann LJP (2012) Sentence production in parkinson disease: Effects of conceptual and task complexity Appl Psycholinguist 33: 22 251 |
11 | Henry JD, Crawford JR (2004) Verbal fluency deficits in Parkinson’s disease: A meta-analysis J Int Neuropsychol Soc 10: 608 622 |
12 | Peran P, Demonet JF, Pernet C, Cardebat D (2004) Verb and noun generation tasks in Huntington’s disease Movement Disord 19: 565 571 |
13 | Macoir J, Fossard M, Merette C, Langlois M, Chantal S, Auclair-Ouellet N (2013) The role of basal ganglia in language production: Evidence from Parkinson’s disease J Parkinsons Dis 3: 393 397 |
14 | Gordon PC (1993) Computational and psychological models of discourse Narrative Discourse in Neurologically Impaired and Normal Aging Adults Brownnell HH, Yves J Singular, San Diego 23 46 |
15 | Hough S, Pierce M (1994) Pragmatics and treatment Language Intervention Strategies in Adult Aphasia 3 Chapey R Williams & Wilkins Baltimore |
16 | Halliday M, Hasan R (1976) Cohesion in English London Longman Group |
17 | Coelho CA (1995) Discourse production deficits following traumatic brain injury: A critical review of the recent literature Aphasiology 9: 409 429 |
18 | Ripich DN, Carpenter BD, Ziol EW (2000) Conversational cohesion patterns in men and women with Alzheimer’s disease: A longitudinal study Int J Lang Com Dis 35: 49 64 |
19 | Davis GA, Coelho CA (2004) Referential cohesion and logical coherence of narration after closed head injury Brain Lang 89: 508 523 |
20 | Andreetta S, Cantagallo A, Marini A (2012) Narrative discourse in anomic aphasia Neuropsychologia 50: 1787 1793 |
21 | DeLong MR, Wichmann T (2006) Parkinson’s disease: A circuit disorder Mov Disord 21: S13 44 45 |
22 | Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology Cell Tissue Res 318: 121 134 |
23 | Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 cases J Neurol Neurosurg Psychiatry 55: 181 184 |
24 | Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician J Psychiatr Res 12: 189 198 |
25 | Kaplan E, Goodglass H, Wientraub S (1983) Boston naming test scoring booklet Lea & Febiger Philadelphia |
26 | Wechsler D (1997) Wechsler Memory Scale III Psychological Corporation New York |
27 | Hunt KW (1965) Grammatical structures written at three grade levels National Council of Teachers of English Champaign |
28 | Van Leer E, Turkstra L (1999) The effect of elicitation task on discourse coherence and cohesion in adolescents with brain injury J Commun Disord 32: 327 348 |
29 | Holtgraves T, Fogle K, Marsh L (2013) Pragmatic language production deficits in Parkinson’s disease Adv Parkinsons Dis 2: 31 36 |
30 | Ripich DN, Terrell BY (1988) Patterns of discourse cohesion and coherence in Alzheimer’s disease J Speech Hear Disord 53: 8 15 |
31 | Glosser G, Deser T (1991) Patterns of discourse production among neurological patients with fluent language disorders Brain Lang 40: 67 88 |
32 | Ford A, Triplett W, Sudhyadhom A, Gullett J, McGregor K, FitzGerald D, Mareci T, White K, Crosson B (2013) Broca’s area and its striatal and thalamic connections: A diffusion-MRI tractography study. article 8 Front Neuroanat 7: 1 12 |
33 | Ullman MT (2004) Contributions of memory circuits to language: The declarative/procedural model Cognition 92: 231 270 |
34 | Benjamin Crosson B, Levy I M (2007) Role of the basal ganglia in language and semantics: Supporting cast Neural Basis of Semantic Memory Hart JJr, Kraut M Cambridge University Press New York 219 243 |
35 | Copland DA, Chenery HJ, Murdoch BE (2000) Processing lexical ambiguities in word triplets: Evidence of lexicalsemantic deficits following dominant nonthalamic subcortical lesions Neuropsychology 14: 379 390 |
36 | Copland D (2003) The basal ganglia and semantic engagement: Potential insights from antic priming in individuals with subcortical vascular lesions, Parkinson’s disease, and cortical lesions J Int Neuropsychol Soc 9: 1041 1052 |
37 | Grossman M, Glosser G, Kalmanson J, Morris J, Stern MB, Hurtig HI (2001) Dopamine supports sentence comprehension in Parkinson’s disease J Neurol Sci 184: 123 130 |
38 | Grossman M, Cooke A, DeVita C, Lee C, Alsop D, Detre J, Gee J, Chen W, Stern MB, Hurtig HI (2003) Grammatical and resource components of sentence processing in Parkinson’s disease: An fMRI study Neurology 60: 775 781 |
Figures and Tables
Fig.1
Mean number of cohesive ties for each narrative. *Controls produced fewer cohesive ties during typical day compared to vacation.
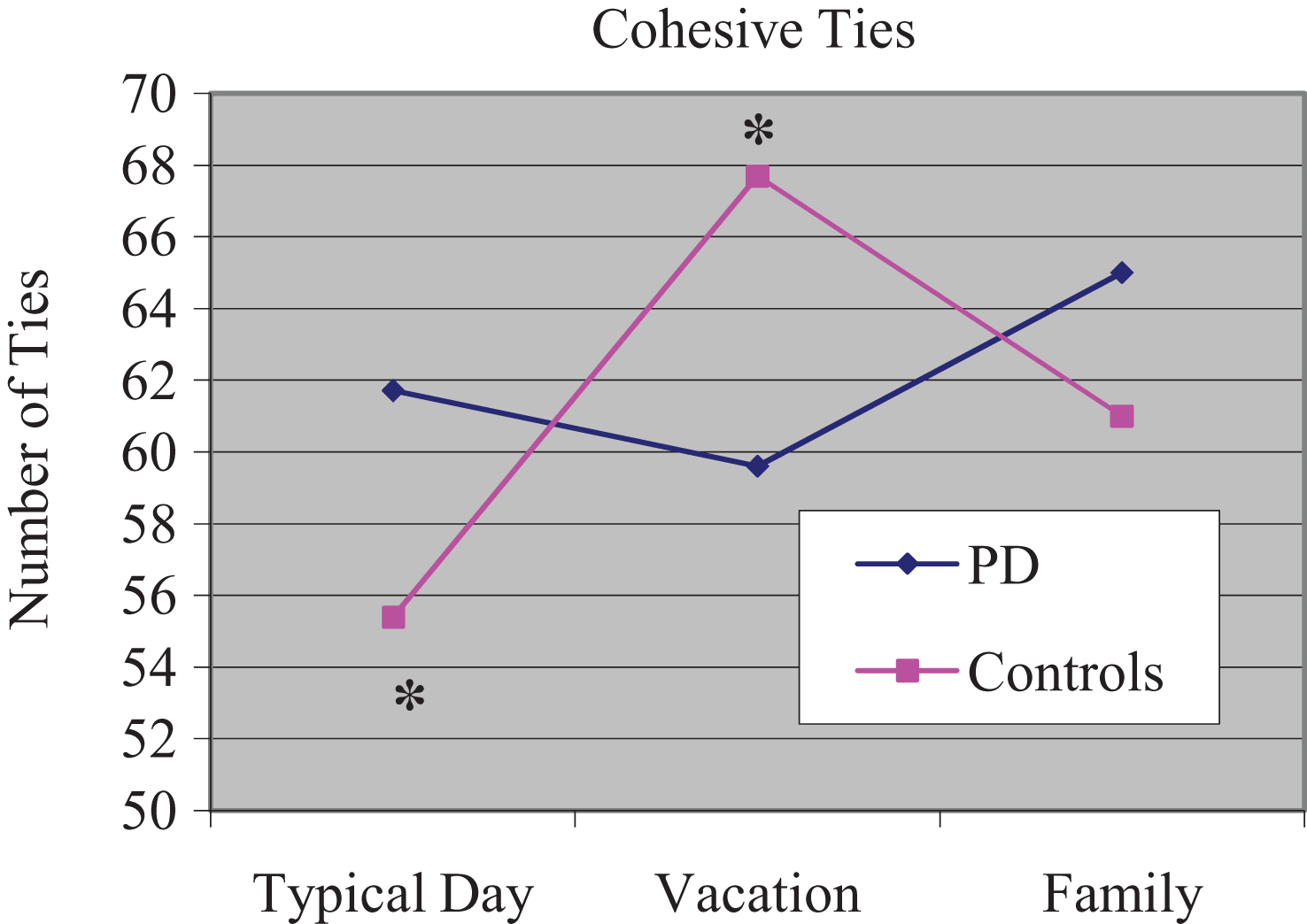
Fig.2
Percentage distribution of each cohesive tie type.
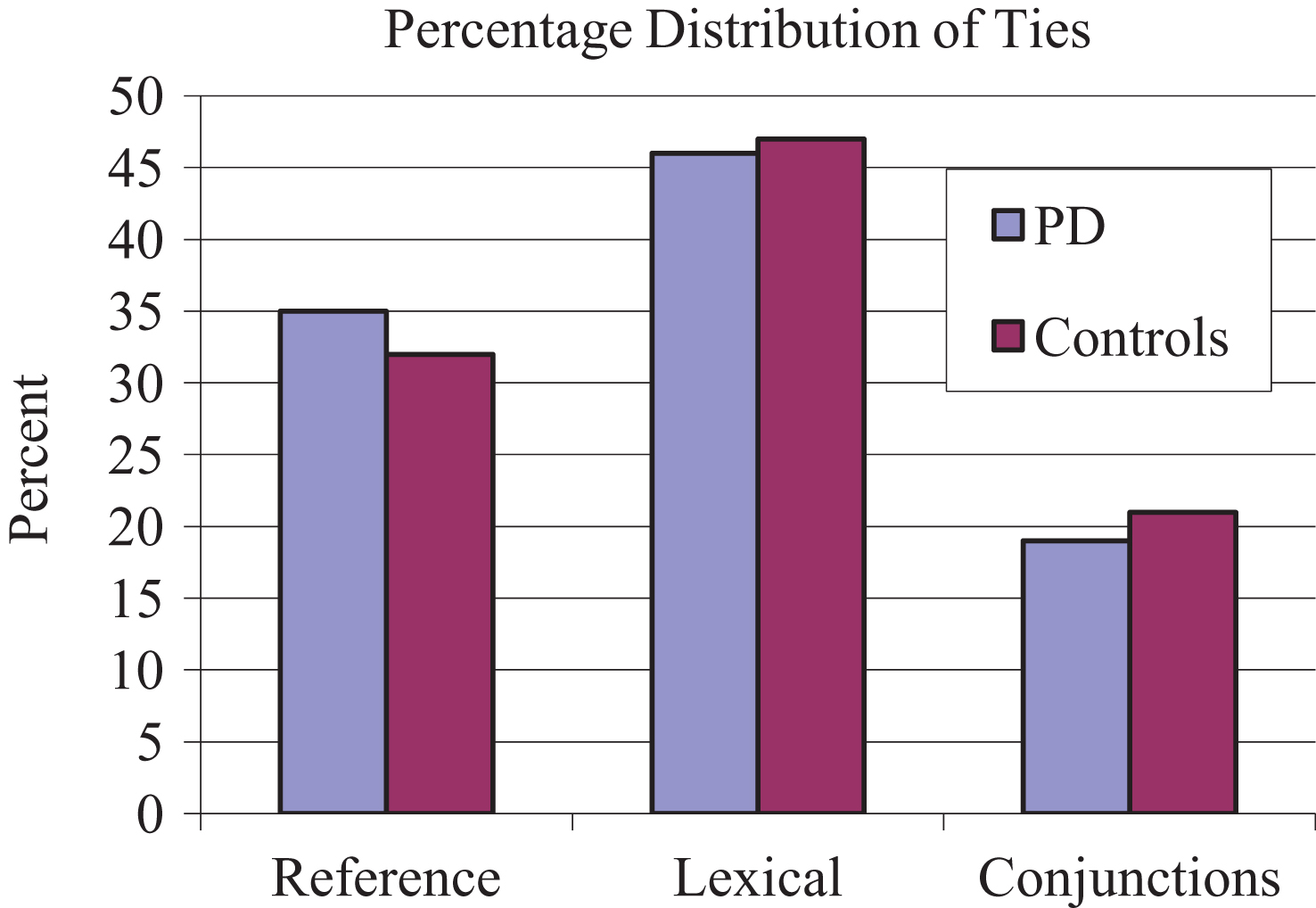
Fig.3
Percent Incomplete and Erroneous Cohesive Ties. *PD subjects produced higher percentage of incomplete and erroneous ties.
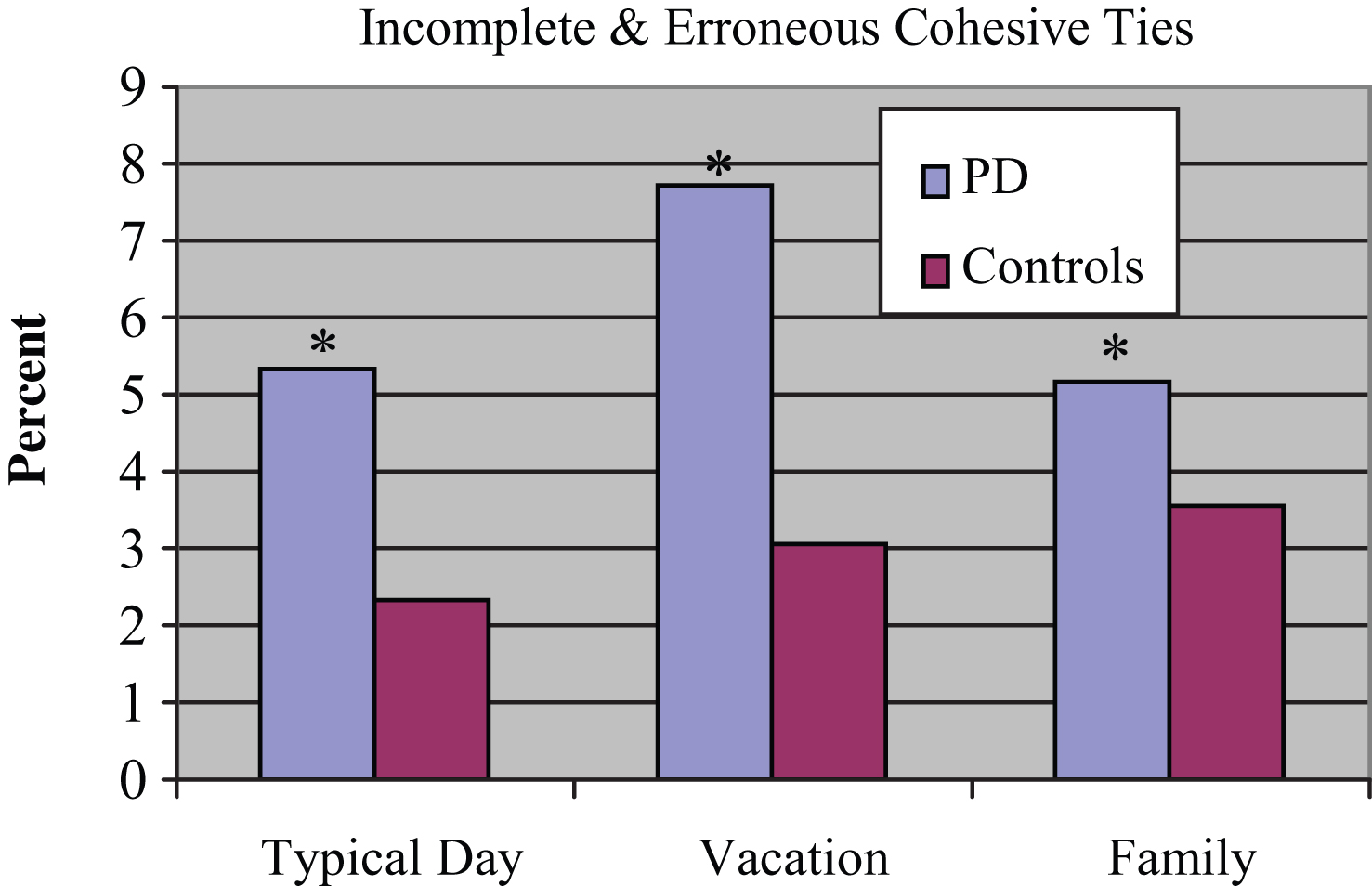
Fig.4
Number of incomplete or erroneous ties for each narrative.
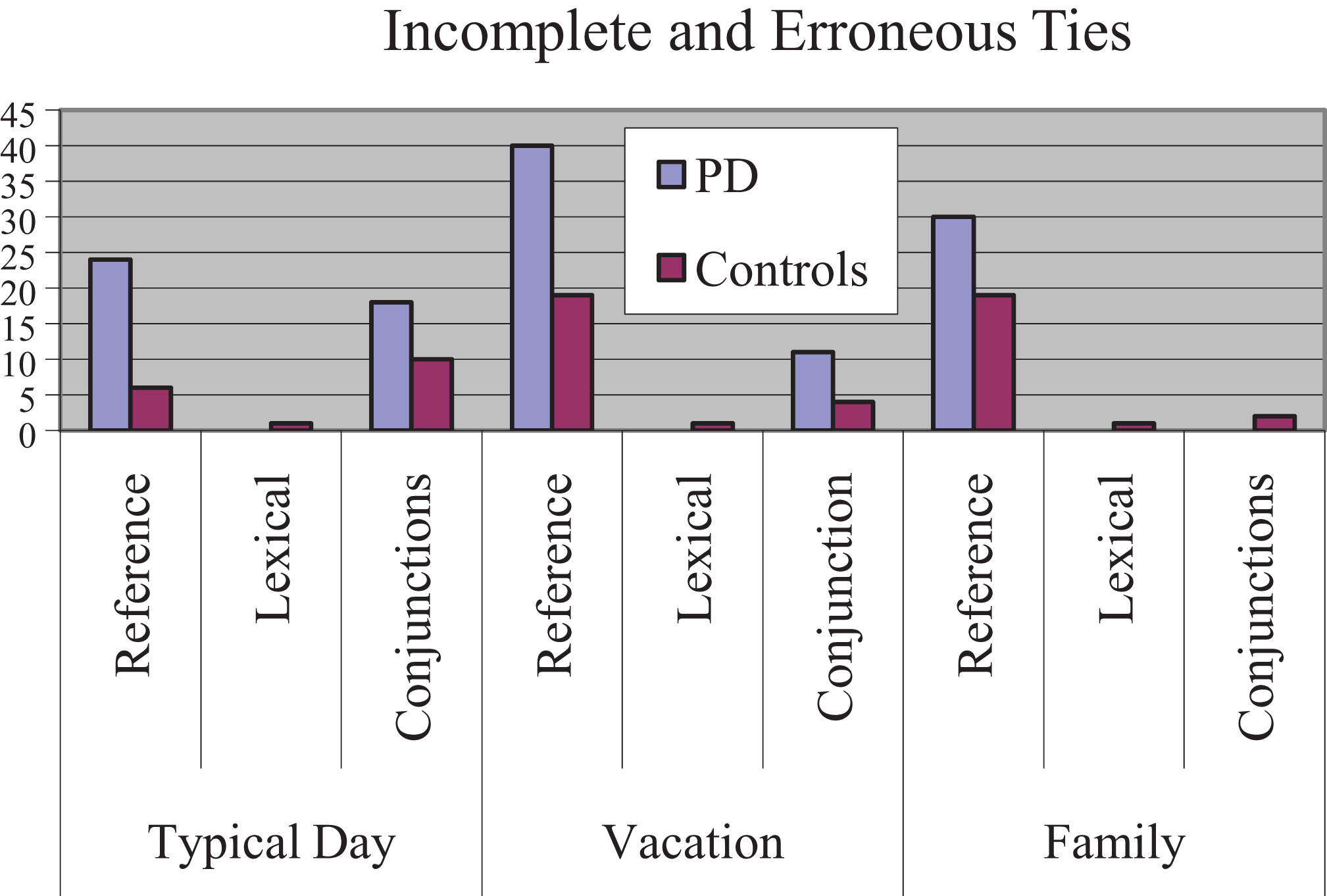
Table 1
Demographic, cognitive and language characteristics of PD subjects
| ID | Age (Years) | Education (Years) | Parkinson Years | H&Y | BNT | MMSE | WMS- LMI |
| P1 | 85 | 12 | 0 | 2 | 53 | 26 | 12 |
| P2 | 74 | 12 | 0 | 3 | 52 | 30 | 43 |
| P3 | 84 | 11 | 4 | 3 | 53 | 29 | 9 |
| P4 | 78 | 12 | 7 | 3 | 51 | 29 | 37 |
| P5 | 64 | 14 | 0.5 | 2 | 60 | 27 | 36 |
| P6 | 84 | 12 | 0 | 2 | 33 | 26 | 13 |
| P7 | 69 | 14 | 0 | 2 | 56 | 30 | 25 |
| P8 | 61 | 12 | 9 | 3 | 55 | 29 | 28 |
| P9 | 76 | 12 | 14 | 3 | 56 | 29 | 38 |
| P10 | 83 | 9 | 0 | 2 | 50 | 30 | 34 |
| P11 | 64 | 12 | 7 | 2 | 58 | 29 | 20 |
| P12 | 40 | 12 | 2 | 2 | 56 | 29 | 35 |
Parkinson Years = the number of years since PD subjects were initially diagnosed with PD. H & Y = Hoehn and Yahr. BNT = Boston Naming Test, all items administered. MMSE = Mini Mental Status Exam. WMS-LMI = Wechsler Memory Scale –Logical Memory I subtest.
Table 2
Demographic, cognitive, and language comparisons
| Variable | PD subjects | Controls | T | p | ||
| M | SD | M | SD | |||
| Age (years) | 71.8 | 13.2 | 72.6 | 13.5 | −0.14 | >0.05 |
| Education (years) | 12.0 | 1.3 | 12.8 | 2.8 | −0.94 | >0.05 |
| Parkinson Years | 3.6 | 4.6 | ||||
| H &Y stage | 2.4 | 0.5 | ||||
| BNT | 52.8 | 6.7 | 51.8 | 8.4 | 0.31 | >0.05 |
| MMSE | 28.6 | 1.4 | 28.8 | 1.7 | −0.26 | >0.05 |
| WMS-LMI | 27.5 | 11.5 | 30.6 | 14.4 | −0.58 | >0.05 |
p values are derived from comparisons of PD subjects to normal controls. Parkinson Years = the number of years since PD subjects were initially diagnosed with PD. H & Y = Hoehn and Yahr. BNT = Boston Naming Test, all items administered. MMSE = Mini Mental Status Exam. WMS-LMI = Wechsler Memory Scale –Logical Memory I subtest.




