Life Expectancy and Causes of Death in Patients with Myotonic Dystrophy Type 2
Abstract
Background:
Myotonic Dystrophy type 2 (DM2) is a dominantly inherited multisystem disease caused by a CCTG repeat expansion in intron 1 of the CNBP gene. Although in the last two decades over 1500 patients with DM2 have been diagnosed worldwide, our clinical impression of a reduced life expectancy in DM2 has not been investigated previously.
Objective:
The aim of this observational study was to determine the life expectancy and the causes of death in patients with genetically confirmed DM2.
Methods:
We identified the data of all deceased patients with DM2 in the Dutch neuromuscular database between 2000 and 2023. Ages and causes of death and the patients’ clinical features during lifetime were determined. Age of death in DM2 was compared to the general population by using life tables with prognostic cohort life expectancy (CLE) and period life expectancy (PLE) data of the Dutch electronic database of statistics (CBS StatLine).
Results:
Twenty-six deceased patients were identified in the Dutch DM2 cohort (n = 125). Median age of death in DM2 (70.9 years) was significantly lower compared to sex- and age-matched CLE (78.1 years) and PLE (82.1 years) in the Netherlands. Main causes of death were cardiac diseases (31%) and pneumonia (27%). Seven patients (27%) had a malignancy at the time of death.
Conclusion:
These results provide new insights into the phenotype of DM2. Life expectancy in patients with DM2 is reduced, possibly attributable to multiple causes including increased risk of cardiac disease, pneumonia, and malignancies. The occurrence of a significantly reduced life expectancy has implications for clinical practice and may form a basis for advanced care planning, including end-of-life care, to optimize quality of life for patients with DM2 and their family. Research in larger cohorts should be done to confirm these findings and to ascertain more about the natural course in DM2.
INTRODUCTION
Myotonic Dystrophy type 2 (DM2) is a dominantly inherited multisystem disease. In 2001, it was discovered to be caused by a CCTG repeat expansion in intron 1 of the CNBP gene on chromosome 3q21.3 [1]. DM2 typically manifests between 20 and 50 years of age and the phenotype of DM2 shows some overlap with Myotonic Dystrophy type 1 (DM1) [2, 3]. Common features are progressive muscle weakness, myotonia, pain, early onset cataracts and multiorgan involvement with cardiac conduction defects, insulin resistance and hypothyroidism. Unlike DM1, in DM2, anticipation and a severe congenital form have not been proven. Cardiac involvement, muscle weakness, and muscle wasting progress more slowly and less severely in DM2 compared to DM1 [4]. Furthermore, DM2 patients present with a predominantly proximal distribution of weakness whereas DM1 is characterized by distal and facial weakness [5]. However, the impact of DM2 on patients’ physical, psychological, and social functioning is significant and as high as in adult-onset DM1 patients [6]. Life expectancy and mean age of death are markedly reduced in DM1 [7], but have not been studied in DM2.
We therefore determined the life expectancy and causes of death in the Dutch DM2 cohort and compared these results with survival and mortality data in the Dutch general population.
METHODS
In this cross-sectional study, all known patients with DM2 in the Dutch neuromuscular database between 2000 and 2023 were identified. Data of all the registered deceased patients with DM2 in the Netherlands at the time of the survey (April 2023) was collected. The age at death, causes of death, and patients’ clinical features during life were ascertained by information from the medical records, general practitioner and/or Registry Office (CBS, B-verklaring). Sex, age at symptom onset, type of initial symptom (or first disease manifestation), and medical history were collected when available.
Life expectancy for the general Dutch population according to age, sex, and calendar year was based on life tables of the Dutch electronic database of statistics (CBS StatLine, 2023) [8, 9]. Analysis of time-trends in life expectancy will differ depending on whether it is calculated by a cohort or a period. We used both frameworks, namely cohort life expectancy (CLE) and period life expectancy (PLE).
CLE tables consider observed and expected improvements in mortality for a cohort throughout its lifetime, showing an average for a cohort, and do not include any individual factors. PLE methods use mortality rates from one single calendar year (e.g. year of birth or year of disease onset) and assume that those rates apply throughout the remainder of a person’s life [10, 11]. To determine PLE, we chose the year of disease onset of DM2 to determine the estimated remaining years of life compared to the Dutch population with the same age, in the same year of disease onset (PLE data of the general Dutch population born before 1950 was not accessible). Therefore, each patient was compared to the prognostic CLE of their own birth cohort, and PLE was determined at the age of symptom onset.
Standard protocol approvals, registrations, and patient consents
The study was approved by the local ethical standards committee (CMO Radboudumc, 2021/13229) who granted a waiver for consent as the (deceased) subjects could not be asked for consent and permission for their medical chart review. In case the deceased patient had objected to the (further) use of human tissue for medical scientific research in the past we refrained from inclusion. The study was in accord with the Helsinki Declaration of 1975. Exclusion criteria were any kind of earlier reported objections from the deceased patient to the (further) use of human tissue for medical scientific research and/or participation in medical scientific research.
Statistical analyses
Continuous data are reported as mean (standard deviation (SD)) and median (inter-quartile range (IQR)) and in nominal variables, numbers and percentages are presented. The deceased patients with DM2 were grouped into men and women, and the data regarding life-expectancy was grouped into data from the patients with DM2 and matched expectations in the Dutch population (CLE and PLE). Between-group differences were analyzed by using unpaired t-tests or Mann-Whitney U tests, as appropriate. The Kaplan-Meier method with log rank test (Mantel-Cox) was used to estimate the survival of the patients and Dutch population with the attained age or life expectancy as survival time.
RESULTS
The Dutch DM2 cohort consists of 125 genetically confirmed patients, of which 26 registered deceased patients (21%) from 16 unrelated families, including one patient who was genetically confirmed post-mortem. Clinical features are shown in Tables 1-3 and survival from the initial symptom of DM2 to death is shown in Figure 1. Cataract was the most common first disease manifestation, followed by muscle weakness and myalgia. Three patients had both muscle weakness and cataract at first presentation. The initial location of muscle weakness was noted in nine patients, which is the proximal lower extremity in all cases. Fifteen patients were wheelchair dependent shortly before their death, of whom 13 were completely wheelchair dependent.
Table 1
Patient characteristics of the included patients with DM2
| Patient | Sex | Age at death | Cause of death | Age of onset | Initial symptom | Mobility before death | Location of death |
| 1 | Male | 60 | Fractures followed a fall (euthanasia) | 23 | Cataract | Wheelchair dependent | Home |
| 2 | Male | 72 | Pneumonia | 59 | Muscle weakness, cataract | Wheelchair dependent | Hospital |
| 3 | Male | 69 | Cardiac arrest (and pneumonia) | 49 | Cataract | Walking | Home |
| 4 | Male | 74 | Heart failure | 30 | Muscle weakness | Walking | Hospital |
| 5 | Male | 39 | Sudden death | 16 | Muscle weakness, myalgia | Walking | Home |
| 6 | Male | 54 | Duodenal carcinoma (euthanasia) | 29 | Fatigue, abnormal liver function | Walking | Home |
| 7 | Male | 64 | Pneumonia | 54 | Myotonia | Walking | Hospital |
| 8 | Male | 77 | Pneumonia | 38 | Muscle weakness, abnormal liver function | Wheelchair dependent | Hospital |
| 9 | Male | 81 | Pneumonia (COVID-19) | 68 | Muscle weakness | Walking | Hospital |
| 10 | Female | 77 | Non-small-cell lung carcinoma | 50 | Muscle weakness, myalgia | Walking | Home |
| 11 | Female | 65 | General decline (weakness, dysphagia) (palliative sedation) | 58 | Muscle weakness | Wheelchair dependent | Home |
| 12 | Female | 69 | Hepatocellular carcinoma | 43 | Cataract | Partial wheelchair dependent | Hospital |
| 13 | Female | 83 | Pneumonia (aspiration) | 51 | Cataract | Wheelchair dependent | Nursing home |
| 14 | Female | 66 | Liver failure | 48 | Muscle weakness, myalgia, cataract | Walking | Hospital |
| 15 | Female | 69 | Pneumonia (aspiration) | 44 | Cataract | Wheelchair dependent | Hospital |
| 16 | Female | 76 | Sudden death | 26 | Myotonia | Wheelchair dependent | Nursing home |
| 17 | Female | 77 | Unknown | 66 | Cataract | Wheelchair dependent | Nursing home |
| 18 | Female | 63 | Cardiac arrest | 58 | Cataract | Walking | Home |
| 19 | Female | 57 | Endocarditis | 38 | Cataract | Wheelchair dependent | Hospital |
| 20 | Female | 59 | Cardiac arrest (ventricular fibrillation) | 43 | Muscle weakness, myalgia, myotonia | Walking | Hospital |
| 21 | Female | 73 | Cerebrovascular event (euthanasia) | 67 | Cataract | Walking | Home |
| 22 | Female | 76 | Heart failure | 58 | Muscle weakness, cataract | Wheelchair dependent | Nursing home |
| 23 | Female | 79 | Cardiac arrest | 65 | Muscle weakness | Wheelchair dependent | Nursing home |
| 24 | Female | 64 | Liver failure | 45 | Muscle weakness, myalgia | Wheelchair dependent | Hospice |
| 25 | Female | 74 | Pneumonia | 42 | Muscle weakness | Wheelchair dependent | Nursing home |
| 26 | Female | 78 | Heart failure | 53 | Cataract | Partial wheelchair dependent | Nursing home |
Table 2
Baseline characteristics, causes and location of death of the patients with DM2. Data shown in numbers (rounded percentages) or years. IQR: interquartile range, SD: standard deviation. *Normal: <200 U/L in men, <170 U/L in women
| Characteristics | Total | Men | Women |
| Sex n (%) | 26 | 9 (35%) | 17 (65%) |
| Age at onset (range in years) | 16–68 | 16–68 | 26–67 |
| Median age at first onset (years, IQR) | 48.5±19.0 | 38.0±25.0 | 50.0±15.0 |
| Mean age at first onset (years, SD) | 47.0±14.1 | 40.7±17.7 | 50.3±11.0 |
| Disease duration (range in years) | 5.6–50.3 | 10.5–44.2 | 5.6–50.3 |
| Median disease duration (years, IQR) | 19.9±13.0 | 23.1±23.6 | 19.5±11.2 |
| Mean disease duration (years, SD) | 22.1±11.5 | 25.1±12.4 | 21.1±11.2 |
| Initial symptom(s), n (%) | |||
| Muscle weakness | 13 (50%) | 5 (56%) | 8 (47%) |
| Cataract | 12 (46%) | 3 (33%) | 9 (53%) |
| Myalgia | 5 (19%) | 1 (11%) | 4 (24%) |
| Myotonia | 2 (8%) | 1 (11%) | 1 (6%) |
| Mobility, n (%) | |||
| Wheelchair dependent | 15 (58%) | 3 (33%) | 12 (71%) |
| Walking | 11 (42%) | 6 (67%) | 5 (29%) |
| Creatine Kinase * (range in U/L) | 62–1464 | 311–1464 | 62–1039 |
| Median Creatine Kinase levels (U/L, IQR) | 376±260 | 429±159 | 296±203 |
| Mean Creatine Kinase levels (U/L, SD) | 441±344 | 561±406 | 358±285 |
| Causes of death, n (%) | |||
| Cardiac | 8 (31%) | 2 (22%) | 6 (35%) |
| Pneumonia | 7 (27%) | 4 (44%) | 3 (18%) |
| Malignancy | 3 (12%) | 1 (11%) | 2 (12%) |
| Liver failure | 2 (8%) | 0 (0%) | 2 (12%) |
| Sudden death | 2 (8%) | 1 (11%) | 1 (6%) |
| Other | 2 (8%) | 0 (0%) | 2 (12%) |
| Fractures | 1 (4%) | 1 (11%) | 0 (0%) |
| Unknown | 1 (4%) | 0 (0%) | 1 (6%) |
| Euthanasia, n (%) | 3 (12%) | 2 (22%) | 1 (6%) |
| Location of death, n (%) | |||
| Hospital | 10 (38%) | 5 (56%) | 5 (29%) |
| Home | 8 (31%) | 4 (44%) | 4 (24%) |
| Nursing home | 7 (27%) | 0 (0%) | 7 (41%) |
| Hospice | 1 (4%) | 0 (0%) | 1 (6%) |
Table 3
Ages at death in DM2 compared to their sex- and age-matched life expectancies based respectively on CLE (from birth to death) and PLE (from symptom onset to death) methods. SD: standard deviation, IQR: interquartile range
| Age at death DM2 | Cohort Life Expectancy (CLE) | Period Life Expectancy (PLE) | |||||||
| Range | Mean | Median | Range | Median | P value | Range | Median | P value | |
| (years) | (years, SD) | (years, IQR) | (years) | (years, IQR) | (DM2–CLE) | (years) | (years, IQR) | (DM2–PLE) | |
| Total n = 26 | 39–83 | 69.4±10.0 | 70.9±12.6 | 71.8–82.4 | 78.1±4.1 | <.001 | 73.8–86.6 | 82.1±5.9 | <.001 |
| Men n = 9 | 39–81 | 65.8±13.1 | 69.2±14,1 | 71.8–82.3 | 72.6±5.8 | 0.122 | 73.8–83.5 | 76.7±2.4 | 0.024 |
| Women n = 17 | 57–83 | 71.4±7.6 | 73.9±11.7 | 76.7–82.4 | 78.2±3.3 | <.001 | 78.0–86.6 | 82.4±1.8 | <.001 |
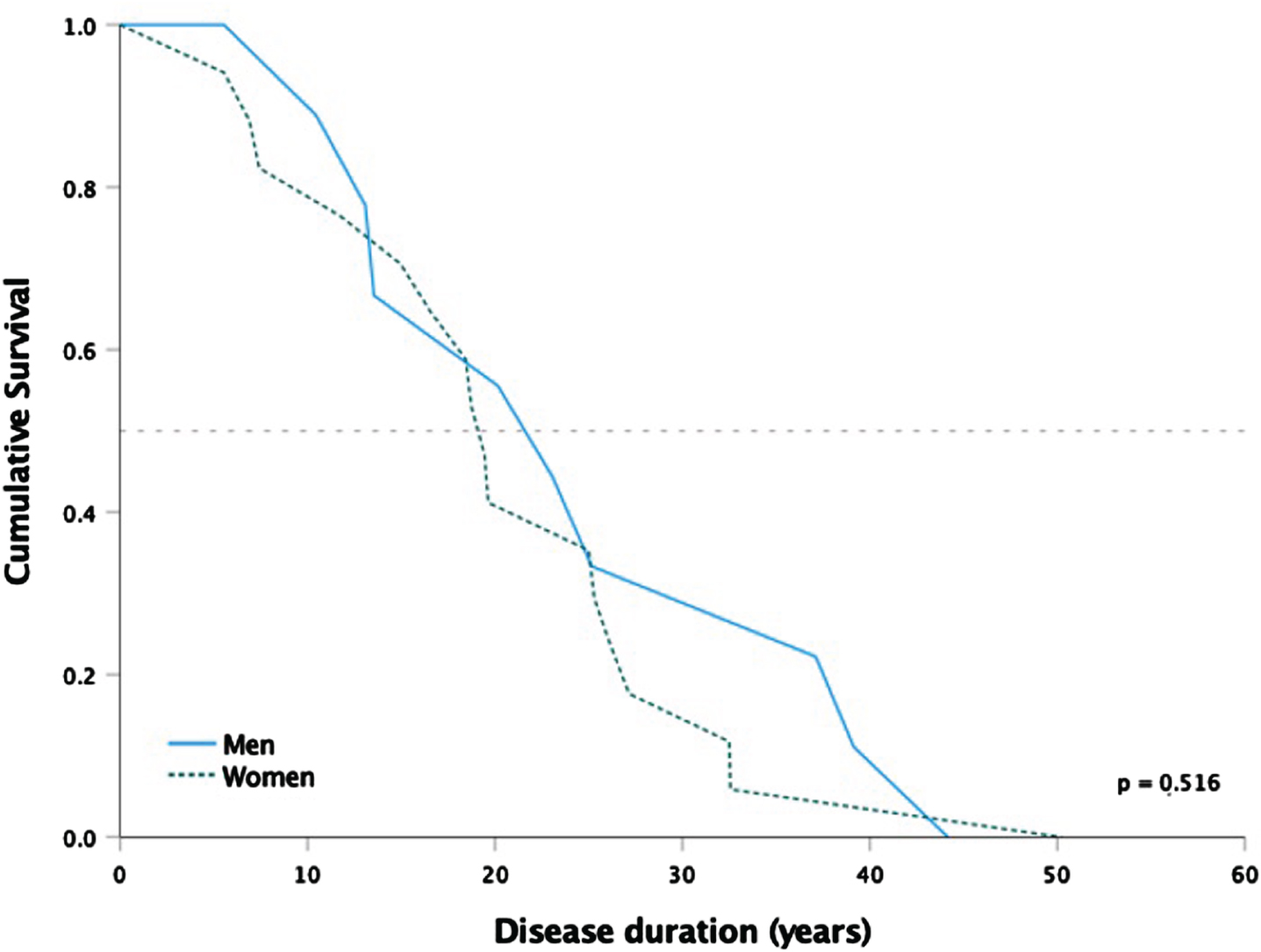
Fig. 1
Kaplan-Meier survival curves of the 26 deceased patients from first symptom to death in years. Median survival is indicated by the horizontal line. P-value from log rank test.
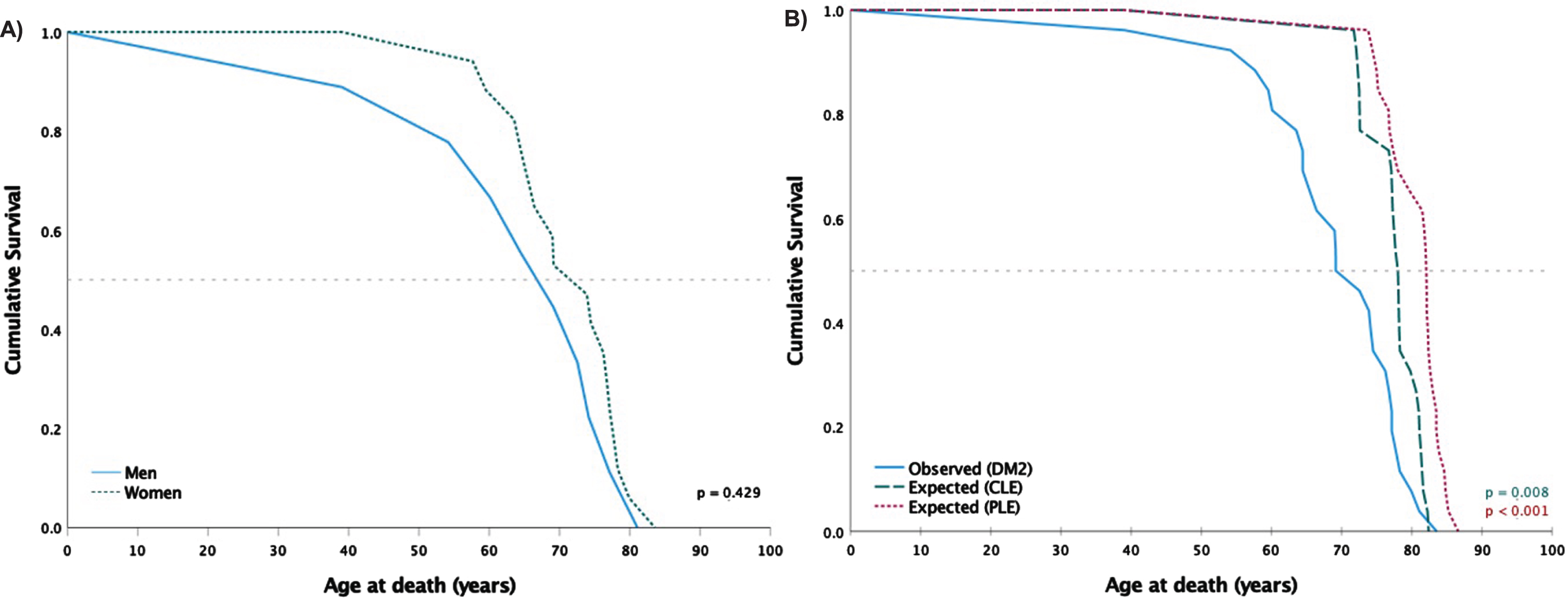
Characteristics of death, including life expectancy in the general Dutch population (CLE and PLE), are shown in Table 3 and Figure 2. The age of death in DM2 was significantly lower compared to sex- and age-matched CLE (p < 0.001) and PLE (p < 0.001) with also significant differences in the survival curves (Table 3, Figure 2B). Patients with DM2 had a mean reduced life expectancy of 8.3 and 11.4 years, respectively. As one patient was a potential outlier because of his younger age of death (39 years), data was additionally analyzed without this patient which resulted in no changes in the significance for both CLE and PLE.
Fig. 2
Kaplan-Meier survival curves of the 26 deceased patients. Median survival is indicated by the horizontal line. P-values from log rank test. A) Deceased men (n = 9) with DM2 compared to women (n = 17). B) Deceased patients with DM2 (n = 26) compared to their sex- and age-matched life expectancies based respectively on CLE (from birth to death) and PLE (from symptom onset to death) methods.
The most common cause of death was cardiac disease (31%), including cardiac arrests (n = 4), heart failure (n = 3) and endocarditis (n = 1). In five patients whom death resulted from cardiac disease, the cardiac history was available; one had a first-degree atrioventricular block, and one had a mild dilated cardiomyopathy. One patient was known to have a pacemaker (Table 1, patient 2). Survival data on the cohort without the cardiac deaths did not change our results (Supplementary Table 1).
The second most marked cause of death was pneumonia (27%) of which three patients suffered from aspiration pneumonia and one patient died due to a COVID-19 pneumonia. One more patient suffered from pneumonia at the time of death but died of a cardiac arrest.
Seven patients (27%) had a registered malignant disease at the time of their death, namely duodenal carcinoma, hepatocellular carcinoma, non-small-cell lung carcinoma, esophageal carcinoma, gastrointestinal stromal tumor, and acute myeloid leukemia, and one patient had chronic lymphocytic leukemia and a history of cutaneous melanoma. Cancer was marked as the leading cause of death in the medical records of three of these seven patients.
Two patients died of liver failure and in one patient the exact cause of death was unknown.
Three patients underwent euthanasia at the age of 54, 60, and 73 years. They suffered from malignancy, multiple complications after a fall, and ischemic stroke respectively. As euthanasia is less common in populations outside the Netherlands, we additionally performed an analysis excluding these three patients. Their removal had no impact on the overall survival scores and the significantly reduced life expectancies (Supplementary Table 2).
At least one patient was treated with palliative sedation and one patient died in a hospice. Most patients died during hospital admission; the reason for admission was not always related to the cause of death.
DISCUSSION
The main finding of this observational study on mortality in DM2 is a significantly reduced life expectancy in DM2. Additionally, we found a high frequency of cardiac disease and pneumonia as the causes of death in these patients. These findings have profound implications for clinical practice.
This study shows that the mean and median age of death in DM2 are significantly lower compared to the life expectancies of the Dutch population. Until now, the lifespan in DM2 was predicted to be normal [2]. However, we demonstrated a reduction in life expectancy of 8.3 to 11.4 years, depending on which method for the estimated life expectancy was used. We chose to use both methods (CLE and PLE) for a more accurate comparison with the life expectancy of the general population as both methods have different uncertainties. Where CLE tables consider observed and expected improvements in mortality for a cohort throughout a lifetime, PLE methods use mortality rates from one calendar year and assume that those rates apply throughout the remainder of a person’s life. PLEs tend to be lower than CLEs because they do not include any assumptions about future improvements in mortality rates [10]. However, we found higher life expectations in using the PLE method compared to the CLE; possibly because the year of symptom onset was used instead of the year of birth. PLE data of the Dutch population born before 1950 was not available, though 6/9 men and 12/17 women in our DM2 group were born before 1950. For determining the PLE, the year of disease onset of DM2 was used and compared to the life expectancy tables corresponding to the same age in the same year. PLE uses the mortality rate in one specific calendar year. Both the median disease onset of 48.5 years in our deceased patients and the fact that pain, as one of the early key symptoms in DM2, is potentially not frequently recognized as the first symptom of DM2, might have caused a higher PLE as these mortality rates improve with time. Nevertheless, the ages of death in DM2 were significantly lower compared to both CLE and PLE methods.
The median age at death in DM2 of 69.2 years for men and 73.9 years for women is higher compared to the earlier reported patients with adult-type DM1 in the Netherlands (median age of death of 60 years for men and 59 years for women) (7). However, data regarding ages at death in DM1 was collected between 1950 and 1997 and presumably medical care in DM1 and general life expectancies increased in the last decades. In addition, DM2 tends to have a more favorable natural course compared to DM1.
In this small cohort, we found a higher mean age at symptom onset (47 years) compared to the earlier reported onset in patients with DM2 (34 to 42 years; no difference between men and women) [5, 12, 13].
The most frequent causes of death in this study were cardiac diseases (31%), pneumonias (27%) and malignancies (12%). These results are different compared to the causes of death among the Dutch general population between 2000 and 2020, in which malignancies are the most frequent causes of death (27–32%), followed by cardiovascular diseases (21–35%), mental disorders and central nervous system diseases (6–14%) and respiratory diseases (6–10%) (14). First, cardiac diseases and pneumonias are well-known systemic complications of myotonic dystrophies (DM). Cardiac involvement is an important cause of premature death in patients with DM and is reported to be different and of a lesser degree in DM2 compared to DM1 [4, 15–17]. In the current study, one patient died of ventricular fibrillation and five other patients died of causes that might be due to cardiac arrhythmias (cardiac arrest and sudden death). While intraventricular and atrioventricular conduction disorders are more common in patients with DM1, patients with DM2 present more frequently with tachyarrhythmias, such as supraventricular and ventricular arrhythmias, left ventricular diastolic dysfunction, and hypertension [18, 19]. Cardiomyopathy (mostly the dilated type) was reported in 18% of the DM2 patients [19]. Sudden cardiac death had been described in patients with DM2, possibly caused by dilated cardiomyopathy and conduction system fibrosis [16]. Two patients in this study suffered sudden nocturnal death (age 39 and 76 years); neither patient had a history of cardiac disease (including screening) or a pacemaker nor did they have epilepsy. Altogether, these cardiac diseases are a potential risk for cardiac death. As this study primarily focused on the age and causes of death including disease characteristics, information about the patients’ complete medical (cardiac) history is lacking. In the Netherlands, patients diagnosed with DM2 have regular cardiac follow-ups according to the DM1 guidelines, making the potential impact of undiscovered cardiac disease as a cause of early cardiac deaths in DM2 of a limited value.
Next, a high percentage of patients died of (aspiration)pneumonia, and one other patient was known to have dysphagia (without pneumonia). Limited data are available regarding respiratory function in DM2 patients; respiratory impairment was found in 6–15% of patients with DM2 [13, 20]. Dysphagia has been described among patients with DM2, however, without leading to aspiration pneumonia [21].
Furthermore, seven patients were known to have eight different malignancies (27%). Five out of these eight malignancies have been reported previously in literature in patients with DM, though hepatocellular carcinoma, gastrointestinal stromal tumor, and duodenal carcinoma were not reported before in DM. Previous literature shows an increased risk of malignancies in patients with DM in general, representing the third leading cause of death with a cancer-related death up to 10–15% [7, 22, 23]. Several potential pathogenic mechanisms underlying the DM carcinogenesis have been described [24]. The cancers in DM most often include the thyroid, endometrium, ovary, colon/rectum, testis, and brain or are melanoma and non-Hodgkin lymphoma [25]. However, cancer risk in specific patients with DM2 is less well studied and probably underestimated, as DM2 is frequently underdiagnosed. Cancer-related death may well be higher in DM2 compared to DM1 as respiratory and cardiac complications in these patients are less frequent. Win et al. showed a high risk of prostate cancer and possibly thyroid cancer in patients with DM2 [26]. We assume that the percentage of patients with malignancies in our cohort (27%) may be underestimated, as this study primarily focused on the ages and causes of death. Additionally, in some cases, a full medical history was lacking in the patients’ medical records. However, malignancies are also common in the general population and the leading cause of death in the general Dutch population (around 30% as well) [14], making it impossible to determine if this percentage is attributable to DM2. More research regarding the pathophysiology, types, and prevalence of malignancies in DM2 specifically is needed.
Two patients died of liver failure and two other patients had abnormal liver function as possible initial symptom of DM2. In both DM1 and DM2, liver problems and liver enzyme abnormalities have been described [27, 28].
Finally, two patients died of suggested muscle-related symptoms caused by DM2; one due to general decline with weakness and dysphagia, and another to the complications of a hip fracture following a fall, possibly caused by muscle weakness. It is quite conceivable that fractures could follow a spontaneous fall, as there is a high incidence of falling in DM and fifty percent of falls result in an injury to these patients [29].
Three patients received euthanasia (12%), which is a higher percentage than reported in the general Dutch population (1.7% in 1990; 4.5% between 2015 and 2021) [30, 31]. In one out of three of these patients, the wish for euthanasia might be indirectly related to DM2 (complications after a fall). The malignant disease might be related as discussed before, though this is uncertain in the ischemic stroke.
One of the strengths of this study is its adequate internal validity. As all registered deceased patients with DM2 in the Netherlands were included, the presence of a selection or observational bias seems unlikely. Nevertheless, there are some limitations as this study was applied to the relatively small Dutch DM2 cohort and primarily focused on the deceased patients and their ages and causes of death. Unfortunately, survival data of the Dutch DM2 cohort overall was not accessible within the parameters of the ethical approval granted for this study but would be of interest in future studies. Ages of death were compared to estimated survival data from life tables instead of human living control groups. Additionally, we considered some outlier bias as one patient was a potential outlier with a younger age of death. To reduce this risk of outlier bias, we also performed statistical analyses without this patient and used medians as well as means. Inclusion of patients with DM2 in the international DM registry and/or further research in larger and complete cohorts should be done to confirm our findings and to learn more about DM2 with respect to the survival and natural course.
In conclusion, the occurrence of a significantly reduced life expectancy in DM2 and cardiac disease and pneumonia as the most frequent causes of death provides new insight into the phenotype of the multisystem disease DM2. These findings highlight the importance for cardiac and pulmonary screening in DM2 and call for more attention for palliative care, end-of-life care, and/or advanced care planning. At this moment, guidelines and literature regarding palliative care and advanced care planning in neuromuscular diseases (except for motor neuron diseases) are scarce (32-34). Additionally, the latest consensus-based care recommendations for adults with DM2 did not discuss this topic (35). To assist medical practitioners, future guidelines should also include recommendations on patient education and advanced care planning, to optimize medical care for patients with DM2.
ACKNOWLEDGMENTS
The authors would like to acknowledge J.G. Blommestein, J.K. Bruins, M.J. Dam-Noordzij, J.A.A. Everts, R.A.J. Hoogervorst, F. de Jongh, E. Marcos, T.J. van der Ploeg, J. Raaphorst, J.P.A. Samijn, L.F. Schipper, I. van Tubergen-Hollander, W.A. van der Vlist and M. Zahrai for providing additional information. Several authors of this publication are members of the Radboudumc Center of Expertise for neuromuscular disorders (Radboud-NMD), the Netherlands, Neuromuscular Center (NL-NMD) and the European Reference Network for rare neuromuscular diseases (EURO-NMD).
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-240089.
REFERENCES
[1] | Liquori CL , Ricker K , Moseley ML , Jacobsen JF , Kress W , Naylor SL , et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. (2001) ;293: (5531):864–7. . |
[2] | Meola G . Myotonic dystrophy type 2: The 2020 update. Acta Myol. (2020) ;39: (4):222–34. |
[3] | Meola G . Clinical and genetic heterogeneity in myotonic dystrophies. Muscle Nerve. (2000) ;23: (12):1789–99. |
[4] | Sansone VA , Brigonzi E , Schoser B , Villani S , Gaeta M , De Ambroggi G , et al. The frequency and severity of cardiac involvement in myotonic dystrophy type 2 (DM2): Long-term outcomes. Int J Cardiol. (2013) ;168: (2):1147–53. |
[5] | Day JW , Ricker K , Jacobsen JF , Rasmussen LJ , Dick KA , Kress W , et al. Myotonic dystrophy type Molecular, diagnostic and clinical spectrum. Neurology. (2003) ;60: (4):657–64. |
[6] | Tieleman AA , Jenks KM , Kalkman JS , Borm G , van Engelen BG . High disease impact of myotonic dystrophy type 2 on physical and mental functioning. J Neurol. (2011) ;258: (10):1820–6. |
[7] | de Die-Smulders CE , Howeler CJ , Thijs C , Mirandolle JF , Anten HB , Smeets HJ , et al. Age and causes of death in adult-onset myotonic dystrophy. Brain. (1998) ;121: (Pt 8):1557–63. |
[8] | CBS StatLine. Life expectancy: Sex and birth generation. 16-12-2022 ed: CBS; (2023) . |
[9] | CBS StatLine. Life expectancy: Sex and ages (per year and period of five years). CBS; (2023) . |
[10] | Buxton J . Period and cohort life expectancy explained. A guide to the two types of life table – cohort and period – used to calculate past and projected life expectancy.: Office for National Statistics (ONS); (2023) [updated 19-01-2023. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/methodologies/periodandcohortlifeexpectancyexplained. |
[11] | van der Meulen A . Life tables and Survival analysis. The Hague/Heerlen: Statistics Netherlands; (2012) . |
[12] | Hilbert JE , Ashizawa T , Day JW , Luebbe EA , Martens WB , McDermott MP , et al. Diagnostic odyssey of patients with myotonic dystrophy. J Neurol. (2013) ;260: (10):2497–504. |
[13] | Montagnese F , Mondello S , Wenninger S , Kress W , Schoser B . Assessing the influence of age and gender on the phenotype of myotonic dystrophy type 2. J Neurol. (2017) ;264: (12):2472–80. |
[14] | Traag T , Hoogenboezem J . Doodsoorzaken 2000-2020.2021. Available from: https://www.cbs.nl/nl-nl/longread/statistische-trends/2021/doodsoorzaken-2000-2020?onepage=true. |
[15] | Ha AH , Tarnopolsky MA , Bergstra TG , Nair GM , Al-Qubbany A , Healey JS . Predictors of atrio-ventricular conduction disease, long-term outcomes in patients with myotonic dystrophy types I and II. Pacing Clin Electrophysiol. (2012) ;35: (10):1262–9. |
[16] | Schoser BG , Ricker K , Schneider-Gold C , Hengstenberg C , Durre J , Bultmann B , et al. Sudden cardiac death in myotonic dystrophy type 2. Neurology. (2004) ;63: (12):2402–4. |
[17] | Benhayon D , Lugo R , Patel R , Carballeira L , Elman L , Cooper JM . Long-term arrhythmia follow-up of patients with myotonic dystrophy. J Cardiovasc Electrophysiol. (2015) ;26: (3):305–10. |
[18] | Bienias P , Lusakowska A , Ciurzynski M , Rymarczyk Z , Irzyk K , Kurnicka K , et al. Supraventricular and Ventricular Arrhythmias Are Related to the Type of Myotonic Dystrophy but Not to Disease Duration or Neurological Status. Pacing Clin Electrophysiol. (2016) ;39: (9):959–68. |
[19] | Peric S , Bjelica B , Aleksic K , Kovacevic M , Cvitan E , Mandic Stojmenovic G , et al. Heart involvement in patients with myotonic dystrophy type 2. Acta Neurol Belg. (2019) ;119: (1):77–82. |
[20] | Sansone VA , Gagnon C , participants of the 207th EW. 207th ENMC Workshop on chronic respiratory insufficiency in myotonic dystrophies: Management and implications for research, 27–29 June 2014, Naarden, The Netherlands. Neuromuscul Disord. (2015) ;25: (5):432–42. |
[21] | Tieleman AA , Knuijt S , van Vliet J , de Swart BJ , Ensink R , van Engelen BG . Dysphagia is present but mild in myotonic dystrophy type 2. Neuromuscul Disord. (2009) ;19: (3):196–8. |
[22] | Fernandez-Torron R , Garcia-Puga M , Emparanza JI , Maneiro M , Cobo AM , Poza JJ , et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology. (2016) ;87: (12):1250–7. |
[23] | Gadalla SM , Pfeiffer RM , Kristinsson SY , Bjorkholm M , Hilbert JE , Moxley RT , 3rd, et al. Quantifying cancer absolute risk and cancer mortality in the presence of competing events after a myotonic dystrophy diagnosis. PLoS One. (2013) ;8: (11):e79851. |
[24] | D’Ambrosio ES , Gonzalez-Perez P . Cancer and Myotonic Dystrophy. J Clin Med. (2023) ;12: (5):1939. |
[25] | Emparanza JI , Lopez de Munain A , Greene MH , Matheu A , Fernandez-Torron R , Gadalla SM . Cancer phenotype in myotonic dystrophy patients: Results from a meta-analysis. Muscle Nerve. (2018) ;58: (4):517–22. |
[26] | Win AK , Perattur PG , Pulido JS , Pulido CM , Lindor NM . Increased cancer risks in myotonic dystrophy. Mayo Clin Proc. (2012) ;87: (2):130–5. |
[27] | Heatwole C , Johnson N , Goldberg B , Martens W , Moxley R , 3rd. Laboratory abnormalities in patients with myotonic dystrophy type 2. Arch Neurol. (2011) ;68: (9):1180–4. |
[28] | Hilbert JE , Barohn RJ , Clemens PR , Luebbe EA , Martens WB , McDermott MP , et al. High frequency of gastrointestinal manifestations in myotonic dystrophy type 1 and type 2. Neurology. (2017) ;89: (13):1348–54. |
[29] | Berends J , Tieleman AA , Horlings CGC , Smulders FHP , Voermans NC , van Engelen BGM , et al. High incidence of falls in patients with myotonic dystrophy type 1 and 2: A prospective study. Neuromuscul Disord. (2019) ;29: (10):758–65. |
[30] | van der Heide A , van Delden JJM , Onwuteaka-Philipsen BD . End-of-Life Decisions in the Netherlands over 25 Years. N Engl J Med. (2017) ;377: (5):492–4. |
[31] | Jaarverslag Regionale Toetsingscommissies Euthanasie 2021. Available from: https://www.euthanasiecommissie.nl/uitspraken/jaarverslagen/2021/maart/31/jaarverslag-2021. |
[32] | Elverson J , Evans H , Dewhurst F . Palliation, end of life care and ventilation withdrawal in neuromuscular disorders. Chron Respir Dis. (2023) ;20: , 14799731231175911. |
[33] | de Visser M , Oliver DJ . Palliative care in neuromuscular diseases. Curr Opin Neurol. (2017) ;30: (6):686–91. |
[34] | Carter GT , Joyce NC , Abresch AL , Smith AE , VandeKeift GK . Using palliative care in progressive neuromuscular disease to maximize quality of life. Phys Med Rehabil Clin N Am. (2012) ;23: (4):903–9. |
[35] | Schoser B , Montagnese F , Bassez G , Fossati B , Gamez J , Heatwole C , et al. Consensus-based care recommendations for adults with myotonic dystrophy type 2. Neurol Clin Pract. (2019) ;9: (4):343–53. |




