Patient-Reported Outcome Measures in Neuromuscular Diseases: A Scoping Review
Abstract
Patient-reported outcome measures (PROMs) are valuable in comprehensively understanding patients’ health experiences and informing healthcare decisions in research and clinical care without clinicians’ input. Until now, no central resource containing information on all PROMS in neuromuscular diseases (NMD) is available, hindering the comparison and choice of PROMs used to monitor NMDs and appropriately reflect the patient’s voice. This scoping review aimed to present a comprehensive assessment of the existing literature on using PROMs in children and adults with NMD. A scoping methodology was followed using Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) and COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines to assess the literature on PROMs in NMDs. Eligibility criteria encompassed articles describing psychometric development or evaluation of generic or disease-specific PROM-based instruments for adults and children with specific NMDs. The data charting process involved extracting measurement properties of included PROMs, comprising validity, reliability, responsiveness, and interpretability information. The review identified 190 PROMs evaluated across 247 studies in individuals with NMDs. The majority of PROMs were disease specific. The physical functioning domain was most assessed. Validity was the most frequently investigated measurement property, with a limited number of PROMs sufficiently evaluated for a range of psychometric characteristics. There is a strong need for further research on the responsiveness and interpretability of PROMs and the development of PROMs on social functioning in NMD.
INTRODUCTION
Patient Reported Outcomes (PROs) enable patients to report their symptoms, daily functioning, quality of life (QoL), and other aspects of their well-being. PROs are the responses patients provide in various forms, such as questionnaires, interviews, or surveys. PROs are valuable for understanding how a patient feels and functions from their perspective [1]. In addition, Patient Reported Outcome Measures (PROMs) are instruments or tools designed to collect, quantify, and evaluate PRO data in a standardized and consistent manner. These measures tend to be questionnaires with standardized questions and response options. They are valuable for comparing and analyzing PRO data across patient populations, treatments, or time points. Using PROMs in clinical practice, research, and health policy assessments ensures that treatment plans and evaluations focus on the patient rather than the disease and adds to the Quintuple Aim by including the patient’s perspective in healthcare decisions. The Quintuple Aim in healthcare is an expansion of the previously established Triple Aim and Quadruple Aim frameworks to enhance the performance of health systems. The original Triple Aim focused on three primary objectives: improving the health of populations, enhancing patient care experiences, and reducing healthcare costs. The Quadruple Aim added a fourth goal to enhance the work life of healthcare providers, including clinicians and staff, addressing issues such as burnout. The Quintuple Aim goes further by incorporating a fifth dimension: advancing health equity. This aims to ensure that every person has an equal opportunity to achieve their full health potential, regardless of their social, economic, or demographic background. The patients are the key experts when considering symptoms, functioning, and perceived health. Therefore, patients must be involved from the beginning when developing measurement instruments, rather than only as people being assessed [2, 3]. Neuromuscular diseases (NMDs), most frequently chronic and progressive diseases, affect and are affected by broader aspects of people’s lives, such as their relationships and social support. When diagnosing an NMD, professionals often focus on biological and physiological variables and symptom status.
In contrast, functional status, general health perceptions, and QoL are valuable because they provide a more holistic assessment of the impact of symptoms on a person’s overall functioning (Vand, [4]. The number of clinical trials and the use of PROMs in clinical practice and research in NMD is expected to continue to increase. This will require more research on the measurement properties of PROMs in patients with NMDs, including rigorous systematic reviews of the existing evidence and new research on determining the measurement properties of current instruments.
In general, PROMs can be generic or disease-specific [5]. Generic PROMs assess the impact of NMD on a patient’s overall health and well-being and are designed for their use in a broad range of diseases or interventions. They often measure health-related quality of life (HR-QoL), e.g., the Short Form Health Survey 36 (SF-36). Contrary to generic PROMs, disease-specific PROMs are focused on a specific disease and designed to target the unique aspects, symptoms, or issues faced by patients of a particular disease or group of diseases (e.g., the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALS-FRS). The disease-specific PROMs are often considered more sensitive to health-status changes than generic PROMs [6].
PROMs may provide a patient-friendly, location-independent, time-efficient, and cost-effective approach to monitoring NMD. Furthermore, implementing PROMs in both children and adult populations enables a direct comparison of outcomes across different age groups, thus enhancing the comprehensiveness of clinical research and improving the tailoring of intervention strategies. The FDA and EMA strongly encourage the integration of patient-reported outcome measures (PROMs) in clinical trials. However, for a PROM to be useful, it must be valid, reliable, responsive, usable, and applicable to the patient’s population of interest [7]. The quality of a measurement instrument is based on the quality of its development process. The fact that an instrument has been used frequently in previous studies does not guarantee its quality. Strong psychometric evidence is essential to establish how a PROM will react in a research setting. When using PROMs not comprehensively validated, there is an increased risk of committing type I and type II errors [8]. There is a tendency for researchers to focus on validity and reliability rather than other equally important measurement properties such as responsiveness and interpretability. Additional studies of responsiveness and interpretability will provide a more in-depth understanding of how PROMs capture patient experiences. An important point to keep in mind is that validation of a scale is not an “all-or-none” process. Still, it is instead an ongoing, iterative process tailored to particular populations and settings.
Moreover, various types of validity, including criterion validity, construct validity, and discriminant validity, as well as different measures of reliability like test–retest and internal consistency, have distinct definitions. [9]. Validation efforts of PROMS in NMD are expected to become more challenging owing to the changing therapeutic environment with increasing numbers of people with NMD receiving disease-modifying treatments, which will require careful study design and patient selection. Developing a PROM is only valuable if a systematic review shows a noticeable gap that cannot be solved using existing PROM(s).
Until now, information about the measurement properties of PROMs in NMDs has been spread across numerous reports, which has hindered the comparison and choice of PROMs in NMDs. It is important to have an overview of the suitable instruments for this task to support the selection of PROMs that can be used in research and clinical care.
This scoping review aims to present a comprehensive assessment of the existing literature on the measurement properties of PROMs in individuals with NMD in children and adult populations by synthesizing the current literature.
MATERIALS AND METHODS
This review utilized a scoping methodology to assess the literature examining PROMs in NMDs. To improve the transparency, reporting, and applicability of the review, the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) and COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) guidelines were followed [10, 11]. The PRISMA-ScR guideline aims to standardize the reporting and methods of scoping reviews. The COSMIN checklist was developed in an international Delphi study to evaluate the methodological quality of health status questionnaires [12]. These measurement properties include content validity, internal consistency, criterion validity, construct validity, reproducibility (consisting of agreement and reliability), responsiveness (also referred to as longitudinal validity), floor and ceiling effects, and interpretability [13]. Due to the large number of expected articles, we focused on assessing the PROMs themselves rather than the quality of the articles. There is no previously published protocol for this scoping review, as the International Prospective Register of Systematic Reviews (PROSPERO) does not accept scoping reviews, literature reviews, or mapping reviews [14].
Eligibility criteria for articles
1. The article describes the psychometric development or evaluation of a generic or disease-specific PROM-based instrument in adults and children with NMD.
2. The following NMDs are included:
a. Myopathies
i. Congenital myopathies
ii. Dystrophic myopathies
iii. Metabolic myopathies
iv. Rare acquired myopathies
b. Rare peripheral nerve diseases: inflammatory and inherited neuropathies
c. Neuromuscular junction disorders: congenital and autoimmune myasthenic syndromes
d. Motor neuron diseases
3. PROMs were required to address one of the following domains:
a. Quality of life
b. Physical functioning
c. Swallowing
d. Pulmonary functioning
e. Fatigue/sleep quality
f. Pain
g. Work/social functioning
h. Psychological well-being and mental health
Any study, including experimental, observational, or review, was considered if inclusion criteria were met. Articles unavailable in English were excluded. If full-text articles or necessary data remained unobtainable, the article was excluded.
Search
The following bibliographic databases were searched to identify potentially relevant scientific articles: MEDLINE, EMBASE, and CINAHL. RP drafted the search strategies with help from an experienced librarian. The final search strategies can be found in the supplementary materials. The literature search was completed in August 2023. The final search results were exported into EndNote, and duplicates were removed. Search results were initially screened by title and abstract, followed by a full-text review of the selected articles. During the full-text review, forward and backward reference searching was used to identify any additional articles not captured during the initial search (NV). Disease domain experts for EURO-NMD reviewed the included PROMs to ensure no PROMs were missing.
Data charting process
The full texts of potentially eligible papers were evaluated. The reviewers extracted measurement properties of included PROMs as recommended by COSMIN. Data extraction was conducted through a single-pass extraction method by CG, BG, JG, RP, and SM, wherein a single reviewer independently extracted relevant information from each included study. Due to the large volume of articles, we opted for a single-reviewer process. Nevertheless, the first author (NV) conducted an integrity check of all the articles. The extraction process involved systematically capturing key data elements. To enhance reliability and minimize bias, the extraction was performed in a standardized manner, with a predefined data extraction form guiding the process (see supplementary materials). The extracted data were subsequently compiled into a comprehensive summary to facilitate the scoping review’s overarching objective of mapping the existing literature. Disagreements on study selection and data extraction were resolved by consensus and discussion with other reviewers if needed. All reviewers were researchers with expertise in NMD.
All authors together developed a data-charting form to determine which variables to extract., This review intended not to evaluate or determine an optimal PROM but to compile the available evidence to inform clinicians and researchers. Hence, the COSMIN risk of bias checklist, Quality Criteria, and GRADE assessments were not applied as part of this study [11]. PROM characteristics included in the review were validity, reliability, responsiveness, and interpretability. The COSMIN panel defines validity as ‘the degree to which an instrument truly measures the construct(s) it purports to measure [12].’ In general, three different types of validity can be distinguished: content validity, criterion validity, and construct validity. Content validity focuses on whether the content of the instrument corresponds with the construct one intends to measure regarding relevance and comprehensiveness. Criterion validity, applicable in situations where there is a gold standard for the construct to be measured, refers to how well the scores of the measurement instrument agree with the scores on the gold standard. Construct validity, applicable in situations with no gold standard, refers to whether the instrument provides the expected scores based on existing knowledge about the construct [12].
Reliability ‘is the extent to which scores for patients who have not changed are the same for repeated measurement under several conditions: e.g., using different sets of items from the same multi-item measurement instrument (internal consistency); over time (test–retest), by different individuals on the same occasion (inter-rater); or by the same individuals (i.e., raters or responders) on different occasions (intra-rater)’ [12].
The COSMIN panel defines responsiveness as ‘the ability of an instrument to detect change over time in the construct to be measured’ [12].
Finally, the COSMIN panel defined interpretability as ‘the degree to which one can assign qualitative meaning. That is clinical or commonly understood connotations to an instrument’s quantitative scores or change in scores. In other words, it is the degree to which it is clear what the score (or change between scores) means. Interpretability is not a measurement property, like validity and reliability, because it does not refer to the quality of an instrument. It refers to what the scores on an instrument mean. However, interpretability was considered sufficiently important by the COSMIN panel to be included in the COSMIN taxonomy [12]. Combined, these measurement properties can be used to describe how well a particular PROM reflects an underlying construct if the PROM can detect changes, and how much error occurs during its use.
Quality of measurement properties
The measurement properties reported by each included study were evaluated using the COSMIN quality criteria [12]. Each measurement property presented in the selected studies was rated as ‘good (++)’ (i.e., when statistical psychometric indexes adequately met COSMIN’s quality criteria), ‘sufficient (+)’ (i.e., when statistical psychometric indexes sufficiently met COSMIN’s quality criteria), ‘insufficient (–)’ (i.e., when the statistical psychometric indexes did not meet COSMIN’s quality criteria), or ‘undetermined (?)’ (i.e., when the appropriate information about measurement indices was not provided) [11]. PROMs with limited information on measurement properties were not further evaluated. Given the potential risks and bias of using outcome measures without documented metrological properties, it was unlikely that such measures would be recommended for use.
RESULTS
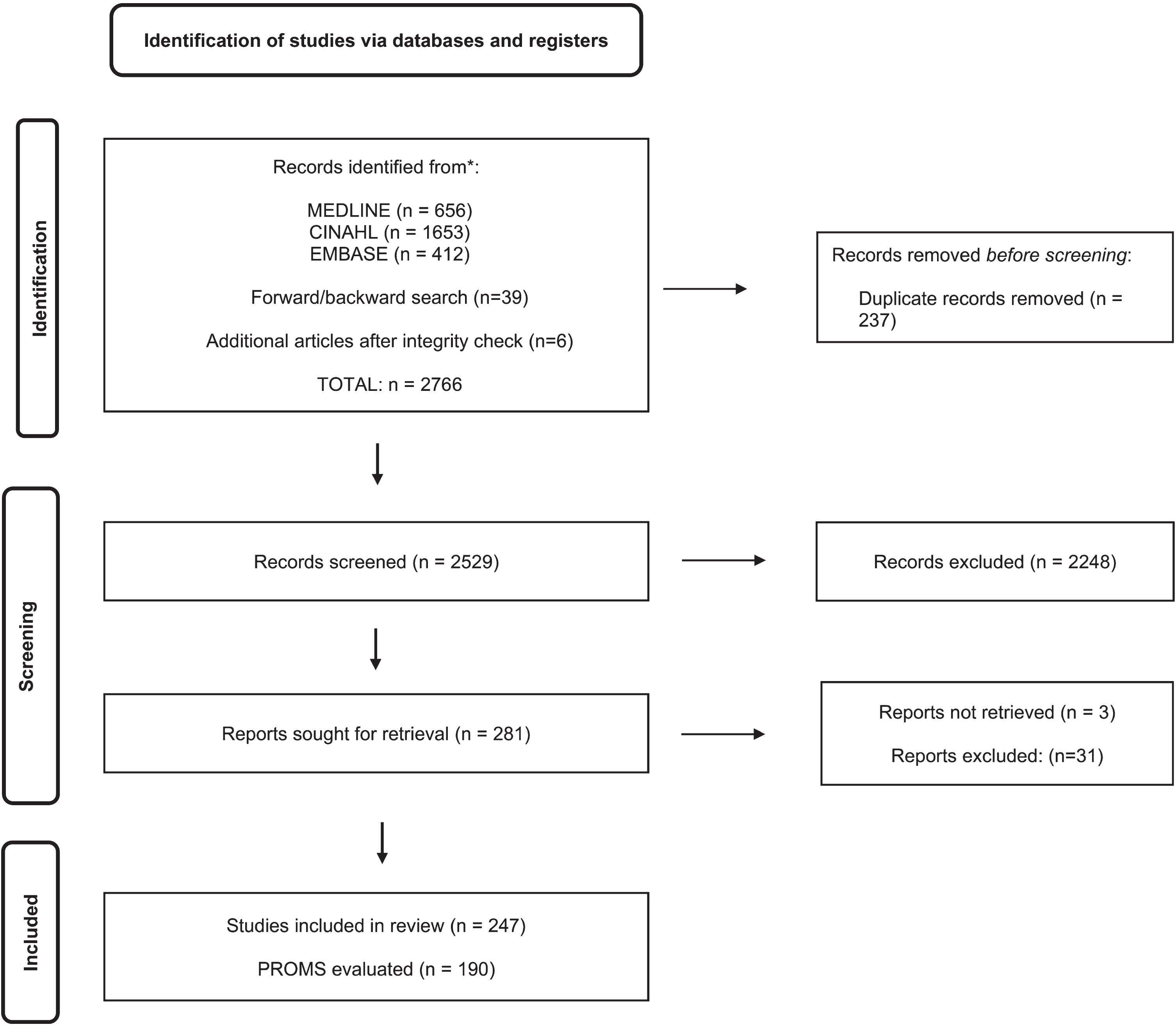
The search strategy identified 2766 publications in total: MEDLINE n = 656, CINAHL n = 1653, EMBASE n = 412, 39 additional reports found during screening reports (via forward and backward searching), and six additional publications after an integrity check [15–20]. After the title and abstract screening, 281 publications were potentially eligible. After the full-text review, 247 studies were included, and 190 PROMs were evaluated. Figure 1 demonstrates the flow for article identification and selection.
Fig. 1
PRISMA Flow diagram for the scoping review process.
In comparison to other NMD, most PROMs were evaluated in ALS. The physical functioning domain was most commonly assessed (Table 1). In total, 106 (56%) PROMs were disease-specific, designed exclusively for one specific type of neuromuscular disease. Regarding the measurement properties investigated, in the 190 PROMs, 151 (79%) were evaluated for validity, 132 (69%) for reliability, 37 (19%) for responsiveness, and 19 (10%) for interpretability.
Table 1
Characteristics of included PROMs
| Characteristic | Number |
| Studies reviewed | 247 |
| PROMs reviewed | 190 |
| Disease-specific PROMs | 106 |
| Generic PROMs | 84 |
| PROMs with sufficient quality in at least three of four measurement properties (validity, reliability, interpretability, responsiveness) | 30 |
| Domain of PROMs | Number |
| Physical functioning | 60 (31%) |
| Quality of life | 52 (27%) |
| Fatigue | 21 (11%) |
| Psychological well-being and mental health | 13 (7%) |
| Swallowing | 4 (2%) |
| Pain | 7 (4%) |
| Pulmonary functioning | 3 (2%) |
| Social functioning | 2 (1%) |
| Other | 3 (2%) |
| Multiple domains | 28 (2%) |
Abbreviation: PROMs: Patiented-Reported Outcome Measures.
We selected the PROMS assessed in the literature for at least three of the four COSMIN measurement properties. This resulted in 17 generic and 14 disease-specific PROMs out of 190, which we have further described in Table 2.
Table 2
Psychometric characteristics of included PROMs
| PROM | Population | Generic (G) or Disease specific (D) | Validity | Reliability | Responsiveness | Interpretability | Domain | Number of questions | Countries |
| Generic PROMs (n = 15) | |||||||||
| ABILHAND (1) | a-NMD | G | ++ | ++ | ? | ++ | PF | 23 | BE |
| ABILHAND-KIDS (2) | c-NMD | G | + | ++ | ? | ++ | PF | 21 | TR |
| ACTIVLIM (3-7) | a-c-NMD; a-BMD, a-LGMD, a-NMD, a-FSHD, a-MD1, | G | ++ | ++ | ++ | + | PF | 56 | BE (5–7), ES (3) |
| ACTIVLIM (8) | a-c-LAMA2-RD; a-c-COL6-RD | G | ++ | ++ | ++ | ? | PF | 56 | USA |
| CHAQ (9-13) | jDM; jIIM | G | ++ | ++ | ++ | ++ | PF | 30 | CA (9, 12, 13), IT (10), USA (11) |
| CHQ-ps (10) | jDM | G | ++ | ++ | ++ | ++ | PW/MW | 6 | IT |
| HAQ-DI (14) | a-Myositis | G | + | ++ | ++ | ++ | PF | 20 | USA |
| INQoL(15-17) | a-GBS; a-MD; a-FSHD; a-LGMD; a-BMD; a-IM | G | ++ | ++ | + | ? | QoL | 23 | JP (16), IT (15), NL (17) |
| INQoL(18-22) | a-NMD | G | ++ | ++ | + | + | QoL | 23 | ES (22), KR (20), NL (19), UK (18, 21), USA (21) |
| I-RODS (23-26) | a-CIDP; a-GBS; a-GM-MGUSP | G | ++ | ++ | ++ | + | PF, SF | 24 | BE (26), BR (26), CA (26), ES (26), FR (26), ME (23), NL (24), RS (23), UK (26), USA (25) |
| NFI-PP (27) | a-PPS | G | + | ++ | + | ? | FAT | 8 | CH, UK |
| Parent’s global assessment of patient’s well-being (10) | jDM | G | ++ | + | + | ? | QoL | 1 | IT |
| PROMIS PF20 (14) | a-Myositis | D | ++ | + | ++ | ++ | PF, QoL | 20 | USA |
| SF-36-PF (14) | a-Myositis | G | ++ | - | ++ | ++ | QoL, PF | 10 | USA |
| SIP (28-30) | a-ALS | G | ++ | + | + | ? | QoL | 12 | USA |
| SWAL-CARE (31) | a-NMD | G | ++ | ++ | ++ | ? | QoL | 15 | SE |
| VAS stiffness (32) | a-Myotonia Congenita | G | ++ | ++ | ++ | ? | Pain | 1 | SE |
| Disease-specific PROMs (n = 14) | |||||||||
| ALSFRS-EX (33, 34) | a-ALS | D | ++ | ++ | ++ | ? | PF | 23 | DE (34), USA (33) |
| ALSAQ-40 (35-44) | a-ALS | D | ++ | ++ | ++ | ++ | PF, QoL | 40 | BR (44), ES (36), IT (37), NL (38), TR (39), UK (35, 40-43) |
| ALSAQ-5 (37, 38, 42, 45) | a-ALS | D | ++ | ++ | ++ | ++ | PF, QoL | 5 | IT (37), NL (38), UK (42, 45) |
| ALSFRS (46, 47) | a-ALS | D | + | ++ | ++ | ? | PF | 10 | CA (47), US (46, 47) |
| ALSFRS-R (48-67) | a-ALS | D | + | ++ | ++ | ? | PF | 12 | AR (60), CN (61), EG (65), ES (51), IT (49, 54, 64), JP, (56) NL (55, 66), PL (50), PT (62), SCT (58), USA (48, 53, 57, 59, 60, 63, 67), ZA (52) |
| DMDSAT (68) | a-c-DMD | D | ++ | ++ | ? | ++ | PF | 24 | UK |
| DYALS (69) | a-ALS | D | ++ | + | + | ? | SWAL | 10 | IT |
| DALS-15 (70, 71) | a-ALS | D | ++ | ++ | ++ | ++ | PULM | 15 | TR (70), DE (71) |
| MG-ADL (72-88) | a-MG | D | ++ | ++ | ++ | ? | PF | 8 | BR (79) CN (86), ES (87), FR (80), IR (78), IT (81), JP (76), KR (83), PL (82, 85), SA (72, 83), USA (73-75, 77), TR (88) |
| MG-ADL-DIS (89) | a-MG | D | + | + | ++ | ? | PF | 31 | IT |
| MG-PRO (90, 91) | a-MG | D | ++ | ++ | ++ | ? | PF | 42 | CN |
| pCMT-QOL (92, 93) | c-CMT | D | ++ | ++ | + | ? | QoL | 60 | AU (92), IT (93), UK (92), US (92) |
| ROADS (48, 94-97) | a-ALS | D | ++ | + | ++ | ? | PF | 27 | CN (95), IT (96, 97), USA (48, 94) |
| SMAIS-ULM (98) | a-SMA2; a-SMA3 | D | ++ | ++ | ++ | ? | PF | 22 | UK |
Abbreviations: ++: good,+: sufficient, -: insufficient, ?: undetermined, ACTIVLIM: Activity Limitations Questionnaire, ALS: Amyotrophic Lateral Sclerosis, ALSAQ-40 : 40-item Amyotrophic Lateral Sclerosis Assessment Questionnaire, ALSAQ-5 : 5-item Amyotrophic Lateral Sclerosis Assessment Questionnaire, ALSFRS-EX: Amyotrophic Lateral Sclerosis Functional Rating Scale Extended, ALSFRS-R: Amyotrophic Lateral Sclerosis Function Rating Scale-Revised ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale, AR: Argentina, AU: Australia, BA: the Republic of Srpska (part of Bosnia and Herzegovina), BE: Belgium, BMD: Becker Muscular Dystrophy, BR: Brazil, CA: Canada, CH: Switzerland, CHAQ: Child health assessment questionnaire, CHQ-ps: Child Health Questionnaire psychosocial summary score, CIDP: Chronic Inflammatory Demyelinating Polyneuropathy, CMT: Charcot- Marie Tooth disease, CN: China, COL6-RD: Collagen VI-related muscular dystrophy, D: Disease-specific, DALS-15 : 15-item Dyspnea Amyotrophic Lateral Sclerosis Scale, DE: Germany, DERM: Dermatomyositis, DMD: Duchenne Muscular Dystrophy, DMDSAT: Duchenne muscular dystrophy Functional Ability Self-Assessment Tool, DYALS: Dysphagia in Amyotrophic Lateral Sclerosis, ES: Spain, FAT: Fatigue (Domain), FR: France, FSHD: Facioscapulohumeral Dystrophy, EG: Egypt, G: Generic, GBS: Guillain Barré Syndrome, GM-MGUSP: Monoclonal Gammopathy of Undetermined Significance, IM: inflammatory myositis, HAQ-DI: Health Assessment Questionnaire Disability Index, I-RODS: Inflammatory Rasch-built Overall Disability Scale, INQoL: Individualized Neuromuscular Quality of Life Questionnaire, IT: Italy, jDM: juvenile Dermatomyositis, IR: Iran, jIIM: juvenile Idiopathic Inflammatory Myopathy, JP: Japan, KR: South-Korea, LAMA2-RD: Laminin alpha 2-related dystrophy, LGMD: Limb Girdle Muscular Dystrophy, MC: Myotonia Congenita, MD1: Myotonic Dystrophy type 1, ME: Montenegro MG-ADL: Myasthenia Gravis Activities of Daily Living, MG-PRO: Myasthenia Gravis Patient Reported Outcome, MG: Myasthenia Gravis, NFI-PP: Neurological Fatigue Index, NL: the Netherlands, NMD: Neuromuscular Diseases, pCMT-QOL: Pediatric Charcot-Marie-Tooth Disease Quality of Life Outcome Measure, PF: Physical Functioning (Domain), PL: Poland, PPS: Post-Polio Syndrome, PR: Portugal, PROM: Patient-reported outcome measure, PROMIS PF-20 20-item Patient Reported Outcomes Measurement Information System physical function, PULM: pulmonary functioning, PW/MHMW: Psychological Well-being and Mental Health, QoL: Quality of Life (Domain), ROADS: Rasch-built overall disability scale, RS: Serbia, SA: Saudi Arabia, SCT: Scotland, SE: Sweden, SF-36-pf: Short Form 36 physical functioning subscale, SF: social functioning, SIP: Sickness Impact Profile, SMA2: Spinal Muscular Atrophy type 2, SMA3: Spinal Muscular Atrophy type 3, SMAIS-ULM: Spinal Muscular Atrophy Independence Scale-Upper Limb Module, SWAL-CARE: Swallowing Quality of Care questionnaire, SWAL: Swallowing (Domain), TR: Turkey, UK: United Kingdom, USA: United States of America, VAS: Visual Analogue Scale, ZA: South Africa.
Only ten PROMs, the Activity Limitations Measure (ACTIVLIM) (in different types of NMD) [21–25], Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) (in ALS) [26–35], ALSAQ-5 (in ALS) [28, 29, 33, 36], Childhood Health Assessment Questionnaire (CHAQ) (in jDM and jIIM) [37–41], Child Health Questionnaire psychosocial summary score (CHQ-ps) (in juvenile dermatomyositis) [38], Dyspnea-ALS-Scale (DALS-15) (in ALS) [42, 43], Health Assessment Questionnaire-Disability Index (HAQ-DI) (in myositis) [44], Individualized Neuromuscular Quality of Life Questionnaire (INQoL) (in different types of NMD) [45–49], Inflammatory Rasch-built Overall Disability Scale ((I-RODS) (in Chronic Inflammatory Demyelinating Polyneuropathy, Guillain Barre Syndrome, Monoclonal gammopathy of undetermined significance) [50–53], and Patient-Reported Outcomes Measurement Information System Physical Function (PROMIS PF20) (in myositis) [44], were sufficiently evaluated for all psychometric characteristics (when statistical psychometric indexes met COSMIN’s quality criteria). Table 2 presents a summary of the quality of these PROMs.
DISCUSSION
To the best of our knowledge, the present review is the first to present a comprehensive assessment of the existing literature on the measurement properties of the available PROMs validated for children and adults with NMDs. Until now, the lack of a centralized resource hindered the selection and comparison of PROMs for monitoring NMDs.
A total of 250 studies and 190 PROMs, covering a wide range of NMDs, were included in this review. The question of which PROM is most suitable to use is difficult to answer. It depends, among other aspects, on the research or clinical question, the population of interest, the setting (e.g., research or clinical practice), the format (e.g., paper or electronic versions), and practical elements such as administration time, patient burden, consent, costs, and availability. And, importantly, whether the scores are meaningful from a patient perspective. The measurement properties of an instrument in one population or setting may differ when applied to another population or context. As NMDs represent a heterogeneous population, it is essential to consider the pros and cons of using generic or disease-specific PROMs. Ideally, a generic PROM that is designed to be relevant across conditions should be validated for different target populations. PROMs are typically developed in one language and subsequently translated into other languages as needed. More than translating an instrument alone is required to apply it in another language. The instrument will also need to be revalidated [54]. The results regarding measurement properties can only be generalized to populations similar to the study sample in which the measurement properties have been evaluated. It is impossible to discuss the quality of a measurement instrument in general, but this should always be considered within the context of a specific study population and purpose. Only ten PROMs were sufficiently evaluated to full analysis of all their measurement properties: validity, reliability, responsivity, and interpretability. A limited number of PROMs have been evaluated for interpretability.
Using generic measures offers a comparative perspective, allowing one to assess where an NMD stands in relation to other conditions or a healthy population evaluated with the same measure. However, they may miss the particular features of each disease [55]. Unfortunately, only 42 (50%) generic PROMs have some documented metrological properties in more than one population.
Based on the results, several observations stand out. In more than 500 studies, the Short Form 36 (SF-36) is used as an outcome measure in NMD. The SF-36 was developed in the 1980s by John E. Ware Jr. and his colleagues at the Health Institute of the New England Medical Center in Boston, Massachusetts, United States of America. It was designed as a general health questionnaire to assess various aspects of HR-QoL. It is one of the most widely used instruments for measuring health outcomes in research and clinical practice. However, the SF-36 is already over 40 years old. The construct of HR-QoL has evolved over decades from a disease-centric to a more patient-centric perspective. This evolution includes an expanded focus on multiple domains, increased attention to psychological well-being and mental health, and efforts to enhance cultural sensitivity and cross-cultural validity. Advances in statistical methods and technology contribute to a more dynamic and comprehensive understanding of quality of life, reflecting a holistic approach that considers physical, psychological, and social well-being. Furthermore, it is important to consider that there have been advancements in the field of HR-QoL assessment since then, including the development of more recent (and patient-reported) QoL assessment tools, such as the Patient-Reported Outcomes Measurement Information System (PROMIS).
In NMDs, psychosocial functioning is essential to a holistic and comprehensive approach to patient care. It helps ensure that the care and support go beyond managing physical symptoms and encompass the broader aspects of a patient’s well-being, quality of life, and functioning [56–58]. Currently, most existing PROMs primarily focus on assessing physical functioning and QoL, while only a limited number of PROMs capture aspects of social functioning. Certain PROMs assess social functioning: 20-item Patient Reported Outcomes Measurement Information System (PROMIS PF20), Assessment of Life Habits (LIFE-H), Facioscapulohumeral muscular dystrophy Rasch-built overall disability scale (FSHD-RODS), Frenchay Activities index (FAI), Inflammatory Rasch-built Overall Disability Scale (I-RODS), Impact on Participation and Autonomy (IPA), MMN-Rasch-built overall disability scale (MMN-RODS), Myotonic Dystrophy type 1 Activity and participation scale (DM1-activ), Neuromuscular Disease Impact Profile (NMDIP), Short Form 36 (SF-36), social withdrawal scale, and the Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-Participation)) but only a few studies (n = 22) have validated these measures.
For this scoping review, three databases recommended by an experienced librarian were searched. The search strategy was developed by a multidisciplinary team, including two patients living with NMD and with expertise in neurology, rehabilitation, and psychology. There are also some limitations to this review. First, only articles published in English were included. Secondly, although we have conducted an integrity check, we cannot be certain that we have been able to include all PROMs. To end, this review aimed to provide a broad overview of the literature; consequently, we did not delve deeply into the methodological quality or the effectiveness of the studies included in the review.
Conclusion
In conclusion, this comprehensive review addresses a critical gap in the literature by offering a thorough assessment of PROMS validated for individuals with NMDs. The extensive inclusion of 250 studies and 190 PROMs underscores the complexity of selecting an optimal instrument, emphasizing the importance of considering various factors such as research or clinical context, patient characteristics, and practical aspects.
FUNDING
None.
CONFLICT OF INTEREST
Christopher Graham receives grant funding from the National Institute for Health and Care Research (NIHR). He undertakes a small amount of consultancy work for National Health Service trusts, involving psychological therapy supervision. Joana Garmendia was funded by University of the Basque Country.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-240003.
REFERENCES
[1] | Patrick DL , Burke LB , Powers JH , Scott JA , Rock EP , Dawisha S , et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. (2007) ;10: (Suppl 2):S125–37. |
[2] | Lavallee DC , Chenok KE , Love RM , Petersen C , Holve E , Segal CD , et al. Incorporating Patient-Reported Outcomes Into Health Care To Engage Patients And Enhance Care. Health Aff (Millwood). (2016) ;35: (4):575–82. |
[3] | Itchhaporia D The Evolution of the Quintuple Aim: Health Equity, Health Outcomes, and the Economy. J Am Coll Cardiol. (2021) ;78: (22):2262–4. |
[4] | Barp A , Ferrero A , Casagrande S , Morini R , Zuccarino R Circulating Biomarkers in Neuromuscular Disorders: What Is Known, What Is New. Biomolecules. (2021) ;11: (8):1246. |
[5] | El Miedany Y Adopting patient-centered care in standard practice: PROMs moving toward disease-specific era. Clin Exp Rheumatol.. (2014) ;32: (5 Suppl 85):S-40–6. |
[6] | Whittal A , Meregaglia M , Nicod E The Use of Patient-Reported Outcome Measures in Rare Diseases and Implications for Health Technology Assessment. Patient. (2021) ;14: (5):485–503. |
[7] | Boateng GO , Neilands TB , Frongillo EA , Melgar-Quinonez HR , Young SL Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front Public Health. (2018) ;6: :149. |
[8] | Hansen CF , Jensen J , Brodersen J , Siersma V , Comins JD , Krogsgaard MR Are adequate PROMs used as outcomes in randomized controlled trials? an analysis of 54 trials. Scand J Med Sci Sports. (2021) ;31: (5):972–81. |
[9] | Burns TM , Graham CD , Rose MR , Simmons Z Quality of life and measures of quality of life in patients with neuromuscular disorders. Muscle Nerve. (2012) ;46: (1):9–25. |
[10] | Tricco AC , Lillie E , Zarin W , O’Brien KK , Colquhoun H , Levac D , et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. (2018) ;169: (7):467–73. |
[11] | Prinsen CAC , Mokkink LB , Bouter LM , Alonso J , Patrick DL , de Vet HCW , et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. (2018) ;27: (5):1147–57. |
[12] | Mokkink LB , Terwee CB , Patrick DL , Alonso J , Stratford PW , Knol DL , et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. (2010) ;63: (7):737–45. |
[13] | Terwee CB , Bot SD , de Boer MR , van der Windt DA , Knol DL , Dekker J , et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. (2007) ;60: (1):34–42. |
[14] | Page MJ , Shamseer L , Tricco AC Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst Rev. (2018) ;7: (1):32. |
[15] | Klingels K , Mayhew AG , Mazzone ES , Duong T , Decostre V , Werlauff U , et al. Development of a patient-reported outcome measure for upper limb function in Duchenne muscular dystrophy: DMD Upper Limb PROM. Dev Med Child Neurol. (2017) ;59: (2):224–31. |
[16] | Cicala G , Pane M , Coratti G , Brogna C , Fanelli L , Norcia G , et al. Patient reported outcome measure for upper limb in Duchenne muscular dystrophy: Correlation with PUL2.0.. Neuromuscul Disord. (2023) ;33: (9):69–73. |
[17] | Vinik EJ , Vinik AI , Paulson JF , Merkies IS , Packman J , Grogan DR , et al. Norfolk QOL-DN: Validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. (2014) ;19: (2):104–14. |
[18] | Elson JL , Cadogan M , Apabhai S , Whittaker RG , Phillips A , Trennell MI , et al. Initial development and validation of a mitochondrial disease quality of life scale. Neuromuscul Disord. (2013) ;23: (4):324–9. |
[19] | Staunton H , Cleanthous S , Teodoro V , Barrett L , Braid J , Ewens B , et al. A Mixed-method Approach to Develop an Ambulatory Module of the SMA Independence Scale. J Neuromuscul Dis. (2023) ;10: (6):1093–109. |
[20] | DiRenzo D , Saygin D , de Groot I , Bingham Iii CO , Lundberg IE , Needham M , et al. Reliability and validity of PROMIS physical function, pain interference, and fatigue as patient reported outcome measures in adult idiopathic inflammatory myopathies: International study from the OMERACT myositis working group. Semin Arthritis Rheum. (2023) ;58: :152111. |
[21] | Pagola I , Torné L , Jericó I , Ibáñez B Transcultural adaptation and validation of the Spanish-language version of ACTIVLIM in adults with inherited myopathies using the Rasch model. Neurologia (Engl Ed). (2021) ;36: (7):514–24. |
[22] | Batcho CS , Van den Bergh PY , Van Damme P , Roy AJ , Thonnard JL , Penta M , et al. How robust is ACTIVLIM for the follow-up of activity limitations in patients with neuromuscular diseases? Neuromuscul Disord. ((2016) ;26: (3):211–20. |
[23] | Vandervelde L , Van den Bergh PY , Goemans N , Thonnard JL ACTIVLIM: A Rasch-built measure of activity limitations in children and adults with neuromuscular disorders. Neuromuscul Disord. (2007) ;17: (6):459–69. |
[24] | Vandervelde L , Dispa D , Van den Bergh PY , Thonnard JL A comparison between self-reported and observed activity limitations in adults with neuromuscular disorders. Arch Phys Med Rehabil. (2008) ;89: (9):1720–3. |
[25] | Vandervelde L , Van den Bergh PY , Goemans N , Thonnard JL Activity limitations in patients with neuromuscular disorders: A responsiveness study of the ACTIVLIM questionnaire. Neuromuscul Disord. (2009) ;19: (2):99–103. |
[26] | Jenkinson C , Fitzpatrick R , Brennan C , Bromberg M , Swash M Development and validation of a short measure of health status for individuals with amyotrophic lateral sclerosis/motor neurone disease: The ALSAQ-40. J Neurol. (1999) ;246: (Suppl 3):Iii16–21. |
[27] | Salas T , Mora J , Esteban J , Rodríguez F , Díaz-Lobato S , Fajardo M Spanish adaptation of the Amyotrophic Lateral Sclerosis Questionnaire ALSAQ-40 for ALS patients. Amyotroph Lateral Scler. (2008) ;9: (3):168–72. |
[28] | Palmieri A , Sorarù G , Lombardi L , D’Ascenzo C , Baggio L , Ermani M , et al. Quality of life and motor impairment in ALS: Italian validation of ALSAQ. Neurol Res. (2010) ;32: (1):32–40. |
[29] | Maessen M , Post MW , Maillé R , Lindeman E , Mooij R , Veldink JH , et al. Validity of the Dutch version of the Amyotrophic Lateral Sclerosis Assessment Questionnaire, ALSAQ-40, ALSAQ-5. Amyotroph Lateral Scler. (2007) ;8: (2):96–100. |
[30] | Alankaya N , Tülek Z , Özakgül A , Kaya A , Dik A Validity and Reliability of the Turkish Version of the Amyotrophic Lateral Sclerosis Assessment Questionnaire. J Neurosci Nurs. (2019) ;51: (5):253–8. |
[31] | Jenkinson C , Fitzpatrick R , Brennan C , Swash M Evidence for the validity and reliability of the ALS assessment questionnaire: The ALSAQ-40. Amyotroph Lateral Scler Other Motor Neuron Disord. (1999) ;1: (1):33–40. |
[32] | Norquist JM , Fitzpatrick R , Jenkinson C Health-related quality of life in amyotrophic lateral sclerosis: Determining a meaningful deterioration. Qual Life Res. (2004) ;13: (8):1409–14. |
[33] | Jenkinson C , Fitzpatrick R , Swash M , Jones G Comparison of the 40-item Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) with a short-form five-item version (ALSAQ-5) in a longitudinal survey. Clin Rehabil. (2007) ;21: (3):266–72. |
[34] | Jenkinson C , Levvy G , Fitzpatrick R , Garratt A The amyotrophic lateral sclerosis assessment questionnaire (ALSAQ-40): Tests of data quality, score reliability and response rate in a survey of patients. J Neurol Sci. (2000) ;180: (1–2):94–100. |
[35] | Pavan K , Marangoni BE , Zinezzi MO , Schmidt KB , Oliveira BC , Buainain RP , et al. Validation of the Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) scale in the Portuguese language. Arq Neuropsiquiatr. (2010) ;68: (1):48–51. |
[36] | Jenkinson C , Fitzpatrick R Reduced item set for the amyotrophic lateral sclerosis assessment questionnaire: Development and validation of the ALSAQ-5. J Neurol Neurosurg Psychiatry. (2001) ;70: (1):70–3. |
[37] | Luca NJ , Feldman BM Health outcomes of pediatric rheumatic diseases. Best Pract Res Clin Rheumatol. (2014) ;28: (2):331–50. |
[38] | Ruperto N , Ravelli A , Pistorio A , Ferriani V , Calvo I , Ganser G , et al. The provisional Paediatric Rheumatology International Trials Organisation/American College of Rheumatology/European League Against Rheumatism Disease activity core set for the evaluation of response to therapy in juvenile dermatomyositis: A prospective validation study. Arthritis Rheum. (2008) ;59: (1):4–13. |
[39] | Rider LG , Aggarwal R , Machado PM , Hogrel JY , Reed AM , Christopher-Stine L , et al. Update on outcome assessment in myositis. Nat Rev Rheumatol. (2018) ;14: (5):303–18. |
[40] | Huber AM , Hicks JE , Lachenbruch PA , Perez MD , Zemel LS , Rennebohm RM , et al. Validation of the Childhood Health Assessment Questionnaire in the juvenile idiopathic myopathies. Juvenile Dermatomyositis Disease Activity Collaborative Study GrouJ Rheumatol. (2001) ;28: (5):1106–11. |
[41] | Feldman BM , Ayling-Campos A , Luy L , Stevens D , Silverman ED , Laxer RM Measuring disability in juvenile dermatomyositis: Validity of the childhood health assessment questionnaire. J Rheumatol. (1995) ;22: (2):326–31. |
[42] | Kolbaşı EN , Açıkbaş E , Akşimşek GP , Aslan GK , Kıyan E Validity and reliability of the Turkish version of “the Dyspnea-ALS-Scale (DALS-15)”. Neurol Sci. (2022) ;43: (3):1823–9. |
[43] | Vogt S , Petri S , Dengler R , Heinze HJ , Vielhaber S Dyspnea in Amyotrophic Lateral Sclerosis: Rasch-Based Development and Validation of a Patient-Reported Outcome (DALS-15). J Pain Symptom Manage. (2018) ;56: (5):736–45.e2. |
[44] | Saygin D , Oddis CV , Dzanko S , Koontz D , Moghadam-Kia S , Ardalan K , et al. Utility of patient-reported outcomes measurement information system (PROMIS) physical function form in inflammatory myopathy. Semin Arthritis Rheum. (2021) ;51: (3):539–46. |
[45] | Vincent KA , Carr AJ , Walburn J , Scott DL , Rose MR Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL). Neurology. (2007) ;68: (13):1051–7. |
[46] | Seesing FM , van Vught LE , Rose MR , Drost G , van Engelen BG , van der Wilt GJ The individualized neuromuscular quality of life questionnaire: Cultural translation and psychometric validation for the Dutch population. Muscle Nerve. (2015) ;51: (4):496–500. |
[47] | Han HJ , Lee SA , Choi YC , Rose MR , Park HJ Validation of the Individualized Neuromuscular Quality of Life Questionnaire in Korean Patients With Genetic Neuromuscular Diseases. J Clin Neurol. (2022) ;18: (5):514–21. |
[48] | Sadjadi R , Vincent KA , Carr AJ , Walburn J , Brooks VL , Pandya S , et al. Validation of the individualised neuromuscular quality of life for the USA with comparison of the impact of muscle disease on those living in USA versus UK. Health Qual Life Outcomes. (2011) ;9: :114. |
[49] | Fagoaga J , Girabent-Farres M , Bagur-Calafat C [Translation and validation of the Individualised Neuromuscular Quality of Life scale for the Spanish population: Quality of life assessment for persons with neuromuscular diseases]. Rev Neurol. (2017) ;64: (5):194–200. |
[50] | Stojanov A , Basta I , Berisavac I , Stojiljkovic-Tamas O , Bozovic I , Arsenijevic M , et al. Responsiveness of 2 Different Ability Outcome Measures in Guillain-Barré Syndrome. Neurologist. (2021) ;26: (6):244–7. |
[51] | Vanhoutte EK , Draak TH , Gorson KC , van Nes SI , Hoeijmakers JG , Van der Pol WL , et al. Impairment measures versus inflammatory RODS in GBS and CIDP: A responsiveness comparison. J Peripher Nerv Syst. (2015) ;20: (3):289–95. |
[52] | Peric S , Bozovic I , Pruppers MHJ , Bjelica B , Stevic Z , Faber CG , et al. Validation of the Serbian version of inflammatory Rasch-built overall disability scale in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. (2019) ;24: (3):260–7. |
[53] | Draak TH , Vanhoutte EK , van Nes SI , Gorson KC , Van der Pol WL , Notermans NC , et al. Changing outcome in inflammatory neuropathies: Rasch-comparative responsiveness. Neurology. (2014) ;83: (23):2124–32. |
[54] | McKenna SP , Doward LC The translation and cultural adaptation of patient-reported outcome measures. Value Health. (2005) ;8: (2):89–91. |
[55] | Wiebe S , Guyatt G , Weaver B , Matijevic S , Sidwell C Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol. (2003) ;56: (1):52–60. |
[56] | Gagnon C , Kierkegaard M , Blackburn C , Chrestian N , Lavoie M , Bouchard MF , et al. Participation restriction in childhood phenotype of myotonic dystrophy type A systematic retrospective chart review. Dev Med Child Neurol. (2017) ;59: (3):291–6. |
[57] | Bendixen RM , Butrum J , Jain MS , Parks R , Hodsdon B , Nichols C , et al. Upper extremity outcome measures for collagen VI-related myopathy and LAMA2-related muscular dystrophy. Neuromuscular Disorders. (2017) ;27: (3):278–85. |
[58] | Kruitwagen-van Reenen ET , van der Pol L , Schroder C , Wadman RI , van den Berg LH , Visser-Meily JMA , et al. Social participation of adult patients with spinal muscular atrophy: Frequency, restrictions, satisfaction, and correlates. Muscle Nerve. (2018) ;58: (6):805–11. |



