Disease Trajectories in the Revised Hammersmith Scale in a Cohort of Untreated Patients with Spinal Muscular Atrophy types 2 and 3
Abstract
Background:
Spinal muscular atrophy (SMA) is a neuromuscular disorder characterised by progressive motor function decline. Motor function is assessed using several functional outcome measures including the Revised Hammersmith Scale (RHS).
Objective:
In this study, we present longitudinal trajectories for the RHS in an international cohort of 149 untreated paediatric SMA 2 and 3 patients (across 531 assessments collected between March 2015 and July 2019).
Methods:
We contextualise these trajectories using both the Hammersmith Functional Motor Scale Expanded (HFMSE) and Revised Upper Limb Module (RULM). At baseline, this cohort included 50% females and 15% of patients had undergone spinal fusion surgery. Patient trajectories were modelled using a natural cubic spline with age, sex, and random effects for each patient.
Results:
RHS and HFMSE scores show similar trends over time in this cohort not receiving disease modifying therapies. The results confirm the strong correlation between the RHS and RULM previously observed in SMA types 2 and 3a. Scoliosis surgery is associated with a reduction of 3 points in the RHS, 4.5 points in the HFMSE for the SMA 2 population, and a reduction of 11.8 points in the RHS, and 13.4 points in the HFMSE for the SMA 3a populations. When comparing the RHS and RULM, there is a lower correlation in the type 3a’s than the type 2 patients. In the SMA 2 population, there is no significant difference between the sexes in either the RHS or HFMSE trajectories. There is no significant difference in the RULM trajectory in the SMA 2 or 3a participants by sex.
Conclusions:
This study demonstrates that the RHS could be used in conjunction with other functional measures such as the RULM to holistically detect SMA disease progression. This will assist with fully understanding changes that occur with treatments, further defining trajectories and therapy outcomes.
INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disorder caused by mutations in the survival motor neuron 1 (SMN1) gene resulting in SMN protein deficiency [1–3]. This leads to muscle atrophy and a progressive decline in motor function due to muscle weakness. Secondary complications include scoliosis, joint contractures, fatigability, and progressive respiratory decline [3, 4].
SMA has historically been classified into types defined by age of onset and the maximum motor milestone achieved. Type 1 children never achieved independent sitting, type 2 children achieved independent sitting but never walked and type 3 achieved independent walking but would lose motor function over time and many became wheelchair dependant [3]. The type 3 has further subgroups of type 3a, with disease onset before 3 years and type 3b with onset after 3 years [5].
The introduction and approval of three disease modifying treatments for SMA (nusinersen, risdiplam and onasemnogene aberparvovec) has underscored the need for a robust understanding of the longitudinal natural history of SMA and how this natural history is represented across functional outcome measures [6–12].
The SMA specific outcome measures commonly used to assess gross motor function for clinical trials in patients with later-onset type 2 and 3 SMA are the Hammersmith Functional Motor Scale Expanded (HFMSE) and more recently the Revised Hammersmith Scale (RHS) [13, 14]. The RHS was developed more recently to expand the HFMSE at both ends of the scale, including an item from the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) which has been shown to reduce floor effect and several items including timed tests from the North Star Ambulatory Assessment (NSAA) to reduce the ceiling effect [13, 15]. Upper limb function is assessed using the Revised Upper Limb Module (RULM) [16, 17]. The RULM and HFMSE when used independently are appropriate for different age and functional groups however it has been shown that when used in conjunction, they are more sensitive to detecting functional changes [18]. As the RHS is being used clinically and more recently as a trial outcome measure it is important to understand the relationship between HFMSE, RULM, and RHS and how the RHS can be used in conjunction with other motor function outcomes.
Aims
With this analysis, we aim to characterise for the first time the longitudinal trajectories for the RHS in an international cohort of SMA 2 and 3 patients not receiving disease modifying therapies (DMT), establish patterns of progression. We contextualise these trajectories by providing the corresponding trajectory in the HFMSE on the same untreated cohort, with the aim of assessing the impact of the differences in items of the two scales to capture disease progression. We also analyse the impact of sex and spinal surgical intervention on these trajectories. We also aim to demonstrate that the RHS score can be used in conjunction with other functional measures such as the RULM to understand this cohort’s disease progression.
MATERIALS AND METHODS
Inclusion criteria
The participants included in this analysis are recruited from the International SMA Consortium (iSMAC) natural history studies (SMA REACH UK, PNCRN USA and Italian Telethon) [19]. Cohort inclusion criteria included no prior or active treatment with any disease modifying therapy, available information on sex, scoliosis surgery status, and a complete RHS and HFMSE assessment. All participants had a genetically confirmed diagnosis of SMA, clinically classified as type 2, 3a or 3b, were receiving SMA Standards of Care treatment [20], had no previous involvement in clinical trials, and had at least two RHS assessments performed between the 17th of March 2015 and the 29th of July 2019. This study is a longitudinal reanalysis of previously published cross-sectional natural history data [15]. The inclusion criteria included those who had all fully completed HFMSE and RHS assessments, known spinal surgery status and known sex. The restriction to complete RHS assessments, as per the instruction manuals, led to the exclusion of 42 assessments and the restriction to complete HFMSE led to a further 4 assessments being excluded.
The RHS, HFMSE, and RULM assessments were conducted by iSMAC trained neuromuscular physiotherapists in the standardised assessment administration. Inter and intra-rater reliability have previously been reported for the HFMSE, RHS and RULM [16, 21–23]. The RHS, HFMSE, and RULM scores were collected in clinics every 6 months as recommended in the international standards of care [20]. The RHS is a 36-item ordinal scale with a maximum score of 69 points (33 items are scored 0–2, and three 0–1) [13]. The HFMSE is a 33-item ordinal scale with a maximum score of 66 points (33 items are scored 0–2) [14]. The RHS and HFMSE can be scored simultaneously, as many items are similar or identical between scales. The RULM is a 20-item scale with a maximum total score of 37. It includes an entry item that does not contribute to the total score but identifies the functional level of the participant. The 19 other items are scores 0–2, with only one exception scored 0–1 [16].
Statistical analysis
Median and range summary statistics are presented for the variables of interest. The correlations between the RHS and HFMSE were performed using Spearman correlations, with p-values calculated using the asymptotic t approximation. A random effects model was fitted separately for both the RHS and the HFMSE by SMA type, with a random intercept for patients. A natural cubic spline was fitted separately for age for both male and female children, and scoliosis surgery was included as a factor. This is a flexible model, which consists of a series of cubic polynomials connected at knots. The polynomials fitted to the youngest and oldest groups are enforced as linear, which prevents overfitting in the sparse RHS and HFMSE assessments in the youngest and oldest patients. The model degrees of freedom were selected as parameter which minimised the Bayesian Information Criterion (a conservative assessment of model fit)–this was 3 across all 4 models (SMA 2 s male and female and SMA 3 s male and female). Significance between nested models was assessed using ANOVA. Residual fits and Quantile-Quantile plots were assessed for the suitability of the model assumptions. No model was fitted for the SMA 3b’s, and instead the trajectories are presented graphically due to the smaller sample size. A binary indicator was added to the model for scoliosis surgery. This leads to a discontinuity when patients undergo scoliosis but due to the visiting process, it wasn’t possible to model the impact of scoliosis surgery on motor function with respect to time. The ages for yearly change estimates are the mid-points of the age group summaries previously presented [15]. RStudio version 2022.07.2 Build 576 was used for this analysis.
RESULTS
Participants
We analysed 531 assessments across 149 paediatric SMA patients. The median number assessments were 3 (IQR: 2–5), this cohort included 96 with SMA type 2, 47 with SMA type 3a and 6 with SMA Type 3b. There were 74 females and 75 males overall. In the patients with type 2 there were 40 female and 56 male participants, in those with type 3a there were 32 female and 15 male participants and there were 2 female and 4 male participants with type 3b.
The median age at the first assessment was 7.2 years [IQR: (3.8, 10.9). range: (1, 17.5)], the median RHS at first assessments was 11 [IQR: (5, 28). range: (0, 69)] and the median HFMSE at first assessments was 17 [IQR: (7, 39). range: (0, 66)]. The RULM total score was recorded in 196 (40%) of assessments and the median RULM at first assessments was 23.5 [IQR: (14.75, 30). range: (0, 37)]. The distribution of age, RHS total score, HFMSE total score and RULM total score by SMA type, sex and spinal surgery status are presented in Table 1.
Table 1
Summary statistics of patient cohort
| Number of | Number of | Age | RHS | HFMSE | RULM | ||||||||
| Participants | Assessments | Median (IQR) | Range | Median (IQR) | Range | Median (IQR) | Range | N | M | Median (IQR) | Range | ||
| All | 149 | 531 | 8.2 (4.8, 11.4) | 1–17.8 | 14 (6, 33) | 0–69 | 19 (7, 41) | 0–66 | 66 (44%) | 196 (40%) | 26 (16, 34) | 0–37 | |
| SMA | 2 | 96 | 324 | 6.45 (3.6, 10.5) | 1–17.7 | 7 (4, 13) | 0–28 | 10 (4, 18.25) | 0–39 | 37 (39%) | 102 (31%) | 16 (11, 20) | 0–33 |
| Type | 3a | 47 | 178 | 9.5 (6.4, 11.6) | 2.7–17.2 | 39 (25, 51.8) | 5–69 | 47 (35, 53) | 6–66 | 29 (62%) | 94 (53%) | 34 (29, 36) | 17–37 |
| 3b | 6 | 29 | 14.8 (10.9, 16.2) | 7.9–17.8 | 59 (55, 66) | 30–69 | 61 (56, 63) | 36–66 | 0 | 0 | |||
| Sex | F | 74 | 266 | 8.8 (4.9, 11.5) | 1.2–17.8 | 17 (7, 36) | 0–68 | 23 (9, 43.75) | 0–64 | 35 (47%) | 106 (40%) | 28 (17.25, 35) | 2–37 |
| M | 75 | 265 | 7.8 (4.7, 11.2) | 1–17.7 | 11 (4, 23) | 0–69 | 15 (5, 32) | 0–66 | 31 (41%) | 90 (34%) | 19.5 (13, 31) | 0–37 | |
| Spinal | Yes | 33 | 74 | 12.4 (10, 15.2) | 2.2–17.7 | 4 (2, 9) | 0–69 | 4 (2, 9.75) | 0–66 | 18 (55%) | 30 (41%) | 16 (9.5, 18.75) | 0–37 |
| Surgery | No | 126 | 457 | 7.4 (4.3, 10.6) | 1–17.8 | 16 (7, 36) | 0–69 | 22 (10, 43) | 0–66 | 55 (44%) | 166 (36%) | 27 (18, 34.75) | 0–37 |
The RHS and HFMSE total scores were very highly correlated overall ρ= 0.99 (p < 0.001). The correlation between the RHS and the HFMSE in the type 2’s was ρ= 0.96 (p < 0.001) and in the type 3a’s ρ= 0.98 (p < 0.001). The correlation between the RHS and the HFMSE in the type 3b’s was ρ= 0.77 (p < 0.001).
In assessments where the RULM was performed, the overall correlation between the RULM and the RHS/HFMSE across the cohort was ρ= 0.895, (p < 0.001) and 0.895, (p < 0.001), respectively. The correlation between the RULM and the RHS/HFMSE in the type 2’s was ρ= 0.799, (p < 0.001) and 0.812, (p < 0.001), respectively. The correlation between the RULM and the RHS/HFMSE in the type 3a’s was ρ= 0.697, (p < 0.001) and 0.711, (p < 0.001), respectively. There was a significant difference in the RHS score of participants who completed the RULM compared to those who did not (median 10, IQR: 4.25–16 in the group with a RULM assessment, median 6, IQR: 4–10 in the group without, p < 0.001).
SMA 2’s
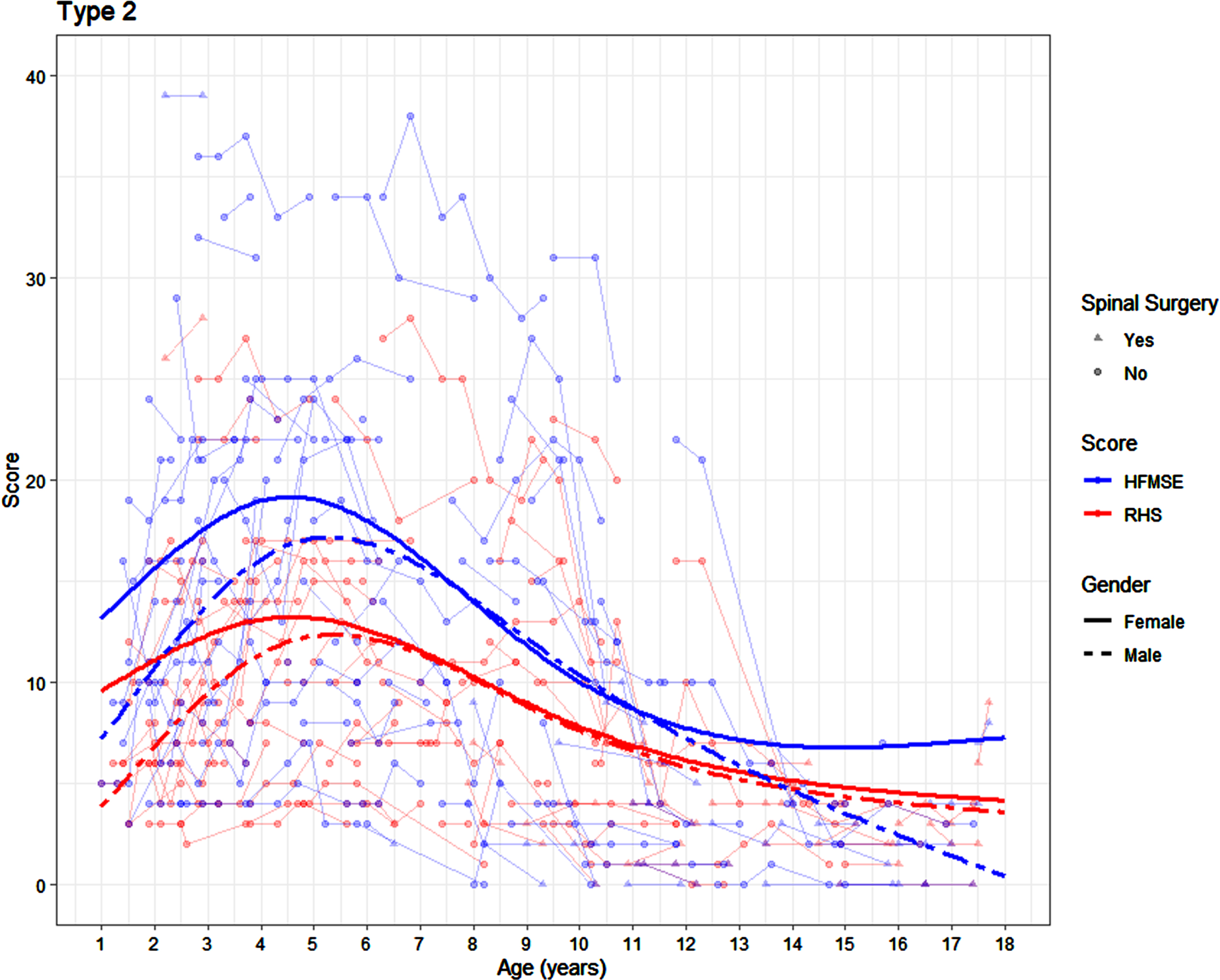
There was no significant difference in either the RHS or the HFMSE trajectory in the SMA 2 participants by sex (p = 0.096 and p = 0.13 respectively). In girls with SMA 2, an average peak RHS score of 13 was achieved between the age of 3.25 and 6.08 years. In the same cohort, an average peak HFMSE score of 19 was achieved in girls between the age of 3.58 and 5.58 years. In boys with SMA 2, the peak RHS score of 12 was achieved slightly later, between the age of 4.08 and 6.83 years. In the same cohort, a peak HFMSE score of 17 was achieved between the age of 4.33 and 6.42 years (Fig. 1). The average yearly change on the RHS in the female SMA 2 patients is +1.04, –0.58, –0.83 and –0.21 at 2.5 years, 5 years, 10.5 years, and 16 years respectively, with corresponding changes of +1.72, –1.06, –1.13 and +0.18 on the HFMSE. The average yearly change on the RHS in the male SMA 2 participants is +2.33, –0.09, –0.89 and –0.25 at 2.5 years, 5 years, 10.5 years, and 16 years respectively, with corresponding changes of +2.74, –0.22, –1.56 and –1.03 on the HFMSE.
Fig. 1
Average SMA 2 trajectories on the RHS and HFMSE by age and sex (average presented for participants who have not undergone scoliosis surgery) surgery).
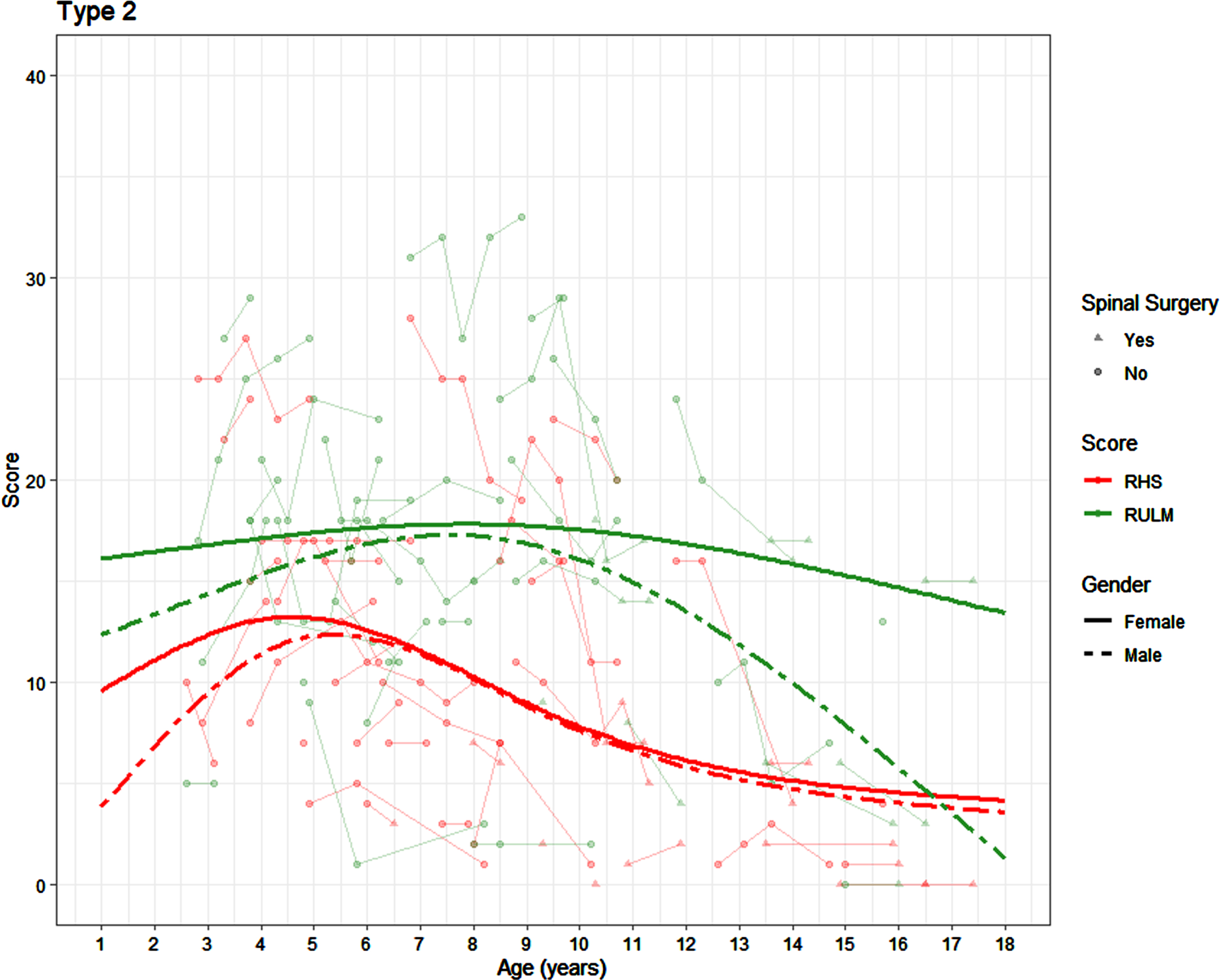
There was no significant difference in the RULM trajectory in the SMA 2 participants by sex (p = 0.302). In girls the average peak RULM score of 18 was achieved between the age of 5.42 and 10.17 years. In boys the average peak RULM score of 17 was achieved between the age of 5.5 and 9.5 years (Fig. 2).
Fig. 2
Average SMA 2 trajectories on the RHS and RULM by age and sex (average presented for participants who have not undergone scoliosis surgery).
Scoliosis surgery in the patients with SMA 2 was associated with a reduction of 2.96 (95% CI: 1.56, 4.36) points in the RHS trajectory, 4.51 (95% CI: 2.55, 6.47) points in the HFMSE and 2.71 (95% CI: 0.08, 5.33) points reduction in the RULM. In the patients with SMA type 2, after scoliosis surgery, RHS items 2 (hands to head in sitting), 3 (sitting to lying), 9 (rolls supine to prone), 10 (lifting head from prone), 11 (props on forearms) and 13 (rolls prone to supine) were mostly scored as a 0 (83%, 94%, 92%, 98%, 92% and 94% respectively), which is significantly different (p < 0.001 for all) to the pre scoliosis surgery cohort (53%, 64%, 52%, 77%, 61% and 56%).
SMA 3a’s
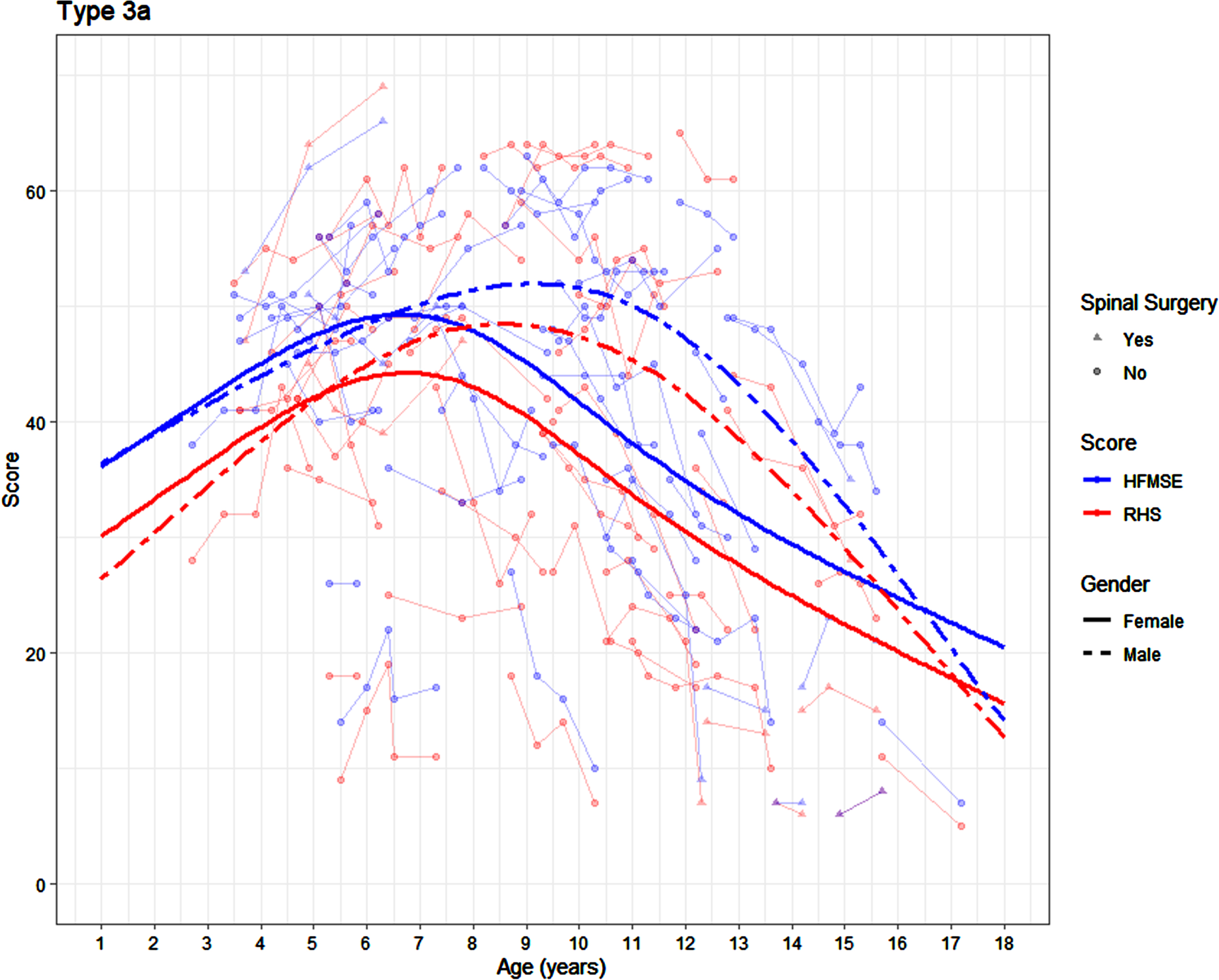
There was no significant difference in the RHS trajectory in the participants with SMA 3a by sex (p = 0.088), but there is a significant difference in the HFMSE trajectory by sex (p = 0.019). In girls with SMA 3a, the peak RHS score of 44 was achieved between the age of 5.83 and 7.67 years. In the same cohort, a peak HFMSE score of 49 was achieved in girls between the age of 5.67 and 7.58 years. In boys with SMA 3a, the peak RHS score of 48 was achieved in boys between the age of 7.25 and 9.92 years. The peak HFMSE score of 52 was achieved in boys between the age of 8.17 and 10.08 years (Fig. 3). The average yearly change on the RHS in the female SMA 3a patients is +3.16, +1.67, –3.36 and –2.28 at 2.5 years, 5 years, 10.5 years and 16 years respectively, with corresponding changes of +3.01, +1.15, –3.34 and –2.16 on the HFMSE. The average yearly change on the RHS in the male SMA 3a participants is +3.98, +3.03, –2.52 and –5.52 at 2.5 years, 5 years, 10.5 years and 16 years respectively, with corresponding changes of +2.53, +2.1, –2.18 and –6.31 on the HFMSE.
Fig. 3
Average SMA 3a trajectory on the RHS and HFMSE by age and sex (average is presented for participants who have not undergone scoliosis surgery).
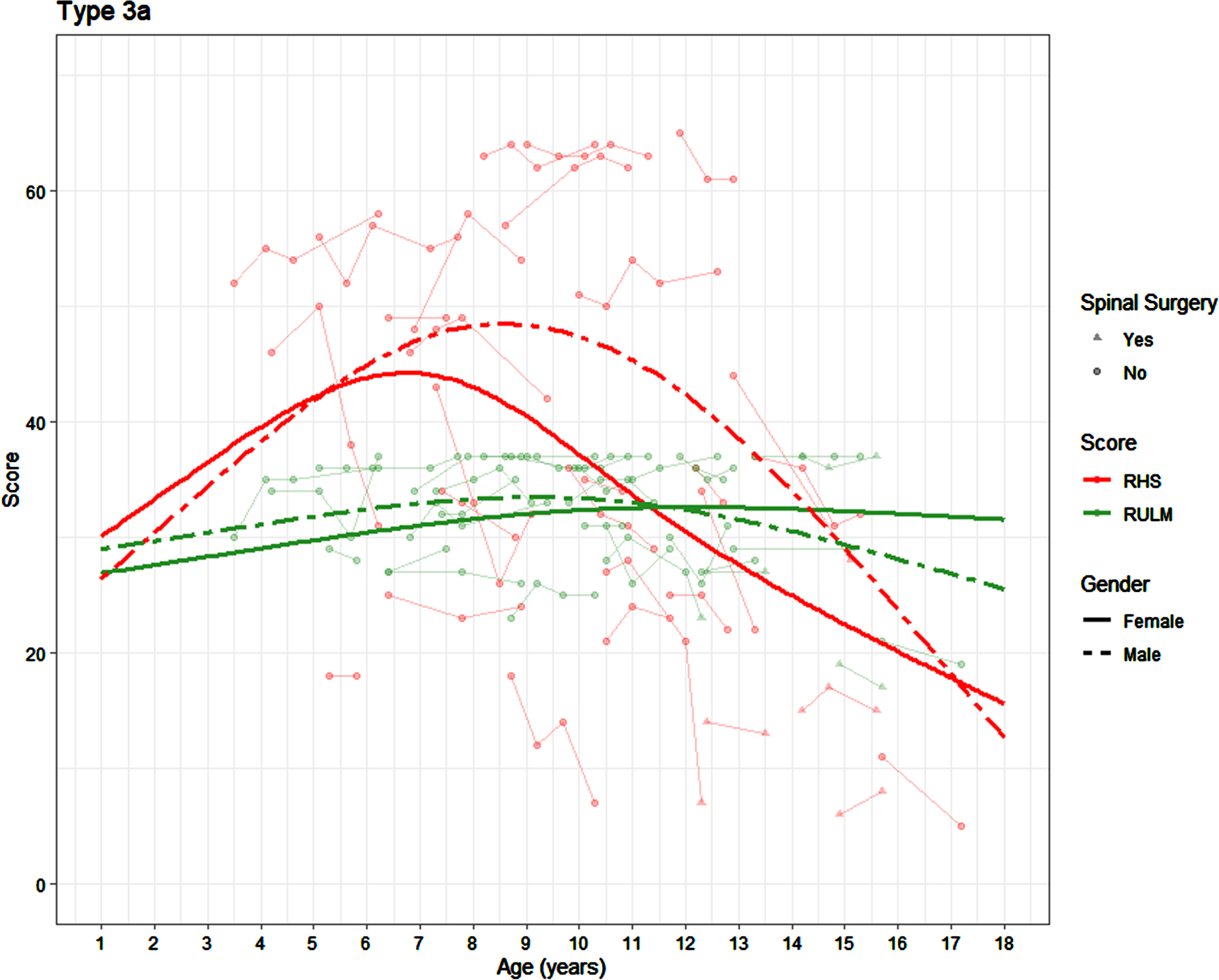
In the assessments of the 3a’s where a RULM was recorded 51% were ambulatory. There was no significant difference in the RULM trajectory in the participants with SMA 3a by sex (p = 0.549)(Fig. 4). In girls with SMA 3a, the peak RULM score of 33 was achieved between the age of 10.75 and 13.67 years, whilst in boys the average peak RULM score of 33 is achieved between the age of 6.17 and 11.83 years.
Fig. 4
Average SMA 3a trajectories on the RHS and RULM by age and sex (average presented for participants who have not undergone scoliosis surgery).
Scoliosis surgery in the SMA 3a patients was associated with a reduction of 11.75 (95% CI: 5.94, 17.56) points in the RHS trajectory, 13.39 (95% CI: 8.34, 18.4) points in the HFMSE and 3.56 (95% CI: 0.87, 6.28) points reduction in the RULM.
In the SMA 3a patients, after scoliosis surgery, RHS items 3 (sitting to lying), 9 (rolls supine to prone), 10 (lifting head from prone), 11 (props on forearms) and 13 (rolls prone to supine) were scored significantly differently (p < 0.001) in the cohort post scoliosis surgery (32%, 32%, 42%, 53% and 32% scored as 0 respectively) compared to pre scoliosis surgery (1%, 3%, 5%, 5% and 4% scored as 0 respectively).
SMA 3b’s
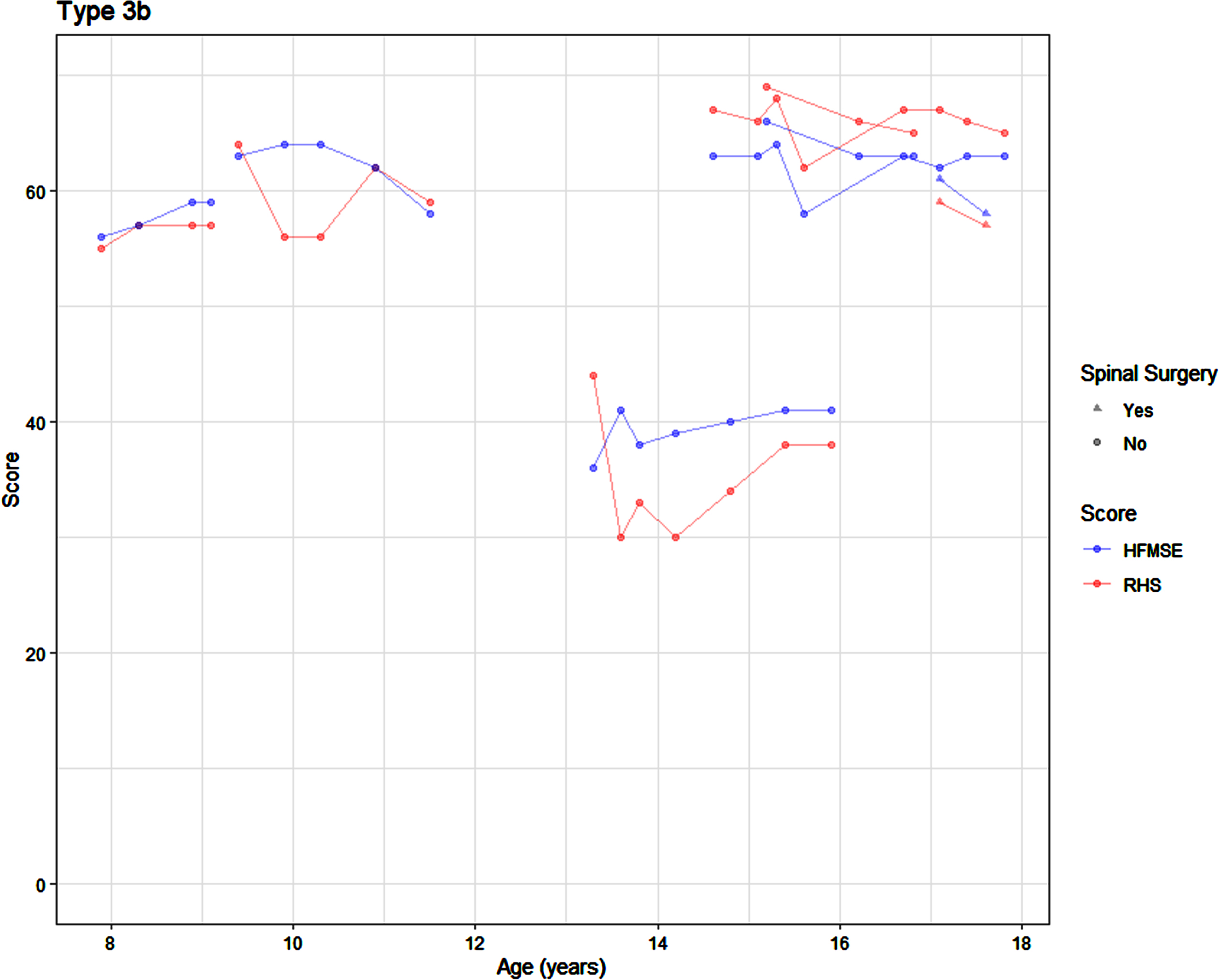
The SMA 3b participants displayed stability over time (Fig. 5), although statistical modelling was not possible due to the small sample size (n = 6).
Fig. 5
Average SMA 3b trajectory on the RHS and HFMSE by age.
DISCUSSION
Our results indicate that this cohort of later-onset SMA demonstrates a time of relative stability prior to a decline in functional skills. The scores on the RHS and HFMSE show similar trends over time in this cohort not receiving DMT. Our analysis has shown a broad age window to achieve the peak score, with deterioration often being linked to growth [3, 24]. In the RHS we observe a stable peak in the SMA type 2 s between 3.25–6 years and in the SMA 3a between 5.6–7.7 years. Previous work on the RHS has reported positive slopes before the age of 5 in those with type 2 SMA followed by a negative slope, whereas the patients with SMA 3a demonstrated an increase in scores before 5 years, stability between ages 5–7 and then a negative trend after 7 years [15].
When comparing the RHS and RULM, there is a lower correlation in the type 3a’s than for the type 2 patients, suggesting a decrease in correlation as function improves. The original upper limb module was specifically designed for a non-ambulant population [25], however the RULM was designed to be suitable for both ambulant and non-ambulant cohorts, which is reflected in this dataset [16, 17]. We identified a significant difference in the RHS score of participants who completed the RULM compared to those who did not. It’s important to consider that this is historical natural history data and prior to the introduction of disease modifying treatments the RULM was not routinely completed in the clinical settings across all sites. We acknowledge that there could be an element of selective bias, as the RULM may not always be completed either in the strongest patients, or in the weakest ones due to lack of perceived usefulness. This limits the interpretation of the results of this study, but as there are very few untreated patients remaining in the respective networks, we consider these results to reflect as accurately as possible the relationship between the RHS and RULM in the natural history of SMA. Despite the limitations these results will help the interpretation of disease modifying therapies in patients who can sit with and without support across the networks. Figures 1 and 2 show that in the SMA type 2 the RHS plateaus at approximately 7 points. This score could be used to inform clinical decision making when selecting the use of scales to capture functional changes and therefore to consider prioritising the RULM at this stage instead.
Overall, the magnitude of the RHS is lower than the HFMSE with more of a marked reduction in the SMA 2 cohort. However, in the SMA 2 cohort we observed a steeper decline in HFMSE, likely representing the larger reduction in score due to the loss of the same item bilaterally. Rasch analysis identified validity issues in some SMA phenotypes for the HFMSE, resulting in the creation of the RHS by remove the duplicated items and reducing the floor and ceiling effect. The RHS is a more compact scale, but this does not reduce its meaningfulness [13, 26]. Indeed, in the type 2 cohort the average peak score of the HFMSE is approximately 5 points higher than in the RHS. This is likely due to items which are tested on both sides in the HFMSE (rolling, items 6–9) and the duplication of items in the HFMSE (sitting independently and hands to head, items 1–4). In the SMA 3a’s, the average peak of the HFMSE is approximately 5 points higher than in the RHS. This is also likely due to other items duplicated (the 4 propping and crawling) items on the HFMSE being merged into 2 items on the RHS. This may indicate either over measurement of certain levels of ability in the HFMSE, or loss of sensitivity in the RHS. As the RHS only collects assessment of one side, further work will need to explore these differences. This change (from bilateral data collection to unilateral collection) was deliberately introduced to avoid discontinuities in the scale and overrepresentation of scores for specific changes. It will nevertheless be important to understand the sensitivity in these cohorts by looking at the relative minimal clinically important difference (MCID) and the minimum detectable change (MDC) in the RHS and compare this to the HFMSE. The MDC for the HFMSE has been reported on but this is likely to be different in the RHS [27, 28].
When analysing the impact of scoliosis surgery, it was clear that this was associated with a reduction in scores in all three scales. It is important to note that also an increase in scoliosis Cobb angle would also negatively impact scores [29]. The reduction due to scoliosis surgery was larger in the HFMSE than the RHS, again due to the bilateral items (4.5 vs 3 in those with SMA 2, 13.4 vs 11.8 in those with SMA 3a), representing similar reductions in total score of between 23% and 27% respectively across SMA type, sex, and total score. In this cohort there were 8 SMA 2 patients for whom an assessment was available both pre and post spinal surgery. Two of these had lost the ability to get their hands to their head in sitting (item 2) and two lost the ability to transition from sitting to lying (item 3). One patient lost the ability to roll from supine to prone and one needed compensation to achieve this (item 9). One patient lost the ability to lift their head from prone and one needed compensation to achieve this (item 10), one lost the ability to prop on their forearms and two needed assistance to achieve this (item 11). One patient lost the ability to roll from prone to supine and one needed compensation to achieve this (item 13).
In our cohort sex did not significantly impact the SMA 2 cohort peak score for either the RHS or HFMSE. The same was true with the RULM trajectory in the SMA 2’s and SMA 3a’s. There was no significant difference in the RHS trajectory in the SMA 3a participants by sex but there is a significant difference in the HFMSE trajectory by sex. In girls with SMA 3a the peak HFMSE score of 49 was achieved between the age of 5.67 and 7.58 years. In boys with SMA 3a the peak HFMSE score of 52 was achieved between the age of 8.17 and 10.08 years. There is a disparity between sex groups in our 3a cohort with 32 females and 15 males, and this small sample size may be the reason for observed differences. Alternatively, some studies have linked differential motor function in SMA to growth. In SMA growth has been found to be more linear in females compared to males, which could explain the later peak for males [30]. However, it’s been previously found that the typical sex differences in strength are not applicable to SMA due to the muscle atrophy, so this might not necessarily explain the higher peak in the male cohort [31].
In paediatric SMA, type 3b is a rare sub-type in an already rare condition, with disease onset anywhere between 3 and 18 years, and our cohort did not have many patients in this category [5, 32]. Due to this the cohort size in this study is very small and statistical analysis could not be performed on the type 3b group which is a limitation. It appears this cohort remains stable in both their RHS and HFMSE scores.
This longitudinal multicentre natural history study showed that the RHS can be used across SMA type and could be used in conjunction with other functional measures such as the RULM to holistically detect disease progression. This will assist with fully understanding changes that occur with treatments, and with defining trajectories and proactive therapy recommendations. Further work is needed to look at the MDC and MCID for the RHS.
ACKNOWLEDGMENTS
In addition to the authors, we would like to acknowledge the contributions of several other institutions. The support of the SMA Trust and of MDUK to the activities of the Dubowitz Neuromuscular Centre is gratefully acknowledged. The support of Famiglie SMA, Telethon (GSP 13002), and ASAMSI to the Nemo Center in Rome and to the Italian network is gratefully acknowledged. The SMA REACH UK working group clinicians, physiotherapists and study co-ordinators: UK (http://www.smareachuk.org/; Clini-calTrials.gov Identifier: NCT03520179) - Great Ormond Street Hospital; University College London; Birmingham Heartlands Hospital; Leeds Children Hospital; Evelina Children’s Hospital, London; The Robert Jones and Agnes Hunt Orthopaedic Hospital, Oswestry; Sheffield Teaching Hospital. The National Institute for Health Research Biomedical Re-search Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. The Pediatric Neuromuscular Clinical Research (PNCR) Network for SMA (Boston Children’s Hospital, Children’s Hospital of Philadelphia, Vagelos College of Physicians and Surgeons, Columbia University, New York; Nemours Children’s Hospital, Orlando; and Stanford University, Palo Alto) gratefully acknowledges the support of the SMA Foundation and Cure SMA.
FUNDING
This study was supported, in the UK, by the SMA REACH UK project (www.smareachuk.org, accessed on 20 January 2023). FM is the chief investigator of the SMA Reach project. Commercial funding for the SMA Reach project is provided by Biogen Inc. and Roche (REC reference: 13/LO/1748, IRAS project ID: 122521), via UCL and GOSH. Historically, funding of the SMA Reach Project has also been provided by the SMA Trust; Muscular Dystrophy UK (07DN02; 37787 http://www.musculardystrophyuk.org/grants/clinical-trial-coordinators/, accessed on 20 January 2023), the MRC Translational Research Centre at UCL and Newcastle (MR/K501074/1), and the National Institute for Health Research Biomedical Research Centre (515048) at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London (http://www.gosh.nhs.uk/research-and-innovation/nihr-great-ormond-street-brc/about-brc, acc-essed on 20 January 2023). The Pediatric Neuromuscular Clinical Research (PNCR) Network for SMA (Boston Children’s Hospital, Children’s Hospital of Philadelphia, Vagelos College of Physicians and Surgeons, Columbia University, New York; Nemours Children’s Hospital, Orlando; and Stanford University, Palo Alto) gratefully acknowledges the support of the SMA Foundation and Cure SMA. The support of Famiglie SMA, Telethon (GSP 13002), and ASAMSI to the Nemo Center in Rome and to the Italian network is gratefully acknowledged.
CONFLICT OF INTEREST
A.D. has received compensation as a consultant on advisory boards for Roche, Biogen, and AveXis. A.M. has served on medical/scientific advisory boards for Biogen and Roche, and has received fees for consulting and training services for Biogen, Roche, Novartis, and Biohaven. A.M.G. receives fees for consulting services for Biogen, Roche, and Audentes, and licensing fees for co-development of the CHOP INTEND. A.P. has served on medical/scientific advisory boards for AveXis, Biogen, and Roche, and has received fees for consulting services for Biogen, Roche, and Audentes. B.T.D. has served as an ad hoc scientific advisory board member for Audentes, AveXis/Novartis Gene Therapies, Biogen, Pfizer, Sarepta, Vertex, and Roche/Genentech; Steering Committee Chair for Roche FIREFISH and MANATEE studies, and DSMB member for Amicus Inc. and Lexeo Therapeutics; B.T.D. has no financial interests in these companies. B.T.D. has received research support from the National Institutes of Health/National Institute of Neurological Disorders and Stroke, the Slaney Family Fund for SMA, the Spinal Muscular Atrophy Foundation, CureSMA, and Working on Walking Fund, and has received grants from Ionis Pharmaceuticals, Inc. for the ENDEAR, CHERISH, CS2/CS12 studies; from Biogen for CS11; and from AveXis, Sarepta Pharmaceuticals, Novartis (AveXis), PTC Therapeutics, Roche, Scholar Rock, and Fibrogen. B.T.D. has also received royalties for books and online publications from Elsevier and UpToDate, Inc. C.B. has served as a consultant, speaker in sponsored symposiums, and principal investigator for Roche, and has received personal fees for AveXis and Roche. C.M. has received consultancy honoraria from Biogen, Roche, and Novartis for participation in educational activities/meetings. C.M. has also received research funding from Roche and Biogen to support Adult SMA REACH activity. D.C.D.V has served as an advisor/consultant for AveXis, Biogen, Cytokinetics, Ionis, METAFORA, Roche, Sanofi, Sarepta, Scholar Rock, SMA Foundation, and Ultragenyx, with no financial interests in these companies; has received grants from Cure SMA, Department of Defense, Glut1 Deficiency Foundation, Hope for Children Research Foundation, National Institutes of Health, and SMA Foundation; has received research funding from Department of Defense, Glut1 Deficiency Foundation, Hope for Children Research Foundation, iSMAC initiative (Biogen), National Institutes of Health, Sanofi, and SMA Foundation; has received clinical trial funding from Ionis, Mallinckrodt, PTC, Santhera, Sarepta, Scholar Rock, and Ultragenyx; serves as the Data Safety Monitoring Committee Chair for Aspa Therapeutics; and is an inventor on a patent for Glut1DS gene therapy. D.R. reports participation to teaching initiatives for Roche. E.A. has served as a consultant and as a speaker in sponsored symposiums for Biogen. E.B. reports participation to Scientific Advisory Boards for Roche, Biogen, PTC Therapeutics, Pfizer, and Novartis. E.B. is involved in clinical trials with Roche, Biogen, Novartis, and PTC Therapeutics. E.Mercuri has participated in advisory boards for SMA studies for AveXis, Biogen, Ionis, Novartis, and Roche; has been a Principal Investigator for ongoing Biogen and Roche clinical trials; and has received research grants from Famiglie SMA Italy, Italian Telethon, Novartis, Scholar Rock, and SMA Europe. E.S.M. reports consultancy fees from Roche, Biogen, Scholar Rock, and Novartis. F.M. reports participation to scientific advisory boards and teaching initiatives for AveXis, Biogen, Roche, and Novartis. E.S.M. is a member of the Rare Disease Scientific Advisory Board for Pfizer. E.S.M. is involved as an investigator in clinical trials from AveXis, Biogen, and Roche. E.S.M. is the principal investigator of the SMA REACH UK clinical network, partially funded by Biogen and by SMA UK. G.B. is principal investigator of clinical trials sponsored by Pfizer, NS Pharma, and Reveragen; has received speaker and/or consulting fees from Sarepta, PTC Therapeutics, Biogen, Novartis Gene Therapies, Inc. (AveXis), and Roche; and has worked as principal investigator of SMA studies sponsored by Novartis Gene Therapies, Inc., and Roche. G.C. reports participation to scientific advisory boards and teaching initiatives for AveXis, Biogen, Roche, and Novartis. J.D. has received personal fees for AveXis, Biogen, Roche, and Novartis. J.M. serves on advisory boards for Biogen, Roche, Genentech, and Sarepta and as a consultant for Scholar Rock and Biogen. She receives grant support from the Muscular Dystrophy Association, Cure SMA, Genentech, and Novartis. M.P. has served as a consultant and as a speaker in sponsored symposiums for Biogen, and has received personal fees for AveXis. M.S. reports participation to scientific advisory boards and teaching initiatives for AveXis, Biogen, and Roche; M.S. is involved as an investigator in clinical trials from AveXis, Biogen, and Roche. R.M.L. has served in advisory boards for Biogen, Roche, and Novartis; has received consulting fees by Roche, Biogen, and Novartis; and research support by Roche and Biogen. R.S.F. has served on medical/scientific advisory boards on SMA-related topics for AveXis, Biogen, Capricor, Families of SMA, Genentech, Ionis Pharmaceuticals, Novartis, Roche, and ScholarRock; received research support from AveXis/Novartis, Biogen/Ionis, Capricor, Roche/Genentech, Scholar Rock, and NIH; and receives license fees for co-development of the CHOP INTEND. S.D.Y. serves on advisory boards for Biogen, Roche, and Scholar Rock, and as a consultant for Biogen, Roche, and Cure SMA. S.D.Y. receives grant support from Cure SMA. S.M. has received honoraria from Biogen, Roche, and Novartis for advisory boards and presentations. T.D. has served on scientific advisory boards for AveXis, Biogen, Roche, Scholar Rock, Novartis, CureSMA, Dyne, and Actigraph; and has received consulting fees Roche, Biogen, Biohaven, Dyne, Sarepta, Solid Bio, Tayjus, Astellas, ATOM, and Trinds. V.S. has served as a consultant and as a speaker in sponsored symposiums for Biogen, and has received personal fees for AveXis. A.W., E.O., E.Milev, G.S., A.R., M.C., M.M., and Z.Z.C. have no conflicting interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
INSTITUTIONAL REVIEW BOARD STATEMENT
This study was conducted in accordance with the Declaration of Helsinki. Each of the International SMA Consortium natural history study sites had ethical approval in place permitting collection of RHS natural history data. SMA REACH UK: National Research Ethics Committee (REC) London Bromley, Health Research Authority REC reference 13/LO/1748. PNCR USA Institutional Review Boards (IRB) and Numbers: IRB Numbers— Columbia University Medical Center Human Research Protection Office IRB reference AAAE8252, The Children’s Hospital of Philadelphia IRB reference 10-007816, Boston Children’s Hospital Office of Clinical Investigations IRB reference 05-02-028, Stanford University Re-search Compliance Office IRB reference 31140, Nemours Children’s Hospital reference number 1004092. The Italian SMA Network is co-ordinated by Catholic University of Sacred Heart, Fondazione Policlinico Universitario Agostino Gemelli IRCCS (IRB proto-col number 2533/18) and includes: University of Messina; IRCCS Bambino Gesù Children’s; University of Milan, Niguarda Hospital; IRCCS Istituto Giannina Gaslini.
REFERENCES
[1] | Petit F , Cuisset JM , Rouaix-Emery N , Cancés C , Sablonnière B , Bieth E , et al. Insights into genotype-phenotype correlations in spinal muscular atrophy: A retrospective study of 103 patients. Muscle Nerve. (2011) ;43: (1):26–30. |
[2] | Verhaart IEC , Robertson A , Leary R , McMacken G , König K , Kirschner J , et al. A multi-source approach to determine SMA incidence and research ready population. J Neurol. (2017) ;264: (7):1465–73. |
[3] | Kaufmann P , McDermott MP , Darras BT , Finkel R , Kang P , Oskoui M , et al. Observational study of spinal muscular atrophy type 2 and 3: Functional outcomes over 1 year. Arch Neurol. (2011) ;68: (6):779–86. |
[4] | Rodriguez-Torres RS , Uher D , Gay EL , Coratti G , Dunaway Young S , Rohwer A , Muni Lofra R , De Vivo DC , Hirano M , Glynn NW , Montes J Measuring Fatigue and Fatigability in Spinal Muscular Atrophy (SMA): Challenges and Opportunities. Journal of Clinical Medicine.. (2023) ;12: (10):3458. |
[5] | Piepers S , Van Den Berg LH , Brugman F , Scheffer H , Ruiterkamp-Versteeg M , Van Engelen BG , Faber CG , De Visser M , van der Pol WL , Wokke JHJ A natural history study of late onset spinal muscular atrophy types 3b and 4. Journal of Neurology. (2008) ;255: :1400–4. |
[6] | Pane M , Coratti G , Pera MC , Sansone VA , Messina S , d’Amico A , Bruno C , Salmin F , Albamonte E , De Sanctis R , Sframeli M Nusinersen efficacy data for 24-month in type 2 and 3 spinal muscular atrophy. Annals of Clinical and Translational Neurology. (2022) ;9: (3):404–9. |
[7] | Darras BT , Chiriboga CA , Iannaccone ST , Swoboda KJ , Montes J , Mignon L , Xia S , Bennett CF , Bishop KM , Shefner JM , Green AM Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology.. (2019) ;92: (21):e2492–e2506. |
[8] | Oskoui M , Day JW , Deconinck N , Mazzone ES , Nascimento A , Saito K , Vuillerot C , Baranello G , Goemans N , Kirschner J , Kostera-Pruszczyk A Two-year efficacy and safety of risdiplam in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA). Journal of Neurology. (2023) 1–16. |
[9] | Mercuri E , Barisic N , Boespflug-Tanguy O , Deconinck N , Kostera-Pruszczyk A , Masson R , Mazzone E , Nascimento A , Saito K , Vlodavets D , Vuillerot C SUNFISH Part 2: Efficacy and safety of risdiplam (RG7916) in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA)(1260) 2020. |
[10] | Dhillon S Risdiplam: First approval. Drugs. (2020) ;80: :1853–8. |
[11] | FDA Center for Biologics Evaluation and Research. (2019). Zolgensma FDA approval.http://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/. |
[12] | Hoy SM Nusinersen: A review in 5q spinal muscular atrophy. CNS Drugs. (2018) ;32: :689–96. |
[13] | Ramsey D , Scoto M , Mayhew A , Main M , Mazzone ES , Montes J , De Sanctis R , Dunaway Young S , Salazar R , Glanzman AM , Pasternak A Revised Hammersmith Scale for spinal muscular atrophy: A SMA specific clinical outcome assessment tool. PloS one. (2017) ;12: (2):e0172346. |
[14] | O’Hagen JM , Glanzman AM , McDermott MP , Ryan PA , Flickinger J , Quigley J , Riley S , Sanborn E , Irvine C , Martens WB , Annis C An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscular Disorders. (2007) ;17: (9-10):693–7. |
[15] | Stimpson G , Ramsey D , Wolfe A , Mayhew A , Scoto M , Baranello G , Muni Lofra R , Main M , Milev E , Coratti G , Pane M 2-year change in revised Hammersmith scale scores in a large cohort of untreated paediatric type 2 and 3 SMA participants. Journal of Clinical Medicine. (2023) ;12: (5):1920. |
[16] | Mazzone ES , Mayhew A , Montes J , Ramsey D , Fanelli L , Young SD , Salazar R , De Sanctis R , Pasternak A , Glanzman A , Coratti G Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle & Nerve. (2017) ;55: (6):869–74. |
[17] | Coratti G , Pera MC , Montes J , Scoto M , Pasternak A , Bovis F , Sframeli M , D’Amico A , Pane M , Albamonte E , Antonaci L Revised upper limb module in type II and III spinal muscular atrophy: 24-month changes. Neuromuscular Disorders. (2022) ;32: (1):36–42. |
[18] | Coratti G , Pera MC , Montes J , Pasternak A , Scoto M , Baranello G , Messina S , Dunaway Young S , Glanzman AM , Duong T , De Sanctis R Different trajectories in upper limb and gross motor function in spinal muscular atrophy. Muscle & Nerve. (2021) ;64: (5):552–9. |
[19] | Mercuri E , Finkel R , Scoto M , Hall S , Eaton S , Rashid A , et al. Development of an academic disease registry for spinal muscular atrophy. Neuromuscular Disorders [Internet]. (2019) ;29: (10)794–9. Available from:https://linkinghub.elsevier.com/retrieve/pii/S0960896619311058. |
[20] | Wang CH , Finkel RS , Bertini ES , Schroth M , Simonds A , Wong B , et al. Consensus Statement for Standard of Care in Spinal Muscular Atrophy Current Problems in the Medical Care of Patients With Spinal Muscular Atrophy Spinal muscular atrophy is a recessively inherited neuro-muscular disease characterized by degeneration of spinal. J Child Neurol [Internet]. [cited Jun 7]. (2022) ;22: :1027–49. |
[21] | Ramsey D , Ramdharry G , Scoto M , Muntoni F , Wallace A , SMA REACH UK network, Revised Hammersmith Scale for spinal muscular atrophy: Inter and intra-rater reliability and agreement. Plos one. (2022) ;17: (12):e0278996. |
[22] | Glanzman AM , Mazzone ES , Young SD , Gee R , Rose K , Mayhew A , Nelson L , Yun C , Alexander K , Darras BT , Zolkipli-Cunningham Z Evaluator training and reliability for SMA global Nusinersen trials. Journal of Neuromuscular Diseases. (2018) ;5: (2):159–66. |
[23] | Glanzman AM , O’Hagen JM , McDermott MP , Martens WB , Flickinger J , Riley S , et al Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. (2011) ;26: (12):1499–507. |
[24] | Kaufmann P , McDermott MP , Darras BT , Finkel RS , Sproule DM , Kang PB , Oskoui M , Constantinescu A , Gooch CL , Foley AR , Yang ML Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. (2012) ;79: (18):1889–97. |
[25] | Mazzone E , Bianco F , Martinelli D , Glanzman AM , Messina S , De Sanctis R , Main M , Eagle M , Florence J , Krosschell K , Vasco G Assessing upper limb function in nonambulant SMA patients: Development of a new module. Neuromuscular Disorders. (2011) ;21: (6):406–12. |
[26] | Cano SJ , Mayhew A , Glanzman AM , Krosschell KJ , Swoboda KJ , Main M , et al. Rasch analysis of clinical outcome measures in spinal muscular atrophy. Muscle & Nerve. (2013) ;49: (3):422–30.Epub 2013/07/10. |
[27] | Stolte B , Bois JM , Bolz S , Kizina K , Totzeck A , Schlag M , Kleinschnitz C , Hagenacker T Minimal clinically important differences in functional motor scores in adults with spinal muscular atrophy. European Journal of Neurology. (2020) ;27: (12):2586–94. |
[28] | Vázquez-Costa JF , Hervás D Minimal detectable change and minimal clinically important difference in spinal muscular atrophy patients. European Journal of Neurology. (2021) ;28: (6):e40–e41. |
[29] | Dunaway Young S , Montes J , Salazar R , Glanzman AM , Pasternak A , Mirek E , Martens W , Finkel RS , Darras BT , De Vivo DC Scoliosis surgery significantly impacts motor abilities in higher-functioning individuals with spinal muscular atrophy. Journal of Neuromuscular Diseases. (2020) ;7: (2):183–92. |
[30] | De Amicis R , Baranello G , Foppiani A , Leone A , Battezzati A , Bedogni G , Ravella S , Giaquinto E , Mastella C , Agosto C , Bertini E Growth patterns in children with spinal muscular atrophy. Orphanet Journal of Rare Diseases. (2021) ;16: (1):1–10. |
[31] | Merlini L , Bertini E , Minetti C , Mongini T , Morandi L , Angelini C , Vita G Motor function–muscle strength relationship in spinal muscular atrophy. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. (2004) ;29: (4):548–52. |
[32] | Zerres K , Rudnik-Schöneborn S , Forrest E , Lusakowska A , Borkowska J , Hausmanowa-Petrusewicz I A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. Journal of the Neurological Sciences. (1997) ;146: (1):67–72. |




