Detection of Autoantibodies Against the Acetylcholine Receptor, Evaluation of Commercially Available Methodologies: Fixed Cell-Based Assay, Radioimmunoprecipitation Assay and Enzyme-Linked Immunosorbent Assay1
Abstract
Background/Objective:
Myasthenia Gravis (MG) is an autoimmune disorder characterized by pathogenic autoantibodies (AAbs) targeting nicotinic acetylcholine receptors (AChR), disrupting neuromuscular communication. RadioImmunoPrecipitation Assay (RIPA) is recommended to detect AChR AAbs, but its complexity and radioactive requirements limit widespread use. We compare non-RIPA anti-AChR immunoassays, including Cell-Based Assay (CBA) and two ELISA kits, against the gold standard RIPA.
Methods/Results:
145 samples were included with medical indication for anti-AChR testing. By the RIPA method, 63 were negative (RIPA-Neg < 0.02 nmol/L), 18 were classified as Borderline (≥0.02 –1 nmol/L), and 64 were positive (RIPA-Pos > 1 nmol/L). The competitive ELISA showed poor agreement with RIPA (Kappa = 0.216). The indirect ELISA demonstrated substantial agreement with RIPA (Kappa = 0.652), with ∼76% sensitivity and ∼94% specificity for MG diagnostic. The CBA, where fixed cells expressing clustered AChR were used as substrate, exhibited almost perfect agreement with RIPA (Kappa = 0.984), yielding ∼98% sensitivity and 96% specificity for MG. In addition, a semiquantitative analysis showed a strong correlation between CBA titration, indirect ELISA, and RIPA levels (r = 0.793 and r = 0.789, respectively).
Conclusions:
The CBA displayed excellent analytical performance for MG diagnostic when compared to RIPA, making it a potential replacement for RIPA in clinical laboratories. Some solid-phase assays (such as the indirect ELISA applied here), as well as CBA titration, offer reliable options to estimate anti-AChR AAb levels after confirming positivity by the CBA.∥
1INTRODUCTION
Autoantibodies (AAb) are immunoglobulins directed against self-antigens and they are valuable biomarkers for autoimmune diseases, playing a relevant role in the clinical diagnosis and management of several of these diseases. Some AAb are directly involved in the pathophysiology of some autoimmune diseases. Myasthenia Gravis (MG) is a neuromuscular autoimmune disease characterized by muscle weakness and fatigue, resulting from AAb directed mostly (∼85% of cases) against the nicotinic acetylcholine receptor (referred to in this manuscript as AChR or nAChR) present at the muscle cell membrane [1]. Anti-AChR AAb hinder the communication between the nerve and muscle fiber. MG can affect any muscle and can be life threatening when swallowing and breathing are impaired. Although there is no cure for MG, appropriate treatment with acetylcholinesterase-inhibitor as well as immunosuppressive drugs [2, 3], or more recently immunobiologicals [4], can substantially improve the patient’s quality of life and prognosis.
In humans, the muscular nAChR exists as an embryonic and an adult isoform, with the adult form predominating after birth. The embryonic form consists of five subunits, α1, β1, γ, and δ, with a proportion of 2 : 1:1 : 1, respectively, arranged in a circular manner to form the cation channel. The adult form is similar but contains the ɛ subunit instead of the γ subunit [5]. The ACh binding sites that promote the “opening” of the cation channel are located at the α1 subunits. The anti-AChR AAb targets preferentially the extracellular part of the α1 subunit, but not necessarily the ACh binding site [5].
When detected in high levels, circulating anti-AChR AAb has 100% specificity for the diagnosis of MG. The laboratory platforms most commonly applied for the detection of anti-AChR AAb are radioimmunoprecipitation assay (RIPA) and solid-phase immunoassays, such as the enzymatic (ELISA) or chemiluminescent (CLIA) types, for which there are available commercial kits. RIPA was developed in the 1970’s and is considered the gold standard [6, 7] for anti-AChR AAb determination. It is based on a mixture of nAChR which can be from various sources, mostly from extract of rhabdomyosarcoma TE671 cells or in some cases muscle tissue [8, 9], with its ligand α-bungarotoxin conjugated to the radioactive isotope 125I (iodine-125). After incubation with the patient’s serum containing anti-AChR AAb, the complex is precipitated with a second anti-human IgG antibody and the radioactivity is quantified by a gamma counter [6, 7]. RIPA is highly sensitive but presents the inherent disadvantage of requiring radioactive materials, which makes its execution costly and restrict to a few centers worldwide. Thus, many clinical labs use solid phase immunoassays, such as ELISA and its variations, which have a poor reputation regarding sensitivity [10].
Over the past decade if has been proposed the use of cell-based assays (CBA) to detect anti-AChR AAb [11, 12]. CBA is an indirect immunofluorescence assay (IFA) that uses cells transfected or transduced with plasmids that encode the protein of interest. Usually, the transfected gene has a cell membrane localizing sequence that ensures the expression of high levels of the native folded protein at the cell membrane, providing an optimal exposure of the relevant epitopes to be targeted by the autoantibodies. Recently a biochip CBA with four fixed transfected cell-lines has been marketed (Euroimmun). The biochip configuration has cells expressing the adult AChR-ɛ, the embryonic AChR-γ, muscle-specific kinase (MuSK), and wild-type cells (EU-90) to be used as negative control in the IFA. The two cell-lines expressing the AChR also contain the other subunits as well as rapsyn, a molecule that clusters the receptors, demonstrated to improve the sensitivity to detect anti-AChR AAb in MG [13]. So far this novel immunoassay has been utilized in only a few studies [11, 14–16], but it has presented an almost perfect Kappa agreement with the RIPA to detect anti-AChR in MG patients.
Our goal in this study was to compare the performance of different commercial assays to detect anti-AChR AAbs: the gold standard RIPA, a recently marketed cell-based assay (CBA), and two solid-phase ELISA kits.
2MATERIALS AND METHODS
2.1Samples
A total of 145 samples with medical request for anti-AChR/anti-Musk AAb testing were retrieved during 2018 until 2021 from the immunology division at Fleury Group Laboratory, Sao Paulo, Brazil. Because our goal was to study anti-AChR reactivity, samples with anti-Musk reactivity were not included in the study, therefore it was expected that none of the 145 samples would show reactivity to MuSK.
Patient’s individual clinical details were not included in the study; however, the MG diagnosis (or the absence of it) was defined based on characteristic clinical and electroneuromyographic findings (when available), or direct contact with the patient’s physician. In no case was the diagnosis of MG established solely on the basis of the autoantibody tests. In addition, the assays performed were as those requested by the patient’s physician; thus, the ethics committee waived the need to collect the patient’s informed consent. The study was approved by the Ethics committee at Fleury Group (Plataforma Brasil CAAE: 57480622.3.0000.5474).
2.2Assays
The following assays were performed in all samples: 1) Anti-AChR by RIPA (Mayo Clinic, USA) on a clinical-service basis; 2) Indirect ELISA (EA 1435-9601 G, Euroimmun, Germany), this kit is registered at the Brazilian regulatory agency to detect anti-AChR (ANVISA 81148560050); 3) Competitive ELISA - Research Use Only (MBS729942, MyBioSource, San Diego, CA, USA); 4) Fixed CBA Myasthenia gravis Mosaic 2 (FA 1435-1010-2, Euroimmun, Germany); 5) Anti-cell antibody (antinuclear antibody) by HEp-2 IFA (FA 1520-2010, Euroimmun, Germany). All assays were performed following the respective manufacturer‘s protocol.
Immunofluorescence slides (the CBA and the HEp-2 IFA) were analyzed for positivity and staining patterns in a fluorescence microscope with 200x or 400x magnification (Axio Imager.M2, Carl Zeiss, Germany). HEp-2 IFA titer was determined with sequential double dilutions starting at 1/80 up to end titer. Semi-quantitative titration of anti-AChR by the CBA was assessed by applying the samples in the following dilutions: 1/10, 1/20, 1/40, 1/100, 1/400 and 1/1000. Presence of a minimum positive signal at a given dilution was considered as the endpoint titer.
2.3Data analysis
Quantitative and semi-quantitative parameters were evaluated for normal distribution with D’Agostino & Pearson normality test, and the distribution was not normal for at least one group in all of them. Thus, when averages of two groups were compared, Mann-Whitney test was applied, when three or more groups were compared, Kruskal-Wallis test was applied. The correlation between anti-AChR levels by the various assays was calculated using Spearman test.
Youden J Index for cutoff determination was calculated with MedCalc Statistical Software version 20.115 (MedCalc Software Ltd, Belgium). The proportion of qualitative variables were compared with two-tailed Chi-squared test (Table 1). To calculate sensitivity and specificity of the assays, positive/negative groups were compared in 2x2 tables by Chi-square with Yates’ correction (Table 2).
Table 1
Reactivity to AChR and HEp-2 cells
| RIPA (n = 145) | Negative | Borderline | Positive | P value | Kappa (f) | |
| (n = 63) | (n = 18) | (n = 64) | (95% CI) | |||
| <0.02 nmol/L | 0.28 (±0.2) | 29.2 (±37) | ||||
| Indirect ELISA (n = 128) | (n = 51) | (n = 15) | (n = 62) | |||
| Cutoff (a) | Ø | 68.6% (n = 35) | 53.3% (n = 8) | 1.6% (n = 1) | <0.001 | 0.688 |
| + | 31.4% (n = 16) | 46.7% (n = 7) | 98.4% (n = 61) | (0.557-0.819) | ||
| Cutoff (b) | Ø | 96.1% (n = 49) | 93.3% (n = 14) | 29.1% (n = 18) | <0.001 | 0.652 |
| + | 3.9% (n = 2) | 6.7% (n = 1) | 70.9% (n = 44) | (0.519-0.785) | ||
| Competitive ELISA (c) | Ø | 87.3% (n = 55) | 88.9% (n = 16) | 65.6% (n = 42) | 0.007 | 0.216 |
| (n = 145) | + | 12.7% (n = 8) | 11.1% (n = 2) | 34.4% (n = 22) | (0.072-0.359) | |
| CBA (n = 145) | CBA-ɛ/γ-Neg | 100% (n = 63) | 72.2% (n = 13) | 1.6% (n = 1) | <0.001 | NA |
| embryonic AChR-γ | 0% (n = 0) | 16.7% (n = 3) | 93.7% (n = 60) | 0.937 (0.876-0.998) | ||
| adult AChR-ɛ | 0% (n = 0) | 27.8% (n = 5) | 98.4% (n = 63) | 0.984 (0.954-1.000) | ||
| HEp-2 IFA | Neg, 48.9% (n = 71) | 47.6% (n = 30) | 38.9% (n = 7) | 53.1% (n = 34) | ||
| Pos, 51.1% (n = 74) | 52.4% (n = 33) | 61.1% (n = 11) | 46.9% (n = 30) | 0.543 | NA | |
| Titer (d) | 1/467 (±483) | 1/240 (±167) | 1/432 (±335) | 0.134# | NA | |
| HEp-2 IFA Patterns (e) | Nuclear speckled | 59% (n = 23) | 62% (n = 8) | 63% (n = 22) | ||
| Nucleolar | 13% (n = 5) | 8% (n = 8) | 17% (n = 5) | |||
| Cytoplasmic | 18% (n = 7) | 15% (n = 2) | 11% (n = 4) | 0.987 | NA | |
| Other patterns | 10% (n = 4) | 15% (n = 2) | 9% (n = 3) |
•Data is presented as % plus n for categorical variables, or averages±S.D in quantitative variables (d). NA: not applicable. (a) Cutoff Youden index J = 0.8409; (b) Cutoff calculated as the average+3 S.D. of the RIPA-Neg group = 4.568; (c) Cutoff calculated as the average+1 S.D. of the RIPA-Neg group = 3.0; (d) HEp-2 IFA titer was compared among the positive samples (# Kruskal-Wallis test); (e) Some samples presented more than one pattern (ICAP www.anapatterns.org); (f) To quantify the agreement with Cohen’s Kappa, only the RIPA negative/positive groups were compared with the negative/positive in the ELISAs and in the CBA, respectively.
Table 2
The anti-AChR immunoassays sensitivity and specificity for MG diagnostic
| Diagnostic (a) | Diagnostic performance | ||||||
| MG (n = 54) | Not-MG (n = 61) | Inconclusive (n = 10) | p | (95% CI) (b) | |||
| RIPA (n = 125) | Negative (n = 56) | 0% (n = 0) | 85.2% (n = 52) | 40% (n = 4) | ***Se | 1.000 (0.933-1.000) | |
| Sp | 1.000 (0.931-1.000) | ||||||
| Borderline (n = 12) | 0% (n = 0) | 14.8% (n = 9) | 30% (n = 3) | PPV | 1.000 (0.933-1.000) | ||
| Positive (n = 57) | 100% (n = 54) | 0% (n = 0) | 30% (n = 3) | NPV | 1.000 (0.933-1.000) | ||
| Indirect ELISA | Ø (n = 65) | 23.1% (n = 12) | 94% (n = 47) | 85.7% (n = 6) | ***Se | 0.769 (0.638-0.862) | |
| (c) (n = 109) | +(n = 44) | 76.9% (n = 40) | 6% (n = 3) | 14.3% (n = 1) | Sp | 0.940 (0.837-0.983) | |
| PPV | 0.930 (0.813-0.976) | ||||||
| NPV | 0.796 (0.677-0.879)) | ||||||
| CBA (d) (n = 125) | CBA-ɛ/γ-Neg (n = 66) | 1,9% (n = 1) | 96.7% (n = 59) | 60% (n = 6) | ***Se | 0.981 (0.902-0.999) | |
| embryonic AChR-γ (n = 55) | 92.6% (n = 50) | 1.6% (n = 1) | 40% (n = 4) | Sp | 0.967 (0.888-0.994) | ||
| PPV | 0.963 (0.876-0.993) | ||||||
| adult AChR-ɛ (n = 59) | 98.1% (n = 53) | 3.3% (n = 2) | 40% (n = 4) | NPV | 0.983 (0.911-0.999) | ||
*** =p < 0.001; Se = sensitivity; Sp = specificity; PPV = Positive Predictive Value; NPV = Negative Predictive Value. (a) For 10 patients, the diagnostic was inconclusive or still under investigation, some with suspicion of MG, but without confirmation; (b) For performance statistical analysis, only the MG and Not-MG groups were considered. Also, only the groups RIPA positive and Negative were considered, and not the Borderline; (c) Cutoff Average of the RIPA-Neg group+3 S.D.=4.568; (d) Only results for CBA-AChR-ɛ were considered for performance analysis, and not the CBA-AChR-γ.
Cohen’s Kappa agreement was quantified using GraphPad online quickcalcs tool, which uses Fleiss equations to compute the standard error (SE) and confidence intervals (CI). All other analysis were performed using GraphPad Prism v7 (Dotmatics, Boston, MA, USA). p values were considered significant when below 0.05, and for all analysis the 95% confidence interval (CI) is also presented when appropriate.
3RESULTS
The concentration of anti-AChR Abs measured by the RIPA is given in nmol/L, and various laboratories and assay manufacturers, as well as the Mayo Clinic where our samples were tested, consider results < 0.02 nmol/L as non-reactive for anti-AChR, or negative (for details, consult < www.mayocliniclabs.com>) [17]. Thus, among the 145 samples included in the study, 63 were non-reactive for anti-AChR by the RIPA. However, some manufacturers of anti-AChR RIPA kits, such as the RSR (Cardiff, UK), Cisbio Bioassays (France) and DIAsource ImmunoAssays (Belgium), to cite a few examples, recommend a cutoff of > 0.4 or 0.5 nmol/L to be consider as positive. Moreover, some publications have recommended a higher cut-off for definition of positive results, as those < 1nmol/L may not be true positives [13, 14]. We then classified our results into three groups: 1)<0.02nmol/L as negative (n = 63, 43.5%) (RIPA-Neg group); 2) between 0.02 and 1nmol/L as borderline (n = 18, 12.4%), and 3)>1nmol/L as positives (n = 64, 44.1%) (RIPA-Pos group). These three groups were considered the reference for anti-AChR reactivity for evaluation of the performance of the other methods throughout the study (Fig. 1A and Table 1).
Fig. 1
Anti-nAChR reactivity by RIPA and ELISA. (A) Anti-nAChR reactivity by RIPA, distribution of samples in the groups according to nmol/L (B) Anti-nAChR reactivity analyzed using an indirect ELISA. Line “a” indicates the Youden index J cutoff = 0.8409, that promotes sensitivity. Line “b” indicates a cutoff that promotes specificity = 4.568, calculated based on the average+3SD of the RIPA-negative group. (C) Analysis using a Competitive ELISA. The cutoff of 3nmol/L was based on the average + 1SD of the RIPA-negative group. **p≤0.01; ***p≤0.001. Error bars = SD.
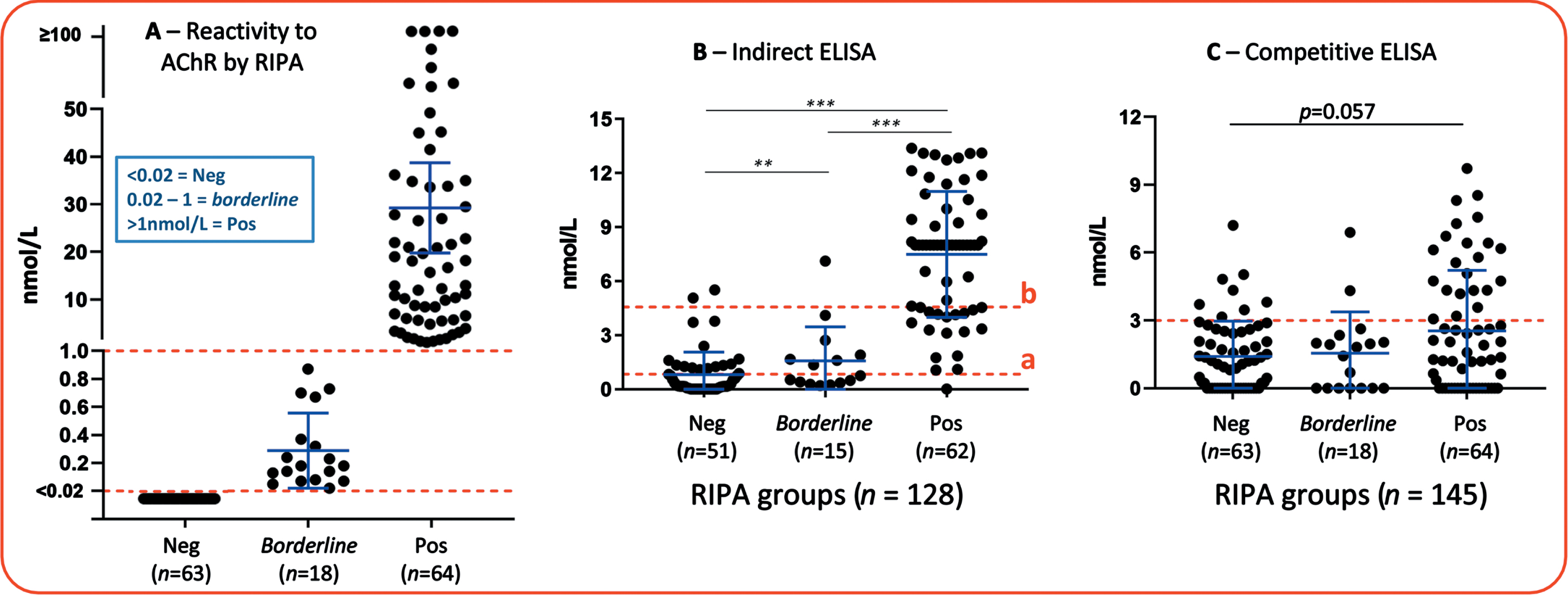
Anti-AChR reactivity was evaluated in 128 samples by indirect ELISA and in 145 samples by competitive ELISA. Among the 128 samples tested in the indirect ELISA, 62 were positive (RIPA-pos) and 51 were negative (RIPA-neg) in the RIPA assay. Using the cutoff recommended by the manufacturer of the indirect ELISA (≥0.5 nmol/L as positives), there was a high proportion of false positives in the RIPA-Neg group (43%, 21 out of the 51 samples), thus we first adjusted the cutoff based in the Youden index J (0.84 nmol/L) [18]. This method for calculating cutoff usually promotes the sensitivity in a given assay [19] (Fig. 1B). From the 62 RIPA-Pos samples, 61 (98.4%) were considered positive by the indirect ELISA with the Youden J cutoff, whereas from the 51 RIPA-Neg samples, only 35 (68.6%). There was a substantial agreement between RIPA and the indirect ELISA with the adjusted cutoff (Kappa = 0.688, 95% CI 0.557-0.819) (Table 1).
Because anti-AChR AAb is considered to be specific for the diagnosis of MG, assay specificity is of tantamount importance to sensitivity [20]. The specificity obtained with the cutoff adjusted using Youden J index was not satisfactory, thus we defined another cutoff (4.56 nmol/L) based on the average+3*SD (+3 times the Standard Deviation) of the RIPA-Neg samples (Fig. 1A and B), this method for stablishing the cutoff promotes the specificity in a given assay. With this cutoff, 44 of the 62 RIPA-Pos samples (70.9%) were considered positive and 49 (96.1%) of the 51 RIPA-Neg samples were considered negative in the indirect ELISA, yielding a substantial agreement with RIPA (Kappa = 0.652, 95% CI 0.519–0.785) (Table 1).
The competitive ELISA gives results for anti-AChR reactivity in ng/mL and the sensitivity of the assay is≥1 ng/mL, however here we converted the values to nmol to facilitate comparison with other assays. Regarding anti-AChR reactivity measured by this assay between the RIPA-Pos and RIPA-Neg groups, the p value (p = 0.057) indicated a strong trend for higher anti-AChR AAb concentration in the RIPA-Pos (Fig. 1C). The manufacturer does not suggest a positive/negative cutoff, thus for this study we defined the cutoff based in the RIPA-Neg average+1*SD (3 nmol/L). The proportion of positives by the competitive ELISA was higher in the RIPA-Pos group when compared to the RIPA-Neg (34.4% versus 12.7%, respectively, p = 0.007), but there was only a fair agreement rate between the competitive ELISA and the RIPA (Kappa = 0.216, 95% CI 0.072-0.359) (Table 1). Overall, the performance of this assay was poor, making this specific kit impractical for clinical application.
The samples were then tested with the fixed CBA biochip, which can individually detect reactivity to the adult AChR-ɛ as well as the embryonic AChR-γ (Fig. 2), visualized by the staining signal in the membrane of the cells expressing AChR (arrows in Fig. 3). Five samples showed reactivity only against the adult AChR-ɛ isoform (example in Fig. 2C), and the additional 63 CBA-positive samples showed reactivity against both isoforms (Figs. 2A-B and 3) meaning none of the samples showed reactivity only against the AChR-γ isoform (Table 1). Among the 64 RIPA-Pos samples, 63 (98.4%) were positive in the fixed CBA. In addition, none of the 63 RIPA-Neg samples showed reactivity in the fixed CBA, meaning 100% specificity for this assay in comparison to RIPA. There was an almost perfect agreement between the RIPA and reactivity to the adult AChR-ɛ CBA (Kappa = 0.984, 95% CI 0.954-1.000). Among the 18 RIPA-Borderline samples, five (27.8%) were positive to adult AChR-ɛ in the CBA (Table 1).
Fig. 2
Anti-nAChR reactivity by the fixed Cell-Based Assay (CBA). Indirect Immunofluorescence analysis with EU90 cells expressing adult AChR-ɛ or embryonic AChR-γ. (A and B) Examples of samples with reactivity for adult and embryonic AChR. (C) Example of a sample with reactivity only for adult ɛ. (D) Example of a sample without any reactivity. Scale bars = 10μm.
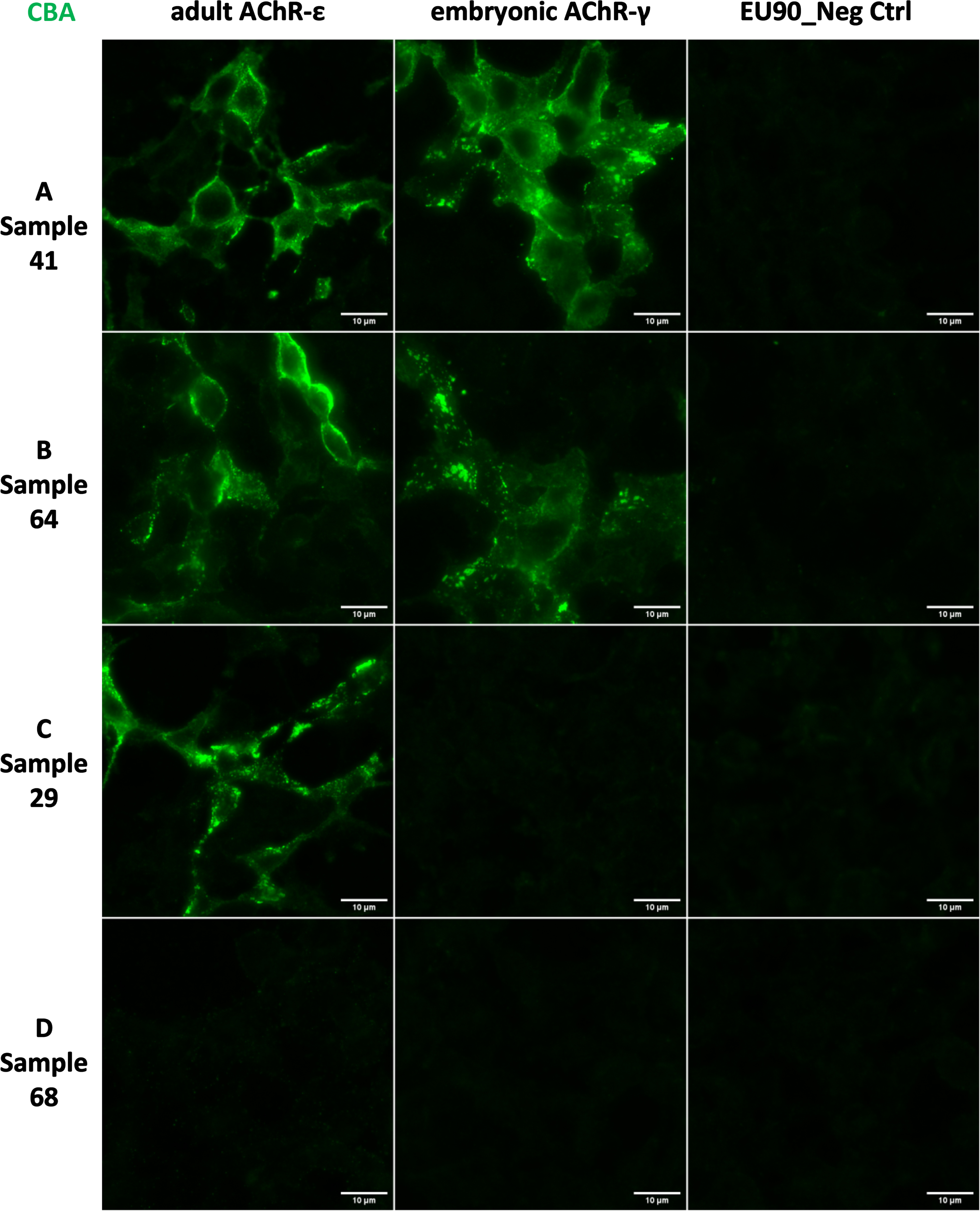
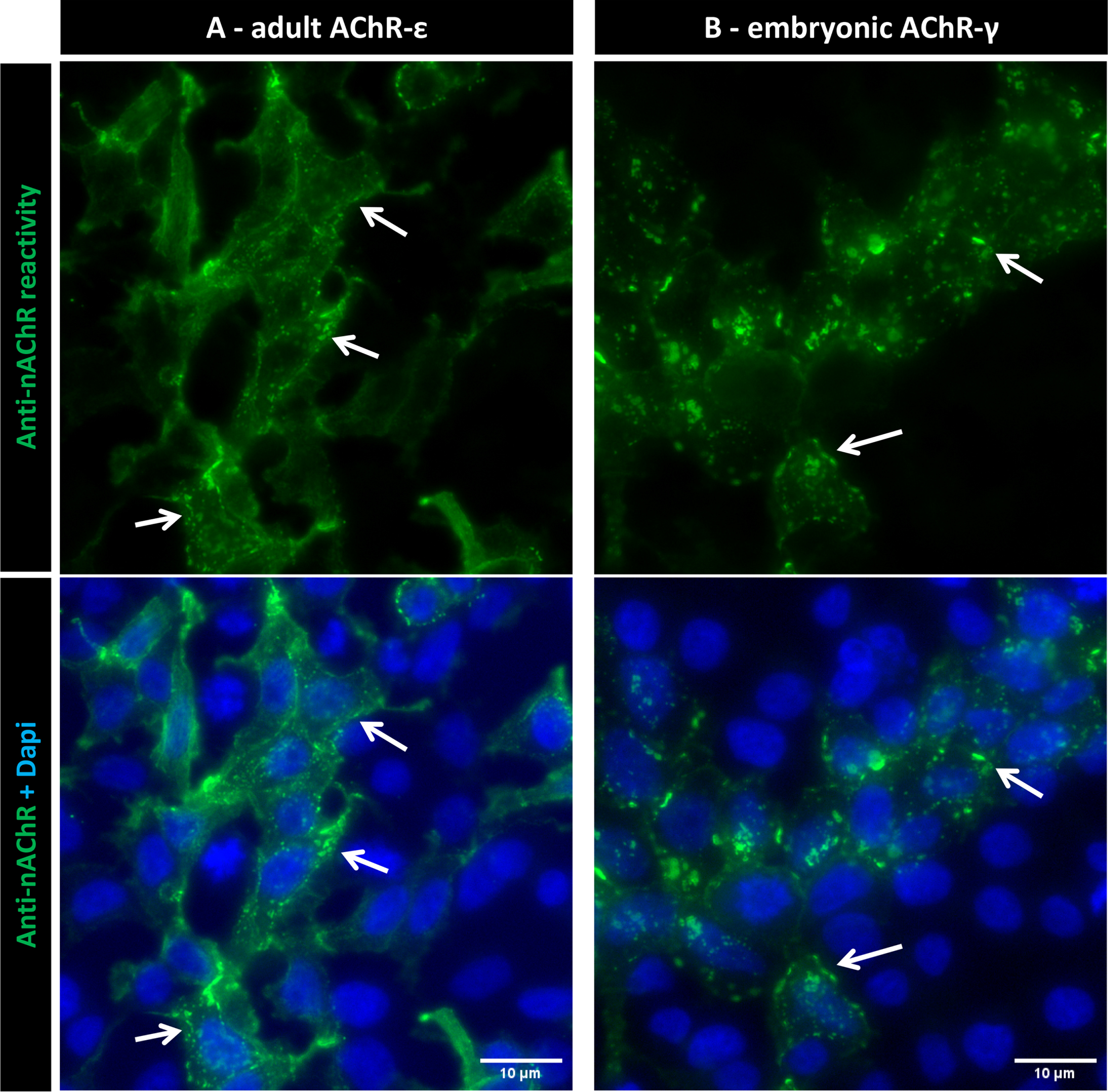
Fig. 3
Anti-nAChR reactivity with Dapi counterstaining. Images from a representative positive sample indicating the localization of AChR clusters in the cytoplasmic membrane (arrows) in both adult (A) and embryonic (B) conformations. Scale bars = 10μm.
From the 145 samples included in the study, information regarding the patient diagnostic was recovered for 115. For 20 samples, no clinical information was available, and for the remaining 10 patients, the diagnostic was inconclusive or still under investigation, some with suspicion of MG, but without confirmation (group “inconclusive”, Table 2). Among those with the diagnostic, 54 (46.9%) had MG, and 61 (53.1%) had other diseases or simple “not MG” (Table 2), the other diseases included but was not limited to: Type 2 diabetes, Sjogren Syndrome, various cancers types, Hashimoto’s hypothyroidism, multiple sclerosis, systemic lupus erythematosus, among others.
All the 54 MG patients belong to the RIPA-Pos group, meaning none of the RIPA-Neg or the borderline were diagnosed with MG, however among the 57 RIPA-Pos, 3 were inconclusive, and none was “Not-MG”. Overall, the RIPA-Pos group, which included samples with levels > 1nmol/L, presented sensitivity and specificity of 100% for MG (Table 2). For the indirect ELISA, sensitivity was 76.9% and specificity was 94%, with a positive predictive value of 93% (Table 2). For the CBA, considering reactivity to the adult AChR-ɛ, sensitivity was 98.1% and specificity was 96.7%. Two samples showed reactivity to AChR-ɛ in the CBA and were “Not-MG”, for both the CBA titration (see below) was 1/10. In addition, both samples were from the RIPA-borderline group, with levels < 1nmol/L. Finaly, one sample was negative in the CBA and presented MG, the RIPA level of anti-AChR for this sample was 35nmol/L (Table 2).
Because the fixed CBA is an indirect immunofluorescence assay, we investigated how the presence of other AAbs would affect the anti-AChR reactivity and interpretation/visualization of the fixed cells at the microscope. Thus, all samples were tested for anti-cell antibody (antinuclear antibody) by the HEp-2 indirect immunofluorescence assay (HEp-2 IFA). The proportion of anti-cell reactivity was similar among the RIPA-Neg, RIPA-Pos and Borderline samples (p = 0.134), with about ∼40–50% positivity in all RIPA groups (Table 1), suggesting that other AAbs in given samples do not interfere with interpretation of anti-AChR reactivity in the CBA. Of special interest, samples with HEp-2 IFA with cytoplasmic staining could be correctly interpreted regarding reactivity to AChR in the fixed CBA. The distribution of HEp-2 IFA patterns, as well as titers, were also similar among the groups (Table 1).
Since anti-AChR titers correlate with the clinical course of MG [21, 22], positive samples should be processed for the determination of anti-AChR serum levels by conventional RIPA, ELISA (solid-phased assays) or by titration in fixed CBA. To evaluate this possibility, we apply the positive samples in sequential dilution (1/10 up to 1/1000) and correlate the CBA end titer with RIPA levels (Fig. 3A). There was a good correlation between the CBA titration and RIPA levels, Spearman r = 0.793 (95% CI 0.693-0.863; p < 0.001) (Fig. 3A). We also compare the RIPA-Pos+Borderline groups with results from the Indirect ELISA, and a similarly good correlation was observed, Spearman r = 0.789 (95% CI 0.682-0.862; p=<0.001) (Fig. 3B). Altogether, these results shows that some solid-phase assays as well as CBA titration by sample dilution are good options to estimate anti-AChR AAb levels after confirming positivity by the CBA.
4DISCUSSION
Anti-AChR antibodies represent the main laboratory parameter for the diagnosis of MG. Due to the practical difficulty in running the gold standard RIPA for anti-AChR in clinical laboratories, solid phase immunoassays, such as ELISA and its variations, have been developed as an alternative approach by the in vitro diagnostic industry. However, detection of anti-AChR autoantibodies for MG diagnosis using ELISA in clinical laboratories has been questioned due to the low accuracy of this technique for this particular autoantibody system [10, 11, 23, 24], and this was not different in our study. Among the two ELISA kits tested, better results were observed with the indirect ELISA with adjusted cutoff that promotes higher specificity (in this case, 94% specificity), but at the expense of sensitivity. According to the kit’s manufacturer, plate-wells are coated with complete recombinant AChR-ɛ/γ, which serve as substrate for the anti-AChR AAbs to bind before a secondary anti-human IgG is applied, thus comprising the indirect ELISA. For the competitive ELISA, both the sample and an anti-AChR-HRP Ab are incubated together in a well pre-coated with AChR (it’s not clear from the manufacturer if it is the whole receptor or just some subunits), but since the number of antibody-binding sites is limited, as more sites are occupied by AAb from the sample, fewer sites are left for the anti-AChR-HRP to bind, allowing the determination of anti-AChR in the given sample. In any case, performance was poor, making this specific competitive ELISA kit impractical for clinical application. Since we only had access to these two ELISA kits, we cannot generalize our findings to other commercially available products that could provide better performance [20], however our data, at least for the indirect ELISA, was similar to other studies using ELISAs from the same or other manufacturers, meaning a high specificity (90–100%) but at the compromise of sensitivity (50–70%), with average kappa agreements of 0.6–0.8 between ELISA and RIPA [11, 16, 25].
The recently developed anti-AChR CBA with fixed cells appears as a promising alternative to RIPA to be used in the clinical laboratory. Confirming recent studies in Italian, Canadian and Chinese cohorts [11, 14–16, 25], our findings with a Brazilian cohort also showed a good performance of the fixed CBA for detection of anti-AChR when compared to RIPA, with an almost perfect agreement (Kappa = 0.984). Sensitivity and specificity for MG were also good for the CBA, 98% and 96%, respectively. This assay has the potential to replace RIPA in the clinical laboratory for detection of anti-AChR AAb because it has the benefit of avoiding radioactive components.
Fig. 4
Correlation of anti-AChR levels. (A) Correlation of anti-AChR levels by RIPA (RIPA-Pos+Borderline groups) with the CBA titration (n = 82). (B) Correlation of levels by RIPA with Indirect ELISA (n = 77). Spearman r and p is shown. Each dot represents one sample and the red line indicates the trend.
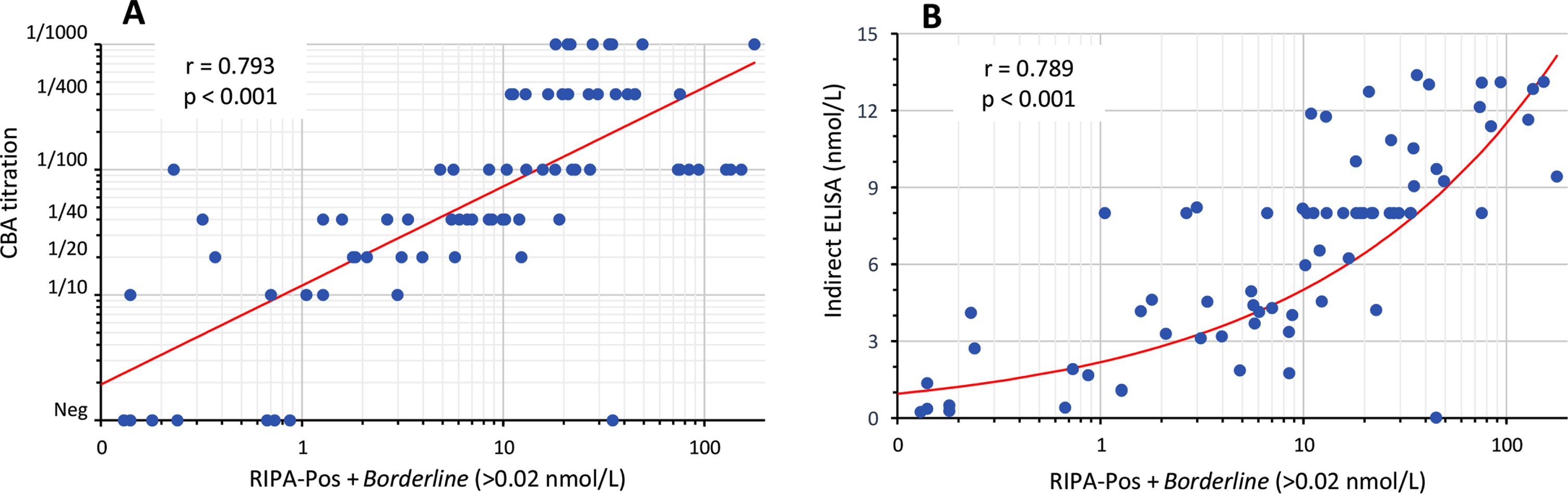
Furthermore, the CBA was less sensitive to detect low titer anti-AChR, as only 5 (<30%) of the samples in the RIPA Borderline group were positive in the CBA. However, this may not be all bad news. From the 12 RIPA borderline we could recover diagnostic data, none has MG. This reinforces previous literature discussion that samples with RIPA anti-AChR results < 1nmol/L may not be true positives and rarely results in MG diagnostics [13, 14].
Although details regarding commercial slides manufacturing are proprietary, the cells are usually dehydrated with alcohol fixatives, to facilitate storage and distribution. Alcohol fixatives precipitate proteins and some of the binding between membrane proteins is lost, as recently demonstrated elsewhere [26]. In the anti-AChR CBA used in this study, cell fixing could affect, up to some degree, the receptor integrity and clustering, which would in turn affect autoantibody binding and impair detection, although here only one patient with MG and RIPA-Pos showed no reactivity in the CBA. It has been suggested that live cells expressing clustered nAChR to detect the AAbs can show a sensitivity even higher than RIPA itself, because the unfixed transfected cells better resembles the physiological expression of the AChR by myocytes [13, 27–29]. However, the maintenance of live-cell cultures expressing AChR requires special facilities and expertise, which also restricts the adoption of this methodology in most clinical laboratories.
A positive reaction on the CBA with fixed cells is given by observing the labeling of the AChR subunits on the membrane of the cells under a microscope. Samples that do not react with the adult AChR-ɛ or the embryonic AChR-γ are considered negative (Figs. 2 and 3) [11]. As observed in other studies [14], we noticed that some samples yielded an excessive non-specific background staining, and curiously a portion of those samples were also positive for anti-cell antibody in the HEp-2 IFA (data not shown). Thus, it is important for any CBA to be performed and analyzed at the microscope by trained personnel to avoid erroneous interpretation.
Recent studies showed that anti-AChR titers correlate with the clinical course of MG [21, 22]. Although we did not perform RIPA titrations, meaning some RIPA values could be substantially higher in some samples, there was a good correlation between RIPA levels and the CBA titration, especially in samples from the RIPA-Pos group (>1nmol/L), suggesting estimating anti-AChR titer by sample dilution in the CBA is feasible.
The samples included in this study arrived with the medical request to test for the presence of anti-AChR/anti-Musk, meaning it’s likely the patients presented neuromuscular symptoms that supported the request. Although we could recover the definitive diagnostic for ∼80% of the samples, patient’s detailed clinical features were not included in the study. This limitation prevented us from investigating a possible association of reactivity to specific adult or embryonic AChR assemblies with the Ocular and Generalized MG forms. A recent study showed a significantly higher sensitivity for both Ocular and Generalized MG in the fixed CBA in comparison with RIPA [14].
In summary, the fixed CBA test presented better performance than the two ELISA products and showed an almost perfect Kappa agreement compared to the gold standard RIPA test. Sensitivity and specificity for MG diagnostic were also good for the CBA, 98% and 96%, respectively. This kit was recently launched commercially and is currently in exclusive use for research purposes, but it has promising potential as an alternative to RIPA in the clinical laboratory, especially due to its radiation-free nature. In addition, employment of some solid-phase assays such as the indirect ELISA used in this study, as well as CBA titration by sample dilution, are good options to estimate anti-AChR AAb levels after confirming positivity by the CBA.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was mainly supported by Sao Paulo Government agency FAPESP (Sao Paulo State Research Foundation) grant numbers #2017/20745-1 and #2021/04588-9, granted to G.D.K. and L.D.S. Also, G.D.K. is supported by the Vicerrectoría de Investigación y Desarrollo Tecnológico (VRIDT) from the Universidad Católica del Norte (UCN) in Chile. Additionally, L.E.C.A. is supported by the Brazilian research agency National Council for Research (CNPq), grant #PQ-1D 310334/2019-5. The funding organizations played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
ETHICS APPROVAL STATEMENT
The study was approved by the Ethics committee at Fleury group, and it is registered at Plataforma Brasil CAAE: 57480622.3.0000.5474.
CONFLICT OF INTEREST DISCLOSURE
Dellavance A, Baldo DC, Gozzi-Silva SC and Andrade LEC were during the development of the study (or still are) paid employees at Fleury Group company. Diogenes L, Gomes K, Prado MS and Keppeke GD declare that they have no conflicts of interest.
DATA AVAILABILITY STATEMENT
All data has been included in the manuscript. Raw data can be made available freely by the corresponding author upon reasonable request.
REFERENCES
[1] | Drachman DB . Myasthenia gravis. The New England journal of medicine. (1994) ;330: (25):1797–810. |
[2] | Mehndiratta MM , Pandey S , Kuntzer T . Acetylcholinesterase inhibitor treatment for myasthenia gravis. The Cochrane database of systematic reviews. (2014) 2014: (10):CD006986. |
[3] | Huang EJ , Wu MH , Wang TJ , Huang TJ , Li YR , Lee CY Myasthenia Gravis: Novel Findings and Perspectives on Traditional to Regenerative Therapeutic Interventions. Aging and Disease. 2022. |
[4] | Kang C . Ravulizumab: A Review in Generalised Myasthenia Gravis. Drugs. (2023) ;83: (8):717–23. |
[5] | Tzartos SJ , Barkas T , Cung MT , Mamalaki A , Marraud M , Orlewski P , et al. Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunological Reviews. (1998) ;163: :89–120. |
[6] | Lindstrom JM , Seybold ME , Lennon VA , Whittingham S , Duane DD . Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. (1976) ;26: (11):1054–9. |
[7] | Patrick J , Lindstrom J , Culp B , McMillan J . Studies on purified eel acetylcholine receptor and anti-acetylcholine receptor antibody. Proceedings of the National Academy of Sciences of the United States of America. (1973) ;70: (12):3334–8. |
[8] | Beeson D , Jacobson L , Newsom-Davis J , Vincent A . A transfected human muscle cell line expressing the adult subtype of the human muscle acetylcholine receptor for diagnostic assays in myasthenia gravis. Neurology. (1996) ;47: (6):1552–5. |
[9] | Stratton MR , Darling J , Pilkington GJ , Lantos PL , Reeves BR , Cooper CS . Characterization of the human cell line TE671. Carcinogenesis. (1989) ;10: (5):899–905. |
[10] | Oger J , Frykman H . An update on laboratory diagnosis in myasthenia gravis. Clinica Chimica Acta; International Journal of Clinical Chemistry. (2015) ;449: :43–8. |
[11] | Villalta D , Fabris M , Verriello L , Grizzo F , Mobilia EM , Lechiara A , et alAcetylcholine receptor and musclespecific tyrosine kinase antibodies detection: is it time for a change? Clinical Chemistry and Laboratory Medicine. 2023. |
[12] | Budhram A . Fixed cell-based assays for autoantibody detection in myasthenia gravis: a diagnostic breakthrough. The Lancet regional health Western Pacific. (2023) ;38: :100876. |
[13] | Leite MI , Jacob S , Viegas S , Cossins J , Clover L , Morgan BP , et al. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain: A Journal of Neurology. (2008) ;131: (Pt 7):1940–52. |
[14] | Mirian A , Nicolle MW , Edmond P , Budhram A . Comparison of fixed cell-based assay to radioimmunoprecipitation assay for acetylcholine receptor antibody detection in myasthenia gravis. Journal of the Neurological Sciences. (2022) ;432: :120084. |
[15] | Spagni G , Gastaldi M , Businaro P , Chemkhi Z , Carrozza C , Mascagna G , et al. Comparison of Fixed and Live Cell-Based Assay for the Detection of AChR and MuSK Antibodies in Myasthenia Gravis. Neurology(R) Neuroimmunology & Neuroinflammation. (2023) 10: (1). |
[16] | Gambino CM , Agnello L , Ciaccio AM , Scazzone C , Vidali M , Di Stefano V ,et al. Detection of Antibodies against the Acetylcholine Receptor in Patients with Myasthenia Gravis: A Comparison of Two Enzyme Immunoassays and a Fixed Cell-Based Assay. Journal of Clinical Medicine. (2023) 12: (14). |
[17] | Shelly S , Paul P , Bi H , Dubey D , Milone M , Sorenson EJ ,, et al. Improving accuracy of myasthenia gravis autoantibody testing by reflex algorithm. Neurology. (2020) ;95: (22):e3002–e11. |
[18] | Youden WJ . Index for rating diagnostic tests. Cancer. (1950) ;3: (1):32–5. |
[19] | Akobeng AKs . Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatrica. (2007) ;96: (5):644–7. |
[20] | Li Y , Peng Y , Yang H . Serological diagnosis of myasthenia gravis and its clinical significance. Annals of Translational Medicine. (2023) ;11: (7):290. |
[21] | Kojima Y , Uzawa A , Ozawa Y , Yasuda M , Onishi Y , Akamine H , et al. Rate of change in acetylcholine receptor antibody levels predicts myasthenia gravis outcome. Journal of Neurology, Neurosurgery, and Psychiatry. (2021) ;92: (9):963–8. |
[22] | Marcuse F , Brandts L , Moens D , Damoiseaux J , Hochstenbag M , Hoeijmakers JGJ , et al. The association between anti-acetylcholine receptor antibody level and clinical improvement in myasthenia gravis. European Journal of Neurology. (2022) ;29: (4):1187–97. |
[23] | Martino G , Twaddle G , Brambilla E , Grimaldi LM . Detection of anti-acetylcholine receptor antibody by an ELISA using human receptor from a rhabdomyosarcoma cell line. Acta Neurologica Scandinavica. (1994) ;89: (1):18–22. |
[24] | Hewer R , Matthews I , Chen S , McGrath V , Evans M , Roberts E , et al. A sensitive non-isotopic assay for acetylcholine receptor autoantibodies. Clinica Chimica Acta; International Journal of Clinical Chemistry. (2006) ;364: (1-2):159–66. |
[25] | Li Z , Zhang C , Chang T , Zhang X , Yang H , Gao F , et al. A multicentre, prospective, double-blind study comparing the accuracy of autoantibody diagnostic assays in myasthenia gravis: the SCREAM study. The Lancet Regional Health Western Pacific. (2023) ;38: :100846. |
[26] | Ichikawa T , Wang D , Miyazawa K , Miyata K , Oshima M , Fukuma T . Chemical fixation creates nanoscale clusters on the cell surface by aggregating membrane proteins. Communications Biology. (2022) ;5: (1):487. |
[27] | Rodriguez Cruz PM , Al-Hajjar M , Huda S , Jacobson L , Woodhall M , Jayawant S ,et al. Clinical Features and Diagnostic Usefulness of Antibodies to Clustered Acetylcholine Receptors in the Diagnosis of Seronegative Myasthenia Gravis. JAMA Neurology. (2015) ;72: (6):642–9. |
[28] | Damato V , Spagni G , Monte G , Woodhall M , Jacobson L , Falso S ,et al. Clinical value of cell-based assays in the characterisation of seronegative myasthenia gravis. Journal of Neurology, Neurosurgery, and Psychiatry. (2022) ;93: (9):995–1000. |
[29] | Cai Y , Han L , Zhu D , Peng J , Li J , Ding J , et al. A Stable Cell Line Expressing Clustered AChR: A Novel Cell-Based Assay for Anti-AChR Antibody Detection in Myasthenia Gravis. Frontiers in Immunology. (2021) ;12: :666046. |




