A Mixed-method Approach to Develop an Ambulatory Module of the SMA Independence Scale
Abstract
Background:
Limited qualitative data exist on the symptoms and impacts of spinal muscular atrophy (SMA) experienced by ambulant individuals. An ambulant module of the SMA Independence Scale (SMAIS) was developed to quantify the assistance required to perform everyday mobility-related activities.
Objective:
The objective of this study was to develop a patient-centered module that provides key insights into what constitutes independence for ambulant and near-ambulant individuals with SMA.
Methods:
A stepwise, mixed-method approach was used. Semi-structured interviews were conducted in three waves with individuals with SMA and caregivers of children with SMA who were ambulant or near-ambulant (can walk ≥5 steps with support). Wave 1 interviews (n = 20) focused on concept elicitation. Wave 2 and 3 interviews (n = 15, both) involved completion and cognitive debriefing of items generated based on Wave 1 interviews. Therapeutic area experts were consulted throughout all key steps of the study. In particular, feedback was provided for item refinement and response option decisions. A macro-level preliminary, exploratory analysis, using Rasch Measurement Theory (RMT), provided insight on measurement properties.
Results:
Wave 1 resulted in 42 mobility and 11 instrumental activity of daily living (iADL) items. During Wave 2, participants defined independence as completing a task with supportive aids but without help from another person, leading to item refinement and modifications to the response scale. Lack of conceptual relevance and ceiling effects led to the removal of all iADL items after Wave 2, and 41 mobility items were tested in Wave 3. Final exploratory RMT and item refinement to reduce overlap led to a 27-item set related to mobility tasks.
Conclusions:
Our study provides preliminary support for using the 27-item SMAIS–Ambulatory Module for ambulant or near-ambulant individuals with SMA. Larger-scale analyses to further assess the psychometric properties of the scale are warranted.
INTRODUCTION
Spinal muscular atrophy (SMA) is a genetic, progressive neuromuscular disease that leads to muscle weakness, loss of motor function and reduced life expectancy [1, 2]. SMA is caused by biallelic mutations of the survival of motor neuron 1 (SMN1) gene. Although a second paralogous SMN gene (SMN2) exists, the levels of functional SMN protein produced by SMN2 are inadequate to compensate for the lack of SMN1 [3], which results in reduced levels of the SMN protein and subsequent motor neuron loss [4]. SMA is traditionally classified into five types (Types 0–4) based on age at disease onset and maximum motor function achieved [5]. Neonates with prenatal-onset SMA (Type 0) do not achieve motor milestones and rarely survive past 6 months of age [5]. Type 1 SMA is the most common form of the disease with symptoms occurring during the first 6 months of life. Without treatment, infants with Type 1 SMA rarely live past 2 years of age. Type 2 SMA manifests between 6 and 18 months of age. Patients can sit independently, but never walk and 70% are alive at 25 years of age [5, 6]. For Type 3 SMA, symptoms will typically appear after 18 months of age. Patients will achieve independent ambulation, but some will lose this ability over time [2, 5]. Adult-onset or Type 4 SMA is the mildest form of the disease and loss of ambulation may not occur until the fifth decade of life, if at all [5, 6]. Three disease-modifying therapies (DMTs) are currently available for the treatment of SMA. These therapies include risdiplam (EVRYSDI®), an orally administered small molecule SMN2 pre-mRNA splicing modifier that increases functional levels of SMN protein; nusinersen (SPINRAZA®), an antisense oligonucleotide designed to modify pre-mRNA splicing of SMN2 given by intrathecal injection; and onasemnogene abeparvovec (ZOLGENSMA®), an intravenously administered adeno-associated virus vector-based gene therapy [7]. Onasemnogene abeparvovec is approved for individuals with SMA < 2 years of age, and risdiplam and nusinersen are approved for pediatric and adult patients with SMA [8–11].
Previous research has highlighted that for individuals with Type 2 and non-ambulant Type 3 SMA, symptoms can ultimately impact an individual’s ability to perform daily activities independently such as eating, dressing, bathing, cleaning, shopping, using technology (i.e. computer keyboard or phone), working or attending school [12, 13]. Functional outcome measures (i.e. the Hammersmith Functional Motor Scale –Expanded, the 32-item Motor Function Measure, and the 6-minute Walk Test) are clinician-reported outcome and performance-outcome scales that assess motor function ability over time by measuring the patient’s ability to complete the items/activities. They were not designed to capture the amount of independence individuals with SMA have when performing activities of daily living (ADLs) [14–16]. Similarly, existing patient- or observer-reported outcome measures (i.e. Assessment of Caregiver Experience in Neuromuscular Disease or the Pediatric Evaluation of Disability Inventory-Computerized Adaptive Testing) assess patient-relevant concepts associated with everyday activities and mobility; however, they do not assess independence nor the level of assistance required to complete activities but include content for a broad range of abilities [17, 18].
With the approval of three DMTs, the natural history of SMA is changing, with individuals stabilizing or achieving new motor abilities. In addition, with the evolution of classical phenotypes of SMA, current clinical care guidelines are shifting from the traditional SMA disease classification towards classification by functional status (non-sitters, sitters, walkers) [19, 20]. Treated individuals with SMA and their families describe changes in ADLs that have historically not been fully captured by existing performance outcome measures [21, 22]. For this reason, the SMA Independence Scale–Upper Limb Module (SMAIS–ULM) was developed to measure the level of independence individuals with Type 2 or non-ambulant Type 3 SMA have when performing such activities [12]. For ambulant individuals, there is a paucity of literature describing their lived experience and no scales that solely assess the level of assistance needed for activities that are relevant to this population. To this end, we describe the findings from a mixed-method study with the goal of developing an ambulatory module for the SMAIS (SMAIS–Amb) suitable for use in future clinical trials and real-world settings to support the assessment of potential treatment benefit in ambulant or near-ambulant patients.
MATERIALS AND METHODS
Sample and recruitment
This study consisted of a non-interventional, descriptive design that involved three waves of participant interviews and a cross-sectional quantitative analysis conducted in the USA (Fig. 1). The total recruitment target was 50 participants, with 20 participants in Wave 1 and 15 participants in Waves 2 and 3 each. Participants with SMA were included in the study if they were aged 12–60 years with a genetic diagnosis of SMA and had an ambulant (defined as able to walk ≥5 steps without support) or near-ambulant (defined as able to walk ≥5 steps with support) self-reported mobility status. Caregivers were only included in the study if they were ≥18 years of age and were the primary caregiver of an ambulant or near-ambulant individual with SMA aged 2–12 years. Participants with SMA were excluded if they had a significant speech impairment. Participants with SMA and caregivers were excluded from the study if they had significant cognitive impairment, hearing difficulty, visual impairment, and/or severe psychopathology. Participants with SMA or caregivers with insufficient knowledge of the English language that could interfere with their ability to provide written consent and complete an interview were further excluded. Participants with SMA or caregivers who had no access to the internet were also excluded, given the online nature of the study. Participant eligibility was determined by self-report based on review of the study advertisement (Supplementary Document 1), which included the aforementioned inclusion and exclusion criteria, and was verified by a researcher from Modus during a screening call.
Fig. 1
A mixed-method approach was used to develop the SMAIS–Amb scale. CE = concept elicitation; CD = cognitive debriefing; RMT = Rasch Measurement Theory; SMAIS–Amb = Spinal Muscular Atrophy Independence Scale Ambulatory Module.
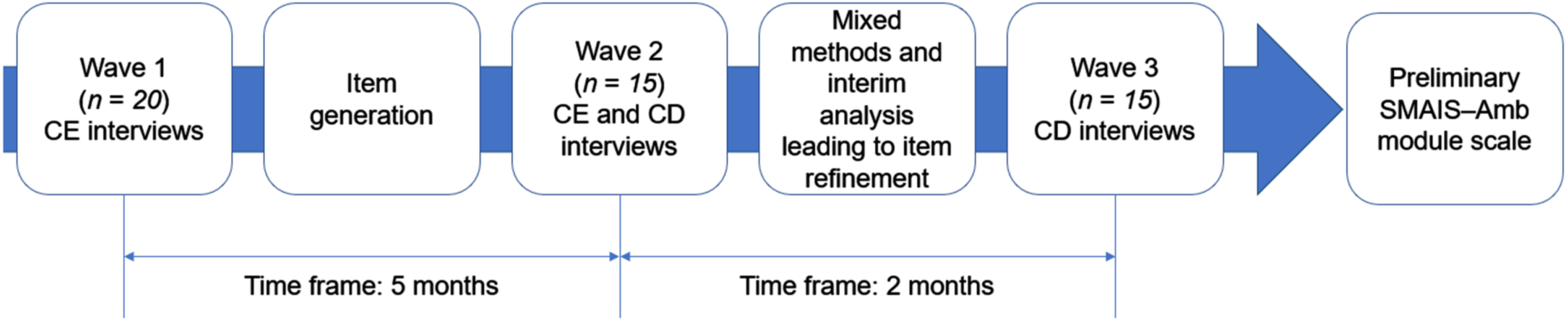
Participants (both individuals with SMA and caregivers) were primarily identified using a general advertisement e-mail that was circulated to the US members of Cure SMA, a patient advocacy group. Members were provided a small description of the study and invited to contact Modus Outcomes staff to participate in the study. Additional recruitment assistance was provided by an agency called Global Perspectives. Eligible participants and caregivers were invited to complete an electronic informed consent form. Pediatric participants aged 12–17 years were invited to complete an electronic assent form with their caregivers, who were also required to complete an electronic informed consent form. Individuals subsequently provided background demographic and clinical information. To ensure that there was not any recruitment overlap between Global Perspectives and participants who responded to the Cure SMA advertisement, eligible patient details following signature of the informed consent form were provided to Modus Outcomes who, following receipt of relevant demographic and clinical information, then scheduled and conducted the interviews. The Copernicus institutional review board reviewed and approved study materials (approval number #20201874) in advance of implementing study-related procedures. Two protocol amendments were submitted to the institutional review board following the first and second waves of interviews (approval number #27937362.1) in order to provide the refined item content for the interviews.
Therapeutic area experts (TAEs)
An expert panel was consulted throughout the study and comprised a group of three clinical TAEs as well as an expert researcher from a patient advocacy organization. The TAEs reviewed all study materials and provided feedback on the interpretation of results and decisions on subsequent steps at the completion of each interview wave.
Study conduct
Interviews
Three rounds of semi-structured interviews were conducted remotely via telephone throughout the course of this study. There was a period of 5 months between the end of Wave 1 and the start of Wave 2, and 2 months between the end of Wave 2 and the start of Wave 3. Interviewers attended training, provided by Modus Outcomes, to review the objectives and general flow of the interviews and ensure consistent data quality (Supplementary Document 2). All interviews lasted approximately 60 minutes, were audio recorded, transcribed and entered into ATLAS.ti, a software package that is designed to facilitate the storage, coding and analysis of qualitative data [23].
Wave 1 concept elicitation (CE) interviews and saturation analysis
Participants engaged in CE interviews, which aimed to explore or elicit the level of support needed when carrying out ADLs. The results from Wave 1 CE interviews were also used to develop a conceptual model (CM), which embodied all information relevant to the ambulatory symptom and impact experience of SMA.
Item generation
The CM developed from Wave 1 CE interviews was used to inform the generation of the first set of items included in the SMAIS–Amb. Item generation followed construction principles and was developed using an iterative process with the TAEs [24, 25]. The items were created using the same language used by the interview participants as much as possible; however, they were adjusted to maintain brevity and minimize semantic overlap. Additionally, the accompanying response scale was initially developed to align with the three-level independence response scale of the SMAIS–ULM, which was defined as 0 (‘I cannot do this at all without help’); 1 (‘I need some help’); and 2 (‘I do not need help’). A ‘not applicable’ response option was also available (12).
Wave 2 and 3 CE and cognitive debriefing (CD) interviews
In line with Wave 1 interviews, Waves 2 and 3 started with a CE section, although shorter, and were subsequently followed with CD of the items generated from the first wave of interviews. Participants were asked to verbalize their thoughts using the ‘think aloud’ process on items newly generated from Wave 2 or Wave 3 interviews. Once CD was complete, participant feedback was compiled into summary tables. The summary tables included participant comments and responses for each item and a list of issues related to the understanding of the item content or retrieval of information. In addition, feedback was compiled on the instructions, format and layout of the SMAIS–Amb.
Interview analysis
All interviews were audio recorded and the digital files transcribed verbatim. The interview transcripts were analyzed by Modus Outcomes Research staff using detailed line-by-line open and inductive coding using ATLAS.ti software [23]. Coding was tailored to key concepts of interest in this study such as independence or level of assistance needed when individuals with SMA completed ADLs. All coding and analyses were conducted in English following each wave of interviews and guided by a coding guidance document that included guidelines for the generation of open codes and a predetermined list of codes or placeholder codes for the cognitive debriefing analyses, which included identifying participant understanding and the relevance of item content. For the Wave 1 CE interviews, saturation analysis was conducted sequentially in four groups of five interviews to determine how much new information on the participants’ experiences was obtained in each group of interviews. If important new concepts were to arise in the last group of interviews, additional interviews would have been considered to ensure all the relevant concepts were elicited [26]. For Wave 2 and 3 CD interviews, the coding was structured where possible and reflected participant comments and feedback on the items in relation to the issues reviewed such as, relevance, clarity, conceptual overlap and ease of completion.
Preliminary and exploratory quantitative analysis
In parallel to Wave 3, a macro-level exploratory quantitative analysis using Rasch Measurement Theory (RMT) was conducted to provide early insight on the measurement properties of the newly generated items [27]. Areas of interest included the comprehensiveness and targeting of the item set, the item quality, and the uniqueness as well as appropriateness of the response scale. Participants with SMA and caregivers (N = 25 target) completed the SMAIS–Amb through a secure web application [28]. Participants previously recruited in Waves 1 and 2 were re-invited to participate to increase the sample of participants completing the version of the item set debriefed in Wave 3 and allow for the preliminary and exploratory quantitative analysis to be finalized. Item-person targeting, person-separation, item-misfit and local dependence analyses were performed to assess the extent to which the observed data ‘fit’ the predictions of the Rasch model.
RESULTS
Wave 1 results
Participants with SMA and caregiver demographics
A total of 20 participants were recruited for Wave 1 interviews (Table 1). Fourteen participants with SMA≥12 years of age were included in Wave 1 (eight ambulant and six near-ambulant). Ages of participants with SMA ranged from 13–58 years with a mean age of 38 years and a mean age at diagnosis of 12 years. The majority of participants with SMA were female (64%) and White Caucasian (86%).
Table 1
Demographics of individuals with SMA and caregivers in Wave 1a
| Participant demographics | Individuals with SMA: | Caregivers of child/children with | ||
| Wave 1 (n = 14) | SMA:b Wave 1 (n = 6) | |||
| Age (years) | ||||
| Mean age (SD), range | 38 (15), 13–58 | 44 (22), 29–88 | ||
| Mean age at diagnosis (SD), range | 12 (11), 2–35 | – | ||
| Mean diagnosis duration (SD), range | 26 (15), 6–56 | – | ||
| Gender, n (%) | ||||
| Male | 5 (36) | 2 (33) | ||
| Female | 9 (64) | 4 (67) | ||
| Caregiver relationship, n (%) | ||||
| Mother | – | 4 (67) | ||
| Father | – | 1 (17) | ||
| Grandparent | – | 1 (17) | ||
| Ethnicity, n (%) | ||||
| White Caucasian | 12 (86) | 4(67) | ||
| Asian | – | 1 (17) | ||
| Biracial | 1 (7) | – | ||
| Hispanic | 1 (7) | – | ||
| Latino | – | 1 (17) | ||
| Employment, n (%) | ||||
| Part time | 3 (21) | 1 (17) | ||
| Retired | 3 (21) | 1 (17) | ||
| Full time | 3 (21) | 4 (67) | ||
| Unemployed | 2 (14) | – | ||
| School | 2 (14) | – | ||
| Unable to work due to disability | 1 (7) | – | ||
| Education, n (%) | ||||
| Secondary/High school | 2 (14) | – | ||
| Technical or Vocational degree | 1 (7) | 1 (17) | ||
| College degree | 9 (64) | 1 (17) | ||
| Graduate degree | 2 (14) | 4 (67) | ||
| Children with SMA (years) | ||||
| Mean age (SD), range | – | 4.0 (2.0), 2.0–8.0 | ||
| Mean age at diagnosis (SD), range | – | 1.5 (0.8), 0.3–3.0 | ||
| Mean diagnosis duration (SD), range | – | 2.5 (1.3), 0.8–4.2 | ||
| Gender of child with SMA, n (%) | ||||
| Male | – | 3 (43) | ||
| Female | – | 4 (57) | ||
| Self- or caregiver-reported ambulatory status, n | ||||
| Ambulantc | 8 | 4 | ||
| Near-ambulantc | 6 | 3 | ||
| Self- or caregiver-reported level of assistance needed, n (%) | In the home | Out of the home | In the home | Out of the home |
| I/They cannot do any activities without help | 0 | 0 | 0 | 1 (14) |
| I/They need a lot of help | 0 | 4 (29) | 4 (57) | 2 (29) |
| I/They need a moderate amount of help | 3 (21) | 3 (21) | 1 (14) | 3 (43) |
| I/They need a little bit of help | 8 (57) | 6 (43) | 1 (14) | 0 |
| I/They do not need any help | 3 (21) | 1 (7) | 1 (14) | 1 (14) |
aA sample of n = 25 participants were included in the RMT analysis, and their demographics are available in Supplementary Table 1. These participants included 11 Wave 3 participants (four Wave 3 participants could not be included as they completed the item set with the initial version of the response options) plus 14 participants who were previously interviewed in Waves 1 and 2. bDemographics included are for the seven children with SMA who the caregivers cared for. cAmbulant is defined as individuals with SMA who can walk≥5 steps without support. Near-ambulant is defined as individuals with SMA who can walk≥5 steps with support (holding a stable object with one or both hands). RMT = Rasch Measurement Theory; SD = standard deviation; SMA = spinal muscular atrophy.
Six individuals were caregivers of children with SMA (three children were ambulant and three children were near-ambulant). Caregiver ages ranged from 29–88 years with a mean age of 44 years. The majority of caregivers were female (67%) and White Caucasian (71%). Caregivers cared for children aged 2–8 years with a mean age of 4 years and a mean age at diagnosis of 1.5 years. In both the ambulant and near-ambulant groups, the level of assistance the individual with SMA needed was variable depending on the location where the activity was being performed.
CE section of interviews and saturation analysis
During the CE interviews, the level of independence or support ambulant or near-ambulant individuals required was explored. A CM (Fig. 2) was created from the CE interviews and included 86 concepts elicited. The content and structure of the CM were finalized following feedback from clinical TAEs. Four primary domains emerged within the CM: symptoms, motor performance, impacts and disease management; these primary domains were further divided into 19 subdomains. The symptoms domain was comprised of neuromuscular, sensory, physical fatigue and physical weakness subdomains.
Fig. 2
Conceptual model of experience of ambulant and near-ambulanta participants with SMA. aAmbulant is defined as individuals with SMA who can walk≥5 steps without support (ambulant group 1). Near-ambulant is defined as individuals with SMA who can walk≥5 steps with support (holding a stable object with one or both hands, ambulant group 2). SMA = spinal muscular atrophy.
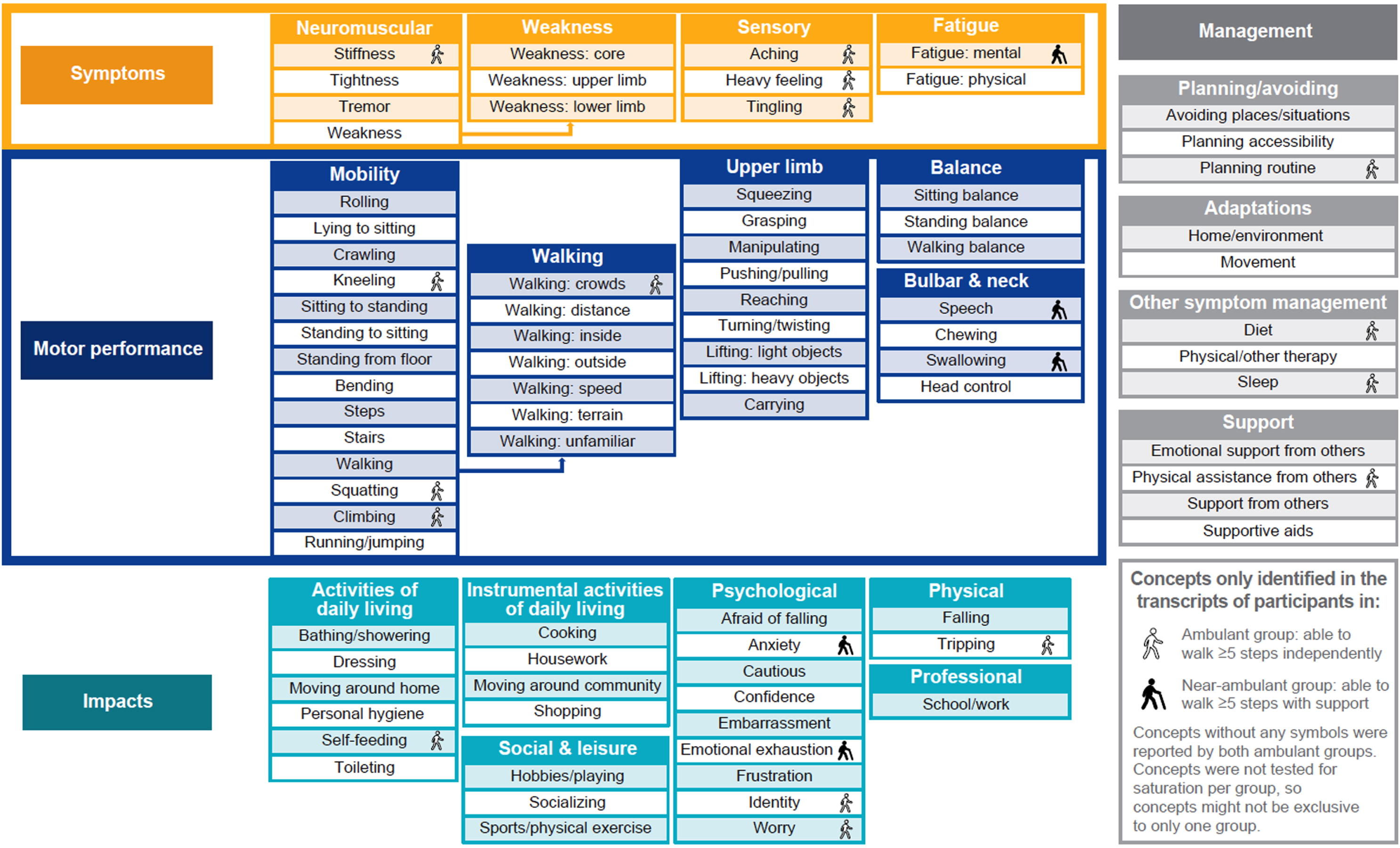
Subdomains within the motor performance domain included overall mobility, walking, upper limb, balance, and bulbar and neck issues. Concepts ranged from ‘rolling over’ to ‘running/jumping’, reflecting the different degrees of challenges in relation to mobility and lower limb performance. Additionally, the breadth of challenges was further reflected in the upper limb subdomain, with items that ranged from ‘squeezing’ to ‘carrying objects’.
The impacts domain was divided into six subdomains. Three of the impacts subdomains were related to issues associated with different activities: ADLs, instrumental ADLs (iADLs), and social and leisure activities. ADLs were related to the six essential activities linked to someone’s independence (i.e. ‘self-feeding’, ‘bathing/showering’, ‘dressing’, ‘personal hygiene’, ‘moving around home’ and ‘toileting’). The iADLs included more complex activities related to the ability to live independently in the community (a full list of iADLs can be found in Supplementary Table 2) [29]. The third subdomain included social as well as leisure activities (including ‘hobbies/playing’, ‘socializing’ and ‘sports/physical exercise’). Outside of these ADLs, participants discussed impacts on physical (‘falling/tripping’), psychological (‘worry/anxiety’) and professional well-being (‘school/work’).
The fourth and final domain, disease management, comprised four subdomains related to planning/avoiding, adaptations, symptom management and support.
The degree to which concepts included in the CM were consistently reported as challenges by the participants was explored. As expected, some participants did not experience difficulty with most of the motor performance and activities concepts, which demonstrated the different levels of disease severity, ambulatory status and motor performance challenges within the sample. For example, all concepts were found to be associated with no difficulty in the bulbar and neck subdomain for at least one participant. Similarly, for concepts in the upper limb subdomain, a relatively higher ratio was associated with no difficulty for at least one participant compared with the mobility subdomain. This is in line with the clinical expectation that these concepts are less relevant for individuals with greater motor function ability. However, some differences were identified at the unique concept level that reflect age and functioning differences. For instance, within the bulbar and neck subdomain, two concepts, ‘speech’ and ‘swallowing’, were reported as more relevant in the near-ambulant group, which is more likely to be an issue for weaker individuals. Concepts of ‘crawling’, ‘self-feeding’, ‘speech’ and ‘swallowing’ were only reflected in the caregiver group which could reflect their relevance to younger individuals with SMA and their developmental stage. These concepts are more commonly affected in younger individuals with more severe forms of the disease, such as Type 1 or Type 2 SMA.
A saturation analysis was conducted at the concept level of the model when CE was completed. Results from the saturation analysis indicated that the 20 interviews in Wave 1 produced a comprehensive set of concepts related to the patient experience of ambulant and near-ambulant individuals with SMA, with 72 out of the 86 unique concepts being identified within the first group of five transcripts. Bulbar and neck motor performance was identified in the third transcript group for the first time. The final group of five transcripts did not identify concepts linked to an additional domain but rather three new concepts linked to bulbar and neck and upper limb issues, which were added. Based on the consistency of the findings, no further interviews were deemed necessary.
Item generation
Items were generated in relation to motor performance, specifically mobility and walking subdomains, and impacts with concepts relating to activities (Supplementary Table 2). The frame of reference of ‘needing help’ and response scale structure were initially developed to be in line with the SMAIS–ULM (i.e. ‘I do not need help’, ‘I need some help’, ‘I cannot do this at all without help’ and ‘not applicable’). However, the physical impact concepts ‘falling and tripping’ were excluded as items because they did not match the target concept of interest or align with the frame of reference of ‘needing help’.
CE findings indicated that many ambulant or near-ambulant individuals with SMA could master the tasks described in this item set without necessarily needing the help of another person. Therefore, to better reflect the patient experience and mitigate a ceiling effect, the instructions on ‘help’ were expanded to refer to help as ‘The amount of assistance you need from another person or from a supportive aid (e.g. a handrail, walking stick, mobility scooter, etc.)’ (Supplementary Table 3). Furthermore, a checklist of 13 supportive aids was developed to capture the range of assistive aids and devices participants reported using (Supplementary Table 4). The checklist was to be completed alongside the new items.
The resulting item set moving into the Wave 2 interviews of the study for testing comprised 42 mobility and 11 iADL items utilizing the SMAIS–ULM response option scale [12]. To capture the iterations of the SMAIS–Amb, a matrix was created that included the initial item, changes to the item and the final item (Supplementary Table 2).
Wave 2 results
Participants with SMA and caregiver demographics
A total of 15 new participants were recruited for the Wave 2 interviews (Table 2). Seven participants with SMA≥12 years of age were included in Wave 2 (six ambulant and one near-ambulant). Ages of participants with SMA ranged from 22–58 years, with a mean age of 40 years and a mean age at diagnosis of 16 years. All of the participants with SMA were White Caucasian females.
Table 2
Demographics of individuals with SMA and caregivers in Wave 2
| Participant demographics | Individuals with SMA: | Caregivers of child/children with | ||
| Wave 2 (n = 7) | SMA:a Wave 2 (n = 8) | |||
| Age (years) | ||||
| Mean age (SD), range | 40 (16), 22–58 | 37 (4), 31–43 | ||
| Mean age at diagnosis (SD), range | 16 (12), 1–31 | – | ||
| Mean diagnosis duration (SD), range | 24 (13), 8–39 | – | ||
| Gender, n (%) | ||||
| Male | – | 1 (12) | ||
| Female | 7 (100) | 7 (88) | ||
| Caregiver relationship, n (%) | ||||
| Mother | – | 7 (88) | ||
| Father | – | 1 (12) | ||
| Ethnicity, n (%) | ||||
| White Caucasian | 7 (100) | 6 (75) | ||
| Asian | – | 1 (12) | ||
| Hispanic | – | 1 (12) | ||
| Employment, n (%) | ||||
| Retired | 1 (14) | – | ||
| Full time | 3 (43) | 5 (62) | ||
| Homemaker | 2 (29) | 3 (37) | ||
| Disabled and able to work part time | 1 (14) | – | ||
| Education, n (%) | ||||
| Secondary/High school | 1 (14) | – | ||
| Technical or Vocational degree | – | 1 (12) | ||
| College degree | 2 (29) | 3 (37) | ||
| Some graduate work | 1 (14) | – | ||
| Graduate degree | 3 (43) | 4 (50) | ||
| Children with SMA (years) | ||||
| Mean age (SD), range | – | 5.0 (3.0), 2.0–10.0 | ||
| Mean age at diagnosis (SD), range | – | 2.0 (2.0), 0.0–5.0 | ||
| Mean diagnosis duration (SD), range | – | 4.0 (3.0), 1.0–8.0 | ||
| Gender of child with SMA, n (%) | ||||
| Male | – | 3 (37) | ||
| Female | – | 5 (62) | ||
| Self- or caregiver-reported ambulatory status, n | ||||
| Ambulantb | 6 | 4 | ||
| Near-ambulantb | 1 | 4 | ||
| Self- or caregiver-reported level of assistance needed, n (%) | In the home | Out of the home | In the home | Out of the home |
| I/They cannot do any activities without help | 0 | 1 (14) | 0 | 3 (37) |
| I/They need a lot of help | 0 | 0 | 3 (37) | 2 (25) |
| I/They need a moderate amount of help | 1 (14) | 1 (14) | 2 (25) | 2 (25) |
| I/They need a little bit of help | 4 (57) | 4 (57) | 3 (37) | 0 |
| I/They do not need any help | 2 (29) | 1 (14) | 0 | 1 (12) |
aDemographics included are for the eight children with SMA who the caregivers cared for. bAmbulant is defined as individuals with SMA who can walk≥5 steps without support. Near-ambulant is defined as individuals with SMA who can walk≥5 steps with support (holding a stable object with one or both hands). SD = standard deviation; SMA = spinal muscular atrophy.
Eight individuals were caregivers of children with SMA (four children were ambulant and four children were near-ambulant). Caregiver ages ranged from 31–43 years with a mean age of 37 years. The majority of caregivers were female (88%) and White Caucasian (75%). Caregivers cared for children aged 2–10 years, with a mean age of 5 years and a mean age at diagnosis of 2 years.
CE section of interviews
CE interviews in Wave 2 focused on both the CM as well as on the exploration of the participants’ definition of help and independence. The CE section of the Wave 2 interviews indicated that the CM of ambulant and near-ambulant patient experience did not need to expand significantly. The only change was the addition of ‘dancing’, an example of a social and leisure activity impacted by SMA.
In terms of the definitions of help and independence, there was a consensus amongst participants to include the use of supportive aids within the broader construct of independence. If the individual with SMA was able to accomplish a task by themselves using an aid but without the help of another person, this was considered independent to them. For example, “My definition of independent would be being able to use an assistive device, but not necessarily need the assistance of another person” (individual with SMA –ambulant). Additionally, types of supportive aids used were elicited. Each aid was endorsed by at least three participants, suggesting that all supportive aids listed were relevant to individuals with SMA. An ‘other’ option was also added that allowed participants to include any aid not listed. This response was chosen least, confirming the comprehensiveness of the list.
CD section of interviews
Item-level feedback on the mobility items
Forty-two mobility and 11 iADL items scored on the SMAIS–ULM response scale were presented. During the CD section of the Wave 2 interview, participants with SMA and caregivers considered the mobility-item set, which included activities performed both in and out of the home, generally relevant. ‘Walk on a slippery surface’ was chosen as the most relevant item (n = 4), whereas ‘getting out of the shower’ and ‘step off a bus/train’ were most frequently reported as the least relevant item (n = 4 each). Three items were flagged as unclear over 10 times: ‘step onto a bus/train’ (n = 15), ‘step off a bus/train’ (n = 13) and ‘walk in an unfamiliar place/environment’ (n = 10). ‘Walk up a single step indoors’ was most frequently chosen to be removed (n = 2), and another 11 items were suggested to be removed at least once (Supplementary Table 2).
Several item combinations overlapped in terms of the underlying concepts that were assessed. Participants (n = 9) most frequently reported that ‘step up on a curb’ conceptually overlapped (n = 9) with the inverse of ‘step down off a curb’. However, six participants considered these two items to be conceptually different. Participants with SMA and caregivers also provided feedback that determined which items within each item combination were the most difficult/relevant. For example, ‘getting off of the toilet’ was reported as more difficult than ‘getting on the toilet’ (n = 11). Eleven concepts were suggested as missing from the item set including items related to ‘dressing oneself’, and items related to ‘walking up an incline or decline’ were most frequently suggested (n = 2, each). The remaining nine items suggested as missing were raised by one participant each.
Item-level feedback on the iADL items
Comparatively, participants considered the 11 iADL-item set less relevant than the mobility item set. Four items, ‘standing while preparing meals/cooking’, ‘cleaning the house’, ‘mowing the lawn’ and ‘going shopping for groceries’ were chosen as the most relevant items, relative to the other item in the pair, on one occasion each despite their association with an adult/older age group. ‘Tidying up or putting things/toys away’ was most frequently reported as unclear (n = 3) since this activity was dependent on the height of an item, situation or size. ‘Setting the table’ was the only item suggested to be removed by two participants. Items related to the amount of weight one can lift and operating a motor vehicle were suggested as missing (n = 1 each).
Instruction and response option feedback
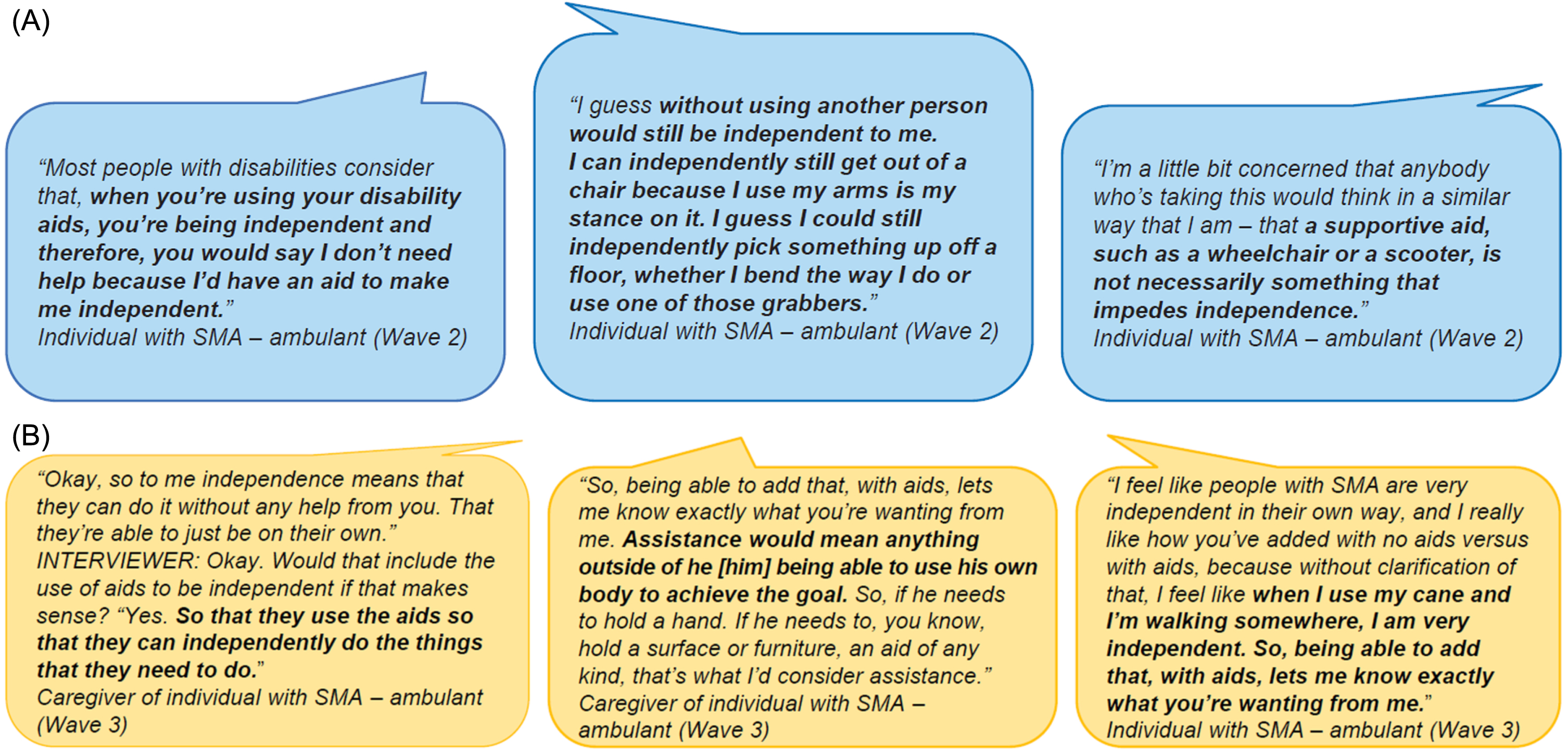
Feedback on the SMAIS–Amb instructions and response options was also elicited. When participants were asked about the instructions of the item sets (Supplementary Table 3), they challenged the idea that using a supportive aid would limit someone’s independence. Participants generally considered help as that from another person and not from a supportive aid (Fig. 3A). Participants were asked to provide alternative suggestions regarding the frame of reference. The most frequent suggestion (n = 4) was to switch to a difficulty frame of reference, meaning the response options would be based on how challenging one perceives a specific task.
Fig. 3
Participant quotes describing what independence means: (A) Instructions and participant quotes from Wave 2. Wave 2 instructions for individuals with SMA: “We would like to learn about your level of independence for doing daily activities. Please select the option that best describes the amount of assistance you need from another person or from a supportive aid (e.g. a handrail, walking stick, mobility scooter, etc) to perform each activity. Please think about the past 7 days and pick only one answer per activity”a,b. (B) Instructions and participant quotes from Wave 3. Wave 3 instructions for individuals with SMA: “We would like to learn about your level of independence. Please select the option that best describes the amount of assistance that you need to perform each activity. Please think about the past 7 days and pick only one answer per activity. If the question is not relevant (e.g. you do not have a bath in your house), please choose the ‘not applicable’ response option”c,d aWave 2 instructions for caregivers were identical. bResponse options in Wave 2 included: 1. ‘I do not need help’; 2. ‘I need some help’; 3. ‘I cannot do this at all without help’; 4. ‘Not applicable’. cWave 3 instructions for caregivers were identical. dResponse options in Wave 3 included: 1. ‘Independent with no aids’; 2. ‘Independent with aids’; 3. ‘Require(s) a little help from another person’; 4. ‘Require(s) a lot of help from another person’; 5. ‘Cannot do this at all’; and 6. ‘Not applicable’. SMA = spinal muscular atrophy.
Descriptive data and endorsement frequencies
Examination of the endorsement frequency (as defined as the proportion of respondents who selected one response option over another), and findings of the mobility-item set showed a good spread of response option endorsement. Out of 42 items, three (‘turn over in bed’, ‘sit up from lying down’ and ‘sit down into a chair’) displayed skewness to the ceiling of the scale (‘I do not need help’) with endorsement frequencies > 80%. No items had aggregated endorsement frequencies < 10%. The ambulant group more frequently endorsed the ceiling categories (‘I do not need help’) versus the near-ambulant group who more frequently endorsed the floor categories (‘I cannot do this at all without help’). The iADL items were more skewed towards the ceiling. Two of 11 iADL items (‘making your bed’ and ‘setting the table’) had 100% endorsement in the ‘I do not need help’ category.
Item refinement
Wave 2 results were presented and discussed at an expert panel with TAEs. The response option continuum was expanded to reflect the participants’ perception of independence with or without the use of supportive aids, while remaining consistent with the SMAIS–ULM concept of interest that related to the level of help needed for ADLs. The instructions were updated to refer to help as ‘the amount of assistance you need to perform each activity’ and reference to help ‘from another person or supportive aid’ was removed (Supplementary Table 3). The instructions were further updated to guide respondents on the use of the ‘not applicable’ option in situations that were not relevant or age appropriate to ensure consistent use of this response option. A selection of mobility-item sets was removed (n = 4) or modified (n = 3) due to poor performance and participant feedback (Supplementary Table 2). Three additional mobility items, ‘standing in the shower’ and ‘walking up/down an incline’, were added after the concepts were suggested as missing by participants and endorsed by the TAEs. No changes were made to this list of supportive aids. Lastly, a decision was made to remove the iADL-item set given the perceived lack of relevance and ceiling effect observed during the Wave 2 interviews (Supplementary Table 2).
Wave 3 results
Participants with SMA and caregiver demographics
A total of 15 new participants were recruited for the Wave 3 interviews (Table 3). Three participants with SMA≥12 years of age were included in Wave 3 (two ambulant and one near-ambulant). Ages of participants with SMA ranged from 19–56 years, with a mean age of 37 years and a mean age at diagnosis of 8 years. All participants with SMA were White Caucasian (two females and one male).
Table 3
Demographics of individuals with SMA and caregivers in Wave 3
| Participant demographics | Individuals with SMA: | Caregivers of child/children with | ||
| Wave 3 (n = 3) | SMA:a Wave 3 (n = 12) | |||
| Age (years) | ||||
| Mean age (SD), range | 37 (15), 19–56 | 36 (5), 30–44 | ||
| Mean age at diagnosis (SD), range | 8 (10), 0–23 | – | ||
| Mean diagnosis duration (SD), range | 29 (7), 19–35 | – | ||
| Gender, n (%) | ||||
| Male | 1 (33) | 2 (16) | ||
| Female | 2 (67) | 10 (84) | ||
| Caregiver relationship, n (%) | ||||
| Mother | – | 10 (84) | ||
| Father | – | 2 (16) | ||
| Ethnicity, n (%) | ||||
| White Caucasian | 3 (100) | 10 (84) | ||
| Asian | – | 1 (8) | ||
| Biracial | – | 1 (8) | ||
| Employment, n (%) | ||||
| Retired | 1 (33) | – | ||
| Full time | – | 7 (58) | ||
| Unemployed | – | – | ||
| Homemaker | – | 5 (42) | ||
| Disabled and able to work part time | 1 (33) | – | ||
| Student | 1 (33) | – | ||
| Education, n (%) | ||||
| Secondary/High school | 1 (33) | – | ||
| Some college | 1 (33) | 1 (8) | ||
| College degree | – | 4 (34) | ||
| Graduate degree | 1 (33) | 7 (58) | ||
| Children with SMA (years) | ||||
| Mean age (SD), range | – | 4.0 (3.0), 2.0–8.0 | ||
| Mean age at diagnosis (SD), range | – | 1.0 (1.0), 0.0–3.0 | ||
| Mean diagnosis duration (SD), range | – | 3.0 (1.0), 0.0–6.0 | ||
| Gender of child with SMA, n (%) | ||||
| Male | – | 9 (75) | ||
| Female | – | 3 (25) | ||
| Self- or caregiver-reported ambulatory status, n | ||||
| Ambulantb | 2 | 8 | ||
| Near-ambulantb | 1 | 4 | ||
| Self- or caregiver-reported level of assistance needed, n (%) | In the home | Out of the home | In the home | Out of the home |
| I/They cannot do any activities without help | 0 | 0 | 1 (8) | 1 (8) |
| I/They need a lot of help | 0 | 0 | 3 (25) | 3 (25) |
| I/They need a moderate amount of help | 1 (33) | 1 (33) | 3 (25) | 4 (33) |
| I/They need a little bit of help | 1 (33) | 2 (67) | 4 (33) | 3 (25) |
| I/They do not need any help | 1 (33) | 0 | 1 (8) | 1 (8) |
aDemographics included are for the 12 children with SMA who the caregivers cared for. bAmbulant is defined as individuals with SMA who can walk≥5 steps without support. Near-ambulant is defined as individuals with SMA who can walk≥5 steps with support (holding a stable object with one or both hands). SD = standard deviation; SMA = spinal muscular atrophy.
Twelve individuals were caregivers of children with SMA (eight children were ambulant and four children were near-ambulant). Caregiver ages ranged from 30–44 years with a mean age of 36 years. The majority of caregivers were female (84%) and White Caucasian (84%). Caregivers cared for children aged 2–8 years, with a mean age of 4 years and mean age at diagnosis of 1 year.
CD section of interviews
Item-level feedback on the mobility items
The feedback indicated that the majority of items were considered relevant to the sample. Specifically, ‘walk from room to room’ was chosen most frequently as the most relevant item (n = 7). ‘Sit down into a high/raised chair’ was chosen most frequently (n = 5) as the least relevant item, suggesting this activity was not applicable to the corresponding participants. Participants flagged a few items as ambiguous and requested further clarity. These included details on ‘height of bed’, ‘starting position when picking something up’ and ‘getting out of car seat vs a car’. In total, 20 items were suggested to be removed mostly on either one or two occasions; however, two items were most frequently suggested for removal on four separate occasions; ‘walk on an uneven surface outside’ and ‘walk on a slippery surface’ (n = 4).
Several item combinations were considered to be overlapping in terms of concepts. The most frequently reported overlap was between ‘getting in the bath’ and ‘getting out of the bath’ (n = 8). The second most frequently reported overlapping item pair was ‘walk on an uneven surface outside’ and ‘walk on an unstable surface outside’ (n = 7). However, these two items were suggested to be conceptually different by more participants (n = 10). Thus, there was conflicting feedback on concepts being conceptually different. Participant feedback relating to overlapping items resulted in the removal of 13 mobility items in Wave 3 (Supplementary Table 2).
Sixteen concepts were suggested as missing from the debriefed item set in Wave 3, which included ‘feeding yourself’, ‘swimming’, ‘dressing yourself’, ‘falls’ and ‘running’. Two of the most frequently reported missing items, ‘feeding yourself’ (n = 4) and ‘dressing yourself’ (n = 2), are both included in the SMAIS–ULM. ‘Falls’ and ‘running’ had previously been disregarded as they did not correspond to the frame of reference of needing help.
Instruction and response option feedback
During Wave 3, participants were positive about expanding the frame of reference to include ‘independent with no aids’ or ‘independent with aids’ and separately having response options relating to ‘requiring help from another person’, commenting that this better reflected their perception of independence (Fig. 3B, Supplementary Table 3). Following additional participant (n = 4) feedback, the response option ‘require(s) help from another person’ was separated into two options for additional testing in the subsequent interviews: ‘requires a little help from another person’ and ‘requires a lot of help from another person’. When the participants were asked for their preference between the two options, the majority (n = 10/15) preferred the response options where help was split between ‘a little’ and ‘a lot’.
Despite endorsement of the expanded definition of independence to include supportive aids and help separately, two issues were identified. Firstly, participants indicated that the range of supportive aids and their associated level of independence may not apply to all response options, such as wheelchairs for walking a long distance. Secondly, some participants indicated confusion over the term ‘aid’, and whether this included another person/the caregiver. To mitigate this concern, there was a suggestion to add the term ‘device’ for clarification purposes.
Descriptive data and endorsement frequencies
Examination of endorsement frequencies of the refined mobility-item set indicated a better spread of response endorsement. Only 1/41 items (‘sit up from lying in bed’) displayed skewness to the ceiling of the scale, with endorsement frequencies > 80% (i.e. selecting ‘I do not need help’). Three items (‘turn over in bed’, ‘sit up from lying down’ and ‘bend over to pick up something’) had aggregated endorsement frequencies < 10%, which could indicate some issues with the participants’ discrimination between the five available response options. Overall, the ambulant group had a higher endorsement to the ceiling categories compared with the near-ambulant group, who had a higher endorsement to the floor categories, thereby following expected patterns.
Preliminary and exploratory macro-level RMT analysis
A preliminary and exploratory RMT analysis was conducted at the end of Wave 3. A total of 25 participants from Wave 1 (n = 3 ambulant and n = 3 near-ambulant), Wave 2 (n = 6 ambulant and n = 3 near-ambulant) and Wave 3 (n = 7 ambulant and n = 3 near-ambulant) were included in the RMT analysis (Supplementary Table 1). While sample sizes as low as n = 30 are considered sufficient for a small-scale RMT analysis [30], the quantitative RMT findings presented in this study are preliminary and will need to be further assessed and confirmed at a later stage. Given the exploratory nature of this macro-level analysis, no decisions were made for the content of the SMAIS-Amb scale simply on the basis of quantitative findings. Disordered thresholds were observed based on the five-level response options. Therefore, for the purposes of the RMT analysis, the five-level response options were collapsed into four levels as the options of ‘requires a little help from another person’ and ‘requires a lot of help from another person’, which were the closest conceptually, were merged to increase precision in the interpretation of findings. The collapsed four-level, 41-item response option pattern decreased disordering and increased precision in the measurement estimates but did not resolve the disordering (Table 4). The scale-to-sample targeting was reasonable (Table 4) but the sample size limited confidence in this result. Item thresholds ranged from –3.44 to 3.99 logits, and person estimates ranged from –5.85 to 2.89 logits (mean 0.92, 88% coverage).
Table 4
Preliminary and exploratory RMT summary findings
| Measurement properties | Wave 3 mobility (41 items) | Wave 3 mobility reduced item set (27 items) |
| Targeting: how adequate is the scale-to-sample targeting?a | 88% | 88% |
| Response thresholds: do the response categories work as intended?b | 41% | 48% |
| Item fit: to what extent do the items work together to define a single measurement construct?c | 93% | 100% |
| Item dependency: to what extent are the items locally independent?d | 105/820 item pairs | 30/351 item pairs |
| PSI: are participants in the sample separated by the scale items (with/without extremes)?e | 0.96/0.98 | 0.96/0.97 |
Higher percentages indicate better findings. aEstimated using the percentage of individual sample measurements (n = 40 in Wave 1 and n = 39 in Wave 2) covered by the scale range. bEstimated based on the percentage of items displaying ordered response thresholds following the collapsing of the ‘a little’ and ‘a lot of help’ response options. cEstimated based on the percentage of items displaying significant chi-square estimates suggesting item misfit. dNumber of item pairs that are locally dependent based on > 0.3 residual correlations indicating > 9% shared variance. ePSI was reported on a scale from 0–1; 0 = all error; 1 = no error. PSI = person separation index; RMT = Rasch Measurement Theory.
Following input from the TAEs, ‘in/out’ and ‘up/down’ item pairs were reduced to limit conceptual overlap. It was further decided to remove the ‘getting into a car’ item, which was raised by participants, and was subsequently agreed by TAEs to be an ambiguous item of poor quality and fit. Thus, the resulting 27-item set was re-examined, and scale-to-sample targeting remained reasonable (Table 4). Although the small-scale RMT analysis was exploratory and preliminary in nature, these findings did not identify any early risk in the measurement properties of the reduced 27-item scale. However, these findings will need to be assessed in a large-scale psychometric validation study for confirmation. The issues on the response option ordering were consistent. There were instances where category probability curves indicated ordered endorsement for each response category (Supplementary Figure 1A) versus other category probability curves that showed disordered response category endorsement (Supplementary Figure 1B). Item fit and dependency marginally improved. The reliability and person separation index were excellent in both item sets, with item dependency markedly reduced (Table 4). Hence, the appropriateness of moving forward with the reduced item set with less conceptual overlap in content was confirmed.
Final item refinement of the provisional SMAIS–Amb
Wave 3 results were presented and discussed at a TAE meeting. There was consensus among the TAEs on the improvement of the response scale and revised frame of reference of independence that included the use of supportive aids and help from another person separately. Given the small sample size from the RMT analyses, interpreting the extent of the quantitative issues with the response scale was limited; therefore, a future quantitative robust psychometric study is needed. This was particularly limited in the case of the ‘requires a little help from another person’ and ‘requires a lot of help from another person’ response options which were qualitatively and clinically endorsed as being separate.
Furthermore, despite the consensus amongst participants on the notion of independence, including the use of supportive aids, the ‘independent with aids’ response option was confounded by the notion that not all supportive aids offer the same level of support. For example, a rail to help someone get onto the toilet likely denotes a higher level of function compared with the help of another person. However, more profound weakness may result in needing an aid such as a rising toilet, which would indicate the need for more help than offered by another person. As the qualitative data supported this gradation of levels of help, no changes were suggested at this stage in relation to these response options as a larger dataset and further psychometric analyses would be required to confirm any revisions. There was agreement on the improved relevance and comprehensiveness of the item set and its potential to discriminate between ambulatory versus near-ambulatory status.
To resolve any ambiguity or inconsistency of response in relation to the walking items (i.e. with participants responding based on their use of a wheelchair), the word ‘walk’ was bolded in each item. Issues on item clarity (qualitative) and item quality/fit (quantitative) were considered.
DISCUSSION
This work adds to the limited patient-centered research in higher-functioning individuals with SMA and introduces comparative patient- and caregiver-centric insights in relation to an individual’s ambulatory status and level of independence [31]. Building on a previous study [12], the 27-item SMAIS–Amb provides the possibility of measuring independence and the level of assistance required to complete everyday lower limb-related mobility tasks in ambulant patients. Developed in conjunction with individuals with SMA, caregivers, a patient advocacy group and clinical TAEs, the SMAIS–Amb fills an important measurement gap for ambulant individuals. The concept of independence and the meaning of independence to participants with SMA and caregivers were carefully considered. Reflecting on the participants’ feedback regarding their perception of independence, which included the use of supportive aids, the SMAIS–Amb response options were refined to ensure the continuum of independence was appropriately captured.
Compared with existing patient- and observer-reported outcome scales in SMA [17, 18], the SMAIS–Amb focuses on independence as the concept of interest and hence builds on the existing measurement scales available. Concepts identified in concept elicitation were not tested for saturation per group (ambulant versus near-ambulant) but rather on the total sample. It is important to consider that although concepts may have only arisen in the near-ambulant or ambulant group in this sample, some individuals may still experience difficulties with these motor functions in a real-world setting (Fig. 2). The SMAIS–Amb is intended for use in clinical trials and potentially in clinics evaluating current treatments in order to allow for the detection of therapy-related changes in mobility-related tasks over time. Maintaining independence when completing activities is critical to both individuals with SMA and their families [32–34]. This measure provides SMA researchers with an important additional assessment in their toolbox for studies evaluating patients with SMA who are ambulant or near-ambulant. The SMAIS–Amb complements the SMAIS–ULM and is intended as a separate but related module such that a broad range of everyday tasks from upper- to lower-limb-related items can be assessed in a clinical trial via these two assessments. Much like the SMAIS–ULM, which was used in the SUNFISH trial (NCT02908685) to measure upper limb motor performance in non-ambulant individuals [12], the SMAIS–Amb can be used in conjunction with gross motor function scales. These motor function scales include the 32-item Motor Function Measure [35], Hammersmith Functional Motor Scale –Expanded [14], Revised Hammersmith Scale [36] and 6-minute Walk Test [15] as well as quality-of-life scales [37] to provide the perspectives of more ambulant patients and caregivers on the level of independence demonstrated when completing everyday mobility related activities. Future studies should examine how data from existing clinician/performance and patient- and caregiver-reported outcome measures, such as the SMAIS–Amb, can be combined to help improve clinical understanding of objective and subjective changes.
Although this study offers unique insight into the perspective of independence for ambulant and near-ambulant individuals with SMA, there were some limitations. Recruitment and screening were based on self-reports of ambulatory status, did not allow for a clinically defined sample and resulted in instances of potentially variable ambulation. Information on participant health literacy, socioeconomic representation, and present and prior SMA DMTs was not collected; thus, participant representativeness regarding these factors is unknown. The overall study sample was not ethnically diverse, such that 86% of participants with SMA in Wave 1 and 100% of participants with SMA in Waves 2 and 3 were White Caucasian, and all interviews were conducted in the USA, which also may bias results. Furthermore, SMA is a rare disease, and flexibility was necessary to fulfill recruitment. Through the efforts of patient advocacy group outreach, the study population had an adequate sample for qualitative research, and conceptual saturation was achieved. However, the RMT analysis was exploratory and due to the small study size, requires replication in a larger dataset. Importantly, the findings of the RMT should be interpreted in the context of the small sample size, as studies have reported more frequent instances of items with mis-ordered parameters when sample sizes were≤50 [38]. Finally, the concept of falls and tripping arose as missing in the first and last set of interviews and although not explicitly matching the frame of reference of needing help, additional outcome measures assessing this concept (used in conjunction with the SMAIS-Amb) would be important for future studies to consider.
The primary focus of this study was on the qualitative development of the SMAIS–Amb scale and corresponding content validity of the scale. In the future, including patients with SMA or caregivers of individuals with SMA as part of the expert panel may help provide additional insights into the content of the SMAIS–Amb scale. Furthermore, larger-scale studies will need to be designed for quantitative analyses to further inform the final item content of the SMAIS–Amb, as conceptual overlap, disordered response options and redundancy issues were reduced but not resolved. Longitudinal studies to assess patients’ independence over time may be useful in informing subsequent versions of the SMAIS–Amb. A better understanding of whether the minimum age for the SMAIS–Amb scale should be raised in future versions is also necessary as it may not be appropriate for caregivers to report on all activities on behalf of patients as young as 2 years of age. Finally, it will be important to validate the SMAIS–Amb cross-culturally and in low-to-middle income countries where access to supportive aids may be variable, and to understand the SMAIS–Amb’s relationship with the SMAIS–ULM.
With the increasing number of patients receiving DMTs worldwide, it is important to capture how treatment impacts independence, particularly in higher-functioning individuals with SMA who are ambulant or near-ambulant. The SMAIS–Amb introduces a mobility-item set with concepts of specific relevance to this patient population. However, the scale presented in this original research is provisional and requires further psychometric testing. The SMAIS–Amb will be made available in the future once additional validation has been conducted. This work adds to the limited patient-centered research in higher-functioning ambulant and near-ambulant individuals with SMA and introduces patient- and caregiver-centered insights into an individual’s level of independence when completing everyday mobility-related activities in and out of the home.
ACKNOWLEDGMENTS INCLUDING SOURCES OF SUPPORT
The authors would like to thank Cure SMA for their recruitment assistance for the interview portion of this study. The authors thank Thread Research for their analytical support and Kathryn Russo, PhD, of Nucleus Global for their medical writing support, which was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland in accordance with the Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Funding was provided by Roche Products Ltd and F. Hoffmann-La Roche Ltd in the form of salaries for Roche employees, and consultancy fees paid to Modus Outcomes LLC, G Baranello, J Kirschner and A Mayhew.
CONFLICT OF INTEREST
H Staunton and J Braid are employees and shareholders of Roche Products Ltd.
S Cano is CSO of Modus Outcomes LLC and L Barrett, S Cleanthous and B Ewens are employees of Modus Outcomes Ltd.
V Teodoro is an employee of F. Hoffmann-La Roche Ltd (Basel).
G Baranello has received speaker and consultancy honoraria from Novartis Gene Therapies, Roche, Biogen, PTC, Pfizer and Sarepta Therapeutics.
J Kirschner has received honoraria for clinical research and/or consultancy activities from Biogen, Novartis Gene Therapies, Roche and Scholar Rock.
L Belter is an employee of Cure SMA, which received funding from Roche/Genentech for other distinct projects and has no competing personal interests.
A Mayhew is a consultant (as well as a trainer) for Roche, Novartis (Avexis), Biogen and Biohaven in the SMA field, as well as for PTC Therapeutics, Sarepta Therapeutics, Italfarmaco, Pfizer, Fibrogen, Modis, Lysogene, Modis, Amicus, Analysis Group and Duchenne UK.
DATA AVAILABILITY STATEMENT
Individual patient-level data generated from this study are not publicly available; aggregated data may be provided by the authors upon reasonable request. Requests to access the aggregated data should be directed to the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230096.
REFERENCES
[1] | Darras BT . Spinal muscular atrophies. Pediatr Clin North Am. (2015) ;62: (3):743–66. |
[2] | Kolb SJ , Kissel JT . Spinal Muscular Atrophy. Neurologic Clinics. (2015) ;33: (4):831–46. |
[3] | Lorson CL , Hahnen E , Androphy EJ , Wirth B . A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America. (1999) ;96: (11):6307–11. |
[4] | Lefebvre S , Burglen L , Reboullet S , Clermont O , Burlet P , Viollet L , et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. (1995) ;80: (1):155–65. |
[5] | Prior TW , Leach ME , Finanger E . Spinal Muscular Atrophy. In: AdamMP, ArdingerHH, PagonRA, WallaceSE, BeanLJH, StephensK, et al., editors. GeneReviews®. Seattle (WA); (1993) . |
[6] | D’Amico A , Mercuri E , Tiziano FD , Bertini E . Spinal muscular atrophy. Orphanet J Rare Dis. (2011) ;6: :71. |
[7] | Waldrop MA , Kolb SJ . Current Treatment Options in Neurology-SMA Therapeutics. Curr Treat Options Neurol. (2019) ;21: (6):25. |
[8] | US Food and Drug Administration (FDA). ZOLGENSMA® (onasemnogene abeparvovec-xioi) [package insert]. Durham, NC: Novartis Gene Therapies, Inc; 2019. |
[9] | US Food and Drug Administration (FDA) EVRYSDI™ (risdiplam) [package insert]. San Francisco, CA: Genentech Inc; 2020. |
[10] | Cure SMA. FDA Approves Genentech’s Evrysdi (risdiplam) For Use in Babies Under Two Months with Spinal Muscular Atrophy. 2022. Available from: https://www.curesma.org/fda-approves-genentechs-evrysdi-risdiplam-for-use-in-babies-under-two-months-with-spinal-muscular-atrophy/ |
[11] | US Food and Drug Administration (FDA). SPINRAZA® (nusinersen) [package insert]. Cambridge, MA: Biogen Inc; 2016. |
[12] | Trundell D , Skalicky A , Staunton H , Hareendran A , Le Scouiller S , Barrett L , et al. Development of the SMA independence scale-upper limb module (SMAIS-ULM): A novel scale for individuals with Type 2 and non-ambulant Type 3 SMA. J Neurol Sci. (2022) ;432: :120059. |
[13] | Duong T , Staunton H , Braid J , Barriere A , Trzaskoma B , Gao L , et al. A Patient-Centered Evaluation of Meaningful Change on the 32-Item Motor Function Measure in Spinal Muscular Atrophy Using Qualitative and Quantitative Data. Front Neurol. (2021) ;12: :770423. |
[14] | O’Hagen JM , Glanzman AM , McDermott MP , Ryan PA , Flickinger J , Quigley J , et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. (2007) ;17: (9-10):693–7. |
[15] | Salvi D , Poffley E , Orchard E , Tarassenko L . The Mobile-Based 6-Minute Walk Test: Usability Study and Algorithm Development and Validation. JMIR Mhealth Uhealth. (2020) ;8: (1):e13756. |
[16] | Bérard C , Payan C , Hodgkinson I , Fermanian J , MFM Collaborative Study GrouA motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. (2005) ;15: (7):463–70. |
[17] | Matsumoto H , Clayton-Krasinski DA , Klinge SA , Gomez JA , Booker WA , Hyman JE , et al. Development and initial validation of the assessment of caregiver experience with neuromuscular disease. J Pediatr Ortho. (2011) ;31: (3):284–92. |
[18] | Pasternak A , Sideridis G , Fragala-Pinkham M , Glanzman AM , Montes J , Dunaway S , et al. Rasch analysis of the Pediatric Evaluation of Disability Inventory-computer adaptive test (PEDI-CAT) item bank for children and young adults with spinal muscular atrophy. Muscle Nerve. (2016) ;54: (6):1097–107. |
[19] | Baranello G , Day J , Klein A , Mercuri E , Servais L , Deconinck N , et al. FIREFISH, a multi-center, open-label trial to investigate the safety and efficacy of RG7916 in babies with Type 1 SMA: Study update and real-life experience of study implementation. Presented at SMA Europe. 2018. |
[20] | Hjartarson HT , Nathorst-Boos K , Sejersen T . Disease Modifying Therapies for the Management of Children with Spinal Muscular Atrophy (5q SMA): An Update on the Emerging Evidence. Drug Des Devel Ther. (2022) ;16: :1865–83. |
[21] | Gusset N , Stalens C , Stumpe E , Klouvi L , Mejat A , Ouillade MC , et al. Understanding European patient expectations towards current therapeutic development in spinal muscular atrophy. Neuromuscul Disord. (2021) ;31: (5):419–30. |
[22] | Belter L , Cruz R , Jarecki J . Quality of life data for individuals affected by spinal muscular atrophy: a baseline dataset from the Cure SMA Community Update Survey. Orphanet J Rare Dis. (2020) ;15: (1):217. |
[23] | ATLAS.ti. Scientific Software Development GmbH. Atlas.ti. software version 7. 2013. |
[24] | Kline P . A Handbook of Test Construction: Introduction to psychometric design. Psychology Revivals. 1986: :274. |
[25] | US Food and Drug Administration (FDA). Guidance for industry. Patient-reported outcome measures: Use in medical product development to support labeling claims; 2009. Available from: https://www.fda.gov/media/77832/download. |
[26] | Saunders B , Sim J , Kingstone T , Baker S , Waterfield J , Bartlam B , et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. (2018) ;52: (4):1893–907. |
[27] | Cano SJ , Mayhew A , Glanzman AM , Krosschell KJ , Swoboda KJ , Main M , et al. Rasch analysis of clinical outcome measures in spinal muscular atrophy. Muscle Nerve. (2014) ;49: (3):422–30. |
[28] | Harvey LA . REDCap: web-based software for all types of data storage and collection. Spinal Cord. (2018) ;56: (7):625. |
[29] | Edemekong PF , Bomgaars DL , Sukumaran S , Schoo C . Activities of Daily Living. StatPearls. Treasure Island (FL) 2022. |
[30] | Linacre J . Sample Size and Item Calibration Stability Rasch Measurement Transactions. (1994) ;7: :328. |
[31] | Mazzella A , Curry M , Belter L , Cruz R , Jarecki J . “I have SMA, SMAdoesn’t have me”: a qualitative snapshot into the challenges,successes, and quality of life of adolescents and young adults withSMA. Orphanet J Rare Dis. (2021) ;16: (1):96. |
[32] | Cruz R , Lenz M , Belter L , Hobby K , Jarecki J . Voice of the patient report: A summary report from an externally led patient focused drug development meeting reflecting the U.S. Food and Drug Administration (FDA) patient-focused drug development initiative. 2018. Available from: http://www.curesma.org/documents/advocacy-documents/sma-voice-of-the-patient.pdf. |
[33] | Cruz R , Belter L , Wasnock M , Nazarelli A , Jarecki J . Evaluating Benefit-risk Decision-making in Spinal Muscular Atrophy: A First-ever Study to Assess Risk Tolerance in the SMA Patient Community. Clin Thera. (2019) ;41: (5):943–60.e4. |
[34] | McGraw S , Qian Y , Henne J , Jarecki J , Hobby K , Yeh WS . A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. (2017) ;17: (1):68. |
[35] | Bérard C , Girardot F , Payan C . User’s Manual: MFM-32 & MFM-20 2019 [updated 2019]. Available from: https://mfm-nmd.org/get-a-user-manual/?lang=en. |
[36] | Ramsey D , Scoto M , Mayhew A , Main M , Mazzone ES , Montes J , et al. Revised Hammersmith Scale for spinal muscular atrophy: A SMA specific clinical outcome assessment tool. PLoS one. (2017) ;12: :e0172346. |
[37] | Iannaccone ST , Hynan LS , Morton A , Buchanan R , Limbers CA , Varni JW . The PedsQL™ in pediatric patients with spinal muscular atrophy: Feasibility, reliability, and validity of the pediatric quality of life inventory™ generic core scales and neuromuscular module. Neuromuscul Disord. (2009) ;19: (12):805–12. |
[38] | Chen WH , Lenderking W , Jin Y , Wyrwich KW , Gelhorn H , Revicki DA . Is Rasch model analysis applicable in small sample size pilot studies for assessing item characteristics? An example using PROMIS pain behavior item bank data. Qual Life Res. (2014) ;23: (2):485–93. |




