Treatment of Symptomatic Spinal Muscular Atrophy with Nusinersen: A Prospective Longitudinal Study on Scoliosis Progression
Abstract
Background:
Nusinersen treatment has demonstrated efficacy in improving clinical outcomes for spinal muscular atrophy (SMA), yet its impact on scoliosis progression remains unclear.
Objective:
This study aimed to assess the progression of scoliosis in pediatric patients with SMA undergoing nusinersen treatment.
Methods:
In this prospective study, data were systematically collected from Hong Kong pediatric SMA patients receiving nusinersen between 2018 and 2023. All patients had longitudinal radiographic studies pre-nusinersen, and at half-yearly or yearly intervals during treatment based on the scoliosis severity. Motor function evaluations were conducted pre-nusinersen, and after starting treatment at 6- and 12-month intervals.
Results:
Twenty-three patients ((SMA type 1 (SMA1) = 8, SMA type 2 (SMA2) = 7, SMA type 3 (SMA3) = 8)) with a median age of 5.8 years (range: 0.4–17.5 years) at nusinersen initiation, and median follow-up duration of 3.4 years (range: 1.1–5.2 years) were included. During the study period, motor scores remained stable or improved in 83% of patients. However, scoliosis progressed across all subtypes, with mean annual progression rates of 5.2, 11.9, and 3.6 degrees in SMA1, SMA2, and SMA3 respectively. Patients initiating nusinersen between ages 5 and 11 years exhibited the most rapid progression, with rates of 11.8, 16.5, and 7.3 degrees per year in SMA1, SMA2, and SMA3 respectively. Positive correlations were observed between the difference in CHOP-INTEND score post-nusinersen and scoliosis progression in SMA1 (rs = 0.741, p = 0.041). Conversely, negative correlations were found between the difference in HFMSE score post-nusinersen and scoliosis progression in SMA2 (rs =−0.890, p = 0.012) and SMA3 (rs =−0.777, p = 0.028).
Conclusions:
This study reveals that nusinersen treatment in symptomatic pediatric SMA patients with motor improvement is linked to increased scoliosis progression in SMA1, whereas it is associated with decreased progression in SMA2 and SMA3. Age, baseline Cobb angle, and motor milestone improvement are influential factors in scoliosis progression.
INTRODUCTION
Spinal muscular atrophy (SMA) is a hereditary condition characterized by proximal muscle weakness due to the homozygous loss of SMN1 gene function, resulting in the deficiency of the survival motor neuron (SMN) protein [1]. The natural history of SMA involved a continual decline of respiratory function and early death in patients with SMA Type 1 (SMA1), and motor function and muscle strength deterioration in patients with SMA Types 2 and 3 (SMA2 and SMA3) [2].
In December 2016, the United States Food and Drug Administration (FDA) approved the first therapeutic drug, nusinersen, for the treatment of SMA. Nusinersen is an SMN2 targeting antisense oligonucleotide (ASO) that affects the splicing of SMN2 mRNA, leading to increased production of functional SMN protein [3]. Significant improvements in motor strength and function and decreased disease burden after treatment initiation that persist over time have been documented in both clinical trials and real-world data [4–7]. Risdiplam is another SMN2 splicing modifier that has been developed as the first oral disease-modifying treatment for SMA. Onasemnogene abeparvovec (AVXS-101), a gene replacement therapy that uses adeno-associated viral (AAV9) vectors containing a copy of the SMN1 gene has also been approved for use. These disease-modifying drugs have revolutionized SMA therapy and altered the natural progressive disease course of SMA.
Scoliosis is a three-dimensional spine deformity characterized by changes in the coronal, sagittal, and axial planes. In pediatric patients with SMA, the presence of worsening axial muscle strength during periods of growth can lead to a collapsing spine and thus result in large magnitudes of scoliosis at a young age [8–10]. Consequently, severe scoliosis in childhood affects patients’ cardiopulmonary development and function as well as truncal balance. A recent study found that the lifetime risk of scoliosis surgery was almost 80% in patients with SMA types 1c and SMA2 [11]. The availability of nusinersen has significantly changed the SMA treatment landscape, and clinical trials have demonstrated its benefits to patients’ motor milestone achievements and functional performance. However, less is known on whether these observed improvements in motor strength alter the natural history of scoliosis progression in type 1 and 2 patients, and is unknown in type 3 patients [12]. This study aimed to investigate the longer-term impact of nusinersen treatment on scoliosis progression in pediatric patients with SMA.
METHODS
Standard protocol and patient consent
This was a prospective study of all eligible SMA patients who received nusinersen in Hong Kong at two designated SMA treatment institutes, the Queen Mary Hospital, and the Hong Kong Children’s Hospital. Since May 2018, nusinersen has been available to all local eligible pediatric SMA1 patients under an Extended Access Program in our territory. In September 2018, the local government approved Community Care Funding to support symptomatic eligible pediatric patients of all three SMA subtypes for nusinersen treatment. All potentially eligible patients underwent pre-treatment multi-specialty team evaluations, similar to those described by Zingariello et al. [13]. All patients who were assessed and deemed eligible for nusinersen fulfilling the following criteria, were included in this study. All patients must be genetically confirmed and symptomatic at the commencement of nusinersen treatment. Medical information, including SMN1 and SMN2 copy numbers, presenting symptoms, respiratory and feeding status and support, and spinal data, at baseline and during serial follow-ups, was systematically collected prospectively. Scoring of functional motor scores before nusinersen treatment and then at six months and twelve months post-treatment in the first year, followed by yearly intervals, were also recorded. All patients received intrathecal nusinersen with a 12 mg dosing regimen according to published protocols, which consisted of 4 loading doses given on day 1, then half a month, one month, and two months post-first dosing, followed by a maintenance dose once every four months thereafter. All the patients were regularly followed-up by multi-specialty teams according to the recommended standard of care. Ethical approval for this prospective study was obtained from the local institutional review board (UW 19-418 & HKCH-REC-2020-075) was obtained. Written informed consent was obtained from the parents of all patients.
Effect of nusinersen on spinal deformity progression
All patients underwent whole spine out-of-brace posteroanterior and lateral radiographs pre-nusinersen treatment and were followed up at 6 months and 12 months after nusinersen initiation, and half-yearly or yearly thereafter depending on the scoliosis severity. Where possible, an erect radiograph was taken in a standing or sitting position without support. In cases where patients could not sit, supine radiographs were taken. The progression rate was consistently monitored in the same position, providing an accurate reflection of curve magnitude in this patient subgroup. The scoliosis curve pattern and magnitude, as measured by the coronal Cobb angle, were documented.
All skeletally immature patients with progressive scoliosis and a Cobb angle of >25 degrees were treated with underarm Boston brace. If surgical threshold was reached, the usual protocol was to offer surgical intervention for growth guidance if the patient was skeletally immature, or early definitive fusion if the triradiate cartilage had closed.
Assessment of motor strength, function, and ambulatory status
The motor outcomes before and after nusinersen initiation were measured by the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) and Hammersmith Infant Neurological Examination (HINE) for SMA1 patients; and Hammersmith Motor Functional Scale Expanded (HMFSE), Revised Upper Limb Module (RULM), and 6-Minute Walk Test (6MWT) for SMA2 and SMA3 patients. The CHOP INTEND axial scores and appendicular motor scores for SMA1 patients were further analyzed to study the correlation between axial muscle strength and scoliosis progression. Trained physiotherapists experienced in using these motor function scales scored all patients.
Statistical analysis
Descriptive statistics were utilized for baseline characteristics, as well as clinical and radiographic data. The rate of scoliosis progression was calculated using the difference between Cobb angles pre-nusinersen treatment and those in the final follow-up radiographs, divided by the duration of follow-up in years. Spearman correlations were conducted to detect the relationship between the rate of scoliosis progression with baseline Cobb angles, and changes in CHOP-INTEND scores for SMA1, and changes in HFMSE scores for SMA2 and SMA3. Statistical significance was accepted at a two-tailed p value of <0.05. Data analyses and graphing were performed using GraphPad Prism version 10.1.0 (GraphPad, La Jolla, CA).
RESULTS
Baseline characteristics
Between January 2018 and October 2023, 26 patients received intrathecal nusinersen treatment at our two institutions under the Community Care Fund support program. Out of these 26 patients, 3 had spinal fusions prior to starting of nusinersen treatment and were excluded. Before the treatment began, all SMA1 patients were non-sitters with stable health conditions, all SMA2 patients could sit independently, and all SMA3 patients were walkers but exhibited signs of motor deterioration.
Among the 23 patients included in this study, 8 had SMA1, 7 had SMA2, and 8 had SMA3. All patients had homozygous SMN1 exon 7 deletion, except for one SMA1 patient who had a point mutation with an exon 7 deletion in SMN1. The baseline demographics of the patients are summarised in Table 1. The median age at the time of nusinersen initiation was 5.8 years, ranging from 0.4 to 17.5 years. The median duration of follow-up was 3.4 years, with a range of 1.1 to 5.2 years. Among the SMA1 patients, they either had 2 or 3 SMN2 copies. All SMA2 patients had 3 SMN2 copies. Half of the SMA3 patients had 3 SMN2 copies and the other half had 4 SMN2 copies. The patient characteristics, including respiratory and feeding status, motor performance, at baseline before nusinersen treatment initiation, and after nusinersen treatment at the latest follow-up, are summarized in Table 2.
Table 1
Baseline characteristics according to SMA subtype
| Characteristics | Type 1 (n = 8) | Type 2 (n = 7) | Type 3 (n = 8) | Total (n = 23) |
| Mean age at starting nusinersen, y (SD) | 3.8 (4.1) | 8.1 (4.8) | 8.4 (5.3) | 6.7 (5.0) |
| Median age starting nusinersen, y (range) | 2.2 (0.4–12.5) | 6.5 (3.2–17.5) | 7.4 (1.6–16.6) | 5.8 (0.4 –17.5) |
| Median duration of follow-up, y (range) | 3.6 (1.1–5.2) | 3.7 (1.3–4.4) | 2.9 (1.1–4.4) | 3.4 (1.1–5.2) |
| Male, n (%) | 3 (38) | 3 (43) | 4 (50) | 10 (43) |
| SMN 2 copy number, n (%) | ||||
| 2 | 5 (63) | 0 (0) | 0 (0) | 5 (22) |
| 3 | 3 (38) | 7 (100) | 4 (50) | 14 (61) |
| 4 | 0 (0) | 0 (0) | 4 (50) | 4 (17) |
Abbreviations: HFMSE = Hammersmith Functional Motor Scale–Expanded; RULM = Revised Upper Limb Module; CHOP-INTEND=The ‘Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; HINE = Hammersmith Infant Neurological Examination; 6MWT = 6-Minute Walk Test.
Table 2
Comparison of patient characteristics at baseline and latest assessment
| Characteristics | Type 1 (n = 8) | Type 2 (n = 7) | Type 3 (n = 8) | |||
| T0 | T1 | T0 | T1 | T0 | T1 | |
| Ventilation status, n (%) | ||||||
| 24 hrs NIV | 3 (38) | 3 (38) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nocturnal NIV | 2 (25) | 5 (63) | 2 (29) | 5 (71) | 1 (13) | 3 (38) |
| Nil | 3 (38) | 0 (0) | 5 (71) | 2 (29) | 7 88) | 5 (63) |
| Feeding status, n (%) | ||||||
| Nasogastric tube feeding | 1 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrostomy feeding | 4 (50) | 7 (88) | 1 (14) | 1 (14) | 0 (0) | 0 (0) |
| Oral | 3 (38) | 1 (13) | 6 (86) | 6 (86) | 8 (100) | 8 (100) |
| Ambulatory status, n (%) | ||||||
| Non-ambulatory | 8 (100) | 8 (100) | 7 (100) | 6 (86) | 0 (0) | 0 (0) |
| Ambulatory | 0 (0) | 0 (0) | 0 (0) | 1 (14) | 8 (100) | 8 (100) |
| Motor function, n (%) | ||||||
| Non-sitter | 8 (100) | 3 (38) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Sitter with support | 0 (0) | 3 (38) | 0 (0) | 1 (14) | 0 (0) | 0 (0) |
| Independent sitter | 0 (0) | 2 (25) | 7 (100) | 5 (71) | 0 (0) | 0 (0) |
| Supported walker | 0 (0) | 0 (0) | 0 (0) | 1 (14) | 1 (13) | 2 (25) |
| Independent walker | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (88) | 6 (75) |
| CHOP-INTEND score, n | n = 8 | n = 8 | ||||
| Mean (SD) | 25.6 (15.4) | 33.0 (23.4) | – | – | – | – |
| Median (range) | 25.5 (5–51) | 28 (3–62) | – | – | – | – |
| Axial CHOP-INTEND score, n | n = 8 | n = 8 | ||||
| Mean (SD) | 6.4 (4.4) | 10.4 (7.5) | – | – | – | – |
| Median (range) | 5.0 (2–14) | 12.0 (0–20) | – | – | – | – |
| Appendicular CHOP-INTEND score, n | n = 8 | n = 8 | ||||
| Mean (SD) | 19.3 (11.8) | 22.6 (16.5) | ||||
| Median (range) | 19.0 (4–7) | 22.0 (2–44) | ||||
| HINE score, n | n = 8 | n = 8 | ||||
| Mean (SD) | 2.5 (3.1) | 5.5 (7.1) | – | – | – | – |
| Median (range) | 1.0 (0–9) | 1.5 (0–9) | – | – | – | – |
| HFMSE score, n | n = 2 | n = 2 | n = 7 | n = 7 | n = 8 | n = 8 |
| Mean (SD) | 2.0 (0) | 9.5 (7.8) | 9.6 (9.3) | 13.1 (14.2) | 41.6 (8.1) | 46 (9.6) |
| Median (range) | 2.0 (2) | 9.5 (4–15) | 4.0 (0–21) | 8.0 (0–30) | 41.5 (30–56) | 47.5 (31–60) |
| RULM score, n | n = 2 | n = 2 | n = 7 | n = 7 | n = 7# | n = 7# |
| Mean (SD) | 5.0 (4.2) | 10.0 (9.9) | 10.0 (8.6) | 12.6 (9.9) | 28.6 (6.2) | 30.4 (5.8) |
| Median (range) | 5.0 (2–8) | 10.0 (3–17) | 8.0 (1–22) | 11.0 (2–26) | 32 (20–36) | 35 (22–35) |
| 6MWT distance | n = 5* | n = 5* | ||||
| Mean (SD) | – | – | – | – | 100.0 (91.0) | 158 (111.8) |
| Median (range) | – | – | – | – | 87 (33.5–243) | 112 (87–335) |
T0: baseline; T1: latest assessment. #Patient 16 with SMA type 3 was 1.6 years old at screening, which was not the appropriate age to complete the RULM assessment. However, at the latest assessment, her RULM score was 31. *Patient 16 with SMA type 3 was 1.6 years old at screening and could not complete the 6MWT at assessment. However, at the latest assessment, her 6MWT distance was 200 m. On the other hand, Patient 17 and 18 with SMA type 3 were able to walk 7 meters and 6.9 meters respectively at baseline screening, but were unable to complete the 6MWT at latest assessment. Abbreviations: HFMSE = Hammersmith Functional Motor Scale–Expanded; RULM = Revised Upper Limb Module; CHOP-INTEND=The ‘Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; HINE = Hammersmith Infant Neurological Examination; 6MWT = 6-Minute Walk Test.
Spinal deformity progression
At baseline, before nusinersen treatment initiation, the mean coronal Cobb angle was 26.3° (range, 0–103°) for SMA1 patients, 45.0° (range, 0–100°) for SMA2 patients, and 6.5° (range, 0–19°) for SMA3 patients (Table 3). At the latest follow-up, the mean Cobb angle for SMA1 patients was 45.4° (range, 0–108°), for SMA2 patients was 75.6° (range 24–141°), and 19.1° (range, 0–41°) for SMA3 patients (Table 3). Therefore, the average rate of scoliosis progression was 5.2°/year for SMA1, 11.9°/year for SMA2, and 3.6°/year for SMA3 (Table 3).
Table 3
Cobb angle progression and motor score changes from baseline to latest assessment
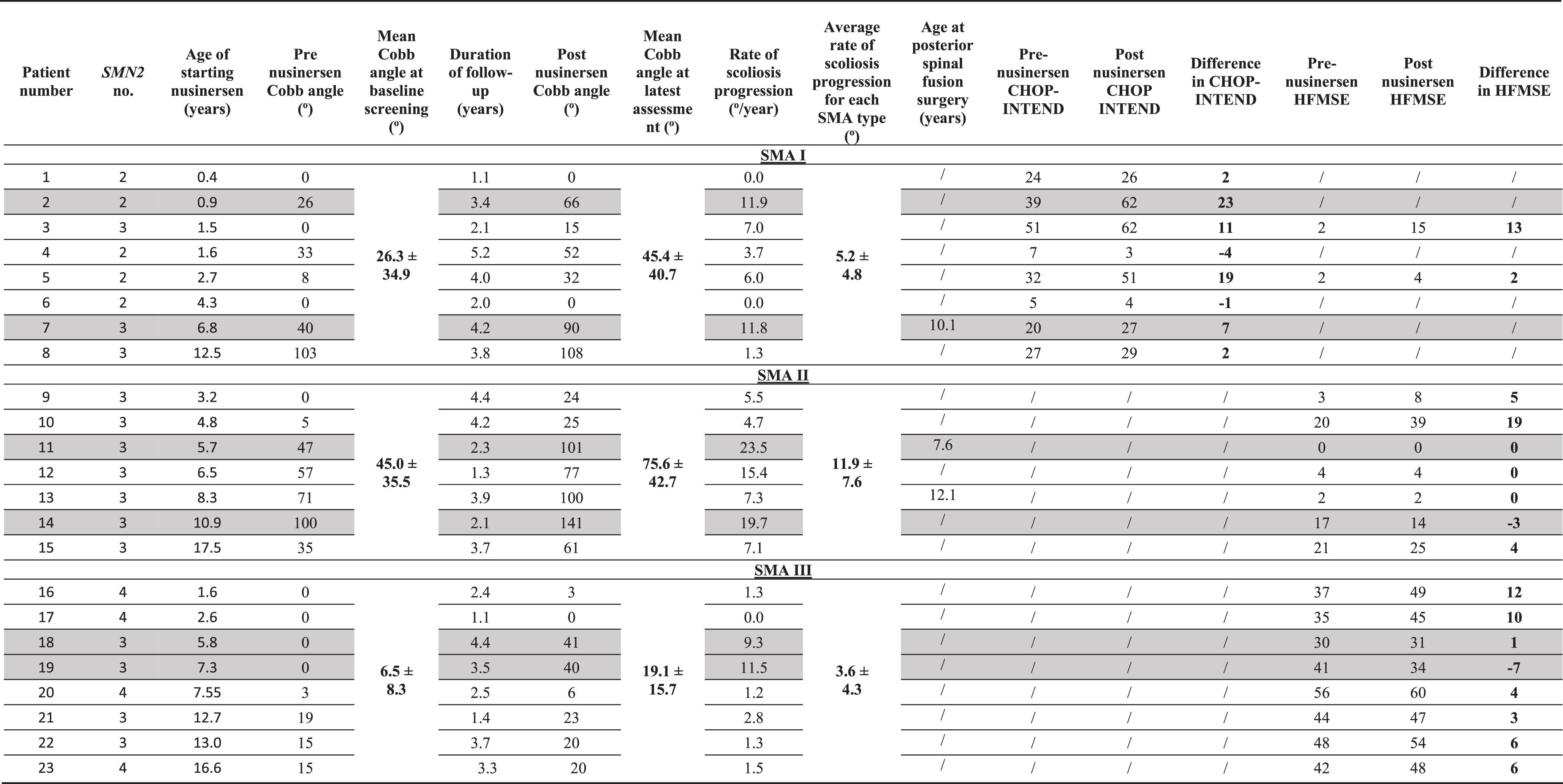 |
Scoliosis progressed in all subtypes. The average rate of scoliosis progression was fastest in SMA2 patients at 11.9°/year compared to SMA1 patients at 5.2°/year and SMA3 patients at 3.6°/year. Within each subtype, the two patients with the fastest rates of scoliosis progression are highlighted in grey. For SMA2 and SMA3, those patients with the fastest rates of progression had nusinersen initiated between 5 to <11 years old. For SMA1, the trend is less consistent with one patient progressing 11.8 degrees/year when nusinersen was initiated at 6.8 years and another patient progressing at 11.9 degrees/year when nusinersen was initiated at 0.9 years old.
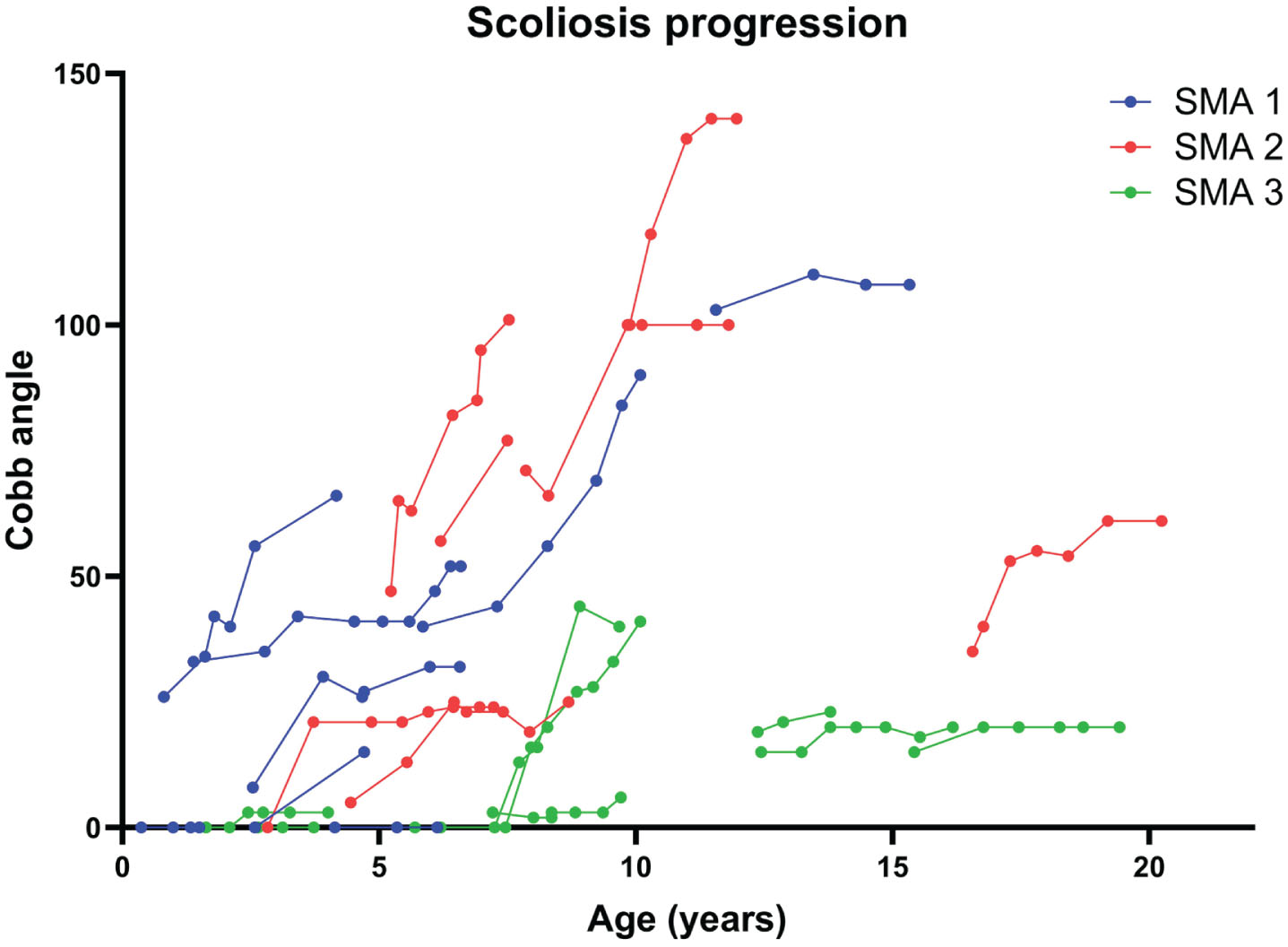
We observed that the Cobb angles in different SMA types progressed most significantly in those patients who started nusinersen between the age of 5 to 11 years (Fig. 1). We therefore performed further analyses by stratifying patients into three age groups at the time of nusinersen initiation: <5 years old, 5 to <11 years old, and ≥11 years old (Table 3). In SMA1 patients, the average rate of progression was 4.8°/year (range from 0–11.9°/year) in those who started nusinersen <5 years old (n = 6), 11.8°/year for 5 to <11 years old (n = 1), and 1.3°/year for >11 years old (n = 1). In SMA2 patients, the average rate of progression in those who started nusinersen <5 years old was 5.1°/year (n = 2), 16.5°/year (range from 7.3 to 23.5 °/year) for 5 to <11 years old (n = 4), and 7.1°/year for >11 years old (n = 1). In SMA3 patients, the average rate of progression in those who started treatment <5 years old was 0.6°/year (n = 2), 7.3°/year for 5 to <11 years old (n = 3), and 1.9°/year for >11 years old (n = 3).
Three patients (patient 7 with SMA1, patient 11 with SMA2, and patient 13 with SMA2) had posterior spinal fusion surgery for scoliosis during follow-up at ages 10.1, 7.6, and 12.1 years old, respectively.
Fig. 1
Cobb angle progression in different SMA types. Scoliosis progressed in all SMA subtypes. The fastest rates of scoliosis progression are observed in patients with SMA1 and SMA2.
Motor strength, motor function, and ambulatory status
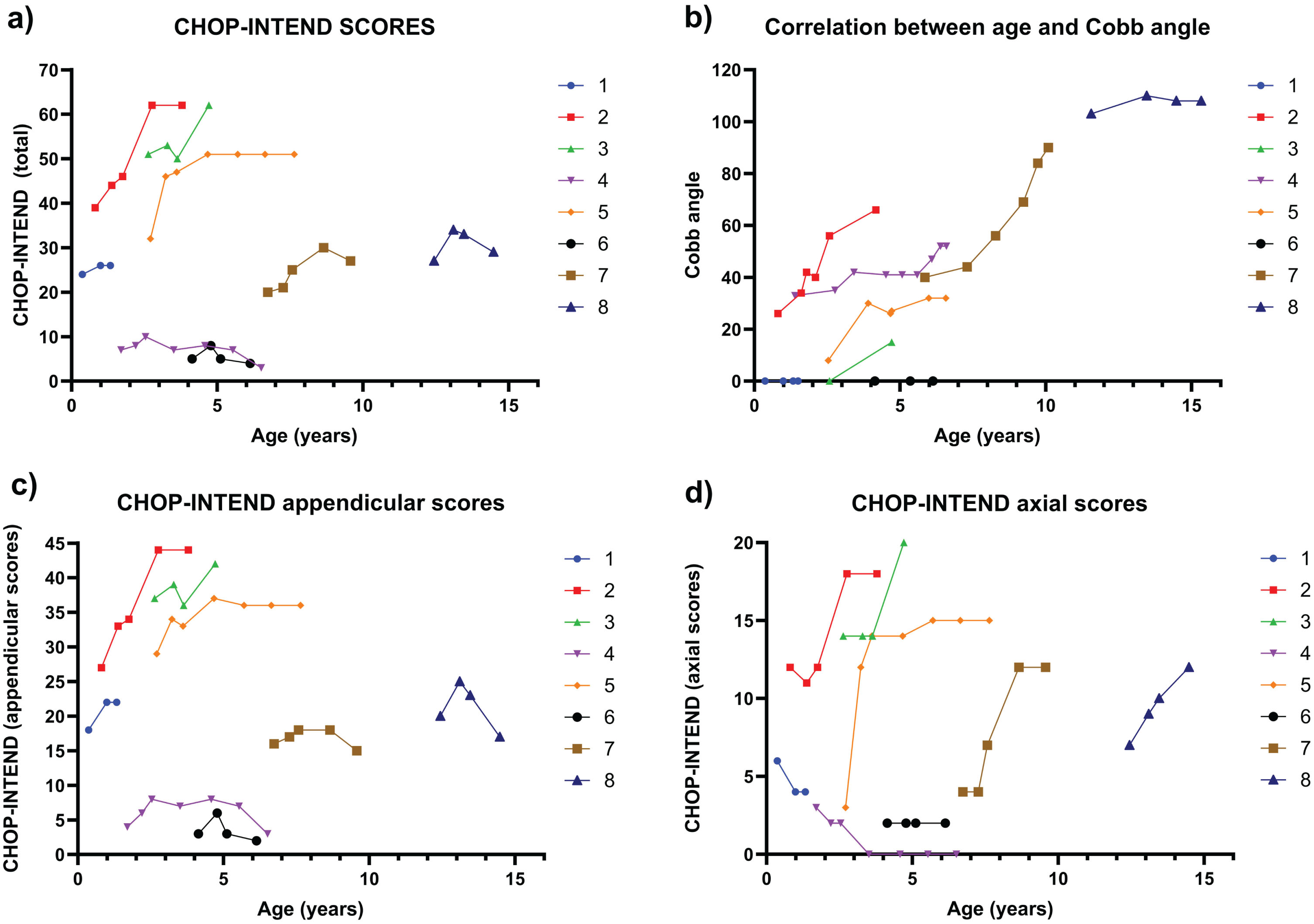
Among the eight SMA1 patients, the median CHOP-INTEND score improved from 25.5 (range 5–51) at baseline, when the median age was 2.2 years (range 0.4–12.5 years old), to 28 (range 3–62) at the latest assessment when their median age was 6.5 years (range 1.5–16.3 years old) (Table 2). Six patients (75%) achieved higher total CHOP-INTEND scores in their latest reviews compared to the baseline. The two patients who experienced decreases in CHOP-INTEND scores both had low baseline scores of 5 and 7, respectively. Further analysis of CHOP-INTEND subscores showed improvement in both median axial CHOP-INTEND score from 5 (range 2–14) to 12 (range 0–20), and the median appendicular CHOP-INTEND score, from 19 (range 3–37) to 22 (range 2–44) (Table 2). Among these 8 patients with SMA1, three patients experienced improvement in both axial and appendicular CHOP-INTEND scores (Patient 2, Patient 3, Patient 5), two patients demonstrated improvements in axial CHOP-INTEND scores but showed a decrement in appendicular CHOP-INTEND scores (Patient 7 and Patient 8), and one patient had a decrease in axial CHOP-INTEND scores and no change in appendicular scores (Patient 4). All these six patients had varying degrees of scoliosis progressions. On the other hand, two patients with low baseline CHOP-INTEND axial scores with either decrease or no change at follow-up showed no scoliosis progression (Patient 1 and Patient 6) (Fig. 2).
Fig. 2
Correlation between age in years (X-Axis) and Total, Appendicular and Axial CHOP-INTEND Scores (Y-Axis) compared with Correlation between age in years (X-Axis) and Cobb Angle (Y-Axis) progression in patients with SMA1. 2a) Correlation between the age in years (X-Axis) and the Total CHOP-INTEND Score (TChS; Total 64 Points) (YAxis), 2b) Correlation between the age in years (X-Axis) and Cobb Angle (Y-Axis), 2c) Correlation between the age in years (X-Axis) and the Appendicular CHOP-INTEND Score (ApS; total 44 Points) (Y-Axis), and 2d) Correlation between the age in years (X-Axis) and the CHOP-INTEND Axial Scores (AxS; 4, 12, 14, 15, and 16; total 20 Points) (Y-Axis).
Overall, the median HINE scores improved from 1 (range 0–9) to 1.5 (range 0–19). All patients achieved higher or the same total HINE scores at the latest assessments compared to the baseline. Regarding ambulatory status, all eight SMA1 patients were non-sitters before treatment. At the latest follow-up, two patients (25%) became independent sitters, and three (38%) became supported sitters.
For the seven SMA2 patients, their median age was 6.5 years (range 3.2–17.5 years old) at baseline and 9.0 years (range 7.6–21.2) at the latest assessment. The median HFMSE increased from 4 (range 0–21) to 8 (range 0–30), and six patient (86%) achieved higher HFMSE scores at the latest assessment. The median RULM scores increased from 8 (range 1–22) at baseline to 11 (range 2–26) at the latest assessment, and six patient (86%) achieved higher RULM scores at the latest assessment. Regarding the motor functional status, one SMA type 2 patient (14%) who was an independent sitter before treatment became a supported walker at the latest assessment.
In the group of eight SMA3 patients, the median HFMSE score improved from 41.5 (range 30–56) at baseline, with patients’ median age of 7.4 years (range 1.6–16.6 years old), to 47.5 (range 31–60) at the latest assessment with patients’ median age of 10.5 years (range 3.7–20.6 years old) (Table 2). Seven patients (88%) achieved improvements in their HFMSE scores at the latest assessment. RULM improved in five out of seven (71%) patients with reported scores, from a baseline median score of 32 (range 20–36) to 35 (range 22–35). Baseline and latest 6MWT distances were recorded in five patients. Four out of five patients recorded an improvement in the walking distances in their 6MWT after nusinersen, improving from a baseline median distance of 87 meters (range 33.5–243 meters) to 112 meters (87–355 meters) at the latest assessment. Two patients who were able to walk 6.9 meters and 7 meters respectively in the 6MWT at baseline were unable to complete 6MWT at the latest assessment.
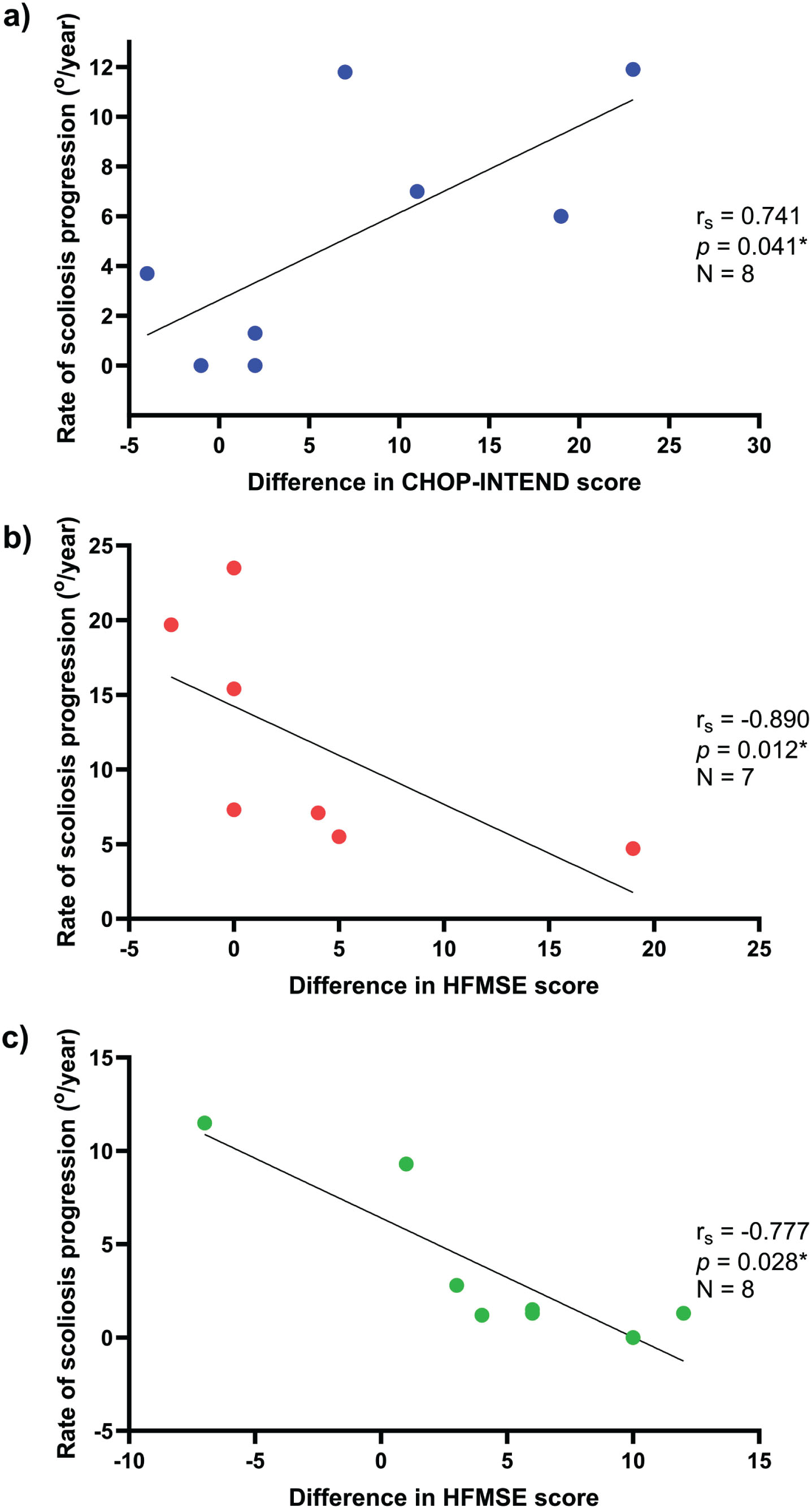
We also discovered a strong positive correlation between the increase in CHOP-INTEND score after nusinersen treatment and the rate of scoliosis progression in SMA1 patients, with a correlation coefficient of rs = 0.741 (p = 0.041) (Fig. 3). Conversely, a strong negative correlation was observed between the increase in HFMSE score after nusinersen treatment and the rate of scoliosis progression in SMA2 and SMA3 patients, with correlation coefficients of rs =−0.890 (p = 0.012) and rs =−0.777 (p = 0.028), respectively (Fig. 3).
Fig. 3
Correlation between the rate of scoliosis progression and the difference in motor scores in patients with SMA1, SMA2, and SMA3. 3a) For SMA1, there is a strong positive correlation (rs = 0.741, p = 0.041) between the increment in CHOP-INTEND scores and the rate of scoliosis progression, which suggests the more improvement in motor function in this group of children after nusinersen, the faster the scoliosis may progress. 3b) For SMA2, there is a strong negative correlation (rs =−0.890, p = 0.121) between the increase in HFMSE scores and the rate of scoliosis progression. 3c) For SMA3, there is a strong negative correlation (rs =−0.777, p = 0.028) between the gain in HFMSE scores and the rate of scoliosis progression. The findings suggest that patients with SMA2 and SMA3 who have a greater degree of motor function improvement have a slower rate of scoliosis progression.
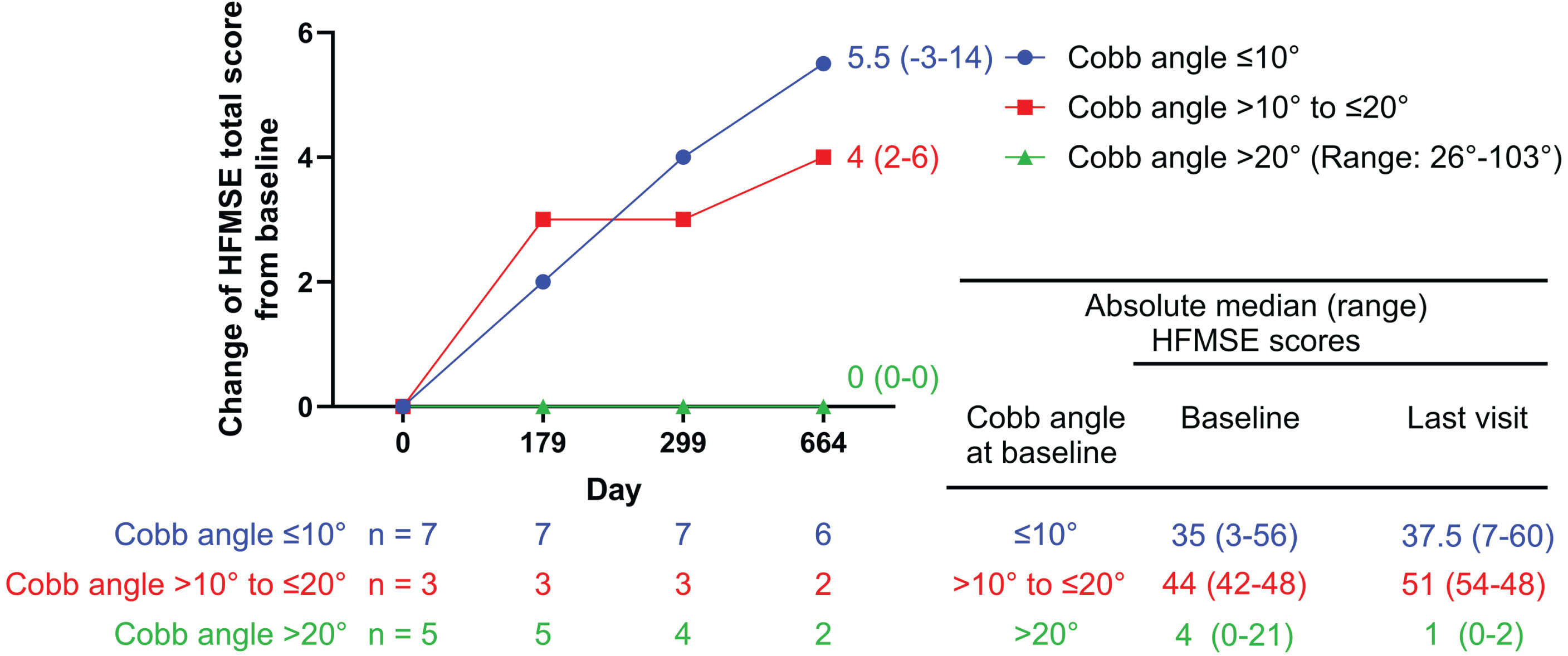
We found the baseline scoliosis severity of our SMA2 and SMA3 cohorts has a relationship with later motor function after nusinersen treatment. Those with no scoliosis (Cobb angle < 10°) or mild scoliosis (Cobb angle 10°– < 20°) have better motor response compared to those with more severe scoliosis (Cobb angle > 20°) (Fig. 4).
Fig. 4
HFMSE scores over time by Cobb angle subgroup in SMA2 and SMA3 patients. SMA2 and SMA3 patients with no/mild scoliosis (Cobb angle ≤ 10°/Cobb angle > 10 to ≤20°) showed greater improvements in HFMSE scores, compared to those with severe scoliosis (Cobb angle > 20°).
DISCUSSION
Spinal deformities are commonly associated with SMA due to the deterioration of axial muscle strength. Progressive curvatures can affect posture, truncal balance, and pulmonary function, necessitating surgical correction and spinal fusion [8, 9, 14–16]. In a previous natural history study on SMA type 2, E Mercuri et al. [17] discovered a non-linear scoliosis progression with age. The 28 SMA2 patients in the cohort initially experienced improvement in HFMSE before the age of 5. They began losing HFMSE scores with scoliosis increasing between ages 5 to 13 years, followed by a relative stabilization phase. The mean age to develop a Cobb angle >20° that required a spinal brace was 6 years 10 months; and the mean age to develop a Cobb angle >50° that required scoliosis surgery was 8 years 10 months. [17]. Coratti et al. [18] reconfirmed the progression of scoliosis over time in SMA type 2 with a larger progression rate in the age between 5 to 12 years. Over 80% of this group of patients require either a spinal brace or spinal surgery. In fact, the lifetime probability of scoliosis surgery was high for both treatment-naïve SMA2 and SMA types 1c patients up to 80%, and also in SMA3 patients up to 40% in those with loss of ambulation [11]. Our study found that despite improvements and stabilization in motor strength and functional scores after nusinersen treatment, spinal deformities progressed over time in our pediatric patients with SMA during the study period. The highest rate of scoliosis progression was noted in those who started nusinersen between ages 5 to 11 years old in SMA type 2, followed by SMA type 1 then SMA type 3. The observed trend is similar to the previous natural history studies.
Nusinersen has improved the clinical outcomes of patients with symptomatic SMA. A multi-national SMA1 study in the Asia-Pacific region showed that earlier treatment led to better clinical outcomes. Those who started nusinersen at ≤2 years old, 36.4% achieved unassisted sitting and increased median CHOP-INTEND scores by 11 points after one year of nusinersen treatment. By contrast, those who started nusinersen > 2 years of age had a median CHOP-INTEND score increase of only 6.5 points [19]. Children with late-onset SMA also experienced clinically significant improvement in motor performance after treatment [5, 7, 20, 21]. Improvements in CHOP-INTEND scores were observed in our SMA1 patients, in HFMSE and RULM in our SMA 2 and SMA3 patients, and in 6 MWT in SMA3 patients. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) is a validated measure to evaluate SMA patients’ motor function [22]. The Hammersmith Motor Functional Scale Expanded (HMFSE) scale includes 33 items with a scoring range from 0 to 66 points and has been validated to evaluate the progression of SMA types 2 and 3 [23]. The Revised Upper Limb Module (RULM) has been validated and shown to be reliable in assessing upper extremity function for SMA [24]. The 6 Minute Walk Test (MWT) has also been shown as valid and reliable as outcome measures for SMA [25].
In the Amrani et al. study [13], seven SMA1 patients who received nusinersen treatment before the age of 6 months demonstrated initial improvement in their CHOP-INTEND scores, correlating with improvement in the Appendicular Score (ApS) but not the Axial Score (AxS). All seven patients developed scoliosis in the first year of life, with Cobb angles ranging between 18° and 60°. The total CHOP-INTEND scores dropped in two patients as their Cobb angle surpassed 40°. In contrast, among our eight SMA1 patients, six experienced scoliosis progression with five showing improvement in AxS, and one exhibiting deterioration in AxS. The girl with the most rapid scoliosis progression (Patient 2, Table 3), who had 2 SMN2 copies and started nusinersen at 10 months old, improved in both AxS and ApS. After nusinersen treatment, she became an independent sitter, and developed scoliosis early required early use of spinal brace. Her scoliosis curve progressed rapidly at 11.9°/year. After 3.4 years of follow-up, her Cobb angle reached 66°, and she is planning to undergo spinal surgery with growing rods soon. Our SMA1 cohort is older compared to the Armani et al SMA1 group. Our findings suggest that other factors, such as age and achievement of sitting milestone also affect the scoliosis progression
When we examined the correlation between the rate of scoliosis progression and the increase in CHOP-INTEND score, we found a strong correlation: a greater improvement in CHOP-INTEND score after nusinersen treatment was associated with faster rates of scoliosis progression in SMA1 patients (Fig. 3). Patient 2 and Patient 7 (Table 3) with SMA1 were non-sitter before nusinersen treatment. Patient 2 achieved independent sitting and Patient 7 became a sitter with support. Both developed scoliosis with significant progression. Patient 2 is now 4 years 8 months old and awaiting spinal surgery. Patient 7 had spinal fusion at the age of 10 years. Drain JP et al. [26], reported a young SMA1 child who received Onasemnogene Abeparvovec gene therapy at 5 months of age. The child experienced rapid scoliosis progression and had magnetic growing rods inserted at 18 months of age. Another prospective observational study from Switzerland [27] involved six symptomatic SMA1 patients with 2 SMN2 copies receiving early Onasemnogene Abeparvovec (n = 4) or bridging nusinersen then Onasemnogene Abeparvovec (n = 2) before 6 months of age. All patients showed meaningful improvement in CHOP-INTEND Scores. Three of them became sitters. However, four patients developed scoliosis (60%) before the age of 2 years. Thus, scoliosis is highly prevalent among young SMA1 patients with meaningful motor gain and improved sitting performance. Their motor performance is similar to SMA2 but on the weaker side of the spectrum. A study in Spain also confirmed that among symptomatic SMA patients receiving the disease-modifying treatments, two-thirds experienced scoliosis complications [28].
On the other hand, the strong negative correlation between the rate of scoliosis progression and the increase in HFMSE score in our SMA2 and SMA3 patients suggests that greater motor function improvements after nusinersen in these 2 SMA types are associated with slower scoliosis progression. For example, one of our SMA2 patients, who became a supported walker after starting nusinersen from an independent sitter has maintained a relatively mild spinal curve of 25° at the age of 9 years old (Patient 10, Table 3). This observation implies a protective effect of nusinersen for SMA2 and SMA3 patients, as it may demonstrate that after a certain threshold of improvement in motor function where the disease is less severe initially, scoliosis progression may indeed be slowed down or halted by nusinersen. These findings correspond to Coratti et al. study [29], which suggests if disease-modifying treatments (DMTs) are initiated earlier in younger infants with type II SMA, especially in those with minimal scoliosis, scoliosis progression may be controlled.
There are several limitations to this study. Firstly, the study’s findings may be limited by the sample size, as this is a small cohort of pediatric patients with SMA receiving nusinersen treatment. Secondly, for SMA1, six children included in the study started nusinersen below the age of 5, and so the progression of scoliosis between 5 to <11 years and >11 years are still not yet known at this stage. Thirdly, our patient group had a wide age range, hence, the baseline of the scoliosis Cobb magnitude was also wide-ranging, particularly for type 1 patients. This may affect the interpretation of the average rates of scoliosis progression because a large Cobb angle is an independent risk factor for curve progression. Additionally, spinal deformities were not uniformly assessed using upright (either standing or sitting) radiographs on all patients because some patients could not sit independently and thus needed to be evaluated with supine radiographs. Nonetheless, the progression rate was monitored using the same position and so should be reflective of the true magnitude of the curve in this subgroup of patients. Lastly, our cohort did not include patients treated with nusinersen at the pre-symptomatic stage. Improvement in SMN protein production with earlier nusinersen treatment in the pre-symptomatic stage may potentially have the biggest effect on preventing scoliosis development and progression, so the findings of this study need to be interpreted in the context of symptomatic patients only. Despite these limitations, this study represents a comprehensive prospective longitudinal review of the effects of nusinersen on scoliosis progression in symptomatic SMA patients. All patients had documentation of their whole spine radiographs pre-and post-nusinersen treatment at regular intervals, and thus this represented a comprehensive study to evaluate the effects of nusinersen treatment on spinal deformities.
Our findings suggest that the highest rate of scoliosis progression was found in children with symptomatic SMA1 and SMA2 who started nusinersen treatment between ages 5 to <11. In children with SMA1, the improved axial and appendicular motor function and sitting performance may also be associated with early scoliosis development and faster rates of scoliosis progression. Although nusinersen has enhance motor control and muscle function, the increased appendicular strength might still be insufficient to provide adequate support to the appendicular skeleton. Moreover, the potential contribution of more frequent upright positioning to the rapid scoliosis progression observed in these patients should be considered. We acknowledge that there is currently no literature supporting any management recommendation, and bracing is typically recommended based on the development of radiographic Cobb angles. However, we propose considering proactive prophylactic bracing use, particularly for young SMA type 1 patients, even in the short-term following disease-modifying treatment, accompanied by thorough longitudinal evaluation for scoliosis development upon follow-up. In contrast to what is observed in SMA1 patients, improved motor function and ambulation in SMA2 and SMA3 patients may slow scoliosis progression. To further investigate scoliosis progression during the entire disease course in SMA patients under DMTs, additional studies are needed, especially for patients who initiate DMTs at a pre-symptomatic stage with longer follow-up periods. Regular surveillance of spinal alignment in patients with SMA using whole spine radiographs, irrespective of the DMTs used, with prompt referral to the orthopaedic surgeon, is recommended.
CONFLICT OF INTEREST
All the authors have nothing to disclose.
ACKNOWLEDGMENTS
We would like to thank Ms. Rachel BY Lee for her professional English Editing Service. We would also like to thank Ms. Cheng Pui Kei, our SMA Treatment Program nurse coordinator for coordinating the regular spinal radiological evaluation for our patients. Last, but not least, we must thank all the multidisciplinary teams currently taking care of our pediatric patients with SMA for the standard of care.
FUNDING
This study was funded by the grant from the donation fund entitled ‘diagnosis and therapy development of rare neurological diseases including neuromuscular diseases’ to Dr SHS Chan (200009121).
REFERENCES
[1] | Lefebvre S , Burglen L , Reboullet S , et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. (1995) ;80: :155–65. |
[2] | Lunn MR , Wang CH . Spinal muscular atrophy. Lancet. (2008) ;371: :2120–33. |
[3] | Passini MA , Bu J , Richards AM , et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med.72ra. (2011) ;3: :18. |
[4] | Finkel RS , Mercuri E , Darras BT , et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N Engl J Med. (2017) ;377: (18):1723–32. |
[5] | Mercuri E , Darras BT , Chiriboga CA , et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N Engl J Med. (2018) ;378: (7):625–35 doi: 10.1056/NEJMoa1710504. |
[6] | Pane M , Coratti G , Sansone VA , et al. Nusinersen in type 1 spinal muscular atrophy: Twelve-month real-world data. Ann Neurol. (2019) ;86: (3):443–51. doi: 10.1002/ana.25533. |
[7] | Szabó L , Gergely A , Jakus R , et al. Efficacy of nusinersen in type 1, 2 and 3 spinal muscular atrophy: Real world data from Hungarian patients. Eur J Paediatr Neurol. (2020) ;27: :37–42. doi: 10.1016/j.ejpn.2020.05.002. |
[8] | Rodillo E , Marini ML , Heckmatt JZ , Dubowitz V . Scoliosis in spinal muscular atrophy: Review of 63 cases. J Child Neurol. (1989) ;4: (2):118–23. doi: 10.1177/088307388900400208. |
[9] | Granata C , Merlini L , Magni E , et al. Spinal muscular atrophy: Natural history and orthopaedic treatment of scoliosis. Spine (Phila Pa 1976). (1989) ;14: :760–2. |
[10] | Mullender M , Blom N , De Kleuver M , et al. A Dutch guideline for the treatment of scoliosis in neuromuscular disorders. Scoliosis. (2008) ;3: :14. doi: 10.1186/1748-7161-3-14. |
[11] | Wijngaarde CA , Brink RC , de Kort FAS , et al. Natural course of scoliosis and lifetime risk of scoliosis surgery in spinal muscular atrophy. Neurology. (2019) ;93: :e149–58. |
[12] | Amrani F , Amin R , Chiang J , et al. Scoliosis in spinal muscular atrophy type 1 in the nusinersen era. Neurology. (2022) ;12: (4):279–87. doi: 10.1212/CPJ.0000000000001179. |
[13] | Zingariello CD , Brandsema J , Drum E , et al. A multidisciplinary approach to dosing nusinersen for spinal muscular atrophy. Neurol Clin Pract. (2019) ;9: :424–32. |
[14] | Aprin H , Bowen JR , MacEwen GD , Hall JE . Spine fusion in patients with spinal muscular atrophy. J Bone Joint Surg Am. (1982) ;64: :1179–87. |
[15] | Chou SH , Lin GT , Shen PC , et al. The effect of scoliosis surgery on pulmonary function in spinal muscular atrophy type II patients. Eur Spine J. (2017) ;26: :1721–31. |
[16] | Fujak A , Raab W , Schuh A , et al. Operative treatment of scoliosis in proximal spinal muscular atrophy: Results of 41 patients. Arch Orthop Trauma Surg. (2012) ;132: :1697–706. |
[17] | Mercuri E , Lucibello S , Pera MC , et al. Long-term progression in type II spinal muscular atrophy: A retrospective observational study. Neurology. (2019) ;93: (13):e1241–7. |
[18] | Coratti G , Pera MC , D’Amico A , et al. Long-term follow-up of scoliosis progression in type II SMA patients. Neuromuscul Disord. (2022) ;32: (11-12):879–85. doi: 10.1016/j.nmd.2022.11.004. |
[19] | Chan SH , Chae JH , Chien YH , et al. Nusinersen in spinal muscular atrophy type 1 from neonates to young adult: 1-year data from three Asia-Pacific regions. J Neurol Neurosurg Psychiatry. (2021) ;92: (11):1244–6. |
[20] | Dunaway Young S , Montes J , Glanzman AM , et al. ; SHINE Study GrouNusinersen treatment of children with later-onset spinal muscular atrophy and scoliosis is associated with improvements or stabilization of motor function. J Clin Med. (2023) ;12: (15):4901. |
[21] | Mendonca RH , Polido GJ , Matsui C , et al. Real-World Data from Nusinersen Treatment for Patients with Later-Onset Spinal Muscular Atrophy: A Single Center Experience. J Neuromuscul Dis. (2021) ;8: (1):101–8. doi: 10.3233/JND-200551. |
[22] | Glanzman AM , Mazzone E , Main M , et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): Test development and reliability. Neuromuscul Disord. (2010) ;20: :155–61. |
[23] | Mercuri E , Finkel R , Montes J , et al. Patterns of disease progression in type 2 and 3 SMA: Implications for clinical trials. Neuromuscul Disord. (2016) ;26: (2):126–31. doi: 10.1016/j.nmd.2015.10.006. |
[24] | Mazzone ES , Mayhew A , Montes J , et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve. (2017) ;55: (6):869–74. doi: 10.1002/mus.25430. |
[25] | Dunaway Young S , Montes J , Kramer SS , et al. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. (2016) ;54: (5):836–42. doi: 10.1002/mus.25120. |
[26] | Drain JP , Iobst CA , Chambers R , et al. Evolving surgical management for early-onset scoliosis in spinal muscular atrophy type 1 given improvements in survival. JBJS Case Connect. (2021) ;11: (1). doi: 10.2106/JBJS.CC.20.00624. |
[27] | Stettner GM , Hasselmann O , Tscherter A , Galiart E , Jacquier D , Klein A . Treatment of spinal muscular atrophy with Onasemnogene Abeparvovec in Switzerland: A prospective observational case series study. BMC Neurol. (2023) ;23: (1):88. doi: 10.1186/s12883-023-03133-6. |
[28] | de-Andrés-Beltrán B , Güeita-Rodríguez J , Palacios-Ceña D , Rodríguez-Fernández ÁL . Clinical and functional characteristics of a new phenotype of SMA type I among a national sample of Spanish children: A cross-sectional study. Children (Basel). (2023) ;10: (5):892. 10.3390/children10050892 |
[29] | Coratti G , Lenkowicz J , Pera MC , et al. Early treatment of type II SMA slows rate of progression of scoliosis. J Neurol Neurosurg Psychiatry. 2023. doi: 10.1136/jnnp-2023-332084. |




