Symptomatic intracranial hypertension in an adult patient with spinal muscular atrophy and arachnoid cysts receiving nusinersen
Abstract
In patients with spinal muscular atrophy (SMA) headache after intrathecal administration of nusinersen is usually attributed to post-lumbar puncture syndrome. However, lumbar puncture opening pressure (LOP) has also been reported to be increased in children with SMA, both before and after treatment with nusinersen, although symptoms associated with increased LOP were not observed. We report to our knowledge the first case of symptomatic intracranial hypertension in an adult SMA patient. This 21-year-old man suffered from headache and vomiting followed by visual disturbances after the 12th injection of nusinersen. Bilateral papilledema was recognized ophthalmologically. MRI of the head showed signs of intracranial hypertension and additionally arachnoid cysts but not hydrocephalus. Symptoms resolved after 8 weeks of treatment with repeated lumbar punctures and acetazolamide. This case raises the possibility of intracranial hypertension as a complication of nusinersen therapy although arachnoid cysts represent another risk factor for intracranial hypertension. We recommend that patients suffering from headache after nusinersen injections should not only be questioned and examined for symptoms suggestive of post-lumbar puncture syndrome, but also intracranial hypertension.
INTRODUCTION
Spinal Muscular Atrophy (SMA) is an autosomal recessive motor neuron disease caused by mutations in SMN1, coding for the survival motor neuron (SMN) protein [1]. SMN deficiency leads to degeneration of spinal cord motor neurons and subsequently to muscle weakness and atrophy with a high variability of disease severity and age of symptom onset. In recent years, therapeutic options, namely nusinersen, risdiplam, and onasemnogene abeparvovec have enabled disease-modifying treatment of patients with SMA [2].
Nusinersen was the first of these drugs approved by the European Medicines Agency (EMA) in June 2017 [3]. Nusinersen is an antisense oligonucleotide (ASO) modifying the splicing of SMN2-RNA which leads to an increase in functional SMN protein. It does not cross the blood brain barrier and therefore must be injected intrathecally. Headache as the most common side effect of nusinersen treatment has largely been attributed to post-lumbar puncture syndrome [4]. A small number of hydrocephalus cases among patients treated with nusinersen have been reported by the company. However, details were not published and it remained unclear whether hydrocephalus was a side effect [5]. Since then, a single case report of a 7-year old child treated with nusinersen showing hydrocephalus with increased lumbar punction opening pressure and optic disc edema has been published [6].
There is a higher incidence of hydrocephalus in patients with SMA compared to controls [7]. Lumbar puncture opening pressure seems to be increased in a relatively large subgroup of SMA patients. One study found LOP>28 cm H2O in 12/34 patients (35.3%) with a maximum LOP of 50cmH2O in patients aged 0.7–20 years receiving intrathecal nusinersen (normal range 10–25cmH2O). However, an increase in LOP was also noted in 2/7 patients before treatment with nusinersen and no symptoms of intracranial hypertension were observed [8]. Hydrocephalus itself is not a sign of idiopathic intracranial hypertension [9].
We report on a patient with symptomatic intracranial hypertension following nusinersen treatment.
CASE REPORT
The 21-year-old male (160cm, 80kg) first showed symptoms of delayed motor development at an age of 6 months. He was able to sit without support but soon lost the ability. He was never able to stand or walk unassisted. At the age of 18 months the diagnosis of 5q-associated SMA was genetically confirmed. The patient has a homozygous deletion of exons 7 and 8 of the SMN1 gene and 3 copies of the SMN2 gene. In May 2019 the patient first presented at our outpatient department for evaluation of treatment options. At that point the patient displayed a severe flaccid tetraparesis. He was mobile with an electric wheelchair which he was able to control with a joystick and could operate touchscreens with his fingers. The patient did not experience problems with speech or swallowing but was on non-invasive ventilation during the night since 2007. Due to severe scoliosis he was wearing a corset when sitting, no spinal surgery had been performed. Preceding the presentation in our outpatient ward the patient had experienced slow but continuous worsening of strength of his hands and fingers with increasing functional impact. He was a second-year aerospace engineering student and had no history of recurrent headaches or visual disturbances. There was no significant weight change in the last 8 months, but the patient gained 10kg of weight between May 2021 and February 2022.
Because of severe scoliosis therapy with CT-guided intrathecal nusinersen was initiated. Between September 2019 and October 2022, the patient received a total of 12 doses of nusinersen without sedation. Initially, the patient reported minor improvement of strength of his left hand which made it easier for him to operate touchscreens. The symptoms remained constant afterwards. After the fourth administration the patient reported minor headaches which lasted a few hours and ceased spontaneously. Following the 11th administration, the patient experienced headache which lasted for a day followed by lumbar pain which lasted for one week, both also ceased spontaneously. No other adverse events occurred.
Fig. 1
Multicolor imaging (color figure available in the electronic version) showing papilledema with hemorrhage and optical coherence tomography (OCT) showing papilledema with secondary macular edema. Written permission to publish these images was granted by the patient.
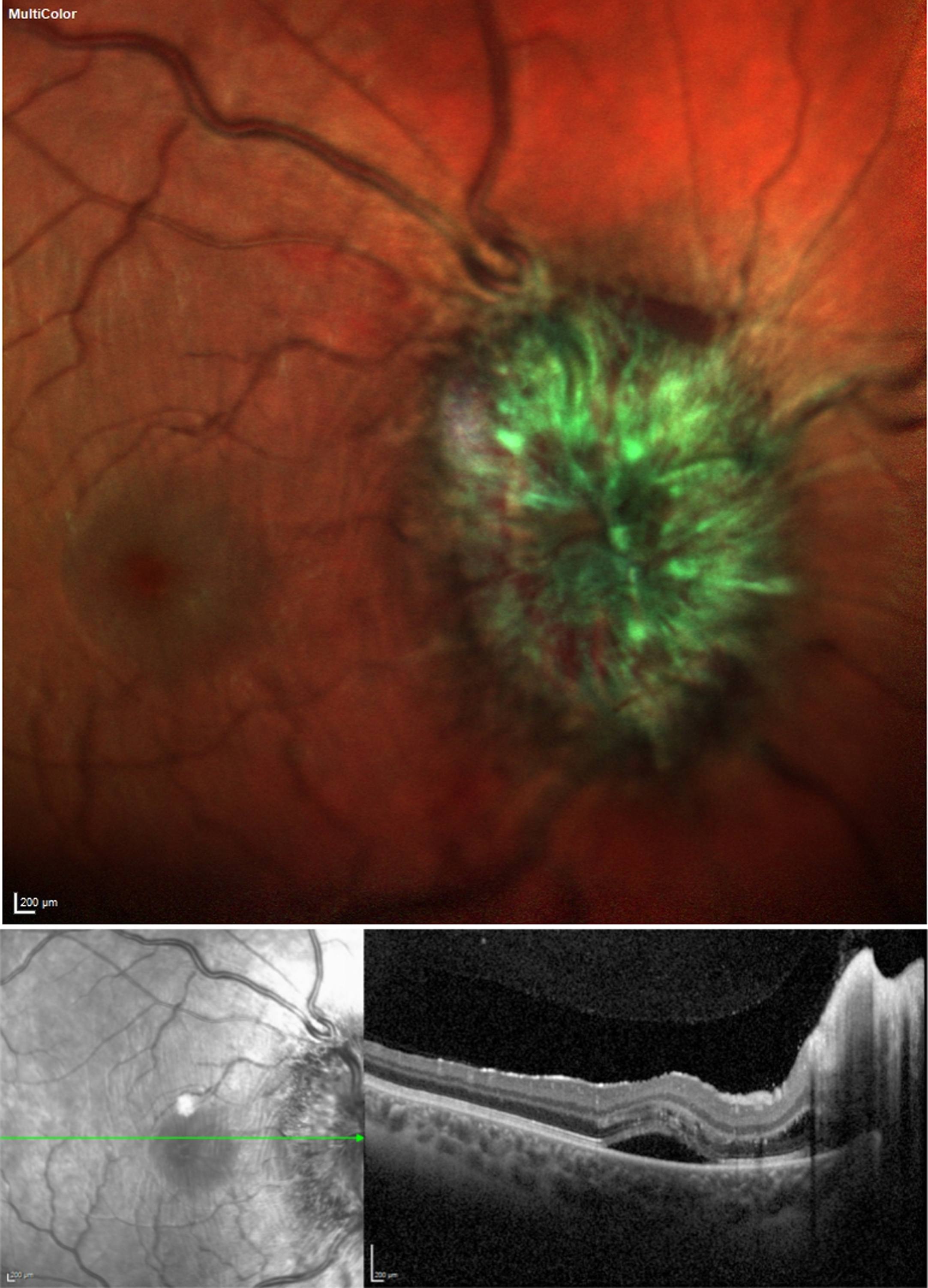
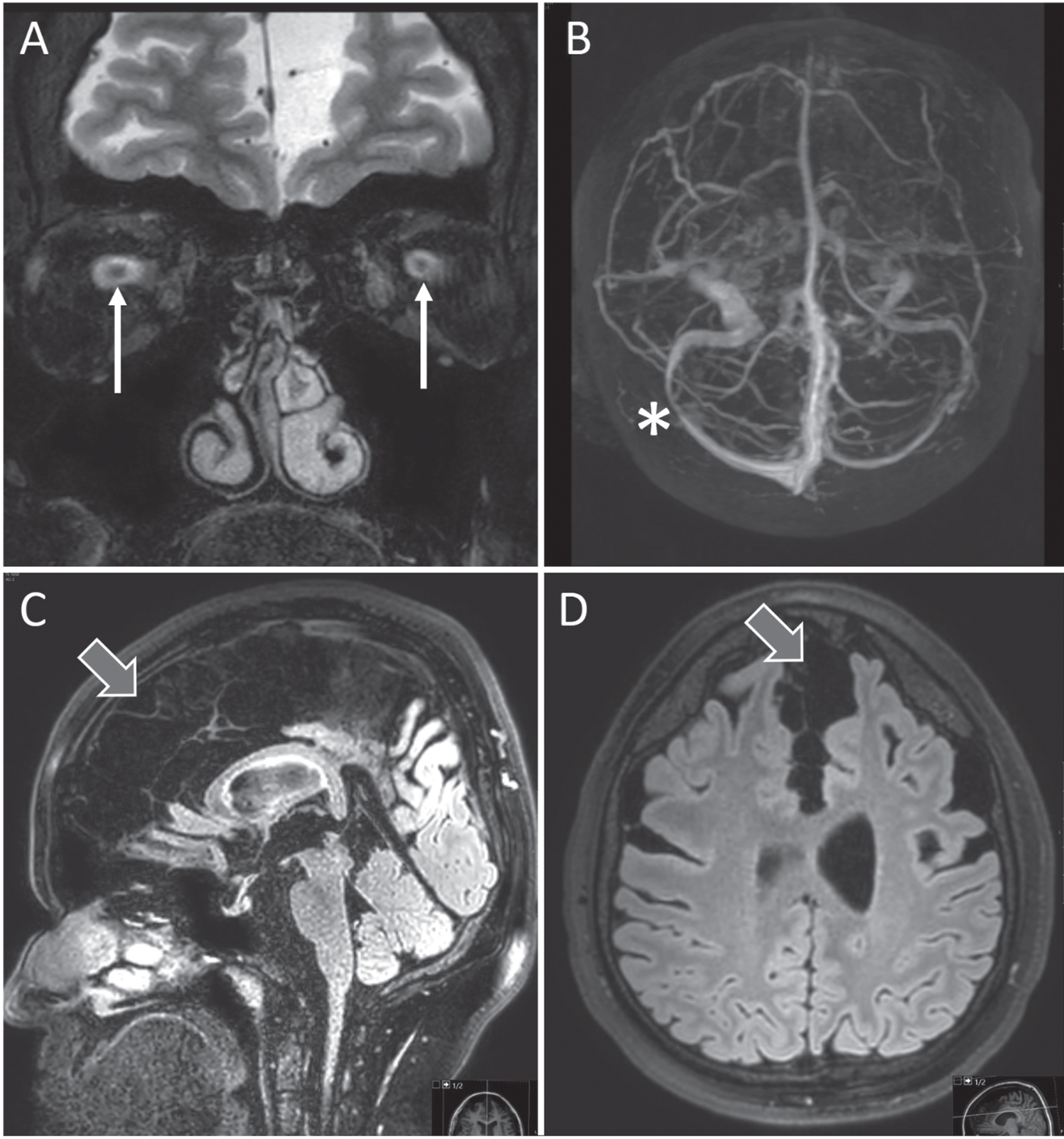
The day after the 12th administration of Nusinersen the patient experienced position independent headache and over the following days developed nausea and repeated vomiting. He presented at our emergency department. He had no fever, did not display clinical signs of meningitis or encephalitis and had only mildly elevated CRP (3.1mg/dl) and leukocytes (9.64G/l). A CT scan with CT angiography of the cerebral venous system did not show signs of cerebral venous thrombosis or intracranial hemorrhage. After administration of intravenous fluids and analgesics the patient reported significant improvement and was discharged. Already at discharge the patient noticed unspecific visual disturbances which he did not find noteworthy at the time. In the following week, however, he noticed an increasingly worsening vision with bilateral central scotoma which prompted him to present at our ophthalmology department 3 weeks after the 12th injection of nusinersen where severe bilateral papilledema with hemorrhage and secondary macular edema was diagnosed (Fig. 1). Visual acuity was markedly reduced (decimal, 0.3 right eye, 0.5 left eye). He received an MRI of the head for suspected intracranial hypertension which showed bilateral stenosis of the transverse sinuses, prominent optic discs, and loss of pituitary volume but again no signs of cerebral venous thrombosis or hemorrhage. The ventricular width was normal without signs of hydrocephalus. There were extensive, most likely congenital, bilateral arachnoid cysts with moderate space-occupying effects (Fig. 2).
Fig. 2
MRI showing classic signs of intracranial hypertension with prominent optic discs (arrows in A), sinus stenosis (asterisk in B) and flattening of pituitary gland (C). Note extensive subarachnoid cysts with local space-occupying appearance (block arrows in C and D). Written consent to publish these images was obtained from the patient.
A CT-guided lumbar puncture showed an elevated opening pressure of 47cmH2O and 40ml of CSF were removed. Cell count was 7/μl, glucose 72mg/dl and lactate 2.02 mmol/l, fluorescence-activated cell sorting (FACS) and levels of CSF protein were normal. The patient was started on acetazolamide with a gradual increase of the dose up to 2×750mg daily which was tolerated well. Subsequent lumbar punctures showed a gradual decrease of opening pressure. CSF cell count was 6/μl three weeks after the first lumbar puncture with slight elevation of CSF protein. Headache had already resolved 10 days after injection of nusinersen and visual acuity and papilledema were significantly improved after two months (Table 1, Fig. 1).
Table 1
Course of clinical symptoms, LOP, CSF parameters and ophthalmological examination. Acetazolamide was started on day 19. (IH –intracranial hypertension, LOP –lumbar puncture opening pressure, CSF –cerebrospinal fluid, OCT - optical coherence tomography)
| Nusinersen injection (day 0) | Day 7 | Day 19 | Day 26 | Day 33 | Day 40 | Day 61 | |
| Clinical Symptoms associated with IH | none | Headache, slight visual disturbances | Central scotoma | Central scotoma | Central scotoma | Central scotoma | none |
| OCT | NA | NA | Bilateral papilledema | Constant papilledema | Constant Papilledema | Papilledema improved | Minimal papilledema |
| Visual acuity right/left (decimal; Snellen) | NA | NA | 0.3/0.5; 20/63 / 20/40 | 0.4/0.8; 20/50 / 20/25 | 0.4/0.7; 20/50 / 20/30 | 1.0/0.9 20/20 / 20/22 | 1.0/0.9 20/20 / 20/22 |
| LOP (cmH2O) | – | – | 47 | 42 | 33 | 23 | – |
| CSF removal (ml) | 5 (before injection of nusinersen) | – | 40 | 30 | 25 | 20 | – |
| CSF cell count (cells/μl) | 2 | – | 7 | – | – | 6 | – |
| CSF Albumin (mg/l) | – | – | 185.0 | – | – | 515.0 | –- |
| CSF IgG (mg/l) | – | – | 34.4 | – | – | 91.0 | – |
| CSF IgA (mg/l) | – | – | 3.7 | – | – | 10.8 | – |
| CSF IgM (mg/l) | – | – | 0.4 | – | – | 1.2 | – |
3DISCUSSION
Our patients presented with typical symptoms and signs of intracranial hypertension. We cannot rule out the possibility of idiopathic intracranial hypertension. However, the observation of increased LOP in children with SMA treated with nusinersen suggests a potential association with treatment.
Repeated lumbar punctures are not usually associated with the development of increased intracranial pressure and there was no sign of causes secondary to the lumbar puncture such as infection, hemorrhage or sinus venous thrombosis in our patient. A correlation between LOP and sedation has been noted in the mentioned cohort [8]. Our patient did not receive general anesthesia or sedation for administration. It was further hypothesized that natural variability in children compared to adults and in the compliance of the ventricular system to small volume changes may contribute to increased LOP. However, our patient was adult and it seems unlikely that a primarily low tolerance to small volume changes in the ventricular system of our patient is the only reason for prolonged symptomatic intracranial hypertension. Also, prior to injecting nusinersen with a volume of 5ml we routinely remove 5ml of CSF. Nusinersen, as an ASO, has the potential for immunogenicity but no significant side effects were reported in patients in whom anti-drug antibodies (ADA) were detected [4]. It is conceivable that an immunogenic reaction may hamper CSF circulation in patients with ADA. The ADA status of our patient is not known. Analysis of CSF did not show signs of a severe intrathecal inflammatory response. This is in line with a recent study where 72 children were reported who received a total of 934 injections. One patient developed aseptic meningitis one day after his first injection but fully recovered and continued treatment with nusinersen but the patient was not described in more detail [10]. Studies looking at routine CSF parameters of patients with SMA receiving nusinersen have consistently shown a mild increase in CSF protein and CSF/serum quotients of albumin without further signs of inflammation. This has been discussed to be the result of either nusinersen treatment or repeated lumbar punctures [11–13]. In rats, using multimodal imaging and radioactively and fluorescently labelled ASO, it was shown that after intrathecal injection they strongly associate with the meninges and are subsequently transported to lymph nodes [14]. The exact mechanisms of CSF elimination of ASO in humans remain elusive and there’s also no data on how repeated injection of intrathecal ASO may have an influence on clearing mechanisms and whether impaired ASO clearing may influence CSF circulation.
CT and MRI scans showed extensive, most likely congenital, bilateral arachnoid cysts with moderate space-occupying effects in our patient. An association between arachnoid cysts and SMA has not been reported. However, arachnoid cysts have been associated with increased intracranial pressure documented by increased LOP, even requiring surgical intervention in few children [15, 16]. While a disequilibrium in CSF circulation is being postulated, the exact mechanism is not well understood [17]. Underlying pathophysiology is also not well understood in idiopathic intracranial hypertension. Not only circulation failure of cerebrospinal fluid but also sinus venous obstruction are being postulated [18].
The cause for intracranial hypertension in our patient receiving intrathecal nusinersen cannot be elucidated clearly. The arachnoid cysts may contribute to a higher susceptibility to develop increased intracranial pressure in our patient. However, the temporal association between nusinersen treatment and symptom onset makes it seem unlikely to be the only causal factor and the treatment potentially contributed to the development of symptoms. Considering the borderline increase in cell count an inflammatory response to intrathecal ASO hampering CSF circulation may have played a role, however, the increase in CSF protein was only apparent 40 days after nusinersen administration. Standard treatment for intracranial hypertension using repeated lumbar puncture with CSF removal and oral acetazolamide led to significant improvement of vision in our patient. After consultation with the patient, he currently refrained from any disease-modifying drug treatment including orally administered risdiplam but will continue regular follow-up examinations every four months.
In conclusion, our case raises the possibility of intracranial hypertension as a complication of nusinersen therapy. However, the arachnoidal cysts represent another risk factor for intracranial hypertension. Possibly the combination of both was relevant. We recommend that patients who receive nusinersen intrathecally should be questioned and examined for symptoms related to intracranial hypertension, even after long treatment. Repeated intrathecal administration of nusinersen may potentially increase the risk to develop symptomatic intracranial hypertension. This might be of special relevance in patients exhibiting post-procedural headache which may be casually attributed to post-lumbar puncture syndrome. Systematic observations in patients receiving ASO in general and nusinersen in particular are necessitated to elucidate a potential association between administration and symptomatic intracranial hypertension. A better understanding of ASO effects in the human central nervous system is needed to be able to grasp the underlying mechanisms.
ACKNOWLEDGMENTS INCLUDING SOURCES OF SUPPORT
The authors would like to thank the patient for granting permission to publish his case and Dr. Isabell Cordts and Dr. Daria Loos for critically reviewing the manuscript.
CONFLICT OF INTEREST
M.D. received personal fees as speaker/consultant from Biogen and Roche. G.M., M.G., L.S., C.M., and M.M. have no conflict of interest to report.
REFERENCES
[1] | Lefebvre S , Bürglen L , Reboullet S , Clermont O , Burlet P , Viollet L , et al. Identification and characterization of a spinal muscular atrophy-determining gene, Cell. (1995) ;80: (1):155–65. |
[2] | Mercuri E , Pera MC , Scoto M , Finkel R , Muntoni F . Spinal muscular atrophy —insights and challenges in the treatment era, Nat Rev Neurol. (2020) ;16: (12):706–15. |
[3] | European Medicines Agency: EMA/736370/2017 -EPAR summary for the public - Spinraza https://www.ema.europa.eu/en/documents/overview/spinraza-epar-summary-publicen.pdfDecember 2022. |
[4] | Hoy SM . Nusinersen: A Review in 5q Spinal Muscular Atrophy, CNS Drugs. (2021) ;35: (12):1317–28. |
[5] | Biogen Idec SPZ-IRL-Spinraza (nusinersen): communicating hydrocephalus not related to meningitis orbleeding reported https://www.hpra.ie/docs/default-source/default-document-library/important-safety-information—spinraza-(nusinersen).pdf December 2022. |
[6] | Falsaperla R , Wenzel A , Raudino G , Sframeli M , Gagliano C , Mazzeo A , et al. Intrathecal Administration of Nusinersen in Patients with SMA Too Little is Known, Neurol Case Re. (2019) ;2: (2):1012. |
[7] | Viscidi E , Wang N , Juneja M , Bhan I , Prada C , James D , et al. The incidence of hydrocephalus among patients with and without spinal muscular atrophy (SMA): Results from a US electronic health records study, Orphanet J Rare Dis. (2021) ;16: (1):207. |
[8] | Becker LL , Weiß C , Tietze A , Martiny V , Kaindl AM . Lumbar Puncture Opening Pressure in Patients with Spinal Muscular Atrophy, Neuropediatrics. (2021) ;52: (3):219–23. |
[9] | Wang Z , Yang L , Djurić PM , Egnor MR . Why don’t ventricles dilate in pseudotumor cerebri? A circuit model ofthe cerebral windkessel, J Neurosurg Pediatr. (2022) ;29: (6):719–26. |
[10] | Scheijmans FEV , Cuppen I , van Eijk RPA , Wijngaarde CA , Schoenmakers MAGC , van der Woude DR , et al. Population-based assessment of nusinersen efficacy in children with spinal muscular atrophy: a 3-year follow-up study, Brain Commun.fcac. (2022) ;4: (6):269. |
[11] | Wurster CD , Koch JC , Cordts I , Dreyhaupt J , Otto M , Uzelac Z , et al. Routine Cerebrospinal Fluid (CSF) Parameters in Patients With Spinal Muscular Atrophy (SMA) Treated With Nusinersen, Front Neurol. (2019) ;10: :1179. |
[12] | Müschen LH , Osmanovic A , Binz C , Jendretzky KF , Ranxha G , Bronzlik P , et al. Cerebrospinal Fluid Parameters in Antisense Oligonucleotide-Treated Adult 5q-Spinal Muscular Atrophy Patients, Brain Sci. (2021) ;11: (3):296. |
[13] | Orbach R , Sagi L , Sadot E , Tokatly Latzer I , Shtamler A , Zisberg T , et al. Cerebrospinal fluid characteristics of patients treated with intrathecal nusinersen for spinal muscular atrophy, Muscle Nerve. (2022) ;66: (6):762–6. |
[14] | Mazur C , Powers B , Zasadny K , Sullivan JM , Dimant H , Kamme F , et al. Brain pharmacology of intrathecal antisense oligonucleotides revealed through multimodal imaging, JCI Insight. (2019) ;4: (20):e129240. |
[15] | Al-Holou WN , Yew AY , Boomsaad ZE , Garton HJL , Muraszko KM , Maher CO . Prevalence and natural history of arachnoid cysts in children: Clinical article, J Neurosurg Pediatr. (2010) ;5: (6):578–85. |
[16] | Prasad S , Avery RA , de Alba Campomanes A , Sutton LN , Liu GT . Symptomatic Increased Intracranial Pressure Due toArachnoid Cysts, Pediatr Neurol. (2011) ;44: (5):377–80. |
[17] | Houlihan LM , Marks C . Cerebrospinal fluid hydrodynamics in arachnoid cyst patients with persistent idiopathic intracranial hypertension: A case series and review, Surg Neurol Int. (2020) ;11: :237. |
[18] | Mollan SP , Davies B , Silver NC , Shaw S , Mallucci CL , Wakerley BR , et al. Idiopathic intracranial hypertension: consensus guidelines on management, J Neurol Neurosurg Psychiatry. (2018) ;89: (10):1088–100. |



