MYH2-related Myopathy: Expanding the Clinical Spectrum of Chronic Progressive External Ophthalmoplegia (CPEO)
Abstract
Chronic progressive external ophthalmoplegia (CPEO) is symptom complex with progressive ptosis and restricted ocular motility without diplopia. MYH2 myopathy is rare disorder presenting with CPEO and muscle weakness. We report two Indian patients of MYH2 myopathy with unique features. Patient-1 presented with early adult-onset esophageal reflux followed by, proximal lower limb weakness, proptosis, CPEO without ptosis. He had elevated creatine kinase along with characteristic muscle MRI findings of prominent semitendinosus and medial gastrocnemius involvement. Patient -2 presented with early adult onset CPEO without limb weakness. His creatine kinase was normal. Both the patients had novel MYH2 mutations: a homozygous 5’splice variation in intron 4 (c.348 + 2dup) in patient 1 and homozygous single base pair deletion in exon 32 (p. Ala1480ProfsTer11) in patient 2. Unique features noted include adult onset, isolated CPEO, proptosis, esophageal reflux disease and absence of skeletal abnormalities. MYH2 myopathy has to be considered in adult patients with CPEO.
INTRODUCTION
Chronic progressive external ophthalmoplegia (CPEO) is a clinical syndrome characterized by symmetric bilateral ptosis with restricted ocular motility. CPEO is seen in various neuromuscular conditions such as mitochondrial disorders, centronuclear myopathies, congenital myasthenic syndromes and oculopharyngeal muscular dystrophy. MYH2 (myosin heavy chain-2) related myopathies are a group of congenital myopathies which present with ptosis and external ophthalmoplegia [1]. They can present as autosomal dominant (AD) mutation with congenital joint contractures, CPEO and proximal muscle weakness [2]. Autosomal recessive (AR) mutations in MYH2 also present with external ophthalmoplegia with proximal weakness but is largely mild and non-progressive [3].
Here we present two interesting cases of homozygous MYH2 myopathy with CPEO and progressive muscle weakness.
This was a retrospective review on two unrelated patients with genetically proven MYH2 myopathy from a neuromuscular division of a quaternary Neurology centre in India. Detailed clinical, laboratory and electrophysiological data was recorded and is being presented. The two patients provided written informed consent for publication of their case details, the investigation results and clinical pictures with unmasked faces. Patient-1 underwent MRI muscle at MRI 1.5 T [Aera; Siemens Healthcare; Erlangen, Germany with body coil at 3 stations - Hip to Upper Thigh, Upper Thigh to Knee, Leg, FOV (Field Of View). Muscle analysis involved visual assessment for fatty infiltration and muscle volume loss [4].
CASE REPORT
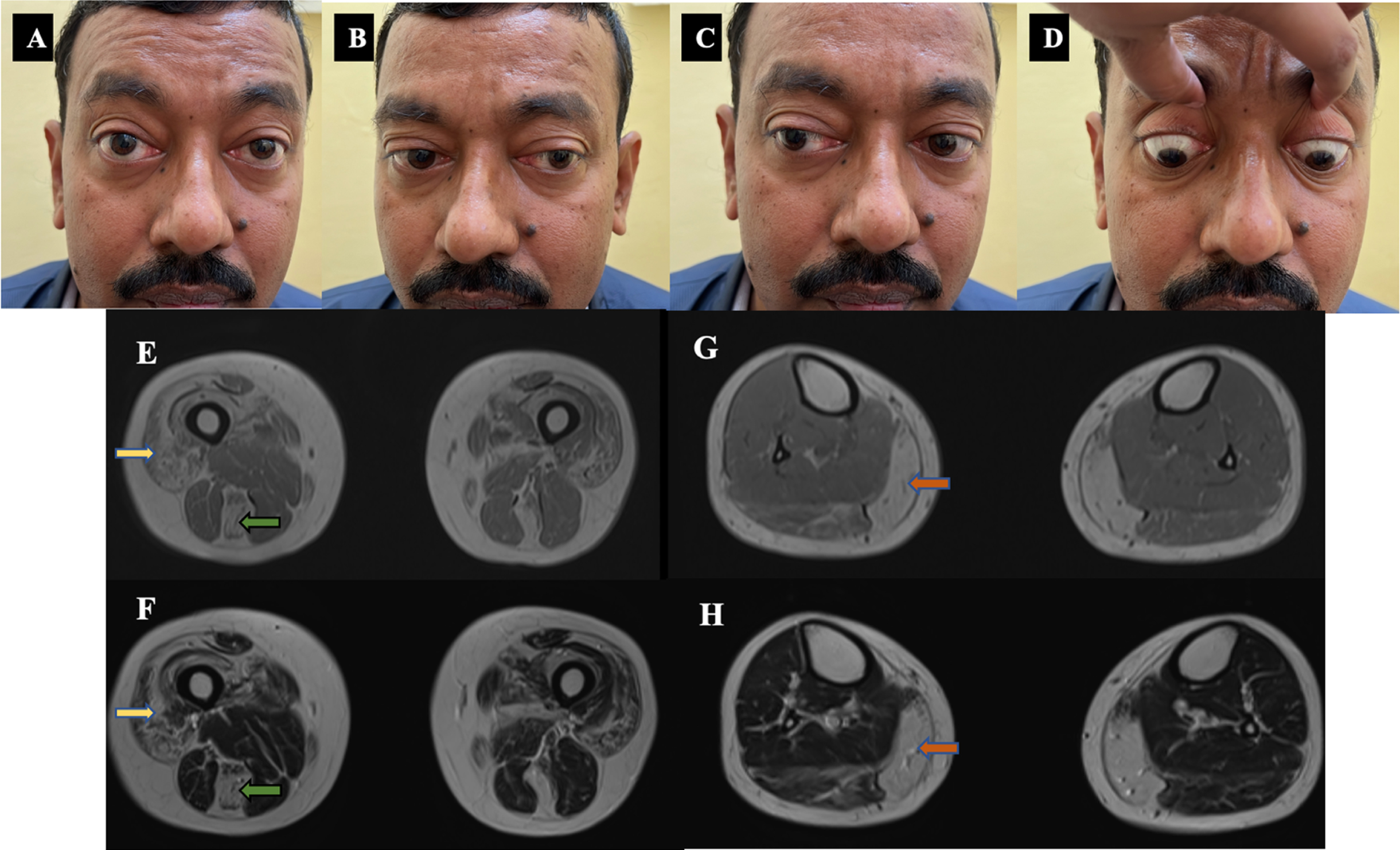
Patient-1: A 47-year-old-man born of non-consanguineous parentage evaluated during 2021, presented with 20 years history of postprandial esophageal reflux followed by 12 years history of difficulty in rising from floor and climbing stairs which was slowly progressive. He had no symptoms of myalgia, easy fatigability or oculo-bulbar symptoms. There was no family history. Examination showed proptosis of 18 mm with restricted eye abduction and elevation without ptosis. (Fig. 1A–D) Facial weakness was noted. Power in the upper limbs was normal with hip weakness of Medical research council (MRC) grade 4. All tendon reflexes were hyperactive. Based on gastrointestinal symptoms with pelvic girdle weakness and ophthalmoparesis a possibility of mitochondrial myopathy was considered. Investigations showed elevated serum creatine kinase of 808 U/L with normal thyroid function test. Repetitive nerve stimulation of orbicularis oculi and trapezius did not show a decremental response. Magnetic resonance imaging of muscles showed prominent fatty infiltration of gluteus maximus, quadriceps and semitendinosus (Fig. 1E–H). Muscle biopsy from quadriceps showed fibre type grouping with secondary myopathic changes.
Fig. 1
Clinical images and MRI muscle of patient –1. A, B, C, D: Clinical images - Ocular motility restriction - Abduction and upgaze restriction with proptosis. E, F, G, H: Muscle MRI images - E- T1 W, F-T2 W images showing quadriceps (yellow arrow), semitendinosus (green arrow) fatty infiltration. G-T1 W, H-T2 W images showing predominant medial gastrocnemius (orange arrow) fatty infiltration with sparing of anterior leg muscles.
Patient-2: A 48-year-old man, born of non-consanguineous parentage, was evaluated during 2022. He presented with 12 years of bilateral drooping of eyelids which was gradually progressive. There was minimal diurnal variation. He did not have diplopia. Fatigable weakness of proximal upper and lower limbs was present. He had no symptoms of dysarthria or dysphagia. There was no positive family history. Examination showed asymmetric ptosis with severe restriction of eye movements in all directions (Fig. 2) and mild facial weakness. Muscle strength was normal. Hyperactive tendon reflexes were noted. Based on the presence of chronic external ophthalmoparesis without diplopia with history of limb fatigability possibilities of mitochondrial chronic progressive external ophthalmoparesis (CPEO) and congenital myasthenic syndromes were considered. Investigations showed serum creatine kinase of 356 U/L. Repetitive nerve stimulation of orbicularis oculi and trapezius did not show significant decrement. Muscle biopsy from quadriceps showed fibre size variation with internalization of nuclei.
Fig. 2
A, B, C, D: Clinical images of patient-2. A –Asymmetric ptosis. B, C- Restricted ocular motility. D,E –Facial weakness.

GENETIC RESULTS
Clinical exome sequencing was performed using custom capture kit in both patients revealing rare homozygous MYH2 variants: In patient 1, a novel single nucleotide duplication c.348 + 2dup (hg38; chr17: g.10547473dupA) at 5’ splice site in intron 4 was identified which was predicted as splice-altering (0.5 score) by splice AI tool [5]. In patient 2, a single nucleotide deletion c.4438del (hg38; chr17: g.10525550del) resulting in frameshift (p. Ala1480ProfsTer11) was identified which was previously reported as pathogenic in clinvar [6]. Both variants are absent in population databases including gnomAD and GenomeAsia 100K as well as internal databases [7]. Based on this evidence both variants are considered as putative disease-causing although further functional validation and segregation in unaffected family members is not performed. Mitochondrial genome sequencing was negative in both patients.
DISCUSSION
Myosinopathies are a group of ultra-rare inherited muscle disorders with varied phenotypic features involving mutation in myosin heavy chain of skeletal muscle (MYH) genes in chromosome 17 [8]. MYH2 is one of the three isoforms of myosin heavy chains expressed mainly in fast twitch 2A and 2B fibres. AD-MYH2 myopathy usually presents with transient congenital arthrogryposis followed by late onset progressive proximal limb weakness and external ophthalmoplegia. It has characteristic muscle biopsy features of reduced type 2A fibres and rimmed vacuoles [9]. AR-MYH2 myopathy is characterised by mild early onset non-progressive proximo-distal weakness of limbs with external ophthalmoparesis with biopsy features of complete absence of type 2A fibres and non-specific myopathic changes [8]. Our patients presented with AR-MYH2 myopathy with unique features in contrast to previously described phenotypic features. Both the patients had adult onset of symptoms. Isolated fatigable CPEO was noted in patient -2 without limb weakness mimicking mitochondrial myopathy and congenital myasthenic syndromes. The prominent ptosis noted in patient - 2 is also rarely described in AR-MYH2 myopathy [8]. Proptosis in our patient (Patient-1) has not been described previously. The prominent postprandial esophageal reflux was noted in patient-1 since early adulthood. This is possibly due to the skeletal muscle MYH2 involvement of the upper esophagus resulting in supine gastric reflux which is a novel manifestation noted [10]. The muscle MRI done in patient -1 showed characteristic differential muscle involvement of antero-medial thigh and medial gastrocnemius. The predominant involvement of semitendinosus with relative sparing of other hamstring muscles has been consistently noted in previous reports which may be a pointer towards MYH2 myopathy diagnosis [1, 11]. Hence MRI muscle can be considered in patients with unexplained external ophthalmoparesis which can help in identifying these patients. Both the variants noted in our patients are novel mutations which may be responsible for the unusual presentations noted in our patients as described. However, functional studies were not performed.
Thus, MYH2 myopathy needs to be considered in the differential list in patients presenting with CPEO even in the absence of proximal myopathy. The absence of decremental response in RNS along with characteristic muscle MRI findings may aid in early diagnosis which can be confirmed with next generation sequencing-based genetic diagnosis.
ACKNOWLEDGMENTS
Nil.
SOURCES OF SUPPORT
Nil.
DISCLOSURE OF FUNDING
Nil.
REFERENCES
[1] | Tajsharghi H , Oldfors A . Myosinopathies: pathology and mechanisms. Acta Neuropathol. (2013) ;125: (1):3–18. |
[2] | Darin N , Kyllerman M , Wahlström J , et al. Autosomal dominant myopathy with congenital joint contractures, ophthalmoplegia, and rimmed vacuoles. Ann Neurol. (1998) ;44: (2):242–8. |
[3] | Tajsharghi H , Hilton-Jones D , Raheem O , et al. Human disease caused by loss of fast IIa myosin heavy chain due to recessive MYH2 mutations. Brain. (2010) ;133: (Pt 5):1451–9. |
[4] | Mercuri E , Pichiecchio A , Counsell S , et al. A short protocol for muscle MRI in children with muscular dystrophies. European Journal of Paediatric Neurology. (2002) ;6: (6):305–7. |
[5] | Jaganathan K , Kyriazopoulou Panagiotopoulou S , McRae JF , Darbandi SF , Knowles D , Li YI , Kosmicki JA , Arbelaez J , Cui W , Schwartz GB , Chow ED , Kanterakis E , Gao H , Kia A , Batzoglou S , Sanders SJ , Farh KK . Predicting Splicing from Primary Sequence with Deep Learning. Cell. (2019) ;176: (3):535–548.e24. |
[6] | National Center for Biotechnology Information. ClinVar; [VCV000959745.4], https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000959745.4. |
[7] | GenomeAsia100K Consortium. The GenomeAsia 100K Project enables genetic discoveries across Asia. Nature. (2019) ;576: (7785):106–111. doi: 10.1038/s41586-019-1793-z. Epub 2019 Dec 4. |
[8] | Oldfors A . Hereditary myosin myopathies. NeuromusculDisord. (2007) ;17: (5):355–67. |
[9] | Cabrera-Serrano M , Fabian VA , Boutilier J , et al. Adult onset distal and proximal myopathy with complete ophthalmoplegia associated with a novel de novo p.(LeuPro) mutation in MYH2. Clin Genet. (2015) ;88: (6):573–8. |
[10] | Hashmi SK , Ceron RH , Heuckeroth RO . Visceral myopathy: clinical syndromes, genetics, pathophysiology, and fall of the cytoskeleton. Am J PhysiolGastrointest Liver Physiol. (2021) ;320: (6):G919–G935. |
[11] | Telese R , Pagliarani S , Lerario A , et al. MYH2 myopathy, a new case expands the clinical and pathological spectrum of the recessive form. Mol Genet Genomic Med. (2020) ;8: (9):e1320. |




