A Mixed-Method Study Exploring Patient-Experienced and Caregiver-Reported Benefits and Side Effects of Corticosteroid Use in Duchenne Muscular Dystrophy
Abstract
Background:
Corticosteroids are recommended to all people with Duchenne as standard of care; patient experience data is important to guide corticosteroid decision making and as a comparator for new treatment options.
Objective:
This study assesses patient and caregiver-reported benefits and side effects from corticosteroids to treat Duchenne muscular dystrophy, their importance, and satisfaction.
Methods:
Using one-on-one interviews (n = 28) and an online survey (n = 236), parents and adults with Duchenne reported corticosteroid benefits and side effects rated as both experienced and important.
Results:
Benefits to breathing, heart function, arm strength, slowing progression of weakness, and getting around were rated as particularly important, regardless of ambulatory status. Important side effects included increased fracture risk, unwanted weight gain, and diabetes/prediabetes. Parents rated behavior issues and adults rated delayed puberty as having high importance. Being ambulatory was independently associated with reporting more net benefit (p = 0.02). For side effects, parent scores were significantly higher than adult score (p = 0.02). Corticosteroid type was not significant. Participants were, overall, satisfied with corticosteroids (means ranging from 6.2 to 7.7 on a scale of 0–10), with no significant differences based on corticosteroid type.
Conclusions:
Overall, most participants were satisfied with the use of corticosteroids. While a range of side effects were rated as important and relatively common, individuals using corticosteroids and their caregivers indicate that benefits outweigh the side effects. Qualitative data indicate that high acceptability is influenced by lack of treatment alternatives. Patient experience data on use of corticosteroids in Duchenne may be relevant to drug development, regulatory assessment of new treatments, and to families making decisions about corticosteroid use.
BACKGROUND
Duchenne and Becker muscular dystrophies are rare X-linked recessive diseases belonging to a group of conditions known as dystrophinopathies. Duchenne and Becker are caused by mutations in the dystrophin gene leading to the absence or reduced production of the dystrophin protein, a protein that is key to stabilization of the muscle cell membranes [1]. This lack of dystrophin in muscle leads to progressive muscle weakness and loss over time causing premature death. Duchenne and Becker are X-linked conditions most commonly diagnosed in males [2].
Duchenne and Becker are characterized by progressive muscle degeneration. Symptoms of the more severe Duchenne type of muscular dystrophy typically appear around age three and lead to the loss of ambulation in the early teens, followed by respiratory and cardiac complications later in life [3]. Corticosteroid therapy has been used for the management of Duchenne for decades with a purpose of slowing disease progression by reducing inflammation in muscle [4–6]. Numerous studies have indicated the benefits and risks of prolonged corticosteroid use in Duchenne. Benefits of corticosteroids include delaying the loss of ambulatory milestones, as well as preserving pulmonary function and reducing the incidence of scoliosis [7–9]. While corticosteroids are known to slow disease progression, chronic use can result in side effects including growth stunting, weight gain, delayed puberty, loss of bone density with consequent fractures, and cataract formation [4, 9, 10].
While the potential benefits and side effects of corticosteroids in Duchenne are well documented, there is a paucity of data regarding how patients and parents think and feel about the benefits and risks of using corticosteroids. In a survey collected in 2010–2012, researchers developed a self-report tool for parents and patients to measure the severity of side effects and importance of benefits of corticosteroids to treat Duchenne. The most severe side effects reported by respondents were weight gain, being short for height, getting upset easily, and having puffy cheeks, while the most important reported benefits were those attributed to breathing and heart function [11]. New treatment and management options are now available for Duchenne. In recent years, drug development efforts have included those targeting muscle inflammation with the goal of finding corticosteroid alternatives that have similar benefits but mitigate or reduce the side effect profile seen in corticosteroids [12].
Eliciting and quantifying patient and caregiver experiences regarding current treatment regimens is a key area of interest of regulators, clinicians, patients, drug developers, and payers. In 2012, the U.S. Food and Drug Administration (FDA) established the Patient-Focused Drug Development (PFDD) initiative aimed at taking a more structured and transparent approach to systematically obtaining the patient perspective on specific diseases and their currently available treatments [13]. Since then, organizations like Parent Project Muscular Dystrophy (PPMD) have been collecting and reporting patient experience data to inform relevant stakeholders and decision-makers within the Duchenne care and drug development ecosystem [14]. Given the paucity of relevant patient experience data, we sought to assess experienced or expected corticosteroid benefits and side effects, the importance that people with Duchenne and their caregivers associated with each, and overall corticosteroid satisfaction. We employed a two-phase study design to obtain data from teens and adults with Duchenne and Becker muscular dystrophy and parents of individuals with Duchenne about their experience using corticosteroids. Data regarding patient and caregiver experiences with corticosteroid use has the potential to provide insights that may inform individual and shared decision-making of patients, parents and treating physicians. This data may also inform the work of drug developers developing potential corticosteroid alternatives, as well as regulators making decisions on the approval of new molecular entities or repurposed medications targeting inflammation.
METHODS
This study utilized a community-engaged approach where an advisory board comprised of physicians, advocates, parents, individuals with Duchenne, and industry partners provided input on the aims and instruments and offered insight on interpretation [15]. The first phase of this mixed-methods study included interviews with parents of children with Duchenne and adult patients with Duchenne or Becker. Our qualitative aim was to explore attitudes and experienced or expected benefits and side effects with using corticosteroids and the impact on participants’ lives. Data generated by the first phase informed the development of a subsequent exploratory survey. The survey aims were to assess respondents’ report of benefits and side effects; factors associated with experiencing more net corticosteroid benefits and more total corticosteroid side effects; and satisfaction with corticosteroid use. Additional data obtained from this study from participants not using steroids and on patient/parent decision-making about the initiation and continuation of corticosteroids are reported elsewhere [16].
The RTI International Institutional Review Board reviewed and approved the study protocol (IRB# STUDY00021154).
ELIGIBILITY AND RECRUITMENT
Recruitment was conducted by PPMD through two existing sources: the Duchenne Registry and their general contact database/social media contacts. The Duchenne Registry (formerly DuchenneConnect) is a patient reported registry for Duchenne and Becker muscular dystrophy. For both study phases, US based participants were recruited directly through the registry via a targeted email, as well as other existing sources including the PPMD website, PPMD general email list, social media, and informal networks. Diagnosis is based on self-report, though a large subset of participants have genetic confirmation of diagnosis through the Duchenne Registry. All other variables (including ambulatory status and corticosteroid regimen) were based on respondent report.
3.1Interview phase
Interview participants were recruited July – August 2020 via direct emails to families from PPMD staff. Those interested completed a screener to determine their eligibility for the study. Eligible individuals included teenagers and young adults with Duchenne or Becker muscular dystrophy and parents of children with Duchenne or Becker (any age). All participants received a $25 gift card for their participation in the study.
3.2Survey phase
Participants for the survey were recruited March – July 2021. They included English-speaking, U.S.-based adult Duchenne patients or parents/guardians of children with Duchenne. We conducted two phases of recruitment for the survey phase of the study. The Duchenne Registry had 279 registrants who met eligibility requirements and had completed the Corticosteroid Survey in Duchenne Registry within 16 months of the survey invitation. All 279 were sent an invitation to participate in the survey.
The second phase of recruitment included an invitation email and reminder email sent through PPMD’s general email list, as well as informal networks and posts placed on social media. At the end of recruitment, participants who had completed the survey were entered into a raffle to win one of five $50 gift cards.
INSTRUMENTS AND DATA COLLECTION
4.1Interviews
Prior to the interview, participants completed a brief survey collecting data on corticosteroid use history and demographics. Interviewers employed a semi-structured interview guide that included: corticosteroid use history, expected and experienced effects of corticosteroids, treatment decision-making, and hypothetical new treatment options (not described here). Interviews were conducted via videoconference over an eight-week period from August – September 2020. Interviews averaged about 45 minutes (range of 28 to 63 minutes). Interviews were audio-recorded and transcribed verbatim.
4.2Survey
The Parent Project Muscular Dystrophy research team members have access to the Duchenne Registry data. Access to this existing data allowed us to develop two versions of the survey. For registry participants, participants consented to the use of the demographic and clinical history information in the Duchenne Registry. Participants who were not in the registry responded to additional demographic and clinical questions in our survey (including corticosteroid type, age at initiation, and whether the individual used corticosteroids continuously) that mirror those collected in the Duchenne Registry. Surveys were programmed using Qualtrics [17]. All surveys included the following components: corticosteroid benefits, corticosteroid side effects, overall experience with corticosteroid, and medical decision-making (to be reported elsewhere).
For the benefit and side effect items, we adapted an existing instrument of items developed by Hendriksen and colleagues [11]. The adaptations were based on findings from the interview study together with advisory committee feedback. The resulting instrument included 11 corticosteroid benefit items (as shown in Figs. 1 and 2) and 16 corticosteroid side effect items (as show in Figs. 3 and 4). Because of the progressive nature of Duchenne and the importance of both experienced and expected outcomes in treatment satisfaction corticosteroid benefit and side effect items used these response options (parent versions of the response options are shown): “Unsure if my child has this benefit/side effect or will ever have it,” “Use to have this benefit/side effect, but not any longer,” “Currently has this benefit/side effect,” “Does not yet have this benefit/side effect but I expect it in the future,” and “Never had this benefit/side effect and I do not expect it in the future.” Participants also indicated the importance for each benefit/side effect on a 5-point Likert type scale ranging from 1 to 5, with higher scores indicating more importance.
Fig. 1
Experienced and expected benefits and rated importance of ambulatory respondents.
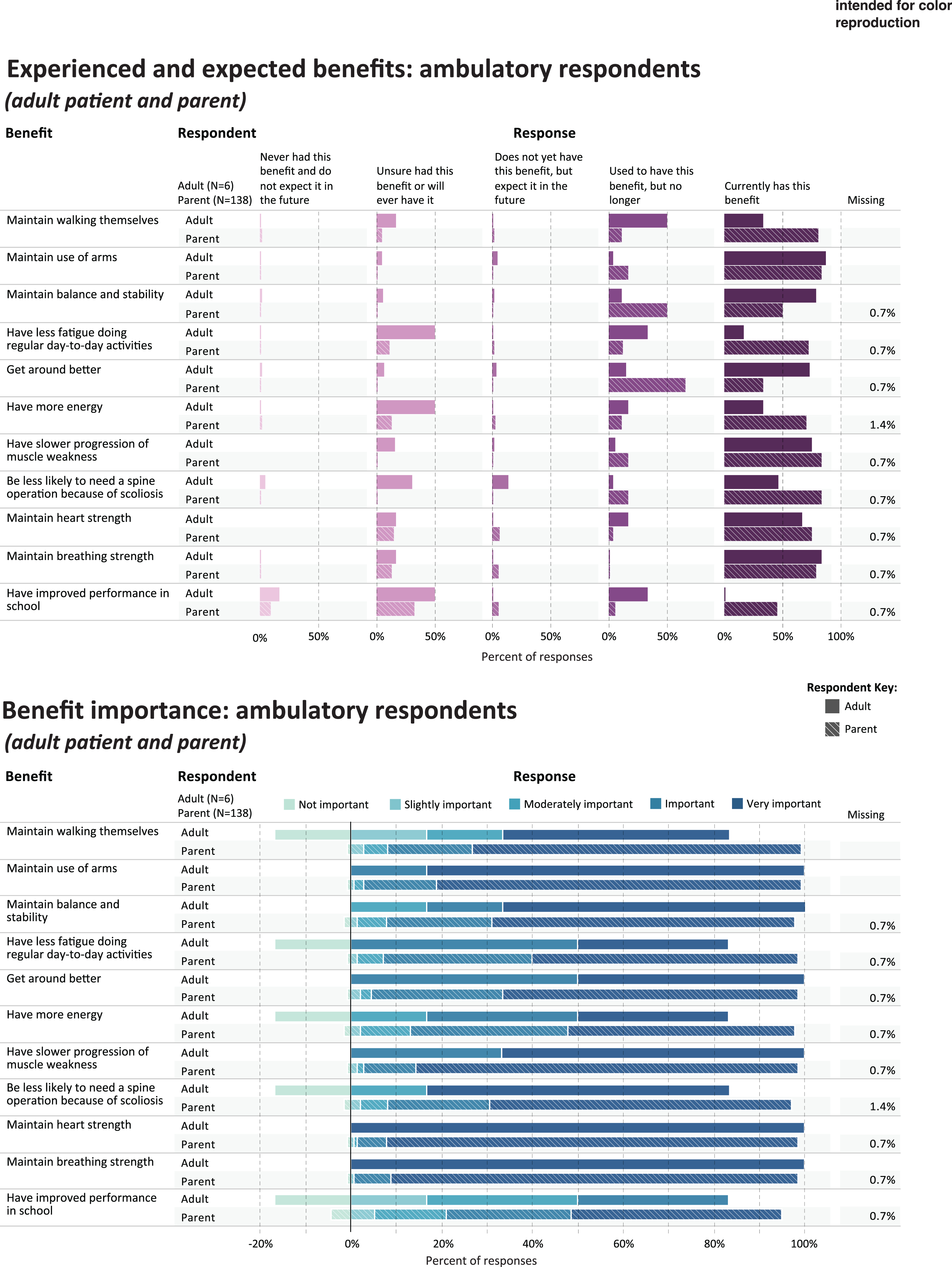
Fig. 2
Experienced and expected benefits and rated importance of non-ambulatory respondents.
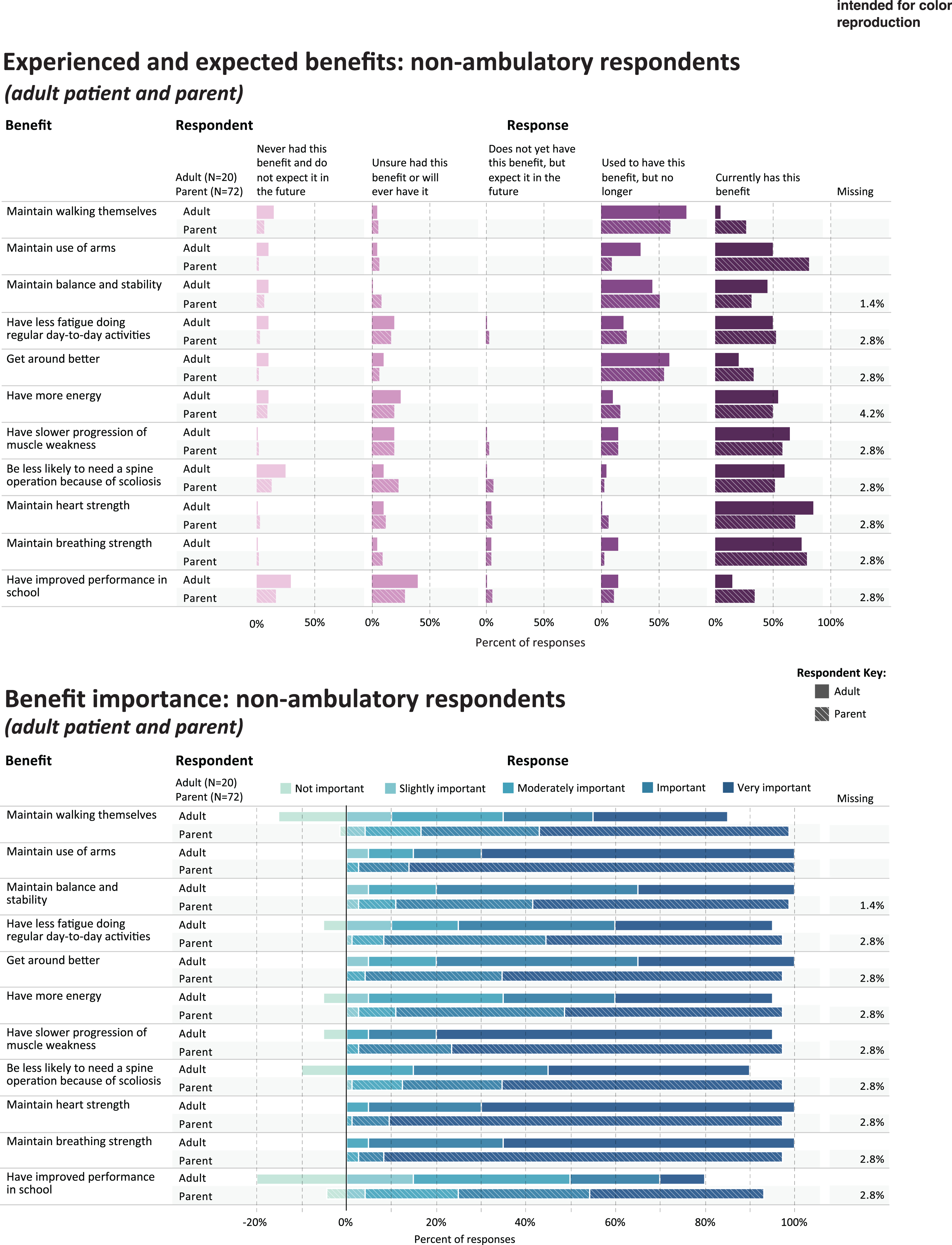
Fig. 3
Experienced and expected side effects and rated importance of ambulatory respondents.
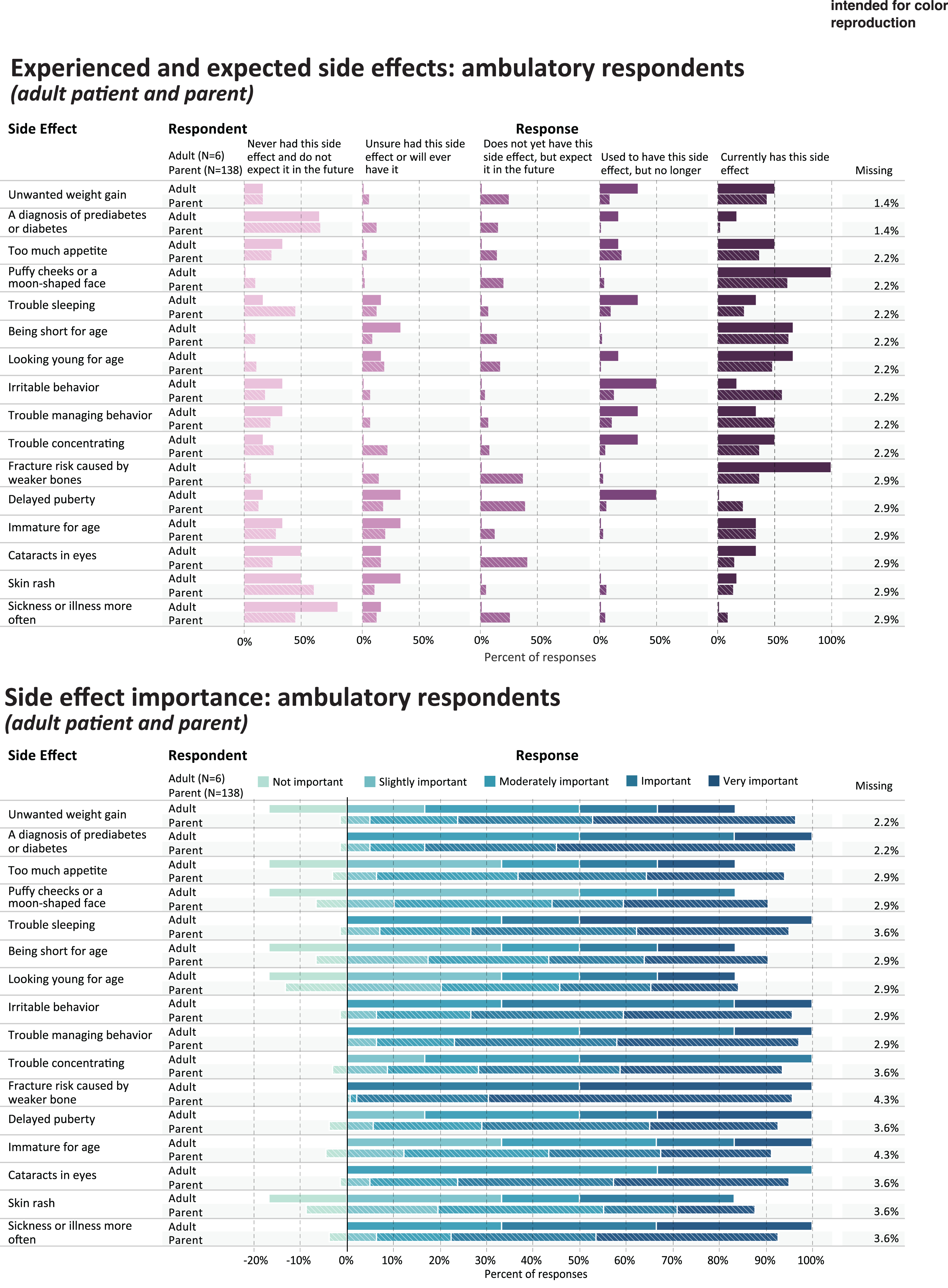
Fig. 4
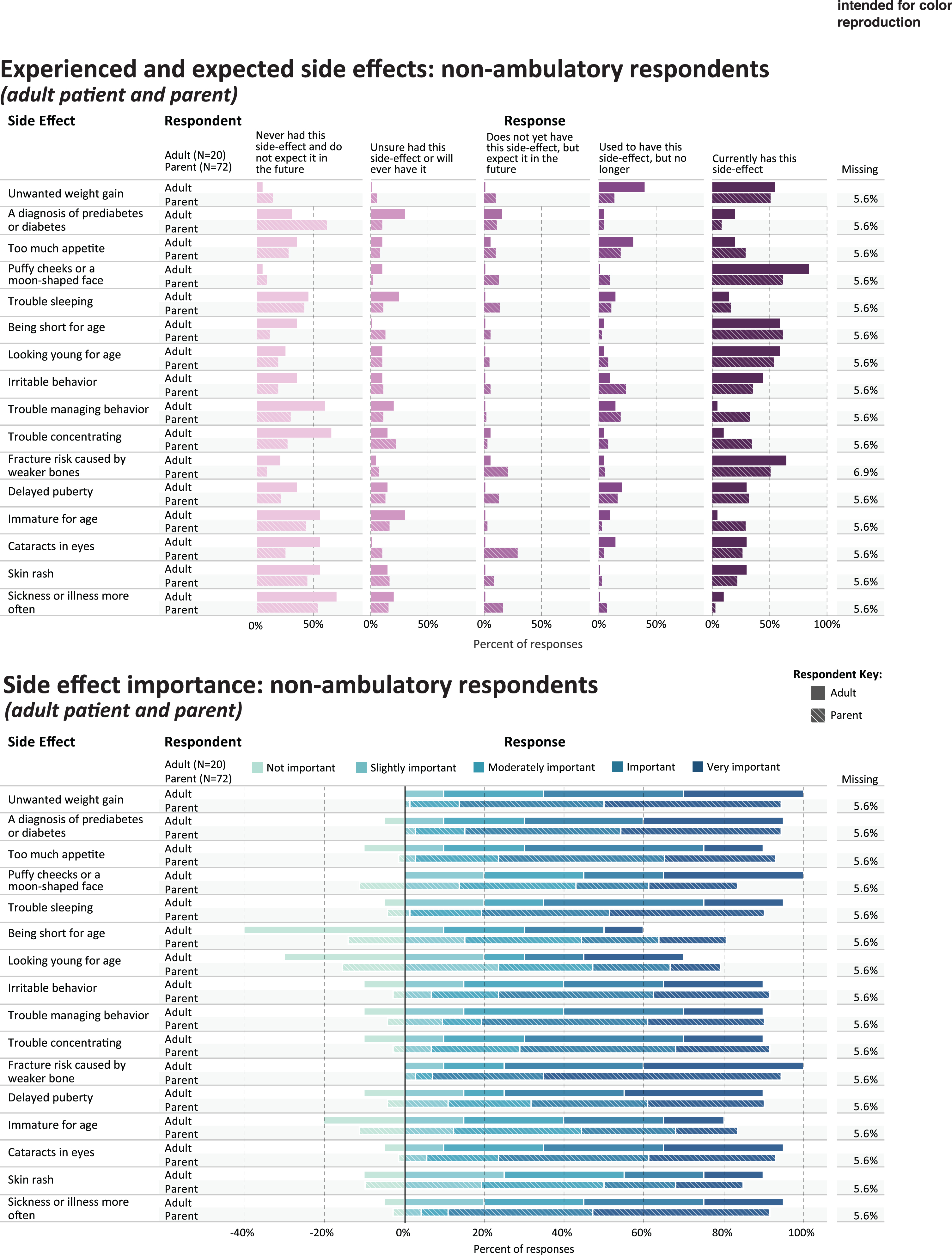
Experienced and expected side effects and rated importance of non-ambulatory respondents.
Participants then reported on overall corticosteroid impact on a 5-point Likert type scale. Questions included degree of overall benefit, overall side effect impact on the person with Duchenne, overall side effect impact on the family, and level of concern about taking corticosteroids long-term (items shown in Table 6). Finally, participants responded using a slider with a scale ranging from 0–10 for items on benefit/side effect balance, satisfaction with corticosteroids, and likelihood to recommend corticosteroids to others, with higher scores indicating more agreement (items shown in Table 7).
ANALYSIS
5.1Interviews
We employed a rapid analytic approach, which involves a team-based process to rapidly develop an understanding of the data [18–20]. Interviewers used a standardized notes template to abridge the data into summary notes, which were then synthesized using a matrix-based approach [18, 20, 21]. For each interview, an analyst (KAP) conducted a quality check of the data by comparing summary notes to transcripts, and then transferred the final summaries into a data matrix that was organized by question domain, survey data, and cohort. The matrix was organized by participant (y-axis) and domain (y-axis). Participant data were summarized for each domain, revealing a range of perceptions, priorities, and attitudes relating to corticosteroid use [22, 23]. A validity check involved a collaborative decision-making process between the Principal Investigator (HLP) and KAP, where the matrix summaries were used to compare content across interviews [22]. Reports of the analyses were shared with the expert advisory board to provide additional interpretation.
5.2Survey
The data analysis was done using SAS® software, Version 7.15 of the SAS System for Windows (Copyright © 2017. SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA).
Ambulation status was determined by response to this question: “How do you or your child (the person with Duchenne or Becker) get around?” Participants were categorized as ambulatory if they responded with “I usually or always walk on my own without help or mobility devices” or “I can get around on my own, but I sometimes need help from a mobility device.” Participants responding with “I use a wheelchair or other mobility device and rarely or never walk” were categorized as non-ambulatory. In subgroups based on respondent type (adult with Duchenne or parent) and ambulatory status, we conducted descriptive analyses to generate frequencies for corticosteroid benefits, side effects, and the importance associated with each benefit or side effect.
We then examined factors contributing to respondents’ total experienced or expected benefits and total experienced or expected side effects. The scores were summed for the benefit items and the side effect items; individuals who did not answer all items were omitted from this analysis. The Cronbach’s alpha coefficient was utilized to determine scale consistency for the benefit and side effect items. The relationship between respondent characteristics (age, corticosteroid type, ambulation status, and respondent type) and net benefit and net side-effect scores were examined using ANCOVA. We used both a visual examination of the residuals as well as Shaprio-Wilk test for normal distribution. If there is normal distribution, a generalized linear model is used with the PROC GLM procedure. If there was not a normal dstirbution, the outcome variable was modeled with log-normal distribution through the PROC GENMOD procedure. For these analyses, age was treated as a covariate while all other characteristics were organized into nominal groups. Interactions were tested but they were not statistically significant so were not included in the model.
Finally, we used cross tabulations to compare responses on the satisfaction items by corticosteroid type (Emflazatrademark/deflazacort vs. prednisone/prednisolone) in subgroups defined by respondent type (adult patient vs parents) and ambulatory status.
RESULTS
6.1Participant characteristics
6.1.1Interviews
The 28 participants reporting on current corticosteroid use included 14 parents of children with Duchenne and 14 teens or adults with Duchenne or Becker (Table 1). Corticosteroid initiation ranged from 2 – 12 years, with a median age of 6 years. The majority (n = 24) indicated continuous corticosteroid use. Six respondents reported currently using Emflazatrademark/deflazacort and 12 reported currently using prednisone/prednisolone.
Table 1
Interview participant characteristics and Duchenne/Becker index cases (n = 28)
| Participant Characteristics | ||
| Median | Range | |
| Parent participant age (years) | 47 | (34–52) |
| Teen/Adult participant age (years) | 25 | (16–36) |
| Sex of participant | Count | |
| Female | 13 (46%) | |
| Male | 15 (54%) | |
| Duchenne or Becker Index Cases | ||
| Median | Range | |
| Age of child with Duchenne or Becker (years) | 14 | (4–16) |
| Age at corticosteroid initiation (years) | 6 | (2–12) |
| Count (%) | ||
| Diagnosis | ||
| Duchenne muscular dystrophy | 27 (96%) | |
| Becker muscular dystrophy | 1 (4%) | |
| Current Corticosteroid Status | ||
| Prednisone/prednisolone | 12 (43%) | |
| Emflazatrademark/deflazacort | 16 (57%) | |
| Corticosteroid Usage History | ||
| Continuous use | 24 (86%) | |
| Took one or more breaks | 4 (14%) |
6.1.2Survey
There were 236 survey participants:138 parents of ambulatory children with Duchenne, 6 adults who reported to be ambulatory and have a diagnosis of Duchenne, 72 parents of non-ambulatory children, and 20 non-ambulatory adults with Duchenne (Table 2). Of participants recruited through the Duchenne Registry, 284 individuals were invited to participate in the survey and 107 participated, excluding 23 missing steroid or ambulatory data, yielding a response rate of 37.7% . A response rate cannot be calculated for individuals recruited from outside of the registry.
Table 2
Survey participant characteristics (all
| Ambulatory (n = 144) | Non-Ambulatory (n = 92) | |||||||||
| Parent (n = 138) | Adult (n = 6) | p-value | Parent (n = 72) | Adult (n = 20) | p-value | |||||
| N or Mean | % or SD | N or Mean | % or SD | N or Mean | % or SD | N or Mean | % or SD | |||
| Age of individual with Duchenne | 9.8 | 4.1 | 25.3 | 6.7 | <.0001 | 15.8 | 6.7 | 27.1 | 5.8 | <.0001 |
| Age at diagnosis | 3.9 | 2.1 | 4.5 | 2.4 | .85 | 4.5 | 2.4 | 4.8 | 6.0 | 0.73 |
| Corticosteroid currently used | .42 | 0.50 | ||||||||
| Emflazatrademark/Deflazacort | 91 | 65.9% | 3 | 50.0% | 49 | 68.1% | 12 | 60.0% | ||
| Prednisone/prednisolone | 47 | 34.1% | 3 | 50.0% | 23 | 31.9% | 8 | 40.0% | ||
| Age at corticosteroid initiation (years) | 5.0 | 2.1 | 5.5 | 2.5 | .11 | 6.8 | 3.0 | 10.0 | 8.4 | 0.55 |
| Steroid use history | 1.00 | 0.16 | ||||||||
| Took one or more breaks | 1 | 0.7% | – | – | 4 | 5.6% | 3 | 15.0% | ||
| Continuous use | 135 | 97.8% | 6 | 100.0% | 67 | 93.1% | 16 | 80.0% | ||
| Missing | 2 | 1.4% | 1 | 1.4% | 1 | 5.0% | ||||
Of corticosteroid users, 155 (65.7%) were using Emflazatrademark/deflazacort and 81 (34.3%) were using prednisone/prednisolone. The average ages at corticosteroid initiation ranged from 5.0 (as reported by parents of ambulatory children) and 10.0 (as reported by non-ambulatory adults). Most respondents indicated they/their child used corticosteroids continuously, without one or more breaks. At the time of this survey, 88 (36.5%) respondents reported that the patient with Duchenne had been through puberty, 32 (13.2%) have taken growth hormone, and 44 (18.3%) have taken testosterone.
CORTICOSTEROIDS BENEFITS
7.1Interview results: Corticosteroid benefits
During the interviews, participants were prompted to identify three benefits that they believe should occur for people who take corticosteroids. The most frequently reported benefits included heart function, lung function, strength, and better ambulation. Protection of the heart and lungs were often reported together as a single unit of benefit from corticosteroid use (n = 20, 71%). Strength was described as better muscular strength and preservation of strength over a longer time (n = 18, 64%). Some participants specifically mentioned arm muscle function and control as an important benefit. Benefits to mobility/ambulation were defined by participants as maintaining walking longer, walking farther with less fatigue, and a general improvement in activities that involve mobility (n = 16, 57%).
Most participants in the teen and adult cohort also reported a general “slowing of the progression of the disease” and living longer (n = 8, 29%). Additionally, several other benefits reported by both cohorts were related to quality of life, including having more confidence, increase in independence, fewer episodes of illness, and maintaining mental health.
When asked to describe benefits they had received from corticosteroids, all participants were able to name specific treatment benefits that were consistent with the list of benefits described above. Some respondents reported being uncertain if they were receiving all the benefits that “should” come from corticosteroid use. Many indicated that they have no way of knowing how their disease would have progressed if they were not on corticosteroids. This was particularly challenging when considering benefits after ambulation ended and for potential benefits to heart and lung function.
“You know, I think when you’re still walking around, it’s easier to see [the benefits of corticosteroids]. I don’t know if I’m like, ‘Oh, wow, they’re still saving my [heart and lung] function.’ Now, I think I’m at better place than it would have been without steroids. So, I think, I have received the benefits to the point that is the most benefits they can give.” -Adult participant
Most parents of children with DMD referenced their child’s current or past maintenance of ambulation as an indicator of corticosteroid benefit. Parents with more than one child with Duchenne and those with a family history compared their children’s/relatives’ progression at specific ages to attempt to define the benefits of corticosteroids. As one parent indicated,
“I feel like he is getting the benefit. I feel like it’s helping him because we can kind of compare a little bit to my brother at the same age; he was actually in wheelchair at this age. And [my son] is still walking [and] getting around well.” -Parent Participant
“We’ll never know what part is the natural course and what is from the corticosteroid … . I can only look back and say, ‘I’m glad that I made the decision and that I did as much as I could.” -Parent participant
7.2Survey results: Corticosteroid benefits
Table 3 shows the five benefits that were most frequently rated as current or prior benefits of corticosteroid use, in each of the study subgroups; and the five benefits that were most frequently rated as important or very important. Figures 1 and 2 provide a more detailed representation of the ratings for each benefit item.
Table 3
Benefits most frequently experienced and most frequently reported as important (top 5), by participant type and ambulatory status
| Benefit Experience: Caregiver Ambulatory | ||||
| Benefit | Currently has benefit (N) | Currently has benefit (%) | Used to have this benefit, but no longer (N) | Used to have this benefit, but no longer (%) |
| Maintain walking themselves | 111 | 80.4% | 15 | 10.9% |
| Maintain use of arms | 120 | 87.0% | 5 | 3.6% |
| Maintain balance and stability | 109 | 79.0% | 16 | 11.6% |
| Get around better | 101 | 73.2% | 20 | 14.5% |
| Have less fatigue doing regular day-to-day activities | 100 | 72.5% | 17 | 12.3% |
| Benefit Importance: Caregiver Ambulatory | ||||
| Benefit | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Maintain breathing strength | 124 | 89.9% | 11 | 8.0% |
| Maintain heart strength | 125 | 90.6% | 9 | 6.5% |
| Maintain use of arms | 111 | 80.4% | 22 | 15.9% |
| Have slower progression of muscle weakness | 116 | 84.1% | 16 | 11.6% |
| Get around better | 90 | 65.2% | 40 | 29.0% |
| Benefit Experience: Adult Ambulatory | ||||
| Benefit | Currently has benefit (N) | Currently has benefit (%) | Used to have this benefit, but no longer (N) | Used to have this benefit, but no longer (%) |
| Maintain use of arms | 5 | 83.3% | 1 | 16.7% |
| Have slower progression of muscle weakness | 5 | 83.3% | 1 | 16.7% |
| Get around better | 2 | 33.3% | 4 | 66.7% |
| Maintain balance and stability | 3 | 50.0% | 3 | 50.0% |
| Be less likely to need spine operation because of scoliosis | 5 | 83.3% | 1 | 16.7% |
| Benefit Importance: Adult Ambulatory | ||||
| Benefit | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Maintain heart strength | 6 | 100.0% | 0 | 0% |
| Maintain breathing strength | 6 | 100.0% | 0 | 0% |
| Maintain use of arms | 5 | 83.3% | 1 | 16.7% |
| Have slower progression of muscle weakness | 4 | 66.7% | 2 | 33.3% |
| Get around better | 3 | 50.0% | 3 | 50.0% |
| Benefit Experience: Caregiver Non-Ambulatory | ||||
| Benefit | Currently has benefit (N) | Currently has benefit (%) | Used to have this benefit, but no longer (N) | Used to have this benefit, but no longer (%) |
| Maintain use of arms | 59 | 81.9% | 7 | 9.7% |
| Get around better | 24 | 33.3% | 40 | 55.6% |
| Maintain walking themselves | 19 | 26.4% | 44 | 61.1% |
| Maintain balance and stability | 23 | 31.9% | 37 | 51.4% |
| Maintain breathing strength | 57 | 79.2% | 2 | 2.8% |
| Benefit Importance: Caregiver Non-Ambulatory | ||||
| Benefit | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Maintain use of arms | 62 | 86.1% | 8 | 11.1% |
| Maintain heart strength | 63 | 87.5% | 6 | 8.3% |
| Maintain breathing strength | 64 | 88.9% | 4 | 5.6% |
| Have slower progression of muscle weakness | 53 | 73.6% | 15 | 20.8% |
| Get around better | 45 | 62.5% | 22 | 30.6% |
| Benefit Experience: Adult Non-Ambulatory | ||||
| Benefit | Currently has benefit (N) | Currently has benefit (%) | Used to have this benefit, but no longer (N) | Used to have this benefit, but no longer (%) |
| Maintain balance and stability | 9 | 45.0% | 9 | 45.0% |
| Maintain breathing strength | 15 | 75.0% | 3 | 15.0% |
| Maintain use of arms | 10 | 50.0% | 7 | 35.0% |
| Maintain heart strength | 17 | 85.0% | 0 | 0% |
| Have slower progression of muscle weakness | 13 | 65.0% | 3 | 15.0% |
| Benefit Importance: Adult Non-Ambulatory | ||||
| Benefit | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Maintain heart strength | 14 | 70.0% | 5 | 25.0% |
| Maintain breathing strength | 13 | 65.0% | 6 | 30.0% |
| Have slower progression of muscle weakness | 15 | 75.0% | 3 | 15.0% |
| Maintain use of arms | 14 | 70.0% | 3 | 15.0% |
| Get around better | 7 | 35.0% | 9 | 45.0% |
PARENTS OF AMBULATORY CHILDREN
For parents (n = 136) reporting through the quantitative survey on their ambulatory child’s experience with corticosteroids, Table 3 shows the percentage that currently has or used to have the most frequently-endorsed benefit items (“ever experienced”): maintain walking (n = 126, 91.3%), maintain use of arms (n = 125, 90.6%), maintain balance and stability (125, 90.6%), get around better (n = 121, 87.7%), and having less fatigue (n = 117, 84.8%). The benefits most frequently rated as “very important” or “important” by parents were maintain breathing strength (n = 135, 97.9%), maintain heart strength (n = 134, 97.1%), maintain use of arms (n = 133, 96.3%), have slower progression of muscle weakness (n = 132, 95.7%), and get around better (n = 130, 94.2%) (Table 3, Fig. 1).
AMBULATORY ADULTS
All ambulatory adults (n = 6) completing the survey rated each of these items as current or prior benefits (“ever experienced”): maintain use of arms (n = 6, 100%), have slower progression of muscle weakness(n = 6, 100%), be less likely to need a spine operation because of scoliosis (n = 6, 100%), maintain balance and stability (n = 6, 100%), and get around better (n = 6, 100%). The benefits most frequently rated as “very important” or “important” by ambulatory adults were maintain breathing strength (n = 6, 100%), maintain heart strength (n = 6, 100%), maintain use of arms (n = 6, 100%), and have slower progression of muscle weakness (n = 6, 100%), and get around better (n = 6, 100%) (Table 3, Fig. 1).
PARENTS OF NON-AMBULATORY CHILDREN
For parents (n = 72) reporting through the survey on their non-ambulatory child’s experience with corticosteroids, Table 3 shows the percentage that currently has or used to have the most commonly-endorsed benefit items (“ever experienced”): maintain use of arms (n = 66, 91.6%), get around better (n = 64, 88.9%), maintain walking (n = 63, 87.5%), maintain balance and stability (n = 61, 83.3%), and maintain breathing strength (n = 59, 82.0%). Benefits reported as most important were maintain use of arms (n = 70, 97.2%), maintain heart strength (n = 69, 95.8%), maintain breathing strength (n = 68, 94.4%), have slower progression of muscle weakness (n = 68, 94.4%), and get around better (n = 67, 93.1%) (Table 3, Fig. 2).
NON-AMBULATORY ADULTS
Among non-ambulatory adults (n = 20), the most frequently-endorsed current or prior benefit items (“ever experienced”) were: maintain balance and stability (n = 18, 90.0%), maintain breathing strength(n = 18, 90.0%), maintain use of arms (n = 17, 85.0%), maintain heart strength (n = 17, 85.0%), and have slower progression of muscle weakness (n = 16, 80.0%). Benefits reported as most important were maintain heart strength (n = 19, 95.0%) maintain breathing strength (n = 19, 95.0%), have slower progression of muscle weakness (n = 18, 90.0%), maintain use of arms (n = 17, 85.0%), and get around better (n = 16, 80.0%) (Table 3, Fig. 2).
CORTICOSTEROID SIDE EFFECTS
12.1Interview results: Corticosteroid side effects
Interview participants were prompted to identify bothersome effects of corticosteroids. Compared to the difficulty described by interviewees with identifying what benefits they experienced, respondents could easily report what side effects they experienced. The most frequently-reported side effects included weight gain, behavior changes, bone health, and impacts on physical appearance.
• Weight gain: This side effect was often attributed to increase in appetite. Many participants cited difficulties with maintaining a feeling of satiation after meals and needing to make healthy food choices to maintain weight. Weight gain was described as negatively affecting self-esteem, causing discomfort in the wheelchair, and negatively impacting long-term health (e.g., higher risk of diabetes or other health problems associated with obesity) (n = 16, 57%).
• Behavior challenges: These were often described as emotional outbursts, anger, and mood changes. Many indicated specific challenges with behavioral impacts in the period following corticosteroid initiation and at younger ages; some participants reported these issues were managed over time or outgrown (n = 15, 54%).
• Bone health: This included concerns about developing osteoporosis/brittle bones that increase risk for fractures and spinal compressions (n = 11, 39%).
• Physical appearance: Impacts on physical appearance were defined several ways. Looking young for age and being short for age were described as having negative psychosocial impacts by some participants (n = 5, 18%). Four respondents reported that delayed puberty influenced social life and treatment by peers. Round-shaped face was also listed as a bothersome effect by a few participants.
“You kind of look like a little kid for a really long time and that’s difficult … .. I think just struggling with self-image is a big one. You know, dating or interpersonal relationships is a big one, because unless you know people, their assumptions about you are not usually accurate.” -Teen/adult participant
Both parent and teen/adult respondents reported monitoring side effects and taking time to manage side effects to the extent possible. Teen/adult respondents and some parent respondents reported that side effects become more manageable or less of an issue as the participant aged. Parents also identified ways corticosteroids impacted others in the family; examples are behavioral issues and impact on the immune system.
“One thing is, we live in fear of him getting sick. Especially with the pandemic because of him taking corticosteroids, we’re afraid to take him out anywhere. And this has affected not only us, but also his sister. I didn’t want her to go back to school because I didn’t want her to get sick and then get him sick, so it’s affecting the whole family.” – Parent Participant
The findings regarding corticosteroid side effects from the semi structured interviews helped to inform the questions related to side effects in the quantitative survey.
12.2Survey results: Corticosteroid side effects
Table 4 shows the five side effect items that were most frequently rated as current or prior side effects of corticosteroid use, in each of the study subgroups; and the five side effects that were most frequently rated as important or very important. Figures 3 and 4 provides a more detailed representation of the ratings for each side effect item.
Table 4
Side effects most frequently experienced and most frequently reported as important (top 5), by participant type and ambulatory status
| Side Effect Experienced: Caregiver Ambulatory | ||||
| Side-Effect | Currently has side effect (N) | Currently has side effect (%) | Used to have this side effect, but no longer (N) | Used to have this side effect, but no longer (%) |
| Irritable behavior | 78 | 56.5% | 17 | 12.3% |
| Puffy cheeks or a moon-shaped face | 85 | 61.6% | 6 | 4.3% |
| Being short for age | 87 | 63.0% | 3 | 2.2% |
| Trouble managing behavior | 69 | 50.0% | 15 | 10.9% |
| Too much appetite | 51 | 37.0% | 26 | 18.8% |
| Side Effect Importance: Caregiver Ambulatory | ||||
| Side-Effect | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Fracture risk caused by weaker bone | 90 | 65.2% | 39 | 28.3% |
| A diagnosis of prediabetes or diabetes | 71 | 51.4% | 39 | 28.3% |
| Trouble managing behavior | 54 | 39.1% | 48 | 34.8% |
| Unwanted weight gain | 60 | 43.5% | 40 | 29.0% |
| Cataracts in eyes | 52 | 37.7% | 46 | 33.3% |
| Side Effect Experienced: Adult Ambulatory | ||||
| Side-Effect | Currently has side effect (N) | Currently has side effect (%) | Used to have this side effect, but no longer (N) | Used to have this side effect, but no longer (%) |
| Puffy cheeks or a moon-shaped face | 6 | 100.0% | 0 | 0% |
| Fracture risk caused by weaker bone | 6 | 100.0% | 0 | 0% |
| Looking young for age | 4 | 66.7% | 1 | 16.7% |
| Unwanted weight gain | 3 | 50.0% | 2 | 33.3% |
| Trouble concentrating | 3 | 50.0% | 2 | 33.3% |
| Side Effect Importance: Adult Ambulatory | ||||
| Side-Effect | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Fracture risk caused by weaker bone | 3 | 50.0% | 3 | 50.0% |
| Irritable behavior | 1 | 16.7% | 3 | 50.0% |
| Trouble sleeping | 3 | 50.0% | 1 | 16.7% |
| Sickness or illness more often | 2 | 33.3% | 2 | 33.3% |
| Delayed puberty | 2 | 33.3% | 1 | 16.7% |
| Side Effect Experienced: Caregiver Non-Ambulatory | ||||
| Side Effect | Current has side effect (N) | Currently has side effect (%) | Used to have this side effect, but no longer (N) | Used to have this side effect, but no longer (%) |
| Puffy cheeks or a moon-shaped face | 45 | 62.5% | 7 | 9.7% |
| Unwanted weight gain | 37 | 51.4% | 10 | 13.9% |
| Being short for age | 45 | 62.5% | 2 | 2.8% |
| Looking young for age | 39 | 54.2% | 6 | 8.3% |
| Irritable behavior | 26 | 36.1% | 17 | 23.6% |
| Side Effect Importance: Caregiver Non-Ambulatory | ||||
| Side-Effect | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Fracture risk caused by weaker bone | 43 | 59.7% | 20 | 27.8% |
| Unwanted weight gain | 32 | 44.4% | 26 | 36.1% |
| Sickness or illness more often | 32 | 44.4% | 26 | 36.1% |
| A diagnosis of prediabetes or diabetes | 29 | 40.3% | 28 | 38.9% |
| Trouble sleeping | 28 | 38.9% | 23 | 31.9% |
| Side Effect Experienced: Adult Non-Ambulatory | ||||
| Side Effect | Currently has side effect (N) | Currently has side effect (%) | Used to have this side effect, but no longer (N) | Used to have this side effect, but no longer (%) |
| Unwanted weight gain | 11 | 55.0% | 8 | 40.0% |
| Puffy cheeks or a moon-shaped face | 17 | 85.0% | 0 | 0% |
| Fracture risk caused by weaker bone | 13 | 65.0% | 1 | 5.0% |
| Being short for age | 12 | 60.0% | 1 | 5.0% |
| Looking young for age | 12 | 60.0% | 1 | 5.0% |
| Side Effect Importance: Adult Non-Ambulatory | ||||
| Side-Effect | Very Important (N) | Very Important (%) | Important (N) | Important (%) |
| Fracture risk caused by weaker bone | 8 | 40.0% | 7 | 35.0% |
| Unwanted weight gain | 6 | 30.0% | 7 | 35.0% |
| A diagnosis of prediabetes or diabetes | 7 | 35.0% | 6 | 30.0% |
| Delayed puberty | 7 | 35.0% | 6 | 30.0% |
| Cataracts in eyes | 6 | 30.0% | 6 | 30.0% |
PARENTS OF AMBULATORY CHILDREN
For parents (n = 138) reporting through the survey on their ambulatory children’s side effect experience, Table 4 shows the percentage that endorsed either currently having or used to have the most common side effect items (“ever experienced”): irritable behavior (n = 95, 68.8%), puffy cheeks (n = 91, 65.9%), being short for age (n = 90, 65.2%), trouble managing behavior (n = 84, 69%), and too much appetite (n = 77, 55.8%). Side effects reported as most important were fracture risk (n = 129, 93.5%), diabetes or prediabetes (n = 110, 79.7%), trouble managing behavior (n = 102, 73.9%), unwanted weight gain (n = 100, 75%), and cataracts (n = 98, 71.0%) (Table 4, Fig. 3).
AMBULATORY ADULTS
Table 4 shows the percentage of ambulatory adults (n = 6) that endorsed either currently having or used to have the most common side effect items (“ever experienced”): puffy cheeks (n = 6, 100%), fracture risk (n = 6, 100%) looking young for age (n = 5,83.4%), unwanted weight gain (n = 5,83.4%), and trouble concentrating (n = 5,83.4%). Side effects reported as most important were fracture risk caused by weaker bones (n = 6, 100%), irritable behavior (n = 4, 66.6%), trouble sleeping (n = 4, 66.6%), sickness more often (n = 4, 66.6%), and delayed puberty (n = 3, 50%) (Table 4, Fig. 3).
PARENTS OF NON-AMBULATORY CHILDREN
For parents (n = 72) reporting on their non-ambulatory children’s side effect experience, Table 4 shows the percentage that endorsed either currently having or used to have the most common side effect items (“ever experienced”): puffy cheeks (n = 52, 72.2%), weight gain (n = 47, 65.3%), being short for age (n = 47, 65.3%), looking young for age (n = 45, 62.5%), and irritable behavior (n = 43, 59.7%). Side effects reported as most important were fracture risk caused by weaker bones (n = 63, 87.5%), unwanted weight gain (n = 58, 80.5%), sickness more often (n = 58, 80.5%), diabetes or prediabetes (n = 57, 79.2%), and trouble sleeping (n = 51, 70.8%) (Fig. 4).
NON-AMBULATORY ADULTS
Table 4 shows the percentage of non-ambulatory adults (n = 20) that endorsed either currently having or used to have the most common side effect items (“ever experienced”): weight gain (n = 19, 95.0%), puffy cheeks (n = 17, 85.0%), fracture risk (n = 14, 70.0%), being short for age (n = 13, 65.0%), and looking young for age (n = 13, 65.0%). Side effects reported as most important were fracture risk caused by weaker bones (n = 15, 75.0%), unwanted weight gain (n = 13, 65.0%), diabetes or prediabetes (n = 13, 65.0%), delayed puberty (n = 13, 65.0%), and cataracts (n = 12, 60.0%) (Table 4, Fig. 4).
FACTORS ASSOCIATED WITH NET BENEFIT AND NET SIDE-EFFECT SCORES
The Cronbach’s Alpha for the benefit item scale was 0.869 (11 items) and for the side effect scale was 0.821 (16 items), indicating good internal consistency (Oppenheim, 1992). The results of the ANCOVA analyses are shown in Table 5.
Table 5
Relationship among corticosteroid type, ambulatory status, respondent type, age and overall benefit score and side effect score
| Benefit summed score | Side effect summed score | |||||
| Summed score | ANCOVA | Summed score | ANCOVA | |||
| F | P | F | P | |||
| Corticosteroid | 0.35 | 0.56 | 1.06 | 0.33 | ||
| Emflazatrademark/Deflazacort | 40.82 | 35.45 | ||||
| Prednisone/Prednisolone | 40.08 | 33.79 | ||||
| Age (in years)a | 0.22 | 0.64 | 1.32 | 0.25 | ||
| 5 | 40.91 | 33.10 | ||||
| 10 | 40.63 | 33.99 | ||||
| 15 | 40.36 | 34.88 | ||||
| 20 | 40.09 | 35.77 | ||||
| 25 | 39.82 | 36.66 | ||||
| 30 | 39.55 | 37.55 | ||||
| 35 | 39.28 | 38.43 | ||||
| 40 | 39.01 | 39.32 | ||||
| Ambulatory status | 5.35 | 0.02b | 0.66 | 0.42 | ||
| Ambulatory | 42.09 | 35.39 | ||||
| Non-Ambulatory | 38.81 | 33.85 | ||||
| Respondent type | 2.62 | 0.11 | 5.17 | 0.02 b | ||
| Adult | 38.46 | 30.21 | ||||
| Parent | 42.43 | 37.95 | ||||
| R2 | 0.079 | 0.032 | ||||
aAge was treated as a covariate; bstatistically significant when p < 0.05. ANCOVA, analysis of covariance.
After controlling for ambulation, respondent type (parent/adult report), and age, there was no significant difference in net benefit score (χ2 (1, 236) = 0.37, p = 0.55)) based on corticosteroid type or based on whether the respondent was the parent or the adult with Duchenne (χ2 (1, 236) = 2.76, p = 0.10)). Although there was a downward trend in benefit score with age, this trend was not statistically significant (χ2 (1, 236) = 0.14, p = 0.71). Only ambulatory status was significant, with the ambulatory group having a modestly higher mean benefit rating (about 3.17 points higher) than the non-ambulatory group (M = 41.85 vs. M = 38.68, p < .05). The R2 value of 0.078 indicate that 7.8% of the variation in net benefit score is explained by the model.
Similar to benefit scores, after controlling for ambulation, respondent type, and age, there was no effect of corticosteroid type on net side effect rating (F (1, 229) = 1.06, p = 0.33). Although there was an upward trend in the net side effect score with age, this trend was not statistically significant (F(1, 229) = 1.32, p = 0.25). There was no effect of ambulatory status on net side effect rating (F (1, 229) = 0.66, p = 0.42). Only respondent type was statistically significant with parents reporting, on average, a nearly 8 point higher side-effect score than adult self-reporters (M = 37.95 vs. M = 30.21, p < .05). The R2 value of 0.032 indicate that 3.2% of the variation in net benefit score is explained by the model.
OVERALL CORTICOSTEROID IMPACT AND SATISFACTION
18.1Interview results
When teen/adult respondents were asked about the overall impact of corticosteroids, good or bad, most reflected on side effects that have impacted or could impact their quality of life (e.g., irritability/mood swings, delayed puberty, weight gain, cataracts, and fracture risk). Some also referenced beneficial impacts such as improved heart and lung function and the ability to do more activities. Several teens/adults noted that taking corticosteroids was “just a part of life”, though one reported concerns about forgetting to take the medicine. No respondents indicated that they regret taking corticosteroids and all but one agreed the benefits outweigh the side effects, though to varying degrees. Most respondents indicated that the side effects were manageable. Several adults reported wanting to change an aspect about their corticosteroid use, such as the dose or regimen.
The quotes below reflect the variation in the degree that benefits were described as outweighing the side effects.
“If it wasn’t for [corticosteroids], I wouldn’t be alive right now.” – Teen/adult participant
“Staying as healthy and stable for as long as possible is much better … you can manage [side effects], you just have to be more careful about weight, it’s not that hard of a thing to do.” – Teen/adult participant
“I think that’s a hard call to make. Especially when you’re in a point in your life where you’re really dealing with the side effects and not seeing so many of the benefits because of the progression. Yeah, but I think it was still the right decision.” – Teen/adult participant
Teen/adult respondents also reflected on the limited treatment options for Duchenne as a reason to accept a treatment that comes with side effects— at the time when most of the respondents started corticosteroids, it was the only treatment available.
“They were the best option … I think the options back then were either not great or terrible. In that case, you’re going to want to choose the one that is ‘not great.’ It’s damage control.” -Teen/adult participant
“I think [starting corticosteroids] was the right thing to do. I think it was the only thing to do.” – Teen/adult participant
Parents focused more on the positive impacts, highlighting benefits that improve quality of life such as strength, energy, mobility, fewer falls, and heart and lung benefits. Some also cited side effects that impact their child’s quality of life, such as cataracts, looking different from peers, and mood changes. Most parents mentioned the ability to manage side effects.
“Well, [corticosteroids] just give him added strength and endurance. I guess, he’s never tired. Even if he’s up all night and up early. He just doesn’t get tired. He’s always got like a positive charge and I mean, he’s positive and it’s good.” – Parent participant
Most parents reported they had no regrets with starting their child on corticosteroids. Two parents reported some regret. One stated, “once you give steroids, his body is never going to go back to normal,” and the other wondered if the large impact of steroids on child’s behavior was “worth it.”
18.2Survey results
When responding to questions about positive and negative corticosteroid impact, all participant groups rated the positive aspects of corticosteroids higher than the negative aspects on the person with Duchenne and on the whole family. Table 6 shows mean scores and standard deviations, revealing no significant differences based on corticosteroid type.
Table 6
Ratings of corticosteroid impact, by corticosteroid (on 1–5 scale)
| Ambulatory | ||||||||||
| Parent | Adult | |||||||||
| Emflaza™/Deflazacort (n = 88) | Prednisone/Prednisolone (n = 46) | Emflaza™/Deflazacort (n = 3) | Prednisone/Prednisolone (n = 3) | |||||||
| Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | p-value | |
| In your opinion, how much do you think taking steroids has helped you/your child? | 3.7 | (0.9) | 3.7 | (1.0) | 1 | 3.7 | (0.6) | 4 | (1.0) | 0.64 |
| In your opinion, how much do steroid side effects bother you/your child? | 3 | (1.1) | 2.8 | (1.2) | 0.22 | 2.7 | (0.6) | 2.7 | (0.6) | 1 |
| In your opinion, how much do steroid side effects bother your whole family? | 2.9 | (1.2) | 2.9 | (1.2) | 0.85 | 2.3 | (0.6) | 2 | (1.0) | 0.64 |
| Non-ambulatory | ||||||||||
| Parent | Adult | |||||||||
| Emflaza™/Deflazacort (n = 46) | Prednisone/Prednisolone (n = 22) | Emflaza™/Deflazacort (n = 12) | Prednisone/Prednisolone (n = 8) | |||||||
| Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | p-value | |
| In your opinion, how much do you think taking steroids has helped you/your child? | 3.8 | (0.9) | 3.5 | (1.4) | 0.42 | 3.9 | (1.2) | 3.6 | (1.1) | 0.52 |
| In your opinion, how much do steroid side effects bother you/your child? | 3.1 | (1.1) | 3 | (0.8) | 0.59 | 3.3 | (0.8) | 2.8 | (0.7) | 0.11 |
| In your opinion, how much do steroid side effects bother your whole family? | 2.8 | (1.2) | 2.7 | (1.0) | 0.53 | 2.8 | (1.2) | 2.3 | (0.9) | 0.21 |
Survey participants rated the degree to which the benefits outweigh the side effects, their overall satisfaction, and the likelihood they would recommend corticosteroids to another patient with Duchenne. Across ambulation, respondent type, and corticosteroid type, participants on average reported that benefits outweighed side effects (means ranging from 7.7 to 8.7 on a scale of 0–10), that they were satisfied with corticosteroids (means ranging from 6.2 to 7.7 on a scale of 0–10), and that they would recommend corticosteroids (means ranging from 7.0 to 9.0 on a scale of 0–10). There were no significant differences based on corticosteroid type (Table 7).
Table 7
Ratings of satisfaction, by corticosteroid (on 0–10 scale)
| Ambulatory | ||||||||||
| Parent | Adult | |||||||||
| Emflaza™/Deflazacort (n = 87) | Prednisone/Prednisolone (n = 46) | Emflaza™/Deflazacort (n = 3) | Prednisone/Prednisolone (n = 3) | |||||||
| Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | p-value | |
| Given your overall experience, which is most true about your experience with steroids: • Side effects outweigh benefits (0) • Benefits outweigh side effects (10) | 7.7 | (1.8) | 7.4 | (2.2) | 0.42 | 8.7 | (0.6) | 7.7 | (1.2) | 0.25 |
| Overall, how satisfied are you with steroids as a treatment? • Not at all (0) • Very much (10) | 6.6 | (1.9) | 6.4 | (2.4) | 0.49 | 7.7 | (1.2) | 7.3 | (2.9) | 0.86 |
| Given your overall experience, how likely are you to recommend steroids to someone like you? • Not at all (0) • Very much (10) | 7.6 | (2.0) | 7.5 | (2.3) | 0.87 | 9 | (1.0) | 8.5 | (0.7) | 0.59 |
| Non-ambulatory | ||||||||||
| Parent | Adult | |||||||||
| Question | Emflaza™/Deflazacort (n = 45) | Prednisone/Prednisolone (n = 22) | Emflaza™/Deflazacort (n = 11) | Prednisone/Prednisolone (n = 8) | ||||||
| Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | p-value | |
| Given your overall experience, which is most true about your experience with steroids: • Side effects outweigh benefits (0) • Benefits outweigh side effects (10) | 7.7 | (1.9) | 7.4 | (2.0) | 0.49 | 7.9 | (1.1) | 7.6 | (1.6) | 0.25 |
| Overall, how satisfied are you with steroids as a treatment? • Not at all (0) • Very much (10) | 7.0 | (2.2) | 6.3 | (2.5) | 0.87 | 6.2 | (2.4) | 7.1 | (2.1) | 0.86 |
| Given your overall experience, how likely are you to recommend steroids to someone like you? • Not at all (0) • Very much (10) | 7.9 | (2.0) | 6.8 | (2.6) | 0.25 | 8.0 | (1.9) | 7.0 | (2.4) | 0.59 |
DISCUSSION
This study provides qualitative and quantitative data related to the benefits and side effects from corticosteroids when used to treat Duchenne muscular dystrophy. Understanding patient and caregiver experience with corticosteroids is important because corticosteroids are the only treatment option available to all people with Duchenne as standard of care, and thus experience data from corticosteroids is an appropriate comparator for new treatment options. Vital benefits were maintaining heart, lung, and arm function, getting around better, and slowing the progression of weakness. Across all groups, participants’ ratings of current or past experience of benefits to heart and lung function were not as high as their ratings of the importance of those benefits, indicating some degree of mismatch between the highly-valued benefits and experienced benefits. Based on our qualitative data, this may be explained by the difficulty that participants had in identifying whether they experienced a heart or lung treatment benefit. Cardiac and pulmonary symptoms become exacerbated later in disease progression, and participants have no benchmark for what progression would have been without corticosteroid intervention. Our data suggest that providers should explore with people with Duchenne and their caregivers any evidence of corticosteroid benefits to heart and lung function, or lack thereof.
Similarly, concern for increased fracture risk and prediabetes/diabetes is high; clinicians should anticipate and address those concerns through regular monitoring (as consistent with Duchenne care guidelines [3.4]) and discuss the results with adults and/or caregivers. While a range of side effects were described as impacting quality of life and rated as important and relatively common, individuals using corticosteroids and their caregivers reported that benefits outweigh the side effects. Interview participants indicated few regrets about corticosteroid use, and survey participants rated that they were satisfied with the treatment. Qualitative data indicate that one component of the acceptability of corticosteroids is that there are few, if any, treatment alternatives available to our study population.
We found no significant difference in net corticosteroid benefit score based on corticosteroid type or based on whether the respondent was the parent or the adult with Duchenne. Only ambulatory status was significant, with the ambulatory group having a modestly higher mean benefit rating than the non-ambulatory group— which is not surprising given that Duchenne progression continues even with corticosteroid use. In addition, there was no effect of corticosteroid type on net side effect rating. Only respondent type was statistically significant with parents reporting, on average, a nearly 8 point higher side-effect score than adult self-reporters. It may be that parents find it more acceptable to report on side effects than people living with Duchenne, or that there is a real difference in perceived side effect burden; our qualitative data suggest the latter, though additional research on this topic is warranted.
LIMITATIONS
This report includes data only from those using corticosteroids. Additional research should be done to explore the reasons why caregivers and individuals with Duchenne decline corticosteroids, especially as new treatment options become available. All data in this study are provided through self-report. Of note, six adults who self-reported with Duchenne rated themselves as ambulatory, which is inconsistent with the adult phenotype of Duchenne. These individuals may have the less-severe form called Becker muscular dystrophy, or they may have inaccurately rated their ambulatory status. Given the desire of Parent Project Muscular Dystrophy to be inclusive to all who report a Duchenne diagnosis, these individuals were maintained in the analysis.
The survey results are limited by a relatively low response rate and a small sample size of adult participants; additional research is needed to confirm the findings. Respondents in the interview phase provided important context in the interpretation of these findings, which is that patients and caregivers must theorize potential treatment benefits (i.e., many of our participants indicated that they have no way of knowing how their disease would have progressed if they were not on corticosteroids) but that treatment-related side effects are more readily attributable to the use of corticosteroids. While this may be true of most treatment experience studies, this is especially important in the context of a progressive disorder.
There may be additional response bias related to reporting on some of the steroid side effects. For example, adult participants may have under-reported behavioral side effects and delayed puberty due to the negative connotation associated with those features. We also ask participants to think back to prior benefits or side effects, and think ahead to anticipated corticosteroid impact, which can lead to additional bias in response. Finally, we did not require a minimal duration of use for inclusion criteria, so some respodents had less experience overall.
CONCLUSIONS
Our data on corticosteroid experience in Duchenne are informative to clinical care and drug development. Corticosteroid use is common in Duchenne, as it is the only FDA approved therapy that is available to all affected individuals; not all parents and people with Duchenne, however, choose to use corticosteroids as a treatment option [24]. These data may also be useful for parents who are deciding whether to initiate corticosteroid use in young children. The results, particularly on benefit and side effect ratings reported by adults, may also be useful to teens/adults who are making choices about initiating, re-initiating, or stopping corticosteroids.
Perceptions of treatment experiences and treatment preferences may vary based on the availability of other treatment options. These results should be interpreted in the context of the current treatment options and the progressive trajectory of Duchenne. New treatment options that may become widely available (e.g., to adults) and that come with a more favorable benefit/risk balance are likely to change how people with Duchenne think and feel about the acceptability of long-term corticosteroid use.
ACKNOWLEDGMENTS
This study was funded by Parent Project Muscular Dystrophy with support from Catabasis Pharmaceuticals, PTC Therapeutics, Mallinckrodt Pharmaceuticals, and Santhera Pharmaceuticals.
REFERENCES
[1] | Hoffman Eric P. , Brown Robert H., Kunkel Louis M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell. (1987) ;51: (6):919–28, ISSN -0092-898 8674. https://doi.org/10.1016/0092-8674(87)90579-4 |
[2] | Florencia Giliberto , Claudia Pamela Radic , Leonela Luce , Verónica Ferreiro , Carlos de Brasi , Irene Szijan Symptomatic female carriers of Duchenne muscular dystrophy (DMD): Genetic and clinical characterization, Journal of the Neurological Sciences. ((2014) ) 336: (1–2):36–41, ISSN 0022--510X. https://doi.org/10.1016/j.jns.2013.09.036 |
[3] | Birnkrant David J , Bushby Katharine, Bann Carla M., Alman Benjamin A., Apkon Susan D., Blackwell Angela, et al., Diagnosis and management of Duchenne muscular dystrophy, part respiratory, cardiac, bone health, and orthopaedic management. The Lancet Neurology. (2018) ;17: (4):347–61, ISSN 1474-4422. https://doi.org/10.1016/S1474-4422(18)30025-5 |
[4] | Birnkrant DJ , Bushby K , Bann CM , Apkon SD , Blackwell A , Brumbaugh D , et al DMD Care Considerations Working GrouDiagnosis and management of Duchenne muscular dystrophy, part diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management, Lancet Neurol. (2018) ;17: (3):251–67. doi: 10.1016/S1474-4422(18)30024-3. |
[5] | Bernd Reitter , Deflazacort vs. prednisone in Duchenne muscular dystrophy: trends of an ongoing study, Brain and Development. ((1995) ) 17: (Supplement 1)39–43, ISSN 0387- 7604. https://doi.org/10.1016/0387-7604(95)00015-1 |
[6] | McDonald Craig M. , Henricson Erik K., Abresch Richard T., Duong Tina, Joyce Nanette C., Hu Fengming, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. The Lancet. (2018) ;391: (10119):451–61, ISSN 0140-6736. https://doi.org/10.1016/S0140-6736(17)32160-8 |
[7] | Sussman MD , Sienko SE , Buckon CE , Hilton C , De Mattos CB , d’Amato C Efficacy of corticosteroid in decreasing scoliosis and extending time to loss of ambulation in a single clinic: an effectiveness trial, Journal of Children’s Orthopaedics. 14: (5):421–432. https://doi.org/10.1302/1863-2548.14.200156. |
[8] | Bylo M , Farewell R , Coppenrath VA , Yogaratnam D A Review of Deflazacort for Patients with Duchenne Muscular Dystrophy, Ann Pharmacother. (2020) ;54: (8):788–794. doi: 10.1177/1060028019900500. Epub 2020 Feb 4. PMID: 32019318. |
[9] | Gloss D , Moxley RT 3rd , Ashwal S , Oskoui M Practice guideline update summary: Corticosteroid treatment of Duchenne muscular dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology, Neurology. (2016) ;86: (5):465–72. doi: 10.1212/WNL.0000000000002337. |
[10] | Birnkrant David J. , Bushby Katharine, Bann Carla M., Alman Benjamin A., Apkon Susan D., Blackwell Angela, et al., Diagnosis and management of Duchenne muscular dystrophy, part respiratory, cardiac, bone health, and orthopaedic management. The Lancet Neurology. (2018) ; 17: (4): 347–61, ISSN 1474-4422. https://doi.org/10.1016/S1474-4422(18)30025-5 |
[11] | Hendriksen RGF , Lionarons JM , Hendriksen JGM , Vles JSH , McAdam LC , Biggar WD Development of a New Self-Reporting Instrument Measuring Benefits and Side Effects of Corticosteroids in Duchenne Muscular Dystrophy: Report from a Pilot Study, J Neuromuscul Dis. (2017) ;4: (3):217–36. doi: 10.3233/JND-170223 |
[12] | Kourakis S , Timpani CA , Campelj DG , Hafner P , Gueven N , Fischer D , Rybalka E Standard of care versus new-wave corticosteroids in the treatment of Duchenne muscular dystrophy: Can we do better? Orphanet J Rare Dis. (2021) ;16: (1):117. doi: 10.1186/s13023-021-01758-9 |
[13] | Chalasani M , Vaidya P , Mullin T Enhancing the incorporation of the patient’s voice in drug development and evaluation, Res Involv Engagem. ((2018) ) 4: :10 doi: 10.1186/s40900-018-0093-3 |
[14] | Crossnohere NL , Fischer R , Crossley E , Vroom E , Bridges JF The evolution of patient-focused drug development and Duchenne muscular dystrophy, Expert Rev Pharmacoecon Outcomes Res. (2020) ;20: (1):57–68. doi: 10.1080/14737167.2020.1734454. |
[15] | Hollin IL , Caroline Young , Hanson C , Bridges JFP , Peay H Developing a Patient-Centered Benefit-Risk Survey: A Community-Engaged Process, Value Health. (2016) ;19: (6):751–7. doi: 10.1016/j.jval.2016.02.014. |
[16] | Porter K , Fischer R , Peay H Decision-making and benefit-risk tradeoffs for Duchenne muscular dystrophy treatment, Value in Health. ((2021) ) 24: :S142. doi: https://doi.org/10.1016/j.jval.2021.04.701 |
[17] | Qualtrics. First release: 2005, Location: Provo, Utah, USA, (2020) . Available at https://www.qualtrics.com |
[18] | Beebe J. Rapid assessment process: An introduction: Rowman Altamira; (2001) . |
[19] | Hamilton A , editor Qualitative methods in rapid turnaround health services 685 research. VA HSR&D Cyberseminar Spotlight on Women’s Health; (2013) ; Online. |
[20] | Vindrola-Padros C , Johnson GA Rapid Techniques in Qualitative Research: A Critical Review of the Literature, Qual Health Res. (2020) ;30: (10):1596–604. doi: 10.1177/1049732320921835 |
[21] | Halcomb EJ , Davidson PM Is verbatim transcription of interview data always necessary? Appl Nurs Res. (2006) ;19: (1):38–42. doi: 10.1016/j.apnr.2005.06.001 |
[22] | Averill JB Matrix analysis as a complementary analytic strategy in qualitative inquiry, Qual Health Res. (2002) ;12: (6):855–66. doi: 10.1177/104973230201200611. |
[23] | Miles MB , Huberman AM Qualitative data analysis. Thousand Oaks, CA: Sage; (1994) . |
[24] | The Food and Drug Administration, FDA approves drug to treat Duchenne muscular dystrophy, https://www.fda.gov/news-events/press-announcements/fda-approves-drug-treat-duchenne-muscular-dystrophy; (2017) [accessed 6 June 2022]. |




