Longitudinal Analysis of PUL 2.0 Domains in Ambulant and Non-Ambulant Duchenne Muscular Dystrophy Patients: How do they Change in Relation to Functional Ability?
Abstract
Background:
The performance of upper limb 2.0 (PUL) is widely used to assess upper limb function in DMD patients. The aim of the study was to assess 24 month PUL changes in a large cohort of DMD patients and to establish whether domains changes occur more frequently in specific functional subgroups.
Methods:
The PUL was performed in 311 patients who had at least one pair of assessments at 24 months, for a total of 808 paired assessments. Ambulant patients were subdivided according to the ability to walk: >350, 250–350, ≤250 meters. Non ambulant patients were subdivided according to the time since they lost ambulation: <1, 1-2, 2–5 or >5 years.
Results:
At 12 months, the mean PUL 2.0 change on all the paired assessments was –1.30 (–1.51––1.05) for the total score, –0.5 (–0.66––0.39) for the shoulder domain, –0.6 (–0.74––0.5) for the elbow domain and –0.1 (–0.20––0.06) for the distal domain.
At 24 months, the mean PUL 2.0 change on all the paired assessments was –2.9 (–3.29––2.60) for the total score, –1.30 (–1.47––1.09) for the shoulder domain, –1.30 (–1.45––1.11) for the elbow domain and –0.4 (–1.48––1.29) for the distal domain.
Changes at 12 and 24 months were statistically significant between subgroups with different functional abilities for the total score and each domain (p < 0.001).
Conclusion:
There were different patterns of changes among the functional subgroups in the individual domains. The time of transition, including the year before and after loss of ambulation, show the peak of negative changes in PUL total scores that reflect not only loss of shoulder but also of elbow activities. These results suggest that patterns of changes should be considered at the time of designing clinical trials.
INTRODUCTION
Duchenne Muscular dystrophy (DMD) is a progressive disease due to mutations in the dystrophin gene on Xp21 [1]. Over the last few years several therapeutical options have been used in clinical trials, with some of them being approved by regulators. Because of this special attention has been paid to the identification of outcome measures that could be used both in clinical and research settings. The Performance of upper limb (PUL) is a functional scale specifically designed to assess upper limb function in both ambulant and non-ambulant patients [2, 3]. The scale is designed to assess functional activities in three domains (shoulder, elbow and distal) with a total score that allows to follow the proximal to distal progression occurring in DMD over the years. A few studies have reported a progressive loss of scores both in the total score and in the three domains suggesting that the progression is not linear [3–6]. As both the original version and the revised 2.0 version have been increasingly used in clinical trials [7], there is the need to ascertain whether the progression in the different domains is related to the overall functional abilities. A previous study from our network has reported that in ambulant patients the correlation between the PUL and the six-minute walk test was not linear (0.499), indicating that the ratio of change is not constant [5].
It has also been reported that the progression in ambulant and non-ambulant patients is different and that ambulation status was associated to the slope of Performance of Upper Limb changes [8]. As both ambulant and non-ambulant patients are quite heterogeneous groups that could be further stratified, the question has arisen whether more specific patterns of progressions in the three domains can be identified in patients with different functional abilities.
The aim of the study was to assess 24-month PUL changes in a large cohort of DMD patients and to establish whether changes in individual domains occurred more frequently in specific functional subgroups.
MATERIAL AND METHODS
Cohort selection and dataset definition
Patients were recruited between September 2011 and January 2022. All patients who had a genetic diagnosis of DMD were included. The study was approved by the institutional review board (ethics committee) of the 14 national tertiary participating centers (Catholic University, Rome; Centro Clinico Nemo, University of Milano, Milan; IRCCS Eugenio Medea Bosisio-Parini, Bosisio-Parini; IRCCS Istituto Giannina Gaslini, Genoa; University of Messina, Messina; IRCCS Ospedale San Raffaele, Milan; Fondazione IRCCS Istituto Neurologico Besta, Milan; Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico, Milan; University of Napoli, Naples; Ospedale Bambino Gesù, Rome; University of Padua, Padua; Istituto Mondino, Pavia; University of Turin, Turin; Neuromuscular Pediatric Unit, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna).
Written informed consent was obtained from all participants (or guardians of participants) in the study.
PUL 2.0
The PUL 2.0 is a functional scale specifically designed to assess upper limb function in DMD patients. It includes an entry item to define the broad starting functional level, and 22 items subdivided into shoulder level (6 items, max score 12), elbow (9 items, max score 17) and distal level (7 items, max score 13) dimension [3, 8]. Each domain (shoulder, elbow, distal) can be scored separately. A total score can be achieved by adding the three level scores (max global score 42). Details of the training sessions and of the reliability studies have already been reported for the original PUL version (ICC of 0.96) and the PUL 2.0 (Person Separation Index (0.95) [9, 10]. New training sessions were performed for the new scale with similar level of agreement [8]. A copy of the PUL 2.0 scoresheet and manual is available at www.opentact.org.
Statistical analysis
A longitudinal dataset with 24-month paired visits was analyzed to quantify differences in 24-month PUL changes among patients with different ambulatory status. Ambulatory status was defined as from the functional status recorded during the 24 month of the study. The ambulant population was subdivided on the basis of meters walked on the 6MWT at baseline >350, ≤350, <250). Patients who were ambulant at baseline but losing ambulation during the duration of the study were also defined as transitioning patients. The non ambulant population was subdivided as follow: patients losing ambulation within 12 months before baseline, patients who lost ambulation between 12 and 24 months before baseline, patients who lost ambulation between 2 and 5 years before baseline, patients who lost ambulation more than 5 years before baseline.
Loss of ambulation was defined as the inability of the patient to walk 10 meters independently.
As the aim of this paper was to describe the PUL changes at each level of ambulatory status, this was assessed by considering each paired assessment as independent from each other and only related to the ambulatory status at the time the paired assessments were performed and not at the very first assessment. Therefore, assessments from patients with a longer follow-up but different ambulatory status overtime are represented in each subgroup. To account for the multiple assessment per participants, mean and 95% CI were obtained by a mixed model (with an unstructured covariance matrix) able to account for correlations of measurements within the same individual. Pairwise comparisons were adjusted with the Tukey-Kramer method.
As patients were only included if they had at least 24 month follow up, missing PUL values at 24 months were excluded from the longitudinal analysis. In case of missing at 12 months, after having assessed the random distribution of missing data, these were replaced by non-linear interpolation (geometric mean, exponential interpolation) (<10% of the included data). Significance level for statistical tests was set at.05. All data processing steps and statistical analysis was performed in SPSS version v27, SAS version 9.4 (Institute Inc., Cary, NC) and RStudio. Version 1.4.1717.
Data sharing and data accessibility
The data that support the findings of this study are available from the corresponding author upon reasonable request.
RESULTS
Whole cohort
Three-hundred-eleven patients had at least one pair of assessments at 24 months, for a total of 808 paired assessments.
At 12 months, the mean PUL 2.0 change on all the paired assessments was –1.30 (–1.51––1.05) for the total score, –0.5 (–0.66––0.39) for the shoulder domain, –0.6 (–0.74––0.5) for the elbow domain and –0.1 (–0.20––0.06) for the distal domain.
At 24 months, the mean PUL 2.0 change on all the paired assessments was –2.9 (–3.29––2.60) for the total score, –1.30 (–1.47––1.09) for the shoulder domain, –1.30 (–1.45––1.11) for the elbow domain and –0.4 (–1.48––1.29) for the distal domain.
There was a significant score difference from baseline to 12 and 24 months (p < 0.001) and from 12 to 24 months (p < 0.001) on the total and on every domain (shoulder, elbow) score. In the distal domain the statistical differences was p < 0.001 between every timepoint beside baseline to 12 m (p = 0.494).
3.2Ambulatory status
Of the 808 paired assessments, 277 were from patients who walked >350 m, 122 were from patients who walked <350 m, 52 were from patients who walked <250 m, 90 from patients who had lost ambulation 12 months before baseline, 38 from patients who had lost ambulation between 12 and 24 months before baseline, 100 from patients who had lost ambulation between 2 and 5 years before baseline, 129 from patients who had lost ambulation more than 5 years before baseline. Details of number of patients involved, changes at 12 and 24 months per total score and each subdomain can be found in Table 1 and Fig. 1. Supplementary table 1 provides details on total score and each subdomain for the non-ambulatory population by PUL 2.0 entry item.
Table 1
12 and 24- month PUL 2.0 changes subdivided by ambulatory status
| >350 mt | <350 mt | <250 mt | <12 m | 12– 24 m | 2– 5 y | >5 y | ||
| (N = 277/105)* | (N = 122/56)* | (N = 52/21)* | (N = 90/30)* | (N = 38/19)* | (N = 100/33)* | (N = 129/46)* | ||
| TRANSITIONING | ||||||||
| No (N, %) | 271 (97.8%) | 85 (69.7%) | 7 (13.5%) | 90 (100%) | 38 (100%) | 100 (100%) | 129 (100%) | |
| Yes (N, %) | 6 (2.2%) | 37 (30.3%) | 45 (86.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| BASELINE | Age | |||||||
| Mean (95% CI) | 8.87 | 9.33 | 10.99 | 12.98 | 14.32 | 16.17 | 19.66 | |
| (8.39– 9.36) | (8.82– 9.84) | (10.38– 11.60) | (12.43– 13.52) | (13.64– 15.01) | (15.61– 16.72) | (19.04– 20.29) | ||
| CS treatment | ||||||||
| No (N, %) | 25 (9.0) | 24 (19.7) | 5 (9.6) | 6 (6.7) | 5 (13.2) | 26 (26.0) | 65 (50.4) | |
| Yes (N, %) | 252 (91.0) | 98 (80.3) | 47 (90.4) | 84 (93.3) | 33 (86.8) | 74 (74.0) | 64 (49.6) | |
| 6MWT | ||||||||
| Mean (95% CI) | 424.21 | 315.09 | 173.62 | |||||
| (417.18– 431.24) | (305.79– 324.39) | (160.04– 187.20) | ||||||
| TOTAL SCORE PUL 2.0 | ||||||||
| Mean (95% CI) | 38.80 | 36.20 | 35.93 | 30.53 | 23.50 | 18.29 | 13.68 | |
| (37.91– 39.70) | (35.22– 37.18) | (34.70– 37.16) | (29.46– 31.60) | (22.11– 24.88) | (17.22– 19.36) | (12.49– 14.87) | ||
| 12 MONTHS | TOTAL | |||||||
| Mean difference from baseline (95% CI) | – 0.17 | – 0.30 | – 2.87 | – 4.20 | – 2.82 | – 1.92 | – 0.93 | |
| (– 0.54 to 0.20) | (– 0.85 to 0.25) | (– 3.71 to – 2.03) | (– 4.83 to – 3.56) | (– 3.78 to – 1.85) | (– 2.53 to – 1.31) | (– 1.48 to – 0.38) | ||
| SHOULDER | ||||||||
| Mean difference from baseline (95% CI) | – 0.10 | – 0.28 | – 1.56 | – 1.93 | – 1.05 | – 0.51 | – 0.10 | |
| (– 0.31 to 0.10) | (– 0.59 to 0.04) | (– 2.05 to – 1.07) | (– 2.30 to – 1.56) | (– 1.61 to – 0.48) | (– 0.86 to – 0.16) | (– 0.40 to 0.22) | ||
| ELBOW | ||||||||
| Mean difference from baseline (95% CI) | – 0.04 | – 0.20 | – 1.23 | – 2.02 | – 1.35 | – 1.07 | – 0.46 | |
| (– 0.23 to 0.15) | (– 0.49 to 0.09) | (– 1.67 to – 0.79) | (– 2.35 to – 1.69) | (– 1.86 to – 0.84) | (– 1.39 to – 0.75) | (– 0.74 to – 0.18) | ||
| DISTAL | ||||||||
| Mean difference from baseline (95% CI) | 0.02 | 0.16 | – 0.09 | – 0.26 | – 0.39 | – 0.38 | – 0.31 | |
| 24 MONTHS | TOTAL | (– 0.10 to 0.13) | (– 0.02 to 0.33) | (– 0.37 to 0.18) | (– 0.47 to – 0.06) | (– 0.71 to – 0.08) | (– 0.58 to – 0.19) | (– 0.48 to – 0.14) |
| Mean difference from baseline (95% CI) | – 1.14 | – 1.79 | – 6.36 | – 7.74 | – 4.85 | – 3.32 | – 2.31 | |
| (– 1.77 to – 0.51) | (– 2.64 to – 0.95) | (– 7.59 to – 5.13) | (– 8.70 to – 6.77) | (– 6.24 to – 3.46) | (– 4.25 to – 2.40) | (– 3.21 to – 1.40) | ||
| SHOULDER | ||||||||
| Mean difference from baseline (95% CI) | – 0.72 | – 1.12 | – 3.86 | – 3.72 | – 1.91 | – 0.60 | – 0.24 | |
| (– 1.59 to – 0.64) | (– 4.55 to – 3.17) | (– 4.26 to – 3.18) | (– 2.69 to – 1.13) | (– 1.12 to – 0.08) | (– 0.75 to 0.26) | |||
| ELBOW | ||||||||
| Mean difference from baseline (95% CI) | – 0.27 | – 0.71 | – 2.26 | – 3.62 | – 2.43 | – 2.05 | – 0.94 | |
| (– 0.56 to 0.02) | (– 1.12 to – 0.30) | (– 2.87 to – 1.65) | (– 4.09 to – 3.16) | (– 3.12 to – 1.75) | (– 2.49 to – 1.60) | (– 1.37 to – 0.51) | ||
| DISTAL | ||||||||
| Mean difference from baseline (95% CI) | – 0.09 | 0.003 | – 0.30 | – 0.44 | – 0.59 | – 0.76 | – 1.08 | |
| (– 0.26 to 0.08) | (– 0.24 to 0.25) | (– 0.67 to 0.07) | (– 0.72 to – 0.15) | (– 1.01 to – 0.17) | (– 1.03 to – 0.49) | (– 1.33 to – 0.82) |
Key to table: * = number of observation/number of patients, CS = Corticosteroid treatment. Mean and 95% CI were obtained by linear mixed models with ambulatory status as dependent variable and unstructured covariance matrix.
Fig. 1
Baseline, 12 and 24 months PUL 2.0 raw score subdivided by ambulatory status. Key to figure: ambulant patients were subdivided according to their baseline 6MWT performance into those walking more than 350 meters, those between 250 and 350 meters and those walking less than 250 meters. Non ambulant patients were subdivided according to the time since loss of ambulation (less than 12 months, between 12 and 24 months, between 2 and 5 years and more than 5 years). Panel coding: A = Total PUL 2.0 score, B = Shoulder PUL 2.0 score, C = Elbow PUL 2.0 score, D = Distal PUL 2.0 score. Color coding: Red = All, Blue = fully ambulant patients, Green = Transitioning, Purple: non ambulant. Error bars = 1 Standard deviation.
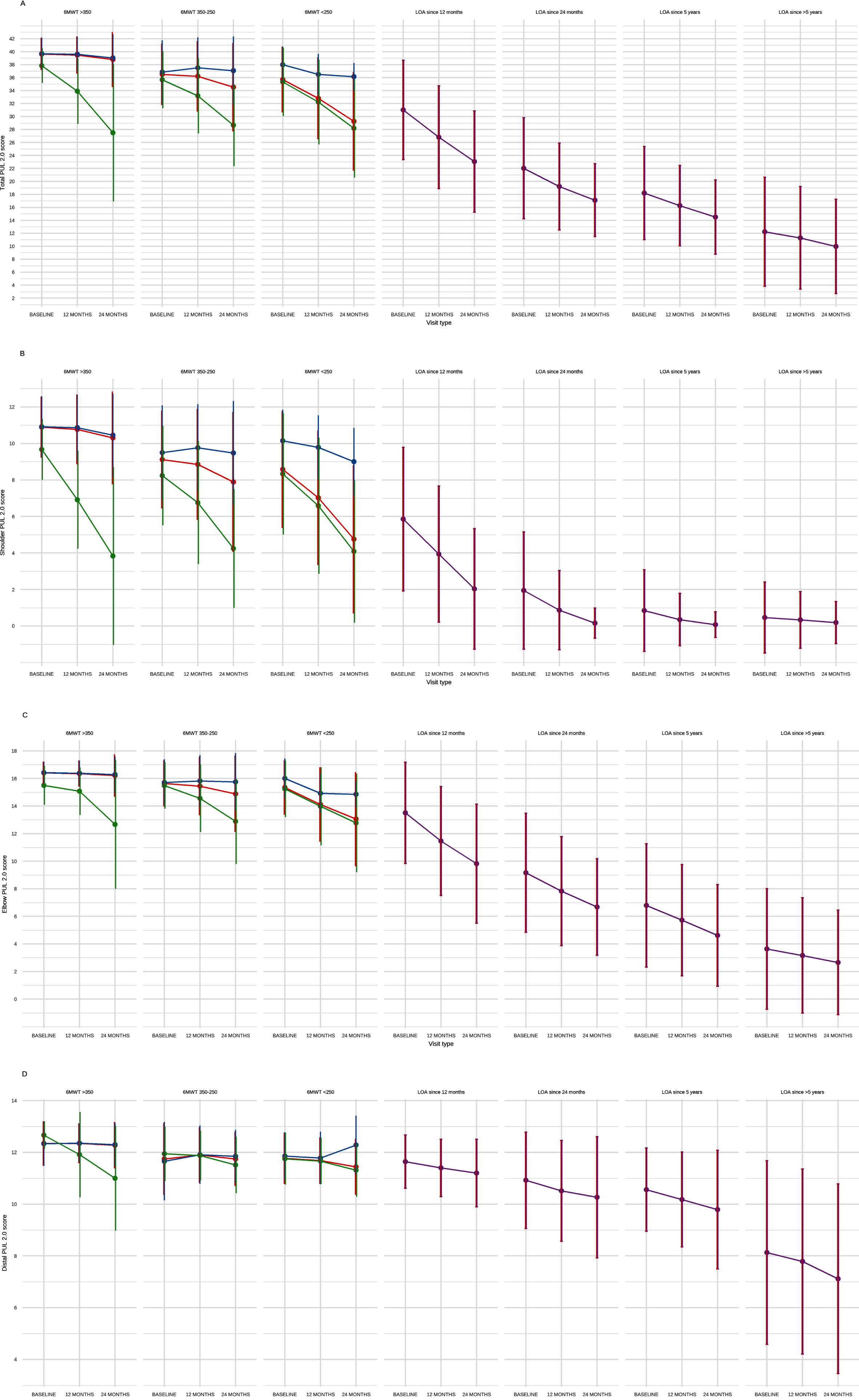
When subdividing the population by ambulatory status, there was a significant difference in raw score at baseline on the total and on every domain (shoulder, elbow, distal) (p < 0.001). Supplementary Figure 1 shows correlation between PUL 2.0 and 6MWT with Spearman correlation r-value.
Changes at 12 and 24 months were statistically significant between ambulatory status subgroup for the total score and for every domain (p x interaction between ambulatory status subgroup and time <0.001). Supplementary Figure 2 shows individual trajectories on the PUL 2.0 subdivided by ambulatory function. Supplementary table 2 shows p-value and confidence intervals for each subgroup on the total score and on every domain.
4DISCUSSION
The PUL test, both in the original version 1.2 and in the revised version 2.0, has been increasingly used both in natural history [3–6] and clinical trials. In clinical trials it is often the primary endpoint for non ambulant patients but it is also used in ambulant cohorts as it allows to identify early signs of upper limb involvement and to follow patients even if loss of ambulation occurs within the duration of the clinical trial. There is a progressive loss of PUL total scores with increasing age and recent studies have suggested that there are different slopes of progression across the three different domains [8]. The non linear progression is a potential challenge for trial design and there is an effort to identify factors that may help to predict the rate of progression for each individual domain, and, more generally, for the total score. This is particularly true if the trials include patients with different levels of functional ability, ranging from young non ambulant boys to patients who have lost ambulation for some time. The aim of our study was to ascertain if the functional abilities at baseline can predict the progression and the magnitude of changes in the different domains and, as a consequence, on the total PUL score. In order to do this, for the ambulant patients we selected two cut off points that have been previously used to identify patients with a more stable progression (>350 m on the 6MWT) [11–14] or who had a more rapid decline and were at higher risk of losing ambulation within two years (<200 m) [15]. Non ambulant patients were classified according to the time since loss of ambulation. The use of cut off points is always somehow limited by the non linear progression of the disease that makes it difficult to obtain a complete segregation of patients in each subgroups. This was partially compensated by the fact that the chosen cut off points were selected based on the distribution of findings from previous natural history studies and clinical trials [11, 12, 15–19].
Our results showed that there is a significant association between functional status and magnitude of PUL changes at 12 and 24 months. Within the group of ambulant boys, those who had a 6MWT >350 meters, who are known to be overall more stable on the 6MWT and the NSAA [14] were also stable on the PUL, with only 6 (2%) showing signs of deterioration on the PUL. With decreasing general functional ability, as measured by the 6MWT, there was an increase both in the number of patients showing deterioration and in the magnitude of the PUL changes. Ambulant boys with 6MWT less than 250 m, who are likely to lose ambulation within 2 years and therefore to transition from ambulant to non ambulant [15], had a decline in PUL that was mainly related to changes in the shoulder domain. These patients had a relatively high mean shoulder score at baseline (>8) and lost an average of –3.8 points at shoulder level over 2 years. As expected, most of these patients (n = 46; 86%) lost ambulation within 2 years. It is of interest that the remaining 14% who maintained ambulation in the two years had lower decline compared to those who lost ambulation suggesting an overall more stable course in both upper and lower limbs.
When we analyzed the non ambulant cohort this was stratified using time from LOA rather than age at assessment as, especially around puberty, the same age can be associated with a wide range of functional abilities [14]. Our results showed a progressive decrease of PUL scores at baseline and a parallel increase in the magnitude of changes with increased time since LOA. Patients who had lost ambulation within 12 months had overall lower total PUL scores at baseline (M: 30.53 (CI: 29.46–31.60)) than those in the most severe ambulant subgroup who were transitioning to non ambulation (<250 m) (M: 35.93 (CI: 34.70–37.16)), with a similar loss of scores mainly on the shoulder level at 24 months (M: –3.86 (CI: –4.55 to –3.17); M: –3.72 (CI: –4.26 to –3.18). Further loss at shoulder level, even if of lesser magnitude, was observed in the boys who had lost ambulation between 12 and 24 months (M: –1.91 (CI: –2.69 to –1.13)) while this was negligible in the groups with longer time since LOA as they already had very low shoulder scores at baseline and little to lose (M: –0.60 (CI: –1.12 to –0.08); M: 0.24 (CI: –0.75 to 0.26)).
It is of note that some loss of scores at elbow level could already be observed in the ambulant patients who were at risk of losing ambulation suggesting that although there is a clear proximal to distal gradient of progression, some involvement of the elbow domain can occur even in boys who still have relatively high shoulder scores. The peak of loss in elbow scores (Mean: –3.7 (SD:3.0)) was observed in the patients who had lost ambulation within 12 months but continued, even if with smaller changes, in those who had lost ambulation for a longer time.
When we analysed the distal domain, small changes were observed across the whole spectrum of functional abilities confirming previous observation that some distal activities, such as prono-supination [20–22], may be affected even in ambulant patients. The peak of loss of scores in the distal domain was in boys who had lost ambulation for more than 5 years (M: –1.08 (CI: –1.33 to –0.82)). It is of note that even in this group of patients, some activities in the distal domain were still preserved, with a mean distal score above 5.
In conclusion, our results confirm the non linear progression of the PUL total scores with different patterns of changes among the functional subgroups that are due to the different patterns of changes in the individual domains. The time of transition, including the year before and after LOA, show the peak of negative changes in PUL total scores that reflect not only loss of shoulder but also of elbow activities, the latter becoming more relevant in those who had already lost ambulation. These results suggest that these patterns of changes should be considered at the time of designing clinical trials for stratification, inclusion criteria or for their interpretation. Further work is in progress to establish the role of different genotypes, such as mutations eligible for skipping individual exons, in the variability of the PUL changes over time.
ACKNOWLEDGMENTS
We are grateful to the Italian Telethon (GUP 21003, Prof Pane; GSP 20001, Prof Mercuri) for the financial support to this study.
CONFLICT OF INTEREST STATEMENT
Senior authors in the study have, over the last few years, been involved in clinical trial as PI or have been involved in advisory boards but there is no conflict of interest and no influence on the topic reported in this study. This does not alter our adherence to policies on sharing data and materials.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-221556.
REFERENCES
[1] | Mercuri E , Muntoni F . Muscular dystrophies. Lancet. (2013) ;381: :845–60. |
[2] | Mayhew A , Mazzone ES , Eagle M , et al. Development of the Performance of the Upper Limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. (2013) ;55: :1038–45. |
[3] | Mayhew AG , Coratti G , Mazzone ES , et al. Performance of Upper Limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. 2019. |
[4] | Pane M , Fanelli L , Mazzone ES , et al. Benefits of glucocorticoids in non-ambulant boys/men with Duchenne muscular dystrophy: A multicentric longitudinal study using the Performance of Upper Limb test. Neuromuscul Disord. (2015) ;25: :749–53. |
[5] | Pane M , Mazzone ES , Sivo S , et al. The 6 minute walk test and performance of upper limb in ambulant duchenne muscular dystrophy boys. PLoS Curr. (2014) ;6: . |
[6] | Hogrel JY , Wary C , Moraux A , et al. Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology. (2016) ;86: :1022–30. |
[7] | McDonald CM , Marban E , Hendrix S , et al. Repeated intravenous cardiosphere-derived cell therapy in late-stage Duchenne muscular dystrophy (HOPE-2): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. (2022) ;399: :1049–58. |
[8] | Pane M , Coratti G , Brogna C , et al. Upper limb function in Duchenne muscular dystrophy: 24 month longitudinal data. PLoS One. (2018) ;13: :e0199223. |
[9] | Pane M , Mazzone ES , Fanelli L , et al. Reliability of the Performance of Upper Limb assessment in Duchenne muscular dystrophy. Neuromuscul Disord. (2014) ;24: :201–6. |
[10] | Mayhew AG , Coratti G , Mazzone ES , et al. Performance of Upper Limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. (2020) ;62: :633–9. |
[11] | Henricson E , Abresch R , Han JJ , et al. The 6-minute walk test and person-reported outcomes in boys with duchenne muscular dystrophy and typically developing controls: Longitudinal comparisons and clinically-meaningful changes over one year. PLoS Curr. (2013) ;5: . |
[12] | McDonald CM , Henricson EK , Abresch RT , et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. (2013) ;48: :343–56. |
[13] | McDonald CM , Henricson EK , Abresch RT , et al. THE 6-minute walk test and other endpoints in Duchenne muscular dystrophy: Longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. (2013) ;48: :343–56. |
[14] | Pane M , Mazzone ES , Sivo S , et al. Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes. PLoS One. (2014) ;9: :e108205. |
[15] | Mazzone ES , Pane M , Sormani MP , et al. 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PLoS One. (2013) ;8: :e52512. |
[16] | Goemans N , Klingels K , van den Hauwe M , et al. Six-minute walk test: Reference values and prediction equation in healthy boys aged 5 to 12 years. PLoS One. (2013) ;8: :e84120. |
[17] | Goemans N , Klingels K , van den Hauwe M , et al. Test-retest reliability and developmental evolution of the 6-min walk test in Caucasian boys aged 5-12 years. Neuromuscul Disord. (2013) ;23: :19–24. |
[18] | Mazzone E , Vasco G , Sormani MP , et al. Functional changes in Duchenne muscular dystrophy: A 12-month longitudinal cohort study. Neurology. (2011) ;77: :250–6. |
[19] | McDonald CM , Campbell C , Torricelli RE , et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD):Amulticentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017. |
[20] | Tartaglione T , Brogna C , Cristiano L , et al. Early involvement of the supinator muscle in Duchenne muscular dystrophy. Neuromuscul Disord. (2018) ;28: :62–3. |
[21] | Brogna C , Cristiano L , Tartaglione T , et al. Functional levels and MRI patterns of muscle involvement in upper limbs in Duchenne muscular dystrophy. PLoS One. (2018) ;13: :e0199222. |
[22] | Brogna C , Cristiano L , Verdolotti T , et al. Longitudinal motor functional outcomes and magnetic resonance imaging patterns of muscle involvement in upper limbs in duchenne muscular dystrophy. Medicina (Kaunas). (2021) ;57: . |




