Advances in Thymidine Kinase 2 Deficiency: Clinical Aspects, Translational Progress, and Emerging Therapies
Abstract
Defects in the replication, maintenance, and repair of mitochondrial DNA (mtDNA) constitute a growing and genetically heterogeneous group of mitochondrial disorders. Multiple genes participate in these processes, including thymidine kinase 2 (TK2) encoding the mitochondrial matrix protein TK2, a critical component of the mitochondrial nucleotide salvage pathway. TK2 deficiency (TK2d) causes mtDNA depletion, multiple deletions, or both, which manifest predominantly as mitochondrial myopathy. A wide clinical spectrum phenotype includes a severe, rapidly progressive, early onset form (median survival: < 2 years); a less severe childhood-onset form; and a late-onset form with a variably slower rate of progression. Clinical presentation typically includes progressive weakness of limb, neck, facial, oropharyngeal, and respiratory muscle, whereas limb myopathy with ptosis, ophthalmoparesis, and respiratory involvement is more common in the late-onset form. Deoxynucleoside monophosphates and deoxynucleosides that can bypass the TK2 enzyme defect have been assessed in a mouse model, as well as under open-label compassionate use (expanded access) in TK2d patients, indicating clinical efficacy with a favorable side-effect profile. This treatment is currently undergoing testing in clinical trials intended to support approval in the US and European Union (EU). In the early expanded access program, growth differentiation factor 15 (GDF-15) appears to be a useful biomarker that correlates with therapeutic response. With the advent of a specific treatment and given the high morbidity and mortality associated with TK2d, clinicians need to know how to recognize and diagnose this disorder. Here, we summarize translational research about this rare condition emphasizing clinical aspects.
DISORDERS OF MITOCHONDRIAL DNA MAINTENANCE
Defects in the replication, maintenance, and repair machinery of mitochondrial DNA (mtDNA), as well as encoding proteins involved in nucleotide metabolism, constitute a growing and genetically heterogeneous group of mitochondrial disorders [1, 2]. Alterations in genes involved in these processes lead to abnormalities in mtDNA quality (multiple deletions and point mutations), quantity (depletion), or both. These disease-associated, gene-encoded proteins can be classified into 4 functional groups: 1) proteins required for mtDNA replication, repair, and maintenance; 2) nucleotide metabolism enzymes; 3) factors involved in mitochondrial dynamics; and 4) proteins with other functions.
Enzymes (genes) directly required for mtDNA integrity include: polymerase γ (POLG) and its processivity subunit (encoded by POLG2), Twinkle helicase (TWNK), DNA replication helicase/nuclease 2 (DNA2), single-stranded DNA binding protein 1 (SSBP1), ribonuclease H1 (RNASEH1), and mitochondrial genome maintenance exonuclease 1 (MGME1) [3–8]. In addition to these factors directly involved in mtDNA upkeep, mtDNA integrity requires balanced pools of its deoxynucleoside triphosphate (dNTP) building blocks, which require enzymes such as: thymidine kinase 2 (TK2), ribonucleotide reductase M2 B subunit (RRM2B), deoxyguanosine kinase (dGK), and thymidine phosphorylase (TYMP) [9–12]. Two deoxyribonucleoside kinases that are expressed in mitochondria are responsible for phosphorylating 4 purine and pyrimidine deoxyribonucleosides: TK2, which phosphorylates deoxythymidine (dThd) and deoxycytidine (dCtd) to deoxythymidine monophosphate (dTMP) and deoxycytidine monophosphate (dCMP), which are further phosphorylated to generate the pyrimidine deoxynucleoside triphosphates (dNTPs) that are incorporated into replicating mtDNA; [13] and deoxyguanosine kinase (dGK), which phosphorylates deoxyguanosine and deoxyadenosine. TK2 is present in virtually all cells [13, 14]. TK2 also phosphorylates a number of pyrimidine nucleoside analogues, such as zidovudine (AZT) used in anti-HIV therapy, and thus may play an important role in the mitochondrial toxicity observed in antiviral and anticancer therapies using nucleoside analogues [15]. Thus, TK2 and dGK catalyze the phosphorylation of all 4 endogenous deoxynucleosides to their respective monophosphates and are therefore essential for mtDNA maintenance and, consequently, mitochondrial functions in tissues.
Autosomal recessive TK2 mutations cause TK2 deficiency (TK2d), a rare mitochondrial disease that manifests primarily as a myopathy. Because of its rarity, most clinicians are not aware of this disease. However, recognition and early diagnosis of this condition have become important as new therapies are being developed and a pharmacological therapy is being evaluated in clinical trials. This review provides a summary of the current understanding of TK2d and addresses the need for greater awareness of this disease.
TK2d: PRECLINICAL INVESTIGATION OF PATHOMECHANISMS AND THERAPY
Mouse models recapitulate the severe early onset human phenotype
In 2008, our group and Zhou et al. reported 2 models of Tk2-deficient mice: a knock-in model for a common pathogenic mutation, p.His121Asn in humans (p.His126Asn in mice) [16], and a knockout mouse using a targeting vector in which exon IV and part of exon V were replaced, producing a truncated protein [17]. In both models, homozygous mutant mice had normal development until 7 to 10 days of age; they then displayed a severe phenotype involving rapidly progressive weakness and fatal encephalomyopathy, initially manifesting as decreased ambulation, unstable gait, coarse tremor, and growth retardation. Most of the Tk2 mutant animals died at 14 to 16 days of age, with no animal surviving more than 30 days. In the knock-in model, mtDNA depletion was expressed in all analyzed organs but, curiously, the brain was the only organ that also showed early defects of oxidative phosphorylation (OXPHOS) complex proteins and activities. In the knock-out model, animals were progressively and severely hypothermic from 10 days of age, possibly due to brown adipose tissue or central nervous system (CNS) involvement.
Although both mouse models did not reproduce the human myopathic phenotype, they recapitulated many aspects of the severe form of TK2 encephalomyopathy, allowing for a deeper pathomechanistic understanding of the disease. These animal models confirmed the hypothesis that the loss of TK2 activity caused dNTP pool imbalances with low deoxythymidine triphosphate (dTTP) and deoxycytidine triphosphate (dCTP) levels and produced mtDNA depletion and defects of mitochondrial respiratory chain (RC) activity. In the knock-out model, mtDNA copy levels were normal at birth and progressively declined during the first 2 weeks of life, suggesting that the dNTP supply for mtDNA replication during fetal development is derived from de novo cytosolic dNTP synthesis. In the knock-in model, despite partial depletion of mtDNA in different tissues like heart, muscle, kidney, and liver, activities of mitochondrial OXPHOS enzymes and levels of mtDNA-encoded polypeptides were maintained at levels similar to controls, indicating transcriptional or translational compensation for the mtDNA defect [18].
Based on the mutant Tk2 mouse model data, Garone et al. developed a deoxycytidine monophosphate (dCMP) and deoxythymidine monophosphate (dTMP) supplementation therapy in the Tk2 knock-in mouse model to bypass the Tk2 biochemical defect [19]. Oral treatment of the Tk2 H126N knock-in mouse (Tk2KI) with dCtd + dThd (each at 260 or 520 mg/kg/day) delayed disease onset and prolonged the median lifespan of the animals by 2- to 3-fold in a dose-dependent manner. Thus, this therapy slowed, but did not stop, the disease process. Although efficacy of dTMP + dCMP therapy in the mouse model was established, rapid catabolism of these deoxynucleotides to dCtd + dThd suggested that deoxynucleosides were the active therapeutic agents.
TK1 and dCK play crucial roles in response to deoxynucleoside therapy
Thymidine kinase activity is present in cells as 2 enzymes: TK1, which is present at the cytosol, and TK2, which resides in the mitochondrial matrix [14]. TK1 converts cytosolic dThd to dTMP whereas cytosolic dCtd is phosphorylated by deoxycytidine kinase (dCK). Both dCMP and dTMP are transported from the cytosol to the mitochondria through pyrimidine nucleotide carriers PNC1 and PNC2 [20–22].
TK2, which is essential for intra-mitochondrial pyrimidine salvage, phosphorylates both dCtd and dThd to generate dCMP and dTMP, which are subsequently phosphorylated to dCTP and dTTP for incorporation into replicating mtDNA. dTTP self-regulates DNA precursor synthesis by negative feedback of TK1 [23]. TK1 activity declines sharply from high activity in S-phase replicating cells to low activity in quiescent cells. In contrast, TK2 expression is not cell-cycle dependent; therefore, its activity becomes functionally relevant after cell-cycle arrest when most of the cytosolic machinery that generates dNTPs is down-regulated. Thus, the period of pyrimidine salvage transition from the predominantly cytosolic TK1 to the mitochondrial TK2 determines when cells in different organs become susceptible to TK2 defects.
Dorado et al. showed that in wild-type mice, Tk2 activity is constant in the second week of life, while Tk1 activity decreases significantly between postnatal days 8 and 13, during the time period that Tk2KI mice begin to manifest weakness and growth retardation [18]. More recently, Lopez-Gomez et al. showed that despite greater bioavailability of dCtd and dThd with parenteral treatment over oral treatment in Tk2KI mice, there was no improvement in the phenotype or survival [24]. These data indicate that down-regulation of Tk1 in most murine tissues during development prevents those tissues from maintaining responses to the dCtd + dThd therapy. Thus, down-regulation of Tk1 activity unmasks Tk2 deficiency in Tk2KI mice and correlates with the early onset of severe mtDNA depletion in the brain and heart. On the other hand, in humans, with the exception of low TK2 activity in muscle [25], little is known about TK1 expression and TK2 activity of mutant proteins in affected tissues from patients. It has been proposed that the low basal activity of TK2 in muscle accounts for the vulnerability of this tissue and/or that the residual activity of the mutant TK2 protein also determines the phenotype.
Efficacy of AAV-mediated gene therapy alone or synergistically with deoxynucleoside in Tk2 knockin mice
In 2021, our group reported that adeno-associated virus serotype 9 (AAV9) delivery of human TK2 cDNA to Tk2KI mice efficiently rescued Tk2 activity in all the tissues tested except the kidneys, delayed disease onset, and increased lifespan [26]. Furthermore, addition of deoxycytidine and deoxythymidine supplementation to AAV9 + adeno-associated virus serotype 2 (AAV2) treated Tk2KI mice dramatically improved mtDNA copy numbers in the liver and kidneys, animal growth, and lifespan beyond changes seen with either deoxynucleoside therapy or gene therapy alone. Further studies are necessary, but these findings indicate that combining gene and pharmacological therapy might be a potent treatment for severe early onset cases.
TK2D: CLINICAL FEATURES
Phenotypic spectrum
Different subtypes of TK2d have been reported based upon retrospective assessments of age-at-onset with early onset variably defined as ≤1, ≤2, or ≤4 years, childhood-onset defined as > 1, > 2 or > 4 year and < 12 years, and late-onset as > 12 years [27–29]. However, in the absence of prospective natural history studies, it is not possible to determine which subtype classification is most accurate. Our clinical experience suggests there are still three forms but expressed on a wider spectrum ranging from early (infantile or ≤1 year), childhood- (> 1 to ≤12 years), and late-onset (> 12 years). (Table 1) In this review, we consider early onset to be ≤1 year. In general, early onset cases are associated with a rapid and progressive phenotype, while the disease progression in the late-onset form is also continuous and debilitating, but generally slower. (Figure 1) Although the isolated myopathic form is the most frequent presentation across age groups, in the early onset cases other organs can be affected, including the brain, heart, kidney, liver, and heart, manifesting as recurrent seizures, encephalopathy, cognitive impairment, episodic coma, sensorineural hearing loss, nephropathy, liver dysfunction/failure, or cardiac ventricular hypertrophy. In the childhood-onset form, TK2d has been associated with a spinal muscular atrophy (SMA)-like lower motor neuron presentation, as well as limb-girdle muscular dystrophy-like presentations [27, 28, 30–32]. In many childhood- and adult-onset cases, limb weakness is accompanied by chronic progressive external ophthalmoplegia (CPEO), ptosis, and severe involvement of oropharyngeal and respiratory muscles (although the brain and heart are spared), with occasional extra-muscular manifestations such as peripheral neuropathy and hearing loss [27–29].
Table 1
Clinical manifestations and muscle histological features of TK2d [27– 29]
| Early Onset (≤1 year) | Childhood-Onset (> 1 through 12 Years) | Late-Onset (> 12 years old) | |
| Clinical symptoms | •Early symptoms preceding evident motor weakness include: intestinal dysmotility, esophageal reflux with recurrent vomiting, and failure to thrive | •Progressive proximal limb myopathy, Gowers’ sign. In some cases, facial diplegia, CPEO, ptosis, and dysphagia | •Progressive proximal limb, axial neck flexor and facial muscle weakness frequently associated with ptosis, ophthalmoparesis, and bulbar weakness |
| •Usually, severe myopathic form. | •< 20% of the patients showed extraskeletal muscle involvement including hearing loss, cognitive decline, encephalopathy, prolonged QT, arrhythmia, multiple bone fractures, renal tubulopathy, and gynecomastia | •Diaphragmatic involvement is very characteristic, occurring in almost all patients with an early onset and slow progression. In some cases, CPEO+ respiratory phenotype | |
| •One-third of patients have extraskeletal muscle manifestations including: dysphagia, multiple bone fractures, nephropathy, rigid spine, coma episodes, and cardiomyopathy | |||
| •30% of patients have CNS involvement: seizures, encephalopathy, lissencephaly, cognitive impairment, microcephaly, bilateral optic atrophy | •Dysphagia and speech disturbances are common | ||
| •In some cases, hearing loss and sensory peripheral neuropathy | |||
| Histopathology | •Combined variable dystrophic alterations with increased fiber size variability, centrally located nuclei, and connective and fat tissue replacement with mitochondrial dysfunction evident as numerous COX-deficient fibers, ragged-red fibers, or both | ||
| •In some cases, “SMA-like” findings with atrophic and hypertrophic fibers distributed as single fibers or in groups and type 1 fiber preponderance | |||
| Laboratory findings | CKa elevation (usually > 1000 U/L), Lactic acidemiab, | CKa elevation (usually > 1000 U/L), Lactic acidemiab, | CKa levels ranging from normal to 2435 U/L |
| Transit elevation of liver enzymes (including ASTc, ALTd, GGTe, bilirubinf), | Transit elevation of liver enzymes (including ASTc, ALTd, GGTe, bilirubinf), | Slightly increased or normal lactate levelsb | |
| Phosphatase alkalineg elevation | Phosphatase alkalineg elevation | ||
| Biomarkers | Baseline GDF-15h (> 10,000 pg/mL, mean) | Baseline GDF-15h (> 1,000 pg/mL, mean) | Baseline GDF-15h (> 1,000 pg/mL, mean) |
| FGF-21i (> 1,000 pg/mL) | FGF-21i (100– 1000 pg/mL) | FGF-21i (100– 1000 pg/mL) | |
| Neuroimaging | In multisystemic forms: cerebral atrophy, and, less frequently, white matter and basal ganglia abnormalities | Usually normal | Usually normal |
| Differential diagnosis | •SMA type 1 or 2 •Pompe disease (acid maltase deficiency) •Congenital myopathies Other mtDNA-depletion syndromes (RRM2B, SUCLA2, SUCLG1) in encephalomyopathic forms | •Limb-girdle muscular dystrophy •SMA type 3 •Pompe disease •Ocular manifestations with facial weakness and dysphagia can mimic congenital myasthenic syndromes | •Autosomal-recessive CPEO due to multiple mtDNA deletions syndromes (primary mutations in POLG1, POLG2, TWNK, MPV17, DGUOK, and RRM2B) •SMA type 3 or 4 •Pompe disease •Limb-girdle muscular dystrophy •MYH2 myopathy |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CNS, central nervous system; COX, cytochrome c oxidase; CPEO, chronic progressive external ophthalmoplegia; FGF-21, fibroblast growth factor 21; GDF-15, growth/differentiation factor 15; GGT, gamma glutamyl transferase; mtDNA, mitochondrial DNA; MYH2, myosin heavy chain 2; RRFs, ragged-red fibers; SMA, spinal muscular atrophy. aCK reference range: males/females ≤3 months: not established; males > 3 months: 39–308 U/L, females > 3 months: 26–192 U/L [54]. bLactate reference range: 0–0.25 mmol/L [54]. cAST reference range: males/females 0–11 months: not established; males 1–13 years: 8–60 U/L, males ≥14 years: 8–48 U/L; females 1–13 years: 8–50 U/L, females ≥14 years: 8–43 U/L [54]. dALT reference range: males/females < 1 year: not established; males ≥1 year: 7–55 U/L; females ≥1 year: 7–45 U/L [54]. eGGT reference range: males/females 0–11 months: < 178 U/L, 12 months to < 6 years: < 21 U/L, 7–12 years: < 24 U/L; males 13–17 years: < 43 U/L, males ≥18 years: 8–61 U/L; females 13–17 years: < 26 U/L, females ≥18 years: 5–36 U/L [54]. fTotal bilirubin reference range: 7–14 days: < 15.0 mg/dL, 15 days to 17 years: ≤1 mg/dL, ≥18 years: ≤1.2 mg/dL [54]. gAlkaline phosphatase reference range: males/females 0–14 days: 83–248 U/L, 15 days to < 1 year: 122–469 U/L, 1 to < 10 years: 142–335 U/L, 10 to < 13 years: 129–417 U/L; males 13 to < 15 years: 116–468 U/L, males 15 to < 17 years: 82–331 U/L, males 17 to < 19 years: 55–149 U/L, males ≥19 years: 40–129 U/L; females 13 to < 15 years: 57–254 U/L, females 15 to < 17 years: 50–117 U/L, females ≥17 years: 35–104 U/L [54]. hGDF-15 reference range: ≥3 months: ≤750 pg/mL [54]. iFGF-21 reference range: < 350 pg/mL as calculated from 95th percentile of control subjects [47].
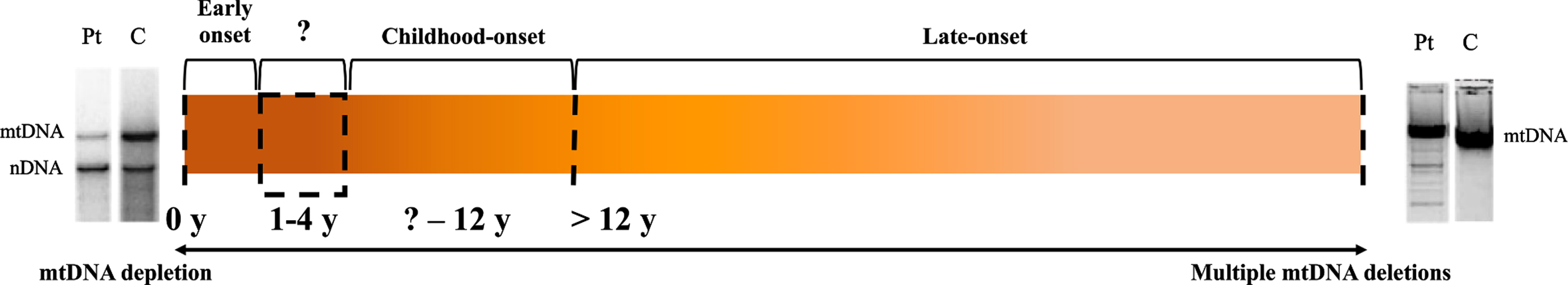
Fig. 1
Spectrum of TK2d Clinical Phenotypes and mtDNA Alterations. C, control; mtDNA, mitochondrial DNA; nDNA, nuclear DNA; Pt, patient; y, years. Varying onset and rates of progression in TK2d are indicated by the spectrum of orange hues. Early and childhood-onset cases characterized by mtDNA depletion are associated with a rapidly progressing, severe myopathy (as represented by the darker orange color). Multiple mtDNA deletions are observed in late-onset cases and patients with this form typically exhibit a slower progressing, yet still debilitating form of TK2d (noted as a progressive change in orange).
Early onset form (≤1 year old)
In the early onset form, symptoms that often precede motor weakness include intestinal dysmotility, esophageal reflux with recurrent vomiting, and failure to thrive. After a normal early motor developmental phase of variable duration, patients predominantly present with motor regression paired with proximal limb weakness and head drop, which progresses rapidly to fatality in the majority of cases within 1 year of follow-up due to respiratory failure [27, 28]. Approximately one-third of the infantile-onset patients have had non-skeletal muscle manifestations including: dysphagia, multiple bone fractures, nephropathy, rigid spine, coma episodes, cardiomyopathy, biventricular hypertrophy, arrhythmia, esophageal atresia, severe peripartum asphyxia, anemia, thrombosis, capillary leak syndrome, bilateral chylothorax, and occipital skin necrosis [27]. In addition, another one-third may have CNS abnormalities, including: seizures, encephalopathy, cognitive impairment, brain atrophy, lissencephaly, microcephaly, and bilateral optic atrophy. Hypertrophic cardiomyopathy is rare in TK2d and has been reported in only 3 cases. Liver steatosis has been reported in 1 patient [27] and has been described in 1 unpublished case; thus, additional studies should be undertaken, including liver ultrasound and gamma glutamyl transferase (GGT) measurement in TK2d associated with increased levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which may be elevated due to myopathy.
Childhood-onset form (> 1 to ≤12 years old)
In the childhood-onset form, which we believe may include patients with disease onset from > 1 to 12 years, moderate to rapidly progressive myopathy evolves and differentiates the intermediate phenotype spectrum of the disease between the rapidly progressive and sometimes multisystemic early onset and the more sluggishly progressive adult-onset phenotype. Typically, progressive proximal limb myopathy with Gowers signs, dropped head, and myopathic histological changes are seen. In many cases, facial diplegia, CPEO, ptosis, dysphagia, and restrictive lung disease are present and may be a clue to the diagnosis. Muscle weakness occasionally resembles SMA type 3 with a progressive clinical course leading to wheelchair-bound status in the majority (60%) of patients, and invasive or non-invasive ventilator dependency in 55% of patients [27]. Less than 20% of the patients showed non-skeletal muscle involvement including hearing loss, as well as 1 patient each with cognitive decline, encephalopathy, prolonged QT interval, arrhythmias, multiple bone fractures, renal tubulopathy, and gynecomastia. CNS involvement is uncommon (10%) [27].
Late-onset form (> 12 years old)
Domínguez-González reported 18 patients with a more homogeneous phenotypic pattern in late-onset TK2d than the early and childhood-onset forms. The patients manifested progressive proximal limb, scapular, neck, and facial muscle weakness frequently associated with ptosis, ophthalmoparesis, and lower bulbar weakness [29]. The mean age-at-onset was 31 years, but in retrospect, most patients had earlier subtle symptoms such as fatigue and exercise intolerance. In addition to typical mitochondrial disease manifestations such as limb myopathic weakness and CPEO, early respiratory muscle involvement was very characteristic occurring in almost all patients with slow progression, leading to non-invasive ventilation (NIV) in 12 of 18 (67%) of patients. In some cases, CPEO, restrictive lung disease, or both were disproportionately severe relative to limb muscle weakness. In fact, isolated CPEO may be the first clinical manifestation in very late-onset cases. Because respiratory muscle weakness is an early and subacute manifestation of TK2d, it is critical to screen for restrictive lung disease and nocturnal hypoventilation during clinical evaluation of these patients, regardless of the severity of the skeletal myopathy. Dysphagia and speech disturbances are common late-onset manifestations resulting in weight loss and anarthria in severe cases. In almost 30% of patients, gastrostromy tube was initiated on average 19.6 years after clinical onset of the disease [29]. Other less frequent manifestations included axonal sensory polyneuropathy, hearing loss, and infrequent cardiac involvement.
Although less severe than the early onset form of TK2d, the late-onset disease causes severe morbidity with muscle weakness impairing functions, restrictive lung disease requiring NIV in most patients, and gastrostomy tube for dysphagia in nearly one-third of patients.
Epidemiology
Mitochondrial diseases (MDs) are rare diseases but, among rare disorders, they are relatively frequent, with an overall estimated prevalence of 11.5:100,000 [33–36]. Mitochondrial DNA depletion/deletion syndrome (MDDS) is comprised of a discrete group of autosomal MDs characterized by significantly decreased mtDNA copy numbers, multiple mtDNA deletions, or both, in affected tissues. As in all ultra-rare disorders, it is challenging to accurately estimate the prevalence of TK2d, a relatively newly characterized and not widely known disease; therefore, this disease is likely to be underdiagnosed. More than 30 distinct TK2 pathogenic variants have been identified, making allele frequency calculations difficult. Additionally, TK2 has only recently been added to nuclear gene panels for MDDS and myopathies, and it is still not included in some gene panels for neuromuscular diseases and muscular dystrophies. We have estimated the prevalence of TK2d in a stepwise manner, applying established epidemiology of larger mitochondrial disease categories and utilizing peer-reviewed publications in which pools of patients from these larger categories were positively genotyped. There is no ethnic predilection for TK2d, allowing for extrapolation across regions, although our cohort of patients suggests an increased predilection for Hispanic ethnicity (Hirano and Berardo, personal observation). Mutation analysis of patients with different forms of MDDs found 12.5% of the screened patients had pathogenic TK2 variants [37]. An additional study that similarly screened a pool of MDDS myopathy patients for specific gene defects revealed 10% had pathogenic TK2 variants [31]. A third study found 18% of a diverse MDDS cohort had pathogenic TK2 mutations, while a minimum of 10% of MDDS myopathy patients and a maximum of 18% of all MDDS patients were found to have TK2d [38]. Based on the established prevalence rates for the larger categories of mitochondrial diseases referenced, one can estimate a minimum prevalence of TK2d at 600 patients and a maximum prevalence of 2700 TK2-deficient patients in the United States.
Diagnosis
Laboratory findings
HyperCKemia is a common finding in almost all patients with TK2d, with significant elevations (> 1000 U/L) in the early onset phenotype (Table 1). Other laboratory abnormalities include venous lactate, aspartate transaminase, alanine aminotransaminase, and lactate dehydrogenase elevations which are more prominent in the early onset form. Garone et al. reported creatine kinase (CK) elevations (272–6500 U/L) among 57 TK2d patients with transient normal values in only 2 patients with early onset myopathy and 4 patients with childhood-onset myopathy, indicating that a normal CK does not exclude TK2d [27]. Temporary CK elevations were observed in the setting of myoglobinuria (1 case) and acute rhabdomyolysis (2 cases).
Neuroimaging
When CNS is involved, neuroimaging may show cerebral atrophy and, less frequently, white matter and basal ganglia abnormalities, particularly in early onset patients [27, 28]. Other less frequent abnormalities include cerebellar (vermis) atrophy, immature myelination, and lissencephaly on brain magnetic resonance imaging or histological postmortem studies [27] and diffuse leukoencephalopathy with rapid loss of supratentorial white and grey matter [39].
Muscle biopsy findings
Unlike other forms of mitochondrial myopathy, muscle histology can show severe dystrophic alterations in TK2d with necrotic and regenerating myofibers, increased centrally located nuclei, fiber-size variability, and connective and fat tissue replacement. Muscle histological analysis of 36 patients summarized by Garone et al. showed myopathic changes, including atrophic and/or necrotic fibers, fiber size variability, increased central nuclei, type 1 predominance, sarcoplasmic vacuoles, lipid droplets or fat replacement of muscle tissue, and fibrosis or increase of connective tissue [27]. Muscle histochemistry typically shows mitochondrial dysfunction with numerous cytochrome c oxidase (COX)-deficient and/or ragged-red fibers (RRFs). “SMA-like” findings with atrophic and hypertrophic fibers, distributed in groups or single fibers, and type 1 fiber preponderance can be seen [30–32].
Levels of mtDNA measured in muscle tissue from 55 cases had an average of 14% mtDNA content. Patients with early onset TK2d manifest severe mtDNA depletion in muscle, while individuals with the more indolent late-onset form typically show multiple mtDNA deletions in muscle [27]. (Figure 1) In the 15 cases where mtDNA content was normal, the onset of disease occurred later, at an average age of 19 years. In contrast, OXPHOS enzyme activities have been shown to be more variable; only 20% to 74% of confirmed TK2d patients have had deficiencies in OXPHOS activities [28].
Molecular Genetics
The TK2 gene is located at chromosome 16q21 and spans 42,410 base pairs with 10 exons encoding a 265 amino acid protein. The TK2 mRNA length is 5114 nucleotides with a 3.8 kilobase long 3’-untranslanted region (NM_004614.5). Since the first description in 2001, there have been more than 100 patients with this gene described worldwide [12, 27–29, 40]. Pathogenic variants are identified in all 10 exons and there is no clear overall genotype–phenotype correlation. Frequent pathogenic variants include p.Thr108Met, p.Asn58Ser, p.Arg130Trp, p.Lys202del, p.His121Asn and p.Arg183Trp. The pathogenic variant p.Arg130Trp appears to be associated with the most severe phenotype, with CNS involvement during the first months of life [41]. Homozygosity for p.Arg183Trp has been associated with myopathy and severe mtDNA depletion restricted to skeletal muscle [42]. p.Lys202del (in either homozygosity or compound heterozygosity) has only been identified in individuals with adult-onset TK2d forms. In addition, p.Ala139Val and p.Phe70Ser were described in a severe-onset form with hypertrophic cardiomyo-pathy.
Differential diagnosis
Although the new era of massive sequencing has led us to a change in diagnostic approach, clinical clues are still important to refine our workup studies. Neck extensor weakness causing dropped head syndrome can also be an early manifestation in all age groups and indicates early axial muscle involvement. In the early onset form TK2 myopathy, alternative differential diagnoses include SMA type 1 or 2, Pompe disease, congenital myopathies, and other mtDNA-depletion syndromes, including RRM2B, SUCLA2, and SUCLG1 in the severe encephalomyopathic forms. Common renal tubulopathy/nephrocalcinosis, sensorineural hearing loss, and abnormal lipid deposition, rather than dystrophic features on muscle biopsy, may differentiate RRM2B from TK2. In contrast, SUCLA2 and SUCLG1 phenotypes frequently manifest as psychomotor delay rather than motor regression and include additional manifestations such as scoliosis/kyphosis, abnormal movement disorders like dystonia, athetoid or chorea, and hearing loss, which distinguish those disorders from the predominantly myopathic presentation of TK2d [44].
Motor neuron conditions such as SMA should be considered, since some reports showed electromyography findings consistent with a motor neuron disorder with active denervation with fibrillations and chronic partial reinnervation with severe loss of motor unit potentials [30, 45]. Although cardiomyopathy is not a frequent manifestation of TK2d, when present, the condition can be confused with Pompe disease. Nevertheless, the unique combination of prominent dystrophic changes and mitochondrial histochemical defects in the muscle biopsy are morphological clues to the diagnosis of TK2d as opposed to Pompe disease and other early onset neuromuscular disorders [27].
In the childhood-onset forms, where ambulation is preserved, SMA type 3 should be considered, as well as ocular manifestations with facial weakness and dysphagia which can mimic congenital myasthenic syndromes. Adult forms of TK2 myopathy, which manifests as CPEO, dysphagia, and muscle weakness, need to be included in the broad spectrum of autosomal CPEO with multiple mtDNA deletions due to mutations in genes such as POLG and POLG2, TWNK, MPV17, DGUOK, and RRM2B, as well as primary mtDNA point mutations and single deletions. The combination of ptosis and severe dysphagia can mimic oculopharyngeal muscular dystrophy, although extraocular and other voluntary muscles are affected later in the disease than in TK2d and display an autosomal dominant inheritance pattern. In late-onset TK2d with prominent respiratory muscle involvement, Pompe disease should always be considered as a differential diagnosis [29]. Prominent scapular winging and facial weakness may mimic fascioscapulohumeral dystrophy.
Treatment
Recently, an open-label, multicenter compassio-nate-use study enrolled 16 TK2d patients to assess the efficacy of oral deoxynucleoside monophosphates and deoxynucleoside treatment [40]. Some patients were initially treated with dTMP and dCMP and later switched to dThd and dCtd (all but one individual), when cell and mouse studies showed that deoxynucleosides (dThd, dCtd) were the active agents. Doses were titrated up to 400 mg/kg/day depending on tolerance. No major adverse effects were observed except for diarrhea, and in a few adult cases, transaminase elevation. The authors conclude that the therapy exerted striking effects on survival in early onset severe myopathy patients by increasing muscle strength, which enabled reduction or discontinuation of mechanical ventilation (2 of 4 patients) and gastrostomy feeding (2 of 3 patients), as well as allowing two patients to walk. In contrast to early onset patients, in late-onset patients therapy produced smaller beneficial effects, with stabilization or mild improvements in motor and respiratory functions, which are clinically important outcomes in this progressive disease. The greater therapeutic response in the early onset compared to late-onset subjects may be due to early intervention before irreversible muscle damage (e.g. fibrosis, fatty tissue replacement of muscle or both) or to greater reversibility of mtDNA depletion relative to mtDNA multiple deletions. Unfortunately, there are no data on muscle biopsies from subjects who have clinically improved on deoxynucleoside or deoxynucleotide therapy. To overcome biases and incomplete data collection inherent in an expanded access program lacking a unitary study protocol, a phase 2 prospective, open-label treatment study to assess the safety and efficacy of the dCtd/dThd compounds (MT1621 Modis/Zogenix Therapeutics) in all forms of TK2d patients is ongoing (NCT03845712) and additional clinical trials are being pursued with the intent to support approval in the US and EU.
Biomarkers
Because a potential treatment is being developed for this particular condition and promising drugs for MDs are under investigation in preclinical and clinical trial studies, specific and sensitive biomarkers are needed to select patients for further investigation and to monitor the disease progression and efficacy of treatment. In 2011, data from 67 patients with confirmed mitochondrial OXPHOS deficiencies and muscle disease suggested that fibroblast growth factor-21 (FGF-21), a member of the FGF family, might be applied as a first-line diagnostic test for these disorders [46]. FGF-21 is involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth, and invasion level. Additional publications suggested another growth factor, growth differentiation factor-15 (GDF-15), may be more sensitive and specific than FGF-21 for the diagnosis of MDs, and even measurement of both GDF-15 and FGF-21 improved disease detection ability, compared to either one independently [47–50]. Nevertheless, another publication showed that combining both biomarkers does not increase the diagnostic utility significantly over that for GDF-15 alone, indicating that there is little synergistic diagnostic benefit in using both biomarkers together [51]. In contrast, a recent prospective cohort study confirmed that induction of FGF-21 and GDF-15 is highly restricted to muscle-manifesting disorders caused by defects in mtDNA expression [52]. Analysis of both muscle and biomarkers showed that only 39% of patients with genetically verified mitochondrial disease showed mitochondrial pathology in muscle, while those biomarkers were elevated in 62% of patients with genetically verified mitochondrial disease [52].
For TK2 myopathy specifically, FGF-15 and GDF-15 levels were tested in serum from 24 patients with TK2d treated with oral deoxynucleosides [53]. Both levels were elevated above normal in all untreated patients at baseline and significantly higher in the infantile and childhood forms. After treatment, GDF-15 levels in all cases showed significant declines with time, which was accompanied by improvement in clinical outcome measures such as a 6-minute walk test and body mass index. Furthermore, in a few cases, discontinuation of the treatment was associated with GDF-15 elevation over time. In contrast, decreases in FGF-21 with treatment were less prominent and consistent. Although more data are required to understand the utility of these biomarkers in mitochondrial disease, for TK2d, GDF-15 appears to be a promising biomarker of disease severity and response to treatment with deoxynucleosides.
CONCLUSIONS
TK2 myopathy is considered an ultra-rare myopathy belonging to the MDDS spectrum. Clinical features include a wide range of manifestations at variable ages-at-onset. The early onset form manifests typically as a pure myopathy combining progressive limb, neck, facial, oropharyngeal, and respiratory muscle weakness, although severe encephalomyopathic forms have been described. In the childhood- and late-onset forms, limb myopathy is usually associated with ptosis, ophthalmoparesis, dysphagia, and respiratory involvement. Early onset TK2 myopathy should be included in the differential diagnosis of SMA, Pompe disease, congenital myopathies, and other MDDS; however, these diagnoses can be distinguished based upon clinical, electrophysiological, and muscle histological findings. Ultimately, genetic testing provides the definitive diagnosis; therefore, TK2 analysis should be included in congenital myopathy and muscular dystrophy panels, in light of recent developments leading towards a promising pharmacological treatment for this particular condition.
FUNDING
This work was supported by research grants from the National Institutes of Health (P01 HD32062), Department of Defense (W81XWH2010807) and Muscular Dystrophy Association (577392). MH is supported by the Arturo Estopinan TK2 Research Fund, Nicholas Nunno Foundation, JDM Fund for Mitochondrial Research, Shuman Mitochondrial Disease Fund, and the Marriott Mitochondrial Disease Clinic Research Fund (MMDCRF) from the J. Willard and Alice S. Marriott Foundation. MH also acknowledges support from NIH U54 NS078059 from NINDS and NICHD.
ACKNOWLEDGMENTS
Medical writing and editorial support, funded by Zogenix, Inc., was provided by Rachel Anantha, PhD, Michelle La Plante, ELS, and Scott Bergfeld, PhD of PharmaWrite, LLC (Princeton, NJ, USA).
DISCLOSURES
AB: None.
CDG: None.
KE: None.
MH: Paid consultant to Modis Therapeutics, a wholly owned subsidiary of Zogenix, Inc., and Entrada Therapeutics. These relationships are de minimus for Columbia University Medical Center. Columbia University has a patent, which is licensed by Modis Therapeutics; this relationship is monitored by an unconflicted external academic researcher. Received honoraria from the AAN and PlatformQ for speaking activities and research support from Modis Therapeutics and Entrada Therapeutics.
REFERENCES
[1] | Hirano M , Marti R , Ferreiro-Barros C , Vilà MR , Tadesse S , Nishigaki Y , et al. Defects of intergenomic communication: autosomal disorders that cause multiple deletions and depletion of mitochondrial DNA. Semin Cell Dev Biol. (2001) ;12: (6):417–27. |
[2] | López-Gómez C , Cámara Y , Hirano M , Martí R . 232nd ENMC international workshop: recommendations for treatment of mitochondrial DNA maintenance disorders [Available from: https://www.enmc.org/download/recommendations-for-treatment-of-mitochondrial-dna-maintenance-disorders/. |
[3] | Jurkute N , Leu C , Pogoda HM , Arno G , Robson AG , Nürnberg G , et al. SSBP1 mutations in dominant optic atrophy with variable retinal degeneration. Ann Neurol. (2019) ;86: (3):368–83. |
[4] | Kornblum C , Nicholls TJ , Haack TB , Schöler S , Peeva V , Danhauser K , et al. Loss-of-function mutations in MGME1 impair mtDNA replication and cause multisystemic mitochondrial disease. Nat Genet. (2013) ;45: (2):214–9. |
[5] | Longley MJ , Clark S , Yu Wai Man C , Hudson G , Durham SE , Taylor RW , et al. Mutant POLG2 disrupts DNA polymerase gamma subunits and causes progressive external ophthalmoplegia. Am J Hum Genet. (2006) ;78: (6):1026–34. |
[6] | Reyes A , Melchionda L , Nasca A , Carrara F , Lamantea E , Zanolini A , et al. RNASEH1 Mutations Impair mtDNA Replication and Cause Adult-Onset Mitochondrial Encephalomyopathy. Am J Hum Genet. (2015) ;97: (1):186–93. |
[7] | Spelbrink JN , Li FY , Tiranti V , Nikali K , Yuan QP , Tariq M , et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet. (2001) ;28: (3):223–31. |
[8] | Van Goethem G , Dermaut B , Löfgren A , Martin JJ , Van Broeckhoven C . Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet. (2001) ;28: (3):211–2. |
[9] | Bourdon A , Minai L , Serre V , Jais JP , Sarzi E , Aubert S , et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet. (2007) ;39: (6):776–80. |
[10] | Mandel H , Szargel R , Labay V , Elpeleg O , Saada A , Shalata A , et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat Genet. (2001) ;29: (3):337–41. |
[11] | Nishino I , Spinazzola A , Hirano M . Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. (1999) ;283: (5402):689–92. |
[12] | Saada A , Shaag A , Mandel H , Nevo Y , Eriksson S , Elpeleg O . Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet. (2001) ;29: (3):342–4. |
[13] | Wang L , Munch-Petersen B , Herrström Sjöberg A , Hellman U , Bergman T , Jörnvall H , et al. Human thymidine kinase molecular cloning and characterisation of the enzyme activity with antiviral and cytostatic nucleoside substrates. FEBS Lett. (1999) ;443: (2):170–4. |
[14] | Eriksson S , Munch-Petersen B , Kierdaszuk B , Arnér E . Expression and substrate specificities of human thymidine kinase 1, thymidine kinase 2 and deoxycytidine kinase. Adv Exp Med Biol. (1991) ;309b: :239–43. |
[15] | Arnaudo E , Dalakas M , Shanske S , Moraes CT , DiMauro S , Schon EA . Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet. (1991) ;337: (8740):508–10. |
[16] | Akman HO , Dorado B , López LC , García-Cazorla Á , Vilà MR , Tanabe LM , et al. Thymidine kinase 2 (H126N) knockin mice show the essential role of balanced deoxynucleotide pools for mitochondrial DNA maintenance. Hum Mol Genet. (2008) ;17: (16):2433–40. |
[17] | Zhou X , Solaroli N , Bjerke M , Stewart JB , Rozell B , Johansson M , et al. Progressive loss of mitochondrial DNA in thymidine kinase 2-deficient mice. Hum Mol Genet. (2008) ;17: (15):2329–35. |
[18] | Dorado B , Area E , Akman HO , Hirano M . Onset and organ specificity of Tk2 deficiency depends on Tk1 down-regulation and transcriptional compensation. Hum Mol Genet. (2011) ;20: (1):155–1564. |
[19] | Garone C , Garcia-Diaz B , Emmanuele V , Lopez LC , Tadesse S , Akman HO , et al. Deoxypyrimidine monophosphate bypass therapy for thymidine kinase 2 deficiency. EMBO Mol Med. (2014) ;6: (8):1016–27. |
[20] | Di Noia MA , Todisco S , Cirigliano A , Rinaldi T , Agrimi G , Iacobazzi V , et al. The human SLC25A33 and SLC25A36 genes of solute carrier family 25 encode two mitochondrial pyrimidine nucleotide transporters. J Biol Chem. (2014) ;289: (48):33137–48. |
[21] | Franzolin E , Miazzi C , Frangini M , Palumbo E , Rampazzo C , Bianchi V . The pyrimidine nucleotide carrier PNC1 and mitochondrial trafficking of thymidine phosphates in cultured human cells. Exp Cell Res. (2012) ;318: (17):2226–36. |
[22] | Palmieri F . The mitochondrial transporter family SLC identification, properties and physiopathology. Mol Aspects Med. (2013) ;34: (2-3):465–84. |
[23] | Munch-Petersen B . Enzymatic regulation of cytosolic thymidine kinase 1 and mitochondrial thymidine kinase a mini review. Nucleosides, Nucleotides & Nucleic Acids. (2010) ;29: (4-6):363–9. |
[24] | Lopez-Gomez C , Hewan H , Sierra C , Akman HO , Sanchez-Quintero MJ , Juanola-Falgarona M , et al. Bioavailability and cytosolic kinases modulate response to deoxynucleoside therapy in TK2 deficiency. EBio Medicine. (2019) ;46: :356–67. |
[25] | Saada A , Shaag A , Elpeleg O . mtDNA depletion myopathy: elucidation of the tissue specificity in the mitochondrial thymidine kinase (TK2) deficiency. Mol Genet Metab. (2003) ;79: (1):1–5. |
[26] | Lopez-Gomez C , Sanchez-Quintero MJ , Lee EJ , Kleiner G , Tadesse S , Xie J , et al. Synergistic deoxynucleoside and gene therapies for thymidine kinase 2 deficiency. Ann Neurol. 2021. |
[27] | Garone C , Taylor RW , Nascimento A , Poulton J , Fratter C , Domínguez-González C , et al. Retrospective natural history of thymidine kinase 2 deficiency. J Med Genet. (2018) ;55: (8):515–21. |
[28] | Wang J , Kim E , Dai H , Stefans V , Vogel H , Al Jasmi F , et al. Clinical and molecular spectrum of thymidine kinase 2-related mtDNA maintenance defect. Mol Genet Metab. (2018) ;124: (2):124–30. |
[29] | Domínguez-González C , Hernández-Laín A , Rivas E , Hernández-Voth A , Sayas Catalán J , Fernández-Torrón R , et al. Late-onset thymidine kinase 2 deficiency: a review of 18 cases. Orphanet J Rare Dis. (2019) ;14: (1):100. |
[30] | Mancuso M , Filosto M , Bonilla E , Hirano M , Shanske S , Vu TH , et al. Mitochondrial myopathy of childhood associated with mitochondrial DNA depletion and a homozygous mutation (T77M) in the TK2 gene. Arch Neurol. (2003) ;60: (7):1007–9. |
[31] | Mancuso M , Salviati L , Sacconi S , Otaegui D , Camaño P , Marina A , et al. Mitochondrial DNA depletion: mutations in thymidine kinase gene with myopathy and SMA. Neurology. (2002) ;59: (8):1197–202. |
[32] | Oskoui M , Davidzon G , Pascual J , Erazo R , Gurgel-Giannetti J , Krishna S , et al. Clinical spectrum of mitochondrial DNA depletion due to mutations in the thymidine kinase 2 gene. Arch Neurol. (2006) ;63: (8):1122–6. |
[33] | Macmillan CJ , Shoubridge EA . Mitochondrial DNA depletion: prevalence in a pediatric population referred for neurologic evaluation. Pediatr Neurol. (1996) ;14: (3):203–10. |
[34] | Rötig A , Kumar D . Mitochondrial genetics and genomics in clinical medicine. In: Kumar D, Eng C, editors. Genomic Med. 2nd ed. United Kingdom: Oxford University Press; (2014) . 131–47. |
[35] | Rötig A , Poulton J . Genetic causes of mitochondrial DNA depletion in humans. Biochim Biophys Acta. (2009) ;1792: (12):1103–8. |
[36] | Sarzi E , Bourdon A , Chrétien D , Zarhrate M , Corcos J , Slama A , et al. Mitochondrial DNA Depletion is a Prevalent Cause of Multiple Respiratory Chain Deficiency in Childhood. J Pediatr. (2007) ;150: (5):531–4.e6. |
[37] | Carrozzo R , Bornstein B , Lucioli S , Campos Y , de la Pena P , Petit N , et al. Mutation analysis in 16 patients with mtDNA depletion. Hum Mutat. (2003) ;21: (4):453–4. |
[38] | Spinazzola A , Invernizzi F , Carrara F , Lamantea E , Donati A , Dirocco M , et al. Clinical and molecular features of mitochondrial DNA depletion syndromes. J Inherit Metab Dis. (2009) ;32: (2):143–58. |
[39] | Mazurova S , Magner M , Kucerova-Vidrova V , Vondrackova A , Stranecky V , Pristoupilova A , et al. Thymidine kinase 2 and alanyl-tRNA synthetase 2 deficiencies cause lethal mitochondrial cardiomyopathy: Case reports and review of the literature. Cardiol Young. (2017) ;27: (5):936–44. |
[40] | Domínguez-González C , Madruga-Garrido M , Mavillard F , Garone C , Aguirre-Rodríguez FJ , Donati MA , et al. Deoxynucleoside therapy for thymidine kinase 2-deficient myopathy. Ann Neurol. (2019) ;86: (2):293–303. |
[41] | Lesko N , Naess K , Wibom R , Solaroli N , Nennesmo I , von Döbeln U , et al. Two novel mutations in thymidine kinase-2 cause early onset fatal encephalomyopathy and severe mtDNA depletion. Neuromuscul Disord. (2010) ;20: (3):198–203. |
[42] | Götz A , Isohanni P , Pihko H , Paetau A , Herva R , Saarenpää-Heikkilä O , et al. Thymidine kinase 2 defects can cause multi-tissue mtDNA depletion syndrome. Brain. (2008) ;131: (Pt 11):2841–50. |
[43] | Finsterer J , Zarrouk-Mahjoub S . Phenotypic and Genotypic Heterogeneity of RRM2B Variants. Neuropediatrics. (2018) ;49: (4):231–7. |
[44] | Carrozzo R , Verrigni D , Rasmussen M , de Coo R , Amartino H , Bianchi M , et al. Succinate-CoA ligase deficiency due to mutations in SUCLA2 and SUCLG phenotype and genotype correlations in 71 patients. J Inherit Metab Dis. (2016) ;39: (2):243–52. |
[45] | Pons R , Andreetta F , Wang CH , Vu TH , Bonilla E , DiMauro S , et al. Mitochondrial myopathy simulating spinal muscular atrophy. Pediatr Neurol. (1996) ;15: (2):153–8. |
[46] | Suomalainen A , Elo JM , Pietiläinen KH , Hakonen AH , Sevastianova K , Korpela M , et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. (2011) ;10: (9):806–18. |
[47] | Davis RL , Liang C , Edema-Hildebrand F , Riley C , Needham M , Sue CM . Fibroblast growth factor 21 is a sensitive biomarker of mitochondrial disease. Neurology. (2013) ;81: (21):1819–26. |
[48] | Kalko SG , Paco S , Jou C , Rodríguez MA , Meznaric M , Rogac M , et al. Transcriptomic profiling of TK2 deficient human skeletal muscle suggests a role for the p53 signalling pathway and identifies growth and differentiation factor-15 as a potential novel biomarker for mitochondrial myopathies. BMC Genomics. (2014) ;15: :91. |
[49] | Koga Y , Povalko N , Inoue E , Ishii A , Fujii K , Fujii T , et al. A new diagnostic indication device of a biomarker growth differentiation factor 15 for mitochondrial diseases: From laboratory to automated inspection. J Inherit Metab Dis. (2021) ;44: (2):358–66. |
[50] | Yatsuga S , Fujita Y , Ishii A , Fukumoto Y , Arahata H , Kakuma T , et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol. (2015) ;78: (5):814–23. |
[51] | Tsygankova PG , Itkis YS , Krylova TD , Kurkina MV , Bychkov IO , Ilyushkina AA , et al. Plasma FGF-21 and GDF-15 are elevated in different inherited metabolic diseases and are not diagnostic for mitochondrial disorders. J Inherit Metab Dis. (2019) ;42: (5):918–33. |
[52] | Lehtonen JM , Auranen M , Darin N , Sofou K , Bindoff L , Hikmat O , et al. Diagnostic value of serum biomarkers FGF21 and GDF15 compared to muscle sample in mitochondrial disease. J Inherit Metab Dis. (2021) ;44: (2):469–80. |
[53] | Dominguez-Gonzalez C , Badosa C , Madruga-Garrido M , Martí I , Paradas C , Ortez C , et al. Growth Differentiation Factor 15 is a potential biomarker of therapeutic response for TK2 deficient myopathy. Sci Rep. (2020) ;10: (1):10111. |
[54] | Mayo Clinic Laboratories. Rochester 2021 Interpretive Handbook 2021 [Available from:https://www.mayocliniclabs.com/test-catalog/pod/MayoTestCatalog-Rochester–SortedByTestName-duplex-interpretive.pdf. |




