Congenital or Early Developing Neuromuscular Diseases Affecting Feeding, Swallowing and Speech – A Review of the Literature from January 1998 to August 2021
Abstract
Background:
The knowledge about the impact of oral motor impairment in neuromuscular diseases (NMDs) is limited but increasing.
Objective:
The aim of this review was to collect and compile knowledge on how muscle weakness in congenital or early developing NMDs directly or indirectly affects feeding, swallowing, speech and saliva control.
Methods:
A literature search was performed in PubMed from January 1, 1998, to August 31, 2021. The keywords “feeding”, “dysphagia”, “swallowing”, “dysarthria”, “speech”, “drooling” and “sialorrhea” were used in combination with “paediatric neuromuscular disease” or specific diagnoses.
Results:
Sixty-five studies were selected for the review, 33 focused on feeding and swallowing, 11 on speech, four on a combination of feeding, swallowing, saliva control or speech and 17 general descriptions. Most of the studies reported on patients with a disorder affecting muscles. These studies show that muscle weakness and impaired motility affecting the muscles innervated by the cranial nerves may influence feeding, swallowing, and speech, and that respiratory function, general health and neurodevelopmental delay also influence these functions. Feeding impairment and breathing difficulties are common in NMDs. Lifesaving interventions such as tube feeding and ventilatory support are common in severe cases.
Conclusions:
Feeding impairment, dysphagia and dysarthria are prevalent in NMDs with congenital or early age of onset. Feeding and swallowing has been studied more than speech and saliva control. More children with NMD survive thanks to new treatment options and it is therefore urgent to follow up how these therapies may impact the development of feeding, swallowing, and speech.
INTRODUCTION
Neuromuscular diseases (NMDs) develop as a result of dysfunction of the motor unit leading to impaired function of peripheral nerves, neuromuscular junctions or muscles. Both spinal nerves and craniofacial nerves may be affected. The diseases can be subdivided into neuropathies, neuromuscular junction disorders and myopathies (including muscular dystrophies). Many of the NMDs have a progressive course. Most NMDs are genetic but could also be due to a hormonal disorder, inflammation, or an autoimmune disease [1, 2]. Some diseases are congenital while others manifest in childhood or have an adult onset. In a comprehensive literature review, Deenen et al. (2015) found an incidence rate lower than 10/100,000 people for most NMDs. The authors conclude that individual NMDs are rare but common as a group [2].
Muscle weakness in one or more muscle groups is the predominant symptom in NMDs and the muscle weakness can cause contractures, scoliosis, problems with walking and hand function, dysphagia, dysarthria, malnutrition and respiratory failure [1, 3]. Breathing difficulties are common [4]. Neurodevelopmental disorders may occur in NMDs affecting children [5–7] and feeding disorder is a frequent finding [8]. In some diseases, the inner organs, such as the heart [9], the hormonal system or the gastro-intestinal system, are affected [10]. At present, there is no cure for NMDs, but drug and gene therapies are effective in certain diagnoses [11].
In some NMDs, the oral motor system is affected [12]. The knowledge about the impact of oral motor impairment on health and wellbeing in NMDs is limited. Updated knowledge about dysphagia and dysarthria is important, especially since increasing numbers of NMD patients survive due to the continuous advances in drug and gene therapies [11]. The specific aim of this literature review was to collect and compile knowledge on how muscle weakness in NMDs directly or indirectly affect feeding, swallowing, saliva control and speech.
MATERIALS AND METHODS
A literature search was performed in the PubMed database and was limited to publications in English between January 1, 1998, to August 31, 2021. Specific keywords were used in combination with “neuromuscular disorder/disease and paediatric” or the name of a specific NMD diagnosis. The keywords were “feeding”, “dysphagia”, “swallowing”, “dysarthria”, “speech” and “drooling/sialorrhea”, “spinal muscular atrophy”, “Charcot-Marie-Tooth disease”, “hereditary motor and sensory neuropathy”, “congenital myasthenic syndromes”, “juvenile myasthenia gravis”, “arthrogryposis multiplex congenita”, “Becker muscular dystrophy”, “congenital myopathy”, “congenital muscular dystrophy”, “Duchenne muscular dystrophy”, “facioscapulohumeral dystrophy”, “juvenile dermatomyositis”, “mitochondrial myopathy”, “Friedreich ataxia”, “Pompe disease”, “Leigh syndrome”, and “myotonic dystrophy type 1”. Only congenital diseases or NMDs with a childhood onset were included in the search. No age groups were excluded but in studies including both childhood and adult onsets of a specific diagnosis, the proportion of patients with childhood onset of the disease was presented. Case reports, intervention studies, reviews, clinical guidelines, and evaluations of examination methods were omitted. If consensus statements for a diagnosis or a diagnosis group are available, these are referred to in a separate paragraph at the end of the section.
RESULTS
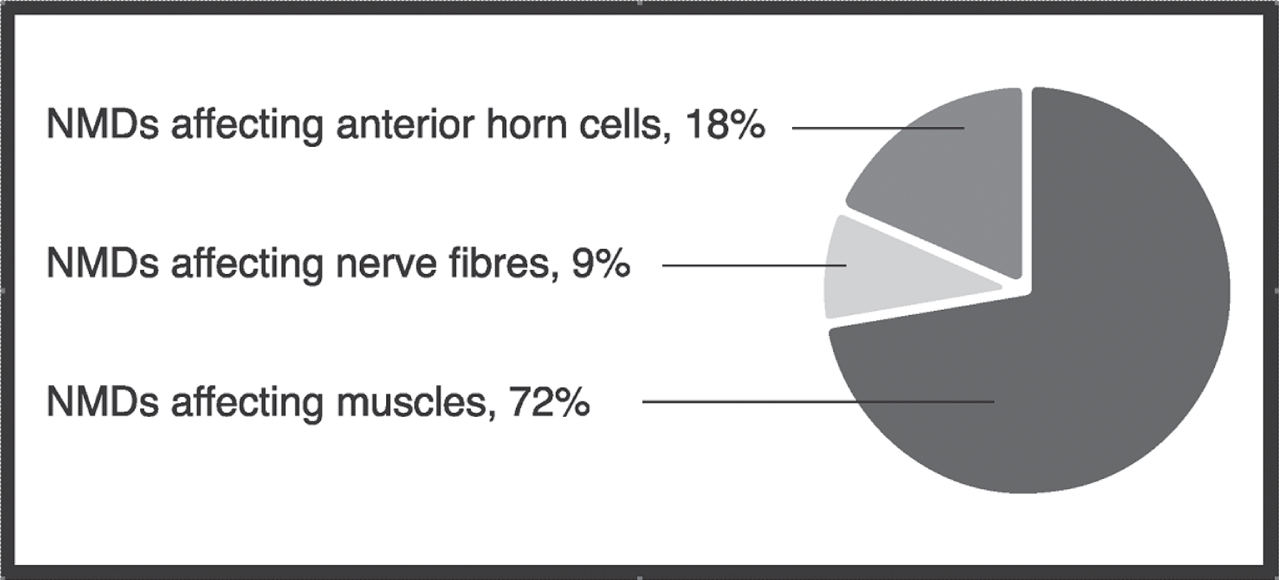
The total search included 1791 articles (after identifying duplicates). Of these, 1512 were removed because they reported on study groups or study aims that were not relevant to the aim of the present review. After this major reduction, 60 reviews and clinical guidelines, 48 intervention studies, 20 methodological studies, and 86 case reports were omitted from the results. Finally, the literature search resulted in 65 clinical studies on feeding, swallowing, saliva control and speech in congenital or early developing NMDs (Table 1). Thirty-three studies focused on feeding and swallowing (51%), 11 on speech (17%), 4 (6%) on a combination of feeding, swallowing, saliva control and/or speech, and 17 (26%) on general diagnostic descriptions that included feeding, swallowing or speech. No study focused on saliva control only. Most of the studies (72%) reported on patients with a disorder affecting the muscles (Fig. 1).
Table 1
Diagnoses and number of participants in clinical studies on feeding, swallowing, saliva control and speech in congenital or early developing NMDs published in the PubMed Database from January 1, 1998, to August 31, 2021
| Title (publication year) | Diagnosis (n) | Age group | Short summary |
| NMDs as a group and comparison between NMDs | |||
| Dysphagia and Dysarthria in Children with Neuromuscular Diseases, a Prevalence Study (2020) [15] | NMD (295) | 2.6–18 yrs. | Dysphagia and dysarthria were present in most of the diagnostic groups. |
| Dysphagia diagnosis with questionnaire, tongue strength measurement, and FEES in patients with childhood-onset muscular dystrophy (2019) [16] | DMD (39) BMD (13) MD:other (6) Controls (56) | 7–27 yrs. 7–27 yrs. 7–12 yrs. 7–27 yrs. | The prevalence of dysphagia was 20.7%. The main complaint was solid food. FEES findings were pharyngeal residue, spillage of food before the swallow and supraglottal penetration. |
| NMDs affecting anterior horn cells | |||
| Feeding and Swallowing Problems in Infants with Spinal Muscular Atrophy Type 1: An Observational Study (2020) [17] | SMA (16) | Infants | Impaired feeding and swallowing remained in infants with SMA type 1 after the start of Nusinersen. |
| Trajectory of change in the swallowing status in spinal muscular atrophy type I (2020) [18] | SMA (11) | 0–2 yrs. | The timeline of deterioration of the swallowing function varied widely in patients with SMA type 1. All subjects were eventually dependent on tube feeding. |
| Feeding problems and malnutrition in spinal muscular atrophy type II (2008) [19] | SMA (122) | 1–47 yrs. | Several patients with SMA type 2 had limited jaw opening, chewing and swallowing difficulties. |
| Feeding difficulties in children and adolescents with spinal muscular atrophy type 2 (2021) [20] | SMA (146) | Clinical record review | Feeding difficulties were present in 60% of children and adolescents with SMA type 2. |
| Dysphagia in spinal muscular atrophy type II: more than a bulbar problem? (2009) [21] | SMA (6) Controls (6) | 6.4–13.4 yrs. | Dysphagia in SMA type 2 was found to be due to a neurological dysfunction influencing the muscle force and efficiency of movement of the tongue and the submental muscle group in combination with compensatory head posture. |
| Prevalence and risk factors for feeding and swallowing diffi–culties in spinal muscular atrophy types II and III (2012) [22] | SMA (108) | 3–45 yrs. | Patients with SMA type 2–3 had a high prevalence of risk factors for feeding and swallowing difficulties. Dysphagia severity was closely related to motor function. |
| Bulbar muscle MRI changes in patients with SMA with reduced mouth opening and dysphagia (2014) [23] | SMA (145) Controls (119) | 1.8–78 yrs. | Reduced maximum mouth opening was common in SMA. |
| Bulbar Problems Self-Reported by Children and Adults with Spinal Muscular Atrophy (2019) [24] | SMA (118) | Register | Fatigue associated with mastication and swallowing, and intelligibility problems were frequently reported. |
| Mastication in Patients with Spinal Muscular Atrophy Types 2 and 3 is Characterized by Abnormal Efficiency, Reduced Endurance, and Fatigue (2021) [25] | SMA (27) | 13–67 yrs. | Non-ambulatory patients demonstrated inefficient mastication. Muscle ultrasound of the mastication muscles showed an abnormal muscle structure. |
| Intellectual abilities, language comprehension, speech, and motor function in children with spinal muscular atrophy type 1 (2021) [26] | SMA (22) | 3–11 yrs. | Children with SMA type 1 showed intelligence and language comprehension in the normal range. Speech was severely compromised. |
| Communication skills among children with spinal muscular atrophy type 1: A parent survey (2019) [27] | SMA | Caregivers’ report | Parents of children with SMA type 1 reported greater receptive than expressive language ability. |
| Patient Reported Impact of Symptoms in Spinal Muscular Atrophy (2018) [28] | SMA (359) | 18–81 yrs. | The prevalence of swallowing and communication difficulties differed among SMA types. |
| NMDs affecting nerve fibres | |||
| Congenital myasthenic syndromes in childhood: diagnostic and management challenges (2008) [36] | CMS (46) | Clinical record review | Feeding difficulties during the neonatal period were common. |
| DOK7 congenital myasthenic syndrome in childhood: early diagnostic clues in 23 children (2013) [37] | CMS (23) | Clinical record review | 48% had stridor and 57% feeding difficulties at birth. Seven of the children with stridor had documented vocal cord palsy. |
| Congenital stridor with feeding difficulty as a presenting symptom of Dok7 congenital myasthenic syndrome (2010) [38] | CMS (11) | Clinical record review | All patients with DOK7 had difficulty with feeding, weak suck, and swallow. |
| Pediatric myasthenia gravis and velopharyngeal incompetence (2004) [39] | CMS (4) | 2.20–14.6 yrs. | Velopharyngeal incompetence affected speech intelligibility. |
| Childhood myasthenia gravis: clinical features and outcomes (2011) [40] | CMS (119) | < 15 yrs. | The prevalence of dysphagia was 7.5%. |
| Clinical Characteristics of Juvenile Myasthenia Gravis in Southern China (2018) [42] | JMS (327) | Clinical record review | Less than 1% had bulbar weakness. |
| NMDs affecting muscles | |||
| Orofacial muscles may be affected in early stages of Becker muscular dystrophy: A preliminary study (2020) [43] | BMD (11) DMD (11) | 10.7±2.4 yrs. 7.7±1.7 yrs. | Clinicians should be aware of dysphagia in both BMD and DMD. |
| A comparison of swallowing dysfunction in Becker muscular dystrophy and Duchenne muscular dystrophy (2018) [44] | BMD (18) DMD (18) | 45.6±15.0 yrs. 17.1±3.7 yrs. | Patients with BMD were found to have dysphagia like patients with DMD if their physical functional status was at the same level. |
| Neurodevelopmental, behavioural, and emotional symptoms in Becker muscular dystrophy (2020) [45] | BMD (70) | 1.0–36.7 yrs. | Language and speech delay was common in BMD. |
| Centronuclear myopathy: clinical aspects of ten Brazilian patients with childhood onset (1998) [46] | CM (10) | 3–25 yrs. | Hypotonia at birth, weak crying and feeding difficulties were common. Patients presented with weakness of masticatory and facial muscles. |
| Nemaline myopathy: a clinical study of 143 cases (2001) [47] | CM (143) | Clinical record review | Feeding difficulties occurred in 79 cases and 75 had significant respiratory disease during the first year of life. |
| Congenital myopathies— clinical features and frequency of individual subtypes diagnosed over a 5-year period in the United Kingdom (2013) [48] | CM (66) | Clinical record review | 35% of all patients required ventilatory support and/or enteral feeding. |
| Congenital myopathies: Natural history of a large pediatric cohort (2015) [49] | CM (125) | Clinical record review/children | Bulbar involvement was present in 46.4% and required gastrostomy placement in 28.8% of the patients. |
| A Cross-Sectional Study of Nemaline Myopathy [50] | CM (57) | 1–57 yrs. | 26% required a wheelchair, tracheostomy, and a feeding tube. |
| Feeding problems in merosin deficient congenital muscular dystrophy (1999) [52] | CMD (14) | 2–14 yrs. | Children with merosin deficient CMD have difficulties at all stages of feeding. |
| National registry of patients with Fukuyama congenital muscular dystrophy in Japan (2018) [53] | CMD (207) | 0–42 yrs. | Dysphagia was observed in 22% of patients with FCMD. |
| Dysphagia in Duchenne muscular dystrophy assessed by validated questionnaire (2013) [55] | DMD + dysph. (9) DMD – dysph. (6) Controls (12) | 21.7±4.2 yrs. 21.0±3 yrs. 24.8±3.1 yrs. | Compared with patients with DMD without dysphagia, the patients with dysphagia report significantly higher scores on different aspects of swallowing and weight gain. |
| Dystrophic changes in masticatory muscles related chewing problems and malocclusions in Duchenne muscular dystrophy (2016) [56] | DMD (72) | 4.8–28 yrs. | Food adaptation and chewing problems were observed in the early stages of the disease. Progressive chewing dysfunction was reflected by an increasingly abnormal echogenicity of the masseter muscle and reduced occlusal contacts. |
| Tongue pressure during swallowing is decreased in patients with Duchenne muscular dystrophy (2014) [57] | DMD (11) Controls (11) | 17–24 yrs. 20–27 yrs. | A correlation was found between tongue pressure and the depth of the palate. |
| Feeding problems and weight gain in Duchenne muscular dystrophy (2006) [58] | DMD (118) | 13.8–35.8 yrs. | Chewing difficulties are common and increase with age. |
| Prevalence of smooth muscle dysfunction among children with Duchenne muscular dystrophy (2020) [59] | DMD (114) | 5–15 yrs. | The prevalence of gastro-oesophageal reflux was 21%. |
| Oral muscles are progressively affected in Duchenne muscular dystrophy: implications for dysphagia treatment (2013) [60] | DMD (24) | 6.3–41.6 yrs. | Oral muscles related to swallowing were progressively affected. Tongue hypertrophy was common in non-ambulatory stages. |
| Predictive factors for masticatory performance in Duchenne muscular dystrophy (2014) [61] | DMD (23) Controls (23) | 6–38 yrs. | Masticatory performance in DMD was severely reduced. |
| Swallowing difficulties in Duchenne muscular dystrophy: indications for feeding assessment and outcome of videofluroscopic swallow studies (2008) [62] | DMD (30) | 6–31.4 yrs. | The oral phase of swallowing was most affected in DMD. Reported swallowing problems were not always associated with difficulties found on VFSS. |
| Dysphagia in patients with Duchenne muscular dystrophy evalu–ated with a questionnaire and videofluorography (2008) [63] | DMD (31) | 9–26 yrs. | VFSS is recommended in all patients with DMD since dysphagia was evident also in those without clinical symptoms. |
| Dysphagia in Duchenne muscular dystrophy assessed objectively by surface electromyography (2013) [64] | DMD + dysph. (9) DMD – dysph. (6) Controls (12) | 21.7±4.2 yrs. 21.0±3.0 yrs. 24.8±3.1 yrs. | Impaired oral motor activity during swallowing was found using surface electromyography. |
| Relationship between Eating and Digestive Symptoms and Respiratory Function in Advanced Duchenne Muscular Dystrophy Patients (2020) [65] | DMD (180) | 22.3±5.0 yrs. | Dysphagia was more closely correlated with respiratory function than with age. |
| Nutritional status, swallowing disorders, and respiratory prognosis in adult Duchenne muscular dystrophy patients (2021) [66] | DMD (117) | 18–39 yrs. | Prevalence of malnutrition, swallowing disorders, and gastrostomy were respectively 62%, 34%, and 11%. |
| Dysphagia in facioscapulohumeral muscular dystrophy (2006) [68] | FSHD (8) | 26–53 yrs. | Dysphagia occurred in patients with advanced FSHD with a mild involvement of the lips, jaw, and lingual muscles. |
| Juvenile dermatomyositis at diagnosis: clinical characteristics of 79 children (1998) [69] | JDM (79) | Caregivers’ report | At diagnosis 35 children (44%) had dysphagia and 34 children (43%) had hoarseness. |
| Oropharyngeal dysphagia in juvenile dermato–myositis (JDM): an evaluation of videofluoroscopy swallow study (VFSS) changes in relation to clinical symptoms and objective muscle scores (2007) [70] | JDM (14) | 2–16 yrs. | VFSS was abnormal in 11 children (79%). |
| Mortality of Japanese patients with Leigh syndrome: Effects of age at onset and genetic diagnosis (2020) [71] | MiM (166) | 35.5±12.2 yrs. | Respiratory dysfunction and feeding impairment were common in neonatal children with Leigh syndrome. |
| Dysphagia in Friedreich Ataxia (2017) [72] | FRDA (60) Controls (59) | 35.5±12.2 yrs. | Dysphagia was common in individuals with FRDA and worsens with disease duration and severity. |
| Changes detected in swallowing function in Friedreich ataxia over 12 months (2019) [73] | FRDA (23) | . | A decline in swallowing function was observed over 12 months. This did not translate into functional decline of swallowing-related health. |
| Dysphagia and swallowing-related quality of life in Friedreich ataxia (2014) [74] | FRDA (36) | 23–58 yrs. | Swallowing function decreases with overall reductions in quality of life. |
| Friedreich ataxia: dysarthria profile and clinical data (2013) [75] | FRDA (20) Controls (10) | 16–71 yrs. 15–68 yrs. | Respiration, voice quality, voice instability, articulation, and tempo were the most affected speech dimensions in FRDA. |
| Voice in Friedreich Ataxia (2017) [76] | FRDA (36) Controls (30) | 37.5±9.4 yrs. 36.5±9.6 yrs. | Mild dysphonia including hoarseness, increased strain and altered pitch variability were characteristic findings. |
| Nasality in Friedreich ataxia (2015) [77] | FRDA (37) Controls (24) | 23–58 yrs. 22–58 yrs. | Participants with FRDA had greater nasality than controls. |
| Differentiating profiles of speech impairments in Friedreich’s ataxia: a perceptual and instrumental approach (2012) [78] | FRDA (7) | 34–56 yrs. | Variability in dysarthria associated with FRDA was found, especially the presence of hypernasality and phonation. |
| Swallow Prognosis and Follow-Up Protocol in Infantile Onset Pompe Disease (2017) [79] | IOPD (12) | Clinical record review | Results on VFSS predicted long-term feeding outcomes in IOPD. |
| Clinical and Molecular Disease Spectrum and Outcomes in Patients with Infantile-Onset Pompe Disease (2020) [80] | IOPD (77) | Clinical record review | > 50% had feeding problems, failure to thrive and malnutrition. |
| A retrospective, multinational, multicentre study on the natural history of infantile-onset Pompe disease (2006) [81] | IOPD (168) | Clinical record review | In IOPD, feeding difficulties (57%), and failure to thrive (53%) appeared after a median age of 4 months. |
| Oropharyngeal dysphagia in infants and children with infantile Pompe disease (2010) [82] | IOPD (13) | Clinical record review | Facial weakness, dysarthria and dysphagia were common in long-term survivors receiving ERT for IOPD. |
| Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy (2012) [83] | IOPD (11) | Clinical record review | Facial weakness, dysarthria and dysphagia were common in long-term survivors receiving ERT for IOPD. |
| Early-onset of symptoms and clinical course of Pompe disease associated with the c.-32-13 T > G variant (2018) [84] | LOPD (84) | Clinical record review | Onset of swallow and feeding difficulties in the first two years of life was not uncommon in LOPD. |
| Analysis of voice quality in patients with late-onset Pompe disease (2016) [85] | LOPD (15) | 15–57 yrs. | Progressive muscle damage in LOPD can lead to changes in the voice and speech. There is a range of pathological voice changes in patients with LOPD. |
| Follow-up analysis of voice quality in patients with late-onset Pompe disease (2018) [86] | LOPD (15) | 15–57 yrs. | At three years follow-up, LOPD patients demonstrated a deterioration in voice quality. |
| Prenatal, Neonatal, and Early Childhood Features in Congenital Myotonic Dystrophy (2018) [88] | DM1 (38) Literature (80) | 0–5 yrs. | Feeding impairment was an early childhood feature in congenital DM1. |
| Orofacial dysfunction in children and adolescents with myotonic dystrophy (2007) [89] | DM1 (56) Controls (56) | 0.5–21 yrs. 2.5–21 yrs. | Intelligibility was moderately or severely reduced in 30 patients (60%). The families reported dysphagia (51.9%) and drooling (37.0%). |
| Orofacial strength, dysarthria, and dysphagia in congenital myotonic dystrophy (2018) [90] | DM1 (41) Controls (29) | 0.5–13.2 yrs. 1.3–13.9 yrs. | Children with congenital DM1 had impaired orofacial functioning that affected communication and swallowing. |
| Speech characteristics in the congenital and childhood-onset forms of myotonic dystrophy type 1 (2018) [91] | DM1 (50) Controls (13) | 7–29 yrs. 15–31 yrs. | Deviant production of bilabials, interdental articulation and hyper nasal speech were characteristic features of dysarthria in congenital and childhood DM1. |
Fig. 1
Type of neuromuscular disease (NMD) and the distribution of clinical studies included in a literature review on congenital or early developing neuromuscular diseases affecting feeding, swallowing, saliva control and speech.
NMDs as a group and comparison between NMDs
Muscle weakness and impaired mobility affecting the muscles innervated by the cranial nerves may cause oral sensorimotor impairment and affect orofacial functions such as feeding, swallowing, saliva control and speech [12, 13]. Feeding and swallowing are generally more severely affected in children with significant weakness before the age of three compared with children with later onset NMD [14]. The prevalence of dysphagia and dysarthria in children with NMDs was investigated in a study from the Netherlands [15]. The study included 295 children with NMD, 2.6–18 years old, who represented 14 different diagnostic groups. Dysarthria was present in 31.5% and dysphagia in 47.2% of the children and were found in nearly all diagnostic groups. Dysphagia was manifested as chewing problems (90%), swallowing problems (43%) and a combination of chewing and swallowing problems (33.5%).
The occurrence of dysphagia in muscular dystrophies was investigated in a study including patients with Becker muscular dystrophy (BMD) (n = 13), DMD (n = 39) and other congenital muscular dystrophies (n = 6) (16). The results were compared with those for 56 age and sex-matched healthy controls. The Eating Assessment Tool-10 (EAT-10) questionnaire was used to identify those with dysphagia and the maximum tongue pressure and tongue endurance were measured. A Flexible Endoscopic Evaluation of Swallowing (FEES) was performed in the patients with dysphagia. The results showed that 20.7% had dysphagia (children 14.6%, adolescents 27.3%, and adults 50%) and the most common complaint was with solid food. Reduced tongue force was common. The main FEES findings were pharyngeal residue, spillage of food before the swallow and supraglottal penetration.
NMDs affecting anterior horn cells
Spinal muscular atrophy (SMA)
The aim of the study by van der Heul et al was to assess feeding and swallowing problems in 16 infants with SMA type 1, including five who were treated with Nusinersen [17]. All eventually developed swallowing problems. Tube feeding had to be initiated when they were 8–12 months old, even though their gross motor function improved during the same period.
A retrospective chart study reviewed the swallowing dysfunction in patients with SMA type 1 [18]. All patients had clinical evidence of oral motor dysfunction because of masticatory and facial muscle weakness, limited mouth opening, drooling, and difficulty chewing solid food. Swallowing deteriorated around six months of age and tube feeding was initiated. All subjects were eventually dependent on a feeding tube. The period from total oral feeding to tube feeding varied between five and twelve months. The authors recommend an individualised approach to the management of swallowing dysfunction in SMA type 1 due to a wide variation among patient degree of deterioration of swallowing function before 12 months of age.
Feeding/eating and weight gain problems have been reported in SMA type 2 and 3 and confirmed in several articles [19–22]. For SMA type 2, the following has been reported: weight more than 2SD above the median (5%), weight less than 2SD below the median (37%), chewing difficulties (28%), limitation of the ability to open the mouth (30%), and swallowing difficulties (25%) [19]. Based on these results, it was concluded that the standards of care should include monitoring and management of feeding difficulties and weight gain. In a study by Wadman et al. (2021), feeding difficulties were present in 60% of children and adolescents with SMA type 2 with a median age at onset of 6.5 years (0–16.5 years) [20]. Underweight and weight gain problems was a major issue in up to 57% of patients. The authors emphasizes that treatment strategies should be tailored to the needs of the individual patient.
The underlying mechanism of dysphagia in children with SMA type 2 has been investigated [21]. The study concluded that it was caused by muscle weakness and impaired mobility of the tongue and submental area in combination with a compensatory head posture. The authors recommend the use of an adapted posture during meals and ending the meal with water.
The prevalence and risk factors of dysphagia in patients with SMA type 2 and 3 have been studied in a questionnaire survey [22]. The most common risk factors reported for dysphagia in patients with SMA type 2 and 3 were choking (30.6%), difficulty transporting food to the mouth (20.4%) and difficulty chewing (20.4%). The severity of the dysphagia was closely related to current overall motor function, and patients with dysphagia had an increased risk of underweight and aspiration pneumonia. In this study, age was not an independent risk factor for feeding and swallowing difficulties.
Studies have been performed on reduced maximum mouth opening and its association with dysphagia in SMA type 1–4 [23]. The results showed that reduced maximum mouth opening is common in SMA types 1–3a. It was associated with dysphagia and concluded to be a sign of bulbar dysfunction. The main cause was found to be a fatty degeneration of specific mouth-opening muscles.
Self-reported bulbar problems such as jaw problems (34%), fatigue associated with mastication (44%), choking (56%) and intelligibility problems were frequently reported in patients with SMA [24]. Bulbar complaints were significantly, although weakly, correlated with reduced mouth opening in patients with SMA type 2.
Van der Heul et al. (2021) have studied mastication in patients with SMA types 2 and 3 [25]. Reduced maximum mouth opening was associated with mastication problems. Non-ambulatory patients demonstrated inefficient mastication. Muscle ultrasound of the mastication muscles showed an abnormal muscle structure in 90% of both ambulatory and non-ambulatory patients. An increase in bulbar problems was reported by patients who were older than 30 years of age and had a milder form of SMA.
A study by Zappa et al. (2021) investigated language comprehension and speech in children with SMA type 1 [26]. Although speech and motor function were severely compromised, children with SMA type 1 presented language comprehension within the normal range. Speech impairment was related to global motor impairment. Parents often report that they perceive their child with SMA type 1 to have greater receptive than expressive language skills [27]. They highlight the importance of speech-generating devices to improve the ability to communicate and thereby also increase quality of life.
One of the largest international studies designed to identify the phenotypic profile and relative importance of individual symptoms in 359 adults with SMA (292 were diagnosed with SMA type 1-3) was performed by Mongiovi et al. (2018) [28]. The symptomatic burden varied based on the patient-reported SMA type. SMA type 1 had a higher prevalence of communication difficulties than all other SMA types and a higher prevalence of choking and swallowing issues compared to types 3 and 4. Gastrointestinal issues, communication difficulties, and choking or swallowing issues all differed between SMA type 2 and 3. Similarly, problems with choking or swallowing issues differed between SMA type 2, 3 and 4. There was no significant difference in the prevalence of these problems between SMA type 3 and SMA type 4. Breathing difficulties, choking or swallowing issues and communication difficulties were significantly less prevalent in the group that walked independently.
The “Consensus statement on standard of care for spinal muscular atrophy” (2007) includes specific recommendations for gastroenterology and nutrition [29]. In “Diagnosis and management of spinal muscular atrophy: Part 1” (2018), the consensus recommendations for nutrition, swallowing and gastrointestinal management are updated [30]. There are also literature reviews regarding feeding and swallowing in SMA [31, 32].
In recent years, there has been very rapid development of drugs in the treatment of SMA with a positive effect of the progression of the disease [33–35]. In 2016, Nusinersen (marketed as Spinraza) was approved for the treatment of SMA. In 2019, the Food and Drug Administration (FDA) approved Onasemnogene abeparvovec (Zolgensma, formerly AXS-101), the first gene therapy approved to treat children younger than two years with SMA. Risdiplam was approved for the treatment of SMA in adults and children two months and older in August 2020. The study by van der Heul et al. (2020) is the only study in this review that includes patients with SMA who have received any of the drugs [17].
NMDs affecting nerve fibres
Charcot-Marie-Tooth disease (CMT)
No study was found that described feeding, swallowing, saliva control or speech in early onset CMT.
NMDs affecting neuromuscular junctions
Congenital myasthenic syndrome (CMS)
Congenital stridor and feeding difficulties during the neonatal period are common in different forms of CMS [36, 37]. Kinali et al. reported on 46 children with CMS, six had stridor (13%), 12 respiratory insufficiency (46%) and 29 (63%) feeding difficulties as neonates [36]. In a study of 23 children with CMS and a mutation in the DOK7 gene, Klein et al. [37] found that 11 (48%) had stridor and 13 (57%) had feeding difficulties at birth [37]. Seven of the children with stridor had documented vocal cord palsy. Stridor and vocal cord palsy were also found in a retrospective case review in 6 of 11 (55%) children with CMS and a mutation in the DOK7 gene [38]. In a retrospective study of 538 children with velopharyngeal inadequacy, four children with myasthenia gravis were identified to have velopharyngeal incompetence [39].
Juvenile myasthenia gravis (JMG)
In a study of 119 children with JMG, 7.5% were diagnosed with dysphagia [40]. Bulbar symptoms may be present in JMG with more generalized skeletal muscle involvement [41]. In a clinical study of JMG in southern China, less than 1% of 327 children had bulbar weakness [42]. No literature describing specific bulbar impairment in JMG was found.
NMDs affecting muscles
Arthrogryposis multiplex congenita (AMC)
No study was found that described feeding, swallowing, saliva control or speech in AMC.
Becker muscular dystrophy (BMD)
If their physical functional status is at the same level, patients with BMD are found to have chewing and swallowing problems like those observed in patients with DMD (see below) [43, 44]. Language and speech delay is common in BMD [45].
Congenital myopathy (CM)
Hypotonia at birth, weak crying, respiratory disease and feeding difficulties are common in infants with CM and many are dependent on ventilatory support and tube feeding during childhood [46, 47]. In a clinical and neurologic examination of 10 patients (3–25 years) with the childhood onset form of centronuclear myopathy, hypotonia at birth, weak crying and feeding difficulties were frequent. Weakness of the masticatory and facial muscles were other characteristic findings [46]. In a report of 143 individuals with nemaline myopathy (NM) (including six with adult onset), 79 (55%) had feeding difficulties and 75 (52%) significant respiratory disease during the first year of life [47]. Of 66 patients with CM assessed over a 5-year period, 35% required ventilatory support and/or enteral feeding [48]. The natural history of 125 patients with CM was studied at a paediatric neuromuscular centre [49]. It was found that bulbar involvement was present in 46.4% and gastrostomy placement in 28.8%.
The clinical history and physical examination of 57 individuals with NM (1–57 years) revealed that 51% required feeding tube [50]. Bulbar function was examined using two drooling scales and a slurp test (measuring the time to drink 4 oz of water through a straw). Thirty-eight individuals participated in the drooling tests and 35% had severe, 16% moderate, 8% mild and 41% no drooling. The results from the drooling frequency test showed that 11% had constant, 32% frequent, and 18% occasional drooling. All 23 individuals who attempted the slurp test had abnormal values.
The “Consensus statement on standard of care for congenital myopathies” (2012) includes recommendations for gastroenterology, nutrition, speech, and oral care [51].
Congenital muscular dystrophy (CMD)
Feeding problems in 14 children (2–14 years) with merosin deficient CMD were investigated in a study by Philpotin et al. [52]. The children had been followed every six months during six years. The examinations included feeding history, mealtime observations, assessment of oral anatomy and VFSS. The children were found to have a deviant oral anatomy and difficulties with chewing and swallowing that progress with age. Six children (43%) were at risk of silent food aspiration and had recurrent chest infections. Gastro-oesophageal reflux was a common finding. In a Japanese study of 207 patients with Fukuyama congenital muscular dystrophy (FCMD), 22% had dysphagia [53].
The “Consensus statement on standard of care for congenital muscular dystrophies” (2010) includes recommendations for gastroenterology, nutrition, and speech [54].
Duchenne muscular dystrophy (DMD)
The Sydney Swallow Questionnaire (SSQ) was used to compare patients with DMD without dysphagia to patients with DMD with dysphagia [55]. The patients with dysphagia reported significantly higher scores on “Difficulty swallowing thick liquids”, “Cough or choke when swallowing liquids”, “Ever cough up or spit out during meals”, “Difficulty starting a swallow”, “Interference with quality of life”, gastrointestinal involvement (gastro-oesophageal reflux and constipation) and weight gain.
In a survey of 118 patients with DMD (13–35 years), the results from a questionnaire showed that chewing difficulties are frequent and increase with age [58]. van den Engel-Hoek et al. (2016) assessed chewing in 72 patients with DMD of different ages (4–28 years) [56]. Food adaptation and chewing problems were observed in the early stages of the disease and mastication became increasingly effortful with age. Progressive chewing dysfunction was reflected by an increasingly abnormal echogenicity of the masseter muscle and reduced occlusal contacts.
The relationship between tongue pressure during water swallowing and the shape of the palate was studied in nine boys with DMD and compared with healthy controls [57]. An intra-oral tongue pressure measuring system was used and the width and depth of the palate were measured from maxillary dental casts. The width of the palate was significantly greater in the patients with DMD compared with controls. A negative correlation was found between tongue pressure magnitude and the depth of the palate.
When patients with DMD answered a questionnaire including questions about feeding difficulties, there were few clinical signs of swallowing difficulties; however, the authors comment that an objective swallowing assessment would be needed to detect minor signs of aspiration [58]. Gastro–oesophageal reflux requiring treatment was rarely reported but almost half the study group complained of constipation. In a study of the prevalence of smooth muscle dysfunction in children with DMD, Manokaran et al. (2020) found that 21% had gastro-oesophageal reflux [59].
Different objective instruments for the assessment of swallowing were used in a study by van Engel-Hoek et al. [60]. Dysphagia in DMD was described as three consecutive stages and the underlying mechanisms of dysphagia were assessed. The results showed that oral muscles related to swallowing were progressively affected. Tongue hypertrophy was common in the early and late non-ambulatory stages. Most problems were found in the late non-ambulatory stage, presenting in both the oral and the pharyngeal phase of swallowing. Other clinical studies have confirmed that the late stage of the disease is the strongest risk factor for masticatory performance and that chewing difficulties in combination with a weak swallow may be the cause of choking [56, 61–63]. Impaired oral motor activity during swallowing has also been found in studies using surface electromyography [55, 60, 64].
The relationship between eating and digestive symptoms and respiratory function has been investigated in patients with DMD who were non-ambulatory and required non-invasive, mechanical ventilatory support [65, 66]. Eating and digestive symptoms were more closely correlated with respiratory function than with age in patients with advanced DMD [65].
The “DMD care considerations –Diagnosis and management of Duchenne muscular dystrophy, part 1” (2018) includes recommendations for nutrition, swallowing and gastrointestinal management [67].
Facioscapulohumeral muscular dystrophy (FSHD)
In their study of dysphagia in FSHD, Wohlgemuth et al. examined eight patients (26–53 years) four whom had childhood onset [68]. An orofacial examination and an evaluation of eating and drinking were carried out. Instrumental assessments with MRI of the tongue and VFSS were included. Weak lips (n = 8), weak tongue (n = 6), weak jaw (n = 6) and delayed oral and pharyngeal transport of food (n = 7) were frequent findings. Four patients had a small tongue.
Juvenile dermatomyositis (JDM)
The caregivers of 79 children with JDM participated in a structured telephone interview, including questions about their child’s clinical symptoms, and 44% reported dysphagia [69]. In a study of oropharyngeal dysphagia in children with JDM by McCann et al. (2007) the aim was to establish predictive factors that could identify children at risk of aspiration [70]. Fourteen children participated in a clinical examination and a VFSS study. Of these, eleven children showed abnormal swallowing on VFSS but two of them could not be identified in the clinical examination alone. The authors therefore recommend that all children with active JDM should be referred for speech and language assessment and VFSS.
Mitochondrial myopathy (MiM)
The age at onset and the prognosis vary between diagnoses. In case of neonatal onset hypotonia, respiratory dysfunction and feeding impairment may be present as in Leigh syndrome [71]. No literature describing specific bulbar impairment in MiM was found.
Friedreich ataxia (FRDA)
Keage et al. studied dysphagia in 60 adults with FRDA, 45 (75%) of whom had childhood onset [72]. The participants were screened for dysphagia based on their case history and a quality-of-life questionnaire, and 59 (98%) of them reported dysphagia. Of these, 35 participated in an oromotor assessment and 38 in a swallowing study (VFSS). Thirty had both assessments. The most frequently affected oromotor domains were laryngeal, tongue, and respiratory function. The airway was significantly compromised in 13/38 patients and all patients who aspirated (n = 10) did so silently. The study results also indicate that dysphagia worsens with the duration and severity of the disease. The progression of dysphagia after one year was assessed in 23 adults with FRDA (87% had childhood onset) [73]. Swallowing-related quality of life, oral motor function and swallowing related health was stable after one year but more impaired tongue, pharyngeal, and cricopharyngeal function was observed in the swallowing examination (VFSS). The impact of dysphagia on quality of life was investigated in 36 adults with FRDA (69% with childhood onset) [74]. The results showed that swallowing function decreases with overall reductions in quality of life.
Twenty patients (16–71 years) with FRDA (42% with childhood onset) and ten controls (15–68 years) participated in a study of speech motor function and intelligibility [75]. Acoustic analyses of audio recordings gave information about speech rate and pitch variation. The most affected speech dimensions were respiration, voice quality, voice instability, articulation, and tempo. Vocal instability could predict ataxia severity. Tempo was correlated with disease duration and articulation could predict the intelligibility score. Speech and voice samples from 36 adults with FRDA (56% with childhood onset) and 30 controls were collected for acoustic and perceptual analysis [76]. Mild dysphonia including hoarseness, increased strain and altered pitch variability were characteristic findings. The onset and severity of the disease correlated with speaking rate and syllabic duration but not with dysphonia severity. The occurrence of nasality was investigated in 37 adults with FRDA (70% with childhood onset) and 20 controls [77]. Perceptual analyses showed that 27 had hypernasality and five hyponasality. A nasometry assessment including eight patients with FRDA and eight controls revealed a significantly higher nasality score in participants with FRDA compared with controls. Folker et al. (2012) investigated the respiratory, laryngeal, velopharyngeal, and articulatory systems of seven patients with FRDA (57% with childhood onset) using both perceptual and instrumental methods [78]. They found a variability in dysarthria associated with FRDA, especially concerning the presence of hypernasality and phonatory dysfunction.
Pompe disease (PD)
Significant feeding difficulties and respiratory distress are common in infant onset Pompe disease (IOPD), and continuous dysphagia monitoring is required [79–81]. Oropharyngeal swallowing was assessed in 13 children with IOPD, 0.5–16.1 months old, before initiation of Enzyme replacement therapy (ERT) [82]. Examination with VFSS showed that all children had dysphagia. The oral stage of swallowing was affected in 77% and the pharyngeal stage in 100%. Common symptoms were weak suck, pharyngeal swallow delay, pharyngeal residue, and airway invasion. Five had silent aspiration.
The frequency and consequences of facial muscle weakness, speech disorders and dysphagia were investigated a follow-up study of 11 long-term survivors of classic IOPD who were treated with ERT [83]. Facial muscle weakness was found in all children. Speech was assessed in four of the children who were found to have disordered articulation, hypernasality and reduced intelligibility. Six children took part in a swallowing examination and five were found to have “ineffective swallowing”, three penetration or aspiration, and two reduced pharyngeal and/or laryngeal sensibility. Regular swallowing assessments and early interventions to improve speech were recommended.
Onset of swallowing and feeding difficulties in the first two years of life occurred in late onset Pompe disease (LOPD) [84]. Szklanny et al. examined voice quality in 19 patients with LOPD, 15–57 years (47% had the juvenile form) [85]. The assessment included otolaryngological examination and electroglottographic, acoustic, and nasalance measurements. Impaired vocal fold closure and weakness of the vocal muscles caused dysphonia in LOPD and speech nasality due to insufficient closure of the soft palate. Instrumental methods revealed that the voice apparatus was most affected in patients with the juvenile form. A deterioration in voice quality was found in a follow-up study three years later [86].
ERT is an approved therapy for PD that aims to improve muscle function [87].
Myotonic dystrophy type 1 (DM1)
Hypotonia, feeding impairment and respiratory problems are early childhood features in congenital DM1 (CDM) [88]. Different aspects of orofacial function were explored in a study of children and adolescents with DM1 [89]. A majority had moderate or severe impairment of lip motility, tongue motility and lip force. Oral motor function was most affected in CDM. Parents reported problems with eating and drinking and saliva control. In an exploratory study of orofacial function, children with CDM were evaluated for speech and swallowing function and for lingual and labial strength [90]. The children had dysarthria (62%) or were non-verbal (38%). A moderate correlation was found between dysarthria, lingual strength, age, and dysphagia and between strength measures and dysphagia.
Speech characteristics, including intelligibility, speech sound production, nasality and compensatory articulation, have been investigated in patients with the congenital and childhood-onset forms of DM1 [91]. The study showed that deviant production of bilabial consonants, interdental articulation and hypernasal speech are characteristic features of dysarthria in CDM and childhood DM1 and that dysarthria is more frequent and more severe in CDM compared with childhood DM1. Based on the findings, it was concluded that most children with DM1 will need speech therapy. In case of incomprehensible speech or severe neurodevelopmental disorder, alternative and augmentative ways of communication could be a part of the treatment.
The “Consensus-based care recommendations for congenital and childhood-onset myotonic dystrophy type 1” (2019) includes recommendations for feeding concerns, dysphagia, and dysarthria [92].
DISCUSSION
The literature search on feeding, swallowing, saliva control and speech in NMDs showed that these topics are noticed more in some diagnoses than in others. Possible explanations could be the rarity of the disease or the fact that feeding impairment, dysphagia or dysarthria are not among the characteristic features. Whether swallowing and speech are affected or not is mostly dependent on the degree of oral motor/bulbar involvement, but respiratory function, general health and neurodevelopmental delay also influence these functions. In NMDs with a progressive course, swallowing and speech may be affected in later stages of the disease. Most studies were on dysphagia/feeding difficulties and only a few on dysarthria and very few on saliva control. There is also a lack of studies following the evolution of these difficulties over time. Almost all the clinical studies were observational studies and about one fifth included healthy controls. Standards of care and comprehensive overviews that contain descriptions of feeding, swallowing, saliva control and speech in NMDs were also mentioned for NMD as a group [12–14], SMA [29–32], JMG [41], CM [51], CMD [54], DMD [66, 67], PD [87] and DM1 [92].
Feeding and breathing difficulties are common in infants with congenital forms of NMDs. Lifesaving interventions such as tube feeding and ventilatory support in common in severe cases. Ventilatory support can have a significant impact on the natural history of NMDs and life expectancy [93]. For this reason, it is important to recognise the onset of signs and symptoms of respiratory failure and to monitor respiratory function in patients with NMD carefully [94, 95].
In several NMDs, gastrostomy tubes are an important tool or aid to achieve good nutritional status. Having a gastrostomy tube as a child or being the parent of a child with a gastrostomy tube could have a major impact on quality of life. It affects not only the meals themselves, but also many other parts of family life. It is important that healthcare professionals ask about eating and eating difficulties as they are not always self-reported [96]. Nutritional management practices vary internationally and there is an urgent need for larger, coordinated, prospective intervention studies of nutrition in NMDs [97, 98]. Salera et al. (2017) [97] highlight the importance of recognising the nutritional status of children with DMD and other NMDs. The authors mention the following nutritional aspects that should be considered: “ . . . the deleterious effects of overnutrition on glucose metabolism, mobility, and respiratory and cardiologic functions; the impact of hyponutrition on muscle and ventilatory function; constipation and other gastrointestinal complications; chewing/swallowing difficulties with an increased risk of aspiration that predisposes to infectious diseases and respiratory complications; as well as osteoporosis with an associated increased risk of fractures.” [97]. Gastrostomy feeding is often recommended to avoid malnutrition in patients with severe dysphagia. However, cardiomyopathy, reduced pulmonary function and other medical risk factors are common in NMDs and postoperative complications have been observed and the perioperative care of patients with NMD who need a gastrostomy therefore requires input from relevant medical specialists [99].
Studies have shown that feeding and swallowing problems occur in most paediatric NMDs and early referral to a speech-language pathologist is therefore recommended [13, 15]. The importance of multidisciplinary and systematic management programmes for patients with NMDs has been emphasized [100–102]. The evidence to determine the effects of dysphagia and dysarthria treatment is insufficient due to a lack of research and validated assessment tools [103–105]. The rapid development of medical treatment in several of the NMDs contributes to the fact that the prognosis for individuals with these diagnoses is constantly evolving. It is a challenge for clinicians and researchers to keep themselves updated about this rapid medical development. For these reasons, literature reviews on NMDs should be repeated after some years. Longitudinal studies are also warranted to provide the right care at the right time for patients with NMD.
The literature search had limitations concerning publication year, language, selected keywords, and choice of search database. Some studies were included even though not all participants belonged to the target group “congenital or early developing NMDs”. To search for studies including intervention and assessment strategies was not a part of the aim but would be interesting subjects for further reviews on dysphagia, and dysarthria in congenital and early developing NMDs.
CONCLUSION
Feeding impairment, dysphagia and dysarthria are common in congenital NMDs or NMDs with early age of onset. Feeding and swallowing have been more extensively studied than speech and saliva control. Feeding impairment and dysphagia can lead to life-threatening symptoms such as malnutrition, dehydration, repeated chest infections or choking. As many patients with NMDs now receive extensive medical interventions to prolong survival, it is essential that these patients are given the best possible opportunity to maintain functions such as feeding, swallowing, saliva control and speech to survive and to preserve their quality of life. To ensure this, speech language pathologists should be included in neuromuscular teams.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Dowling JJ , H DG , Cohn RD , Campbell C ,Treating pediatric neuromuscular disorders: The future is now. Am J Med Genet A (2018) ;176: (4):804–41. DOI: 10.1002/ajmg.a.38418. |
[2] | Deenen JC , Horlings CG , Verschuuren JJ , Verbeek AL , van Engelen BG , The epidemiology of neuromuscular disorders: A comprehensive overview of the literature. J Neuromuscul Dis (2015) ;2: (1):73–85. |
[3] | Mary P , Servais L , Vialle R , Neuromuscular diseases: Diagnosis and management. Orthop Traumatol Surg Res (2018) ;104: (1S):.S89–95. DOI: 10.1016/j.otsr.2017.04.019. |
[4] | Panitch HB , Respiratory implications of pediatric neuromuscular disease. Respir Care (2017) ;62: (6):826–48. DOI: 10.4187/respcare.05250. |
[5] | Ricotti V , Mandy WP , Scoto M , Pane M , Deconinck N , Messina S , et al., Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev Med Child Neurol (2016) ;58: (1):77–84. DOI: 10.1111/dmcn.12922. |
[6] | Astrea G , Battini R , Lenzi S , Frosini S , Bonetti S , Moretti E , et al., Learning disabilities in neuromuscular disorders: A springboard for adult life. Acta Myol (2016) ;35: (2):90–5. |
[7] | Ekström AB , Hakenäs-Plate L , Tulinius M , Wentz E , Cognition and adaptive skills in myotonic dystrophy type A study of 55 individuals with congenital and childhood forms. Dev Med Child Neurol (2009) ;51: (12):982–90. DOI: 10.1111/j.1469-8749.2009.03300.x. |
[8] | Chou E , Lindeback R , Sampaio H , Farrar MA , Nutritional practices in pediatric patients with neuromuscular disorders. Nutr Rev. 2020. DOI: 10.1093/nutrit/nuz109. |
[9] | Feingold B , Mahle WT , Auerbach S , Clemens P , Domenighetti AA , Jefferies JL , et al., Management of cardiac involvement associated with neuromuscular diseases: A scientific statement from the American heart association. Circulation (2017) ;136: (13):e200–e31. DOI: 10.1161/CIR.0000000000000526. |
[10] | Bushby K , Finkel R , Birnkrant DJ , Case LE , Clemens PR , Cripe L , et al., Diagnosis and management of Duchenne muscular dystrophy, part Implementation of multidisciplinary care. Lancet Neurol (2010) ;9: (2):177–89. DOI: 10.1016/S1474-4422(09)70272-8. |
[11] | Roy B , Griggs R , Advances in treatments in muscular dystrophies and motor neuron disorders. Neurol Clin (2021) ;39: (1):87–112. DOI: 10.1016/j.ncl.2020.09.005. |
[12] | Sjögreen L Oromotor disorders arising from neuromuscular diseases. In: Roig-Quilis M, Pennington L, editors. Oromotor Disorders in Childhood. Barcelona, Spain: Viguera; 2011. |
[13] | van den Engel-Hoek L , de Groot IJ , de Swart BJ , Erasmus CE , Feeding and swallowing disorders in pediatric neuromuscular diseases: An overview. J Neuromuscul Dis (2015) ;2: (4):357–69. DOI: 10.3233/JND-190465. |
[14] | Chou E , Lindeback R , D’Silva AM , Sampaio H , Neville K , Farrar MA , Growth and nutrition in pediatric neuromuscular disorders. Clin Nutr (2021) ;40: (6):4341–8. DOI: 10.1016/j.clnu.2021.01.013. |
[15] | Kooi-van Es M , Erasmus CE , de Swart BJM , Voet NBM , van der Wees PJ , de Groot IJM , et al., Dysphagia and dysarthria in children with neuromuscular diseases, a prevalence study. J Neuromuscul Dis (2020) ;7: (3):287–95. DOI: 10.3233/JND-190436. |
[16] | Printza A , Goutsikas C , Triaridis S , Kyrgidis A , Haidopoulou K , Constantinidis J , et al., Dysphagia diagnosis with questionnaire, tongue strength measurement, and FEES in patients with childhood-onset muscular dystrophy. Int J Pediatr Otorhinolaryngol (2019) ;117: :198–203. DOI: 10.1016/j.ijporl.2018.12.005. |
[17] | van der Heul AMB , Cuppen I , Wadman RI , Asselman F , Schoenmakers M , van de Woude DR , et al., Feeding and swallowing problems in infants with spinal muscular atrophy type An observational study. J Neuromuscul Dis (2020) ;7: (3):323–30. DOI: 10.3233/JND-190465. |
[18] | Choi YA , Suh DI , Chae JH , Shin HI , Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int J Pediatr Otorhinolaryngol (2020) ;130: . 1098–18. DOI: 10.1016/j.ijporl.2019.109818. |
[19] | Messina S , Pane M , De Rose P , Vasta I , Sorleti D , Aloysius A , et al., Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord (2008) ;18: (5):389–93. DOI: 10.1016/j.nmd.2008.02.008. |
[20] | Wadman RI , De Amicis R , Brusa C , Battezzati A , Bertoli S , Davis T , et al., Feeding difficulties in children and adolescents with spinal muscular atrophy type 2. Neuromuscul Disord (2021) ;31: (2):101–12. DOI: 10.1016/j.nmd.2020.12.007. |
[21] | van den Engel-Hoek L , Erasmus CE , van Bruggen HW , de Swart BJ , Sie LT , Steenks MH , et al., Dysphagia in spinal muscular atrophy type II: More than a bulbar problem? Neurology (2009) ;73: (21):1787–91. DOI: 10.1212/WNL.0b013e3181c34aa6. |
[22] | Chen YS , Shih HH , Chen TH , Kuo CH , Jong YJ , Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types II and III. J Pediatr (2012) ;160: (3):447–51 e1. DOI: 10.1016/j.jpeds.2011.08.016. |
[23] | Wadman RI , van Bruggen HW , Witkamp TD , Spar-reboom-Kalaykova SI , Stam M , van den Berg LH , et al., Bulbar muscle MRI changes in patients with SMA with reduced mouth opening and dysphagia. Neurology (2014) ;83: (12):1060–6. DOI: 10.1212/WNL.0000000000000796. |
[24] | van der Heul AMB , Wijngaarde CA , Wadman RI , Asselman F , van den Aardweg MTA , Bartels B , et al., Bulbar problems self-reported by children and adults with spinal muscular atrophy. J Neuromuscul Dis (2019) ;6: (3):361–8. DOI: 10.3233/JND-190379. |
[25] | van der Heul AMB , van Eijk RPA , Wadman RI , Asselman F , Cuppen I , Nievelstein RAJ , et al., Mastication in patients with spinal muscular atrophy types 2 and 3 is characterized by abnormal efficiency, reduced endurance, and fatigue. Dysphagia. 2021. DOI: 10.3233/JND-190379. |
[26] | Zappa G , LoMauro A , Baranello G , Cavallo E , Corti P , Mastella C , et al., Intellectual abilities, language comprehension, speech, and motor function in children with spinal muscular atrophy type 1. J Neurodev Disord (2021) ;13: (1):9. DOI: 10.1186/s11689-021-09355-4. |
[27] | Ball LJ , Chavez S , Perez G , Bharucha-Goebel D , Smart K , Kundrat K , et al., Communication skills among children with spinal muscular atrophy type A parent survey. Assist Technol (2019) ;1: :1–11. DOI: 10.1080/10400435.2019.1586788. |
[28] | Mongiovi P , Dilek N , Garland C , Hunter M , Kissel JT , Luebbe E , et al., Patient reported impact of symptoms in spinal muscular atrophy (PRISM-SMA). Neurology (2014) ;91: (13):e1206–e14. DOI: 10.1212/WNL.0000000000006241. |
[29] | Wang CH , Finkel RS , Bertini ES , Schroth M , Simonds A , Wong B , et al., Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol (2007) ;22: (8):1027–49. DOI: 10.1177/0883073807305788. |
[30] | Mercuri E , Finkel RS , Muntoni F , Wirth B , Montes J , Main M ,et al., Diagnosis and management of spinal muscular atrophy: Part Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord (2018) ;28: (2):103–15. DOI: 10.1016/j.nmd.2017.11.005. |
[31] | Corsello A , Scatigno L , Pascuzzi MC , Calcaterra V , Dilillo D , Vizzuso S , et al., Nutritional, gastrointestinal and endo-metabolic challenges in the management of children with spinal muscular atrophy type 1. Nutrients (2021) ;13: (7). DOI: 10.3390/nu13072400. |
[32] | McGrattan KE , Graham RJ , DiDonato CJ , Darras BT , Dysphagia phenotypes in spinal muscular atrophy: The past, present, and promise for the future. Am J Speech Lang Pathol (2021) ;30: (3):1008–22. DOI: 10.1044/2021_AJSLP-20-00217. |
[33] | Baranello G , Darras BT , Day JW , Deconinck N , Klein A , Masson R , et al., Risdiplam in type 1 spinal muscular atrophy. N Engl J Med (2021) ;384: (10):915–23. DOI: 10.1056/NEJMoa2009965. |
[34] | Al-Zaidy SA , Mendell JR , From clinical trials to clinical practice: Practical considerations for gene replacement therapy in SMA type 1 Pediatr Neurol , (2019) ;100: :1–1110.1016/j.pediatrneurol.2019.06.007. |
[35] | Pane M , Palermo C , Messina S , Sansone VA , Bruno C , Catteruccia M , et al., Nusinersen in type 1 SMA infants, children and young adults: Preliminary results on motor function. Neuromuscul Disord (2018) ;28: (7):582–5. DOI: 10.1016/j.nmd.2018.05.010. |
[36] | Kinali M , Beeson D , Pitt MC , Jungbluth H , Simonds AK , Aloysius A , et al., Congenital myasthenic syndromes in childhood: Diagnostic and management challenges. J Neuroimmunol (2008) ;202: :202:6–12. DOI: 10.1016/j.jneuroim.2008.06.026. |
[37] | Klein A , Pitt MC , McHugh JC , Niks EH , Sewry CA , Phadke R , et al., DOK7 congenital myasthenic syndrome in childhood: Early diagnostic clues in 23 children. Neuromuscul Disord (2013) ;23: (11):883–91. DOI: 10.1016/j.nmd.2013.06.002. |
[38] | Jephson CG , Mills NA , Pitt MC , Beeson D , Aloysius A , Muntoni F , et al., Congenital stridor with feeding difficulty as a presenting symptom of Dok7 congenital myasthenic syndrome. Int J Pediatr Otorhinolaryngol (2010) ;74: (9):991–4. DOI: 10.1016/j.ijporl.2010.05.022. |
[39] | Rieder AA , Conley SF , Rowe L , Pediatric myasthenia gravis and velopharyngeal incompetence. Int J Pediatr Otorhinolaryngol (2004) ;68: (6):747–52. DOI: 10.1016/j.ijporl.2004.01.006. |
[40] | Sri-udomkajorn S , Panichai P , Liumsuwan S , Childhood myasthenia gravis: Clinical features and outcomes. J Med Assoc Thai (2011) ;94: (Suppl 3), S152–7. |
[41] | O’Connell K , Ramdas S , Palace J , Management of juvenile myasthenia gravis. Front Neurol (2020) ;11: :743. DOI: 10.3389/fneur.2020.00743. |
[42] | Huang X , Li Y , Feng H , Chen P , Liu W , Clinical characteristics of juvenile myasthenia gravis in Southern China. Front Neurol (2018) ;9: :77. DOI: 10.3389/fneur.2018.00077. |
[43] | Lagarde MLJ , van Alfen N , Geurts ACH , de Groot IJM , van den Engel-Hoek L , Orofacial muscles may be affected in early stages of Becker muscular dystrophy: A preliminary study. Muscle Nerve (2020) ;61: (2):213–7. DOI: 10.1002/mus.26771. |
[44] | Yamada Y , Kawakami M , Wada A , Otsuka T , Muraoka K , Liu M , A comparison of swallowing dysfunction in Becker muscular dystrophy and Duchenne muscular dystrophy. Disabil Rehabil (2018) ;40: (12):1421–5. DOI: 10.1080/09638288.2017.1298680. |
[45] | Lambert JT , Darmahkasih AJ , Horn PS , Rybalsky I , Shellenbarger KC , Tian C , et al., Neurodevelopmental, behavioral, and emotional symptoms in Becker muscular dystrophy. Muscle Nerve (2020) ;61: (2):156–62. DOI: 10.1002/mus.26750. |
[46] | Zanoteli E , Oliveira AS , Schmidt B , Gabbai AA , Centronuclear myopathy: Clinical aspects of ten Brazilian patients with childhood onset. J Neurol Sci (1998) ;158: (1):76–82. DOI: 10.1016/s0022-510x(98)00091-4. |
[47] | Ryan MM , Schnell C , Strickland CD , Shield LK , Morgan G , Iannaccone ST , et al., Nemaline myopathy: A clinical study of 143 cases. Ann Neurol (2001) ;50: (3):312–20. DOI: 10.1002/ana.1080. |
[48] | Maggi L , Scoto M , Cirak S , Robb SA , Klein A , Lillis S , et al., Congenital myopathies–clinical features and frequency of individual subtypes diagnosed over a 5-year period in the United Kingdom. Neuromuscul Disord (2013) ;23: (3):195–205. DOI: 10.1016/j.nmd.2013.01.004. |
[49] | Colombo I , Scoto M , Manzur AY , Robb SA , Maggi L , Gowda V , et al., Congenital myopathies: Natural history of a large pediatric cohort. Neurology (2015) ;84: (1):28–35. DOI: 10.1212/WNL.0000000000001110. |
[50] | Amburgey K , Acker M , Saeed S , Amin R , Beggs AH , Bonnemann CG , et al., A cross-sectional study of nemaline myopathy. Neurology (1425) ;96: (10):e–e36. DOI: 10.1212/WNL.0000000000011458. |
[51] | Wang CH , Dowling JJ , North K , Schroth MK , Sejersen T , Shapiro F , et al., Consensus statement on standard of care for congenital myopathies. J Child Neurol (2012) ;27: (3):363–82. DOI: 10.1177/0883073812436605. |
[52] | Philpot J , Bagnall A , King C , Dubowitz V , Muntoni F , Feeding problems in merosin deficient congenital muscular dystrophy. Arch Dis Child (1999) ;80: (6):542–7. DOI: 10.1136/adc.80.6.542. |
[53] | Ishigaki K , Ihara C , Nakamura H , Mori-Yoshimura M , Maruo K , Taniguchi-Ikeda M , et al., National registry of patients with Fukuyama congenital muscular dystrophy in Japan. Neuromuscul Disord (2018) ;28: (10):885–93. DOI: 10.1016/j.nmd.2018.08.001. |
[54] | Wang CH , Bonnemann CG , Rutkowski A , Sejersen T , Bellini J , Battista V , et al., Consensus statement on standard of care for congenital muscular dystrophies. J Child Neurol (2010) ;25: (12):1559–81. DOI: 10.1177/0883073810381924. |
[55] | Archer SK , Garrod R , Hart N , Miller S , Dysphagia in Duchenne muscular dystrophy assessed by validated questionnaire. Int J Lang Commun Disord (2013) ;48: (2):240–6. DOI: 10.1111/j.1460-6984.2012.00197.x. |
[56] | van den Engel-Hoek L , de Groot IJ , Sie LT , van Bruggen HW , de Groot SA , Erasmus CE , et al., Dystrophic changes in masticatory muscles related chewing problems and malocclusions in Duchenne muscular dystrophy. Neuromuscul Disord (2016) ;26: (6):354–60. DOI: 10.1016/j.nmd.2016.03.008. |
[57] | Hamanaka-Kondoh S , Kondoh J , Tamine K , Hori K , Fujiwara S , Maeda Y , et al., Tongue pressure during swallowing is decreased in patients with Duchenne muscular dystrophy. Neuromuscul Disord (2014) ;24: (6):474–81. DOI: 10.1016/j.nmd.2014.03.003. |
[58] | Pane M , Vasta I , Messina S , Sorleti D , Aloysius A , Sciarra F , et al., Feeding problems and weight gain in Duchenne muscular dystrophy. Eur J Paediatr Neurol (2006) ;10: (5-6):231–6. DOI: 10.1016/j.ejpn.2006.08.008. |
[59] | Manokaran RK , Aggarwala S , Kumar R , Gupta AK , Chakrabarty B , Jauhari P , et al., Prevalence of smooth muscle dysfunction among children with Duchenne muscular dystrophy. Muscle Nerve (2020) ;62: (6):699–704. DOI: 10.1002/mus.27077. |
[60] | van den Engel-Hoek L , Erasmus CE , Hendriks JC , Geurts AC , Klein WM , Pillen S , et al., Oral muscles are progressively affected in Duchenne muscular dystrophy: Implications for dysphagia treatment. J Neurol (2020) ;260: (5):1295–303. DOI: 10.1007/s00415-012-6793-y. |
[61] | van Bruggen HW , van de Engel-Hoek L , Steenks MH , Bronkhorst EM , Creugers NH , de Groot IJ , et al., Predictive factors for masticatory performance in Duchenne muscular dystrophy. Neuromuscul Disord (2014) ;24: (8):684–92. DOI: 10.1016/j.nmd.2014.05.011. |
[62] | Aloysius A , Born P , Kinali M , Davis T , Pane M , Mercuri E , Swallowing difficulties in Duchenne muscular dystrophy: Indications for feeding assessment and outcome of videofluroscopic swallow studies. Eur J Paediatr Neurol (2008) ;12: (3):239–45. DOI: 10.1016/j.ejpn.2007.08.009. |
[63] | Hanayama K , Liu M , Higuchi Y , Fujiwara T , Tsuji T , Hase K , et al., Dysphagia in patients with Duchenne muscular dystrophy evaluated with a questionnaire and videofluorography. Disabil Rehabil (2008) ;30: (7):517–22. DOI: 10.1080/09638280701355595. |
[64] | Archer SK , Garrod R , Hart N , Miller S Dysphagia in Duchenne muscular dystrophy assessed objectively by surface electromyography. Dysphagia (2013) ;28: (2):188–98. DOI: 10.1007/s00455-012-9429-6. |
[65] | Lee JW , Oh HJ , Choi WA , Kim DJ , Kang SW , Relationship between eating and digestive symptoms and respiratory function in advanced duchenne muscular dystrophy patients. J Neuromuscul Dis (2020) ;7: (2):101–7. DOI: 10.3233/JND-190435. |
[66] | Fayssoil A , Chaffaut C , Prigent H , Laforet P , Clair B , Orlikowski D , et al., Nutritional status, swallowing disorders, and respiratory prognosis in adult Duchenne muscular dystrophy patients. Pediatr Pulmonol (2021) ;56: (7):2146–54. DOI: 10.1002/ppul.25430. |
[67] | Birnkrant DJ , Bushby K , Bann CM , Apkon SD , Blackwell A , Brumbaugh D , et al., Diagnosis and management of Duchenne muscular dystrophy, part Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol (2018) ;17: (3):251–67. DOI: 10.1016/S1474-4422(18)30024-3. |
[68] | Wohlgemuth M , de Swart BJ , Kalf JG , Joosten FB , Van der Vliet AM , Padberg GW . Dysphagia in facioscapulohumeral muscular dystrophy. Neurology (2006) ;66: (12):1926–8. DOI: 10.1212/01.wnl.0000219760.76441.f8. |
[69] | Pachman LM , Hayford JR , Chung A , Daugherty CA , Pallansch MA , Fink CW , et al., Juvenile dermatomyositis at diagnosis: Clinical characteristics of 79 children. J Rheumatol (1998) ;25: (6):1198–204. |
[70] | McCann LJ , Garay SM , Ryan MM , Harris R , Riley P , Pilkington CA , Oropharyngeal dysphagia in juvenile dermatomyositis (JDM): An evaluation of videofluoroscopy swallow study (VFSS) changes in relation to clinical symptoms and objective muscle scores. Rheumatology (Oxford) (2007) ;46: (8):1363–6. DOI: 10.1093/rheumatology/kem131. |
[71] | Ogawa E , Fushimi T , Ogawa-Tominaga M , Shimura M , Tajika M , Ichimoto K , et al., Mortality of Japanese patients with Leigh syndrome: Effects of age at onset and genetic diagnosis. J Inherit Metab Dis (2020) ;43: (4):819–26. DOI: 10.1002/jimd.12218. |
[72] | Keage MJ , Delatycki MB , Gupta I , Corben LA , Vogel AP , Dysphagia in friedreich ataxia. Dysphagia (2017) ;32: (5):626–35. DOI: 10.1007/s00455-017-9804-4. |
[73] | Keage M , Delatycki MB , Dyer J , Corben LA , Vogel AP . Changes detected in swallowing function in Friedreich ataxia over 12 months. Neuromuscul Disord (2019) ;29: (10):786–93. DOI: 10.1016/j.nmd.2019.08.013. |
[74] | Vogel AP , Brown SE , Folker JE , Corben LA , Delatycki MB , Dysphagia and swallowing-related quality of life in Friedreich ataxia. J Neurol (2014) ;261: (2):392–9. DOI: 10.1007/s00415-013-7208-4. |
[75] | Brendel B , Ackermann H , Berg D , Lindig T , Scholderle T , Schols L , et al., Friedreich ataxia: Dysarthria profile and clinical data. Cerebellum (2013) ;12: (4):475–84. DOI: 10.1007/s12311-012-0440-0. |
[76] | Vogel AP , Wardrop MI , Folker JE , Synofzik M , Corben LA , Delatycki MB , et al., Voice in friedreich ataxia. J Voice (2017) ;31: (2):243 e9–e19. DOI: 10.1016/j.jvoice.2016.04.015. |
[77] | Poole ML , Wee JS , Folker JE , Corben LA , Delatycki MB , Vogel AP , Nasality in Friedreich ataxia. Clin Linguist Phon (2015) ;29: (1):46–58. DOI: 10.3109/02699206.2014.954734. |
[78] | Folker JE , Murdoch BE , Rosen KM , Cahill LM , Delatycki MB , Corben LA , et al., Differentiating profiles of speech impairments in Friedreich’s ataxia: A perceptual and instrumental approach. Int J Lang Commun Disord (2012) ;47: (1):65–76. DOI: 10.1111/j.1460-6984.2011.00078.x. |
[79] | Swift G , Cleary M , Grunewald S , Lozano S , Ryan M , Davison J . Swallow prognosis and follow-up protocol in infantile onset pompe disease. JIMD Rep (2017) ;33: :11–7. DOI: 10.1007/8904_2016_576. |
[80] | Gupta N , Kazi ZB , Nampoothiri S , Jagdeesh S , Kabra M , Puri RD , et al., Clinical and molecular disease spectrum and outcomes in patients with infantile-onset pompe disease. J Pediatr (2020) ;216: :44–50 e5. DOI: 10.1016/j.jpeds.2019.08.058. |
[81] | Kishnani PS , Hwu WL , Mandel H , Nicolino M , Yong F , Corzo D , et al., A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr (2006) ;148: (5):671–6. DOI: 10.1016/j.jpeds.2005.11.033. |
[82] | Jones HN , Muller CW , Lin M , Banugaria SG , Case LE , Li JS , et al., Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia (2010) ;25: (4):277–83. DOI: 10.1007/s00455-009-9252-x. |
[83] | van Gelder CM , van Capelle CI , Ebbink BJ , Moor-van Nugteren I , van den Hout JM , Hakkesteegt MM , et al., Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy. J Inherit Metab Dis (2012) ;35: (3):505–11. DOI: 10.1007/s10545-011-9404-7. |
[84] | Herbert M , Case LE , Rairikar M , Cope H , Bailey L , Austin SL , et al., Early-onset of symptoms and clinical course of Pompe disease associated with the c-32-13T > G variant.. Mol Genet Metab (2019) ;126: (2):106–16. DOI: 10.1016/j.ymgme.2018.08.009. |
[85] | Szklanny K , Gubrynowicz R , Iwanicka-Pronicka K , Tylki-Szymanska A , Analysis of voice quality in patients with late-onset Pompe disease. Orphanet J Rare Dis (2016) ;11: (1):99. DOI: 10.1186/s13023-016-0480-5. |
[86] | Szklanny K , Tylki-Szymanska A , Follow-up analysis of voice quality in patients with late-onset Pompe disease. Orphanet J Rare Dis (2018) ;13: (1):189. DOI: 10.1186/s13023-018-0932-1. |
[87] | Salabarria SM , Nair J , Clement N , Smith BK , Raben N , Fuller DD , et al., Advancements in AAV-mediated gene therapy for pompe disease. J Neuromuscul Dis (2020) ;7: (1):15–31. DOI: 10.3233/JND-190426. |
[88] | Zapata-Aldana E , Ceballos-Saenz D , Hicks R , Campbell C , Prenatal, neonatal, and early childhood features in congenital myotonic dystrophy. J Neuromuscul Dis (2018) ;5: (3):331–40. DOI: 10.3233/JND-170277. |
[89] | Sjögreen L , Engvall M , Ekström AB , Lohmander A , Kiliaridis S , Tulinius M , Orofacial dysfunction in children and adolescents with myotonic dystrophy. Dev Med Child Neurol (2007) ;49: (1):18–22. DOI: 10.1111/j.1469-8749.2007.0060a.x. |
[90] | Berggren KN , Hung M , Dixon MM , Bounsanga J , Crockett B , Foye MD , et al., Orofacial strength, dysarthria, and dysphagia in congenital myotonic dystrophy. Muscle Nerve (2018) ;58: (3):413–7. DOI: 10.1002/mus.26176. |
[91] | Sjögreen L , Mårtensson A , Ekström AB , Speech characteristics in the congenital and childhood-onset forms of myotonic dystrophy type 1. Int J Lang Commun Disord (2018) ;53: (3):576–83. DOI: 10.1111/1460-6984.12370. |
[92] | Johnson NE , Aldana EZ , Angeard N , Ashizawa T , Berggren KN , Marini-Bettolo C , et al., Consensus-based care recommendations for congenital and childhood-onset myotonic dystrophy type 1. Neurol Clin Pract (2019) ;9: (5):443–54. DOI: 10.1212/CPJ.0000000000000646. |
[93] | Simonds AK , Recent advances in respiratory care for neuromuscular disease. Chest (2006) ;130: (6):1879–86. DOI: 10.1378/chest.130.6.1879. |
[94] | Hutchinson D , Whyte K Neuromuscular disease and respiratory failure. Pract Neurol (2008) ;8: (4):229–37. DOI: 10.1136/pn.2008.152611. |
[95] | Hull J , Aniapravan R , Chan E , Chatwin M , Forton J , Gallagher J , et al., British thoracic society guideline for respiratory management of children with neuromuscular weakness. Thorax (2012) ;67: (Suppl 1):i1–40. DOI: 10.1136/thoraxjnl-2012-201964. |
[96] | Backman E , Granlund M , Karlsson AK . Parental perspectives on family mealtimes related to gastrostomy tube feeding in children. Qual Health Res. 2021:104973232 1997133. DOI: 10.1177/1049732321997133. |
[97] | Salera S , Menni F , Moggio M , Guez S , Sciacco M , Esposito S , Nutritional challenges in duchenne muscular dystrophy. Nutrients (2017) ;9: (6). DOI: 10.3390/nu9060594. |
[98] | Moore GE , Lindenmayer AW , McConchie GA , Ryan MM , Davidson ZE , Describing nutrition in spinal muscular atrophy: A systematic review. Neuromuscul Disord (2016) ;26: (7):395–404. DOI: 10.1016/j.nmd.2016.05.005. |
[99] | Boivin A , Antonelli R , Sethna NF , Perioperative management of gastrostomy tube placement in Duchenne muscular dystrophy adolescent and young adult patients: A role for a perioperative surgical home. Paediatr Anaesth (2018) ;28: (2):127–33. DOI: 10.1111/pan.13295. |
[100] | Tilton AH , Miller MD , Khoshoo V , Nutrition and swallowing in pediatric neuromuscular patients. Semin Pediatr Neurol (1998) ;5: (2):106–15. |
[101] | Paganoni S , Nicholson K , Leigh F , Swoboda K , Chad D , Drake K , et al., Developing multidisciplinary clinics for neuromuscular care and research. Muscle Nerve (2017) ;56: (5):848–58. DOI: 10.1002/mus.25725. |
[102] | LaDonna KA , Koopman WJ , Venance SL , Myotonic dystrophy (DM1) and dysphagia: The need for dysphagia management guidelines and an assessment tool. Can J Neurosci Nurs (2011) ;33: (1):42–6. |
[103] | Hill M , Hughes T , Milford C , Treatment for swallowing difficulties (dysphagia) in chronic muscle disease. Cochrane Database Syst Rev.CD03 (2004) (2):CD004303. DOI: 10.1002/14651858.CD004303.pub2. |
[104] | Jones K , Pitceathly RD , Rose MR , McGowan S , Hill M , Badrising UA , et al., Interventions for dysphagia in long-term, progressive muscle disease. Cochrane Database Syst Rev (2016) ;2: ::CD004303. DOI: 10.1002/14651858.CD004303.pub4. |
[105] | Pilz W , Baijens LW , Kremer B , Oropharyngeal dysphagia in myotonic dystrophy type A systematic review. Dysphagia (2014) ;29: (3):319–31. DOI: 10.1007/s00455-013-9510-9. |




