Healthcare Utilisation and Satisfaction with Care in Patients with Amyotrophic Lateral Sclerosis - An Observational Study
Abstract
Background:
Patients with amyotrophic lateral sclerosis (ALS) need a large amount of healthcare services. Knowledge on use of and satisfaction with healthcare is, however, scarce.
Objective:
The objectives were to explore use and satisfaction of healthcare in patients with ALS.
Methods:
The sample consisted of patients with ALS, recruited from the ALS clinic at the Karolinska University Hospital, Stockholm, Sweden, participating in a three-year observational study. Data on healthcare utilisation were retrieved from the computerised register at Region Stockholm, Sweden. Information regarding disability, contextual factors and satisfaction with care was collected by home visits.
Results:
Over time, half, or less of the patients used inpatient care, whereas all used outpatient care. Half of all outpatient contacts were with providers of advanced healthcare in the home and one-fifth with allied health professionals. Nurses performing home visits composed the largest proportion of outpatient contacts. A small amount of the utilised outpatient care emerged from the ALS clinic. Patients with severe disease and longer time since diagnosis had fewer contacts with the ALS clinic. Satisfaction with care was in general stable over time with around two-thirds or more of patients being satisfied. Most patients wanted to participate in care planning, but few had.
Conclusion:
Patients with ALS use hospital-based specialist care and other outpatient care in parallel with many healthcare providers involved. Our findings highlight the need for implementation of person-centred care to improve both coordination of care, care transitions and satisfaction with healthcare services.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease primarily affecting upper and lower motor neurons and characterized by progressive muscle weakness and wasting. Besides muscular symptoms, cognitive and behaviour changes can occur, being more frequent in severe stages of the disease [1]. Other commonly reported impairments include fatigue, anxiety, depression, and pain [2]. The European incidence rate is approximately 3 per 100 000 people-years, with a slightly higher rate among men [3, 4]. Depending on the site of onset, ALS can be described as spinal, i.e., starting with muscular signs in the limbs, or bulbar which starts with speech and swallowing difficulties. Bulbar-onset ALS is present in about 25%of patients and is associated with worse prognosis [5, 6]. Although most patients with ALS die from respiratory failure within 2–4 years of diagnosis, the inter-individual variety is substantial and some survive for decades [7, 8].
There is no cure for ALS and clinical management focus on treatment and care to alleviate symptoms, and to improve survival and health-related quality of life (HRQL). Published guidelines recommend, inter alia, regular support from a multidisciplinary care team, symptomatic and disease-modifying medical treatment, percutaneous endoscopic gastrostomy feeding to improve nutrition and mechanical ventilation to improve respiratory function [9, 10]. Since ALS has a vast impact on functioning with activity limitations and participation restrictions in social and lifestyle activities [11, 12], patients will need a large amount of healthcare services. Thus, besides the need for medical care, patients with ALS have a need for other healthcare and social services, such as rehabilitation, advanced healthcare care in the home, special transport services, home care services and housing with special services due to disability.
The organisation of healthcare and social services differs internationally. Thus, the study results from different countries might not be extrapolated to other countries due to differences in healthcare systems and policies. Swedish citizens have, based on their needs, access to healthcare and social services to a low cost for the individual. However, the organisation and delivery of services vary between regions and municipalities. In the Stockholm county (Region Stockholm), the outpatient ALS clinic at the department of neurology at the Karolinska University Hospital has the main responsibility for the ALS specialist care. A cohort of patients, and their next of kin, from this ALS clinic has been followed every six-months during a three-year period [2, 13, 14]. The results have shown that patients, regardless of disease severity, experienced fatigue, anxiety, depression, and pain, and that about one-third experienced two or more of these impairments concurrently, i.e., at the same time point [2]. Further, activity limitations and participation restrictions were common, and the use of aids and services increased over time [2]. In addition, despite the provided social services, next of kin served as informal caregivers and devoted a significant amount of time to care duties [14].
However, for the assessment of healthcare needs and the planning and organisation of good quality care for patients with ALS, it is important to gain knowledge not only on functioning but also on which healthcare services they currently use, and their stated need and satisfaction with care. The literature on healthcare utilisation in patients with ALS is scarce and mainly focus on costs [15–17], especially at the end of life [18, 19]. The need for including the patient perspective, i.e., patient satisfaction with healthcare services has been highlighted to yield a fuller picture [20], but studies are scarce [21]. There is, further, a dearth of studies combining these aspects, i.e., use of and satisfaction with healthcare. Thus, the aims of the present study were to: a) describe the use of healthcare in this cohort of patients with ALS; b) explore differences in use of healthcare based on personal and disease-related factors c) describe satisfaction with care from the patients’ perspective, and d) explore differences in use of healthcare based on satisfaction with healthcare.
MATERIAL AND METHODS
Participants and procedures
The present study is based on data from our three-year observational study in patients with ALS for which the case-finding procedure has been previously described [2]. In brief, patients were recruited from the ALS clinic at the Karolinska University Hospital, Stockholm, Sweden. The inclusion criteria were being≥18 years of age and having an ALS diagnosis according to the revised El Escorial criteria [22]. Exclusion criteria were inability to understand Swedish, dementia or being in a terminal stage. Sixty patients were enrolled at baseline (T1) and these were thereafter contacted every six months up to three years (T2-T7) after baseline unless they declined participation or deceased. Informed signed consent was retrieved at each time point. The study was approved by the ethical review board in Stockholm, registration number: 2012/842-31/2, and procedures were conducted in accordance with the Declaration of Helsinki.
The data collection procedure regarding disability, contextual factors and satisfaction with care has been previously described [2]. In brief, data were collected during home visits and comprised study-specific protocols administered as interviews and self-reported standardised questionnaires. Each home visit lasted approximately 2.5 hours and was performed by healthcare professionals with clinical experience of ALS and trained for the purpose of data collection. Medical records were used to retrieve information on time since diagnosis and site of onset (spinal or bulbar). Study-specific protocols were used for data collection on age, sex, civil status, education level, aids, and services. Use of aids were reported for nutrition, communication, ventilation, mobility, and activities of daily living (ADL), and services concerned home adaptations, homecare by personal assistants or homecare services, and transportation services. The ALS Functioning Rating Scale-Revised (ALSFRS-R) was used as a proxy for disease severity [23]. The total scale score ranges from 0 to 48 and higher scores indicate less severity.
Data on utilisation of healthcare were obtained from the computerised register at Region Stockholm. The register contains information regarding all care, i.e., inpatient and outpatient care (inpatient days, outpatient clinical visits, home visits, telephone contacts, administrative contacts) with healthcare providers within Region Stockholm. We retrieved data on patients’ utilisation of healthcare during one year before (T0) the baseline assessment (T1) and thereafter during the time the patients remained in the study (T2-T7), i.e., up to each individual patient’s last data collection.
Satisfaction with care was assessed by a previously used questionnaire [24–26] based on the taxonomy of Ware [27]. The following dimensions were included in the questionnaire: art of care, availability, accessibility, continuity, participation in care planning, efficacy/outcome of care and finances. The questionnaire’s items were constructed as statements with which the patients with ALS had to agree or disagree (satisfied-dissatisfied) on a five-graded Likert scale. Patients also had the option of responding “not applicable” or “no need” to each statement. The answers were dichotomised into satisfied (1-2 on the Likert scale) or not satisfied (3–5 on the Likert scale). The dichotomised item “contact with all expertise needed” was used as a proxy for satisfaction with healthcare in analyses of use of healthcare.
Statistics
Descriptive statistics were used to present and analyse data. Number of inpatient days and outpatient contacts were summarised for each patient until termination in the study, and the cohort’s total number of inpatient days and outpatient contacts were summarised. Mann-Whitney U tests were used to analyse between-group (age, sex, education level, time since diagnosis, and disease severity) differences in total number of inpatient days and outpatient contacts. Inpatient data were summarised by department, i.e., neurology, medicine, geriatrics, rehabilitation and other which included surgery, orthopaedics, and palliative care. Outpatient contacts were summarised by outpatient healthcare providers, i.e., advanced healthcare in the home, allied health professionals (including rehabilitation personnel such as occupational therapists, dieticians, physiotherapists, speech therapists, welfare officers and psychologists), primary care, the ALS clinic, and others. Other outpatient care included contacts with medicine, surgery, orthopaedic, psychiatry, geriatrics, respiratory, ear/nose/throat and radiology clinics. Baseline data were used for group dichotomisation, i.e., age (< 65 years/≥65 years), sex (man/woman), education (university level / below), time since diagnosis (< 24 months/≥24 months) and disease severity (ALSFRS-R scores 0–24/25–48). Changes in satisfaction with care between baseline (T1) and the first six-month follow-up (T2) in patients reporting a need on both occasions were analysed with the McNemar test. Difference in use of healthcare at each timepoint, i.e., number of inpatient days and outpatient contacts, between patients reporting being satisfied versus those not satisfied (on the item “contact with all expertise needed) were analysed with Mann-Whitney U tests. Analyses were performed with IBM SPSS Statistics 27 (for Windows). The level of significance was set to p < 0.05.
RESULTS
Twenty-five of the 60 patients included at baseline died between data collections and 23 declined participation at some time-point during the three-year study. Thus, 39 patients participated in data collections at T2, 29 at T3, 17 at T4, 14 at T5 and T6, and 12 patients participated in data collections at T7. Information on patient characteristics is presented in Table 1. The median age was around 60 years, the majority lived with a family member, around a third had a university education, aids and services were frequently used, and this use appeared to increase over time.
Table 1
Patient characteristics at each data collection (T1–T7) during the three-year study period. N represents the number of patients participating at each time point
| T1 (N = 60) | T2 (N = 39) | T3 (N = 29) | T4 (N = 17) | T5 (N = 14) | T6 (N = 14) | T7 (N = 12) | |
| Age median | 61 (55–69) | 61 (55–68) | 61 (53–66) | 62 (49–69) | 63 (58–68) | 62 (51–68) | 63 (48–68) |
| years (IQR) | |||||||
| Sex n (%) | |||||||
| Female | 28 (47) | 17 (44) | 12 (41) | 6 (35) | 4 (29) | 6 (43) | 6 (50) |
| Civil status n (%) | |||||||
| Living alone | 15 (25) | 7 (18) | 6 (21) | 3 (18) | 2 (14) | 2 (14) | 2 (17) |
| Education n (%) | |||||||
| University | 22 (37) | 12 (31) | 9 (31) | 6 (35) | 5 (36) | 5 (36) | 5 (42) |
| ALS onset n (%) | |||||||
| Spinal | 45 (75) | 31 (79) | 25 (86) | 16 (94) | 13 (93) | 13 (93) | 11 (92) |
| Time since diagnosis | |||||||
| median months (IQR) | 17 (8–35) | 24 (14–55) | 35 (20–82) | 52 (32–107) | 60 (36–123) | 65 (45–129) | 77 (50–152) |
| Disease severity | |||||||
| ALSFRS-R, median (IQR) | 29 (20–37) | 29# (18–34) | 25 (12–33) | 27 (16–33) | 23 (11–32) | 19 (3–26) | 15 (5–27) |
| Aids n (%) | |||||||
| Nutrition | 18 (30) | 12 (31) | 11 (38) | 5 (29) | 6 (43) | 7 (50) | 7 (58) |
| Communication | 36 (60) | 24 (61) | 17 (59) | 11 (65) | 11 (79) | 13 (93) | 11 (92) |
| Ventilation | 22 (64) | 15 (38) | 8 (28) | 6 (65) | 6 (43) | 8 (57) | 7 (58) |
| Mobility | 49 (82) | 36 (92) | 28 (97) | 16 (94) | 13 (93) | 14 (100) | 12 (100) |
| ADL | 43 (72) | 32 (82) | 24 (83) | 15 (88) | 12 (86) | 13 (93) | 12 (100) |
| Services n (%) | |||||||
| Home adaptations | 34 (57) | 28 (72) | 23 (79) | 13 (77) | 12 (86) | 13 (93) | 12 (100) |
| Home care* | 31 (52) | 25 (64) | 23 (79) | 12 (71) | 10 (71) | 11 (79) | 10 (83) |
| Transport services | 40 (67) | 33 (85) | 27 (93) | 17 (100) | 14 (100) | 14 (100) | 12 (100) |
ADL: activities of daily living, IQR: interquartile range. *Home care provided by personnel from community-based social services, or community and government salaried personal assistants. ALSFRS-R: ALS Functioning Rating Scale-Revised. # N = 38.
Approximately half of the patients (52%) had the year before baseline been an inpatient with a median stay of 12 days (Table 2). Between 24%and 44%received inpatient care during each six-months period, i.e., from baseline until the last data-collection (Table 2). The total amount of inpatient days (i.e., from one year before baseline to the last data collection) was 1316 days where care at the neurology and rehabilitation departments composed two thirds of all inpatient care (Fig. 1). Altogether 37 individual patients received inpatient care at the neurology department and 10 at a rehabilitation department.
Table 2
Use of inpatient and outpatient care during one year before (T0) the first data-collection (T1), and from each data-collection until the next 6 months later (T2–T7). N = patients included at each timepoint
| Time points | Inpatient days total | Users | Inpatient days/user | |||
| n | (%) | median | min | max | ||
| T0–T1, N = 60 | 630 | 31 | (52) | 12 | 1 | 136 |
| T1–T2, N = 39 | 207 | 13 | (33) | 8 | 2 | 94 |
| T2–T3, N = 29 | 106 | 7 | (24) | 11 | 1 | 42 |
| T3–T4, N = 17 | 91 | 5 | (29) | 19 | 8 | 28 |
| T4–T5, N = 16 | 103 | 7 | (44) | 11 | 3 | 42 |
| T5–T6, N = 14 | 68 | 6 | (43) | 6 | 1 | 35 |
| T6–T7, N = 12 | 111 | 4 | (33) | 31 | 7 | 42 |
| Outpatient contacts total | Outpatient contacts/user | |||||
| T0–T1, N = 60 | 5600 | 60 | (100) | 52 | 4 | 1012 |
| T1–T2, N = 39 | 2338 | 39 | (100) | 32 | 1 | 512 |
| T2–T3, N = 29 | 2379 | 29 | (100) | 31 | 3 | 497 |
| T3–T4, N = 17 | 877 | 17 | (100) | 32 | 3 | 255 |
| T4–T5, N = 16 | 752 | 16 | (100) | 22 | 1 | 292 |
| T5–T6, N = 14 | 994 | 14 | (100) | 37 | 2 | 292 |
| T6–T7, N = 12 | 1096 | 12 | (100) | 115 | 1 | 240 |
Fig. 1
Hospital inpatient care from one year before study start (T0) until the last participated data collection (T1–T7), proportion of inpatient days at different departments. Other inpatient care includes surgery, orthopaedics, and palliative care.
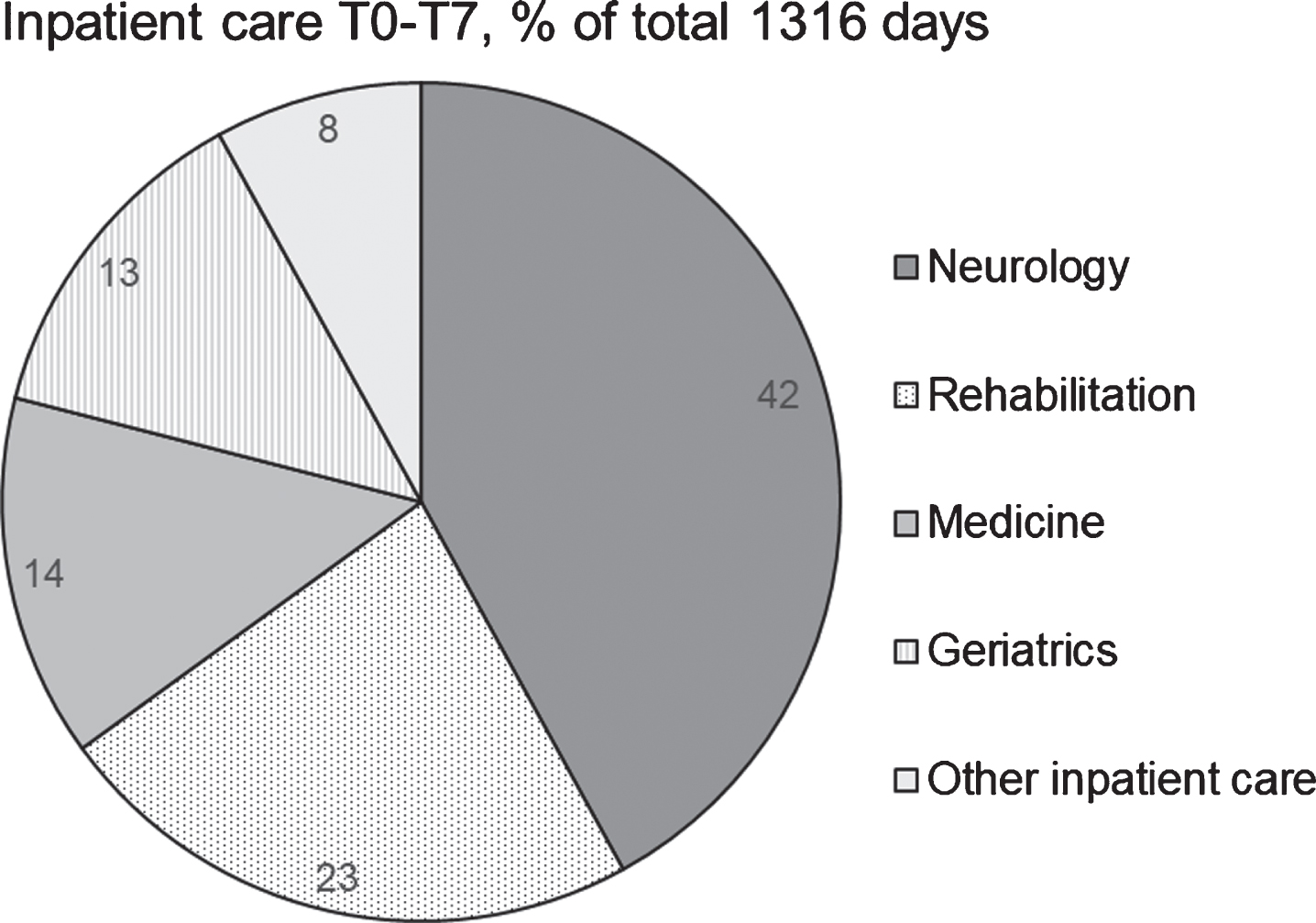
There were no significant between-group differences (age, sex, education level, time since diagnosis, and disease severity) in use of inpatient care at the departments of neurology, medicine and geriatrics or the summarised others which included surgery, orthopaedics, and palliative care. There was a significant difference in use of inpatient care at rehabilitation departments depending on time since diagnosis, p = 0.008. The median (minimum-maximum) stay for those with < 24 months since diagnosis was 18 (11–19) days compared to 37 (28–84) days for those with≥24 months since diagnosis.
All patients had registered outpatient contacts during the year before baseline (T0-T1), and all received outpatient care during the study period (Table 2). The median number of contacts per user varied over time, from 52 the year before baseline to 115 at T7 (Table 2). A total of 14036 outpatient contacts (i.e., from one year before baseline to the last data collection) were registered of which half were registered by providers of advanced healthcare in the home and a fifth by allied health professionals (Fig. 2). Distributions of outpatient contacts by healthcare personnel and sort of contacts are presented in Table 3. Over half of all outpatient contacts consisted of home visits, mainly performed by nurses. Outpatient contacts with physicians were mainly clinic visits followed by administrative contacts, i.e., without patient. Over half of the outpatient contacts with occupational therapists were home visits while half of the contacts for physiotherapists were visits at the clinic. Over time, all patients had registered outpatient contacts with physicians, 58 with nurses, 55 with physiotherapists, 50 with occupational therapists, 42 with dieticians, 36 with speech therapists, 33 with welfare officers and 5 patients had outpatient contacts with psychologists.
Fig. 2
Hospital outpatient care from one year before study start (T0) until the last participated data collection (T1–T7), proportion of contacts with different outpatient healthcare providers. Other outpatient care included contacts with medicine, surgery, orthopaedic, psychiatry, geriatrics, respiratory, ear/nose/throat and radiology clinics.
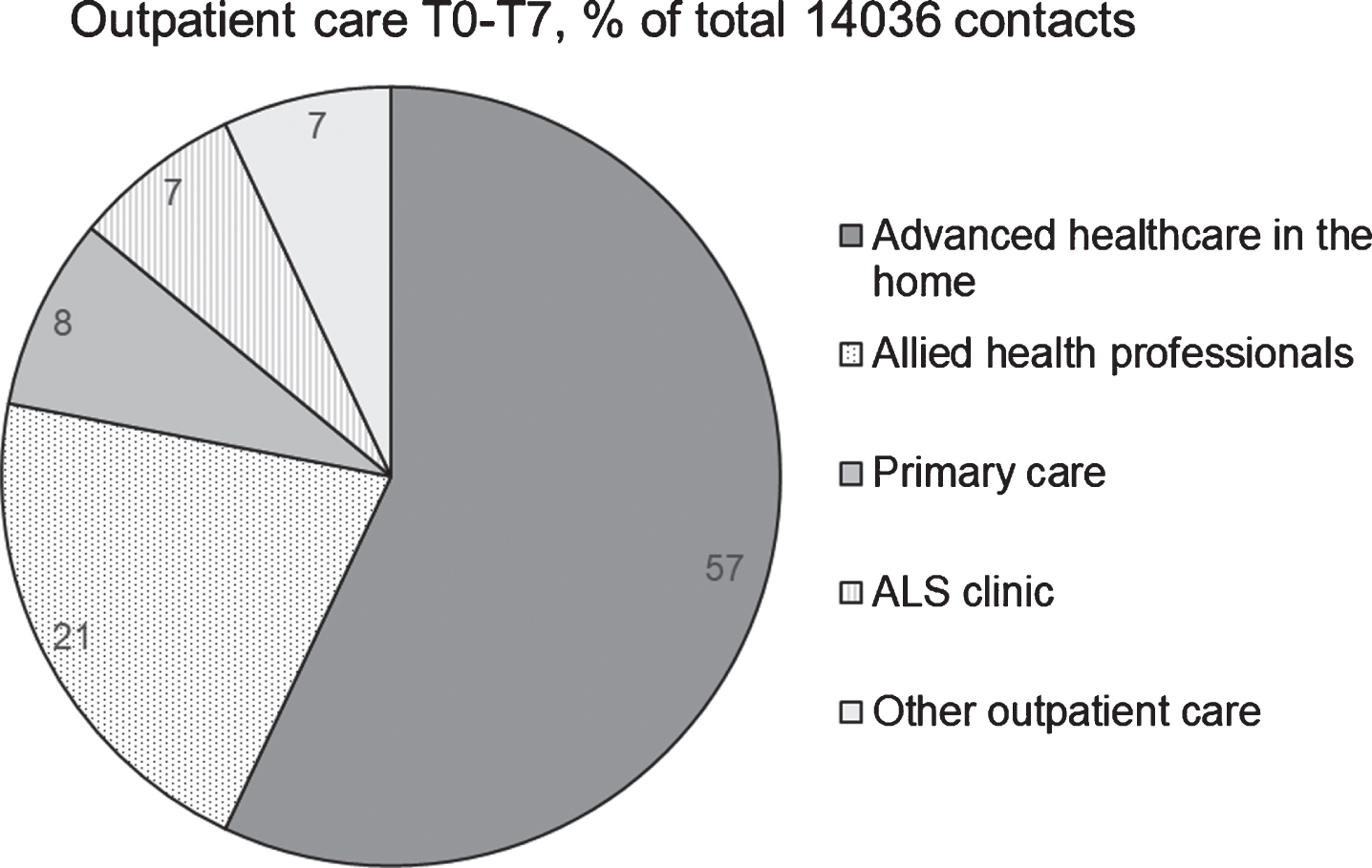
Table 3
Distribution of outpatient contacts by healthcare personnel and sort of contact from one year before study start (T0) until the last participated data collection during the three-year study period (T1–T7)
| Sum contacts n (%) | Clinic visits n (%) | Home visits n (%) | Telephone contacts n (%) | Administrative contacts n (%) | |
| Nurses | 8012 (57) | 344 (4) | 6767 (84) | 417 (5) | 484 (6) |
| Physicians | 2233 (16) | 1327 (59) | 288 (13) | 163 (7) | 455 (20) |
| Occupational therapists | 1305 (9) | 262 (20) | 757 (58) | 33 (3) | 253 (19) |
| Physiotherapists | 1169 (8) | 585 (50) | 396 (34) | 145 (12) | 43 (4) |
| Speech therapists | 494 (4) | 331 (67) | 104 (21) | 37 (7) | 22 (4) |
| Dieticians | 333 (2) | 69 (21) | 63 (19) | 187 (56) | 14 (4) |
| Welfare officers, Psychologists | 236 (2) | 107 (45) | 110 (47) | 6 (3) | 13 (6) |
| Others* | 254 (2) | 204 (80) | 20 (8) | 1 (1) | 29 (11) |
| Total | 14036 (100) | 3229 (23) | 8505 (61) | 989 (7) | 1313 (9) |
*Others include contacts with foot-care specialists, and radiology and physiology laboratory technicians.
There were no significant between-group (age, sex, education level, time since diagnosis, and disease severity) differences in use of outpatient care from providers of advanced healthcare in the home, allied health professionals, and primary care. There were significant differences in use of outpatient ALS clinic care between groups based on time since diagnosis (p = 0.046) and disease severity (p = 0.006). The median (minimum-maximum) contacts for those with < 24 months since diagnosis was 15 (2–95) compared to 11 (2–37) for those with≥24 months since diagnosis. The median (minimum-maximum) contacts for those with an ALSFRS-R score between 0–24 (severe disease) was 10 (2–25) compared to 15 (2–95) for those with ALSFRS-R score between 25–48 (less severe). There was a significant difference in use of other outpatient care (i.e., including contacts with medicine, surgery, orthopaedic, psychiatry, geriatrics, respiratory, ear/nose/throat and radiology clinics) between groups based on age (p = 0.044). The median (minimum-maximum) contacts for those < 65 years was 8 (1–111) compared to 5 (2–33) for those≥65 years.
Satisfaction with the various dimensions of health care (i.e., art of care, availability, accessibility, continuity, participation in care planning, efficacy/outcome of care and finances) for those with a manifested need or being applicable from the perspective of patients with ALS was relatively stable over time (Table 4). In general, two thirds or more of the patients were satisfied with the art of care (sympathy or engagement from staff and kind treatment) and the continuity, i.e., meeting the same staff. As for availability, most patients were satisfied although less were satisfied with the availability of physicians. The proportion of patients being satisfied with access to welfare officers and psychologists was in general lower than for other healthcare personnel, even though a majority expressed that they had had contact with all expertise needed. Almost all patients wanted to participate in care planning, but few perceived that they had taken part. Regarding efficacy or outcome of care, over time around two-thirds to all patients expressing a need were satisfied.
Table 4
Satisfaction with healthcare by patients with ALS at baseline (T1) and at each follow-up during the three-year study period (T2–T7). Number and proportion satisfied of those with a manifested need or applicable from the perspective of patients with ALS
| T1 (N = 60) Satisfied n (%) | T2 (N = 39) Satisfied n (%) | T3 (N = 29) Satisfied n (%) | T4 (N = 17) Satisfied n (%) | T5 (N = 14) Satisfied n (%) | T6 (N = 14) Satisfied n (%) | T7 (N = 12) Satisfied n (%) | |
| Art of care | |||||||
| Sympathy/engagement from staff | |||||||
| Physicians | 43 (78) | 30 (83) | 17 (71) | 14 (82) | 12 (100) | 13 (100) | 11 (92) |
| Nurses | 33 (75) | 23 (74) | 20 (91) | 11 (73) | 10 (91) | 12 (100) | 9 (82) |
| Occupational therapist | 41 (85) | 29 (85) | 21 (88) | 13 (87) | 7 (70) | 8 (80) | 7 (64) |
| Dieticians | 22 (76) | 17 (85) | 9 (69) | 6 (60) | 5 (83) | 7 (100) | 7 (100) |
| Welfare officer | 30 (79) | 21 (78) | 13 (76) | 7 (88) | 5 (83) | 5 (83) | 3 (75) |
| Speech therapist | 21 (72) | 18 (90) | 10 (91) | 5 (63) | 5 (100) | 6 (100) | 4 (80) |
| Physiotherapists | 32 (78) | 18 (90) | 13 (87) | 9 (82) | 6 (100) | 6 (75) | 7 (70) |
| Psychologists | 4 (57) | 5 (100) | 2 (67) | 1 (100) | 1 (100) | ||
| Kind treatment | |||||||
| Physicians | 51 (93) | 30 (83) | 19 (73) | 15 (88) | 12 (100) | 13 (100) | 11 (92) |
| Nurses | 45 (94) | 26 (84) | 20 (91) | 14 (93) | 11 (100) | 12 (100) | 10 (91) |
| Occupational therapist | 47 (96) | 30 (91) | 20 (83) | 14 (93) | 9 (90) | 8 (89) | 8 (73) |
| Dieticians | 26 (90) | 18 (90) | 12 (75) | 7 (78) | 6 (100) | 7 (100) | 7 (100) |
| Welfare officer | 34 (87) | 22 (81) | 13 (76) | 8 (100) | 5 (100) | 5 (100) | 3 (75) |
| Speech therapist | 27 (84) | 17 (85) | 10 (83) | 7 (88) | 5 (100) | 5 (100) | 5 (100) |
| Physiotherapists | 38 (86) | 25 (89) | 13 (81) | 10 (91) | 6 (100) | 7 (88) | 6 (60) |
| Psychologists | 3 (60) | 4 (80) | 3 (100) | 1 (100) | |||
| Availability | |||||||
| Physicians | 30 (64) | 23 (72) | 15 (68) | 9 (60) | 10 (83) | 12 (92) | 11 (100) |
| Nurses | 33 (73) | 24 (86) | 18 (86) | 12 (80) | 10 (91) | 11 (100) | 10 (100) |
| Occupational therapist | 34 (83) | 28 (88) | 16 (70) | 11 (79) | 10 (91) | 8 (80) | 9 (90) |
| Dieticians | 17 (77) | 15 (83) | 10 (77) | 4 (57) | 5 (83) | 7 (100) | 7 (100) |
| Welfare officer | 26 (81) | 21 (84) | 10 (67) | 7 (100) | 5 (100) | 5 (83) | 3 (75) |
| Speech therapist | 15 (65) | 14 (88) | 8 (73) | 4 (67) | 4 (80) | 4 (100) | 3 (75) |
| Physiotherapists | 29 (81) | 20 (83) | 12 (80) | 10 (100) | 5 (100) | 7 (88) | 8 (89) |
| Psychologists | 5 (100) | 2 (67) | |||||
| Accessibility | |||||||
| Physicians | 36 (73) | 27 (87) | 18 (75) | 14 (82) | 11 (85) | 10 (100) | 10 (91) |
| Nurses | 32 (84) | 16 (70) | 16 (89) | 11 (79) | 10 (100) | 6 (86) | 9 (100) |
| Occupational therapist | 37 (84) | 24 (83) | 18 (82) | 12 (86) | 6 (60) | 6 (75) | 8 (73) |
| Dieticians | 23 (72) | 16 (94) | 9 (64) | 9 (82) | 6 (100) | 6 (100) | 6 (100) |
| Welfare officer | 22 (65) | 14 (61) | 11 (73) | 7 (78) | 2 (67) | 3 (60) | 3 (75) |
| Speech therapist | 20 (77) | 15 (79) | 10 (77) | 6 (86) | 2 (50) | 3 (60) | 2 (67) |
| Physiotherapists | 28 (64) | 17 (68) | 12 (75) | 11 (85) | 6 (67) | 5 (71) | 6 (75) |
| Psychologists | 3 (33) | 4 (40) | 1 (20) | 1 (100) | |||
| Contact with all expertise needed | 36 (68) | 26 (72) | 18 (72) | 13 (76) | 12 (86) | 11 (85) | 10 (83) |
| Continuity | |||||||
| Meeting same staff | 48(91) | 29 (81) | 19 (76) | 14 (82) | 11 (79) | 11 (85) | 12 (100) |
| Participation in care planning | |||||||
| Want to participate | 44 (81) | 27 (75) | 22 (85) | 13 (76) | 12 (86) | 11 (85) | 12(100) |
| Have participated | 25 (50) | 15 (44) | 14 (56) | 10 (63) | 7 (58) | 7 (58) | 8 (67) |
| Efficacy/outcome of care | |||||||
| ALS clinic care | 43 (77) | 30 (94) | 21 (78) | 14 (82) | 9 (75) | 10 (91) | 11 (100) |
| Hospital inpatient care | 24 (65) | 25 (86) | 16 (76) | 10 (77) | 5 (100) | 7 (88) | 5 (71) |
| Hospital outpatient care | 11 (58) | 17 (94) | 8 (67) | 8 (80) | 8 (89) | 6 (86) | 6 (100) |
| Other outpatient care | 22 (65) | 14 (67) | 11 (79) | 4 (36) | 5 (63) | 3 (100) | |
| Advanced healthcare in the home | 7 (64) | 7 (64) | 9 (90) | 3 (100) | 3 (100) | 3 (60) | 5 (63) |
| Rehabilitation/convalescence care | 9 (75) | 11 (92) | 9 (90) | 10 (100) | 3 (75) | 4 (100) | 2 (67) |
| Finances | 35 (69) | 24 (73) | 19 (83) | 10 (63) | 13 (93) | 11 (85) | 10 (83) |
There were no significant differences in satisfaction with care in those patients reporting a need at both baseline (T1) and at the first follow-up (T2). There were no statistically significant differences in use of healthcare between groups satisfied or not satisfied with healthcare except for outpatient contacts at T3. At that timepoint, the median (min-max) number of contacts were 22 (3–107) for those satisfied (n = 18) and 40 (6–278) for those not satisfied (n = 7), p = 0.017.
DISCUSSION
We have explored utilisation of healthcare and satisfaction with care in a cohort of patients with ALS who participated in our three-year observational study [2]. Over time, between a quarter and half of the patients used inpatient care, and two-thirds of the total number of inpatient days were spent at neurology and rehabilitation departments. All patients used outpatient care, and over half of the total number of contacts were with providers of advanced healthcare in the home and one-fifth with allied health professionals. Nurses performing home visits composed the largest proportion of outpatient contacts. Analyses revealed that patients with less severe disease and shorter time since diagnosis had significantly more contacts with the outpatient ALS clinic compared to those with severe disease and longer time since diagnosis. Satisfaction with the various dimensions of care was in general stable over time with around two-thirds or more of patients being satisfied. However, few patients perceived that they had taken part in care planning. Overall, there seemed to be no significant difference in use of healthcare between patients being satisfied with healthcare versus those not satisfied.
Healthcare utilisation data imply that patients with ALS mainly had outpatient contacts and that half, or less of the participating patients, utilised inpatient care. This is in line with findings from other neurological conditions in Sweden such multiple sclerosis [26] and stroke [28], but differ from the reported large amount of inpatient care in patients with Guillain-Barré syndrome [24]. Another striking difference is the amount of inpatient rehabilitation, where only 10 patients with ALS (16%) had any inpatient rehabilitation days registered in comparison to 31%of patients with multiple sclerosis [26] and 83%of patients with Guillain-Barré syndrome [24]. These differences can be due to the diverse courses of these diseases, or just mirror changes in the organisation of healthcare where the trend during the last decades is to reduce inpatient care in favour of outpatient care.
Most of the outpatient care consisted of contacts with personnel providing advanced healthcare in the home. In fact, almost two-thirds of all registered outpatient contacts were home visits. In addition to these visits, the patients also had home care provided by personnel from community-based social services, or community and government salaried personal assistants. Although all these services make it possible for patients to remain in their home even at the end of life, it may also induce a significant amount of strain for both patients and their families. The wish and struggle to maintain roles within the family and a sense of normality, as well as to retain control of personal and domestic space are factors highlighted by patients with ALS and their families [29–32]. However, the burden of care experienced by family caregivers might not depend on utilisation of healthcare and social services but on the subjective experience and psychological composition of the family caregivers [33].
Current guidelines on clinical management of ALS recommend that patients have contact with an experienced neurologist and receive regular support from a multidisciplinary care team [9, 34]. The ALS clinic at the department of neurology Karolinska University Hospital was developed over 30 years ago with the aim to improve the management and care for patients with ALS and their families in the Region Stockholm. Besides neurologists and nurses, team members from other hospital departments include occupational therapists, physiotherapists, welfare officers, speech therapists and dieticians. However, it is clear from our results that healthcare emerging from the ALS clinic only composed a small amount of the utilised outpatient care. Further, that contacts with the ALS clinic was reduced as patients became worse and needed advanced healthcare in the home. Studies have shown that it is important to individualise and involve patients and their caregivers, both through information and joint decision-making, to avoid experienced difficulties during care transitions [35, 36]. It would therefore be interesting to study these care transitions from one healthcare provider to another, for example from the ALS clinic to advanced healthcare in the home, from both the patients’ and the healthcare system perspective. Further, to adhere to the guidelines on clinical management of ALS, here is a need to develop a structured multidisciplinary care organisation among healthcare providers in outpatient care, beyond the multidisciplinary care provided by the ALS-clinic.
The management and care of ALS incorporate many different healthcare providers and there is a need for care coordination, a topic which has been reported to receive little attention in the literature [37]. Perhaps the found discrepancy between wanting and having participated in care planning illustrates the problem with care coordination and formulation of coherent care plans. Even though the Swedish Patient Act (2014:821) state the patient’s right to participate in all decisions about the care he/she will be receiving, many patients with ALS stated that they had not participated. Our findings of extensive use of outpatient care beside the rather small amount of care from the ALS clinic, clearly indicate the need of coordination between these entities and with the individual patient participating. An issue previously raised by patients with motor neuron diseases [38, 39]. Thus, there is a need for strategies to enhance the coordination of care and to incorporate the patients with ALS as full partners to their care-providers with a role in healthcare decisions [40], i.e., to establish a person-centered care [41] where care is based on the patient’s preferences, needs, and values, taking patient resources into account [42]. This may be facilitated by interdisciplinary or transdisciplinary care models which have shown to improve health outcome, resource utilisation and satisfaction in patients with ALS [43].
Satisfaction with care in our cohort of patients with ALS resembled previous findings in patients with multiple sclerosis [25, 26], where lower satisfaction with availability of physicians and psychologists, and accessibility to psychosocial support /counselling was found. Studies on satisfaction with care in patients with ALS and their family caregivers report concerns about inadequate emotional support [38], lack of continuity and coordination of services [38, 39], unmet needs [44] and difficulties to accessing services [45]. Even though most of our patients expressed being satisfied with the various dimensions of healthcare, patients being dissatisfied varied from around a third to a fifth depending on timepoint and dimensions. This indicate that there is room for improvement. Patients who receive tailored services seem more satisfied [46]. Thus, person-centered care should be adopted to increase both satisfaction with and the quality of healthcare services.
The major strength with this study is that data on healthcare utilisation came from the computerised register where all in- and outpatient care can be identified and linked to the individual patient and, thus, is not dependent on the patients’ memory. In addition, the prospective design with repeated data-collections by home visits to the patients, and the possibility to link patient characteristics and their perceived satisfaction with healthcare to all register-based data make this study unique. However, studying patients with ALS over time has its challenges due to the progressive nature of the disease. Even though recruitment was performed at an ALS clinic with probably the largest patient cohort in Sweden, the study sample was reduced already after one year which is a limitation of the study. Also, studying satisfaction with healthcare by use of a questionnaire does not give the full picture and should preferably be complemented by qualitative studies.
In conclusion, patients with ALS use hospital-based specialist care and other outpatient care in parallel with many healthcare providers involved. Our findings highlight the need for implementation of person-centred care to improve both coordination of care, care transitions and satisfaction with healthcare services.
ACKNOWLEDGMENTS
The authors thank the patients with ALS who participated in the study. We acknowledge help from Anne Zachau, Rayomand Press and Lars-Olof Ronnevi with the recruitment of patients with ALS and Gunilla Cröde Widsell in data collections.
FUNDING
The work was supported by the Swedish Research Council under grant 521-2014-3196; Neuro Sweden; KID funding under grant 3-1233/2013; and the Strategic Research Programme in Care Sciences, Karolinska Institutet.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Crockford C , Newton J , Lonergan K , Chiwera T , Booth T , Chandran S , et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology. (2018) ;91: :e1370–e8080. |
[2] | Sandstedt P , Littorin S , Johansson S , Gottberg K , Ytterberg C , Kierkegaard M . Disability and Contextual Factors in Patients with Amyotrophic Lateral Sclerosis - A Three-Year Observational Study. J Neuromuscul Dis. (2018) ;5: :439–49. |
[3] | Logroscino G , Traynor BJ , Hardiman O , Chio A , Mitchell D , Swingler RJ , et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. (2010) ;81: :385–90. |
[4] | Longinetti E , Fang F . Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr Opin Neurol. (2019) ;32: :771–6. |
[5] | Moura MC , Novaes MR , Eduardo EJ , Zago YS , Freitas Rdel N , Casulari LA . Prognostic Factors in Amyotrophic Lateral Sclerosis: A Population-Based Study. PLoS One. (2015) ;10: :e0141500. |
[6] | Kiernan MC , Vucic S , Cheah BC , Turner MR , Eisen A , Hardiman O , et al. Amyotrophic lateral sclerosis. Lancet. (2011) ;377: :942–55. |
[7] | Pupillo E , Messina P , Logroscino G , Beghi E . Long-term survival in amyotrophic lateral sclerosis: A population-based study. Ann Neurol. (2014) ;75: :287–97. |
[8] | Lavernhe S , Antoine JC , Court-Fortune I , Dimier N , Costes F , Lacour A , et al. Home care organization impacts patient management and survival in ALS. Amyotroph Lateral Scler Frontotemporal Degener. (2017) ;18: :562–8. |
[9] | Andersen PM , Abrahams S , Borasio GD , de Carvalho M , Chio A , Van Damme P , et al. EFNS guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS) - revised report of an EFNS task force. Eur J Neurol. (2012) ;19: :360–75. |
[10] | Miller RG , Jackson CE , Kasarskis EJ , England JD , Forshew D , Johnston W , et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. (2009) ;73: :1218–26. |
[11] | De Groot IJ , Post MW , Van Heuveln T , Van Den Berg LH , Lindeman E . Measurement of decline of functioning in persons with amyotrophic lateral sclerosis: Responsiveness and possible applications of the Functional Independence Measure, Barthel Index, Rehabilitation Activities Profile and Frenchay Activities Index. Amyotroph Lateral Scler. (2006) ;7: :167–72. |
[12] | Van Groenestijn AC , Schröder CD , Kruitwagen-Van Reenen ET , Van Den Berg LH , Visser-Meily JMA . Participation restrictions in ambulatory amyotrophic lateral sclerosis patients: Physical and psychological factors. Muscle Nerve. (2017) ;56: :912–8. |
[13] | Sandstedt P , Johansson S , Ytterberg C , Ingre C , Holmqvist LW , Kierkegaard M . Predictors of health-related quality of life in people with amyotrophic lateral sclerosis. J Neurol Sci. (2016) ;370: :269–73. |
[14] | Sandstedt P , Littorin S , Crode Widsell G , Johansson S , Gottberg K , Ytterberg C , et al. Caregiver experience, health-related quality of life and life satisfaction among informal caregivers to patients with amyotrophic lateral sclerosis: A cross-sectional study. J Clin Nurs. (2018) ;27: :4321–30. |
[15] | Gladman M , Zinman L . The economic impact of amyotrophic lateral sclerosis: A systematic review. Expert Rev Pharmacoecon Outcomes Res. (2015) ;15: :439–50. |
[16] | Moore A , Young CA , Hughes DA . Health Utilities and Costs for Motor Neurone Disease. Value Health. (2019) ;22: :1257–65. |
[17] | Song H , Liu JC , Cao ZP , Luo WJ , Chen JY . Medical cost and healthcare utilization of amyotrophic lateral sclerosis in China: A cohort study based on hospital data from 2015 to 2018. Medicine (Baltimore). (2020) ;99: :e23258. |
[18] | Zwicker J , Qureshi D , Talarico R , Bourque P , Scott M , Chin-Yee N , et al. Dying of amyotrophic lateral sclerosis: Health care use and cost in the last year of life. Neurology. (2019) ;93: :e2083–e93. |
[19] | Maetens A , Deliens L , De Bleecker J , Caraceni A , De Ridder M , Beernaert K , et al. Healthcare utilization at the end of life in people dying from amyotrophic lateral sclerosis: A retrospective cohort study using linked administrative data. J Neurol Sci. (2019) ;406: :116444. |
[20] | Foley G , Timonen V , Hardiman O . Experience of services as a key outcome in amyotrophic lateral sclerosis (ALS) care: The case for a better understanding of patient experiences. Am J Hosp Palliat Care. (2012) ;29: :362–7. |
[21] | Foley G , Timonen V , Hardiman O . Patients’ perceptions of services and preferences for care in amyotrophic lateral sclerosis: A review. Amyotroph Lateral Scler. (2012) ;13: :11–24. |
[22] | Brooks BR , Miller RG , Swash M , Munsat TL . El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. (2000) ;1: :293–9. |
[23] | Cedarbaum JM , Stambler N , Malta E , Fuller C , Hilt D , Thurmond B , et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. (1999) ;169: :13–21. |
[24] | Forsberg A , de Pedro-Cuesta J , Widén Holmqvist L . Use of healthcare, patient satisfaction and burden of care in Guillain-Barre syndrome. J Rehabil Med. (2006) ;38: :230–6. |
[25] | Gottberg K , Einarsson U , Ytterberg C , Fredrikson S , von Koch L , Holmqvist LW . Use of health care services and satisfaction with care in people with multiple sclerosis in Stockholm County: A population-based study. Mult Scler. (2008) ;14: :962–71. |
[26] | Chruzander C , Johansson S , Gottberg K , Einarsson U , Hillert J , Holmqvist LW , et al. A 10-year population-based study of people with multiple sclerosis in Stockholm, Sweden: Use of and satisfaction with care and the value of different factors in predicting use of care. BMC Health Serv Res. (2015) ;15: :480. |
[27] | Ware JE Jr , Snyder MK , Wright WR , Davies AR . Defining and measuring patient satisfaction with medical care. Eval Program Plann. (1983) ;6: :247–63. |
[28] | Minet LR , Peterson E , von Koch L , Ytterberg C . Healthcare Utilization After Stroke: A 1-Year Prospective Study. J Am Med Dir Assoc. (2020) ;21: :1684–8. |
[29] | Gottberg K , Ytterberg C , Sandstedt P , Johansson S , Kierkegaard M . Experiences of next of kin to patients with amyotrophic lateral sclerosis using invasive ventilation via tracheostomy. Disabil Rehabil. 2019: 1–8. |
[30] | Foley G , Timonen V , Hardiman O . Acceptance and decision making in amyotrophic lateral sclerosis from a life-course perspective. Qual Health Res. (2014) ;24: :67–77. |
[31] | O’Brien MR , Whitehead B , Murphy PN , Mitchell JD , Jack BA . Social services homecare for people with motor neurone disease/amyotrophic lateral sclerosis: Why are such services used or refused? Palliat Med (2012) ;26: :123–31. |
[32] | Foley G , Timonen V , Hardiman O . Exerting control and adapting to loss in amyotrophic lateral sclerosis. Soc Sci Med. (2014) ;101: :113–9. |
[33] | Burke T , Galvin M , Pinto-Grau M , Lonergan K , Madden C , Mays I , et al. Caregivers of patients with amyotrophic lateral sclerosis: Investigating quality of life, caregiver burden, service engagement, and patient survival. J Neurol. (2017) ;264: :898–904. |
[34] | Miller RG , Jackson CE , Kasarskis EJ , England JD , Forshew D , Johnston W , et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. (2009) ;73: :1227–33. |
[35] | Hesselink G , Flink M , Olsson M , Barach P , Dudzik-Urbaniak E , Orrego C , et al. Are patients discharged with care? A qualitative study of perceptions and experiences of patients, family members and care providers. BMJ Qual Saf. (2012) ;21: (Suppl 1):i39–49. |
[36] | Verhaegh KJ , Jepma P , Geerlings SE , de Rooij SE , Buurman BM . Not feeling ready to go home: A qualitative analysis of chronically ill patients’ perceptions on care transitions. Int J Qual Health Care. (2019) ;31: :125–32. |
[37] | Janssens AIWA , Ruytings M , Al-Chalabi A , Chio A , Hardiman O , McDermott CJ , et al. A mapping review of international guidance on the management and care of amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener. (2016) ;17: :325–36. |
[38] | Brown JB . User, carer and professional experiences of care in motor neurone disease. Prim Health Care Res Devel. (2003) ;4: :207–17. |
[39] | Hugel H , Grundy N , Rigby S , Young CA . How does current care practice influence the experience of a new diagnosis of motor neuron disease? A qualitative study of current guidelines-based practice. Amyotroph Lateral Scler. (2006) ;7: :161–6. |
[40] | The Swedish Agency for Health and Care Services Analyses. Patient-centeredness in Sweden’s health system-an external assessment and six steps for progress. https://www.vardanalys.se/rapporter/patient-centeredness-in-swedens-health-system/2012. |
[41] | Ekman I , Swedberg K , Taft C , Lindseth A , Norberg A , Brink E , et al. Person-centered care–ready for prime time. Eur J Cardiovasc Nurs. (2011) ;10: :248–51. |
[42] | Institute of Medicine Committee on Quality of Health Care in A. Crossing the Quality Chasm: A New Health System for the 21st Century.Washington (DC): National Academies Press (US); (2001) . pp. 39–60. |
[43] | Howard I , Potts A . Interprofessional Care for Neuromuscular Disease. Current treatment options in neurology. (2019) ;21: :35. |
[44] | van Teijlingen ER , Friend E , Kamal AD . Service use and needs of people with motor neurone disease and their carers in Scotland. Health Soc Care Community. (2001) ;9: :397–403. |
[45] | Hughes RA , Sinha A , Higginson I , Down K , Leigh PN . Living with motor neurone disease: Lives, experiences of services and suggestions for change. Health Soc Care Community. (2005) ;13: :64–74. |
[46] | Kristjanson LJ , Aoun SM , Yates P . Are supportive services meeting the needs of Australians with neurodegenerative conditions and their families? J Palliat Care. (2006) ;22: :151–7. |




