E-Health & Innovation to Overcome Barriers in Neuromuscular Diseases. Report from the 1st eNMD Congress: Nice, France, March 22-23, 2019
Abstract
By definition, neuromuscular diseases are rare and fluctuating in terms of symptoms; patients are often lately diagnosed, do not have enough information to understand their condition and be proactive in their management. Usually, insufficient resources or services are available, leading to patients’ social burden. From a medical perspective, the rarity of such diseases leads to the unfamiliarity of the medical staff and caregiver and an absence of consensus in disease assessment, treatment, and management. Innovations have to be developed in response to patients’ and physicians’ unmet needs.
It is vital to improve several aspects of patients’ quality of life with a better comprehension of their disease, simplify their management and follow-up, help their caregiver, and reduce the social and economic burden for living with a rare debilitating disease. Database construction regrouping patients’ data and symptoms according to specific country registration on data privacy will be critical in establishing a clear consensus on neuromuscular disease treatment.
Clinicians also need technological innovations to help them recognize neuromuscular diseases, find the best therapeutic approach based on medical consensus, and tools to follow patients’ states regularly. Diagnosis also has to be improved by implementing automated systems to analyze a considerable amount of data, representing a significant step forward to accelerate the diagnosis and the patients’ follow up. Further, the development of new tools able to precisely measure specific outcomes reliably is of the matter of importance in clinical trials to assess the efficacy of a newly developed compound.
In this context, creation of an expert community is essential to communicate and share ideas. To this end, 97 clinicians, healthcare professionals, researchers, and representatives of private companies from 9 different countries met to discuss the new perspective and challenges to develop and implement innovative tools in the field of neuromuscular diseases.
GOALS OF THE CONGRESS
Given the complexity of symptoms, management, and assessment of neuromuscular diseases (NMD), it is essential to build a community of experts who can share resources and knowledge. Further, developing new tools to help with patients’ care, follow-up, and disease assessment is important to improve the way patients’ are treated. The eNMD congress is a milestone in achieving this objective, bringing together internationally recognized experts on NMD and innovations able to make tomorrow’s medicine to treat, manage, and monitor complex diseases such as NMD. The meeting was attended by 97 participants with 24 speakers from 9 different countries: France, Canada, Slovenia, Germany, Italy, USA, Spain, Denmark, and Belgium.
This first eNMD congress aimed to present new tools and innovations developed to overcome barriers in NMD and explore the opportunity to use remote medicine for patients’ follow-up or connected devices to establish new outcomes measures. Overall, this meeting was divided into five different sessions, each addressing how new technologies will change the face of NMD assessment, follow-up, and monitoring. The first session was on the challenges and strategies of e-health and innovations. The second session was on e-registries, telemedicine, and m-health apps. The third one was on robotics and domotics, the fourth on connected devices. The last session was on various new developments in the field of NMD.
INTRODUCTORY TALKS
After the introduction by Charles Guepratte, the Director of Nice University Hospital, Jean-Marc Gambaudo, the President of Côte d’Azur University, and Mathilde Demory-Zory, the city of Nice representative, Serge Braun, the AFM-Téléthon Chief Scientific Officer, open the congress with an explanation of ten ways in which neuromuscular diseases can benefit from Digital Health. Digital health technology (DHT) may help to solve issues such as those expressed by patients through a barometer published in 2015 by Maladies rares info service: delay to obtain a diagnosis, improper care, stop working for parent careers, insufficient knowledge of health professionals outside the hospital, and patients’ social isolation.
DHT can be used to optimize medical and social resources, ease treatment access, organize multidisciplinary daily- and social healthcare, speed-up expertise, improve patient autonomy (visual recognition devices, brain-computer interfaces, deep learning), and reduce costs (potential 60% in chronic diseases), travels, and need of emergency services. Conditions are met to implement such tools. Even though 41% of French people have doubts about data security, 75% are ready to communicate through digital channels with their general practitioner, 81% of clinicians also accept such technologies, and expected reduced costs are close to 100 billion € in the European Union with e-health technologies.
Examples in the NMD field were presented, showing tools (data mining of online databases, genetic, and omics) to speed-up massive diagnostic delays (11 months in ALS, up to 20 years in late-onset acid maltase deficiency). Similar approaches can be used to identify new treatment opportunities (ex: combinatorial pharmacology). Examples of tele-expertise application in the multidisciplinary management of NMD were presented, which improve care management and break the isolation of patients affected by NMD. Useful E-learning tools were presented, together with clinical and monitoring tool-based medico-economic NMD cohort studies, as well as patient support program solutions and remote health monitoring systems (examples including the ActiMyo or Feetme and wearable intelligent devices). Brain-computer interfaces, communication technologies, and speech software were also addressed, which are useful for accelerated diagnosis and management. Concluding remarks included the need for electronic patient records, agreed standards for data protection, funding, and reimbursement models in a highly fragmented market, which should be designed to contribute to the people they are aimed at.
The second introductory talk presented policies. Anna Kole and Ines Hernando presented “E-Health Strategies and Roadmaps supporting Rare Disease Policies”. Sharing and pooling data between patients and health service providers, hospitals, health professionals, and health and research information networks is essential for rare diseases and any area requiring a specific concentration of expertise. Only through data aggregation can one attain a critical mass, which generates the knowledge needed to provide the best care quality and drive forward research leading to more innovative treatments: electronic health records, telemedicine services, and portable patient-monitoring devices. Patient data sharing is of central importance to the ERN concept that expertise travels instead of patients and rests upon data’s ability to travel. For the data to travel, it has to be electronic. The current obstacles in making this happen are 1) technical, due to continued lack of interoperable data platforms; 2) legal due to a lack of clarity and interpretation of data sharing legislation; 3) financial due to inadequate financial support of infrastructures, tools, and expertise needed to meet demand; 4) social/political due to a continued need for a paradigm shift in attitudes and the political will required for real electronic sharing of patient data. Several legislations provide a policy basis to guide activities to overcome these obstacles, including Article 14 of the Directive on Patients’ Rights in Cross Border Healthcare (2011), the General Data Protection Regulation (2015), and the Communication on enabling the digital transformation of health and care in the Digital Single Market (2018). Several multi-stakeholder initiatives are addressing current challenges as well. Out of necessity, people living with rare diseases may be the most motivated to ensure that their health data are used as effectively as possible to improve their own health outcomes and well-being and those of future generations, as evidenced by a EURORDIS Rare Barometer Survey on data sharing. Several patient-centered policy initiatives in eHealth and data sharing aim to maximize the implementation of comprehensive e-health in Europe, most recently EURORDIS’ paper on a Mature ERN System (http://download2.eurordis.org/documents/pdf/EURORDIS_vision_on_mature_ERNs_FINAL.pdf) and the multi-stakeholder Rare 2030 Foresight Study on rare disease policy
TOPIC 1: CHALLENGES AND STRATEGIES OF E-HEALTH AND INNOVATION
New technologies have transformed the way we communicate and live our lives. A few years ago, a meeting could only be done in person. Still, one can now attend a virtual meeting worldwide without moving from our location, allowing communication and exchange internationally. Digital tools are part of our daily practice and have evolved alongside progress in robotics, artificial intelligence, and others. This technology integrates into our daily lives through the ability to control our home with a smartphone, unlock our car without keys and phone applications, and change our social behavior. New technologies can change the way patients are diagnosed, care is managed, and disease progression is understood. Some incurable illnesses are now manageable, and treatments exist to help patients.
Sabrina Sacconi started the meeting with an overview of the recent technological progress and their application in NMD in terms of diagnosis and care. Genetic tools’ evolution revolutionized genetic disease diagnosis, allowing identifying many genes involved in neuromuscular or other diseases’ pathophysiology, leading to the elaboration of new treatments and improving patients’ quality of life. Routine analysis has also evolved through the implementation of new or already existing tools amelioration. The level of details and the imaging machine’s resolution are now so refined that it is possible to classify disease severity or detect tiny muscle changes based on MRI analysis [1]. An electromyogram can be performed using bracelet-type sensors to stimulate and detect muscle contraction. Muscle pathology also benefited from technological progress to identify reliable biomarkers for disease evolution and severity, with machine learning and automated tissue pattern recognition approaches to quantify the muscular dystrophies, inflammation, and fat infiltration. The emergence of new devices to precisely measure and record specific outcomes such as distance, speed, movement, and mobile application [2] or telemedicine development eased monitoring and follow-up by making them remote.
Despite all this significant progress, these approaches present some limitations that have to be overcome. First, the level of details and the amount of data generated by automated analysis require specific analysis and interpretation tools. Data storage, collection, and sharing can be a problem due to ethical issues as each country has its registration on data privacy. These concerns have to be addressed before large-scale deployment of such approaches.
Hanns Lochmüller talked about the RD-Connect’s approach to identify genetic causes in rare neuromuscular diseases. Indeed, 6-8% of the European population –between 27 and 36 million people –are affected by one of the 5000-8000 distinct rare diseases. RD-Connect (www.rd-connect.eu) is a unique global infrastructure project that links up databases, registries, biobanks, and clinical bioinformatics data used in rare disease research into a central resource for researchers worldwide. It has developed an integrated research platform in which complete clinical profiles are combined with -omics data and sample availability for rare disease research, particularly research funded under the International Rare Diseases Research Consortium (IRDiRC; www.irdirc.org).
RD-Connect’s mechanism for sharing and analyzing rare disease genomic data begins with submitting the raw.bam or fastq files, which is essential to allow data from multiple sequencing providers to be processed through a standard pipeline to ensure comparability. The raw data are stored for long-term access at the European Genome-phenome Archive (EGA), a secure, controlled-access repository. In contrast, the processed data are made accessible online for real-time analysis in the RD-Connect genomics analysis interface. Thanks to the initial collaboration with the two partner projects, NeurOmics and EURenOmics, the platform is a rich resource containing whole exomes and genomes of a large number of individuals with rare neuromuscular and rare renal disease. It is still growing rapidly for other RD areas such as mitochondrial, neurogenetic, and immunological disorders. Several thousand more datasets are planned for submission in the coming year through submissions by Solve-RD and the European Reference Networks (ERNs), now that the platform is open for submission of data from all rare disease projects [3–5].
These talks presented exciting perspectives in the NMD diagnosis, care, and management, bringing hope to improve patients’ quality of life, monitoring, and treatment. Further, all this technological progress paved the way to new medical approaches in which medical care will be personalized to integrate differences among people (genetic, proteomic, metabolomics [6]), predictive to treat people the earliest possible, proactive, to empower patients and preventive to keep people informed.
TOPIC 2: E-REGISTRIES –TELEMEDICINE –M-HEALTH APPS
Neuromuscular diseases are rare disorders, i.e., with a prevalence lower than 1:2000. Usually, doctors are unfamiliar with these diseases because of the rarity and are unaware of such disorder’s signs and symptoms, leading to a delayed diagnosis and medical care. Further, access to care can be challenging for people with a severely debilitating disease or living far from the hospital. To counter these problems, telemedicine appears to be a good alternative.
First, Julij Šelb discussed the results of studies examining the acceptance, safety, and efficiency of telemedical solutions in different NMD (i.e., FSHD, ALS). Due to their physical disabilities, NMD patients preferred telemedicine to in-person visits, especially when living far from the doctor’s office [7–10]. Telemedicine is also useful in 1) patients with advanced cancer where telemedical monitoring improved the overall patients’ survival [11]; 2) hypertension where the use of telemedicine was associated with reductions in systolic and diastolic blood pressure [12]; 3) diabetes where telemedicine significantly improves the levels of HbA1c [13] and 4) heart failure where the use of telemedicine was associated with improvements in overall mortality and heart failure-related hospitalizations [14]. Overall, telemedicine proves to be adequate to help to assess diagnosis and improve patients’ medical care.
Pauline Lahaut presented the PITEM-PROSOL project to develop and experiment innovative solutions in the healthcare and social welfare sectors (https://www.interreg-alcotra.eu/fr/decouvrir-alcotra/presentation-generale-du-programme, https://www.interreg-alcotra.eu/fr/decouvrir-alcotra/les-projets-finances/pro-sol). This project is funded by the ALCOTRA Program (Alpes Latines COopération TRAnsfrontalière), a European program dedicated to the French-Italian Cooperation to improve the quality of life of the population, sustaining the development of the territory, the economy, and the social systems (Directive 2011/24/EU: DIRECTIVE 2011/24/UE DU PARLEMENT EUROPÉEN ET DU CONSEIL du 9 mars 2011 relative à l’application des droits des patients en matière de soins de santé transfrontaliers, 4.4.2011 Journal officiel de l’Union européenne L 88/45). The PITEM-PROSOL program triggers vulnerable populations, such as elderly, women (family responsibilities and a longer life prevent a proper care of themselves, and increase age-related issues), and pediatric population (adapted schooling to enable proper care is not always possible). This project, led by Prof. Sacconi’s team in France (https://www.systeme-nerveux-peripherique-muscle.chu-nice.fr/la-telemedecine/), aims to set up a telemedicine platform, and provide education through digital tools. Patients living in the remote Alpine area will thus be able to consult their specialized practitioner either from home or from the nearest healthcare facility. Through this platform, patients, caregivers, and healthcare professionals (HCP) will also have access to educational content such as e-learning and MOOCS.
An ongoing clinical study on the French border side ensures patients’ follow-up’s feasibility via teleconsultation by assessing the users’ satisfaction (patients, caregivers, HCP) based on technical and medical criteria. The platform will then be tested for cross-border teleconsultations. The study results are not known yet, but this project’s success relies on technical standards to ensure interoperability and good network coverage, patient engagement, caregivers, HCP, and continuous telemedicine services’ quality monitoring. To summarize, telemedicine is not a regular medical practice replacement and must comply with the same safety and quality requirements as in routine practice.
Erika Schirinzi illustrated mobile applications’ growing implications in healthcare and disease management. In recent years digital technologies have generated a new relationship paradigm between clinicians and patients, in different clinical settings, particularly in the continuity of care for patients suffering from chronic diseases. A mobile app, called AIGkit, specifically designed for adult patients with Pompe disease, was developed as a prototype of a high-risk frailty population, in which the phenotypic complexity requires to refine monitoring and clinical data collection. The aim is to provide clinicians a continuous tracking of each patient in real-time and real-ambient of everyday life. The app is structured as a sort of diary, in which the patient data entered, such as respiratory parameters or motor training programs, are shared by synchronization with a web platform monitored by physicians. The app could represent an innovative tool for disease management, clinical data collection, and the definition and evaluation of outcome measures towards trial readiness. The app creation was supported by the Italian patients Association of Glycogenoses (AIG) and the Italian scientific Society of Myology (SIM) and technically designed by Vidiemme Consulting.
During this session, the talks were articulated around telemedicine’s use and benefits to monitor patients’ quality of life and disease evolution remotely. Telemedicine seems to be a good compromise between classical medical care, where the patient has to come to the hospital and see different specialists, and home care, where the medical staff has to go to the patient’s home. Telemedicine combines the advantages of both approaches: patients can receive classical medical care without going to the hospital, and medical staff can take care of the patients without going to their homes. However, as telemedicine development is very recent, there are currently some issues. For example, telemedicine setting up requires technical training and equipment and reduces in-person interaction. Further, there is no long-term hindsight on the telemedicine benefits in patients’ care. To conclude, telemedicine is a promising approach to renew classical medical care, but such practices have not yet found their final place in the landscape of health care.
TOPIC 3: ROBOTICS, DOMOTICS
Recent years have seen the emergence of robotics and domotics fields, aiming to automate and facilitate daily life. When applied to medicine, these technologies contribute to the apparition of robotic prostheses, exoskeleton, assistive tools, or automated solutions to help patients with disabilities.
Gabriele Siciliano pointed out that technology-driven assistive solutions to support patients with neuromuscular diseases have seen rapid development in the last years. However, integrating sensors to monitor and adapt the patients’ existing motor schemes continuously recognizes some limits. One critical point is that a fundamental background of neurophysiological insights is necessary to distinguish the normal and the pathological aspects of the sensorimotor functions to help the adaptive mechanisms adopted by the impaired neuromuscular system. Consequently, the assistive tool’s design requires a combination of specialized engineering and neurophysiological knowledge to set up devices able to apply a mere copy strategy in compensating the deficit and understand, translate, and model the physiological sensorimotor synergies. When appropriately used, technological tools can integrate the conventional therapeutic approach providing adjustable replacement supports and, when possible, targeted training systems.
The next topic, presented by Tina Duong, highlighted the application of powered clothing to improve functional mobility for Duchenne Muscular Dystrophy (DMD) utilizing wearable technology from Seismic Company that integrates robotics with clothing to augment and support the movement. The design approach is based on the human kinetics’ mechanics with a base garment for support, power, and strength through conformal textile springs, flex grips to distribute the load, inertial measurement units (IMU) to measure body orientation, flex anchors, and actuators for power. The Solid suit has 3 prototype developments: 1) Ankle stretch system for active and passive range of motion, 2) Mobility for the trunk and hip support to improve standing stability and assisted sit to stand 3) Reach for chest and arm support for upper limb function. The presentation focused on the sit-to-stand prototype for DMD. The suit design integrates human movement knowledge based on muscle imbalances from DMD disease progression with a multidisciplinary team of software and mechanical engineers, industrial designer, and physical therapist. With a user-centric design approach, all team members were introduced to individuals with DMD, understood the disease’s clinical progression, and reviewed videos for movement analysis. All stages of early prototype testing involved individuals with DMD as users. DMD movement patterns were based on compensations seen in middle to late ambulatory stages of the disease. Understanding disease progression was essential in this modular concept to address changing needs for don/doff, comfort and mobility with disease progression. Sit-to-stand movement analysis was broken down into 7 stages based on DMD muscle compensations with focal areas of assist and stabilization defined for each stage driving lines of action and degree of assistance required to reduce weight and bulk of the suit. The suit objective was to augment functional movement by working with compensations seen in a typical DMD sit-to-stand, including trunk momentum to initiate movement, use of upper limbs to compensate for hip extension weakness, and abduction of lower limbs. Stability was provided with significant consideration to not inhibit or make more difficult sitting and trunk movements more difficult. Functional movement requires repeated movement from initiation, sustain of range of motion and return to baseline. The DMD functional apparel is an adjustable modular suit that allows for refinement of elements with motor declines based on disease progression.
Jean-Yves Hogrel discussed the potential exoskeleton use to compensate for disability in NMD. Since the beginning of the century, the number of studies involving exoskeletons has grown exponentially. Technologies evolution for compensating motor disabilities caused by varying conditions can explain this. Exoskeletons are defined as wearable machines. They can be either active (implying electric motors, pneumatics, levers, hydraulics, mechanics, or a combination of technologies) or passive (using elastics, straps, or springs), which allow movement with increased strength or endurance. Initially, they were primarily developed for military applications, but now they are used for industrial and medical purposes. As technical aids for neuromuscular disorders, they can be classified into different categories, i.e., rehabilitation, substitution, and assistive devices. For rehabilitation, robot-assisted therapy enables effective and intensive training and ensures optimal neuroplasticity and recovery potential. For substitution, such devices replace a completely lost function (for example, paralysis) and act instead of the person. Assistive devices partly compensate for impairments and help individuals perform complicated movements. Several exoskeletons have been approved to be on the market, but only in the field of rehabilitation [15, 16].
Exoskeletons are required to interact with an individual. A very precise cohabitation is necessary for the device to adapt to its symptoms, morphology, and abilities. In the beginning, using an exoskeleton may be costly (from a metabolic point of view) and exhausting. Particularly in NMD, an individual often has a modified motor schema, which becomes very established over the years, progressively encoded in the motor cortex to adapt to his/her impairments (particularly muscle weakness). The interference of another decision loop may add difficulties. Since the aim is to regain mobility and independence, the balance between benefits and risks must be carefully studied. The clinical trials’ design is thus mandatory and must assess the short, mid, and long-term safety, feasibility, usability, and efficacy of such technologies.
José Javier Serrano Olmedo presented the eGlance methodology, a procedure to help blind people to obtain information about the physical context “at a glance.” With eGlance, blind users can choose the physical direction to acquire data on which physical objects are located. This augmented reality tool informs in real-time blind people on the surrounding context, providing information on physical events or the presence of objects not accessible by hand or with the white cane help. This tool has two main features: remotely visiting and real-time accompanying. The interior spaces, previously virtualized as 3D scenarios, can be seen and memorized from home, helping people move when physically present. Further, if electromagnetic beacons were placed in the room(s), blind people can visit new, unfamiliar indoor areas. In a non-intrusive way, eGlance can describe on-demand or proactively, especially in case of risk, which objects are around, closer or further away, while the user walks. It can also guide the user, if requested, to specific locations [17–19].
This session presented complex applications of robotics and domotics to improve patients’ quality of life. These applications rely on too sophisticated sensors to precisely measure body position, limb angle, or recorded physical objects location to help blind people move. However, it is crucial to fully characterize the sensors system catching all the physical variables used to help patients. Indeed, such a system’s reliability has to be assessed to ensure that the sensors measure what it is supposed to measure. In other terms, the system has to be accurate and reliable. This can be tested, for example, by correlating movement recorded by the sensors with motion analysis to ensure the correctness of the system
TOPIC 4: CONNECTED DEVICES
Connected devices are tools that can help to monitor patients’ condition. While some devices are developed for clinical trials to precisely measure some outcomes, reduce variability or harmonize the results, others are developed to benefit the patients and assessed specific functions such as motor neuron, ability to walk a certain distance in a defined amount of time. Data are then wirelessly transmitted to a mobile application, and results can be analyzed remotely by the clinician.
To illustrate the benefits of connected devices in monitoring patients’ conditions, John Vissing presented his experience with the 6-Minute Walking Test (6MWT). This test is a common outcome in follow-up and clinical trials in patients with NMD. However, this test’s reliability is weakened by first a learning effect (patients push themselves further once they know the test) and second a day-to-day variation caused by unstable motivation. Correction of performed walking distance by heart pulse rate can eliminate the learning effect and weed out the day-to-day variation that does not reflect a real change in a patient’s clinical condition [20]. Based on these observations, the 6MWT+ project is under development and aimed to improve the monitoring system of chronic neuromuscular diseases through a mobile application and the integration of biomechanical sensors into the test while providing clinicians more information on the disease progression. The pilot project integrates several sensors to evaluate walking function in patients affected by DMD, Becker muscular dystrophy, and Myotonic Dystrophy.
Laurent Servais presented the story behind the European Medical Agency (EMA) approval of the 95th centile of the stride speeds, which is a digital outcomes measure. Magneto inertial technology offers researchers the opportunity to continuously assess patients’ movement quantity and quality in a controlled [21] and non-controlled environment [22, 23]. Several challenges are associated with such measures: the first one is related to the quality of the sensor and the required level of precision in the calibration to ensure reliable measures, the second is to deal with real-life hazard and events, the third is to reduce the amount of data provided into a single outcome that is clinically meaningful. In DMD, the 95th centile of the stride speeds recorded during 180 hours has been proven reliable, clinically meaningful, sensitive to change, and poorly sensitive to real-life events such as weather or timing in the day. It has been qualified as a valid secondary outcome by the EMA [24]. Similar work is ongoing in non-ambulant patients and other diseases, such as Spinal Muscular Atrophy [21, 25, 26], FSHD, ALS, and MS.
Dominique Vincent-Genod presented on the role of connected devices to measure motor function in patients with Spinal Muscular Atrophy (SMA). Indeed, given the progress of research and management in SMA and the parallel development of technology in motion capture and virtual reality, interactive and child friendly validated tools are needed to assess patients’ motor function. The Motor Function Measure assessment (MFM) is a validated and sensitive tool designed for patients with neuromuscular disorders. Using a Microsoft Kinect and a digital tablet, the objectives are to improve the MFM acceptability and reliability and improve children’s participation by adding the digital assessment into a serious game. The feasibility study assessed the system’s relevance to capture postures and motions during an MFM test. Among the 32 items of the MFM, 14 were recognized by the Kinect, and 3 items were performed using a tablet. MFM scoring provided by a therapist looking directly at the patients was compared to MFM scoring based on the Kinect sensor’s digital data. On 21 MFM records, a high concordance was found between the two independent scorings (76%). To compare the scoring of 3 items using paper versus a digital tablet, a study enrolling 100 patients was performed to report on a high or good concordance for items 18, 19, and 22 of the MFM. An ongoing step is to include the assessment into a playful scenario to improve children’s participation [27–29].
This session’s talks highlighted the auspicious and powerful use of connected devices in patients’ healthcare, whether it be to monitor patients or the reliability of clinical trial results. However, two main questions arise from this session. First, what are the primary outcomes of connected devices? Are they made to measure changes in patients’ condition with treatment or be used as a self-rehabilitating tool? This depends on the type and role of the devices. For example, 6MWT+ has a role in clinical trials to have reliable and standardize results, while digital MFM with Kinect aimed to monitor and assess patients’ disease progression.
Second, is it possible to monitor several diseases/functions with the same devices? Even if a device is designed for a specific disease, it should be possible to use it for another disease, provided that they are similar and without modification. If the diseases are too different, it should still be possible to use the same device, but the algorithm must be adapted to the new disease.
PITCH SESSION: WHAT IS NEW ON NEUROMUSCULAR DISEASES?
The last session was concentrated on the advance on NMD knowledge. As these diseases are rare, a better understanding of their pathophysiology is crucial to improve patients’ healthcare and quality of life.
Sylvie Bannwarth presented MitoDIAG, the French network for mitochondrial diseases. Mitochondrial diseases are amongst the most genetically and phenotypically diverse groups of inherited diseases. This heterogeneity, the vast phenotypic overlap with other disease entities, and the absence of reliable biomarkers make molecular diagnosis difficult. Mitodiag, the French network of 11 laboratories for diagnosing mitochondrial diseases, has been created to improve the diagnosis. MitoDIAG has developed (i) a new bioinformatic tool, eKLIPse, to detect and quantify mtDNA deletions from NGS data [30] and (ii) a specific database, Mitomatcher, to combine phenotypic and genomic information of French mitochondrial disease patients.
Damien Eggenspieler talked about ActiMyo, a digital biomarker platform developed for NMD. While a very active space, drug development in rare neuromuscular diseases has led to very few drug approvals in the past three decades [31]. The lack of a reliable and meaningful motor outcome, which is objective (limiting traditional bias like motivation & fatigue) and sensitive (enough to change to pick up early signs of efficacy), is one factor hindering drug development.
The advance of digital outcome has proven its potential to fill this gap by providing devices to capture objective Real World motor evidence from patients. Sysnav took the challenge to develop a holter of movement, with the appropriate level of precision & accuracy, and disease optimized outcomes. The first outcome, Stride Velocity 95th centile, has been approved by EMA and reviewed by the FDA for ambulant patients living with DMD. New partnerships & collaborations with leading institution & drug developers are starting to qualify outcomes beyond DMD and expand the benefits to other diseases and with both ambulant & non-ambulant patients.
Savine Vicart showed the design of a clinical and medico-economic national study involving an m-health monitoring tool on a rare disease cohort on periodic paralysis (RaDiCo-PP). Periodic paralysis is a rare disease characterized by episodic muscle weakness attacks. The collection of information necessary for patient follow-up and therapeutic adaptations is complicated because of the symptoms’ episodic and variable expression [32–35]. Patients struggle to report the characteristics of their crisis accurately. Presently, there is no validated tool to assess paralytic attacks in a standardized way. Physical disability during attacks directly impacts the social and professional life of patients and caregivers [36, 37].
They proposed a clinical trial to study the feasibility, acceptability, and utility of a smartphone application to collect, in real-time, precise and comparable data and evaluated the disease’s medico-economic and social impact. These collected data will allow a characterization of the periodic paralysis population [38].
Jordi Diaz-Manera presented a study in which the authors have developed a new way to guide the diagnosis of muscular dystrophies, called “MYO-GUIDE.” They have developed an artificial intelligence tool to analyze fat replacement patterns in muscle MRIs of 10 different genetically confirmed muscular dystrophies. In detail, they have applied a machine learning strategy using Random Forest to analyze the results of fat replacement quantification in patients’ muscles. The authors developed 2000 different models and got the one with the highest accuracy. The final model has an accuracy of 95% with very high specificity and sensibility values. When compared with human experts on the field, the tool had higher accuracy than humans. In summary, this study demonstrates that machine learning is useful in analyzing muscle MRIs to help guide the genetic diagnosis of patients with muscular dystrophies [39].
Overall, this pitch session brought exciting insight into the next era in diagnosing and managing neuromuscular disease from patients’ and clinicians’ sides. Establishing new ways to investigate neuromuscular disease pathophysiology and management will allow better patient care and a faster diagnosis establishment. It is critical to use the latest progress in artificial intelligence and machine learning to design new tools to improve patients’ quality of life.
CONCLUSIONS
By their rarity, many patients with NMD experience a long diagnostic odyssey or are misdiagnosed, leading to inadequate care or treatment. No cure exists for these diseases, although significant progress was made in recent years. NMD management is also complicated by multi-system involvement of disease and inconsistent standards of care with annual or bi-annual basis patient visits. These visits do not necessarily reflect the patient’s health state in between clinic visits. With evolving technology, there is still a gap in the technological resources to decrease care management burdens, leading to the need for family or caregiver assistance to perform daily living activities. There is a real need to improve and introduce new technologies and innovations to complement patients’ classical medical care (Fig. 1).
Fig. 1
Patients’ unmet needs and potential solutions using innovative technologies.
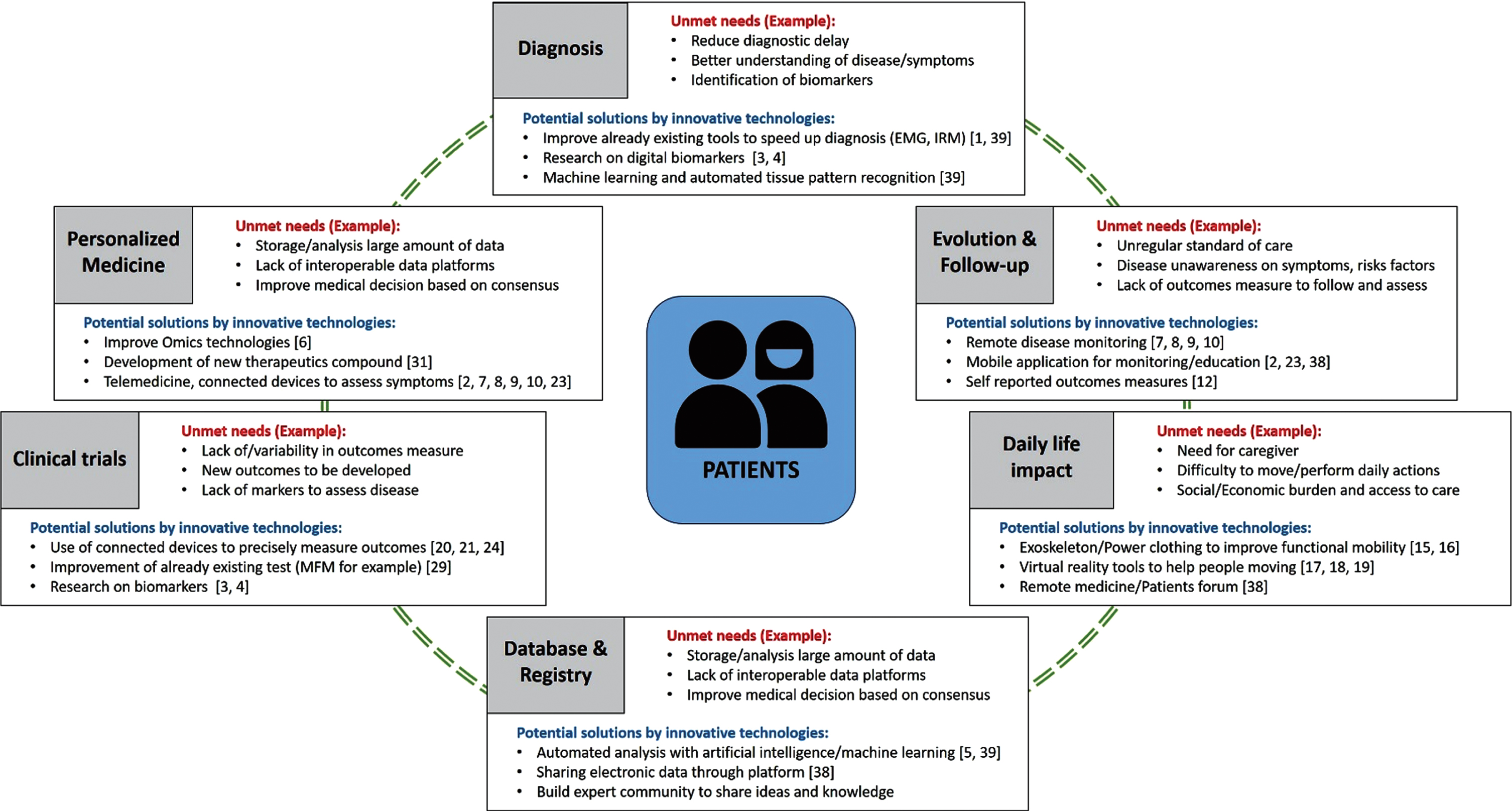
During this meeting, many promising approaches were shown to improve patients’ management and quality of life, ranging from telemedicine to mobile applications to avoid unnecessary patient travel for clinic visits that do not require physical examinations. Technological advances may decrease caregiver burden, increase patient autonomy with robotic prostheses or powered clothing. However, these applications will only reach their full potential when we will provide access to these innovations to all patients. Indeed, patients’ do not have to search for innovations, but innovations have to reach them. Discussions at the meeting attempted to address translation of innovation to patients by increasing clinicians’ awareness of the disease, its symptoms, how to manage it, and up to date on the recent technological advancements. This can be done with innovative tools such as mobile applications and congresses such as this one. Sharing information is crucial to improve patients’ wellness and transform the way medical practices are done today: shifting from the classical medical care to an anticipator personalized medicine care paradigm to avoid delay in diagnosis and long term preventative healthcare.
With this meeting, NMD specialists and innovations experts convened to build a community to contribute to the world-wide effort to improve patients care outcomes, quality of life, disease assessment, and monitoring, with a particular effort on the development of new tools to help to diagnose; assess and monitor NMD.
CONFLICTS OF INTERESTS
T. Duong received consultancy and advisory boards from Solid Bio, Biogen, Sarepta, Roche, and Novartis.
J.Y. Hogrel received consultancy fees from Biogen, Minoryx, and Sarepta.
L. Servais received consultancy from Biogen, Novartis, Roche, Santhera, Sarepta, RegenexBio, Pfizzer, Affinia, Dynacure. He chairs the DSMB of Lupin and Fibrogen
J. Vissing has received consultancy fees from Argenx, Roche, Viela Bio, Biogen, Amicus Therapeutics, Genethon, PTC Therapeutics, Sanofi/Genzyme, Ultragenyx Pharmaceutical, Fulcrum Therapeutics, ML Bio Solutions, Sarepta Therapeutics, Novartis Pharma AG, Stealth Biotherapeutics, Zogenix, Regeneron, UCB Pharma, Viela Bio, Lundbeck Pharma, and Lupin Limited.
S. Sacconi served as a consultant for Sanofi-Genzyme.
All other authors declare no conflict of interests.
ACKNOWLEDGMENTS
We would like to thank the University Hospital of Nice for hosting the congress, the Centre d’Innovation et d’Usages en Santé for their precious help in the organization of the whole event, the European Reference Network for Neuromuscular Disease (ERN Euro-NMD), and the City of Nice for their help in the achievement of this event. We would also like to thank the different industrial partners, the AFM-Téléthon and the Conseil Départmental des Alpes-Maritimes for their financial supports.,
REFERENCES
[1] | Smith AC , Parrish TB , Abbott R , Hoggarth MA , Mendoza K , Chen YF , et al. Muscle-fat MRI: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve. (2014) ;50: :170–6. https://doi.org/10.1002/mus.24255. |
[2] | Ricci G , Baldanzi S , Seidita F , Proietti C , Carlini F , Peviani S , et al. A mobile app for patients with Pompe disease and its possible clinical applications. Neuromuscul Disord NMD. (2018) ;28: :471–5. https://doi.org/10.1016/j.nmd.2018.03.005. |
[3] | Thompson R , Johnston L , Taruscio D , Monaco L , Béroud C , Gut IG , et al. RD-Connect: An integrated platform connecting databases, registries, biobanks and clinical bioinformatics for rare disease research. J Gen Intern Med. (2014) ;29: (Suppl 3):S780–787. https://doi.org/10.1007/s11606-014-2908-8. |
[4] | Lochmüller H , Badowska DM , Thompson R , Knoers NV , Aartsma-Rus A , Gut I , et al. RD-Connect, NeurOmics and EURenOmics: Collaborative European initiative for rare diseases. Eur J Hum Genet EJHG. (2018) ;26: :778–85. https://doi.org/10.1038/s41431-018-0115-5. |
[5] | Austin CP , Cutillo CM , Lau LPL , Jonker AH , Rath A , Julkowska D , et al. Future of Rare Diseases Research 2017-2027: An IRDiRC Perspective. Clin Transl Sci.. (2018) ;11: :21–7. https://doi.org/10.1111/cts.12500. |
[6] | Johnston L , Thompson R , Turner C , Bushby K , Lochmüller H , Straub V . The impact of integrated omics technologies for patients with rare diseases. Expert Opin Orphan Drugs. (2014) ;2: :1211–9. https://doi.org/10.1517/21678707.2014.974554. |
[7] | Bashiri M , Greenfield LJ , Oliveto A . Telemedicine Interest for Routine Follow-Up Care Among Neurology Patients in Arkansas. Telemed J E-Health Off J Am Telemed Assoc. (2016) ;22: :514–8. https://doi.org/10.1089/tmj.2015.0112. |
[8] | Nijeweme-d’Hollosy WO , Janssen EPF , Huis in ’t Veld RMHA , Spoelstra J , Vollenbroek-Hutten MMR , Hermens HJ . Tele-treatment of patients with amyotrophic lateral sclerosis (ALS). J Telemed Telecare. (2006) ;12: (Suppl 1):31–4. https://doi.org/10.1258/135763306777978434. |
[9] | Portaro S , Calabrò RS , Bramanti P , Silvestri G , Torrisi M , Conti-Nibali V , et al. Telemedicine for Facio-Scapulo-Humeral Muscular Dystrophy: A multidisciplinary approach to improve quality of life and reduce hospitalization rate? Disabil Health J. (2018) ;11: :306–9. https://doi.org/10.1016/j.dhjo.2017.09.003. |
[10] | Pinto A , Almeida JP , Pinto S , Pereira J , Oliveira AG , de Carvalho M . Home telemonitoring of non-invasive ventilation decreases healthcare utilisation in a prospective controlled trial of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. (2010) ;81: :1238–42. https://doi.org/10.1136/jnnp.2010.206680. |
[11] | Basch E , Deal AM , Dueck AC , Scher HI , Kris MG , Hudis C , et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. (2017) ;318: :197–8. https://doi.org/10.1001/jama.2017.7156. |
[12] | Tucker KL , Sheppard JP , Stevens R , Bosworth HB , Bove A , Bray EP , et al. Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis. PLoS Med. (2017) ;14: :e1002389. https://doi.org/10.1371/journal.pmed.1002389. |
[13] | Lee SWH , Chan CKY , Chua SS , Chaiyakunapruk N . Comparative effectiveness of telemedicine strategies on type 2 diabetes management: A systematic review and network meta-analysis. Sci Rep. (2017) ;7: :12680. https://doi.org/10.1038/s41598-017-12987-z. |
[14] | Koehler F , Koehler K , Deckwart O , Prescher S , Wegscheider K , Kirwan B-A , et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet Lond Engl. (2018) ;392: :1047–57. https://doi.org/10.1016/S0140-6736(18)31880-4. |
[15] | Mcleod JC , Ward SJ , Hicks AL . Evaluation of the KeeogoTM Dermoskeleton. Disabil Rehabil Assist Technol. (2019) ;14: :503–12. https://doi.org/10.1080/17483107.2017.1396624. |
[16] | McGibbon CA , Sexton A , Jayaraman A , Deems-Dluhy S , Gryfe P , Novak A , et al. Evaluation of the Keeogo exoskeleton for assisting ambulatory activities in people with multiple sclerosis: An open-label, randomized, cross-over trial. J Neuroengineering Rehabil. (2018) ;15: :117. https://doi.org/10.1186/s12984-018-0468-6. |
[17] | Cobo A , Guerrón NE , Martín C , del Pozo F , Serrano JJ . Differences between blind people’s cognitive maps after proximity and distant exploration of virtual environments. Comput Hum Behav. (2017) ;77: :294–308. https://doi.org/10.1016/j.chb.2017.09.007. |
[18] | Guerrón NE , Cobo A , Serrano Olmedo JJ , Martín C . Sensitive interfaces for blind people in virtual visits inside unknown spaces. Int J Hum-Comput Stud. (2020) ;133: :13–25. https://doi.org/10.1016/j.ijhcs.2019.08.004. |
[19] | Cobo A , Serrano Olmedo J , Guerron N . Methodology for building virtual reality mobile applications for blind people on advanced visits to unknown interior spaces., 2018. |
[20] | Prahm KP , Witting N , Vissing J . Decreased variability of the 6-minute walk test by heart rate correction in patients with neuromuscular disease. PloS One. (2014) ;9: :e114273. https://doi.org/10.1371/journal.pone.0114273. |
[21] | Le Moing A-G , Seferian AM , Moraux A , Annoussamy M , Dorveaux E , Gasnier E , et al. A Movement Monitor Based on Magneto-Inertial Sensors for Non-Ambulant Patients with Duchenne Muscular Dystrophy: A Pilot Study in Controlled Environment. PloS One. (2016) ;11: :e0156696. https://doi.org/10.1371/journal.pone.0156696. |
[22] | Zemel MB . Proposed role of calcium and dairy food components in weight management and metabolic health. Phys Sportsmed. (2009) ;37: :29–39. https://doi.org/10.3810/psm.2009.06.1707. |
[23] | Lilien C , Gasnier E , Gidaro T , Seferian A , Grelet M , Vissière D , et al. Home-Based Monitor for Gait and Activity Analysis. JVis Exp JoVE. 2019. https://doi.org/10.3791/59668. |
[24] | Haberkamp M , Moseley J , Athanasiou D , de Andres-Trelles F , Elferink A , Rosa MM , et al. European regulators’ views on a wearable-derived performance measurement of ambulation for Duchenne muscular dystrophy regulatory trials. Neuromuscul Disord NMD. (2019) ;29: :514–6. https://doi.org/10.1016/j.nmd.2019.06.003. |
[25] | Chabanon A , Seferian AM , Daron A , Péréon Y , Cances C , Vuillerot C , et al. Prospective and longitudinal natural history study of patients with Type 2 and 3 spinal muscular atrophy: Baseline data NatHis-SMA study. PloS One. (2018) ;13: :e0201004. https://doi.org/10.1371/journal.pone.0201004. |
[26] | Annoussamy M , Seferian AM , Daron A , Péréon Y , Cances C , Vuillerot C , et al. Natural history of Type 2 and 3 spinal muscular atrophy: 2-year NatHis-SMA study. Ann Clin Transl Neurol. 2020. https://doi.org/10.1002/acn3.51281. |
[27] | Bérard C , Payan C , Hodgkinson I , Fermanian J , MFM Collaborative Study Group. A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord NMD. (2005) ;15: :463–70. https://doi.org/10.1016/j.nmd.2005.03.004. |
[28] | Vuillerot C , Payan C , Iwaz J , Ecochard R , Bérard C , MFM Spinal Muscular Atrophy Study Group. Responsiveness of the motor function measure in patients with spinal muscular atrophy. Arch Phys Med Rehabil. (2013) ;94: :1555–61. https://doi.org/10.1016/j.apmr.2013.01.014. |
[29] | Da Gama A , Fallavollita P , Teichrieb V , Navab N . Motor Rehabilitation Using Kinect: A Systematic Review. Games Health J. (2015) ;4: :123–35. https://doi.org/10.1089/g4h.2014.0047. |
[30] | Goudenège D , Bris C , Hoffmann V , Desquiret-Dumas V , Jardel C , Rucheton B , et al. eKLIPse: A sensitive tool for the detection and quantification of mitochondrial DNA deletions from next-generation sequencing data. Genet Med. (2019) ;21: :1407–16. https://doi.org/10.1038/s41436-018-0350-8. |
[31] | Sakate R , Fukagawa A , Takagaki Y , Okura H , Matsuyama A . Trends of Clinical Trials for Drug Development in Rare Diseases. Curr Clin Pharmacol. (2018) ;13: :199–208. https://doi.org/10.2174/1574884713666180604081349. |
[32] | Charles G , Zheng C , Lehmann-Horn F , Jurkat-Rott K , Levitt J . Characterization of hyperkalemic periodic paralysis: A survey of genetically diagnosed individuals. J Neurol. (2013) ;260: :2606–13. https://doi.org/10.1007/s00415-013-7025-9. |
[33] | Fontaine B . Periodic paralysis. Adv Genet. (2008) ;63: :3–23. https://doi.org/10.1016/S0065-2660(08)01001-8. |
[34] | Fontaine B , Fournier E , Sternberg D , Vicart S , Tabti N . Hypokalemic periodic paralysis: A model for a clinical and research approach to a rare disorder. Neurother J Am Soc Exp Neurother. (2007) ;4: :225–32. https://doi.org/10.1016/j.nurt.2007.01.002. |
[35] | Fialho D , Griggs RC , Matthews E . Periodic paralysis. Handb Clin Neurol. (2018) ;148: :505–20. https://doi.org/10.1016/B978-0-444-64076-5.00032-6. |
[36] | Phillips L , Trivedi JR . Skeletal Muscle Channelopathies. Neurother J Am Soc Exp Neurother. (2018) ;15: :954–65. https://doi.org/10.1007/s13311-018-00678-0. |
[37] | Statland JM , Fontaine B , Hanna MG , Johnson NE , Kissel JT , Sansone VA , et al. Review of the Diagnosis and Treatment of Periodic Paralysis. Muscle Nerve. (2018) ;57: :522–30. https://doi.org/10.1002/mus.26009. |
[38] | Wang J , Wang Y , Wei C , Yao NA , Yuan A , Shan Y , et al. Smartphone interventions for long-term health management of chronic diseases: an integrative review. Telemed J E-Health Off J Am Telemed Assoc. (2014) ;20: :570–83. https://doi.org/10.1089/tmj.2013.0243. |
[39] | Verdú-díaz J , Alonso-Perez J , Nuñez-Peralta C , Tasca G , Vissing J , Straub V , et al. P.301Myo-Guide: A new artificial intelligence MRI-based tool to aid diagnosis of patients with muscular dystrophies. Neuromuscul Disord. (2019) ;29: :S155. https://doi.org/10.1016/j.nmd.2019.06.415. |




