Oral and Swallowing Abilities Tool (OrSAT) for Type 1 SMA Patients: Development of a New Module
Abstract
We describe the development of a new tool specifically designed to record oral abilities, swallowing and, more generally, feeding in young type 1 SMA patients, to be used during the first 24 months of life.
The tool is composed by a checklist and a separate section summarizing the functional abilities into levels of feeding/swallowing impairment. The checklist includes 12 questions assessing aspects thought to be clinically meaningful for a type 1 SMA population and developmentally appropriate for infants during the first months of life. Each item is graded with a score of 0 or 1, depending on the child’s ability to perform the activity. As some items are age-dependent, the number of items to be used, and therefore the maximum score, changes with increasing age. The levels of feeding/swallowing impairment include four levels that can be identified using easily identifiable clinical criteria.
In an attempt to validate the tool in an untreated population we applied it to 24 type 1 SMA patients (age range: 2.3–24.1 months, mean: 10.8) in whom the same information collected by the new tool had been previously recorded using a less-structured format.
When patients were classified in three groups according to the Dubowitz decimal classification, there was a significant difference both at baseline and at follow-up (p < 0.001). The items assessing fatigue during the nursing sessions were the most frequently impaired even in infants who did not have any other obvious clinical sign of swallowing difficulties.
LIST OF ABBREVIATIONS
SMA | Spinal muscular atrophy |
SMN1 | Survival of Motor Neuron 1 |
CHOP INTEND | Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders |
OrSAT | Oral and Swallowing Abilities Tool |
ASHA NOMS | American Speech-Language-Hearing Association National Outcomes MeasurementSystem |
VFSS | VideoFluoroscopic Swallow Study |
NdSSS | Neuromuscular disease Swallowing Status Scale |
INTRODUCTION
Spinal muscular atrophy (SMA) is a neuromuscular disorder caused by mutations in the SMN1 gene, leading to motor neuron loss and subsequent muscular atrophy and weakness. Type 1 is the most severe form of SMA, characterized by severe hypotonia, weakness and increasing respiratory, swallowing and feeding difficulties over time [1]. As type 1 includes patients with different degree of severity, it has been proposed that it could be subdivided into three main subgroups: 1.1, the more severe end of the spectrum with early, severely reduced mobility, respiratory and bulbar difficulties; 1.5, the most common phenotype, with inability to raise the legs against gravity or to maintain the head posture but no overt feeding or respiratory difficulties at diagnosis; 1.9, the mildest phenotypes, infants may achieve head control [2, 3]. The three subgroups grossly overlap with the subtypes 1 A, B and C, proposed in another classification more based on age of onset [3, 4].
Improvements in standards of care [5, 6] and the advent of new therapeutic approaches have resulted in a dramatic improvement in survival and functional abilities in these patients, changing the course of the disease [7–10]. So far, most of the attention has been focused on survival and motor or respiratory outcome [11]. Despite feeding being one of the most important aspects in the care of type 1 SMA, less has been reported about possible changes in oral and swallowing abilities in the treated patients. This is at least partly due to the paucity of available specific tools to assess these aspects, especially in the first years. While there are a number of outcome measures designed to assess motor function, such as the CHOP INTEND [12] or scales assessing motor developmental milestones [13, 14] there are no dedicated tools for the assessment of swallowing and oral motor skills in type 1 SMA or in general, in weak young infants in the first 24 months of life. One of the challenges in this age group, especially in the first year after birth, is that the level of abilities increases with age, with changes in feeding modalities and in the consistency of food texture [15]. Also, the volume of food needs to increase as the child grows to provide adequate nutrition to support growth. As such, even in the absence of overt dysphagia there may be an element of oral-motor fatigue that precludes adequate caloric and fluid intake. In the handful of studies reporting feeding related issues in SMA patients, with few exceptions, do not include or only include few type 1 infants [16–20]. A very recent study used an observation list and recorded different parameters during a feeding session [21]. In another study nutritional aspects were assessed as part of a survey [22].
The Neuromuscular disease swallowing status scale (NdSSS) has recently been used in a large neuromuscular cohort including 11 type 1 infants [23]. The NdSSS is an 8-stage scale, designed to evaluate swallowing conditions that are frequently encountered in progressive neuromuscular disorders in various clinical settings, that can be used by nonprofessionals without special knowledge [24]. However, it was not specifically designed for infants and does not capture some aspects such as fatigue during the nursing sessions or need of specific positioning during meals, that are relevant for type 1 patients.
In this paper we describe the development of a new tool specifically designed to record different aspects related to oral and swallowing abilities in type 1 SMA patients that can be easily used in a clinical setting, in infants from the first months after birth until 24 months of age. We also report the application of the new tool to a cohort of typically developing infants of the same age and to a cohort of type 1 SMA patients.
MATERIALS AND METHODS
The aim of our effort was to develop a tool that could be easily used in the clinical routine, not requiring expensive instruments or invasive diagnostic procedures, but still able to capture a number of aspects related to oral abilities and swallowing, recorded using a structured format. As some of these aspects are likely to change over time with increasing age or for other reasons, such as disease progression or possible intervention, we stratified the items according to age and added an easy scoring system in an attempt to quantify the possible changes in the individual items at different ages. The new tool includes a checklist assessing individual aspects of swallowing or related to the use of orofacial muscles. A separate section summarizes the functional abilities into levels of impairment.
The checklist was based on our clinical experience, including the questions that are routinely asked in our clinical setting by clinicians at each visit as part of the clinical history, but adding a more structured format and a scoring system.
The second part reporting a description of levels of impairment was based on clinical experience and review of the literature.
The development of the tool followed a number of steps:
1. Review of the notes and of the information regarding oral and swallowing abilities collected as part of our clinical routine.
2. Literature search: we performed a systematic review of tools used in SMA, with a comprehensive search of the following electronic databases: MEDLINE, CINAHL, PsycINFO, and EMBASE. The primary search terms ‘spinal muscular atrophy’ a ‘neuromuscular disorder’ were combined with keywords ‘swallowing’, ‘feeding’, ‘dysphagia’ and ‘bulbar’. All electronic searches were limited to the English language and to publication years 1980 to 2020. The titles and abstracts of articles were screened by the first authors (BB, LF). As it was not always possible to ascertain details of the questionnaires from the abstract, we first identified all papers of interest and the full text of articles were then examined to obtain details. Only papers reporting tools used in type 1 were selected.
3. Identification of the items and finalization of the tool.
4. Assessing clinical meaningfulness of the items with type 1 families via interviews and focus groups.
5. Preliminary validation in a cohort of typically developing infants and inter and intra-observer reliability.
6. Application of the tool to a type 1 SMA cohort.
In order to preliminarily assess the tool’s sensitivity to detect changes over time, the OrSAT was also used in a retrospective of a recent cohort of type 1 infants. The information collected using the OrSAT structured format had been previously collected using a less structured format. First, we used both the structured and the unstructured format in a small number of prospectively enrolled type 1 infants to look at the consistency of responses and to establish the possibility of using our retrospective data collected with the unstructured format. Once this was achieved, we used the OrSAT structured format in a retrospective cohort of 24 consecutive type 1 SMA patients (8 females and 16 males) younger than 2 years, admitted to our Center between January 2011 and December 2016. All patients had a genetically confirmed diagnosis of SMA, with homozygous deletion of exon 7 in the SMN1 gene and a clinically confirmed diagnosis of type 1 SMA. Infants were classified according to the Dubowitz’s decimal classification [2]: 1.1 (8 patients); 1.5 (8 patients); 1.9 (8 patients).
7. Correlation between functional levels and checklist scores.
Statistical analysis
Intercorrelation coefficient was used to establish inter and intra-observer reliability as part of the development of the scale.
In the retrospective group of type 1 infants the difference in baseline scores and progression in the 3 subgroups was calculated using the Kruskall Wallis test with level of significance set at 0.05.
A correlation between the total scores obtained on the checklist and the functional levels was performed using correlation coefficient test.
Table 1
Details of the papers reporting feeding/swallowing abilities in type 1 SMA
| Reference | Study design | Sample size | Aim | Assessment |
| van der Heul (2020) | Prospective cohort | 16 | To study feeding and swallowing problems in type 1SMA patients and their relation with functional motor scores. | Evaluation during feeding session; VFSS; CHOP INTEND |
| Ah-Choi et al. (2020) | Retrospective cohort | 11 | To evaluate the change in progressive swallowing dysfunction from birth up to years | NdSSS; VFSS |
| van der Heul (2019) | Cross-sectional | 11 | To investigate self-reported bulbar problems in patients with SMA and their relationship to age | DDD(p)NMD |
| van den Engel-Hoek etal. (2015) | Systematic review | |||
| Wadman et al. (2014) | Cross-sectional | 11 | To evaluate: | Non-standardized questionnaire |
| 1. Eating problems (difficulties with biting, swallowing, and/or chewing). | ||||
| 2. Dysphagia (occurring problems with swallowing, i.e., problems moving food or fluids from the oral cavity to the throat or delayed passage of food or drinks through the esophagus). | ||||
| 3. Choking (blockage of the throat by food or drinks). | ||||
| Davis et al. (2014) | Cross-sectional | 44 | To provide a current snapshot of the nutritional practices of children with SMA type 1. | Non-standardized questionnaire |
| Durkin et al. (2008) | Retrospective cohort | 9 | To assess the risks and benefits of early referral anti-reflux procedure shortly after diagnosis of SMA type 1. | VFSS |
VFSS: VideoFluoroscopic Swallow Study. CHOP INTEND: Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders. NdSSS: Neuromuscular Disease Swallowing Status Scale. DDD(p)NMD: Diagnostic List of Dysphagia and Dysarthria in (pediatric) patients with Neuromuscular Diseases. GER: gastroesophageal reflux.
Development of the checklist
1. Review of the notes
Clinical data regarding feeding and swallowing were available on the clinical notes in all patients. As part of our clinical routine, all the information required were consistently collected asking always the same questions even if without a structured form. Data were available for all the assessments. As part of our routine, in agreement with care recommendations, type 1 patients are generally seen at least every 3 months until the age of one year and at least every 6 months after that. After revision of the notes we identified 10 items.
2. Literature search and development of the final version of the checklist
No scale appeared to be specifically designed for type 1 SMA and most of the items included were either similar to those we already used in clinical practice or were not suitable for the construct of our tool that was designed as a checklist rather than an observer rated scale.
3. Identification of the items and finalization of the tool
We initially also explored the possibility to add items generally recorded by the speech therapists, that could record the child’s behavior during the observation of a meal when the family came for their routine assessments. The original idea was to develop a scale, similar to the Egen Klassification scale [25], that could combine patient reported and observer rated items. We therefore included measurements of heart rate and of O2 saturation during a meal but these items added a level of complexity that was beyond the scope of our scale, so, even though important as part of the speech therapist assessment, they were not included in the final version. We also excluded items or additional investigations such as video-fluoroscopy, that should be specifically managed by speech therapists or other trained specialists. In contrast, following increasing evidence of infants treated with the new available drugs, showing an improvement in speech, rarely present in untreated type 1 infants [26] we added two items exploring the ability to produce syllables in the first months or clear words around one year and beyond. These items were meant to record the use of orofacial muscles and strength of respiratory muscles rather than as a neurodevelopmental or cognitive measure of speech and comprehension.
The final version of the scale includes 12 items assessing swallowing and oral skills that were thought to be relevant for type 1 SMA population and developmentally appropriate for infants since the first months of age (0–5, 6–9 and 10–24 months) (Table 2).
Table 2
OrSAT checklist and levels of impairment
| 0–5 m | 6–9 m | 10–24 m | ||||
| Score* | 1 | 0 | 1 | 0 | 1 | 0 |
| 1. Able to swallow thin liquids (for example: milk, water) | Y | N | Y | N | Y | N |
| 2. Able to swallow semi liquids (for example: yogurt, pureed fruits and vegetables) | Y | N | Y | N | ||
| 3. Able to swallow semisolids (for example: rice cereal, mashed banana, cooked egg) | Y | N | Y | N | ||
| 4. Able to swallow solids (requires chewing and then swallowing, for example: meat, apple pieces) | Y | N | ||||
| Score | 1 | 0 | ||||
| 5. Need for intervention | No need for intervention | Need for intervention | ||||
| •thickening food | ||||||
| •positioning | ||||||
| 6. Cough/signs of stagnation during meal | No cough/signs of stagnation during meal | Cough/signs of stagnation during meal | ||||
| 7. Able to swallow without tiring | Able to swallow without tiring | Able to swallow but easily tired, needs to rest periodically during a meal | ||||
| 8. Able to complete a meal | Able to complete a meal | Unable to complete a meal | ||||
| 9. Duration of main meals (< 45 min for semisolids and 25 min for breastfeeding) | (< 45 min for semisolids and 25 min for breastfeeding) | Longer | ||||
| 10. Need for suctioning during mealtime | No need for suctioning during mealtime | Need for suctioning during mealtime | ||||
| 11. Able to speak one or more syllables (if age > 6 months) | Yes | No | ||||
| 12. Able to speak correctly one or more words (if age > 12 months) | Yes | No | ||||
| TOTAL SCORE | ||||||
| Levels of impairment | ||||||
| No impairment: the individual’s ability to eat is not limited by swallow function. Swallowing is reported as safe and efficient for all consistencies (when age appropriate), without choking episodes or other clinical signs such as retching or cough. | ||||||
| Mild impairment: swallowing is safe, but usually requires moderate cues to use compensatory strategies or more careful posturing or other intervention (thickening food). | ||||||
| Moderate impairment: swallowing for thin liquids is safe but the infant gets easily tired and unable to complete a full meal and takes less than 50%of nutrition and hydration by mouth. These children may require need for oral supplements or alternative method of feeding (NG tube or G-tube). | ||||||
| Severe impairment: individual is not able to swallow anything safely by mouth. All nutrition and hydration is received through non-oral means (e.g. nasogastric or G-tube). | ||||||
In the four first items, assessing the ability to take different food consistencies by mouth, these were defined according to the IDDSI food texture [15]: thin liquids, semi-liquids, semisolids and solids.
Checklist scoring system
The main caregiver, generally the mother, was asked structured questions to assess the items of the checklist. The responses were graded by using a simple scoring system: each item was scored as 0 or 1 depending if the child was able or unable to perform the activity (Table 2). As some items are age dependent, the number of items, and therefore the maximal score, increases with increasing age. In the infants younger than 6 months who cannot be assessed on swallowing semisolids or solids the maximum score is 7 while in those between 6 and 9 months, who can also be assessed on semi-liquids/semisolids the maximum score is 10. For infants of 10 months or older who can be assessed for all food consistencies including solids, the maximum total score is 12.
Development of the levels of feeding/swallowing impairment
An additional separate section was added to define levels of feeding/swallowing impairment. This was loosely based on the criteria used in the American Speech-Language-Hearing Association National Outcomes Measurement System (ASHA NOMS) [27], a multidimensional instrument assessing the supervision required for feeding and the type of diet. Our classification is much simpler and uses levels of impairment rather than abilities. This was simplified for general use both by reducing the number of levels, as some were not relevant for our age group and excluding activities that could only be collected by speech therapists or trained professionals, in order to be easily assessed in routine clinical practice. The functional levels assessed are scored separately from the checklist (Table 2).
Functional levels were classified as follows:
No impairment: the individual’s ability to eat is not limited by swallow function. Swallowing is reported as safe and efficient for all consistencies (when age appropriate), without choking episodes or other clinical signs such as retching or cough.
Mild impairment: swallowing is safe, but usually requires moderate cues to use compensatory strategies or more careful posturing or other intervention (thickening food or other edit in texture).
Moderate impairment: the individual is able to swallow some food consistencies safely by mouth but gets easily tired and/or is unable to complete a full meal and/or takes less than 50%of nutrition and hydration by mouth. These children may require need for oral supplements or tube feeding (NG tube or G-tube).
Severe impairment: individual is not able to swallow anything safely by mouth. All nutrition and hydration is received through non-oral means (e.g. nasogastric or G-tube).
4. Assessing clinical meaningfulness of the items with type 1 families via interviews and focus groups
After the final version of both checklist and functional levels was designed, we conducted interviews with 56 individual caregivers and 4 focus groups with caregivers of type 1 infants who were asked if the aspects included were relevant for their child or more generally for SMA infants. All the activities included were considered to be clinically meaningful for the families with a concordance of 100%.
5. Preliminary validation in a cohort of typically developing infants and inter and intra-observer reliability
The checklist and the functional levels were piloted by the same examiners in 60 typically developing infants of age between 3 and 24 months. Less than 5%of the typically developing infants had a score of 0 on individual items and always only in one item of the checklist. The items that were found to have occasional scores of 0 were those assessing choking and duration of the meal. The maximal functional score was generally the full score according to the age and in less than 5%the total scores were different but within one point only from the age appropriate maximum score. When the levels of impairment were assessed, more than 95%of the children had a normal level and less than 5%had a level labelled as mild impairment as they required some posture or texture modifications generally related to gastroesophageal reflux.
The OrSAT test-retest reliability was evaluated on a sample group of 10 typically developing and in 10 type 1 patients. The OrSAT was given twice at a distance of 5 to 14 days showing a Cronbach alfa estimated value of 0.93.
6. Application of the tool to a type 1 SMA cohort
Although the new tool was based on our previous routine assessment including the same questions used in the checklist, the modality of data collection used in the past was less structured.
In order to establish whether the new scoring system could be applied to a retrospective cohort in whom the data had been routinely collected over the last years using similar questions but without a structured form, we first investigated possible concordance between our previous routine assessment and the new structured approach. The two methods were used independently by two examiners, each blind to the results of the other, to the same families on consecutive days, randomly starting with one or the other assessment. A correlation between the two tools showed a very high concordance in the responses (ICC > 0.9) suggesting that the old data could be scored with the new scoring system.
RESULTS
The OrSAT was used in 24 type 1 patients who had been attending our Clinic in the last few years in whom the same information collected by the new tool had been previously recorded using a less-structured format.
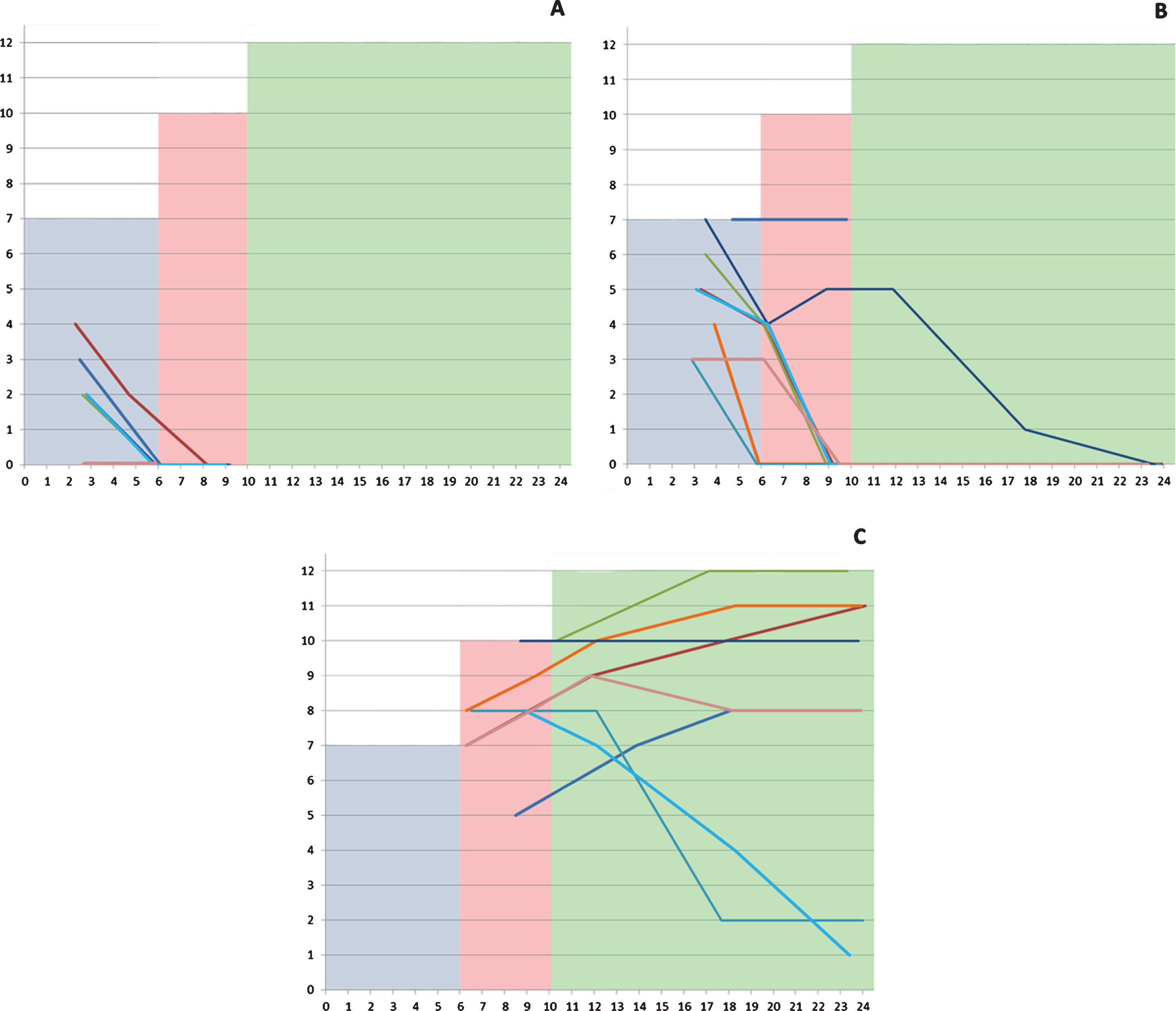
The scores on the checklist ranged between 0 and 7 in the assessments performed before 6 months (mean: 3.43, SD: 2.15), between 0 and 10 in those performed between 6 and 10 months (mean: 3.16, SD: 3.64), and between 0 and 12 in those older than 10 months (mean: 5.39, SD: 4.38) (Fig. 1).
Fig. 1
Individual details of the scores in the different subgroups according to severity highlighted by age range (0–5; 6–9; 10–24 months). The shaded areas corresponds to the maximum total score of the tool corresponding to what is expected in neurotypical infants.
1.1
All 8 patients had an assessment at 3 months. Their CHOP INTEND scores at 3 months ranged between 12 and 22. Only 1 of the 8 required tube feeding and 4 had a weight below 2 SD. The OrSAT checklist total score was 0 in 2/8, 2 in 4/8, 3 in 1/8 and 4 in the remaining infant. Most items had a score of 0 with the exception of able to swallow liquids, able to complete a meal and, even if less frequently, the items assessing the presence of cough or the need for suctioning.
At the age of 6 months the CHOP INTEND scores in the 7 surviving infants ranged between 9 and 16, none of the infants was able to swallow liquids and to complete a meal and all had a weight below 2 SD. All had a score of 0 in all the checklist items.
At 9 months, the CHOP INTEND scores in the surviving 5 infants ranged between 3 and 6. Figure 1 shows details of the individual changes.
1.5
All 8 patients had an assessment at 3 months. At the age of 3 months 1/8 required tube feeding and 3/8 had a weight below 2 SD. The OrSAT checklist total score was 3 in 2/8, 4 in 1/8, 5 in 2/8, 6 in 1/8 and 7 in 2/8. The items assessing meal duration and tiredness during the nursing session were the most frequently impaired.
Table 3
Mean scores for each individual item and visit subdivided by type 1 SMA decimal classification
| Item | SMA decimal classification | OrSAT checklist scores | |||||
| 3 months (Mean) | 6 months (Mean) | 9 months (Mean) | 12 months (Mean) | 18 months (Mean) | 24 months (Mean) | ||
| Item 1 | 1.1 | 0.75 | 0.14 | 0 | -* | -* | -* |
| Able to swallow liquids | 1.5 | 1 | 0.75 | 0.25 | 0.2 | 0 | 0 |
| 1.9 | - | 1 | 1 | 1 | 0.75 | 0.75 | |
| Item 2 | 1.1 | NA | 0 | 0 | -* | -* | -* |
| Able to swallow semi liquids | 1.5 | NA | 0.375 | 0.125 | 0.2 | 0 | 0 |
| 1.9 | - | 1 | 1 | 1 | 0.875 | 0.75 | |
| Item 3 | 1.1 | NA | 0 | 0 | -* | -* | -* |
| Able to swallow semisolids | 1.5 | NA | 0.25 | 0 | 0 | 0 | 0 |
| 1.9 | - | 0.75 | 0.875 | 1 | 0.875 | 0.75 | |
| Item 4 | 1.1 | NA | NA | NA | -* | -* | -* |
| Able to swallow solids | 1.5 | NA | NA | NA | 0 | 0 | 0 |
| 1.9 | - | NA | NA | 0.25 | 0.5 | 0.625 | |
| Item 5 | 1.1 | 0 | 0 | 0 | -* | -* | -* |
| Need for intervention | 1.5 | 0.75 | 0.125 | 0.125 | 0 | 0 | 0 |
| 1.9 | - | 0.5 | 0.5 | 0.25 | 0.25 | 0.25 | |
| Item 6 | 1.1 | 0.25 | 0 | 0 | -* | -* | -* |
| Cough/signs of stagnation during meal | 1.5 | 0.75 | 0.25 | 0.25 | 0.2 | 0 | 0 |
| 1.9 | - | 0.75 | 0.75 | 0.75 | 0.625 | 0.625 | |
| Item 7 | 1.1 | 0 | 0 | 0 | -* | -* | -* |
| Able to swallow without tiring | 1.5 | 0.375 | 0.125 | 0.125 | 0 | 0 | 0 |
| 1.9 | - | 0.75 | 0.625 | 0.5 | 0.5 | 0.5 | |
| Item 8 | 1.1 | 0.5 | 0.14 | 0 | -* | -* | -* |
| Able to complete a meal | 1.5 | 0.875 | 0.5 | 0.125 | 0 | 0 | 0 |
| 1.9 | - | 1 | 1 | 1 | 0.625 | 0.625 | |
| Item 9 | 1.1 | 0 | 0 | 0 | -* | -* | -* |
| Duration of main meals | 1.5 | 0.25 | 0.25 | 0.25 | 0.2 | 0 | 0 |
| 1.9 | - | 0.5 | 0.625 | 0.5 | 0.375 | 0.375 | |
| Item 10 | 1.1 | 0.375 | 0 | 0 | -* | -* | -* |
| Need for suctioning during mealtime | 1.5 | 1 | 0.625 | 0.25 | 0 | 0 | 0 |
| 1.9 | - | 1 | 1 | 1 | 0.75 | 0.75 | |
| Item 11 | 1.1 | NA | 0 | 0 | -* | -* | -* |
| Able to speak > 1 syllable | 1.5 | NA | 0 | 0 | 0.2 | 0.25 | 0.25 |
| 1.9 | - | 0.25 | 0.875 | 1 | 1 | 1 | |
| Item 12 | 1.1 | NA | NA | NA | -* | -* | -* |
| Able to speak correctly > 1 word | 1.5 | NA | NA | NA | 0 | 0 | 0 |
| 1.9 | - | NA | NA | 0.5 | 0.875 | 0.875 | |
Key to legend: NA: not available; *: death; -: not evaluated.
By 6 months the CHOP INTEND scores ranged between 21 and 32. Two of the 8 infants required tube feeding and 3/8 had a weight below 2 SD. The OrSAT checklist scores decreased on all items in 7 of 8 infants. At 9 months the CHOP INTEND scores ranged 17 and 28, on the OrSAT only two of the infants had a score respectively of 7 and 5 and did not require tube feeding while all the others had 0 and required tube feeding. Figure 1 shows details of the individual changes.
1.9
Type 1.9 infants were generally assessed after 6 months. At 9 months the CHOP INTEND scores ranged between 28 and 41. None of the infants required tube feeding. The OrSAT checklist total score was more than 9 in 3/8, 8 in 4/8 and 5 in one infant.
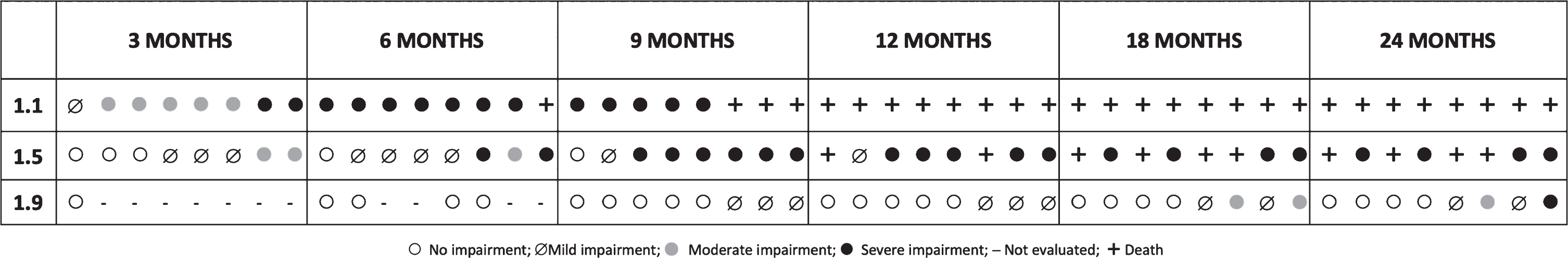
Fig. 2
Change in levels of impairment in the study cohort subdivided by SMA 1 decimal classification and visits: Each symbol corresponds to a single patient at each given age (open dot ○: no impairment; barred dot ø: mild impairment; grey dot •: moderate impairment; full dot •: severe impairment; the symbol –was used when an evaluation was not performed).
At one year of age, the CHOP INTEND scores ranged between 25 and 39. The OrSAT scores showed minimal changes, with only a few infants requiring intervention, tiring during the nursing session and showing a longer duration. Tiredness during the nursing session and duration of the meal were also the items most frequently impaired from 12 months onwards. At 24 months the total score at the CHOP INTEND ranged between 20 and 35, and the total score at the OrSAT was more than 9 in 4/8, 8 in 2/8, 2 in one and 1 in another one infant. None of the 1.9 infants had weight below 2SD or required tube feeding with exception of one at the age of 18 months.
The difference between 1.1, 1.5 and 1.9 was significant both at baseline (p < 0,001) and when the follow up changes were considered (p = 0,001).
Levels of impairment
1.1
Seven of the 8 patients had a moderate or severe impaired swallowing by 3 months of age. By 6 months all of the surviving ones had severe impairment.
4.61.5
Six of the 8 patients had no impairment (n = 3) or only mild impairment (n = 3) and none had severe involvement by 3 months with a progressive deterioration over time.
1.9
Before the age of 9 months all the patients who had been diagnosed and could be assessed had no impairment. At 9 months 7 of the 8 had no impairment and 1 had mild impairment. Moderate or severe involvement occurred only in 2 patients by 18 months.
7. Correlation between functional levels and checklist scores
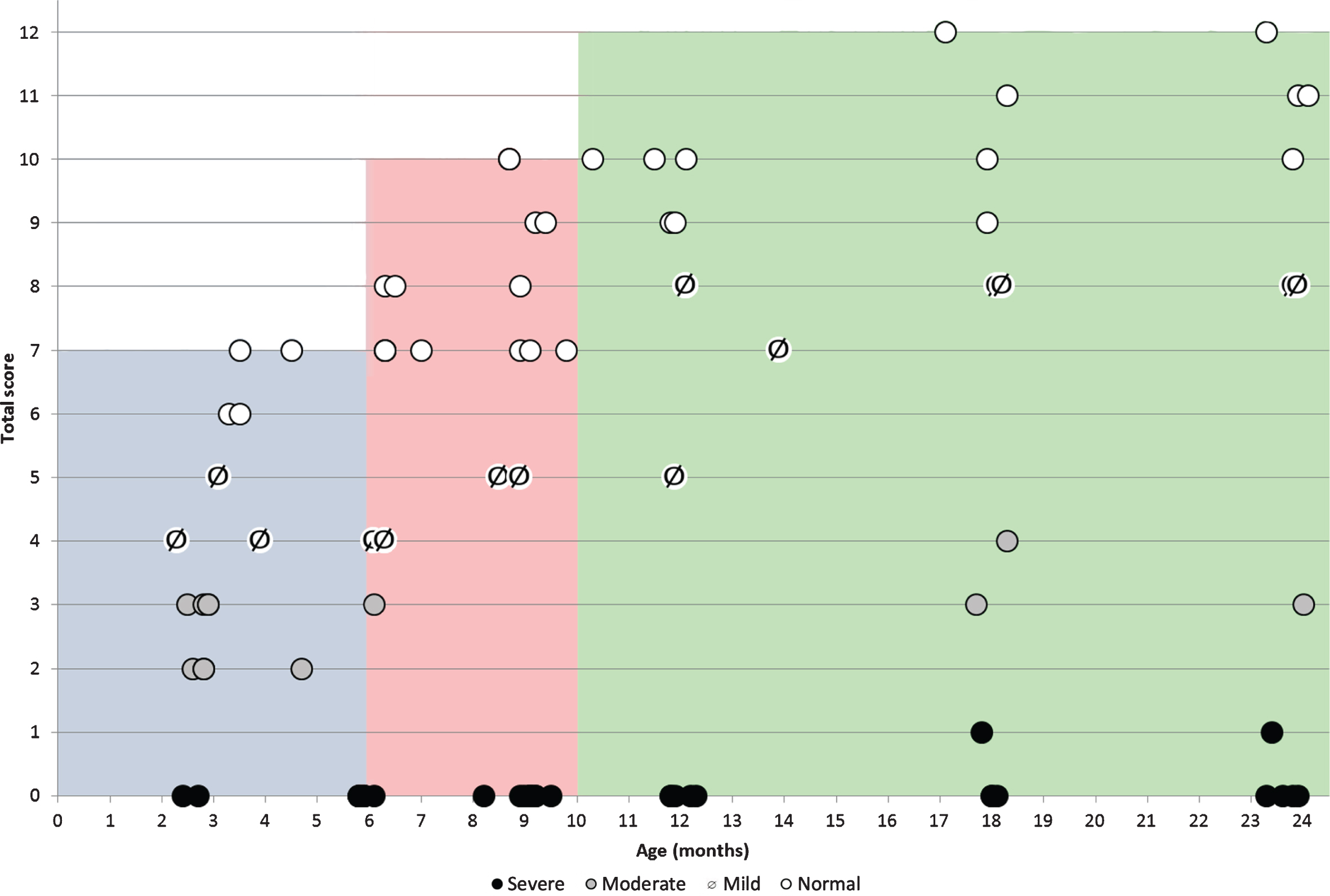
In infants below the age of 6 months, in whom the possible maximum score on the checklist is 7, all infants labeled as with no impairment on the classification of level impairment had scores of 6 and 7 on the checklist. Those labeled as with mild impairment had scores of 4 and 5, those with moderate impairment had scores of 2 and 3 and those with severe impairment all had a score of 0. The correlation coefficient was 0.984.
In infants between 6 and 9.99 months, in whom the possible maximum score on the checklist is 10, all infants labeled as with no impairment had checklist scores between 7 and 10, those with mild impairment had scores of 5, those with moderate impairment had scores of 3 and those with severe impairment all had as a score of 0. The correlation coefficient was 0.951.
In infants > 10 months, in whom the possible maximum score on the checklist is 12, all infants labeled as with no impairment had scores between 9 and 12, those with mild impairment had scores between 5 and 8, those with moderate impairment had scores between 3 and 4 and those with severe impairment all had as a score of 0 (Fig. 3). The correlation coefficient was 0.983.
Fig. 3
Correlation between total OrSAT checklist scores and age in different subgroups subdivided according to the OrSAT level of impairment. (○: no impairment; barred dot ø: mild impairment; grey dot \color grey•: moderate impairment; full dot •: severe impairment. Each symbol corresponds to a single patient. The shaded areas identify the maximum total OrSAT checklist score expected in typically developing infants at different ages (grey 0–5 months; Pink 6–9 months green: 10–24 months).
DISCUSSION
Dysphagia is one of the most critical aspects of care of type 1 SMA patients, who often present weakness affecting the various phases of feeding abilities, that may involve not only the pharyngeal phase (decreased airway protection in combination with weak swallowing) but also sucking and handling of oral secretion [19]. Both the first version and the revised care recommendations [5, 6, 28] highlight the importance of regular speech therapist assessments to monitor these aspects, suggesting to observe feedings and a careful evaluation of possible difficulties related to feeding [5, 6]. For non-sitters, a swallow study is recommended shortly after diagnosis and, if the initial test is normal, closely monitored to detect possible early signs of feeding difficulties.
An effort has been made, at least in tertiary care centers, to implement regular clinical and radiological assessments in clinical practice, as they were not always routinely available for the lack of specialized speech and language therapists, physicians, and radiologists with interest and expertise in this field. As type 1 infants have very frequent visits to the centers for the administration of the new therapies and other assessments, there has been an increasing need to regularly collect structured information by mean of an easy clinical tool that could be easily updated at each appointment and used as a complement to the full speech therapist assessments performed at fixed intervals.
Despite the clinical relevance of dysphagia in type 1 infants, it is surprising how little effort there has been to develop disease specific tools aimed to identify different aspects of oral motor or swallowing difficulties. Even in the recent trials, swallowing was not formally investigated with a structured assessment and was often defined by the need for tube feeding [7, 29]. Only in the gene replacement trial the request to report the ability to swallow thin liquids was used [8].
We report the development of a new tool providing a simple assessment of oral abilities and swallowing in type 1 infants by using a checklist aimed to systematically record aspects of oral motor and swallowing function that are often assessed in clinical routine but not in a structured format. The checklist also provides the possibility to quantify the severity of impairment by using a very simple scoring system.
While this checklist is not meant to replace the more structured observer rated speech therapist assessment internationally recommended, it provides a measure of the parents’ perception of their child’s swallowing ability that also covers possible episodes occurring at home that may not be seen during a single observation in clinic. The use of patient reported measures has been strongly encouraged both by regulatory authorities both in Europe (‘Reflection Paper on the Regulatory Guidance for the use of Health Related Quality of Life (HRQL) Measures in the Evaluation of Medicinal Products’) and US (Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. US DHHS, FDA) as these tools can provide better understanding of the impact caused by disease and treatment on the patient.
Our checklist was developed using a template of questions that were routinely asked in our clinical setting, adding two items assessing the oral motor aspects of language. While language was in the past rarely observed in type 1 infants, following the advent of the new therapies there is an increasing number of patients in whom is possible to monitor language development over the first years. One of the challenges with developing an assessment for infants as young as newborns as well as infants in the first years after birth is that it should take into account age related changes, especially at the time when infants switch from a fully liquid diet to the introduction of new textures. Because of this, we defined age specific items and maximum possible scores for different ages.
We also designed a separate classification reporting functional levels, as a complement to the checklist, based on other tools used in other disorders and in a wider age range, simplified for the exclusive use in young infants in a clinical setting.
In order to explore the ability of the checklist and of the functional levels to detect possible changes over time in type 1, we retrospectively applied these tools to a cohort of type 1 infants longitudinally followed in our Unit. The results show a clear separation in swallowing abilities among infants with different severity of SMA, both at baseline and at follow up (p < 0.01). Infants with the most severe form, nearly invariably had some swallowing difficulties. Their follow up showed that already by six months, in all the survivors there was the indication for tube feeding. All but one had a score of zero at all time points and only at three months very few infants were able to swallow liquids even though they tired very easily and required compensatory postures and liquid thickening. Even in those with relatively better scores there was already an indication for introducing tube feeding.
At the other end of the spectrum, infants with type 1.9 not only had high scores at the time of diagnosis, that was often after 3 months, but had an increase in their total scores with increasing age, often reaching the ceiling of the age appropriate maximum scores. Interestingly, despite the good scores, some of these infants were still reported to be tired and had an increased duration of the nursing session time. There was also some ‘delay’ in the time when solid food was introduced as reflected by the changes in the scores related to this activity. Patients with the ‘intermediate’ type 1 phenotype, 1.5, had a more variable progression and most of them reached a score of 0 between 6 and 12 months. It is of note that in the first 6 months in most patients their carers did not report problems with swallowing liquids, choking episodes or other concern, but often reported that the children tired easily during feeding and they often needed short and frequent meals. Tiredness and need for intervention often preceded more obvious difficulties requiring more structured intervention after the age of 6 months. These findings were quite consistent even in the milder type 1, clearly indicating that they should be regularly assessed and discussed with families and other operators involved in the care of the patients.
The subdivision of our cohort into clinical subtypes allowed a more precise definition of the trajectories and to reduce the variability reported in previous studies. A recent study, using the Neuromuscular Disease Swallowing Status Scale (NDSSS) and Videofluoroscopy swallowing studies in type 1 infants in the first 2 years, reported that on average swallowing function deteriorated at 6 months with a large variability of the time when tube feeding was needed [23]. A previous survey in type 1 patients with a wider age range also reported similar variability but with an average age of feeding tube placement of 11 months [22].
Not surprisingly, the checklist scores were associated with the functional levels that were independently recorded capturing other aspects, such as posturing or use of alternative ways of feeding that are not captured in the checklist. By using the two tools together, we were able to establish which checklist total scores were associated with each level in the different age groups.
These results should be interpreted with caution as these findings are strongly limited by the retrospective use of the tool. While we would have ideally started a new data collection to prospectively establish the possible changes and natural history of swallowing abilities, at the time we developed the final version, all our type I patients were on treatment with Nusinersen or in clinical trials. The only chance to observe possible changes in untreated patients was therefore to use the recent retrospective data. The use of both the not structured format used in clinical practice in the past and the structured OrSAT checklist in a small sample of prospectively enrolled patients showed a good concordance. This suggests that, even if with caution, these results may provide at least an indication of the ability of the new tool to capture changes over time in type 1 infants and some reference data in untreated type 1 patients.
Our findings indicate that, in addition to the items assessing choking and possible unsafe swallowing, particular attention should be paid to those assessing fatigue during the nursing session, as this was the most frequent finding even in infants who did not have any other obvious sign of swallowing difficulties. These results are in agreement with a recent study using a more structured observer rated approach who also showed that shortened nursing sessions were extremely frequent [19]. These aspects should therefore be systematically explored with specific questions as parents often increase the frequency of feeding to compensate the short duration of nursing sessions, underestimating the importance of these findings.
Further work is needed to complete the validation of the new tool and to fully explore its possible use in clinical and research settings. In our study the checklist was always performed by a clinical examiner interviewing the carers and further work is required to establish the reliability of the tool as a self-administered patient reported measure.
Further studies exploring the new tool, also in relation to the more structured and complete assessments performed by speech therapists, will provide additional information on the sensitivity of this new tool as a clinical routine screening tool. Our preliminary results suggest that our structured examination reporting parents’ perception could be potentially used at each clinical appointment to provide some general information, especially when time and resources for more structured assessments are limited. Further work is also requested to further explore the ability to talk that is becoming increasingly achieved in treated patients. This will become particularly important at the time the scale will be used in infants treated with the new available therapies.
ACKNOWLEDGMENTS
Not applicable.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
ETHICS COMMITTEE AGREEMENT
The study is part of a larger natural history study on SMA approved by the Ethics Committee of our Institution (CEG1032218/08), and fully informed consent from the parents of participant was obtained before the study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Individual data are shown in tables and figures. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
CONFLICT OF INTEREST
Eugenio Mercuri has received funding as a member of advisory boards for SMA studies for Scholar Rock, AveXis, Biogen, Ionis Pharmaceuticals, Inc., Novartis, and Roche; principal investigator for ongoing Ionis Pharmaceuticals, Scholar Rock, Inc./Biogen and Roche clinical trials; funding from Famiglie SMA Italy, Italian Telethon, and SMA Europe.
Giorgia Coratti received funding from Biogen, Roche, AveXis and Genesis Pharma as speaker in sponsored symposia.
Roberto de Sanctis received funding from Biogen as speaker in sponsored symposia.
Marika Pane received funding as a member of advisory boards from Biogen and AveXis and as speaker in sponsored symposia.
Richard S. Finkel Richard Finkel has received personal compensation for activities with Ionis Pharmaceuticals, Biogen, AveXis, Capricor, Catabasis, Lilly, Roche, Novartis; and the SMA Foundation, SMA Europe and Cure SMA as a consultant or advisor. Dr. Finkel has received research support from Ionis Pharmaceuticals, Biogen, Lilly, Cytokinetics Sarepta, NIH, MDA, and Summit.
Beatrice Berti, Lavinia Fanelli, Roberta Onesimo, Concetta Palermo, Daniela Leone, Sara Carnicella, Giulia Norcia, Nicola Forcina, Antonella Cerchiari, Valentina Giorgio, Simona Lucibello have nothing to disclose.
AUTHOR’S CONTRIBUTIONS
BB, LF, MP, EM: Conceptualization, Formal Analysis, Investigation, Methodology, Writing Original Draft Preparation.
RDS: Data Curation.
RO, CP, DL: Data Curation, Formal Analysis, Investigation.
GC: Data Curation, Formal Analysis, Methodology.
SC, GN, NF, VG: Data Curation.
AC: Conceptualization, Methodology.
SL: Data Curation, Methodology.
RSF: Data Curation, Methodology, Formal Analysis, Investigation.
REFERENCES
[1] | D’Amico A , Mercuri E , Tiziano FD , Bertini E . Spinal muscular atrophy. Orphanet J Rare Dis. (2011) ;6: :71. |
[2] | Dubowitz V . Chaos in the classification of SMA: A possible resolution. Neuromuscul Disord. (1995) ;5: (1):3–5. |
[3] | De Sanctis R , Pane M , Coratti G , Palermo C , Leone D , Pera MC , et al. Clinical phenotypes and trajectories of disease progression in type 1 spinal muscular atrophy. Neuromuscul Disord. (2018) ;28: (1):24–8. |
[4] | Finkel R , Bertini E , Muntoni F , Mercuri E , Group ESWS 209th ENMC International Workshop: Outcome Measures and Clinical Trial Readiness in Spinal Muscular Atrophy 7-9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord. (2015) ;25: (7):593–602. |
[5] | Mercuri E , Finkel RS , Muntoni F , Wirth B , Montes J , Main M , et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. (2018) ;28: (2):103–15. |
[6] | Finkel RS , Mercuri E , Meyer OH , Simonds AK , Schroth MK , Graham RJ , et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. (2018) ;28: (3):197–207. |
[7] | Finkel RS , Mercuri E , Darras BT , Connolly AM , Kuntz NL , Kirschner J , et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N Engl J Med. (2017) ;377: (18):1723–32. |
[8] | Mendell JR , Al-Zaidy S , Shell R , Arnold WD , Rodino-Klapac LR , Prior TW , et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N Engl J Med. (2017) ;377: (18):1713–22. |
[9] | Pane M , Coratti G , Sansone VA , Messina S , Bruno C , Catteruccia M , et al. Nusinersen in type 1 spinal muscular atrophy: Twelve-month real-world data. Ann Neurol. (2019) ;86: (3):443–51. |
[10] | Pechmann A , Langer T , Schorling D , Stein S , Vogt S , Schara U , et al. Evaluation of Children with SMA Type 1 Under Treatment with Nusinersen within the Expanded Access Program in Germany. J Neuromuscul Dis.. (2018) ;5: (2):135–43. |
[11] | Sansone VA , Pirola A , Albamonte E , Pane M et al. Respiratory Needs in Patients with Type 1 Spinal Muscular Atrophy Treated with Nusinersen. J Pediatr. (2020) ;223: :227–8. doi: 10.1016/j.jpeds.2020.04.069. Epub 2020 May 4 |
[12] | Glanzman AM , McDermott MP , Montes J , Martens WB , Flickinger J , Riley S , et al. Validation of the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND). Pediatr Phys Ther. (2011) ;23: (4):322–6. |
[13] | Haataja L , Mercuri E , Regev R , Cowan F , Rutherford M , Dubowitz V , et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. (1999) ;135: (2 Pt 1):153–61. |
[14] | De Sanctis R , Coratti G , Pasternak A , Montes J , Pane M , Mazzone ES , et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord. (2016) ;26: (11):754–9. |
[15] | Cichero JA , Lam P , Steele CM , Hanson B , Chen J , Dantas RO , et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia. (2017) ;32: (2):293–314. |
[16] | van der Heul AMB , Wijngaarde CA , Wadman RI , Asselman F , van den Aardweg MTA , Bartels B , et al. Bulbar Problems Self-Reported by Children and Adults with Spinal Muscular Atrophy. J Neuromuscul Dis. (2019) ;6: (3):361–8. |
[17] | Messina S , Pane M , De Rose P , Vasta I , Sorleti D , Aloysius A , et al. Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord. (2008) ;18: (5):389–93. |
[18] | Wadman RI , van Bruggen HW , Witkamp TD , Sparreboom-Kalaykova SI , Stam M , van den Berg LH , et al. Bulbar muscle MRI changes in patients with SMA with reduced mouth opening and dysphagia. Neurology. (2014) ;83: (12):1060–6. |
[19] | van den Engel-Hoek L , de Groot IJ , de Swart BJ , Erasmus CE . Feeding and Swallowing Disorders in Pediatric Neuromuscular Diseases: An Overview. J Neuromuscul Dis. (2015) ;2: (4):357–69. |
[20] | Audag N , Goubau C , Toussaint M , Reychler G . Screening and evaluation tools of dysphagia in children with neuromuscular diseases: A systematic review. Dev Med Child Neurol. (2017) ;59: (6):591–6. |
[21] | van der Heul AMB , Cuppen I , Wadman RI , Asselman F , Schoenmakers M , van de Woude DR , et al. Feeding and Swallowing Problems in Infants with Spinal Muscular Atrophy Type 1: An Observational Study. J Neuromuscul Dis. 2020. |
[22] | Davis RH , Godshall BJ , Seffrood E , Marcus M , LaSalle BA , Wong B , et al. Nutritional practices at a glance: Spinal muscular atrophy type I nutrition survey findings. J Child Neurol. (2014) ;29: (11):1467–72. |
[23] | Choi YA , Suh DI , Chae JH , Shin HI . Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int J Pediatr Otorhinolaryngol. (2020) ;130: :109818. |
[24] | Wada A , Kawakami M , Liu M , Otaka E , Nishimura A , Liu F , et al. Development of a new scale for dysphagia in patients with progressive neuromuscular diseases: The Neuromuscular Disease Swallowing Status Scale (NdSSS). J Neurol. (2015) ;262: (10):2225–31. |
[25] | Steffensen BF , Lyager S , Werge B , Rahbek J , Mattsson E . Physical capacity in non-ambulatory people with Duchenne muscular dystrophy or spinal muscular atrophy: A longitudinal study. Dev Med Child Neurol. (2002) ;44: (9):623–32. |
[26] | Bach JR , Saltstein K , Sinquee D , Weaver B , Komaroff E . Long-term survival in Werdnig-Hoffmann disease. Am J Phys Med Rehabil. (2007) ;86: (5):339–45. |
[27] | American Speech-Language Hearing Association: NOMS: American Speech-Language Hearing Association National Outcome Measurement System. Adult Speech-Language Pathology training manual.Rockville:. 1998. |
[28] | Wang CH , Finkel RS , Bertini ES , Schroth M , Simonds A , Wong B , et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. (2007) ;22: (8):1027–49. |
[29] | Finkel RS , Chiriboga CA , Vajsar J , Day JW , Montes J , De Vivo DC , et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet. (2016) ;388: (10063):3017–26. |




