The Role of Nutrition and Physical Activity as Trigger Factors of Paralytic Attacks in Primary Periodic Paralysis
Abstract
Background:
Primary periodic paralysis (PPP) are rare inherited neuromuscular disorders including Hypokalemic periodic paralysis (HypoPP), Hyperkalemic periodic paralysis (HyperPP) and Andersen-Tawil syndrome (ATS) characterised by attacks of weakness or paralysis of skeletal muscles. Limited effective pharmacological treatments are available, and avoidance of lifestyle related triggers seems important.
Objective:
Our aim was to search and assess the scientific literature for information on trigger factors related to nutrition and physical activity in PPP.
Methods:
We searched Ovid Medline and Embase database for scientific papers published between January 1, 1990, to January 31, 2020.
Results:
We did not identify published observation or intervention studies evaluating effect of lifestyle changes on attacks. Current knowledge is based on case-reports, expert opinions, and retrospective case studies with inadequate methods for description of nutrition and physical activity. In HypoPP, high carbohydrate and salt intake, over-eating, alcohol, dehydration, hard physical activity, and rest after exercise are frequently reported triggers. Regarding HyperPP, fasting, intake of potassium, alcohol, cold foods or beverages, physical activity, and rest after exercise are frequently reported triggers. No nutrition related triggers are reported regarding ATS, exercise can however induce ventricular arrhythmias.
Conclusions:
Our results support that dietary intake and physical activity may play a role in causing paralytic attacks in PPP, although the current scientific evidence is weak. To provide good evidence-based patient care, several lifestyle aspects need to be further assessed and described.
INTRODUCTION
Primary periodic paralysis (PPP) are rare inherited neuromuscular disorders characterised by attacks of weakness or paralysis of skeletal muscles [1–3]. Affected individuals normally present with symptoms in the first or second decade of life [1–3]. Symp-tomatic attacks may vary from minutes to days, and frequency ranging from daily to only a few in a lifetime [1–3]. Permanent weakness may develop and typically occur in the fourth to fifth decade of life, independently of severity and frequency of paralytic attacks [1–3]. The disorder is divided into three subtypes and include Hypokalemic periodic paralysis (HypoPP), Hyperkalemic periodic para-lysis (HyperPP) and Andersen-Tawil syndrome (ATS) [1–3]. Important differential diagnoses whose clinical features overlap with HypoPP and Hyper-PP, includes paramyotonia congenita (PMC) and normokalemic PP which in most cases are caused by mutations of the sodium channel [4]. HypoPP is caused by mutations in either a calcium channel gene (CACNA1S; 40–60% of kindreds) or a sodium channel gene (SCN4A: 7–20% of kindreds) [1, 4]. HyperPP is caused by mutations in a sodium channel gene (SCN4A: 80% of kindreds) [2], and ATS is caused by mutations in the potassium channel gene (KCNJ2:60% of kindreds) [4]. In the remaining cases, the causes are unknown [1, 2, 4]. The common feature of the involved genes is that they code for voltage-gated ion channels that are primarily expressed in skeletal muscle. An exception from this is the KCNJ2 gene found in ATS, which codes for potassium channels that are additionally expressed in the heart and brain [1–3]. The disease prevalence is estimated to be 1:100 000, 0.17:100 000 and 0.10-0.08:100 000 for HypoPP, HyperPP and ATS, respectively [2–4].
Affected individuals with HypoPP experience attacks of weakness or paralysis of skeletal muscle often with concomitant hypokalemia (serum potassium < 3.5 mmol/L) [1]. In individuals with HyperPP, attacks of weakness or paralysis occur with concomitant hyperkalemia (serum potassium > 5 mmol/L) or an increase in serum potassium concentration of minimum 1.5 mmol/L during an attack [2]. Compared to HypoPP, attacks in HyperPP tend to be more frequent but shorter in duration, and they can also affect muscles of the eyes, throat and trunk [2, 4]. In many cases of HyperPP, mild muscle stiffness (myotonia) is present, and muscle stiffness is aggravated by exercise (paramyotonia) [2]. ATS is characterized by cardiac arrhythmias, prolonged QT interval and physical dysmorphologies in addition to weakness or paralytic attacks [3]. The attacks in ATS can be associated with high, low or normal serum potassium levels [4].
All forms of PPP, regardless of mutation, share a common final mechanism, a long-lasting depolarization of the sarcolemma leading to inactivation of sodium (Na+) ion channels, thereby inhibiting action potentials and rendering the muscle fibres electrically inexcitable [5–7]. The human body has however several compensation mechanisms for ion channel malfunction, meaning that exogenous or endogenous triggers are often required to produce symptoms in affected individuals [5–7]. Examples of these are triggers that change the serum potassium (K +) level, the ion responsible for degree of muscle excitability [5–7]. Hyperkalemia can develop as a result of physical activity, muscle cell damage, excessive oral or intravenous administration of potassium [6]. Treatment with nonselective beta blockers, K + sparing diuretics, ACE inhibitors and angiotensin-II-receptor antagonists can also induce hyperkalemia [6]. Hypokalemia can be induced by catecholamines (adrenalin, noradrenalin, dopamine) and be a side effect of many drugs such as beta-2-agonist (Salbutamol), diuretics (loop, thiazides, carbonic anhydrase inhibitors), glucocorticoids, laxatives, and some antimicrobial drugs [6]. Hypokalemia can also be induced by promoting transcellular potassium shifts from serum into the muscles by stimulating the sodium-potassium (Na+, K +) pumps as seen post-exercise, or by insulin administration [6]. This can explain why lifestyle elements such as stress, nutrition or physical activity are frequently reported to trigger episodes in PPP [1–3, 5].
The currently available treatment options for PPP aims to abort or reduce the frequency and severity of the paralytic attacks [4]. Pharmacological prophylactic treatments for all three subtypes of PPP are the carbonic anhydrase inhibitors Dichlorphenamide or Acetazolamide [1–4]. Dichlorphenamide is however the only drug with authorized indication in PPP [8]. Some patients experience however worsening of paralytic episodes, and others complain of side effects. If carbonic anhydrase inhibitors are not an option, aldosterone antagonists (K + sparing diuretic) can be helpful for individuals with HypoPP or ATS prone to hypokalemia [1, 3, 4]. Regarding HyperPP, loop diuretics such as Hydrochlorothiazide can be an option [2, 4]. To prevent ventricular arrhythmias in ATS, Flecainide (antiarytmika), beta-blockers or calcium channel blockers are used [3]. Combination therapy is also possible for all three types of PPP [1–4].
As there are limited effective pharmacological treatment options for PPP, avoidance of lifestyle elements that trigger attacks is important. Review articles recommend identifying triggers and subsequently avoid them as an initial step to prevent attacks [1–4]. Prophylactic potassium supplementation should be considered in individuals with Hypo-PP, where trigger avoidance is either unfeasible or unsuccessful [4]. Although avoidance of lifestyle related triggers and potassium supplementation is described to be important prophylactic initial measures, few previous reviews have assessed and described these aspects in any detail in PPP.
Our aim was to search and assess the scientific literature for information on nutritional and physical activity related trigger factors in HypoPP, HyperPP and ATS. Physical activity and nutrition related to development of permanent weakness was not within the scope of our assessment. Based on our findings we aimed to systematize lifestyle advices for patients with these disorders and possibly provide recommendations for future scientific studies.
METHOD
We searched the Ovid Medline and Embase database for scientific papers published between January 1, 1990, to January 31, 2020. This period was considered relevant because of the vast advances in genetics during the last three decades, improving the identification and understanding of rare hereditary disorders. We conducted two separate searches. For both searches, the following diagnosis related open-ended search terms were used: “familial periodic paralysis” OR “hypokalemic periodic paralysis” OR “hyperkalemic periodic paralysis” OR “periodic paralysis” OR “sodium channel muscle disease” OR “Andersen-Tawil”. To identify articles related to nutritional aspects, we combined the diagnosis specific search with the following open-ended search terms: “carbohydrate” OR “sugar” OR “fructose” OR “sucrose” OR “fiber” OR “starch” OR “glucose” OR “syrup” OR “dietary potassium” OR “fasting” OR “dietary sodium” OR “dietary salt” OR “natrium” OR “dietary” OR “nutrition”. Similarly, to identify articles related to physical activity aspects, we combined the diagnosis specific search with the following open-ended search terms: “exercise” OR “physical activity” OR “physical inactivity” OR “training” OR “rest” OR “biking” OR “swim” OR “run” OR “jog” OR “climb” OR “gymnastic” OR “exertion” OR “lift” OR “muscle”.
After excluding duplicates within each search category, the search revealed 431 and 284 articles related to nutritional intake and physical activity, respectively. Two clinical dietitians independently assessed each title and abstract from the search results on nutrition related aspects. The same procedure was conducted by three physiotherapists with respect to the search results on physical activity. We included original studies with all types of study designs, but only the most recent relevant reviews and expert opinions. Articles in English or Scandinavian languages, describing PPP and nutritional intake or physical activity related factors as triggers or relievers of attacks were included. Articles concerning differential diagnosis, animal, muscle fiber or cell studies, neurophysiological tests, or other outcomes or exposure that were not in our scope of interest, were excluded. Any discrepancy between reviewers was resolved through group discussions and in some cases by assessment of the full text of the article before the final group decision was done. Once the included abstracts were identified, full-text papers were retrieved and evaluated by two groups of reviewers. Manual searches were also conducted using the reference lists from included articles.
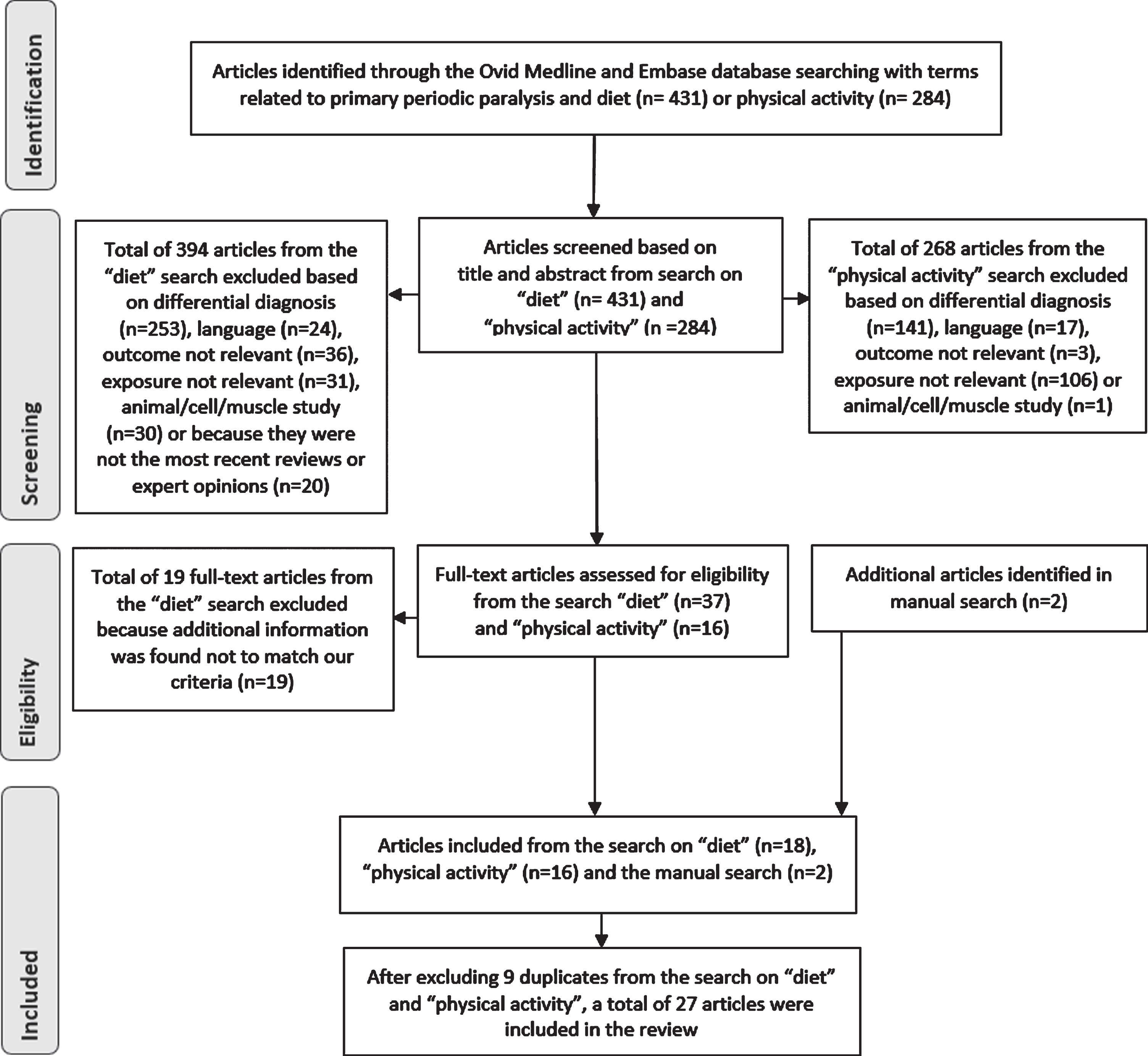
We included a total of 36 articles, 18 articles from the search on nutritional intake, 16 articles from the search on physical activity and 2 articles from the manual search. When the included articles from the different searches on diet and physical activity were compared, 9 duplicates were identified. Additionally, several of the included articles in the search on nutritional intake had descriptions of both nutritional intake and physical activity. As a result, we ended up with a total of 27 articles after excluding the duplicates. Figure 1 summarizes the selection prosses in detail.
Fig. 1
Flow diagram of identification, screening, selection and elimination process.
RESULTS
Hypokalemic periodic paralysis
Nutritional related trigger factors and treatment strategies
Paralytic episodes in HypoPP are commonly reported to be triggered by high carbohydrate intake [9–15]. Even so, in the retrieved studies we did not identify any in-depth description of the meal composition like the amount and type of carbohydrates, or other sources of energy in the meals suspected to trigger a paralytic episode [9–15]. Carbohydrate intake was typically described as either intake of sweets, sources of high amount of carbohydrates [9], carbohydrate-heavy meals [10–14] or high sugar intake [15]. Only a few studies gave some details on the type of carbohydrate-rich meal leading to paralysis [10, 11, 14], for details see Table 1. A high sodium intake [9, 16, 17], over-eating [18], alcohol consumption and dehydration [16] were also reported to trigger paralytic episodes. Eating in the evening or irregular meals could lead to attacks as well [11, 19].
Table 1
Studies describing triggers of attacks in primary periodic paralysis
| Study design and number of cases | Diagnosis | Age and gender | Triggers of attacks | ||
| Nutritional factors | Physical activity factors | ||||
| Kantola et al. (1992) | Cross-sectional study (n = 21, from seven families) | HypoPP Not genotyped | Data on age not shown 71% males, 29% females | Carbohydrate intake (n = 11); eating sweets and chocolate (n = 5); evening meals (n = 4); irregular meals (n = 3) | Hard exercise (n = 8) |
| Links et al. (1994) | Case-series study (n = 44 kindreds) | HypoPP Not genotyped | – | Chinese food; carbohydrate rich meals (2–12 hours prior attacks) | Exercise; combination of little movement and cold (2–12 hours prior attacks) |
| Kumar et al. (2018) | Case-report study Siblings (n = 2) | HypoPP NM_000069.3 (CACNA1S):c.3716G>A (p.Arg1239His) | 16 (case 1) and 14 (case 2) years of age Males | Large carbohydrate consumption and irregular mealtime led to episodic weakness lasting <2 hr (case 1); large fast-food meal, or a Chinese meal and a bacon sandwich led to episodes of muscle stiffness (case 2) | Reduced physical activity led to episodic weakness lasting <2 hr in the morning (case 1); stiffness of muscles at the end of exercise (case 2) |
| Kageyama et al. (2006) | Case-report study Two cases | HypoPP CACNA1S heterozygous mutation, variant not specified | 21 (case 1) and 33 (case 2) years of age Males | Over-eating (case 1) | Athletic activity (case 2) |
| Lewis et al. (2019) | Case-report study One case | HypoPP Not genotyped | 21 years of age Male | High-carbohydrate snack in the day prior to acute paralytic attack | Vigorous physical activity (basketball) the day prior to a paralytic attack |
| Stapleton (2018) | Case-report study One case | HypoPP No match to known genetic mutations | 33 years of age Male | Carbohydrate-heavy meal (pasta with a large quantity of sugary, carbonated drinks) evening prior to generalised weakness and dysarthria upon waking | – |
| Alhasan et al. (2019) | Case-report study One case | HypoPP NM_000069.3 (CACNA1S): c.1583g>A; (p.Arg528His) | 14 years of age Male | – | Football game and physical exhaustion the day prior to a paralytic attack |
| Dogan et al. (2015) | Case-report study One case | HypoPP Not genotyped | 33 years of age Male | – | Heavy physical activity the previous week |
| Andersen et al. (2014) | Case-report study One case | HypoPP NM_000069.3 (CACNA1S): c.1583g>a; (p.Arg528His) | 13 years of age Male | Carbohydrate rich meals the evening prior to paralytic episodes in the morning | Hard physical activity the evening prior to paralytic episodes in the morning |
| Damallie et al. (2000) | Case-report study One case | HypoPP Not genotyped | 20 years of age Female (27 weeks gestation) | 1-hour 50 g glucose screening test resulted in hypokalemia (2,1 mEq/L) and gradual bilateral lower extremity weakness with numbness and pain development the following day, unable to stand within 24 hours | – |
| Levitt (2008) | Expert Opinion | HypoPP | – | High carbohydrate meals and sodium; Chinese food (with and without monosodium glutamate); alcohol; dehydration | Rest after exercise; strenuous physical activity; change in daily activity patterns |
| Charles et al. (2013) | Cross-sectional study (n = 94) | HyperPP Genetically identified, but data not shown | 19-84 years of age 53% males and 47% females | Hunger (43%); potassium in food (35%); specific foods or beverages (35%); potassium supplements (14%); alcohol (45%); | Rest after exercise (67%), changes in activity level (41%) |
| Bradley et al. (1990) | Case-series study (n = 20, from four families) | HyperPP Not genotyped | Data on age and gender not shown for all cases | Up to 120 mEq (9 g) of potassium chloride produced attacks in at least one member of each family; fasting induced attack (reported by 40-year-old man) | Rest after exercise and prolonged sitting (especially in a warm room) trigger attacks of limb weakness (reported by same 40-year-old man) |
| Cleland and Tawil (2014) | Letter to editor Case report One case | HyperPP NM_000334.4 (SCN4A): c.2111C>T (p.Thr704Met) | 17 years of age Male | – | Upon awakening or during rest after exercise |
| Inoue et al. (2018) | Case-control study (n = 26) | ATS KCNJ2 mutations, variants not specified | 10-33 years of age 27% males and 73% females | – | Exercise triggered cardiac symptoms in 9 of 26 ATS patients |
| Fernlund et al. (2013) | Case-report study One case (10 family members with ATS) | ATS NM_000891.2 (KCNJ2): c.271.282del (p.Ala91_Leu94del) | 22 years of age Female | – | Near drowning episode at 15; syncope triggered by physical exercise at 16 in school gymnastics; 7 of 10 affected family members had exercise-induced VT |
| Efremidis et al. (2006) | Case-report study One case | ATS Not genotyped | 42 years of age Female | – | Recreational swimming caused a cardiac event |
| Cavel-Greant et al. (2012) | Cross-sectional study (HypoPP group n = 46, HyperPP group n = 6, ATS group n = 6,PC group n = 4, and PP with unknown mutation n = 4) | HypoPP: NM_000069.3 (CACNA1S): c1583G>A (p.Arg528His) (n = 8); NM _000069.3 (CACNA1S):c.3716G>A (p.Arg1239His) (n = 4); NM_000069.2 (CACNA1S): c.2691G>T (p.Arg897Ser) (n = 1)HyperPP: NM_000334.4(SCN4A):c.2111C>7 (p.Thr704Met) (n = 1)ATS: NM_000891.2 (KCNJ2): c.199C>T (p.Arg67Trp) (n = 2) | 41-82 years of age30% males, 70% females | – | High intensity training (as prescribed by some of the physiotherapists) to the point of fatigue made 40% of the respondents weaker (unknown subclassification)No mention of exercise- induced episodic attacks. |
| Miller et al. (2004) | Cross-sectional study (HypoPP group n = 71, 56 kindreds, HyperPP group n = 99, 47 kindreds, and PC group n = 57, 26 kindreds) | HypoPP: NM_000069.3(CACNA1S): c.1583G>A (p.Arg528His) (n = 15); NM_000069.3 (CACNA1S): c.3716G>A (p.Arg1239His) (n = 15); NM_000069.3 (CACNA1S): c.3715C>G (p.Arg1239Gly) (n = 1); SCN4A mutation, variation not specified (n = 5); and without mutations (n = 20) HyperPP: NM_000334.4 (SCN4A): c.2111C>T (p.Thr704Met) (n = 10); NM_000334.4 (SCN4A): c.4774A>G (p.Met1592Val) (n = 10); other SCN4A mutations with variations not specified (n = 10); and without mutations (n = 17) | Data on age not shown 62% males and 38% females (HypoPP group); 50% males and 50% females (HypoPP and PC group) | HypoPP: intake of high carbs/sweets (18-80%) or salt (0-24%) HyperPP: hunger (6–29%), missing meals (-%) or potassium rich foods (21–32%) and supplement (100%). | HypoPP: exercise/rest after exercise (50–93%) HyperPP: exercise/rest after exercise (69–83%) |
| Levitt et al. (2004) | Expert option | HypoPP, HyperPP and ATS | – | HypoPP: “Sweets”, high carb, and salt HyperPP: hunger, fasting, potassium-rich food (e.g. bananas, orange juice) and potassium supplements | Exercise, rest after exercise (Hypo- and HyperPP) |
Hr, hours; Min, Minutes; VT, ventricular tachycardia; PC, Paramyotonia congenita. For the description of sequence variants, the HCVS nomenclature recommendations were used (den Dunnen, J.T., Dalgleish, R., Maglott, D.R., Hart, R.K., Greenblatt, M.S., McGowan-Jordan et al. (2016). HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Human Mutation, 37: 564-569. https://doi.org/10.1002/humu.22981).
A diet low in carbohydrate [11–13, 16] and sodium [10], and avoidance of foods like pizza, pasta, candy [17] and large carbohydrate rich meals before bedtime [19] are recommended for individuals with HypoPP in several reports and reviews. A 2000 calorie diabetic diet has also been suggested based on one case-study [15]. According to an expert opinion by Levitt, avoidance of high carbohydrate meals needs to be explicitly outlined to individuals with HypoPP as a treatment strategy. More complex carbohydrates were suggested to be better tolerated than starch and sugar rich carbohydrate sources [16]. Individuals who are salt-sensitive should identify the amount of salt that induces attacks. Salts with potassium chloride rather than sodium chloride, is suggested as a salt alternative [16].
In HypoPP, potassium supplementation is frequently reported to prevent, relieve, or abort paralytic episodes [9, 10, 12–15, 17, 19]. In the majority of the studies, a description of dose, form and route of administration was however lacking [9, 10, 12, 14, 17, 19]. To prevent attacks, some studies suggested daily oral potassium chloride supplementation in doses varying from 30 mEq (2,25 g) to 60 mEq (4,50 g) potassium [13–15]. To relieve attacks, the potassium chloride dose given at onset differed slightly between case-report studies, from 15–20 mEq (1,125–1,5 g) oral potassium chloride supplement [14] to 1-2 tablets Sando K (12–24 mEq / 0.9–1.8 g potassium chloride) [11]. To abort ongoing attacks the potassium dose administered in two case-reports, was 40 mEq (3 g) [15] and 140 mEq (10.5 g) potassium chloride [13], respectively. The doses in mEq potassium chloride per kg bodyweight were not shown [13, 15]. In his expert opinion, Levitt describes how potassium can be used as a preventer, reliever, and treatment of paralytic attacks [14]. The interventions suggested by Levitt are based on personal and clinical experiences.
Physical activity related trigger factors and treatment strategies
Physical activity is a commonly reported trigger of paralytic attacks in HypoPP [9, 10, 12–14, 17, 20, 21]. Various forms of physical activity could lead to an attack (see Table 1), but the amount and intensity of activity was poorly described in the literature. Typically, activity was described as hard/strenuous/vigorous without any further definition of intensity or duration [12, 13, 20, 21], or just as exercise [9, 14, 17, 18]. One expert opinion highlights rest after exercise as a trigger [16], correlating well with case reports describing attacks occurring at night or in the morning the day after vigorous activity [10, 12, 13, 20, 21].
Some authors recommend to avoid strenuous activity to reduce the frequency of attacks [10, 12, 13]. Hard physical activity seems to have a different effect on attacks compared to mild physical activity. In a five-generation family screening, several patients reported that walking off an incipient attack was possible, especially during the night [14].
Hyperkalemic periodic paralysis
Nutritional related trigger factors and treatment strategies
Fasting and intake of potassium supplements or potassium rich foods are commonly reported as triggers of attacks in HyperPP [9, 22, 23]. In one cross-sectional study based on medical records, hunger and missing meals, or eating potassium rich foods often gave rise to weakness in around 1/3 of participants with genetically confirmed HyperPP (NM_000334.4 (SCN4A): c.2111C>T (p.Thr704Met) n = 50, NM_000334.4 (SCN4A): c.4774A>G (p.Met1592Val) n = 13, all HyperPP mutations n =82) [9]. Potassium supplements caused weakness in all participants, regardless of genetic subtype [9]. Alcohol, cold foods or beverages, and certain foods (not specified) are recognized as additional triggers in individuals affected by HyperPP [22].
Some studies recommend carbohydrate rich foods, frequent meals and evasions of nutrition related trigger factors to prevent attacks in HyperPP [17, 22]. The cross-sectional study by Charles et al. describe that a majority of participants used a combination of medication and carbohydrate-rich foods as their treatment strategy [22]. Most participants ate carbohydrate-rich snacks every two to three hours and three to five medium- to smaller-sized meals a day. They also reported drinking plenty of fluids, but avoided alcohol, a range of high potassium foods (not specified), and cold foods and beverages [22]. To abort acute attacks participants recommended specific carbohydrate rich foods and beverages [22]. They detected no difference between the impact of solid versus liquid carbohydrates, and a relieving effect was noted within an hour by most participants [22]. Surprisingly, 13 % reported that potassium reduced the weakness during paretic episodes [22].
Physical activity related trigger factors and relievers
Physical activity and rest after exercise is also commonly reported as triggers of paralytic attacks in individuals with HyperPP [9, 17, 22–25]. None of the included studies described the duration or intensity of activity in any detail. Changes in activity level [23] and prolonged sitting [22] was also mentioned as triggers.
Gentle exercise and muscle relaxation are reported to mitigate an upcoming attack [22, 23]. Patients reported doing activities like walking, biking and swimming to avoid attacks [23].
Anderson-Tawil syndrome
In ATS, we did not identify any case reports or studies describing nutritional factors as triggers or treatment strategies of attacks.
We identified two case reports describing exercise-induced ventricular arrhythmias in ATS [26, 27]. Intensity and duration of the exercise were unspecified. In a case-control study of 26 ATS patients, Inoue et al. found increased ventricular arrhythmias with increasing heart rate in 35% of cases. The arrhythmias disappeared as the heart rate approached its maximum but increased again in the recovery phase [28].
We found no case reports or studies describing physical activity related relievers of paralytic attacks in ATS.
DISCUSSION
Although lifestyle factors related to nutritional intake and physical activity frequently are reported as triggers of attacks in PPP, this topic has to a limited extent been studied.
We did not identify any published observation or intervention studies in this group of neuromuscular disorders regarding lifestyle change and effect on paralytic attacks. The current knowledge on nutrition and physical activity related trigger factors is based on case-reports, expert opinions, and a few retrospective case studies with inadequate methods for description of nutritional intake and physical activity level. The majority of the studies retrieved information from questionnaires or medical journals leading to incomplete information. Moreover, most of the studies identified included a small number of participants with a wide age range and various genetic profiles. There are more case-report studies on men compared to women, which likely reflects a higher number of asymptomatic females because of decreased penetrance. The lack of knowledge inhibits the possibility to develop evidence-based lifestyle advices for patients.
To summarize, individuals affected by HypoPP most commonly report a high carbohydrate intake, hard physical activity and rest after exercise as triggers of attacks. In HyperPP, fasting, intake of pota-ssium supplements or potassium rich foods, physical activity and rest after exercise are the most reported triggers of attacks. In the literature, no nutritional related trigger factors are reported regarding ATS. It is however described that exercise can induce ventricular arrhythmias in ATS.
To prevent attacks, it is recommended that individuals with PPP identify their individual triggers and subsequently avoid them. Individuals with HypoPP are explicitly recommended to use daily potassium supplements to prevent paralytic episodes, and to take extra potassium supplements to relieve attacks or abort ongoing attacks. Several cases with HypoPP also report that walking off an incipient attack is possible. Regarding HyperPP, individuals are recommended to eat carbohydrate rich foods and frequent meals, and to drink a lot of fluids to prevent attacks. Gentle exercise and muscle relaxation are also reported to mitigate an upcoming attack in HyperPP.
A high carbohydrate intake seems to play an important role in triggering (HypoPP) and preventing (HyperPP) attacks in PPP, which may be related to the glucose-insulin homeostasis. In healthy individuals, a meal with carbohydrates causes a rise in blood glucose levels and consequently a spike in endogenous insulin which stimulate cells absorption of glucose and lowering of serum glucose levels [29]. In addition to lowering serum glucose levels, insulin furthermore leads to decreased potassium levels in serum by activating sodium-potassium ATPase pumps in muscle cells, causing a flux of potassium into muscles [7, 30, 31]. This potassium shift may explain the possible triggering effect of high carbohydrate intake in HypoPP and ATS prone to hypokalemia. This insulin driven inward shift of K + ions may have the potential to trigger inappropriate activity of mutated voltage-gated ion channels, resulting in membrane depolarization and consequently paralysis or weakness, but the evidence is lacking. Contrarily, it has been proposed that insulin reduces inward rectifier K + conductance in HypoPP patients and K + depleted rat model, thus promoting membrane depolarization that causes inexcitability. The membrane depolarization was not prevented by inhibition of sodium or calcium channels [32, 33]. On the contrary, in HyperPP and ATS prone to hyperkalemia, intake of carbohydrates could relieve attacks as the following raise in blood insulin levels can lead to a shift of potassium from serum into muscle cells and lead to a reduction of serum potassium levels [6]. Future research is needed to prove these hypotheses.
To date no studies have systematically investigated the triggering or relieving effects of different amounts and types of carbohydrates, nor carbohydrates in combination with protein and fat. Some studies in HypoPP indicate that the type of carbohydrate rich meal may affect the risk of triggering an attack [11, 14, 16]. A possible explanation for this is that the postprandial (after meal) insulin and glucose response (PPGR) varies in healthy subjects depending on the type of carbohydrate and amounts of fat and protein consumed with it [32–37]. Eating a mixed meal consisting of fat, protein, or both, in addition to carbohydrates results in a lower post prandial glucose response (PPGR) and insulin response compared to eating a meal with the same calorie content consisting of exclusively carbohydrates [34, 35]. Another interesting topic regarding nutritional intake is that several food items (fruit, juice, vegetables, chocolate, potato chips, liquorice) contain both carbohydrates and potassium, and may be more potent as a trigger factor than judged by the carbohydrate or potassium content alone. Consideration should be given that it might be beneficial for all subgroups of PPP to avoid food items with a significantly high amount of potassium to maintain a balanced serum potassium level. This might help prevent attacks developing in individuals with HyperPP and make it easier to administer the correct doses of daily potassium supplementation recommended to individuals with HypoPP. This leads to nutritional recommendations that are hard to navigate without having certain knowledge of food composition. Case reports suggest that there are likely individual differences in the sensitivity to triggers related to nutritional intake, even within the same family. This demonstrates that there is a need to investigate the effect of different carbohydrates and meal compositions on the risk of paralytic attacks, and the need for individually adapted nutritional advices.
Strenuous exercise and rest after exercise are commonly reported to trigger attacks in PPP. This phenomenon can be explained by the massive shifts in potassium homeostasis observed during exercise and the subsequent stimulation of the Sodium-Potassium ATPase pumps in skeletal muscles seen post-exercise [6, 7, 30, 31, 38]. Mild physical activity and muscle relaxation is on the other hand reported to postpone or prevent an attack in both HypoPP and HyperPP [22, 23]. Patients report doing activities like walking, biking and swimming to avoid attacks [23]. Cleland & Tawil (2014) suggest a partial restoration of membrane excitability as a possible explanation for this phenomenon [24]. This could be related to the prominent role skeletal muscles have in the extrarenal K + homeostasis [6, 7, 30, 31, 38]. Physical activity also contributes to insulin-independent glucose uptake into muscle cells which has been demonstrated to reduce blood glucose levels and postprandial insulin release [39, 40]. This may contribute to the beneficial effect of mild to moderate physical activity in people with HypoPP.
The fact that some patients describe that they become worse from exercise therapy with a physiotherapist might indicate that physiotherapists may lack knowledge of the diagnoses and use training principles adapted to healthy people [24]. Our clinical experience, supported by some case reports, indicate large individual variations in exercise tolerance, even within the same family. This emphasizes the need for further research to understand the mechanisms behind the triggering and relieving effects of physical activity. Patients with PPP should be followed up individually by a physiotherapist to explore the physical activity possibilities and intensity boundaries of the individual, and particularly in cases of permanent weakness to find optimal movement strategies.
It was demanding to conduct a literature search regarding nutrition and physical activity in PPP, as few articles clearly and exclusively describe these aspects. As we read the full text articles included from the two sperate searches, we identified several articles that contained information on both nutrition and physical activity, which was not initially indicated based on the title and abstract. Therefore, we can not rule out that articles have been excluded based on the title and abstract, that in full text include information on nutrition or physical activity. Nevertheless, due to our systematic approach, we are confident that we have not missed major studies that have examined and described relevant factors in more detail.
CONCLUSION
Our results support that dietary intake and physical activity may play a role in causing and preventing paralytic episodes in PPP, although the current evidence is weak. To provide good evidence-based patient care, several lifestyle aspects need to be described in more detail using validated scientific methods, representative samples, and controlled study designs.
ACKNOWLEDGMENTS
We would like to thank Hilde Flaatten, librarian at the University of Oslo Medical Library, for assisting with the composition and implementation of the literature search. We also want to thank the management of Frambu Resource Centre for Rare Disorders for their support of this literature study.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to all of the following: (1) the conception and design of the literature study, (2) drafting the article or revising it critically, and (3) final approval of the version to be submitted.
REFERENCES
[1] | Weber F , Lehmann-Horn F . Hypokalemic Periodic Paralysis. 2002 Apr 30 [Updated 2018 Jul 26]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®[Internet]. Seattle (WA): University ofWashington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1338/. |
[2] | Weber F , Jurkat-Rott K , Lehmann-Horn F . Hyperkalemic Periodic Paralysis. 2003 Jul 18 [Updated 2016 Jan 28]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1496/ |
[3] | Veerapandiyan A , Statland JM , Tawil R . Andersen-Tawil Syndrome. 2004 Nov 22 [Updated 2018 Jun 7]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1264/. |
[4] | Statland JM , Fontaine B , Hanna MG , Johnson NE , Kissel JT , Sansone VA , et al. Review of the Diagnosis and Treatment of Periodic Paralysis. Muscle Nerve. (2018) ;57: (4):522–30. doi: 10.1002/mus.26009 |
[5] | Lehmann-Horn F , Jurkat-Rott K , Rüdel R . Periodic paralysis: Understanding channelopathies. Curr Neurol Neurosci Rep. (2002) ;2: (1):61–9. doi: 10.1007/s11910-002-0055-9 |
[6] | Clausen T . Hormonal and pharmacological modification of plasma potassium homeostasis. Fundam Clin Pharmacol. (2010) ;24: (5):595–605. doi: 10.1111/j.1472-8206.2010.00859.x |
[7] | Cannon SC . Channelopathies of skeletal muscle excitability. Compr Physiol. (2015) ;5: (2):761–90. doi: 10.1002/cphy.c140062 |
[8] | Sansone VA , Burge J , McDermott MP , Smith PC , Herr B , Tawil R , et al. Randomized, placebo-controlled trials of dichlorphenamide in periodic paralysis. Neurology. (2016) ;86: (15):1408–16. doi: 10.1212/WNL.0000000000002416 |
[9] | Miller TM , Dias da Silva MR , Miller HA , Kwiecinski H , Mendell JR , Tawil R , et al. Correlating phenotype and genotype in the periodic paralyses. Neurology. (2004) ;63: (9):1647–55. doi: 10.1212/01.wnl.0000143383.91137.00 |
[10] | Stapleton LJ . Hypokalaemia periodic paralysis. Scott Med J. (2018) ;63: (1):28–31. doi: 10.1177/0036933017727420 |
[11] | Kumar S , Offiong EE , Sangita S , Hussain N . Phenotypical Variation with Same Genetic Mutation in Familial Hypokalemic Periodic Paralysis. J Pediatr Neurosci. (2018) ;13: (2):218–20. doi: 10.4103/jpn.JPN_44_17 |
[12] | Andersen AH , Hansen B , Hermansen MN . Hereditary hypokaliaemic periodic paralysis in a 13-year-old boy. Ugeskr Laeger. (2014) ;176: (25A):V12120754. Danish. |
[13] | Lewis KL , Malouff TD , Kesler AM , Harris DM . Hypokalemic periodic paralysis - the importance of patient education. Rom J Intern Med. (2019) ;57: (3):263–5. doi: 10.2478/rjim-2019-0004 |
[14] | Links TP , Smit AJ , Molenaar WM , Zwarts MJ , Oosterhuis HJ . Familial hypokalemic periodic paralysis. Clinical, diagnostic and therapeutic aspects. J Neurol Sci. (1994) ;122: (1):33–43. doi: 10.1016/0022-510x(94)90049-3 |
[15] | Damallie KK , Drake JG , Block WA . Hypokalemic periodic paralysis in pregnancy after 1-hour glucose screen. Obstet Gynecol. (2000) ;95: (6 Pt 2):1037. doi: 10.1016/s0029-7844(00)00874-7 |
[16] | Levitt JO . Practical aspects in the management of hypo-kalemic periodic paralysis. J Transl Med. (2008) ;6: ;18. doi: 10.1186/1479-5876-6-18. Erratum in: J Transl Med. 2014; 12:198. |
[17] | Levitt J , Cochran P , Jankowiak J . Patient page. Attacks of immobility caused by diet or exercise? The mystery of periodic paralyses Neurology. (2004) ;63.: :E17–8. 10.1212/01.WNL.0000146275.91221.65 |
[18] | Kageyama K , Terui K , Tsutaya S , Matsuda E , Shoji M , Sakihara S , et al. Gene analysis of the calcium channel 1 subunit and clinical studies for two patients with hypokalemic periodic paralysis. J Endocrinol Invest. (2006) ;29: (10):928–33. doi: 10.1007/BF03349199 |
[19] | Kantola IM , Tarssanen LT . Familial hypokalaemic periodic paralysis in Finland. J Neurol Neurosurg Psychiatry. (1992) ;55: (4):322–4. doi: 10.1136/jnnp.55.4.322 |
[20] | Alhasan K , Abdallah M , Kari J , Bashiri F . Hypokalemic periodic paralysis due to CACNA1S gene mutation. Neurosciences. (2019) ;24: :225–30. 10.17712/nsj.2018.3.20180005 |
[21] | Dogan NO , Avcu N , Yaka E , Isikkent A , Durmus U . Weakness in the Emergency Department: Hypokalemic Periodic Paralysis Induced By Strenuous Physical Activity. Turk J Emerg Med. (2016) ;15: (2):93–5. doi: 10.5505/1304.7361.2015.57984 |
[22] | Charles G , Zheng C , Lehmann-Horn F , Jurkat-Rott K , Levitt J . Characterization of hyperkalemic periodic paralysis: A survey of genetically diagnosed individuals. J Neurol. (2013) ;260: (10):2606–13. doi: 10.1007/s00415-013-7025-9 |
[23] | Petruzewicz I , Adelman LS , Jenkison M , Jedrzejowska H , Drac H , Pendlebury WW . Progressive myopathy in hyperkalemic periodic paralysis. Arch Neurol. (1990) ;47: (9):1013–7. doi: 10.1001/archneur.1990.00530090091018 |
[24] | Cavel-Greant D , Lehmann-Horn F , Jurkat-Rott K . The impact of permanent muscle weakness on quality of life in periodic paralysis: A survey of 66 patients. Acta Myol. (2012) ;31: (2):126–33. |
[25] | Cleland JC , Tawil R . Post-exercise increment in compound muscle action potential amplitude in hyperkalemic periodic paralysis. Clin Neurophysiol. (2014) ;125: (10):2134–5. doi: 10.1016/j.clinph.2014.02.005 |
[26] | Efremidis M , Pappas LK , Sideris A , Letsas KP , Gavrielatos GD , Kardaras F . Swimming-triggered aborted sudden cardiac death in a patient with Andersen-Tawil syndrome. Int J Cardiol. (2006) ;112: (2):e45–7. doi: 10.1016/j.ijcard.2006.02.028 |
[27] | Fernlund E , Lundin C , Hertervig E , Kongstad O , Alders M , Platonov P . Novel mutation in the KCNJ2 gene is associated with a malignant arrhythmic phenotype of Andersen-Tawil syndrome. Ann Noninvasive Electrocardiol. (2013) ;18: (5):471–8. doi: 10.1111/anec.12074. PMID: 24047492; PMCID: PMC6932293 |
[28] | Inoue YY , Aiba T , Kawata H , Sakaguchi T , Mitsuma W , Morita H , et al. Different responses to exercise between Andersen–Tawil syndrome and catecholaminergic polymorphic ventricular tachycardia. EP Europace. 2017;20. 10.1093/europace/eux351 |
[29] | Sand O , Vigleik Sjaastad Ø , Haug E . Human physiology. The endocrine system. 2014. 2. edition. Language: Norwegian (Bokmål). Page 283-285. ISBN/EAN: 9788205423411. |
[30] | Palmer BF , Clegg DJ . Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am J Kidney Dis. (2019) ;74: (5):682–95. doi: 0.1053/j.ajkd.2019.03.427 |
[31] | Ho K . A critically swift response: Insulin-Stimulated Potassium and Glucose Transport in Skeletal Muscle. CJASN. (2011) ;6: (7):1513–516; DOI: 10.2215/CJN.04540511 |
[32] | Tricarico D , Pierno S , Mallamaci R , Brigiani GS , Capriulo R , Santoro G , et al. The Biophysical and Pharmacological Characteristics of Skeletal Muscle ATP-Sensitive K+ Channels Are Modified in K+ Depleted Rat, an Animal Model of Hypokalemic Periodic Paralysis. Molecular Pharmacology. (1997) ;54: (1):197–206; doi: https:doi.org/10.1124/mol.54.1.197 |
[33] | Ruff RL . Insulin acts in hypokalemic periodic paralysis by reducing inward rectifier K+current. Neurology. (1999) ;53: (7):1556–63. doi: 10.1212/wnl.53.7.1556 |
[34] | Imamura F , Micha R , Wu JH , de Oliveira Otto MC , Otite FO , Abioye AI , et al. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. (2016) ;13: (7):e1002087. doi: 10.1371/journal.pmed.1002087 |
[35] | Augustin LS , Kendall CW , Jenkins DJ , Willett WC , Astrup A , Barclay AW , et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. (2015) ;25: (9):795–815. doi: 10.1016/j.numecd.2015.05.005 |
[36] | Brand-Miller JC , Thomas M , Swan V , Ahmad ZI , Petocz P , Colagiuri S . Physiological validation of the concept of glycemic load in lean young adults. J Nutr. (2003) ;133: (9):2728–32. doi: 10.1093/jn/133.9.2728 |
[37] | Jenkins DJ , Wolever TM , Taylor RH , Barker H , Fielden H , Baldwin JM , et al. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am J Clin Nutr. (1981) ;34: :362–6. |
[38] | Sejersted OM , Sjøgaard G . Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. (2000) ;80: (4):1411–81. doi: 10.1152/physrev.2000.80.4.1411 |
[39] | Francois ME , Baldi JC , Manning PJ , Lucas SJ , Hawley JA , Williams MJ , et al. ‘Exercise snacks’ before meals: A novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. (2014) ;57: (7):1437–45. doi: 10.1007/s00125-014-3244-6 |
[40] | Dunstan DW , Kingwell BA , Larsen R , Healy GN , Cerin E , Hamilton MT , et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. (2012) ;35: (5):976–83. doi: 10.2337/dc11-1931 |




