Clinical Experience with Eculizumab in Treatment-Refractory Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis
Abstract
Background:
Although established therapies are effective in most patients with generalized myasthenia gravis (gMG), some patients do not respond or they experience intolerable adverse events, highlighting the need for better tolerated, targeted therapies for treatment-refractory gMG.
Objective:
To describe real-world experience with eculizumab in patients with treatment-refractory acetylcholine receptor antibody-positive (AChR+) gMG.
Methods:
Retrospective chart review of 15 patients with treatment-refractory AChR+ gMG treated for 12 months with eculizumab (900 mg/week for 4 weeks then 1200 mg every 2 weeks). Outcome measures were Myasthenia Gravis–Activities of Daily Living (MG-ADL) scores, number of exacerbations, single-breath count test (SBCT) score, medication changes, selected Quantitative Myasthenia Gravis (QMG) evaluations, and adverse events. Data collected at 3-monthly intervals for 12 months before and after eculizumab initiation were analyzed.
Results:
Clinically meaningful reductions in total MG-ADL scores were observed at 3 months following eculizumab initiation and maintained up to 12 months in all patients. After 12 months’ eculizumab treatment, there was a significant reduction in the number of acute exacerbations; mean (SD) SBCT score improved from 28.13 (0.33) to 50.26 (2.86); all patients achieved a ‘none’ or ‘mild’ rating for QMG evaluations; all patients reduced their daily prednisone dose; and nine patients had discontinued pyridostigmine. At the end of treatment, intravenous immunoglobulin was discontinued in all six patients receiving this therapy at eculizumab initiation. Eculizumab was well tolerated.
Conclusions:
This real-world study demonstrated improvement in outcome measures and decreased concomitant drug requirement within 12 months of eculizumab initiation in patients with treatment-refractory AChR+ gMG.
INTRODUCTION
Myasthenia gravis (MG) is an autoimmune, neuro-muscular-junction disorder characterized by skeletal muscle weakness and fatigability [1]. Although established therapies are effective in most patients with MG, 10–20% of patients are refractory to treatment due to inadequate response or intolerance to therapy [2]. Patients with refractory generalized MG (gMG) are at risk of frequent exacerbations that can cause episodes of respiratory failure requiring mechanical ventilation [3]. The quality of life for such patients is poor and there is a need for new, better tolerated, targeted therapies for this condition [4, 5].
A high proportion of patients with MG produce antibodies that bind to the acetylcholine receptor (AChR), thereby reducing receptor activity by blocking acetylcholine binding; causing the receptor to degrade; or activating the complement cascade. The latter mechanism produces membrane attack complexes (MACs) that damage the postsynaptic membrane of the neuromuscular junction and reduce the number of functional AChRs [3, 6]. Eculizumab is a recombinant humanized monoclonal antibody that binds to complement component C5 and prevents conversion to the proinflammatory C5a and MAC component C5b. In a Phase 3, randomized, double-blind, placebo-controlled study (REGAIN) and its open-label extension, eculizumab was shown to be effective in patients with treatment-refractory AChR antibody-positive (AChR+) gMG [3, 7, 8]. Eculizumab has been approved for the treatment of AChR+ gMG in the USA (refractory not required) [9], Europe (treatment-refractory AChR+ gMG) [10], and Japan (inadequate response to high-dose intravenous immunoglobulin [IVIg] therapy and plasma exchange) [11].
This report describes our clinical experience in managing 15 patients with treatment-refractory AChR+ gMG during the first 12 months of their treatment with eculizumab.
PATIENTS AND METHODS
Aim
This study was approved by the institutional review board of University of Missouri-Columbia (approval number 2023301) to describe clinical experience with eculizumab when added to existing therapy in patients with treatment-refractory AChR+ gMG.
Design
A retrospective chart review was conducted of data from patients with gMG treated for ≥3 years between December 2016 and January 2019 in the Neurology Clinic at the University of Missouri, Columbia, USA. Electronic medical records were used to review patient demographic information. Data recorded at 3-monthly intervals in the 12 months before and after eculizumab initiation were evaluated for the following parameters: Myasthenia Gravis–Activities of Daily Living (MG-ADL) scores [12]; number of exacerbations, as assessed by the on-call board-certified neurologist, based on the presence of dysphagia, acute respiratory failure, or major functional disability precluding physical activity and other objective examination findings [13]; physical assessments of selected items (routinely conducted in our clinical practice) from the Quantitative Myasthenia Gravis (QMG) evaluation (degree of ptosis, double vision, and eye closure, and the duration of ability to stretch out arms and legs, classified as none, mild, moderate, or severe) [14]; and respiratory function, using the single-breath count test (SBCT), performed by asking patients to take a deep breath and count as far as possible in their normal voice at an approximate rate of two counts per second [15]. In addition, medication regimen at initiation of eculizumab, changes in medication over 12 months, and final medication regimen at the end of 12 months of eculizumab therapy were reviewed. Adverse events assessed by the treating physician as being associated with eculizumab administration were also reviewed.
Patients
Patients were eligible for inclusion if they were: ≥18 years old; diagnosed with gMG that was refractory to conventional treatments; seropositive for AChR antibodies (radioimmunoassay, Mayo Clinic Laboratories, Rochester, MN, USA; Test ID: MGA1); treated with eculizumab for ≥12 months; and vaccinated against Neisseria meningitidis before initiating eculizumab, as recommended in the prescribing information [9]. Refractory MG was defined as treatment with ≥2 immunosuppressants for ≥12 months without symptom control, or ≥1 immunosuppressant for ≥12 months with IVIg or plasma exchange given ≥4 times/year without symptom control. All patients had an MG-ADL score ≥6 before eculizumab initiation, which was administered at an induction dose of 900 mg per week for 4 weeks (at Weeks 0, 1, 2, and 3), then at 1200 mg at Week 4, followed by 1200 mg every 2 weeks thereafter, as per the prescribing information for the product [9].
RESULTS
General characteristics
A total of 15 patients with treatment-refractory AChR+ gMG were identified on retrospective chart review. The mean (standard deviation [SD]) age was 47.4 years (14.9 years); nine were female and six were male; 14 were White and one was Black. The Myasthenia Gravis Foundation of America disease classification was IIa in five patients (33.3%), IIb in four patients (26.7%), IIIa in four patients (26.7%), and IIIb in two patients (13.3%). Thymectomy had been performed in nine patients (60.0%) before initiation of eculizumab therapy. During the 12–15 months before eculizumab initiation, patients had received combinations of IVIg therapy, the immunosuppressants prednisone, mycophenolate mofetil, and azathioprine, and the acetylcholinesterase inhibitor pyridostigmine; none of the patients had received rituximab. None of these therapies were stopped at the time of eculizumab initiation. The demographic data at the time of selection of patients included in the analysis are summarized in Table 1.
Table 1
Baseline demographics of patients included in the analysis
| Patient | Age (years) | Sex | Ethnicity | MGFA class | Thymectomy | Therapy at eculizumab initiation |
| 1 | 45 | F | White | IIa | Yes | Prednisone 35 mg/day + pyridostigmine 60 mg TID + IVIg 1g/kg q4w + mycophenolate mofetil 1000 mg BID |
| 2 | 36 | F | White | IIIa | Yes | Prednisone 30 mg/day + pyridostigmine 60 mg TID + azathioprine 200 mg/day |
| 3 | 56 | M | White | IIIb | Yes | Prednisone 40 mg/day + mycophenolate mofetil 1000 mg BID + pyridostigmine 60 mg TID |
| 4 | 72 | M | White | IIa | No | Prednisone 40 mg/day + mycophenolate mofetil 1000 mg BID + pyridostigmine 60 mg TID |
| 5 | 65 | F | White | IIb | No | IVIg 1g/kg q4w + pyridostigmine 60 mg TID + prednisone 30 mg/day |
| 6 | 26 | F | White | IIIa | Yes | IVIg 1g/kg q4w + prednisone 40 mg/day + pyridostigmine 60 mg TID |
| 7 | 66 | M | White | IIb | No | Prednisone 30 mg/day + mycophenolate mofetil 1000 mg BID + pyridostigmine 60 mg TID |
| 8 | 38 | F | White | IIa | No | Prednisone 40 mg/day + mycophenolate mofetil 1500 mg BID + pyridostigmine 60 mg TID |
| 9 | 30 | F | White | IIb | Yes | Prednisone 20 mg/day + mycophenolate mofetil 1500 mg BID + pyridostigmine 60 mg TID |
| 10 | 60 | M | White | IIa | No | Prednisone 40 mg/day + azathioprine 200 mg/day + pyridostigmine 60 mg TID |
| 11 | 61 | F | Black | IIIb | Yes | IVIg 1g/kg q5w + prednisone 30 mg/day + pyridostigmine 60 mg TID |
| 12 | 38 | F | White | IIIa | Yes | IVIg 1g/kg q4w + prednisone 20 mg/day + pyridostigmine 60 mg TID |
| 13 | 24 | F | White | IIIa | Yes | IVIg 1g/kg q4w + prednisone 45 mg/day + pyridostigmine 60 mg TID + mycophenolate mofetil 1000 mg BID |
| 14 | 43 | M | White | IIb | Yes | Prednisone 40 mg/day + pyridostigmine (dose unknown) |
| 15 | 51 | M | White | IIa | No | Prednisone 40 mg/day + pyridostigmine 60 mg TID + mycophenolate mofetil 1000 mg BID |
BID, twice daily; F, female; IVIg, intravenous immunoglobulin; M, male; MGFA, Myasthenia Gravis Foundation of America; qnw, every n weeks; TID, three times daily.
MG-ADL score
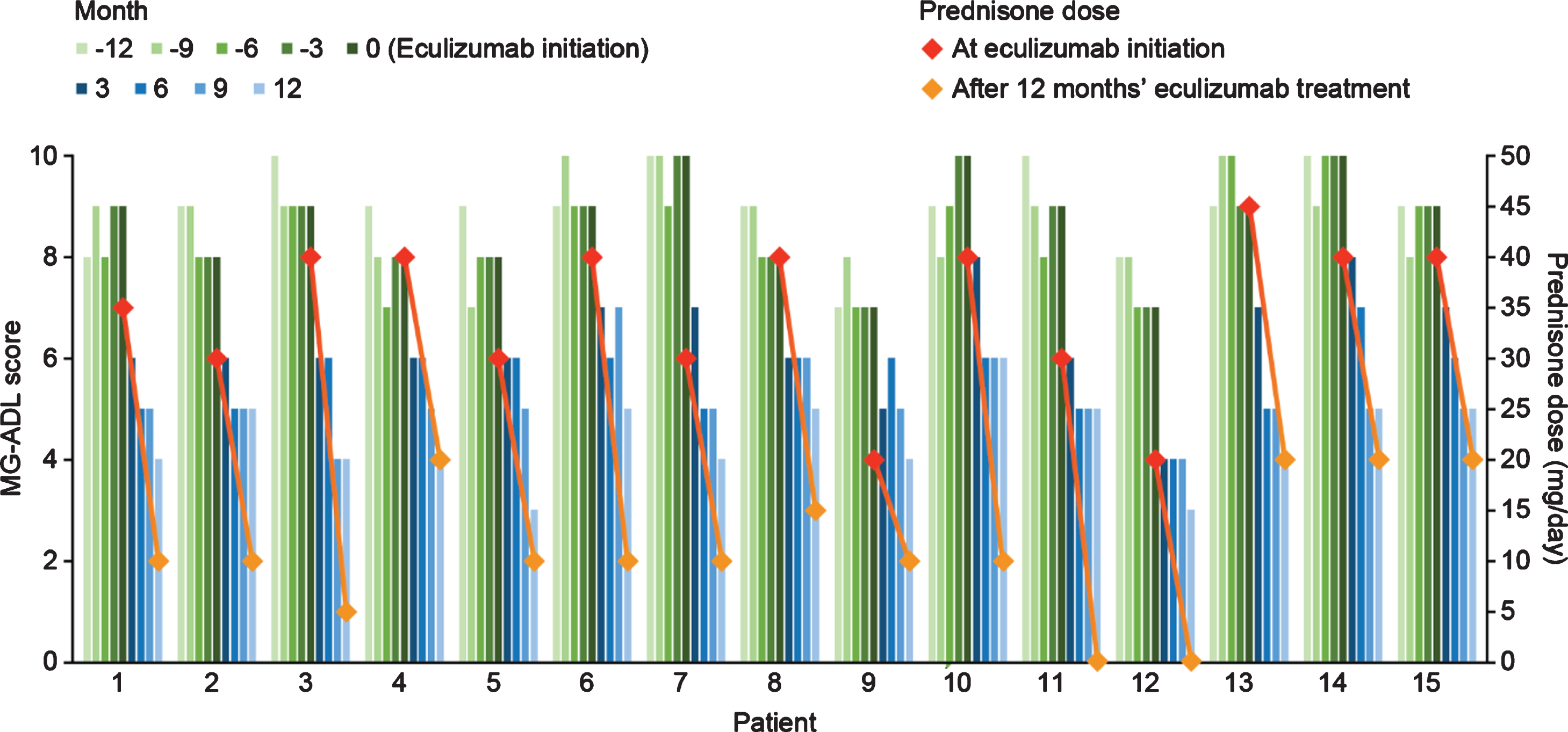
After eculizumab treatment was started, there was a gradual reduction in MG-ADL total scores up to the end of the study period (Month 12) in all patients (Fig. 1). The mean (SD) total MG-ADL score at the time of initiation of eculizumab therapy was 8.66 (0.94). Clinically meaningful reductions (≥2 points) in total MG-ADL scores were observed 3 months after initiation of eculizumab and were maintained through Month 12 in all patients [16]. The mean (SD) MG-ADL score was 6.33 (1.01), 5.53 (0.71), 5.13 (0.74), and 4.40 (0.83) at 3, 6, 9, and 12 months after eculizumab initiation, respectively.
Fig. 1
Quarterly MG-ADL scores 12 months before and after eculizumab initiation and prednisone doses at eculizumab initiation and 12 months after eculizumab initiation for individual patients with acetylcholine receptor antibody-positive generalized myasthenia gravis (n = 15). MG-ADL, Myasthenia Gravis–Activities of Daily Living.
Acute exacerbations
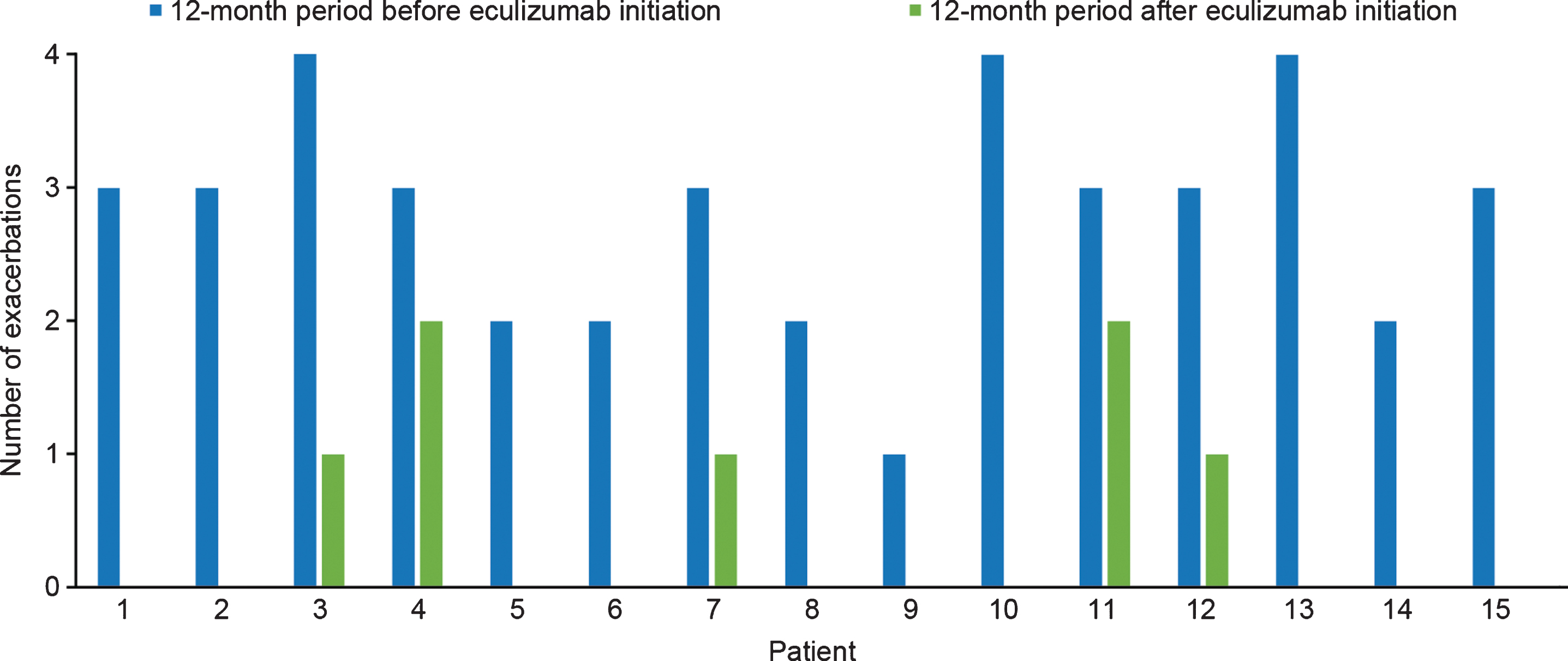
A total of 42 acute exacerbations were reported in 15 patients in the 12 months before initiation of eculizumab treatment, with all patients experiencing at least one exacerbation and a mean (SD) of 2.8 (0.83) exacerbations per patient per year. After initiation of eculizumab therapy, a total of seven exacerbations were reported in the patient group in 12 months, with a mean (SD) of 0.46 (0.71) exacerbations per patient per year. Ten of the patients had no acute exacerbations after eculizumab initiation (Fig. 2). In the 12 months following eculizumab initiation the mean number of exacerbations per patient was reduced by 2.33 (SD 0.98; p < 0.001 [two-tailed paired t test]) compared with the 12 months before eculizumab treatment.
Fig. 2
Number of acute exacerbations before and after eculizumab initiation for individual patients with acetylcholine receptor antibody-positive generalized myasthenia gravis (n = 15).
Single-breath count test
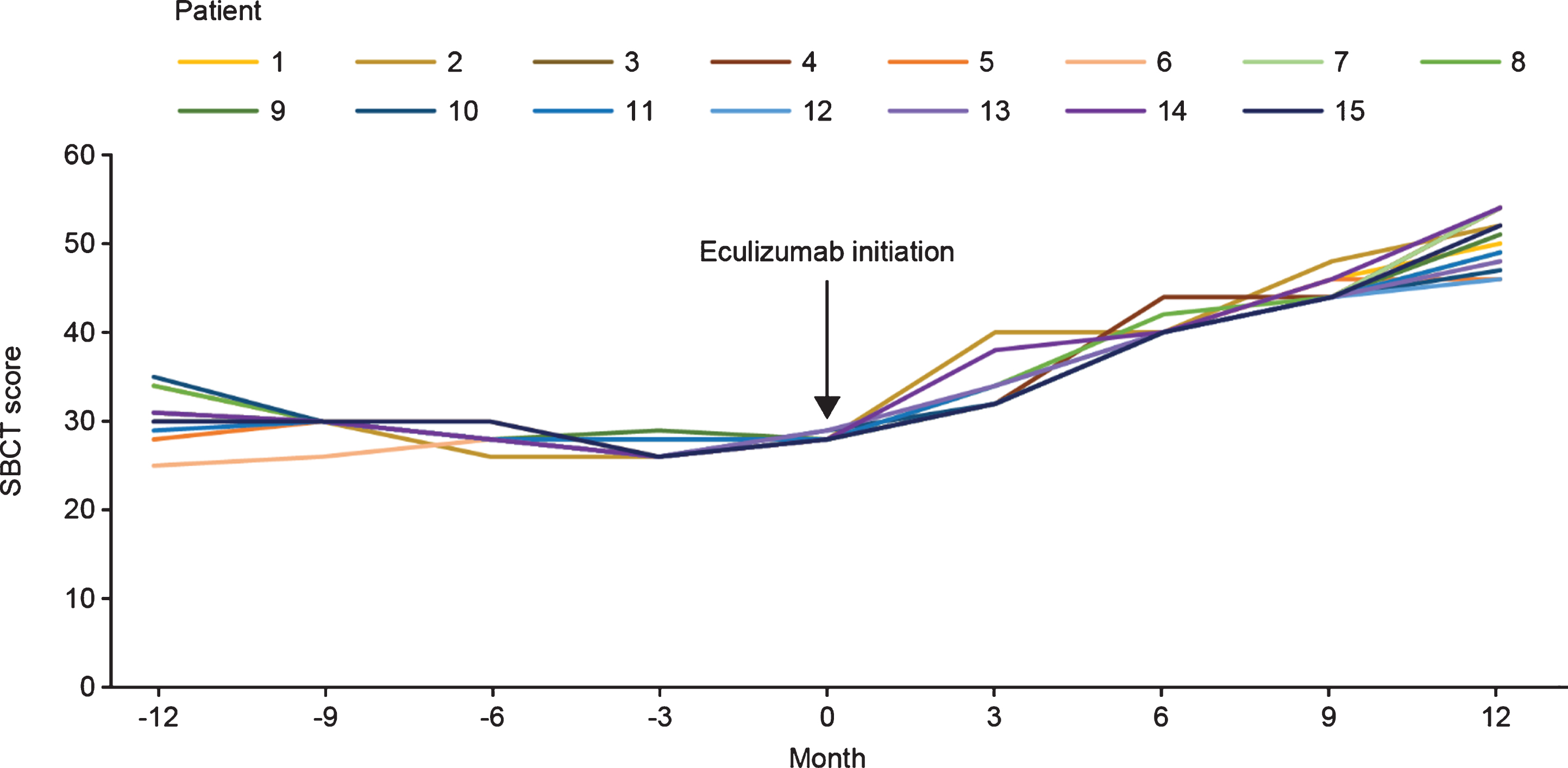
The mean (SD) SBCT score at the time of initiation of eculizumab therapy was 28.13 (0.33). After eculizumab was started, there was a gradual increase in SBCT total score up to Month 12 in all patients. Mean (SD) SBCT score was 33.33 (2.38), 40.40 (1.08), 44.67 (1.23), and 50.26 (2.86) at 3, 6, 9, and 12 months, respectively, after initiation of eculizumab therapy (Fig. 3).
Fig. 3
SBCT scores before and after eculizumab initiation for individual patients with acetylcholine receptor antibody-positive generalized myasthenia gravis (n = 15). SBCT, single-breath count test.
QMG-based assessments
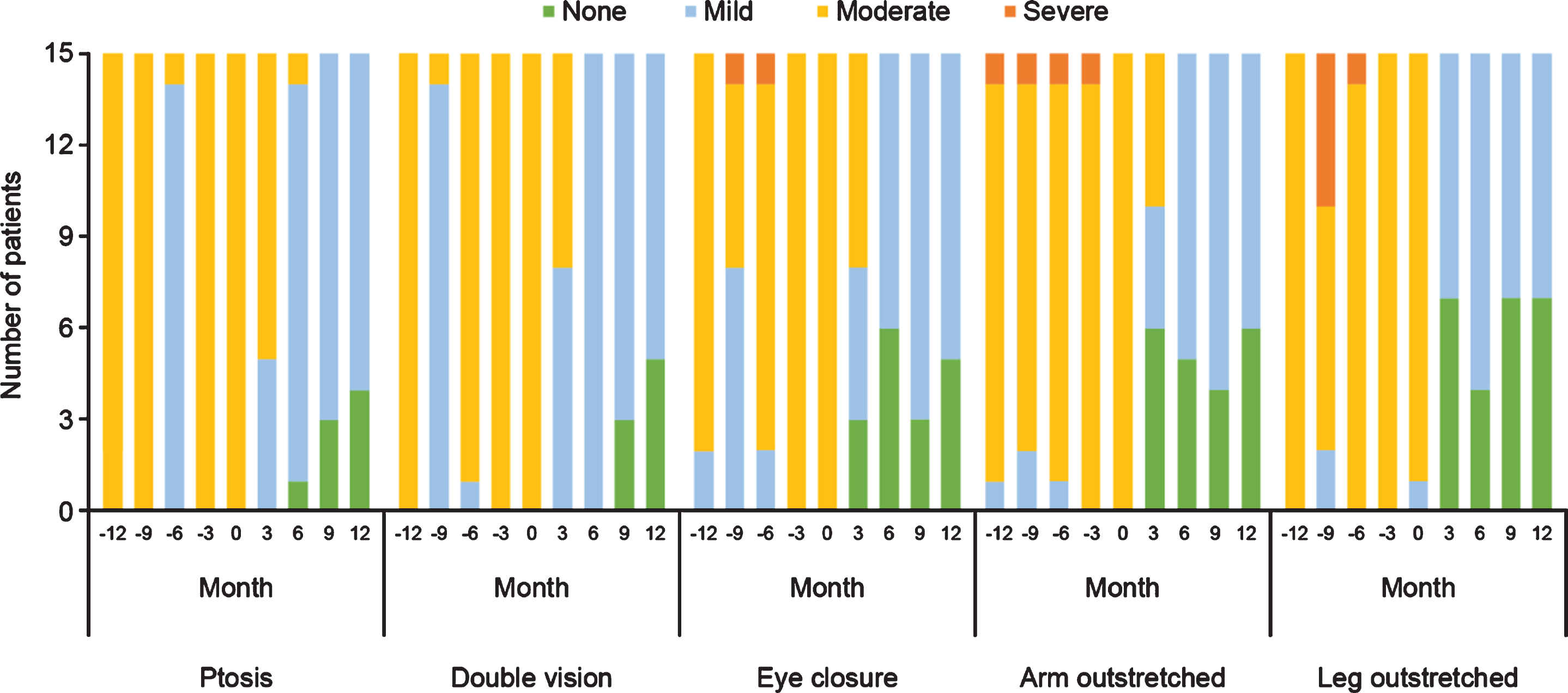
With regard to physical assessments, the severity of ptosis, double vision, eye closure, and limb-stretching impairment was relatively stable in the 12 months before eculizumab initiation, and improved during eculizumab treatment. All patients maintained or achieved a classification of ‘none’ or ‘mild’ in all parameters within 12 months of eculizumab therapy commencing and none experienced ‘severe’ symptoms. From 6 months onwards, 14 patients had ‘mild’ or ‘none’ ratings for all symptoms, with only one patient having ptosis assessments of ‘moderate’ at Month 6 followed by ‘mild’ and ‘none’ at Months 9 and 12, respectively (Fig. 4).
Fig. 4
Qualitative physical assessments of selected items from the QMG evaluation before and during treatment with eculizumab in patients with acetylcholine receptor antibody-positive generalized myasthenia gravis (n = 15). QMG, Quantitative Myasthenia Gravis.
Dose reductions of concomitant medications
The therapies being administered to patients at the beginning of eculizumab administration were IVIg (n = 6), prednisone (n = 15), pyridostigmine (n = 15), mycophenolate mofetil (n = 8), and azathioprine (n = 2). Twelve months after starting eculizumab, all patients had undergone reductions in concomitant medications. All patients had reduced their daily prednisone dose as follows: prednisone was reduced by 5 mg every month in nine patients, 5 mg every other month in three patients, 5 mg at 3 and 9 months in one patient, and 5 mg every month after 6 months on eculizumab by one of the patients who stopped without medical advice; the schedule of the other patient who decided to stop is unknown. After 12 months of treatment with eculizumab the mean prednisone dose was reduced by 23.33 mg/day (SD 6.17; p < 0.001 [two-tailed paired t test]) compared with the dose at the start of eculizumab treatment. All six patients receiving IVIg therapy before eculizumab initiation were successfully tapered off at a mean (SD) time of 5 (2.23) months after eculizumab initiation. In addition, nine patients had discontinued pyridostigmine by the end of 12 months of eculizumab therapy.
Two patients were receiving eculizumab alone by the end of the study, one for 2 months and one for an unknown length of time. At the start of eculizumab treatment one had been receiving IVIg every 5 weeks, prednisone 30 mg/day, and pyridostigmine 60 mg three times a day; the other patient had been receiving IVIg every 4 weeks, prednisone 20 mg/day, and pyridostigmine 60 mg three times a day. IVIg treatment was stopped under medical supervision at 3 months after eculizumab initiation in both cases. Both patients stopped pyridostigmine and prednisone without medical advice: one at a time unknown, while the other discontinued pyridostigmine after 3 months of eculizumab treatment and reduced their prednisone dose from 20 mg/day by 5 mg/month after 6 months of eculizumab therapy.
Safety
Ten patients had adverse events that were considered to be related to eculizumab during treatment. Three patients had myalgia, treated with acetamino-phen; three patients had upper respiratory tract infection; two patients had post-transfusion headache, treated with acetaminophen; one patient had nausea; and one patient had elevated aspartate transaminase (AST) and alanine transaminase (ALT) levels. All events except the AST/ALT elevations resolved completely and were considered mild in severity; no changes were made to the eculizumab treatment schedule as a result of adverse events.
DISCUSSION
In this real-world study, eculizumab was associated with improvements in all aspects of gMG assessed. Following initiation, clinically meaningful reductions were observed in total MG-ADL scores at 3 months, which were maintained through Month 12 in all patients. During the same period, there was a gradual increase in SBCT total score in all patients and acute exacerbations were significantly reduced, with ten patients exacerbation free. Due to time constraints the full QMG test is not performed in our clinic; however, we assessed the severity of the QMG components that could be readily evaluated: ptosis, double vision, eye closure, and impairment to holding the arm and leg outstretched. The severity of each of these symptoms was reduced with eculizumab treatment: all patients maintained or achieved a classification of ‘none’ or ‘mild’ in all parameters within 12 months of eculizumab therapy and none experienced ‘severe’ symptoms during this time.
Our study showed similar improvements in MG-ADL scores, SBCT scores, number of acute exacerbations, and severity of QMG parameters to those seen in previous studies of eculizumab in patients with treatment-refractory gMG [3, 7, 8, 17].
The 26-week Phase 3 REGAIN study demonstrated clinical benefits of eculizumab in patients with treatment-refractory AChR+ gMG [3, 18]. A greater proportion of patients who were treated with eculizumab than those receiving placebo achieved clinically meaningful responses, as determined by the MG-ADL total score (≥3-point improvement [60 vs 40%; p = 0.0229]) and the QMG total score (≥5-point improvement [45 vs 19%; p = 0.0018]), regardless of background immunosuppressant administration [3]. In patients who were switched from placebo in REGAIN to eculizumab in the extension study, a significant improvement from baseline in the MG-ADL score was seen as early as Week 1 of therapy, which was maintained up to at least 52 weeks (p < 0.0001) [18, 19]. An interim analysis of the open-label extension study reported that following treatment with eculizumab, improvements in activities of daily living, muscle strength, functional ability, and quality of life were maintained through 3 years [7]. Patients who had received placebo during REGAIN experienced rapid and sustained improvements during the open-label administration of eculizumab; 55.2% patients had improvements from baseline of ≥3 points in MG-ADL scores and 39.7% had ≥5-point improvement from baseline in QMG total score at a median of 22.7 months [7]. The acute-exacerbation rate for all reported events was reduced significantly by 75% compared with the year before patients entered the REGAIN study (p < 0.0001) [7].
In addition to improvements in efficacy outcomes, the current study reports a significantly decreased requirement for prednisone therapy within 12 months after eculizumab therapy, signaling that eculizumab therapy in patients with gMG may have immunosuppressant-sparing benefits, alongside its clinical effectiveness. All patients had their daily prednisone dose reduced and two stopped treatment completely of their own volition. Once patients experienced an improvement of ≥2 on their MG-ADL score that persisted for ≥3 months, IVIg was discontinued. All six patients initially receiving IVIg had stopped by 9 months after eculizumab initiation, the mean (SD) time to discontinuation being 5 (2.23) months. Nine patients had stopped pyridostigmine by 12 months after eculizumab initiation according to their own preference. At the end of 12 months’ therapy with eculizumab, two patients were being successfully treated with eculizumab monotherapy, one for 2 months and one for an unknown length of time. No unwanted effects were seen in any patient during reduction or discontinuation of concomitant medications.
Reduction in concomitant medications, especially immunosuppressants, is a critical goal in the treatment of gMG as they have well-documented unwanted side effects. Prolonged treatment with glucocorticoids can cause weight gain, diabetes, hypertension, osteoporosis, and cataracts [20, 21]. The short-term adverse effects of IVIg are numerous, including headache, fever, nausea, hypertension, dermatitis, pulmonary edema, venous thrombosis, aseptic meningitis, and hemolysis, with an increased risk of HIV or viral hepatitis in the long term [20, 22]. In addition, pyridostigmine is associated with gastrointestinal effects, miosis, heavy perspiration, and muscle cramps [20, 21].
Chronic IVIg administration in combination with eculizumab could potentially reduce serum levels of eculizumab [9]. Previous studies have avoided concomitant administration; for example, patients who had received IVIg within the 4 weeks before randomization were excluded from the REGAIN trial [3]. In a case series by Levine [17], eculizumab was initiated in 13 patients with treatment-refractory AChR+ gMG 10–14 days after their last IVIg infusion without safety concerns. Six patients receiving IVIg were included in the current study, enabling observation of the level and length of response to eculizumab before discontinuing IVIg; no adverse effects were observed in these patients over a mean of 5 months. Such information is a welcome addition to the sum of knowledge about the safety of co-administration of eculizumab and IVIg and provides useful information about when and how to stop IVIg in patients receiving both treatments.
Eculizumab was well tolerated in this study, reinforcing results seen in previous studies, in which minimal adverse events were reported in the majority of patients [3, 7, 8, 17–19, 23]. The good tolerability profile of eculizumab and its potential to reduce the burden of immunosuppressants make this therapy a promising addition in the treatment of MG.
This study has several limitations. This was a retrospective study of medical records at a single institution, with a small sample size and lack of control group, which limits the overall generalizability of the findings. Larger controlled studies with a more robust design are warranted to confirm these additional potential benefits of eculizumab in treatment-refractory AChR+ gMG, particularly to shed light on tapering schedules for other immunosuppressive therapies and the place of eculizumab monotherapy.
CONCLUSION
Our real-world study in 15 patients with treatment-refractory AChR+ gMG has further consolidated our understanding of the benefits of eculizumab observed in the REGAIN trial and other clinical studies. Over a period of 12 months, eculizumab improved key efficacy outcome measures and decreased immunosuppressant requirements, while being well tolerated.
ACKNOWLEDGMENTS
Editorial support was provided by Jackie Mayne of Anthemis Consulting Ltd, funded by Alexion Pharmaceuticals, Inc. Alexion Pharmaceuticals provided a medical-accuracy review of the final manuscript.
CONFLICT OF INTEREST
Nakul Katyal and Naureen Narula report no conflicts of interest. Raghav Govindarajan has served on advisory committees for Alexion Pharmaceuticals, Argenx, and Catalyst Pharmaceuticals, and is a member of the speakers’ bureau for Alexion Pharmaceuticals and Catalyst Pharmaceuticals.
REFERENCES
[1] | Juel VC , Massey JM . Myasthenia gravis. Orphanet J Rare Dis. (2007) ;2: :44. doi: 10.1186/1750-1172-2-44 |
[2] | Schneider-Gold C , Hagenacker T , Melzer N , Ruck T . Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. (2019) ;12: :1756286419832242. doi: 10.1177/1756286419832242 |
[3] | Howard JF Jr , Utsugisawa K , Benatar M , Murai H , Barohn RJ , Illa I et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) ;16: (12):976–86. doi: 10.1016/S1474-4422(17)30369-1 |
[4] | Dunand M , Botez SA , Borruat FX , Roux-Lombard P , Spertini F , Kuntzer T . Unsatisfactory outcomes in myasthenia gravis: influence by care providers. J Neurol. (2010) ;257: (3):338–43. doi: 10.1007/s00415-009-5318-9 |
[5] | Sanders DB , Evoli A . Immunosuppressive therapies in myasthenia gravis. Autoimmunity. (2010) ;43: (5-6):428–35. doi: 10.3109/08916930903518107 |
[6] | Thomas TC , Rollins SA , Rother RP , Giannoni MA , Hartman SL , Elliott EA , et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. (1996) ;33: (17-18):1389–401. doi: 10.1016/s0161-5890(96)00078-8 |
[7] | Muppidi S , Utsugisawa K , Benatar M , Murai H , Barohn RJ , Illa I , et al. Long-term safety and efficacy of eculizu-mab in generalized myasthenia gravis. Muscle Nerve. (2019) ;60: (1):14–24. doi: 10.1002/mus.26447 |
[8] | Howard JF Jr , Barohn RJ , Cutter GR , Freimer M , Juel VC , Mozaffar T et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. (2013) ;48: (1):76–84. doi: 10.1002/mus.23839 |
[9] | Alexion Pharmaceuticals Inc.: SOLIRIS® (Eculizumab) Injection Prescribing Information; 2019. https://alexion.com/Documents/Soliris_USPI.pdf Accessed October 2019. |
[10] | Alexion Europe: Soliris (eculizumab) Summary of Product Characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/soliris Accessed October 2019. |
[11] | Pharmaceuticals andMedicalDevicesAgency: Eculizumab (Genetical Recombination) SOLIRIS® for Intravenous Infusion 300mg (Japanese Package Insert), Revised: February 2019 (Version 11); 2019. http://www.soliris.jp/common/pdf/tempu_bunsho.pdf Accessed November 2019. |
[12] | Wolfe GI , Herbelin L , Nations SP , Foster B , Bryan WW , Barohn RJ . Myasthenia gravis activities of daily living profile. Neurology. (1999) ;52: (7):1487–9. doi: 10.1212/wnl.52.7.1487 |
[13] | Gajdos P , Tranchant C , Clair B , Bolgert F , Eymard B , Stojkovic T , et al. Treatment of myasthenia gravis exacerbation with intravenous immunoglobulin: a randomized doubleblind clinical trial. Arch Neurol. (2005) ;62: (11):1689–93. doi: 10.1001/archneur.62.11.1689 |
[14] | Barnett C , Katzberg H , Nabavi M , Bril V . The quantitative myasthenia gravis score: comparison with clinical, electrophysiological, and laboratory markers. J Clin Neuromuscul Dis. (2012) ;13: (4):201–5. doi: 10.1097/CND.0b013e31824619d5 |
[15] | Elsheikh B , Arnold WD , Gharibshahi S , Reynolds J , Freimer M , Kissel JT . Correlation of single-breath count test and neck flexor muscle strength with spirometry in myasthenia gravis. Muscle Nerve. (2016) ;53: (1):134–6. doi: 10.1002/mus.24929 |
[16] | Muppidi S , Wolfe GI , Conaway M , Burns TM . MG-ADL: still a relevant outcome measure. Muscle Nerve. (2011) ;44: (5):727–31. doi: 10.1002/mus.22140 |
[17] | Levine TD . Safety of an abbreviated transition period when switching from intravenous immunoglobulin to eculizumab in patients with treatment-refractory myasthenia gravis: a case series. Am J Case Rep. (2019) ;20: :965–70. doi: 10.12659/AJCR.916424 |
[18] | Dhillon S . Eculizumab: a review in generalized myasthenia gravis. Drugs. (2018) ;78: (3):367–76. doi: 10.1007/s40265-018-0875-9 |
[19] | Howard JF Jr , Wang JJ , O’Brien F , Mantegazza R . Efficacy of eculizumab on myasthenia gravis-activities of daily living and its respiratory, bulbar, limb and ocular domains in patients with ACHR+ refractory generalized myasthenia gravis. Muscle Nerve. (2017) ;56: (3):106. |
[20] | Melzer N , Ruck T , Fuhr P , Gold R , Hohlfeld R , Marx A ,et al.. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol. (2016) ;263: (8):1473–94. doi: 10.1007/s00415-016-8045-z |
[21] | Farmakidis C , Pasnoor M , Dimachkie MM , Barohn RJ , Treatment of myasthenia gravis. Neurol Clin. (2018) ;36: (2):311–37. doi: 10.1016/j.ncl.2018.01.011 |
[22] | Guo Y , Tian X , Wang X , Xiao Z , Adverse effects of immunoglobulin therapy. Front Immunol. (2018) ;9: :1299. doi: 10.3389/fimmu.2018.01299 |
[23] | Andersen H , Mantegazza R , Wang JJ , O’Brien F , Patra K , Howard JF Jr. Eculizumab improves fatigue in refractory generalized myasthenia gravis. Qual Life Res. (2019) ;28: (8):2247–54. doi: 10.1007/s11136-019-02148-2 |




