Decision-Making And Selection Bias in Four Observational Studies on Duchenne and Becker Muscular Dystrophy
Abstract
Background:
Natural history data are essential for trial design in Duchenne (DMD) and Becker muscular dystrophy (BMD), but recruitment for observational studies can be challenging.
Objective:
We reviewed reasons why patients or caregivers declined participation, and compared characteristics of participants and non-participants to assess possible selection bias in four observational studies, three on DMD and one on BMD.
Methods:
Three pediatric DMD studies focused on cross-sectional cognitive function and brain MRI (DMDbrain, n = 35 and DMDperfusion, n = 12), and on longitudinal upper extremity function and muscle MRI (DMDarm, n = 22). One adult BMD study assessed longitudinal functioning (n = 36). Considerations for non-participation were retrospectively reviewed from screening logs. Age, travel-time, DMD gene mutations and age at loss of ambulation (DMDarm and BMD study only), of participants and non-participants were derived from the Dutch Dystrophinopathy Database and compared using nonparametric tests (p < 0.05).
Results:
The perceived burden of the protocol (38.2%), use of MRI (30.4%), and travel-time to the study site (19.1%) were the most frequently reported considerations for non-participation. Only few patients reported lack of personal gain (0.0– 5.9%). Overall, participating patients were representative for the studied sub-populations, except for a younger age of DMDarm study participants and a complete lack of participants with a mutation beyond exon 63.
Conclusion:
Optimizing patient involvement in protocol design, improving MRI experiences, and integrating research into clinics are important factors to decrease burden and facilitate participation. Nationwide registries are essential to compare participants and non-participants and ensure representative observational research. Specific effort is needed to include patients with distal mutations in cognitive studies.
INTRODUCTION
Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are caused by mutations in the DMD gene [1]. This leads to absence of dystrophin in DMD, and to a truncated and partly functional protein in BMD muscles. These neuromuscular diseases form a spectrum in which DMD patients lose ambulation around their early teens, while BMD patients have a milder but more variable disease course [2, 3]. In both diseases, a higher prevalence of learning and behavioral disabilities has been reported [4– 6]. In DMD, this is associated with absence of different dystrophin isoforms in the brain [7, 8].
Although the first drugs in DMD have now received regulatory approval, there is no cure yet [9]. Currently, many studies worldwide are recruiting DMD patients simultaneously: 21 interventional clinical trials and 15 observational studies (ClinicalTrials.gov accessed on February 2nd 2020). BMD patients are being recruited for three interventional clinical trials and two observational studies worldwide (ClinicalTrials.gov accessed on February 2nd 2020). These clinical trials are challenging because of the rarity of the diseases and a variable rate of progression [9], which stresses the importance of detailed knowledge of the natural history [10]. The possibility to use historical controls reduces the required number of participants per study [11], but even further highlights the need for high quality natural history data. In observational studies however, direct benefit to patients is lacking, while the added burden of research on top of the disease and clinical care could be perceived as high. Knowledge of factors that influence the decision-making process for participation can be used when designing study protocols in order to increase the participation rate and avoid selection bias. Such detailed and high quality natural history data would enable their use for placebo arms, and for determination of primary and secondary outcome measures in interventional trials. While considerations for not participating have been described for interventional trials [12– 14], only one observational study reported on this topic [5].
In the present study, we reviewed the decision-making considerations reported by eligible patients and compared patient characteristics of participants and non-participants in three DMD and one BMD observational studies conducted at our institute.
METHODS
DMD and BMD patients were recruited in the following observational studies at the Leiden University Medical Center (LUMC): ‘Non-invasive assessment of brain involvement in DMD’ (DMDbrain; ABR number NL23184.058.09; onset of recruitment in 2010), ‘The background of the reduced cerebral blood flow in DMD’ (DMDperfusion; ABR number NL58182.058.16; onset of recruitment in 2017), ‘Upper extremity outcome measures in non-ambulant DMD patients’ (DMDarm; ABR number NL63133.058.17; onset of recruitment in 2018), and ‘The natural history study of BMD’ (BMD; ABR number NL50171.058.14; onset of recruitment in 2014). All studies are registered at ToetsingOnline (https://www.toetsingonline.nl). For recruitment, the Dutch Dystrophinopathy Database (DDD) was used (‘Epidemiology, natural course and registration of dystrophinopathies in the Netherlands’; ABR number NL21411.058.08) (3). This nationwide registry, initiated in 2008, provided the opportunity for all Dutch DMD and BMD patients to list their names and contact details together with details on comorbidities, medication use, disease history and current functional status. The local ethics committee approved all studies and the registry in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent had been obtained from all patients and from legal representatives for patients under 16 years of age.
DMDbrain – non-invasive assessment of brain involvement in DMD
Thirty-five DMD patients were recruited from the DDD and through the Duchenne Parent Project Netherlands (DPP NL) newsletter. Inclusion criteria were: male, genetically confirmed DMD patients ≥8 years old. Exclusion criteria were: MRI contra-indications such as scoliosis surgery, daytime artificial ventilation or the inability to lie supine for 45 minutes. Patients were included in the study from March 2010 until October 2012. The cross-sectional study design consisted of a single visit and included a one-hour neuropsychological assessment and two 30 minute MRI scans (at 3 Tesla and at 7 Tesla) of the brain. Results have previously been reported (15– 18).
DMDperfusion – the background of the reduced cerebral blood flow in DMD
Thirteen DMD patients were recruited from the DDD, the LUMC outpatient clinic, and through a poster at the DPP NL annual conference. Inclusion criteria were: ambulant male, genetically confirmed DMD patients ≥10 years old. Exclusion criteria were MRI contraindications, a medical history of cardiovascular disease, diabetes mellitus, neurological disease (other than DMD), recurrent syncope, and joint contractures preventing the use of the tilting table. Patients were included in the study beginning January 2017 and recruitment is ongoing. The cross-sectional protocol includes a one-and-a half hour tilting table experiment with transcranial doppler and blood pressure measurements, 30 minute (task-based) MRI of the brain and cerebral vasculature, brief neuropsychological assessment (20 minutes), and cardiac ultrasound if this was not available from a recent clinical care visit.
DMDarm – upper extremity outcome measures in non-ambulant DMD patients
Twenty-two DMD patients were recruited from the DDD, via Dutch neurologists and rehabilitation specialists, and through the Spierziekten Nederland (SN) website, the DPP NL website and Facebook page, and a poster at the DPP NL and SN annual conferences. Inclusion criteria were male, non-ambulant genetically confirmed DMD patients ≥8 years old. Exclusion criteria were: MRI contra-indications, exposure to an investigational drug ≤6 months prior to participation and recent (≤6 months) upper extremity surgery or trauma. Patients were included in the study from April 2018 until June 2019. This ongoing longitudinal study consists of three half-day visits at 0– 12– 18 months and the protocol includes functional upper extremity outcome measures and a 45 minute MRI scan of the upper extremity.
BMD – the natural history study of BMD
Thirty-six BMD patients were recruited from the DDD and the LUMC outpatient clinic. Inclusion criteria were male BMD patients ≥18 years old. BMD was defined as follows: an in-frame mutation in the dystrophin gene, or a reduced amount of dystrophin in a muscle biopsy, or an out-of-frame mutation with a mild disease course (>16 years old at loss of ambulation). Patients were included in the study from November 2014 until June 2016. The longitudinal study required four half-day to full-day visits at 0– 12– 24– 36 months including functional tests, cardiac ultrasound and pulmonary function tests, and a single neuropsychological assessment. Optionally, patients could also participate in the following sub studies: 1) yearly blood sample collection for biomarker studies, 2) muscle biopsies at one time-point, and 3) lower extremity muscle MRI at two time-points. Last follow-up visit took place in August 2019.
Data collection
Review of considerations for non-participation
All considerations for non-participation were obtained during the telephone calls used for the inclusion. For the DMDbrain study, the study information letter was sent first, and potential participants or their legal representatives were called within a few weeks to discuss inclusion. If patients or their legal representatives decided not to participate, they were not actively asked for reasons for non-participation as this could be perceived as pressure to participate. When they volunteered a reason, this was recorded. For the other three studies, the decision not to participate could either be made at the first telephone call, before the study information letter was sent, or at the second telephone call after reading the study information letter. At the time of these studies, more thorough implementation of Good Clinical Practice (GCP) guidelines led to more in depth logging of the screening and enrollment process. Therefore, information on considerations for non-participation in these three studies was actively requested, although patients were always allowed to not answer this question.
All considerations for non-participation that had been recorded in the screening and enrollment logs of all studies were gathered retrospectively by one observer (KJN) and checked by a second observer (ND). Patients or parents could provide one or more considerations, and these considerations were divided in the following groups: ‘Burden of protocol’, ‘Travel-time’, ‘Burden of clinical care’, ‘Other research’, ‘No advantage’, ‘Not interested’, ‘MRI’. Definitions and examples of these considerations can be found in Table 1.
Table 1
Definitions and examples of considerations for non-participation
| Consideration | Definition | Examples |
| Burden of protocol | All characteristics of the protocol, except the MRI, that could lead to a burden for patients or parents. | ∗Amount of time participation would cost patients or parents, needing to take time off from school or work. |
| ∗Amount of physical energy the study would cost. | ||
| ∗Psychological stress patients and parents already had due to the disease to which participation would add. | ||
| ∗Behavioral difficulties that would lead to strain of patients and parents when taking part in the study. | ||
| ∗Stress that neuropsychological testing might cause due to the potential diagnosis of a cognitive impairment. | ||
| Travel-time | The time needed for traveling. | ∗Travel-time needed when for instance private wheelchair transportation was used. |
| Burden of clinical care | Number of tests and visits already required for clinical care to which the study would add. | ∗Number of tests, cardiac MRIs and visits already required for clinical care which made some patients not want to undergo an extra MRI or go to the hospital for an extra visit for the study. |
| ∗In the Netherlands patients visit the hospital one average once a year, and upon publication of the revised standards of care in 2018, a cardiac MRI after the age of ten has been added to this. | ||
| Other research | Patients already having participated in previous or participating in current interventional trials or observational studies. | ∗Previous and current interventional trials. ∗Previous observational studies including MRI studies. |
| No advantage | Patient or parent did not want to participate due to a lack of potential personal benefit for the patient. | |
| Not interested | Patient was not interested to participate in research, but gave no further specification. | |
| MRI | Patient not wanting to undergo an MRI or a predicted difficulty for the patient to conform to the MRI protocol. | ∗Predicted difficulty for the patient to lie still or maintain the supine (DMDbrain and DMDperfusion) or lateral MRI position (DMDarm). |
| ∗If patients gave ‘MRI’ as consideration because they did not want to undergo an MRI, it was asked whether patients had previously undergone an MRI. |
Assessment of patient characteristics
Age for both participants and non-participants was defined as the age at which study information was received. For the DMDbrain study this exact date was unavailable for 30 subjects, resulting in a maximal uncertainty of nine months. Travel-time to the LUMC was derived with registered postal codes from the DDD, using ‘https://www.google.nl/maps’ and setting the date and time at a Monday in June 2019 outside rush hour.
All DMD gene mutations were derived from the DDD. For DMD, the mutation locations within the DMD gene predicted the absence of the following dystrophin isoforms in the brain: absence of only Dp427 (mutation in exon 1– 44), absence of Dp427 and possibly Dp140 (mutation in exon 45– 50), absence of Dp427 and Dp140 (mutation in exon 51– 62), and absence of Dp427, Dp140 and Dp71 (mutation in exon 63– 79) [7]. The same locations were used to group mutations in the BMD patients although a similar prediction of isoform expression cannot be made.
At the time of registration in the DDD, patients or their caregivers had received a general questionnaire about their disease, including a question concerning comorbidities. This self-reported neurological and psychiatric comorbidity was used in the analysis for the DMDbrain and BMD studies only, as these studies started less than five years after most patients registered in the DDD. Age at loss of ambulation was derived from the DDD and included in the analysis for the DMDarm and BMD studies only, as these were the studies assessing motor performance.
Statistical analysis
For each study, the considerations for non-participation were summed per consideration group and adjusted for the total number of participants from whom a consideration was recorded per study. To compare the incidences of the different considerations for non-participation over all studies, percentages from the different studies were averaged to get an overall percentage. The three considerations with the highest overall percentage are reported here.
Age, travel-time and age at loss of ambulation (for DMDarm and BMD), were compared between participants and non-participants per study using the Mann-Whitney U test. Presence of a distal mutation upstream of exon 51 or 63 was compared between participants and non-participants per study using the Fisher’s exact test. Statistical significance was set at p < 0.05. Bonferroni-Holm correction was used to correct for the multiple comparisons of presence of a distal mutation upstream of exon 51 or 63 within each observational study.
RESULTS
Participation
After pre-screening for age and diagnosis using the DDD, the patients who were reached by phone were registered per study as the first quantifiable step in the inclusion process (Table 2). The participation rate for the different studies was 35.4% (n = 35) for DMDbrain, 40.0% (n = 12) for DMDperfusion, 21.6% (n = 22) for DMDarm, and 48.6% (n = 36) for the BMD study. Characteristics of participants and non-participants for each study are shown in Table 3.
Table 2
Participation in the four observational studies
| Eligible and reached by phone | Did not meet inclusion criteria | Inclusion criteria met | Did not want to participate | Participated | % of patients willing to participate | |
| DMDbrain | 116 | 17 | 99 | 64 | 35 | 35.4% |
| DMDperfusion | 44 | 14 | 30 | 18 | 12 | 40.0% |
| DMDarm | 122 | 20 | 102 | 80 | 22 | 21.6% |
| BMD | 92 | 19 | 74 | 38 | 36 | 48.6% |
Table 3
Characteristics of participants and non-participants of the four observational studies
| DMDbrain | DMDperfusion | DMDarm | BMD | |
| (n = 35/n = 64) | (n = 12/n = 18) | (n = 22/n = 80) | (n = 36/n = 38) | |
| Age at study information, years | ||||
| participants | 12 (10– 15) | 10.6 (10.1– 11.8) | 13.2 (12.1– 16.1)∗ | 42.3 (31.5– 52.4) |
| non-participants | 13 (11– 15) | 11.3 (10.4– 12.7) | 16.1 (13.2– 20.4)∗ | 42.5 (33.7– 54.4) |
| Travel-time, minutes | ||||
| participants | 65 (35– 85) | 35 (22– 76)∗ | 73 (34– 86) | 48 (30– 80) |
| non-participants | 63 (40– 87) | 73 (40– 110)∗ | 65 (35– 85) n = 75 | 65 (45– 90) |
| Age at loss of ambulation, years | ||||
| participants | Not recorded | Not applicable | 11.5 (10.1– 13.1) | 32 (22– 41) n = 6 |
| non-participants | 11.0 (9.1– 12.5) n = 72 | 13 (11– 42) n = 13 |
Data are median (1st quartile; 3rd quartile). After the study acronym the total number of participants and non-participants is shown as follows: (participants/non-participants). In case of missing data the number of patients for whom the data was available was presented after the result with n = number. ∗=p-value <0.05 for difference between participants and non-participants per study.
Considerations for non-participation
Considerations for not participating in the four observational studies are shown in Fig. 1 and underlying data are given in Supplementary Tables 1, 2 and 3. The number of patients for whom a consideration for non-participation was recorded differed per study and was 44 (68.8%) for DMDbrain, 17 (94.4%) for DMDperfusion, 80 (100.0%) for DMDarm, and 29 (76.3%) for the BMD study. The consideration ‘Burden of protocol’ was provided most often by a mean of 37.9% (range 11.8– 55.2%) of responders. This was followed by ‘MRI’ reported by 30.0% (range 23.8– 41.2%) averaged over the three MRI studies, and ‘Travel-time’ reported by 19.0% (range 9.1– 26.3%). Regarding the three MRI studies, 40.5% gave ‘MRI’ as a reason to decline participation because of a predicted difficulty for the participant to conform to the MRI protocol, while 59.5% gave this consideration because they did not want to undergo an MRI. Of this last group, 62.5% noted that this was due to a previous MRI experience, which had either been scary, unpleasant or long. The consideration ‘No advantage’ was not provided in the DMDbrain and BMD studies, and only by 2.5% of the non-participants in the DMDarm and 5.9% in the DMDperfusion studies.
Fig. 1
Considerations for not participating in the four observational studies. The presented percentage of non-participants who provided a consideration is adjusted for the percentage of patients for whom a consideration for non-participation was recorded: 68.8% for DMDbrain (black), 94.4% for DMDperfusion (dark gray), 100.0% for DMDarm (gray), and 76.3% for the BMD study (light gray). ∗MRI was an optional part of the BMD study.
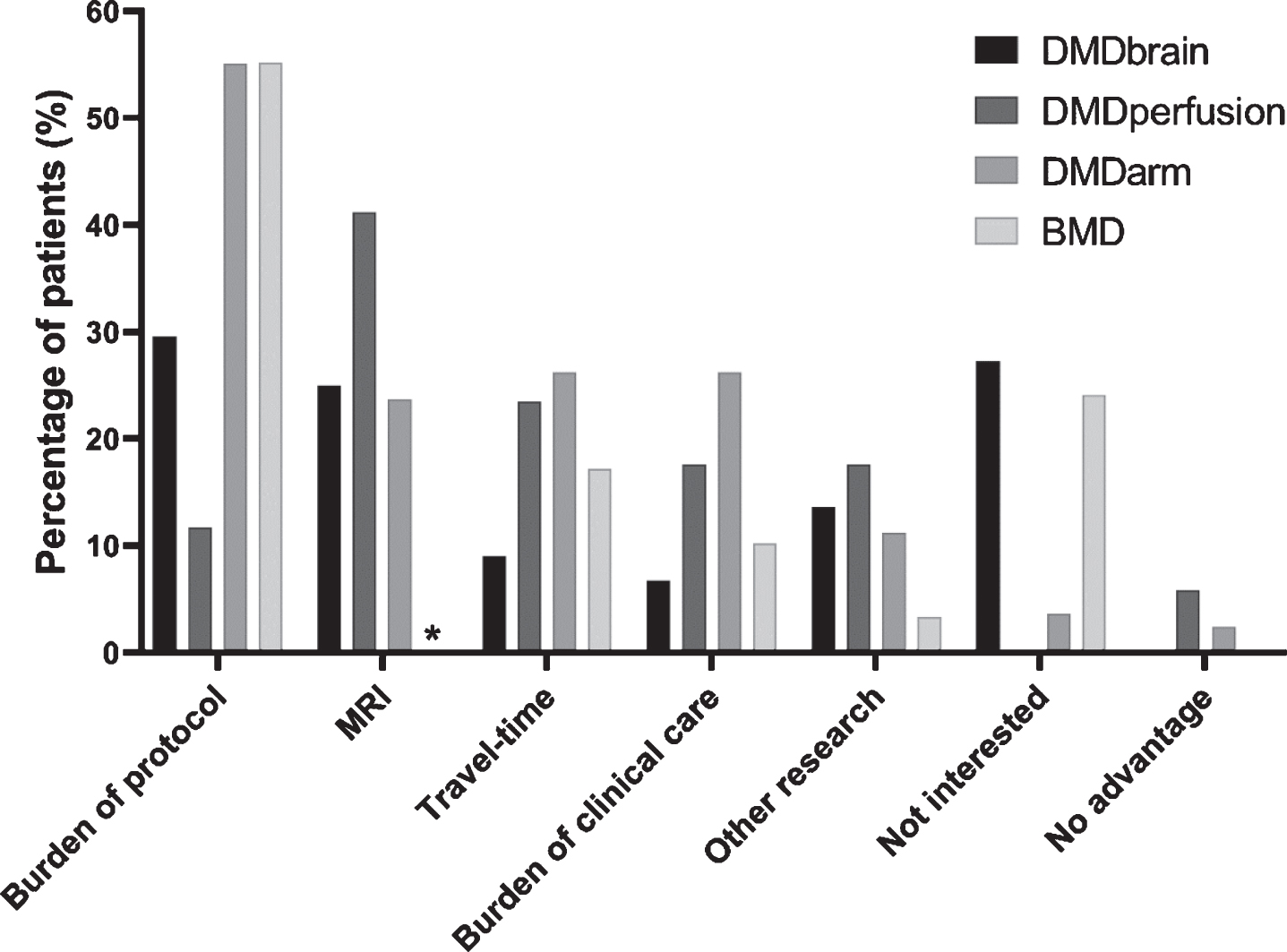
The number of participants who provided more than one consideration differed per study and was 3 for DMDbrain (6.8%), 3 for DMDperfusion (17.7%), 34 for DMDarm (42.5%) and 3 for the BMD study (10.3%). The most often occurring combinations of two considerations were: ‘Burden of the protocol’ and ‘Travel-time’ (n = 19), ‘Burden of the protocol’ and ‘Burden of clinical care’ (n = 10), and ‘Burden of the protocol’ and ‘MRI’ (n = 6).
Patient characteristics
Age at receipt of study information was comparable between participants and non-participants for all studies except the DMDarm study, where non-participants were 2.9 years older (p = 0.012; Table 3).
Travel-time only differed between participants and non-participants in the DMDperfusion study (p = 0.016; Table 3), where it was 38 minutes longer for non-participants. There was missing travel-time data for five DMDarm non-participants who had not registered in the DDD.
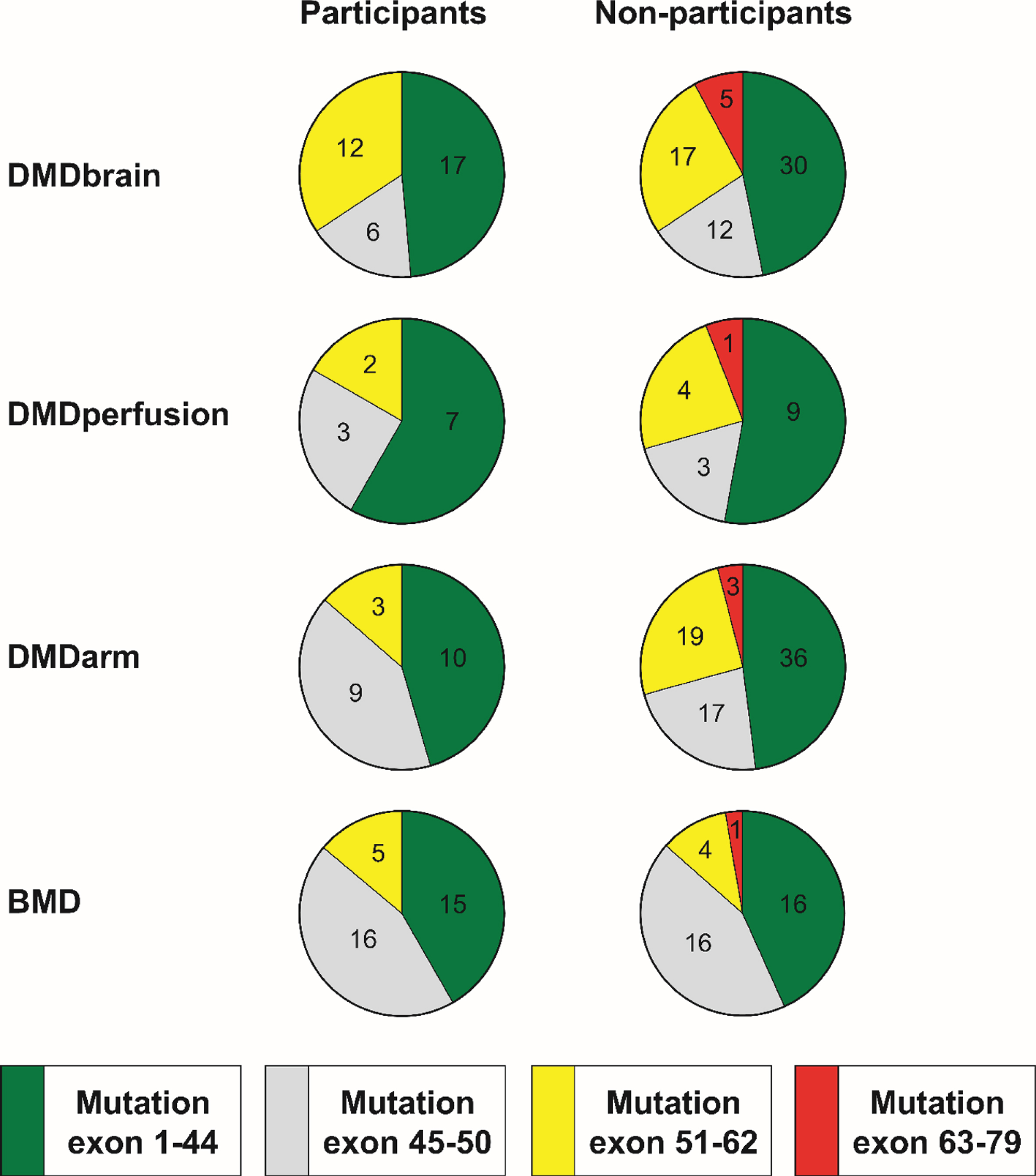
The presence of the different mutations in participants and non-participants of all studies is presented in Fig. 2. While there were some patients with exon 63– 79 mutations in the non-participant group of all studies, no patients with this mutation participated in any of the studies. However, the proportion of exon 63– 79 mutations or exon 51– 79 mutations did not differ significantly between participants and non-participants for any of the studies. There were missing mutation data for some non-participants: one (5.6%) from DMDperfusion whose mutation was not registered in the DDD, five (6.3%) from DMDarm who had not registered in the DDD, and one (2.6%) from the BMD study in whom the diagnosis had been based on a muscle biopsy only.
Fig. 2
Position of the mutation within DMD gene in participants and non-participants of the four observational studies. In DMD, this predicts the following isoforms to be absent: exon 1– 44 mutations affect Dp427, exon 45– 50 mutations affect Dp427 and possibly Dp140, exon 51– 62 mutations affect Dp427 and Dp140, and exon 63– 79 mutations affect Dp427, Dp140 and Dp71. The same locations were used to group mutations in the BMD patients although a similar prediction of isoform expression cannot be made. The numbers in the pie charts represent the number of participants and non-participants in the different studies that have a certain mutation.
Neurological and psychiatric comorbidity was self-reported by two participants (5.7%) or their caregivers in the DMDbrain study. For one patient this was autism spectrum disorder (ASD), intellectual disability, and oppositional defiant disorder (ODD), and for the other patient this was attention deficit hyperactivity disorder (ADHD). In the DMDbrain non-participant group, such comorbidity was self-reported by five patients (7.8%) or their caregivers: for one patient this was ASD, for two patients ASD and intellectual disability, for one patient ASD and attention deficit disorder (ADD), and for one patient ADHD. In the BMD study, similar comorbidity was self-reported by one participant (2.8%) as ADHD, while in the non-participants group five patients (13.2%) reported this: two reported ASD, two ADHD, and one dyslexia.
In the DMDarm study, age at loss of ambulation was comparable between participants and non-participants (p = 0.231; Table 3). For the BMD study, loss of ambulation had occurred in six (16.7%) participants and 14 (36.8%) non-participants. Here too, age at loss of ambulation was comparable between participants and non-participants (p = 0.333). Loss of ambulation data was missing for one non-participant from the BMD study and eight non-participants from the DMDarm study, of whom five had not registered in the DDD and three had not yet lost ambulation at the time of the DDD questionnaire.
DISCUSSION
In this study, we assessed which considerations played a role in decision-making for participation in four observational studies in DMD and BMD. We found that the following three considerations for not participating were most often provided: ‘Burden of protocol’, ‘MRI’, and ‘Travel-time’. Additionally, we compared patient characteristics between participants and non-participants, and showed that the included cohorts were representative for the currently studied variables, except for a younger age of participants in the DMDarm study and a lack of patients with the most distal mutations.
An important example of protocol burden was the amount of time that participation would cost patients or parents, needing to take time off from school or work. This was also underscored by a previous observational study in BMD patients that reported lack of time as main reason for non-participation [5]. The four studies at our institute mainly took place on week days, mostly outside of school hours. Many participating patients, their parents, and the local ethical committee also expressed that they preferred holidays and weekends, while other patients and parents actually preferred schooldays. Other examples of protocol burden in our studies were the amount of physical energy the study costs, extra psychological stress due to participation, and behavioral difficulties of patients that lead to extra strain when taking part. Both examples of study burden and the timing of study days could be ameliorated by obtaining the patient and parent perspective on the study design and its feasibility early-on in the protocol development. This is increasingly being advocated by many stakeholders in the field [19– 22], and was recently summarized in a report by the European Neuromuscular Centre (ENMC) [23].
A previous MRI experience was mentioned by a relatively large proportion of patients as the reason to decline participation. Examples were “a painful cardiac MRI”, “MRI was too long and very noisy”, “stress before the MRI due to ASD”, and “scared to fall off the MRI table”, highlighting that both the duration and the patient experience play a role. The duration of the MRI protocol can be reduced by scan acceleration techniques and combining different scan contrasts into one acquisition. While 30– 45 minutes of MRI was sometimes considered as long by our non-participants, another large MRI study showed that yearly MRI sessions of 75– 90 minutes in pediatric DMD patients are possible [24]. Understanding differences between these MRI studies and implementing corresponding adjustments could improve the MRI experience. To this end, more international collaboration and exchange of not only protocols, but also personal experience is needed. MRI vendors are developing methods to improve patient comfort as well, such as up to 99% sound reduction, calming visual themes projected on the MRI, using wider bores, and more comfortable coils [25, 26]. In research, stress reduction by showing videos, having a parent present in the MRI room during the scan, and using a mock scanner beforehand is already used. In regular clinical practice, this is more difficult due to time and budget constraints, potentially leading to negative subjective experiences. It is thus essential to dedicate specific attention to these vulnerable and rare patient categories when performing assessments in clinic.
‘Travel-time’ was also an important consideration for non-participation. While we reported travel-times outside of the rush hour for consistency and these may seem low, participants were often unable to avoid the busy rush hour of the Netherlands and this could cause the actual travel-times to be twice as high. For the DMDperfusion study, travel-times were also longer for non-participants than participants, which supports that travel-times influenced the decision to participate. In some observational studies the travel-time and time cost of participation has been minimized by performing these studies during regular visits as part of the outpatient clinical care [27– 30]. This can both reduce the burden of research and limit the number of visits to the hospital, and should therefore be explored for all future studies.
‘Other research’ was mentioned more often as consideration for non-participation in our DMD studies (13.6% in DMDbrain, 17.7% in DMDperfusion, 11.3% in DMDarm) compared to our BMD study (3.5%). At the time of inclusion a much larger number of studies had been performed or were ongoing in DMD compared to BMD, which is likely the reason for this difference. This also could have influenced the lower participation rates in the DMD studies compared to BMD.
Interestingly, the lack of personal gain was only rarely reported in our studies (2.5% in DMDarm, 5.9% in DMDperfusion). This is in contrast to the study by Peay et al. where an online survey was used to assess barriers for parents to have their child with DMD or BMD for the first time participate in an interventional trial [12]. Since most parents wanted their child to participate, only the barrier “my child could receive placebo” was deemed more true than untrue on a Likert-type scale. Personal gain therefore seems more important in the decision to participate in an interventional trial than an observational study.
Representativeness of included cohorts is of utmost importance in any study, both interventional and observational. Details on this are often lacking because most studies extensively describe the included, but not the excluded patients in primary tables. Although screening logs are getting more comprehensive due to GCP regulations, data are hardly ever analyzed or published. In our studies, assessment of selection bias was possible because of the DDD registry [3], as this contained characteristics of the non-participants. While participants in the DMDarm study were 2.9 years younger than the non-participants, age at loss of ambulation, as a proxy for rate of disease progression, was comparable. Therefore, participants were probably less progressed at the time of inclusion. While age and disease progression were taken into account in the design and analyses of this study, this bias could be problematic in a study targeting older and more severely affected patients. We also found that a few non-participants in all studies had an exon 63– 79 mutation, while no patients with this rare mutation participated in any of the studies. This was not statistically significant, which could have been caused by the rarity of this very distal mutation. DMD patients with exon 63– 79 mutations have absent Dp427, Dp140 and Dp71 in the brain and a higher occurrence of learning and behavioral disabilities [7, 8]. This could cause them to opt out of research in general or be unable to follow study specific instructions especially regarding the MRI protocols. To prevent selection bias, future observational studies in DMD and BMD should aim to include patients with exon 63– 79 mutations, especially when assessing cognitive and behavioral aspects of the diseases.
A more detailed study of selection bias would be possible if registries contained more extensive information than contact details and clinical information that is provided on inclusion. We are currently improving the DDD with an optional yearly update of important clinical items via a short questionnaire, and a formal collaboration with patient organizations to ensure nationwide participation. Future Dutch studies can use this extensive data to study selection bias in more detail. Furthermore, every observational study and interventional trial should use a similar registry to analyze selection bias in their cohort and publish the results.
There are limitations to our study. Due to the retrospective study design, considerations for not participating were not equally available for all studies. Furthermore, only limited data was available on non-participants via the previous version of the DDD registry. Finally, we reported a much lower prevalence of neurologic and psychiatric comorbidity (2.8% – 13.2%) compared to literature (up to 67% of BMD and 90% of DMD patients) [4, 6]. As our results were self-reported at the time of registering in the DDD, any diagnoses made after that have not been automatically recorded. This underestimation supports more consistent screening and assessment of cognitive diagnoses, as well as the need for regular updates of registries.
In summary, we reviewed the considerations provided for not taking part in four DMD and BMD observational studies and found that ‘Burden of protocol’, ‘ MRI’, and ‘Travel-time’ were most frequently reported. Participating patients were overall representative of the studied sub-populations, except for age in the DMDarm study which may point to the challenge of studying more advanced stages of these conditions and the lack of distal mutations upstream of exon 63.
Optimizing the involvement of patients while designing protocols, improving the MRI experience, and integrating observational research and clinical care are all factors that need to be addressed to facilitate and increase patient participation. Nationwide registries that enable the recruitment of patients are essential for the comparison of participants and non-participants to ensure that observational research is representative.
DECLARATIONS
Funding
The DMDbrain study was supported by Duchenne Parent Project Netherlands (DPP NL) and the Gratama Foundation (grant number 10.13). The DMDperfusion study was funded by DPP NL. The DMDarm study was supported by Stichting Spieren for Spieren (grant number SvS15). The BMD study was funded by the Netherlands Organization for Health Research and Development (ZonMw) (grant number 113302001).
Conflict of interest
The authors declare that they have no conflicts of interest in regard to this study.
ACKNOWLEDGMENTS
The authors are members of the European Reference Network for Rare Neuromuscular Diseases [ERN EURO-NMD].
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-200541.
Supplementary table 1, 2 and 3: All data on considerations for not participating in the four observational studies.
REFERENCES
[1] | Hoffman EP , Brown RH Jr , Kunkel LM . Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. (1987) ;51: (6):919–28. doi:10.1016/0092-8674(87)90579-4 |
[2] | Bushby KM , Gardner-Medwin D . The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I. Natural history. Journal of Neurology. (1993) ;240: (2):98–104. doi:10.1007/BF00858725 |
[3] | van den Bergen JC , Ginjaar HB , van Essen AJ , Pangalila R , de Groot IJ , Wijkstra PJ , et al. Forty-Five Years of Duchenne Muscular Dystrophy in The Netherlands. J Neuromuscul Dis. (2014) ;1: (1):99–109. |
[4] | Banihani R , Smile S , Yoon G , Dupuis A , Mosleh M , Snider A , et al. Cognitive and Neurobehavioral Profile in Boys With Duchenne Muscular Dystrophy. J Child Neurol. (2015) ;30: (11):1472–82. doi:10.1177/0883073815570154 |
[5] | Mori-Yoshimura M , Mizuno Y , Yoshida S , Ishihara N , Minami N , Morimoto E , et al. Psychiatric and neurodevelopmental aspects of Becker muscular dystrophy. Neuromuscul Disord. (2019) ;29: (12):930–9. doi:10.1016/j.nmd.2019.09.006 |
[6] | Young HK , Barton BA , Waisbren S , Portales Dale L , Ryan MM , Webster RI , et al. Cognitive and psychological profile of males with Becker muscular dystrophy. J Child Neurol. (2008) ;23: (2):155–62. doi:10.1177/0883073807307975 |
[7] | Taylor PJ , Betts GA , Maroulis S , Gilissen C , Pedersen RL , Mowat DR , et al. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS One. (2010) ;5: (1):e8803. doi:10.1371/journal.pone.0008803 |
[8] | Pane M , Lombardo ME , Alfieri P , D’Amico A , Bianco F , Vasco G , et al. Attention deficit hyperactivity disorder and cognitive function in Duchenne muscular dystrophy: Phenotype-genotype correlation. J Pediatr. (2012) ;161: (4):705–9 e1. doi:10.1016/j.jpeds.2012.03.020 |
[9] | Verhaart IEC , Aartsma-Rus A . Therapeutic developments for Duchenne muscular dystrophy. Nature Reviews Neurology. (2019) ;15: (7):373–86. doi:10.1038/s41582-019-0203-3 |
[10] | Straub V , Balabanov P , Bushby K , Ensini M , Goemans N , De Luca A , et al. Stakeholder cooperation to overcome challenges in orphan medicine development: The example of Duchenne muscular dystrophy. Lancet Neurol. (2016) ;15: (8):882–90. doi:10.1016/S1474-4422(16)30035-7 |
[11] | CDER, CBER. Duchenne Muscular Dystrophy and Related Dystrophinopathies: Developing Drugs for Treatment. Guidance for Industry. https://www.fda.gov/media/92233/download. Accessed on May 1, 2019. US Food & Drug Administration (FDA). 2018. |
[12] | Peay HL , Biesecker BB , Wilfond BS , Jarecki J , Umstead KL , Escolar DM , et al. Barriers and facilitators to clinical trial participation among parents of children with pediatric neuromuscular disorders. Clin Trials. (2018) ;15: (2):139–48. doi:10.1177/1740774517751118 |
[13] | Tromp K , Zwaan CM , van de Vathorst S . Motivations of children and their parents to participate in drug research: A systematic review. Eur J Pediatr. (2016) ;175: (5):599–612. doi:10.1007/s00431-016-2715-9 |
[14] | Akmatov MK , Jentsch L , Riese P , May M , Ahmed MW , Werner D , et al. Motivations for (non)participation in population-based health studies among the elderly - comparison of participants and nonparticipants of a prospective study on influenza vaccination. BMC Med Res Methodol. (2017) ;17: (1):18. doi:10.1186/s12874-017-0302-z |
[15] | Doorenweerd N , Dumas EM , Ghariq E , Schmid S , Straathof CS , Roest AA , et al. Decreased cerebral perfusion in Duchenne muscular dystrophy patients. Neuromuscul Disord. (2017) ;27: (1):29–37. doi:10.1016/j.nmd.2016.10.005 |
[16] | Doorenweerd N , Hooijmans M , Schubert SA , Webb AG , Straathof CS , van Zwet EW , et al. Proton Magnetic Resonance Spectroscopy Indicates Preserved Cerebral Biochemical Composition in Duchenne Muscular Dystrophy Patients. J Neuromuscul Dis. (2017) ;4: (1):53–8. doi:10.3233/JND-160201 |
[17] | Doorenweerd N , Straathof CS , Dumas EM , Spitali P , Ginjaar IB , Wokke BH , et al. Reduced cerebral gray matter and altered white matter in boys with Duchenne muscular dystrophy. Ann Neurol. (2014) ;76: (3):403–11. doi:10.1002/ana.24222 |
[18] | Straathof CS , Doorenweerd N , Wokke BH , Dumas EM , van den Bergen JC , van Buchem MA , et al. Temporalis muscle hypertrophy and reduced skull eccentricity in Duchenne muscular dystrophy. J Child Neurol. (2014) ;29: (10):1344–8. doi:10.1177/0883073813518106 |
[19] | Lochmuller H , Torrent IFJ , Le Cam Y , Jonker AH , Lau LP , Baynam G , et al. The International Rare Diseases Research Consortium: Policies and Guidelines to maximize impact. Eur J Hum Genet. (2017) ;25: (12):1293–302. doi:10.1038/s41431-017-0008-z |
[20] | EURORDIS Open Academy. https://openacademy.eurordis.org/. Accessed on March 26, 2020. EURORDIS-Rare Diseases Europe. |
[21] | Witteman HO , Chipenda Dansokho S , Colquhoun H , Fagerlin A , Giguere AMC , Glouberman S , et al. Twelve Lessons Learned for Effective Research Partnerships Between Patients, Caregivers, Clinicians, Academic Researchers, and Other Stakeholders. J Gen Intern Med. (2018) ;33: (4):558–62. doi:10.1007/s11606-017-4269-6 |
[22] | ’Patiëntenparticipatie’ (patient participation). https://subsidieportaal.spierfonds.nl/nl/soorten%20subsidies. Accessed on March 26, 2020. Princess Beatrix Muscle Foundation (Prinses Beatrix Spierfonds). |
[23] | Lochmuller H , Ambrosini A , van Engelen B , Hansson M , Tibben A , Breukel A , et al. The Position of Neuromuscular Patients in Shared Decision Making. Report from the 235th ENMC Workshop: Milan, Italy, January 19-20, 2018. J Neuromuscul Dis. (2019) ;6: (1):161–72. doi:10.3233/JND-180368 |
[24] | Forbes SC , Walter GA , Rooney WD , Wang DJ , DeVos S , Pollaro J , et al. Skeletal muscles of ambulant children with Duchenne muscular dystrophy: Validation of multicenter study of evaluation with MR imaging and MR spectroscopy. Radiology. (2013) ;269: (1):198–207. doi:10.1148/radiol.13121948 |
[25] | MRI in-bore experience. https://www.philips.nl/healthcare/educatie/technologies/mri/mri-in-bore-experience. Accessed on March 26, 2020. Philips Healthcare. |
[26] | MRI patient experience. https://www.siemens-healthineers.com/magnetic-resonance-imaging/patient-experience. Accessed on April 7, 2020. Siemens Healthineers. |
[27] | Pane M , Mazzone ES , Sivo S , Sormani MP , Messina S , D’Amico A , et al. Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes. PLoS One. (2014) ;9: (10):e108205. doi:10.1371/journal.pone.0108205 |
[28] | Goemans N , Vanden Hauwe M , Signorovitch J , Swallow E , Song J , Collaborative Trajectory Analysis P. Individualized Prediction of Changes in 6-Minute Walk Distance for Patients with Duchenne Muscular Dystrophy. PLoS One. (2016) ;11: (10):e0164684. doi:10.1371/journal.pone.0164684 |
[29] | Wong BL , Rybalsky I , Shellenbarger KC , Tian C , McMahon MA , Rutter MM , et al. Long-Term Outcome of Interdisciplinary Management of Patients with Duchenne Muscular Dystrophy Receiving Daily Glucocorticoid Treatment. J Pediatr. (2017) ;182: :296–303 e1. doi:10.1016/j.jpeds.2016.11.078 |
[30] | Muntoni F , Domingos J , Manzur AY , Mayhew A , Guglieri M , Network UKN , et al. Categorising trajectories and individual item changes of the North Star Ambulatory Assessment in patients with Duchenne muscular dystrophy. PLoS One. (2019) ;14: (9):e0221097. doi:10.1371/journal.pone.0221097 |




