Outcomes of peripherally inserted double lumen central catheter in very low birth weight infants
Abstract
OBJECTIVE:
In order to evaluate safety and usefulness of peripherally inserted double lumen central catheter (PIDLCC) in very low birth weight (VLBW) infants, outcomes of VLBW infants who had PIDLCC was studied.
SUBJECTIVE:
Thirty-nine VLBW infants who were admitted to our NICU in 2013 were retrospectively analyzed.
RESULTS:
Mean birth weight and gestational age was 1042.7 gram and 28.5 weeks, respectively. Total duration of indwelling PIDLCC was 1121 days (mean 28.5+18.2 days) with 85 PIDLCCs used. Dressing at the insertion site was done twice weekly with 10% povidone iodine. Four (10.3% with mean of 48 days) infants had catheter-related blood stream infection (CRBSI), with a 3.57 infection per 1000 catheter-day. The mean for days of PIDLCC in 35 infants without CRBSI was 26.5 days. Organisms isolated were Staphylococcus epidermidis, Staphylococcus aureus and Staphylococcus capitis ureolytic. Our study showed significant difference in the duration of indwelling catheter (p = 0.023) and intraventricular hemorrhage (p = 0.043) between the CRBSI group and non-CRBSI group. Five (12.8%) infants had abnormal thyroid function test, in which two infants required thyroxine supplementation upon discharge. However, duration of PIDLCC and abnormal thyroid function test was not statistically significant (p = 0.218). One (2.5%) infant died (death was not related to CRBSI). There was no serious adverse effects secondary to PIDLCC.
CONCLUSION:
It is concluded that the use and maintenance of PIDLCC is safe for VLBW infants, but close monitoring should be observed to detect early signs of infection.
1Introduction
Even though outcomes of VLBW infants have been improving constantly, care of prematurity has always been a challenge, especially in reducing morbidities [1]. Numerous factors play important roles in achievingthis, one of the factors is nutritional support. As these premature infants require longer duration to achieve full enteral feeding, they require central line for parenteral nutrition. Central lines are also used for intravenous medication, such as inotrope, as securing intravenous access is not easy especially in very low birth weight (VLBW) and extremely low birth weight (ELBW) infants.
Peripherally inserted double lumen central catheter (PIDLCC) is recently available in neonatal medicine, and commonly used in Neonatal Intensive Care Unit (NICU), apart from umbilical vein cannulation. Ever since venous cannulation using silicone catheter was introduced by Shaw in 1973, peripherally inserted central catheters (PICCs) have been the better choice compared to surgically inserted, tunneled central venous catheters. Both methods are equally safe and effective [2], and PICC is less costly and no surgical intervention needed [3]. Catheter-related blood stream infection (CRBSI) is a recognized complication [4–11]. CRBSI infection rate per 1000 catheter-day has been reported ranging from 1.1 to 8.3 [6, 9–11].
The objective of this study was to assess our current practice of PIDLCC, as well as complications related to it in VLBW infants in our NICU. In addition, we also wanted to identify risk factors for developing CRBSI in these infants.
2Patients and methods
2.1Study population
This study was conducted by reviewing medical records of 46 VLBW infants (birth weight <1500 gram) who were admitted to NICU, Tokyo Women’s Medical University (TWMU) Hospital in 2013, retrospectively.
We excluded 7 infants from this study because of reasons indicated in Fig. 1. A total of 85 PIDLCCs were used in 39 infants who were included in this study.
2.2PIDLCC
PIDLCC is a routine procedure in our NICU for all infants below 1500gram upon admission. ArgyleTM 27G×20 cm double lumen catheter (CovidienTM) was used in all infants who had PIDLCC (Fig. 2). This procedure was carried out while the infant was housed inside incubator, and performed by the attending doctor under standard barrier precautions (i.e. surgical mask, sterile gloves and small sterile drape). Sterile field for placement of items needed for this procedure was created by draping the table top with sterile sheet. The area around the insertion site was identified, disinfected with 10% povidone iodine (sterile packed 3 cotton balls in 10% povidone iodine manufactured by Hakujuji Co. Ltd.), and then draped with sterile sheet. Next the vein was cannulated using either 24G Jelco® Plus (Smiths Medical) or introducer provided by the kit. Bleeding from the insertion site was secured with sterile cotton ball. PIDLCC was secured with 3M Steri-StripTM. The area was then wrapped with sterile gauze. Placement of the tip was confirmed by radiograph.
Dressing was done twice a week (Tuesday and Friday) by the attending doctor. The cotton ball was removed and the area around the insertion site was disinfected with 10% povidone iodine. Steri- StripTM was only replaced if needed. New sterile gauze was then used to wrap the area.
PIDLCC was changed if there was obstruction, phlebitis, extravasation, leaking or suspected infection. PIDLCC was removed totally when infants had achieved enteral feeding of minimum 100 ml/kg/day or no longer needed for intravenous medication. All PIDLCC tips (approximately 3 cm) were routinely sent for culture upon removal.
2.3Definitions
CRBSI was diagnosed when a laboratory-confirmed blood culture grew the same organism as the catheter tip (PIDLCC must be in-situ for more than 2 days), and infant was clinically septic [4, 5, 7]. Phlebitis was diagnosed when erythema occurred at the surrounding area of the insertion site. Obstruction was diagnosed when there was difficulty in infusing medication via perfusor pump. Extravasation was diagnosed when the surrounding area of the insertion site became swollen.
2.4Statistical methods
Statistical Package for the Social Sciences (SPSS) version 17 was used to analyze our data. Chi-square test or Fisher’s Exact Test was performed for parametric data, and Mann-Whitney Test was performed for non-parametric data. A p value of less than 0.05 was considered statistically significant.
2.5Ethics approval
University’s Institutional Review Board approved the conduct of this retrospective study. Parental written consent (for all treatment and procedures) is a routine for all infants who are admitted to our NICU.
3Results
Table 1 shows summary of the clinical characteristics of VLBW neonates with PIDLCCs. A total of 39 neonates were included in this study, in which 85 PIDLCCs were used. Birth weight and gestational age ranged from 436g to 1496g, and 23 weeks to 34 weeks, respectively. Out of 39 neonates, 24 (61.5%) had PIDLCCs inserted more than once, due to suspected infection, phlebitis, extravasation and obstruction. Total duration of indwelling PICC was 1121 days, with mean of 28.5+18.2 days.
There was no statistically significant result found in maternal associated problems (Group B Streptococcus, meconium-stained amniotic fluid, preterm rupture of membranes, clinical chorioamnionitis, antenatal steroid use, non-reassuring fetal status) in regards to CRBSI (p > 0.05). Among the risk factors summarized in Table 2, only spontaneous intestinal perforation, intraventricular hemorrhage (IVH) and duration of indwelling PIDLCC were statistically significant with p value of 0.003, 0.043 and 0.023, respectively.
Catheter-related complications were listed in Table 3. Four neonates were diagnosed with CRBSI, which were 3.57 infections per 1000 catheter-day. Fourteen PIDLCCs (17%) were changed electively due to suspected infection. Organisms isolated were Staphylococcus epidermis (2 neonates), Staphylococcus aureus (1 neonate) and Staphylococcus capitis ureolytic (1 neonate). One infant who had CRBSI at Day 27 of life, died at Day 59 of life due to chronic lung disease with grade 4 IVH. There was no major catheter-related complications such as pericardial effusion, cardiac tamponade or pneumothorax documented.
There were 5 (12.8%) neonates who had abnormal thyroid function, in which 2 of them required thyroxine supplement upon discharge. None of these neonates’ mothers had abnormal thyroid function. Mean number of PIDLCC used in normal thyroid function group vs abnormal thyroid function group was 2+1.2 and 3.4+1.1, respectively (p = 0.022). Mean duration of indwelling PIDLCC in normal thyroid function group vs abnormal thyroid function group was 27.5+18.3 days and 38.2+16.2 days, respectively (p = 0.218).
4Discussion
Our infection rate of 3.57 infections per 1000 catheter-day (10.3%) was even lower as compared to those reported before in VLBW infants using single lumen catheters [9, 10]. This result was also almost similar to those previously reported studies that included all infants [6, 9–11], even though we did not change the catheter electively every fortnightly. Ohki et al. [11] recently reported that the CRBSI infection rate per 1000 catheter-day for 19 NICUs in Japan was 1.1 with maximum barrier precautions, 1.2 with standard precautions and 1.8 with no specific precautions. However, in that study, the authors included all neonates who had PICCs. Taking these background risks, the routine use of the double lumen catheter towards VLBW infants can be considered as a safe procedure, and recommended in NICU. Of course, the benefits of PIDLCC use among VLBW infants are invaluable.
It has been shown in some studies [9–12] that the risk of acquiring CRBSI has strong correlation with duration of the indwelling catheter. Our study also showed similar result even with the double lumen catheter. The difference in mean duration of indwelling PIDLCC between 2 groups was 11.5 days. Hsu et al. [9] reported that relative risk for PICC infection rate for the duration of 11–20 days and more than 20 days was 1.72 and 4.66, respectively as compared to less than 10 days duration. Due to the small study group, we observed that there was no statistically significant result (p = 0.057) when we further grouped the duration of indwelling catheters according to 2-weekly duration. As it was a small study group, there was no significant result between 2 groups in term of gestational age and birth weight.
We found that neonates who needed more than one PIDLCC inserted had longer duration of indwelling catheter, which increased the risk of catheter-related complication, particularly CRBSI (p = 0.04). However, there was no liner correlation between the duration of PIDLCC and the CRBSI rate. This result indicates there was no limited duration for PIDLCC use among VLBW infants.
There was also concern regarding use of 10% povidone iodine in neonates that could cause hypothyroidism [13, 14]. However Brown et al. [15] concluded that transient neonatal hypothyroidism was not a common cause of routine skin cleansing with povidone iodine in North America, where iodine-deficiency was not an issue. In addition, Majidipour et al. [16] showed 10% povidone iodine was more effective on reducing bacterial skin colonies as compared to chlorhexidine 2% . There was also concern regarding contact dermatitis with chlorhexidine in VLBW infants [17–19]. From our study, we found that by routinely dressing the insertion site twice weekly with 10% povidone iodine, the incidence of hypothyroidism in those neonates was not statistically significant. There was no skin changes seen as well. Even though there was a significant result in the mean number of PIDLCC used with abnormal thyroid function, we attributed it to the wider area exposed to 10% povidone iodine as compared to only dressing alone.
In study conducted by Linder et al. [20] it was concluded that IVH was associated with early sepsis and failure to give antenatal steroid treatment. In our study, there were 6 neonates with IVH, in which 2 had CRBSI (one of them had early sepsis). Out of the 6, 3 of them had low Apgar score of below 5 at 5 minutes of life (23 weeker 436g, 25 weeker 656g and 26 weeker 1031g). Our study showed significant result between IVH and CRBSI, and the relative risk in developing IVH in CRBSI group as compared to non-CRBSI group was 5.5. As IVH is inversely related with gestational age, the adjusted relative risk by gestational age was further increased to 6.7.
Adrenal insufficiency of prematurity (AOP) which occurred in 4% of VLBW infants in Japan as reported in Masumoto et al. [21] AOP causes late-onset circulatory collapse (LCC) that present after one week of life, in which a replacement dose of hydrocortisone (1-2mg/kg/dose) is administered. From our study, there was only 1 infant (3.8%) out of 26 infants diagnosed with LCC had CRBSI. The use of glucocorticoid in these infants did not increase the risk of CRBSI (p = 0.062).
All infants in this study received total parenteral nutrition through the double lumen catheter. This is the major advantage of the double lumen catheter. None of the infants developed necrotizing enterocolitis. One infant (25%) with CRBSI had spontaneous intestinal perforation in the first week of life, which was statistically significant (p = 0.003). This infant had early onset sepsis and was very ill, as compared to the other 3 infants who did not develop early onset sepsis.
Our study was conducted retrospectively and limited by its small study group. Hence a study conducted prospectively with a larger study group would be more informative.
In conclusion, our study demonstrated that the use of the double lumen catheter is safe in VLBW infants, but close monitoring should be observed to detect early signs of infection.
References
[1] | Kusuda S , Fujimura M , Uchiyama A , Totsu S , Matsunami K , Neonatal Research Network, Japan. Trends in morbidity and mortality among very low birth weight infants from to in Japan. Pediatr Res (2012) ;72: :531–538. |
[2] | Hosseinpour M , Mashadi MR , Behdad S , Azarbad Z . Central venous catheterization in neonates: Comparison of complications with percutaneous and open surgical methods. J Indian Assoc Pediatr Surg (2011) ;16: (3):99–101. |
[3] | Shulman RJ , Pokorny WJ , Martin CG , Petitt R , Baldaia L , Roney D . Comparison of percutaneous and surgical placement of central venous catheters in neonates. J Pediatr Surg (1986) ;21: (4):348–350. |
[4] | Centers for Disease Control and Prevention’s National Healthcare Safety Network Device-associated Module CLABSI January 2014. http://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf. |
[5] | Dobbins BM , Kite P , Wilcox MH . Diagnosis of central venous catheter related sepsis - a critical look inside. J Clin Pathol (1999) ;52: :165–172. |
[6] | Taylor JE , McDonald SJ , Tan K . A survey of central venous catheter practices in Australian and New Zealand tertiary neonatal units. Aust Crit Care (2014) ;27: :36–42. |
[7] | Abad CL , Safdar N . Catheter-related bloodstream infections. Infectious Disease Special Edition (2011) ;84–98. |
[8] | Lee JH . Catheter-related bloodstream infections in neonatal intensive care units. Korean J Pediatr (2011) ;54: (9):363–367. |
[9] | Hsu JF , Tsai MH , Huang HR , Lien R , Chu SM , Huang CB . Risk factors of catheter-related bloodstream infection with percutaneously inserted central venous catheters in very low birth weight infants: A center’s experience in Taiwan. Pediatr Neonatol (2010) ;51: (6):336–342. |
[10] | Jha K , Joseph T , Jacobs NM , Pyati S , Khilfeh M . Very low birth weight infants (<g) with indwelling peripherally inserted central venous catheters: Colonization and risk of nosocomial bloodstream infections. Neonatology Today (2014) ;9: (1):1–6. |
[11] | Ohki Y , Maruyama K , Harigaya A , Kohno M , Arakawa H . Complications of peripherally inserted central catheter in Japanese neonatal intensive care units. Pediatr Int (2013) ;55: :185–189. |
[12] | Milstone AM , Reich NG , Advani S , et al. Catheter dwell time and CLABSIs in neonates with PICCs: A multicentre cohort study. Pediatricse (2013) ;132: :1609–1615. |
[13] | Yilmaz D , Tezic HT , Zorlu P , Firat S , Bilaloglu E , Kutlu AO . Single dose povidone-iodine on thyroid functions and urinary iodine excretion. Indian J Pediatr (2003) ;70: :675–677. |
[14] | Pinsker JE , McBayne K , Edwards M , Jensen K , Crudo DF , Bauer AJ . Transient hypothyroidism in premature infants after short-term topical iodine exposure: An avoidable risk? Pediatr Neonatol (2013) ;54: :128–131. |
[15] | Brown RS , Bloomfield S , Bednarek FJ , Mitchell ML , Braverman LE . Routine skin cleansing with povidone-iodine is not a common cause of transient neonatal hypothyroidism in North America: A prospective controlled study. Thyroid (1997) ;7: (3):395–400. |
[16] | Majidipour N , Abdeyazdan Z , Zargham-Boroujeni A . Chlorhexidine or povidone-iodine on skin colonization in neonates? Iran J Nurs Midwifery Res (2013) ;18: (1):54–58. |
[17] | Garland JS , Alex CP , Mueller CD , et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics (2001) ;107: :1431–1436. |
[18] | Garland JS , Alex CP , Uhing MR , Peterside IE , Rentz A , Harris MC . Pilot trial to compare tolerance of chlorhexidine gluconate to povidone-iodine antisepsis for central venous catheter placement in neonates. J Perinatol (2009) ;29: :808–813. |
[19] | Linder N , Prince S , Barzilai A , et al. Disinfection with 10% povidone-iodine vs 0.5% chlorhexidine gluconate in 70% isopropanol in the neonatal intensive care unit. Acta Pediatr (2004) ;93: :205–210. |
[20] | Linder N , Haskin O , Levit O , et al. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: A retrospective case-control study. Pediatricse (2003) ;111: :590–595. |
[21] | Masumoto K , Kusuda S , Aoyagi H , et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr Res (2008) ;63: (6):686–690. |
Figures and Tables
Fig.1
Study population of TWMU from 1st January 2013 to 31st December 2013.
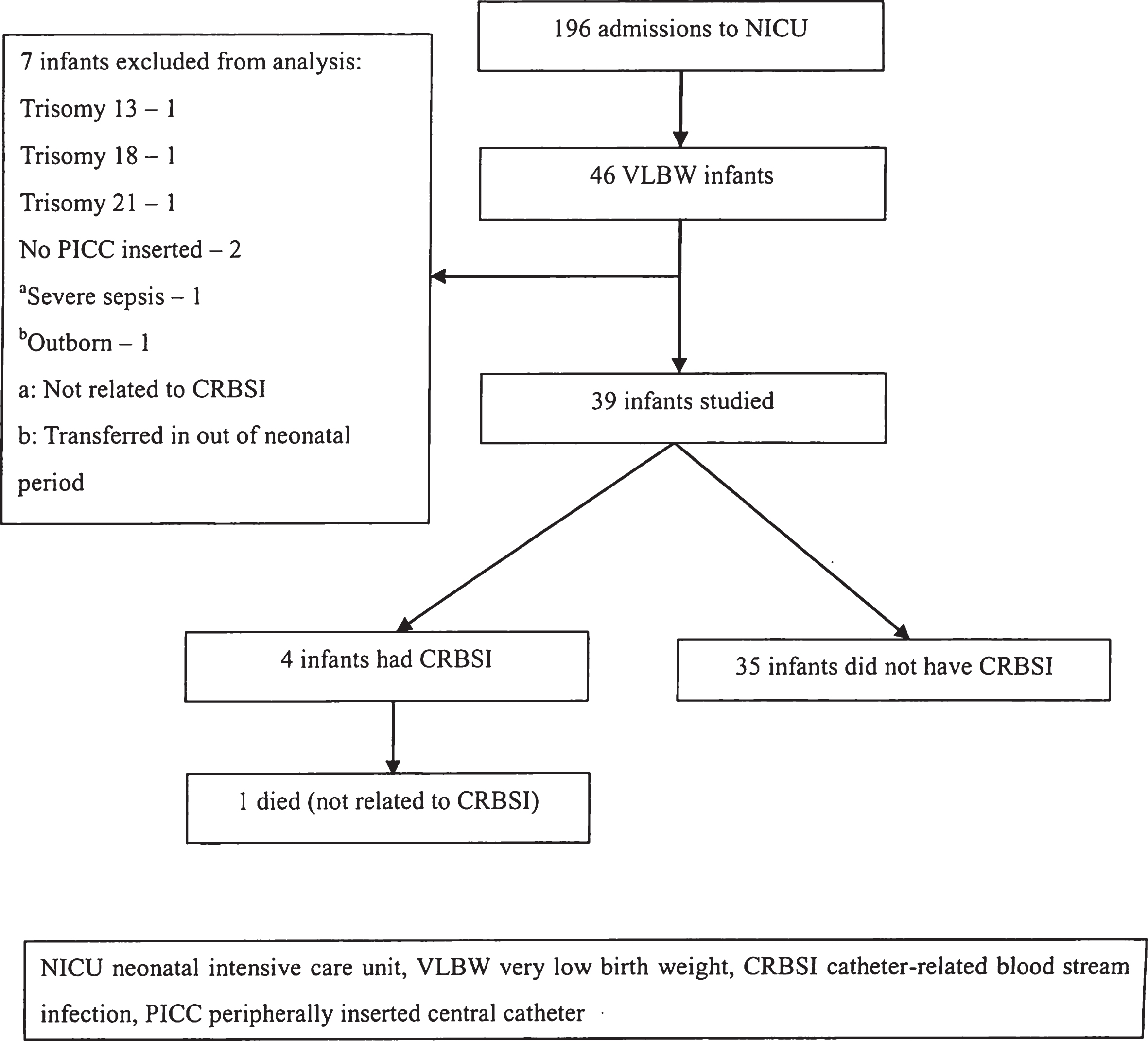
Fig.2
Double lumen catheter (Argyle™).
Table 1
Summary of study group
| CRBSI n (%) | Total n (%) | p value | ||
| Yes | No | |||
| Gender: | ||||
| Male | 2 (10) | 18 (90) | 20 (51.3) | 0.957 |
| Female | 2 (10.5) | 17 (89.5) | 19 (48.7) | |
| Mean birth weight (g) | 842.7±163.7 | 1065.5±300.2 | 0.156 | |
| Birth weight (g) | ||||
| <750 | 1 (12.5) | 7 (87.5) | 8 (20.5) | 0.224 |
| 750–999 | 2 (22.2) | 7 (77.8) | 9 (23.1) | |
| 1000–1249 | 1 (9.1) | 10 (90.9) | 11 (28.2) | |
| 1250–1499 | 0 | 11 (100) | 11 (28.2) | |
| Mean gestational age (week) | 27.5±2.6 | 28.7±2.4 | 0.362 | |
| Gestational age (week) | ||||
| <24 | 0 | 3 (100) | 3 (7.7) | 0.348 |
| 25–26 | 2 (50) | 2 (50) | 4 (10.3) | |
| 27–28 | 1 (10) | 9 (90) | 10 (25.6) | |
| 29–30 | 0 | 14 (100) | 14 (35.9) | |
| 31–32 | 1 (14.3) | 6 (85.7) | 7 (17.9) | |
| 32–34 | 0 | 1 (100) | 1 (2.6) | |
| Growth: | ||||
| AFD | 1 (4.2) | 23 (95.8) | 24 (61.5) | 0.113 |
| LFD | 3 (20) | 12 (80) | 15 (38.5) | |
| Median Apgar score at 1 min (25thQ –75thQ) | 5 (2–7) | 7 (5–8) | 0.107 | |
| Median Apgar score at 5 min (25thQ –75thQ) | 6 (3–9) | 8 (7–9) | 0.314 | |
CRBSI catheter-related blood stream infection, n number, g gram, AFD appropriate for date, LFD light for date.
Table 2
Risk factors
| RDS: | ||||
| Yes | 3 (14.3) | 18 (85.7) | 21 (53.8) | 0.37 |
| No | 1 (5.6) | 17 (94.4) | 18 (46.2) | |
| PDA: | ||||
| Yes | 2 (14.3) | 12 (85.7) | 14 (35.9) | 0.535 |
| No | 2 (8) | 23 (92) | 25 (64.1) | |
| # Indomethacin: | ||||
| Yes | 3 (17.6) | 14 (82.4) | 17 (43.6) | 0.181 |
| No | 1 (4.5) | 21 (95.5) | 22 (56.4) | |
| IVH: | ||||
| Yes | 2 (33.3) | 4 (66.7) | 6 (15.4) | 0.043 |
| No | 2 (6.1) | 31 (93.9) | 33 (84.6) | |
| LCC: | ||||
| Yes | 1 (3.8) | 25 (96.2) | 26 (66.7) | 0.062 |
| No | 3 (23.1) | 10 (76.9) | 13 (33.3) | |
| Early onset sepsis: | ||||
| Yes | 1 (50) | 1 (50) | 2 (5.1) | 0.057 |
| No | 3 (8.1) | 34 (91.9) | 37 (94.9) | |
| NEC: | ||||
| Yes | 0 | 0 | 0 | NA |
| No | 4 (10.3) | 35 (89.7) | 39 (100) | |
| Spontaneous intestinal perforation: | ||||
| Yes | 1 (100) | 0 | 1 (2.6) | 0.003 |
| No | 3 (7.9) | 35 (92.1) | 38 (97.4) | |
| aMean days to achieve | 3 (7.9) 36.7±32.7 | 35 (92.1) 24.7 ±16.4 | 38 | 0.270 |
| enteral feeding 100 ml/kg/day | ||||
| Mean duration of | 48±17.6 | 26.5±17.2 | 0.023 | |
| indwelling PIDLCC (day) | ||||
| Duration of indwelling PIDLCC (day) | ||||
| 1–14 | 0 | 7 (100) | 7 (17.9) | 0.057 |
| 15–28 | 1 (6.3) | 15 (93.8) | 16 (41) | |
| 29–42 | 0 | 8 (100) | 8 (20.5) | |
| 43–56 | 2 (50) | 2 (50) | 4 (10.3) | |
| 57–70 | 1 (33.3) | 2 (66.7) | 3 (7.7) | |
| 71–85 | 0 | 1 (100) | 1 (2.6) | |
| Mean number of PIDLCC used/patient | 3.75±1.7 | 2±1.1 | 0.009 | |
| Number | ||||
| 1 | 0 | 15 (100) | 15 (38.5) | 0.04 |
| 2 | 1 (8.3) | 11 (91.7) | 12 (30.8) | |
| 3 | 1 (20) | 4 (80) | 5 (12.8) | |
| 4 | 1 (20) | 4 (80) | 5 (12.8) | |
| 5 | 0 | 1 (100) | 1 (2.6) | |
| 6 | 1 (100) | 0 | 1 (2.6) |
RDS respiratory distress syndrome, PDA patent ductus arteriosus, IVH intraventricular hemorrhage, LCC late-onset circulatory collapse, NEC necrotizing enterocolitis, PIDLCC peripherally inserted double lumen central catheter, () number in percentage, # 2 had indomethacin for IVH prophylaxis, a1 was excluded because did not achieve enteral feeding 100 ml/kg/day.
Table 3
Catheter-related complications
| Complications | n (%) | n/1000 catheter-days |
| CRBSI | 4 (10.3) | 3.57 |
| Phlebitis | 10 (12) | 8.92 |
| Obstruction | 8 (9) | 7.14 |
| Extravasation | 12 (14) | 10.7 |
Total duration of indwelling PIDLCC: 1121 days, Number PDLICC used: 85.




