Proceedings of the 15th International Newborn Brain Conference: Neuro-imaging studies
Consensus brain injury classification predicts long-term outcomes in hypoxic ischemic encephalopathy infants after therapeutic hypothermia
Doctor Alia Embaireeg1, Doctor Sarfaraz Momin1, Doctor Prashanth Murthy1, Doctor Amina Benlamri1,3, Doctor Elsa Fiedrich1,3, Doctor Lara Leijser1, Doctor Hussein Zein1, Doctor James Scott2, Doctor Zarina Assis2, Doctor Sujith Gurram Venkata1, Selphee Tang3, Jun Li4, Doctor Khorshid Mohammad1
1Department of Pediatrics, Section of Neonatology, University of Calgary , 2Department of Diagnostic Imaging, Division of Neuroradiology, University of Calgary , 3Department of Pediatrics, Neonatal Follow - Up Clinic, Alberta Children’s Hospital, University of Calgary, 4Cumming School of Medicine, University of Calgary
BACKGROUND: Hypoxic Ischemic Encephalopathy (HIE) is a significant cause of perinatal encephalopathy, affecting a substantial number of neonates. Therapeutic hypothermia (TH) has become a standard treatment, significantly reducing mortality leaving a portion of survivors with varying degrees of neurodevelopmental impairment. Neuroimaging, specifically magnetic resonance imaging (MRI), plays a crucial role in prognostication. A standardized MRI scoring system to assess brain injury severity and extent is crucial for predicting long term neurodevelopmental outcome.
OBJECTIVES: This study aimed to investigate the predictive value of the Canadian Standardized consensus classification of brain injury diagnosed with MRI on long – term neurodevelopmental outcomes at 18 – 24 months of corrected age in infants with HIE post therapeutic hypothermia.
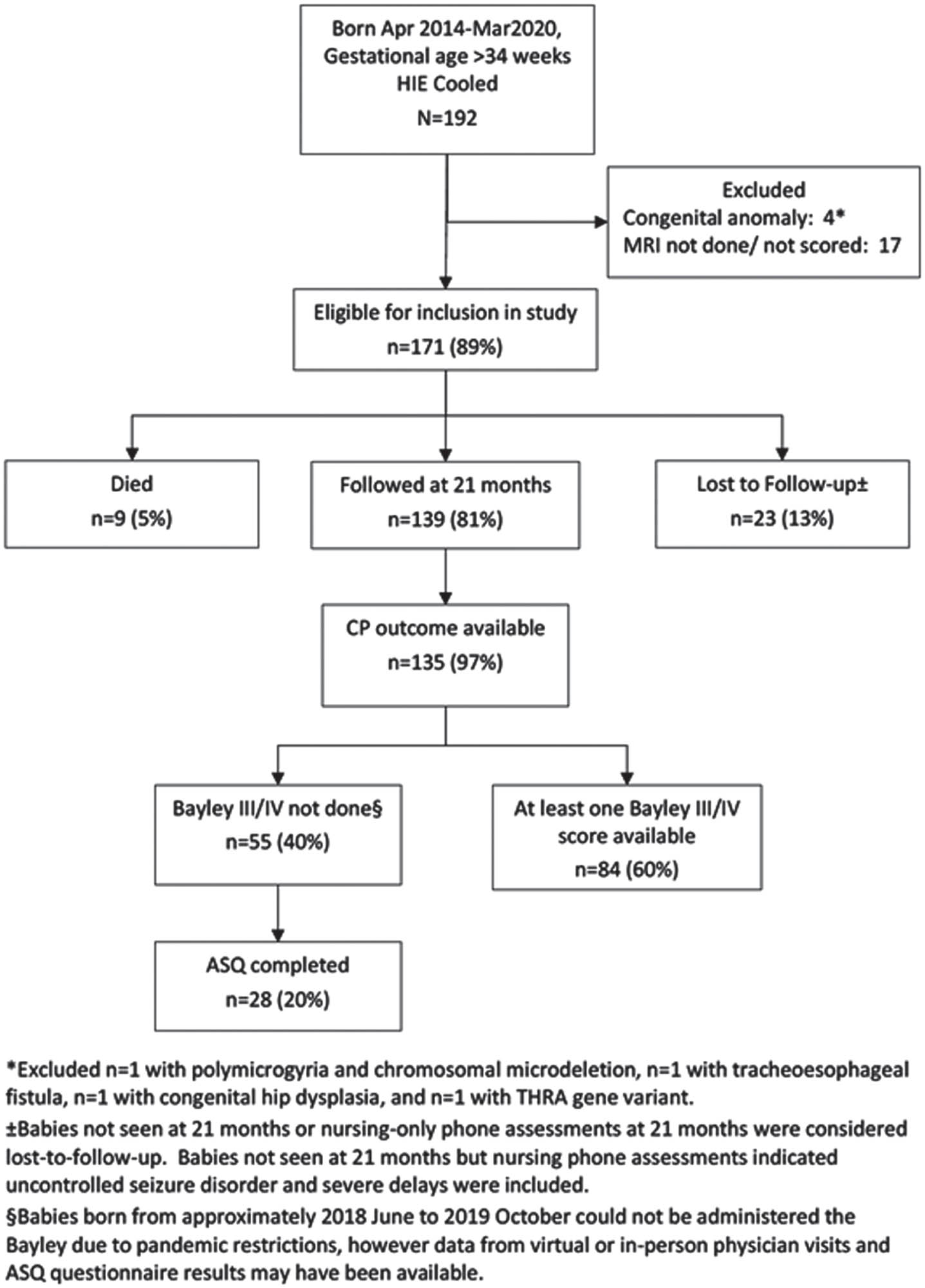
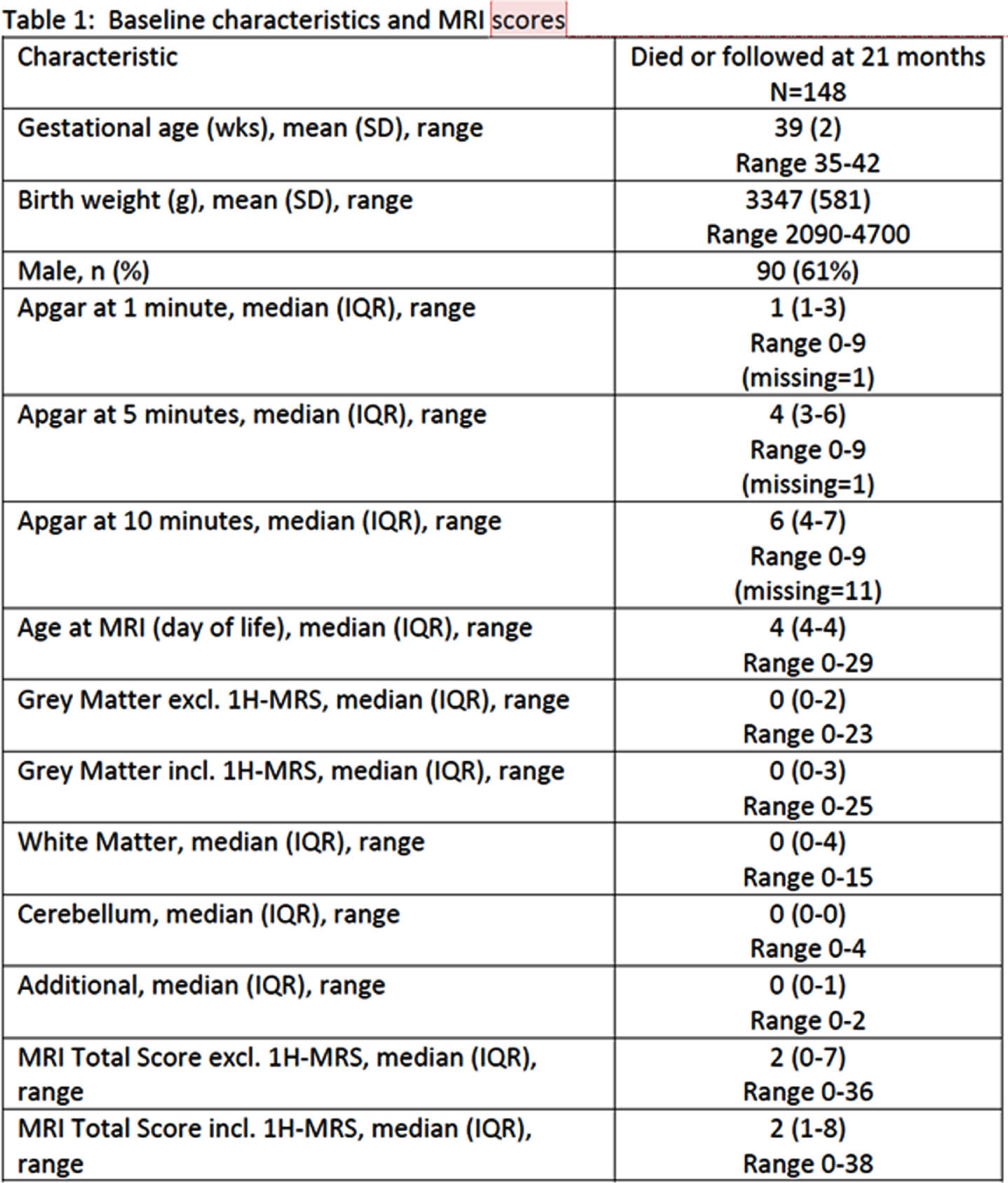
METHODS: A Retrospective observational cohort study conducted between April 2014 and March 2020 included term and near – term neonates with moderate to severe HIE who underwent TH and had MRI imaging to evaluate brain injury who were admitted to a tertiary care center in Calgary, Alberta. Institutional criteria for TH eligibility were defines. A Canadian consensus scoring system, categorizing various patterns of brain injury, was applied to the MRI scans. Follow up at 18 – 24 months adjusted age assessed neurodevelopmental outcomes. The data was compiled and underwent statistical analysis using SAS software, Version 9.4. Tests used were two sided and significance define as a p-value <0.05
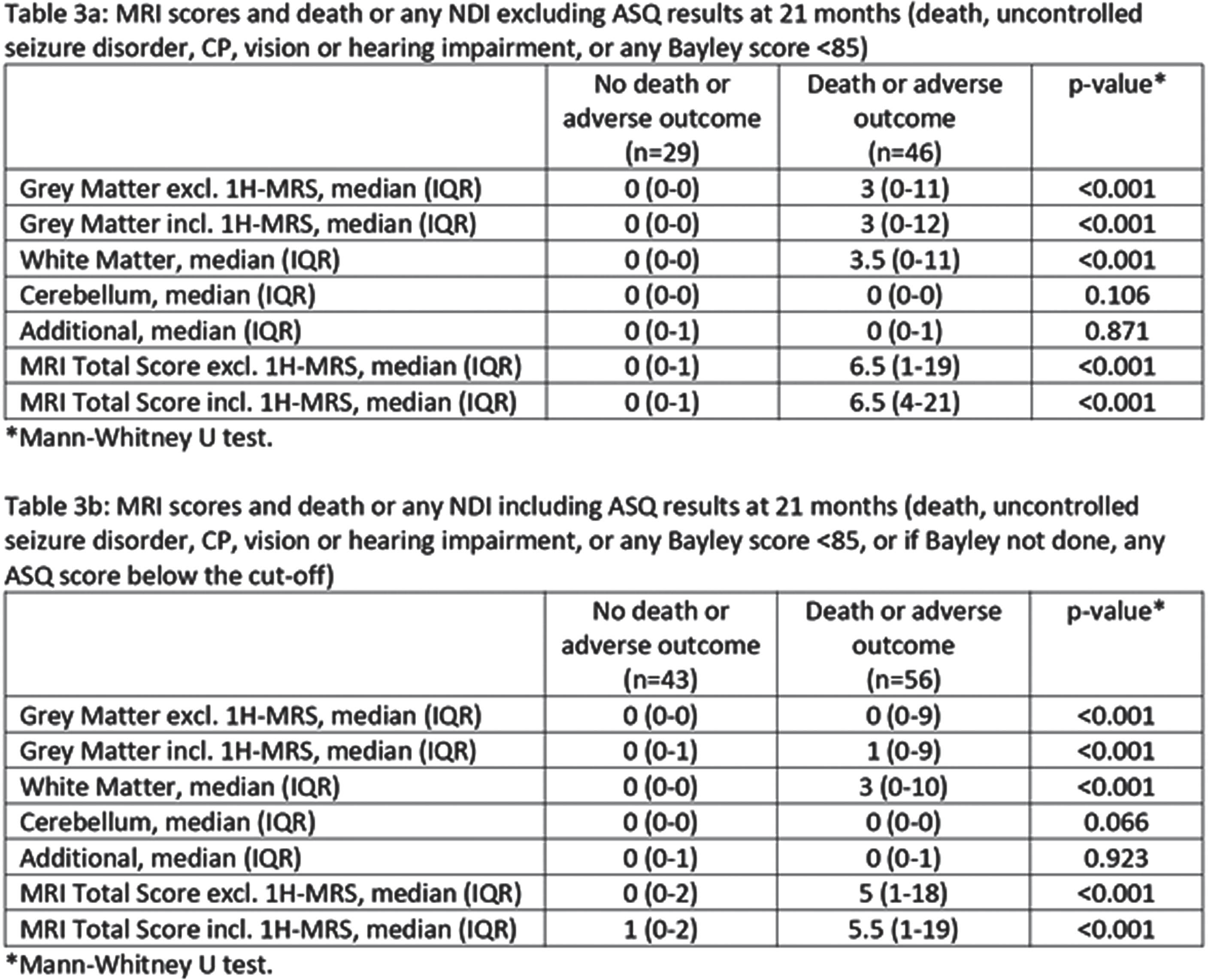
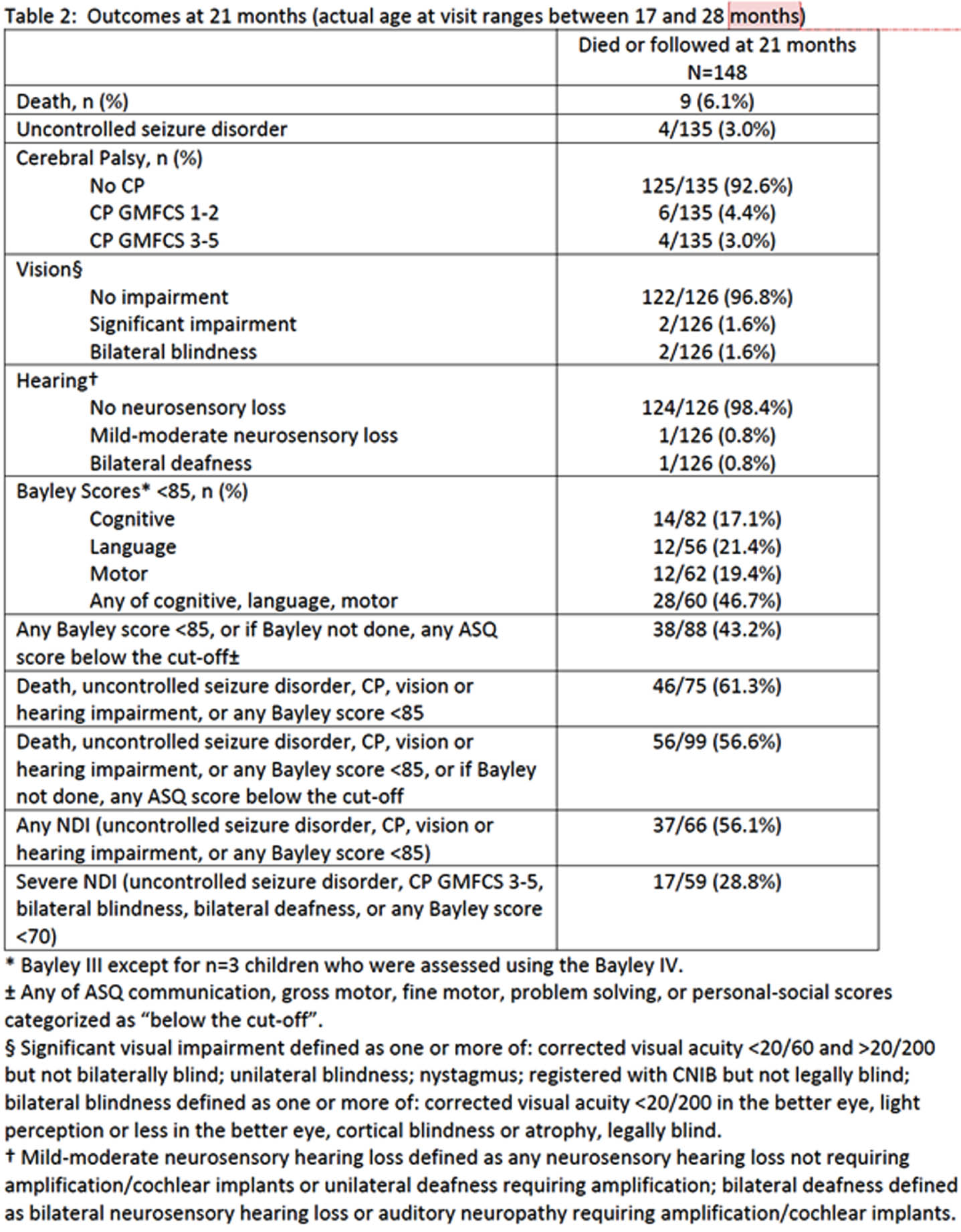
RESULTS: Of the 171 infants included, 5% died and 81% were assessed for neurodevelopmental outcomes. Among surviving infants, a substantial proportion exhibited various degrees of neurodevelopmental impairment, including severe NDI (28.8%), any NDI (56.1%), severe cerebral palsy (3%) and uncontrolled seizure disorder (3%). Lower composite Bayley scores were observed in 46.7% of infants. MRI scores were associated with these outcomes, underscoring the utility of the MRI scoring system for predicting adverse neurodevelopmental outcomes.
CONCLUSION: This study demonstrates the significant long – term consequences of moderate to severe HIE, even after therapeutic hypothermia. The standardized Canadian consensus MRI classification offers a valuable tool for predicting these outcomes and can guide treatment decisions and support families. Further research and validation of these findings are necessary to enhance care and management for infants with neonatal encephalopathy, providing improved prognostication and potentially better ling term outcomes.
Integrating clinical and neuroimaging markers to predict onset of post-hemorrhagic ventricular dilatation in preterm neonates
Dr AbdulAziz Al-Garni1, Ms Avneet Mazara1, Dr Nina Stein1,2, Doctor Ipsita Goswami1,2
1Mcmaster Children’s Hospital, 2Hamilton Health Sciences
BACKGROUND AND OBJECTIVE: Posthemorrhagic ventricular dilatation (PHVD) is one of the major complications of intraventricular hemorrhage (IVH) and accounts for almost 30-40% of infants with Grade 2-4 IVH. PHVD is associated with higher risk of death or developmental impairment such as cerebral palsy, cognitive, functional, and attention deficits compared to infants with IVH without ventricular dilatation. All preterm infants with IVH are diagnosed within a week of life and subsequent serial ultrasounds (US) are needed to detect the infants who develop PHVD. This study aims to explore the early perinatal risk factors in addition to the Grade of IVH associated with the progression to PHVD, with the ultimate goal of developing a predictive model using key clinical and neuroimaging-based predictors.
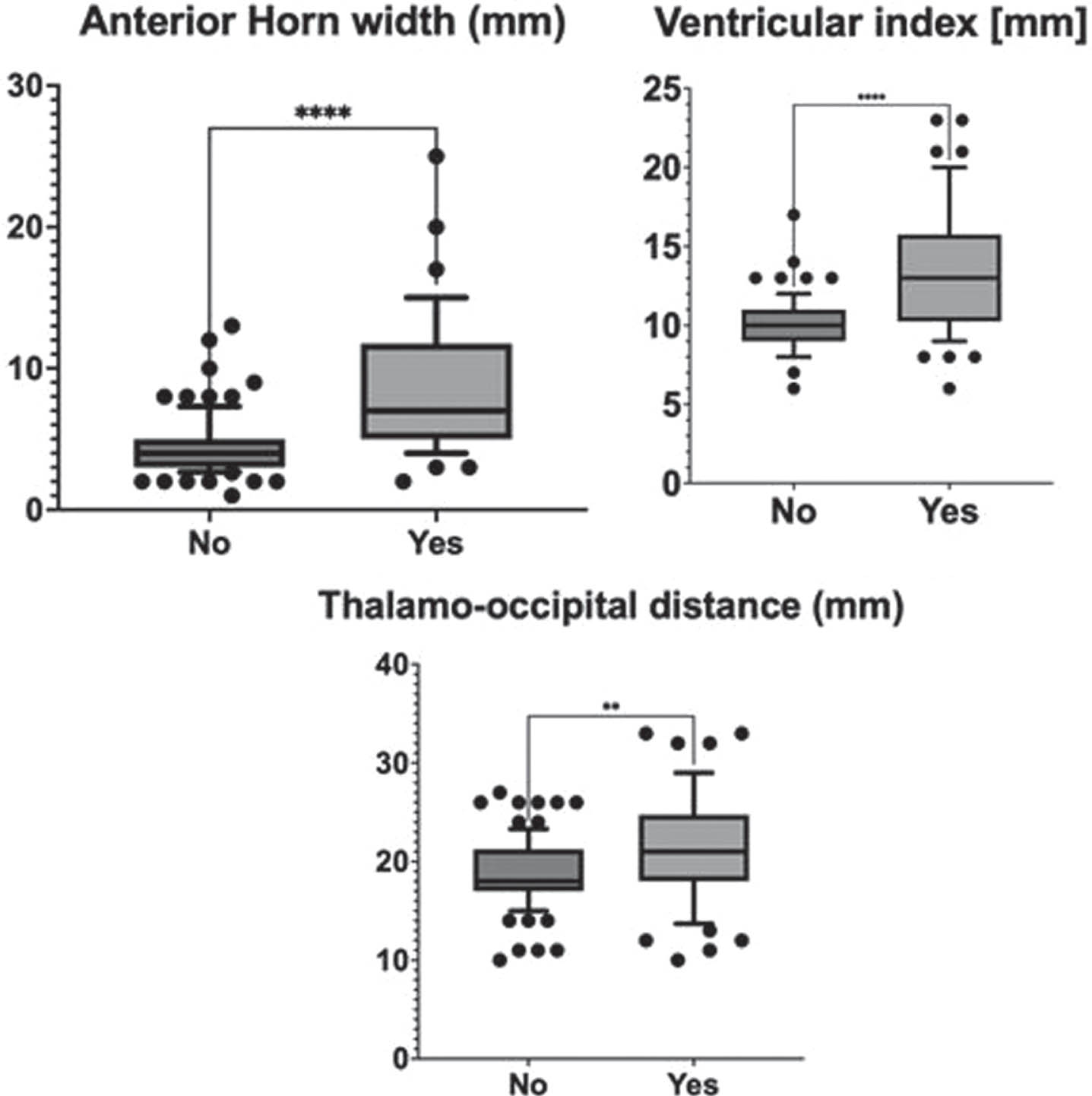
METHODOLOGY: A retrospective chart review of preterm infants ≤29 weeks gestational age (GA) and birthweight (BW) ≤1500g with Grade 2,3,4 IVH, admitted between January 2015-December 2021 were included. All cranial US done within 2weeks postnatal age were assessed for Grade of IVH, Anterior Horn Width (AHW), Ventricular Index (VI) and Thalamooccipital Index (TOD) according to the Canadian consensus statement. Only the highest grade and dimension on either side were considered for data analysis. Primary composite Outcome was defined as death or any US with VI or AHW or TOD ≥ moderate risk based on Eldib et al[1] between 2weeks postnatal age or 40 weeks postmenstrual age.
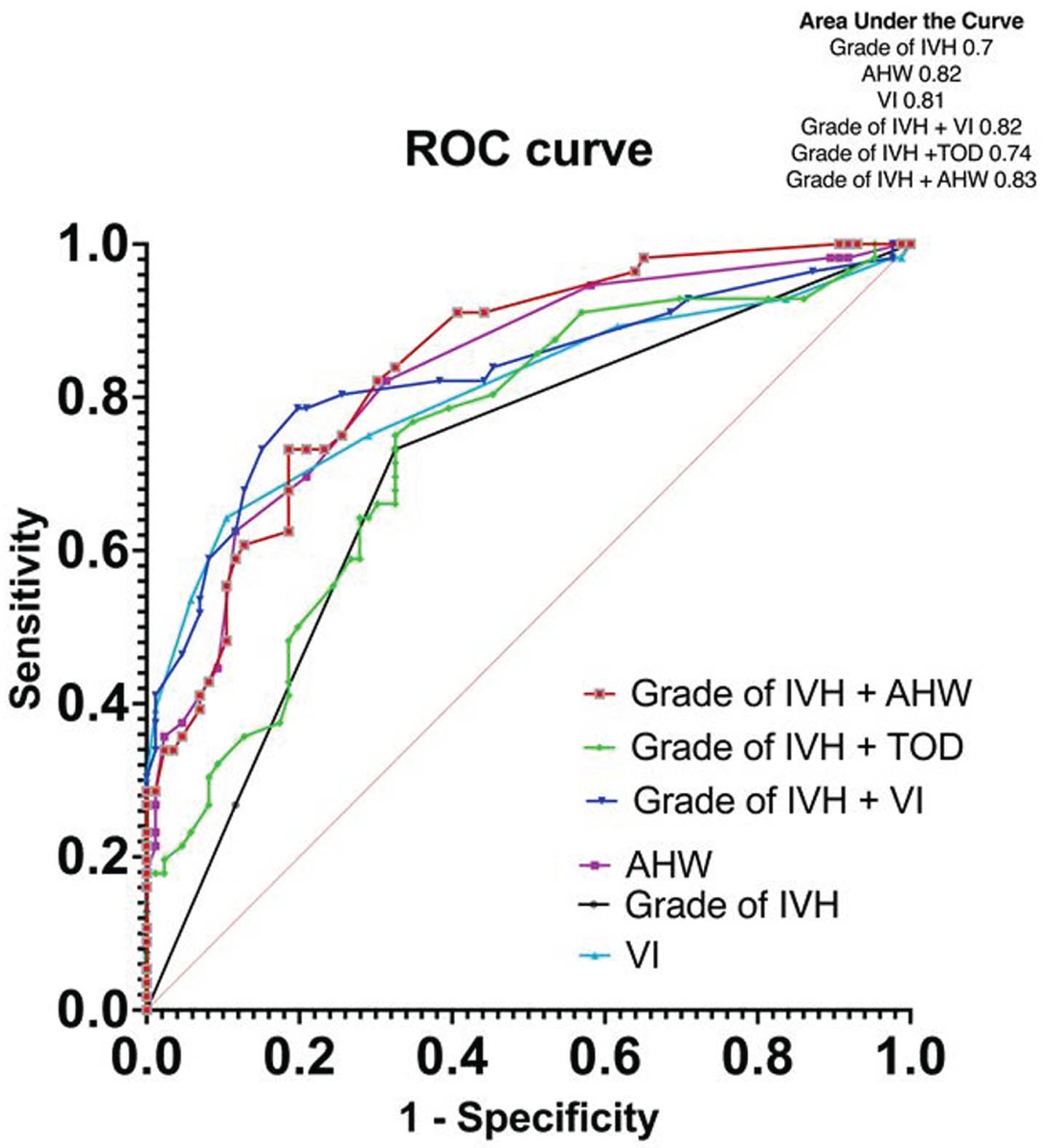
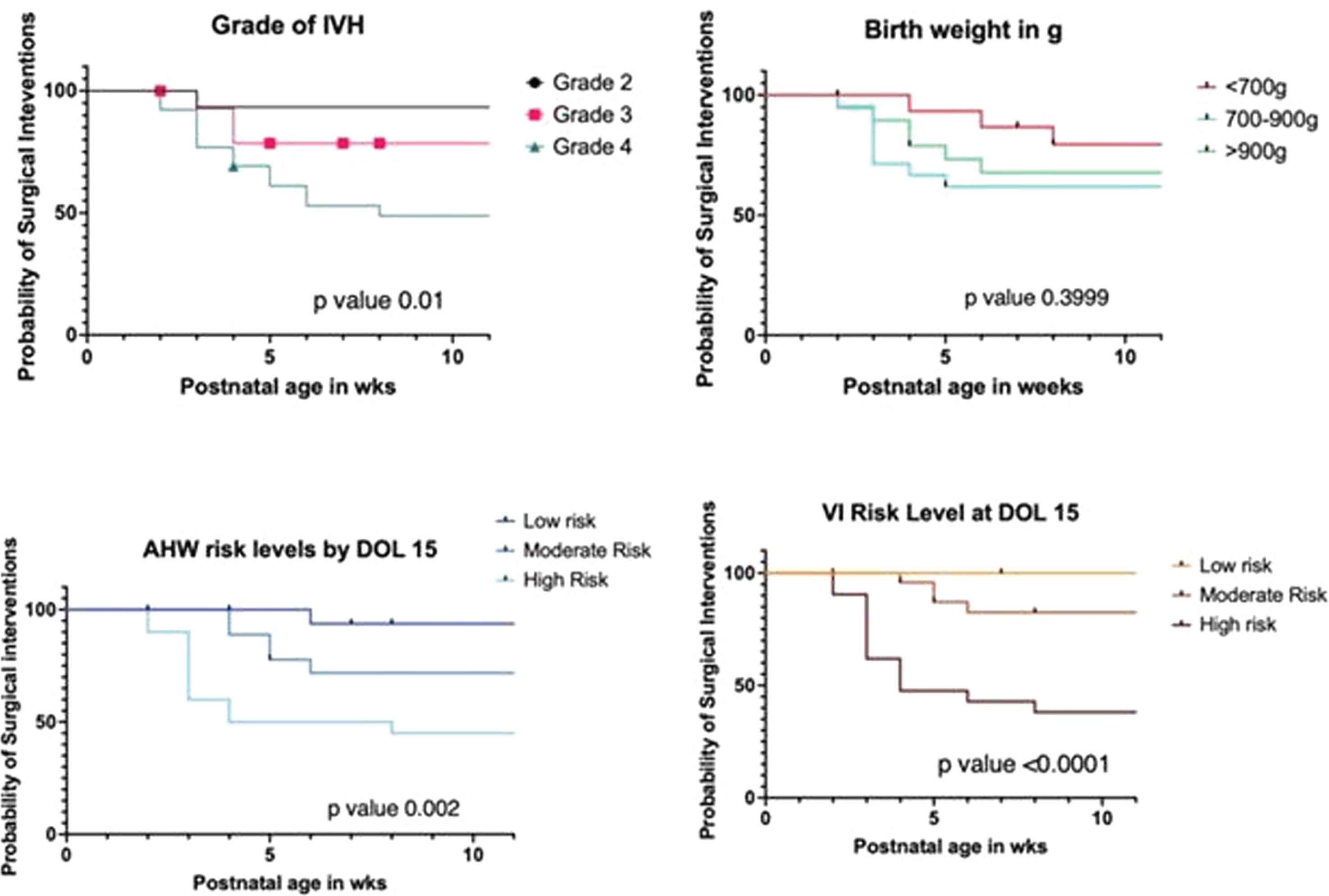
RESULTS: A total of 146 infants with a mean (SD) GA of 26 (1.8) weeks, BW 900 (234)g and female (46%) were included (excluded 4 for missing data). The primary outcome occurred in 56 (39%), ventricular reservoir was needed in 17 (30%) and Shunt insertion was needed in 11 (20%). The clinical characteristics of infants with and without the composite outcome were comparable except that the former were significantly smaller and received >2 doses of surfactant [Table 1]. On multivariate logistic regression, the risk factors present within 2 weeks postnatal age that significantly increased the odds of developing PHVD were hemodynamically significant duct arteriosus [OR 6.1 (95%CI 1.9-22)], culture-proven sepsis [OR 5.4 (95%CI 1.8-18)], Grade 3 IVH [OR 4.6 (95%CI 1.1-22)], Grade 4 IVH [OR 3 (95%CI 0.9-10)], and VI(mm) [OR 2.1 (95%CI 1.6-2.9)] [Table 2, Figure 1]. On survival analysis, surgical interventions were noted to be done at earlier postnatal age in neonates whose VI and AHW were at high-risk levels by 2weeks postnatal age and those with Grade 4 IVH [Figure 2].
CONCLUSION: In addition to the Grade of IVH, clinical predictors such as hemodynamically significant duct arteriosus and bacterial septicemia along with risk levels of US-based markers such as AHW and VI measured within 2weeks of life are potential predictors of later onset of PHVD. Validity of the proposed predictor model will need to be tested in a larger cohort.
BIBLIOGRAPHY:
1. El-Dib M et al. J Pediatr. 2020 Nov;226:16-27.e3
Cerebellar pathologies in extremely preterm infants with intraventricular hemorrhage in relation to neurodevelopmental outcome
Doctor Julia Buchmayer1, Doctor Gregor Kasprian2, Doctor Sophie Stummer1, Doctor Renate Fuiko1, Doctor Patric Kienast2, Doctor Angelika Berger1, Doctor Katharina Goeral1
1Comprehensive Center for Pediatrics, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Intensive Care and Neuropediatrics, Medical University of Vienna, 2Department of Radiology, Division of Neuroradiology and Musculoskeletal Radiology, Medical University of Vienna
OBJECTIVES: Extremely preterm birth and intraventricular hemorrhage (IVH) are established risk factors for adverse neurodevelopmental outcome. As IVH is often associated with cerebellar hemorrhage (CBH), the additional influence on development of these posterior fossa abnormalities is warranted.
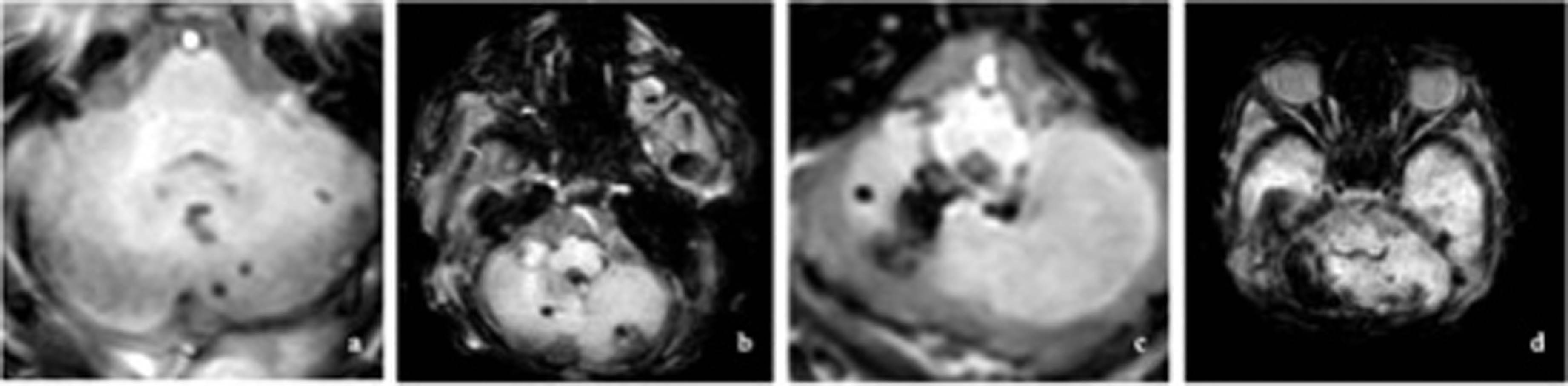
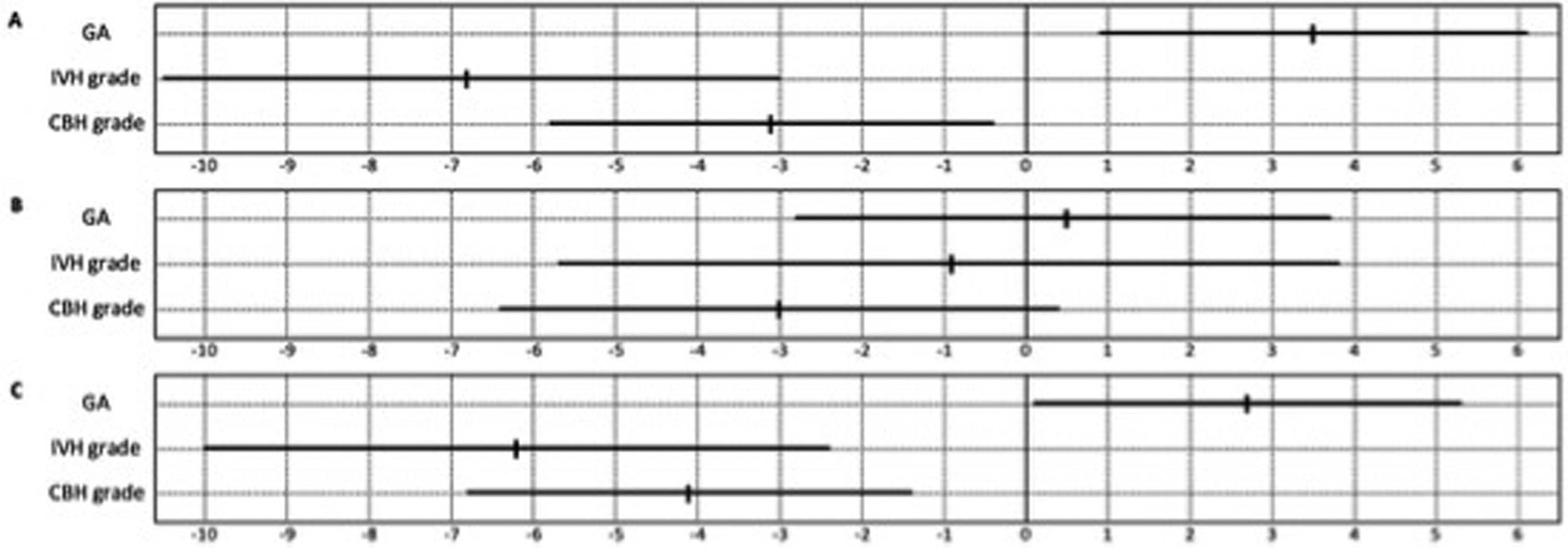
METHODS: This observational cohort study analyzed cerebral magnetic resonance imaging data at term-equivalent age of patients <28 weeks of gestation with IVH born between 2011 and 2021 at the level IV Neonatal Intensive Care Unit of the Medical University of Vienna. Cerebellar hemorrhage was graded according to Kidokoro et al(1) as shown in Figure 1, with grade I consisting of unilateral punctate lesions ≤3 mm in size, grade II consisting of bilateral punctate lesions ≤3 mm, grade III consisting of a unilateral lesion >3 mm and grade IV was diagnosed when extensive lesions were observed bilaterally. In addition, a category of “cerebellar atrophy” was introduced, including atrophy of at least one third of one hemisphere due to hemorrhage or intracranial pressure (e.g. trapped fourth ventricle). Additionally, cerebellar growth was categorized by measurement of transverse cerebellar diameter. Finally, the impact of injuries on outcome at two-years corrected age (tested with Bayley-III) was evaluated, incorporating multivariable regression analysis.
Figure 1
Classification of cerebellar hemorrhage, with a unilateral punctate lesions (≤3 mm); b bilateral punctate lesions (≤3 mm); c unilateral extensive hemorrhage (>3 mm); d bilateral extensive hemorrhage (>3 mm).
RESULTS: Among 103 patients, 69 (67%) showed CBH with a median grade of 1 (IQR 0-3). At two-years corrected age CBH was significantly associated with impaired cognitive and motor outcome. Also, multivariable regression models identified CBH as an independent predictor of poor cognitive, language and motor development at two-years corrected age, as shown in Figure 2. Cerebellar atrophy, affecting 30 (29%) infants, was linked to significantly impaired outcome across all domains (cognitive, language and motor). Conversely, increasing cerebellar size correlated with better motor development (p=0.04).
Figure 2
Forest plot representing unstandardized coeffi cients (B) along with their 95% confi dence intervals of predictor variables on A cognitive, B language and C motor outcome calculated using multivariable regression analysis.
CONCLUSION: Extremely preterm infants with IVH and concomitant CBH showed significant cognitive and motor impairment. The extent of CBH was associated with the grade of developmental delay, mainly regarding motor development. These findings may support prediction of long-term outcome and parental counseling in patients with IVH.
REFERENCE:
(1) Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics. Aug 2014;134(2):e444-53. doi:10.1542/peds.2013-2336
Early versus late brain magnetic resonance imaging and spectroscopy in infants with neonatal encephalopathy
Doctor Tatiana Nuzum, Doctor Pradeep Mally, Doctor Elena Wachtel
1Hassenfeld Children’s Hospital at NYU Langone, 2NYCH+H Bellevue Hospital Center
BACKGROUND: Magnetic Resonance (MR) Imaging is the current gold standard for early neurodevelopmental prognostication of infants with Neonatal Encephalopathy (NE). Recent studies have suggested that obtaining MR imaging (MRI) at a single time-point may underestimate the degree of neurologic injury. MR Spectroscopy (MRS) is a newer modality of neuroimaging that may be more sensitive in the detection of hypoxic-ischemic injury.
OBJECTIVE: To evaluate the utility of early and late MRI in infants with NE, and to determine the concordance between MRS and initial MRI.
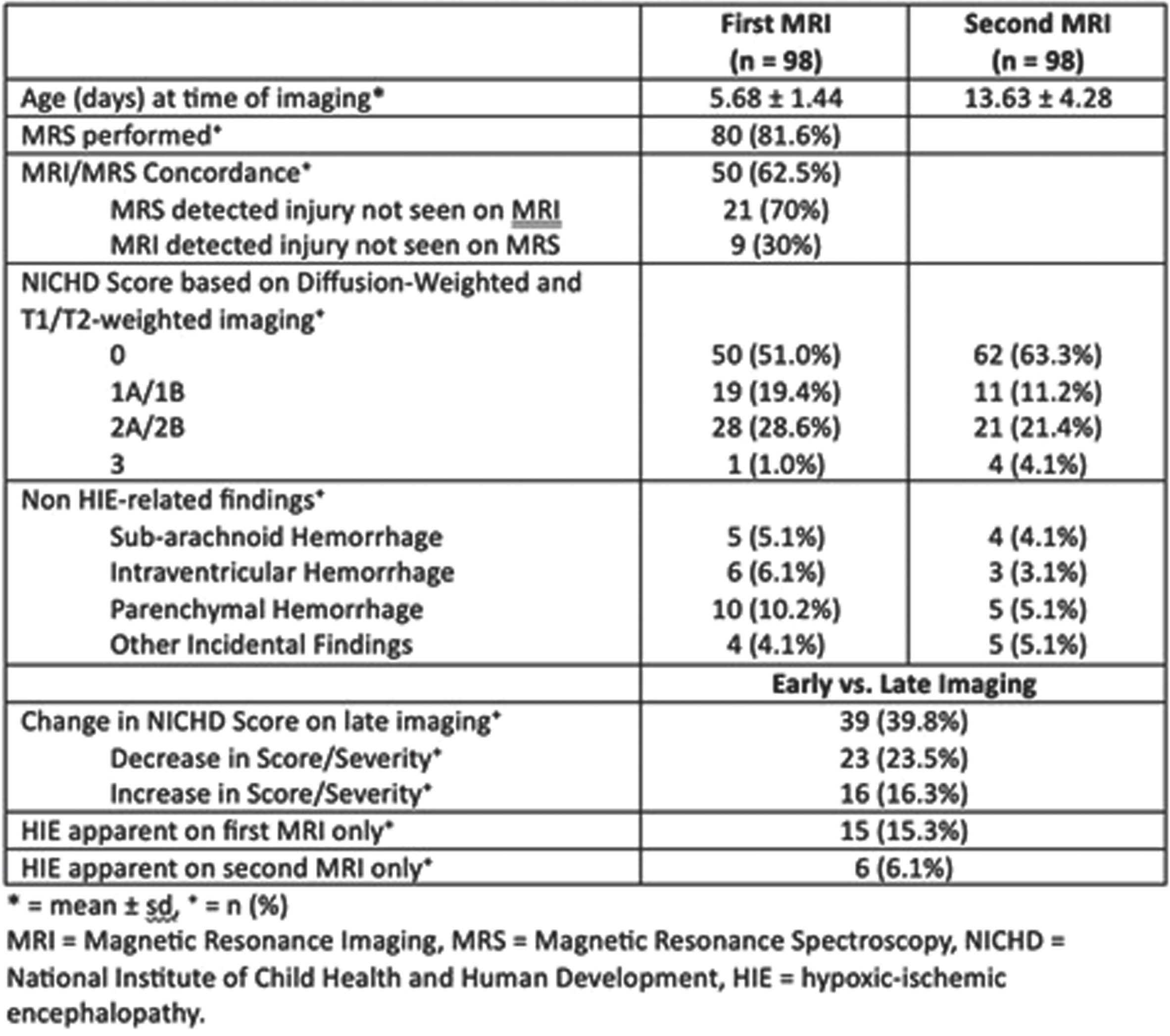
METHODS: This was a retrospective chart review including infants with NE born between 2017 and 2023 at two regional perinatal centers, NYU Langone Health and Bellevue Hospital Centers. Demographic and clinical information including MRI results were collected. All infants underwent early diffusion-weighted MRI with MRS, and late conventional T1/T2-weighted imaging.
RESULTS: Ninety-eight infants were included. Subject demographics are described in Table 1. The majority (78%) were moderately encephalopathic. Early and late MR imaging was performed at an average of 5.7 and 13.6 days of life respectively. Both MRIs were assigned an injury severity score based on the NICHD NRN pattern of injury. Differences in these scores between first and second MRIs are outlined in Table 2. Forty percent of infants exhibited a change in severity of injury between early and late MRI. Twenty-three infants (24%) were found to have milder injury and 16 (16%) were found to have more severe injury according to late imaging. Fifteen percent of infants had evidence of hypoxic-ischemic injury on early imaging only, and 6% on late imaging only. Eighty infants (82%) underwent MRS with early imaging. Concordance of injury severity between MRS and MRI was 62.5%. Among the cases of discordant MRI/MRS, MRS detected additional injury in 70% of cases, and MRI detected additional injury in 30% of cases. MRI findings unrelated to hypoxic-ischemic injury are also outlined in Table 2.
CONCLUSION: Both early and late imaging are necessary to fully define neurologic injury and provide accurate neurodevelopmental prognoses in cases of neonatal encephalopathy. Had imaging not been performed at two intervals, radiologic diagnoses of hypoxic-ischemic injury would have been missed in 6-15% of cases, and characterization of injury may have been inaccurate in up to 40% of cases. Further analysis of MRS results with an independent reviewer may test the superiority of this modality in comparison to conventional MR imaging, given the discrepancy found in this study.
Methodological considerations on diffusion MRI tractography in infants aged 0-2 years: A scoping review
Ms Anouk Verschuur1,2,3, Ms Regan King2, dr. Chantal Tax3,4, Dr. Martijn Boomsma1, Dr. Gerda van Wezel-Meijler1, Dr. Alexander Leemans3, Dr. Lara Leijser2
1Isala Hospital, 2University of Calgary, 3Univerisity Medical Center Utrecht, 4Cardiff University
OBJECTIVE: Understanding the intricate processes of typical and atypical brain development holds significant clinical importance, as many neurological and neurobehavioral disorders have their origins in the preterm period [1]. Diffusion magnetic resonance imaging (dMRI) tractography is a dedicated method to study the micro-structural architecture of the brain’s white matter [2]. Despite a keen interest to increase the understanding of the rapidly developing young brain through tractography, detailed descriptions on how to approach tractography in infants are limited. The purposes of this review were 1) to describe current tractography methods as used on young infant dMRI data and summarize their limitations, and 2) to provide recommendations for advanced tractography in young infant brains.
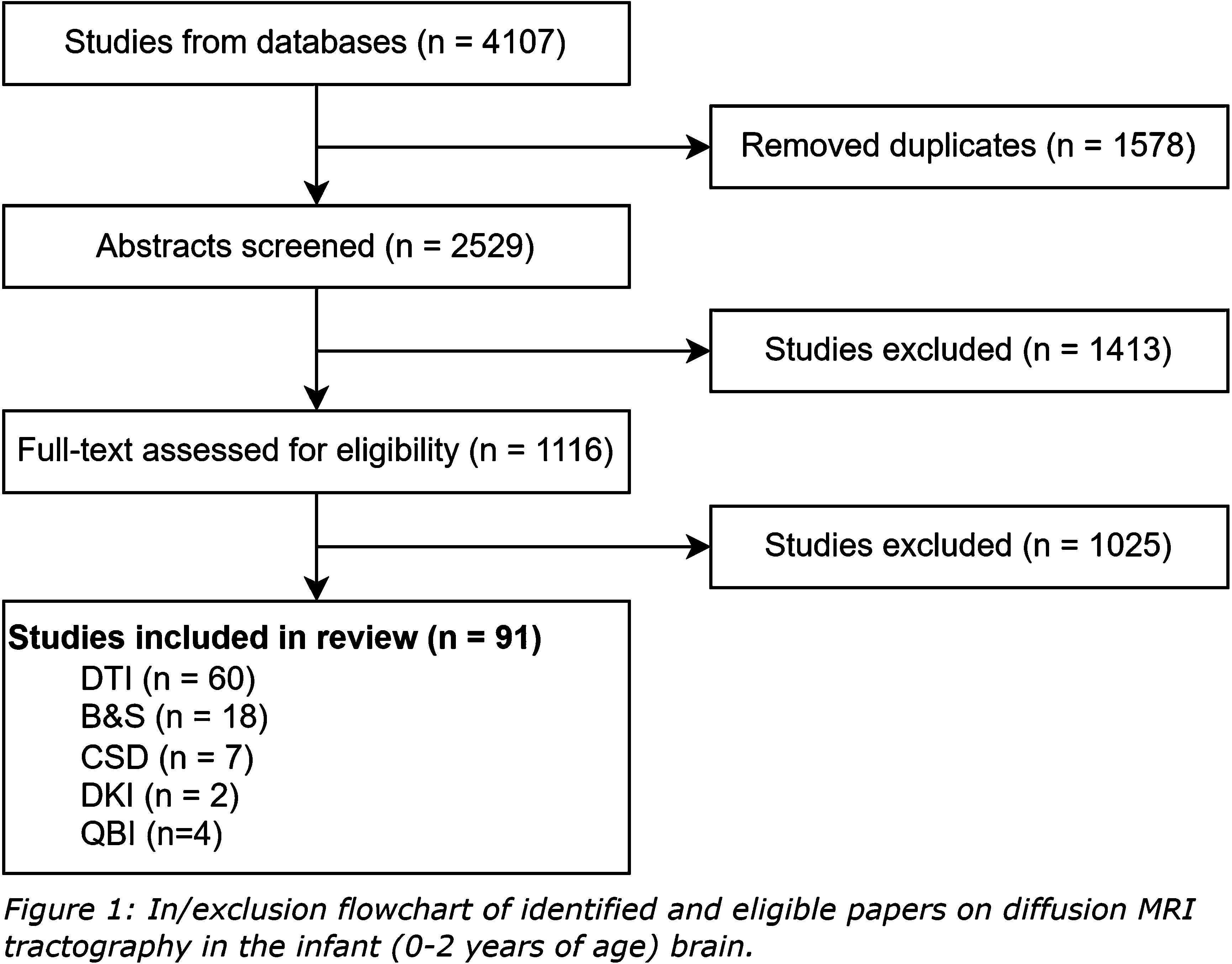
METHODS: Through a scoping review, methodological approaches to white matter tractography and their commonly reported limitations in infants aged 0-2 years are evaluated. A search was performed in July 2022. Studies applying white matter tractography on brain scans of infants who underwent dMRI between birth and two years of age were included. Papers that described tractography on a single fiber bundle, single scan, or for whole brain connectome analysis were excluded. Ongoing research, studies with missing details on tractography, and re-use of tractography data were also excluded. Screening of papers was performed independently by two researchers and discussed in a consensus meeting. A third researcher was consulted if consensus could not be reached.
RESULTS: A total of 2529 papers were identified, of which 1413 were excluded based on title and abstract. An additional 1025 papers were excluded after full text screening (Figure 1). The 91 remaining papers were considered eligible for this review. The majority (66%) of included tractography literature described the use of diffusion tensor imaging (DTI; Figure 2) [2]. Despite having the potential for more detailed reconstruction of the organizationally complex white matter, especially in crossing nerve bundles, and extensive knowledge gained from application on the adult brain. Only few studies described advanced tractography approaches, including constrained spherical deconvolution (CSD; Figure 3), diffusion kurtosis imaging (DKI), ball-and-sticks model (B&S) and Q-ball imaging (QBI) [3-7]. This may be partly attributed to technical challenges with imaging, image processing and the need for short scan times in young children.
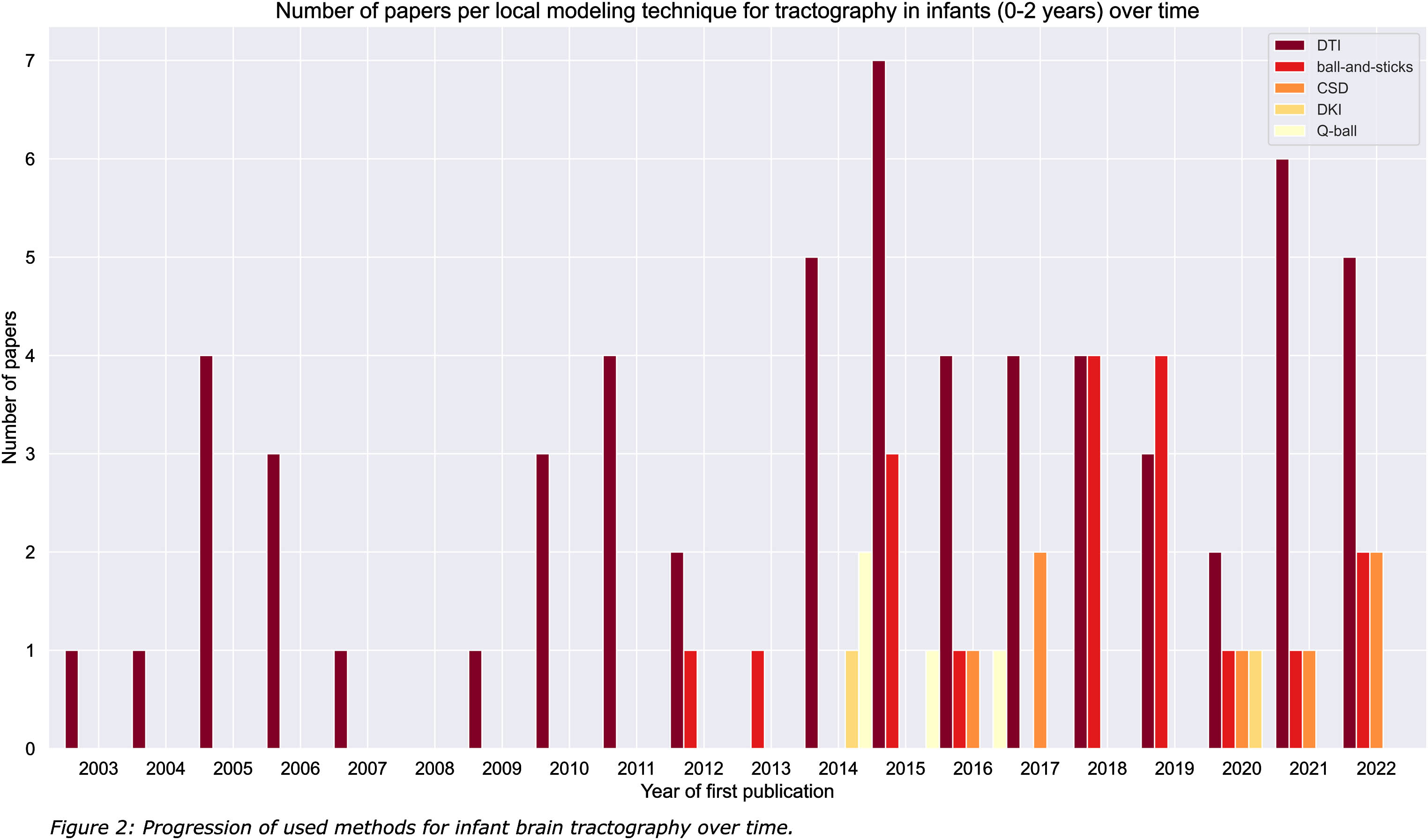
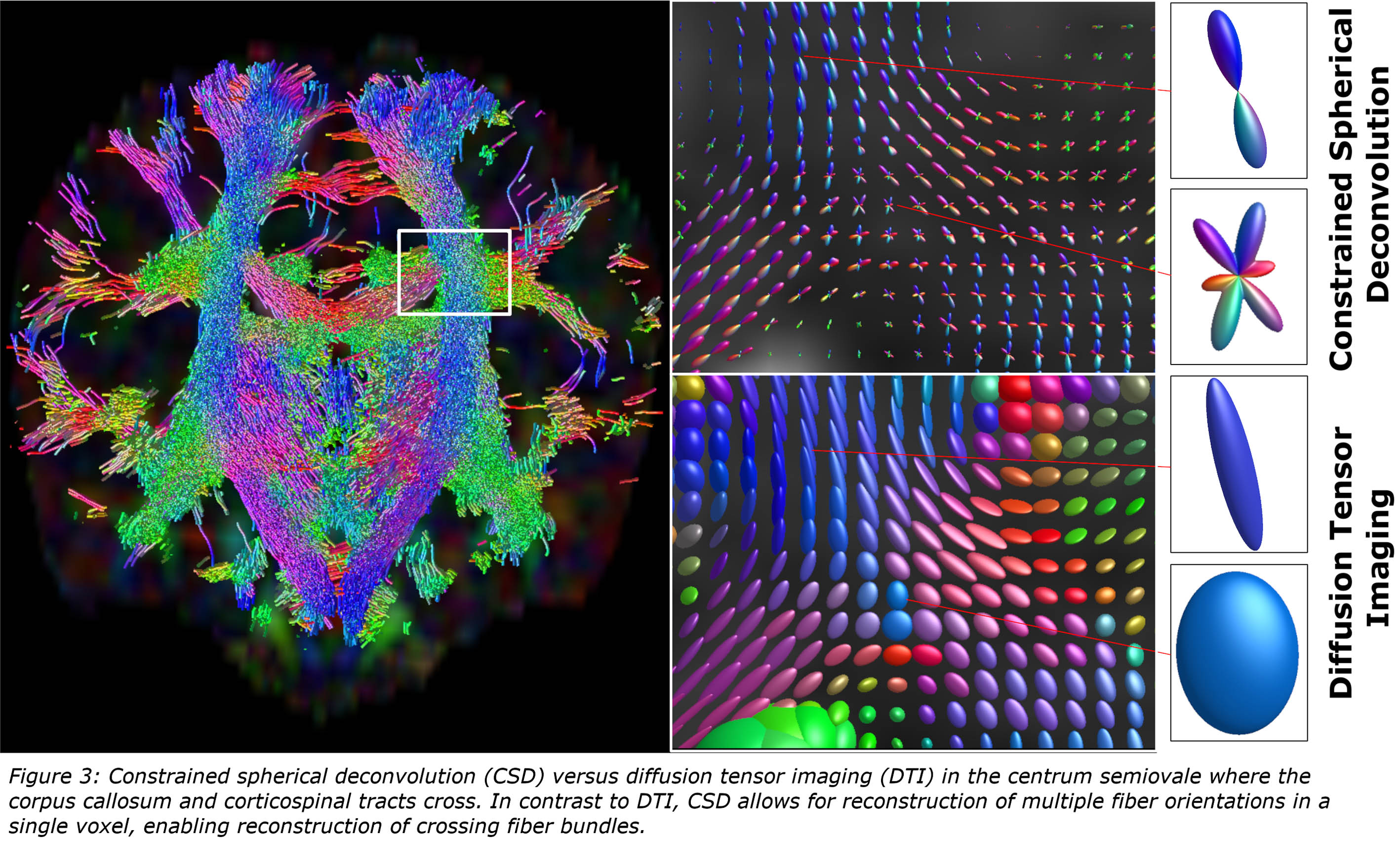
CONCLUSION: Optimization of the largely unexplored advanced tractography approaches for use in young infants is warranted. More reliable reconstruction of the developing and vulnerable white matter contributes to distinguishing subtle differences between typical and atypical brain development, and may contribute to early recognition, personalized treatment plans and improved prognosis and monitoring of developmental disorders in young infants.
Evaluation of brain extraction techniques in preterm neonatal MRI at term: implications for intracranial volumes
Tânia F. Vaz1, Nuno Canto Moreira2, Lena Hellström-Westas3, Nima Naseh3, Nuno Matela1, Hugo A. Ferreira1
1Instituto De Biofísica E Engenharia Biomédica, Faculdade De Ciências Da Universidade De Lisboa, Portugal, 2Department of Neuroradiology, Karolinska University Hospital, 3Department of Women’s and Children’s Health, Uppsala University
BACKGROUND AND PURPOSE: Magnetic resonance imaging (MRI) plays a crucial role in evaluating early brain development and injuries in newborns [1]. For automated volumetric analysis, brain tissue segmentation is necessary, which requires brain extraction (BE) to exclude non-brain tissues. However, BE is challenging in neonatal brain MRI due to lower spatial resolution, lower signal-to-noise ratio, lower contrast-to-noise ratio, greater intensity variation within tissues, smaller brain size, evolving shape, and motion artifacts compared to adult brain images [2]. While various methods exist for BE, manual segmentation remains the standard approach. This study aims to evaluate different BE methods in preterm neonatal MRI and their impact on intracranial volumes (ICVs) at term-equivalent age (TEA).
METHODOLOGY: The study involved twenty-two premature neonates (mean gestational age ± standard deviation: 28.4±2.1 weeks) with brain MRI performed at TEA, without detectable lesions or congenital conditions. Manual segmentation of ICVs was performed using 3DSlicer on 2D T2-weighted scans to establish reference brain masks. Additionally, four automated BE methods were employed: HD Brain Extraction Tool (HD-BET) [3], Simple Watershed Scalping (SWS) [4], SynthStrip [5], and Brain Extraction Tool (BET2) [6]. Comparisons between BE methods were performed using voxel overlap-based and surface distance-based metrics. The Friedman’s test was used to assess significant differences, followed by post hoc tests corrected for multiple comparisons (Bonferroni-adjusted). Agreement between ICVs was reported using Bland-Altman analysis and volume-based metrics, whilst statistical analyses were conducted using IBM®SPSS® v27 software with a significance level of 5%.
RESULTS: The automated segmentations most similar to the manual segmentation were observed with the HD-BET and SWS, followed by the SynthStrip and BET2 methods (Table 1, Figure 1). Additionally, the root mean square error and respective coefficients of variation were: 13.8 mL (3.0%) for HD-BET, 10.5 mL (2.3%) for SWS, 13.6 mL (2.9%) for SynthStrip, and 33.8 mL (7.3%) for BET2.
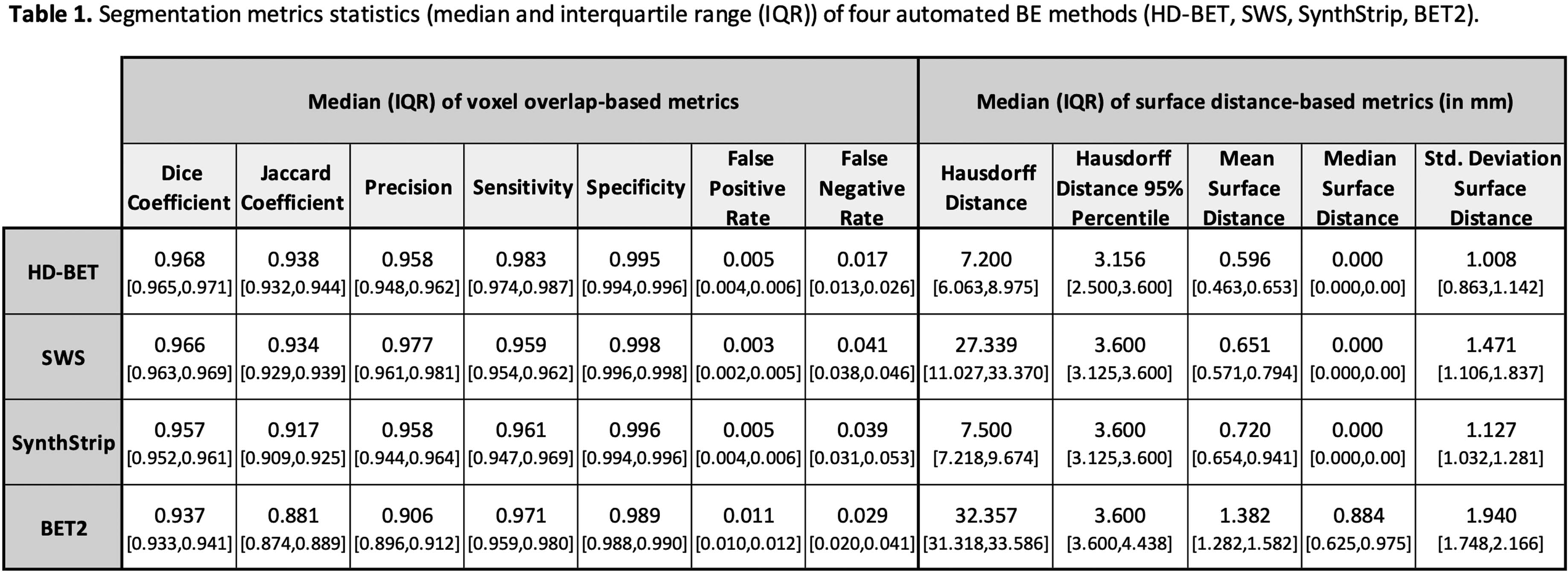
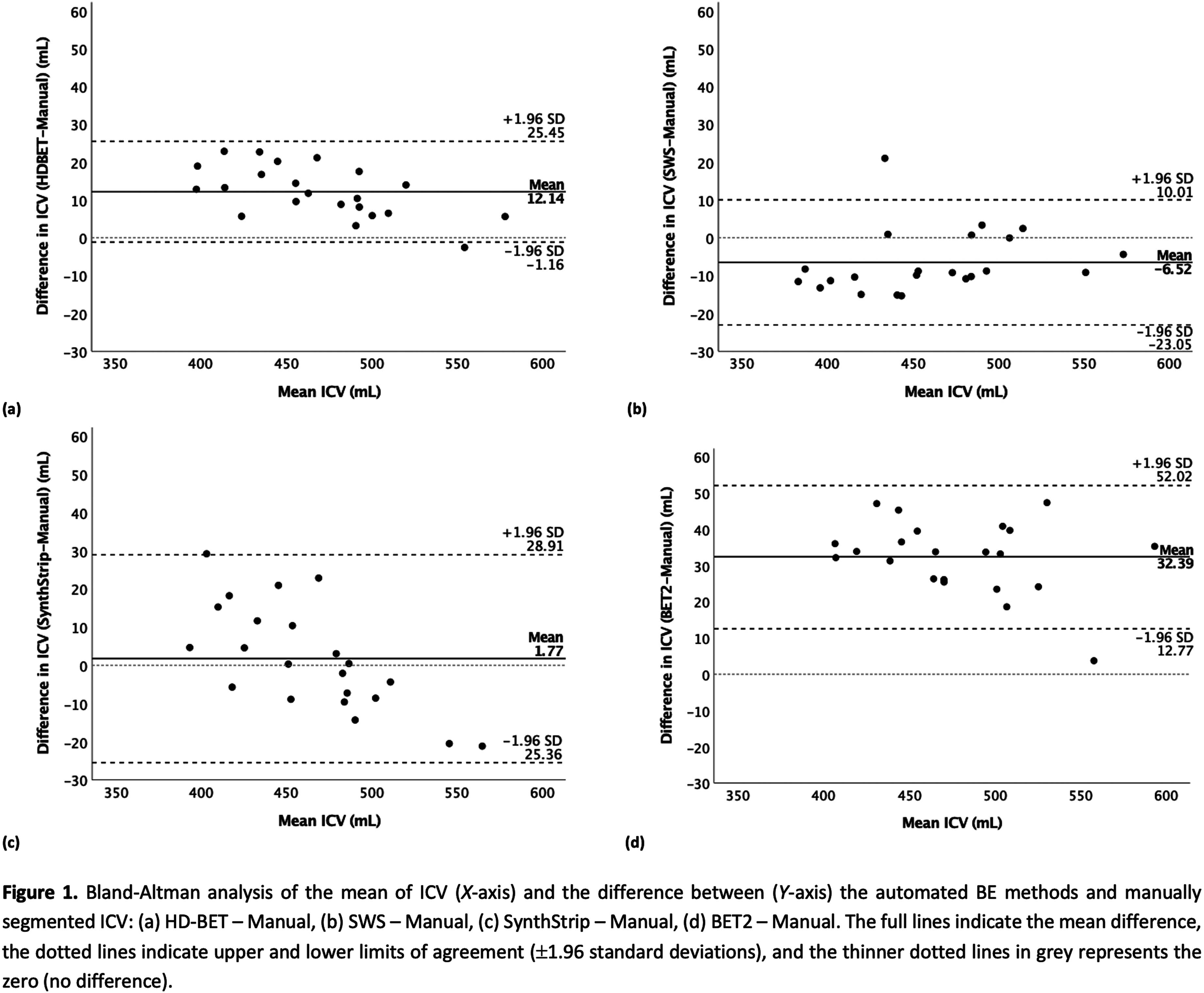
CONCLUSION: HD-BET and SWS are the most similar to the manual segmentation, showing estimated ICVs with clinically-acceptable errors <3%.
BIBLIOGRAPHY:
[1] Volpe JJ. Neurology of the Newborn. 5th ed. Saunders; 2008. p. 172-177.
[2] Devi CN, Chandrasekharan A, Sundararaman VK, Alex ZC. Neonatal brain MRI segmentation: A review. Comput Biol Med. 2015;64:163–78.
[3] Isensee F, Schell M, Pflueger I, Brugnara G, Bonekamp D, Neuberger U, et al. Automated brain extraction of multisequence MRI using artificial neural networks. Hum Brain Mapp. 2019;40(17):4952–64.
[4] Beare RJ, Chen J, Kelly CE, Alexopoulos D, Smyser CD, Rogers CE, et al. Neonatal Brain Tissue Classification with Morphological Adaptation and Unified Segmentation. Front Neuroinform. 2016;10:12.
[5] Hoopes A, Mora JS, Dalca AV, Fischl B, Hoffmann M. SynthStrip: skull-stripping for any brain image. NeuroImage. 2022;260:119474.
[6] Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–55.
A comparative study between in-NICU 1T MRI and conventional 3T MRI
Danielle Sharon1, MSN, RN, BSN Elizabeth Singh1, MD Camilo Jaimes2, MD, MSc Patricia Ellen Grant3, MD, MBChB Terrie Inder4, MD Mohamed El-Dib1,5
1Brigham And Women’s Hospital, 2Massachusetts General Hospital, 3Boston Children’s Hospital, 4Childrens Hospital of Orange County, 5Harvard Medical School
BACKGROUND: In the neonatal period, brain magnetic resonance imaging (MRI) is a valuable clinical tool that offers the ability to visualize brain injury and assess brain growth and development. At the Brigham and Women’s Hospital Neonatal Intensive Care Unit (NICU), we began using the Embrace scanner, a 1-Tesla (1T), in-unit MRI clinically in March of 2020. This scanner allows for easier and safer access to brain MRIs for high-risk infants. This retrospective study aims to assess the added diagnostic utility and clinical value of a 3T MRI after obtaining a 1T MRI.
METHODOLOGY: We identified a cohort of infants with an initial MRI in the 1T scanner that had repeat imaging within 14 days in a 3-Tesla (3T) scanner. All infants were admitted to the level III NICU at Brigham and Women’s Hospital. The MRIs were done for clinical purposes (per BWH clinical practice guidelines) or as part of research protocols. Follow-up 3T MRIs were performed to obtain higher resolution imaging, magnetic resonance angiogram (MRA), magnetic resonance venography (MRV), or as part of a research protocol. Both clinical and research MRIs were reported in the same format and were interpreted by pediatric neuroradiologists at Boston Children’s Hospital.
RESULTS: 15 clinical and 19 research MRIs were performed on 1T with 13 scans in full-term infants and 21 in preterm infants (half prior to term equivalent). 26/34 (76%) of the 1T MRIs were interpreted as abnormal. 28 clinical and 6 research MRIs were performed on 3T with 13 scans in full-term infants, 4 in preterm infants prior to TEA, and 17 in preterm infants at TEA. 27/34 (79%) of the 3T scans were found to have an abnormality. In 31 cases (91%), the 3T MRI showed similar or expected evolution of known findings found at 1T (examples in Figure 1). Variation existed in three cases where either an abnormality was defined as artifact, or a more extensive recognition of injury occurred (Table 1, Figure 2). There appeared to be no clinical impact of the change between the 1T and 3T imaging results.
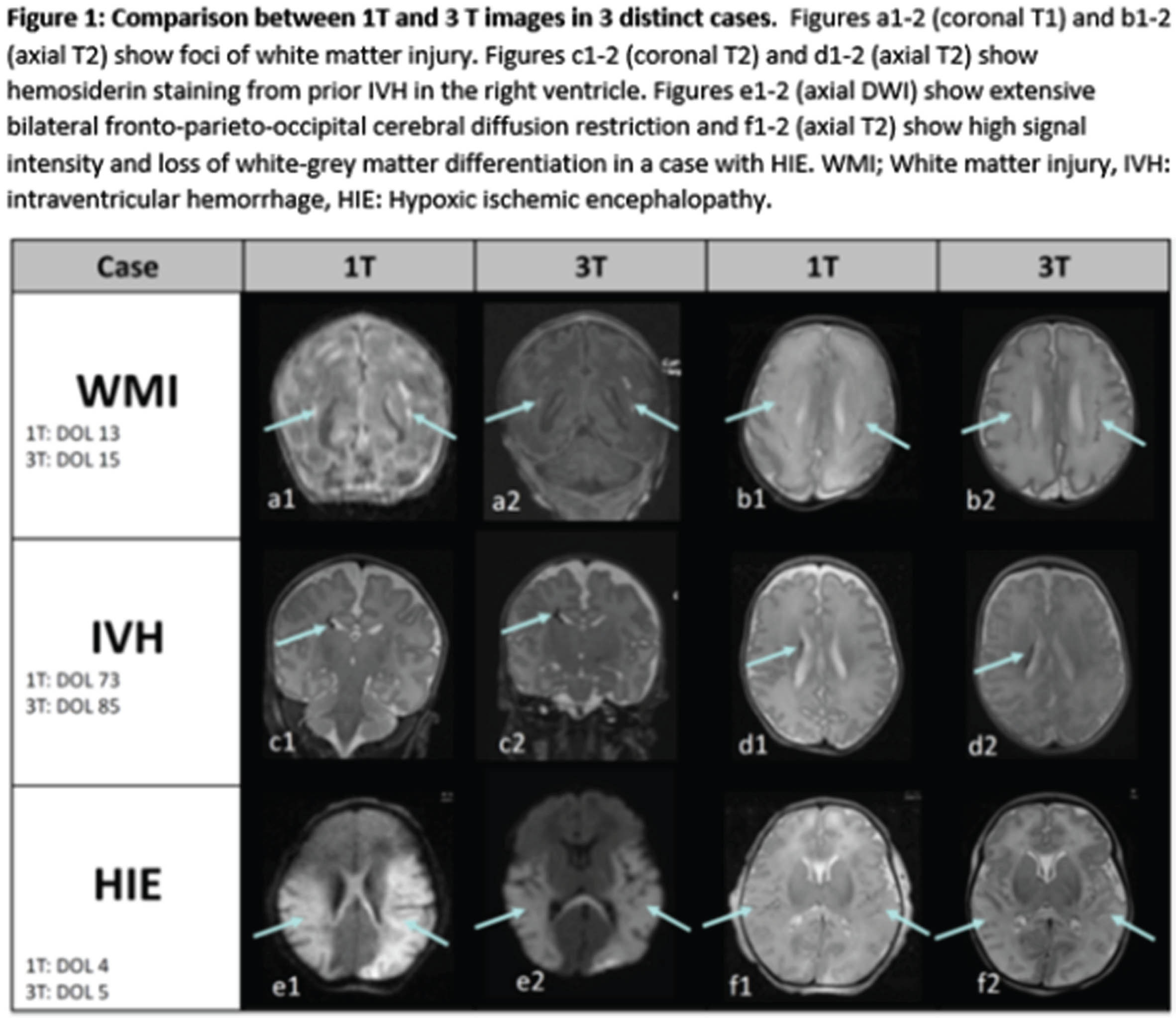
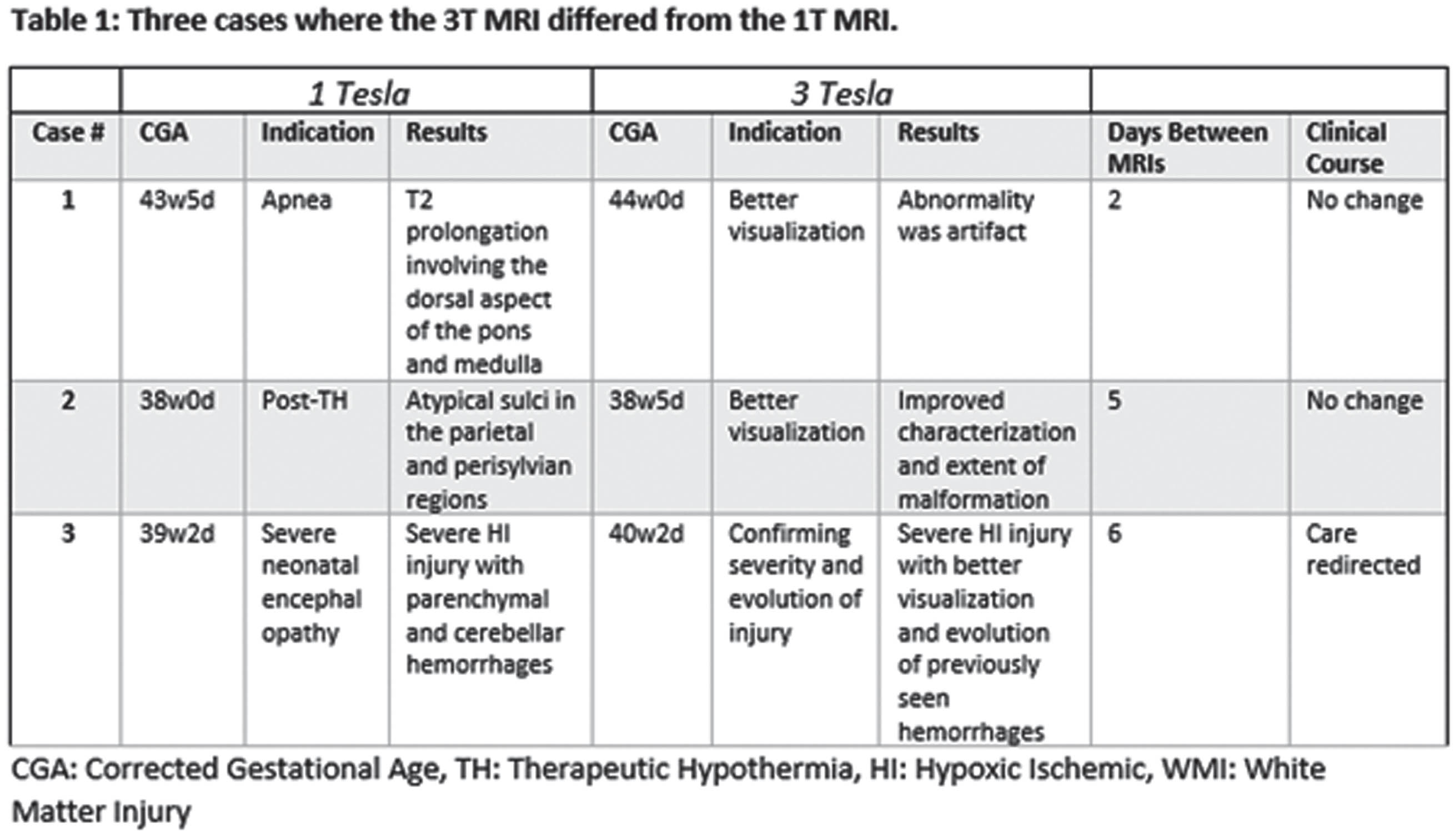
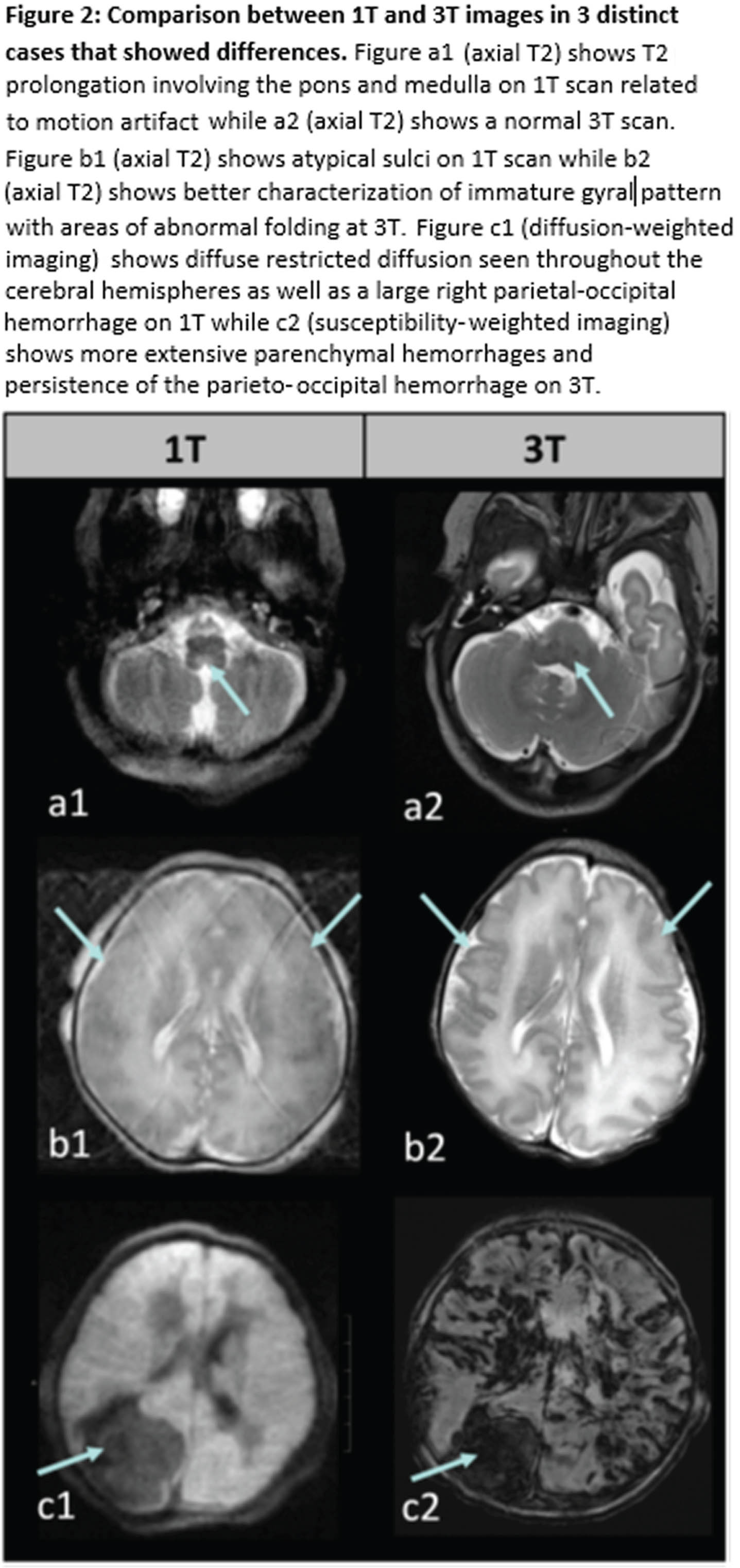
CONCLUSION: Images from 1T MRI are sufficient for characterizing a wide range of neonatal brain injuries and abnormalities. During the study period, there were limitations associated with the 1T scanner, such as the absence of susceptibility-weighted imaging (SWI) and MRA/MRV. Moreover, in cases involving known or suspected malformations, 3T MRI offers increased signal-to-noise ratio (SNR) allowing increased spatial resolution. However, within our cohort, these limitations did not impact clinical management.
BIBLIOGRAPHY:
Thiim et al., J Perinatol Off J Calif Perinat Assoc. 2022.
Berson et al., Front Neurosci. 2023.
Inder et al., J Pediatr. 2021.
The role of cerebral ultrasound in the identification of potential SARS-CoV-2-associated neonatal brain lesions
Dr. Sofia Pellizzari1, Doctor Arianna Zuccato1, Doctor Maria Chiara Bagnato1, Doctor Laura Pecoraro1, Doctor Carlo Alberto Forcellini1, Doctor Mariela Adriana Ventola1, Doctor Elena Bonafiglia1, Doctor Francesca Bissolo1, Doctor Rossella Frassoldati1, Doctor Renzo Beghini1
1Neonatal Intensive Care Unit, University Hospital of Verona
BACKGROUND: Background and Purpose: Since the onset of the SARS-CoV-2 pandemic, concerns have arisen regarding the effects of COVID-19 infection during pregnancy. Previous studies have suggested an increased risk of severe complications for both mothers and neonates. However, the specific impact of SARS-CoV-2 on fetal brain development remains unclear. This study aims to investigate the influence of SARS-CoV-2 infection during pregnancy on neonatal neurological outcomes, with a focus on neuroradiological features detectable through a non-invasive method, such as cerebral ultrasound. The research also seeks to assess the risk of specific brain lesions in neonates exposed to the virus, considering various factors, including gestational age at the time of infection, the severity of maternal COVID-19 symptoms, maternal vaccination status, and the presence of placental histological lesions.
METHODOLOGY: Conducted at the Ospedale della Donna e del Bambino in Verona, this prospective observational cohort study included neonates born between January 2022 and May 2023, born at or after 34 weeks of gestation and without diagnoses of TORCH infections or congenital malformations. All newborns underwent cerebral ultrasound within ten days of birth. A follow-up examination was conducted at one month for SARS-CoV-2-exposed neonates. Ultrasound images of standard sagittal and coronal planes were obtained. Statistical analysis was performed using STATA software.
RESULTS: The study enrolled a total of 240 subjects, including 118 neonates exposed to SARS-CoV-2 during pregnancy and 122 unexposed neonates. Among the observed brain lesions, lenticulostriate vasculopathy was the most common in exposed neonates (8,5%). The frequent occurrence of this vasculitic and progressive brain lesion supports the hypothesis of a correlation with inflammation-related placental pathology caused by SARS-CoV-2. Other abnormalities, such as focal echogenicities, intraventricular hemorrhages, and subependymal cysts, were also identified. The study revealed that in utero exposure to SARS-CoV-2 is significantly associated with presence of these ultrasound brain lesions (p-value=0.007, a=0.05). The relative risk of brain injury in the exposed population was 3.87, with an Odds Ratio of 4.2. The SARS-CoV-2 exposure was also significantly associated with the presence of placental histopathological lesions (p-value <0.001), and these were significantly associated with the presence of ultrasound lesions (p-value=0,023).
CONCLUSION: This study’s findings suggest that neonates exposed to SARS-CoV-2 in utero face an increased risk of developing brain abnormalities detectable through cerebral ultrasound, including lenticulostriate vasculopathy, subependymal cysts, and intraventricular hemorrhages. These results underline the importance of preventing SARS-CoV-2 infection during pregnancy, potentially necessitating closer monitoring of virus-exposed fetuses. Moreover, they emphasize the need for postnatal neurological follow-up for neonates exposed to the virus at birth. These findings open new research perspectives into the effects of COVID-19 and congenital viral infections on fetal, neonatal, and infant health, encouraging further studies for a better understanding of long-term effects.
Neural mechanisms underlying emotion processing and autistic traits during task-based fMRI following very preterm birth
Ms Marguerite Leoni1,2, Dr Lucy Vanes1,2, Ms Anais Durand1,2, Ms Laila Hadava1,2, Dr Chiara Nosarti1,2
1Centre for the Developing Brain, School of Biomedical Engineering & Imaging Sciences, King’s College London, 2Department of Child and Adolescent Psychiatry, Institute of Psychiatry Psychology and Neuroscience, King’s College London
BACKGROUND: Very preterm birth (VPT; <32 weeks’ gestation) is associated with altered brain development and behavioural problems such as facial emotional processing. In addition, VPT born participants are at significantly greater risk of being diagnosed with autism spectrum disorder (ASD) compared to their full-term (FT) born peers. In this study, whole brain activation and its association with dimensional ASD traits was assessed in VPT and FT children in a implicit facial emotional processing task-based fMRI paradigm.
METHODOLOGY: 83 VPT and 61 age-matched FT participants (7-11 years old) underwent an implicit facial emotion processing functional magnetic resonance imaging (fMRI) task to explore hemodynamic responses associated with facial emotion processing in association with autistic-like difficulties with social communication and interaction (SCI). These were assessed using the Social Responsiveness Scale – 2nd edition (SRS-2).
RESULTS: VPT participants displayed hyperactivation in the right superior frontal and mid occipital gyri when processing angry and fearful faces (compared to neutral faces), respectively. Whole brain analyses revealed that increased social communication difficulties in both VPT and FT participants were associated and with reduced activation of the left superior and inferior occipital gyri when processing faces (compared to shapes), and with increased activation in the right fusiform gyrus when processing angry (compared to neutral) faces. In contrast, VPT participants showed a positive association between social communication deficit and activation in left mid-temporal gyrus when processing fearful (compared to neutral) faces, while this association was reversed in FT participants.
CONCLUSION: Our results highlight both overlapping and distinct mechanisms underlying social communication deficits in preterm and full-term children. Group differences between FT and VPT emerged particularly in brain areas associated with processing emotions and first-level face processing. On the other hand, differences related to increased social communication difficulties in whole brain analyses were seen in VPT children in brain regions involved in the “social brain” and face-specific areas. VPT brain activation during facial emotional processing seems unique to this population as it differs significantly from FT.
Risk of severe intraventricular hemorrhage in premature infants with prenatal exposure to illicit substance/s.
Doctor Divya Rana1, Dr. Mimily Harsono, Dr. Massroor Pourcyrous1
1University Of Tennessee Medical Science Center
BACKGROUND: Maternal substance use disorder (SUD) is a major public health concern, particularity in united States with rising use of illegal synthetic opioids as well as legalization of marijuana. SUD during pregnancy increases risk of stillbirth, birth defects, low birth weight and premature birth. Use of one or more substance/s. which easily cross placenta, have neurovascular pharmacodynamic effects and can potentially increase risk of severe intraventricular hemorrhage (IVH). Published reports on infants with gestational age (GA) <32 weeks or birth weight (BW) <1500 grams showed incidence of all grades IVH remains at ~25 % and severe-IVH at ~7.7%. However, the incidence of IVH in premature neonates exposed to illicit substance/s is unknown.
OBJECTIVE: We hypothesized that fetal exposure to illicit substance/s will increase risk of IVH in premature infants.
METHODOLOGY: This is a prospective cohort study of Cranial Ultrasound (CUS) findings in preterm infants who had in-utero illicit substance/s exposure. Serial CUS are obtained within first 14 days, at 21-30 days-of-life and at corrected-GA of 36 weeks. We included all live birth with GA <32wk or BW <1,500g that were born in 2014 - 2020. In-utero illicit drug exposed infants were identified from maternal history, maternal urine toxicology test and confirmed with post-natal umbilical cord tissue toxicology test. We excluded complex congenital and chromosomal anomalies infants.
RESULTS: 1,164 infants (GA <32wk or BW <1,500g) were born in our maternity Hospital from 2014 - 2020. We considered CUS to be abnormal based on the worst grade identified in the first 30 days-of-life. The incidence of IVH in non-exposed infants was as follows: grade I 19.6%, grade II 7.8%, grade III 2.9% and grade IV 6.3%. We identified 55 infants that were exposed prenatally to illicit substance/s. Of the exposed infants, 58.2% (32/55) had abnormal CUS; compared to non-exposed; the incidence of IVH was as follows: grade I 43.6% [p<0.01; OR 3.89, 95% CI 2.11-7.17], grade II 9.1% [p=0.09; OR 2.02, 95% CI 0.74-5.52], grade III 1.8% [p=0.46; OR 1.1, 95% CI 0.14-8.44] and no grade IV IVH [p=0.33; OR 0.24, 95% CI 0.01-4.07]. Maternal-Infant characteristics (Table 1).
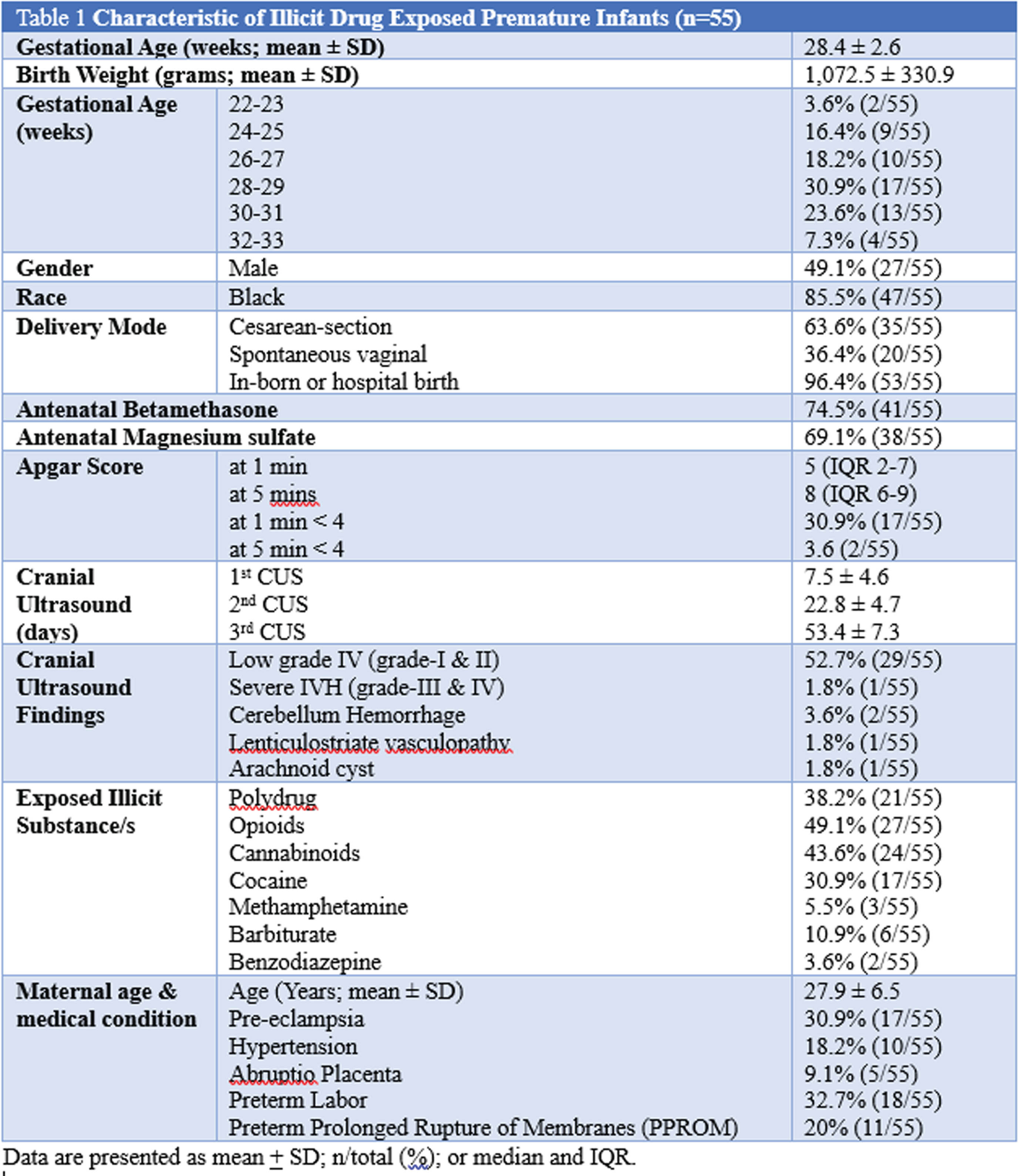
CONCLUSION: Low grade IVH (grade 1 and 2) was noted in majority of the illicit substance/s-exposed premature infants, and severe IVH (grade 3 and 4) was lower compared to non-exposed infants. We plan to further investigate the severity of IVH and its correlation with the drug of abused, umbilical cord drug concentrations, maternal lifestyle, and other relevant medical-social issues.
Early and term-equivalent age MRI in detection of white matter injury in very preterm infants
Doctor Sriya Roychaudhuri1,2, Doctor Gabriel Cote-Corriveau1,3, Doctor Carmina Erdei1,4, Doctor Terrie Inder1,4,5
1Division of Newborn Medicine, Department of Pediatrics, Brigham and Women’s Hospital, 2Division of Neonatal-Perinatal Medicine, Department of Pediatrics, Surrey Memorial Hospital, University of British Columbia, 32Department of Pediatrics, Sainte-Justine University Hospital Center, 4Harvard Medical School, 5Division of Neonatology, Children’s Hospital of Orange County and Department of Pediatrics, University of California
BACKGROUND: White matter injury (WMI) in infants born preterm is the most prevalent form of injury and associated with adverse neurodevelopmental outcomes. Magnetic resonance imaging (MRI) has distinct advantages over routine cranial ultrasound to visualize WMI in the initial weeks following preterm birth and at term-equivalent age (TEA). Systematic MRI scoring methods to assess the extent of WMI have been proposed for both early and TEA times. Early MRI interpretation focuses on characterization of the number and size of the WMI lesions,(1) whereas TEA MRI captures sequelae of prior injury such as cystic lesions, signal abnormalities, delayed myelination and white matter volume loss.(2)
OBJECTIVE: To compare WMI on early brain MRI, at 30 to 34 weeks post-menstrual age (PMA), with WMI assessment at TEA (37-42 weeks PMA) MRI, using two standardized scoring systems, in infants born < 33 weeks gestational age (GA).
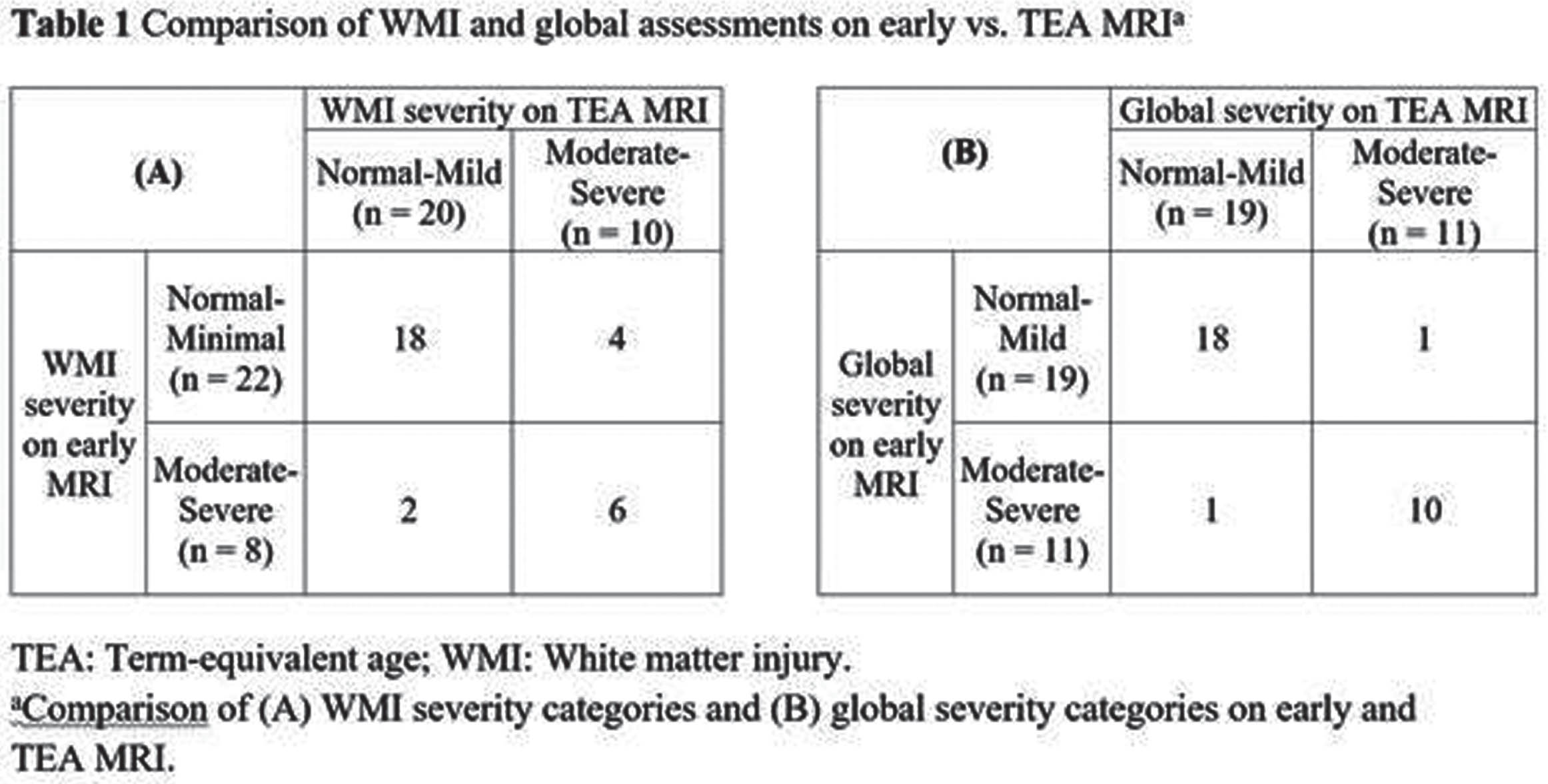
MATERIALS AND METHODS: The Miller et al (1) scoring was used for categorization of injury on early MRI. TEA MRIs were assessed for WMI and its sequelae using the Kidokoro et al (2) score. The degree of WMI and global injury were categorized as normal-mild and moderate-severe. Global injury scores consider presence and degree of intraventricular hemorrhage and ventricular dilation along with WMI. We assessed the relationship between early and TEA classifications using Fisher’s exact tests.
RESULTS: The cohort consisted of 30 infants with mean GA of 28.7 weeks and median birthweight of 1220 grams. The early MRIs were performed at an average of 33.0 weeks (SD = 1.4), and the TEA MRIs at 38.6 weeks (SD = 1.4) PMA (Figure 1). There was a strong association between the systematic assessments of global injury severity (p < 0.001) and WMI (p=0.007) at the two time points (Table 1)(Figure 2). There was no instance of newly detected WMI or increased lesion severity noted on TEA when compared to early MRI scans.
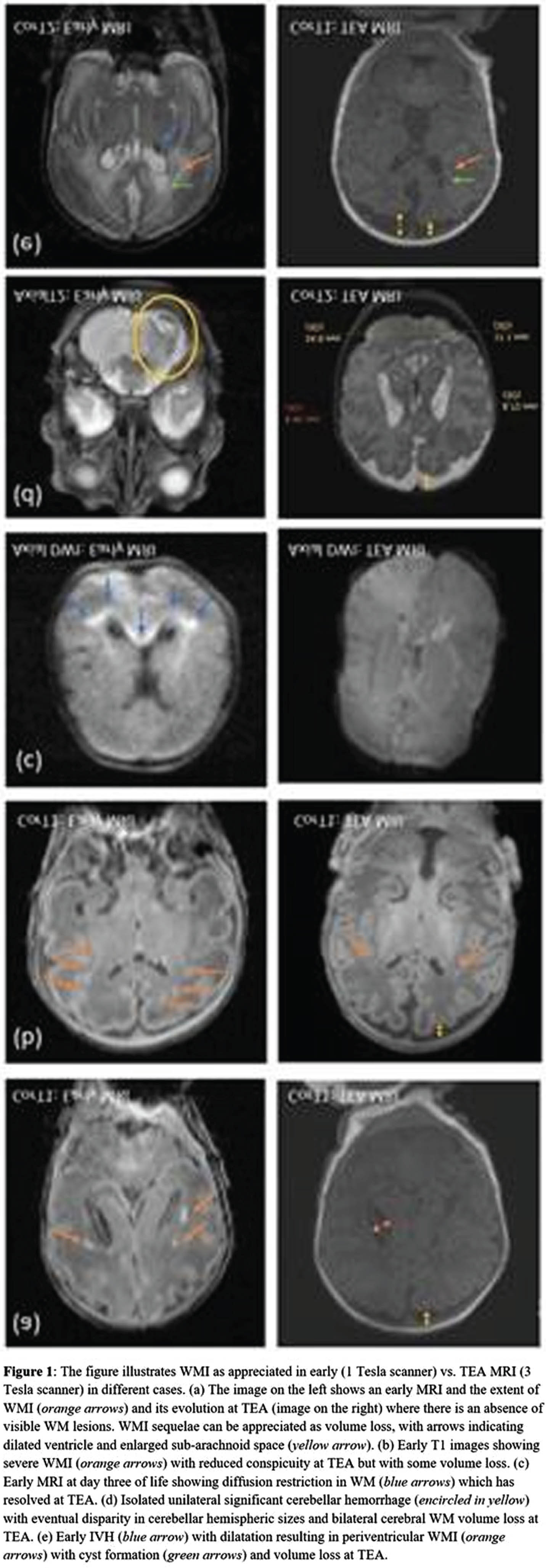
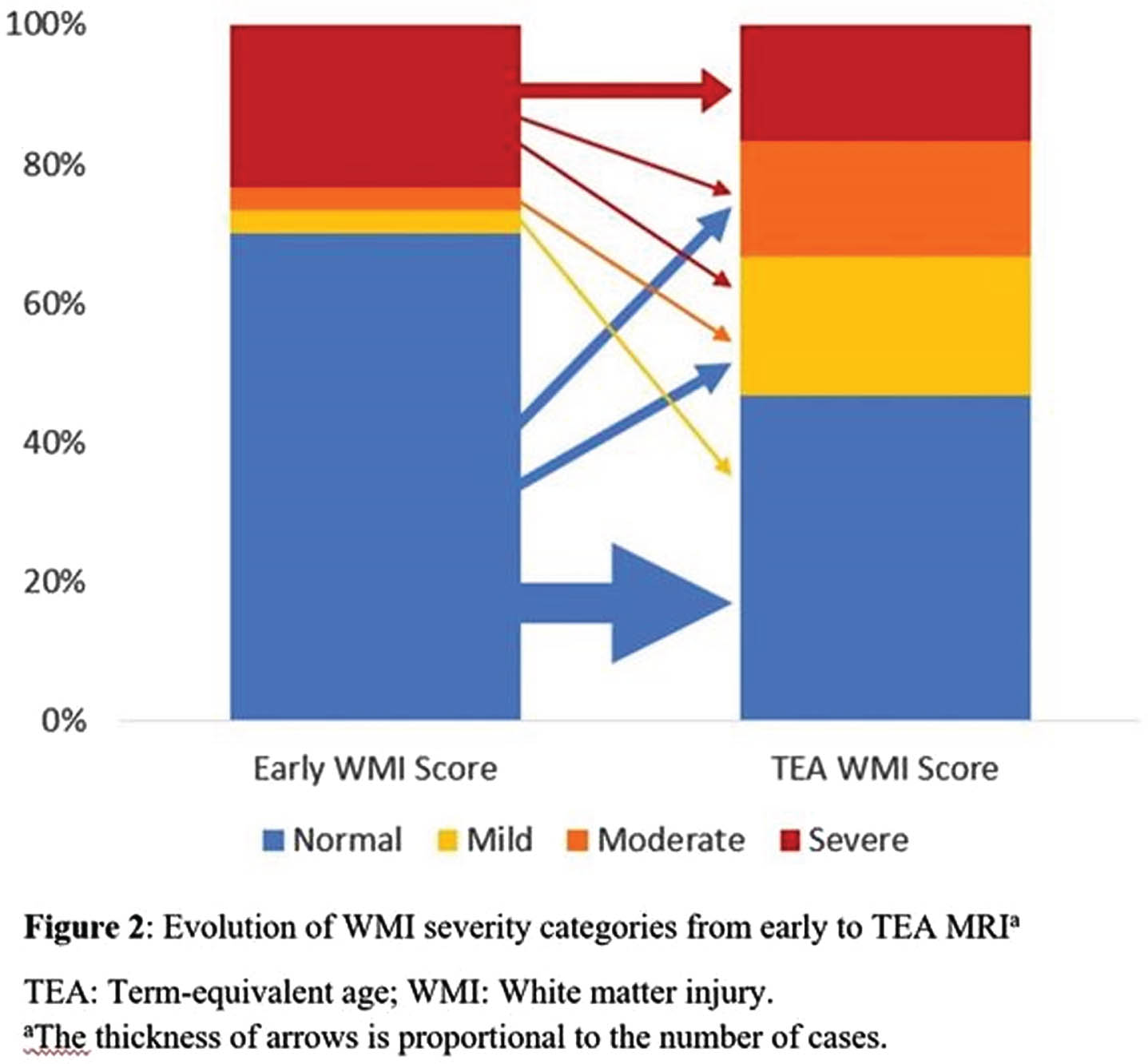
CONCLUSION: Evaluating brain injury using standardized scoring systems enables clinicians and researchers to analyze images in a systematic manner and follow WMI longitudinally in very preterm infants. Although the optimal timing to undertake neuroimaging in the preterm infant remains to be determined, early (30-34 weeks) and TEA MRI may independently provide valuable information on WMI and risk for associated sequelae.
BIBLIOGRAPHY:
1. Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005 Nov;147(5):609–16.
2. Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34(11):2208–14.
Intraventricular brain hemorrhage detection through transfontanelle photoacoustic imaging: An innovative approach
Ms Juliana Benavides1, Dr Rayyan Manwar1, Dr. Laura S. McGuire2, Mr Md. Tarikul Islam1, Dr Anthony Shoo3, Dr Fady T. Charbel2, Dr Martha G. Menchaca3, Dr Amanda P. Siegel1, Dr De-Ann M. Pillers3, Dr. Juri G. Gelovani4, Dr Karman Avanaki1
1Richard and Loan Hill Department of Bioengineering, Richard and Loan Hill Department of Bioengineering, University of Illinois at Chicago, 2Department of Neurological Surgery, University of Illinois at Chicago – College of Medicine, 3Section of Neonatology, Department of Pediatrics, UIHealth Children’s Hospital of the University of Illinois at Chicago, 4Provost Office, College of Medicine and Health Sciences, United Arab Emirates University
SUMMARY: The capability of transfontanelle photoacoustic imaging (TFPAI) to detect intraventricular and periventricular hemorrhages has been demonstrated in a sheep brain model in-vivo.
BACKGROUND AND PURPOSE: In preterm neonates, intraventricular (IVH) and periventricular (PVH) hemorrhages are common due to the fragility of undeveloped periventricular blood vessels. While transfontanelle ultrasound has been utilized for initial screenings of the brain through the anterior fontanelle, its reliability in diagnosing mild hemorrhages remains uncertain. Inconclusive diagnoses are common in those cases, leading to potential oversight and increased risks of moderate to severe neurodevelopmental complications. This study demonstrates the capability of photoacoustic (PA) imaging to detect IVH/PVH volumes as small as 0.3 mL of blood in the brain.
METHODOLOGY: A cranial window, simulating the neonatal fontanelle, was created in an adult sheep model with a diameter of 2.5 mm. The hemorrhage was induced by injecting heparinized arterial blood into the left lateral ventricle in volumes ranging from 0.1 to 1.0 mL in increments of 0.1 mL. Photoacoustic images were acquired at a 798 nm wavelength (isosbestic point for oxyhemoglobin and deoxyhemoglobin) after each injection.
RESULTS: In both IVH and PVH models, photoacoustic average intensity showed a linear increase corresponding to the increment of the injected volumes of blood, effectively detecting mild hemorrhages. For IVH model, it was detected volumes as small as 0.3 mL and 0.2 mL for the two animals used in the study. In contrast, the PVH mode showed detection at 0.2 mL and 0.3 mL respectively. Comparative analysis with ultrasound data revealed that IVH signals were detectable in cerebrospinal fluid (CSF) at 0.5 mL, which corresponds to 5% bleeding, aligning with clinical neonatal studies. For the PVH model, ultrasound detection started at an injection of 0.4 mL. While both methods identified IVH and PVH in vivo, TFPAI demonstrated superior sensitivity in detecting smaller hemorrhages.
CONCLUSION: The results demonstrate that the TFPAI signal intensity is strongly correlated with the concentration of blood in ventricular CSF and volume of blood in a periventricular lesion. In addition, the comparison with the results from the ultrasound imaging showed that the photoacoustic imaging modality has higher sensitivity for the detection of mild hemorrhages in both models IVH and PVH. Future steps aim to refine blood measurement accuracy in CSF through alternative image reconstruction algorithms, compensating for non-linear effects to enhance the precision of weak PA signals. We expect that the TFPAI probe will be a valuable monitoring tool especially for neonates with critical conditions and admitted into intensive care unit (NICU).
Hemodynamic responses associated with spontaneous neural activity in the early developing brain
Doctor Anna Shiraki1,2, Doctor Hama Watanabe3, Professor Gentaro Taga3, Doctor Misa Hashimoto1, Doctor Misae Yamada1, Doctor Hajime Narita1, Doctor Takamasa Mitsumatsu1, Doctor Sumire Kumai1, Doctor Ryosuke Suzui1, Doctor Fumi Sawamura1, Doctor Takashi Maeda4, Doctor Yuji Ito1, Doctor Hiroyuki Yamamoto1, Doctor Tomohiko Nakata1, Professor Yoshiaki Sato4, Professor Jun Natsume1,5, Professor Hiroyuki Kidokoro1
1Department of Pediatrics, Nagoya University Graduate School of Medicine, 2Department of Pediatrics, Nagoya Memorial Hospital, 3Graduate School of Education, The University of Tokyo, 4Division of Neonatology, Center for Maternal-Neonatal Care, Nagoya University Hospital, 5Department of Developmental Disability Medicine, Nagoya University Graduate School of Medicine
BACKGROUND AND PURPOSE: Delta brushes are prominent intrinsic activities observed in the electroencephalogram (EEG) of preterm infants, typically between 32 and 36 weeks of postmenstrual age (PMA). These spontaneous neural activities are believed to be associated with the activity of subplate neurons, which is crucial for early brain development. However, it remains unclear whether the specific brain regions respond to these localized neural activities. We aimed to explore hemodynamic responses associated with delta brushes in preterm EEG using simultaneous EEG and functional near-infrared spectroscopy (fNIRS) recordings.
METHODOLOGY: We enrolled 17 infants whose gestational ages ranged from 30 to 34 weeks. EEG-fNIRS recordings were conducted at two time points: 34 and 36 weeks of PMA, with a median analysis duration of 50 min. EEG was recorded polygraphically with eight electrodes (Fp1, Fp2, C3, C4, O1, O2, T3 and T4) to distinguish active sleep (AS) from quiet sleep (QS). To identify the brush component of delta brushes in the bipolar EEG envelope, we developed an automated detection algorithm. This algorithm filtered EEG at 8–25 Hz and detected brushes with durations of 0.3–1.5 s and average amplitudes exceeding 10 μV within a sliding window of 0.3 s. An eight-channel NIRS device was placed around the head to measure changes in oxy- and deoxy-hemoglobin concentrations (Fig. 1). Hemodynamic grand averages were calculated for localized brushes in each NIRS channel during both AS and QS in each recording. Subsequently, we conducted group analyses based on the PMA at the time of recording (Fig. 2). The group analysis data were classified into three responses according to the relationships between oxy- and deoxy- hemoglobin changes: in-phase, anti-phase, and no response. We also investigated the associations between these hemodynamic responses, the PMA at the time of recording, and NIRS channel positions, including frontal, temporal-anterior, temporal-posterior, and occipital areas.
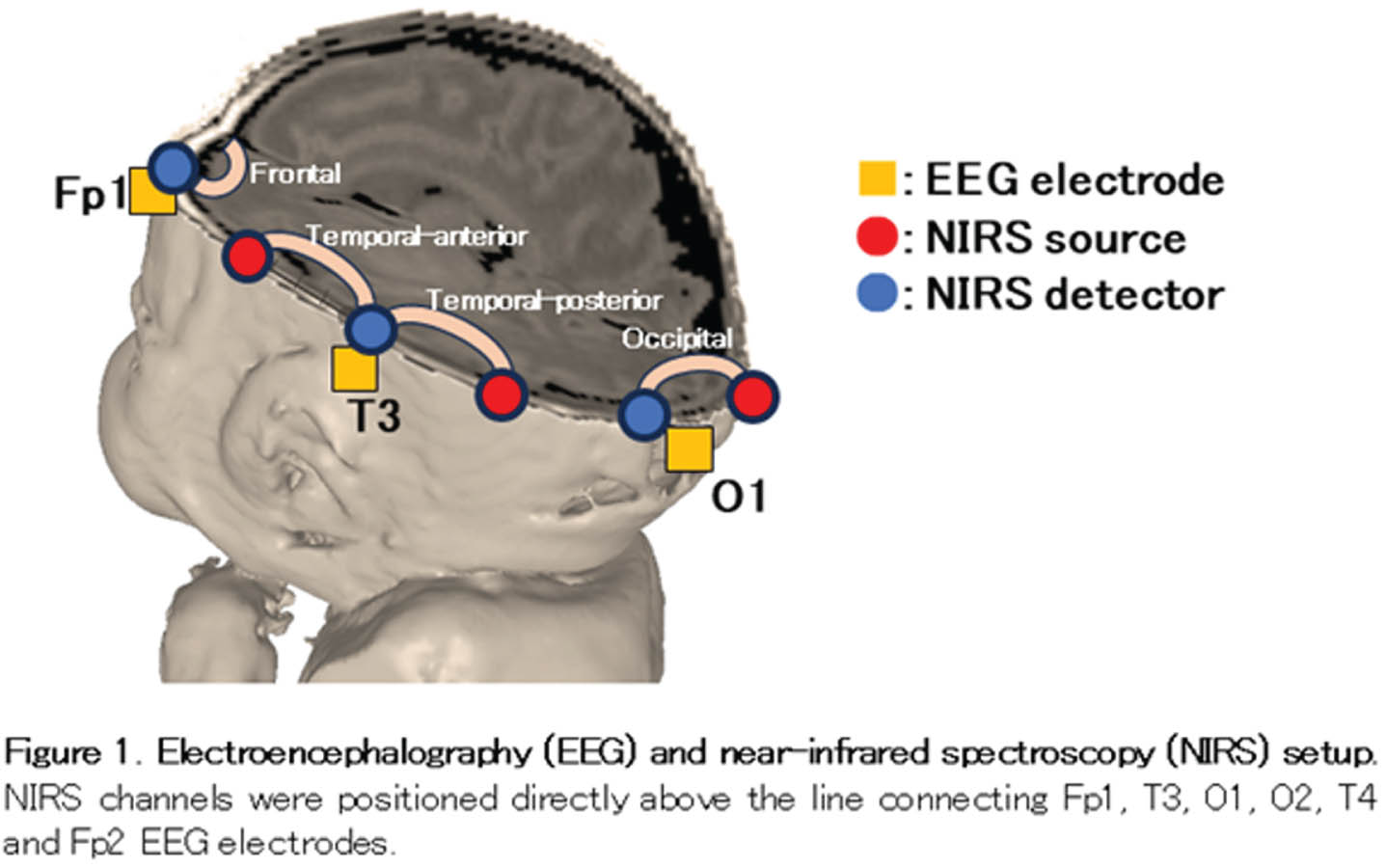
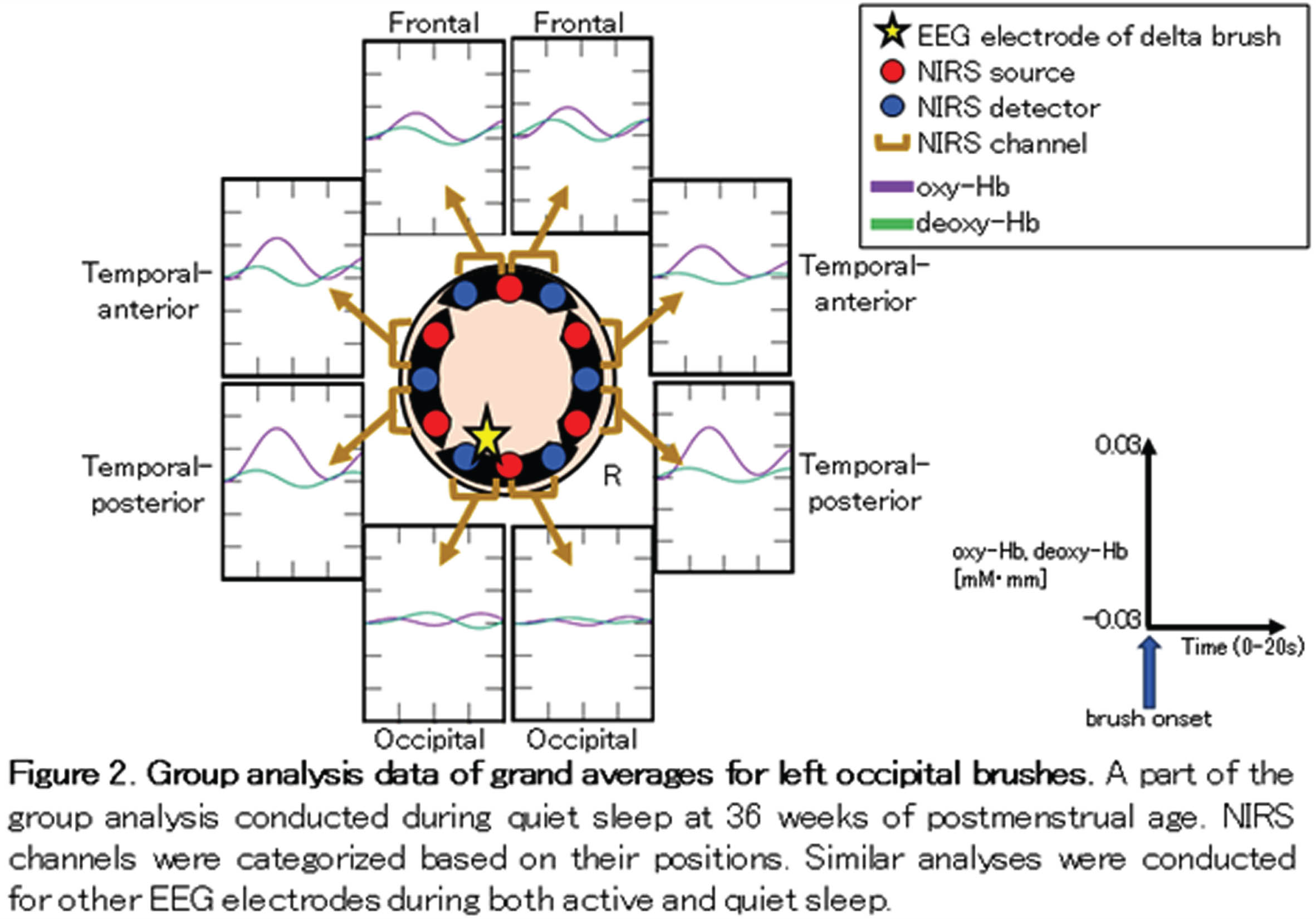
RESULTS: Among the 64 grand average data sets for each PMA group and sleep state, over 75 % displayed hemodynamic changes associated with localized delta brushes (Fig. 3). At 36 weeks of PMA, there was a higher prevalence of anti-phase responses observed in both AS and QS. Considering NIRS channel positions, the temporal-anterior regions exhibited a more pronounced hemodynamic response rate in both the 34 and 36-week PMA groups (Fig. 4). Notably, the increased prevalence of anti-phase responses in the temporal-anterior regions at 36 weeks of PMA.
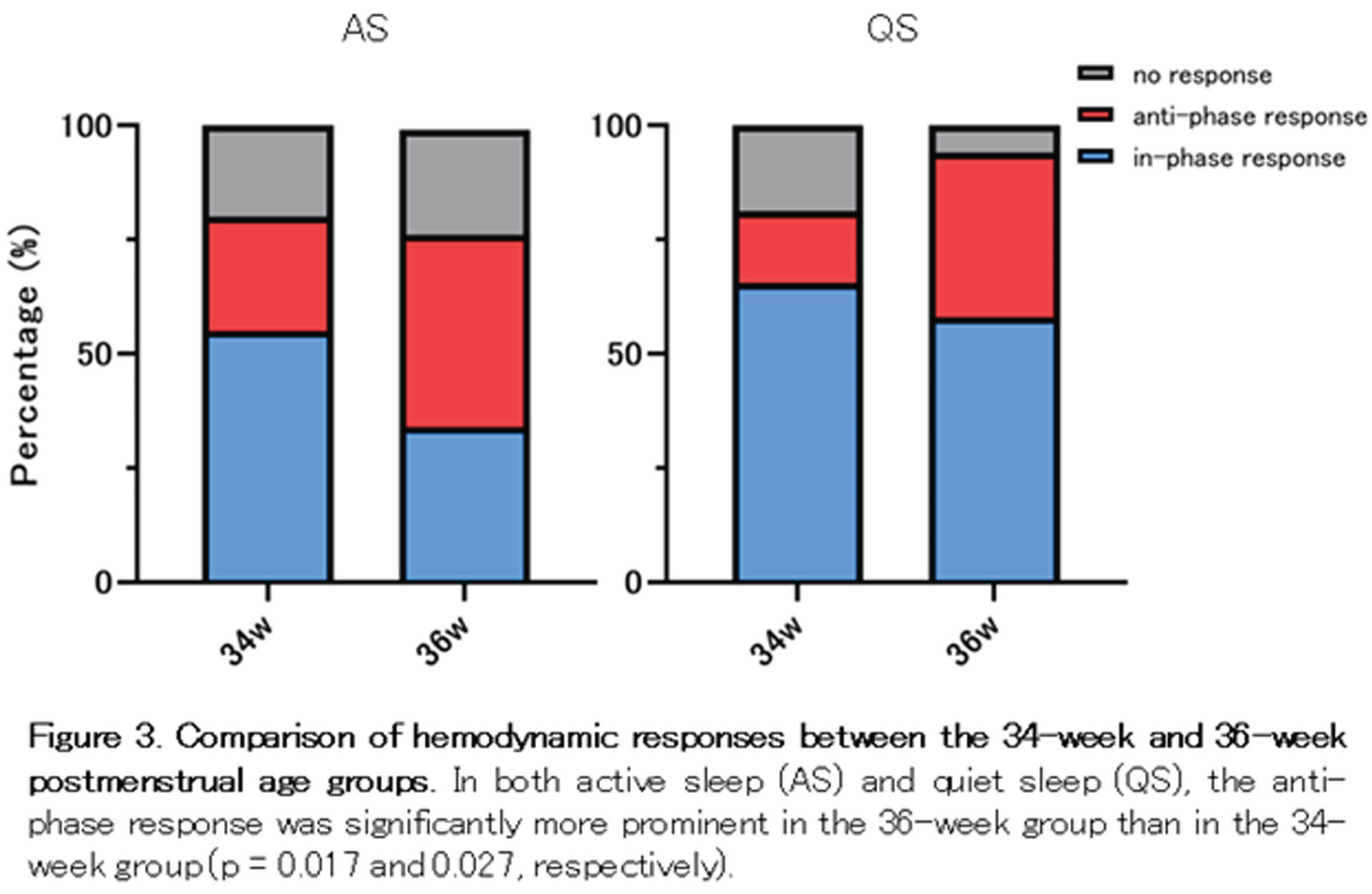
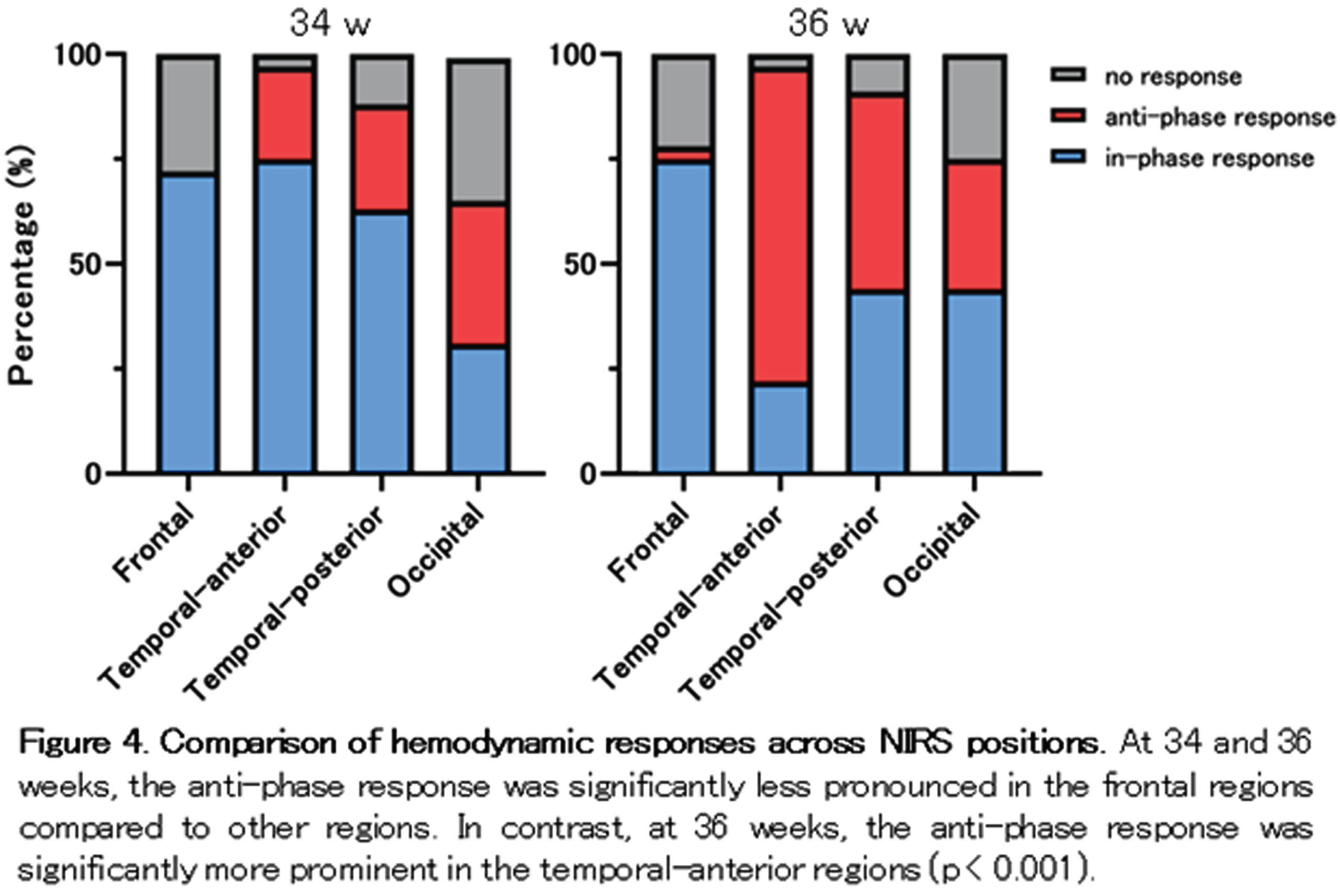
CONCLUSION/IMPACT: Delta brushes were associated with hemodynamic responses in broad areas as early as 34 weeks of PMA. These responses shifted from an in-phase to an anti-phase pattern as the brain matured. The increased rate and prevalence of anti-phase responses in the temporal-anterior regions indicate the involvement of the insula, a most strongly interconnected hub in the developing brain.
Contemporary patterns of brain injury in infants with neonatal encephalopathy in the therapeutic hypothermia era
Aisling Garvey1,2, Eniko Szakmar2,3, Hoda El-Shibiny2, Hélène Meunier4, Brian Walsh1,2,5, Sara Cherkerzian2, Mohamed El-Dib2, Terrie Inder2,6
1Infant Research Centre, 2Department of Pediatrics, Division of Newborn Medicine, Brigham and Womens Hospital, Harvard Medical School, 31st Department of Pediatrics, Semmelweis University, 4Department of Neonatology, Alix de Champagne, 5Department of Neonatology, Cork University Maternity Hospital, 6Childrens Hospital of Orange County, University of California Irvine
BACKGROUND: Brain injury in Neonatal Encephalopathy (NE) has historically been classified into injury to the deep grey matter, white matter/watershed area or global injury (1). However, injury in other areas have been described (2,3). This study aimed to describe contemporary patterns of brain injury in infants with NE in the era of therapeutic hypothermia (TH) and to examine potential risk factors associated with different patterns of injury.
METHODOLOGY: Retrospective analysis of all infants with NE admitted for TH to the Brigham and Women’s Hospital, a tertiary NICU (January 2016-December 2021). MRIs were performed after rewarming and scored independently by 3 reviewers blinded to outcome and grade of encephalopathy using the Weeke scoring system (2). Mann-Whitney U Tests were performed to compare risk factors in infants with and without different patterns of injury.
RESULTS: Two-hundred and eighty-nine infants with all grades of NE were included with a median gestational age of 39.3weeks (IQR 37.5-40.1) and median birthweight of 3.13kg (IQR 2.72-3.54). Of these, 196 (68%) had abnormal MRI findings. Locations of injury are outlined in Table 1. Injury to the grey matter was associated with the requirement for extensive resuscitation (chest compressions p<0.001, epinephrine p<0.001), lower 10-minute Apgar scores (p=0.003), worse post-natal gas measurements (pH p=0.043, base deficit p=0.033, lactate p=0.031) and the requirement for inotropes in the NICU (p=0.013). Injury to the white matter was associated with a lower birthweight (p=0.005), risk of chorioamnionitis (p=0.033), lower umbilical pH values (p<0.001), lower 5- and 10-minute Apgar scores (p=0.021 and 0.015 respectively) and the requirement for intubation (p=0.004). Infants with grey or white matter injury had a higher clinical (p=0.003, p=0.001) and EEG grade (p=0.002, p=0.048) of NE and were more likely to require intubation (p=0.022, p=0.004). These infants had a higher incidence of seizures (p<0.001) and death (p=0.003), and had longer hospital stays (p<0.001). Cerebellar injury was associated with vaginal delivery (p=0.043) and incidence of PPV (p=0.043).
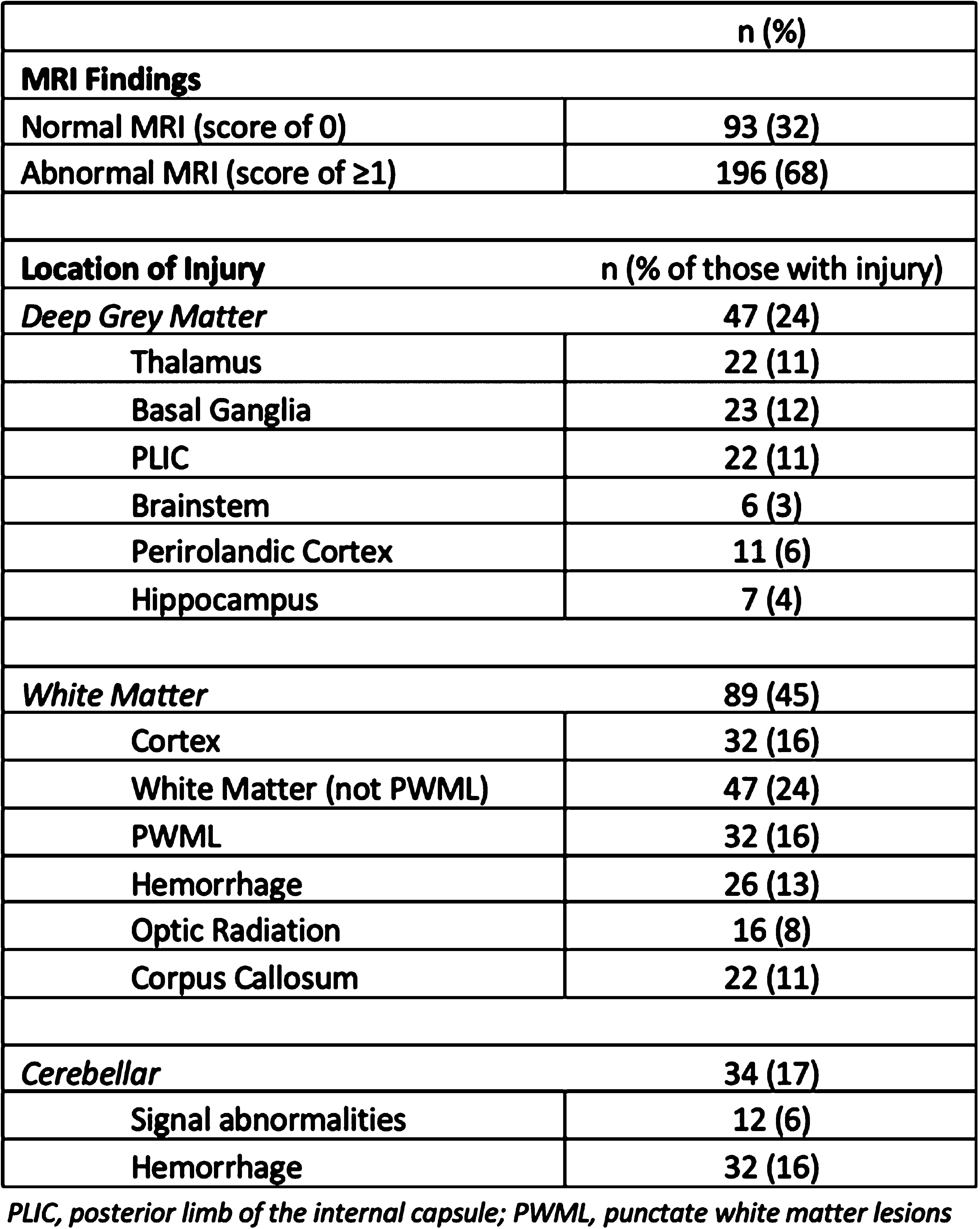
CONCLUSION: Areas of brain injury noted in infants with NE extend beyond the hallmark patterns of basal ganglia/thalamus and watershed areas and are associated with different risk factors which may aid in the short-term prediction of outcome.
BIBLIOGRAPHY:
1. Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR. 1998;19(1):143-9
2. Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr. 2018;192:33-40.e2
3. Wisnowski JL, Wintermark P, Bonifacio SL, Smyser CD, Barkovich AJ, Edwards AD, et al. Neuroimaging in the term newborn with neonatal encephalopathy. Semin Fetal Neonatal Med. 2021;26(5):101304
Magnetic resonance imaging and proton spectroscopy predicts neurodevelopmental impairment outcomes after neonatal encephalopathy in Uganda
Dr Samantha Sadoo1, Ms Carol Nanyunja1, Dr Ivan Mambule2, Professor Emily Webb1, Dr Jamiir Mugalu3, Dr Alan Bainbridge4, Dr Kelly Pegoretti Baruteau5, Dr Francisco Torrealdea4, Dr Latha Srinivasan5, Ms Allena Nabawanuka6, Dr Gilbert Gilbert7, Dr Sean Mathieson8, Mr Joe Bwambale6, Dr Samson K Lubowa6, Mr Huzair Ssesembo6, Dr Pia Wintermark10, Professor Geraldine Boylan8, Professor Moffat Nyirenda2, Professor Nicola Robertson11, Professor Michael Kawooya13, Professor Frances Cowan12, Dr Annettee Nakimuli9, Doctor Cally Tann1
1London School of Hygiene & Tropical Medicine, 2MRC/UVRI & LSHTM Uganda Research Unit , 3Kawempe National Referral Hospital, 4University College London , 5University College London Hospital, 6Kampala MRI Centre, 7MRC Clinical Science, Philips Healthcare, 8INFANT Research Centre, University College Cork, 9Makerere University, 10McGill University, 11University of Edinburgh, 12Imperial College London, 13Ernest Cook Ultrasound Research and Education Institute (ECUREI)
BACKGROUND: Intrapartum-related neonatal encephalopathy (NE) is a leading cause of child death and disability globally, especially in low-income countries where therapeutic hypothermia is frequently unavailable (1). Neonatal brain magnetic resonance imaging and spectroscopy (MRI/MRS) are key tools for brain injury detection, assessment, and prognostication (2). We assessed feasibility and acceptability of MRI/MRS and predictive validity for adverse neurodevelopmental outcome after NE in Uganda.
METHODOLOGY: Neonates were recruited to a prospective cohort study (“Baby BRAiNS”) at a tertiary referral facility in Kampala, Uganda(3). Participants had NE (Thompson score ≥5; ≥36weeks; ≥1.8kg; resuscitation at birth) and underwent MRI/MRS at around day 10 of life (1.5 Tesla) after collaborative protocol development and training (3). Images were evaluated for diagnostic quality, injury pattern and severity, and scored using Rutherford and NICHD criteria (4,5). Basal ganglia and thalamus (BGT) MRS were analysed for peak area metabolite ratios, including lactate, N-acetylaspartate (NAA), creatine, and choline, using Tarquin. Adverse outcome (18-24 months) was defined as death or moderate-severe neurodevelopmental impairment; Bayley-III cognitive/motor score <70, Hammersmith Infant Neurological Examination <67, and/or GMFCS level 3-5. Optimal MRI score/MRS ratio thresholds for prediction of outcomes were identified through ROC curve analysis, and predictive validity assessed.
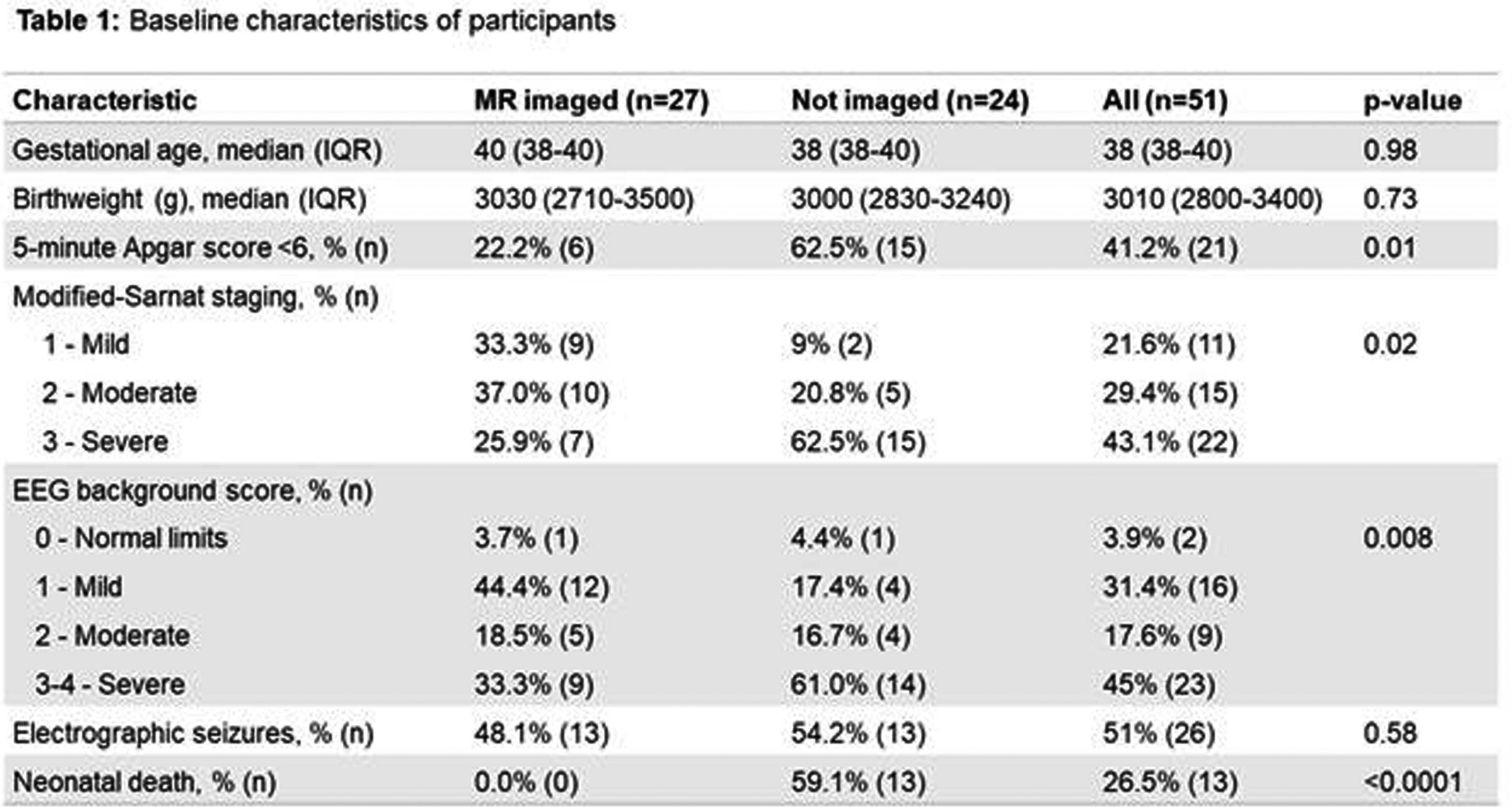
RESULTS: Of 51 participants, 27 consented for and underwent MRI, and 24 MRS (median 11 days, IQR 11-16.4), all of diagnostic quality: providing evidence of feasibility and acceptability. Twenty-four were not imaged due to death before scan (13), Covid-19 lockdown (6), failure to attend (3), and withdrawal (2). Of those imaged, 21 had outcome data (1 death; 5 adverse; 15 favourable). Non-imaged infants had more severe NE, EEG background, and higher mortality (Table 1). Abnormalities were seen in 93% (26); 84% (22) showed hypoxia-ischaemia, none showed antenatal injury. Severe abnormalities were seen equally in the BGT and white matter (14.8%). Of the 7 (26%) predictive of adverse outcome (Rutherford), 6 had adverse outcome on follow-up (Figure 1). Rutherford score ≥7 and NICHD ≥2A were both predictive of adverse outcome (Figure 2). MRS metabolite ratios were significantly different in the adverse compared to favourable outcome groups (p=0.01, 0.006, 0.009). Sensitivity and specificity of Lac/NAA ≥0.15 for adverse outcome were 83% and 92%, respectively (AUC 0.88) (Figure 3).
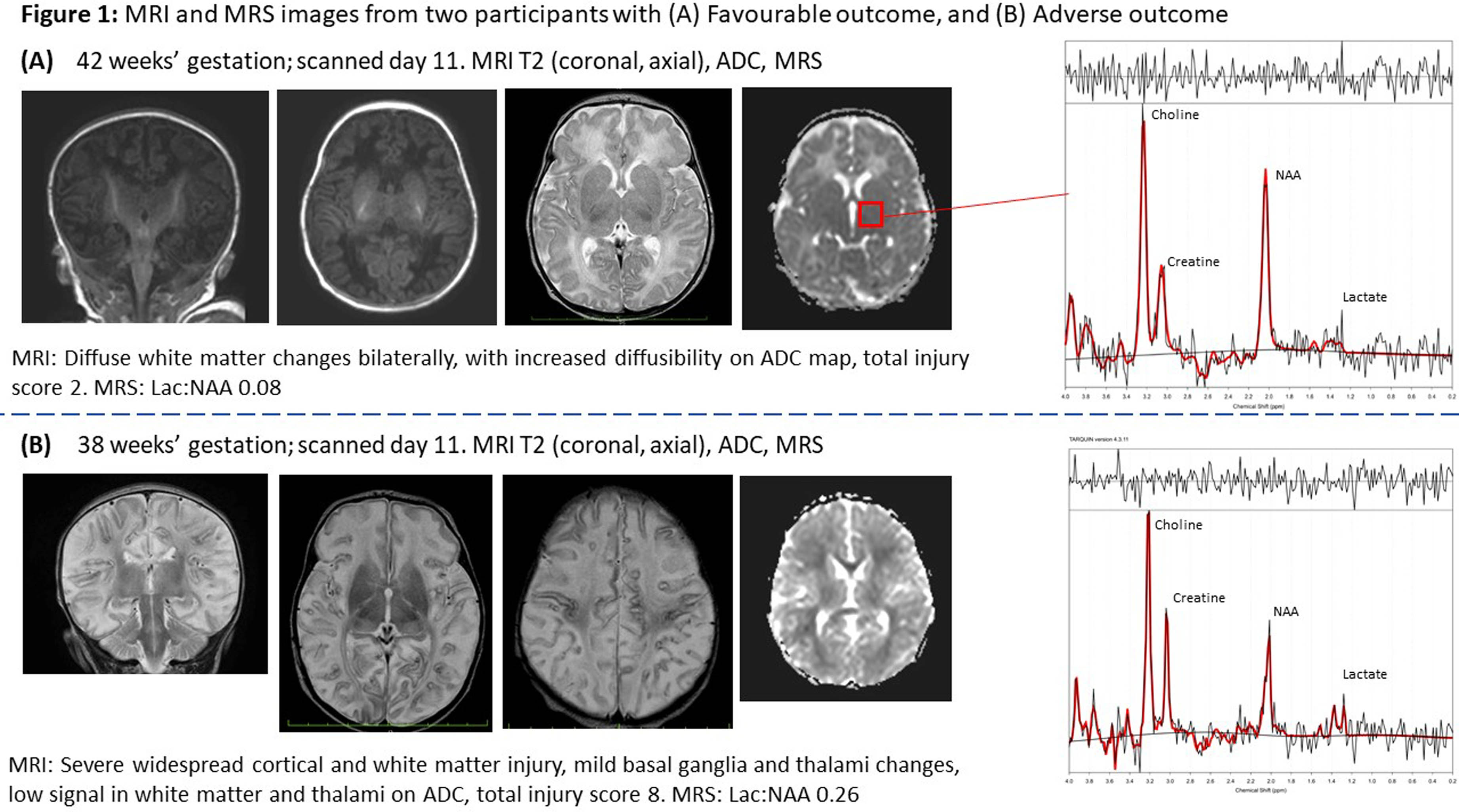
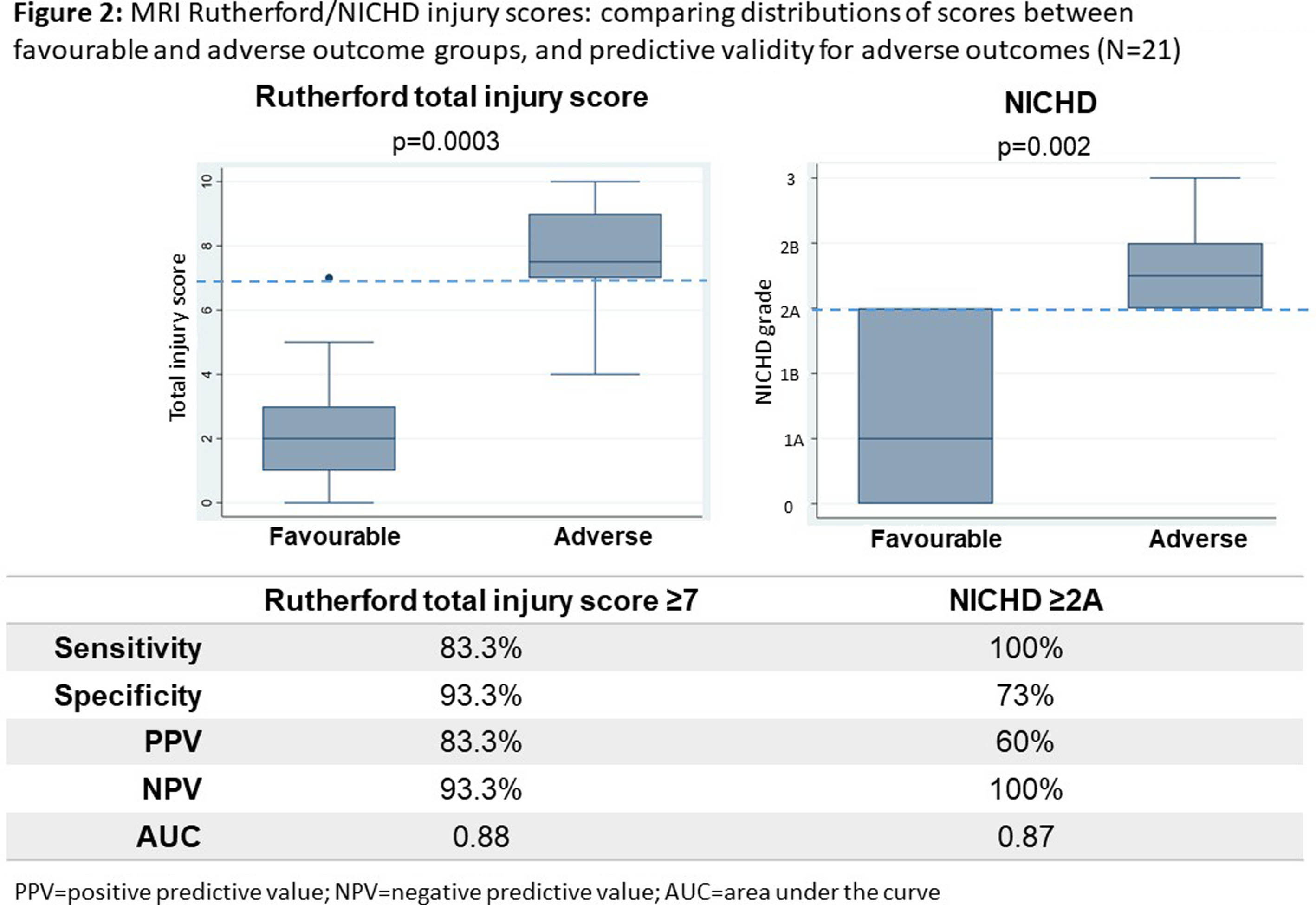
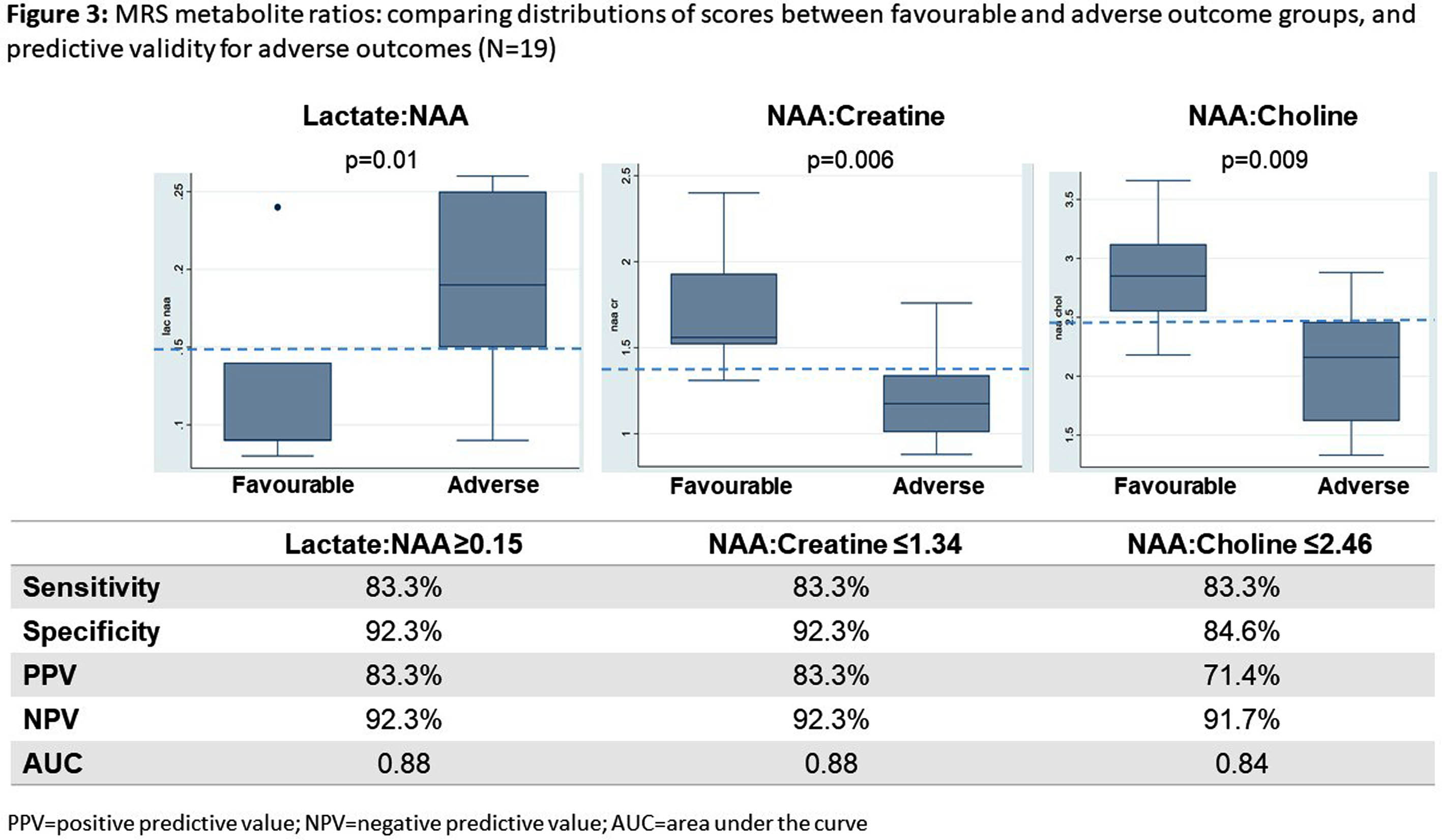
CONCLUSION: We found neonatal MRI/MRS to be feasible in this Ugandan research population. The majority had evidence of hypoxia-ischaemia on MRI. Patterns of brain injury and Lac/NAA ratios differed from UK cohorts, reflecting high early mortality or differences in exposures and aetiology in this resource-limited care context. MRI and MRS were predictive of 18-month neurodevelopmental outcomes and could be utilised in future trials of novel neuroprotective strategies.
BIBLIOGRAPHY:
1. UN. Levels and Trends in Child Mortality Report 2020.
2. van Laerhoven.Pediatrics.2013;131(1):88-98.
3. Nanyunja.Gates Open Res.2022,6:10
4. Rutherford,Lancet Neurol.2010;9(1):39-45.
5. Shankaran.NEJM.2012;366(22):2085-92.
Comparison of clinical and brain MRI findings between Canadian and Dutch moderate-late preterm infant cohorts
Ms Regan King1, Dr Emma Hofland-Burry1, Dr Gerda Meijler2, Dr Vivanne Boswinkel2, Mrs Jeanne Scotland2, Dr Martijn F. Boomsma2, Dr Hussein Zein1, Dr Leonora Hendson1, Dr Khorshid Mohammed1, Dr Lara M. Leijser1
1University Of Calgary, 2Isala Hospital
BACKGROUND: Approximately 12.5 million infants worldwide are born moderate to late preterm (MLPT) at 32-36 weeks’ gestation each year. While at risk for brain lesions and neurodevelopmental problems, MLPT infants mostly do not qualify for the neurological surveillance (e.g., cranial ultrasound, developmental follow-up) routinely provided to very preterm infants (<32 weeks), resulting in often late diagnosis of problems (1). As recently shown by our team, clinical care guidelines for MLPT infants are limited and, if present, vary across neonatal centers (2). A better understanding of population characteristics and short- and long-term outcomes may enable advances in care and outcomes of MLPT infants. Our objectives were to study the occurrence of neonatal morbidity and brain lesions in a large cohort of MLPT infants born in Canada and The Netherlands, and compare occurrences between Canadian and Dutch infants.
METHODOLOGY: As part of the Brain Imaging in Moderate-late Preterm infants (BIMP) study, brain Magnetic Resonance Imaging (MRI) around term-equivalent age (40-44 weeks PMA) was performed in 121 Canadian and 127 Dutch MLPT infants. For all infants, perinatal and neonatal data, such as mode of delivery, sex, small for gestational age (GA), need for respiratory support, sepsis, feeding difficulties and age at discharge, were collected. Conventional brain MR images (T1, T2, susceptibility) were assessed by three experts for brain lesion types common in very preterm infants, including hemorrhages (intraventricular [IVH], cerebellar [CBH], punctate), white matter injury, cystic lesions, infarction and signs of atrophy (irregular ventricles, wide extracerebral spaces). Perinatal and neonatal characteristics and occurrence brain lesions were calculated for all infants combined and compared between the Canadian and Dutch cohort using appropriate statistical tests.
RESULTS: The infants (61% male) had a mean GA of 34.4 weeks and birthweight of 2234 grams. All were admitted to neonatal units, and most frequently showed respiratory distress requiring support (40%). No significant differences in perinatal and neonatal characteristics were present between the Canadian and Dutch cohort, except for Canadian infants more often requiring respiratory support (53% vs 30%) and caffeine (56% vs 21%) (both P <0.05). The most frequently detected brain lesions in overall cohort were signs of atrophy (28%), IVH (11%) and CBH (11%), with most lesions considered to be mild and no significant differences in occurrence between the two cohorts (P >0.05).
CONCLUSION: Our study confirms that MLPT infants, regardless of continent of birth, frequently experience neonatal morbidities and brain lesions. Also, the study suggests a differing approach to respiratory management and/or an effect of altitude between continents. Large cohort studies on the association between morbidities and later adverse neurodevelopmental outcomes, and the underlying mechanisms, are needed to standardize and advance the clinical care and therewith potentially outcomes of MLPT infants globally.
Lack of association between neurodevelopmental outcomes and MRI and inflammatory cytokines in neonatal encephalopathy
Professor Arun Bokde, Dr Deirdre Sweetman, Dr Saima Aslam, Dr Megan Ni Bhroin, Dr Lynne Kelly, Dr Mary O’Dea, Dr Tim Hurley, Dr Marie Slevin, Dr Angela Byrne, Dr Gabrielle Colleran, Professor Eleanor Molloy
1Trinity College Dublin
BACKGROUND: The objective is to evaluate whether neurodevelopmental outcomes at two years of age in NE are predicted by MRI brain scores and inflammatory cytokines. The MRI brain scores were the Barkovich, NICHD NRN and Weeke scoring systems, the four inflammatory cytokines were granulocyte macrophage-colony stimulating factor (GM-CSF), erythropoietin (EPO), interleukins (IL-8), and Vascular Endothelial Growth Factor (VEGF) and neurodevelopmental outcome at two years of age was measured with the Bayley-III.
METHODOLOGY: The infants for this study were part of three programmes: MODE [1], NEBULA [2], and NEPTuNE [3]. Data obtained from infants in these cohorts were used for retrospective analysis in the present study. Ethical approval was granted by the Ethics Committee at National Maternity Hospital, Holles Street, Dublin. Informed consent for participation in study was granted by legal guardian after the study was explained to them. Infants with NE were included according to the criteria of Huang et al [4]. Neuroimaging was carried out on or before 14 days of life. Serial blood samples in the first week of life were evaluated for multiplex cytokines. Therapeutic hypothermia (TH) was administered according to the TOBY criteria [5], with infants treated for a duration of 72 hours. Statistical analyses included general linear model (GLM) to assess the association between inflammatory cytokines (EPO, GM-CSF, IL-8, VEGF) and MRI brain scores (Barkovich, NICHD NRN, Weeke, normal/abnormal MRI). This was performed separately for each cytokine and each scoring system. Log values for each cytokine were used for this analysis, in order to transform the data from skewed to normally distributed.
RESULTS: There were 70 infants included in analysis from the 136 recruited for the original studies – the 70 had complete MRI scores, inflammatory markers, and Bayles-III scores – see Table 1 and 2 for demographics and clinical scores, and MRI scores, respectively. No statistical association was detected between the independent factors MRI score and inflammatory markers (EPO, IL-8, VEGF) with outcome variable Bayless-III scores in GLM model. There was a trend to statistical significance (table 3) in the model that included GM-CSF as independent factor with all 3 MRI scoring systems.
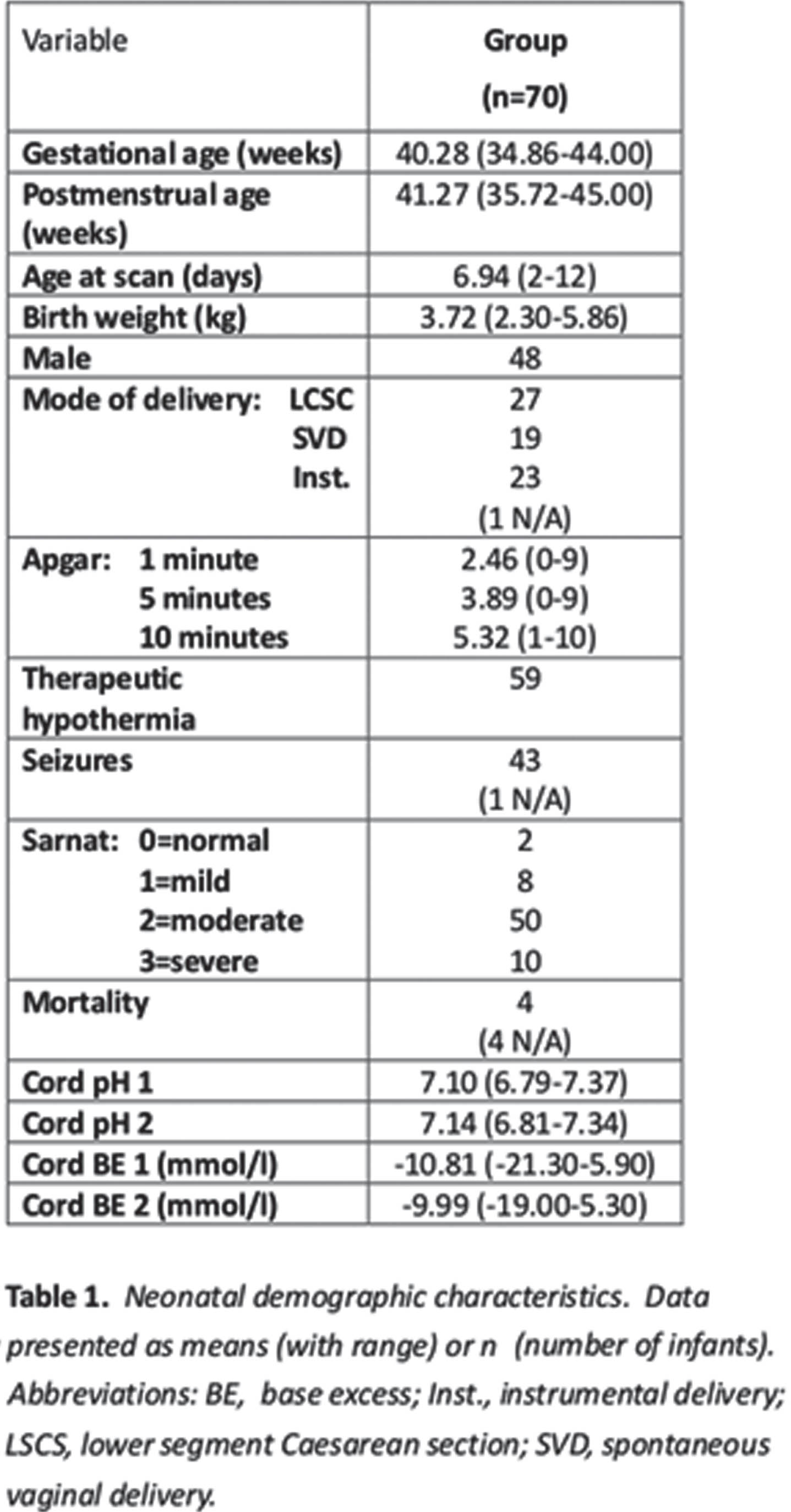
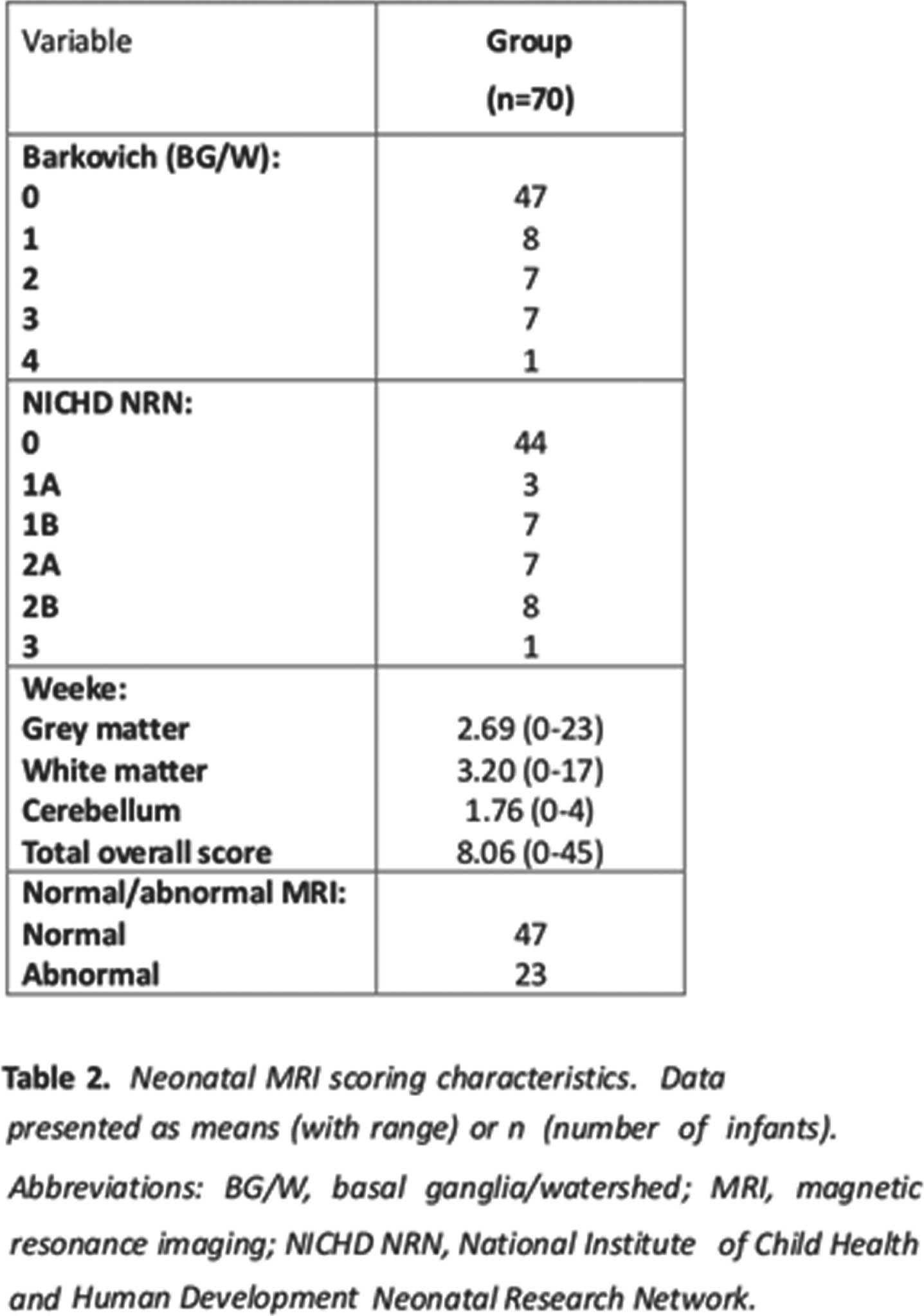
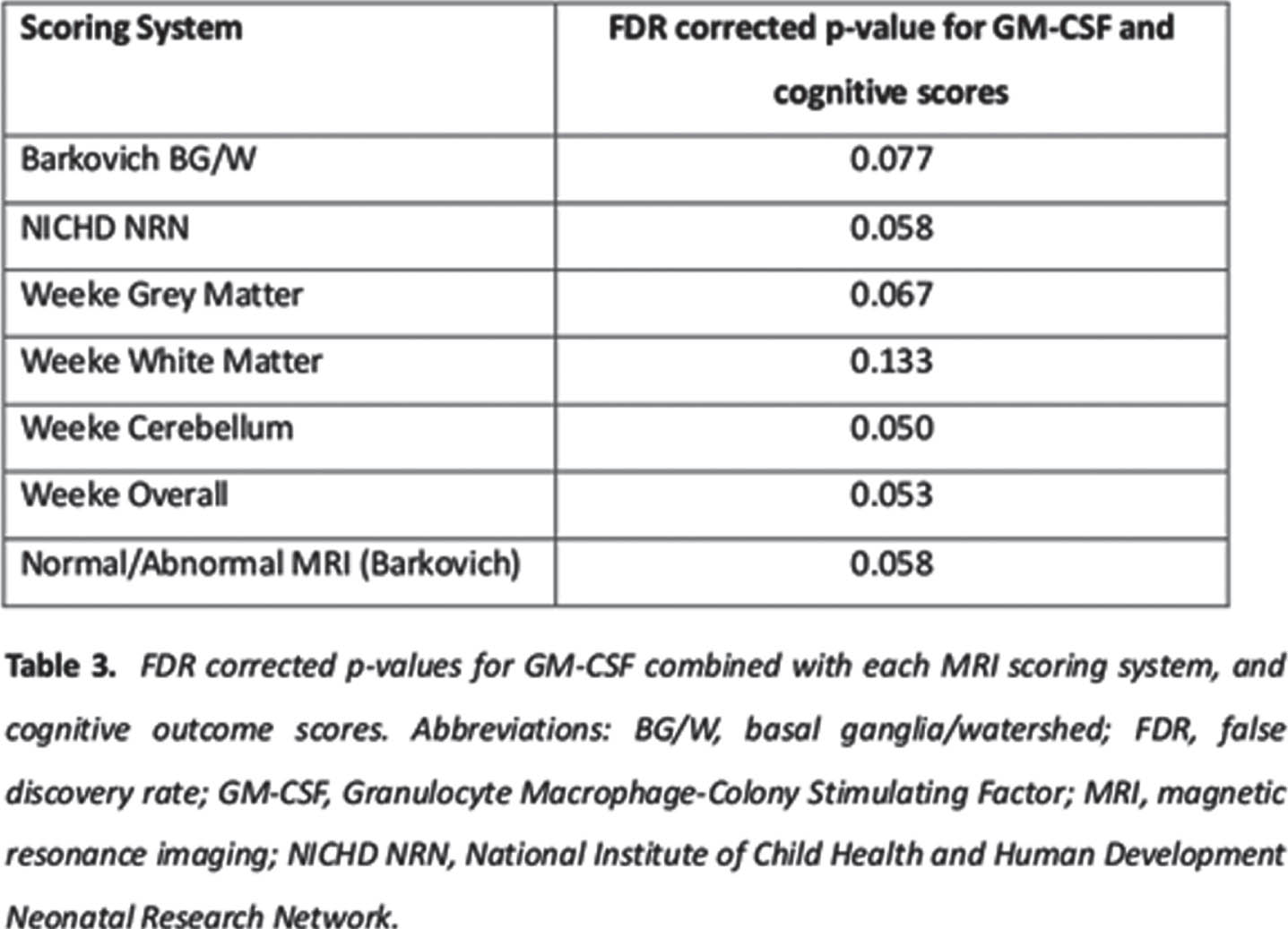
CONCLUSION: It may be that the Weeke grey matter scoring system may be the most sensitive to inflammatory markers in NE in a model to predict outcome. Further studies with larger sample sizes are needed.
REFERENCES:
[1] Sweetman D. 2014. PhD thesis, Royal College of Surgeons in Ireland.
[2] Aslam S. 2017. PhD thesis, Royal College of Surgeons in Ireland.
[3] Molloy EJ et al. HRB Open Res. 2020;3(40):40.
[4] Huang CC et al. N Engl J Med. 1999 ;341(5):328-35.
[5] Azzopardi DV et al. N Engl J Med. 2009 Oct 1;361(14):1349-58.
Do measurements matter?: Impact of 2d lateral ventricular size on outcome in preterm infants
Doctor Raphaela Jernej1, Doctor Renate Fuiko1, Univ.-Prof. Doctor Gregor Kasprian2, Univ.-Prof. Doctor Angelika Berger1, Doctor Katharina Goeral1
1Medical University of Vienna, Comprehensive Center for Pediatrics, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Intensive Care and Neuropediatrics, 2Medical University of Vienna, Department of Radiology, Division of Neuroradiology and Musculoskeletal Radiology
BACKGROUND: Bedside cranial ultrasound (cUS) is cost-effective, widely available, and is performed routinely on a daily basis. Studies showed a correlation between anterior horn with (AHW) measured in 2D ultrasound and neurodevelopmental outcome in infants with PHVD, but little is known about the role of ventricular index (VI), AHW and thalamo-occipital distance (TOD) in preterm neonates.
METHODOLOGY: Retrospective, single-center study of serial cUS scans in preterm neonates born <32 weeks applying 2D measurement techniques (VI, AHW, TOD). A composite score was calculated using the median for a certain gestational age as a cutoff for the maximum value of every parameter and patient (maximum score of 3). Our aim was to investigate the impact of the mentioned parameters on neurodevelopmental outcome at 12 months corrected age.
RESULTS: A total of 79 serial cUS scans in a cohort of 32 patients were available for analysis. Median GA at birth was 25 weeks and 35 weeks at scan. In 80% of scans, all measured parameters were <97th percentile. Median composite score was 1 (IQR 0-2). Median outcome scores were 96 for cognition, 90 for language and 95 for motor development. Considering every single parameter, as well as the composite score, there was no significant impact on specific outcome domains. Regarding outcome categories, a significant impact of our composite score on motor outcome (p=0.042) was observed.
CONCLUSION: There was no significant impact of ventricular measurements in serial cranial ultrasound on neurodevelopmental outcome at 12 months corrected age. However, using a composite score for each patient a significant impact on motor outcome categories was found. Data with a higher number of patients is in progress and will be available until the conference (aim = 200 patients).




