Proceedings of the 15th International Newborn Brain Conference: Other forms of brain monitoring, such as NIRS, fMRI, biochemical, etc.
Machine learning predicts outcomes in preterm neonates with intraventricular hemorrhage using targeted proteomics
Gabriel A. Vignolle2, Priska Bauerstätter2, Silvia Schönthaler2, Christa Nöhammer2, Monika Olischar1, Angelika Berger1, Gregor Kasprian3, Georg Langs4, Klemens Vierlinger2, Doctor Katharina Goeral1
1Medical University of Vienna – Comprehensive Center for Pediatrics, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Intensive Care and Neuropediatrics, 2Austrian Institute of Technology - Center for Health & Bioresources, Competence Unit Molecular Diagnostics, Austrian Institute of Technology, 3Medical University of Vienna – Department of Radiology, Division of Neuroradiology and Musculoskeletal Radiology, 4Medical University of Vienna – Department of Radiology, Computational Imaging Research Lab
BACKGROUND AND OBJECTIVE: Preterm neonates with intraventricular hemorrhage (IVH) are at risk for posthemorrhagic ventricular dilatation (PHVD). In recent years targeted proteomics has developed into a powerful protein quantification tool in biomedical research, systems biology, and clinical applications. This study aims to inform therapeutic decision-making and parental counseling using proteomics in this high-risk group.
METHODS: In this prospective study, we investigated preterm neonates born <34 weeks of gestation between 2011 to 2023 with intraventricular hemorrhage (IVH). We performed targeted proteomics analysis on different biological matrices (blood, urine, and CSF), derived from a longitudinal neonatal cohort spanning a decade. We employed explainable machine learning (ML) algorithms to predict PHVD development, patient survival, and identify disease-specific protein-biomarkers. The targeted approach applied in this study was a Proximity Extension Assay (PEA), which combines the specificity of dual antibody recognition with qPCR readout and enables the detection of low concentrations of proteins in biofluids as previously shown in several publications. We applied various ML methods from different domains (statistics, regularization ML, deep learning, decision trees, Bayesian) to the targeted proteomic data for biomarker detection to understand PHVD development and predict the survival of IVH patients. We trained and evaluated 600 models using cross-validation techniques.
RESULTS: A total of 100 patients were included in the analysis, 29 of whom did not survive. Median GA was 25.6 (24.1-27.1), and median IVH grade was 3 (3-4), with bilateral IVH (n=89) in the majority. A significant number of survivors required neurosurgical intervention for PHVD (n=45 of 71 (63%)). We identified both known (e.g. NEFL (neurofilament light chain)) and novel biomarkers that serve as key indicators for PHVD development and survival prediction. Figure 1 shows a feature plot in CSF of NEFL, while Figure 2 shows NPX values of NEFL in different biofluids between neonates who survived vs. died (distribution shown as heatmap).
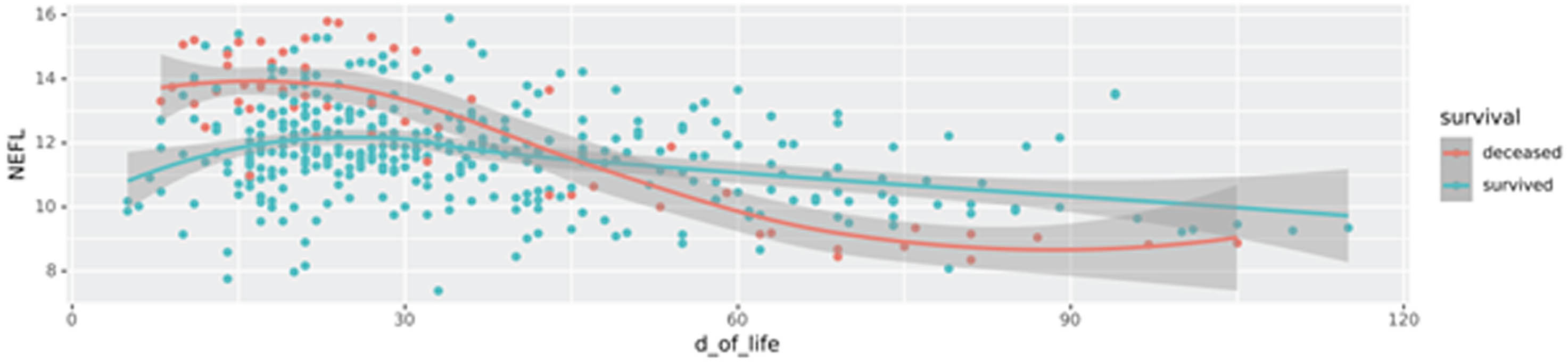
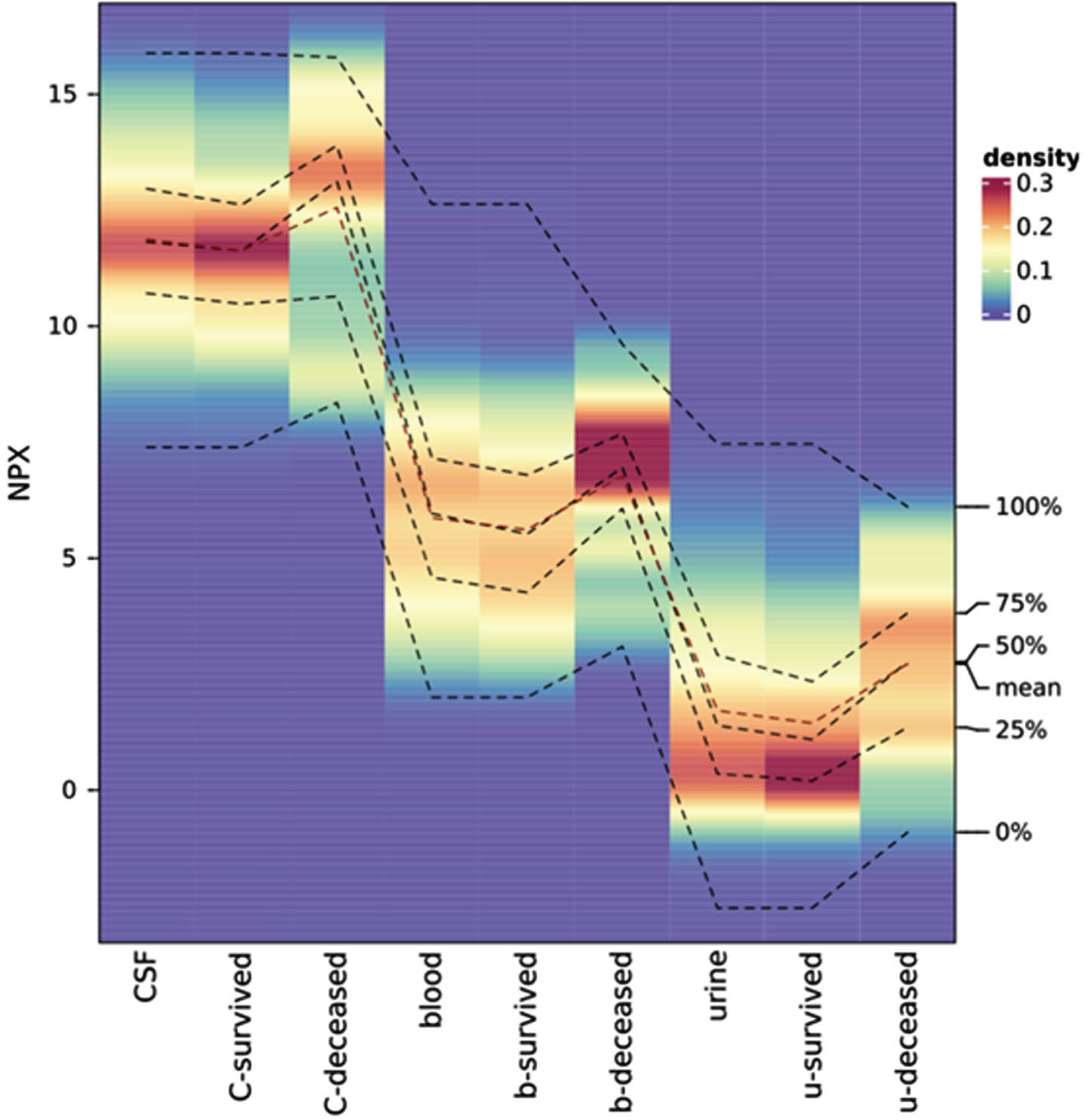
CONCLUSION: Our study provides new insights into clinically relevant biomarkers within defined time frames. It demonstrates the robustness of our machine learning models and benefits from a unique neonatal patient cohort.
Blood lactate levels increases prediction of clinical outcomes of neonates with hypoxic ischemic encephalopathy
Nicholas Nicoletti1, Rakesh Lavu1, Wei Liu1, Sreenivas Karnati1, Subhash Puthuraya1, Hany Aly1, Ceyda Acun1
1Cleveland Clinic Children’s Hospital
BACKGROUND: Neonatal hypoxic–ischemic encephalopathy (HIE) is one of the most common causes of severe neurological deficit in children. The severity of lactic acidemia reflects the degree of fetal hypoxia–ischemia, but a single lactate measurement gives no definitive information regarding the duration of asphyxia. It is currently unknown if prolonged duration of normalization of lactate level associated with poor neurodevelopmental outcomes in infants with HIE undergoing therapeutic hypothermia.
OBJECTIVE: The objective of this study is to assess the association between duration of blood lactate normalization, severity of lactic acidosis, and short-term outcomes in neonates with HIE treated with therapeutic hypothermia (TH).
METHODS: This is a retrospective, single-center cohort study of HIE neonates, gestational age ≥36 weeks who underwent TH between January 2012 and December 2022. Neonates with lethal congenital malformations, chromosomal anomalies, or need for extracorporeal membrane oxygenation were excluded.
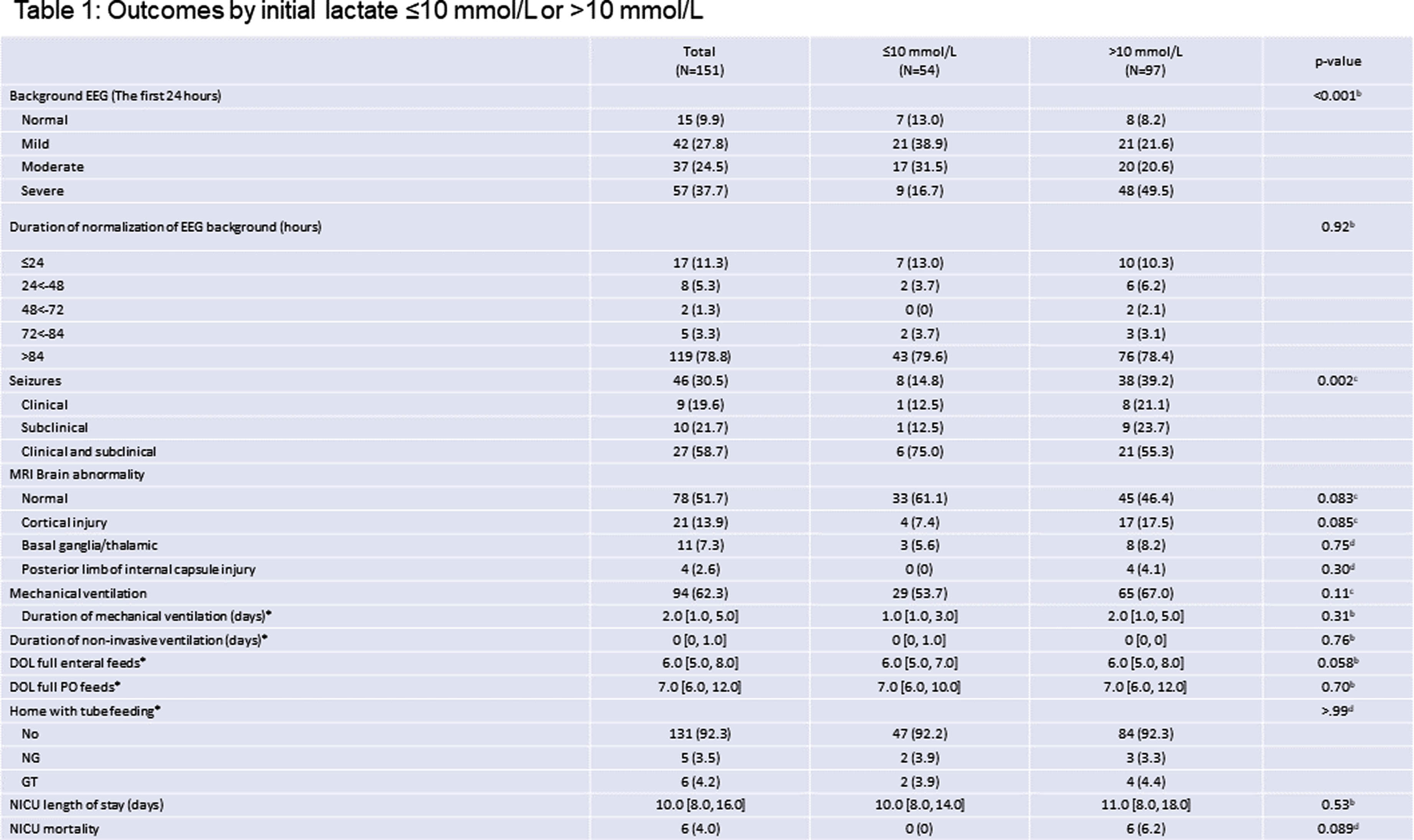
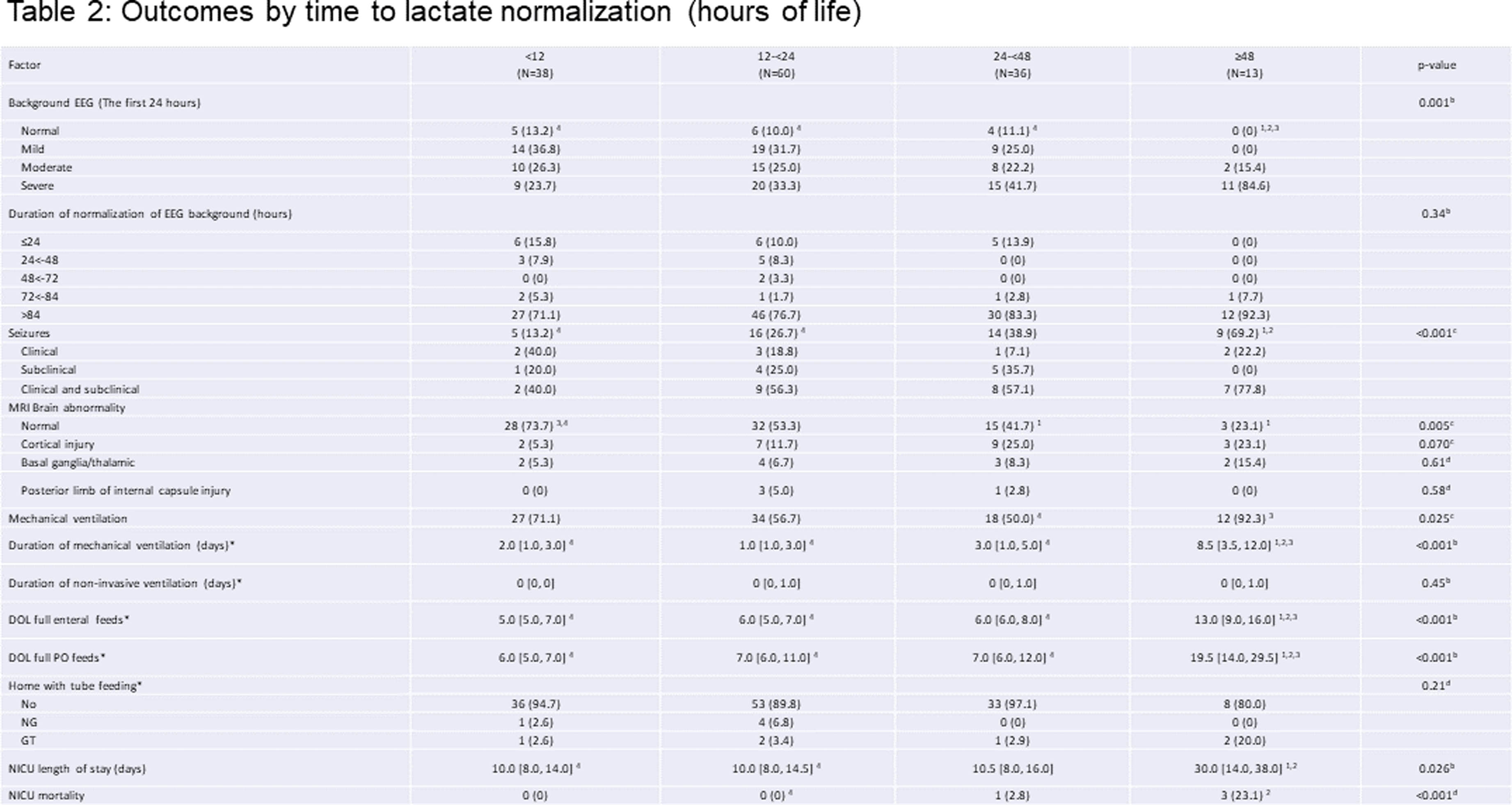
RESULTS: Overall, 151 patients were studied, with 56.3% males, 60.3% cesarean section delivery, 42.4/14.6% moderate/severe HIE, and median gestational age at 39.3 weeks. Of all neonates, 66.7% of patients’ lactate levels were normalized in the first 24 hours. Only 11.3% of neonates had background EEG normalization in the first 24 hours of life. Initial lactate levels of >10 mmol/L was associated with abnormal EEG background in the first 24 hours of life (p<0.001), and seizures (p=0.002). The duration of lactate normalization ≥48 hours were associated with higher level of background EEG abnormality in the first 24 hours of life (p=0.001) and higher likelihood of seizures (p<0.001). Besides, the duration of lactate normalization ≥48 hours were associated with higher likelihood of abnormal MRI results, mechanical ventilation support and NICU mortality, as well as longer days ventilation support, full enteral (PO) feeds, and NICU stay (all p<0.05).
CONCLUSION: Duration of blood lactate normalization ≥48 hours was associated with increased seizure burden, abnormal EEG and MRI findings, prolonged mechanical ventilation, and increased mortality in infants with HIE who underwent TH.
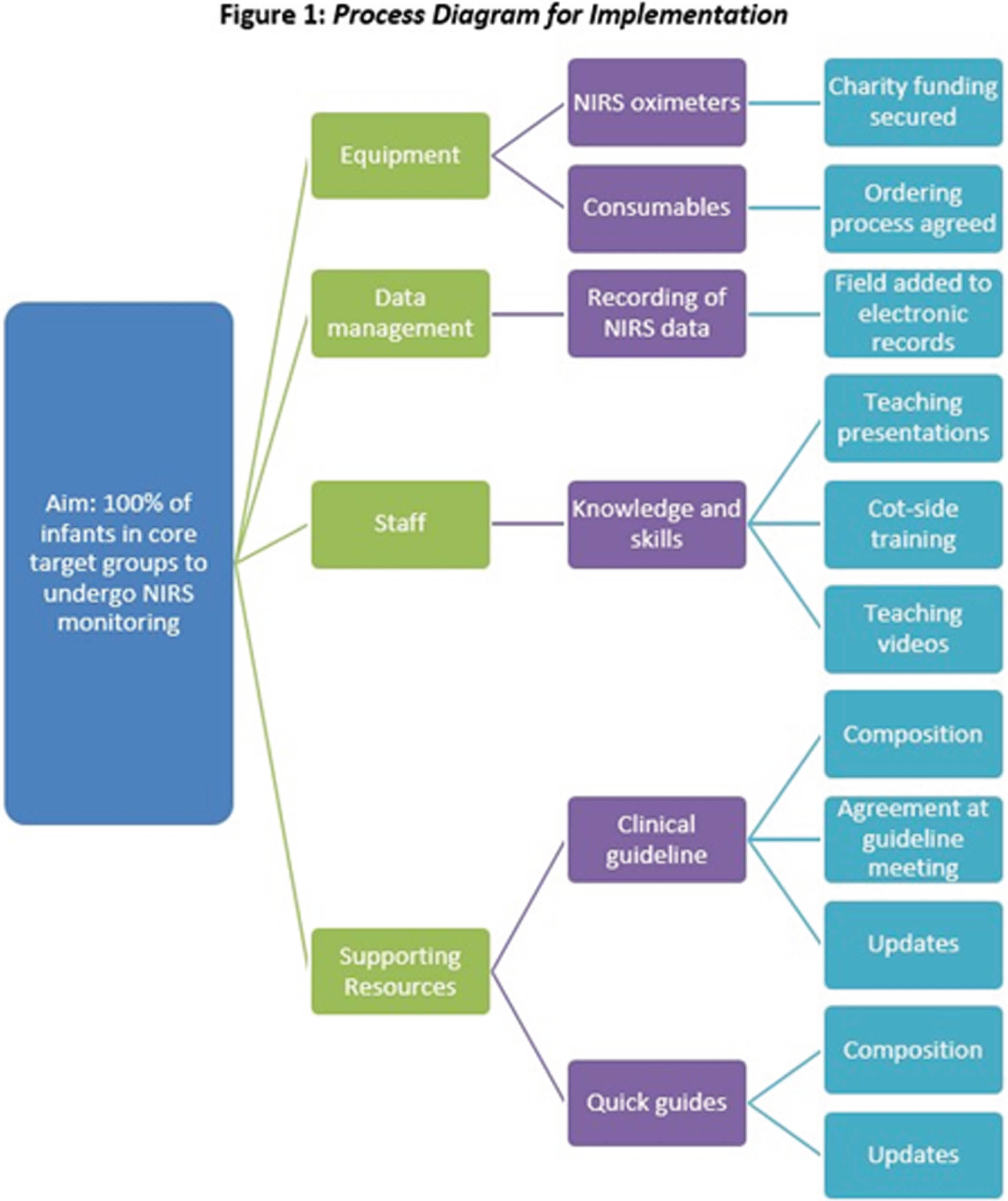
Implementation of cerebral near-infrared spectroscopy in a tertiary neonatal intensive care unit in the UK
Helen L Turner1,2, James P Boardman1,3, Nicola J Robertson1,4
1Centre for Clinical Brain Sciences, The University of Edinburgh, 2Simpson Centre for Reproductive Health, The Royal Infirmary of Edinburgh , 3Centre for Reproductive Health, The University of Edinburgh, 4EGA Institute for Women’s Health, University College London
BACKGROUND: Monitoring cerebral oxygenation saturation using near-infrared spectroscopy (NIRS) at the cot-side may contribute to the improved neurocritical care of high-risk newborns and prevention of secondary injury. Our aims are to describe the implementation of cerebral NIRS within our NICU; to assess compliance in target groups; and to monitor for adverse events.
METHODOLOGY: This project was set in the Royal Infirmary of Edinburgh, the tertiary referral centre for South East Scotland, where between 45-60 infants born at <28 weeks gestational age (extremely preterm) and 12-15 term infants with moderate-severe neonatal encephalopathy (NE) receive care annually. NIRS monitoring, using O3 Regional Oximeters (Masimo, California, USA) with neonatal sensors, was introduced in December 2021.
QI methodology was used to plan and assess implementation. The implementation process, including production of a clinical guideline and multi-disciplinary teaching, is illustrated in Fig 1. Compliance was used as a process measure in two key groups: extremely preterm infants for the first 72 hours after birth and infants undergoing therapeutic hypothermia for NE. The secondary outcome measure was NIRS-associated adverse events.
Eligible infants were identified through screening of electronic admission records (BadgerNet). Individual electronic records (clinical notes and observations charts) were reviewed manually and data extracted for analysis using Microsoft Excel.
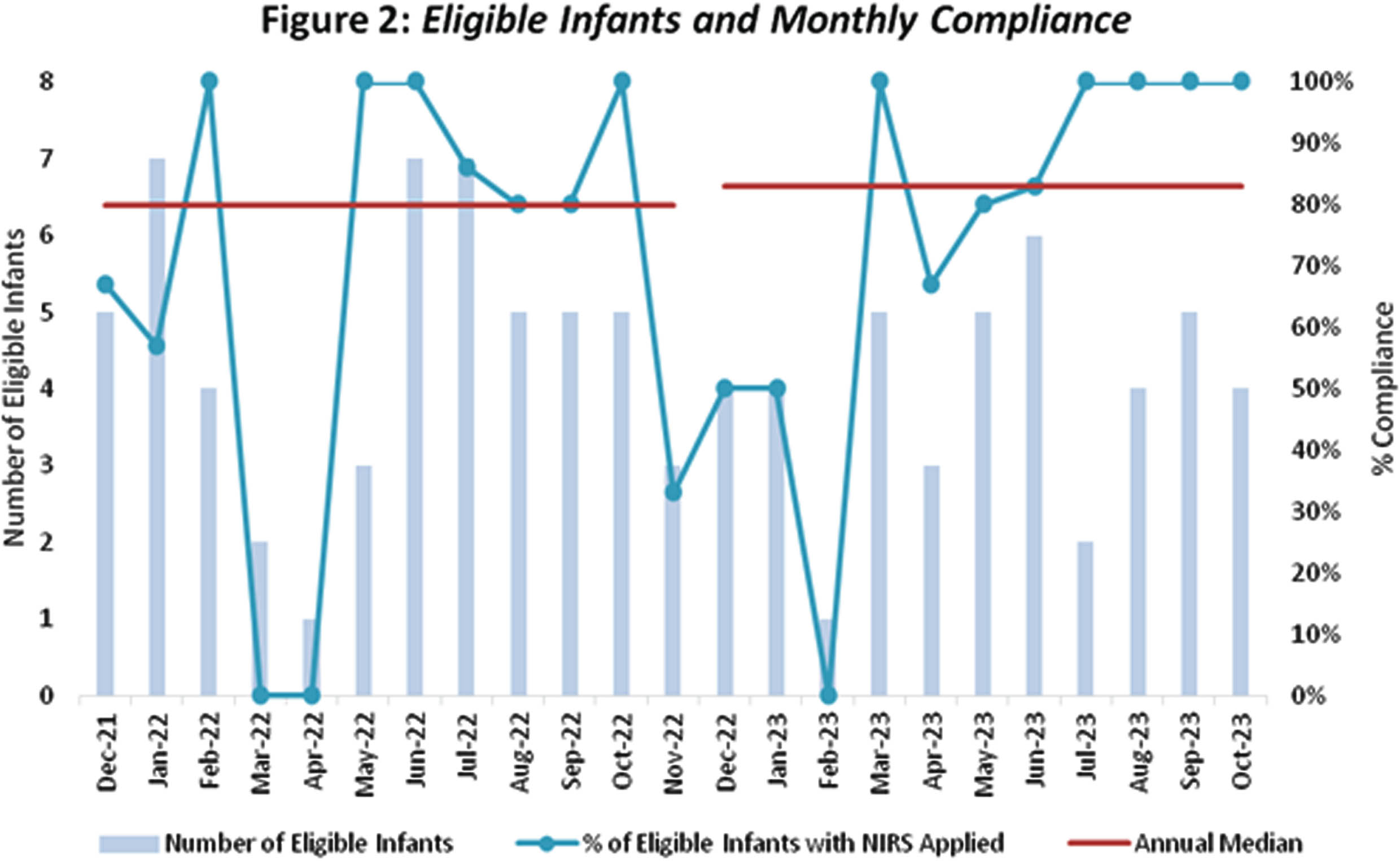
RESULTS: NIRS monitoring was applied to 76 (78%) of the 97 eligible infants admitted over 23 months. Sixty-one (80%) infants were extremely preterm: mean gestational age (GA) 25.74 weeks (range 23.00 – 27.84) and mean birthweight 798 grams (range 515 – 1200). Fifteen (20%) were infants with NE: mean GA 38.99 weeks (range 36.14 – 41.84) and mean birthweight 3447 grams (range 2370 – 4170).
Monthly compliance with application of NIRS monitoring is shown in Fig 2. Median compliance was 80% over the first 12 months, increasing to 86% for the next 11 months.
Reasons for non-compliance were examined. Amongst 21 cases where infants were not monitored, there were equipment issues in seven (33%) cases and concerns regarding skin fragility in five (24%) infants of 22 weeks GA. The need for further education regarding eligibility and troubleshooting equipment issues was identified and actioned. No reason was documented in eight (38%) cases. Regarding adverse events, the probe was removed early in four (5%) infants. There was bruising in one infant, who had a bleeding diathesis. In the other three, skin traction was a concern, but no skin damage was noted.
CONCLUSION: We have successfully implemented cerebral NIRS in our tertiary NICU, with 80% median compliance. Using QI methodology allowed us to identify and target reasons for non-compliance. Amongst our predominantly extremely preterm population, adverse events were uncommon. Using cerebral NIRS to target specific research questions is our next priority, and recruitment to prospective observational studies has begun.
Quantitative analysis of cry acoustics and neurophysiological signals for assessing the infant health status
Ana Laguna Pradas1, Sandra Pusil1, Anna Lucia Paltrinnieri2, Angel Bazan1, Phd Silvia Orlandi3
1ZOUNDREAM AG, 2Neonatology Department, Barcelona Centre for Maternal-Fetal and Neonatal Medicine (BCNatal), Hospital Clínic, Universitat de Barcelona, 3Department of Electrical, Electronic and Information Engineering “Guglielmo Marconi”(DEI), University of Bologna
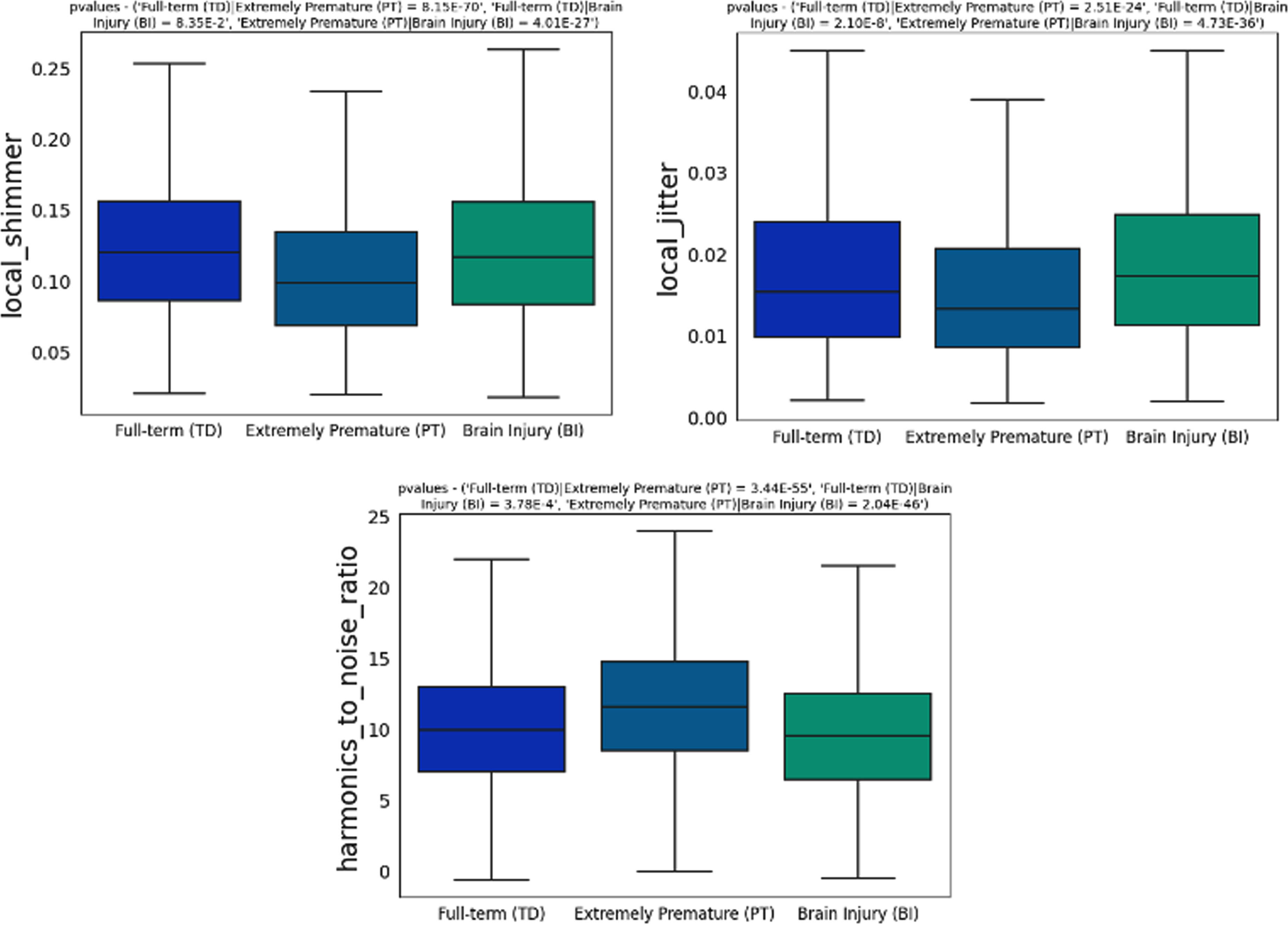
BACKGROUND AND OBJECTIVE: In the early stages of life, infant crying stands as the first proper attempt by which humans engage with their caregiver. Hence, cry is not solely recognized as a communicative signal, but also as a concrete indication and/or manifestation of symptoms or signs providing insights into the health status and development of the child. During cry production, the vagal system is the central factor influencing the variations in cry acoustics. Impairments in either brainstem function or higher-level brain functions can impact the regulation of crying by the vagal system, leading to irregularities in the characteristics of cry sounds. This preliminary study aimed to explore differences in the acoustic features of cries and neurophysiological patterns among three groups of infants: full-term, extremely premature, and infants with brain injuries. The purpose was to ascertain the viability of cry analysis as an objective non-invasive technique contributing to the early identification of infants vulnerable to developmental challenges improving neonatal care prognosis.
METHODOLOGY: A dataset comprising audio recordings of spontaneous infant cries and neurophysiological signals were collected from the three aforementioned groups of infants at Hospital Clinic (Barcelona, Spain). Acoustic features, including time, frequency, and spectral characteristics, were extracted from the cry recordings. Neurophysiological signals were processed to analyze brain activity patterns. Statistical analyses, including ANOVA and correlation tests, were conducted to assess differences and relationships among the groups and variables.
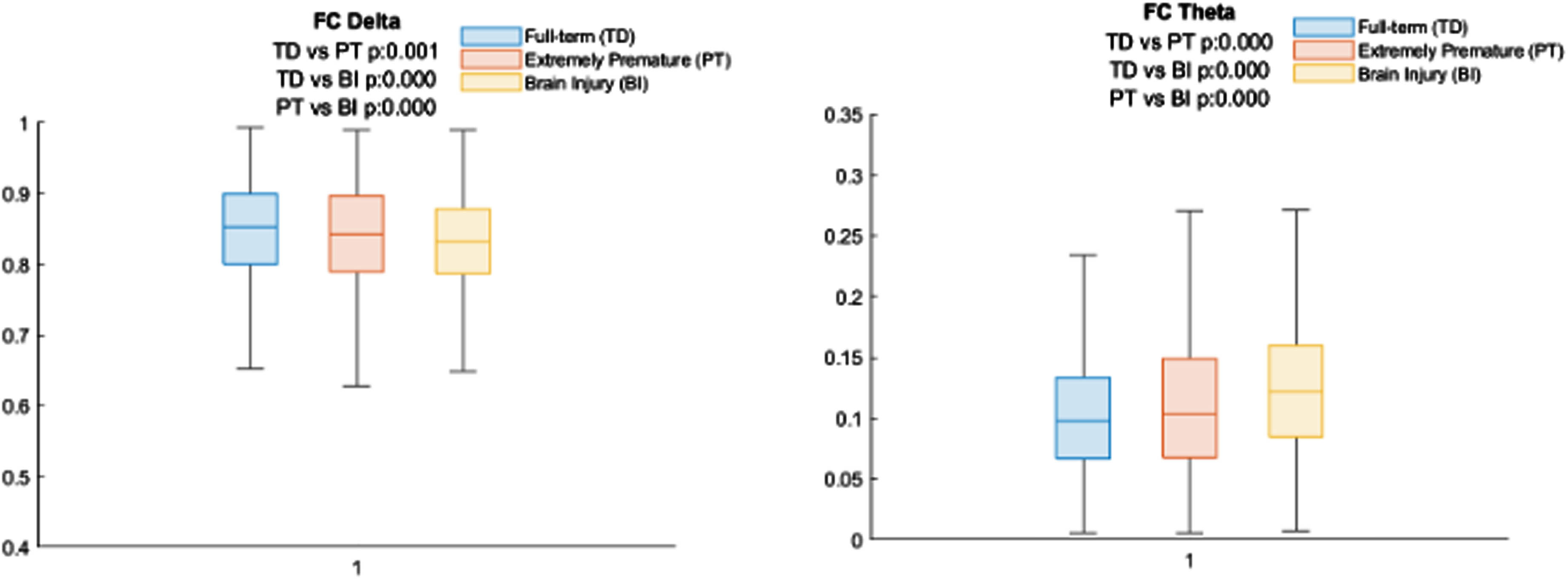
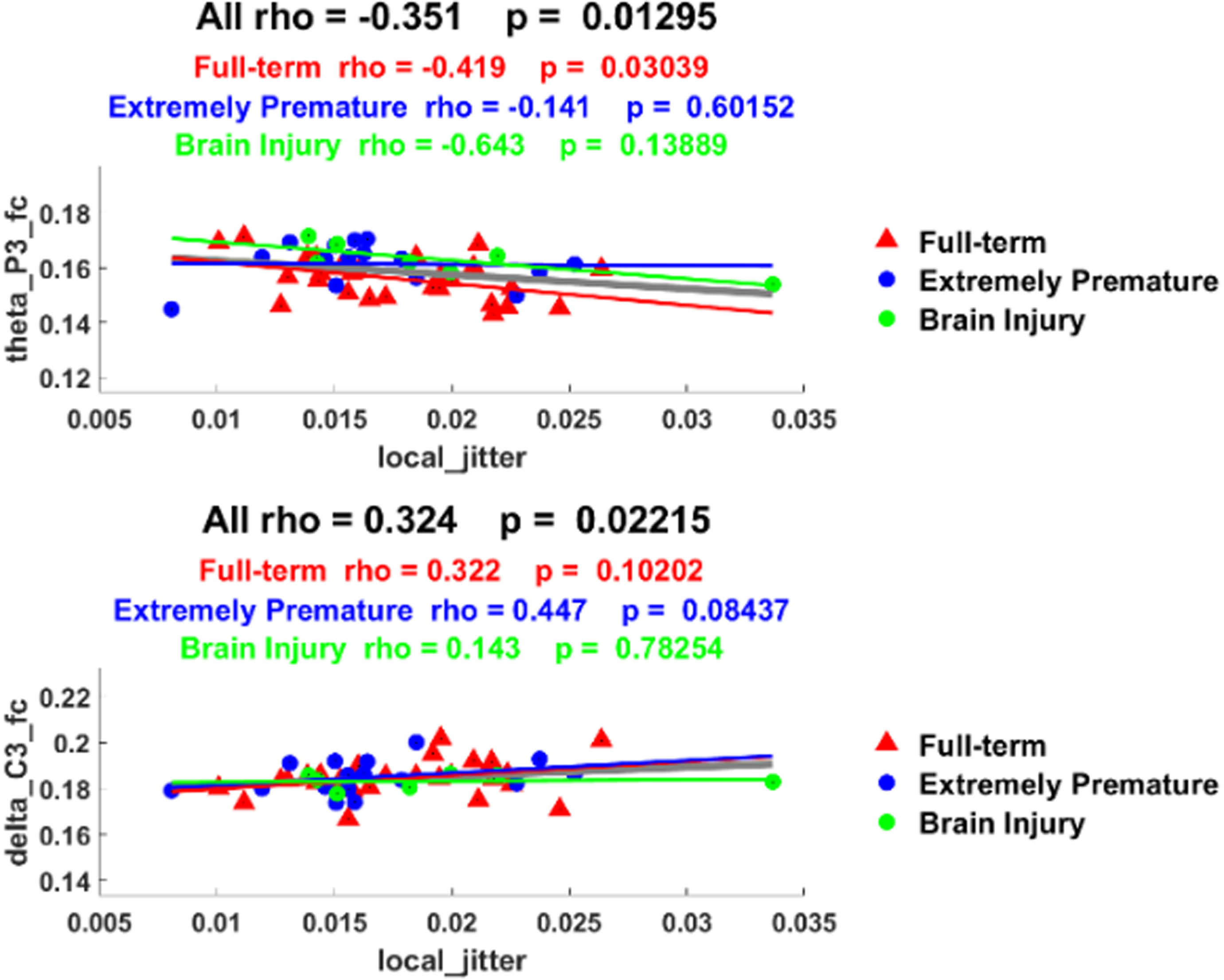
RESULTS: Significant statistical differences were identified among groups based on both acoustic and neurophysiological patterns. Specifically, pathological infants exhibited higher levels of jitter, shimmer, and noise (p<0.001). On the neurophysiological signals, infants with brain injuries exhibited a reduced delta band power (p<0.001), whereas theta band power demonstrated an inverse trend by increasing in affected infants (p<0.001). Significant correlations were also identified among different data signals (p<0.05). Delta and theta were significantly correlated with jitter, shimmer and harmonic noise to ratio.
CONCLUSION: The observed differences in cry acoustics and neurophysiological patterns suggest the potential of cry to be used as a non-invasive biomarker in the neurological assessment of the newborn. This research paves the way for the incorporation of data-driven analysis into neonatal care, potentially improving early diagnosis and intervention for at-risk infants, thereby improving their overall well-being and developmental outcome.
BIBLIOGRAPHY:
1. Crying as a sign, a symptom, & a signal: Clinical emotional and developmental aspects of infant and toddler crying. viii, 228 (Cambridge University Press, 2000).
2. Wasz-Höckert, O., Michelsson, K. & Lind, J. Twenty-Five Years of Scandinavian Cry Research. in Infant Crying: Theoretical and Research Perspectives (eds. Lester, B. M. & Zachariah
Monitoring parameters of premature neonates <32Weeks’ gestation with&without adverse outcome-A secondary analysis of the COSGOD-III-trial
Bernhard Schwaberger1,2,3, Berndt Urlesberger1,2,3, Christina H. Wolfsberger1,2,3, Nariae Baik-Schneditz1,2,3, Daniel Pfurtscheller1,2,3, Katharina Goeral4, Marlene Hammerl5, Tina Perme6, Eugene M Dempsey7, Laila Springer8, Gianluca Lista9, Tomasz Szczapa10, Hans Fuchs11, Jenny Bua12, Lukasz Karpinski10, Alexander Avian13, Brenda Law14,15, Julia Buchmayer4, Ursula Kiechl-Kohlendorfer5, Lilijana Kornhauser-Cerar6, Christoph E Schwarz7, Kerstin Gründler8, Ilaria Stucchi9, Katrin Klebermass-Schrehof4, Georg M Schmölzer14,15, Gerhard Pichler1,2,3
1Division of Neonatology, Department of Pediatrics and Adolescent Medicine, Medical University of Graz, Austria, 2Research Unit for Microcirculation and Macrocirculation of the Newborn, Medical University of Graz, 3Research Unit for Cerebral Development and Oximetry Research, Medical University of Graz, 4Comprehensive Center for Pediatrics, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Intensive Care and Neuropediatrics, Medical University of Vienna, 5Department of Pediatrics II, Neonatology, Medical University of Innsbruck, 6NICU, Department for Perinatology, Division of Gynaecology and Obstetrics, University Medical Centre Ljubljana, 7INFANT Research Centre, University College Cork, Cork University Maternity Hospital, 8Department of Neonatology, University Children’s Hospital of Tübingen, 9Neonatologia e Terapia Intensiva Neonatale (TIN) Ospedale dei Bambini “V Buzzi”, 10II Department of Neonatology, Neonatal Biophysical Monitoring and Cardiopulmonary Therapies Research Unit, Chair of Neonatology, Poznan University of Medical Sciences, 11Division of Neonatology and Pediatric Intensive Care Medicine, Center for Pediatrics and Adolescent Medicine, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, 12Neonatal Intensive Care Unit, Institute for Maternal and Child Health, “IRCCS Burlo Garofolo”, 13Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, 14Centre for the Studies of Asphyxia and Resuscitation, Neonatal Research Unit, Royal Alexandra Hospital, 15Department of Pediatrics, University of Alberta
BACKGROUND: To identify differences in demographic characteristics and monitoring parameters between premature neonates <32weeks’ gestation who died or had cerebral injury (adverse outcome) compared to those who survived without cerebral injury (favorable outcome).
METHODOLOGY: This ancillary study is a post-hoc analysis of secondary outcome parameters of a multicenter randomized clinical trial (COSGOD-III) in which neonates were randomized either to cerebral oxygen saturation (crSO2) monitoring by near-infrared spectroscopy (NIRS) in addition to routine monitoring within the first 15 minutes after birth to guide stabilization or a control group with standard care. In the present study all included premature neonates were assigned either to adverse outcome group (composite of mortality and cerebral injury defined as any grade of intraventricular hemorrhage or cystic periventricular leukomalacia) or favorable outcome group (survival without cerebral injury). Group differences of demographic characteristics, and monitoring parameters (heart rate [HR], arterial oxygen saturation [SpO2] and crSO2) were assessed.
RESULTS: In total, 607 premature neonates were included in the COSGOD-III-Trial. In the present study 117(19%) and 490(81%) were assigned to adverse outcome group and favorable outcome group, respectively. In the adverse outcome group, premature neonates had lower median gestational age [26.7(25.5-28.9) vs. 29.3(27.3-30.7); p<.001], birth weight [900(690-1195) vs. 1150(885-1435); p<.001] and Apgar scores (p<.001) compared to the favorable outcome group. The adverse outcome group had significantly lower HR from 2 to 4min and SpO2 from 2 to 8min, thereafter both parameters were similar in both groups. crSO2 was similar in both groups within the first minutes and was significantly lower in the adverse outcome group from 4 to 15min after birth compared to the favorable outcome group (Table 1).
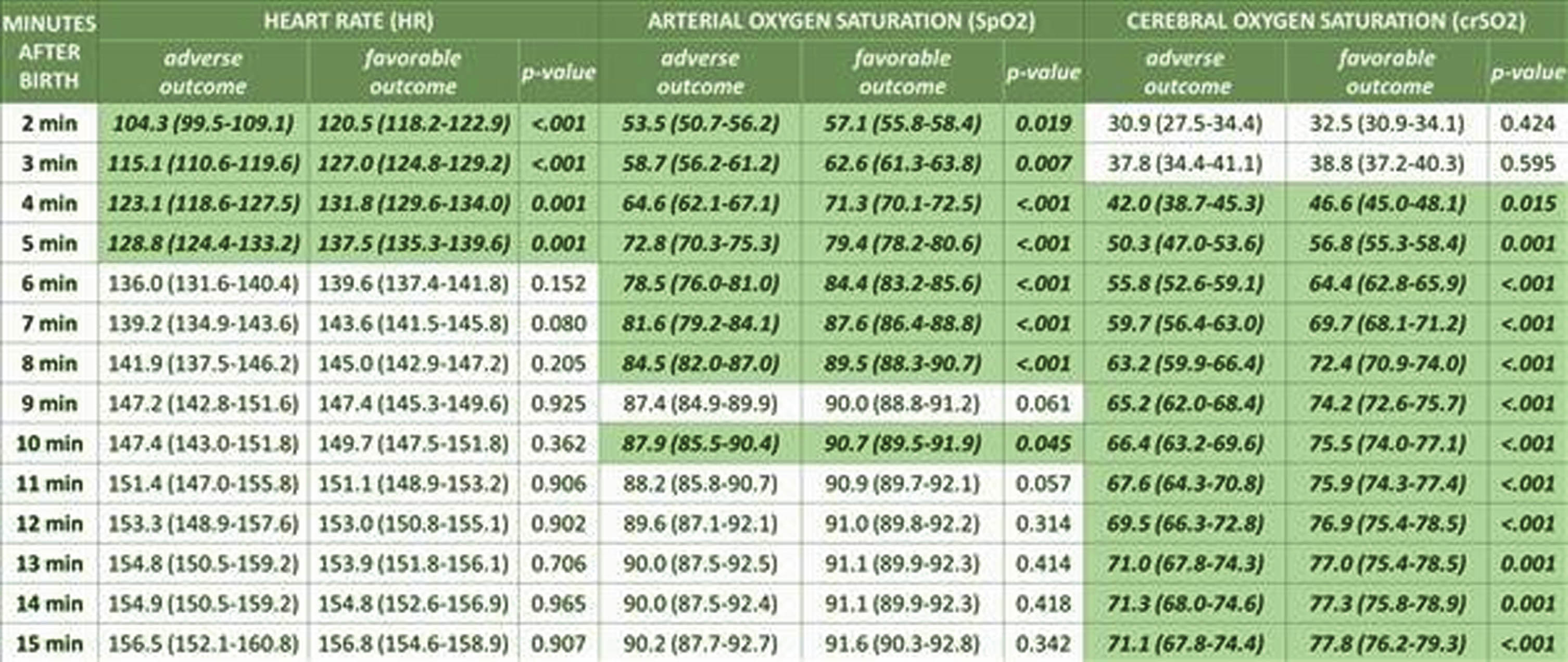
CONCLUSION: Beside lower gestational age, birth weight and Apgar scores, premature neonates <32weeks’ gestation with adverse outcome had lower HR, SpO2 and crSO2 after birth. While routine parameters (HR and SpO2) equalized between groups shortly after birth (4 and 8 minutes, respectively), crSO2 values continued to be lower in the adverse outcome group until minute 15 after birth demonstrating a potential benefit of using NIRS monitoring for identifying neonates in need for medical interventions to potentially prevent adverse outcome.
Persistent pulmonary hypertension affects cerebral oxygenation in neonates with neonatal encephalopathy
Dimitrios Rallis1, Hoda El-Shibiny2, Eniko Szakmar3, Aisling Garvey2, Helen Christou2, Mohamed El-Dib2
1University of Ioannina, 2Division of Newborn Medicine, Department of Pediatrics, Brigham and Women’s Hospital, Harvard Medical School, 3Division of Neonatology, 1st Department of Pediatrics, Semmelweis University
BACKGROUND AND OBJECTIVE: Persistent pulmonary hypertension of the newborn (PPHN) affects systemic oxygenation and may worsen brain injury in neonates with neonatal encephalopathy (NE). Our aim was to evaluate the impact of PPHN on cerebral regional oxygenation (crSO2), as measured with near-infrared spectroscopy (NIRS), in neonates with NE treated with therapeutic hypothermia (TH).
MATERIALS AND METHODOLOGY: We retrospectively evaluated neonates with NE in our institution, between 2018-2022, comparing neonates with vs without PPHN. Overall, 164 (76%) neonates were analyzed, including 19 (12%) neonates with PPHN and 145 (88%) neonates without. crSO2 was expressed as the mean of the 24-hour recording, within the first, second and third day of hypothermia, the rewarming period, and the period after rewarming. Weeke score was applied for the evaluation of the brain magnetic resonance imaging (MRI). To adjust for the severity of NE, we calculated the predicted probability based on meconium-stained amniotic fluid, Apgar score at 10 minutes, umbilical arterial pH, the initial aEEG pattern, and the highest NE score, and a propensity score matching was performed between neonates with and without PPHN, with nearest neighbor and caliper set to 0.2. Linear regression analysis was then performed to evaluate the impact of PPHN on crSO2 and total MRI injury score, adjusted for the predicted probability.
RESULTS: Neonatal characteristics and outcomes are depicted in Table 1. During the first 3 phases of hypothermia, no differences were recorded in crSO2 between PPHN and non-PPHN groups; however, neonates with PPHN had significantly higher crSO2 during the rewarming period, and the period after rewarming compared to the non-PPHN group (87±6 vs 80±6, p=0.001, and 87±5 vs 80±7, p=0.008, respectively), (Figure 1). Also, neonates with PPHN had a significantly higher total MRI injury score [7(2-19) vs 1(0-3), p<0.001]. After adjusting for the predicted probability, PPHN was significantly associated with higher cerebral rSO2 on the rewarming period (b 6.89, 95%CI 3.10-10.68, p=0.001) and the period after rewarming (b 9.92, 95%CI 1.17-18.67, p=0.029), and the total MRI injury score (b 6.64, 95%CI 0.36-12.92, p=0.039), (Table 2).
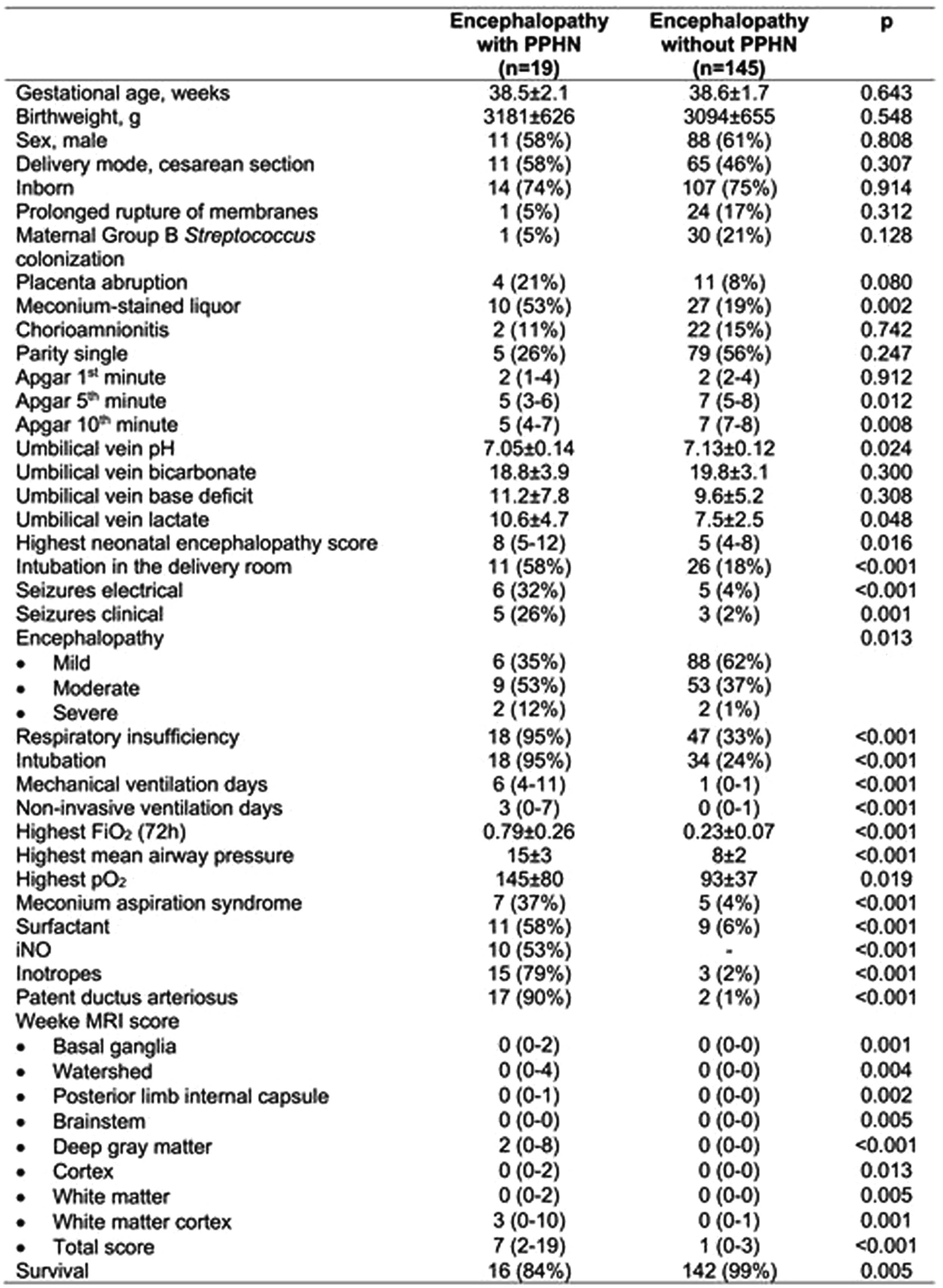
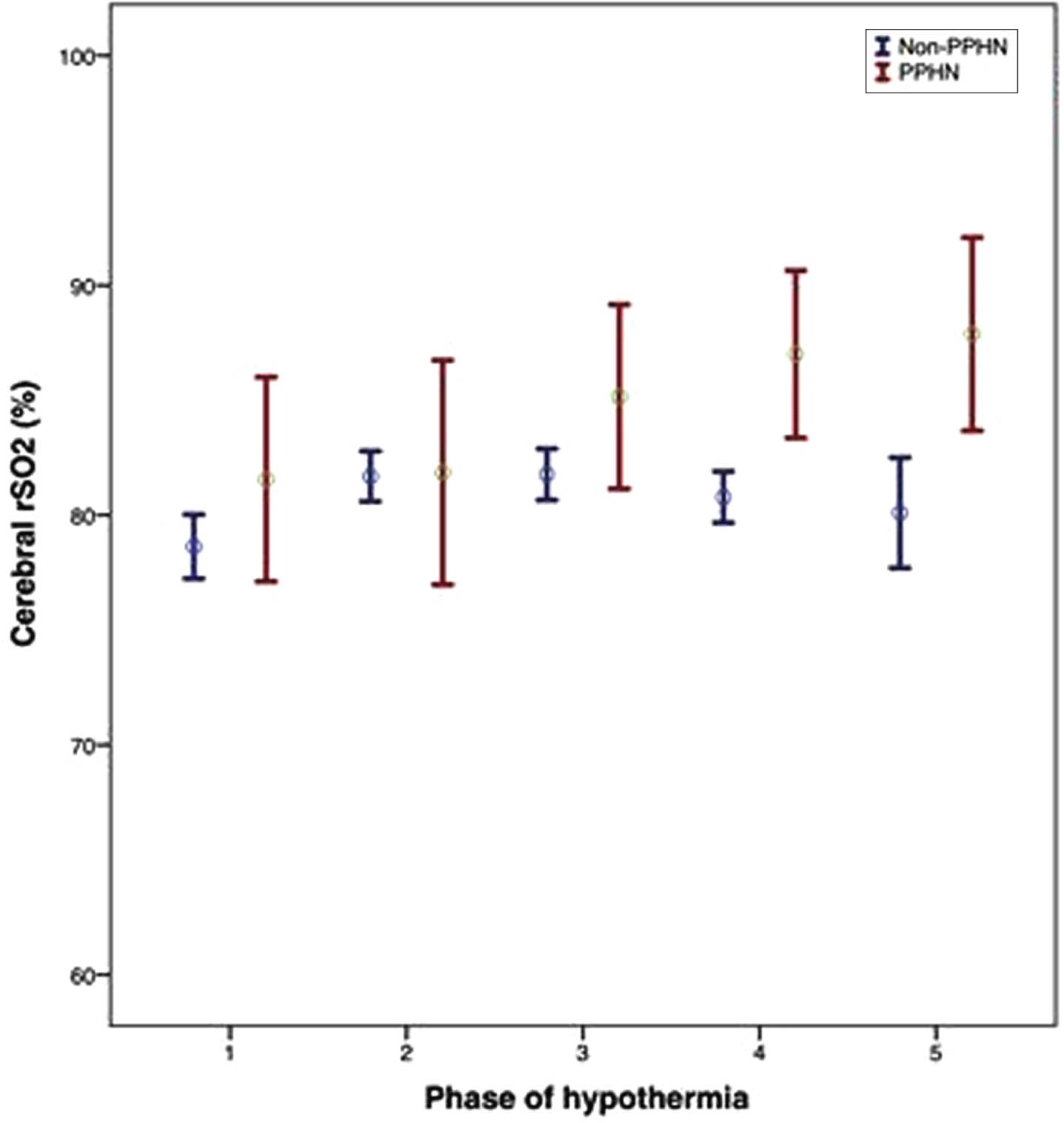
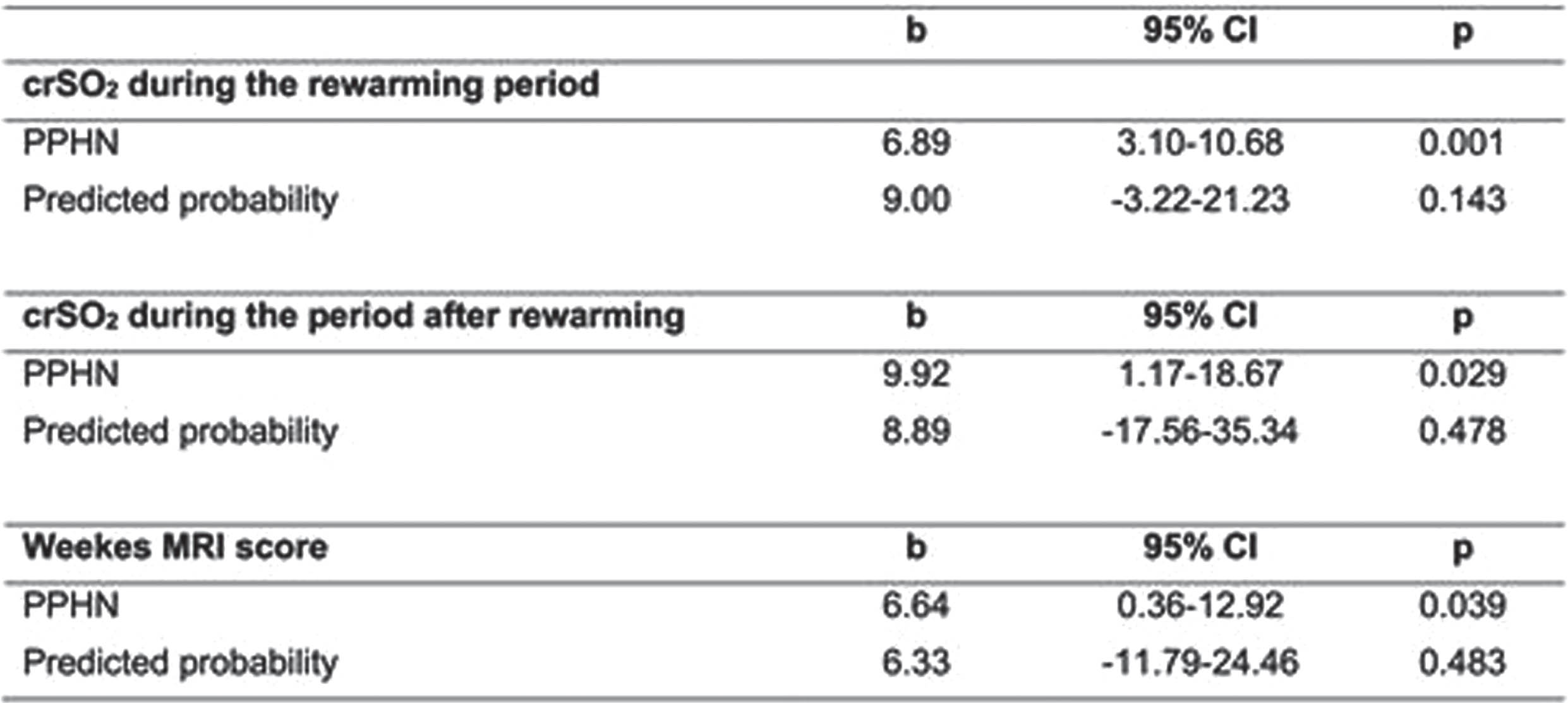
CONCLUSION: PPHN was associated with higher cerebral crSO2 during and after the rewarming periods, and worse brain MRI injury score, indicating a significant impact of PPHN on brain injury in neonates with NE undergoing TH.
REFERENCES:
1. Joanna R G V, Lopriore E, Te Pas AB, Rijken M, van Zwet EW, de Bruine FT, Steggerda SJ. Persistent pulmonary hypertension in neonates with perinatal asphyxia and therapeutic hypothermia: a frequent and perilous combination. J Matern Fetal Neonatal Med. 2022 Dec;35(25):4969-4975.
2. Gagnon MH, Wintermark P. Effect of persistent pulmonary hypertension on brain oxygenation in asphyxiated term newborns treated with hypothermia. J Matern Fetal Neonatal Med. 2016;29(13):2049-55.
Cerebral pulsatile NIRS: Heart rate monitoring during neonatal transition in term neonates-an observational study
Bernhard Schwaberger1,2,3, Christoph Schlatzer1,2,3, Marlies Bruckner1,2,3, Berndt Urlesberger1,2,3, Gerhard Pichler1,2,3, Lukas Mileder1,2,3, Nariae Baik-Schneditz1,2,3
1Division of Neonatology, Department of Pediatrics and Adolescent Medicine, Medical University of Graz, Austria, 2Research Unit for Microcirculation and Macrocirculation of the Newborn, Medical University of Graz, 3Research Unit for Cerebral Development and Oximetry Research, Medical University of Graz
BACKGROUND: Recent guidelines recommend continuous monitoring of heart rate (HR) and arterial oxygen saturation (SpO2) during neonatal transition using electrocardiography (ECG) and/or pulse oximetry. Recently, Hamamatsu Photonics K.K. (Japan) introduced a new pulsatile near-infrared spectroscopy mode (pNIRS), enabling the measurement of the cerebral pulse rate (cPR). The aim of the study was to evaluate the feasibility and reliability of HR monitoring using pNIRS compared to the two routine monitoring methods.
METHODOLOGY: HR was measured in term neonates delivered by cesarean section using ECG, pulse oximetry, and pNIRS during the first 10 minutes after birth. Analysis of variance (ANOVA) was conducted for normally distributed values or Kruskal-Wallis tests for non-normally distributed values to compare the minute-to-minute mean values of the three monitoring methods. Data analysis commenced from the third minute after birth due to missing values in minutes 1 and 2 after birth.
RESULTS: A total of 55 term neonates (gestational age median [IQR]: 38+5 [38+2-39+1]) were included. Cerebral pulse rate measurement using pNIRS was feasible in every newborn. Cerebral pulse rate correlated well with ECG data. Until the fifth minute after birth, the HR from pNIRS was on average higher than that from pulse oximetry. However, a significant difference among the three methods was observed only in the fourth minute (p=0.016), [Minute 4: ECG versus pulse oximetry (p=0.030), pulse oximetry versus pNIRS (p=0.036), ECG versus pNIRS (p=1.000)]. No significant differences were noted among the three monitoring methods from the fifth minute onwards.
DISCUSSION: Cerebral pulse rate measurement using pNIRS was feasible in every newborn. The pNIRS-derived HR was closer to ECG data than to pulse oximetry in the first minutes after birth. The ability to measure cerebral pulse rate provides continuous information about cerebral perfusion for the first time. The significance of pNIRS in neonates with respiratory failure and hemodynamic instability needs further evaluation in consecutive studies.
Reference ranges for cerebral oxygen saturation during neonatal transition in extremely and very preterm neonates
Bernhard Schwaberger1,2,3, Christina H. Wolfsberger1,2,3, Berndt Urlesberger1,2,3, Katharina Goeral4, Marlene Hammer5, Alexander Avian6, Tina Perme7, Eugene M Dempsey8, Laila Springer9, Gianluca Lista10, Tomasz Szczapa11, Hans Fuchs12, Lukasz Karpinski11, Jenny Bua13, Brenda Law14,15, Julia Buchmayer4, Ursula Kiechl-Kohlendorfer5, Lilijana Kornhauser-Cerar7, Christoph E Schwarz8, Kerstin Gründler9, Ilaria Stucchi10, Katrin Klebermass-Schrehof4, Georg M Schmölzer14,15, Gerhard Pichler1,2,3
1Division of Neonatology, Department of Pediatrics and Adolescent Medicine, Medical University of Graz, Austria, 2Research Unit for Microcirculation and Macrocirculation of the Newborn, Medical University of Graz, 3Research Unit for Cerebral Development and Oximetry Research, Medical University of Graz, 4Comprehensive Center for Pediatrics, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Intensive Care and Neuropediatrics, Medical University of Vienna, 5Department of Pediatrics II, Neonatology, Medical University of Innsbruck, 6Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, 7NICU, Department for Perinatology, Division of Gynaecology and Obstetrics, University Medical Centre Ljubljana, 8INFANT Research Centre, University College Cork, Cork University Maternity Hospital, 9Department of Neonatology, University Children’s Hospital of Tübingen, 10Neonatologia e Terapia Intensiva Neonatale (TIN) Ospedale dei Bambini “V Buzzi,” Milano, Italia, 11II Department of Neonatology, Neonatal Biophysical Monitoring and Cardiopulmonary Therapies Research Unit, Chair of Neonatology, Poznan University of Medical Sciences, 12Division of Neonatology and Pediatric Intensive Care Medicine, Center for Pediatrics and Adolescent Medicine, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, 13Neonatal Intensive Care Unit, Institute for Maternal and Child Health, “IRCCS Burlo Garofolo”, 14Centre for the Studies of Asphyxia and Resuscitation, Neonatal Research Unit, Royal Alexandra Hospital, 15Department of Pediatrics, University of Alberta
OBJECTIVE: To define reference values and centile charts for arterial oxygen saturation (SpO2), heart rate (HR) and cerebral oxygen saturation (crSO2) during the first 15 minutes after birth in extremely and very low gestational age preterm neonates with favourable outcome.
METHODOLOGY: Preterm neonates <32 weeks of gestational age, included in the COSGOD III trial with visible NIRS measurements and with favourable outcome, defined as surviving without cerebral injuries (any intraventricular haemorrhage or cystic periventricular leucomalacia) or inflammatory morbidities (culture proven sepsis or necrotizing enterocolitis) until term age, were eligible for inclusion to define reference ranges and centile charts. Pulse oximetry was used for measurements of SpO2, and electrocardiography was used for measurements of HR. crSO2 was assessed using the INVOS 5100c monitor. Measurements were performed during the first 15 minutes after birth. Reference ranges and centile charts (10th to 90th centile) were defined for each minute starting at minute two.
RESULTS: A total of 210 preterm neonates with a median gestational age of 29.7 (27.6-30.9) weeks and a birth weight of 1200 (900-1460) grams were eligible for analysis. The 10th centiles of SpO2 were 32%, 52%, 83% and 85% at minute two, five, ten and 15, respectively. The 10th centiles of HR at minute two, five, ten and 15 were 70bpm, 109bpm, 126bpm and 134bpm, respectively. The 10th centiles of crSO2 at minute two, five, ten and 15 were 15%, 27%, 59% and 63%, respectively.
CONCLUSION: This study provides reference ranges and centile charts for SpO2, HR and crSO2 for extremely and very low gestational age preterm neonates with favourable outcome. These centile charts might help to target oxygenation during resuscitation after birth in very preterm neonates.
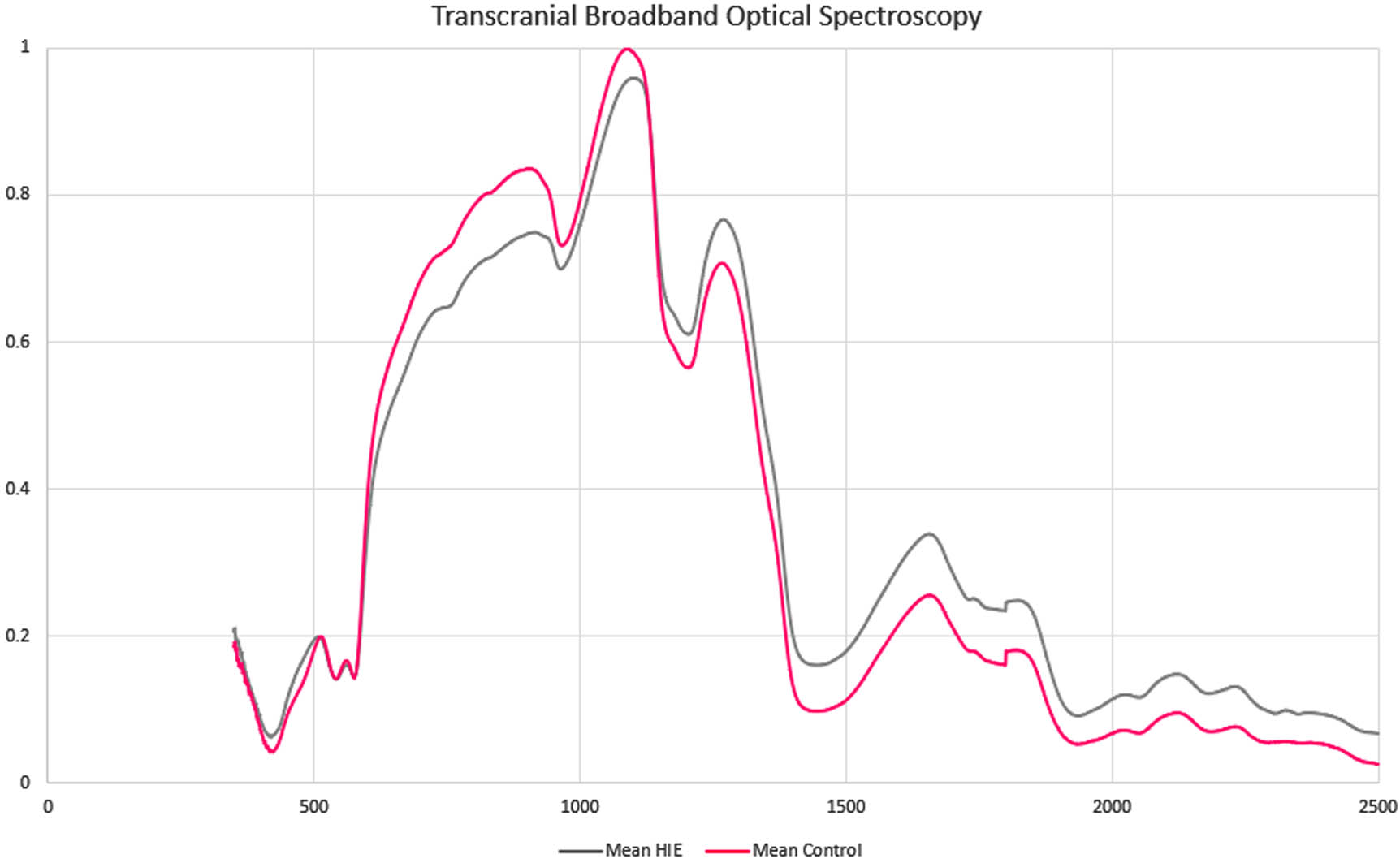
Using broadband optical spectroscopy to characterize newborn hypoxic ischemic injury: A pilot study
Imran Ilahi1, Janine Khan1, Andrea Pardo1, Seth Goldstein1
1Ann & Robert H. Lurie Children’s Hospital of Chicago
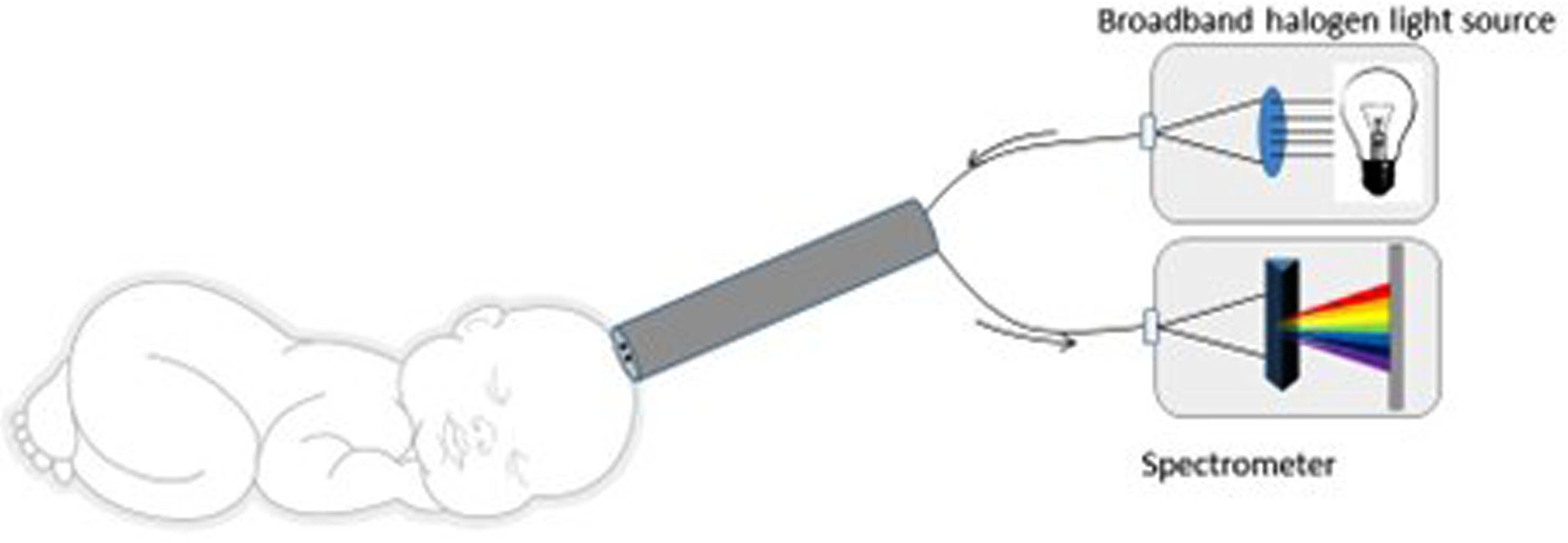
BACKGROUND: Hypoxic ischemic encephalopathy (HIE) is a common cause of mortality and severe disability. HIE is diagnosed with a combination of biochemical and clinical findings (1). Neonates with moderate to severe HIE benefit from therapeutic hypothermia (TH) starting within 6 hours of life to prevent death or severe disability (2). Prompt diagnosis of HIE is key to initiating therapeutic interventions within the appropriate time window. Broadband optical spectroscopy (BOS) uses a noninvasive infrared reflectance light measurement, consisting of a handheld probe connected to a broadband halogen light source via fiberoptic cables, coupled with a spectroradiometer that detects a wide range of wavelengths (Figure 1). BOS offers 500 times greater granularity than commercially available near-infrared oximeters. BOS involves contact with the skin for less than a second and does not generate warmth or discomfort. It does not expose the infant to ultraviolet rays or ionizing radiation. BOS may improve the early diagnosis of neonates that may benefit from TH.
METHODOLOGY: Neonates were identified as candidates for TH on admission to the NICU, and healthy controls underwent transfontanellar BOS. Controls were age-matched neonates without critical illness or intracranial pathology. Inclusion criteria consisted of neonates >/= 36 weeks gestational age (GA) with moderate to severe HIE, eligible for TH. Exclusion criteria were neonates < 36 weeks GA, birth weight <1800g, and suspected subgaleal hemorrhage.
RESULTS: BOS was obtained from 12 neonates within the first 24 hours of life. There were 6 neonates with HIE eligible for TH, and 6 healthy controls; the median GA was 39.4 weeks and the median weight was 3.5 kg. No safety concerns or changes to clinical care were noted. Average spectroscopy signatures are shown in Figure 2. There were differences between the near-infrared and short-wave infrared portions of the curves.
CONCLUSION: Our pilot study presents differences in BOS data in neonates with HIE vs. healthy controls. This safe, non-invasive diagnostic tool may help early HIE detection and monitoring of at-risk neonates in the NICU. These findings suggest that BOS could be an adjunct to current clinical practices for HIE assessment in neonates, improving the identification of neonates eligible for TH and potentially other neuroprotective interventions.
BIBLIOGRAPHY:
1. Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123(4):896-901. doi:10.1097/01.AOG.0000445580.65983.d2
2. Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574-1584. doi:10.1056/NEJMcps050929
3. Goldstein SD, Beaulieu RJ, Niño DF, et al. Early detection of necrotizing enterocolitis using broadband optical spectroscopy. J Pediatr Surg. 2018;53(6):1192-1196. doi:10.1016/j.jpedsurg.2018.02.083
Cerebral oxygenation changes in neonates under anesthesia
Vera Balog1, Sarolta Trinh1, Magdolna Barra2, Balazs Hauser3, Miklos Szabo1, Agnes Jermendy1
1Division of Neonatology, Pediatric Center, MTA Center of Excellence, Semmelweis University, 2Department of Nuclear Medicine, Semmelweis University, 3Department of Anesthesiology and Intensive Therapy, Semmelweis University
BACKGROUND: Intraoperative cerebral hypoxia can increase the risk of adverse postoperative outcomes. Cerebral NIRS monitoring can provide early warning signs of impaired tissue oxygenation (rSO2), however, it is not part of the routine monitoring setup, and normal values and lower safety limits are not well-defined. We aimed to describe rSO2 changes during neonatal non-cardiac surgeries and their relation to other physiological parameters.
METHODOLOGY: A prospective observational study was conducted in the operating room of the Pediatric Centre, Semmelweis University. Neonates under 6 kg were enrolled, who were admitted to our level III NICU due to a condition that required surgical intervention between 2020-2023. The monitoring setup included a patient monitor, an anesthesia machine (Drager Infinity DeltaXL and Perseus A500), and a Medtronic Invos 5100C NIRS monitor. NIRS events were categorized based on the difference from the median values of the first recorded minute. A mild decrease was defined as an 11-20% decrease from the baseline or an absolute rSO2 value of 60-69. A moderate decrease was considered a 21-30% decrease or an absolute rSO2 value between 50-59. Events were labeled severe decrease if more than a 30% decrease occurred or the absolute rSO2 value was below 50. Event severity was described based on the lowest value. Events with severe rSO2 decrease were analyzed separately, and synchronous changes in heart rate (HR), peripheral oxygen saturation (SpO2), end-tidal carbon dioxide (etCO2), and mean arterial pressure (MAP) were assessed. Wilcoxon singed-rank test was used to analyze the differences between the rSO2 nadirs and HR, SpO2, MAP, and etCO2 nadirs.
RESULTS: 95 patients were enrolled in the final analysis, with a median 36 [28; 38] weeks gestational age and 2500 [1125; 3030] grams birth weight. 55% of the patients were premature. The length of NIRS measurements was a median of 120 [79; 190] minutes with a 98 [43; 100] % ratio of data points in each patient. We observed 59 events of mild decrease in the NIRS values, that lasted for a median of 2.5 [1.5; 5.7] minutes, while moderate decrease appeared 30 times, with a median of 18 [7.0; 42.8] minutes length. Interestingly, severe decreases appeared 40 times, but they lasted much longer: 39.2 [15; 85] minutes. During severe events, we compared the lowest HR, SpO2, MAP, and etCO2 values. We found that only etCO2 showed a significant decrease (median -22.5 [-60.9; 4.3] %) at the time of nadir rSO2 compared to levels 5 mins before the severe rSO2 events (p=0.034).
CONCLUSION: Undesired rSO2 changes were frequent during neonatal anesthesia. EtCO2 showed association with the rSO2 changes during severe events, suggesting that ventilation has a marked influence on cerebral oxygenation.
Neurointensive monitoring during neonatal anaesthesia: Synchronous cerebral oxygenation and mean arterial pressure changes
Luca Laura Bogner1, Sarolta H. Trinh1, Magdolna Barra2, Vera Balog1, Miklos Szabo1, Melinda Kis-Tamas3, Balazs Hauser3, Agnes Jermendy1
1Division of Neonatology, Paediatric Centre, Semmelweis University, Budapest, Hungary, 2Department of Nuclear Medicine, Medical Imaging Centre, Semmelweis University, Budapest, Hungary, 3Department of Anaesthesiology and Intensive Therapy, Semmelweis University, Budapest, Hungary
BACKGROUND: Safeguarding children’s brains is of great importance during paediatric anaesthesia. Near-infrared spectroscopy (NIRS) is nowadays widely used in neonatal care, however it is seldom applied during non-cardiac surgeries of newborns. Since blood pressure does not reflect end-organ perfusion precisely, the addition of NIRS monitoring may aid hemodynamic management during neonatal anaesthesia. We aimed to describe hypotensive events and their relation to low cerebral oxygenation (crSO2) episodes during general anaesthesia of newborns in a tertiary neonatal surgical center.
METHODOLOGY: This was a prospective observational study, conducted between June 2021 and March 2023 in the Paediatric Centre, Semmelweis University, Budapest, Hungary. A total of 100 patients (61 preterm and 39 term neonates) were enrolled who required general anaesthesia for surgical care. Cerebral oxygenation were recorded with a NIRS monitor (Medtronic INVOS 5100C) with a 5 second frequency and non-invasive blood pressure measurement were taken with a 5 minute frequency. Low cerebral oxygenation was defined as crSO2 events lasting longer that 1 minute, relative decrease of crSO2 values 11 to 20% below baseline value or absolute crSO2 value below 70%. For preterm infants <7 days old mean arterial pressure (MAP) less than their gestational age in weeks was defined as hypotension, for infants >7 days old MAP was defined according to de Graaff’s recommendations. Data are shown in median [IQR] and %.
RESULTS: Gestational age of the total population was 32[28;38] with a bodyweight of 2.7[1.9;3.4] kg. The age at the time of surgery was 26[6;68] days of life. We recorded a median of 116[75;173] minutes of anesthesia. The length of anesthesia was longer in term vs preterm neonates (126[104;180] vs 95[68;157] minutes, respectively, p<0.01). We found a weak correlation between MAP and crSO2 values (r=0.241). In our population, the majority of patients, 79 neonates, had crSO2 decrease during anaesthesia. It lasted a median 10[5;25] minutes, corresponding to 39% of the anesthesia time in term neonates, while it was 12[2;32] minutes in preterm neonates, corresponding to a longer median period, 46% (p‹0.01). Hypotension occurred in 62 patients. In the term population, hypotensive periods were shorter, 22[15;36] minutes, 38% of the recorded time. In the preterm group the length was 29[15;40] minutes, 49%. A total of 53 patients had both decreased crSO2 and hypotensive events during their general anaesthesia. Synchronous decrease lasted for median 29 minutes, which corresponded to 22% of the total anaesthesia time, but only 44% of the crSO2 decrease time and 51% of the hypotension time.
CONCLUSION: In summary, crSO2 decrease occurs frequently during neonatal anaesthesia, especially among preterm neonates, but it coincides with hypotension only half of the time. NIRS could be a useful addition to the monitoring of newborns undergoing general anaesthesia and may aid therapy.
Multi-modal monitoring of infants with hypoxic-ischaemic encephalopathy within 12-hours of birth and prediction of outcome
Aisling Garvey1,2,3, Brian Walsh1,2,3, Sean Mathieson1, Michael Moore4, John O’Toole1,2, Vicki Livingstone1,2, Andreea Pavel1,2, Daragh Finn1,2, Geraldine Boylan1,2, Deirdre Murray1,2, Eugene Dempsey1,2
1Infant Research Centre, 2Department of Pediatrics and Child Health, University College Cork, 3Department of Pediatrics, Division of Newborn Medicine, Brigham and Women’s Hospital, Harvard Medical School, 4Department of Radiology, Cork University Hospital
BACKGROUND: Hypoxic-ischaemic encephalopathy (HIE) carries a significant risk of brain injury and adverse neurodevelopmental outcome (1, 2). Early identification of at-risk infants is critical to optimise intervention. We aimed to investigate the ability of currently available bedside monitoring techniques to predict short-term MRI and long-term neurodevelopmental outcome in infants with HIE.
METHODOLOGY: Prospective observational study conducted in a tertiary NICU, Ireland. Infants with all grades of HIE had continuous electroencephalography (EEG), non-invasive cardiac output monitoring (NICOM) and near-infrared spectroscopy (NIRS) commenced within the first 6 hours of admission to the NICU. One-hour epochs of time-synchronised data were selected at 6 and 12 hours. Abnormal short-term outcome was defined as an abnormal MRI (Barkovich scoring) and/or death in the first week after birth. Abnormal long-term outcome was defined as a score of <1SD below the mean in any of the developmental domains of the Bayley’s Developmental Assessment at approximately 2 years of age or death of the infant.
RESULTS: Fifty-seven infants with HIE were included (27 mild, 24 moderate, 6 severe). Median gestational age was 39.9 weeks (IQR 38.1–40.7) and birthweight was 3.4 kgs (IQR 3.0-3.7). Three infants died in the first week and neurodevelopmental outcome was available in 42 infants at a median age of 25 months (IQR 23-26) . At 6 hours, quantitative EEG features of relative spectral power and spectral difference at the higher frequency bands were significantly associated with abnormal long-term outcome: AUC 0.83, 95%CI 0.66-0.99 and AUC 0.78, 95%CI 0.55-1.00 respectively (Table 1). At 12 hours, quantitative EEG features of spectral power significantly predicted abnormal short-term outcome (AUC 0.68, 95%CI 0.53-0.84). Quantitative EEG features of spectral power and cerebral oxygenation (cSO2) significantly predicted long-term outcome: AUC 0.75, 95%CI 0.57-0.93 and AUC 0.73, 95%CI 0.55-0.90 respectively. Combining different modalities did not improve prediction. NICOM measurements were not helpful in identifying infants with abnormal outcome at either time point.
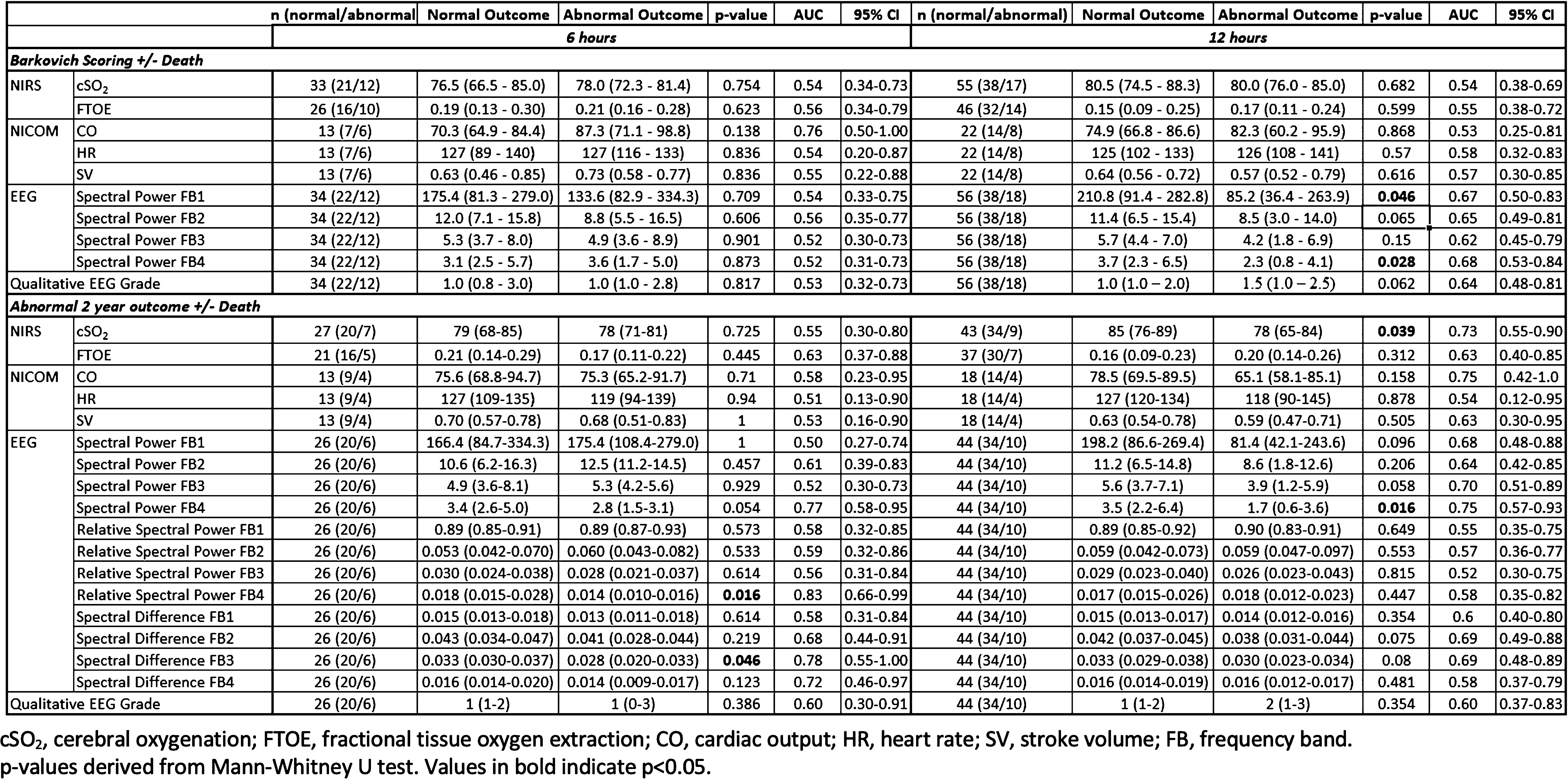
CONCLUSION: EEG remains the best individual predictor of outcome in infants with HIE. Quantitative EEG features at 6 and 12 hours successively predicted both short- and long-term outcome in infants with HIE.
REFERENCES:
1. Conway, J. M., Walsh, B. H., Boylan, G. B. & Murray, D. M. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome – a systematic review. Early Hum. Dev. 120, 80–87 (2018).
2. Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD003311. doi: 10.1002/14651858.CD003311.pub3.
Long-term impact of posthemorrhagic ventricular dilatation on cerebral oxygenation in preterm infants with intraventricular hemorrhage
Julia Elis1, Mirjam Steiner1, Lisa Klein1, Vito Giordano1, Monika Olischar1, Angelika Berger1, Katharina Goeral1
1Medical University of Vienna
BACKGROUND AND OBJECTIVE: Intraventricular hemorrhage (IVH) and its complication, posthemorrhagic ventricular dilatation (PHVD), remain significant contributors to morbidity and mortality in premature infants. Despite prior research demonstrating the short-term effects of IVH on cerebral oxygenation, this study seeks to investigate the long-term consequences. Specifically, we will explore the implications of consecutive PHVD development as well as the impact of neurosurgical interventions (NSI) in patients with IVH and PHVD. Utilizing near-infrared spectroscopy, we aim to gain a better understanding of their long-term impact.
METHODOLOGY: In this prospective study preterm neonates with a gestational age <33 weeks born from 2013 to 2023 were investigated. Regional cerebral oxygen saturation (rScO2) was monitored repetitively starting at the time of IVH diagnosis for nearly 20 weeks of life. Further analysis involved assessing the duration of rScO2 values falling outside the established normal range (<55% or >85%) as well as the computation of fractional cerebral tissue oxygen extraction (cFTOE). The median values of those parameters were analyzed by postnatal age and injury group (IVH only; IVH+PHVD without NSI; IVH+PHVD with NSI).
RESULTS: This study included 146 preterm infants with a median gestational age of 25.7 (24.3-27.6) weeks and the diagnosis of IVH. Among them, 52.8% (n=77) were diagnosed with PHVD, of which 72.7% (n=56) required NSI. The analysis revealed substantial disparities among these groups. Specifically, patients with IVH+PHVD requiring NSI, exhibited lower rScO2 values (66.1 % vs. 55,7%, p<0.001), longer durations of rScO2 outside the established normal range (4.6% vs. 46.7%, p<0.001), and higher levels of cFTOE (0.29 vs. 0.42, p<0.001) compared to IVH infants who were not affected by PHVD. Conversely, IVH+PHVD patients without the need for NSI did not show significant difference compared to IVH patients without ventricular dilatation.
CONCLUSION: Our findings emphasize the profound cerebral effects in neonates affected by IVH and PHVD and highlight the enduring physiologic consequences. In particular, infants who required NSI, showed a continuous decline in rScO2 during the first 20 weeks of life, indicating persistent inadequate cerebral oxygenation, whereas their elevated cFTOE levels reflect increased oxygen demand.
Oxidant and antioxidants following neonatal hypoxic ischemia
Nur Aycan1, Derya Cay Demir2, Serap Karaman1, Eyyup Yurekturk1, Murat Basaranoglu1, Oguz Tuncer1
1Van Yuzuncu Yil University Pediatrics, 2Van Yuzuncu Yil University Chemistry
BACKGROUND: Neonatal hypoxic-ischemic encephalopathy (HIE) is a significant cause of perinatal and postnatal morbidity and mortality worldwide. No specific marker of neonatal hypoxia has been reported with good predictive effect still today. Therapeutic hypothermia is established as the standard of care to improve neurologic recovery in infants with HIE. Catalase (CAT) is the most crucial hydrogen peroxide-scavenging enzyme in the body, and catalase activity detection is used to detect levels of inflammation and oxidative stress. Lipid peroxidation levels marked after hypoxia can be detected based on the level of Malondialdehyde (MDA). In the neonatal period, glutathione (GSH) is the most critical non-enzymatic endogenous antioxidant. Magnetic resonance imaging (MRI) is a valuable tool for predicting long-term outcomes of neonates with HIE, in which hypothermia and systemic supportive care form the cornerstone of therapy. We aim to explain the pathways and relations between the oxidant and antioxidant status to detail oxidant-antioxidant balance and cellular mechanisms.
METHODOLOGY: After ethics committee approval and informing the families, serum levels of malondialdehyde, as an oxidative stress marker, and catalase and glutathione as antioxidant enzymes were measured in first blood samples of fifty-two neonates diagnosed with neonatal hypoxic-ischemic encephalopathy in our neonatal intensive care unit. The mean, standard error, and correlations of the oxidant and antioxidant parameters were calculated.
RESULTS: Older than 37 weeks of gestation and hypoxic, fifty-two neonates, 84% of whom were born vaginally delivery (28 boys, 24 girls), were included in our research. Gestation weeks were 38.03±0.13, and the birth weight of patients was 3202.34±96.202. Compared to healthy newborns (n=23), catalase was statistically significantly lower than in the healthy group (p=0.0001), while MDA serum level was significantly higher in hypoxic newborns (p=0.01). The negative correlation between GSH and MDA was statistically significant (r=-0.32, p=0.02). The evaluation of the relationship of early (at postnatal fifth day)diffusion MRI findings with oxidants and antioxidants is ongoing.
CONCLUSION: HIE pathophysiology involves oxidative stress, mitochondrial energy production failure, glutaminergic excitotoxicity, and apoptosis. Explaining the pathways between oxidant-antioxidant balance and cell death, which will explain the pathophysiology of HIE, is essential in order to develop life-saving treatment strategies that will minimize the effects of oxygen deprivation on other body organs, especially the brain.
BIBLIOGRAPHY:
1. Greco P, Nencini G, Piva I, et al. Pathophysiology of hypoxic-ischemic encephalopathy: a review of the past and a view on the future. Acta Neurol Belg. 2020;120(2):277-288. doi:10.1007/s13760-020-01308-3
2. Natarajan G, Pappas A, Shankaran S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE). Semin Perinatol. 2016;40(8):549-555. doi:10.1053/j.semperi.2016.09.007
3. Torres-Cuevas I, Corral-Debrinski M, Gressens P. Brain oxidative damage in murine models of neonatal hypoxia/ischemia and reoxygenation. Free Radic Biol Med. 2019;142:3-15. doi:10.1016/j.freeradbiomed.2019.06.011
How does neonatal hypoxia affect thyroid hormones?
Nur Aycan1, Eyyup Yurekturk1, Murat Basaranoglu1, Serap Karaman1, Oguz Tuncer
1Van Yuzuncu Yil University
BACKGROUND: Thyroid hormone plays an essential role in brain development and fetal, neonatal, and adult brain function and may influence neuronal recovery after hypoxia and traumatic brain injury. We aimed to see whether there is a relationship between the effects of hypoxia at the cellular level and the stages of the disease.
METHODOLOGY: After ethics committee approval and informing the families, demographic characteristics, laboratory parameters, diffusion MRI images, and TSH and free T4 levels from thyroid function tests on a postnatal day five were recorded retrospectively in newborns who were hospitalized with the diagnosis of hypoxic-ischemic encephalopathy in the neonatal intensive care unit of our hospital in the last two years.
RESULTS: A total of 81 patients over 37 weeks of gestation were included in the study. Of the babies, 35 (43.2%) were female and 46 (56.8%) were male. Their mean gestational age was 38.26±1.87, and their mean birth weight was 3010±75.28. Sarnat staging (stage 1 n=23, stage 2 n=47, and stage 3 n=11) was performed according to the clinical status of the patient. According to Sarnat scoring, there was no statistical difference between TSH levels on postnatal day 5 (p=0.17), but free T4 level was statistically significantly higher in stage 2 than in stage 1 and stage 3 (p=0.001).
CONCLUSION: Thyroid hormone is known to have significant effects on energy catabolism, thermoregulation, growth, development, bone and central nervous system development. The fact that thyroid hormones can be detected differently in different clinical conditions of hypoxia suggests whether they can be used as a parameter in the staging of hypoxia.
BIBLIOGRAPHY:
1 Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696-705. doi:10.1001/archneur.1976.00500100030012
2 Tani N, Ishikawa M, Watanabe M, Ikeda T, Ishikawa T. Thyroid-related hormones as potential markers of hypoxia/ischemia. Hum Cell. 2020;33(3):545-558. doi:10.1007/s13577-020-00341-x
3 Li J, Abe K, Milanesi A, Liu YY, Brent GA. Thyroid Hormone Protects Primary Cortical Neurons Exposed to Hypoxia by Reducing DNA Methylation and Apoptosis. Endocrinology. 2019;160(10):2243-2256. doi:10.1210/en.2019-00125
4 Li J, Donangelo I, Abe K, et al. Thyroid hormone treatment activates protective pathways in both in vivo and in vitro models of neuronal injury. Mol Cell Endocrinol. 2017;452:120-130. doi:10.1016/j.mce.2017.05.023
Heart rate variability during therapeutic hypothermia in neonatal encephalopathy
John Sunwoo1, Seh Hyun Kim2, Katie Hannon2, Eniko Szakmar2,3, Aisling Garvey2,4, Chelsea Munster2, Sara Cherkerzian2, Hoda Elshibiny2, Helen Christou2, Terrie Inder2,5, Maria Angela Franceschini1, Mohamed El-Dib2
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, 2Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Harvard Medical School, 3Division of Neonatology, 1st Department of Pediatrics, Semmelweis University, 4INFANT Research Centre, Cork, Ireland; Department of Paediatrics and Child Health, University College Cork, 5Center for Neonatal Research, Children’s Hospital of Orange County, Orange; Department of Pediatrics, University of California Irvine
BACKGROUND: Continuous monitoring of autonomic nervous system (ANS) responses during therapeutic hypothermia (TH) in neonates offers important markers for hypoxic-ischemic encephalopathy. Heart rate variability (HRV) indices, which reflect ANS activity, are particularly informative during TH. The high frequency component of HRV—a marker of parasympathetic tone—is known to increase with cooling[1], promoting the recovery of cerebral tissues and circulatory functions; However, only few studies have reported a comparative analysis of HRV changes throughout the entire cooling and subsequent rewarming periods, especially in the context of adverse brain injury outcomes. We hypothesized that infants with brain injury would exhibit a blunted HRV response to TH.
METHODOLOGY: EKG was collected from infants who underwent TH for neonatal encephalopathy at Brigham and Women’s Hospital, MA. Post-TH brain injury was assessed using Weeke et al.’s MRI-based grading (PMID:29246356). A Weeke score >3 was considered ‘brain injury’. The R-to-R interval was extracted and cleaned using automated algorithms and visual examinations, and analyzed across high (HF=0.2-2Hz), low (LF=0.04-0.2Hz), and very-low frequency (VLF=0.01-0.04Hz) bands using a wavelet transform technique. HF indices were combined into 1-hour medians, represented by each point in Figure 1, and further grouped into 6-hour bins for the cooling stage and 3-hour bins for the rewarming stage. A mixed model was used to account for repeated measures in each bin.
RESULTS: We analyzed 43 infants in this report. Absolute HF values were log10-transformed to achieve normality. In both groups, we found the anticipated increases in HF during cooling, and in heart rate during rewarming (Figure 1). In the brain injury group, we found a consistently lower log10HF (Figure 1-a), while their normalized HF was not different from the normal group (Figure 1-b). Both groups exhibited a gradual increase in Total Power, LF, and VLF during cooling, and a decrease upon rewarming (not shown). Although visually subtle, the brain injury group maintained a statistically higher heart rate throughout TH (Figure 1-c).
CONCLUSION: We report HRV indices and heart rate changes during therapeutic hypothermia, confirming the anticipated responses to cooling and rewarming. Cooling-induced HF increases suggested enhanced parasympathetic activity in both brain injury and normal groups. However, the brain injury group showed consistently lower HF levels and higher heart rates, suggesting diminished parasympathetic tone and its capacity. Insignificant differences in relative HF power may be due to a proportional increase in Total Power during cooling. Future work will incorporate cerebral oxygenation, and covariates that can induce parasympathetic withdrawal, such as modes of ventilation. This work provides additional insights into HRV changes during TH and their possible association with brain injury outcomes.
BIBLIOGRAPHY:
[1] R. M. Goulding et al., “Heart rate variability in hypoxic ischemic encephalopathy during therapeutic hypothermia.,” Pediatr. Res., vol.81, no.4, pp.609–615, Apr. 2017.
NIRS for prediction of adverse neurological outcomes in infants with HIE receiving therapeutic hypothermia
Alam Randhawa1, Pauline De Jesus1, Kristine Woodward2, Khorshid Mohammad2, Michael J. Esser2
1University of Calgary, 2Alberta Children’s Hospital
BACKGROUND: Hypoxic Ischemic Encephalopathy (HIE) in term and near-term infants can result in brain injury due to oxygen and/or blood flow deprivation (1). Despite, therapeutic hypothermia (TH) treatment, consequences of HIE still include death or life-long disability. Current tools may not accurately predict the long-term outcomes (2,3). Near-infrared spectroscopy (NIRS) measures of cerebral regional oxygenation (rSO2) is a growing area of interest for prognostication (4).
OBJECTIVE: To determine if changes in NIRS (rSO2) in neonates with HIE, and receiving TH, were associated with other markers of brain injury and neurodevelopmental outcomes.
METHODOLOGY: A retrospective cohort study of 36 term infants (>35 weeks gestation) born between March 01, 2018, and March 21, 2020, meeting HIE and TH criteria. The rSO2 values were measured via one sensor in the left frontal region, in conjunction with EEG, MRI and other clinical variables. Statistical analysis and machine learning (ML) algorithms were used to determine which variables were associated with the short-term outcome of brain injury on MRI or longer-term outcomes of neurodevelopmental impairments within 3 years of life.
RESULTS: The distribution of our cohort was skewed towards mild HIE. HIE severity was not associated with short-term brain injury or longer-term neurodevelopmental outcomes. The mean rSO2 was not associated with MRI results which were also not associated with neurodevelopmental outcomes. Although not statistically significant, higher mean rSO2 values, with concomitant less variability, were associated with greater likelihood of neurodevelopmental impairments within 3 years of life. Using ML techniques, rSO2, measured by NIRS was the most important predictor for neurodevelopmental outcomes in models where only EEG and MRI were included, and remained the most important predictor when other clinical variables were considered (sex, arterial pH, resuscitation etc.).
CONCLUSION: Infants with HIE and poor neurodevelopmental outcomes may have persistent dysregulation in cerebral oxygenation during TH. Future work should include larger sample sizes, enhancing data capture to facilitate greater utility of machine learning (ML) algorithms, expanding follow-up to school age children expanding follow-up to school age children to determine potential effect on higher cortical functioning and wider coverage of NIRS probes to better ascertain regional differences.
BIBLIOGRAPHY:
1. Herrera, C. A., & Silver, R. M. (2016). Perinatal Asphyxia from the Obstetric Standpoint: Diagnosis and Interventions. Clinics in Perinatology, 43(3), 423–438. https://doi.org/10.1016/j.clp.2016.04.003
2. Higgins, R. D., & Shankaran, S. (2009). Hypothermia for hypoxic ischemic encephalopathy in infants ≥36weeks. Early Human Development, 85(10, Supplement), S49–S52. https://doi.org/10.1016/j.earlhumdev.2009.08.015
3. Vannucci, R. C. (1990). Current and potentially new management strategies for perinatal hypoxic-ischemic encephalopathy. Pediatrics, 85(6), 961-968.
4. Mitra, S., Bale, G., Meek, J., Tachtsidis, I., & Robertson, N. J. (2020). Cerebral Near Infrared Spectroscopy Monitoring in Term Infants With Hypoxic Ischemic Encephalopathy—A Systematic Review. Frontiers in Neurology, 11. https://www.frontiersin.org/articles/10.3389/fneur.2020.00393
Transcranial doppler ultrasonography in assessing intervention effects in hydrocephalus infants in Uganda
Marvin Seruwu2, Rutvi Vyas1, Davis Natukwatsa2, Ronald Mulondo3, Starlin Tindimwebwa2, Collins Akugizibwe2, Moses Wabukoma2, Enock Kainja2, Joshua Magombe2, Isaac Emurang2, Esther Nalule2, Michael Woglom1, Simeo Ochieng2, Chuan-Heng Hsiao1, Ajay Rajaram1, German Figueroa1, Braulio Ramírez1, Julia Tatz1, Steven Schiff3, P. Ellen Grant1, Benjamin Warf1, Miriah Kemigisha Katugi2, Edith Mbabazi Kabachelor2, Jason Sutin1, Pei-yi Lin1
1Boston Children’s Hospital, 2CURE Children’s Hospital of Uganda , 3Yale School of Medicine, Yale University
BACKGROUND: Neonatal hydrocephalus is a major global health issue, especially in low- and middle-income countries (LMICs) where post-infectious hydrocephalus (PIH) is endemic yet access to neurosurgical care is limited. CURE Children’s Hospital of Uganda (CCHU) developed endoscopic third ventriculostomy/choroid plexus cauterization (ETV/CPC) as the only implant-free treatment for infant hydrocephalus. ETV/CPC is as effective as the traditional treatment of implanting a ventriculoperitoneal shunt (VPS) (Kulkarni et al. 2017), but about a third of treatments fail within six months for reasons not well understood. Transcranial Doppler Ultrasound (TCD) is a readily available point of care tool for measuring large artery cerebral blood flow velocity that is not currently a uniform standard of care. Our objective is to investigate perioperative changes in TCD variables in infants treated with ETV/CPC and their relationship with surgical outcome.
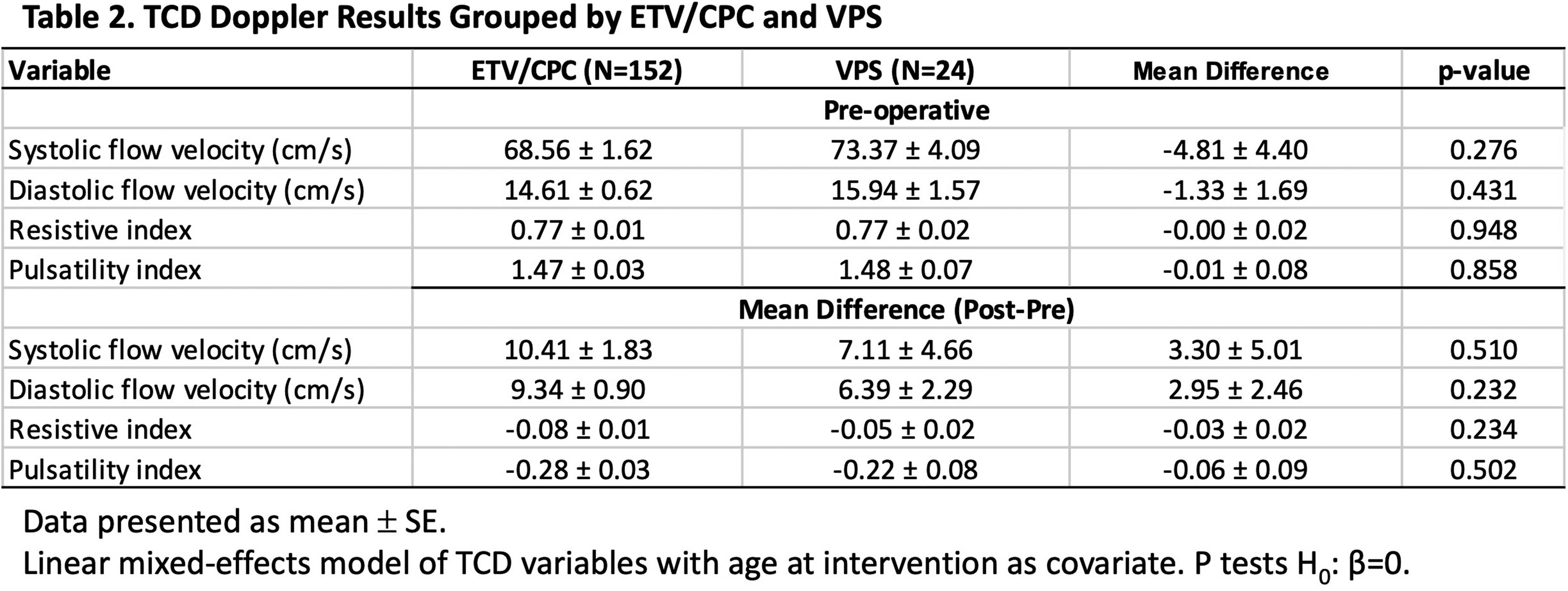
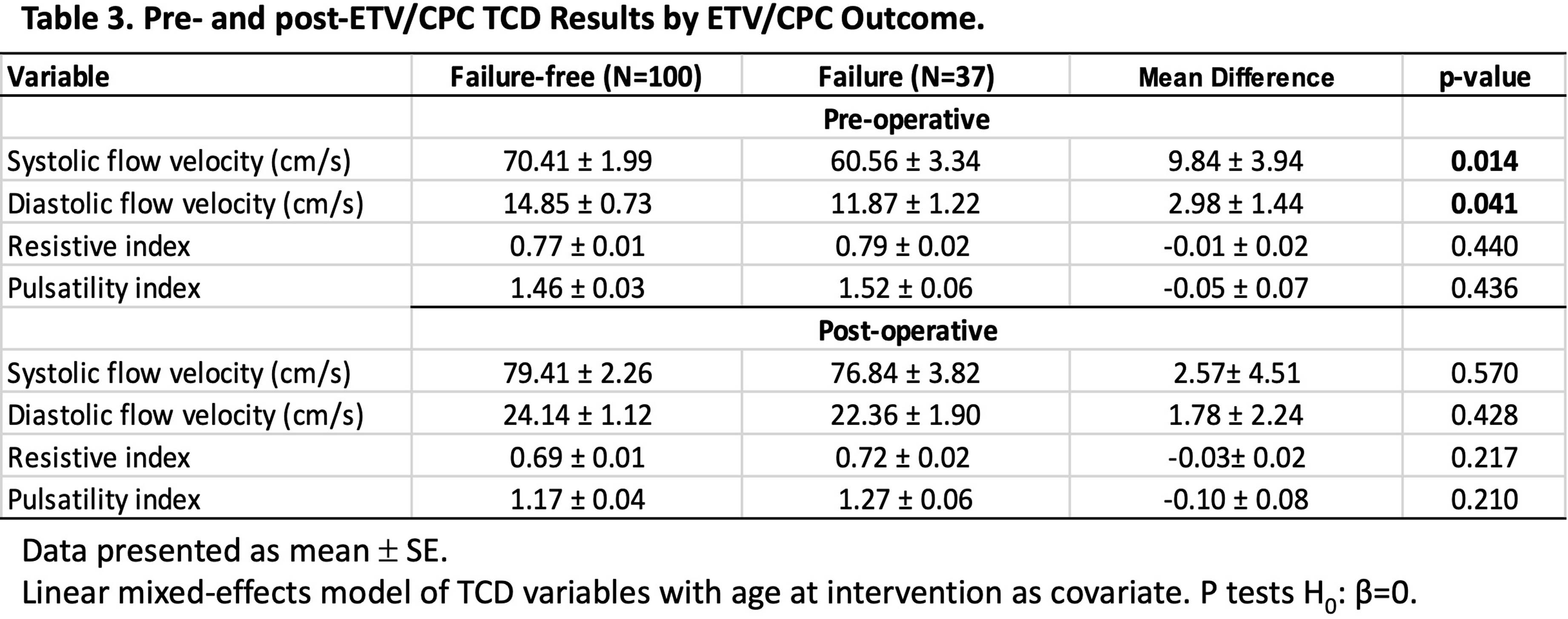
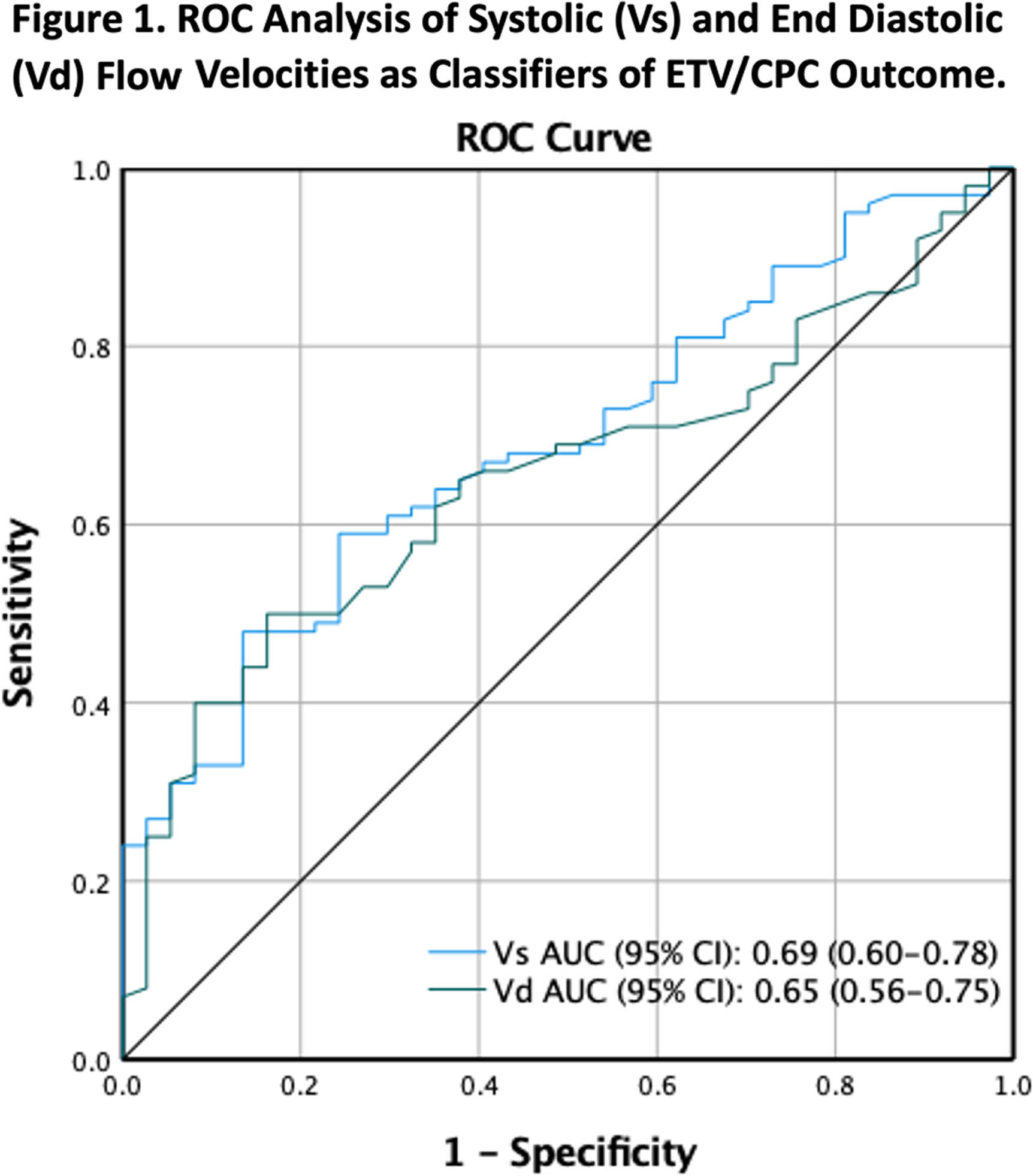
METHODOLOGY: This is a subgroup analysis of a prospective, observational study of patients at risk for ETV/CPC treatment failure (NCT 03650101) (Vadset et al. 2022). Infants under six months old considered to be treated with ETV/CPC for hydrocephalus were eligible for the study. Systolic (Vs) and end diastolic (Vd) flow velocities, resistive (RI) and pulsatility (PI) indices of the anterior cerebral artery were measured preoperatively and postoperatively by TCD within a few days of surgery. ETV/CPC treatment failure is defined as the need for any re-operation related to hydrocephalus within six months. Paired t test was used to test the intervention effect of TCD variables. Linear mixed model with age at intervention as a covariate was used to compare TCD results sub-grouped by intervention and ETV/CPC outcome. ROC analysis was used to evaluate the performance of the TCD assessment in ETV/CPC risk prediction.
RESULTS: Two-hundred patients were enrolled and 190 (71F/119M; median (IQR) age: 66 (31-110) days) were included in this analysis. Of those intended for ETV/CPC, 33 (17%) crossed to VPS, with crossovers more likely to have PIH, wait longer to receive their definitive surgery and 39% had a prior ventriculosubgaleal shunt (Table 1). Despite these differences in clinical characteristics, neither pre-operative TCD variables, nor their change following intervention differed significantly between patients treated with ETV/CPC or VPS. (Table 2). Patients with ETV/CPC failure are younger than patients with successful outcome (failure-free) (Mean difference: 34.2 ± 10 days, p<0.001). After ETV/CPC, Vs and Vd improved and RI and PI decreased, regardless of the treatment outcome (p<0.001, T-test). However, both pre-op Vs and Vd are higher in the failure-free group than those in failure group after controlling for age (Table 3). ROC analysis demonstrated a moderate discriminatory ability in predicting ETV/CPC treatment success (Figure 1).
CONCLUSION: Our results suggest TCD has potential for risk assessment and clinical decision making in the management of hydrocephalus.
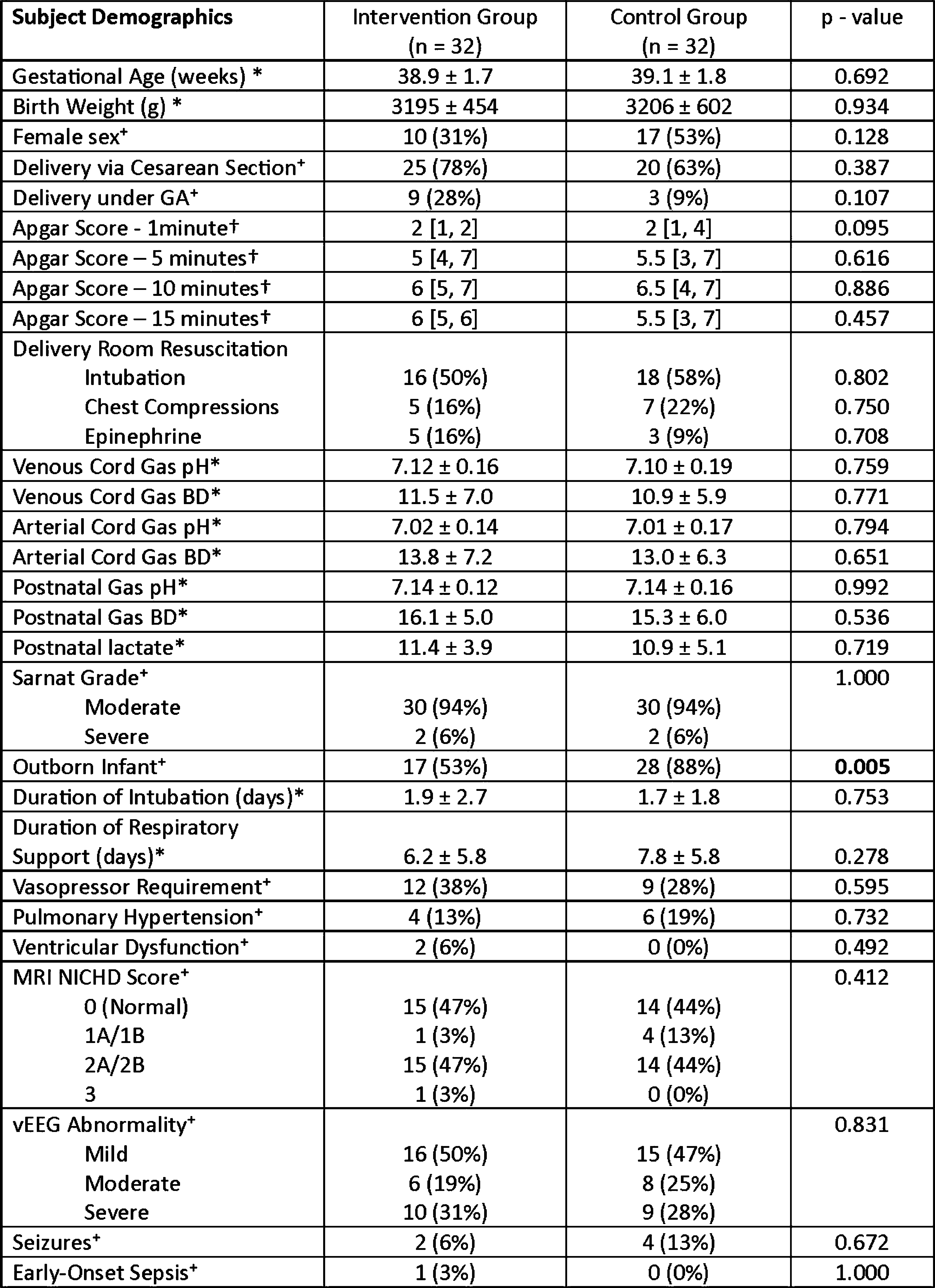
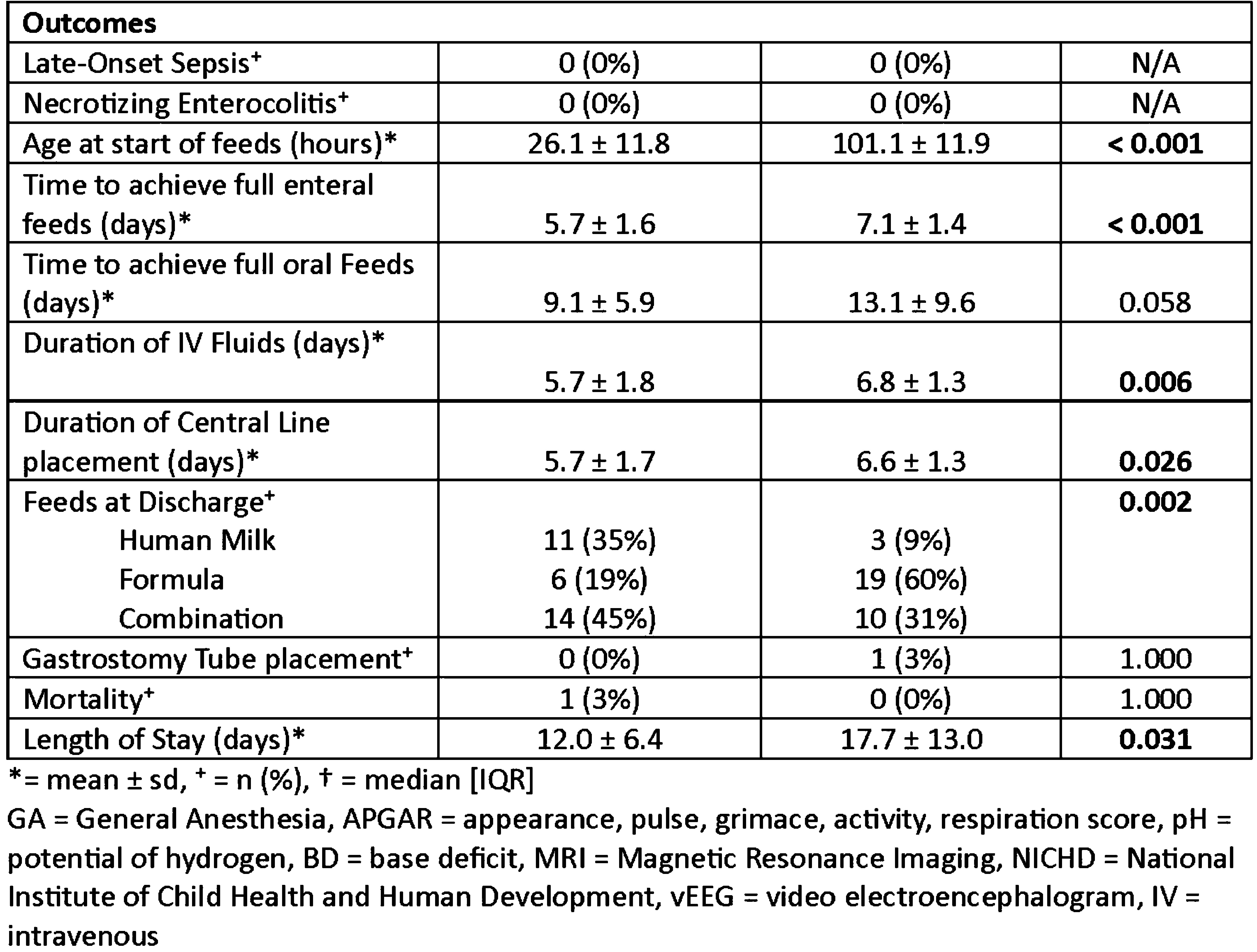
A prospective study describing splanchnic NIRS in infants receiving minimal enteral nutrition during therapeutic hypothermia
Tatiana Nuzum, Martha Caprio, Sean Bailey, Elena Wachtel
1Hassenfeld Children’s Hospital at NYU Langone, 2NYCH+H Bellevue Hospital Center
BACKGROUND: Therapeutic Hypothermia (TH) is the standard of care for moderate to severe neonatal encephalopathy (NE). Historically, minimal enteral nutrition (MEN) has been withheld during TH due to concerns about splanchnic perfusion. Recent observational studies have shown that providing MEN during TH is safe and is associated with several benefits. Human milk is known to contain mesenchymal stem cells which are potentially neuroregenerative. Splanchnic perfusion measured by near-infrared spectroscopy (NIRS) has not yet been assessed in this population.
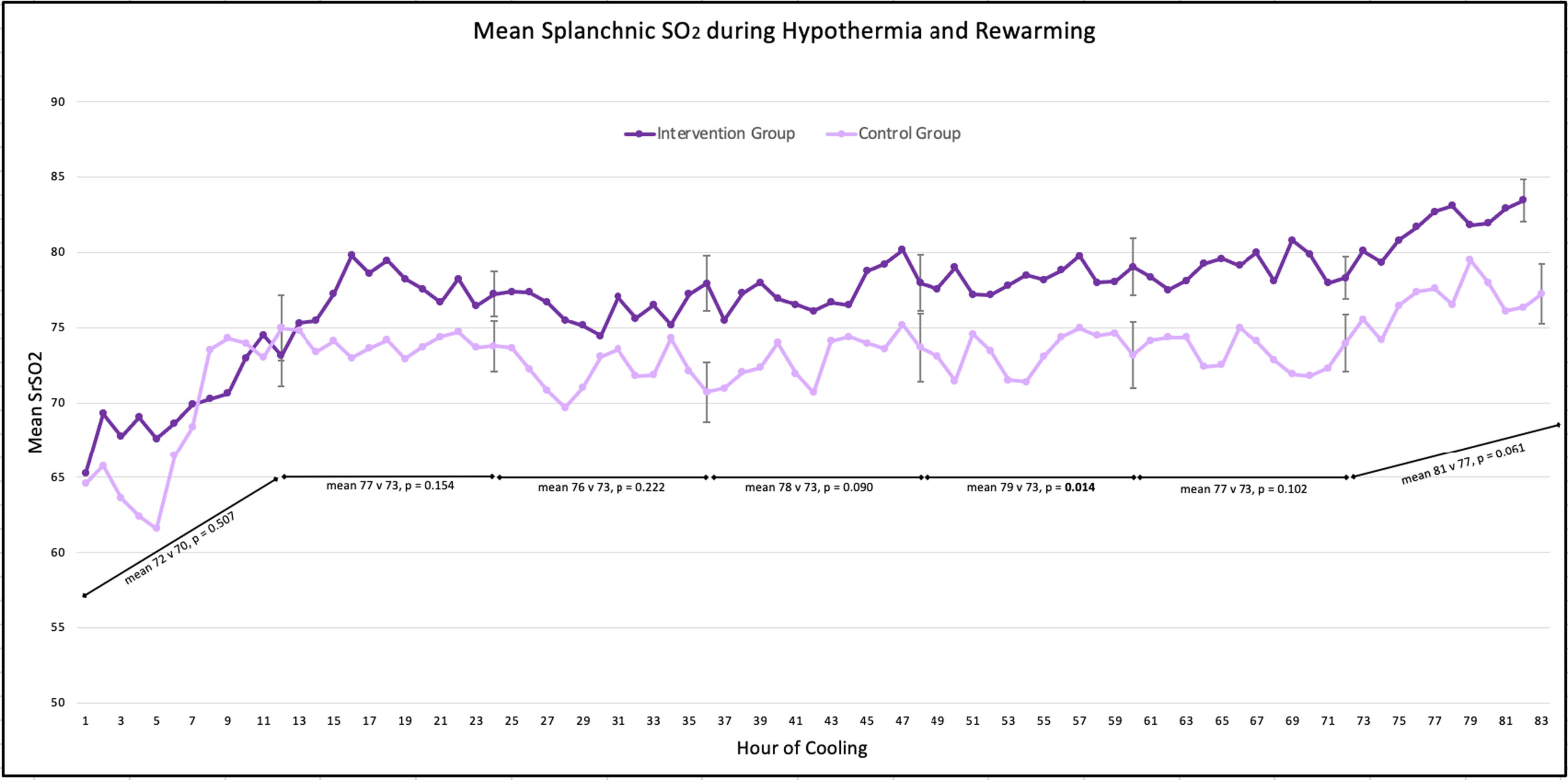
AIM: To compare clinical outcomes and splanchnic regional oxygen saturation (SrSO2) levels between infants who received MEN during TH, and those who did not.
METHODS: Infants were prospectively enrolled after informed consent was obtained. All infants that qualified for TH based on NICHD criteria were eligible. Exclusion criteria included dopamine infusion > 10mcg/kg/min or 2 vasoactive agents simultaneously, or baseline SrSO2 < 45%. Infants received 10-15 mL/kg/day of expressed or donor human milk for the duration of TH and rewarming. SrSO2 was recorded hourly. A group of historic controls who were NPO during TH was also compiled after applying the same exclusion criteria. Clinical data for the infant’s NICU course was collected.
RESULTS: Infants were prospectively enrolled after informed consent was obtained. All infants that qualified for TH based on NICHD criteria were eligible. Exclusion criteria included dopamine infusion > 10mcg/kg/min or 2 vasoactive agents simultaneously, or baseline SrSO2 < 45%. Infants received 10-15 mL/kg/day of expressed or donor human milk for the duration of TH and rewarming. SrSO2 was recorded hourly. A group of historic controls who were NPO during TH was also compiled after applying the same exclusion criteria. Clinical data for the infant’s NICU course was collected.
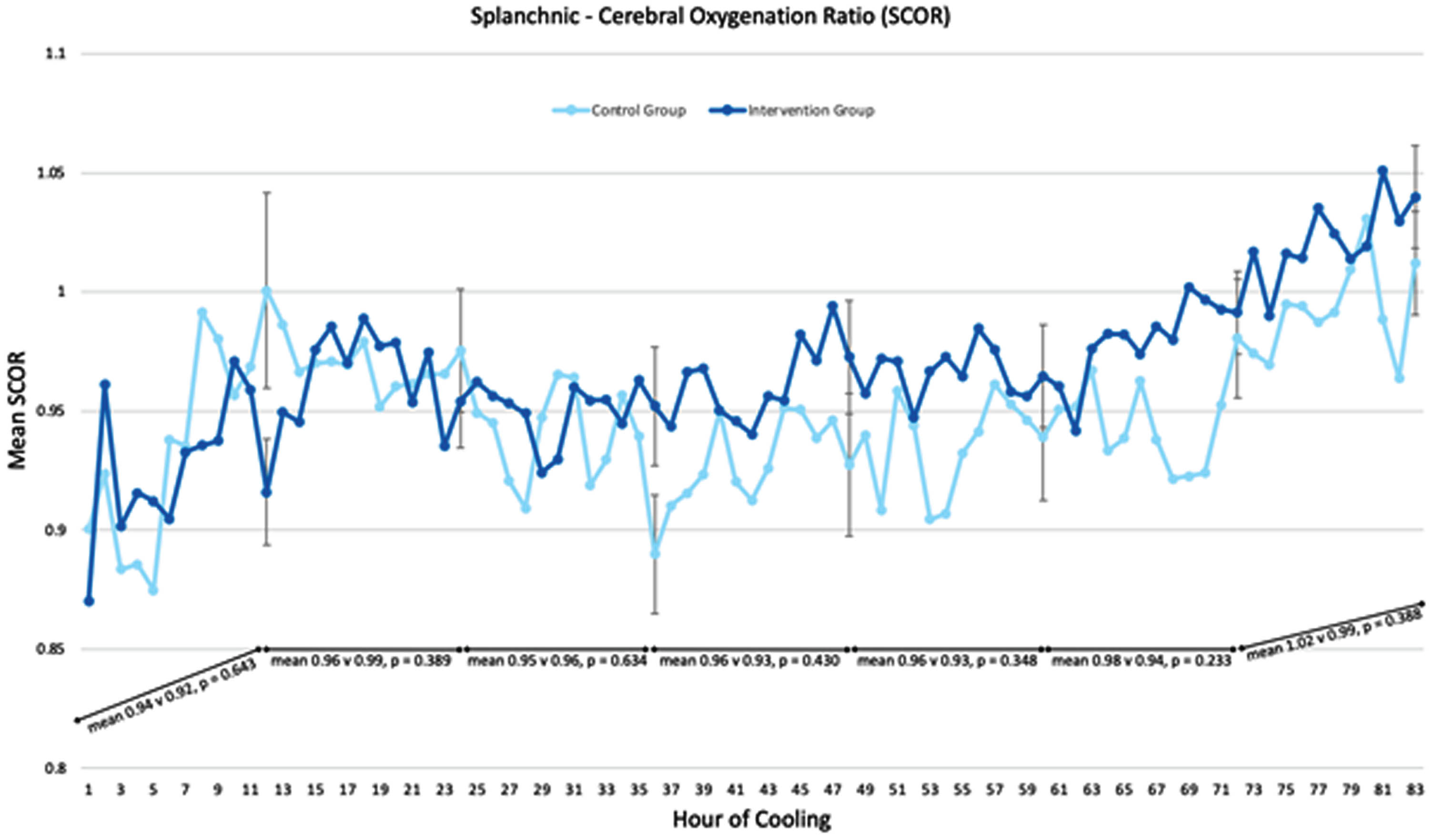
CONCLUSION: SCORs were within normal ranges throughout hypothermia and rewarming and did not differ between groups. Providing MEN during TH is safe and results in several important benefits including fewer central line days, shorter hospital stays and greater human milk feeding success. Future directions will include assessing long-term neurodevelopmental outcomes.
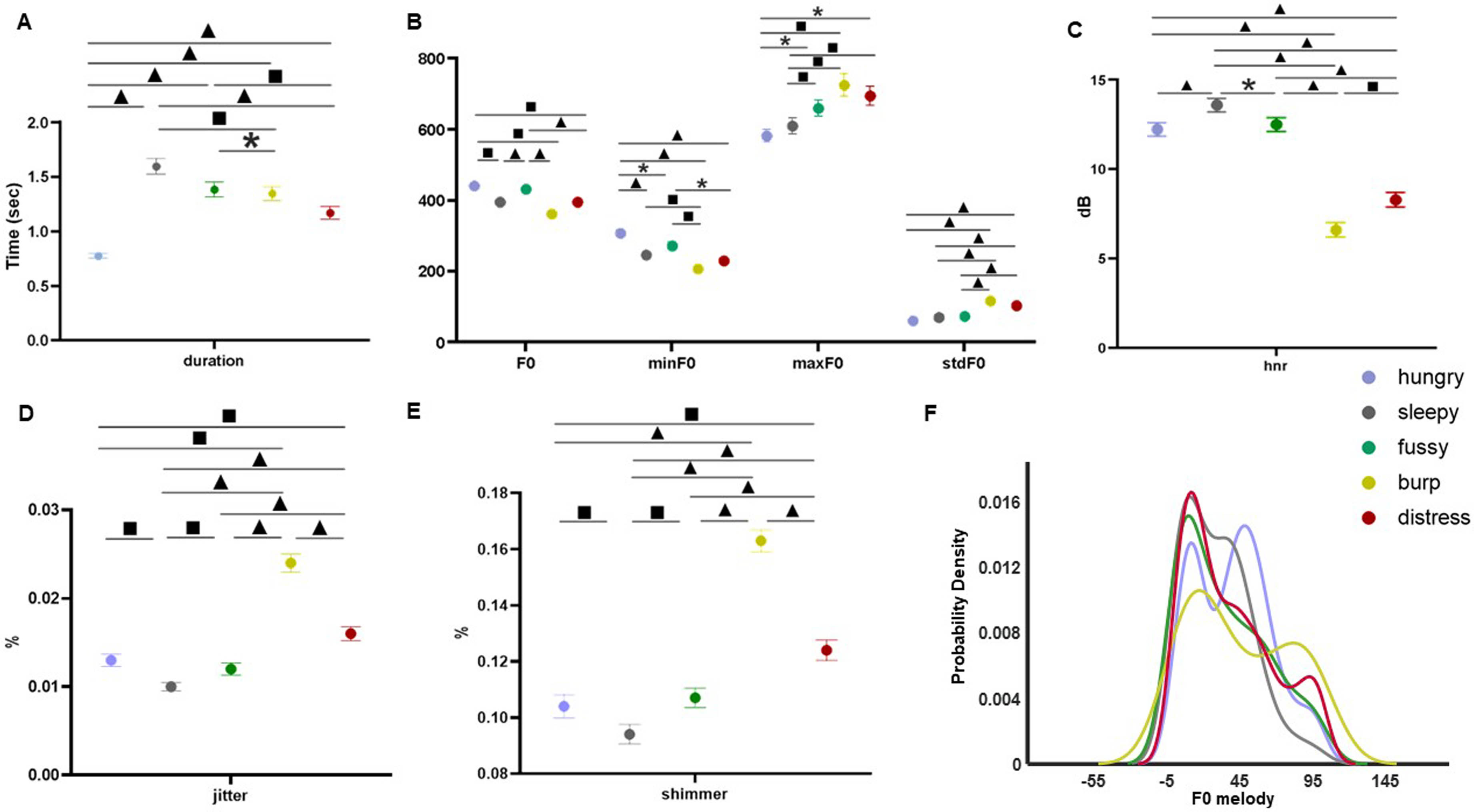
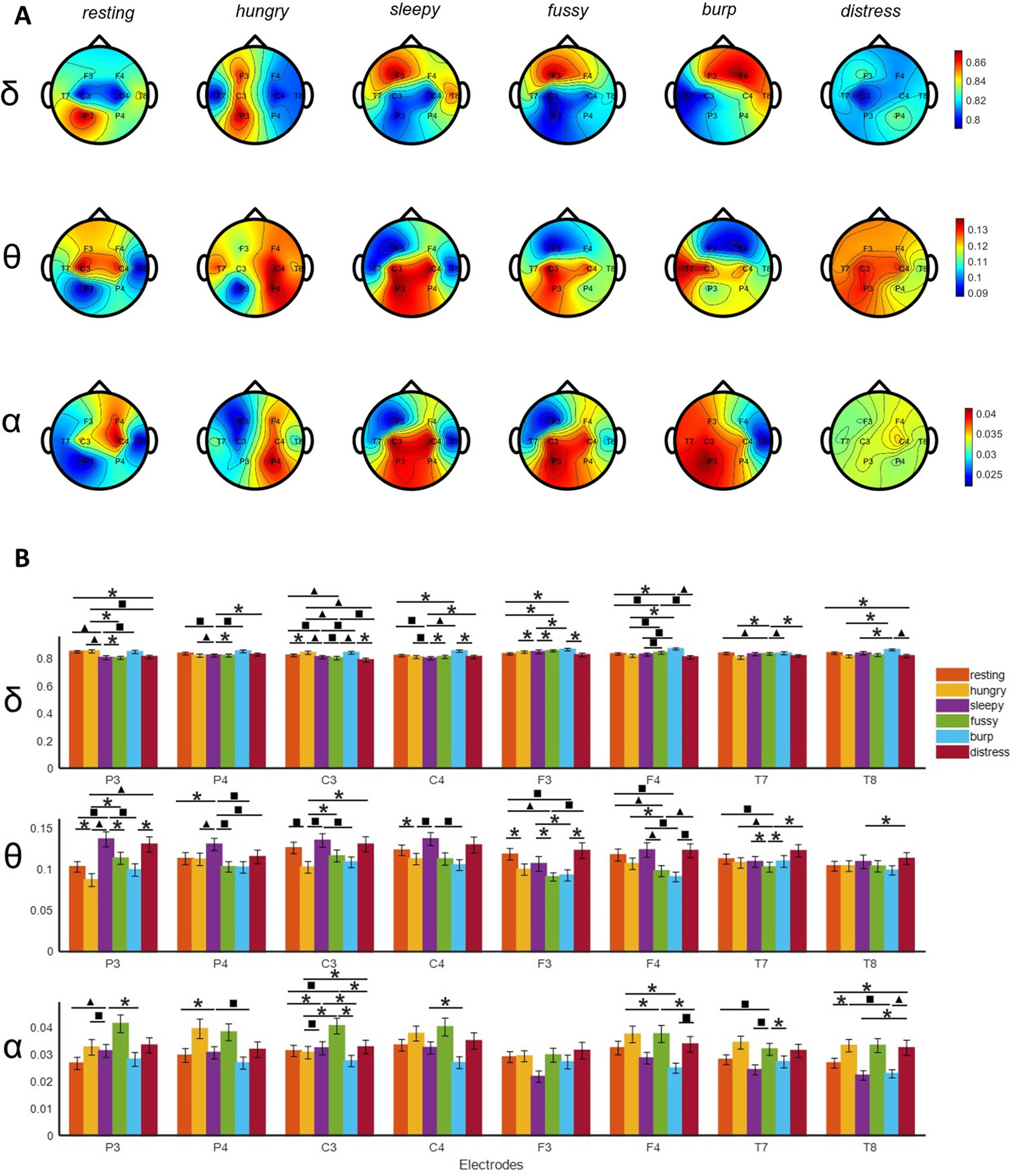
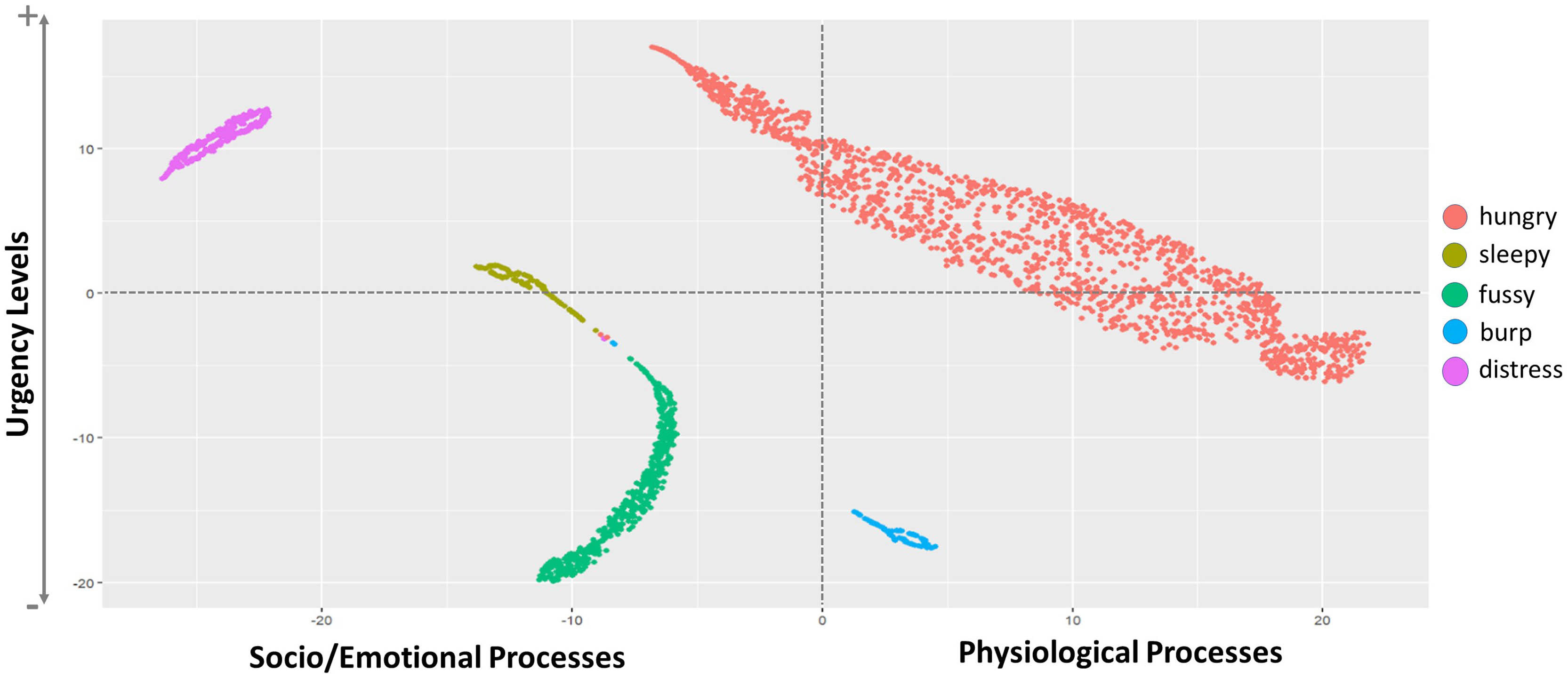
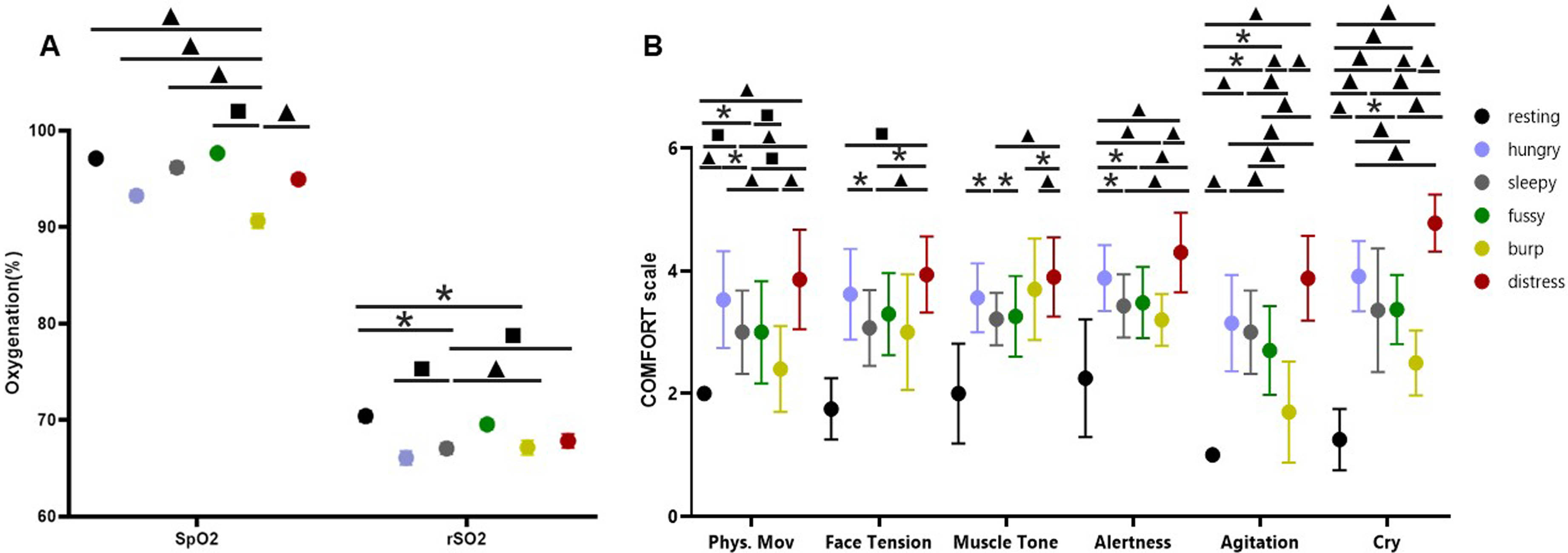
Multi-modal analysis of infant cry types characterization: Acoustics, body language and brain signals
Ana Laguna Pradas1, Sandra Pusil1, Anna Lucia Paltrinieri2, Angel Bazan1, Silvia Orlandi3
1ZOUNDREAM AG, 2Neonatology Department, Barcelona Centre for Maternal-Fetal and Neonatal Medicine (BCNatal), Hospital Clínic, Universitat de Barcelona, 3Department of Electrical, Electronic and Information Engineering “Guglielmo Marconi”(DEI), University of Bologna
BACKGROUND AND OBJECTIVE: Infant crying is the first attempt babies use to communicate during their initial months of life. A misunderstanding of the cry message can compromise infant care and future neurodevelopmental process.
METHODOLOGY: An exploratory study collecting multimodal data (i.e., crying, electroencephalography (EEG), near-infrared spectroscopy (NIRS), facial expressions, and body movements) from 38 healthy full-term newborns was conducted at Hospital Clinic-Maternitat Barcelona. Cry types were defined based on different conditions (i.e., hunger, sleepiness, fussiness, need to burp, and distress). Statistical analysis, Machine Learning (ML), and Deep Learning (DL) techniques were used to identify relevant features for cry type classification and to evaluate a robust DL algorithm named Acoustic MultiStage Interpreter (AMSI).
RESULTS: Significant differences were found across cry types based on acoustics, EEG, NIRS, facial expressions, and body movements. Acoustics and body language were identified as the most relevant ML features to support the cause of crying. The DL AMSI algorithm achieved an accuracy rate of 92%.
CONCLUSION: This study set a precedent for cry analysis research by highlighting the complexity of newborn cry expression and strengthening the potential use of infant cry analysis as an objective, reliable, accessible, and non invasive tool for cry interpretation, improving the infant-parent relationship and ensuring family well-being.
BIBLIOGRAPHY:
1. Crying as a sign, a sympton, & a signal: Clinical emotional and developmental aspects of infant and toddler crying. viii, 228 (Cambridge University Press, 2000).
2. Reggiannini, B., Sheinkopf, S. J., Silverman, H. F., Li, X. & Lester, B. M. A flexible analysis tool for the quantitative acoustic assessment of infant cry. J. Speech Lang. Hear. Res. JSLHR 56, 1416–1428 (2013).
3. Laguna, A. et al. How can cry acoustics associate newborns’ distress levels with neurophysiological and behavioral signals? Neonatol. Rev. (2023) doi:10.21203/rs.3.rs-2238719/v1.
4. Wasz-Höckert, O., Michelsson, K. & Lind, J. Twenty-Five Years of Scandinavian Cry Research. in Infant Crying: Theoretical and Research Perspectives (eds. Lester, B. M. & Zachariah Boukydis, C. F.) 83–104 (Springer US, 1985). doi:10.1007/978-1-4613-2381-5_4.
5. Teixeira, J. P., Oliveira, C. & Lopes, C. Vocal Acoustic Analysis – Jitter, Shimmer and HNR Parameters. Procedia Technol. 9, 1112–1122 (2013).
6. Toole, J. M. O. & Boylan, G. B. NEURAL: quantitative features for newborn EEG using Matlab. (2017) doi:10.48550/arxiv.1704.05694.
7. Orlandi, S., Bocchi, L., Donzelli, G. & Manfredi, C. Central blood oxygen saturation vs crying in preterm newborns. Biomed. Signal Process. Control 7, 88–92 (2012).
8. Ingraito, P. F. & Laguna Pradas, A. A Computer-Implemented Method of Providing Data For An Automated Baby Cry Assessment.
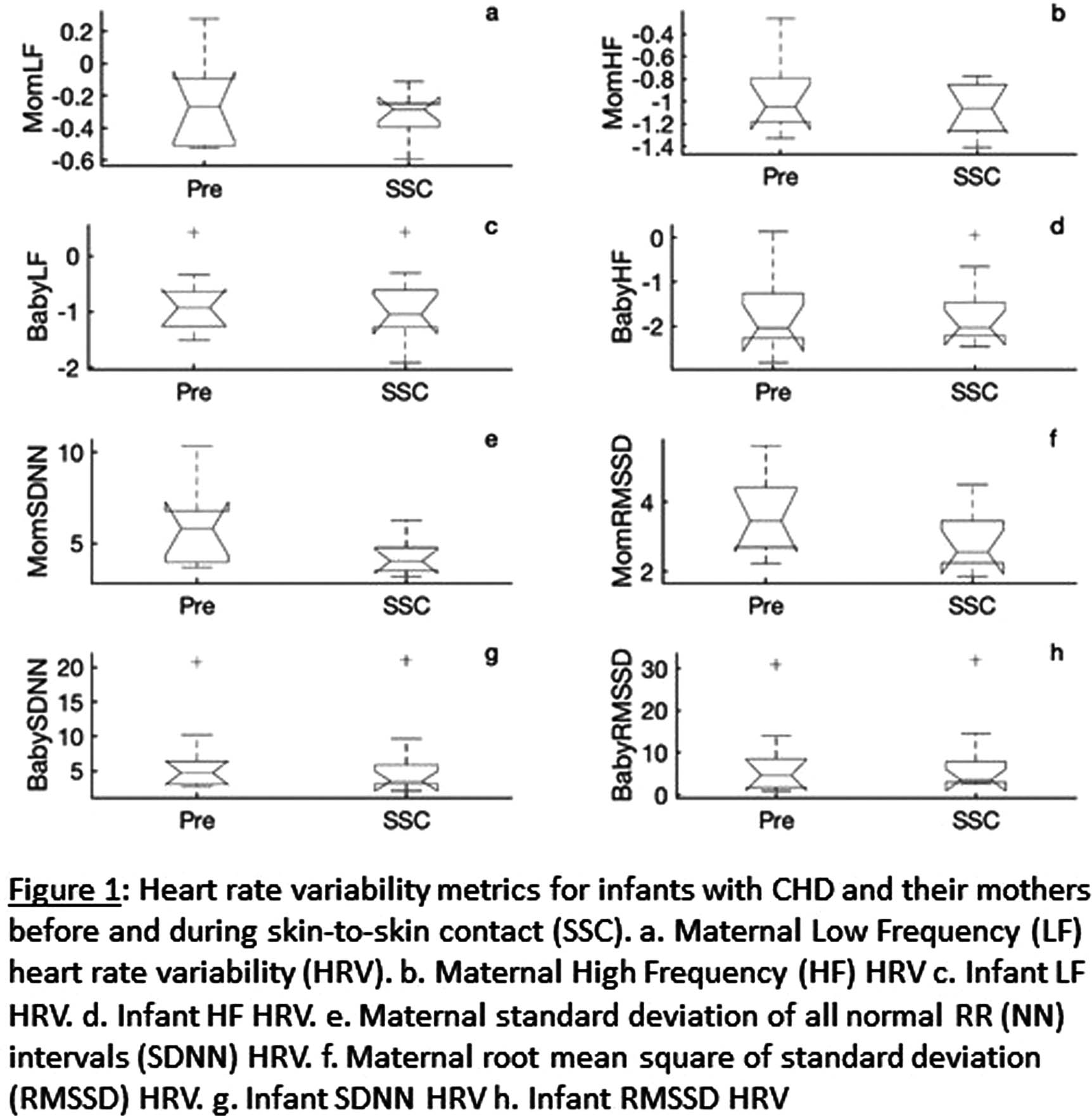
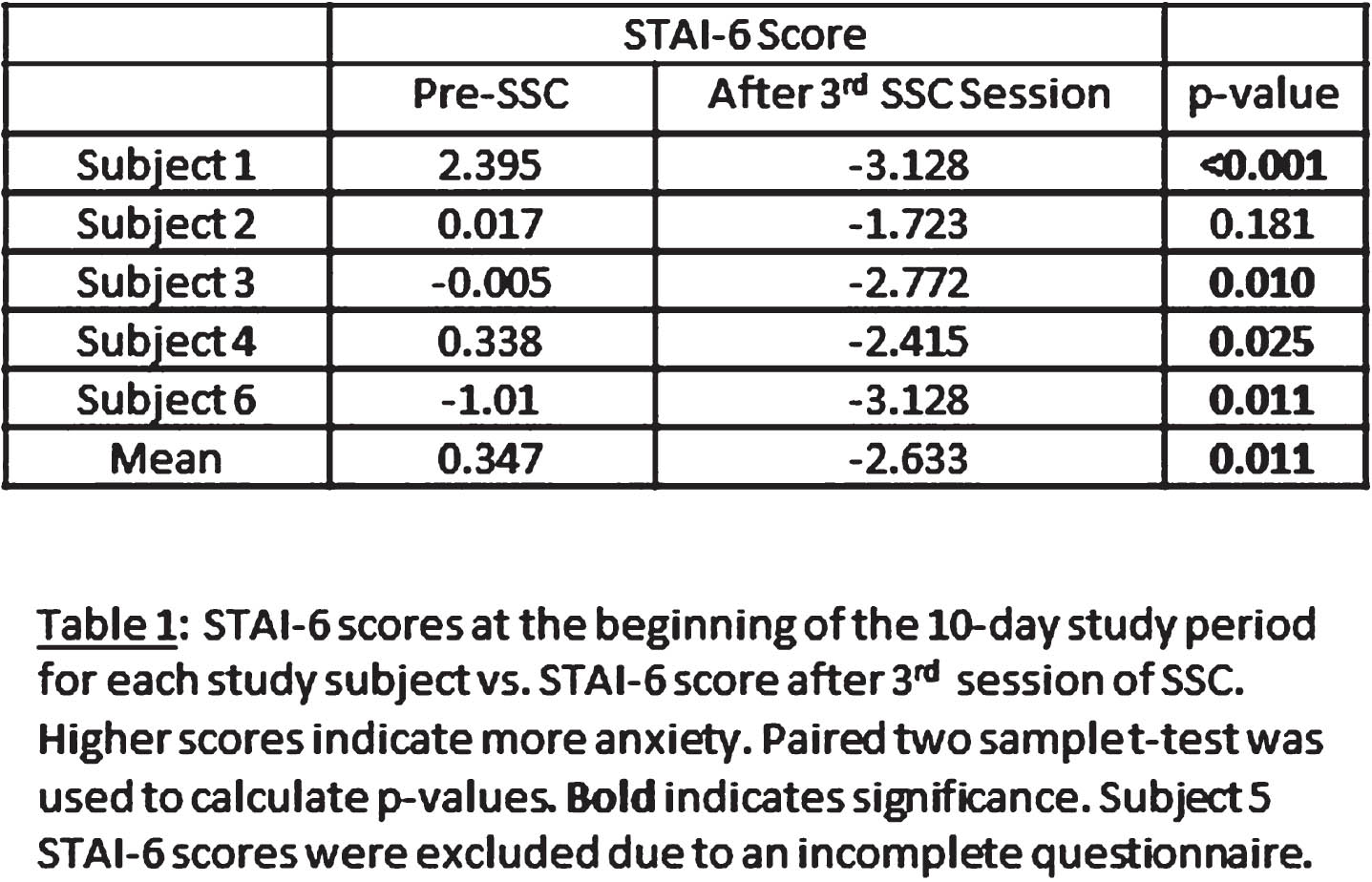
Skin-to-skin in congenital heart disease: impact on mother and infant autonomic function and maternal anxiety
Alexa Asch1, Rathinaswamy B Govindan1,4, Ellen Bartolini1,2, Venkata Chirumammilla1, Cara Pleau5, Mandana O’Donnell6, Sarah Schlatterer1,3,4
1Prenatal Pediatrics Institute, Children’s National Hospital, 2 Developing Brain Institute, Children’s National Hospital, 3 Department of Neurology, George Washington University School of Medicine and Health Sciences, 4Department of Pediatrics, George Washington University School of Medicine and Health Sciences, 5Division of Cardiology, Children’s National Hospital, 6Children’s National Hospital
BACKGROUND AND PURPOSE: Skin-to-skin contact (SSC)—a holding technique in which the diapered infant is held against the mother’s bare chest—has been extensively studied in the preterm population and found to have significant benefits for both mothers and preterm infants. SSC has been understudied in the congenital heart disease (CHD) population, which consists primarily of term neonates, and is often not routinely practiced in pediatric cardiac intensive care units (CICU). In this single-center prospective pilot study, we aim to evaluate the impact of low-dose post-operative SSC on maternal stress and autonomic function, measured through heart rate variability (HRV), in infants with CHD in the Children’s National Hospital CICU and their mothers. We also evaluated feasibility of SSC post-operatively.
METHODOLOGY: Six mother-infant dyads participated in three sessions of SSC within a 10-day post-operative period. Mother and infant heart rates were simultaneously recorded using the Biopac MP-160® device for 15 minutes before SSC and for an hour minimum during SSC. We calculated HRV metrics, including high frequency (HF), low frequency (LF), standard deviation of all normal RR (NN) intervals (SDNN), and root mean square of standard deviation (RMSSD) of RR intervals, using 5-minute epochs of EKG for each mother and infant. The mothers completed the State Trait Anxiety Inventory-6 (STAI-6) at the beginning of the study period and after each SSC session.
RESULTS: Table 1 shows that STAI-6 scores significantly decreased after SSC. Figure 1 illustrates our HRV data. SDNN significantly decreased during SSC compared to pre-SSC in our cohort (p=0.02 in mothers, p=0.04 in infants). RMSSD significantly decreased in mothers during SSC (p=0.03) but not in infants. A reduction in SDNN has been associated with decreased stress in healthy individuals in other studies.1 There was no significant change in HF or LF metrics of HRV during SSC. Neither mothers nor CICU nurses reported any significant challenges to feasibility of SSC during our study.
CONCLUSION: These findings demonstrate that SDNN and RMSSD decreased after SSC, and there were significant improvements in anxiety (STAI-6 scores) after SSC. Our data suggest that SSC does impact maternal and fetal autonomic function and reduce maternal anxiety. This study is limited by a small sample size, and a larger n will be required to validate these preliminary data and further characterize the impact of SSC on HRV and maternal anxiety in the CHD population. Our data suggest that further study of SSC in CHD is warranted, and that SSC is likely beneficial to both mothers and infants.