Proceedings of the 15th International Newborn Brain Conference: Neonatal Neurocritical Care, seizures, and continuous aEEG and /or EEG monitoring
The Efficacy of an AI-based seizure-detection algorithm for term vs. preterm neonates
Hiroyuki Kidokoro1, Anna Shiraki1, Toshiki Takeo2, Tatsuya Fukasawa2, Misa Hashimoto1, Misae Yamada1, Hajime Narita1, Takamasa Mitsumatsu1, Sumire Kumai1, Ryosuke Suzui1, Yuji Ito1, Hiroyuki Yamamoto1, Tomohiko Nakata1, Yoshiaki Sato1, Tetsuo Kubota2, Jun Natsume1
1Nagoya University Hospital, 2Anjo Kosei Hospital
OBJECTIVE: The development and clinical application of EEG devices equipped with seizure-detection algorithms using artificial intelligence in Neonatal Intensive Care Units has grown. Although some of these algorithms, trained using term seizure data, have reported sensitivities of approximately 0.5, their adaptability to seizures observed in preterm infants remains uncertain. This study examined the validity of automatic seizure detection in term neonates using a seizure-detection algorithm in our cohort and assessed its validity for seizures in preterm infants.
METHODS: Between 2019 and 2020, EEG recordings from two institutions that had been recorded with a nine-channel bipolar montage, and included seizure patterns, were collected. The EEG records were classified into those before 37 weeks postmenstrual age (preterm EEGs) and those from 37 weeks onward (term EEGs). EEG records without seizure patterns were also collected. Using the Nihon Kohden seizure-detection program (model QL-162A), these recordings were retrospectively re-analyzed to assess the seizure-detection capability. Experienced investigators evaluated all EEGs for the presence or absence of a seizure pattern. The performance of the program at detecting seizures was investigated.
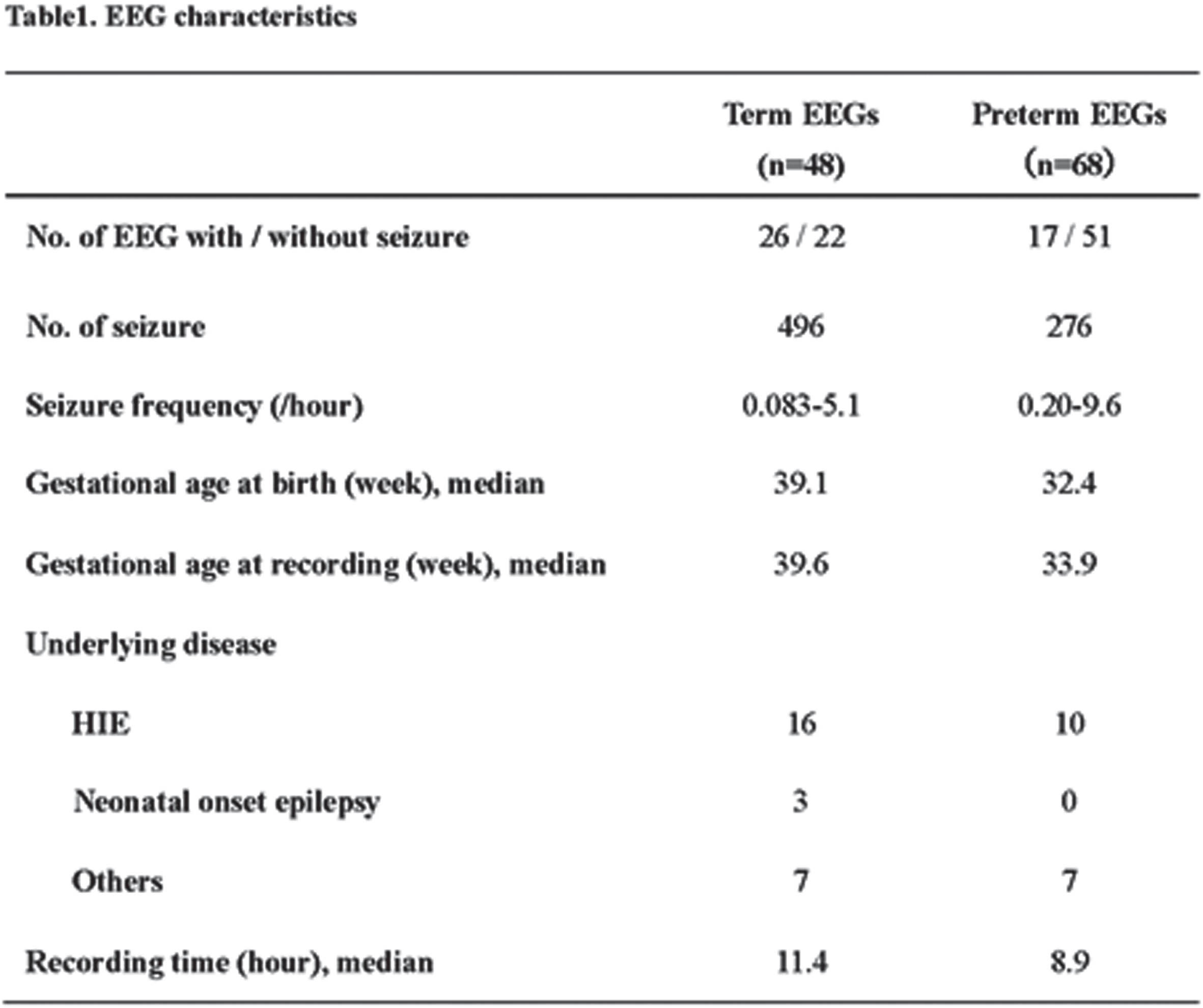
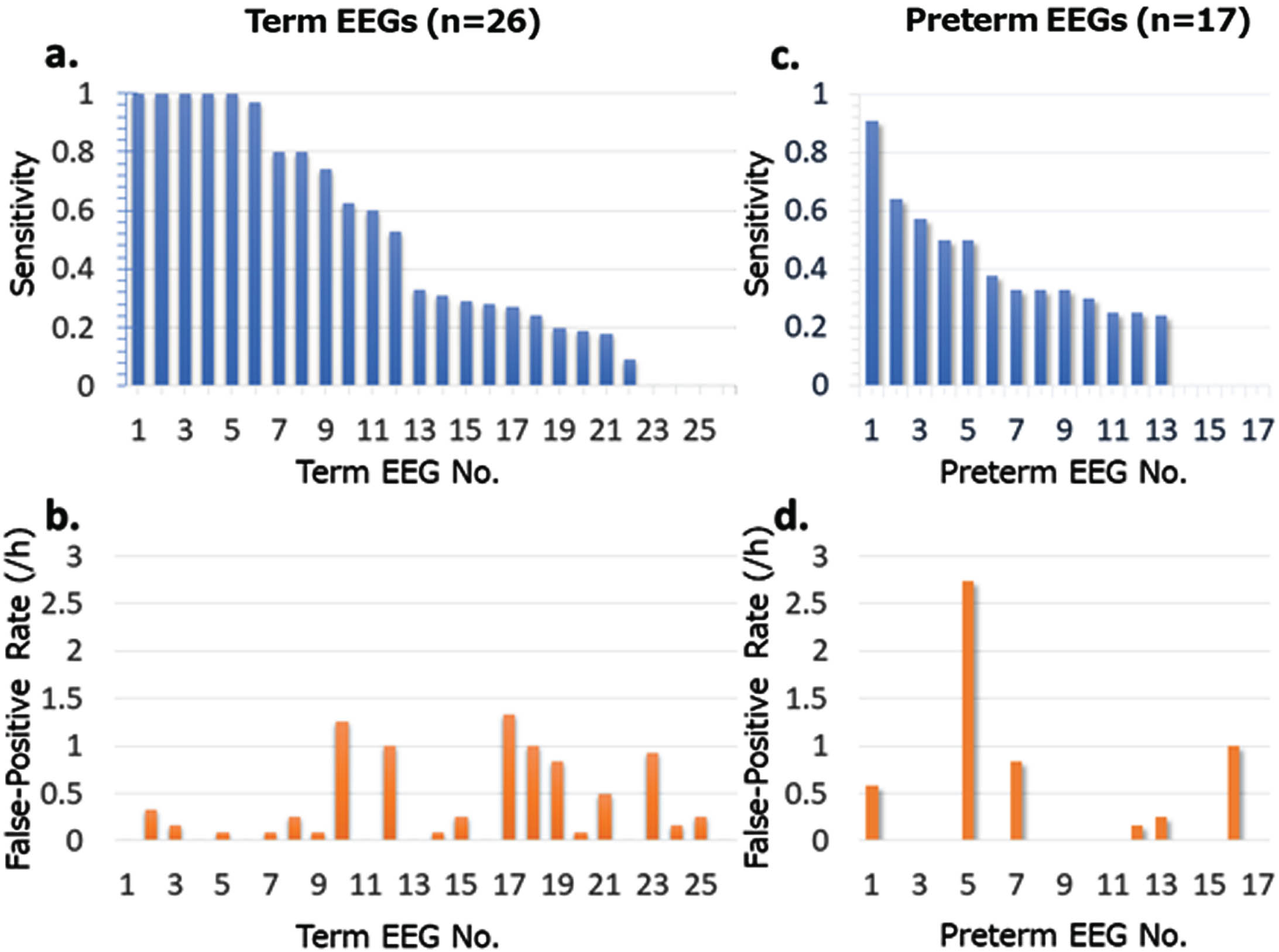
RESULTS: Of the 48 term EEGs, 26 contained 496 seizure episodes. Of the 68 preterm EEGs, 17 included 276 seizure episodes. The average gestational age at recording was 39.6 weeks for the term EEGs and 33.9 weeks for the preterm EEGs. Among the term recordings, hypoxic–ischemic encephalopathy was identified in 16 of the 26 cases, and 3 had neonatal-onset epilepsy. In comparison, 10 of the 17 preterm infants were diagnosed with hypoxic–ischemic encephalopathy. The seizure-detection program accurately identified seizures in 25 of the 26 term infants with seizures, for a sensitivity of 0.96 and specificity of 0.41. At the individual seizure level, the sensitivity averaged 0.48, with a false-positive rate of 0.33 (/hour). For preterm infants, 14 of 17 were correctly identified with a sensitivity of 0.82 and specificity of 0.49. The seizure detection sensitivity was 0.33 and the false-positive rate was 0.33 (/hour). Notable characteristics of undetected seizures in preterm infants included rhythmic delta activity and brief seizure episodes. Events often misclassified as seizures encompassed delta activity specific to preterm infants and various artifacts.
CONCLUSION: Compared to term infants, the seizure-detection sensitivity was lower in preterm infants. There is a need to develop an artificial intelligence algorithm specifically trained on the unique background EEG patterns and seizure waveforms of preterm infants.
Feasibility and quality assessment of an 8-channel EEG in emergency transport
Mark O’Sullivan1, Colm Murphy1, Alison O’Shea2, Geraldine Boylan1, Brian Walsh1
1Infant Research Centre, 2Munster Technological University
BACKGROUND: EEG monitoring of neonates with suspected brain injury is a critical tool to inform clinical care. However, conventional EEG monitoring requires significant expertise and setup time that typically limits its usage to tertiary-level hospitals. The use of neonatal EEG monitoring in both regional hospitals and during transport may be of clinical benefit to ensure timely treatment of neonatal seizures and escalation of care at the receiving hospital. A feasibility study to set up and analyse the data quality of a custom-designed portable and wireless EEG monitor during emergency transport was conducted.
METHODOLOGY: The study was conducted on a healthy adult volunteer in a fully functional training ambulance operated by an emergency transport technician (EMT) at Parkview Health Mirro Center, Indiana, US. A qualified EEG technician completed the EEG setup. The time to place all EEG electrodes and commence the EEG recording was measured while in transit. 10 minutes of EEG was then recorded while in transit and 10 minutes of EEG was recorded while stationary in order to assess the comparative data quality between stationary versus transit.
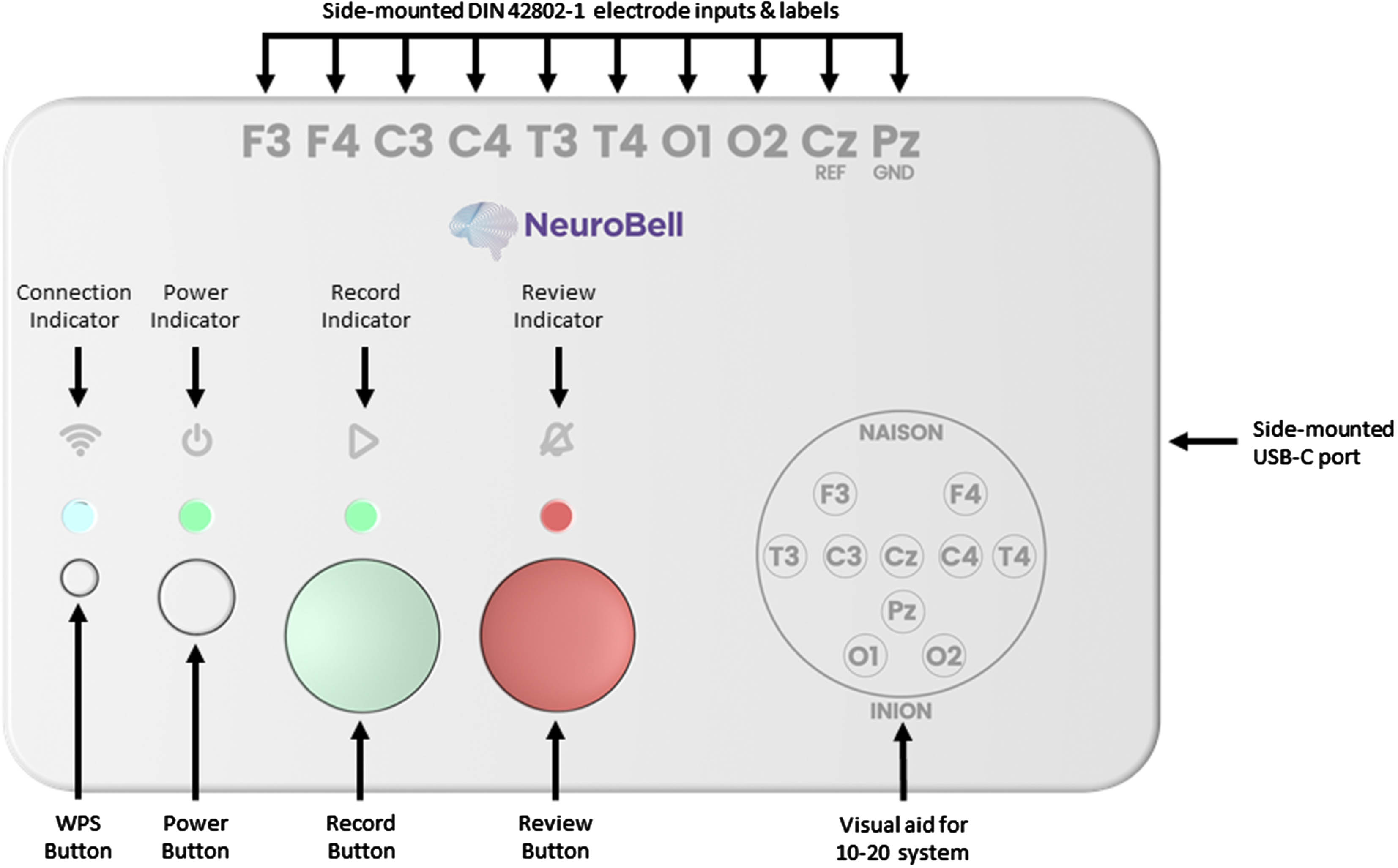
RESULTS: Figure 1 presents the custom EEG monitor, which requires the placement of 10 EEG electrodes at the following locations: F3, F4, C3, C4, T3, T4, O1, O2, Cz (Reference), and Pz (Ground). The system can be configured for recording with three button presses: 1) “Power”, 2) “Record” which starts impedance checks on all electrodes, and 3) “Record” again after the impedance light is green to begin the EEG recording. Ambu Neuroline Cup electrodes with NuPrep adhesive and 10-20 Conductive gels were used for each electrode to achieve impedance values lower than 50kO. The time taken to place the electrodes, achieve suitable impedance and begin EEG recording, while in transit was 11 minutes. Figure 2 and Figure 3 display segments of the EEG in transit and stationary, respectively. Figure 4 displays the spectrogram of the EEG during transit and stationary. There was an increase in low-frequency activity during transit, due to motion artefacts of the patient and equipment. The additional artefacts were particularly evident in the occipital channels.
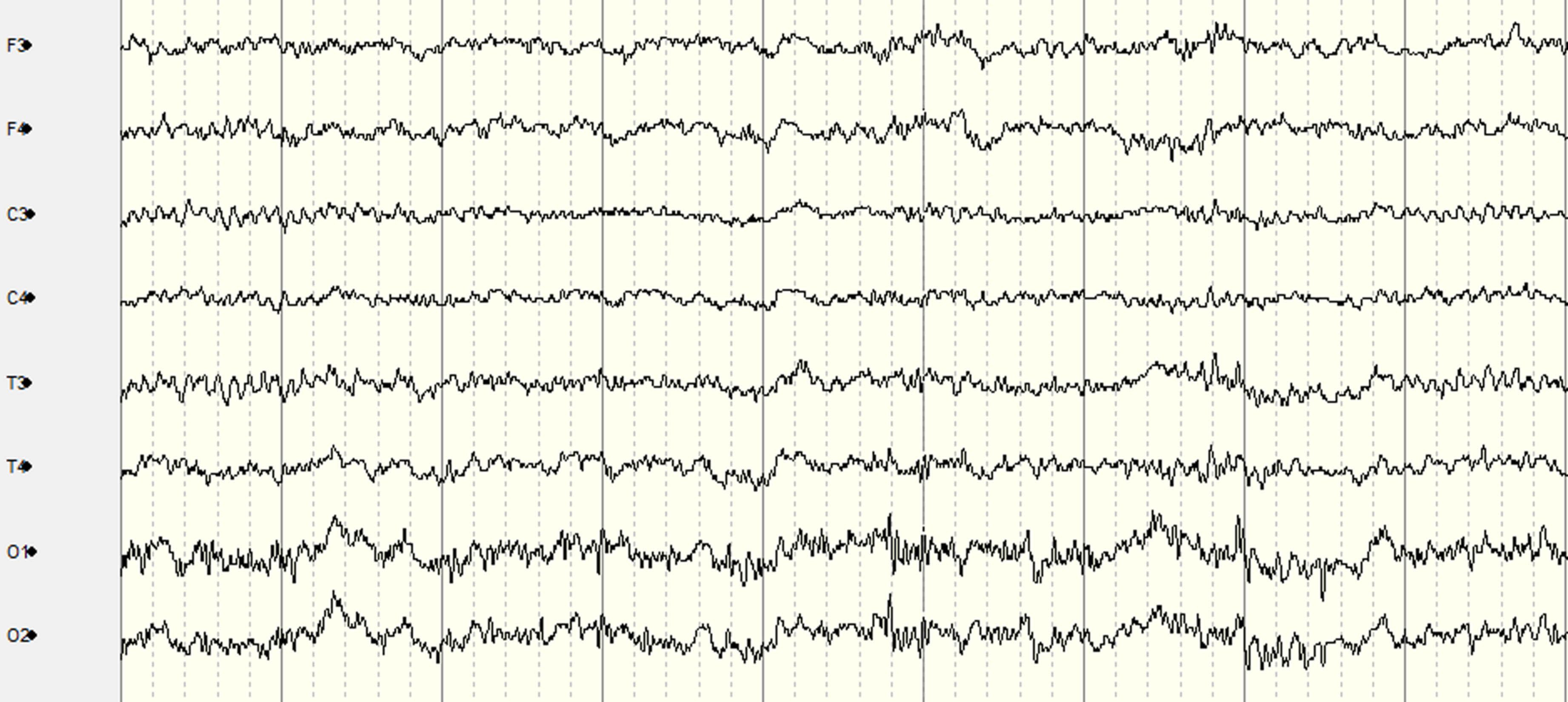
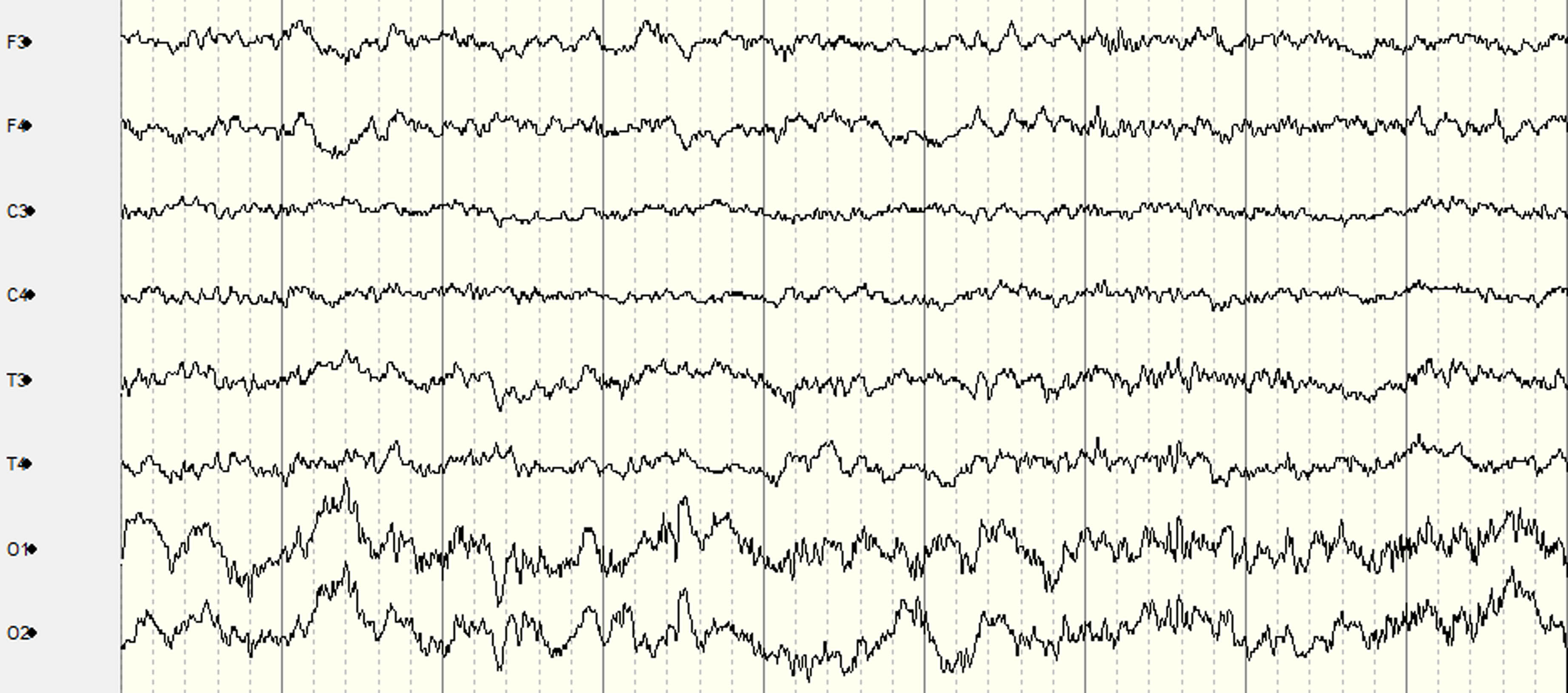
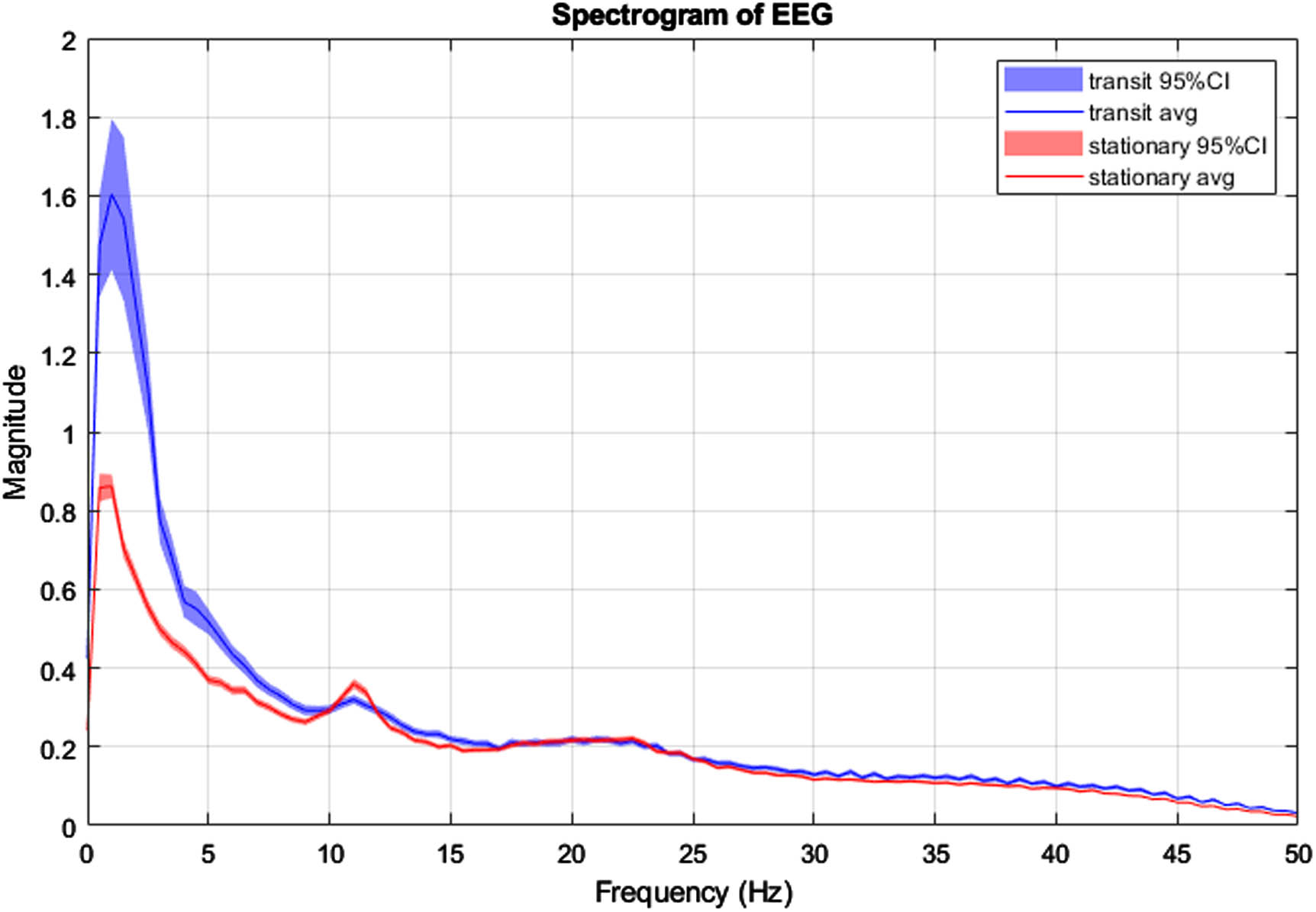
CONCLUSION: This feasibility study demonstrates that it is practical to obtain accurate EEG recordings using the 10-20 EEG placement montage modified for neonates in an emergency transport setting. A healthy adult volunteer on a standard ambulance trolley was used in this feasibility study. In neonates, the time for setup and quality of EEG recordings may be further improved as there is typically less hair, less movement, and also specialised neonatal transport trolleys with vibration damping that may reduce artefacts further.
Validation of neonatal encephalopathy scores using neurophysiology measures
Emily Herzberg1,2, Sara Bates1,2, Jason Boulanger3, Ivana Culic4,5, Mohamed El-Dib2,6, Hoda El-Shibiny6, Munish Gupta2,4, Anne Hansen2,7, Terrie Inder6,8,9, Kyoung Joung10, Carol Keohane11, Jessica Landers12, Silvia Patrizi2,6,13, Arnold Sansevere14, Brian Walsh6,15, Janet Soul2,12
1Massachusetts General Hospital, 2Harvard Medical School, 3Department of Patient Safety, CRICO/Risk Management Foundation of the Harvard Medical Institutions, 4Beth Israel Deaconess Medical Center, 5Beverly Hospital , 6Brigham and Women’s Hospital , 7Boston Children’s Hospital, Division of Newborn Medicine, 8Center for Neonatal Research, Children’s Hospital of Orange County, 9University of California, Irvine-College of Medicine, 10New York-Presbyterian Morgan Stanley Children’s Hospital, 11South Shore Health, 12Boston Children’s Hospital, Department of Neurology, 13Newton Wellesley Hospital, 14Children’s National Hospital, 15INFANT Research Centre, University College Cork
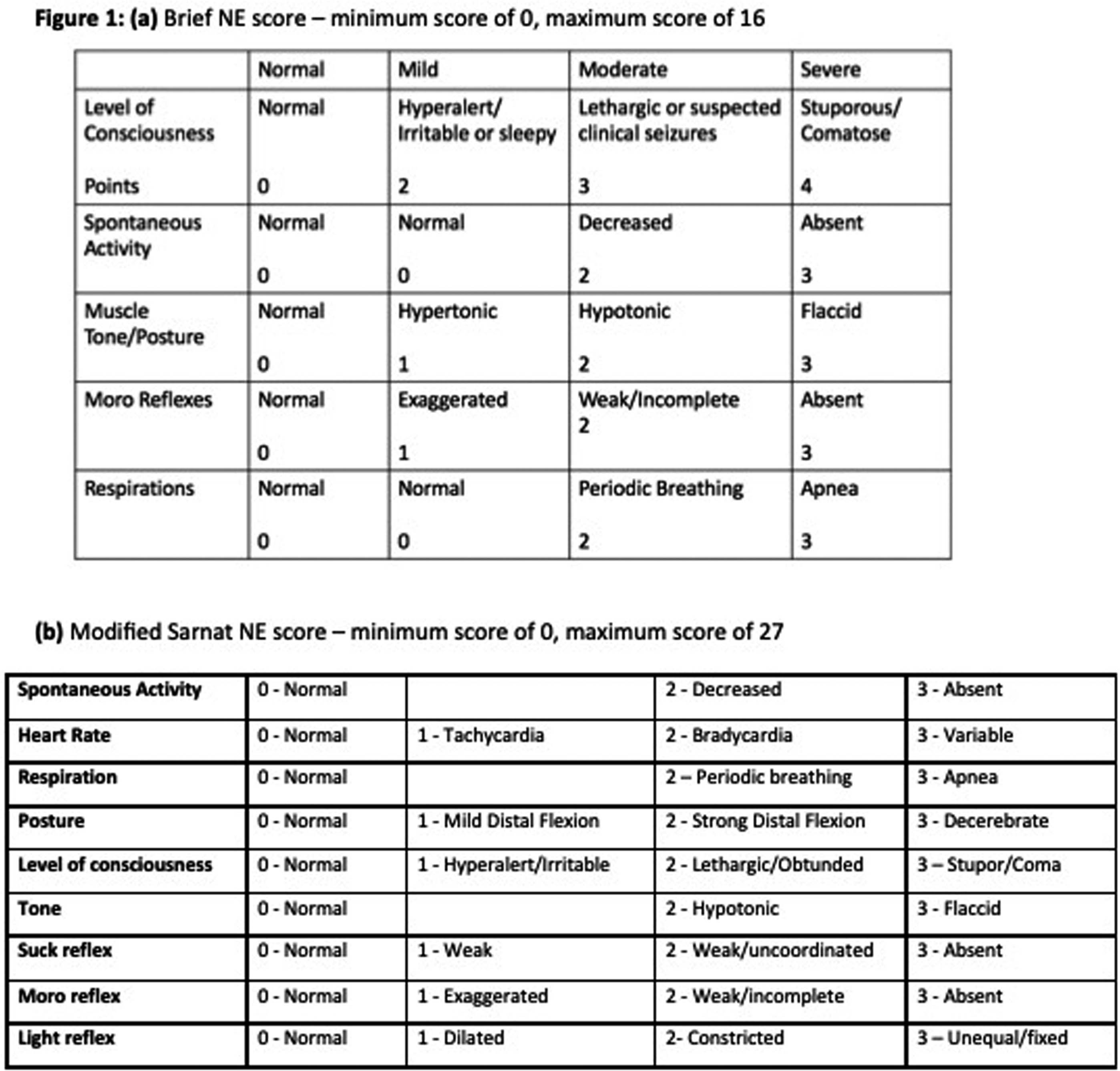
BACKGROUND: Therapeutic hypothermia (TH) is standard therapy for neonates with perinatal asphyxia and moderate-severe neonatal encephalopathy (NE). Traditionally, NE severity is classified by neurologic exam, and sometimes by electroencephalogram (EEG). Neurologic exams are subjective and variable, whereas EEG is a more objective, reproducible measure of acute NE. We validated two NE scores (Brief NE and Modified Sarnat NE scores, Fig. 1) used at three academic NICUs that provide TH in Massachusetts by determining their relationship with amplitude-integrated EEG (aEEG) and/or conventional EEG (cEEG) data.
METHODS: From a cohort of 816 neonates enrolled from 04/2018-04/2021 in a collaborative NE Registry of 14 centers in Massachusetts, 321 neonates were included in our analysis; 272 were treated with TH. We compared serial NE scores, aEEG,1 and cEEG² data with ANOVA and analyzed associations between NE score components and aEEG/cEEG with Fischer’s exact test.
RESULTS: Clinical characteristics are shown in Table 1. Most neonates had normal early aEEG (176/253, 70%), while a minority had a normal later cEEG (117/270, 43%). Seizures were uncommon, occurring in 49/321 (15%) neonates. There was a significant association between both NE scores and aEEG background pattern (p<0.001 for both) and cEEG classification (p<0.001 for both, Fig. 2). For 65 neonates with a Brief NE score of 1-3 and 51 neonates with a Modified Sarnat NE score of 1-3 (mild NE), 16% and 24% had an abnormal aEEG (discontinuous or worse), respectively and 36% and 43% had an abnormal cEEG (excessively discontinuous or worse), respectively. NE score components with significant associations with aEEG/cEEG were level of consciousness (p=0.03), spontaneous activity (p<0.001), suck (p=0.002), Moro (p=0.008) and pupillary reflex (p=0.04), but not tone, posture, or heart rate. Most neonates with serial NE scores (52/64, 81%) had evolution of NE in the first 6 hours, with most neonates showing an improvement in NE score (36/64, 56%).
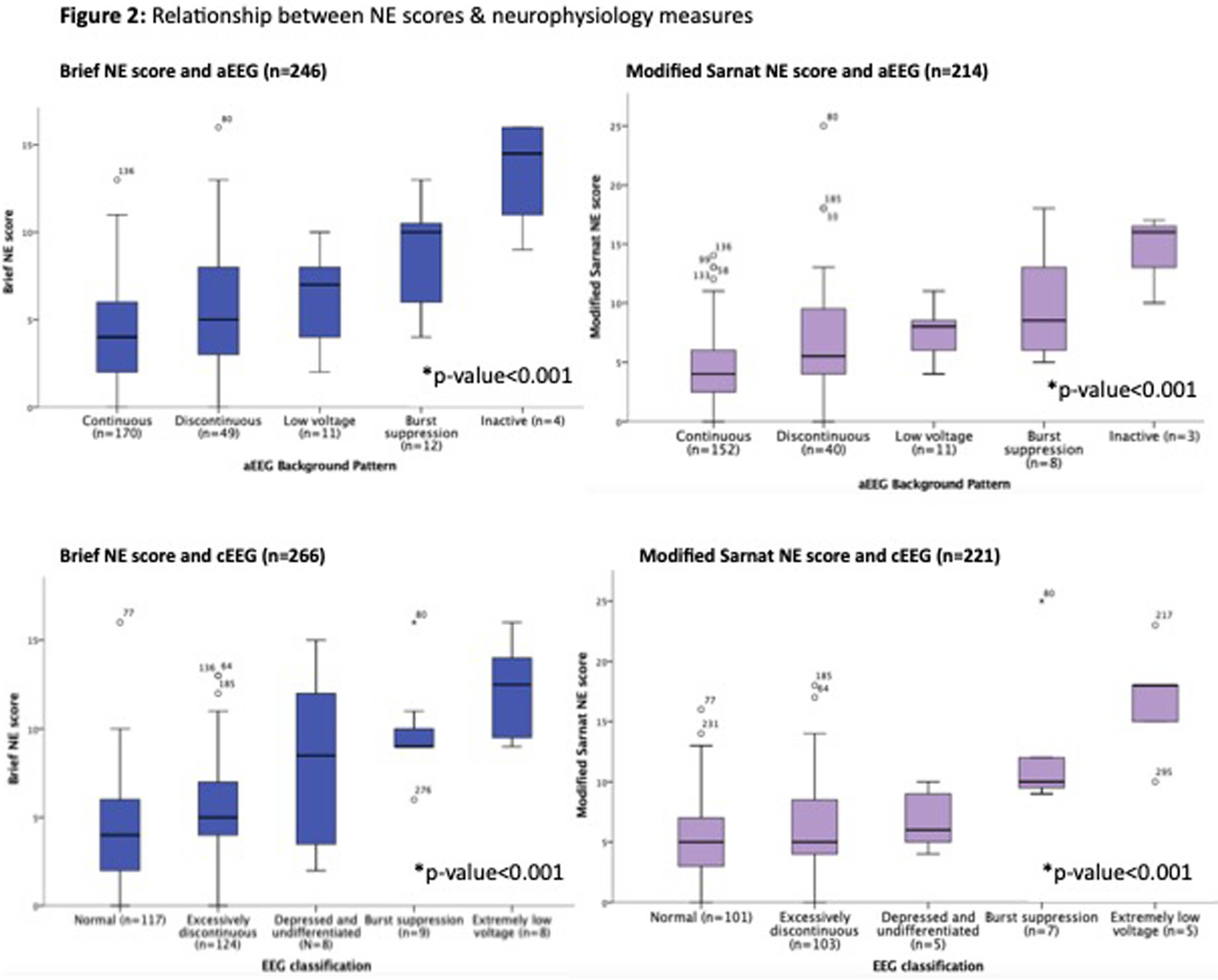
CONCLUSION: demonstrate a statistically significant association between two NE scores and aEEG/cEEG, supporting their validity in diagnosing NE. We also demonstrate limitations of NE scores, as neonates with mild NE by NE score may have moderate-severe NE by neurophysiology measures. Most neonates have an evolution in NE within the first 6 hours, underscoring importance of serial NE exams. These findings support standardized use of neurophysiology in NE evaluation for TH treatment decision-making in neonates with mild-severe NE by exam.
BIBLIOGRAPHY:
1. Hellström-Westas, L. et al. Amplitude-integrated EEG Classification and Interpretation in Preterm and Term Infants. NeoReviews 7, e76–e87 (2006).
2. Tsuchida, T. N. et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 30, 161–173 (2013).
A multicentre validation of the ANSeR automated seizure detection algorithm for neonatal EEG
Sean Mathieson1,2, Denis Dwyer1,3, Linda De Vries4, Mats Blennow5,6, Adrienne Foran7, Divyen Shah8,9, Vicki Livingstone1,2, Alexander C Van Huffelen10, Elena Pavlidis1,2,14, Janet Rennie11, Jacopo Proietti1,2, Deirdre Murray1,2, Ronit Pressler12, Olga Kapellou13, William Marnane1,3, Geraldine Boylan1,2
1INFANT Research Centre, University College Cork, 2Department of Paediatrics & Child Health, University College Cork, 3Department of Electrical Engineering, University College Cork, 4Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, 5Department of Neonatal Medicine, Karolinska University Hospital, 6Division of Paediatrics, Department CLINTEC, Karolinska Institutet, 7Rotunda Hospital, 8Royal London Hospital, 9Queen Mary University of London, 10Clinical Neurophysiology, University Medical Centre Utrect, 11Institute of Women’s Health, University College London, 12Department of Clinical Neurophysiology, Great Ormond Street Hospital for Children NHS Trust, 13Homerton University Hospital NHS Foundation Trust, 14Child Neurology and Neurorehabilitation Unit, Central Hospital of Bolzano
BACKGROUND AND OBJECTIVE: Automated seizure detection algorithms for neonatal EEG offer the potential to detect and treat seizure promptly. Datasets to test these algorithms however, are often small and may not reflect ‘real world’ circumstances. The objective of this study was to test the ANSeR (Algorithm for Neonatal Seizure Recognition) algorithm on two very large datasets of continuous unedited EEG recordings derived from 8 recording sites across 4 countries, and compare performance against a smaller validation dataset previously published (1).
METHODS: The ANSeR algorithm uses machine learning to detect seizures and has previously been described by our group(2). Continuous EEG data from term neonates (>36 weeks GA) at risk of seizures with multiple aetiologies, was recorded in 2 consecutive projects (ANSeR I, II) between January 2011 and February 2017. All EEGs were annotated for seizures twice by experts and a consensus annotation derived as the gold standard. ANSeR annotations were compared with expert consensus annotation across the full range of ANSeR sensitivity thresholds (0.1-0.9), using event-based and time-based metrics. The area under the receiver operator curve (AUROC) of sensitivity vs 1-specificity was calculated and compared between the 3 datasets.
RESULTS: In ANSeR I 214 infants were included and total of 13828.57 hours of EEG was recorded with a median recording length of 63.2 hours, and a median recording onset of 8.1 hours. One or more seizure was detected in 71/214 (33.18%) infants. In ANSeR II 258 infants were included and a total of 13548.6 hours of EEG was recorded with a median recording length of 49.35 hours, and a median recording onset of 29.65 hours after birth. One or more seizures was detected in 71/258 (27.52%) neonates. The median AUC values (for all ANSeR sensitivity thresholds) for ANSeR performance on the 3 datasets are comparable: 2016 validation set 0.945 (IQR: 0.921-0.971); ANSeR 1 dataset 0.939 (IQR 0.880-0.984); ANSeR II dataset 0.924 (IQR 0.834-0.973) (table 1, figure 1). We consider ANSeR sensitivity thresholds between 0.3 and 0.5 to be clinically relevant, giving a reasonable trade-off between seizure detection and false detection. For these sensitivity threshold, ANSeR performs best on the ANSeR I dataset (threshold 0.3: SDR 76.19%, FD/h 0.173. Threshold 0.5: SDR 54.84%, FD/h 0.014) (Table 1).
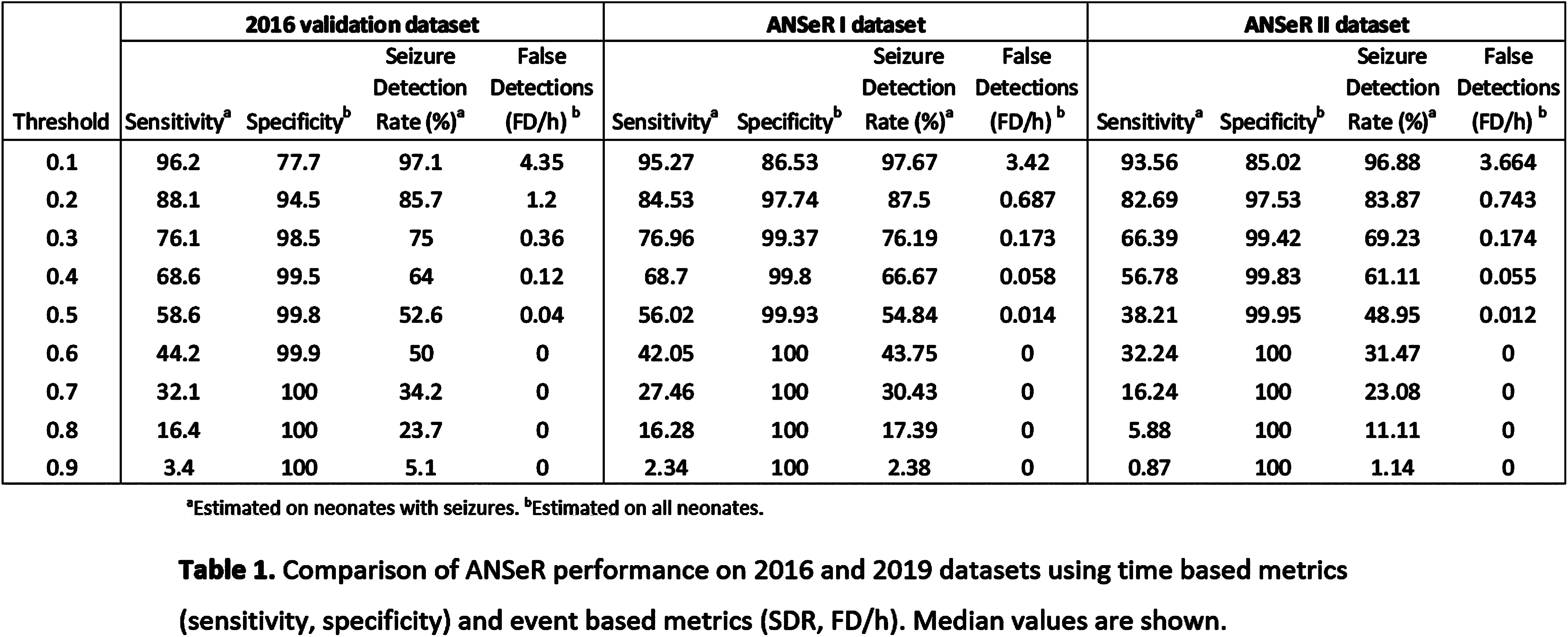
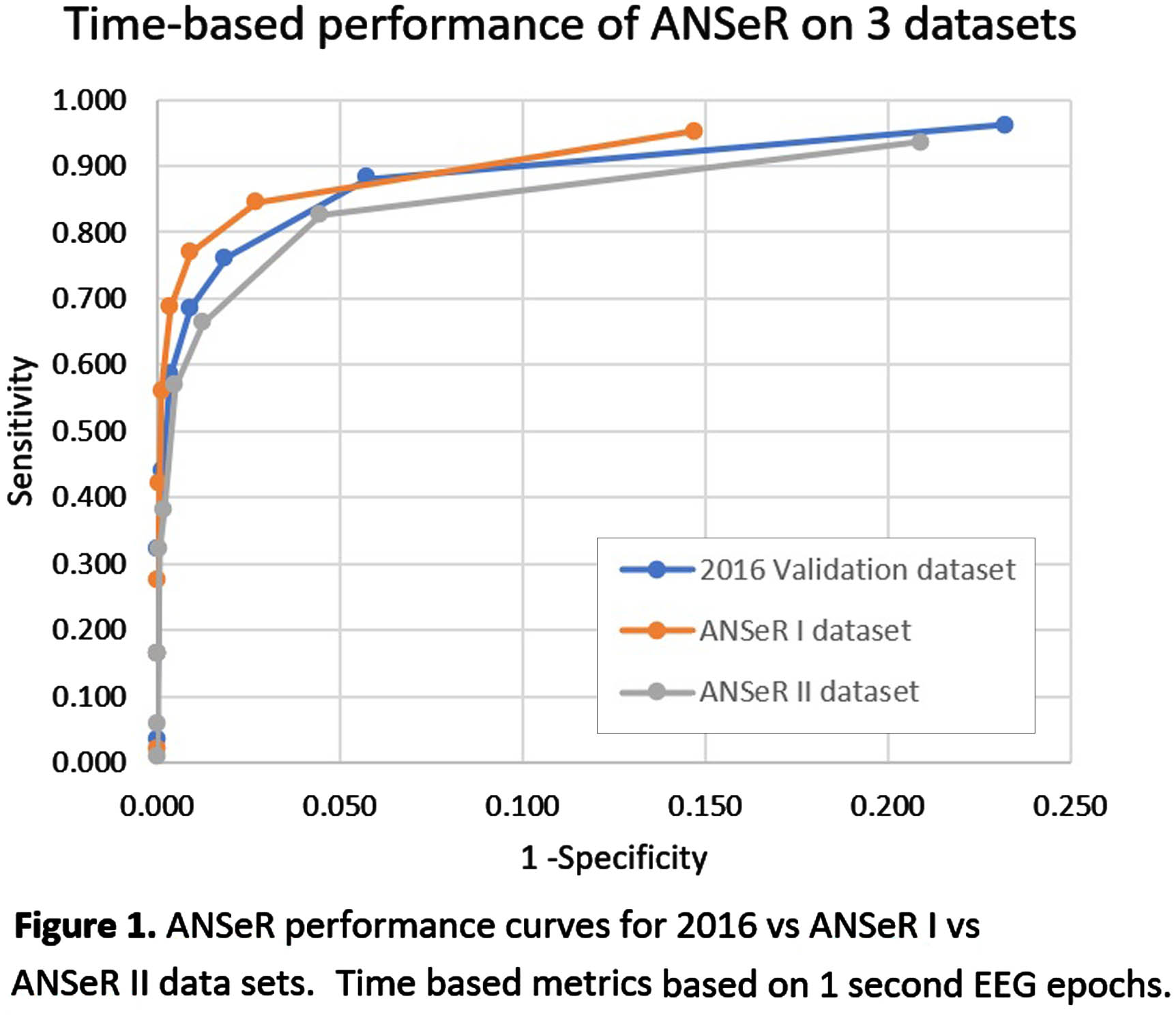
CONCLUSION: ANSeR performs well on these two very large datasets and is comparable to previous results. We have shown that ANSeR performance is robust to data from diverse populations at multiple sites and recording techniques.
BIBLIOGRAPHY:
1. Mathieson SR, Stevenson NJ, Low E, et al. Validation of an automated seizure detection algorithm for term neonates. Clin Neurophysiol. Jan 2016;127(1):156-168. doi:10.1016/j.clinph.2015.04.075
2. Temko A, Thomas E, Marnane W, Lightbody G, Boylan G. EEG-based neonatal seizure detection with Support Vector Machines. Clin Neurophysiol. Mar 2011;122(3):464-473. doi:10.1016/j.clinph.2010.06.034
Relationship between severity of clinical encephalopathy and EEG background in neonates with hypoxic-ischemic encephalopathy
Marie Cornet1, Yvonne Wu1, Adam Numis1, Sarah Monsell2, Sandra Juul2, Hannah Glass1
1UCSF Benioff Children’s Hospital, 2University of Washington
BACKGROUND: Treatment with therapeutic hypothermia (TH) improves neurodevelopmental outcomes in neonates with moderate and severe hypoxic-ischemic encephalopathy (HIE). Assessing eligibility for TH in the first hours of life is challenging. The EEG background quantifies brain dysfunction. Some centers rely on clinical encephalopathy alone, while others also require an abnormal EEG. Yet, the association between the severity of clinical encephalopathy assessed by a modified Sarnat exam and EEG dysfunction assessed by clinical EEG reads is seldom described.
OBJECTIVE: To assess the association between the severity of clinical encephalopathy and severity of brain dysfunction by EEG on the first day of life (DOL1).
METHODOLOGY: Infants enrolled in the High-dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial with EEG or aEEG report available on DOL1 were included. EEG background continuity was categorized as normal, excessively discontinuous, or severely abnormal based on EEG reports. The severity of clinical encephalopathy was assessed by Sarnat exam performed by trained investigators on DOL1. We assessed the association between severity of clinical encephalopathy and EEG background using descriptive statistics and proportional odds regression.
RESULTS: Among 500 infants in the HEAL trial, 478 (96%) had interpretable EEG reports. The EEG background was normal in 186 (39%), excessively discontinuous in 171 (36%), and severely abnormal in 121 (25%). EEG background severity was associated with birth location, gestational age, Apgar score, resuscitation at birth, and acidosis (Table 1). There was a significant association between Sarnat score and EEG severity (Figure 1) or number of severe findings on exam (Figure 2A). Infants who scored normal or mild in at least one category had >50% chance of having a normal EEG (Figure 2B). Some infants had discordant exam and EEG findings. Among infants with milder encephalopathy (<2 severe exam findings), the ones with a severe EEG had lower Apgar scores (p=0.002) and more sentinel events (RR 2.3 [95%CI 1.2-4.6]) compared to those with more severe clinical encephalopathy.
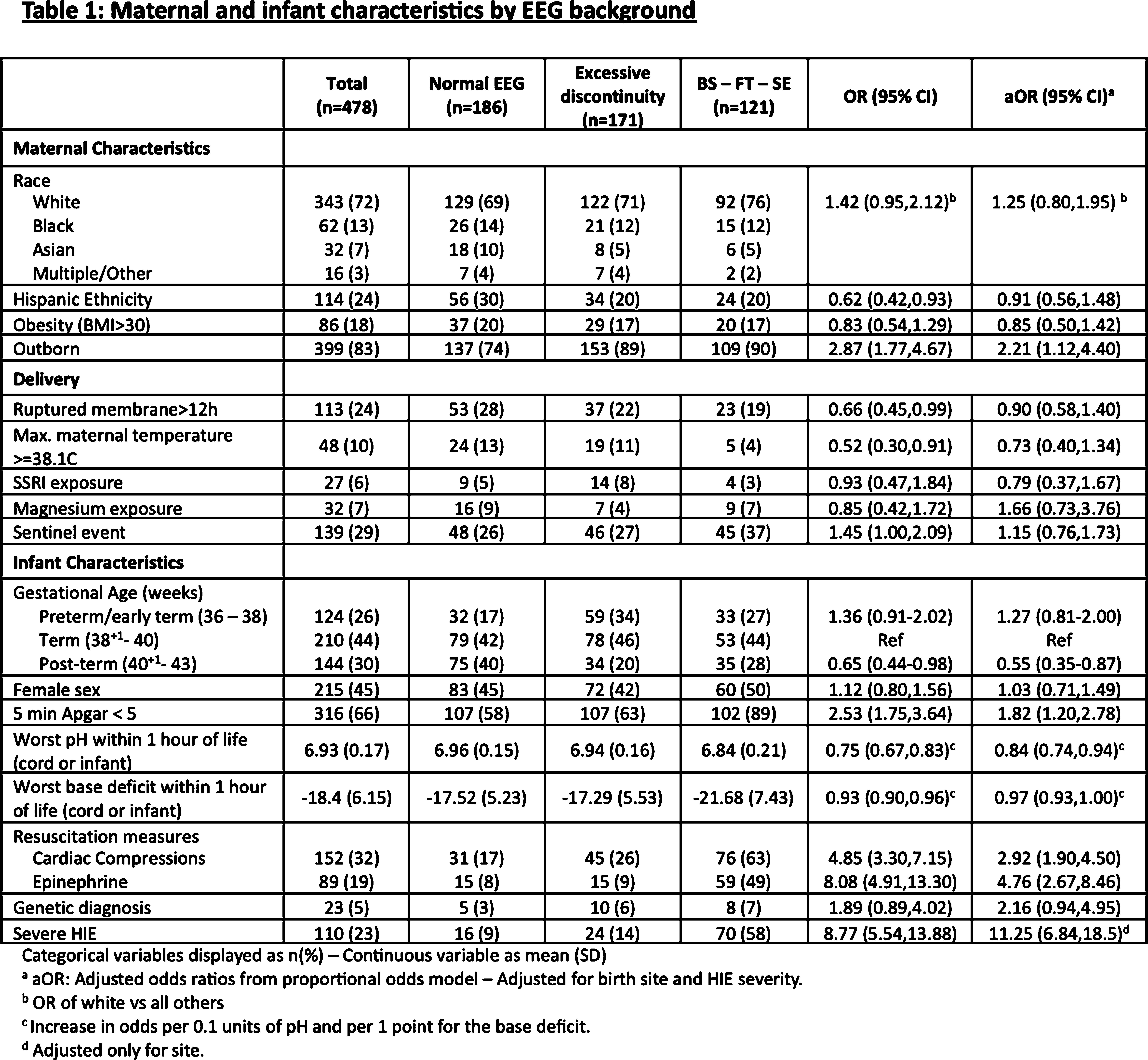
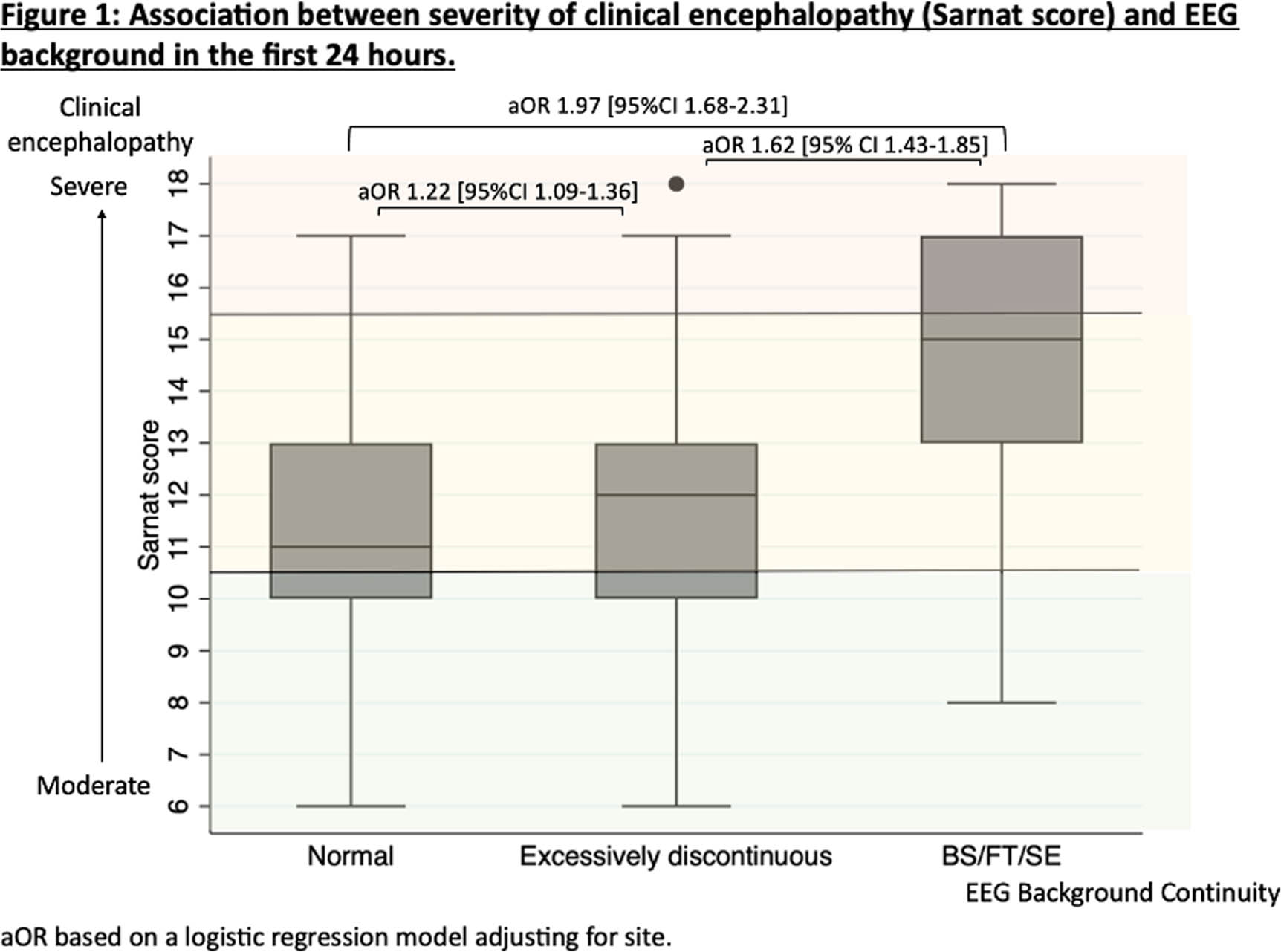
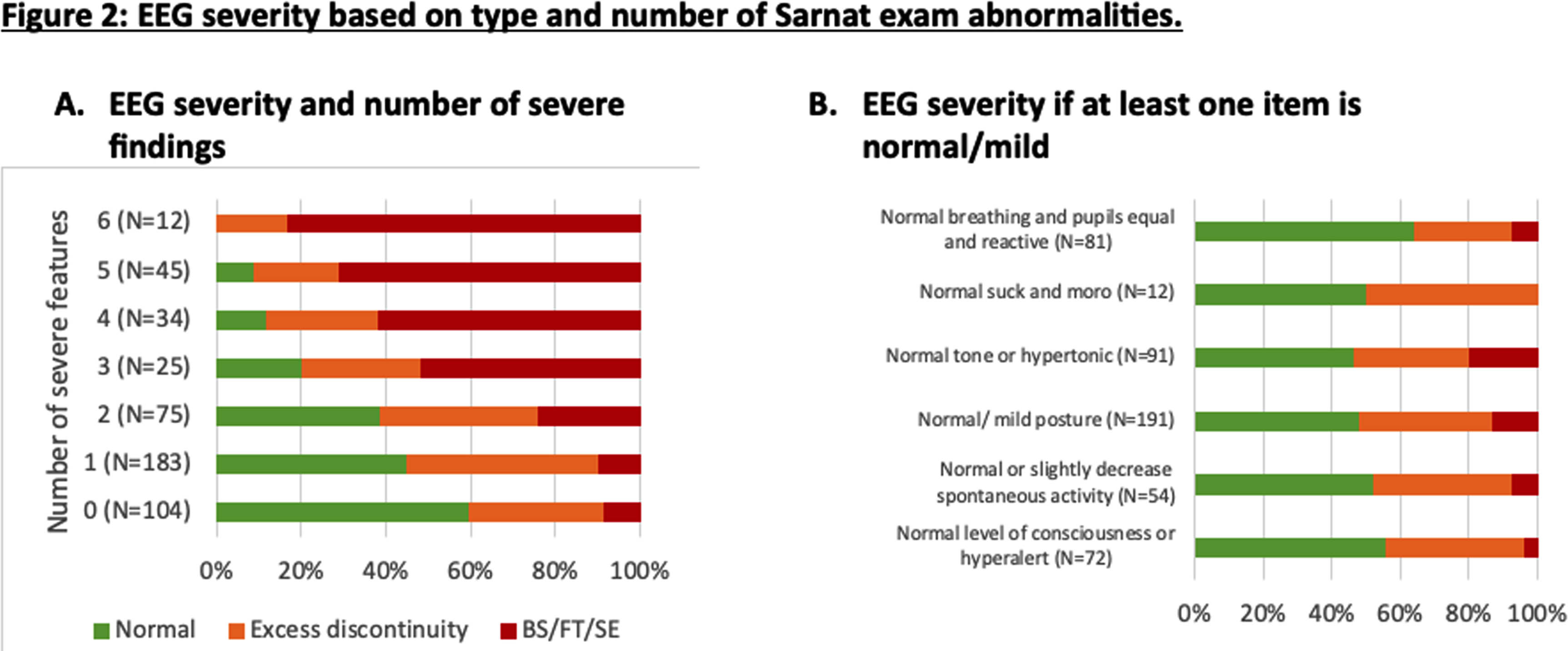
CONCLUSION: In this cohort, over a third of infants with moderate or severe clinical encephalopathy had a normal EEG background. While the clinical severity of encephalopathy and EEG background abnormalities are closely associated, further studies are needed to determine which is better for predicting outcomes and whether infants with a normal EEG background do benefit from cooling.
Evaluation of the diagnostic accuracy of the Persyst P14 Seizure Detector in the neonatal population
Eleanor Duckworth1, Ronit Pressler1, Maria Chalia1
1Great Ormond Street Hospital for Children
BACKGROUND: Neonatal seizures are predominantly electrographic-only and challenging to diagnose(1). Multichannel, video continuous electroencephalogram (cEEG) is the gold standard investigation. However, this is not available in all neonatal units and the out of hours neurophysiology support is variable(2). Automated seizure detection algorithms (SDAs) are designed to analyse cEEG data to determine the probability of a seizure and alert clinicians to potential seizures(3). The aim of this study was to evaluate the accuracy of the automated Persyst P14 Seizure Detector to identify neonatal seizures.
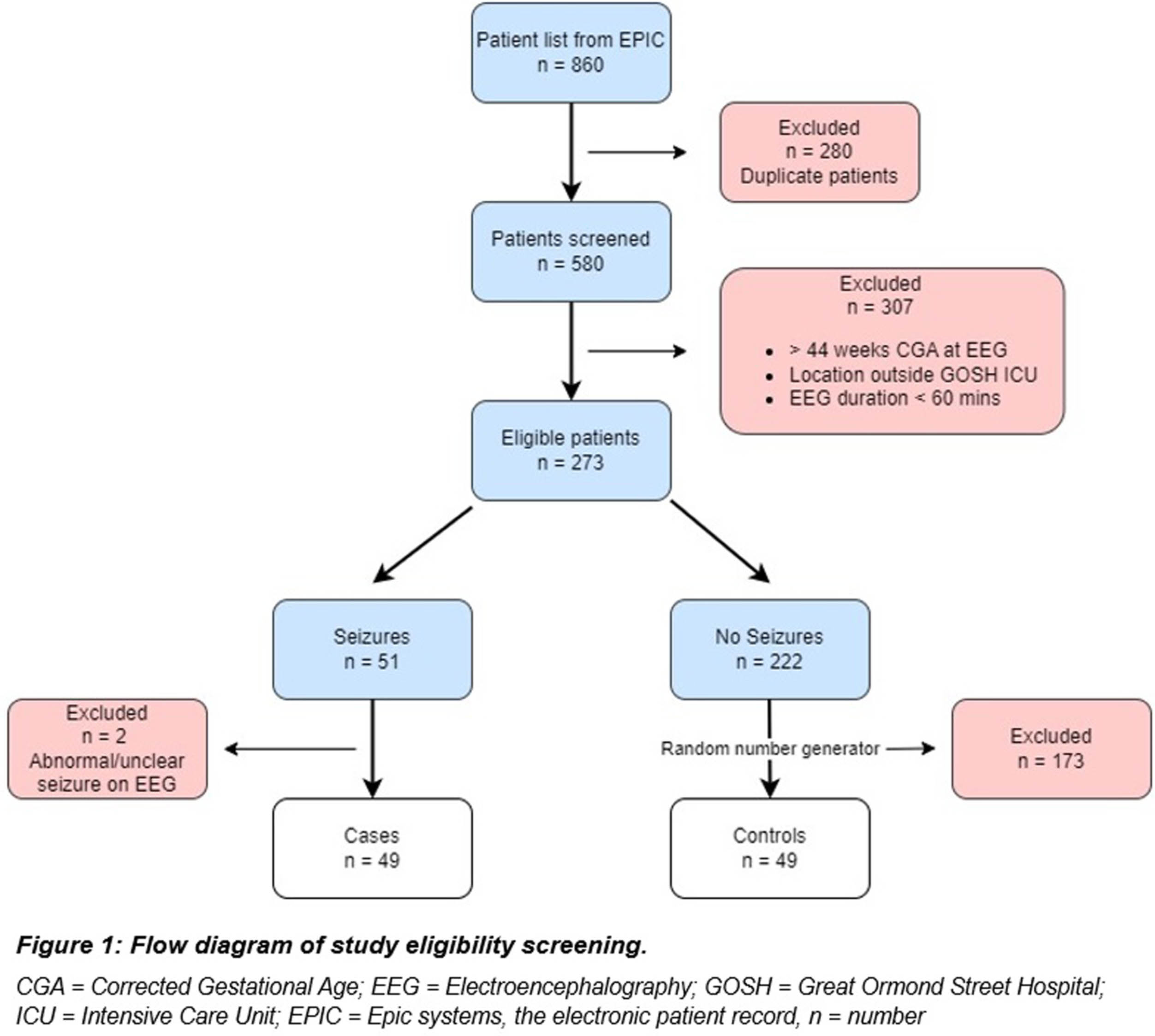
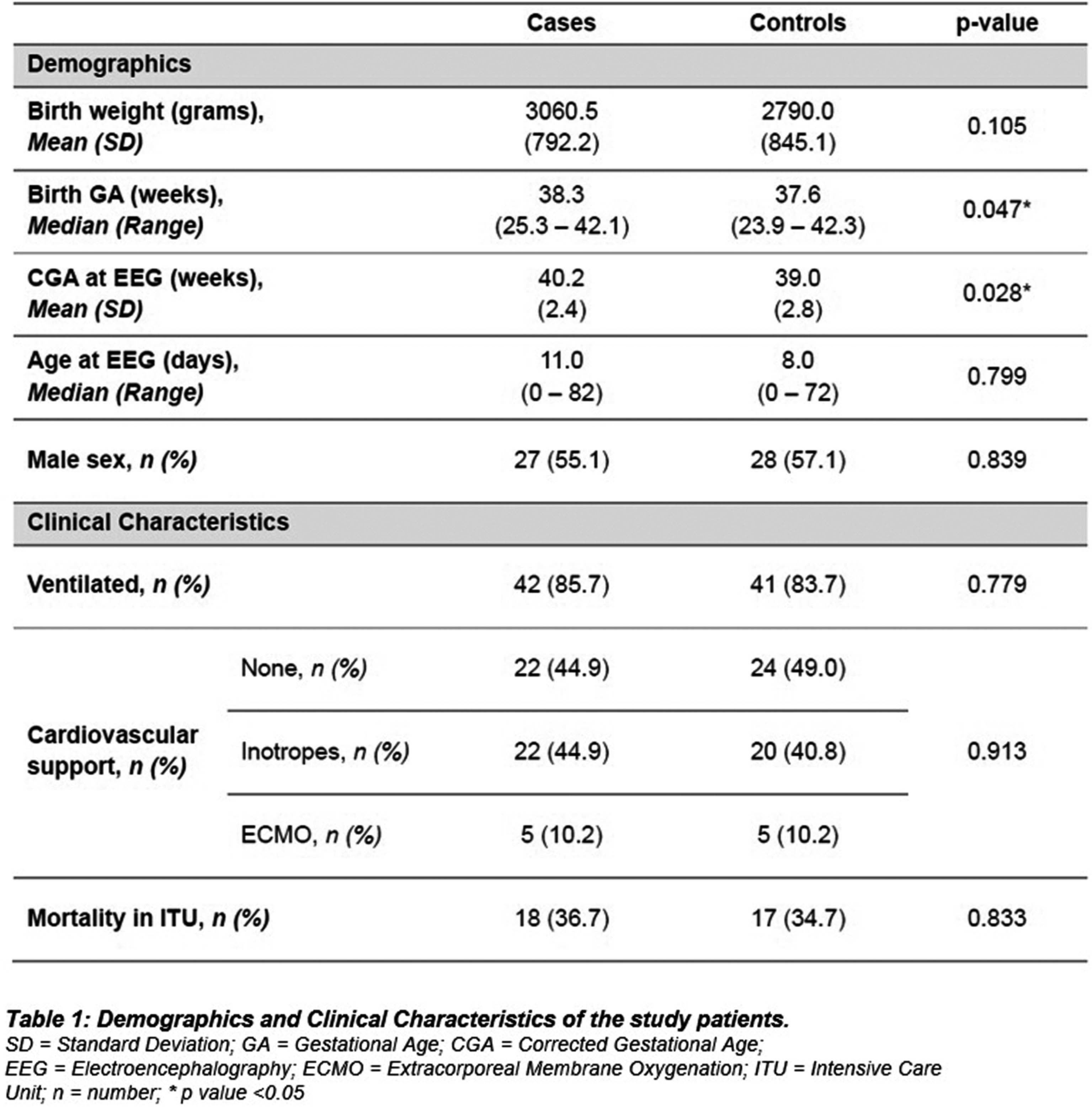
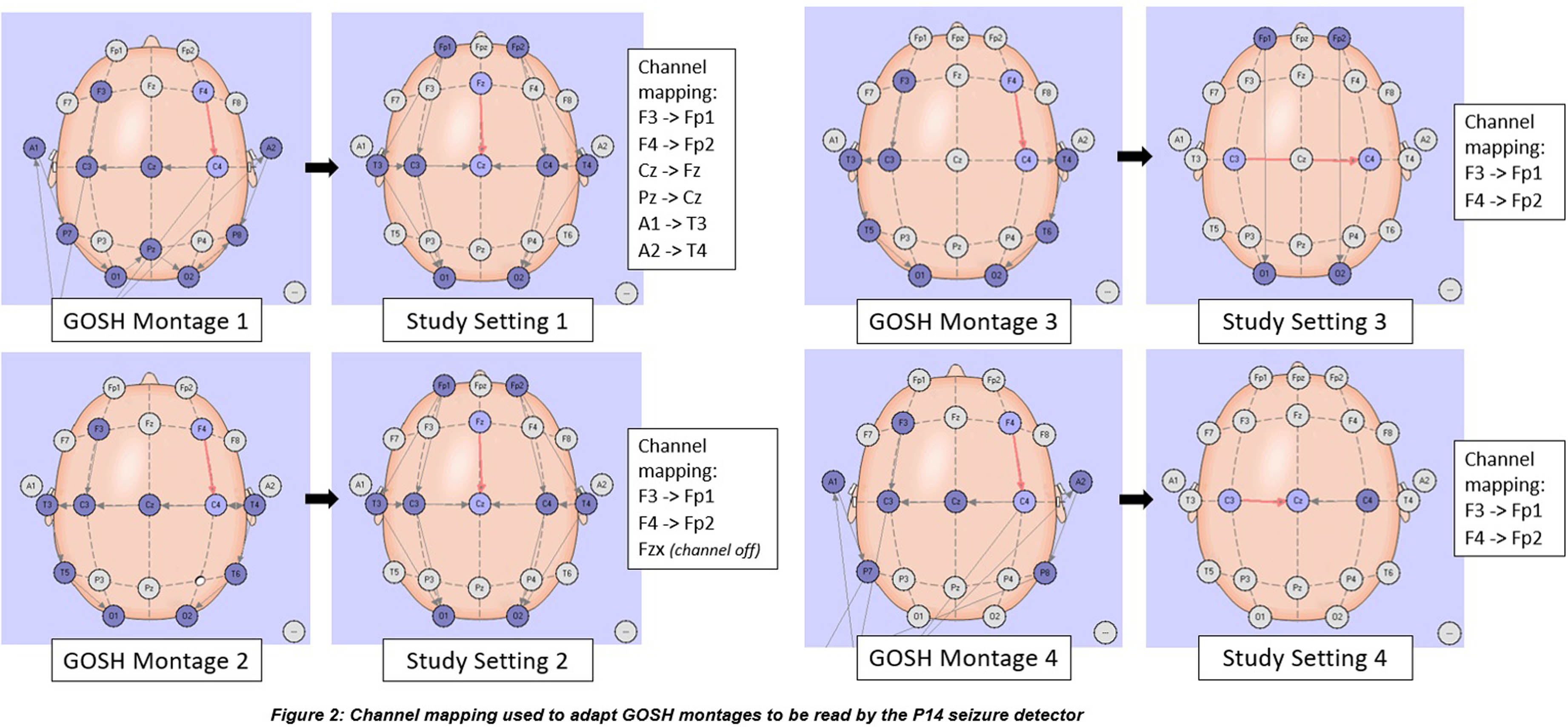
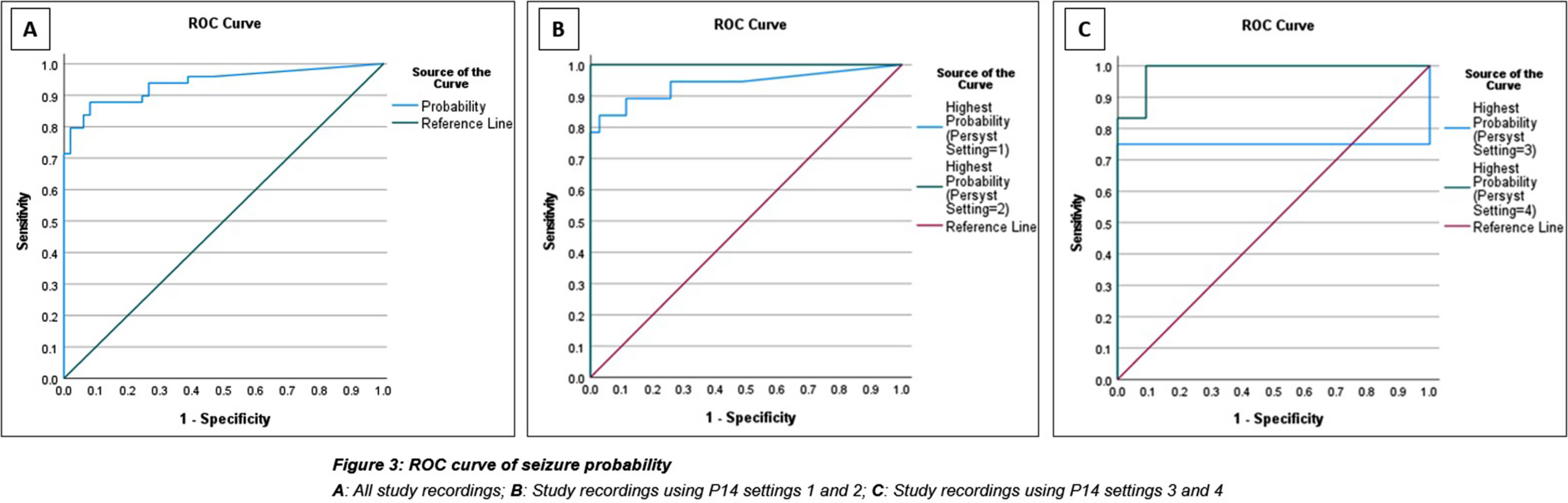
METHODOLOGY: This was an evaluation case control study in neonates undergoing cEEG recording during admission to any of the neonatal, paediatric, or cardiac intensive care units at Great Ormond Street Hospital (GOSH) from May 2019 to December 2022. One-hour clips of cEEGs were analysed by the P14 detector producing a seizure probability trend for 49 cases and 49 controls. Channel mapping was required to adapt the original GOSH recording montage to the P14 detector requirements (Figure 2). A ROC curve was constructed and the optimal probability threshold for sensitivity and specificity was identified.
RESULTS: The area under the curve for ROC analysis was 0.939. At a probability threshold of 0.6, the sensitivity was 79.6%, and specificity 98.0%. Further analysis after subdividing the probability results by P14 settings for small groups, showed some difference in accuracy. (Figure 3). The most common seizure aetiology was hypoxic ischaemic injury (34.7%), followed by inborn errors of metabolism (14.3%), trauma (8.2%) and intracranial haemorrhage (8.2%). There was an overall high mortality of 35% for both cases and controls, during their intensive care admission.
CONCLUSION: The Persyst P14 seizure detector shows good diagnostic accuracy for neonatal seizure detection, similar to reported sensitivities for P14 in adult populations and other SDAs. Further investigation to test the P14 detector’s accuracy for individual seizure detection, as well as confirming the optimal seizure probability thresholds in a larger cohort of patients, is needed to fully determine its clinical utility. However, the results of this study are encouraging and support its use in the neonatal population.
BIBLIOGRAPHY:
1. Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93(3):F187-91.
2. Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol. 2012;46(2):111-5.
3. Temko A, Lightbody G. Detecting Neonatal Seizures With Computer Algorithms. J Clin Neurophysiol. 2016;33(5):394-402.
Neonatal cardiac arrest following lacosamide treatment: A case report
Melissa Huberman1, Carolina Mallar1, Paige Kalika1
1University of Miami Miller School of Medicine
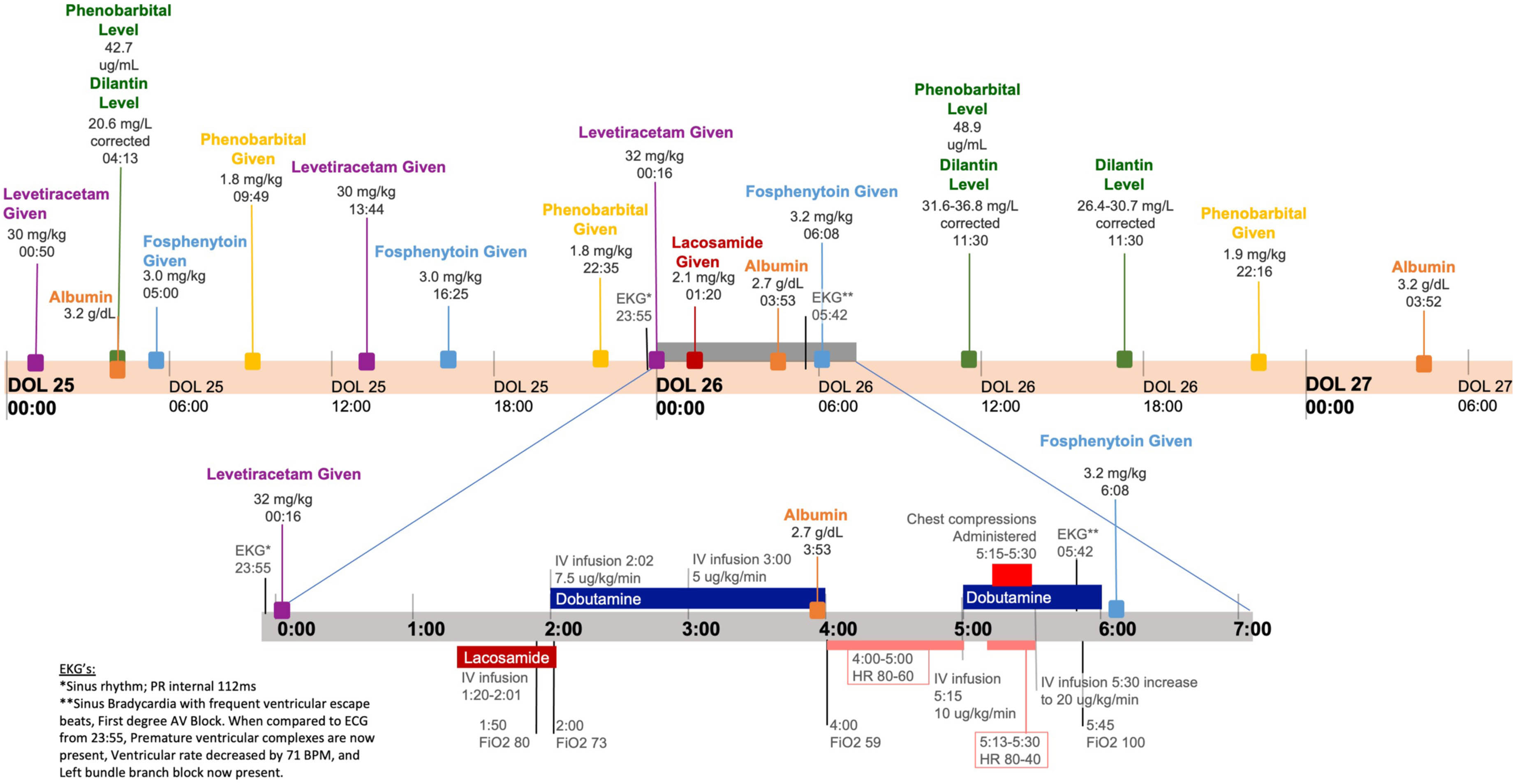
BACKGROUND AND OBJECTIVE: Lacosamide is an anti-seizure medication (ASM) with FDA approval for treatment of partial-onset seizures in patients above the age of 1 month with current evaluation for expansion to neonatal patients. Lacosamide works by selective enhancement of collapsin response mediator protein 2 (CRMP-2) which induce preferential slow promotion of sodium channels to the hyperpolarized inactive state (1). Lacosamide is generally well tolerated; however, clinical and nonclinical studies have linked its use with cardiac side effects including PR Prolongation and AV block. We report a case of AV heart block in a 3-week-old female neonate.
RESULTS: We present the case of a 3-week-old female neonatal patient born at 25 weeks gestation who developed 2nd degree AV heart block and cardiac arrest after initiating lacosamide therapy. The patient was being treated for neonatal seizure complicated by intraventricular hemorrhage (grade II) and electrolyte disturbances with phenobarbital, levetiracetam, and phenytoin. Prior to addition of lacosamide therapy, the patient had an unremarkable EKG and no known cardiac risk factors for lacosamide. After medication discontinuation, the patient experienced no reoccurring episodes or other cardiac events and in follow-up visits, patient was meeting developmental milestones appropriately.
CONCLUSION/IMPACT: Use of lacosamide for neonatal populations is currently under evaluation in a multi-center, open label randomized active comparator study (NCT04519645). Such studies may take the drug from a promising novel therapy to a standard in care (2). To our knowledge, this is the first report of adverse cardiac event (AV block) in the setting of neonatal lacosamide use. This report is limited by use of other ASMs, as well as by retrospective genetic information and post-ligation cardiac syndrome. Although this event was likely multifactorial, the timing of lacosamide administration in relation to the cardiac event, the unremarkable ECG obtained prior to administration, and the absence of recurrent events since medication discontinuation suggests that lacosamide may have played a role in the patient’s arrest. Risk of future adverse cardiac events should be evaluated when determining the safety and efficacy of lacosamide in the neonatal population. Additionally this case may inform future use of pre-initiation EKG, careful review of family and gestational history, and careful informed consent to future patients.
BIBLIOGRAPHY:
1. Errington AC, Stohr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol. Jan 2008;73(1):157-69. doi:10.1124/mol.107.039867
2. Ziobro JM, Eschbach K, Shellhaas RA. Novel Therapeutics for Neonatal Seizures. Neurotherapeutics. Jul 2021;18(3):1564-1581. doi:10.1007/s13311-021-01085-8
The association of electrographic-seizure characteristics in infants 24 to 72 old with two-year developmental outcomes
Steve Mehrkanoon, Paul Colditz, Rod Hunt
1Monash University - Department of Paediatrics, 2The University of Queensland Centre for Clinical Research , 3Monash University - Department of Paediatrics
BACKGROUND: Encephalopathic term or near-term infants often present with seizures. In the NEST (1), we found no significant differences in 2-year outcomes between those infants whose electrographic + clinical seizures were treated, compared to those infants who only received anticonvulsant therapy for clinically detected seizures alone. The NEST recruited many infants beyond 24 hours of age, at a time when the bulk of the seizure burden may have already occurred. Here we examined the association between aEEG seizure characteristics collected beyond 24 hours of life and 2-year outcomes.
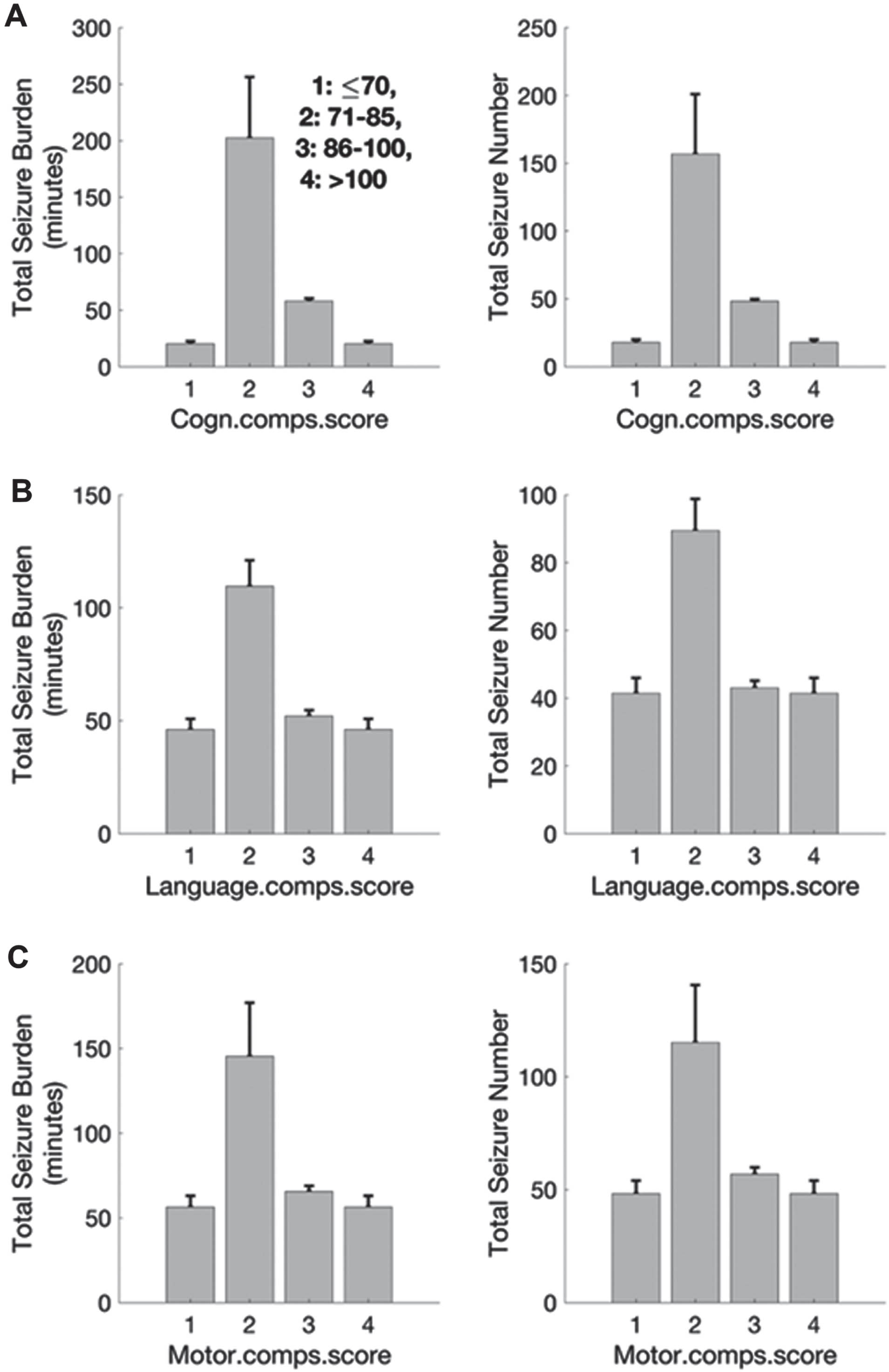
METHODOLOGY: 95 infants’ data recruited to the NEST were analysed – mean GA was 37±1.5 weeks, and 45 infants were male. The aEEG was used to detect seizures between [2 ~ 14] Hz (2,3). Total seizure burden (TSB) and total seizure number (TSN) were calculated. The primary outcome was neurodevelopmental performance assessed with BSID-III collected around 24 months of age, and divided into four bands: <70 (>-2SD below the mean), 71 – 85 (-1 to -2 SD below the mean), 86 – 100 and >100 (published mean).
RESULTS: The number of infants with outcomes in the first band (<70) was small. A strong correlation was found for the remainder of the cohort for both TSB and TSN (Figure 1). The highest and lowest TSB measurements were associated respectively with the lower scores (71 – 85) and the highest scores on the BSID-III (>100) (left column). The TSB measurements associated with the low composite scores (between 71-85) were significantly different to the higher composite scores on the BSID-III (86-100 and ≥100) (Z=6, p<0.01). The highest and lowest TSN measurements were associated respectively with the lower scores (71 – 85) and the higher scores (86 – 100) on the BSID-III (A-C, right column). The TSN measurements associated with the low composite scores (between 71-85) in primary outcomes were significantly different to the higher composite scores on the BSID-III (86-100 and ≥100) (Z≥6, p<0.01).
CONCLUSION: Despite application of aEEG beyond 24 hours of life, total seizure burden and total seizure number between 24 and 72 hours of age correlated with outcomes measured at 2 years with BSID-III.
BIBLIOGRAPHY:
1. Hunt RW, Liley HG, Wagh D, Schembri R, Lee KJ, Shearman AD, Francis-Pester S, deWaal K, Cheong JYL, et al, for the Newborn Electrographic Seizure Trial Investigators. Effect of treatment of clinical seizures vs electrographic seizures in full-term and near-term neonates: A randomized controlled trial. JAMA Network Open 2021; 4(12): e2139604.
2. Tao JD, Mathur AM. Using amplitude-integrated EEG in neonatal intensive care. J Perinatol. 2010 Oct;30 Suppl:S73-81.
3. Mehrkanoon S, et al. Identifying Emergent Mesoscopic–Macroscopic Functional Brain Network Dynamics in Infants at Term-Equivalent Age with Electric Source Neuroimaging, Brain Connectivity, 2021, 11(8), 663-677.
Exploring a novel use case: A 3-D embroidered-textrode smart hat for infantile epilepsy EEG monitoring
Komal Komal1,2, Frances Cleary2, John Stephen Gary Wells1, Louise Bennett1
1School of Health Sciences, South East Technological University, Cork Road, 2Walton Institute, South East Technological University, Cork Road
BACKGROUND: Infantile epilepsy is a challenging neurological disorder that necessitates innovative diagnostic solutions for early detection and intervention. For long-term monitoring of seizures, the traditional portable EEG headsets that utilise electrodes can be problematic due to their rigid structure, weight, and interconnecting wires.
AIM: Advances in textile electrodes (textrodes) are driving new innovative smart wearable epilepsy monitoring applications. This research aims to design a layered 3D embroidered textrode capable of providing a more usable, comfortable, and effective method for EEG monitoring of infants with epilepsy.
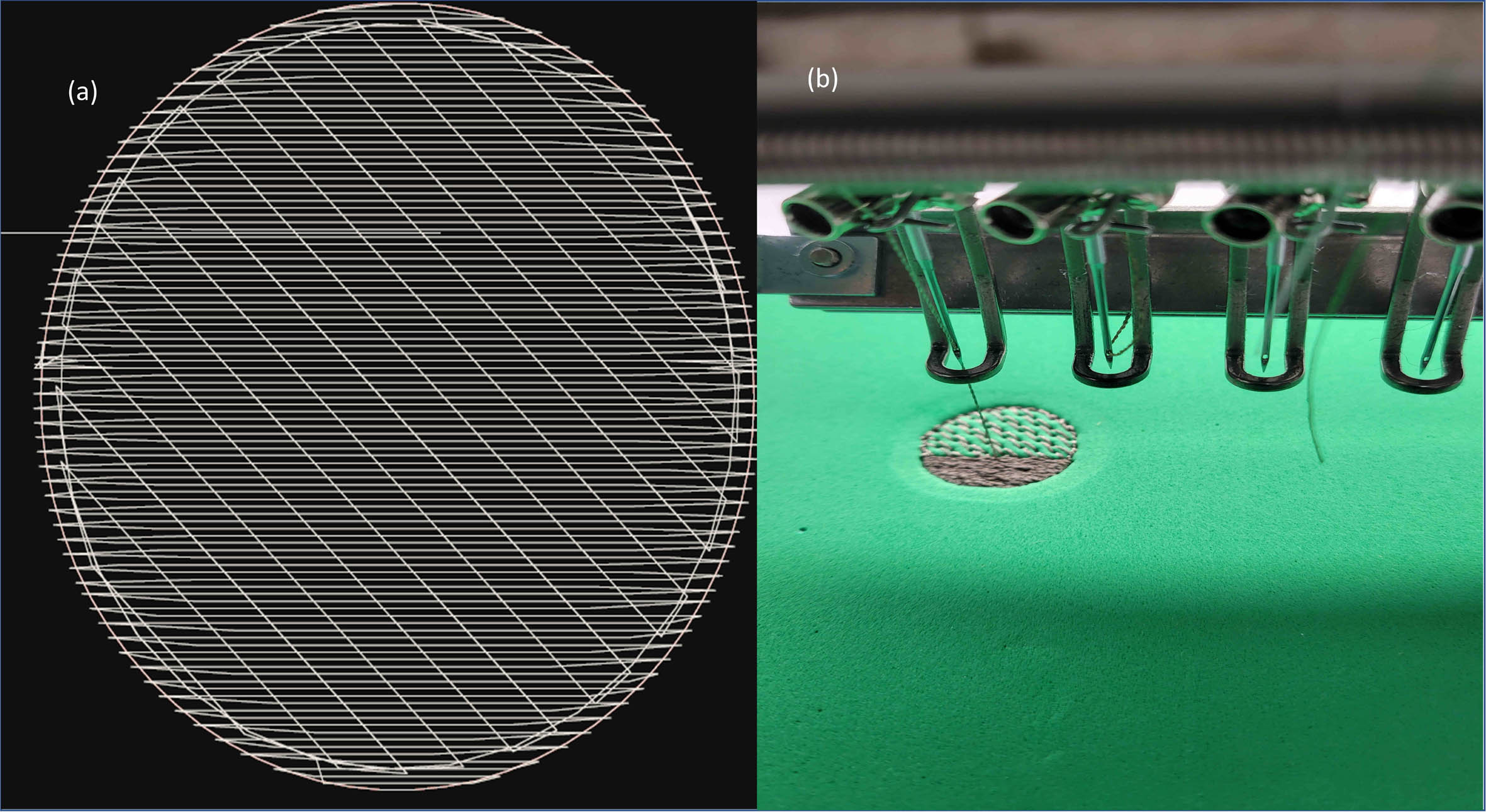
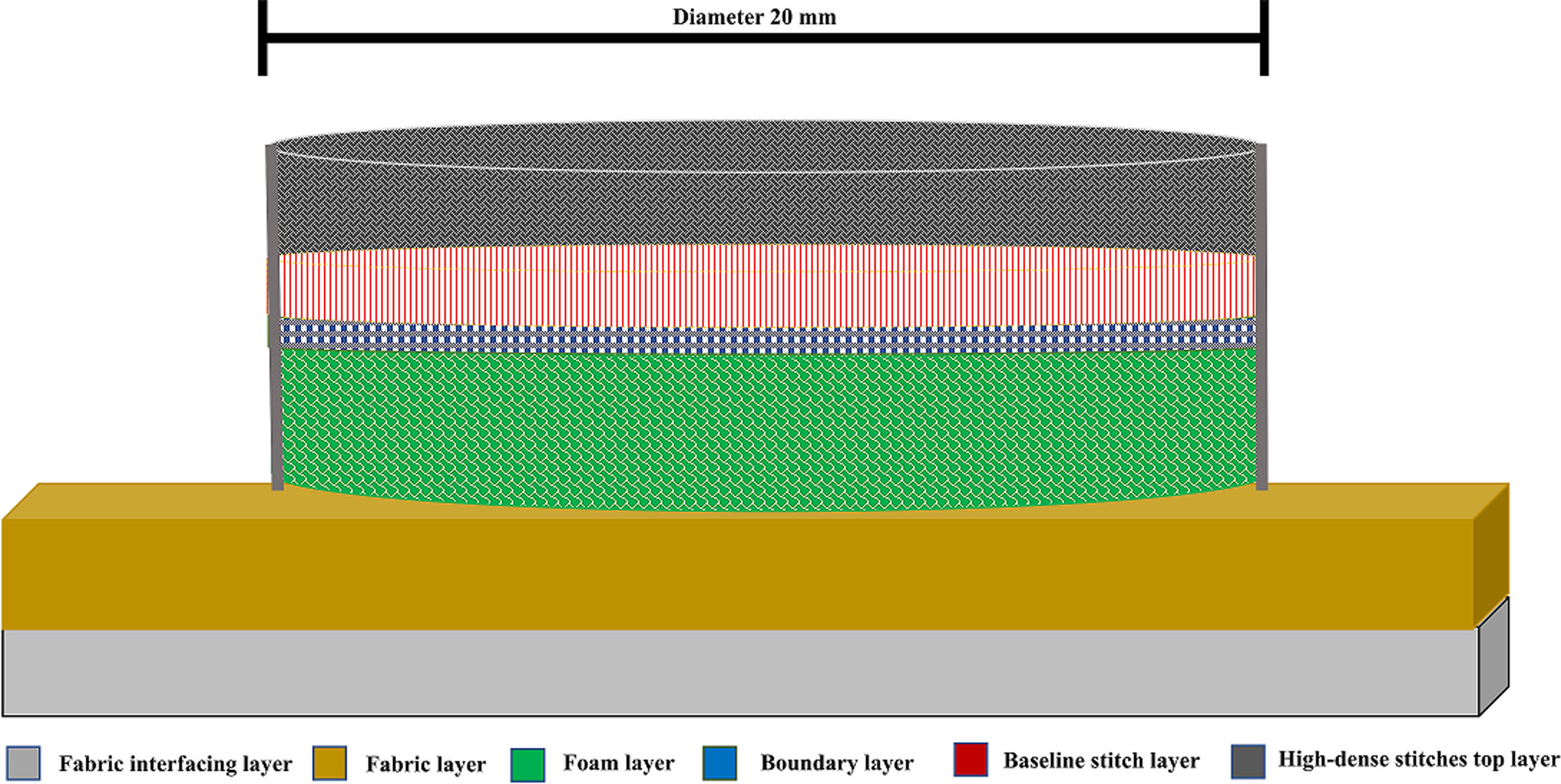
METHODOLOGY: A textrode design pattern was created using EPC win digitized software. The software design of the textrode consisted of three main stitching layers. Layer one created boundary stitches to outline the textrode’s shape. The second layer consisted of a baseline of noncompact stitches required to stabilize the structure. Finally, the top conductive thread stitching layer consisted of dense and compact embroidered stitching providing the necessary surface area density for the operation of the textrode. To fabricate the software design of the textrode, a ZSK technical embroidery machine was utilised as seen in Figure 1. The embroidery fabrication process involved the use of three distinctive layers: (1) fabric and interfacing layer; (2) 3-D foam layer; and (3) a conductive thread stitching layer, as seen in Figure 2. The addition of the foam layer provides a three-dimensional elevation to the overall textrode design and enhances skin-electrode contact for higher precision EEG recording. The textrodes were integrated into an infant’s 100% cotton, comfortable, and breathable hat. Placement points of the textrodes were located at FP1 and FP2 (as per 10-20 electrode system) along with a reference electrode connected at ear A1 location as highlighted in Figure 3.
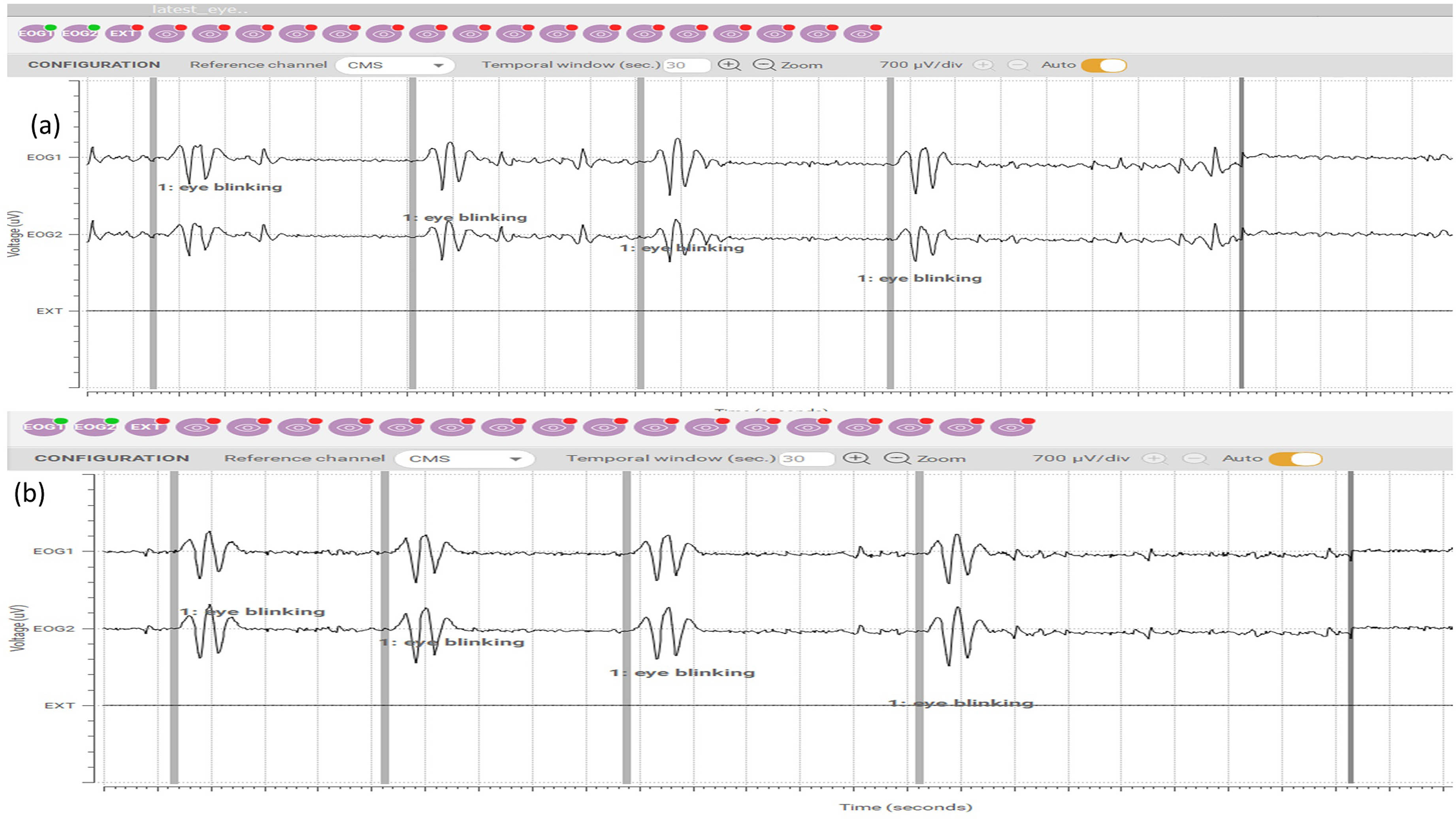
RESULTS: Epileptic seizures can be identified by contralateral (abnormal) blinking of an eye, offering a diagnostic clue for epilepsy and other central nervous system disorders. A cross-comparison of the textrode against a medical-grade EEG electrode was completed. Observation of ‘eye blinking’ actions were conducted. A high amplitude of the positive peak indicates eyelid closure, while a high amplitude of the negative peak signifies eyelid opening. Figure 4 (a) demonstrates eye blinking activity monitored using a smart hat with embedded 3D textrodes while Figure 4(b) presents EEG readings obtained using medical-graded electrodes.
CONCLUSION: The recording of contralateral ‘eye blinking’ is a key biomarker supporting epilepsy diagnosis. The 3-D textrode smart wearable hat application enables effective monitoring of EEG bio-signals in infants. The textrode solutions not only provide excellent skin contact and offer wearer comfort during long-term seizure monitoring but also can be reused and washed providing enhanced hygiene compliance. Future research is required in the form of clinical trials to gain feedback and assess further improvements required in textrode design and functionality.

Multivariate EEG functional connectivity analysis in newborns: Preliminary results on painful procedures and neurodevelopmental outcomes
Lorenzo Frassineti1, Caterina Coviello2, Silvia Lori3, Giovanna Bertini4, Simonetta Gabbanini3, Cesarina Cossu3, Maria Bastianelli3, Clara Lunardi4, Simona Montano2, Valentina Guarguagli1, Antonio Lanata1, Carlo Dani4
1Department of Information Engineering, University of Florence, 2Division of Neonatology, University of Florence, Florence, Italy, 3Neurophysiology Unit, Neuro-Musculo-Skeletal Department, Careggi University Hospital, Florence, Italy, 4Department of Neurosciences, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy
BACKGROUND AND PURPOSE: Preterm children are at risk for language development delays. Early shared reading has been demonstrated to enhance language development and functional brain connectivity in full-term children. Therefore, recent research examines the potential benefits of NICU reading programs for the language development of preterm children. Indeed, early reading to preterm newborns improves their direct exposure to parental voices and facilitates contingent communication. Children’s books provide parents with a more structured framework for interacting with their infants, offering enriched linguistic input characterized by richer language and grammar. It also enhances parental involvement and sense of control. Furthermore, NICU reading programs may have lasting effects by encouraging ongoing reading practices after discharge. Consequently, this study aims to evaluate the impact of reading habits during the first two years of life following NICU discharge (including a NICU reading program) on language development.
METHODOLOGY: Preterm neonates born < 32 weeks gestational age (GA) between September 2018 and May 2021, and admitted to the NICU of the Careggi University Hospital of Florence, Italy, were recruited for this prospective cohort study. Infants with severe brain injury defined as the occurrence of intraventricular hemorrhage > 3 grade and cystic periventricular leukomalacia were excluded. An 8-channel EEG recording was performed at term equivalent age (TEA). The exposure to neonatal painful procedures was evaluated recording the number of skin-breaking procedures from birth to EEG recording. Moreover, the BAYLEY-III neurodevelopmental scores at 24 months were collected. Several EEG-based indexes were investigated: connectivity hemispheric measures [3], EEG entropy indexes [1], and multivariate phase synchrony indexes such as Circular Omega Complexity (COC) or Hyper-Tours Synchrony (HTS) [4]. The multivariate statistical analysis was performed using generalized linear mixed-effects (GLME) models, considering also the false discovery rate correction for multiple comparisons.
RESULTS: Seventy-seven preterm newborns were enrolled. GA at birth was 27±2 weeks, the postmenstrual age (PMA) at the recording was 38±1.5 weeks. The cumulative number of painful procedures was 101±65. The multivariate statistical analysis showed that several quantitative EEG indexes were associated with painful procedures. Specifically, multivariate indexes on generalized functional connectivity, such as COC related to delta and theta waves (p-values < 0.02), suggest a sort of short-term effect related to painful procedures that might alter the brain dynamics. Moreover, the same EEG parameters were found as significant predictors of infants with neurodevelopmental delay. In fact, alterations on COC indexes were associated with low cognitive and motor BAYLEY scores: p-values 0.02 and 0.007, respectively.
CONCLUSION: Preliminary results suggest that quantitative multivariate EEG indexes may be helpful to characterize the neurodevelopmental outcomes and to monitor the impact of painful procedures on the brain dynamics of infants.
BIBLIOGRAPHY:
[1] Lavanga, M., et al., (2021). Pain, 162(5), 1556.
[2] Lammertink, F., et al., (2022). Translational psychiatry, 12(1), 256.
[3] Toole, J. M. & Boylan, G. B., (2017). arXiv preprint arXiv:1704.05694.
[4] Baboukani, P. S., et al., (2019). Digital Signal Processing, 84, 59-68.
Bronchopulmonary dysplasia (BPD) and amplitude-integrated electroencephalogram (aEEG): Pilot study
Divya Rana1, Mimily Harsono1, Massroor Pourcyrous1
1University of Tennessee Medical Science Center
BACKGROUND: Bronchopulmonary dysplasia (BPD) is a chronic lung disease in preterm infants who require oxygen supplementation at 36 weeks postmenstrual age. Infants with BPD have an increased risk for poor neurodevelopmental outcome. Previous studies have shown that normal neonatal sleep pattern plays an important role for brain activity and neuronal development. Using aEEG monitoring, presence of sleep-wake cycle (SWC) has been shown to be an important marker of neurodevelopmental outcome. However, the assessment of neonatal SWC status in infants with BPD is still lacking. In this study, we used aEEG in infants with BPD to monitor the presence of SWC.
OBJECTIVE: We hypothesized that infants with BPD have abnormal SWC detected by aEEG.
METHODOLOGY: This is an observational study. This study was performed from August 2018 to November 2022 in NICU at ROH birthing hospital. Infants born <32 weeks gestational age (GA) with diagnosis of BPD were recruited and informed consents from legal guardians or parents were obtained. Study was approved by the institutional IRB. Infants’ demographics, clinically relevant information and blood gas results were documented. A bedside two-channel (C3-C4 and P3-P4) aEEG with Olympic Brainz CFM (Natus) was applied. All aEEG recordings were obtained for 18 to 24 hours while infants received routine care. All aEEGs were read using aEEG classification from the Department of Pediatric Newborn Medicine at Brigham and Women’s Hospital (www.phscpd.org/BWH NeonatalaEEG). The aEEG monitoring can detect background pattern (continuous, discontinuous, burst-suppression, low voltage, inactive, flat), cycling (no cycling, imminent cycling, established cycling) and seizures, status epilepticus.
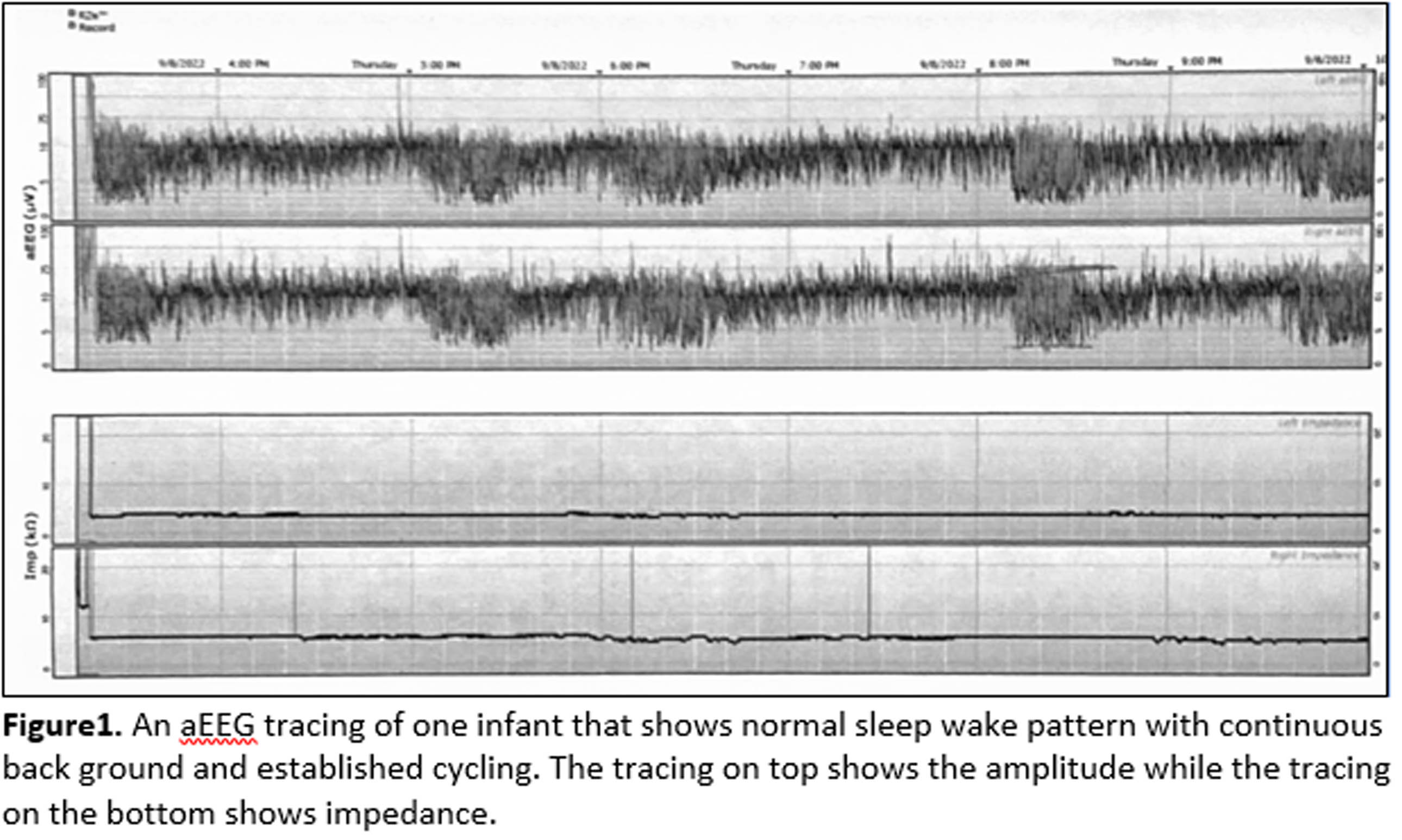
RESULTS: A total of 13 infants were enrolled. The mean BW and GA were 26 ± 2 weeks and 718 ± 190 grams, respectively. The average postmenstrual age when the first aEEG obtained was 37 ± 3 weeks at day of life 77 ± 28. All enrolled infants met the criteria for BPD with no other identifiable major medical conditions. At the time of aEEG monitoring, four infants were on invasive mechanical ventilator; five infants were on non-invasive ventilator, four infants were on oxygen via bi-nasal cannula. The blood gas had mean pH of 7.35 ± 0.03, pCO2 of 52 ± 9 mmHg, and base excess of 4 ± 3 (Table 1). All 13 infants had a normal aEEG background pattern and a normal SWC. An example of an aEEG recording on one infant is shown on Figure 1.
CONCLUSION: In this study, we found that infants with BPD have no identifiable SWC abnormalities on aEEG recording. We aim to enroll more infants with BPD for our study. We also plan to correlate the aEEG findings with the neurodevelopmental outcomes.
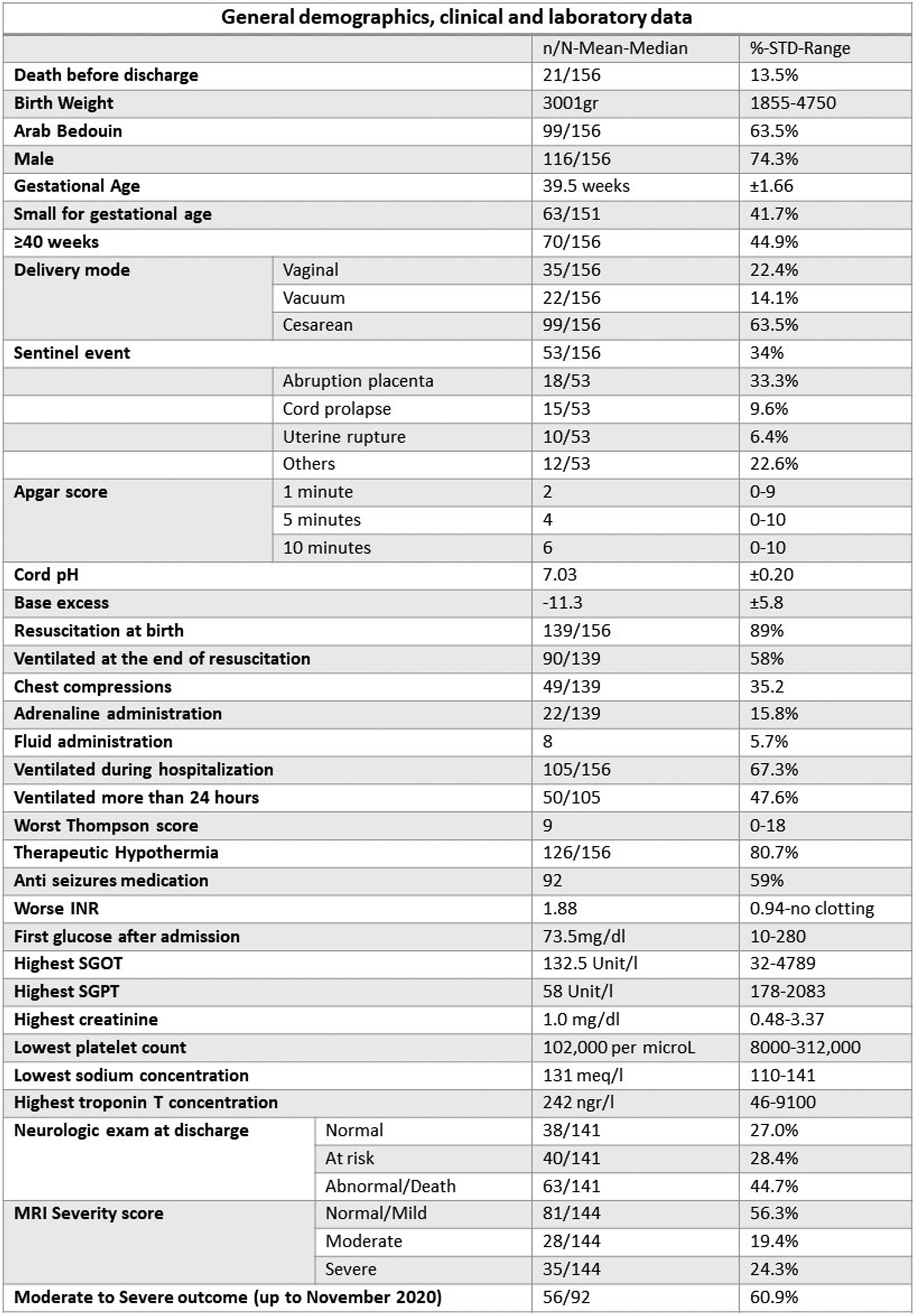
Neonatal encephalopathy in the diverse population of Southern Israel: 8 year single center experience
Kyla Marks1, Ramy Abramsky1, Ilan Shelef1, Analia Michaelovski1, Amid Zahalka1, Miri Goldshtein1, Doreen Ozalvo1, Lior Wilk1, Eilon Shany1
1Soroka University Medical Center/Ben Gurion University of the Negev
BACKGROUND: The incidence of neonatal hypoxic ischemic encephalopathy (NHIE) and associated clinical, laboratory and imaging parameters vary according to clinical settings and geographic locations. Accordingly, to improve the care of infants with NHIE in a specific population, local data collection and follow-up programs are invaluable.
OBJECTIVE: The objective of this study was to evaluate the association of clinical and laboratory data with short- and long-term outcome in a cohort of infants with NHIE.
METHODOLOGY: This study derives from a prospective NHIE database collected between November 2014 and July 2023. Included were infants over 35 weeks gestation with NHIE. Demographic, clinical, laboratory, neurophysiologic parameters and therapeutic modalities were prospectively collected. MRI scans were assessed according to a modified Rutherford classification that included diffusion weighted imaging. Outcome data was collected from the child developmental center, medical files and parental questionnaires. Univariable analysis is presented with multivariable analysis to be presented at the conference. P values < 0.003 were considered significant to correct for multiple comparisons.
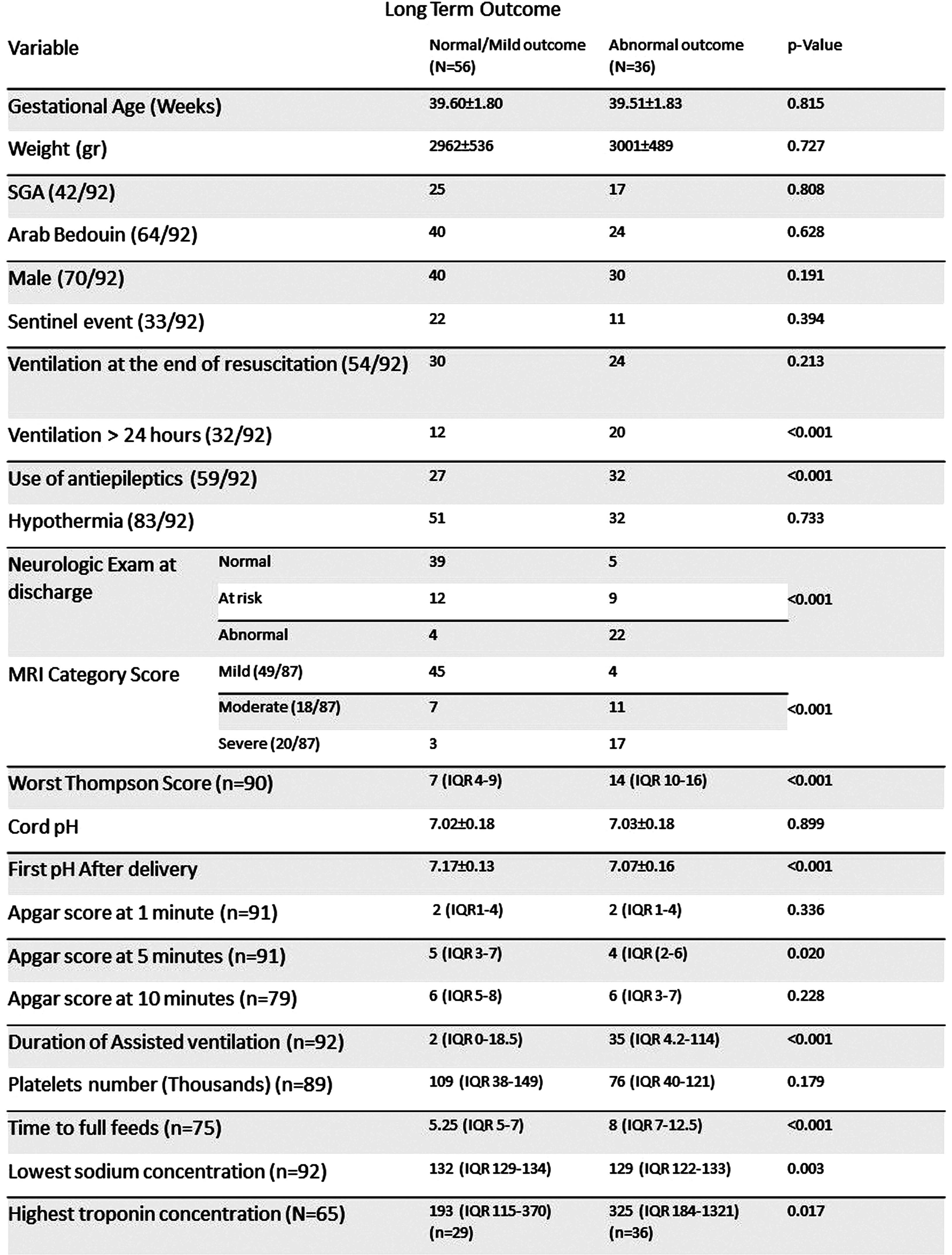
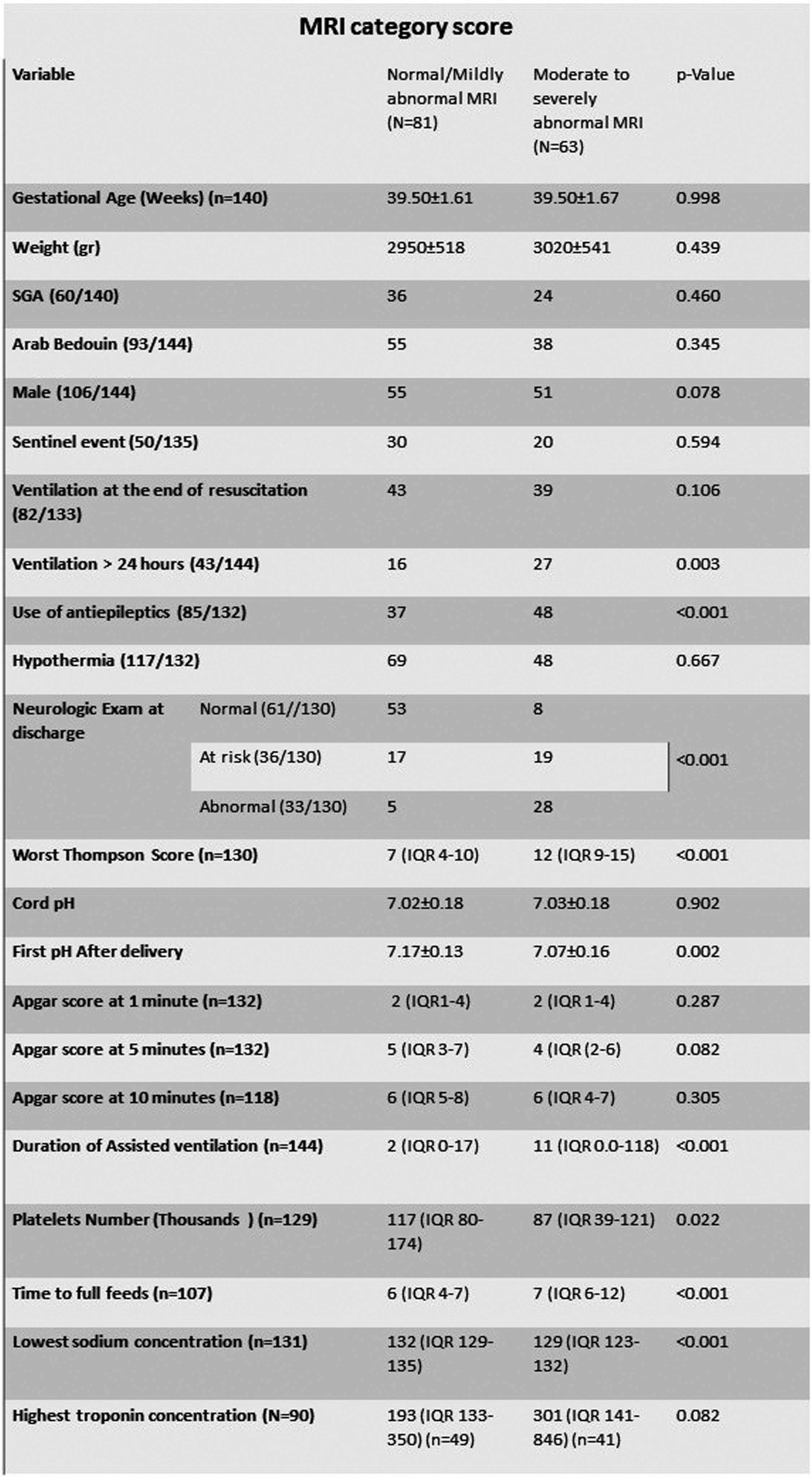
RESULTS: During the study period 188 infants with neonatal encephalopathy were assessed. Thirty infants were excluded (no consent (n=16), refuted NHIE diagnosis (n=5), infarct (n=5), less than 35 weeks (n=4), intraventricular hemorrhage (n=1), trisomy 21 (n=1)). Six of the excluded infants died before discharge. Overall, 156 infants (1.15 per 1000 deliveries) were included in the study, 126 (80.7%) were treated with therapeutic hypothermia and 92 (59.0%) with antiepileptic drugs. Clinical and laboratory variables are presented in table 1. MRI scans were available in 144 infants, in 35 (24.3%), no abnormalities were detected. The MRI severity score was mild/normal in 81 (56.3%), moderate in 28 (19.4%) and severe in 35 (24.3%). Neurocognitive outcome at 2-3 years was available for 92 infants born before November 2020. Twenty-six (28.2%) infants had normal outcome, 30 (32.6%) were categorized with mildly abnormal outcome and 31 (33.7%) were categorized with either moderate or severe outcome (of them 8 (25.8%) died). Univariable analysis of the associations of different variables with long term outcome and MRI severity scores are presented in tables 2 and 3. First blood pH on NICU admission, ventilation more than 24 hours, duration of ventilation, utilization of antiepileptic drugs, low sodium concentration (p=0.003 for the association with long term outcome), time to full feeds and neurologic examination at discharge were associated with long term outcome and MRI severity scores (p<0.001 for all). MRI category score was significantly associated with long term outcome (p<0.001). Amplitude integrated EEG association with outcome and multivariable analysis will also be presented.
CONCLUSION: In this population, clinical and laboratory variables during hospitalization in infants with NHIE were significantly associated with long term outcome and can be compared to other international cohort outcome studies of NHIE.
Early EEG in infants with mild hypoxic-ischaemic encephalopathy and 2-year outcome
Aisling Garvey1,2,3, Geraldine Boylan1,3, Andreea Pavel1,3,4, John O’Toole1,3, Brian Walsh1,2,3,4, Eugene Dempsey1,3,4, Deirdre Murray1,3
1Infant Research Centre, 2Department of Pediatrics, Division of Newborn Medicine, Brigham and Womens Hospital, Harvard Medical School, 3Department of Pediatrics and Child Health, University College Cork, 4Department of Neonatology, Cork University Maternity Hospital
BACKGROUND: Infants with mild hypoxic-ischaemic encephalopathy (HIE) are at significant risk of poor long-term developmental outcome.(1) Early identification of infants at risk may allow for targeted interventions in the neonatal period and beyond. Early EEG before 6 hours of age demonstrates differences in qualitative and quantitative measures in infants with mild HIE compared to healthy term infants.(2) This study aimed to examine if detailed EEG analysis (in the first 6 hours after birth) in infants with mild HIE is associated with neurodevelopmental outcome at 2 years.
METHODOLOGY: Infants > 36 weeks with mild HIE, not undergoing therapeutic hypothermia (TH), with EEG recording within 6 hours of age and standardised neurodevelopmental follow-up at approximately 2 years were identified from 4 previous prospective studies in Cork, Ireland (2003-2019). EEGs within 6 hours of birth were independently analysed by 2 neonatal neurophysiologists blinded to outcome using a previously published grading system and quantitatively assessed using multiple features of amplitude, spectral shape and inter-hemispheric connectivity (2, 3).
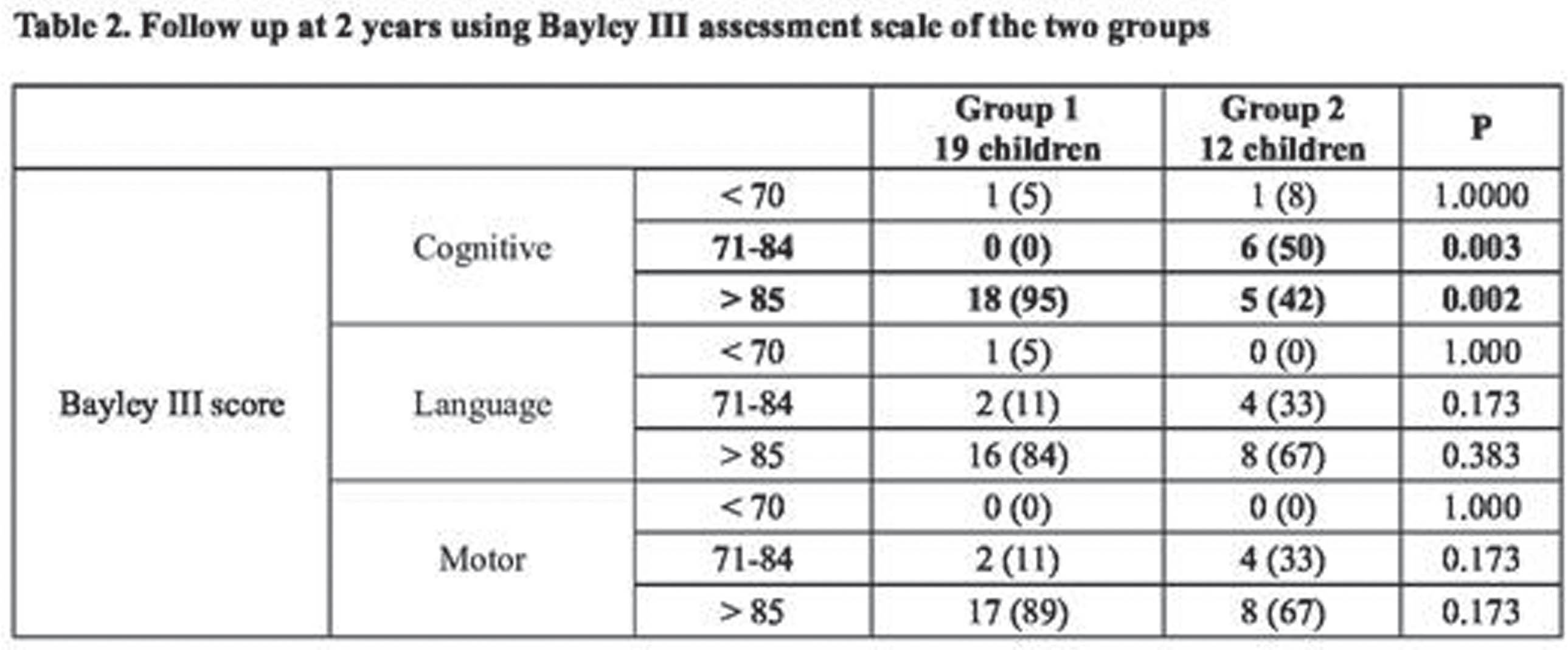
RESULTS: Forty-eight infants with mild HIE were included. Infants were followed up at a median age of 24 months (IQR 22-29). Twelve infants (25%) had an abnormal outcome at follow up. Seventy-three percent (35/48) of infants had at least one abnormal feature on EEG at 6 hours on qualitative analysis, 9 of whom (26%) had an abnormal outcome. Of the 13 infants with normal early qualitative EEG, 3 (23%) had an abnormal outcome. Qualitative EEG features at 6 hours were not associated with outcome. Quantitative analysis of spectral difference was significantly associated with adverse outcome at the lower frequency bands (correlation coefficient -0.37, p=0.01).
CONCLUSION: Early quantitative EGG features may be better in identifying infants with mild HIE at risk of poor neurodevelopmental outcome than qualitative EEG analysis alone. Infants with mild HIE have an increased risk of learning, emotional and behavioural difficulties which may not be evident until school age or beyond and longer term follow up will be important in this cohort (4).
REFERENCES:
1. Conway, J, et al. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome – a systematic review. Early Hum. Dev. 120, 80–87 (2018).
2. Garvey AA, et al. Multichannel EEG abnormalities during the first 6 hours in infants with mild hypoxic-ischaemic encephalopathy. Pediatr Res. 2021 Jul;90(1):117-124. doi: 10.1038/s41390-021-01412-x. Epub 2021 Apr 20. Erratum in: Pediatr Res. 2021 Jun 4;: PMID: 33879847; PMCID: PMC8370873.
3. O’ Toole, J. & Boylan, G. NEURAL: quantitative features for newborn EEG using Matlab. Preprint at arXiv. https://arxiv.org/abs/1704.05694 (2017).
4. Murray, DM., et al. Early EEG grade and outcome at 5 years after mild neonatal hypoxicischemic encephalopathy. Pediatrics 138, e20160659 (2016).
Seizures in mild neonatal encephalopathy and short-term MRI outcome
Aisling Garvey1,2, Brian Walsh1,2,3, Hoda El-Shibiny2, Terrie Inder2,4, Mohamed El-Dib2
1Infant Research Centre, 2Department of Pediatrics, Division of Newborn Medicine, Brigham and Womens Hospital, Harvard Medical School, 3Department of Neonatology, Cork University Maternity Hospital, 4Children’s Hospital of Orange County, University of California Irvine
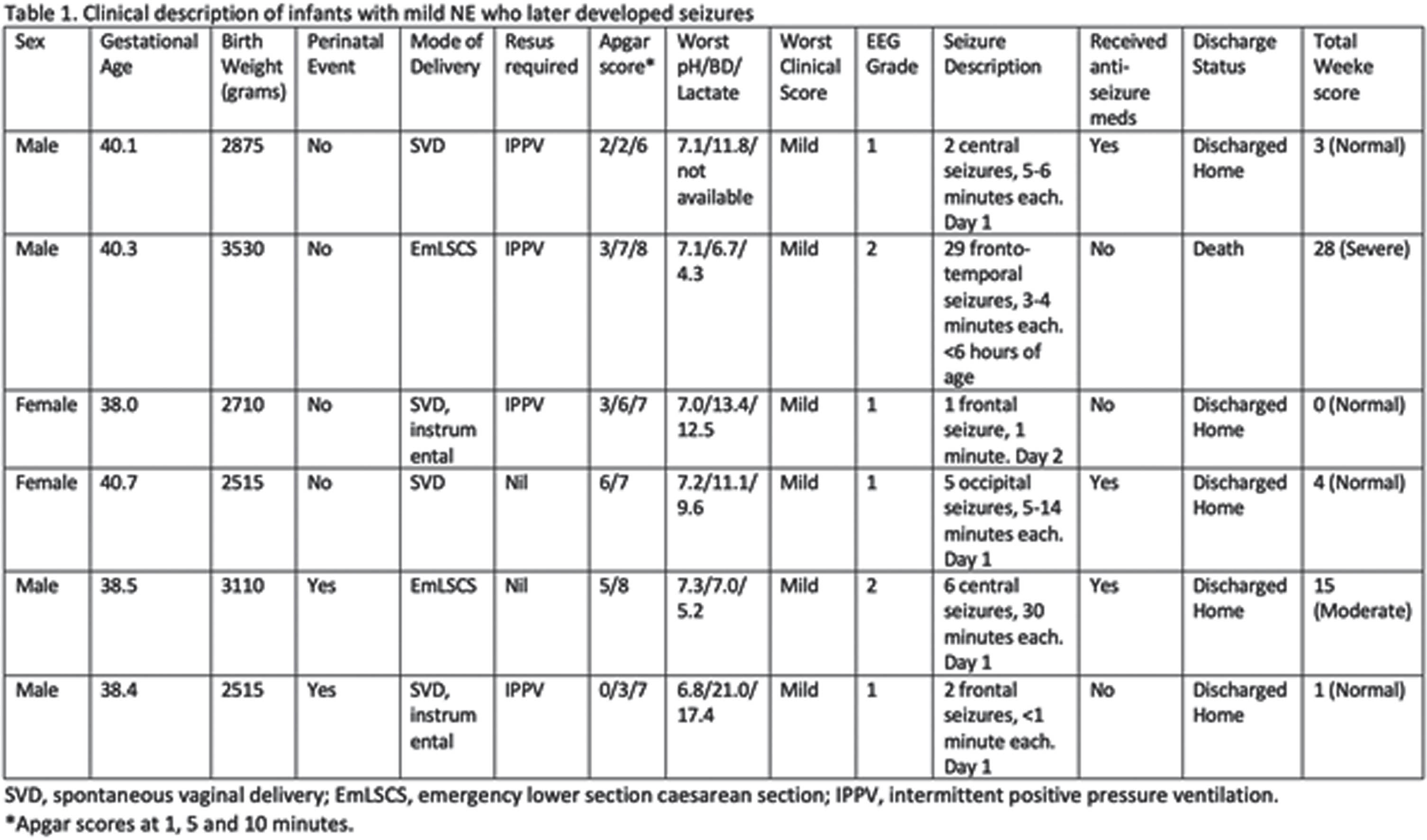
BACKGROUND: Many clinical grading systems have been developed to categorize and define Neonatal Encephalopathy (1, 2, 3). Initially designed to serially examine infants over the first week of life, they are now used to inform clinical practice and guide intervention in the first 6 hours after birth (4, 5). NE continues to be one of the leading causes of seizures in term neonates and, if present, implies a moderate-severe grade of NE (1, 2, 3). The aim of this study is to describe MRI outcomes in infants initially classified as having mild NE who later developed seizures.
METHODOLOGY: Retrospective cohort study of infants receiving therapeutic hypothermia (TH) for NE at the Brigham and Women’s Hospital, Boston, a tertiary level Neonatal Unit, between 2016-2021. From this cohort, infants with mild NE with seizures were identified. EEGs and MRIs were independently graded according to previously published grading systems by 2 Neonatologists with Neurology subspecialisation (6, 7).
RESULTS: During this time, 291 infants received TH for NE. Twenty-six infants (9%) had seizures confirmed electrographically, of which 6 had a clinical diagnosis of mild NE. Table 1 outlines the relevant EEG and clinical findings. Four of the six infants had a normal MRI Brain prior to discharge. Two infants had moderate to severe injury on their MRI, both of whom had moderate abnormalities noted on their EEG in the first day. One of these infants had profound hypoglycaemia suggestive of an underlying metabolic condition and seizures commenced in the first 6 hours after birth. This infant died on day 11.
CONCLUSION: In our cohort, 67% (4/6) infants with mild NE who later had seizures had a normal MRI Brain. Infants with injury noted on their MRI (2/6) had abnormal features on their early EEG. Presence of seizures does not, by default, imply a poor outcome however further research and long-term follow up is required. EEG may be helpful in identifying infants at risk.
REFERENCES:
1. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress-A clinical and electroencephalographic study. Arch Neurol.1976;33(10):696-705.
2. Levene MI, et al. Comparison of two methods of predicting outcome in perinatal asphyxia. Lancet.1986;1(8472):67-9.
3. Thompson CM, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr.1997;86(7):757-61.
4. Shankaran S, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med.2005;353(15):1574-84.
5. Azzopardi D, et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr.2008;8:17.
6. Murray DM, et al. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics.2009;124(3):e459-67.
7. Weeke LC, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr.2018;192:33-40.e2.
Feeding problems and associated short-term outcomes in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia
Ceyda Acun1, Rakesh Lavu1, Nicholas Nicoletti1, Wei Liu1, Sreenivas Karnati1, Subhash Puthuraya1, Hany Aly1
1Cleveland Clinic Children’s Hospital
BACKGROUND: Hypoxic Ischemic encephalopathy (HIE) is a major cause of morbidity and mortality in neonates. Early brain injury is known to have a deleterious impact on oral motor functions, which may lead to feeding and speech problems.
OBJECTIVE: To evaluate the prevalence of swallowing impairment and its association with brain magnetic resonance imaging (MRI) and electroencephalogram (EEG) abnormalities in neonates with HIE and treated with therapeutic hypothermia (TH).
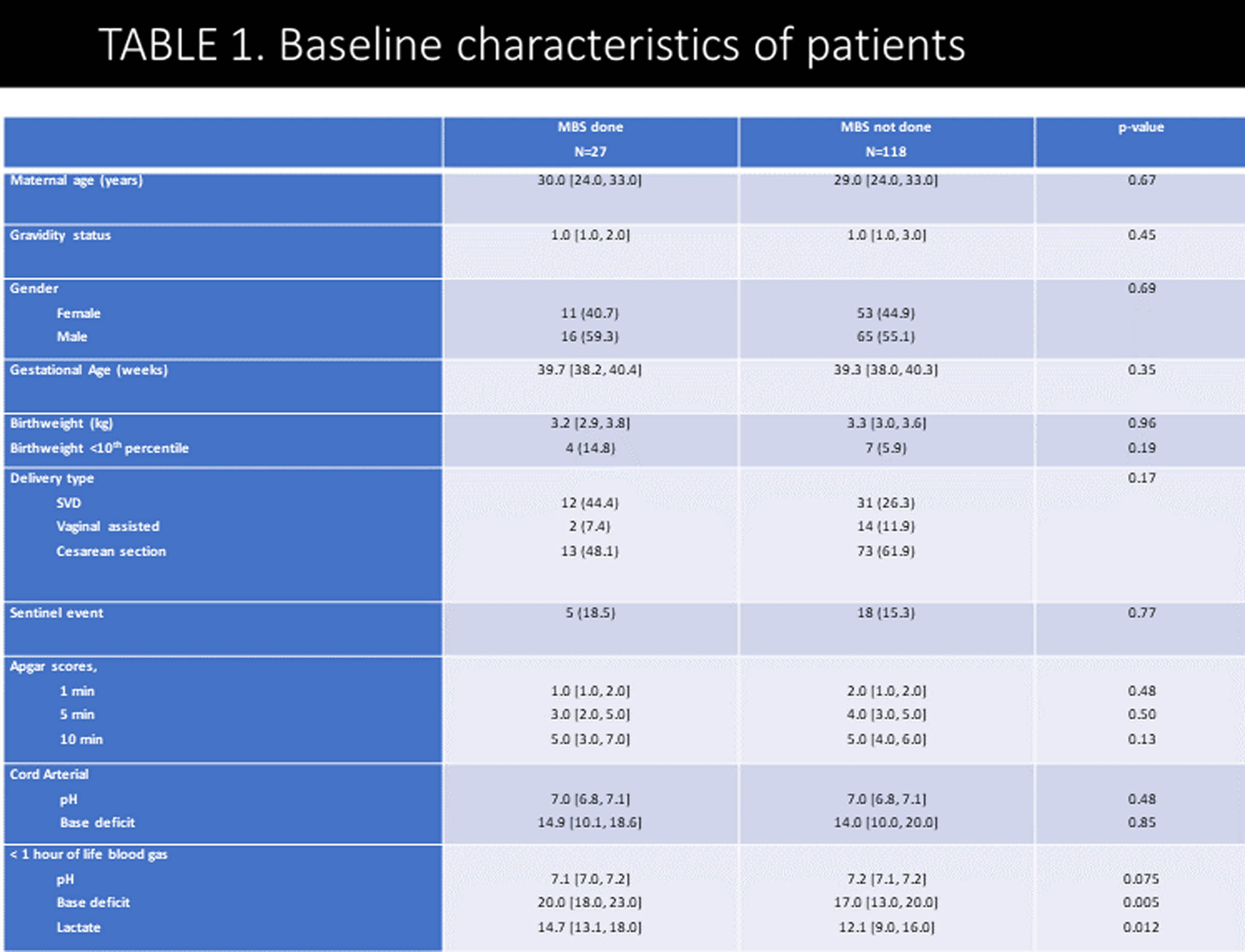
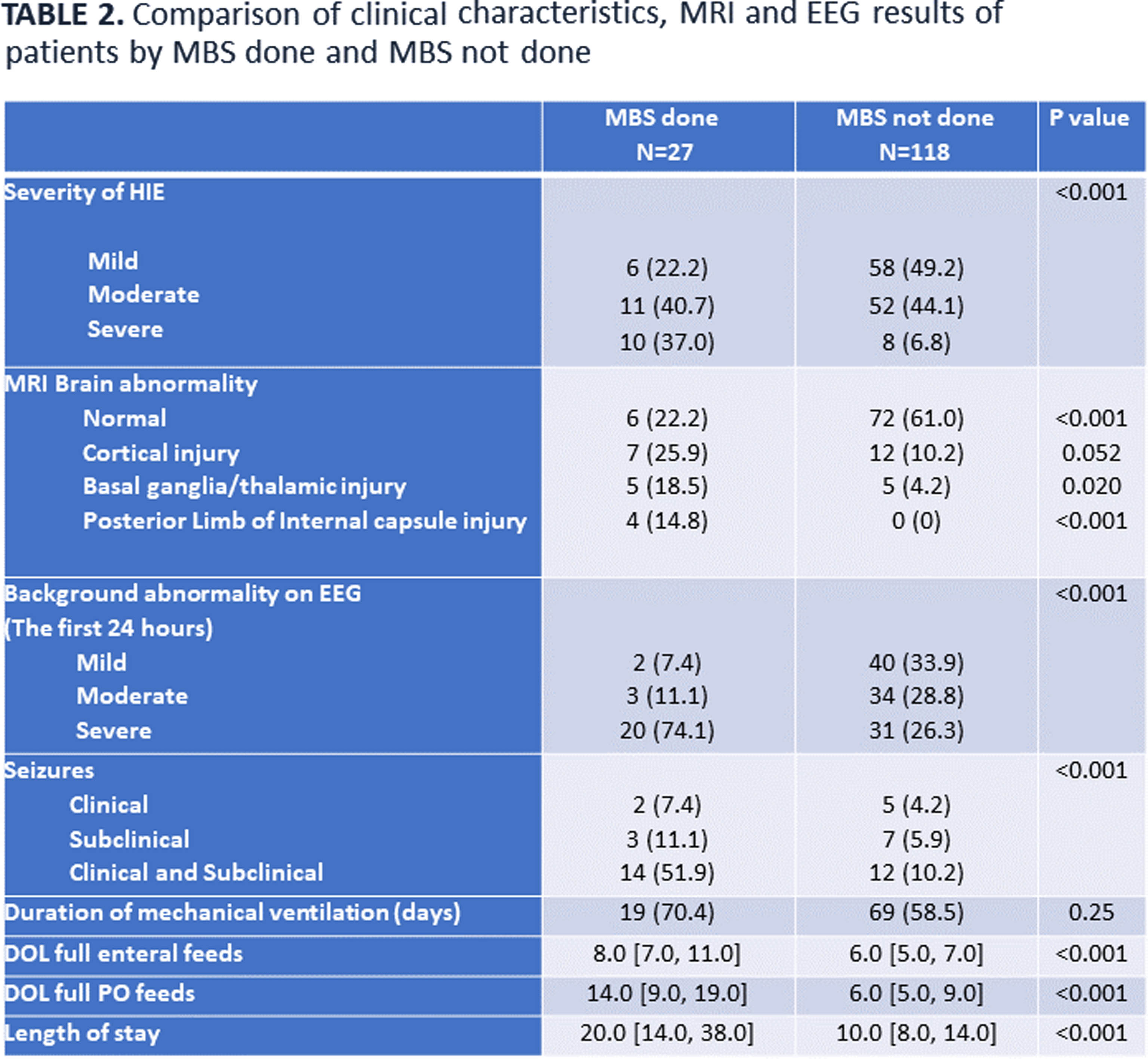
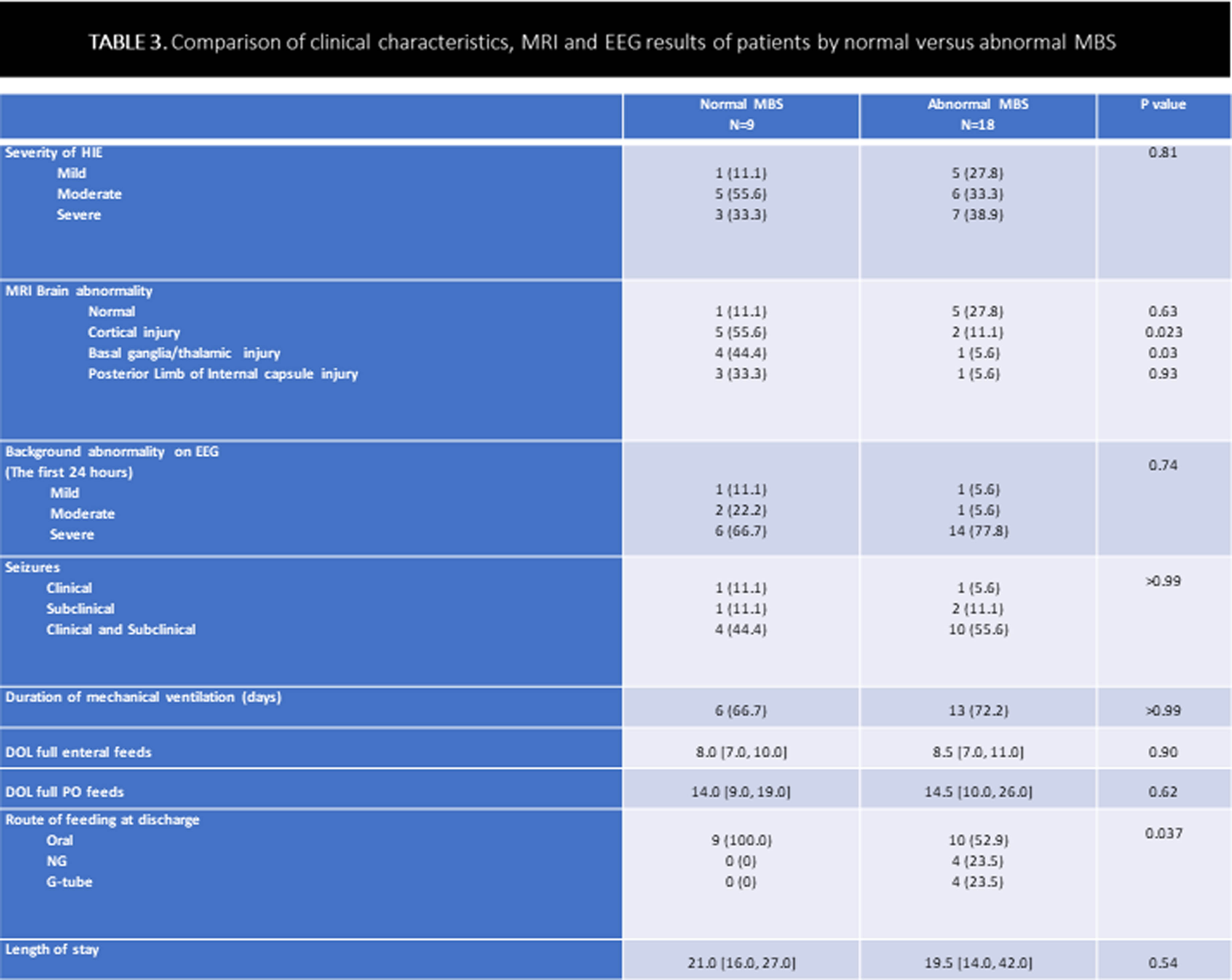
METHODOLOGY: This was a retrospective, single-center cohort study of HIE neonates (gestational age ≥ 36 weeks) who underwent TH between January 2012 and December 2022. Neonates with other significant congenital anomalies, genetic conditions, need for extracorporeal membrane oxygenation, or in-hospital mortality were excluded. Following rewarming, a modified barium swallow (MBS) was performed on neonates considered at risk for aspiration to identify swallowing impairment; brain MRI was performed in clinically stable babies (usually between 5-7 days of life). EEG was continuously performed from TH initiation till at least 80 hours of life.
RESULTS: In total, 145 neonates were included, with 55.9% males, 59.3% born by cesarean section, and 43.4/12.4% moderate/severe HIE. Overall, 27/145 (18.6%) MBS evaluations were performed: 9 (33.3%) normal, 8 (29.6%) dysphagia without aspiration, and 10 (37.0%) dysphagia with aspiration. Prevalence of abnormal swallow study was 7/18 (39%) in severe HIE, 6/63 (9%) in moderate HIE and 5/64 (8%) in mild HIE. Infants who required MBS had a significantly higher incidence of seizures, abnormal MRI, EEG, and longer hospital stay. Infants with abnormal MBS had more cortical, basal/thalamic ganglia injury compared to neonates with normal MBS. Among the infants who had abnormal MBS, only 8/18 (44 %) were discharged home on tube feeds: 4 with nasogastric and 4 with gastrostomy tube.
CONCLUSION: In this cohort of babies with HIE treated with TH, the prevalence of dysphagia was lower (overall 12.4%) than previously reported. Dysphagia improved in most babies by the time of discharge. Infants with all degree of HIE undergoing TH should be assessed by speech pathologist before initiating oral feeds.
BIBLIOGRAPHY:
1. Malan R, Van der Linde J, Kritzinger A, Graham MA, Krüger E. Evolution of Feeding and Developmental Outcomes in Infants With Moderate Hypoxic-Ischemic Encephalopathy: A Pilot Study. Neonatal Network 2023; 42: 264-275.
2. Jadcherla SR, Khot T, Moore R, Malkar M, Gulati IK, Slaughter JL. Feeding methods at discharge predict long-term feeding and neurodevelopmental outcomes in preterm infants referred for gastrostomy evaluation. J Pediatr. 2017;181:125–130.
3. Adhikari S, Rao KS. Neurodevelopmental outcome of term infants with perinatal asphyxia with hypoxic ischemic encephalopathy stage II. Brain Dev. 2017;39:107–111.
Relationship between EEG spectral power and dysglycemia with neurodevelopmental outcomes after neonatal encephalopathy
Janie Damien1,2,3, Phetsamone Vannasing1,3, Julie Tremblay1,3, Laurence Petitpas1,2,3, Bohdana Marandyuk1, Thameya Balasingam1, Ramy El Jalbout1, Natacha Paquette1,2,3, Gianluca Donofrio1,4, Anne Gallagher1,2,3, Elana F Pinchefsky1,2,3
1Sainte-Justine University Hospital Center, 2University of Montreal, 3Neurodevelopmental Optical Imaging Laboratory (LION Lab), 4University of Genoa
BACKGROUND AND OBJECTIVE: Identification of early markers of brain function is essential to aid in the prediction of neurodevelopmental outcomes following neonatal encephalopathy (NE). Potentially modifiable risk factors such as dysglycemia are frequent in the first hours of life in newborns with NE and may contribute to impaired brain function and long-term adverse outcomes. The relationship between dysglycemia and brain function after therapeutic hypothermia and at follow-up in NE needs to be further investigated to improve the prediction of neurodevelopmental outcomes. Therefore, we studied how dysglycemia and brain function on electroencephalography (EEG) following therapeutic hypothermia relate to neurodevelopmental outcomes in children with NE.
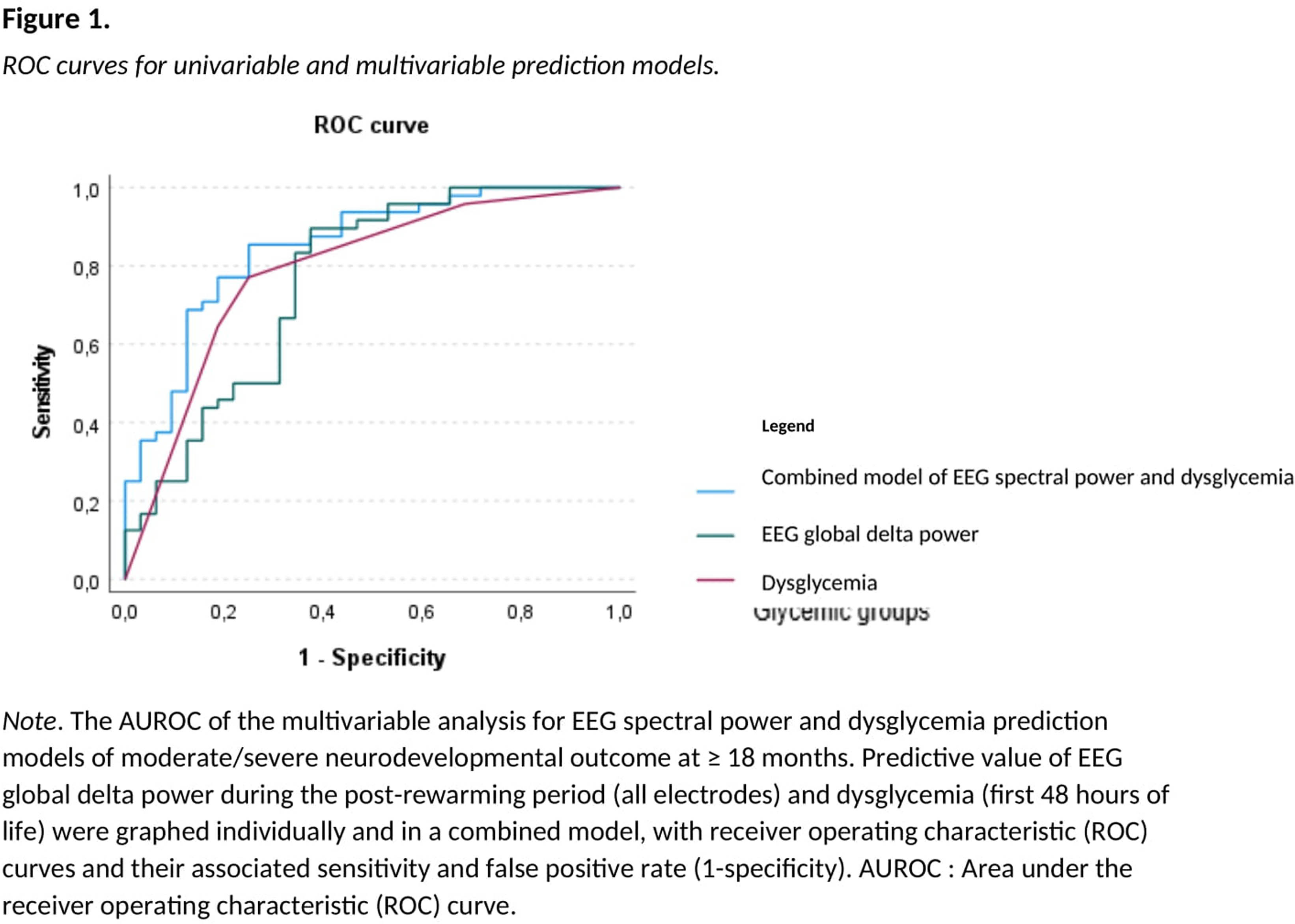
HYPOTHESIS: We hypothesized that neonatal dysglycemia (hypo- or hyperglycemia in the first 0-48 hours of life) and EEG spectral power (measured during the post-rewarming period and the routine 2-month follow-up) in NE are related to neurodevelopmental outcomes at ≥18 months. Methodology. This retrospective study included 90 neonates with encephalopathy who received therapeutic hypothermia. EEG absolute spectral power was calculated during the 6-hour post-rewarming period and the 30-minute routine follow-up at 2-months, within all frequency bands (delta, theta, alpha, beta, total) and brain regions (frontal Fp1\2, central C3\4, temporal T3\4, occipital O1\2, and global average of all electrodes). Measures of dysglycemia (hypoglycemia, hyperglycemia, and glycemic lability) and glucose variability (mean absolute glucose change) were computed for the first 48 hours of life. Neurodevelopmental outcomes included motor, language or global developmental delays, visual impairments, auditory deficits, cerebral palsy, mortality, and a composite measure of normal/mild or moderate/severe outcome at ≥18 months. Using logistic regressions with area under receiver operating characteristic (AUROC) curves, we evaluated EEG and glucose variables in separate and combined models to predict neurodevelopmental outcomes, adjusting for NE severity and MRI brain injury and using Bonferroni correction for multiple comparisons.
RESULTS: Global delta power during post-rewarming and dysglycemia episodes (hyperglycemia and glycemic lability) during the first 0-48 hours of life were the variables with the highest predictive values for moderate/severe neurodevelopmental outcome at ≥18 months (AUROC=0.8, 95%CI 0.7;0.9 for both models). The combined model including global delta power and dysglycemia more accurately predicted moderate/severe neurodevelopmental outcome (AUROC=0.9, 95%CI [0.8,0.9], p<.001). After adjusting for NE severity and MRI brain injury, only higher global delta power remained significantly associated with lower odds of moderate/severe neurodevelopmental outcome (OR=0.9, 95%CI [0.8,1.0], p=.04), motor delay (OR=0.9, 95%CI [0.8,1.0], p=.04), global developmental delay (OR=0.9, 95%CI [0.8,1.0], p=.04), and auditory deficits (OR=0.9, 95%CI [0.8,1.0], p=.03).
CONCLUSION: This study identified quantitative EEG markers of brain function after therapeutic hypothermia that are associated with neurodevelopmental outcomes. Global delta power measured during post-rewarming accurately predicted unfavorable neurodevelopmental outcomes at ≥18 months, even after adjusting for NE severity and MRI brain injury.
Hypoxic ischemic encephalopathy: 2-years’ follow-up comparing clinical criteria and aEEG as criteria for therapeutic hypothermia
Maria Elena Cavicchiolo1, Nicoletta Mainini1, Benedetta Bua1, Marta Meneghelli1, Elena Priante1, Giulia Soravia1, Chiara Lasagni1, Giovanna Verlato1, Eugenio Baraldi1
1Padova University Hospital
BACKGROUND: Neonatal hypoxic ischemic encephalopathy (HIE) is a leading cause of death and neurodevelopmental impairment in neonates with an incidence from 2 to 3/1,000 newborns. Therapeutic hypothermia (TH) is a recommended regimen for newborn infants who are at or near term with evolving moderate-to-severe HIE. Current guidelines for the decision on starting therapeutic hypothermia are based only on clinical criteria, such as Sarnat & Sarnat score of 2 and 3 evaluated at 30 and 60 minutes of life. Recent evidences demonstrated possible better outcomes of patients with Sarnat 1 (i.e. mild HIE) who received TH compared with whom did not receive such treatment regimen. aEEG, which is a bed side, simple to read method for the evaluation of the neurological electric activity has a central role in monitoring the baby during TH and to prognostic considerations.
OBJECTIVE: We aimed to assess the outcome of patients at 2-years of age who received TH for HIE comparing the selection criteria to start TH.
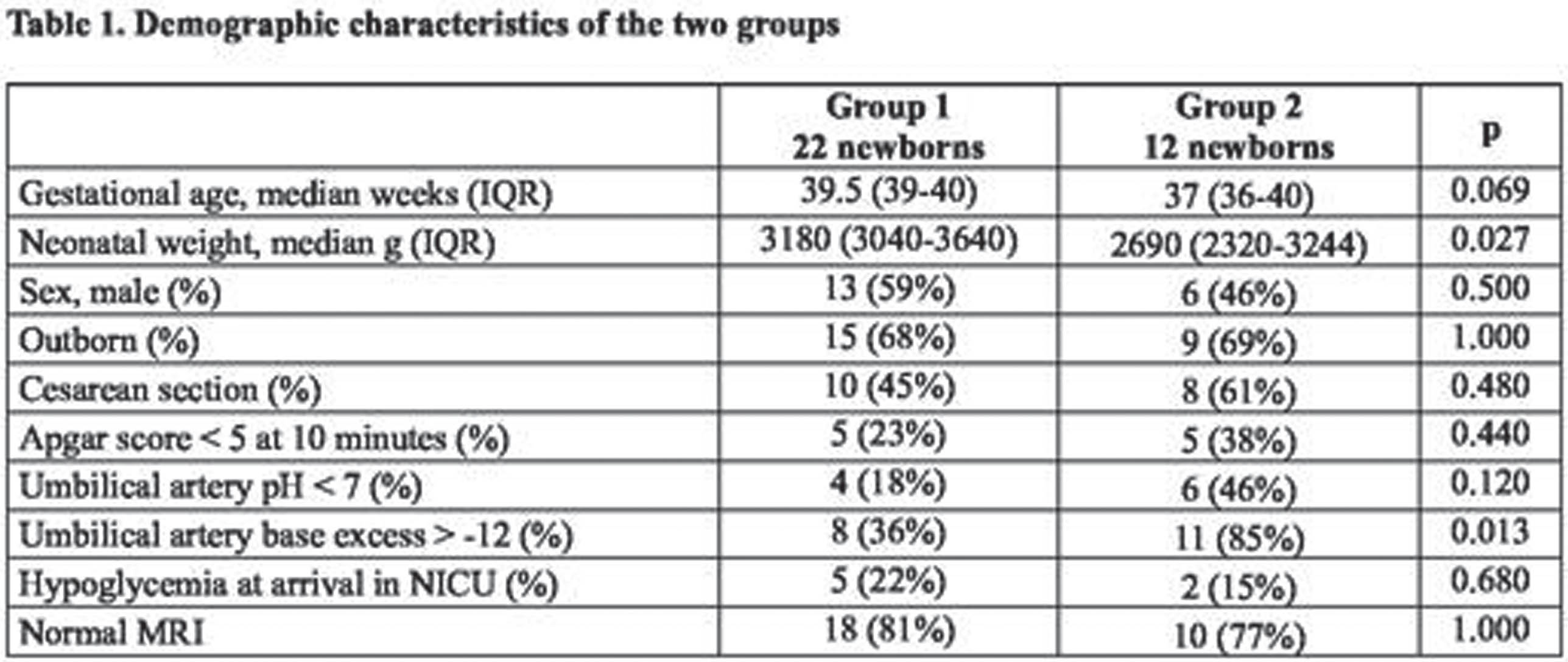
METHODS: We retrospectively revised records from babies who suffered HIE and underwent TH at the NICU of Padua Hospital in a 7-year period (January 2015-December 2022). We compared Bayley III scores performed at 2 years of age of such babies comparing two groups: Group 1) TH was offered based on Sarnat score 2 or 3; and Group 2) TH was offered to patients with Sarnat score 1 but with an abnormal aEEG during the initial evaluating phase before starting TH.
RESULTS: In 7 years, 31 patients who received TH had available Bayley III score at 2 years of age. Sixty-eighty percent were outborn babies and 61% were male. Comparing Group 1 and Group 2, the two groups were homogeneous for demographic characteristics and criteria for asphyxia and following Sarnat score evaluation. At 2 years of age, motor and language Bayley III scores were similar in both groups, but cognitive Bayley III score was statistically significant higher in the Group 1 (cognitive > 85 in 95% vs 42%, p=0.002).
CONCLUSION: Patients with abnormal aEEG before starting TH even with a milder HIE demonstrated a worse cognitive outcome at 2-years of age than patients with a moderate-severe HIE. Our study demonstrated the role of early aEEG as a decision tool to start TH also in newborns without definitive clinical criteria for such treatment. Larger studies are needed to evaluate the possible integration of aEEG into the decision-making process to initiate TH.
Automated EEG background classification of long-duration neonatal recordings to predict 2-year outcome
Fabio Magarelli1,2, Geraldine Boylan1,3, John O’Toole1,3
1Infant Research Centre, 2The SFI Centre for Research and Training in Artificial Intelligence, CRT in AI, 3Department of Paediatrics and Child Health
BACKGROUND: Background abnormalities in EEG have been shown to predict neurodevelopmental outcomes in neonates with hypoxic ischaemic encephalopathy (HIE)[1,2,3]. However, how this association evolves during the first days after birth is unclear. We aim to use an automated algorithm to examine the relationship between background abnormality assessment in continuous, long-duration EEG and neurodevelopmental outcomes at 2 years of age.
METHODOLOGY: We used a previously developed convolutional neural network [4], to grade the severity of background abnormalities from continuous, long-duration EEG of 183 babies [5,6] with HIE up to 100 hours after birth. The algorithm produces a continuous output score, ranging from 0 to 4, to indicate normal EEG (0) up to isoelectric and status epilepticus (4). The score was calculated every 5 minutes of EEG with 50% overlap, and the median value per hour was taken to smooth this estimate. We examined the relationship between EEG severity at each hour of recording and 2 year neurodevelopmental outcome. The outcome was classified as either typical or adverse. We also examined the possibility of developing a Machine Learning (ML) model trained on features extracted from the temporal evolution of the algorithm’s score to predict neurodevelopmental outcomes.
RESULTS: We found an association between EEG score and neurodevelopmental outcome, in agreement with previous studies with a median AUC (area under the receiver operating characteristic curve) of 0.67 (interquartile range: 0.652, 0.685) between 10 and 80 hours after birth (see Image 1). The maximum AUC value was found at 16h from birth (0.722), closely followed by another peak at 24h (0.720). When considering the mean grade across time, we found an AUC of 0.70. Beyond the maximum AUC value, we found a slight but consistent decrease in AUC values over time (see Table 2). Extracting quantitative features from the continuous EEG score and combining these features in a logistic regression model did not improve AUC (0.69) over selecting 1-hour epochs around the 16 or 24 hour timepoint
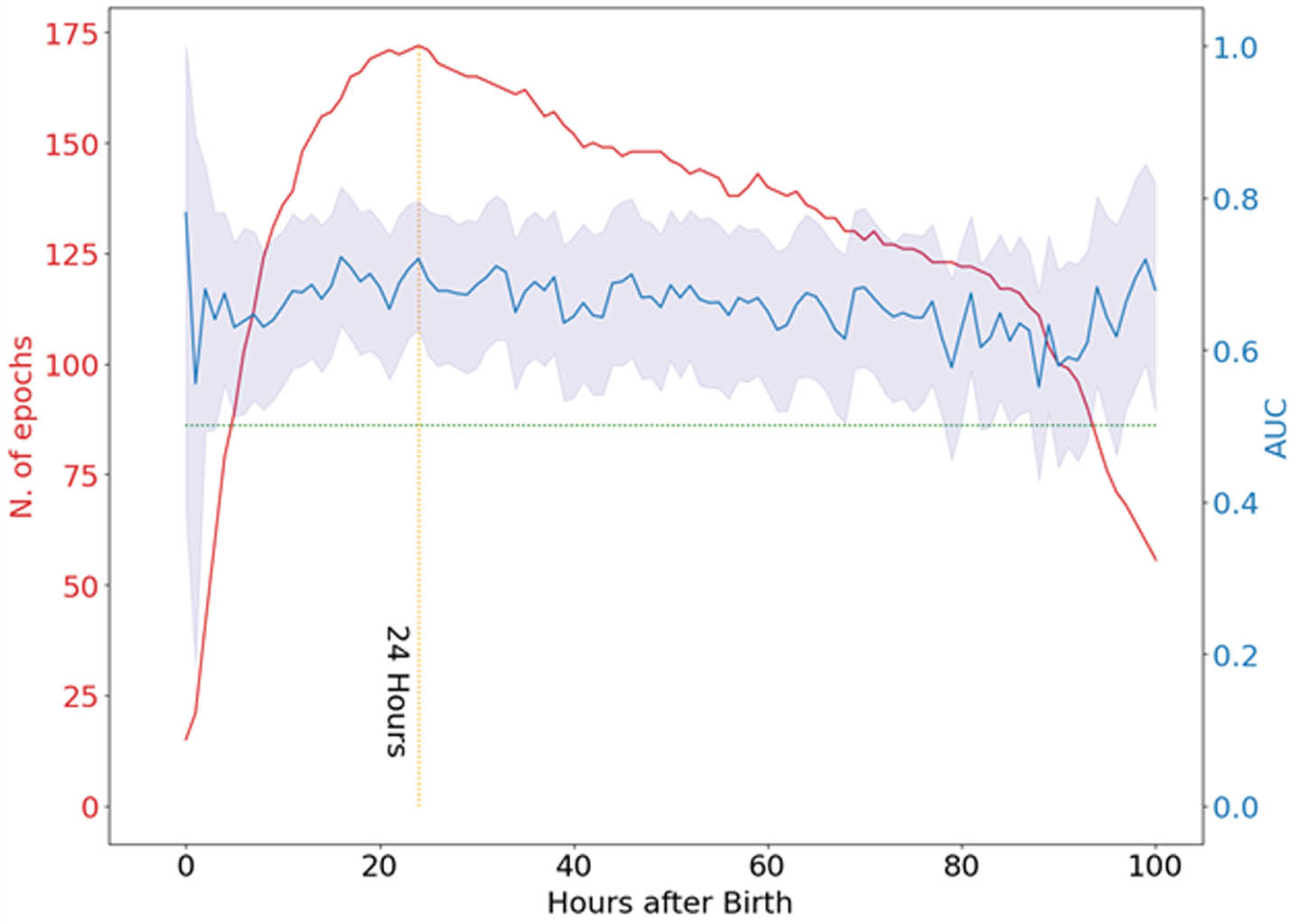
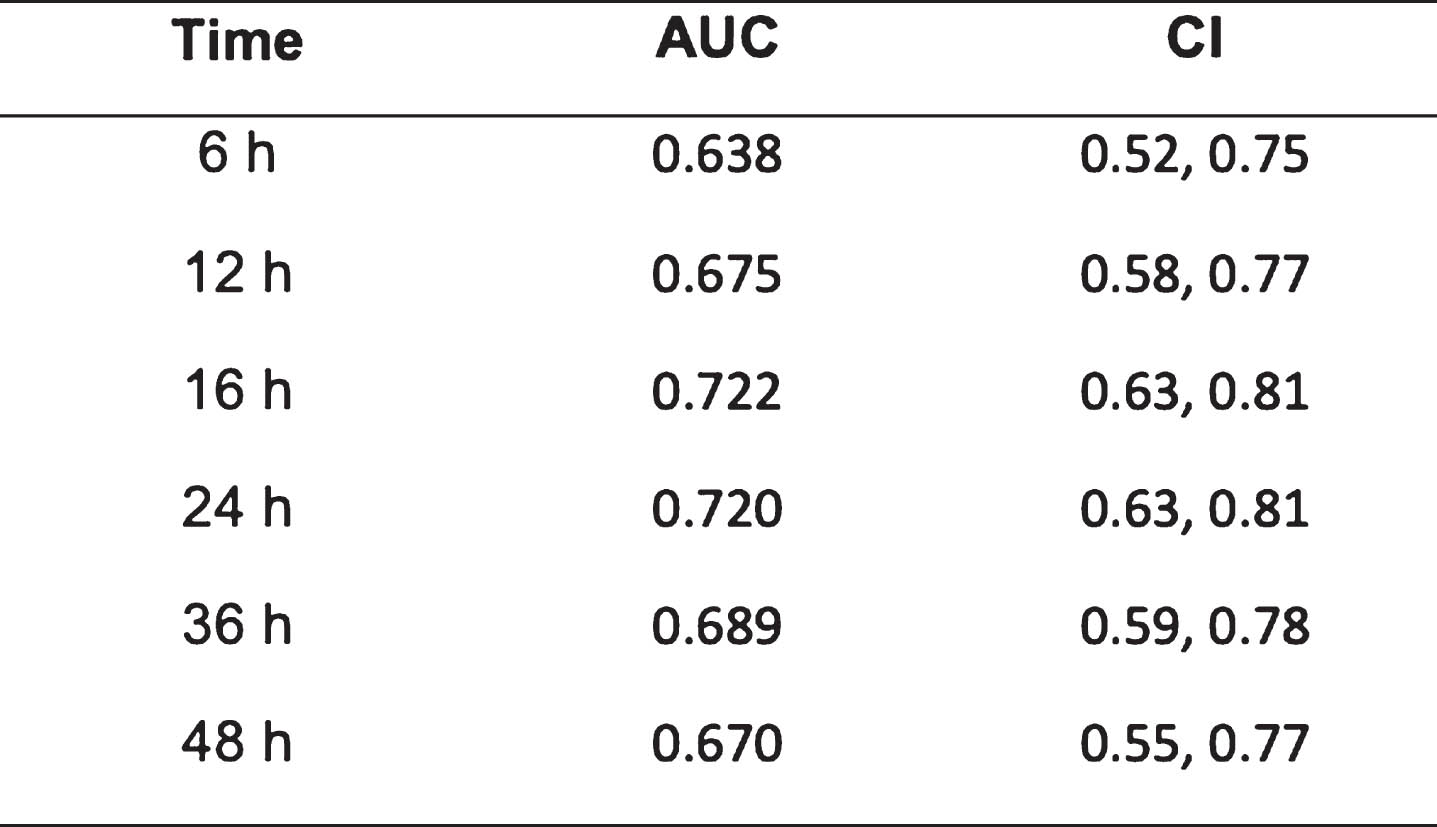
CONCLUSION: The assessment of continuous, long-duration EEG abnormalities did not provide an improvement in predicting neurodevelopmental outcomes at 2 years over the use of 1-hour epochs EEG around 24h from birth.
Status epilepticus and epileptic seizures in newborns with congenital heart diseases
Mariarita Capizzi1, Massimo Mastrangelo1, Giuseppe Isgró2, Angela Satriano2, Alessandro Giamberti3, Alessandro Varrica3, Luigi Moro1, Giada Sauchella1, Marco Ranucci2
1Pediatric Neurology Service, IRCCS Policlinico San Donato, 2Pediatric Cardiac Intensive Care Unit, IRCCS Policlinico San Donato, 3Pediatric Cardiac Surgery Unit, IRCCS Policlinico San Donato
BACKGROUND: The ACNS’s Guideline (1) in 2011 stated that Continuous Electroencephalography Monitoring is mandatory in neonates in different Scenarios conferring High Risk of Neonatal Seizures. Congenital Heart Disease (CHD) is a condition that affects newborns within this population. Nevertheless, recently Feldmann et al. (2) demonstrated that in Europe the EEG monitoring in CHD is infrequent. For this reason, in newborns and infants up to 12 months old we performed a quality improvement EEG study (that is still ongoing) from December 2020 to December 2023 evaluating 1) epileptic seizure and status epilepticus incidence, 2) clinical and cEEG/aEEG characteristics. The data reported here pertains solely to newborns.
METHODOLOGY: at the IRCCS Policlinico San Donato Intensive Care and Pediatric Cardiosurgery Units, a total of 175 neonates (35-44 weeks CA) with CHD needing corrective surgery underwent electroencephalographic (cEEG/aEEG) monitoring, with the following timeframes:
- T0: before surgery: 90 - 180 minutes videoEEG/aEEG recording;
- T1: 12-48 hours after surgery: long-term (12-24 hours) combined cEEG/aEEG recording;
- T2: 7 – 10 days after surgery: 90 - 180 minutes videoEEG/aEEG recording;
- T extra: further videoEEG and or long term cEEG/aEEG recordings, based on clinical needs.
EEG tracings were interpreted using standardized ACNS terminology (1). Epileptic seizures were classified as electroclinical and electrographyc only events according to Pressler et al., (3). Status Epileticus was defined according to Wusthoff (4).
RESULTS: 169 newborns underwent EEG monitoring project. The median duration of EEG recordings was for T0: 2,27 H (1,34- 3,41) , for T1: 15,15 H (4-24,5), for T2: 7 H (3-17), for Textra: 12,89 H (1-23,30). 14/169 (8,3%) had epileptic seizures, 10/14 (71,4%) had electrical only seizures; status epilepticus occurred in 9/14 (64,3%). Seizure onset occured in T0 in 4/14, in T1 in 6/14 in T2 in 3/14, in Textra in 1/14.
CONCLUSION: in Newborns with CHD, seizures and Status Epilepticus can occur both before and soon after surgery as well as in the following days. Since Status Epilepticus and electrographic-only events are overrepresented, being often the only seizure type, electroencephalographic monitoring is mandatory for their identification: most seizures would not have been identified without monitoring.
BIBLIOGRAPHY:
1) Renée A. Shellhaas et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol 2011;28: 611–617.
2) Maria Feldmann., et al. Neuromonitoring, neuroimaging, and neurodevelopmental follow-up practices in neonatal congenital heart disease: a European survey. Pediatric Research (2023) 93:168–175.
3) Ronit M. Pressler, et al. The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia 2021;62:615–628.
4) Courtney J. Wusthoff. Diagnosing Neonatal Seizures and Status Epilepticus. J Clin Neurophysiol 2013;30: 115–121)
Improving neurological assessment and documentation for neonates with hypoxic-ischaemic encephalopathy: a quality improvement project
Mani Singla1, Akpoembele Deborah Madise-Wobo2, Stephanie Redpath2
1University of Alberta, 2University of Ottawa
BACKGROUND: Therapeutic hypothermia (TH) is a standard of care for neonates ≥36 weeks gestational age (GA) with moderate-to-severe hypoxic-ischemic encephalopathy (1). Timely and complete neurological examination in neonates with suspicion of hypoxic-ischemic encephalopathy (HIE) is a key determinant for selecting the appropriate neonates for TH within the first six hours of age or presumed asphyxia insult (2). All patients considered for TH at our hospital are out-born and therefore require referral and transport.
PROBLEM STATEMENT: Quality improvement methodology was used to identify opportunities for improvement in the patient inclusion process for TH. Given the variety of clinical healthcare providers involved in the referral process, we identified an inconsistent approach to the neurological assessment of the patient at the time of first call when seeking to determine appropriate patient eligibility for TH.
METHODOLOGY: In response, we created a standardized neurological assessment tool for documentation at three distinct time-points; the time of the initial call for referral, arrival of the transport team (TT) bedside to the patient and the patient arrival at the tertiary level hospital NICU. Monthly audits, educational and awareness sessions were conducted to reinforce the tool and improvement process. After the period of fifteen months (June 2022-Sept 2023), statistical analysis was performed to assess for the impact for best patient care.
RESULTS: We had 35 neonates referred with a primary diagnosis of HIE. The revised standardized neurological assessment tool was used in 75% of referrals. Almost 70% HIE referrals included a documented neuro-assessment at the time of the first call, 82% had 2nd assessment completed on TT arrival and 84% had 3rd assessment documented on arrival at the receiving hospital. In those who had all three-serial neuro-assessments complete, the MRI results appeared to align with abnormal results in 90% of those clinically determined as moderate to severely encephalopathic neonates. In the incomplete assessment group, approximately 50% of neonates who were classified as moderate to severe HIE, had a normal MRI.
CONCLUSION: Through this quality improvement study, we have demonstrated an improvement in timely documentation process of the neurological examination in neonates with suspicion of HIE, allowing us to ensure appropriate patient selection for TH and considering the risks of treatment to those who may not need it. Determining neonates as eligible or not for TH, at a single time point in time and pre-transport to a level 3 NICU, is exceptionally challenging and requires further review.
BIBLIOGRAPHY:
1. Chalak LF, Adams-Huet B, Sant’Anna G. A Total Sarnat Score in Mild Hypoxic-ischemic Encephalopathy Can Detect Infants at Higher Risk of Disability. J Pediatr. 2019 Nov;214:217-221.e1.
2. Carlton K, Cabacungan E, Adams SJ, Cohen SS. Quality improvement for reducing utilization drift in hypoxic-ischemic encephalopathy management. J Perinat Med. 2020 Nov 3;49(3):389-395.
USP9X-female syndrome: an underrecognized cause of neonatal seizures?
Elena Pavlidis1, Elisabetta Chiodin2, Elisa Boni1, Alex Staffler2, Alessandra Ponta1, Aurora Curró3, Anita Wischmeijer3, Maria Chiara Zanotti4, Federica Verdi5, Lucio Parmeggiani1
1Child Neurology and Neurorehabilitation Unit, Department of Pediatrics, Regional Hospital of Bolzano, Bolzano, Italy., 2Neonatal Intensive Care Unit - Division of Neonatology, Regional Hospital of Bolzano, Bolzano, Italy., 3Clinical Genetics Service and South Tyrol Coordination Center for Rare Diseases, Department of Pediatrics, Regional Hospital of Bolzano, Bolzano, Italy., 4Department of Radiology, Regional Hospital of Bolzano, Bolzano, Italy., 5Obstetrics and Gynecology, Regional Hospital of Bolzano, Bolzano, Italy
BACKGROUND: Variants in USP9X are associated with female-restricted X-linked mental retardation (MRXS99F), a rare syndrome characterized by intellectual disability and a wide variety of additional congenital anomalies (1,2). Although USP9X variants may predispose to seizures, and epilepsy in those patients has been reported in 24% of case (3,1), no clear description of these features is provided and only one case of transient seizures in the first day of life is reported (2). We provide the first description of the electroclinical phenotype in a patient with USP9X female-syndrome showing neonatal seizures.
METHODOLOGY: Serial video-EEG polygraphic recordings of the neonate (5 recordings for a total of 570 minutes, mean duration 114 minutes; minimum 43 minutes maximum 3 hours and 8 minutes) were obtained during the first weeks of life (first EEG at 5 days and last at 18 days after birth).
RESULTS: A diagnosis of ventriculomegaly, verticalized hyppocampi and cerebellar vermis malrotation was made during pregnancy by means of fetal MRI. Karyotype and array-CGH were normal. The female neonate was the first-born of consanguineous parents. No perinatal sufferance was reported. At birth she was dysmorphic, hypotonic, showed poor motricity/sucking and episodes of desaturations. The first EEG showed two brief focal electrographic-only seizures involving the right occipital region and the right temporal region (30 and 88 seconds duration respectively) (Figure 1). The background EEG showed the presence of sleep-wake cycles, a clear differentiation between quiet and active sleep, moderate depression of interbursts intervals (Figure 2). She was treated with a bolus of phenytoine and subsequent maintenence treatment. The serial EEG recordings during the neonatal period showed another short electrographic-only brief seizure involving the right occipital region at day 10 after birth. Brain MRI showed pontal and cerebellar vermis hypoplasia, bilateral hyppocampal malrotation and ventriculomegaly (Figure 3). Ecochardiography detected a patent foramen ovale and an abdomen ultrasound evidenced a urachal cyst. She also showed a left eye coloboma.
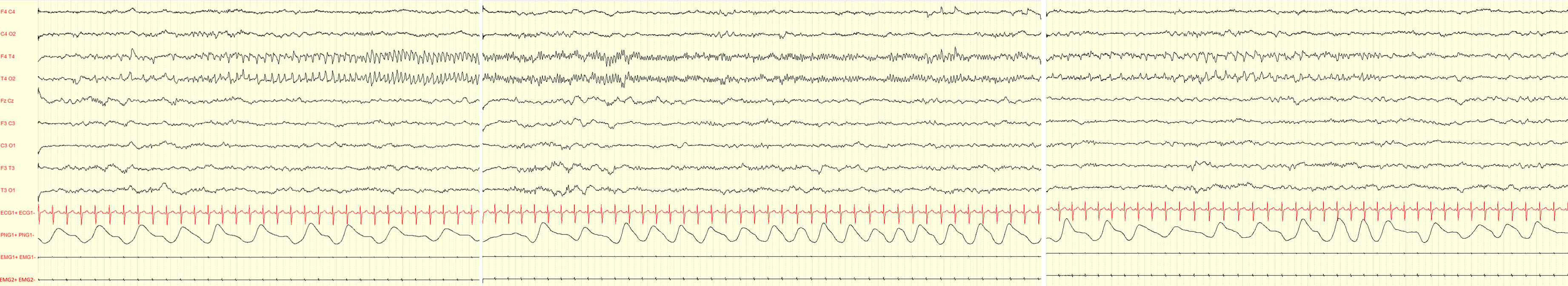
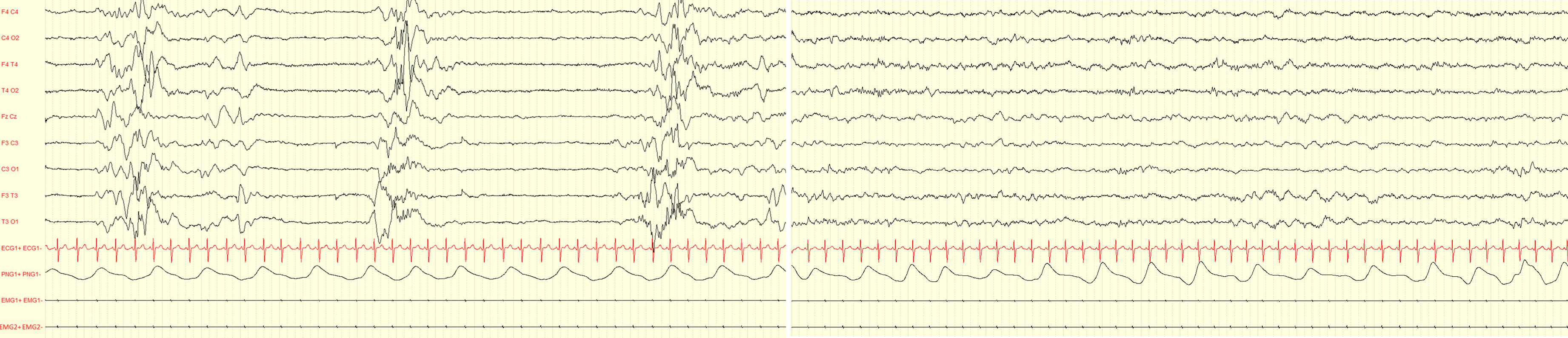
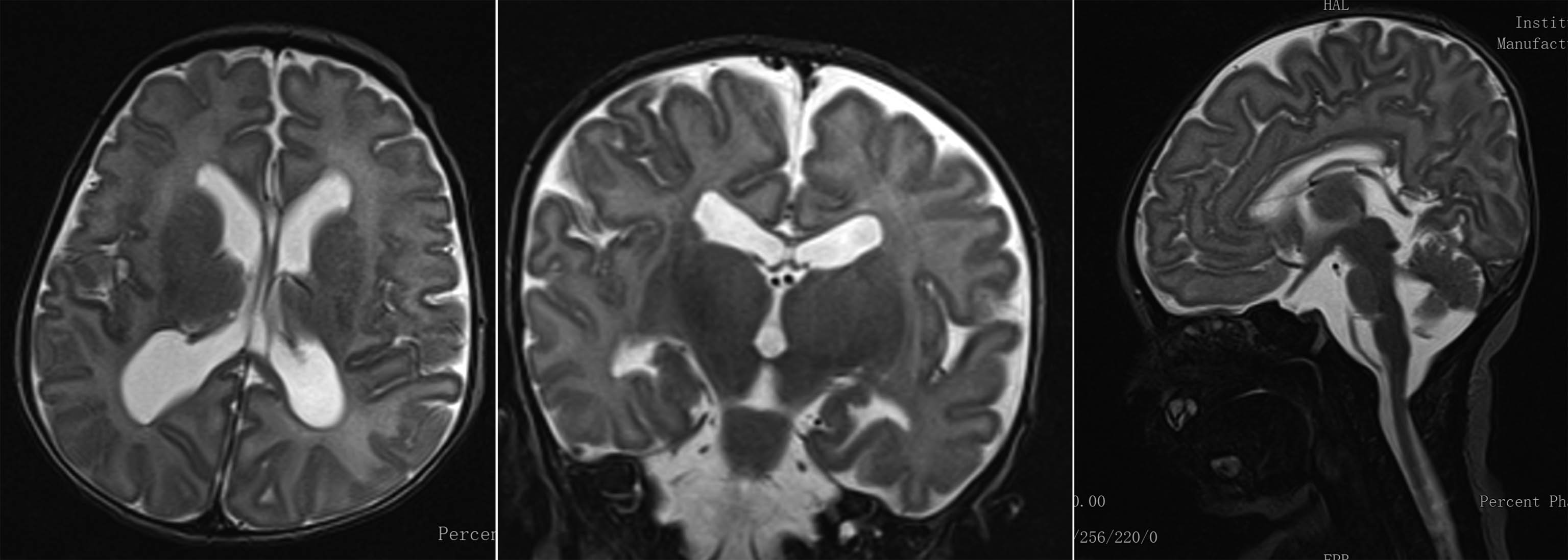
CONCLUSION: Neonates with USP9X variants might experience electrographic-only neonatal seizures. Although USP9X variants are known to be related to epilepsy, existing reports are mainly focused on the intellectual disability and congenital anomalies associated; little is known about seizures and their possible occurrence in neonatal life. The present case further highlights the importance of early EEG recordings in infants at risk for neonatal seizures, including those with brain malformations and/or dysmorphic features.
BIBLIOGRAPHY:
1. Reijnders MR et al. De Novo Loss-of-Function Mutations in USP9X Cause a Female-Specific Recognizable Syndrome with Developmental Delay and Congenital Malformations.Am J Hum Genet. 2016;98(2):373-81.
2. Jolly LA et al. Missense variant contribution to USP9X-female syndrome. NPJ Genom Med. 2020;5(1):53.
3. Paemka L et al. Seizures are regulated by ubiquitin-specific peptidase 9 X-linked (USP9X), a de-ubiquitinase. PLoS Genet. 2015;11(3):e1005022.
Exploring the relationships between arterial blood pressure and quantitative EEG features in extremely preterm Infants
Minoo Ashoori1,2, John M. O’Toole1,3, Ken D. O’Halloran1,2, Geraldine B. Boylan1,3, Eugene M. Dempsey1,3,4, Fiona B. McDonald1,2
1Infant research centre, University College Cork, 2Department of Physiology, School of Medicine, College of Medicine and Health, University College Cork, 3Department of Paediatrics and Child Health, University College Cork, 4Department of Neonatology, Cork University Maternity Hospital
BACKGROUND: Hypotension is an ongoing clinical concern in preterm infants, as it has been associated with adverse short- and long-term consequences. We sought to examine the trend of blood pressure in the first days of life and investigate the relationship between quantitative electroencephalography (EEG) characteristics and mean arterial blood pressure (MABP) in extremely preterm infants.
METHODOLOGY: We analyzed a subset of infants enrolled in the Management of Hypotension in Preterm Infants (HIP) trial (n = 28). All were <28 weeks of gestational age (GA). For each infant, we examined one hour of EEG records at 6-hour intervals and documented MABP for the first 60 hours of life. The EEG signals were pre-processed, first automatically using a MATLAB code, and then screened manually by a neonatal EEG expert for artifacts. Quantitative features were extracted from the EEG signals using previously published methods. Changes in MABP over time were analyzed by repeated measures analysis of variance (rANOVA) examining two factors GA (<26 or >26 weeks), and if they were hypotensive or normotensive. Putative correlations between EEG features and MABP were assessed using partial correlation adjusting for GA.
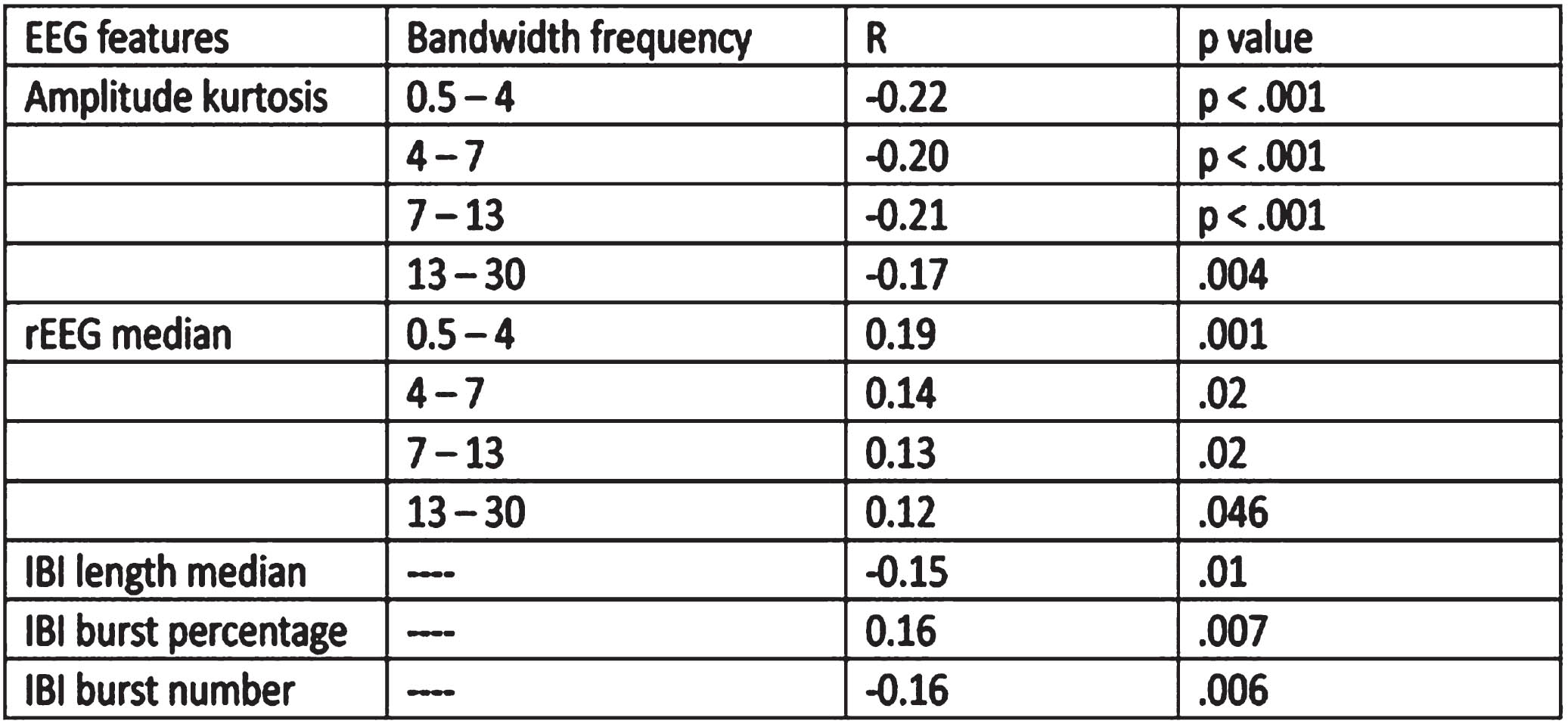
RESULTS: MABP increased in the first hours of life (p= 0.02). We demonstrated weak but significant correlations between MABP and EEG quantitative features, after adjusting for GA. Amplitude kurtosis in all bandwidths, inter-burst interval (IBI) length, and IBI number were negatively correlated with MABP, while range EEG in all bandwidths and IBI burst percentage were positively correlated with MABP. See Table 1.
CONCLUSION: We identified a correlation between MABP and a number of quantitative EEG features in extremely preterm infants, independent of gestational age, suggesting MABP may be an important biomarker of brain activity.
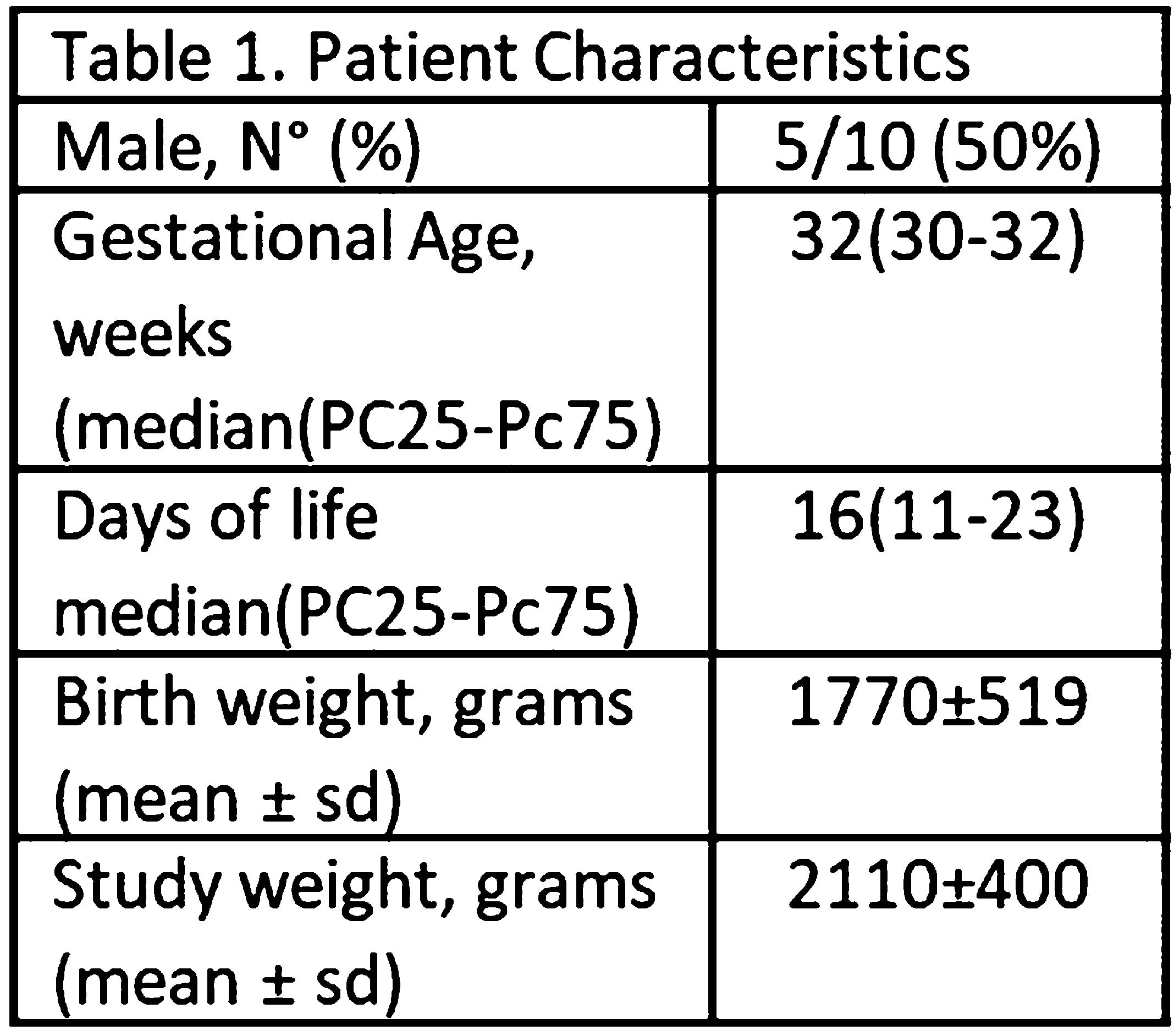
Effects of two rates of mechanical dorsal stimuli on biomarkers in healthy preterm: pilot study
Paulina Toso1, Miriam Faunes1, María Jesús Alvarez1, Stephany Campbell1, Alberto Toso1, Alvaro Gonzalez1
1 Escuela de Medicina, Pontificia Universidad Catolica De Chile
BACKGROUND: Mechanical Kinesthetic stimuli could be an intervention to treat apneas and hypoxic events in preterm infants, however its biomarkers impacts are not well known.
PURPOSE: To describe the effects on vital signs, pain scale and aEEG sleep-wake cycle of dorsal mechanical stimuli at 2 rates in healthy preterm infants
METHODOLOGY: Cross over pilot study on 10 preterm with BW >1550g and GA between 28-33 weeks, stable and without respiratory support (Table 1), from March 2020 to September 2021. A surgical glove connected to a mechanical ventilator was placed under patients back on supine position. The glove was sequentially inflated and deflated with a peak and plateau pressures of 12 and 5 cm H2O. A two hour baseline period without external stimuli was followed by two 2-hours random study periods with glove inflation with a rate of 20 and 40 per minute (rpm), respectively. Infants were continuous monitored and vital signs were saved every 10 minutes; respiratory rate (RR), heart rate (HR), oxygen saturations (Sats) and respiratory synchronicity with the mechanical stimuli (RS). Neonatal-Infant Pain Scale (NIPS) was evaluated and registered every 10 minutes. The presence of Sleep-wake Cycle (SWC) by Amplitude Integrated Electroencephalography monitor (aEEG) was evaluated by two blinded neonatologists, on a delayed basis (Figure 1). Each of 2-hours recording periods occurred between feedings, assuring infants were clean and comfortable. A written consent was obtained from parents. NCT04584814. Descriptive and analytic statistics (95%CI-α=0, 05) were calculated with XLSTAT13.
RESULTS: RR and Sats serial measures demonstrates changes by ANOVA between basal and the two rates of mechanical dorsal stimuli (p<0,0001). At 20rpm stimuli, RR mean decreases in a range of 19-50% of the basal period, and at 40rpm RR decreases 24-40%. Sats mean increases up to 2-7% points during interventions ( Figure 2). 18 %( 0-77) of the study time, the respiratory drive synchronizes with the mechanical stimuli, without differences between 20 or 40 rpm. 94% of the record, babies seem relaxed and quiet (NIPS score ≤2). 9/10 patients don’t alter the basal sleep structure on aEEG during the stimulation protocol, with 85% of interobserver agreement (Figure 3). No adverse events were reported.
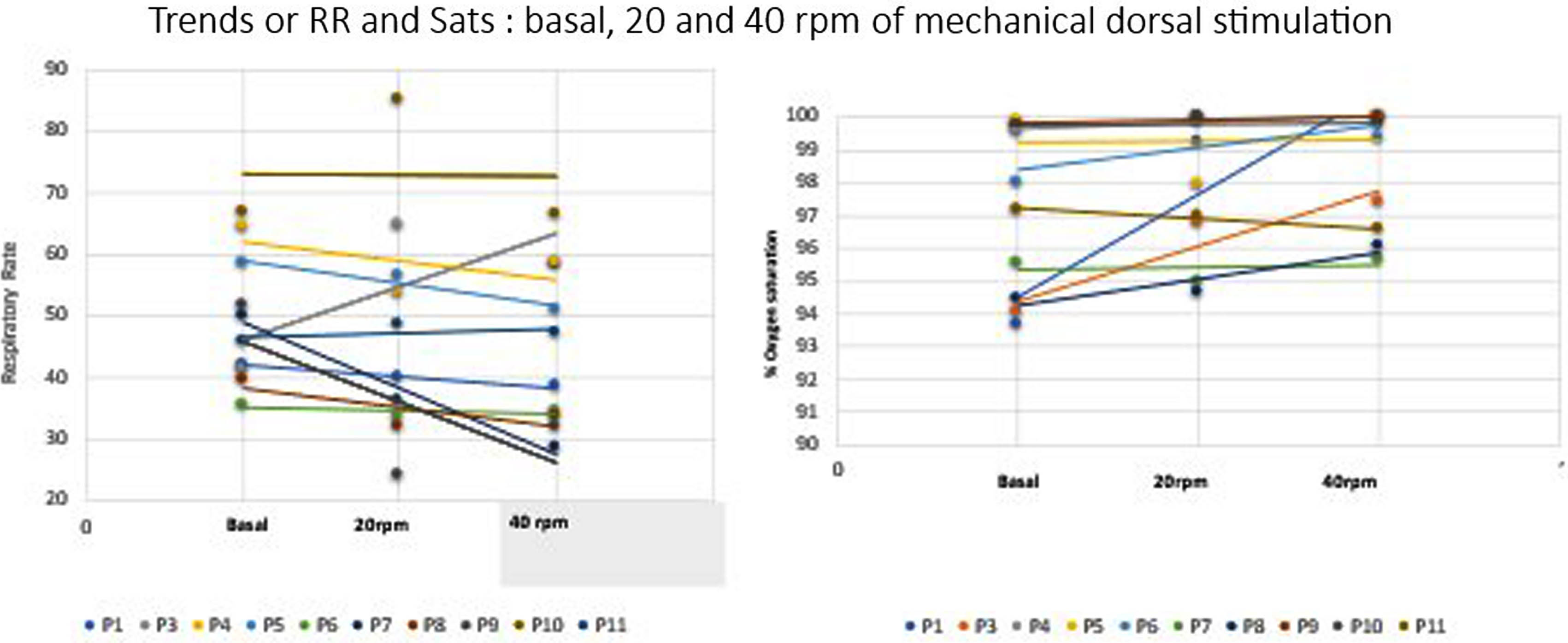
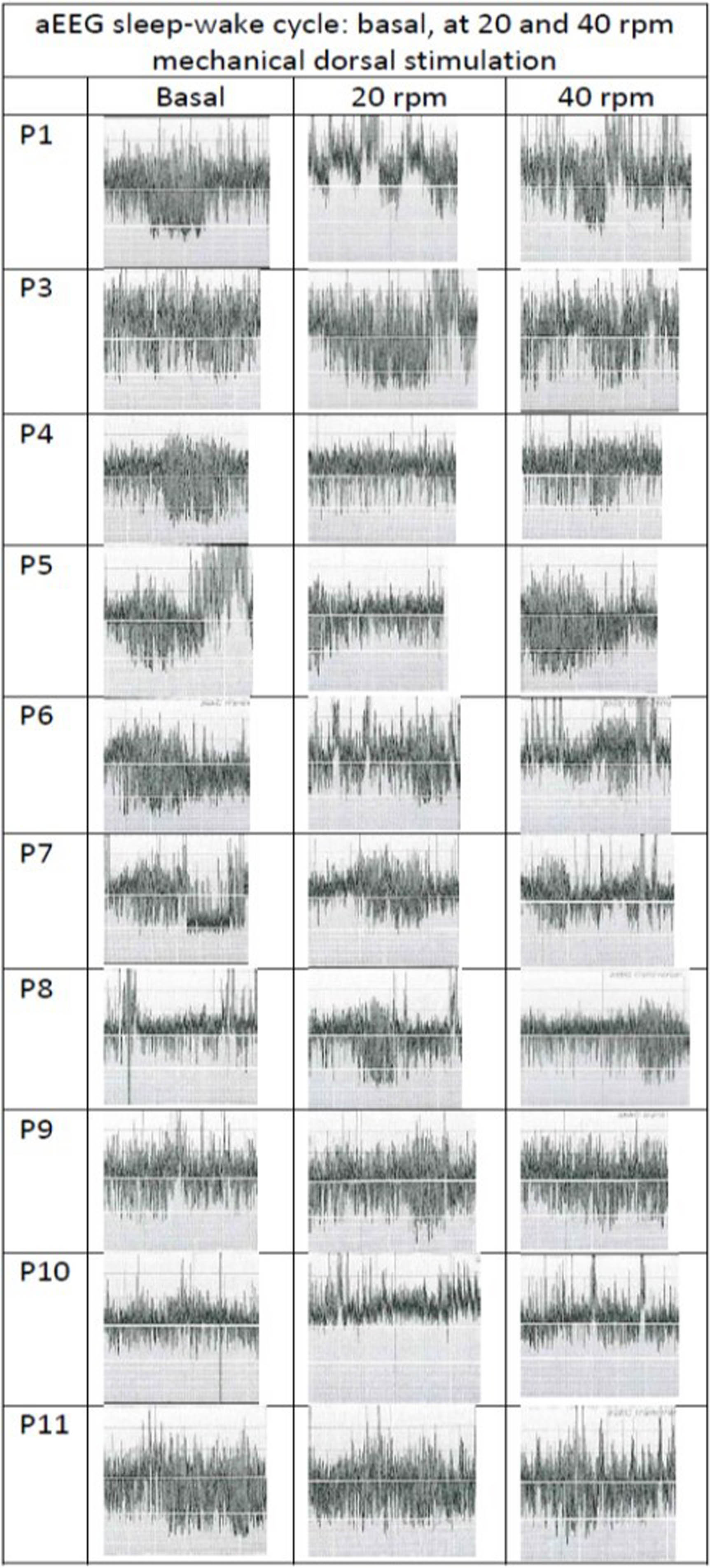
CONCLUSION: Mechanical dorsal stimuli interferes the respiratory drive of this healthy preterm. Main findings were a marked decrease of the basal RR, increase of the basal oxygen saturation and some respiratory synchronization with the external stimuli. On the other hand, this impulse not caused discomfort or altered sleep structure. Next steps should correlate this with respiratory volumes measures.
Retrospective, multicenter study of lacosamide to treat neonatal seizures
Alexandra Santana Almansa1, Jessica Landers1, Nicholas Abend2, Giulia Benedetti3, Catherine Chu4, Andrew Knox5, Shavonne Massey2, Steffany Moen6, Andrea Pardo7, Renee Shellhaas8, Cameron Thomas9, Tammy Tsuchida10, Sonya Wang11, Arnold Sansevere10, Janet Soul1
1Boston Children’s Hospital, 2Children’s Hospital of Philadelphia, 3University of Michigan, 4Mass General Hospital, 5University of Wisconsin School of Medicine and Public Health, 6Sanford Health Medical Group , 7Ann & Robert H. Lurie Children’s Hospital of Chicago, 8Washington University in St. Louis School of Medicine, 9Cincinnati Children’s Hospital Medical Center, 10Children’s National Hospital, 11University of Minnesota
BACKGROUND: Anti-seizure medications (ASMs) for neonatal seizures are off-label except for phenobarbital. Lacosamide has efficacy in infants and is available in intravenous formulation. We aim to evaluate the safety and efficacy of lacosamide for neonatal seizures.
METHODOLOGY: We conducted a 10-center, retrospective study of lacosamide for neonatal seizures. Neonates born between 2008-2020, with seizure onset <44 weeks postmenstrual age (PMA) and lacosamide treatment initiated by ≤48 weeks PMA were included. Clinical data were collected from medical records.
RESULTS: We identified 62 eligible neonates lacosamide (Table 1). Adverse events (AEs) were reported in 53%, none of which were attributed to lacosamide. The most common AEs were cytopenias (15%) and cardiac disorders (8%), including atrial arrhythmia (2%), bradycardia (8%) and cardiac arrest (3%). No instances of ventricular arrhythmias, atrioventricular block, or atrial fibrillation or flutter were observed. No deaths (16) were attributed to lacosamide. The first lacosamide dose (median 5.0 mg/kg, IQR 2.5, 10.0) was given at a median PMA of 40.3 weeks (IQR 39.1, 43.1). Lacosamide was administered as the 4th line ASM or later in 93%. A median of 4 (3,5) ASMs were administered prior to LCM and 55% did not receive another ASM after lacosamide administration. Seizure cessation, defined as permanent end of clinical and/or EEG-proven seizures during the inpatient stay, occurred after lacosamide administration in 37% of neonates, including 21% for whom no other ASM was administered and 16% who had one or more additional ASMs administered. There was a decrease in seizure burden at 4 hours after lacosamide administration, compared with 2 hours before lacosamide administration (p=0.009), as well as 24 hours after lacosamide administration, compared with 24 hours before lacosamide administration (p= 0.007). (Figure 1A) For neonates who did not have seizure cessation, there was nonetheless a decrease in seizure burden 4 hours after lacosamide administration when compared with 2 hours before lacosamide administration (p=0.007). For those neonates who had seizure cessation, when compared with seizure burden 24 hours before lacosamide administration, there was a decrease in seizure burden at 24 hours (p=0.028) and 7 days (p=0.043) after lacosamide administration, as well as at the time of highest daily maintenance lacosamide dose. Lacosamide was continued at hospital discharge for 72% of neonates. Among the 28% with LCM discontinued, the reasons included lack of efficacy (69%), seizure resolution/simplification of ASM regimen (23%), and replacement with a different ASM (8%).
CONCLUSION: Despite high rates of intractable seizures in this neonatal cohort, AEs were not attributed to lacosamide, some neonates experienced seizure cessation after lacosamide administration, and most neonates continued on lacosamide at hospital discharge. These results suggest potential benefit without adverse events and a rationale for future prospective studies.
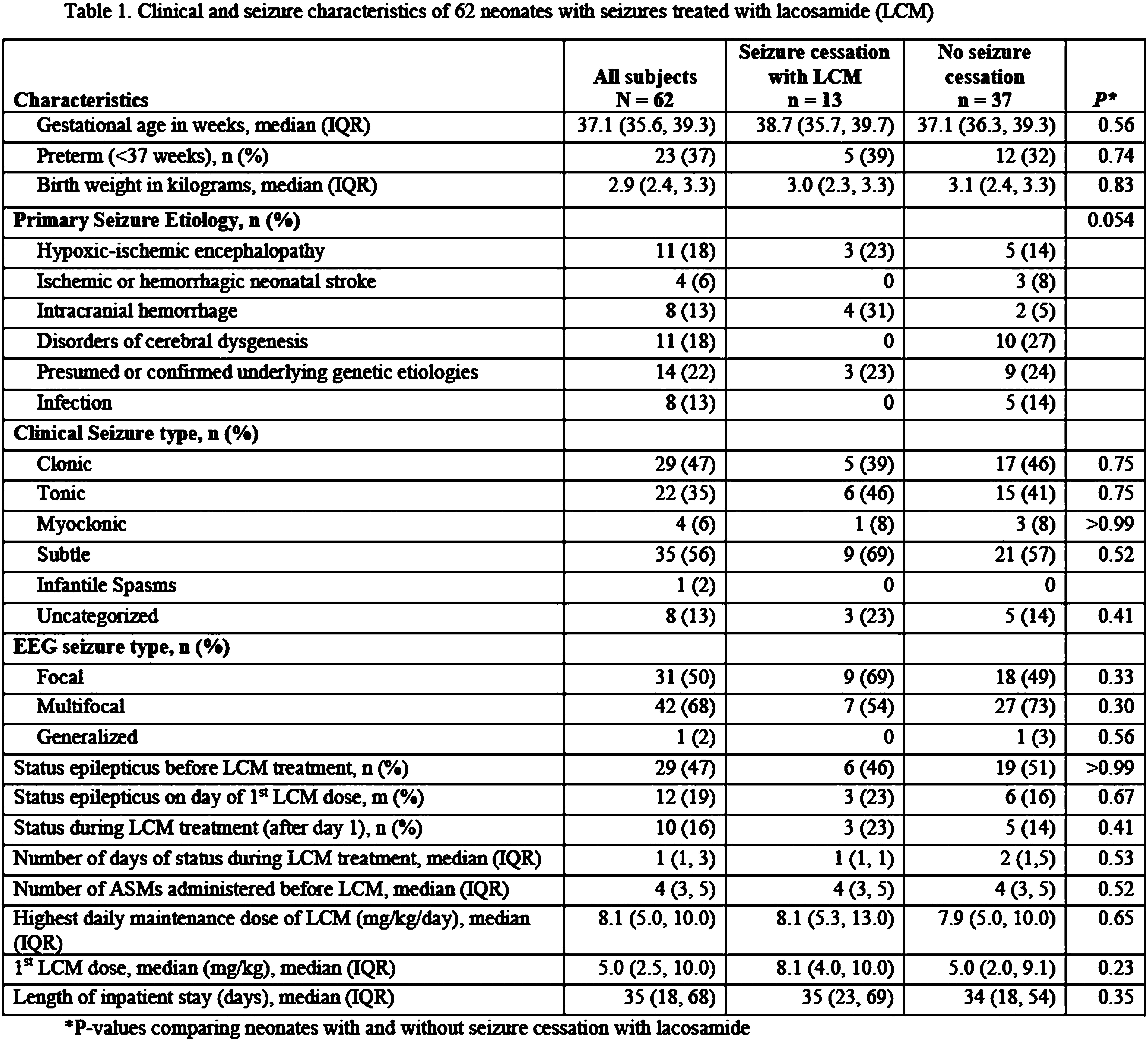
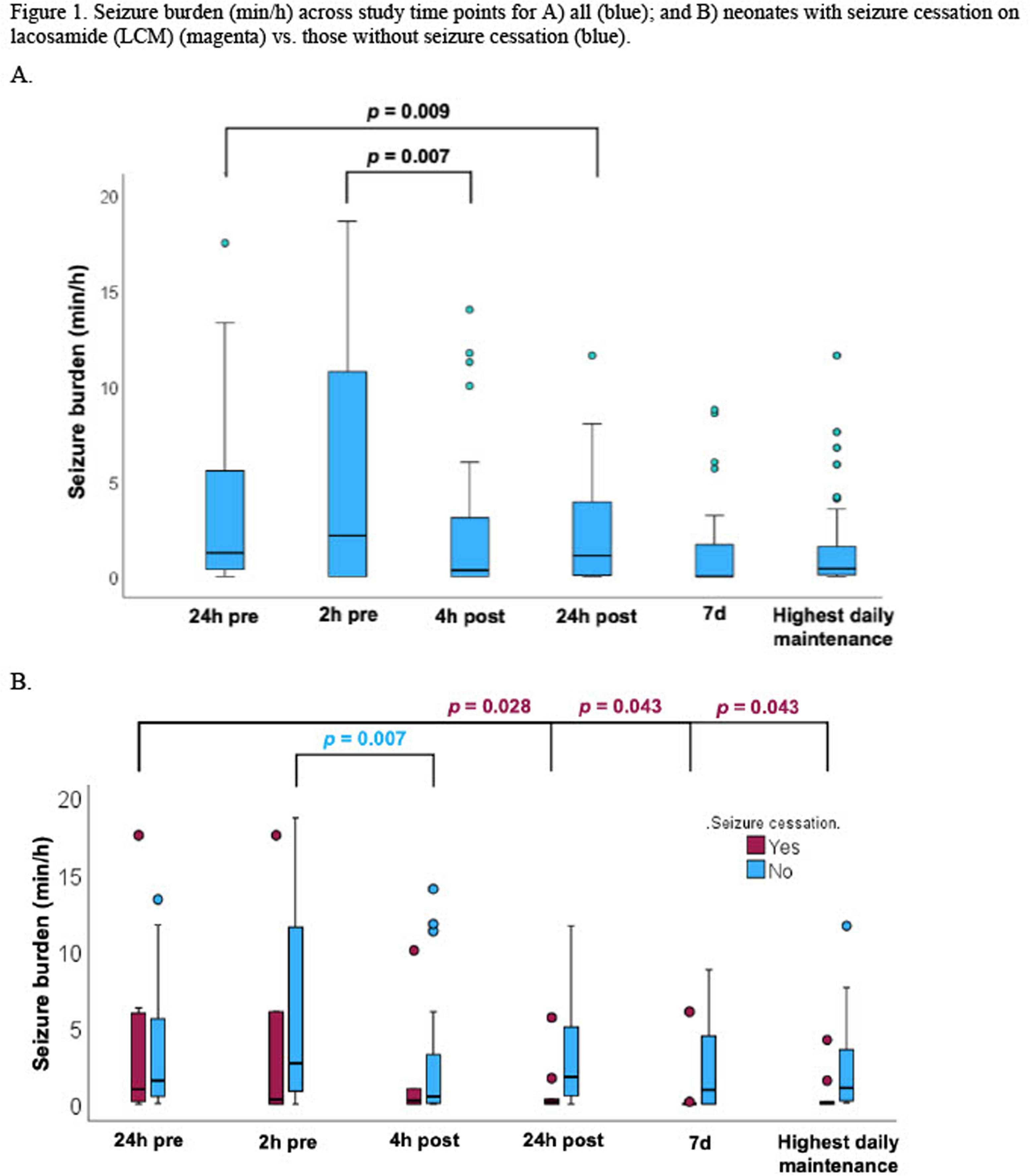
Sleep state organisation at term equivalent age in different neonatal cohorts
Anneleen Dereymaeker1, Tim Hermans2, Katrien Jansen3, Gunnar Naulaers1, Maarten De Vos3
1Neonatology, Department of Development and Regeneration, University Hospitals KU Leuven, 2 KU Leuven Esat Stadius, 3Child Neurology, Department of Development and Regeneration, University Hospitals KU Leuven
BACKGROUND: Understanding the contextual framework of neonatal sleep in the organization of brain networks carries clinical potential. This study describes the sleep architecture of extreme preterm (EP), very preterm (VP), moderate preterm (MP), neonates with congenital heart disease (CHD) and term newborns (TN) using fully automated sleep staging.
METHODOLOGY: 195 multichannel EEGs recorded between 35 and 43 weeks postmenstrual age (PMA), were analyzed with an automated sleep staging algorithm. The sleep staging algorithm consists of five sequential steps: artefact detection, data cleaning, sleep state classification, reliability analysis and hypnogram construction. It classifies each 30s-segment as one of four sleep states: Active Sleep (AS), with low voltage irregular pattern (LVI or AS2) and Active Sleep state 1 (AS I), Quiet sleep (QS) with Quiet Sleep Tracé-Alternant (QS-TA), and Transitional Sleep (TS). The method checks whether predictions are reliable by identifying artefacts and uncertain predictions. The distribution of sleep states, sleep bout durations, percentage of total sleep and unexpected transitions are quantified.
RESULTS: Sleep data from 57 EP, 49 VP, 38 MP, 18 CHD and 33 TN were analyzed. Since there is a significant effect of PMA on sleep state maturation, groups were alle matched for a mean PMA of 39 weeks. Interesting, EP babies and neonates with CHD have comparable sleep architecture. Compared to term newborns, EP, VP and CHD have a lower percentage QS-HVS and a lower percentage of AS1. Compared to moderate preterms, EP also have a lower percentage of AS1. EP and VP have longer LVI sleep bout durations, also reflected in a higher percentage of LVI compared to TN. All groups have la lower percentage of mature QS-HVS and more immature QS-TA compared to TN. Sleep cycles last a little less than one hour, and the sleep time is around 55 minutes. Neonates with CHD have the shortest sleep time/cycle.
CONCLUSION: This quantitative sleep modeling approach contributes to a pipeline for automated reporting of sleep parameters by mapping datasets from different neonatal cohorts, which offers improved diagnostic characterization of neonatal sleep-wake architecture and might help to identify biomarkers of neurodevelopmental outcome.
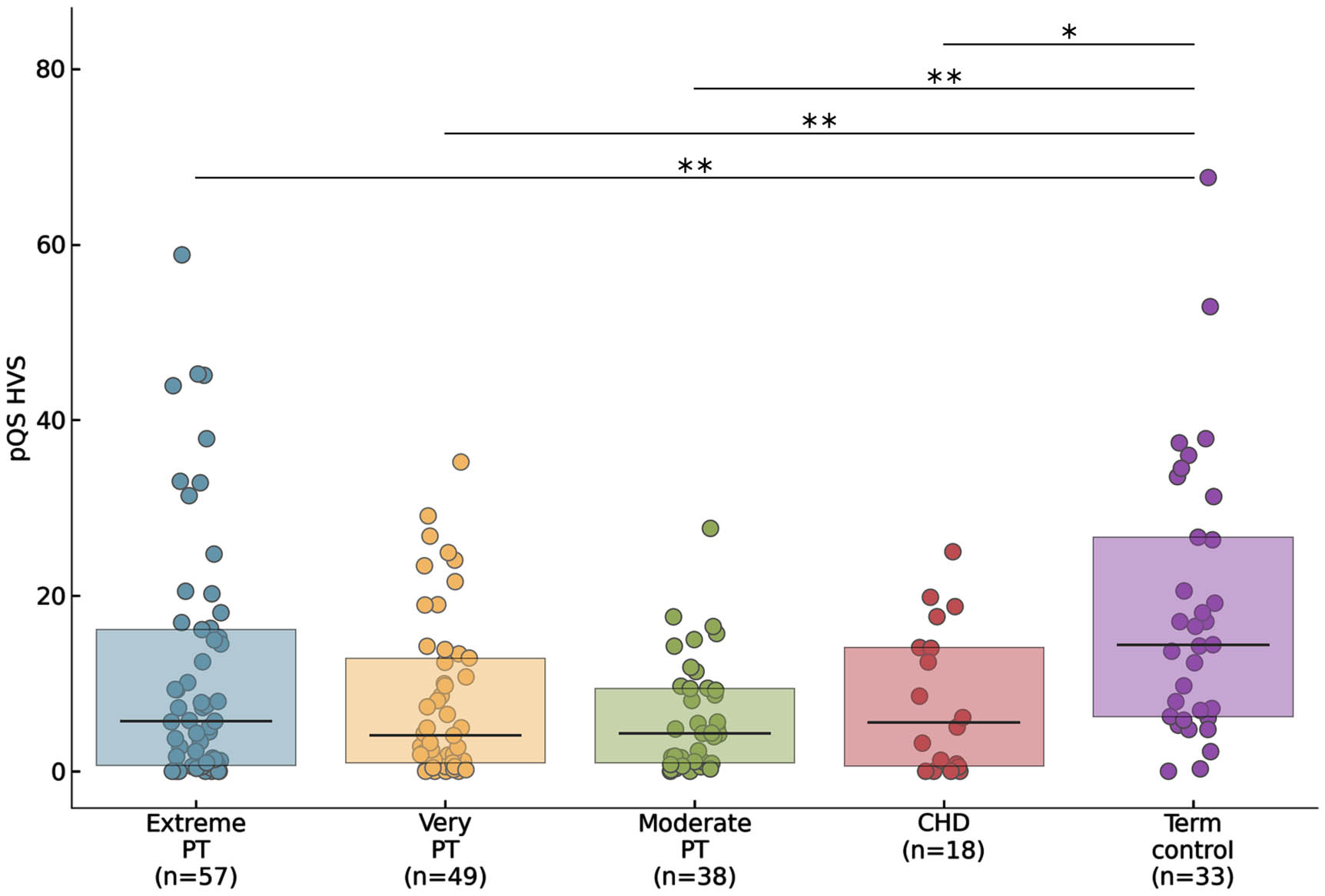
Quantification of discontinuous activity in the EEG of term neonates
John M O’ Toole1, Sean R Mathieson1, Robert Hogan1, Geraldine B Boylan1,2
1Cergenx, 2INFANT Research Centre, University College Cork
BACKGROUND: Abnormal activity in the background pattern of neonatal EEG is indicative of impaired cerebral function. Excessive discontinuous activity is a key abnormality in grading EEG background activity. Quantification of many aspects of discontinuous activity, and its relation to EEG grades, is largely unexplored. We examine periods of discontinuous activity (PDA) across time and EEG channels and explore the relation to EEG background grades.
METHODOLOGY: EEG was collected from historic EEG datasets of term neonates recorded at the Cork University Hospital, Ireland. After review, 1-hour epochs were selected at different time points across the long-duration multi-channel EEG recordings. Each EEG channel was annotated for PDAs by a clinical physiologist with expertise in neonatal EEG. PDAs were defined as periods of low-voltage activity (<25 μV peak-to-peak) with varying duration. Five features were generated from the per-channel PDAs: maximum (estimated from the 95th centile) and median PDA duration, percentage of PDAs, number of PDAs, and a complexity measure of PDA. EEG epochs were graded for major abnormalities in the background pattern across 5 grades (Murray et al., 2009). Grading was performed before PDA annotation.
RESULTS: Eighty 1-hour EEG epochs from 50 neonates were annotated for PDA, resulting in 31,021 annotated PDA events. As illustrated in Figure 1, agreement among channels for PDA was variable: Fleiss’ kappa ranged from 0.041 to 0.863 with a median value of 0.381. This agreement increased with an increasingly abnormal EEG grade: correlation (95% confidence interval) was 0.311 (0.09 to 0.50) with p=0.006. Despite a large disagreement in PDA among channels in some instances, there was no significant difference (p>0.510) in PDA features generated from different channels. See Table 1 for more details. All 5 PDA features were correlated with the EEG background grades, with correlation ranging from r=0.741 for number of PDAs to r=0.875 for percentage of PDAs. This association remained when the features were estimated on a reduced 2-channel montage. See Table 2 for details.
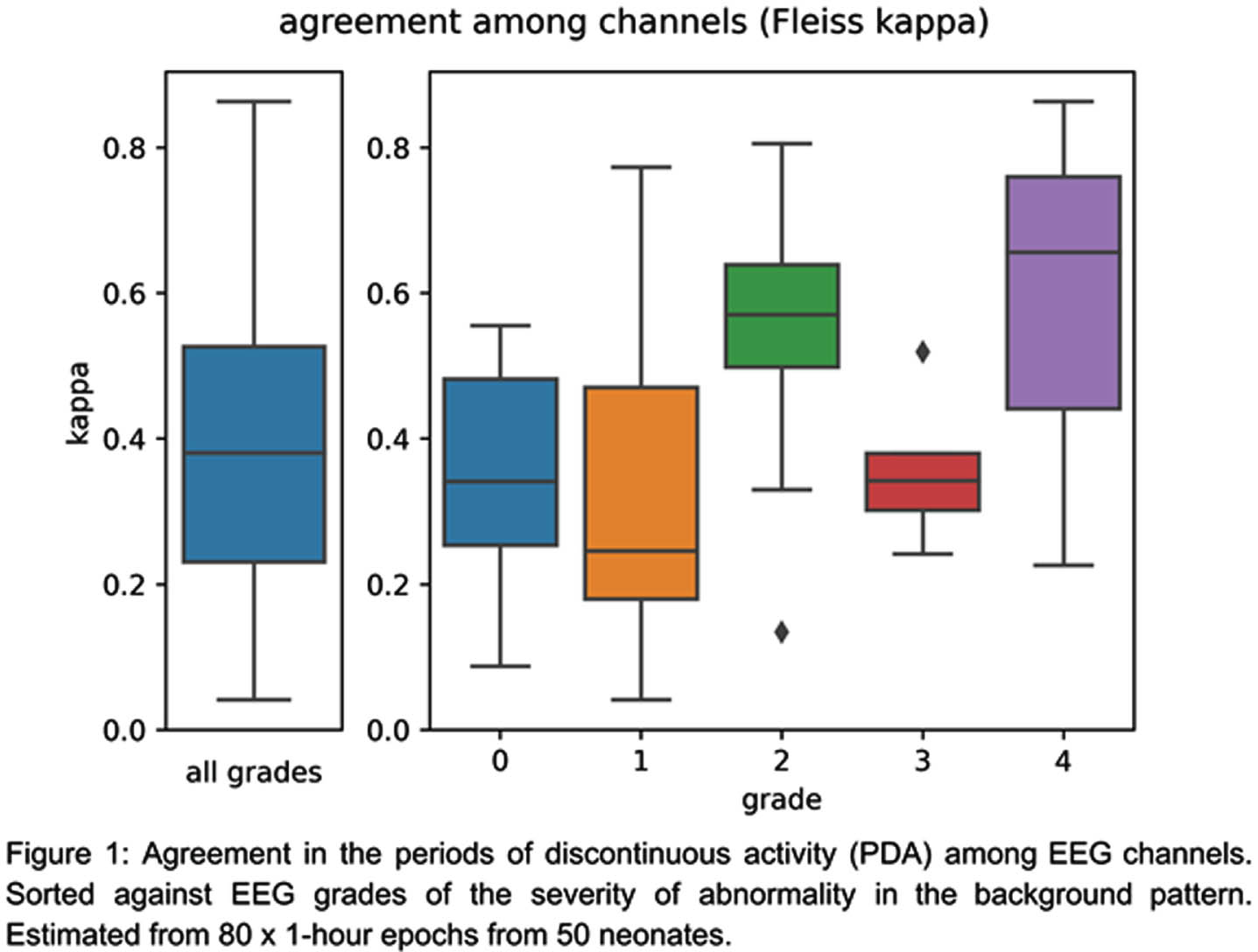
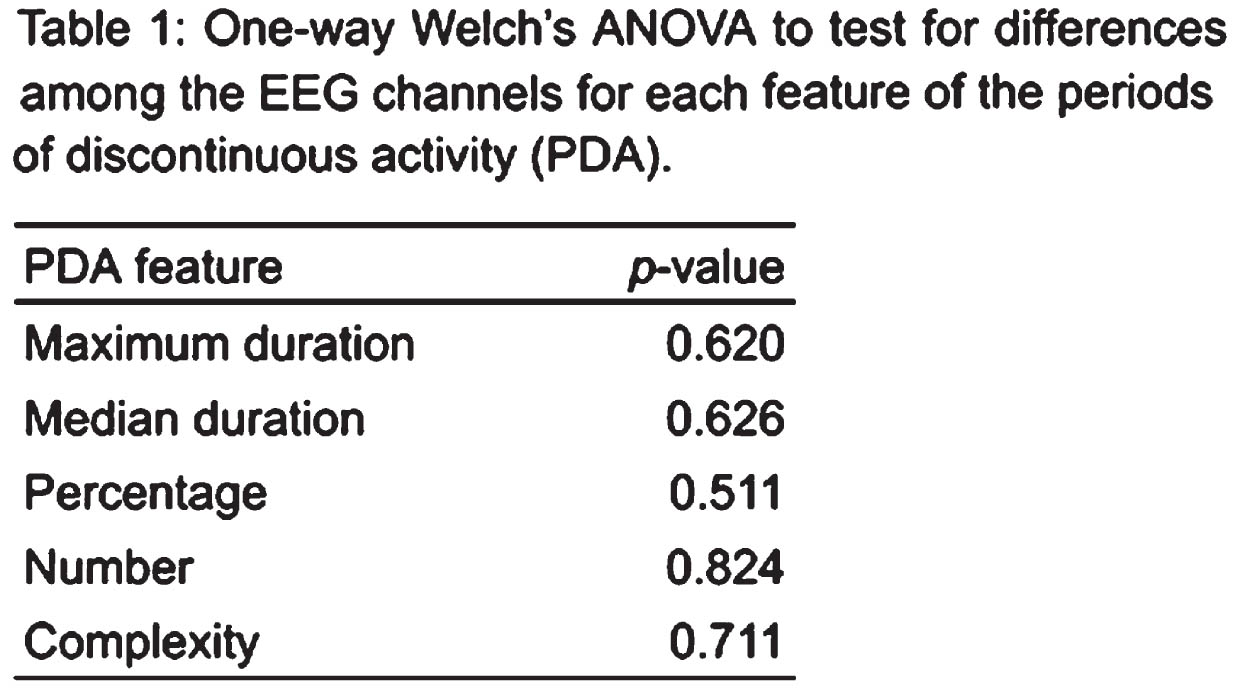
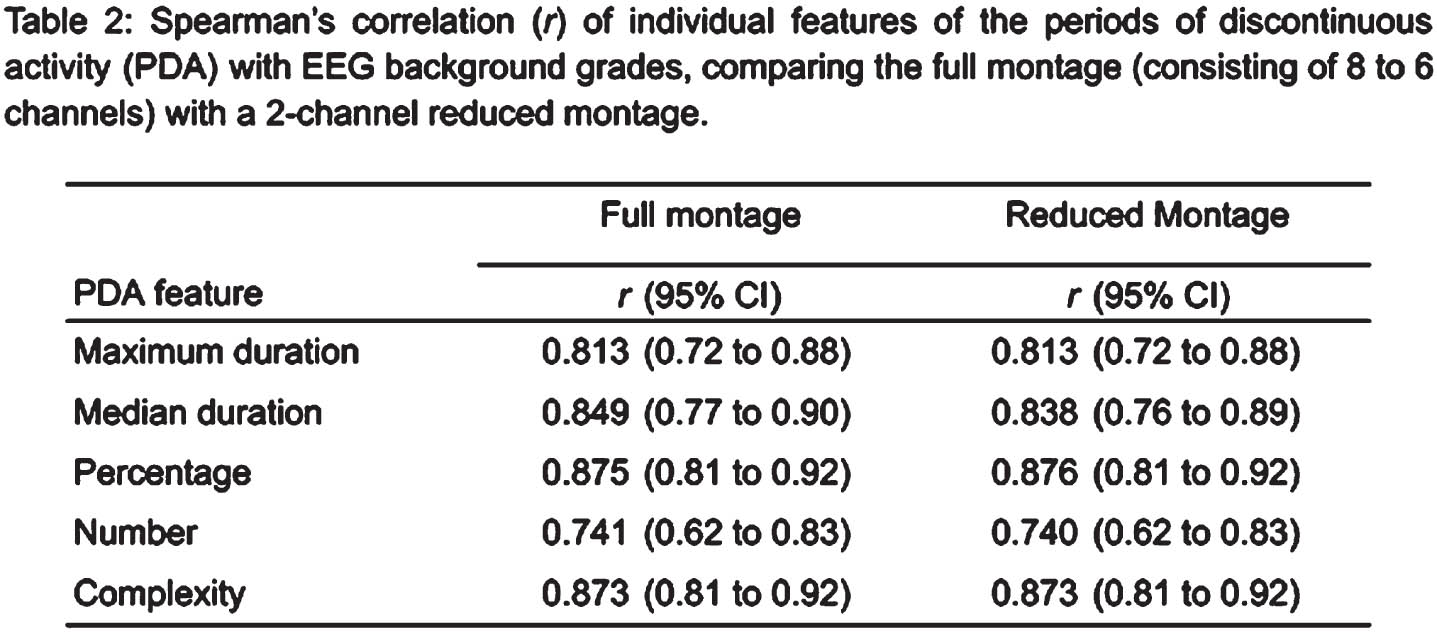
CONCLUSION: Similarity of PDA across EEG channels in term neonates is highly variable, suggesting the importance of per-channel annotations for potential algorithm development or in the evaluation of discontinuities for EEG background grading. A reduced EEG montage may be as informative for EEG grading as a full montage: features summarizing PDA over the 1-hour epoch did not significantly differ among channels and a full-channel and 2-channel estimate of these features were both as strongly associated with the background grades.
REFERENCES:
Murray DM, Boylan GB, Ryan CA, & Connolly S. (2009). Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics, 124(3), e459-e467.
Automated detection of discontinuous activity in neonatal EEG
John M O’ Toole1, Robert Hogan1, Sean R Mathieson1, Aurel Luca1, Geraldine B Boylan1,2, Sean Griffin1
1Cergenx, 2INFANT Research Centre, University College Cork
BACKGROUND: EEG monitoring can indicate cerebral dysfunction in real-time at the cotside. Grading of major abnormalities in the background pattern of EEG is a useful tool to quantify the level of potential cerebral injury. Automated assessment of this background pattern can provide an objective and scalable marker of EEG activity and assist in EEG review. Multiple algorithms have been proposed to classify the EEG grading. We develop an algorithm to detect one specific but important background abnormality, namely periods of discontinuous activity (PDA) in multi-channel EEG.
METHODOLOGY: This study uses existing EEG data recorded from term neonates in Cork University Hospital, Ireland. One-hour epochs of multi-channel EEG, with minimal artefact, were selected for review. A clinical physiologist, with expertise in neonatal EEG, annotated PDAs on a per-channel basis across the entire epoch. PDAs were defined as low-voltage (<25 μV peak-to-peak) activity which ranged in duration from short periods of quiescence to prolonged intervals. Machine-learning methods were applied to develop a model that automatically annotates the EEG for PDAs. The method, using a modern convolutional neural-network architecture, was trained and tested using cross-validation. Four features were constructed from the algorithm output over the 1-hour epochs: median duration and maximum (estimated from the 95th-centile) duration of PDAs, and the number and percentage of PDAs. A subset of the EEG epochs were graded for background abnormalities, using a 5-grade scheme proposed in Murray et al. (2009). The PDA features were combined, using a logistic regression model, to produce a dichotomous classification from the 5 EEG grades.
RESULTS: Eighty 1-hour epochs of EEG were selected for review from 50 neonates. Cross-validation testing performance of the PDA model produced an area-under the receiver operator characteristic curve (AUC) of 0.980 and a Matthews correlation coefficient of 0.793. The features from the PDA predictions were correlated with EEG background grades, as illustrated in Figure 1. Combining these features to detect normal or mildly-abnormal background versus moderately abnormal, severely abnormal, or isoelectric activity resulted in a cross-validation testing AUC of 0.896, with a sensitivity/specificity of 92.0/83.9%.
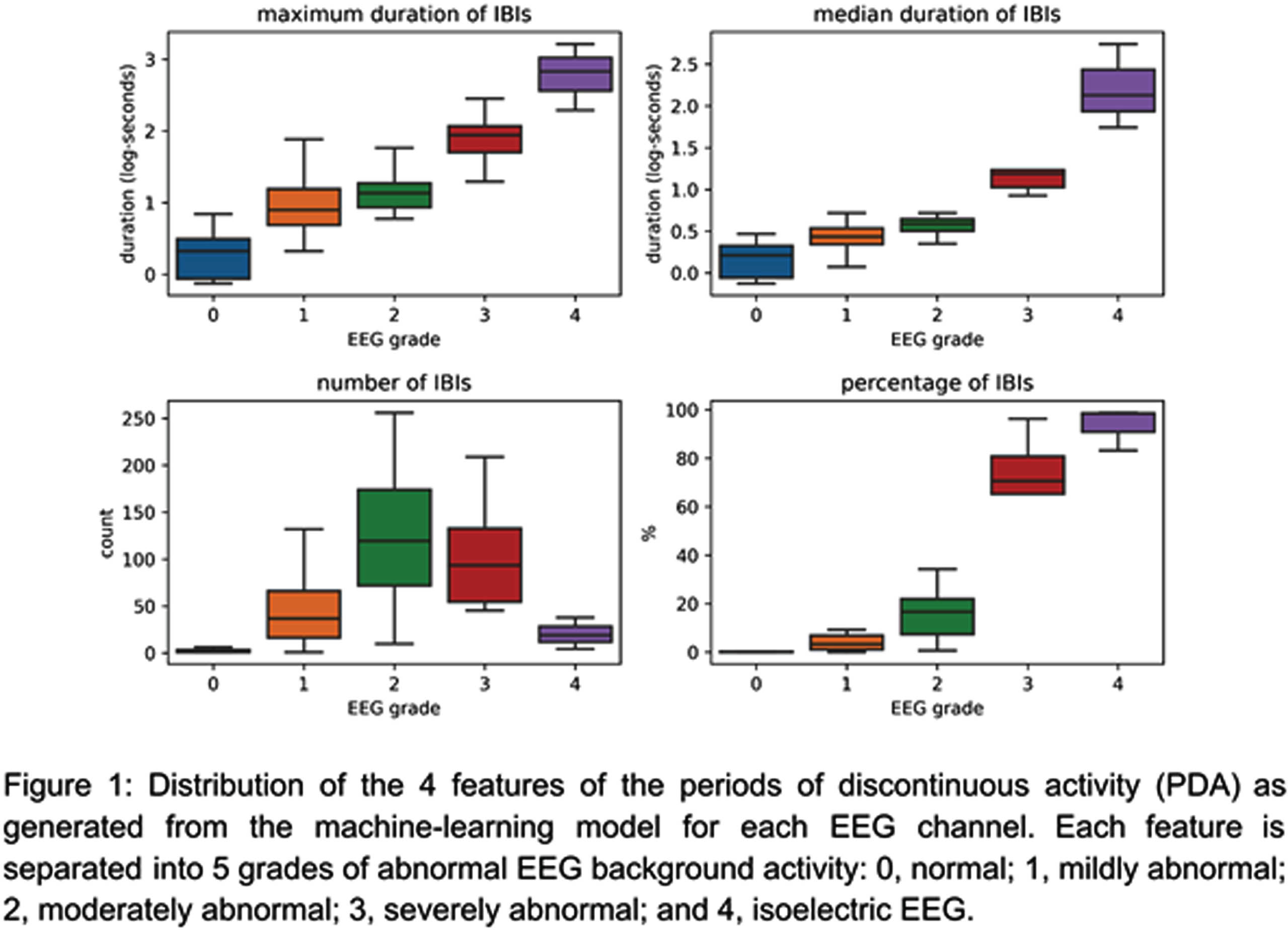
CONCLUSION: Our proposed machine-learning model can detect PDAs with a high level of accuracy (AUC=0.980, MCC=0.793) on a per-channel basis. Features of the PDA are strongly associated with the different grades of background abnormalities and can be combined to differentiate normal/mildly abnormal EEG from the more severe grades.
REFERENCES:
Murray DM, Boylan GB, Ryan CA, & Connolly S. (2009). Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics, 124(3), e459-e467.
Early clinical and neurophysiological multimodal assessment with EEG and SEPs in neonatal hypoxic-ischaemic encephalopathy
Jacopo Proietti1, Sean Mathieson1, Mary Anne Ryan1, Eugene Dempsey1, Elena Ponzetto2, Sofia Ferri2, Stefano Seraglio2, Martina Marangone2, Gaetano Cantalupo2, Brian Walsh1, Geraldine Boylan1
1INFANT Research Centre, University College Cork, 2Child Neuropsychiatry Unit, University Hospital of Verona (full member of the European Reference Network EpiCARE)
BACKGROUND: Early clinical and neurophysiological assessment is crucial to the management of neonates with hypoxic-ischaemic encephalopathy (HIE). Clinical and EEG scores are the most frequently used methods for early stratification of HIE severity. Somatosensory evoked potentials (SEPs) have been shown to be associated with long-term outcome following HIE, but there is less data available on the association of SEPs and HIE severity in the immediate neonatal period. The objective of this study was to describe early neurophysiological changes in HIE, using continuous multichannel video-EEG (vEEG) and simultaneously recorded evoked potentials, complemented by clinical examination at the same stage, and to evaluate their relationships with short-term outcome.
METHODOLOGY: Term neonates with HIE were prospectively recruited in Verona University Hospital and Cork University Maternity Hospital between July 2021 and September 2023, and evaluated after admission to the NICU. A clinical and EEG score were assigned, according to the PRIME stratification system [1] and to Murray grading system [2] respectively. Cortical SEPs were also recorded when possible, and classified as present or absent [3]. Healthy controls were recruited for comparison. An abnormal short-term outcome was defined as development of seizures, abnormal MRI findings or abnormal neurological findings at discharge.
RESULTS: 25 HIE cases were recruited. The median age at vEEG start, followed by neurological examination, was 4.86 hours (IQR 2.54). The median total Sarnat Score was 6 (IQR 6.5, range 0-14/18): ≤4 in 36%, between 5 and 7 in 32%, ≥8 in 32%. The background EEG was mildly abnormal (score 0-1) in 56%, moderately abnormal (score 2) in 20%, severely abnormal (score 3-4) in 24%. SEPs of diagnostic quality were obtained in 64% (16/25) patients with HIE and in 100% (12/12) of controls. Cortical responses were bilaterally present in 100% (12) of control patients, and 87,5% (14) of HIE cases. They were bilaterally absent in 12,5% (2) of HIE cases. The 3 cases with abnormal short-term outcome had total Sarnat score ≥8 and severe background EEG alteration; SEPs were bilaterally absent in 2 of them.
CONCLUSION: A combined clinical and neurophysiological multimodal assessment with EEG and SEPs is feasible and reliable in the earliest hours after birth.
BIBLIOGRAPHY:
[1] Chalak LF, Nguyen KA, Prempunpong C et al. Prospective research in infants with mild encephalopathy identified in the first six hours of life: neurodevelopmental outcomes at 18-22 months. Pediatric Research 2018 Dec;84(6):861-868.
[2] Murray DM, Ryan CA, Boylan GB et al. Prediction of seizures in asphyxiated neonates: correlation with continuous videoelectroencephalographic monitoring. Pediatrics 2006;118:41–6.
[3] Nevalainen P, Marchi V, Metsäranta M, et al. Evoked potentials recorded during routine EEG predict outcome after perinatal asphyxia. Clinical Neurophysiology 128(2017);1337–1343.
Figure 1.
Multimodal neurophysiological monitoring in the NICU, with video-EEG, aEEG, and simultaneously recorded SEPs.
Data collection variability: A global profile of neonatal hypoxic-ischemic encephalopathy registries
Eric Peeples1,2, Ulrike Mietzsch3,4, Eleanor Molloy5,6, Gabrielle deVeber7,8, Khorshid Mohammad9, Janet Soul10,11, Danielle Guez-Barber12, Betsy Pilon13, Vann Chau7,8, Sonia Bonifacio14,15, Jehier Afifi16,17, Alexa Craig18, Pia Wintermark19,20
1University Of Nebraska Medical Center, 2Children’s Nebraska, 3University of Washington, 4Seattle Children’s Hospital, 5Trinity College Dublin, 6Children’s Hospital Ireland, 7The Hospital for Sick Children, 8University of Toronto, 9University of Calgary, 10Boston Children’s Hospital, 11Harvard Medical School, 12The Children’s Hospital of Philadelphia, 13Hope for HIE, 14Lucile Packard Children’s Hospital, 15Stanford University School of Medicine, 16IWK Health Centre, 17Dalhousie University, 18Maine Medical Center, 19McGill University, 20Montreal Children’s Hospital
BACKGROUND: Neonatal encephalopathy (NE) – including hypoxic-ischemic encephalopathy (HIE) – is prevalent worldwide and is associated with significant morbidity and mortality. Registries can provide hypothesis-generating data and monitor trends in outcomes (1-3), but variability between registries limits data pooling across registries for stronger analyses. This study sought to survey neonatal NE/HIE data registries to assess similarities and variability and support future harmonization of standard data elements.
METHODOLOGY: Cross-sectional study collecting data elements from current or recent NE/HIE registry data forms. Registries were identified by literature search and email inquiries to investigators worldwide. Data were categorized by group consensus. Descriptive statistics summarized characteristics and variability in data elements between registries.
RESULTS: 1100 total data elements were abstracted from 21 registries, representing 14 countries, including 3 middle-income countries (Table 1). Registries had a median of 106 distinct data elements per registry (range 59-367). Table 2 demonstrates the element stratification by category. The most commonly collected data were related to pregnancy, hypothermia therapy, and short-term hospital outcomes. Least consistently collected data were non-acid/base laboratory values. Only 4 of 1100 (0.4%) variables were consistently collected in every registry (the top 20 data elements are shown in Table 3). Even when elements were collected by multiple registries, the format of the individual data element (numeric, categorical, free text, etc) often differed across registries. Eighteen of 21 (85.7%) registries included at least one free text response element, with a median of 2 free text response elements (range 0-18) per registry. Only 3 of 21 (14.3%) registries included developmental follow-up fields and 2 others (9.5%) linked their data to a separate follow-up registry.
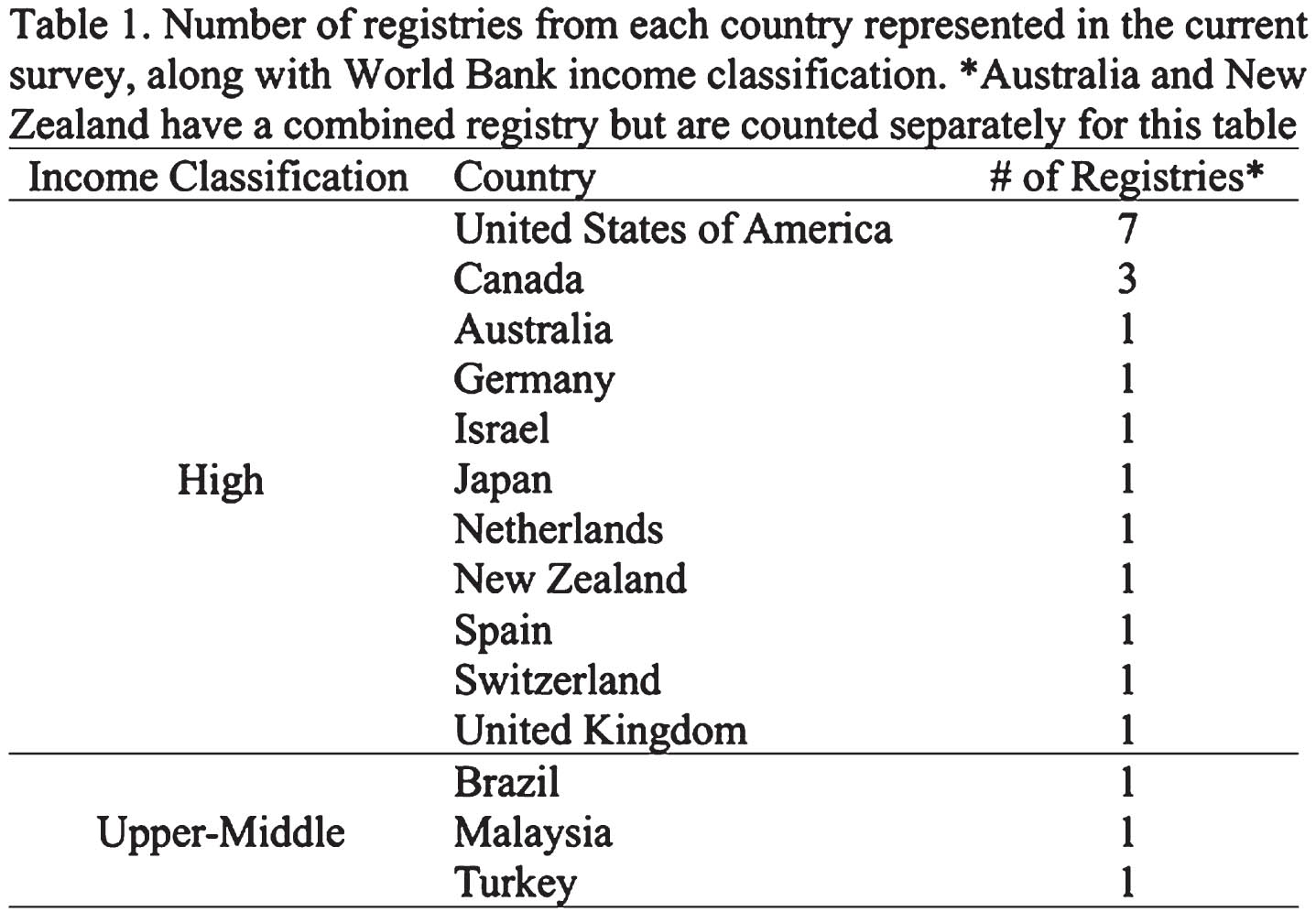
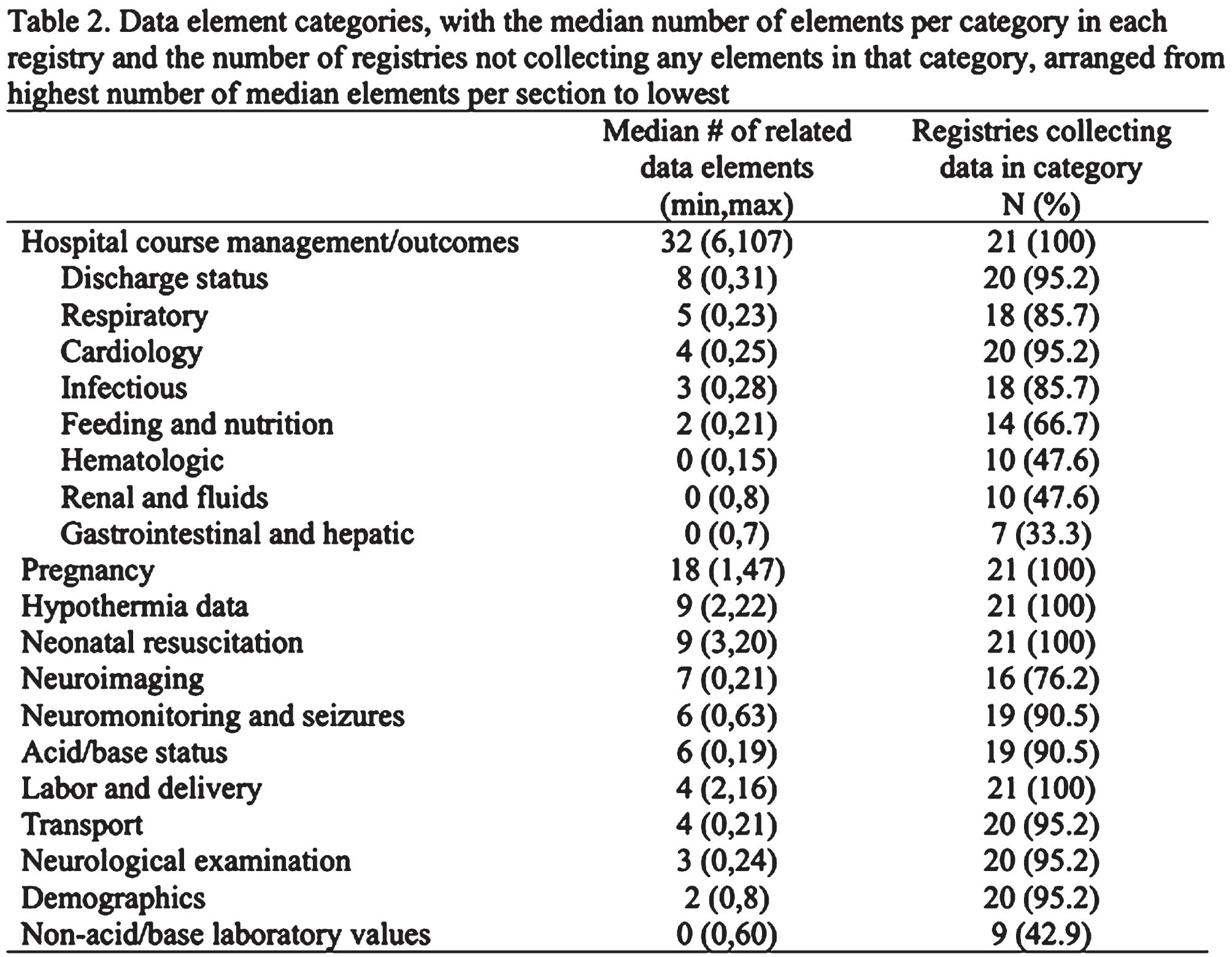
CONCLUSION/IMPACT: This study identified many ongoing NE/HIE registries around the world and demonstrated considerable variability in the number, type, and format of data collected. Future attempts to develop standard data elements and harmonize data collection will be crucial to facilitate collaboration between registries.
Eye mirror of the brain: from a pale eye reflex to an atypical stroke
Sara Monaco1, Pier Luigi Bacchini1, Ferdinando Avellis2, Serafina Perrone3
1Vaio Hospital, 2Vaio Hospital, 3Parma Hospital, 4Parma Hospital
BACKGROUND: The American Academy of Pediatrics recommends red reflex assessment in the neonatal period (1). The red reflex has become a screening test in the Emilia Romagna Region from 2018 (2). The examination appears useful in the early diagnosis of ocular diseases (cataracts, glaucoma, retinoblastoma, retinal abnormalities), but also as the first sign of a neurogenic dysfunction (3). In a first-level hospital, performing an ultrasound examination of the brain is essential for good assistance to newborns.
CASE REPORT: C.A., male, was born from spontaneous delivery at 37 weeks, weighing 2740 g, and without malformations. At birth the newborn required ventilation with NeoPuff (FiO2 0.21) for 1 minute (Apgar at 1’= 5, 5’= 7, 10’=9). There were no criteria for Hypoxic-Ischemic Encephalopathy. On the third day of life, in a state of apparent well-being, the newborn showed an ocular reactive asymmetry: in the right eye normal red reflex, instead in the left eye a pale red reflex and a fixed pupil unresponsive to light stimuli. Transfontanellar ultrasound showed a hemorrhagic hyperechoic area of the brain parenchyma involving the right temporal lobe and insula. Transferred to a second-level hospital, the ophthalmological and RETCAM examination identified signs of thrombosis of the retinal vein. MRI and MRI angiography diagnosed edema of the optic nerve in the left hemisphere and the alteration of the flow of the arterial segment operculum temporal-insular in the right hemisphere and associated venous stasis. The EEG showed no alterations consistent with seizures.
RESULTS: KS immunoreactivity occurs in both radial and tangential migratory pathways of neuroblasts as early as 9wk and expressed throughout the remainder of gestation, before neuronal maturation or synaptogenesis. KS also occurs early in long and short axonal fascicles before axonal growth into fascicles. The earliest telencephalic grey matter structure to exhibit KS is the globus pallidus, followed by the thalamus. Synaptophysin reactivity is absent or minimal and neuronal protein markers immature when KS first appears.
CONCLUSION: The diagnosis of stroke in the neonatal population can be complicated by the absence of localization signs especially if the location is atypical. The red reflex test is a screening test for eye diseases. Still, it can be very useful for the early recognition of neurological pathologies involving the eye. The screening should be promoted, including through courses in collaboration between ophthalmologists and pediatricians. The diagnostic gold standard for ischemic or hemorrhagic stroke is MRI (4). However, transfontanellar ultrasound remains valid, as an exam that is easily performed, has good sensitivity, can be repeated in the short term, and has early identification at least as regards hemorrhagic lesions (5-6). Delays in diagnosis of stroke could be reduced by increasing the use of brain ultrasound among medical staff members in the first-level hospital, where magnetic resonance imaging is not immediately available.
Catecholamine exposure supports high-grade intracerebral haemorrhage and death in extremely preterm infants
Ricarda Will1, Christian Gille1, Bernd Beedgen1, Christoph E. Schwarz1
1Clinic for Neonatology, University Hospital Heidelberg
BACKGROUND AND OBJECTIVE: Intracerebral haemorrhages (ICH) represent a complication in preterm infants (PI) relevant to mortality and neurological outcomes [1]. The incidence of high-grade ICH in extremely PI varies internationally (5 to 52%) [2]. Pathophysiological factors include the immature germinal matrix and immature cerebral autoregulation [3]. Therefore, factors influencing cerebral blood flow, such as arterial hypotension or its treatment with catecholamines, represent key risk factors [4-6]. In contrast to international practice, the study center follows a proactive circulatory management approach (e.g., target mean arterial pressure of 30 mmHg on day 1 of life) [7]. The aim of this study was to investigate the association between catecholamine therapy and the combined endpoint of ICH ≥ grade 3 or death in extremely low gestational age (GA) or birth weight (BW) PI.
METHODOLOGY: This monocentric retrospective study included all PI born in the perinatal center from 2010 to 2022 with BW < 1000 g or GA < 28 weeks of gestation. Infants who died in the delivery room or primarily received palliative care and them with severe underlying diseases (e.g., hydrops fetalis) were excluded. Statistical analysis was performed using binary logistic regression in relation to the combined endpoint (significance level 0.05).
RESULTS: A total of 638 PI with a median GA of 26.3 weeks (IQR 24.9; 27.7) and a median BW of 790 g (IQR 600; 950) were included, of which 332 infants (52.0%) were exposed to catecholamines. ICH of any grade occurred in n=156 (24.5%) of infants (ICH Grade 1, 2, 3, 4: n=85 (13.3%), n=31 (4.9%), n=12 (1.9%), n=28 (4.4%)). Mortality rate was 6.4% (n=41). The combined endpoint ICH ≥ grade 3 or death occurred in 9.1% (n=58) of cases. Catecholamine exposure was significantly associated with the combined endpoint (odds ratio (OR) 9.3 [95% CI 3.9-22.0], p<0.001). Adjusted for the variables gender, multiples, BW and GA, this association remained significant (aOR 4.9 [95% CI 2.0-12.2], p<0.001).
CONCLUSION: Comparable to the current literature an independent association between catecholamine exposure and the combined endpoint ICH ≥ 3 / death was shown in our study cohort [6]. While a higher proportion of 52% of infants received catecholamines, the rate of high-grade ICH does not appear elevated in international comparisons [2, 6]. The exact relationship between catecholamine therapy and ICH should be subject of prospective studies in the near future.
BIBLIOGRAPHY:
[1] Vohr et al Pediatrics 2000
[2] Siffel et al J Perinat Med 2021
[3] Gilard et al J Clin Med 2020
[4] Garvey et al Semin Perinatol 2022
[5] Szpecht et al Childs Nerv Syst 2016
[6] Abdul Aziz et al J Matern Fetal Neonatal Med 2020
[7] Dempsey et al J Perinatol 2006
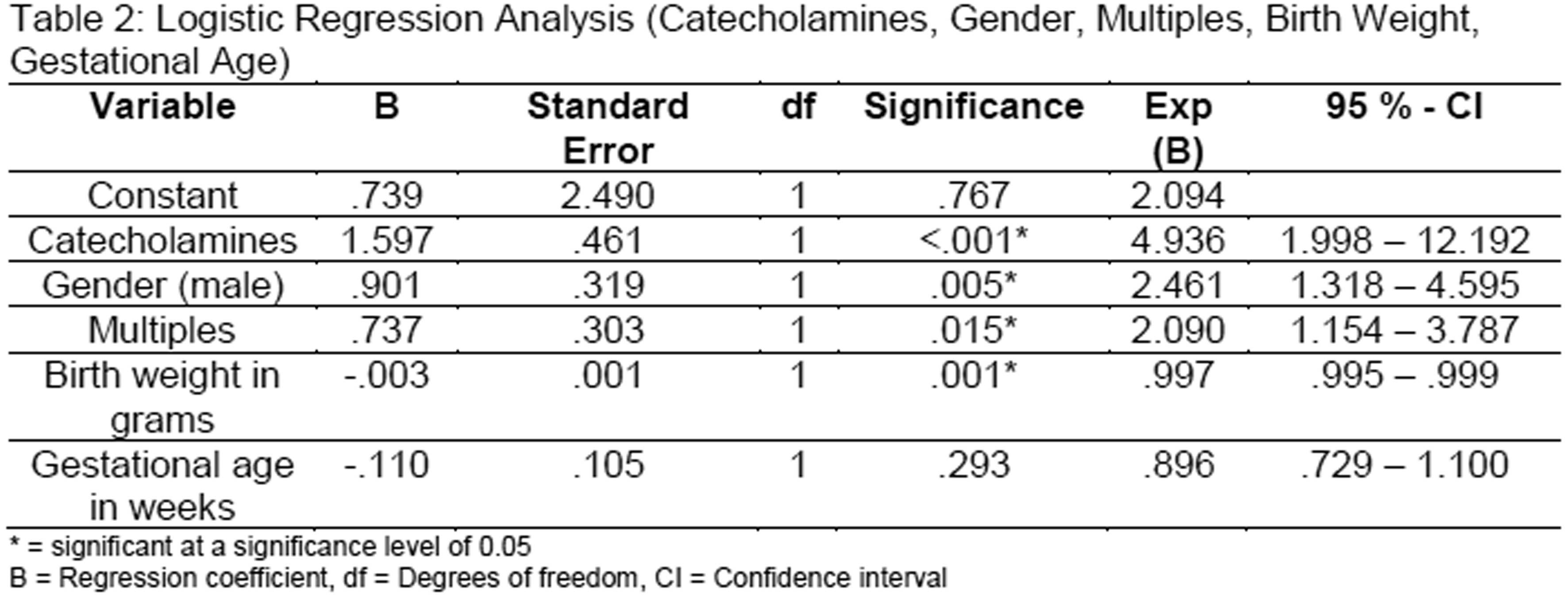
Irish neonatal encephalopathy PhD training network (NEPTuNE) poster presentation
Beth Corcoran1, Eleanor Molloy1
1Trinity College Dublin, 2Infant Centre, UCC, 3University of Galway
INTRODUCTION: Neonatal encephalopathy (NE) is one of the most common causes of neonatal brain injury in full-term infants. For every baby that dies from NE, another will survive with significant lifelong disability.A recent systematic review estimated that in 2010, 1.15 million babies developed neonatal encephalopathy directly related to intrapartum asphyxia with 287,000 deaths, 233,000 infants surviving with moderate/severe disability and 181,000 living with mild impairment. There is an urgent need to study this population in greater detail and to establish the cause of brain injury so that ultimately, it can be prevented.
BACKGROUND: The HRB Neonatal Encephalopathy PhD Training Network (HRB NEPTuNE) is a major collaborative structured PhD research programme led by Professor Eleanor Molloy, (Consultant Neonatologist, Chair and Professor of Paediatrics, TCD and Tallaght Hospital) and co-lead Professor Geraldine Boylan (Professor of Neonatal Physiology and Director of the INFANT Research Centre, UCC). The programme is funded by the Health Research Board Ireland (HRB) Collaborative Doctoral Award scheme. HRB NEPTuNE programme has created a unique national collaborative multidisciplinary research group that includes parents to optimise the investigation and management of neonatal brain injury. It brings together researchers with internationally recognised expertise in neonatology, paediatrics, neurodevelopment, family-centred care, clinical trials and methodology, pharmacology, epidemiology, biostatistics, translational research, and neuroimaging in neonatal brain injury.
The programme has trained seven PhD scholars in multidisciplinary research projects in premier research centres in Ireland. The joint supervision of HRB NEPTuNE scholars allows a new generation of expertise to be developed in Ireland that will join international groups to further integrated care and progress future research in this field. PhD students have experienced a holistic overview of research in this area involving the entire translational paradigm from basic science research, translational clinical research, and clinical trials to epidemiology and population health while getting in-depth expertise in their chosen discipline.
Parental and patient and public involvement (PPI) has been a key element of the HRB NEPTuNE programme. Throughout its lifecycle, PPI representatives from our parent collaborator, the Irish Neonatal Health Alliance, have been critical to the development of the programme from the application stage where they advised on the key research questions. They have been involved in PhD supervision and have met regularly with scholars to guide research. Their input has also been integral to our governance structures, publications, study days and educational outreach.
Key outputs from the programme include seven PhD graduates, over fifty publications, national clinical guidelines, and parent information material.
Using quality improvement methodology to facilitate early medication discontinuation in neonates with acute provoked seizure
Jaime Twanow2, Rae Leonor Gumayan1, Darrah Haffner2, Margie Ream2, Laurel Slaughter2, Jason Kovalcik2, Trina Anthony2, Jacqueline Magers2, Megan Rose2, Adam Ostendorf2
1Childrens National Hospital, 2Nationwide Children’s Hospital
BACKGROUND: Acute provoked seizures occur with an incidence of 1-3 per 1,000 infants during the first week of life. There is growing recognition of the need to balance the benefits of seizure management with the risks of anti-seizure medications (ASM). Phenobarbital, the recommended first-line treatment, has been shown to lead to neuroapoptosis with unknown impacts on neurodevelopment. Recent recommendations provided management guidelines and a framework for weaning ASM in the acute provoked seizure population once the risk of seizures is decreased. Using Quality Improvement (QI) methodology, we aimed to decrease the percentage of neonates in the Nationwide Children’s Hospital (NCH) Neonatal Intensive Care Unit (NICU) with acute provoked seizures discharged with an anti-seizure medication from a baseline of 88% to < 15% and maintain for 12 months.
METHODOLOGY: A multidisciplinary quality improvement (QI) team developed a process map, a key driver diagram, and Pareto charts to focus interventions. Plan Do Study Act (PDSA) cycles were utilized and are ongoing. Data were tracked utilizing electronic medical record data queries and trends were identified employing accepted QI principles and chart review. Diagnoses of febrile seizure or initiation of an ASM during follow up period (as a marker for new diagnosis of epilepsy) were tracked as balancing measures.
RESULTS: The 2-year baseline population included 78 infants who received ASM, 69 (88%) of which were determined to have acute provoked seizures. Of those infants, 61 (88%) were discharged home on ASM. Of the few infants weaned prior to discharge, 1 infant later presented to the ER with febrile seizures, and none of the infants were subsequently diagnosed with epilepsy. In the 2 years since implementing the intervention, 56 infants received an ASM, with 48 infants (86%) judged to have acute provoked seizures. Of these 48 infants, 6 (10%) were discharged home on ASM and weaned as an outpatient. Of the infants weaned prior to discharge, 1 infant developed seizures while in the NICU, and ASMs were restarted. None of the infants in the intervention group have been diagnosed with febrile seizures or epilepsy. No infants with provoked seizures have been discharged home on an ASM for 12 months.
CONCLUSION: Quality improvement methodology allowed our team to standardize medication choices and the optimization of ASM duration in neonates with acute provoked seizure, aligning care in the NCH NICU with recent recommendations. Widespread education of providers and parents regarding the evolving evidence in the field of neonatal neurology fostered acceptance and contributed to the successful implementation of the new neonatal seizure management guidelines. This project demonstrates that QI methodology is an effective tool for achieving large-scale practice improvements in the NICU setting.
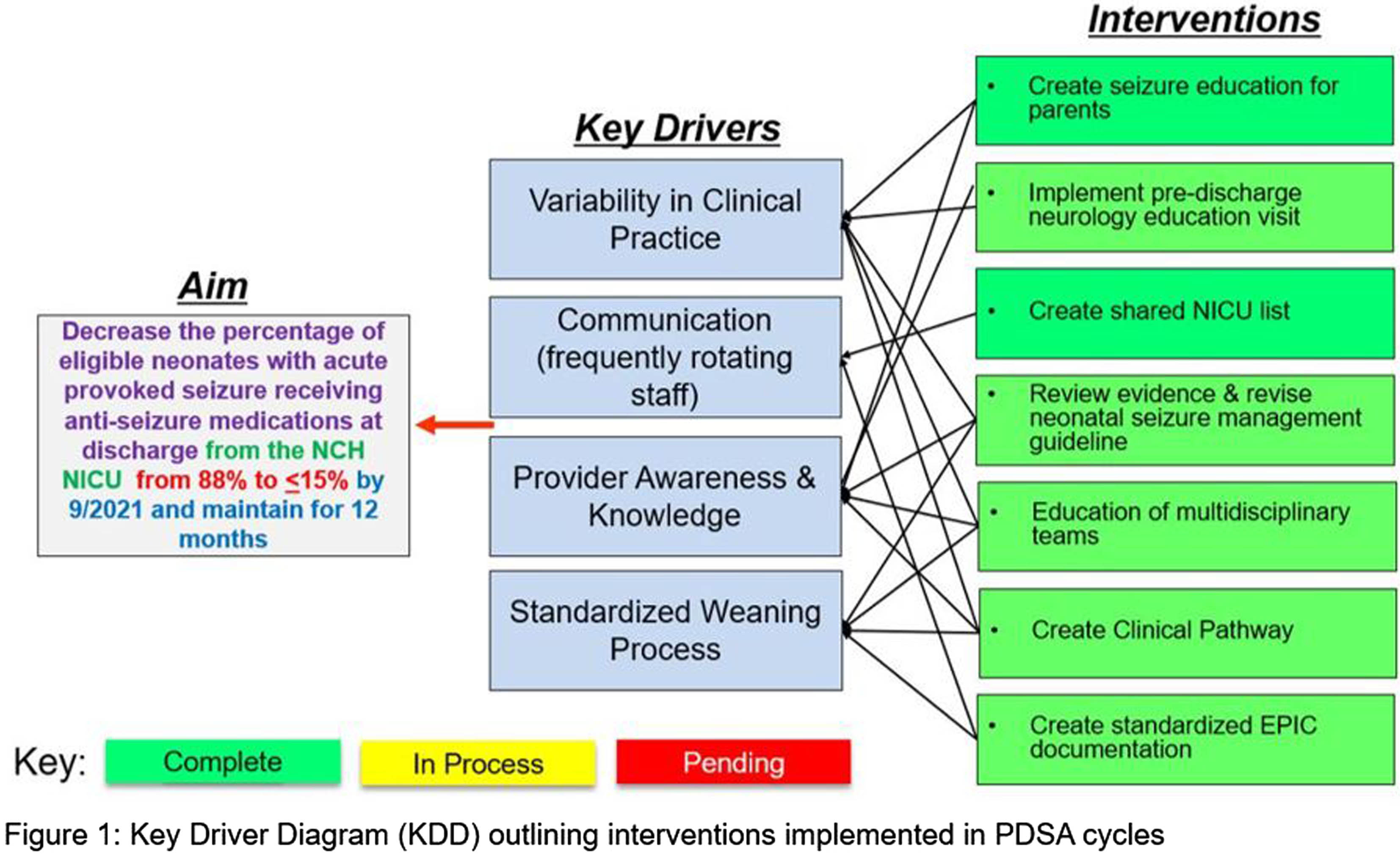
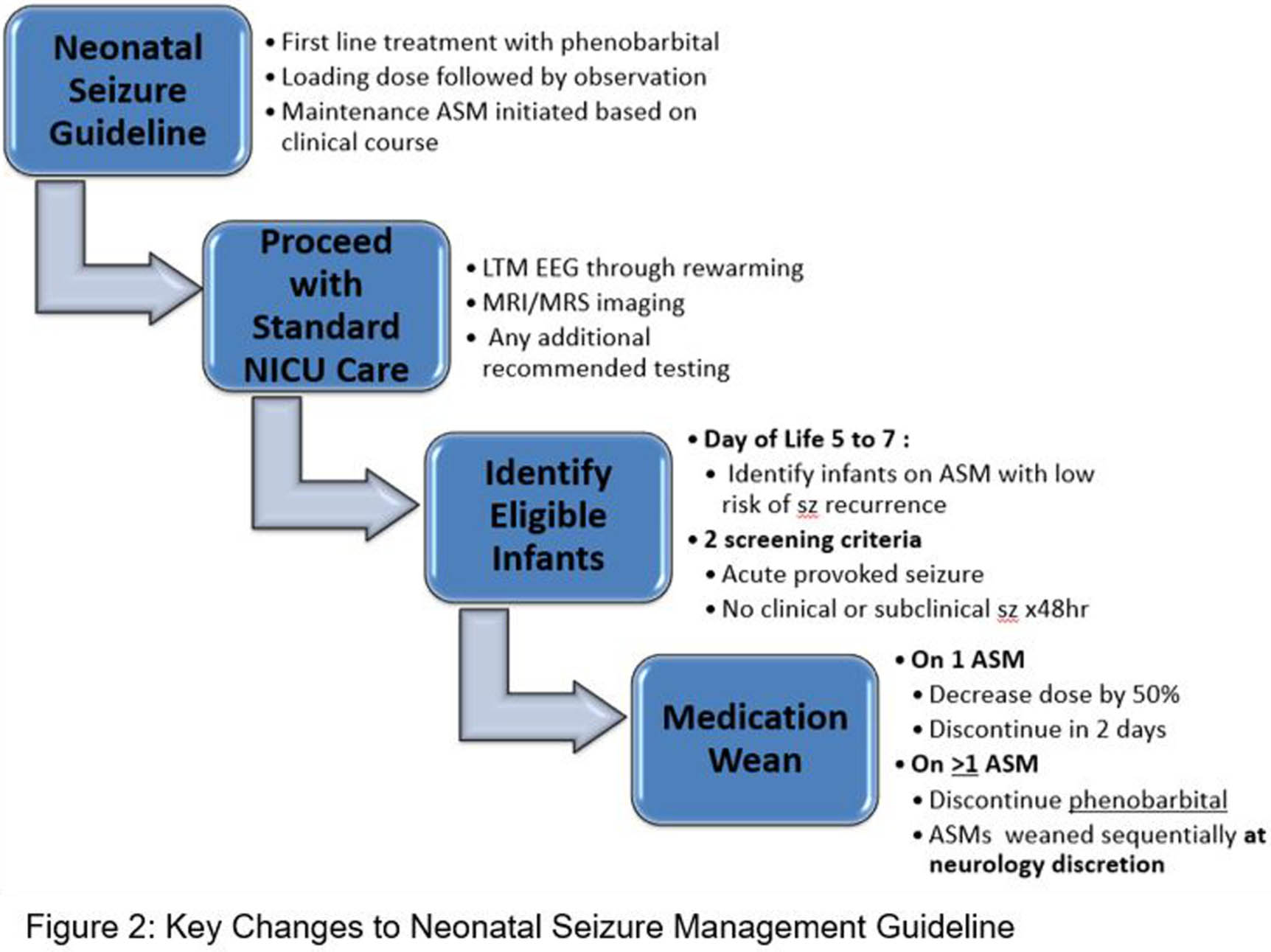
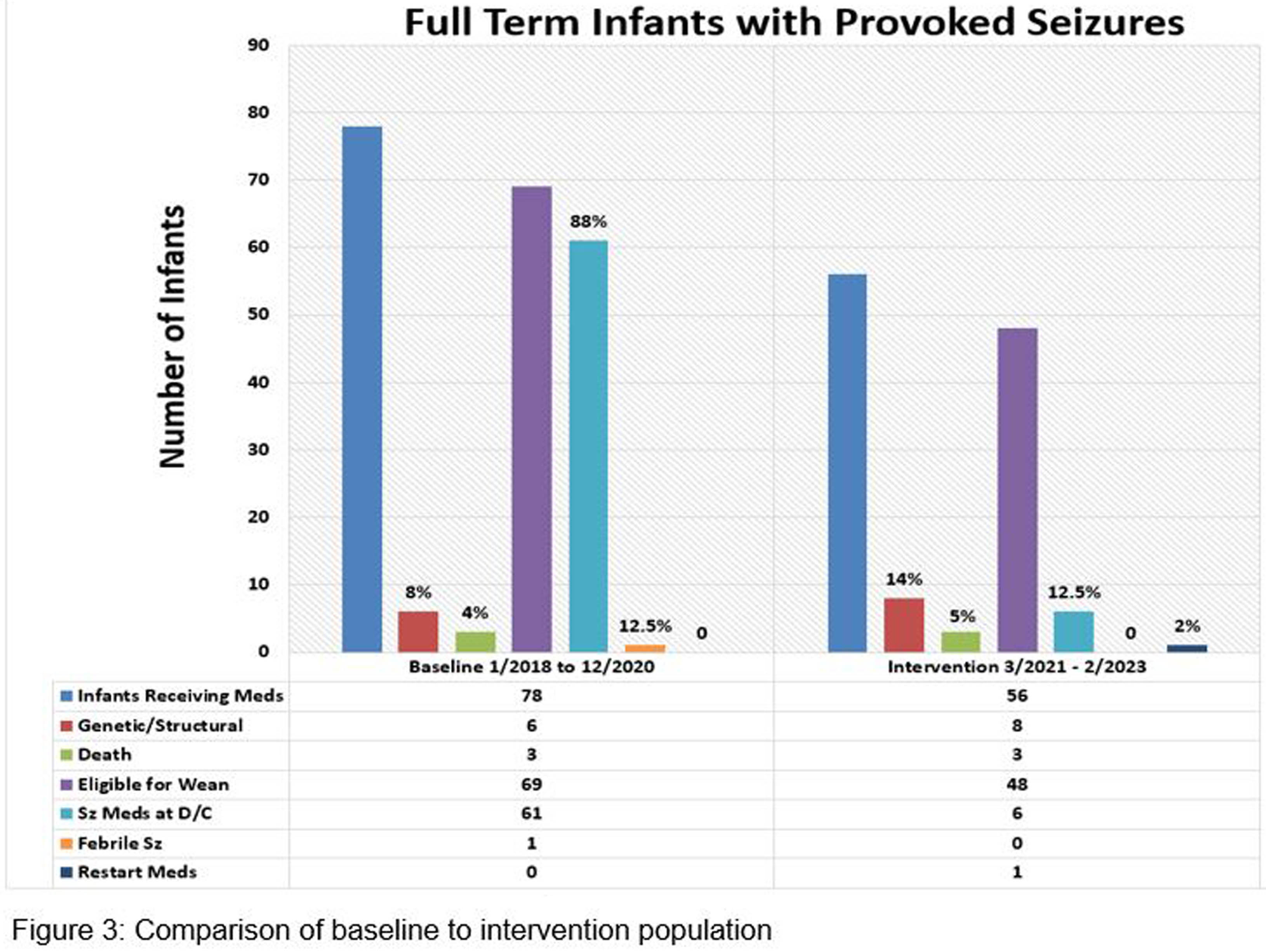
Ventriculitis in neonate: A devastating CNS infection
Anita Singh1, Kirti Naranje, Akanksha Verma, Fajia Farhath
1Sanjay Gandhi Postgraduate Institute Of Medical Sciences
INTRODUCTION: Neonatal ventriculitis, most commonly post meningitis is an important cause of neonatal morbidity and mortality. The incidence ranges from 52-94% post gram negative meningitis. Though etiologies are multifactorial sepsis and meningitis are important causes. Hydrocephalus is one of the important complications of ventriculitis and can lead to poor neurodevelopmental sequelae, so early diagnosis and prompt treatment are of paramount importance. Data on exact incidence, risk factors and early birth and hospital characteristics of neonatal ventriculitis are lacking.
OBJECTIVE: To explore the birth and early hospital characteristics ,trends of ventricular tap findings ,USG &MRI findings as well as outcome of newborns with ventriculitis who were admitted in our center from 2014-2023.
METHODOLOGY: We did a retrospective observational study on 17 patients (inborn & outborn) who were admitted in NICU,SGPGIMS,Lucknow between 2014 -2022. Data was retrospectively collected from the hospital records of all patients who were diagnosed with ventriculitis. The patients demographic data(age ,gender, gestational age, birth weight, antenatal risk factors, mode of conception & delivery, birth weight) were collected. Initial symptoms as well as serial ventricular tap findings were collected from records. Initial sepsis work up and blood culture reports and antibiotic sensitivity were collected from hospital information system data. Serial USG findings & MRI findings were recorded. Antibiotics type, duration as well as hearing vision and neurological examination findings were recorded.
RESULTS: The median gestational age was 32.1 ±8.7(24-40) weeks,82.3% male babies & 17.7% were females, 76.5% were outborn. Only one out of 17 patients was IVF conceived, median maternal age was 28 ±5 (23-37) years, Risk factor for sepsis was present in 11.7%, out of 17 patients 10 patients were born by LSCS(58.8%) & 13 patients required delivery room resuscitation. The median birthweight was 1400±1126(875-3600) grams & APGAR scores were not known in 64.7%.The median age at first presenting complaint was 10±10 (4-35) days and the median age at admission was 11±19(1-60) days. Most common presenting complaint was increasing head size followed by seizures, fever was least common presenting complaint. CSF culture was positive in 2 cases however blood culture was positive in all cases. Hydrocephalus was observed in three fourth cases. The overall survival was 53%.
CONCLUSION: Neonatal ventriculitis is associated with high mortality rates. Acinetobacter & Klebsiella pneumoniae were common isolates in the case series.