Proceedings of the 15th International Newborn Brain Conference: Fetal and/or neonatal brain development, both normal and abnormal
A histopathological study of perinatal hypoxic-ischemic brain damage
Masayuki Itoh1
1National Center of Neurology and Psychiatry
BACKGROUND AND OBJECTIVE: Perinatal hypoxic–ischemic brain damage is a major cause of neuronal and behavior deficits, in which the onset of injury can be before, at or after birth, and the effects may be delayed. Pontosubicular neuron necrosis (PSN) is one of perinatal hypoxic–ischemic brain injury and its pathological peculiarity is neuronal apoptosis. In this study, we investigated whether apoptotic cascade of PSN used a caspase-pathway or not, and whether hypoglycemia activated apoptosis or not.
MATERIALS: Sections of the pons of PSN with and without hypoglycemia were stained using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) and immunohistochemistry for glial fibrillary acidic protein (GFAP), Bcl-2, Bcl-x and activated caspase 3. Additionally, we performed immunoblot analysis of Bcl-2, Bcl-x and activated caspase 3.
RESULTS: TUNEL-positive cell was closely associated with the presence of karyorrhexis. Under combination of karyorrhectic and TUNEL-positive cells, number of apoptotic cells in premature brains was significantly more than in mature brains
CONCLUSION: Hypoxic–ischemic brain injury was considered to easily lead to apoptosis in premature infants. Moreover, as this pathophysiology, caspase-pathway activation contributed to neuronal death from caspase-immunohistochemistry analyses. PSN with hypoglycemia showed large number of apoptotic cells and higher expression of activated caspase 3. The result may be more severe with the background of hypoglycemia and prematurity complicated by hypoxia and/or ischemia.
Yield of genetic testing in posterior fossa anomalies
Usha Appalaneni1, Ana Cristancho2, Erica Schindewolf2, Kendra Miller2, Ingrid Lee2
1Advocate Children’s Hospital, 2Children’s Hospital of Philadelphia
BACKGROUND AND OBJECTIVE: Following abnormal anatomy scan, fetal MRI is often performed, now with increased identification of posterior fossa anomalies (PFAs). Genetic testing is strongly recommended in these high-risk pregnancies. Non-invasive prenatal testing (NIPT) is useful for detecting chromosomal aberrations. Expanded testing, such as amniocentesis or chorionic villus sampling, is recommended following negative NIPT. When expanded prenatal genetic testing is recommended for patients with PFAs, we seek to understand which genetic tests would be most useful in making a prenatal genetic diagnosis.
METHODS: A retrospective chart review of mothers referred to the Center for Fetal Diagnosis and Treatment at the Children’s Hospital of Philadelphia from 2015 – 2022 was conducted. A query of 18 terms was used to identify fetuses with posterior fossa abnormalities. 40 patients were identified following the initial query. Manual abstraction of prenatal and postnatal imaging findings, results of genetic testing, and presence of other CNS or body findings was conducted. 6 patients were excluded after review revealed the fetus did not have PFAs.
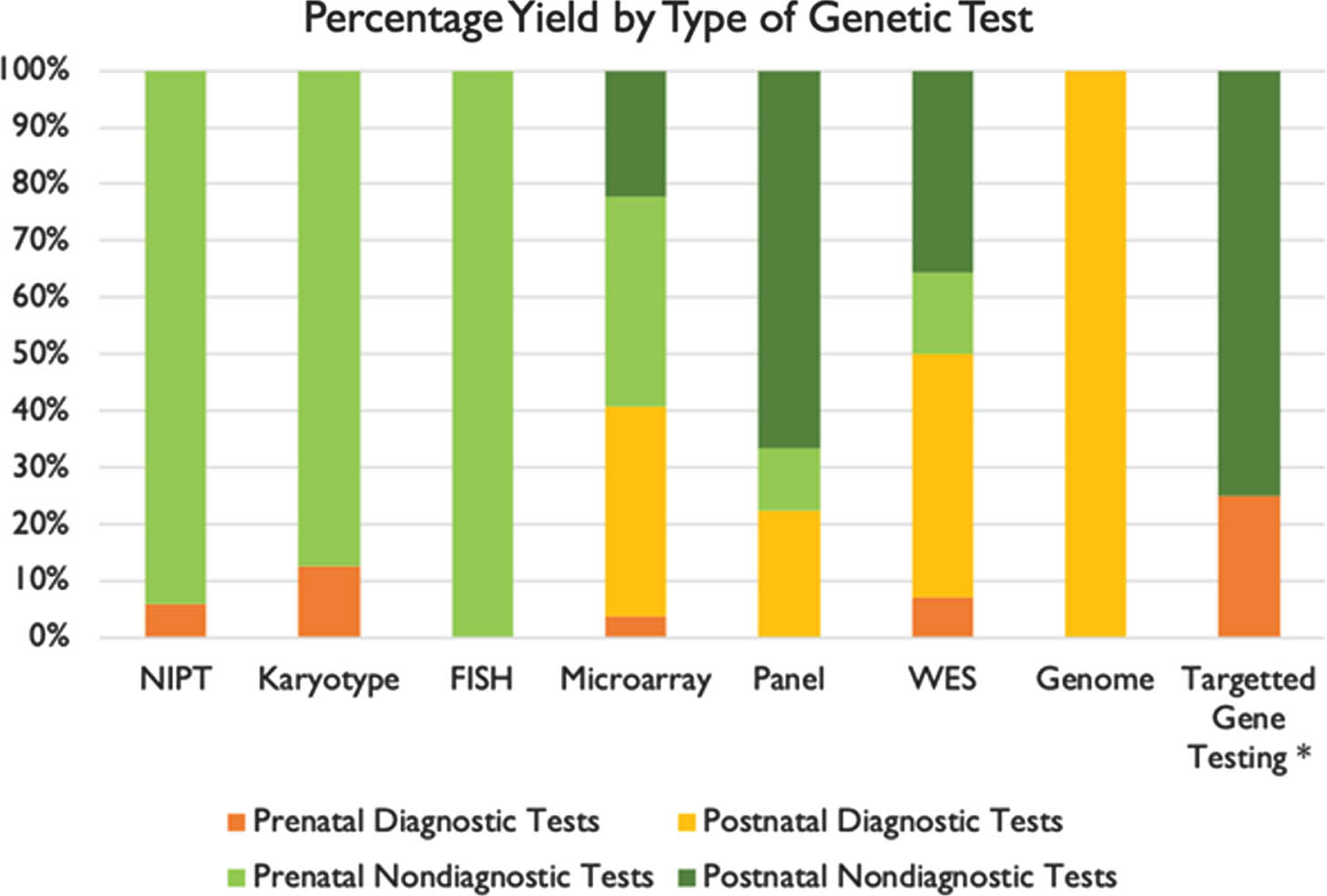
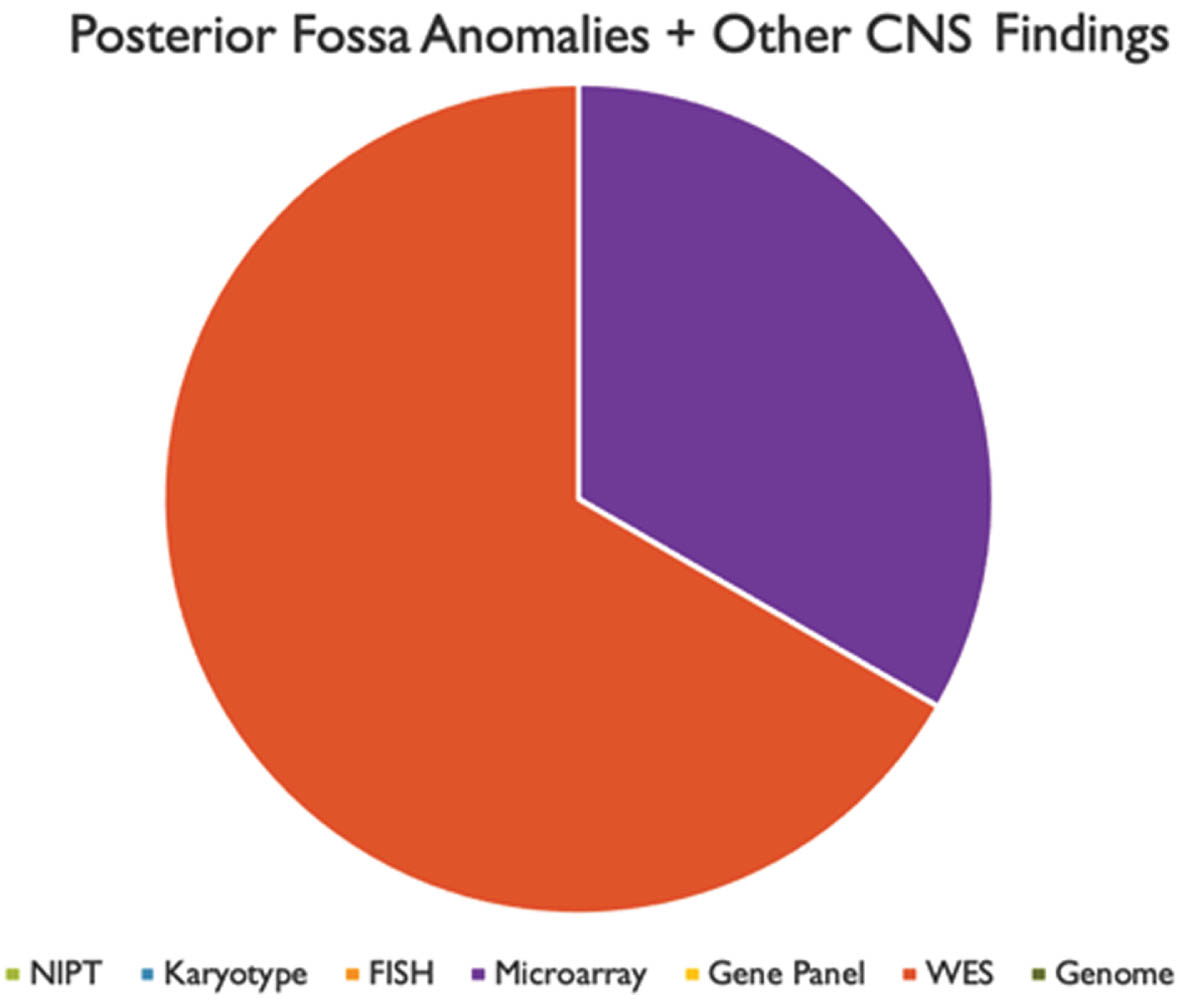
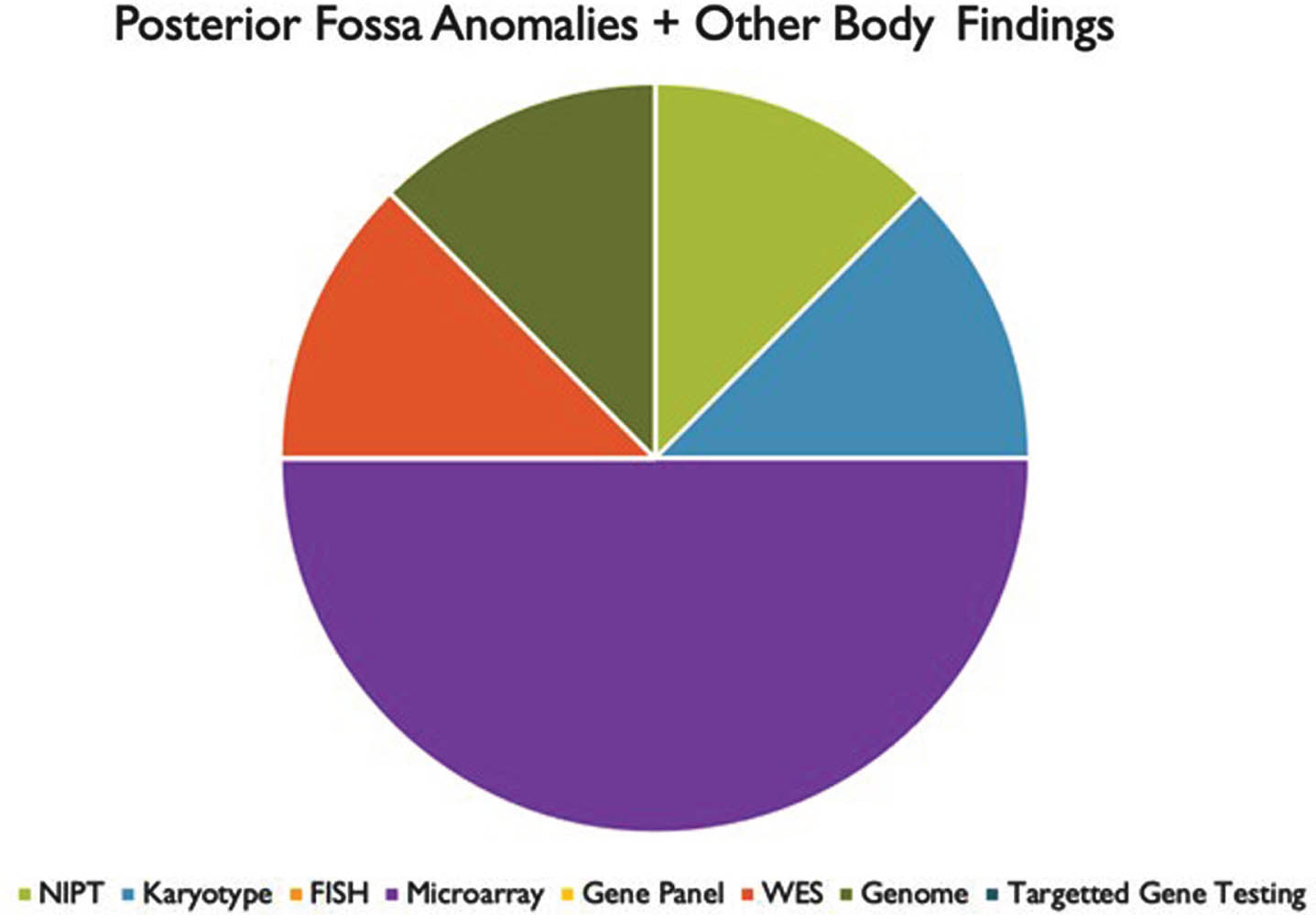
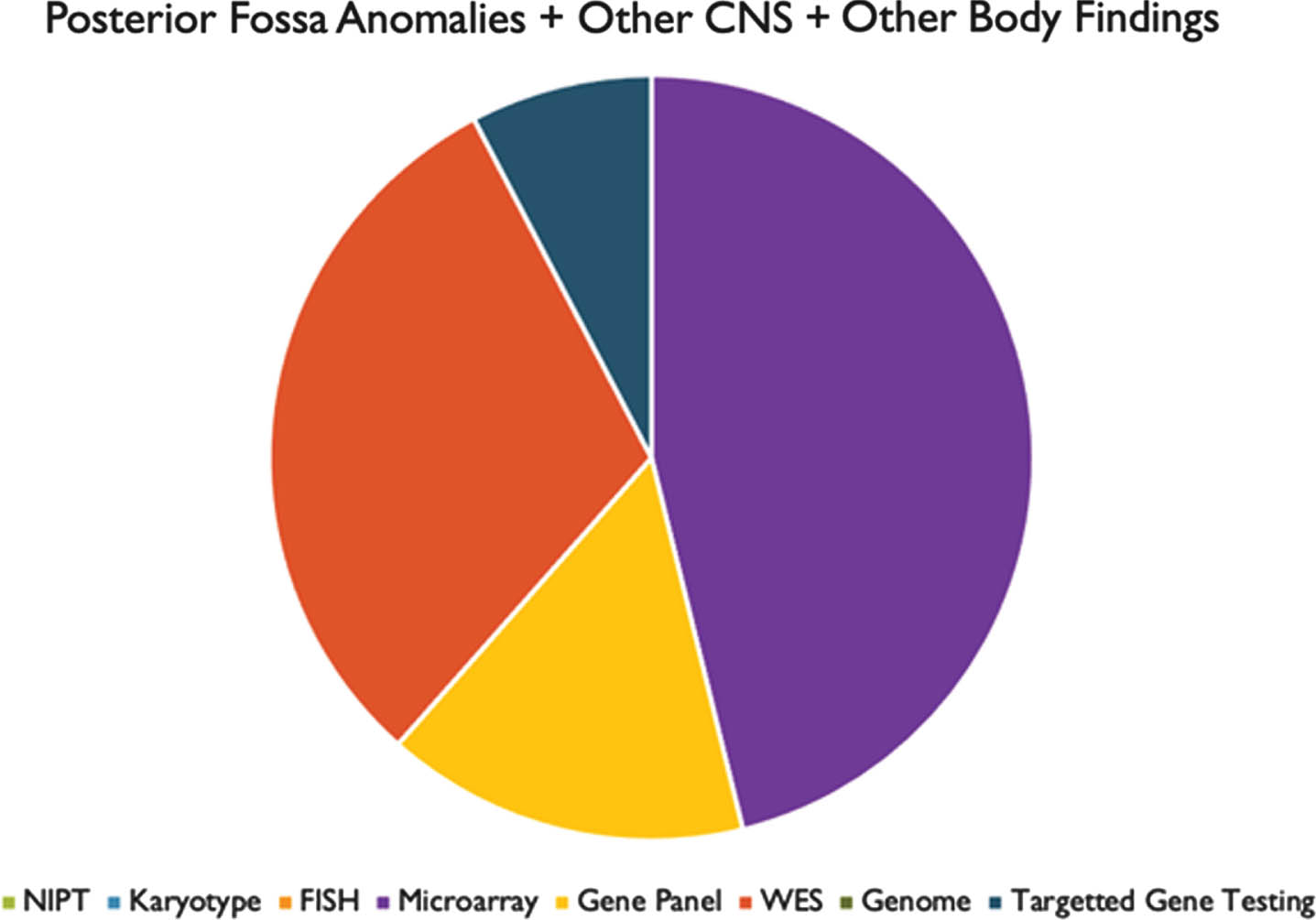
RESULTS: We found that prenatal and postnatal MRI findings were typically congruent. Genetic testing was performed in 33 of 34 patients. A positive genetic diagnosis was made in 22 patients (64.7%). Overall, the highest yield genetic tests were whole exome sequencing (50%) and chromosomal microarray (41%). We also found that most patients had other abnormalities in association with their PFA.
CONCLUSIONS: These results suggest that there is a high likelihood of ascertaining a genetic diagnosis in patients with PFA, particularly in patients with other associated congenital anomalies. The highest yield tests, both in prenatal and postnatal evaluations, were microarray and WES. However, these tests were rarely performed in the prenatal setting. Our analysis would indicate that in mothers undergoing invasive procedures such as amniocentesis or CVS, microarray and WES should be utilized more frequently to make a genetic diagnosis.
BIBLIOGRAPHY:
1. D’Antonio, F, et al. (2016). Systematic review and meta-analysis of isolated posterior fossa malformations on prenatal ultrasound imaging (part 1): nomenclature, diagnostic accuracy and associated anomalies. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology, 47(6), 690–697. https://doi.org/10.1002/uog.14900
2. D’Antonio, F., et al. (2016). Systematic review and meta-analysis of isolated posterior fossa malformations on prenatal imaging (part 2): neurodevelopmental outcome. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology, 48(1), 28–37. https://doi.org/10.1002/uog.15755
3. Lord, J., et al. Prenatal Assessment of Genomes and Exomes Consortium (2019). Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet (London, England), 393(10173), 747–757. https://doi.org/10.1016/S0140-6736(18)31940-8
4. Shekdar, K. (2011). Posterior fossa malformations. Seminars in ultrasound, CT, and MR, 32(3), 228–241. https://doi.org/10.1053/j.sult.2011.02.003
Management of the second stage of labor: Fetal and maternal outcomes
Stephen Wood1, Mattew Hicks2, Selphee Tang1, Khorshid Mohammad1
1University of Calgary, 2University of Alberta
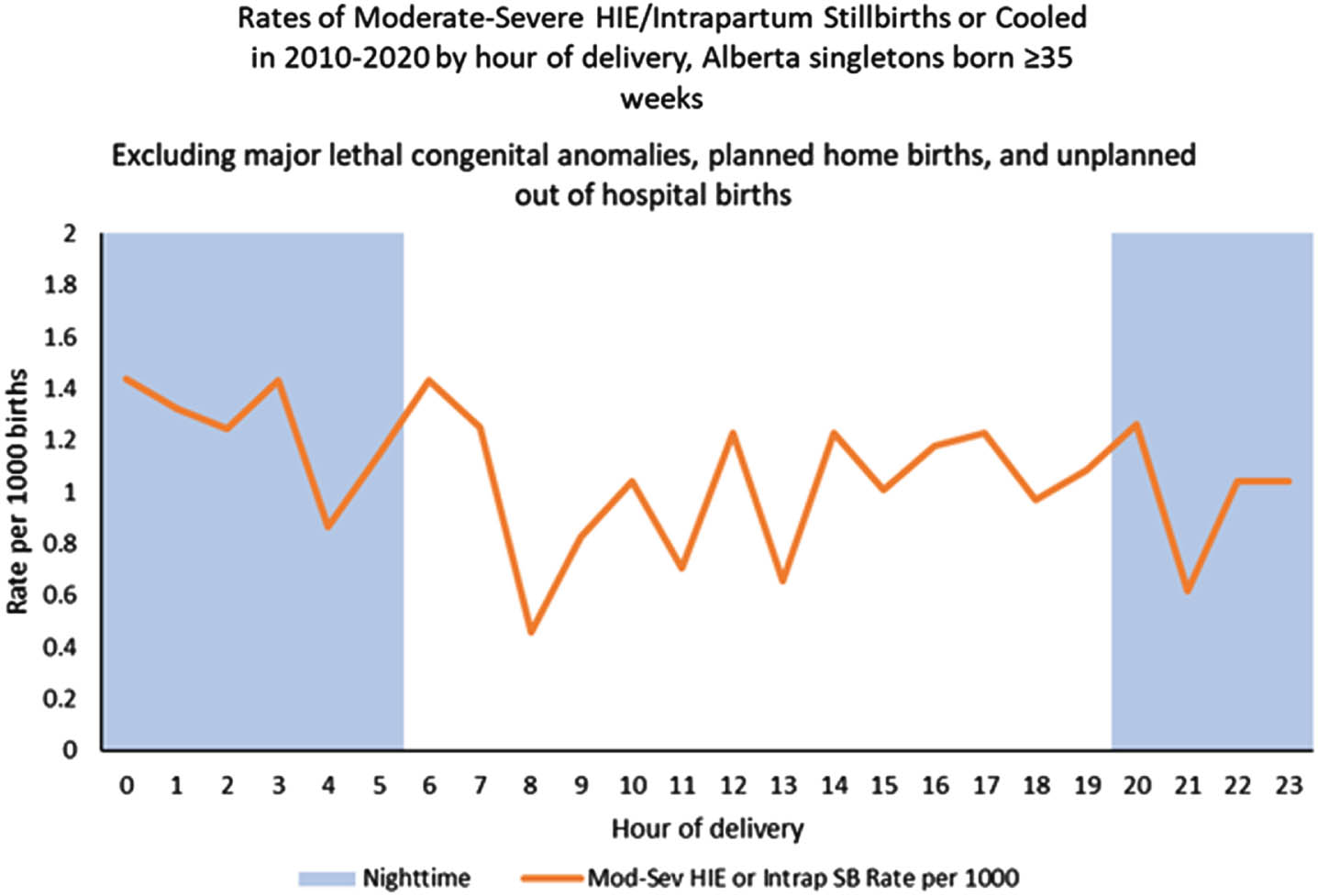
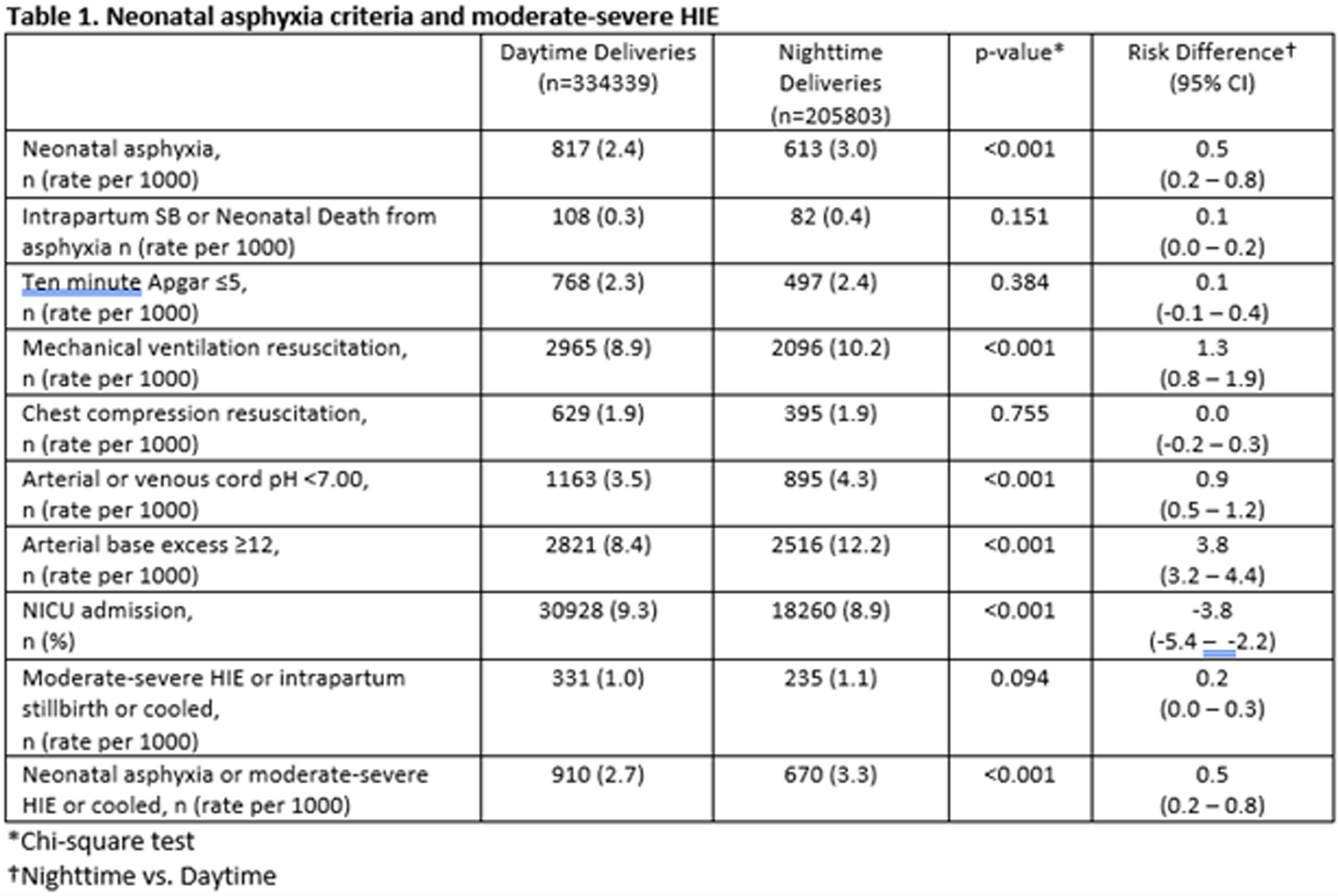
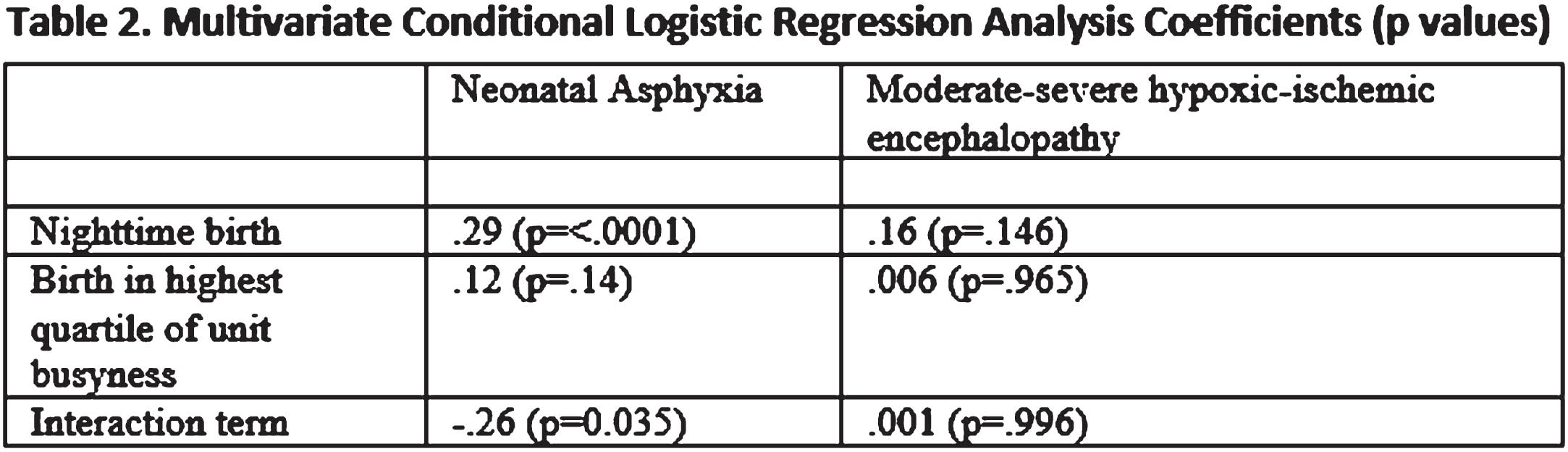
OBJECTIVES: To evaluate the possible association between nighttime births and unit busyness and neonatal asphyxia or moderate-severe hypoxic ischemic encephalopathy
METHODS: Retrospective cohort study with nested case control study of all singleton births ≥ 35-week gestation, in Alberta, a province of Canada, from 2010–20. Asphyxia was defined as intrapartum stillbirth or neonatal death from asphyxia or Neonatal Intensive Care Unit admission and at least two of the following: a. Apgar score of ≤ 5 at 10 minutes; b. mechanical ventilation or chest compressions for resuscitation within 10 minutes; c. cord pH < 7.00 (venous or arterial), or arterial base excess ≥ 12 at birth. Moderate-severe hypoxic ischemic encephalopathy was defined as per Sarnat criteria. Nighttime birth was defined as between 2000 and 0559. Unit busyness was characterized by number of births by site per shift and was described by quartiles.
RESULTS: Data was available on 540,142 births and 1580 cases of neonatal asphyxia or moderate-sever hypoxic ischemic encephalopathy. The risk of neonatal asphyxia was slightly higher for nighttime births, OR 1.21 (1.09, 1.35), but moderate-severe hypoxic ischemic encephalopathy was not, OR 1.17 (0.94, 1.45). We did not observe an increase in the risk of neonatal asphyxia or moderate-severe hypoxic ischemic encephalopathy with delivery in the highest quartile of unit busyness.
CONCLUSION: Nighttime birth is associated with neonatal asphyxia but not moderate-severe hypoxic ischemic encephalopathy. Unit busyness is not associated with either outcome. This lack of association with unit busyness may be due to difficulty in measuring it precisely.
Hypoxic-ischemic encephalopathy: A longitudinal cohort study assessing multiple organ involvement
Lynn Bitar1, Yu-lun Liu1, Rachel Leon1, Lina Chalak1, Srinivas Kota
1Division of Neonatal-Perinatal Medicine, University of Texas Southwestern Medical Center, USA
BACKGROUND: Hypoxic ischemic encephalopathy (HIE) potentially affects multiple organs including the brain, heart, liver, and kidneys, which can lead to long-term complications. To date, few studies have evaluated the presence of multi-organ dysfunction (MOD) in neonates with mild HIE.
OBJECTIVES: To evaluate the incidence of MOD in neonates with mild, moderate, and severe HIE; and to determine the correlation between the HIE severity and the degree of organ involvement.
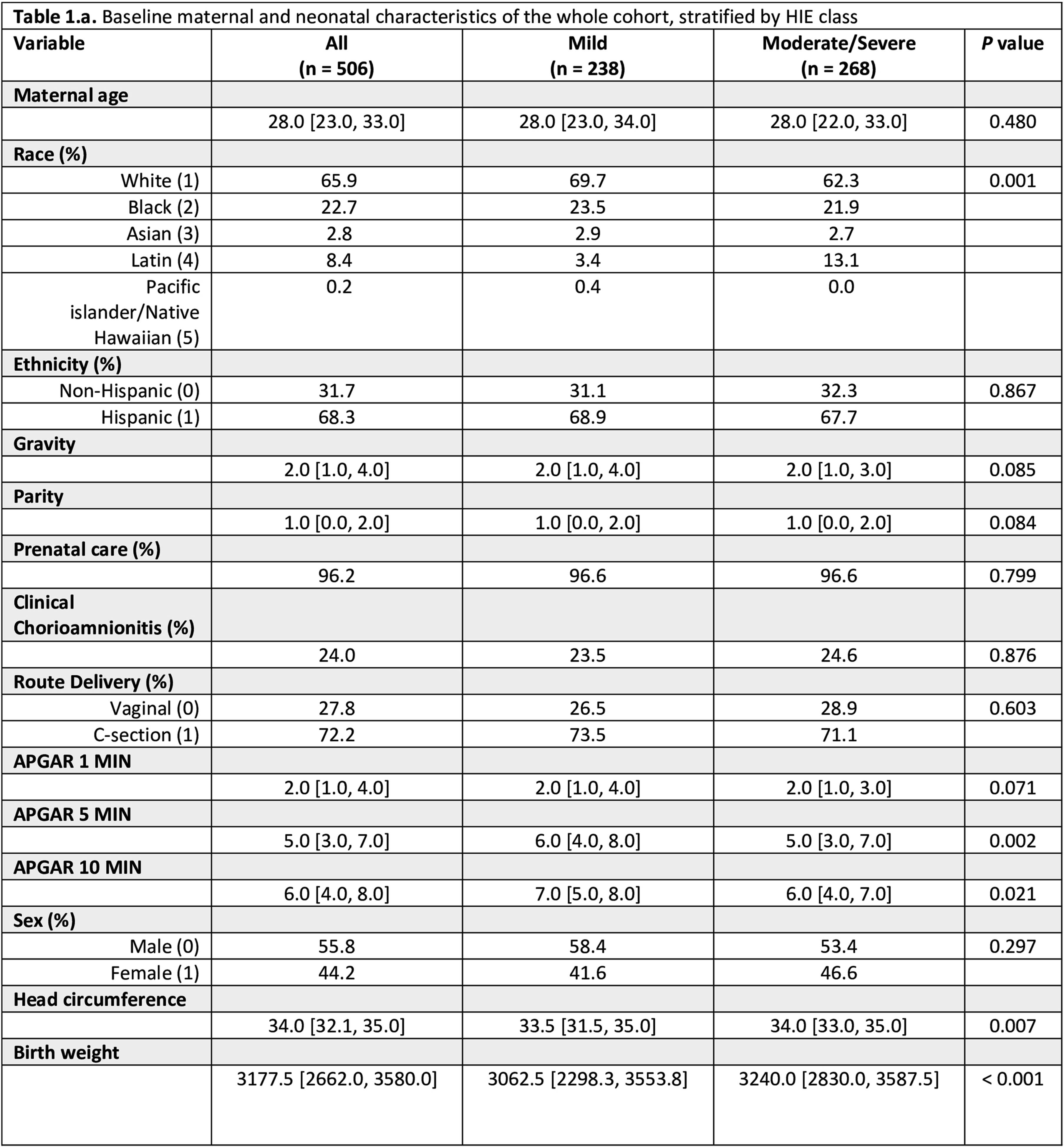
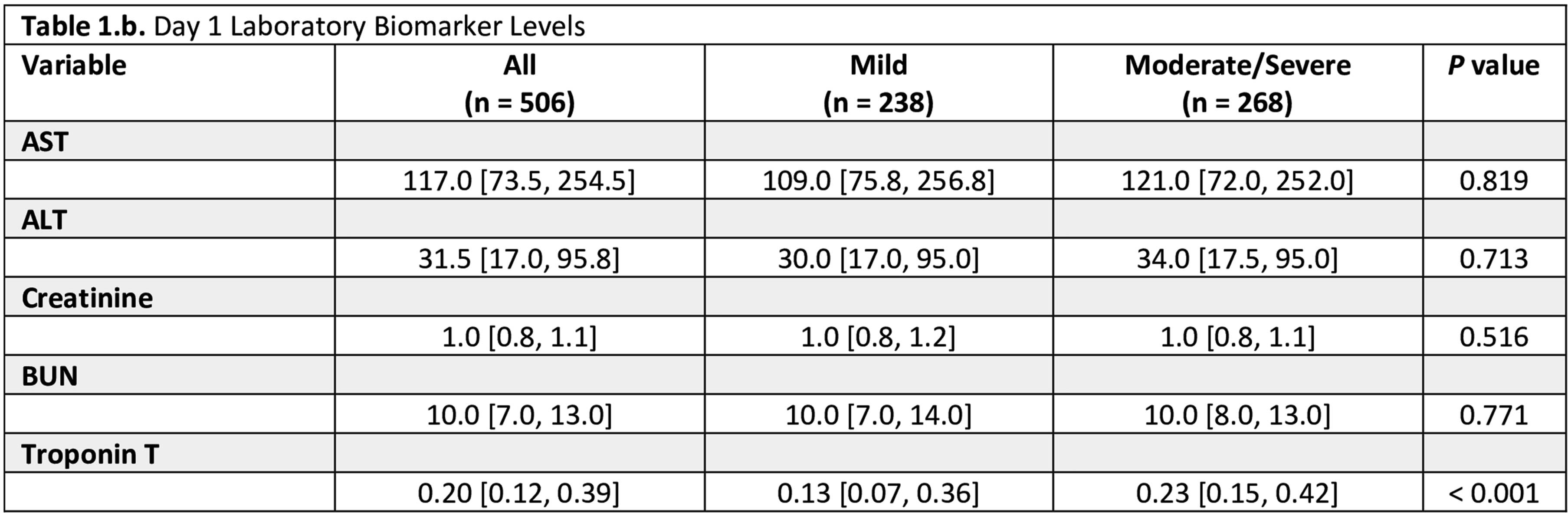
METHODS: In this retrospective cohort study, we included neonates with HIE admitted to the intensive care unit at Parkland Memorial hospital, between 2009-2023. Our cohort was divided into two groups based on Sarnat staging: one for newborns with mild HIE and the other for newborns with moderate to severe HIE who received therapeutic hypothermia. To assess MOD, we evaluated cardiac function using echocardiography and troponin T, renal function using creatinine and blood urea nitrogen (BUN), liver function using alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Markers of MOD were collected on days 1 and 3 after birth.
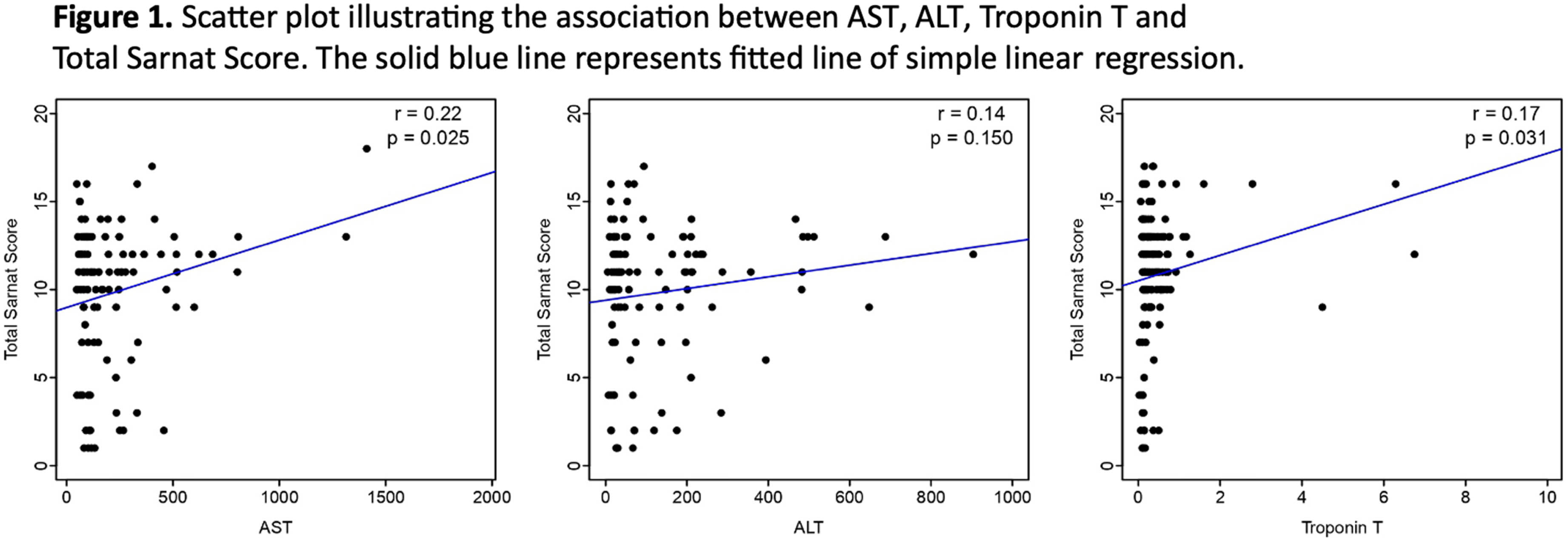
RESULTS: KS We included 506 neonates: 238 with mild HIE, and 268 with moderate to severe HIE (Table 1). Among the whole cohort, 46% had cardiac injury, 34% had kidney injury, and 29% had liver injury. In the mild HIE group 33% exhibited MRI abnormalities, while the moderate to severe group had a significantly higher rate (69%; p=0.04). The number of organs involved was also higher in the moderate to severe group (38% vs. 27%; p=0.010). Notably, the rates of kidney (35% vs. 33%; p=0.631) and liver injury (30% vs. 28%; p=0.606) were comparable between the two groups while neonates with moderate to severe HIE showed a significantly higher incidence of cardiac injury compared to those with mild HIE (65% vs. 26%; p<0.001). The Sarnat score was not significantly different with the extent of organ injury (AST, ALT, and Troponin T levels, Figure 1).
CONCLUSION: This study highlights the high risk of MOD among neonates with mild HIE despite their lower degree of encephalopathy. It emphasizes the importance of close monitoring of MOD-related biomarkers in neonates, regardless of their HIE severity.
Prenatal MRI brain findings associated with epilepsy by 2 years of age
Leah Ferrante1, April Gorman2, Michelle Machie2
1University of Texas at Austin, 2University of Texas Southwestern Medical Center
BACKGROUND: Fetal neurology is an evolving field that involves counseling prospective parents about the risks for neurological and developmental disabilities. Certain neuroanatomical abnormalities are known to be associated with a higher risk of developing epilepsy when associated with a postnatal MRI of the brain. Prenatal MRI cannot always detect these abnormalities. Prior studies done on this topic looked at short term outcomes or do not use epilepsy as an outcome measure. The purpose of this study was to determine if prenatal imaging can guide fetal neurologists to be more accurate in predicting postnatal epilepsy risk. The objective of this study was to determine if there are specific prenatal imaging findings that predict developing epilepsy by 2 years of age.
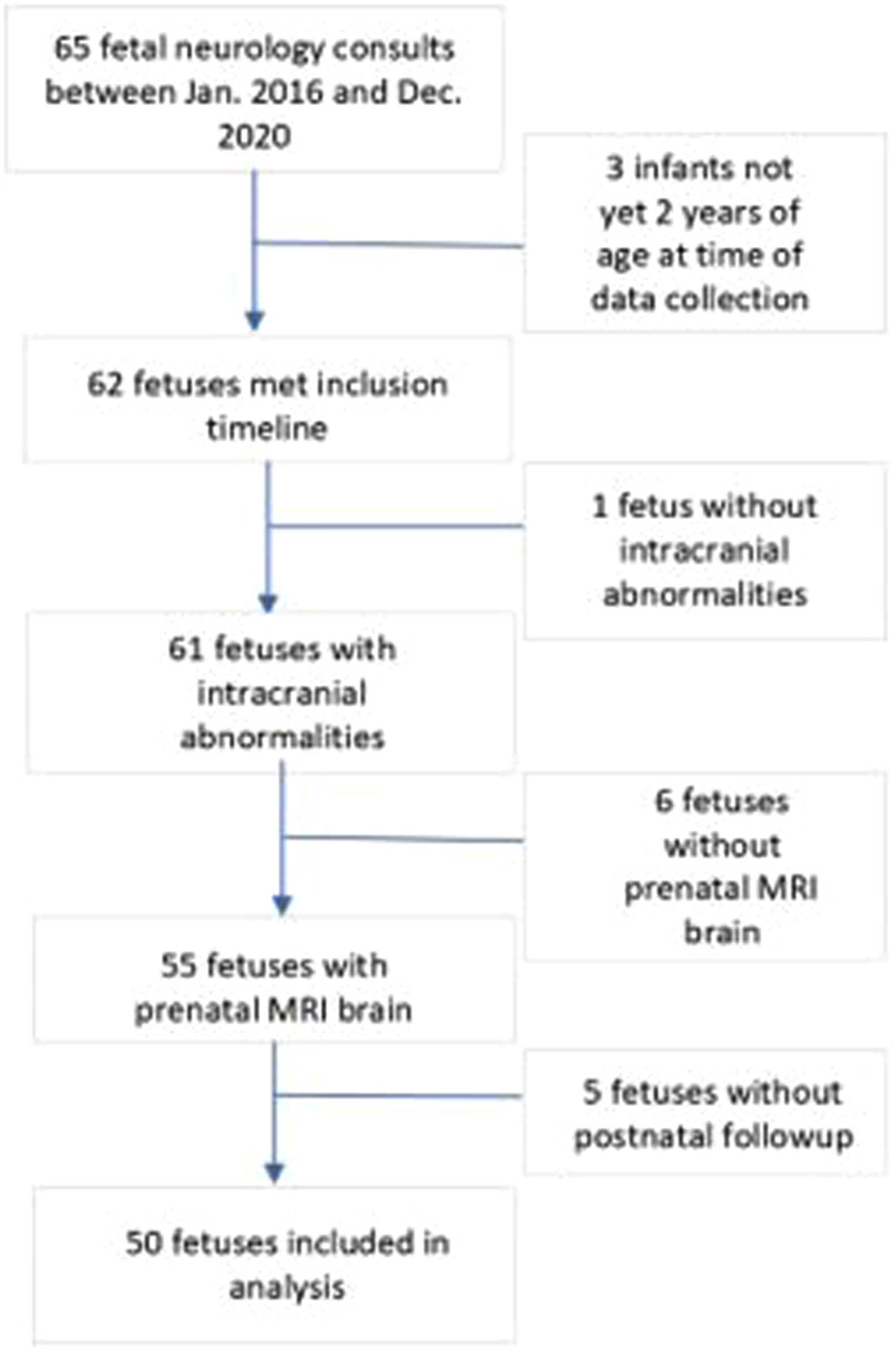
METHODOLOGY: This is a single center retrospective cohort chart review study. All fetal neurology consults between January 2016 and December 2020 were included so that patients were at least 2 years of age by December 2022. Inclusion criteria were presence of intracranial abnormalities, prenatal MRI, and postnatal follow-up with a neurologist. Exclusion criteria were consultation done outside of the defined time window, child not 2 years old by December 2022, no intracranial abnormalities, no prenatal MRI, and no postnatal follow-up with neurology (Figure 1). Primary outcome measure was epilepsy diagnosis by 2 years. Charts were reviewed for epilepsy diagnosis or daily antiseizure medications prescribed in absence of explicit diagnosis in chart (for children following an outside neurologist). Pertinent information was derived from maternal chart review, and radiologic findings were based on both the “impressions” and “findings”.
RESULTS: The three most common CNS findings in our fetal cohort were ventriculomegaly (22/50), agenesis/dysgenesis of the corpus callosum (12/22), and absence of the septum pellucidum (12/50). In total, 14/50 of infants in this cohort developed epilepsy by 2 years. All infants with perinatal seizures (4/50) developed epilepsy by 2 years. 75% (3 /4) of infants with microcephaly developed epilepsy by 2 years with OR 15.05 (95% CI=0.798-283.8). Hispanic ethnicity seemed to be protective with OR 0.136 (95% CI=0.022-0.858) compared to other races/ethnicities.
CONCLUSION: A significant proportion of the cohort developed epilepsy. Hispanic infants were best represented compared to other racial/ethnic groups, and likely represents a truer estimation of risk for development of epilepsy. Larger numbers are needed for statistically significant findings but there are meaningful trends in this dataset.
BIBLIOGRAPHY:
1. Ream MA, Mulkey SB. A neurologist’s Practical Guide to conducting a fetal consultation. Seminars in Pediatric Neurology. 2022;42:100957. doi:10.1016/j.spen.2022.100957
2. Arroyo MS, Hopkin RJ, Nagaraj UD, Kline-Fath B, Venkatesan C. Fetal Brain MRI findings and neonatal outcome of common diagnosis at a tertiary care center. Journal of Perinatology. 2019;39(8):1072-1077. doi:10.1038/s41372-019-0407-9
3. Cardenas AM, Whitehead MT, Bulas DI. Fetal neuroimaging update. Seminars in Pediatric Neurology. 2020;33:100801. doi:10.1016/j.spen.2020.100801
Neuromonitoring at term-equivalent age is associated with post-menstrual age and neurodevelopmental outcome in preterm infants
Anurudhya Karthikeyan1,2, Marie-Noëlle Simard1,3, Rasheda Chowdhury1,2, Olivia Beaulieu1, Gabriel Côte-Corriveau1,4,5, Marie-Michèle Gagnon1, Mélanie Gagnon1, Catherine Bernard1, Anne-Monique Nuyt1,5, Thuy Mai Luu1,5, Mathieu Dehaes1,2,6
1Research Center, Sainte-Justine University Hospital Centre, 2Institute of Biomedical Engineering, University of Montreal, 3School of rehabilitation, University of Montreal, 4Department of Epidemiology, Biostatistics, and Occupational Health, Faculty of Medicine, McGill University, 5Department of Pediatrics, Sainte-Justine University Hospital Centre, Université de Montréal, 6Department of Radiology, Radio-oncology and Nuclear Medicine, University of Montreal
BACKGROUND AND RANDOM: In Canada, 8% of infants are born prematurely every year1 and the majority is born at 29-36 weeks’ of gestational age. A deeper understanding of early preterm brain development may aid in identifying biomarkers associated with later neurodevelopment. Our objective was to examine the associations between cerebral metabolism/hemodynamic parameters measured at term-equivalent age (TEA) with post-menstrual age (PMA) and 2-year neurodevelopmental outcome.
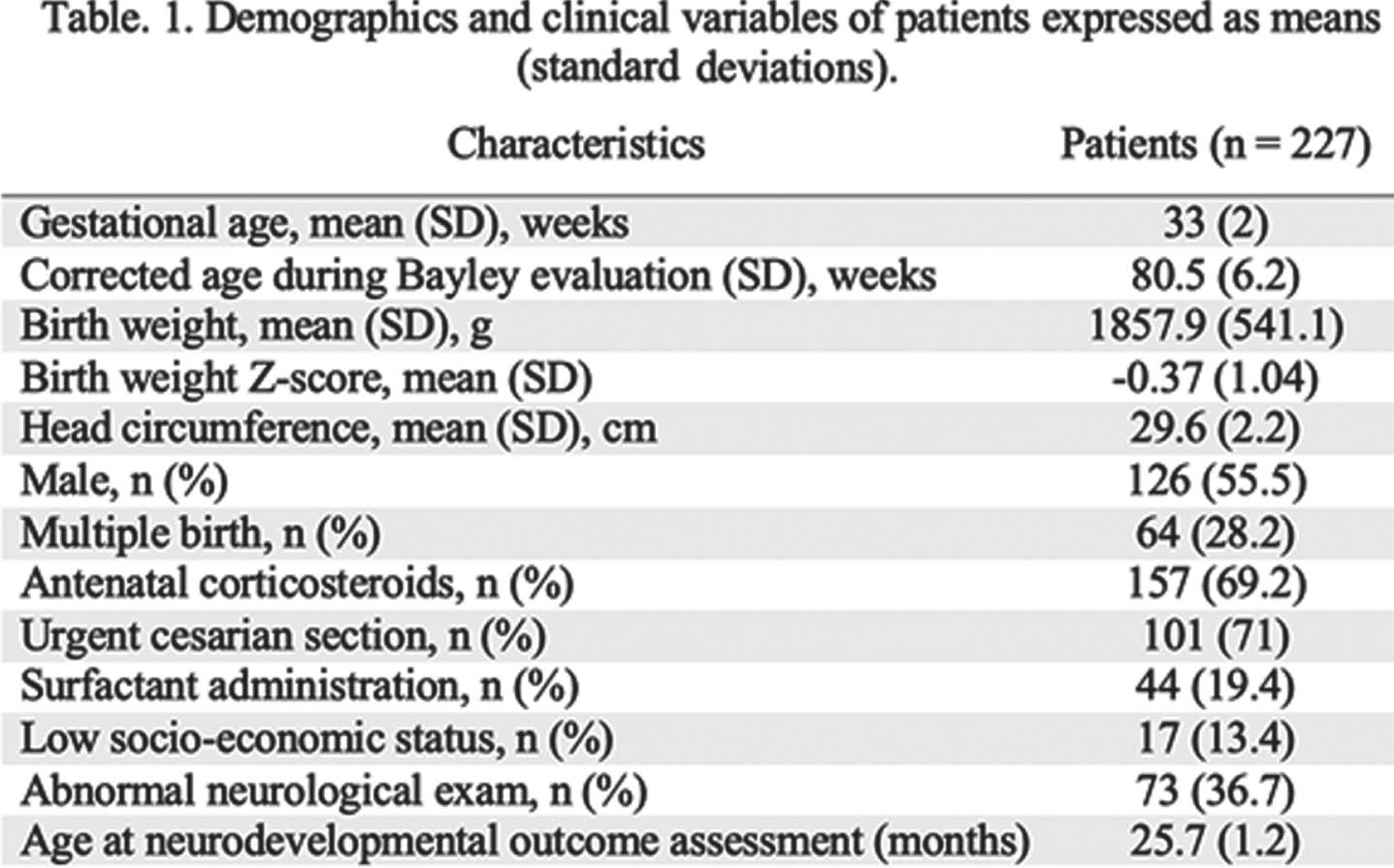
METHODOLOGY: In this single-center prospective observational study, 227 infants born at 29-36 weeks’ of gestational age and admitted for at least 48h in the neonatal intensive care unit were recruited between 2018 and 2021. Infants with major conditions such as chromosomal or congenital abnormality, neonatal stroke, hypoxic-ischemic encephalopathy, moribund infants, and infants under child protection services were excluded. Non-invasive bedside optical neuromonitoring was used to measure cerebral hemoglobin oxygen saturation (SO2) and an index of microvascular cerebral blood flow (CBFi) at TEA (defined between 35 and 43 weeks postmenstrual age - PMA). Peripheral arterial oxygen saturation and blood hemoglobin concentration (HGB) were retrieved from medical charts during this period and used to estimate cerebral oxygen extraction fraction (OEF) as well as indices of cerebral oxygen metabolism (CMRO2i) and delivery (CDO2i). Neurodevelopmental outcome was assessed at 2 years of corrected age with the Bayley scales of infant and toddler development, 4th edition. Pearson’s and Spearman’s correlation tests were used to assess the associations of neuromonitoring parameters with PMA and neurodevelopmental outcome, respectively. Benjamini-Hochberg method was used to correct for multiple comparisons.
RESULTS: Demographics and clinical variables are showed in Table 1. Figure 1 shows the distribution of neuromonitoring parameters and hemoglobin concentration with respect to PMA. Increases in CBFi, CMRO2i, CDO2i and OEF were observed while SO2 and HGB decreased with increasing PMA. At TEA, CDO2i, CMRO2i, SO2 and OEF were significantly associated with cognitive and language composite scores as well as with receptive and expressive communication (except for CDO2i) scales (Table 2).
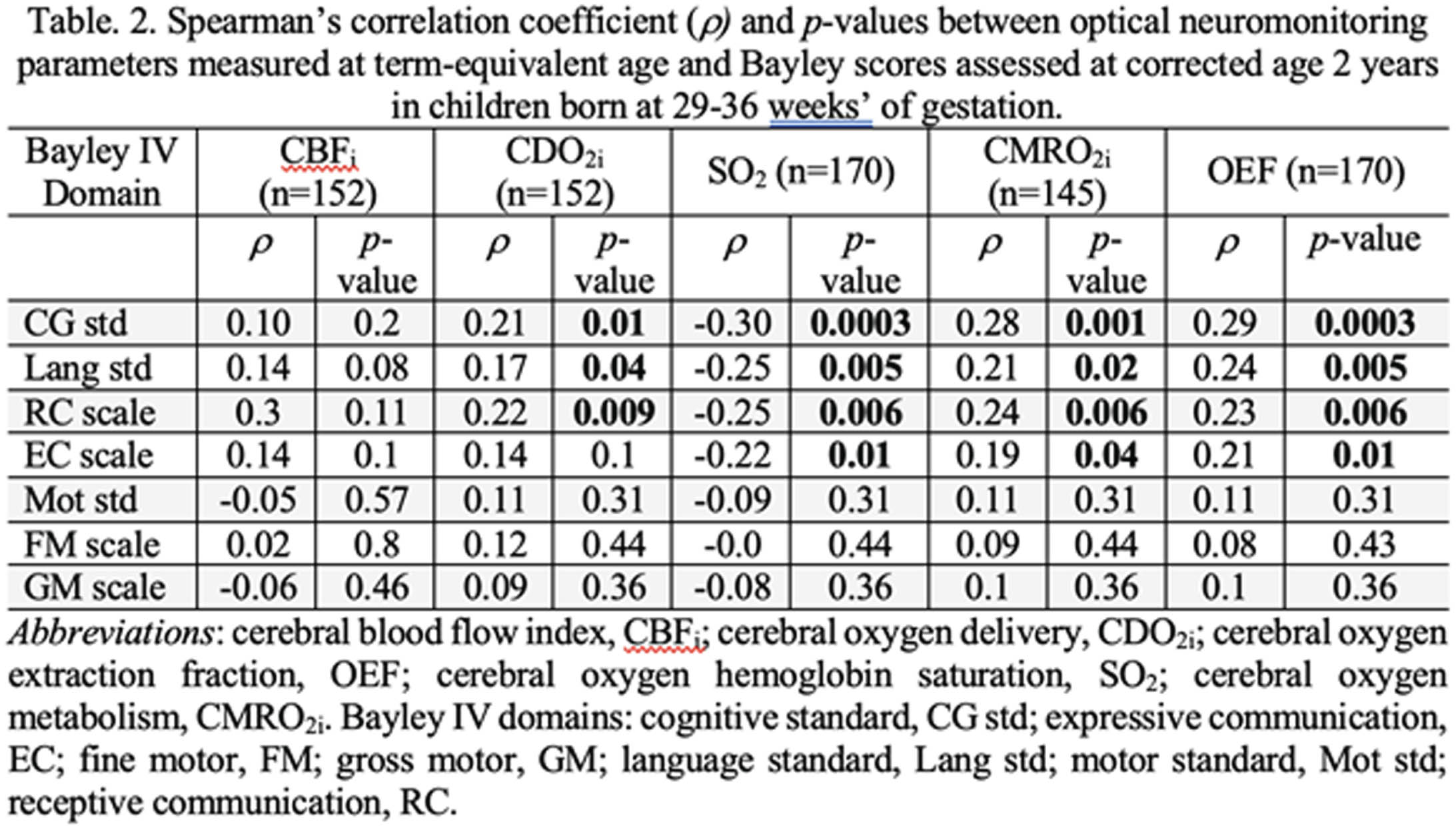
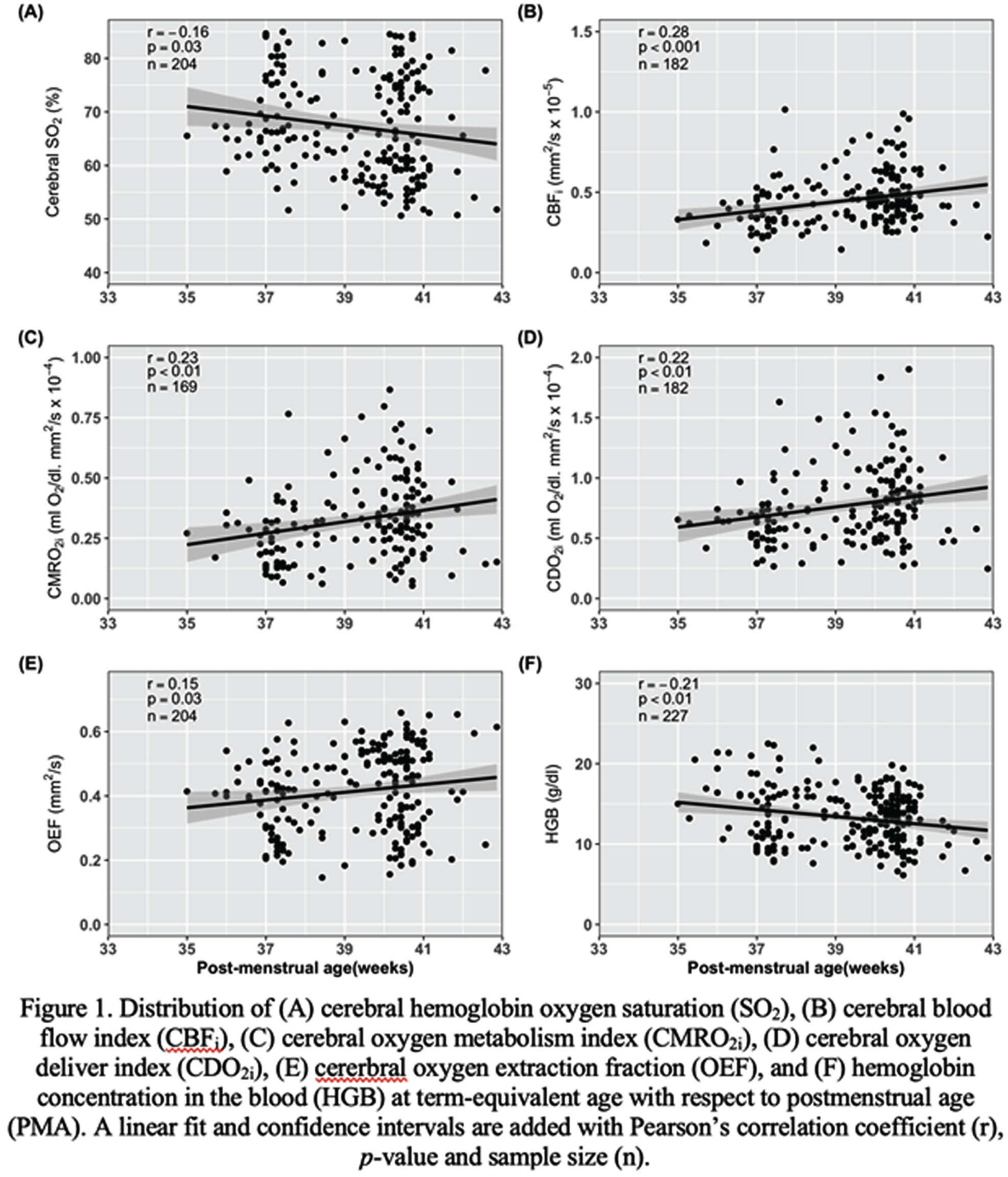
CONCLUSION: Increased cerebral oxygen availability (via increases in CBFi and CDO2i) and increased cerebral oxygen use (via increases in CMRO2i, OEF and a decrease in SO2) over PMA may indicate increased metabolic demand to support maturational processes involved in brain development2, 3. These parameters (except CBFi) were also associated with cognitive and language domains. These associations demonstrate the potential role of bedside optical neuromonitoring as a tool to identify early biomarkers of neurodevelopment in premature infants.
BIBLIOGRAPHY:
1. Statistics Canada. Live births, by weeks of gestation [cited 2022 07 December].
2. Côté-Corriveau, G., et al., Frontiers in Neuroscience, 2023. 17.
3. Ouyang, M., et al., Neuroimage, 2017. 147: p. 233-242.
Endocrine, ophthalmic and developmental outcomes of children prenatally diagnosed with midline brain defects
McKenna Coletti1, Jen Keene1, Allison Smego1, Marielle Young1, Betsy Ostrander1
1Primary Children’s Hospital
BACKGROUND: Midline brain malformations (MBMs) are common prenatally diagnosed brain malformations associated with endocrinologic, ophthalmic and developmental adverse outcomes [1]. These comorbidities are imperative to identify early to optimize neurodevelopmental outcomes and avoid medical complications. The post-natal evaluation of infants with MBMs is poorly studied with a paucity of information available on the optimal timing and frequency of post-natal studies [2,3]. Our regional children’s hospital has had a multi-specialty pathway in place to guide evaluation of children with prenatally identified MBMs for the last five years. The goal of this study is to evaluate is the effectiveness of this approach at identifying complications of prenatally identified MBMs in order to support prenatal counseling and multidisciplinary care.
METHODS: A retrospective review was conducted of all neonates identified prenatally with suspected MBMs between 2018 and 2022 at the only multidisciplinary referral center in a multistate region. Abnormalities were categorized as isolated vs complicated absent cavum septum pellucidum(aCSP) (N=11 vs N=13) or corpus callosum abnormalities (aCC) (N=11 vs N=43) or holoprosencephaly spectrum (N=12). We assessed subsequent diagnoses and outcomes using a standardized assessment pathway (Fig 1).
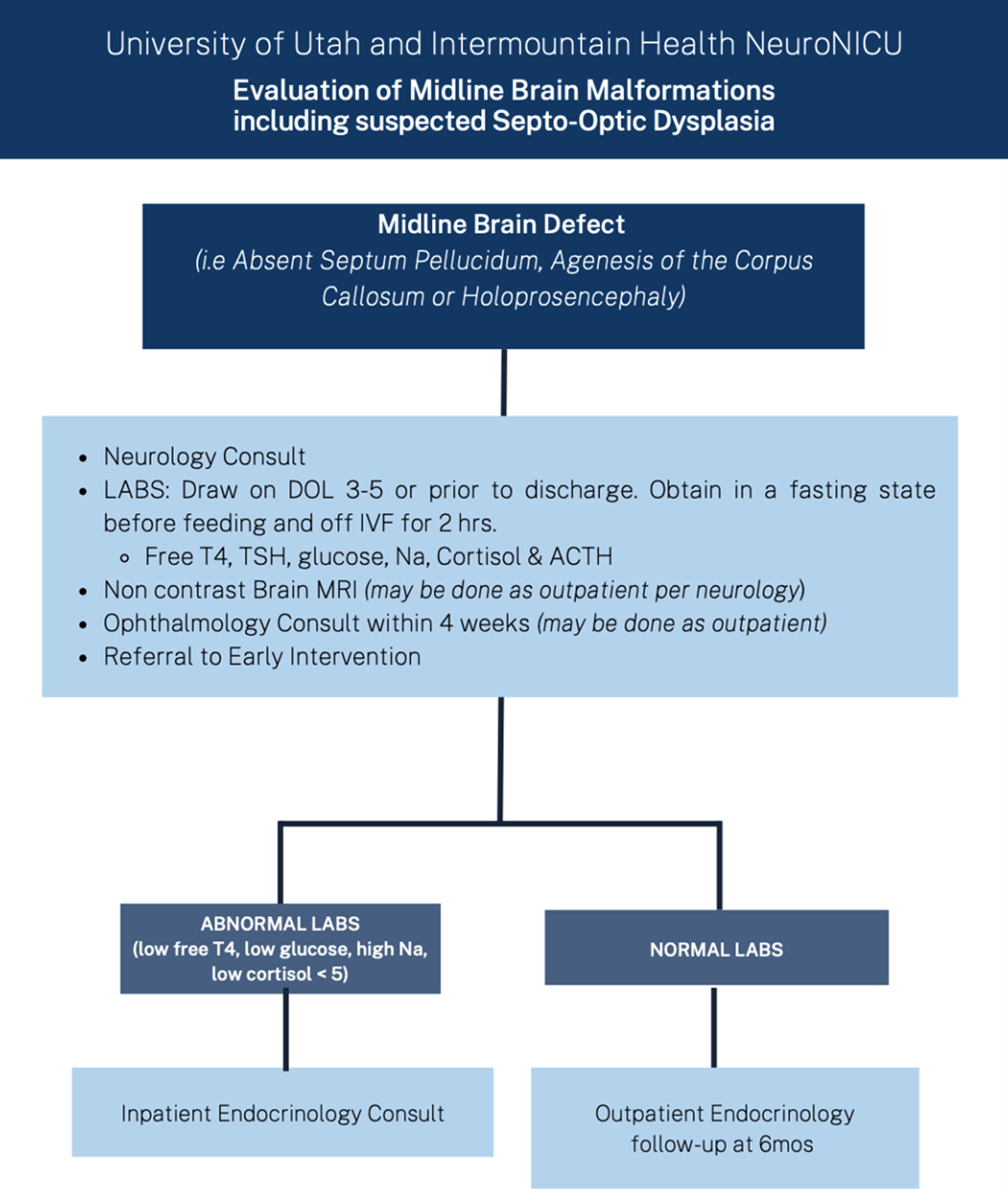
RESULTS: Of the 100 patients that were evaluated, 90 were seen in follow-up. Infants with holoprosencephaly were significantly more likely to die than patients with isolated aCSP or aCC (p=0.02) (Fig 2). There were no significant differences between rates of diagnosis for endocrine, ophthalmic, or epileptic complications between groups, with all types of defects demonstrating a risk for complications. The median time to diagnosis of optic nerve hypoplasia (ONH) was 3 days and initial identification of endocrine concerns was 7 days (Table 1). Surviving infants with holoprosencephaly had universal developmental delay, significantly more than the 10% seen in isolated aCSP or aCC (p=0.007) and infants with isolated MBMs were significantly more likely to be alive and without endocrine, ophthalmic, developmental, or epileptic diagnoses at last follow-up than other groups (isolated aCSP =67%, aCC 82%) (Fig 2).
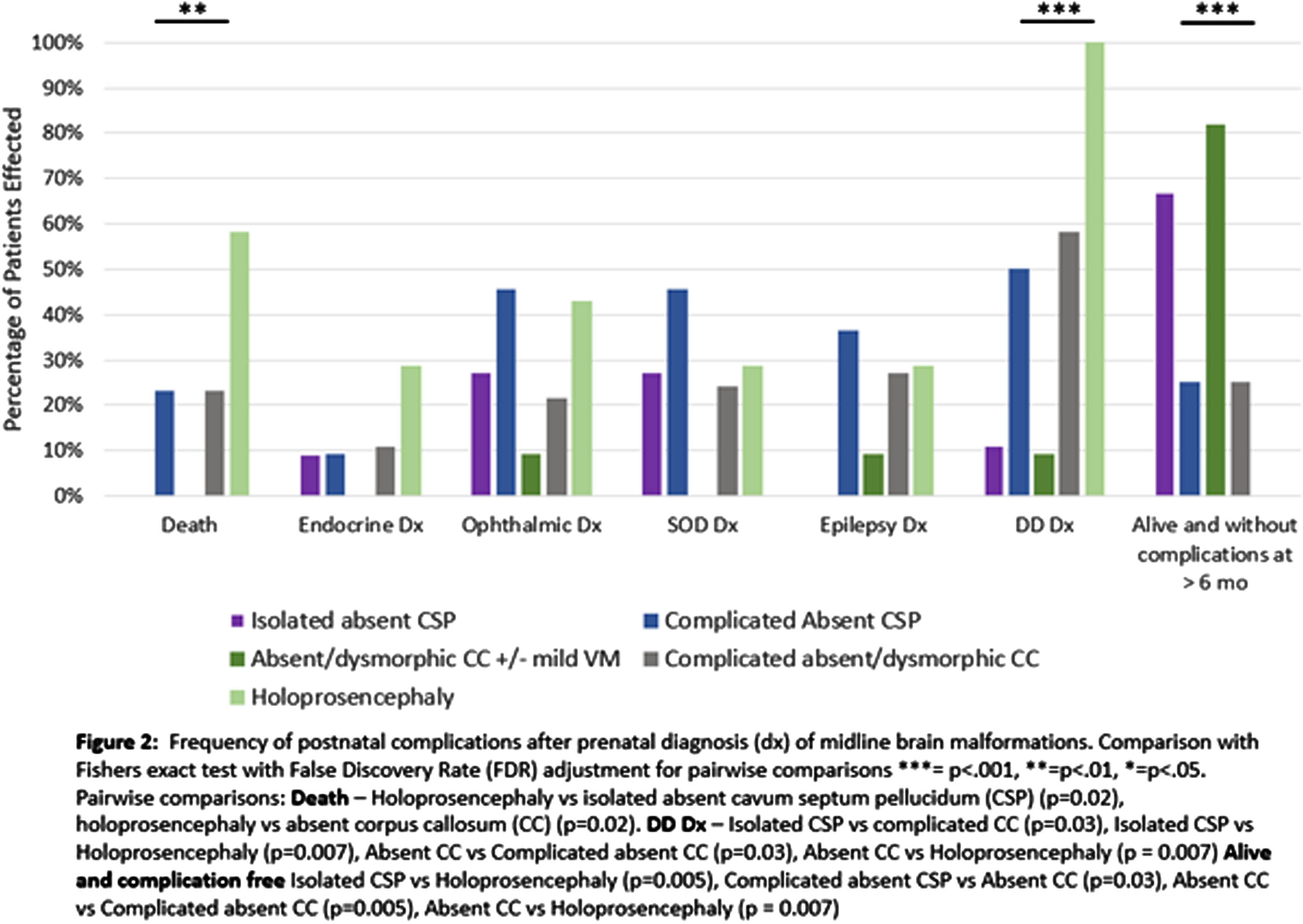
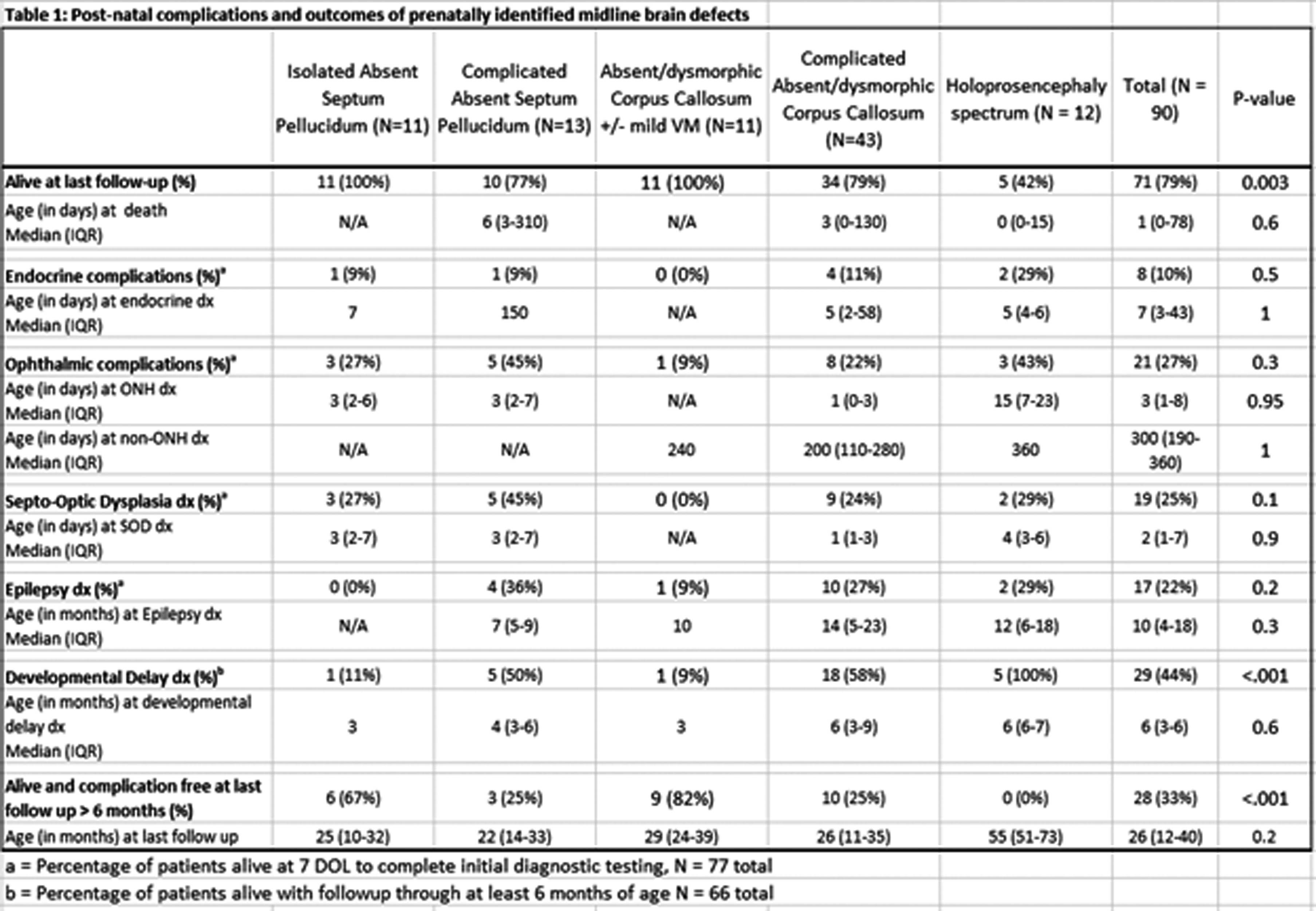
CONCLUSION: Our study shows the importance of endocrine and ophthalmic screening in all infants with midline brain defects, ideally shortly after birth. The majority of infants with isolated aCSP or aCC did not have any MBM associated diagnoses at last follow-up but all groups had a risk of comorbidities and would benefit from multispecialty post-natal monitoring.
BIBLIOGRAPHY:
[1] Webb and Dattani. European Journal of Human Genetics 2010 18:4 2009;18:393–7
[2] Cerbone et al. EClinicalMedicine 2020;19:100224
[3] Ganau et al. Eye Brain 2019;11:37
Understanding the influence of placental pathology on the development of neonatal encephalopathy: A case-control study
Áine Fox1,2, Adam Reynolds1,2, Emma Doyle1,3, Michael Geary1,4, Rocco Cuzzilla1,2,5, Breda Hayes1,2
1Royal College of Surgeons (RCSI), 2Department of Neonatology, Rotunda Hospital, 3Department of Pathology, Rotunda Hospital, 4Department of Obstetrics, Rotunda Hospital, 5Department of Neonatology, The Women’s
BACKGROUND: Neonatal encephalopathy (NE) secondary to hypoxic ischaemia (HI) remains incompletely understood. The role of the placenta in the pathogenesis of NE has come into the spotlight recently however the relationship between NE and the placenta has yet to be clearly defined [1]. Understanding the influence of the placenta on NE could enable improved identification of fetuses at-risk with individualised delivery plans for higher risk maternal/placental/fetal triads. It could open up new avenues for therapeutic interventions for babies with NE with placental pathology. There is limited research comparing the differences in placenta histology for babies with NE compared with controls [2, 3]. This is limiting our ability to understand the impact of placental histology on the pathogenesis of NE. This study hypothesized that placental pathology is more common in babies born with NE compared with healthy controls.
METHODOLOGY: This was a retrospective case control study performed in single tertiary NICU. Cases were (near-) term babies with moderate or severe NE born between 2006-2021.
Controls were healthy babies born between Jan 2022-Jan 2023 >=36+0 gestation, 5-minute Apgar score>=8 and no resuscitation after 5-minutes.
Placental histology was reviewed by one perinatal histopathologist (ED) using the 2016 Amsterdam consensus guidelines for reporting placental histology [4].
RESULTS: Seventy-one cases and 98 controls were included. Twenty-seven (38%) cases had a sentinel event and 15(21%) cases died. Thirty-five (49%) had moderate encephalopathy and 36(51%) had severe encephalopathy.
Cases had a higher mean placental weight with a wider standard deviation(SD) however both cases and controls had a birth weight to placenta weight ratio within acceptable limits. Cases had a higher incidence of pathology compared with controls with higher incidence of histological chorioamnionitis, fetal vascular malperfusion(FVM) and delayed villous maturation (Table 1).
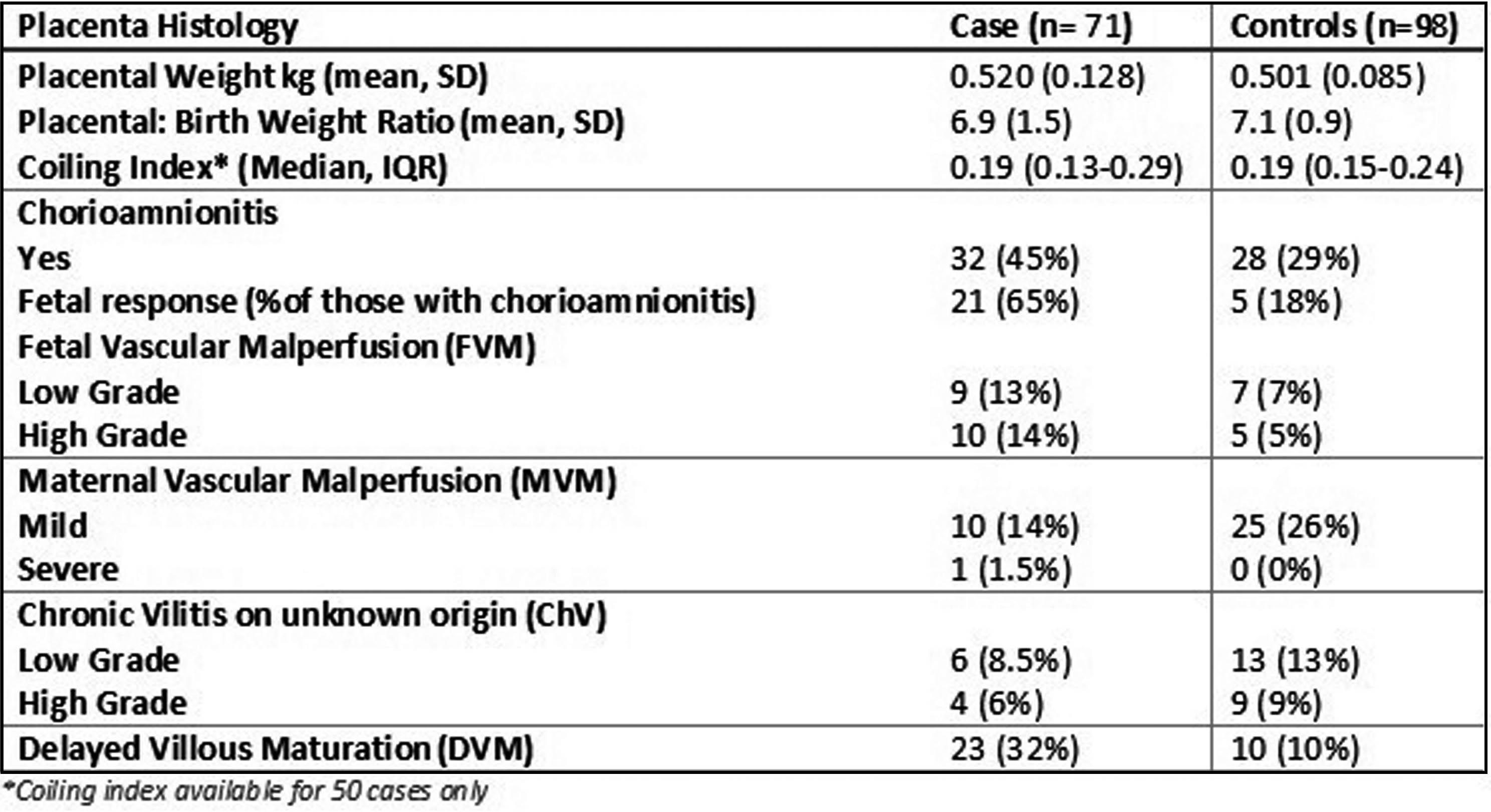
CONCLUSION: This case-control study demonstrates that there is a higher incidence of placental pathology for babies born with NE compared with controls. This suggests that placental pathology is either a cause of neonatal encephalopathy or leads to reduced tolerance of labour leading to NE. Further research is required to further delineate the role of the placenta in the pathogenesis of NE.
BIBLIOGRAPHY:
1. Penn, A.A., et al., Placental contribution to neonatal encephalopathy. Semin Fetal Neonatal Med, 2021. 26(4): p. 101276.
2. Vik, T., et al., The Placenta in Neonatal Encephalopathy: A Case-Control Study. J Pediatr, 2018. 202: p. 77-85.e3.
3. Hayes, B.C., et al., The placenta in infants >36 weeks gestation with neonatal encephalopathy: a case control study. Arch Dis Child Fetal Neonatal Ed, 2013. 98(3): p. F233-9.
4. Khong, T.Y., et al., Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med, 2016. 140(7): p. 698-713.
How does placental inflammation impact tolerance of labour at term?
Áine Fox1,2, Adam Reynolds1,2, Aisling Dunne2, Emma Doyle3, Ailbhe Tarrant4, Miriam Martinez-Biarge5, Michael Geary1,6, Rocco Cuzzilla1,2,7, Breda Hayes1,2
1Royal College of Surgeons (RCSI), 2Department of Neonatology, Rotunda Hospital, 3Department of Pathology, Rotunda Hospital, 4Department of Radiology, Rotunda Hospital, 5Department of Paediatrics, Hammersmith Hospital, 6Department of Obstetrics, Rotunda Hospital, Dublin, 7Department of Neonatology, The Royal Women’s Hospital
BACKGROUND AND OBJECTIVES: Histological chorioamnionitis (HCA) is associated with increased neonatal morbidity and mortality. HCA likely causes fetal inflammatory response (FIR) in the fetus and leads to altered perfusion which in turn decreases the capacity of the fetus to tolerate hypoxic-ischaemia (HI) in the perinatal period.
This study hypothesized that placental inflammation is associated with increased severity of brain injury for babies with neonatal encephalopathy (NE) compared to those with NE without placental inflammation.
METHODOLOGY: This was a retrospective cohort study of (near-) term babies with moderate or severe NE born between 2006-2021 in a single tertiary NICU.
Placental histology was reviewed using the 2016 Amsterdam consensus guidelines for reporting placental histology [1]. MRIs were scored using a validated scoring system [2].
RESULTS: Seventy-one babies had placenta available. Twenty-seven (38%) cases had a sentinel event and 15 (21%) cases died. Thirty-six (51%) had severe NE.
Thirty-two (45%) babies had HCA with 21 (65%) having fetal response. HCA was associated with higher intrapartum fetal heart rates and higher white cell counts after birth compared with those without.
Those with HCA had higher gestational age and less intrapartum sentinel events. HCA was also more prevalent in those with fetal vascular malperfusion (n=19) with ten (53%) having HCA. There was no association with other clinical characteristics (Table 1).
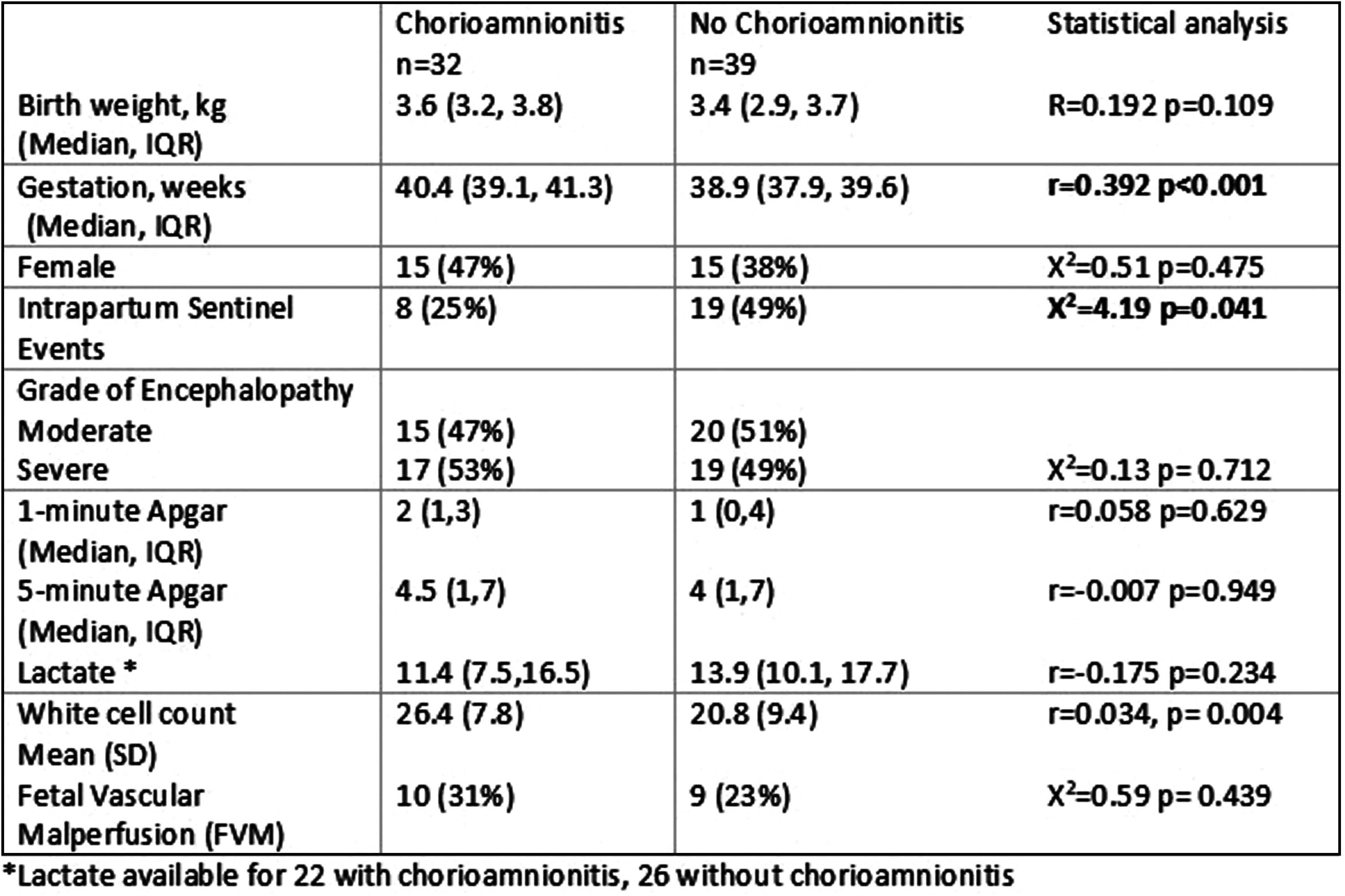
Of those with MRI available (n=55), there was no relationship between injury score and presence of HCA. There was a trend for less HCA for those who died or had higher brain injury scores, however this was not significant (Table 2).
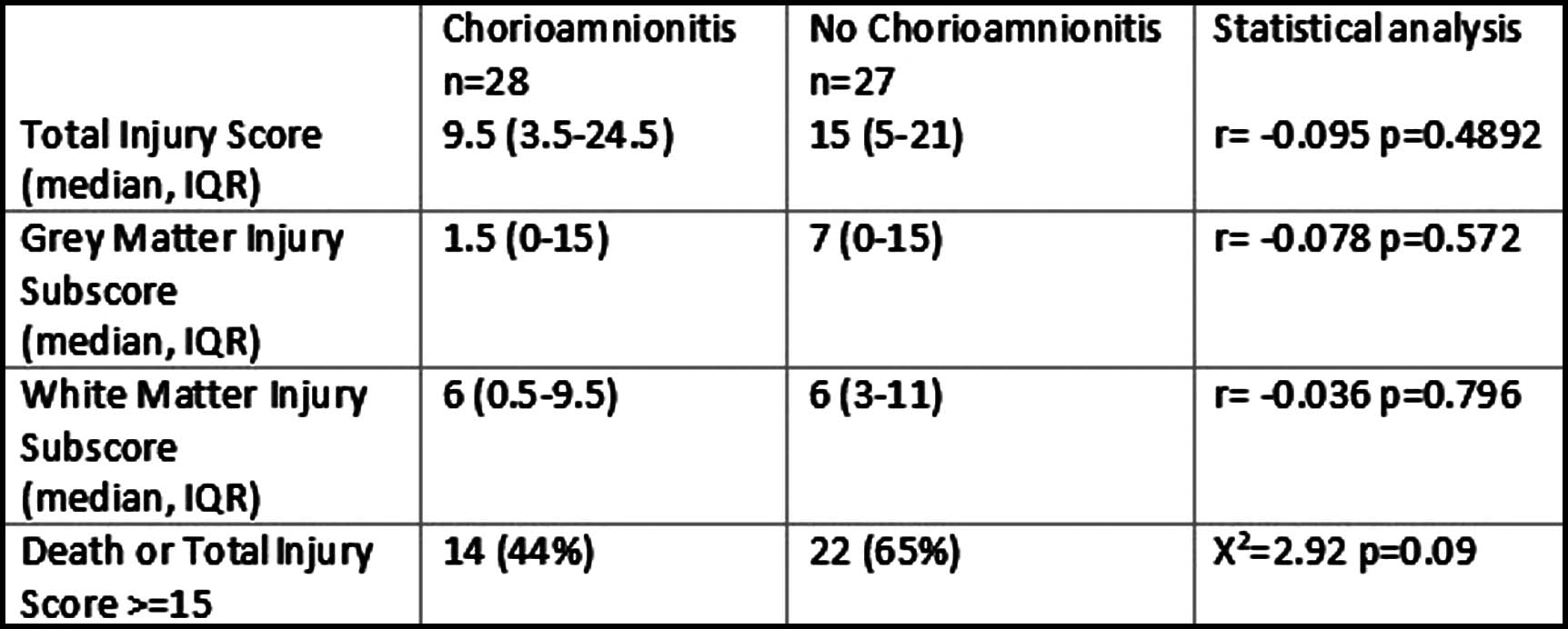
CONCLUSION: This cohort study demonstrates an increased incidence of HCA compared to reported incidence in a healthy population [3]. HCA is associated with increased risk of NE however it is not associated with grade of encephalopathy, death or brain injury scores. Given the lower incidence of HCA in cases with a history of sentinel event, HCA may play a greater role where the cause of poor tolerance of labour is less well understood. Although not associated with short term outcomes following NE, follow on studies are required to assess the role of HCA on longer term outcomes.
BIBLIOGRAPHY:
1. Khong, T.Y., et al., Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med, 2016. 140(7): p. 698-713.
2. Weeke, L.C., et al., A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr, 2018. 192: p. 33-40.e2.
3. Conti, N., et al., Term histologic chorioamnionitis: a heterogeneous condition. European Journal of Obstetrics & Gynecology and Reproductive Biology, 2015. 188: p. 34-38.
The emergence of consciousness-state dependent complexity: SEPs and perturbation complexity index in newborns and infants
Lorenzo Riboldi1, Chiara Peila1, Irene Ronga2,3, Karol Poles2, Simone Russo4, Angela Comanducci, Alice Rossi Sebastiano2, Alessandra Coscia1, Francesca Garbarini2
1Neonatal Unit, Department of Public Health and Pediatrics, University of Turin, 2Manibus Lab, Department of Psychology, University of Turin, 3BIP Research Group, Department of Psychology, University of Turin, 4Department of Biomedical, Surgical and Dental Sciences, University of Milano, 5RCCS Fondazione Don Carlo Gnocchi Onlus
BACKGROUND: The comparison between sleep and wakefulness has largely been employed as a model to investigate unconscious and conscious sensory processing. When consciousness fades, EEG responses are stronger as the disruption of effective cortical connectivity occurs. The Perturbational Complexity Index (PCI-st) has recently been proposed as an objective measure of the brain response complexity, that correlates well with the level of consciousness and quantifies the responses to a perturbation of the EEG signal, indexing whether the signal remains segregated or if it spreads in a large network.
Previous studies have shown that somatosensory evoked potentials (SEPs) are influenced by sleep-stages with larger SEPs during sleep compared to during wakefulness, which have been demonstrated from birth.
In the present study we aimed to evaluate whether the complexity of brain responses in term of event-related potentials could discriminate between conscious states early in life, capitalizing on the recently devised approach based on the computation of PCI-st on the EEG responses elicited by peripheral stimulations.
METHODOLOGY: A between and within subjects analysis was performed in two samples: 46 newborns (12-76 hours of age) vs 22 infants (2-4 months of age). EEG responses to median-nerve tactile stimulation during wakefulness and sleep were collected. All subjects didn’t present any medical problem. SEPs’ amplitude and PCI-st values were compared between conscious states in each developmental sample by means of matched paired t-tests. Furthermore, we used Pearson correlation coefficient between the age (in hours for newborns and in days for infants) and the delta between the PCI-st values in wakefulness and sleep.
RESULTS: Both samples showed an enhancement of SEPs amplitude during sleep as compared to wakefulness [newborns: centroparietal channels, in a 140-160 ms time-window, t 16 =-3.37, p=0.004 (CP6); infants: frontocentral and parietal channels, in a 185-220 ms time-window, t 16 =3.69, p=0.002 (FC5)]. By comparing PCI-st values, a reversed pattern was found between the two samples. While infants, similarly to adults, showed higher PCI-st values during wakefulness than sleep (t=4.76; p=0.0006), newborns showed significantly higher PCI-st values during sleep than wakefulness (t=3.91; p=0.001). Moreover, while the age correlated with the difference between wakefulness and sleep in infants (r=0.58; p=0.02), this was not observed in newborns (r=-0.1; p=0.71).
CONCLUSION: We can state that while SEP values show greater segregation of brain responses during sleep already at birth, PCI-st values suggest that the working structure related to brain complexity is fully developed only in the postnatal period: newborns, whose functional connectivity maturation is mainly triggered by endogenous drives, show greater complexity in sleep than in wakefulness, whereas, infants whose brain maturation is mainly experience-dependent show greater brain complexity in the awake state, presenting the same pattern as that seen in adults.
Cranial ultrasound evaluation of brain growth in infants conceived through medically assisted reproduction
Valentina Rizzardi1, Ida Sirgiovanni1, Elisabetta Ghezzi1, Milena Bray1, Andrea Busnelli2, Fabrizio Ciralli1
1U.O. di Neonatologia e Patologia Neonatale, Humanitas San Pio X, Humanitas University, Milan, Italy, 2U.O. di Ginecologia e Medicina Riproduttiva, Centro di Fertilizzazione, IRCCS Humanitas Research Hospital, Humanitas University Milan, Italy
BACKGROUND: The interest regarding the impact of ART (Assisted reproductive Technologies) on neurodevelopment is increasing, due to the susceptibility1 of the CNS to external stimuli during fetal life. However, studies show inconsistencies in the ART role in neurodevelopment2 3.
In the postnatal period, brain linear measurements at cranial ultrasound (cUS) can be used as quantitative markers of brain growth, which is an important predictor of neurodevelopmental outcomes4 5 6.
OBJECTIVES: The aim of the study was to assess brain measurements of infants conceived by ART compared with those conceived spontaneously (SC) using cUS.
METHODOLOGY: We conducted a retrospective study at Humanitas San Pio X Hospital of Milan. We enrolled term singletons born from January 2002 to the end of April 2023 conceived through ART and SC infants who underwent a cUS scan within the first 7 days of life. Exclusion criteria were prematurity, gemellarity, cerebral cysts, intraventricular hemorrhage, congenital cerebral anomalies, head circumference <3rd or >97th pct, transfer to a neonatal intensive care unit. cUS scans were performed through the anterior fontanelle by senior neonatologists and fourteen brain structures (Figure 1) were compared between ART and SC infants. Socio-demographic maternal variables and basic infants’ characteristics were collected from clinical records.
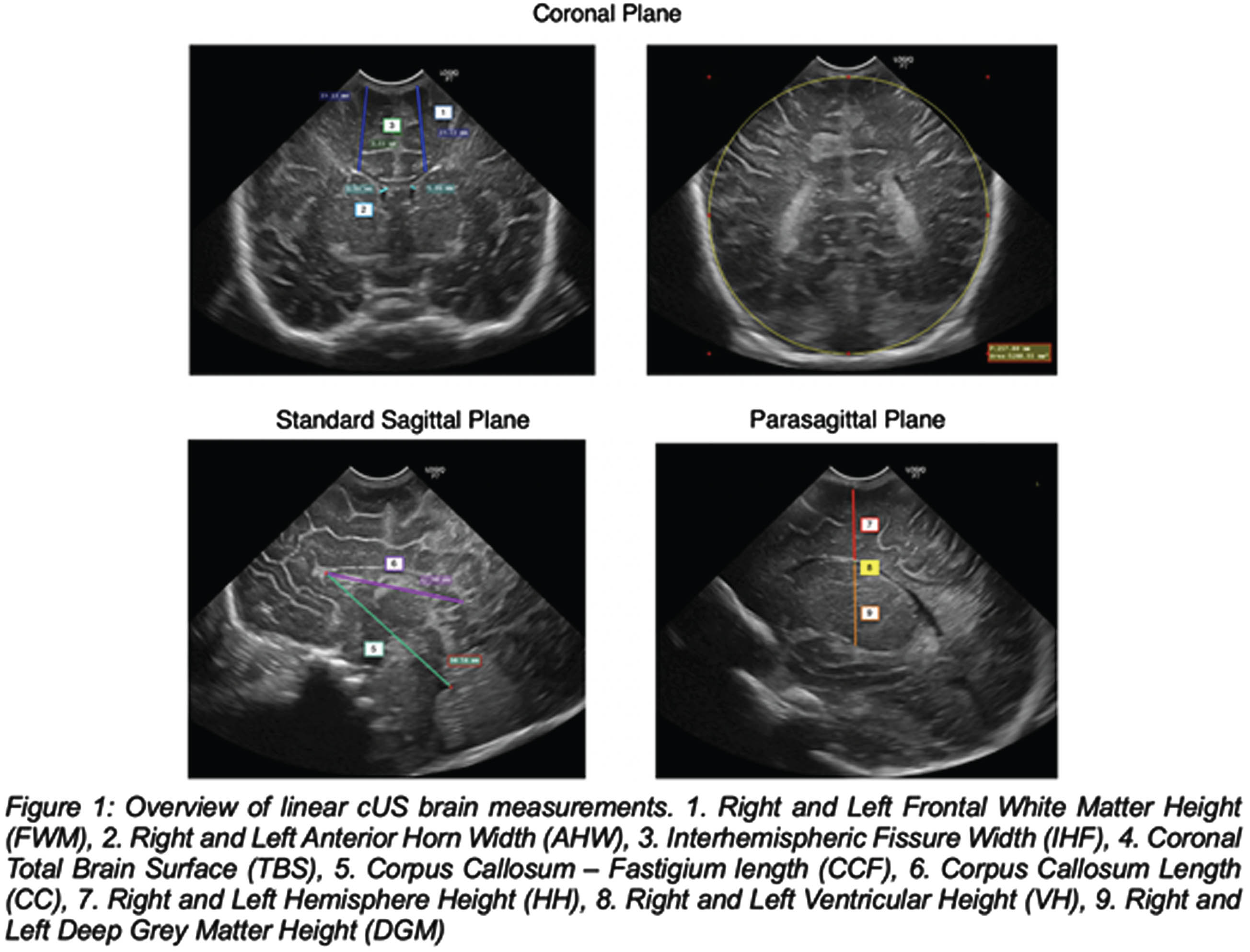
Quantitative and qualitative variables were compared through independent t-test and Fisher’s Exact Test respectively. HC at birth and cUS measurements relationship was assessed via linear regression.
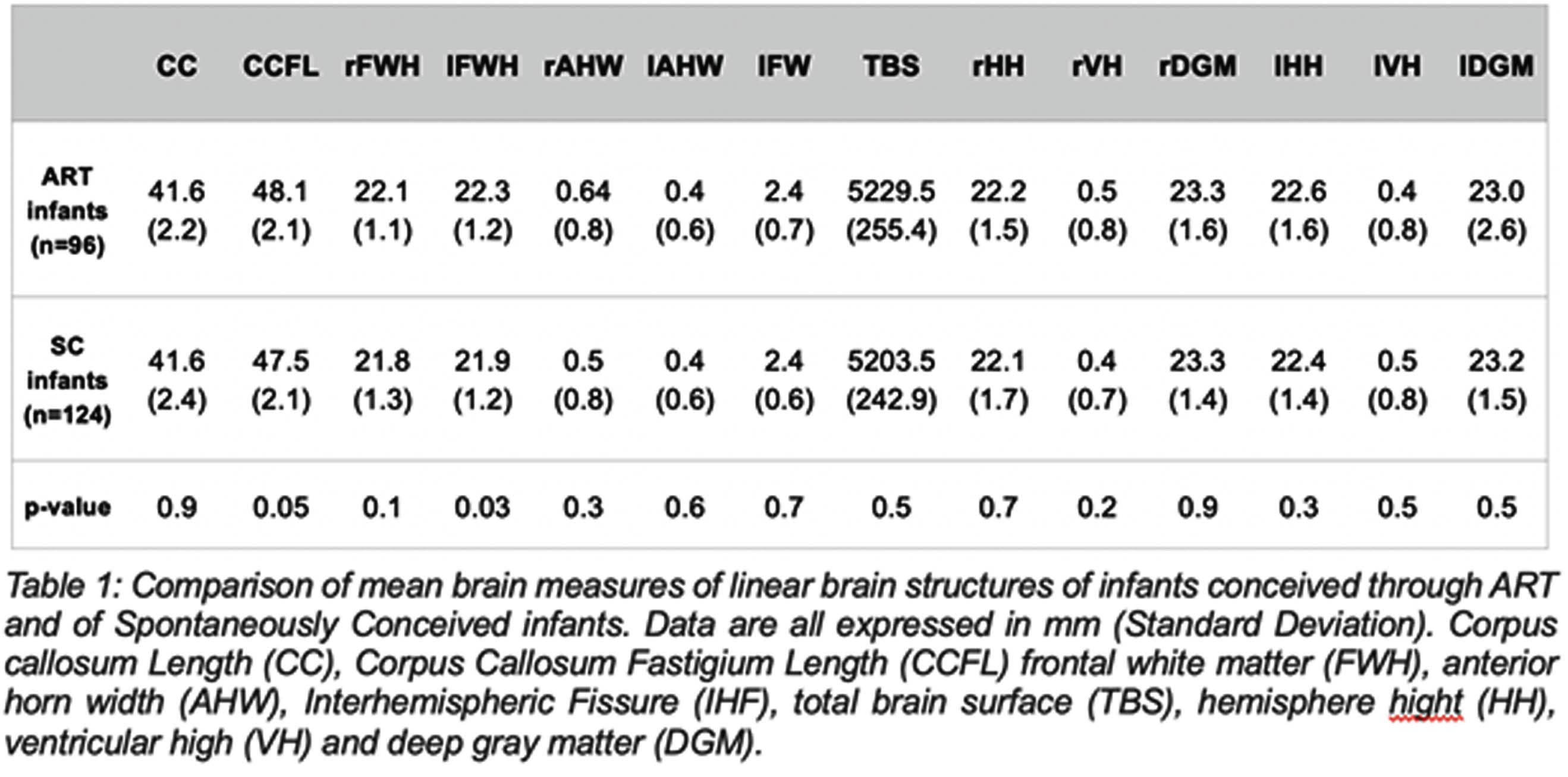
RESULTS: Brain measurements of 96 ART infants and 124 term SC infants were determined on cUS (Table 2). The corpus callosum – fastigium length (48.1 mm vs 47.5 mm, p = 00.5) and the left frontal white matter height (22.3 mm vs 21.9 mm, p = 0.03) were greater in the case group than in the controls. However, after adjusting for HC at birth these differences became non-significant (p = 0.2 and p = 0.1, respectively). No statistically significant differences were found in corpus callosum length, right frontal white matter height, anterior horn width, total brain surface, interhemispheric fissure, ventricular and deep grey matter height.
CONCLUSION: This is the first study to analyze linear brain measurements in ART-conceived infants and to compare them to naturally conceived ones. Results show similar brain growth, implying ART doesn’t affect it. The main limitation of the study is the retrospective design. More research is needed to link ART-conceived infants’ brain growth to their neurodevelopment.
BIBLIOGRAPHY:
1 J. C. Silbereis et al, 2016, Neuron
2 M. L. Boutet et al., 2022, Ultrasound in Obstet & Gyne 60 (5)
3 A. Pinborg et al, 2019, Int J Obstet
4 R. Arena et al., 2021, Am J Perinatol
5 V. Boswinkel et al., 2021, Early Human Development
6 R. Cuzzilla et al., 2023, Arch Dis Child Fetal Neonatal Ed, p. fetalneonatal-2022-324660
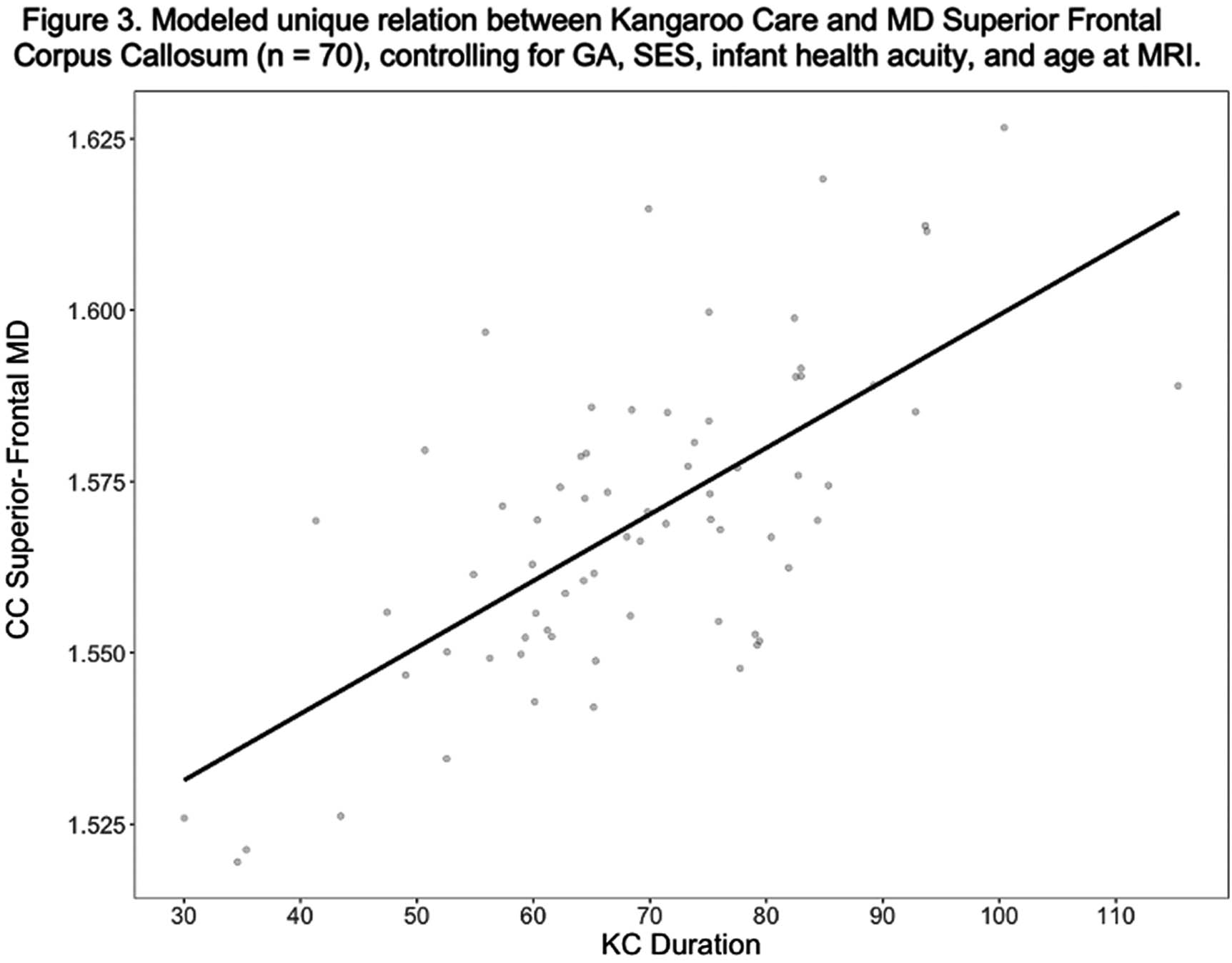
Relations between kangaroo care and neonatal white matter connectivity in infants born very preterm
Katherine Travis1, Molly Lazarus1, Lisa Bruckert1, Virginia Marchman2, Sarah Dubner1, Rocio Poblaciones1, Heidi Feldman1, Melissa Scala3
1Stanford University Department of Pediatrics, Division of Developmental-Behavioral Pediatrics
2Stanford University Department of Psychology
3Stanford University Department of Pediatrics, Division of Neonatology
BACKGROUND AND OBJECTIVES: Kangaroo Care (KC), or skin-to-skin care, is a developmental care method beneficial to health outcomes in preterm infants. Children born very preterm (VPT <32 weeks gestational age (GA)) are at-risk for white matter injuries and abnormal white matter development.1 Aberrant white matter development is linked to adverse neurodevelopmental outcomes in VPT children.² KC is often encouraged in the neonatal intensive care unit as a neuroprotective strategy, yet evidence linking hospital-based KC practices to neonatal structural brain development is limited. The present study examined relations between KC and brain white matter connectivity from near-term diffusion MRI (dMRI) scans.
METHODOLOGY: We performed a retrospective cohort study of male and female VPT infants (N=100). Infants underwent diffusion MRI scanning prior to hospital discharge as part of the standardized near-term imaging protocol. Post-menstrual age (PMA) at scan was 34.4 - 41.2 weeks. White matter connectivity was assessed with mean diffusivity (MD) and fractional anisotropy (FA) measured from four fronto-limbic white matter tracts (superior frontal corpus callosum (CC), cingulate, anterior thalamic radiation, uncinate). Medical staff recorded daily minutes of parent-administered KC in the electronic medical record. We expressed KC as duration (minutes of KC / instance). We performed hierarchical linear regressions to assess the unique contribution of KC to white matter connectivity of each pathway after controlling for GA, socio-economic status (SES), health acuity and PMA at scan.
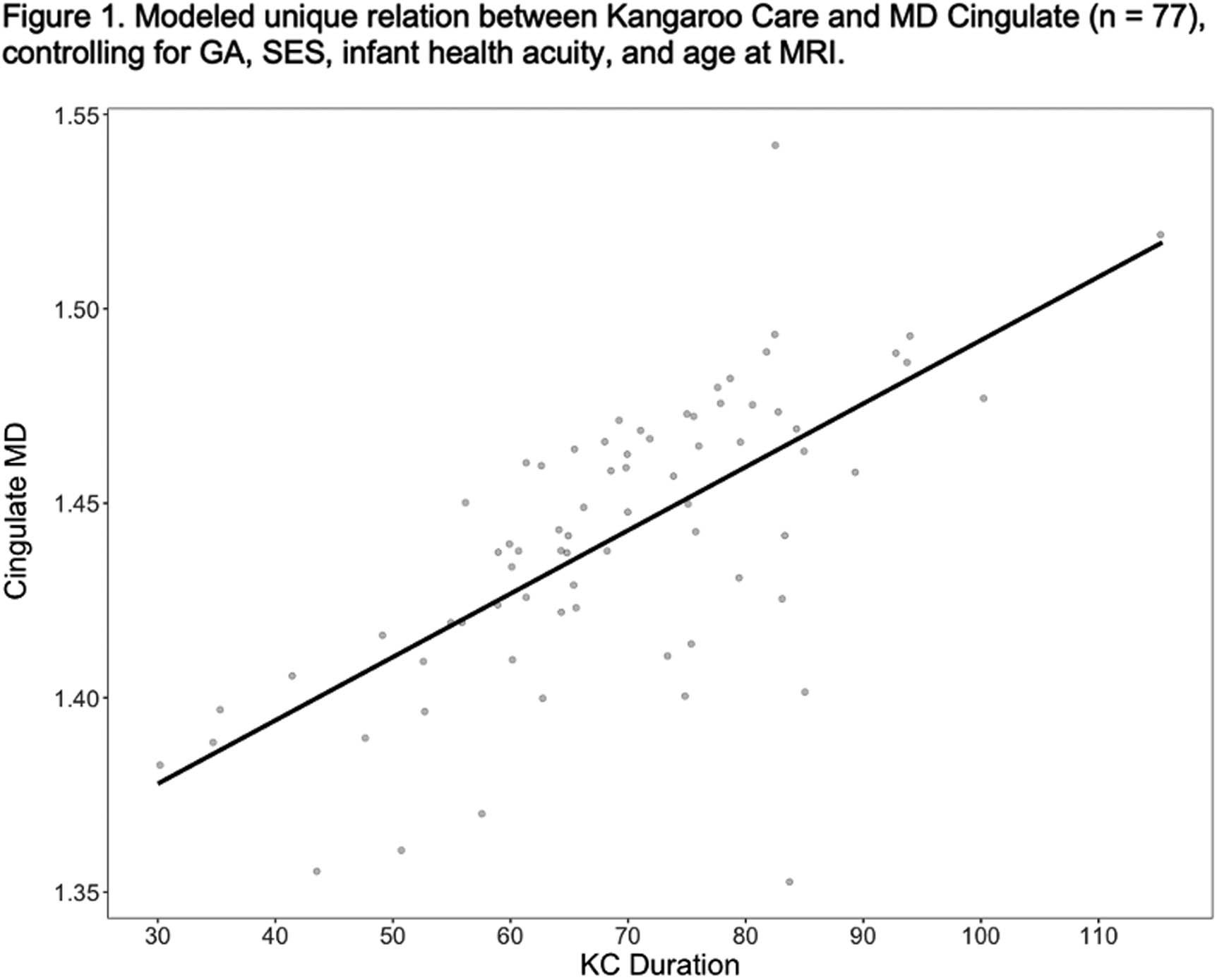
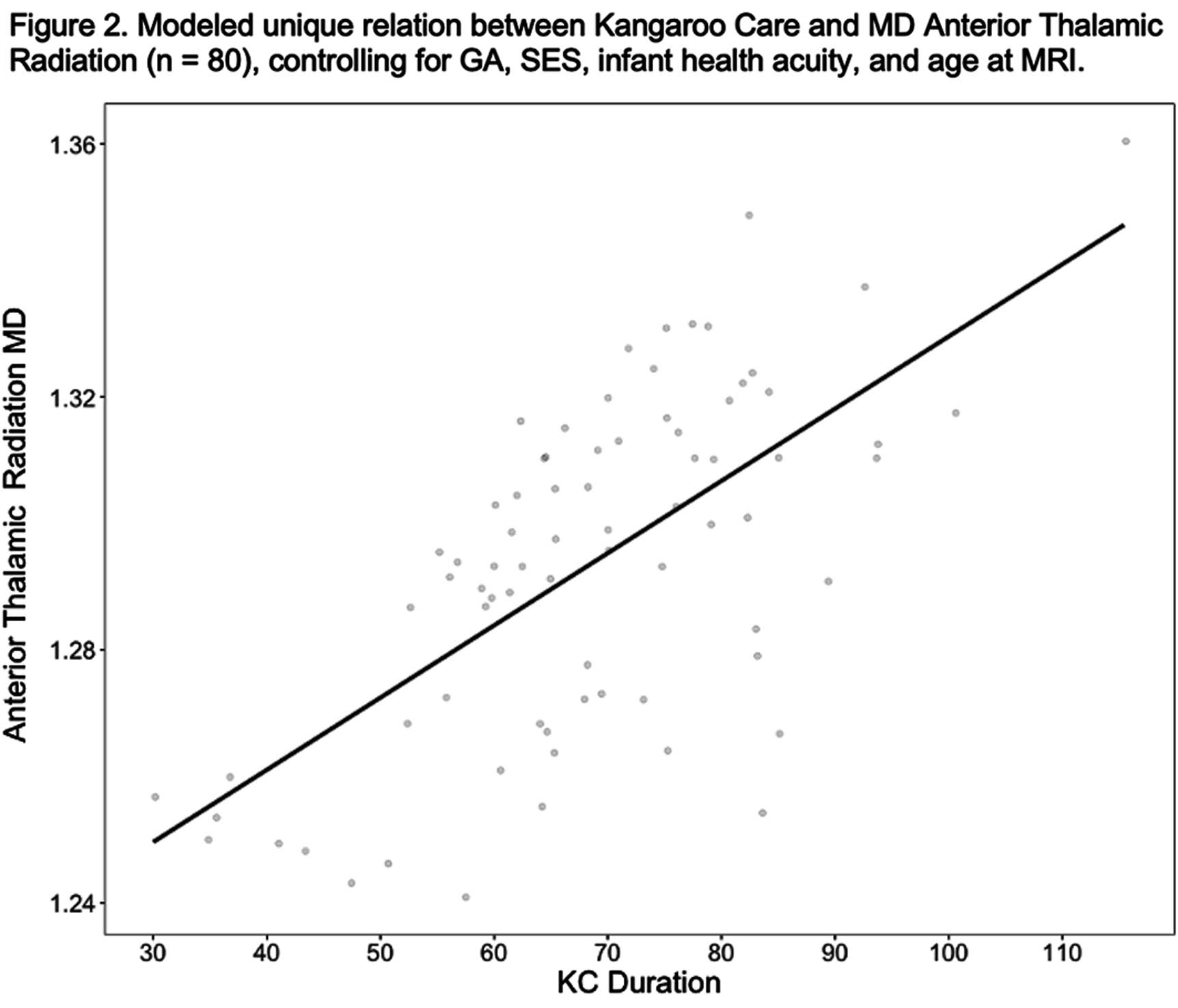
RESULTS: KC predicted cingulate (B = .002; p =.005) and anterior thalamic (B = .002; p = .043) mean MD over and above GA, SES, health acuity, and PMA at scan. KC marginally predicted superior frontal CC MD (B =.001, p = .097) and did not predict uncinate mean MD or mean FA from any of these tracts.
CONCLUSION: Variations white matter brain development in VPT infants related to the duration of KC instances lasted during hospitalization. These associations suggest that the experience of KC may be a unique contributor to fronto-limbic white matter connectivity. These findings also indicate that longer durations of KC instances beyond the minimum recommended time of 60-90 minutes may be important for brain development. Ongoing analyses will explore whether fronto-limbic white matter connectivity mediates relations between KC and long-term neurodevelopmental outcomes.
BIBLIOGRAPHY
1 Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017 Sep;134(3):331-349. doi: 10.1007/s00401-017-1718-6. Epub 2017 May 22. PMID: 28534077; PMCID: PMC5973818.
2 Inder TE, Volpe JJ, Anderson PJ. Defining the Neurologic Consequences of Preterm Birth. N Engl J Med. 2023 Aug 3;389(5):441-453. doi: 10.1056/NEJMra2303347. PMID: 37530825.
Type of intravenous lipid emulsion affects very preterm brain development
Katherine Ottolini1, Sudeepta Basu1, Kushal Kapse1, Tameka Watson1, Patricia Saulino1, Jonathan Murnick1, Adre DuPlessis1, Catherline Limperopoulos1, Nickie Andescavage1
1Children’s National Hospital
BACKGROUND AND OBJECTIVES: Intravenous lipid emulsions (ILE) are an essential component of early preterm nutritional support. Multicomponent lipid emulsions (MLE) containing soybean, olive, coconut, and fish oil are being increasingly utilized over traditional soybean-only lipid emulsions (SLE); MLE contains higher omega-3 fatty acid and vitamin E levels with anti-inflammatory and antioxidant properties. The impact of MLE compared to ILE on preterm brain development has not been previously studied using multi-modal quantitative brain magnetic resonance imaging (qMRI) and magnetic resonance spectroscopy (MRS) techniques. The aim of this study was to compare early brain growth and metabolism in very preterm infants receiving MLE versus SLE using qMRI and MRS at term equivalent age (TEA).
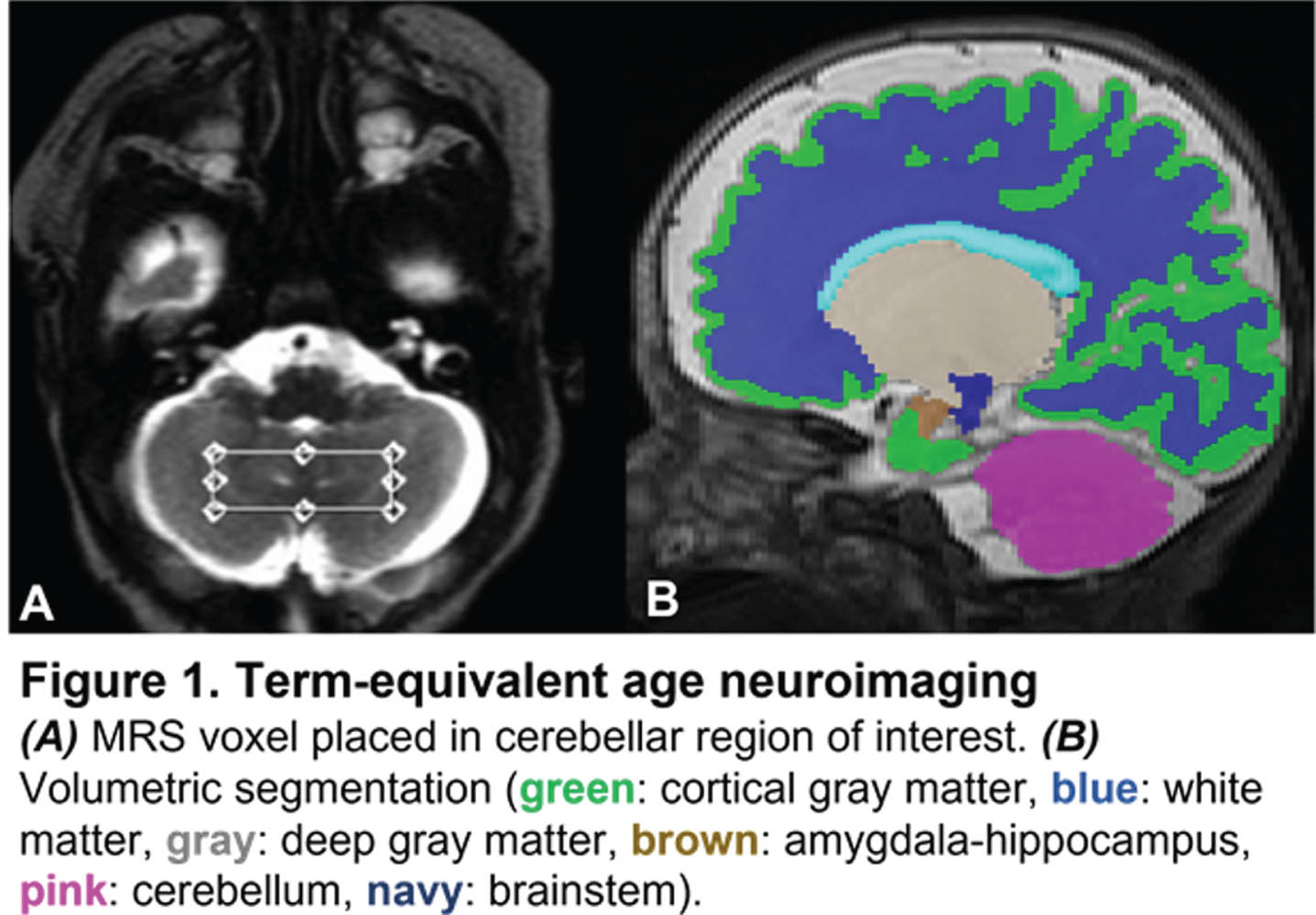
METHODOLOGY: Preterm infants were prospectively enrolled as part of an observational study. Human milk-fed infants born at very low birth weight (VLBW, <1500g) and gestational age (GA) ≤ 32 weeks admitted to our level IV neonatal intensive care unit within the first week of life were included for analysis. Infants with IVH (greater than Grade 2) and parenchymal brain injury were excluded. Infants born prior to January 2019 received SLE (Intralipid), while infants born after January 2019 received MLE (SMOFLipid) per unit nutritional policy. Brain MRI and MRS (cerebellum, TE = 35ms, TR = 2000ms) were performed at TEA. 3-D volumetric MRI data were acquired on a 3T scanner, from which regional (cortical gray matter, white matter, deep gray matter, amygdala-hippocampus, cerebellum, brainstem) and total brain volumes were calculated (Figure 1). Analysis of covariance was used to evaluate differences in TEA brain volumes and cerebellar metabolite levels based on ILE type, controlling for birth GA, age at MRI, and number of TPN days.
RESULTS: Nutritional and MRI data were acquired for 80 preterm infants. On average, infants who received MLE underwent TEA MRI later than SLE (40.6 ± 1.6 vs. 39.3 ± 2.1 weeks, p <0.01), with no other significant differences in baseline patient characteristics (Table 1A). Infants receiving SLE demonstrated larger deep gray matter and amygdala-hippocampal volumes, whereas those receiving MLE demonstrated larger brainstem volumes (Table 1B). Cerebellar MRS revealed significantly higher creatine, lactate, inositol, gamma-aminobutyric acid, and glutamate levels in infants who received SLE compared to MLE (Table 1C).
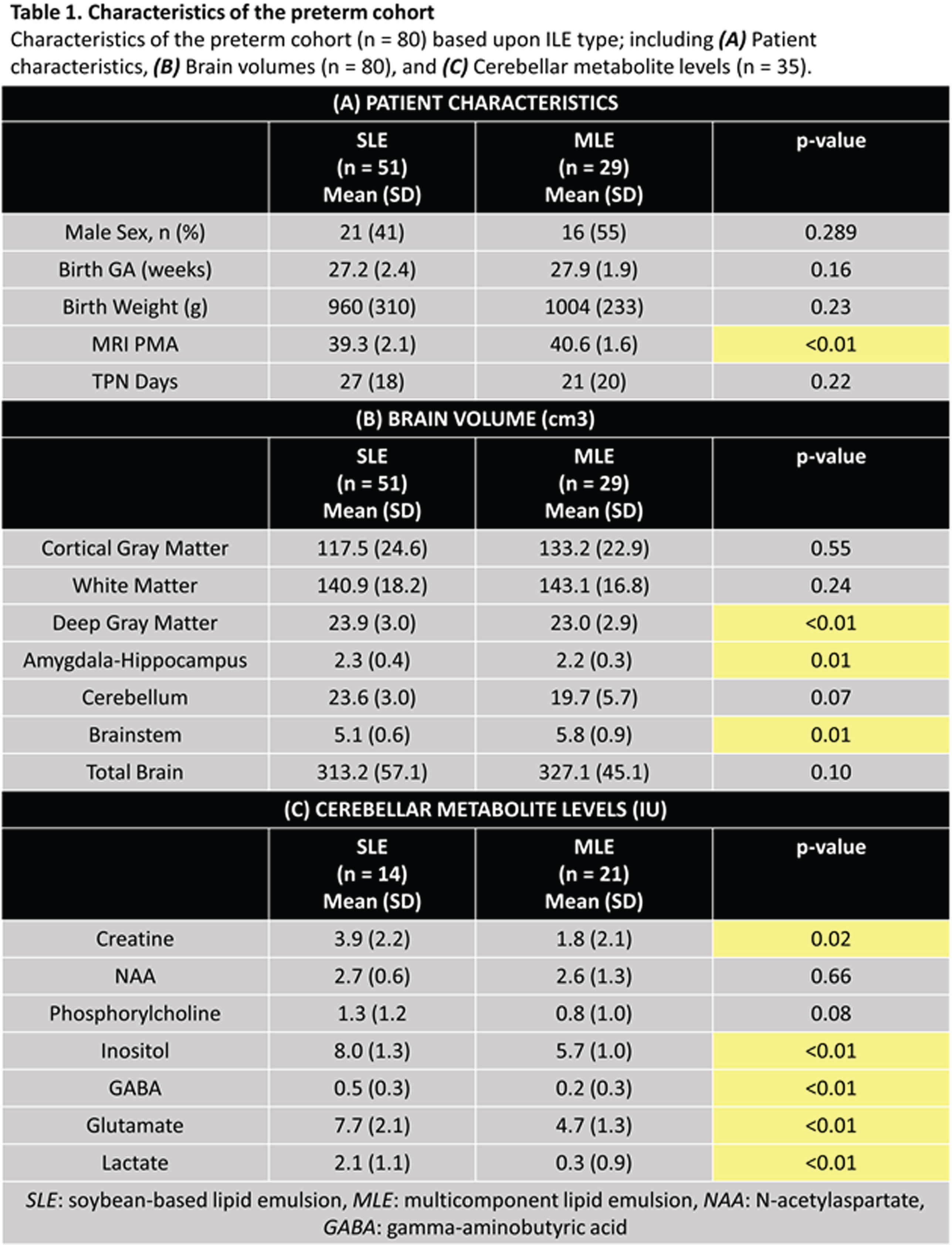
CONCLUSION: Preterm infants receiving MLE demonstrated distinct patterns of regional brain growth and cerebellar metabolism at TEA compared to SLE. Although infants receiving SLE demonstrated larger deep gray matter and amygdala-hippocampus volumes, they also exhibited increased lactate, a metabolite associated with brain injury, and inhibitory and excitatory neurotransmitters that could potentially signify disruptions in metabolic development. Further investigation is necessary and warranted to elucidate the effects of different types of ILEs on preterm brain development and assess the implications for long-term neurodevelopment.
Timing of growth failure in very premature infants and implications for brain development
Tameka Watson1, Katherine Ottolini1, Sudeepta Basu1, Kushal Kapse1, Jonathan Murnick1, Patricia Saulino1, Nickie Andescavage1, Adre Du Plessis1, Catherine Limperopoulos1
1Children’s National Hospital
BACKGROUND AND OBJECTIVES: Adequate nutrition and growth are essential for the developing human brain, which more than doubles in size during the third trimester. For preterm infants, postnatal growth throughout this critical developmental window has major implications for neurodevelopmental outcomes. Up to half of very preterm infants experience growth failure (GF), however the precise timing of this GF and associated implications for brain growth have not been well-described. The objective of this study was to determine the timing of postnatal GF in very preterm infants and then explore the relationship between somatic growth and brain development by term-equivalent age (TEA).
METHODOLOGY: We enrolled preterm infants born at ≤ 32 weeks’ gestation admitted within the first 2 weeks of life, excluding infants with parenchymal brain injury and small for gestational age (GA) at birth (weight z-score less than -2). Weekly growth data were recorded from birth to TEA and converted to z-scores. GF was defined as a decrease in weight z-score by greater than 1 from birth to TEA. Regional and total brain volumes were calculated from TEA brain MRI (3T). Growth trajectories of preterm infants with and without GF were assessed to identify differences in weight z-score at 4-week intervals from birth through TEA (Figure 1). For the time periods during which weight z-scores demonstrated significant divergence between the two groups, we then explored the relationship between weight z-score and brain volumes at TEA.
RESULTS: Growth data were acquired for 70 infants; 43 had GF. There were no significant differences in sex (49% vs. 44% male), birth weight (944±290g vs. 986±299g), birth GA (27.1±2.4 vs. 27.8±2.1 weeks), or corrected age (CA) at TEA MRI (39.5±1.8 vs. 39.9±2.4 weeks) between infants with and without GF. Infants with GF had lower weight z-scores at 36 weeks CA (-1.8±0.8 vs. -1.3±0.9, p = 0.028) and TEA (-2.1±0.9 vs. -1±0.8, p <0.001) (Figure 1). Weight z-scores at 36 weeks and TEA were significantly associated with TEA brain volumes. Weight z-score at 36 weeks CA positively correlated with deep gray matter (β = 0.274, p = 0.027) and amygdala-hippocampus (β = 0.250, p = 0.045) volumes. Weight z-score at TEA was positively associated with deep gray matter (β = 0.247, p = 0.042), white matter (β = 0.351, p = 0.003), and brainstem (β = 0.4, p <0.001) volumes.
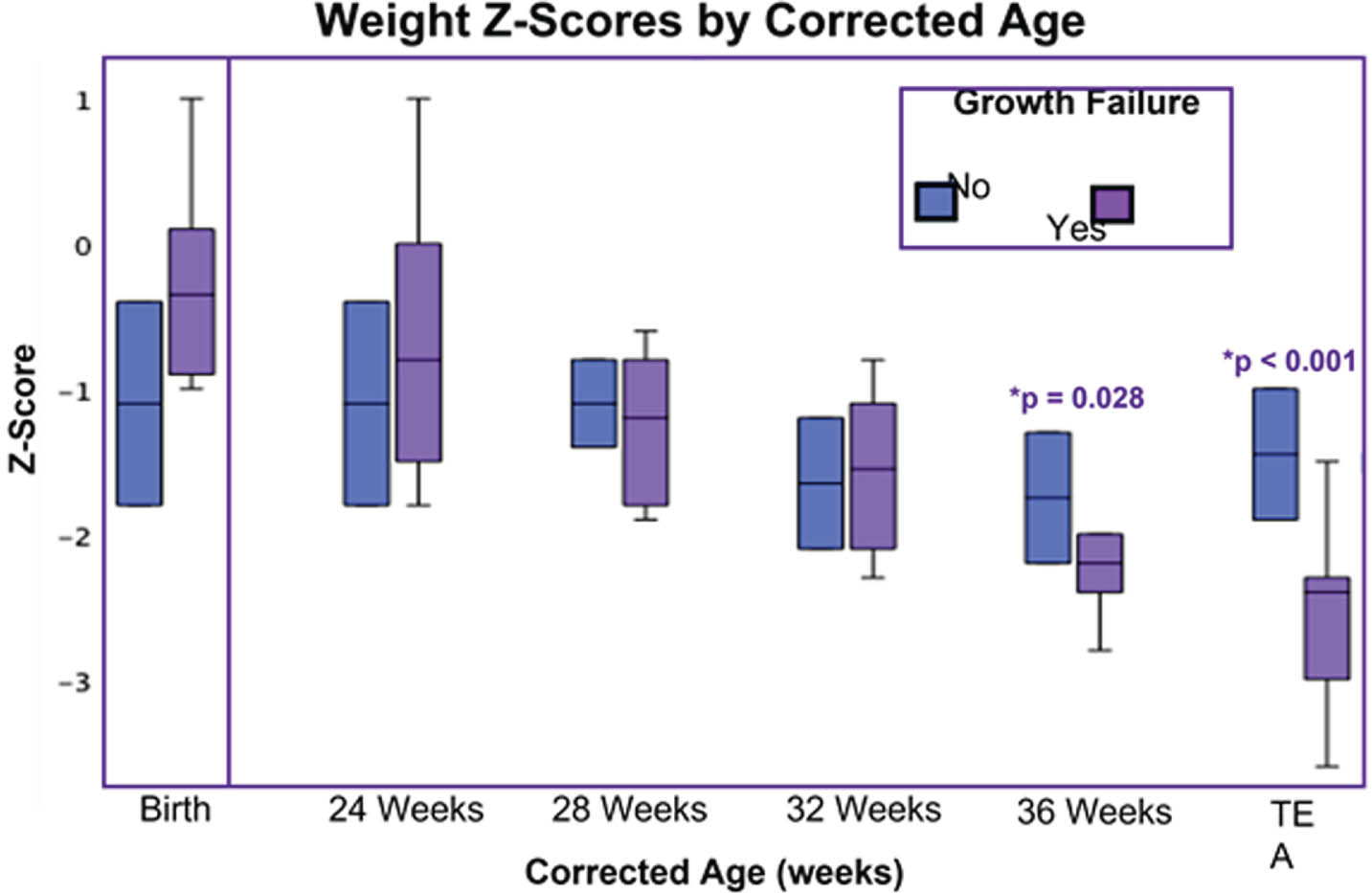
CONCLUSION: These findings suggest the time between 32 weeks CA and TEA represents a window for targeting nutrition to prevent GF in very preterm infants. Somatic growth during this time was associated with brain volumes at TEA, emphasizing the importance of adequate nutrition to support brain growth.
Longitudinal zinc concentrations in whole blood of preterm infants: associations with brain and body growth
Christopher Elitt1,2, Sara Cherkerzian2,3, Katherine Bell2,3, Jordan O’Brien3, Meghana Iragavarapu3, Silvi Minga3, Rachel Suttin3, Jianlin Wang1, Madeline Ross1, Samantha Remis1, Jane Shanahan1, Bogdan Fedeles4, Terrie Inder5,6, Helen Christou2,3, Mandy Belfort2,3, Paul Rosenberg1,2
1Boston Children’s Hospital, Neurology, 2Harvard Medical School, 3Brigham and Women’s Hospital, Newborn Medicine, 4Massachusetts Institute of Technology, 5Children’s Hospital of Orange County, 6University of California, Irvine School of Medicine
BACKGROUND AND OBJECTIVES: Dietary zinc is essential for brain development. Preterm infants are at high risk for zinc deficiency due to absent 3rd trimester transfer from the placenta, impaired absorption, and, potentially, inadequate dietary intake. The objectives of this study were twofold: (1) to quantify longitudinal whole blood zinc concentrations among very preterm infants (VPIs) and compare with full term infants (FTIs), and (2) to determine whether zinc status was associated with brain and body size in preterm infants measured at term equivalent age.
METHODOLOGY: We studied 43 VPIs [gestational age (GA) range, 24-30 weeks] and 15 FTIs (GA range, 37-39 weeks). We used inductively coupled plasma-mass spectrometry (ICP-MS) to measure zinc isotope concentrations (64Zn, 66Zn, 68Zn, 70Zn) in peripheral whole blood samples collected from VPIs at 4 time points [day of life (DOL) 1, DOL14, DOL30, hospital discharge] and from FTI cord blood. Total zinc concentrations were calculated using 64Zn. Zinc isotope ratios (66Zn/64Zn, 68Zn/64Zn, and 70Zn/64Zn) were also determined and compared to natural abundance ratios. Outcome measures in VPIs at term equivalent age included anthropometric indicators (weight, length, head circumference), fat-free mass determined by air displacement plethysmography, and quantitative brain metrics from magnetic resonance imaging. A high zinc group consisting of infants with 1 or more outlier values [defined as 75th%ile + 1.5*(IQR)] was identified within the VPI population.
RESULTS: Among VPIs median zinc concentrations decreased over the first month of the NICU hospitalization as previously reported and then increased by hospital discharge. Compared to FTI cord blood [714 ug/L; interquartile range (IQR): 674-866 ug/L], VPIs had higher zinc concentrations at discharge [943 ug/L; IQR: 736-1176 ug/L; p<0.05; Wilcoxon rank-sum test to compare medians]. Infants in the high zinc group had higher median weight z-scores at discharge (p=0.0271) and showed a trend towards larger cerebellar diameters (p=0.0723). Interestingly, median isotopic ratios (66Zn/64Zn, 68Zn/64Zn) in the FTIs and VPIs were all greater than natural abundance ratios, indicating differential zinc isotopic absorption or cellular transport in both VPIs and FTIs.
CONCLUSION: Our results suggest that higher whole blood zinc concentrations than normally found in the NICU population may be associated with improved somatic and brain growth in the NICU. These studies have important implications for understanding the nutritional basis for optimal neurodevelopment among VPIs and for devising NICU-based zinc supplementation strategies.
Persistent glutamatergic neuron dysfunction is predicted by early single-nucleus multi-omics after prenatal hypoxic brain injury
Ana Cristancho1, Donald Joseph1, Margaret Cassidy1, Preeti Chauhan1, Ethan Gadra1, Elyse Gadra1, Donya Zarrinnegar1, Bianca Rodriguez1, Eric Marsh1
1Children’s Hospital of Philadelphia/University of Pennsylvania
BACKGROUND: Prenatal and perinatal hypoxic injury affects over a million births annually, eventually leading to neurodevelopmental disability in one-third of those children. Yet, we lack targeted interventions for improving outcomes. A limitation toward developing therapeutics is that we lack understanding of the multifaceted, cell type-specific molecular consequences of this transient insult on the developing brain.
METHODOLOGY: To address this gap, we performed joint single nucleus RNA-sequencing and assay for transposase-accessible chromatin sequencing from the cortex of mice immediately after prenatal hypoxia exposure (8 hours of 5% inspired oxygen at embryonic day 17.5). This animal model phenocopies mild hypoxic injury seen in perinatal hypoxic injury. Seurat, Signac, and ArchR were used to process this data for cell identification and analyze differential gene expression and chromatin accessibility. We used Golgi staining, whole-cell patch-clamp electrophysiology, and cortical bulk-RNA sequencing in juvenile mice to examine glutamatergic neuron structure, function, and molecular signature.
RESULTS: Over 140,000 nuclei were sequenced from 16 total samples evenly divided between normoxia and hypoxia as well as males and females. We identified clusters of known neuronal and glial cell populations. Prenatal hypoxia led to a slight increase in endothelial cells but no further changes in cell number for other cell types. We found several cell type-specific disruptions in gene expression and regions of chromatin organization after prenatal hypoxia. Many of the cell type-specific genes dysregulated by hypoxia were associated with pathogenic variants that cause neurodevelopmental disabilities. Most remarkably, we discovered that hypoxic glutamatergic neurons had a selective disassociation between global chromatin organization and gene expression. Glutamatergic neurons, which develop synapses postnatally, also demonstrated dysregulation of genes associated with neuron structure and synapse function after prenatal hypoxia. We found that glutamatergic neurons had decreased dendritic spine density and prolonged action potential hyperpolarization one month after the hypoxic insult. Bulk RNA-seq analysis in juvenile animals revealed dysregulation of potassium channel-related genes. Notably, many of the potassium channels associated with hyperpolarization were not expressed in fetal glutamatergic neurons at baseline, but about 80% of these genes had abnormalities in nearby chromatin accessibility after prenatal hypoxia.
CONCLUSION: Together, these findings suggest that prenatal hypoxia disrupts the organization of chromatin and the transcriptome in glutamatergic neurons, leading to persistent disruption of neuronal maturation and structure that may contribute to lasting behavioral deficits. Ongoing analyses will test (1) whether the shifts in the chromatin organization after prenatal hypoxia are persistent in juvenile mice and (2) which motifs are present at sites of differential accessibility that may suggest pathways that are amenable to pharmacologic intervention in brain injury. An important implication of identifying epigenetic regulators in pre/perinatal hypoxic injury is the potential for expanding the therapeutic window past the typical newborn period to improve neurodevelopmental outcomes.
Impaired microglia repair responses after injury in the developing brain: Emerging potential therapeutic strategies
Sophie Tremblay1
1CHU Sainte-justine
BACKGROUND: Very preterm infants are exposed to multiple inflammatory stressors, including perinatal cerebellar hemorrhage (CBH) and postnatal infections, both of which are significant risk factors for neurodevelopmental challenges (1,2,3). This study aims to assess the fundamental properties of microglial cells to gain a profound understanding of their involvement in cerebellar injury resulting in cerebellar underdevelopment and neurodevelopmental delay.
METHODOLOGY: We developed a model of cerebellar underdevelopment (4) in mice using a combination of perinatal insults including a systemic inflammatory stress (LPS) with intraparenchymal cerebellar hemorrhages (collagenase) at postnatal day 2 (P2). Alterations of microglial states after injury were analyzed at different time points (P2, P3, P7, and P15) using flow cytometry along with cerebellar atrophy and behavioral outcomes. Additionally, the residual phagocytic capacity of microglial cells was assessed through a standardized bead assay and immunostaining techniques.
RESULTS: Our data showed that two weeks after being exposed to perinatal insults (P15), the overall normalized percentage of microglial cells featuring a reparative state are decreased in mouse pups exposed to a systemic inflammatory stress alone (LPS: 8.9±0.8%, ***P=0.0004, n=11) or exposed to a combination of perinatal insults (CBH+LPS: 12.5±1.7%, **P=0.0025, n=10) compared to controls (35.0±7.4%, n=10). In addition, there are significant alterations of the temporal progression towards proportion between microglia repair subtypes involved in brain tissue recovery. The normalized percentage of microglial cells still displaying an early anti-inflammatory phenotype remains significantly increased (*P=0.0119) along with an abnormal lower percentage of cells favoring debris scavenging and tissue homeostasis (**P=0.0200) two weeks after the insults compared to controls. Furthermore, primary cell cultures of microglial cells exposed in vivo to perinatal insults displayed a decreased residual phagocytic capacity after exposure to a systemic inflammatory stress alone (LPS: 31.67±8.36%, P=0.871, n=6), to cerebellar hemorrhage alone (CBH: 13.75±3.86%, *P=0.035, n=8) or exposed to combined insults (CBH+LPS: 21.60±7.58%, P=0.312, n=5) compared to controls (33.50±6.99%, n=10).
CONCLUSION: Exposure to perinatal insults induces alterations in tissue repair function of microglial cells following injury. Anti-inflammatory and tissue remodeling properties of microglial cells seems altered and may potentially create a window of vulnerability during the recovery phase post-injury. By identifying further specific alterations of microglia repair responses, we may identify potential therapeutic target to favor a complete recovery after injury by modulating microglia responses.
BIBLIOGRAPHY:
1. Spoto, G. et al. Frontiers Syst Neurosci 15, 655164 (2021).
2. Brossard-Racine, M. & Limperopoulos, C. Semin Perinatol 45, 151470 (2021).
3. Garfinkle, J. et al. Ann Neurol 88, 1095–1108 (2020).
4. Tremblay, S. et al. Brain Behav Immun 1–20 (2017).
Two-dimensional cranial ultrasound-based biomarkers of brain growth in extreme and very preterm infants
Medha Goyal1, Meagan Quigley1, Nina Stein1, Ipsita Goswami1
1McMaster Children’s Hospital
BACKGROUND: Preterm infants are susceptible to white matter injury and altered brain growth. Importantly, smaller brain size was found to be associated with poor neurodevelopment outcomes at 2 years. Multiple factors are known to impact brain growth such as systemic inflammation, fetal growth restriction, and postnatal steroid exposure.
OBJECTIVE: The study aims to compare linear brain metrics at term equivalent age (TEA) between neonates born ≤28weeks gestational age (GA) and 28+1-32weeks GA. We hypothesize that neonates born ≤ 28 weeks GA have smaller linear measurements in certain regions of the brain.
METHODOLOGY: A retrospective chart review of consecutive infants born at GA≤32 weeks between January-May 2019 was conducted. We excluded infants with major brain injury [Grade 3-4 intraventricular hemorrhage, periventricular leukomalacia, post-hemorrhagic ventricular dilatation]. The cranial two-dimensional ultrasound (cUS) performed at TEA (36-40 weeks) was assessed at mid-coronal, parasagittal, and mid-sagittal planes. Regional linear brain metrics were quantified under the following categories: (i) Cerebrum [Biparietal diameter and depth of cingulate sulcus]; (ii) Cerebral White Matter [Corpus callosum length, thickness, and fastigial distance]; (iii) Deep Grey Matter [Basal ganglia and Caudate Nucleus width]; (iv) Cerebellum [height, width, transverse diameter] (Figure 1).
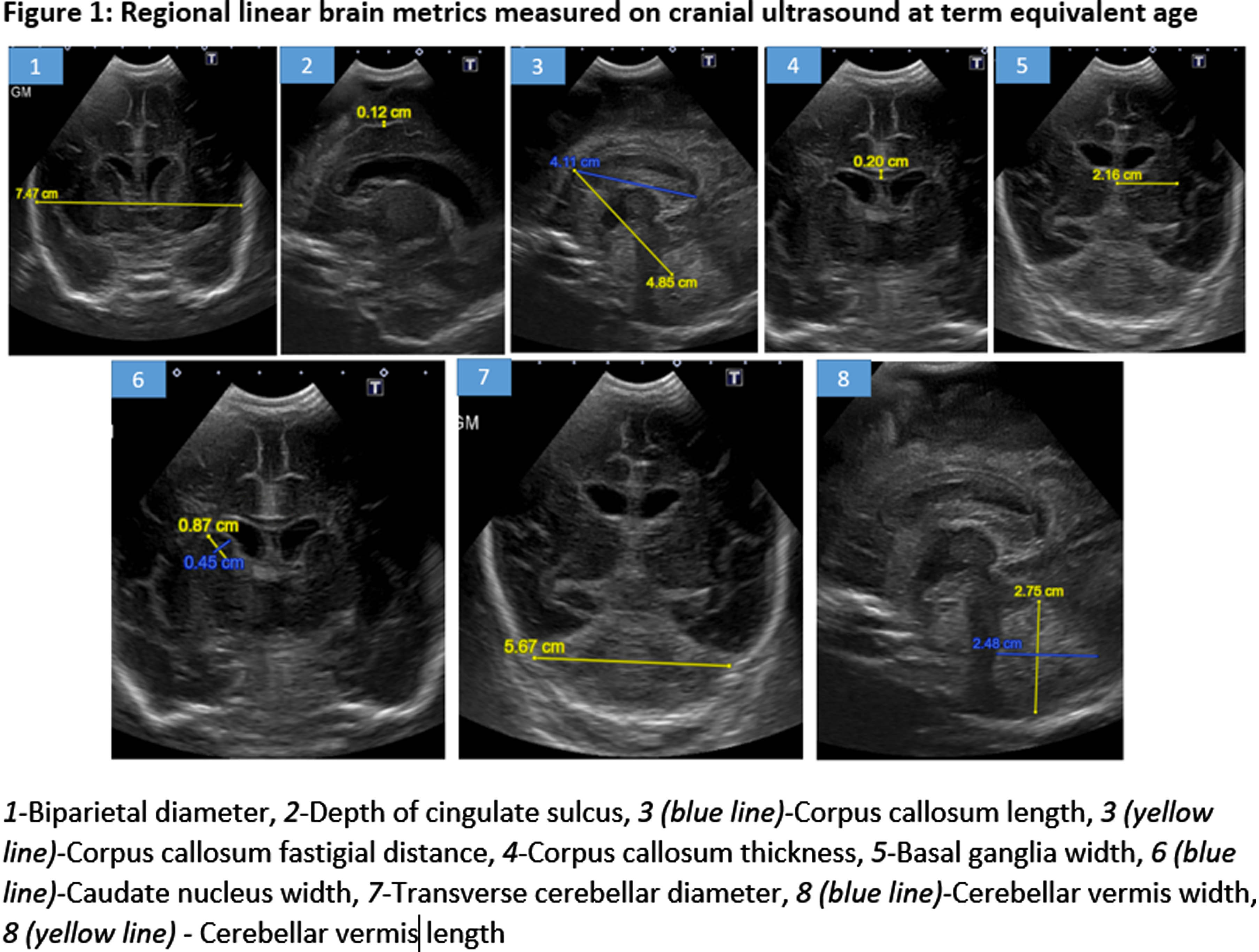
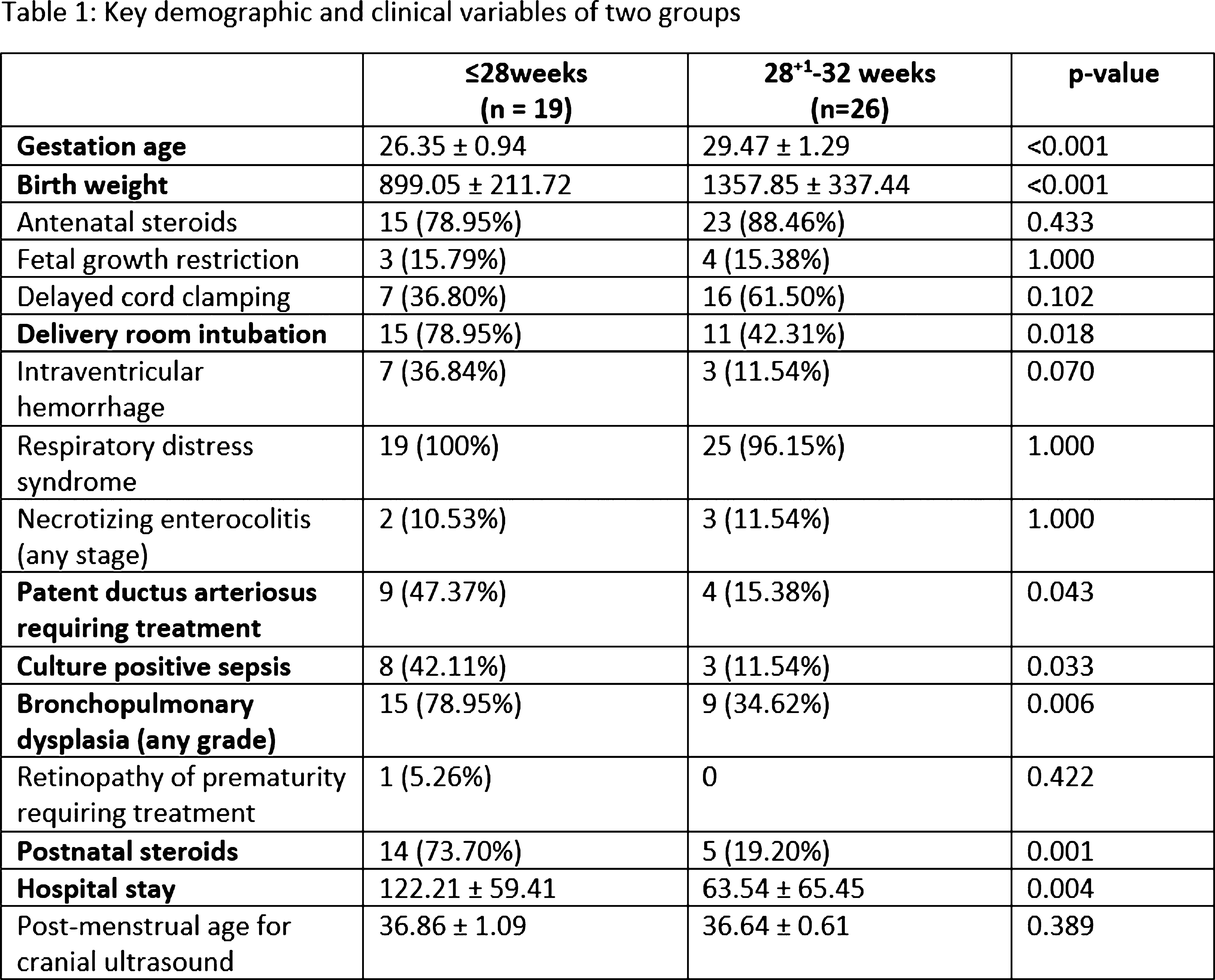
RESULTS: We enrolled 48 neonates who met the inclusion criteria, 3 neonates were excluded [2 had post-hemorrhagic ventricular dilatation, and 1 diagnosed with Prader-villi syndrome]. Of the 45 included, the mean GA and birth-weight were 28 ± 1.9 weeks and 1164 ± 368 grams, and 22 (49%) were males. The mean maternal age was 32 ± 4 years, 12 (27%) twins, and 2 (4%) triplets, and 31 (69%) were delivered by C-section. Key neonatal morbidities included respiratory distress syndrome (98%), intraventricular hemorrhage (22%), necrotizing enterocolitis (22%), patent ductus arteriosus (PDA) requiring treatment (29%), culture-positive sepsis (25%), meningitis (4%), bronchopulmonary dysplasia (53%), ROP requiring treatment (2%). The median hospital stay was 80 days (34.5, 124). The neonates ≤28 weeks had higher rates of delivery room intubation, PDA, culture-proven sepsis, and bronchopulmonary dysplasia of any grade (Table 1). The mean GA for cUS was 36.74 ± 0.84 weeks. There was statistically significant difference in median (IQR) corpus callosum length [≤28 weeks 4.12(3.9-4.5)mm versus 28-32 weeks 4.5(4.3-4.7)mm, p=0.006]. (Figure 2, 3) Biparietal diameter, depth of cingulate gyrus, corpus-callosum width, corpus-callosum-fastigial length, basal ganglia, caudate head width, transverse cerebellar diameter, and vermis were not significantly different.
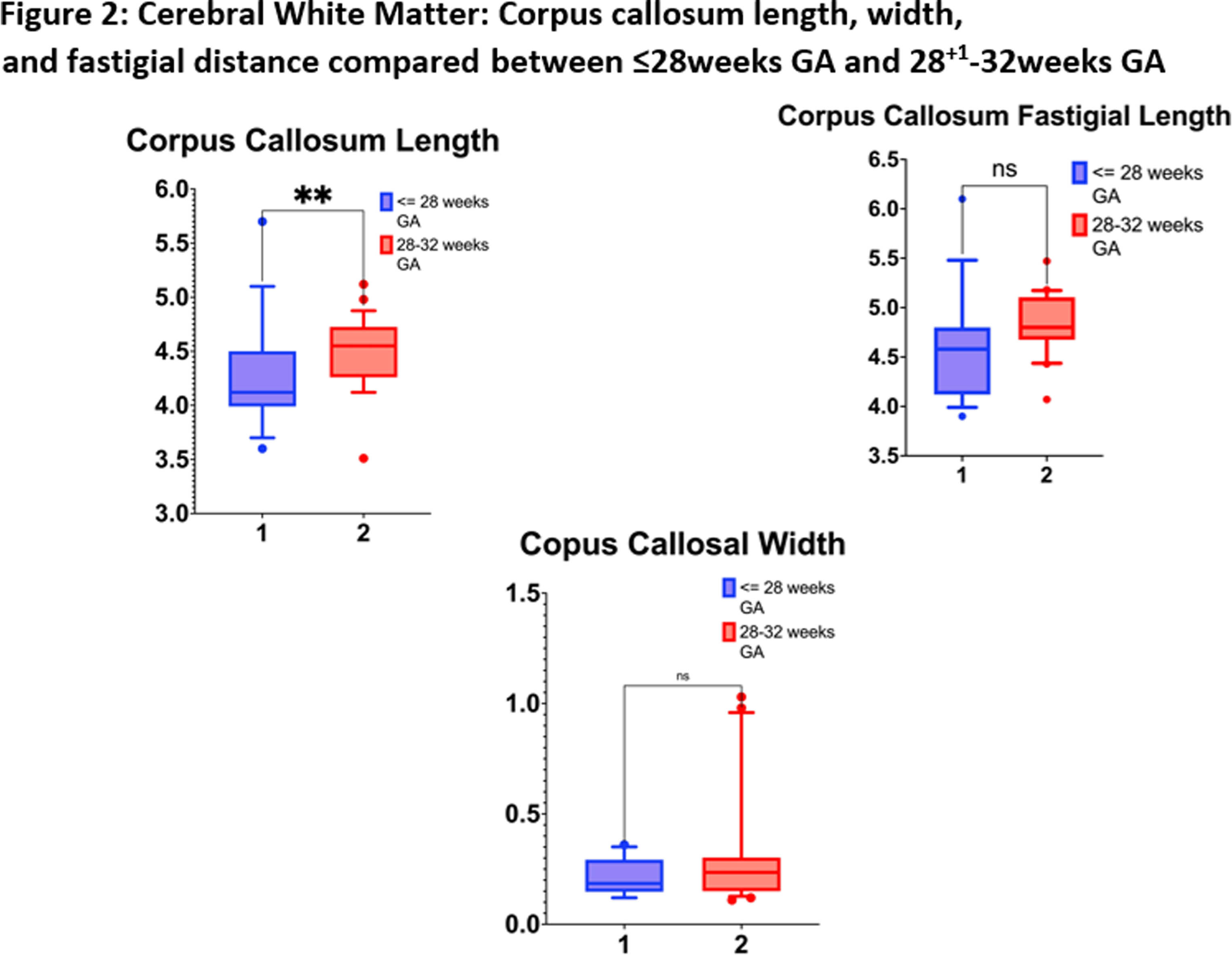
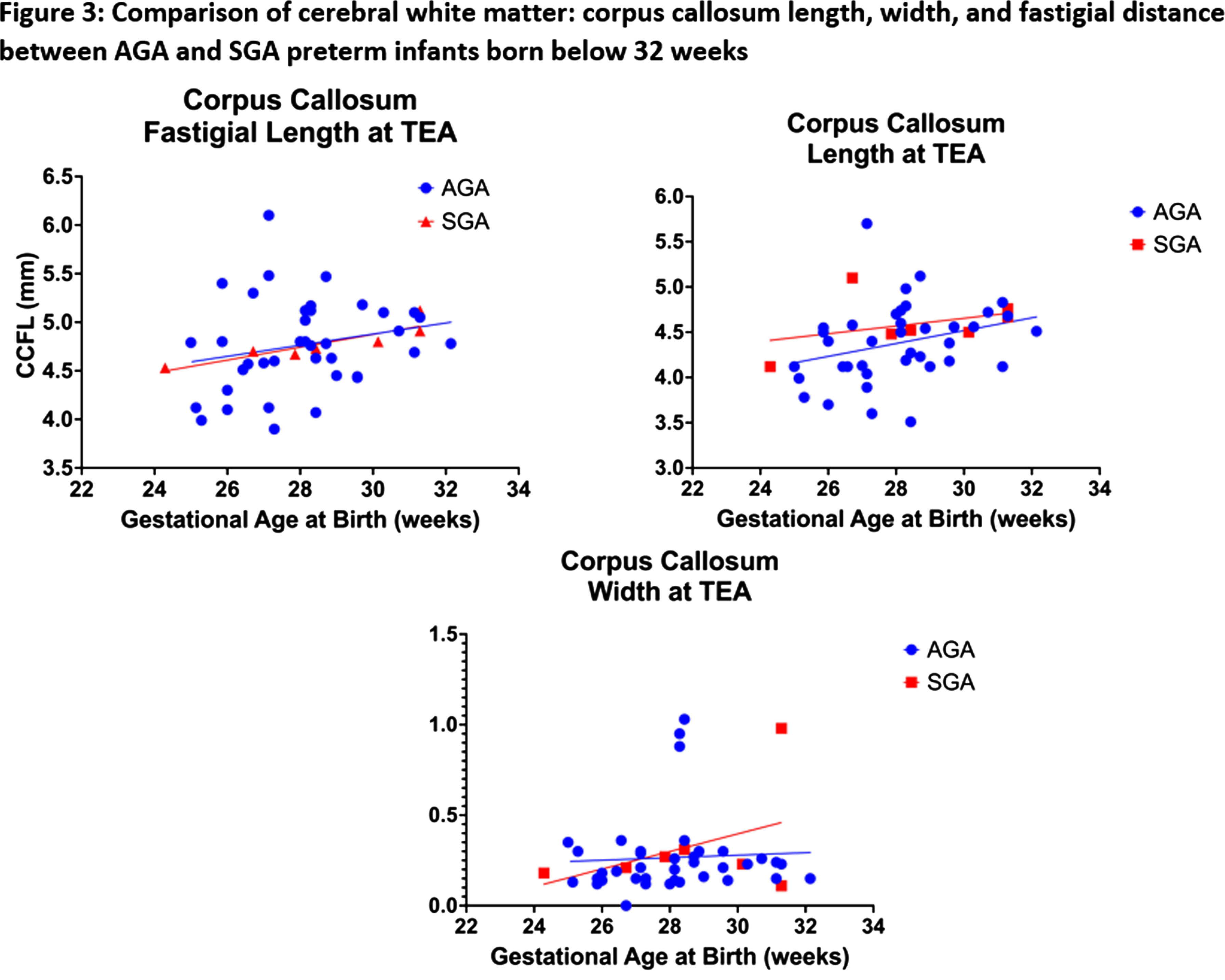
CONCLUSION: Compared to preterm neonates born after 28 weeks GA, those born before 28 weeks have shorter mean corpus-callosum length at TEA, indicative of poor postnatal white matter growth, despite being free from major brain injuries. The implications of shorter corpus callosal width on neurodevelopmental outcomes of the cohort will need to be further explored in future studies. This study is limited due to the small sample size.
Assessment of selective motor control in very low birth weight infants using BabyOSCAR
Denise Kao1, Colleen Peyton1,2, Theresa Sukal Moulton1,2
1Department of Physical Therapy and Human Movement Sciences, Feinberg School of Medicine, Northwestern University , 2Department of Pediatrics, Feinberg School of Medicine, Northwestern University
BACKGROUND: Selective motor control (SMC), or the ability to isolate one joint at a time, is impaired in children with spastic cerebral palsy (CP), contributing to difficulties with functional mobility and dexterous tasks. Recently we developed and validated a tool that measures spontaneous SMC in 3-month-old infants, called BabyOSCAR (Observational Selective Control Appraisal) which was predictive of spastic CP at age 2 years. The General Movement Assessment (GMA) is another clinical tool which is highly predictive of CP. Because SMC may develop differently in infants born preterm, we investigated the validity of BabyOSCAR in a prospective sample of infants born very low birth weight (VLBW). Our aims were to determine if BabyOSCAR score was related to gestational age (GA) and if there were relationships between BabyOSCAR scores and GMA.
METHODOLOGY: 22 VLBW infants were prospectively video recorded while awake, alert, and spontaneously moving. These video recordings occurred longitudinally between 49 and 60 wks postmenstrual age (PMA) and were used to score both the BabyOSCAR and the GMA. BabyOSCAR Total Score is a composite of observed SMC in the joints of the arms (Arm Score) and legs (Leg Score). We used regression modeling to determine associations between 1) BabyOSCAR scores and GA and 2) BabyOSCAR scores and GMA.
RESULTS: Our sample had a median birthweight of 983g (IQR 805, 1160), median GA of 27 wks (IQR 26, 29), was 36% female (n=8), and had a 38% incidence of intraventricular hemorrhage of any grade. In total, 55 videos were included for rating. Among all scored videos, higher BabyOSCAR Leg Scores were significantly related to having normal GMA (p=0.002), but Total Scores and Arm Scores were not significantly related to GMA. When comparing each baby’s best performance on BabyOSCAR (n=22 videos), both higher BabyOSCAR Total Scores and higher Leg Scores were related to a normal GMA (p=0.004; p=0.004). However, BabyOSCAR Arm Scores were not significantly related to GMA. BabyOSCAR Total Scores and Leg Scores were not significantly related to GA, however lower Arm Scores were related to a younger GA (p=0.011).
CONCLUSION: These preliminary findings suggest that BabyOSCAR Leg Score is concurrently valid with GMA. While we previously demonstrated a stronger concurrent validity between BabyOSCAR Scores and GMA, the children tested were not exclusively born VLBW, as in our current sample. Taken together, these findings may indicate that SMC, especially in the arms, may emerge on a different trajectory in very preterm infants. Additional follow-up is warranted to understand long-term trajectories and emergence of SMC in infants born VLBW.
Microglial depletion worsens lesion in female but not male mice after P10 hypoxia-ischemia
Danielle Guez-Barber1, Sofia E. Nicolayevsky, Kaya J.D. Johnson, Sanghee Yun, Amelia J. Eisch
1Children’s Hospital of Philadelphia
BACKGROUND: Rodent models for perinatal hypoxic ischemic (HI) encephalopathy have reported sex differences such as males having larger brain lesions than females after the same injury. Microglia, the resident immune cells of the brain that have distinct developmental trajectories and gene expression patterns by sex, likely play a different role in males and females following HI. However, there is conflicting literature on whether depletion of microglia worsens or improves HI-induced lesions, and whether this differs by sex. We tested the effect of pharmacologic microglial depletion on lesion size and developmental milestones.
METHODOLOGY: C57BL/6J mouse pups received daily intraperitoneal injections from postnatal day 7 (P7) to P12 of either 25 mg/kg PLX3397 (a CSF1R inhibitor) or vehicle (Veh). At P10, pups either underwent Hypoxic-Ischemic (HI) insult using a modified Vannucci procedure, or a Sham insult. This resulted in four groups tested (Veh-Sham, Veh-HI, PLX-Sham, PLX-HI); all groups included both male and female mice. Behavioral testing was performed both pre-HI (forelimb grasping [P8, P9]) and post-HI (open field traversal [P12], gross behavior and appearance in homecage and new environment [P13]). Brains were collected at P13, fixed, and sectioned, then either immunolabeled for Iba1 or stained with cresyl violet for injury scoring.
RESULTS: Immunohistochemistry for Iba1 demonstrated >95% depletion of microglia at either P10 or P13 in all PLX3397-treated mice; depletion did not differ by sex (Fig. 1). In the hippocampus, Female PLX-HI mice had worse cresyl violet lesion scores than Female Veh-HI mice; this was not true in male mice, where there was a trend in the opposite direction. Female PLX-HI mice also had worse lesion scores than Male PLX-HI mice (Fig. 2). There were no differences among groups in forelimb grasp, open field traversal times, or weight gain.
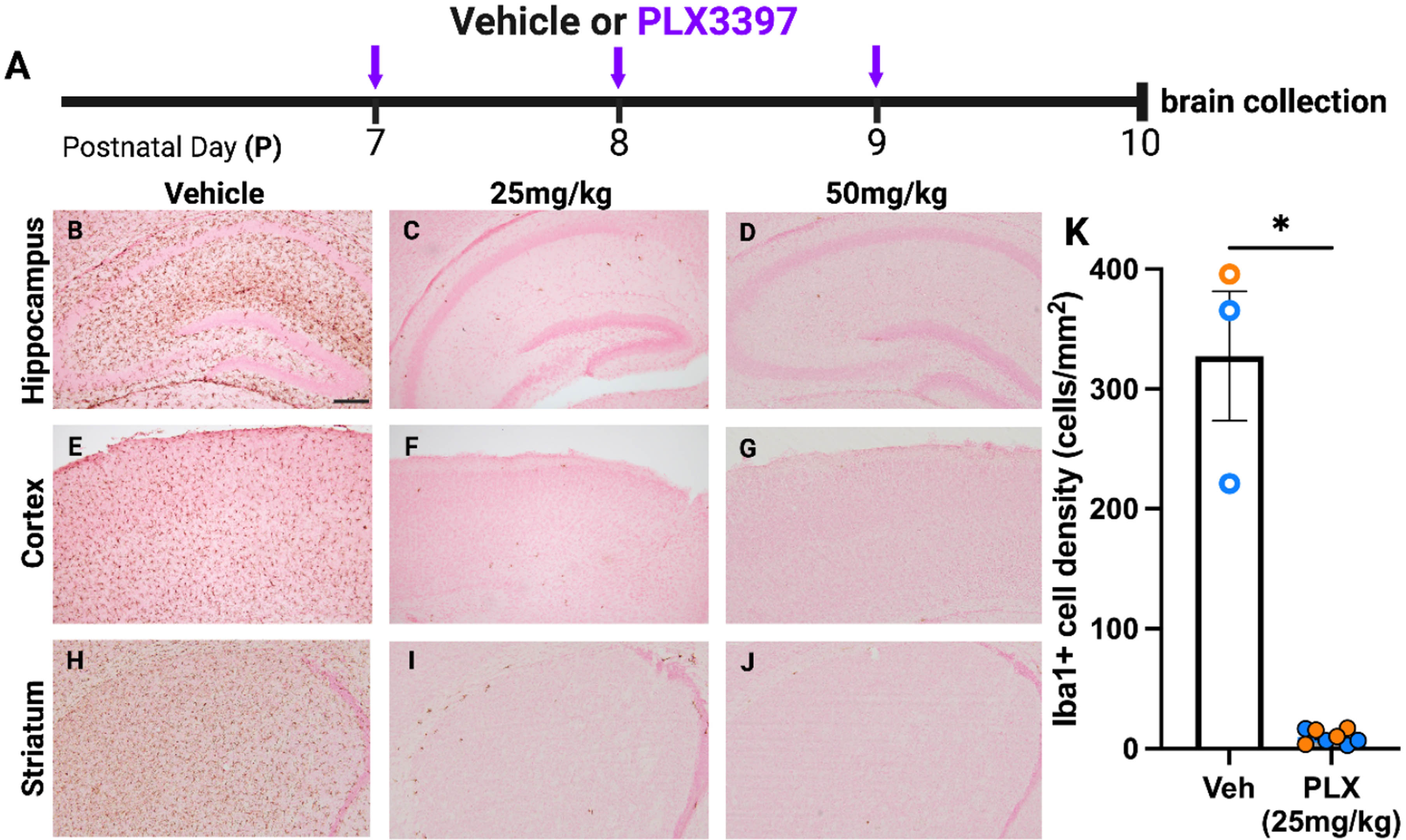
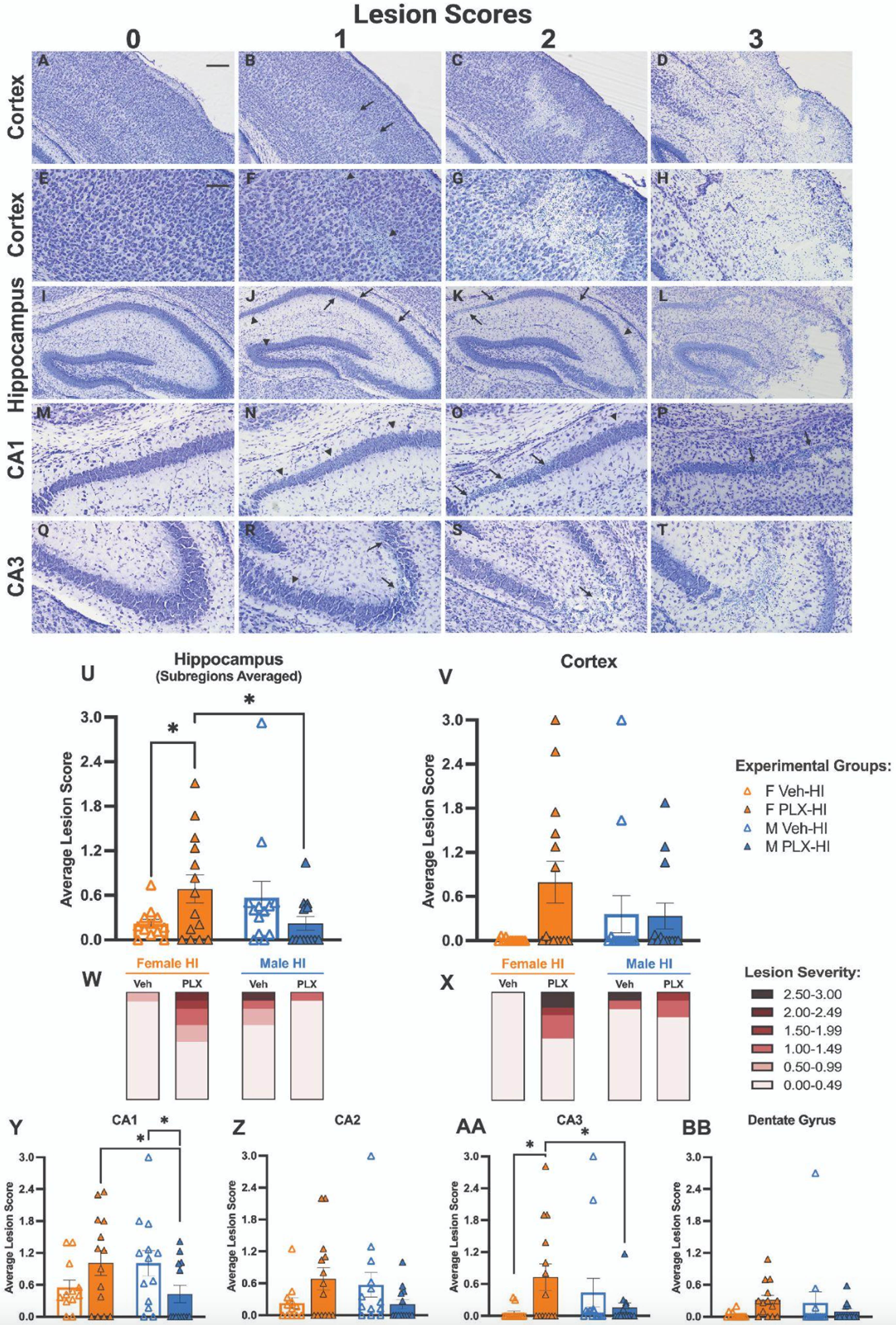
CONCLUSION: Microglial depletion worsens HI-induced injury in female mice but not in male mice.
IMPACT: Brain injury after hypoxia-ischemia may proceed via different immune mechanisms in males and females.
Persistent neuroinflammation after viral clearance in a mouse model of congenital Zika virus
Shannon Agner, Ethan Liu, Robyn Klein
1Washington University School of Medicine
BACKGROUND: One in 7 babies born to mothers in the United States and its territories that were infected with Zika virus (ZIKV) during pregnancy have neurodevelopmental problems associated with ZIKV exposure [1]. Recent data also indicate that ZIKV-exposed children without major structural brain abnormalities at birth may still have cognitive and behavioral deficits, with the complete extent and causes still poorly understood [2]. Neurotropic viruses cause damage not only through the process of viral infection and dissemination, but also as a result of antiviral host immune responses [3]. Adult models of Zika infection have demonstrated persistent neuroinflammation and injury, far beyond recovery from infection [4]. In this study, we tested the hypothesis that microglial and astroglial activation persists beyond viral infection in mice exposed to Zika virus in utero.
METHODOLOGY: Timed pregnancies of humanized STAT2 knock-in mice on a C57Bl/6J background were performed. Pregnant dams were either infected with 104 focus forming units of Zika-mouse adapted Dakar strain of virus or 0.5% fetal bovine serum in phosphate-buffered saline (i.e., mock solution) at embryonic day 6.5. At postnatal day 10 (P10), pup brains were harvested and collected as 10 mm sections. Antibodies for were used for immunohistochemical staining for Iba1 (microglia and macrophages) and GFAP (astrocytes). Images were acquired using a Nikon confocal microscope, and data were analyzed in a single plane. Quantification of each antibody stain was performed using percent area of intensity.
RESULTS: Mice exposed to Zika in utero at E6.5 had increased GFAP and Iba1 positive cells throughout cortical and subcortical regions at P10 compared to mock-infected mice. This indicates persistent inflammation in the pup brain after viral clearance of ZIKV, which occurs around P0.
CONCLUSION: At P10 in a mouse model of congenital Zika virus, which is commonly thought to approximate term-equivalent neurodevelopment of the human infant, microglial and astroglial activation persists beyond viral clearance. Persistent neuroinflammation after viral clearance has been shown to contribute to neurocognitive deficits in adult animal models of ZIKV infection. These findings suggest that persistent neuroinflammation also occurs in congenital ZIKV infection, but it is unknown how this may contribute to neurocognitive differences in observed in congenital ZIKV. Future work will include repeat experiments to confirm results and additional assays to further understand the role of and downstream implications for persistent neuroinflammation.
BIBLIOGRAPHY:
1. CDC. Data & Statistics on Zika and Pregnancy, https://www.cdc.gov/pregnancy/zika/data/index.html (2019).
2. Adams Waldorf, K. M. et al. Nat Med 24, 368-374 (2018).
3. Jurado, K. A. et al. Nature Microbiology (2017).
4. Garber, C. et al. Nat Neurosci 22, 1276-1288 (2019).
Neonatal Hypoxic Ischemic Encephalopathy: Sex Differences in the Acute Response of Neutrophils to Brain Injury
Viola Caretti1, Janelle Korf1, Laura Harrington1, Tina Findley1, Priya Ganesh1, Louise McCullough1
1UTHealth Houston
BACKGROUND: Worldwide, neonatal hypoxic-ischemic brain injury (HIE) is a leading cause of childhood death and disability1. Male infants are at increased risk for HIE and death1 2. The etiology of this sexual dimorphism is not understood but could stem from differences in inflammatory response to acute brain injury. Recent research suggests that the aryl hydrocarbon receptor (AHR) is crucial in the inflammatory response to stroke in adults3 and has a key role in sexually dimorphic effects in the developing brain4. AHR’s role in the inflammatory response to neonatal brain injury is unknown.
OBJECTIVE: To investigate the role of AHR in mediating sex differences in acute inflammatory response to HIE.
METHODOLOGY: To assess immune differences in humans with HIE, we did a retrospective study at a single academic institution from 2011 to 2021 (n=310). Inclusion criteria were gestational age ≥ 36 weeks, moderate to severe HIE by Sarnat staging, CBC reported within the 1st day of life (n= 201), and exclusion of concomitant infection. To study HIE acute inflammatory responses, we leveraged the modified Rice-Vannucci model, where permanent ligation of the right common carotid artery in pups is followed by 50 minutes of hypoxia (N=6, per sex). Controls received sham surgeries followed by normoxia or hypoxia (N=4, per sex, per group). Spleens, ischemic, and contralateral brain hemispheres were collected for flow cytometry 72 hours after surgery. We determined AHR levels in neutrophils, lymphocytes, monocytes and microglia. Statistics were performed by 2-way ANOVA with Tukey’s multiple comparisons and Mann-Whitney t-test for preclinical and clinical results, respectively; a p<0.05 was significant.
RESULTS: Acutely, in the periphery of human neonates with HIE, the CBC of females had significantly higher neutrophil, lymphocyte, and monocyte count than males, with the most remarkable sex-difference in neutrophil count (p<0.01). Similarly, in pups, females had a significant increase in neutrophils in the spleen compared to males and controls acutely after injury (p = 0.03). AHR was significantly increased in the ischemic hemisphere of females when compared to the contralateral hemisphere (p<0.01), normoxia (p<0.01) and hypoxia (p<0.01) controls, and the ischemic hemisphere of males (p = 0.02). For both sexes, the majority of AHR+ cells were neutrophils (67%), with significantly more AHR+ neutrophils in females than male (p=0.03). The absolute count of neutrophils in the ischemic hemisphere did not differ between sexes, indicating AHR+ sex-dimorphism in the injured brain acutely after HIE.
CONCLUSION: Sex-specific differences in the neutrophil peripheral count and AHR expression within the ischemic hemisphere suggest a sex-dimorphic role of AHR in the acute response of neutrophils to HIE.
BIBLIOGRAPHY:
1. Korf et al. 2023, PMCID: PMC10485647
2. Chalak et al. 2023, PMCID: PMC10394577
3. Wan-Ci Chen et al. 2019, PMCID: PMC6790016
4. Haque et al. 2022, PMC9322714
Placental findings from newborns with hypoxic-ischemic encephalopathy
Daniil Kamyshanskiy1, Yevgeniy Kamyshanskiy1, Liana Chernova2, Yasminur Turdybekova3, Irina Kopobayeva3
1Department of Pathology, Karaganda Medical University, 2Gala-Clinic, 3Center for Family Planning and Reproductive Health
BACKGROUND: Neonatal hypoxic-ischemic encephalopathy (HIE) is a significant cause of morbidity and mortality of newborns worldwide. Expanding the evidence on placental findings will provide additional information on the mechanisms and timing of HIE.
OBJECTIVE: To determine whether pre-determined placental findings differ in newborns with HIE compared to newborns without HIE.
METHODS: A retrospective “case-control” study of placentas from single pregnancy at 35 weeks or more. The data were extracted from electronic database of third-level hospital in Karaganda (Kazakhstan) (2016-2023). HIE was classified according to the Sarnat scale [Sarnat HB et al., 1976]. Placentas from children with HIE were compared with randomly selected children from the control group of clinically healthy full-term newborns (1:1 ratio). Placentas were examined by a perinatal pathologist who was unaware of the case status, using internationally recommended definitions and terminology [Khong TY et al., 2016]. The data were analyzed using the Chi-square test.
RESULTS: Placentas of 371 newborns with HIE (245 with Sarnat 1, 87 with Sarnat 2, and 39 with Sarnat 3) were compared with 371 placentas of control group newborns without HIE. Maternal vascular malperfusion (MVM), Villitis of unknown etiology (VUE), and Distal villous immaturity (DVI) did not differ between the groups, but placental injuries with Fetal vascular malperfusion (FVM) were more common in placentas of newborns with moderate (24.1%) and severe (25.6%) HIE than in the control group (5.7%) (p = 0.001). Stage of hypoxic damage of more than 30% of placental volume was more often associated with moderate (46.0%) and severe (53.8%) HIE than in the control group (4.8%) (p = 0.001). Prolonged placental damage was more common in the placentas of newborns with HIE, including mild (27.8%), moderate (50.6%), and severe (53.8%) HIE than in the control group (4.0%).
CONCLUSION: HIE and its severity are associated with antenatal hypoxic damage of placenta. Moderate and severe HIE is very often associated with FVM and hypoxic damage of more than 30% of placental volume. HIE is often associated with prolonged placental damage caused by long-term or frequently recurring episodes of antenatal hypoxic-ischemic events, which can be explained by “Domino effect” when pathological processes overlap and reinforce each other. FVM, damage of more than 30% of placental tissue and prolonged placental hypoxia are associated with severe forms of neonatal HIE and can be diagnostic predictors of severe antenatal damage or fetal distress, allowing stratification of a group of fetuses/newborns with unfavorable intrauterine conditions of development and risk of disease in the postnatal period.
Genetic and congenital anomalies in newborns with hypoxic-ischemic encephalopathy
Adriana Morell1, Marie-Coralie Cornet1, Sarah Monsell2, Bryan Comstock2, Doctor Hannah Glass1, Fernando Gonzalez1, Dennis Mayock2, Patrick Heagerty2, Sandra Juul2, Yvonne Wu1
1University of California, San Francisco
2Univeristy of Washington
BACKGROUND AND OBJECTIVE: Infants diagnosed with HIE may have underlying conditions predisposing them to sustaining hypoxic-ischemic injury during the labor and delivery process. It remains unclear how the presence of a genetic or congenital anomaly impacts the clinical presentation and outcomes of HIE. We determined the frequency of diagnosed and unsuspected genetic or congenital anomalies in infants with HIE, and whether such anomalies are associated with severity of encephalopathy or with neurodevelopmental outcomes.
METHODOLOGY: Infants with moderate or severe HIE enrolled in the High-include Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial (NCT02811263) underwent genetic testing when clinically indicated (e.g., dysmorphic features, family history, organ anomaly). Congenital anomalies, defined as structural malformation of a major organ system, were diagnosed prior to hospital discharge, and were prospectively recorded. The primary outcome, i.e., death or neurodevelopmental impairment (NDI), was determined at 22-36 months of age by a standardized neurologic examination, Bayley Scales of Infant Development-III (BSID-III), and the Gross Motor Function Classification Scales. Secondary outcomes included cerebral palsy, a 5-level ordinal outcome (no NDI, mild NDI, moderate NDI, moderate NDI, severe NDI, or death), gross motor function classification system (GMFCS) >= 1 indicating inability to walk 10 feet, and BSID-III motor, cognitive and language scores at age 22-36 months.
RESULTS: Of 500 infants with HIE, 24 (5%, 95% CI 3-7%) were diagnosed with a genetic or congenital anomaly. Of these 24 infants, 10 (2%) had only a genetic anomaly, 9 (2%) had only a congenital anomaly, and 5 (1%) had both (Table 1). Infants with and without genetic or congenital anomalies had similar rates of severe encephalopathy(17% vs. 23%, P=0.62) (Table 2) and similar findings on neonatal EEG and brain MRI (Table 3). However, infants with genetic or congenital anomalies were more likely to have the primary outcome of death or NDI (75% vs 50%, P= 0.02) (Table 3). Among survivors, those with a genetic or congenital anomaly were more likely to be diagnosed with cerebral palsy (32% vs. 13%, p =0.02), and to have a low (<=85) BSID-III score in cognitive (65% vs. 13%, P=0.02), language (65% vs 13%, P=0.02) and motor (70% vs 13%, P=<0.001) domains than HIE survivors without such anomalies (Table 3).
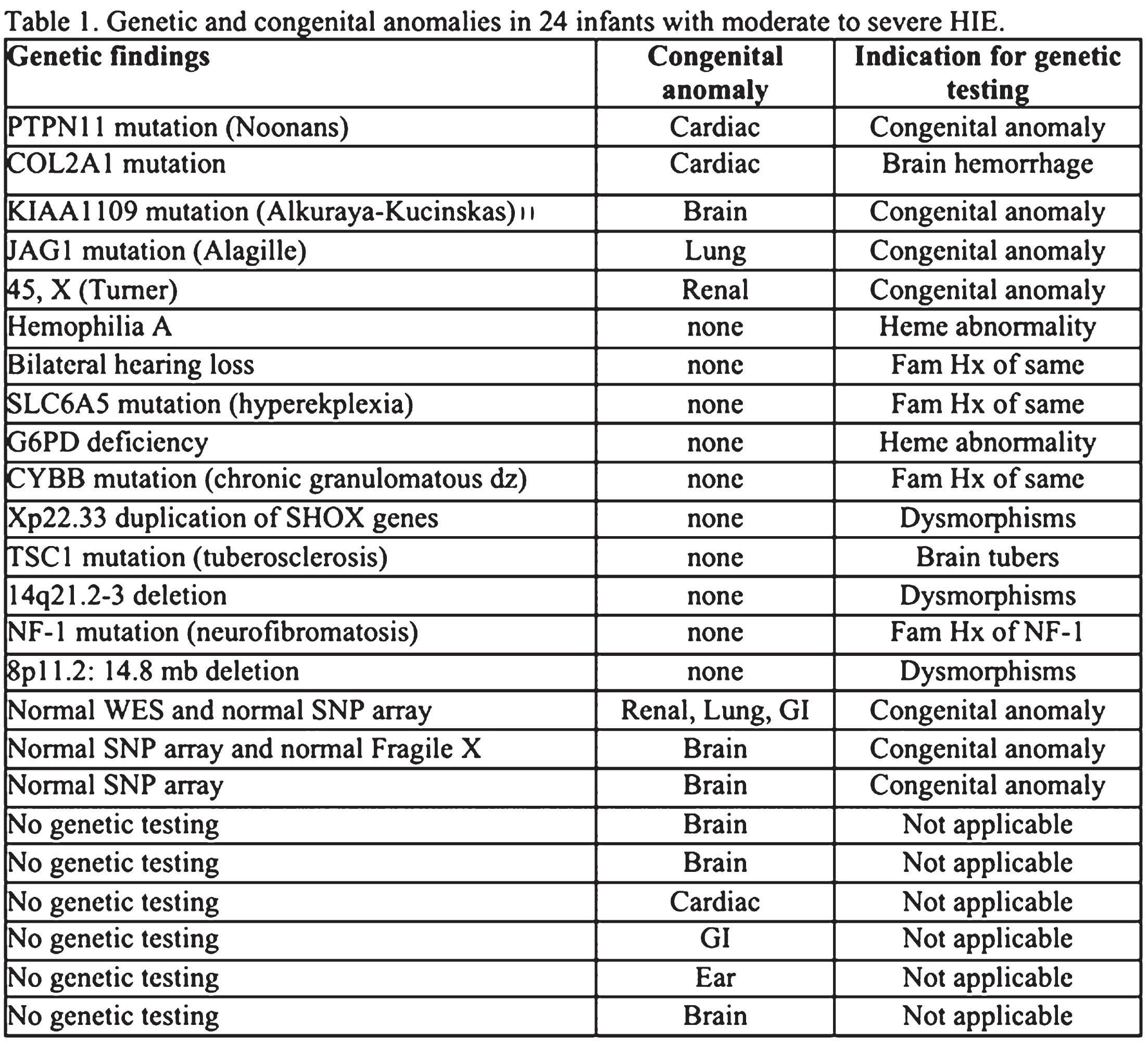
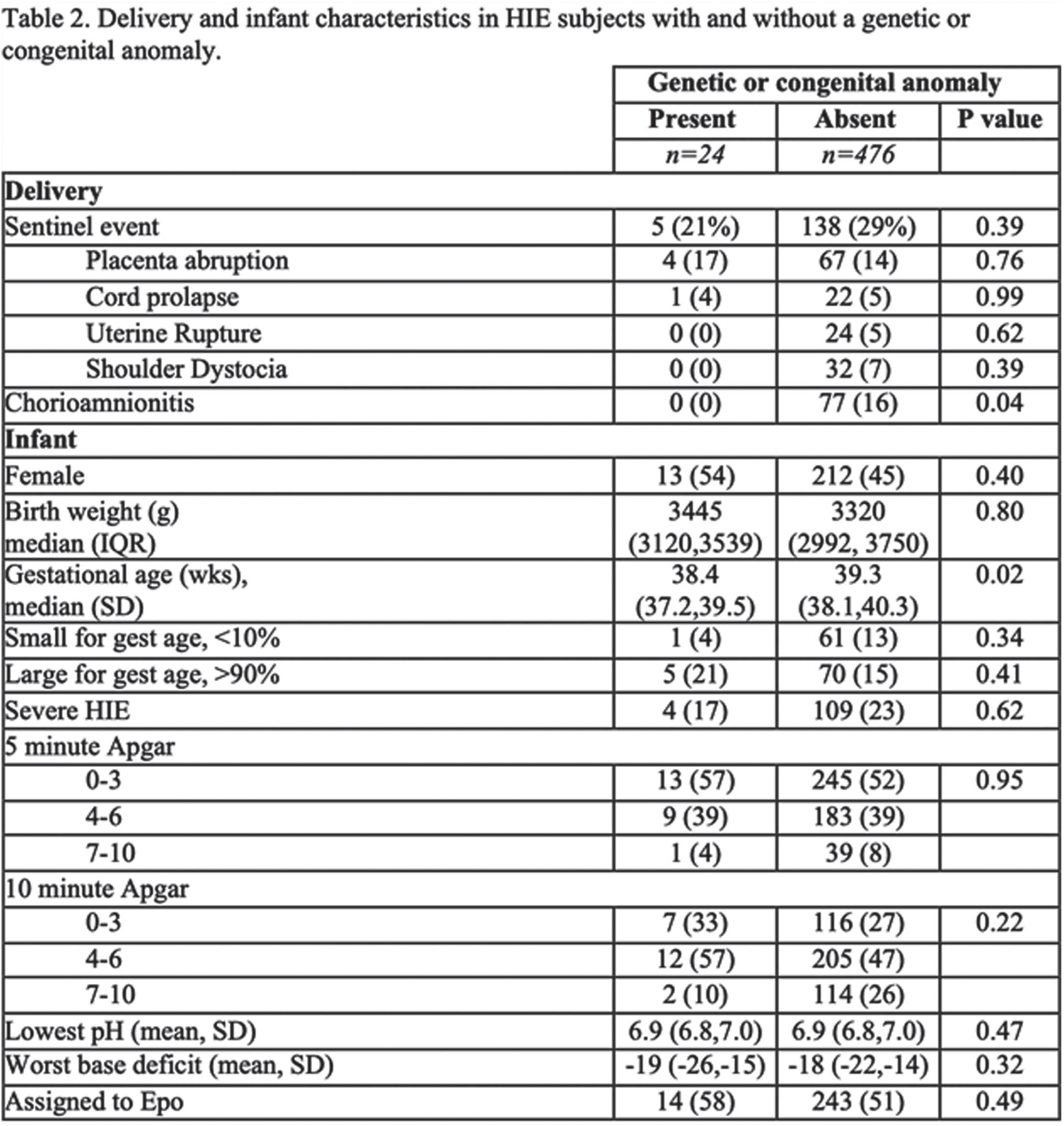
CONCLUSION: Among infants with HIE the frequency of diagnosed genetic or congenital anomalies was 5%. Despite similar clinical markers of HIE severity, infants with HIE and a genetic or congenital anomaly had worse neurodevelopmental outcomes than infants with HIE alone. This study points to the importance of having a low threshold for preforming genetic testing in infants who have both HIE and clinical features suggestive of a genetic disorder.
Lethal congenital contracture arthrogryposis - 11 (LCCS11)
Lekarz Malgorzata Bochenska1, Witold Blaz1, Lekarz Pawel Zapolnik1, Lekarz Magdalena Swider2
1Klinika Noworodków z Oddzialem Intensywnej Terapii Noworodka Kliniczny Szpital Wojewódzki Nr 2 W Rzeszowie Polska, 2Klinika Anestezjologii z Oddzialem Intensywnej Terapii Kliniczny Szpital Wojewódzki Nr 2 W Rzeszowie Polska
BACKGROUND: Arthrogryposis is rare disorder occuring in 1 out of every 3000 live births comprising congenital joint contracture in two or more areas of the body. Lethal congenital contracture arthrogryposis-11 (LCCS11) is caused by homozygous or compound heterozygous mutation in the GLDN gene (608603) on chromosome 15q21. This gene encodes GLDN (Gliomedin) a protein which exists in both transmembrane and secreted forms, promotes formation of the nodes of Ranvier in the peripheral nervous system. We present a patient, in whom variant within the GLDN gene was identified.
OBJECTIVE: The aim of our presentation was to analyze the clinical and diagnostic features leading to final diagnosis
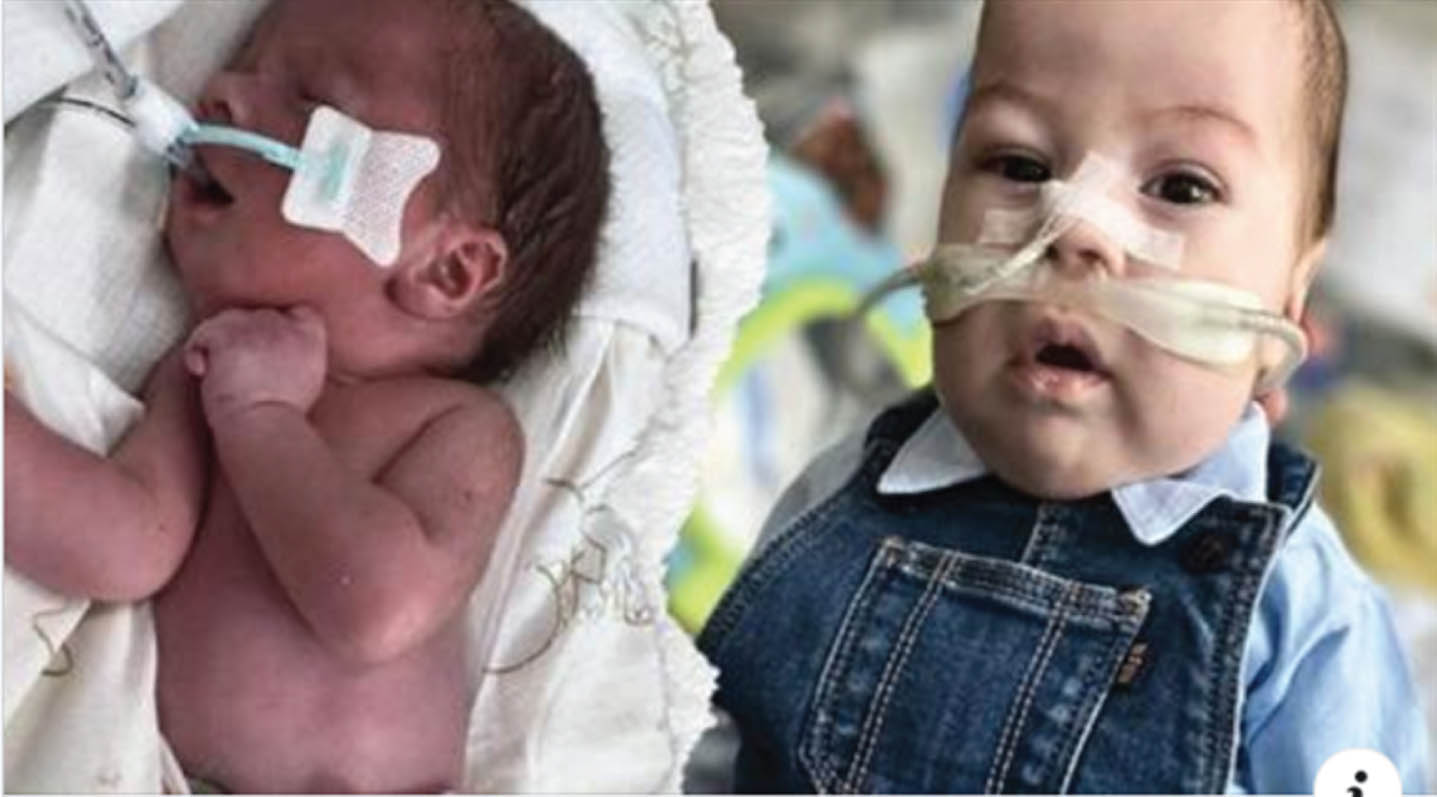
METHODOLOGY: The boy G1, P1, was born by Caesarean section (due to polyhydramnios, hypertensive pregnancy) in 37th GA with BW of 2400g, head circumference 35cm (50-90th percentile). In the Apgar scale, he received 3/4/7/7 points. The family history for miscarriages, deaths in early childhood as well as for neurological and genetic diseases was negative. The perinatal period was complicated by respiratory failure, pulmonary hypertension, congenital infection and difficulties in feeding (lack of sucking). Prolonged mechanical ventilation was used to stabilize the child’s condition.
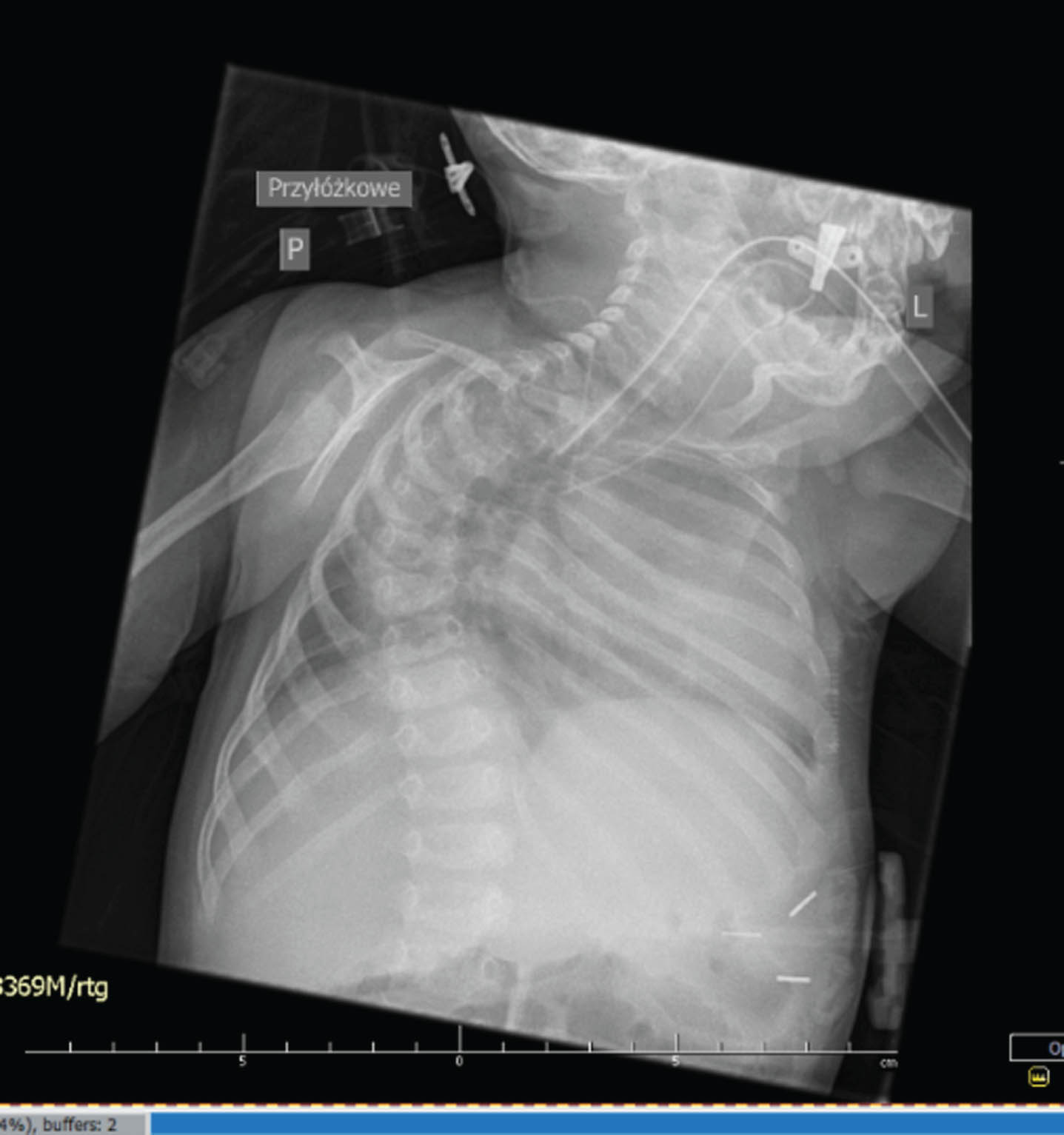
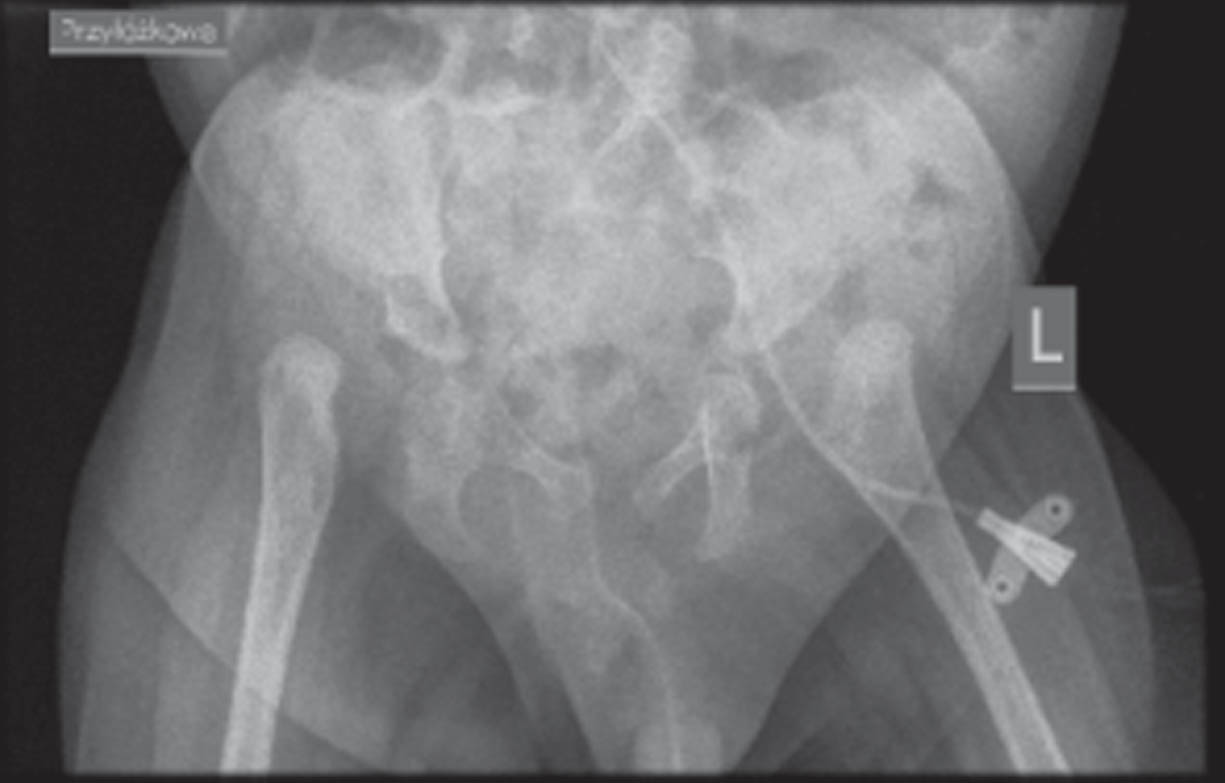
RESULTS: Physical examination revealed long face, frontal bossing, slight retrognatia, low-set auricles, cleft palate, short neck, limited neck mobility (stiff spine), long phalanges, overlapping fingers, clinodactyly: 3rd, 4th and 5th fingers, pectoral hump on the right side, scoliosis, extra hair in the lumbar-sacral area, muscle atrophy in the lower limbs, long feet, flexion and extension contractures of the upper and lower limbs, respectively, as well as flexion of the wrists and fingers, areflexia. Electroneurography ( 17th DOL) indicates generalized axonal damage to the nerve trunks (polyneuropathy). Without any signs of muscle damage, either myopathic or neurogenic. Magnetic Resonance Imaging (MRI) brain (16 th DOL) - enlarged paracerebral and paracerebellar fluid spaces, narrow cavity of the septum pellucidum and cavity of Verga ( up to 4 mm wide). X-ray examination show right-convex kyphoscoliosis of the thoracic spine, failure to close vertebral arches in the entire range of the spine, subluxation in the left hip joint. Array-based comparative genomic hybridization (aCGH) – (11th DOL) was negative. Whole Exome Sequencing(WES)-(35 th DOL) led to identification of the homozygous variant c1093C > T on GLDN gene from chromosome 15
CONCLUSION: In this paper, we present clinical and structural evidence of pathogenicity of the homozygous variant c1093C > T on GLDN gene from chromosome 15. We emphasize the need for rapid genetic diagnosis - WES in congenital diseases, especially in cases with unclear clinical picture or when the prognosis is poor.
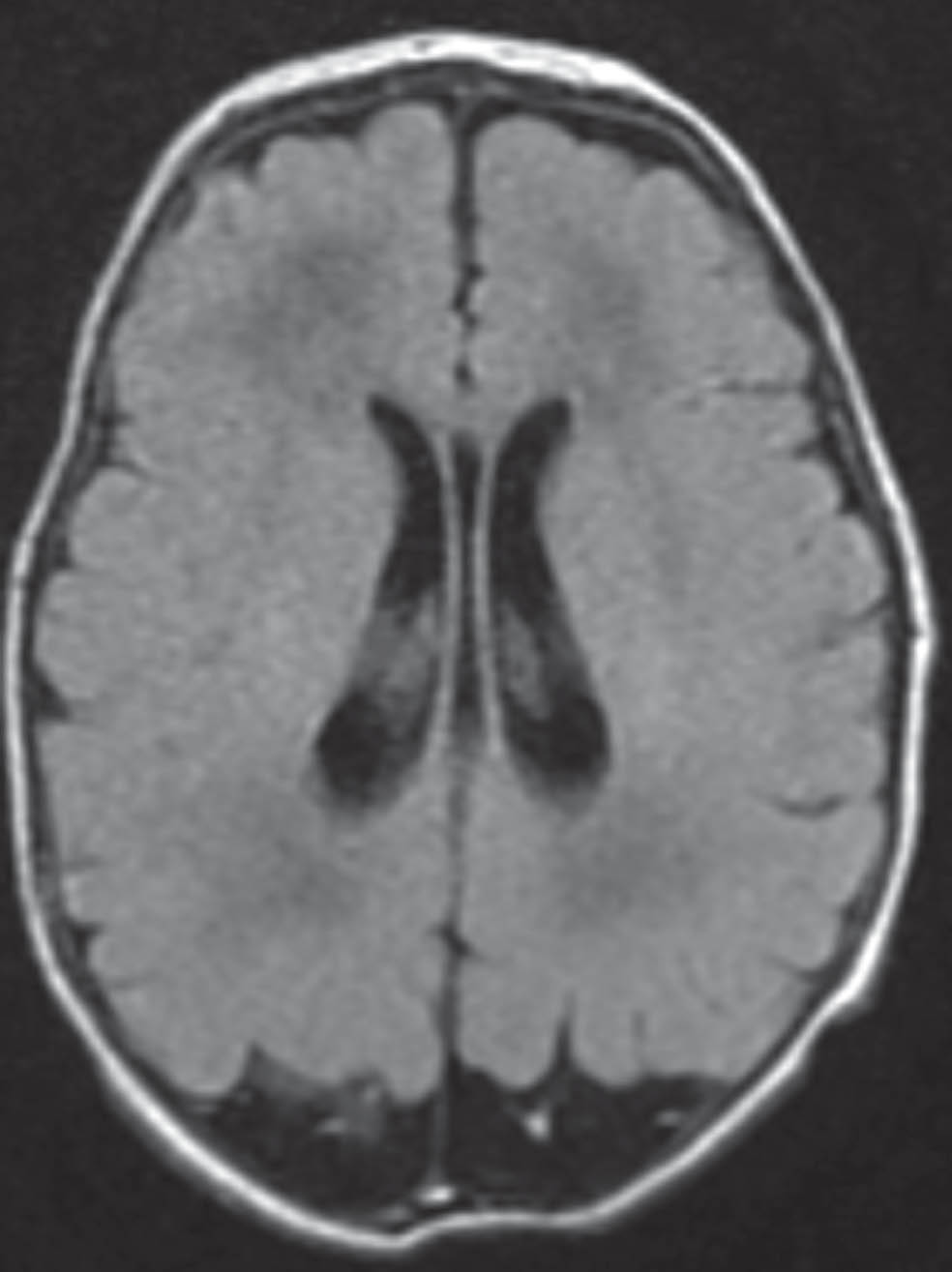
Challenges in diagnosis and management of a D-bifunctional protein deficiency case in a low-middle-income country
Ioana Bianca Mutica1, Maria Livia Ognean1,3, Gabriela Vişa2, Ioana Mătăcuţă2,3, Claudiu Matei3,4
1Clinical County Emergency Hospital, Neonatology Dept., 2Paediatric Clinical Hospital , 3Faculty of Medicine, Lucian Blaga University, 4Medlife Polisano Hospital Sibiu
BACKGROUND: D-bifunctional protein(DBP) deficiency is a single peroxisomal enzyme deficiency, with an estimated prevalence of 1:100.000, an autosomal recessive inborn error of very long-chain fatty acids metabolism. The most common symptoms are severe hypotonia(98%) and seizures(93%), usually occurring within the first month of life. The prognosis is poor, most patients with DPB type I die in the first 2 years of life.
METHODOLOGY: We are presenting the case of a female newborn, delivered vaginally at 40 gestational weeks, birth weight of 2590g, and an Apgar score of 6/8/9. From the first day of life(DOL) the infant presented generalized hypotonia, weak sucking reflex, and transient hypoglycemia. Additionally, in the 2nd DOL, she developed seizures with aEEG correspondence. The seizures were difficult to manage throughout the entire period of life, regardless of the antiepileptic treatment adjustments. Besides moderate elevated CRP (due to an early onset sepsis), no other blood abnormalities were found. Electrolytes and serum lactate were within normal range, the extended metabolic and neonatal epilepsy syndromes screening were negative; the newborn was also tested for Pompe disease and Spinal Muscular Atrophy, both with negative results. Mild ventriculomegaly was observed at brain ultrasound while cranial MRI showed callosal hypoplasia and diffuse cerebral hypomyelination. After 42 days in the neonatology department, the infant was transferred to the paediatric neurology and had multiple episodes of pneumonia with respiratory failure and urinary tract infection, which led to hydronephrosis. Exome sequencing was performed and a final diagnosis of D-bifunctional protein deficiency type I was made - two mutations were described HSD17B4 c.788del, p. (Pro263Glnfs*2) and the unpublished homozygous missense variant SUOX c.913G>A, p. (Ala305Thr)(at that moment, a variant of uncertain significance). At 11 months the patient developed obstructive hydrocephalus, ventriculoperitoneal shunt was inserted. Shortly after the procedure, she presented generalized seizure, respiratory failure with cardiac arrest, and died at 1 year old.
RESULTS: With any financial support from the public health services for the screening workups, neuro-muscular diseases, and genetics tests, as well as for the neurosurgical intervention we faced many challenges in the diagnosis and management of our patient. These obstacles added to poor health infrastructure, low human resources, and low financial resources of the family. Grace to an interdisciplinary approach and collaboration we were able to offer a diagnosis and proper treatment and support to an infant and a family in a very difficult situation.
CONCLUSION: D-bifunctional protein deficiency is a rare, life-limiting disease, with severe symptoms and life-threatening complications. Thus, proper management implies a good functional health system accessible and affordable, for both medical caregivers and families.
BIBLIOGRAPHY:
Ferdinandusse S, Denis S, et al. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann Neurol. 2006 Jan;59(1):92-104. doi: 10.1002/ana.20702. PMID: 16278854.




