Proceedings of the 14th International Newborn Brain Conference: Neuro-imaging studies
Cardiorespiratory instability and reactivity correlate with brain volume and surface area at term-equivalent age in very low birth weight infants
Vesoulis Z1, Kausch S2, Alexopoulos D3, Smyser C3, Sullivan B2
1Department of Pediatrics, Washington University, St. Louis, United States, 2Department of Pediatrics, University of Virginia, Charlottesvlle, United States, 3Department of Neurology, Washington University, St. Louis, United States
BACKGROUND: Cardiorespiratory deterioration is common in very low birth weight (<1500g) infants. This period of critical brain growth and development may be impacted by insults such as sepsis and hypoxia. The physiological response to illness manifests through autonomic signaling and is detected as changes in heart rate (HR) and oxygenation (SpO2). We hypothesized that limited autonomic activity and reactivity may indicate poor cardiovascular autoregulation, increasing the risk of cerebral hypoxic-ischemia, the fundamental basis for preterm brain injury. The cumulative hypoxic insult may be correlated with decreased brain growth at term equivalent age (TEA).
METHODS: Infants born <32 weeks were recruited for a pilot study of longitudinal vital sign collection and term-equivalent imaging. All included infants (n=12) had continuous HR/SpO2 data from the entire NICU stay and a non-sedated, non-contrast MRI performed at term-equivalent age. Autonomic signaling was quantified in 10-min windows across the entire NICU stay using a validated multi-feature logistic regression model called pulse oximetry warning score (POWS) where low mean POWS represents decreased autonomic activity and low standard deviation of POWS represents decreased reactivity or responsiveness.
Using T2-weighted images, total cortical surface area (CSA), gray matter, white matter, and total brain volume were calculated using a semi-automated pipeline. We used Spearman correlation to evaluate the relationship between the mean and SD of POWS with brain volumetrics.
RESULTS: Two example infants are shown in Figure 1; infant A has a low mean POWS with limited variance (low SD POWS) while infant B has a much high mean POWS with significantly greater variance.
As shown in Figure 2, a higher mean POWS during NICU hospitalization was associated with increased gray matter volume (R=0.71, p=0.01), total brain volume (R=0.69, p=0.02), and total cortical surface area (R=0.78, p<0.01). An even stronger association was noted between the variance (SD) of POWS and gray matter volume (R=0.72, p=.01), total brain volume (R=0.76, p<0.01), and total cortical surface area (R=0.78, p<0.01) shown in Figure 3. There was no association between white matter volume and mean or SD POWS.
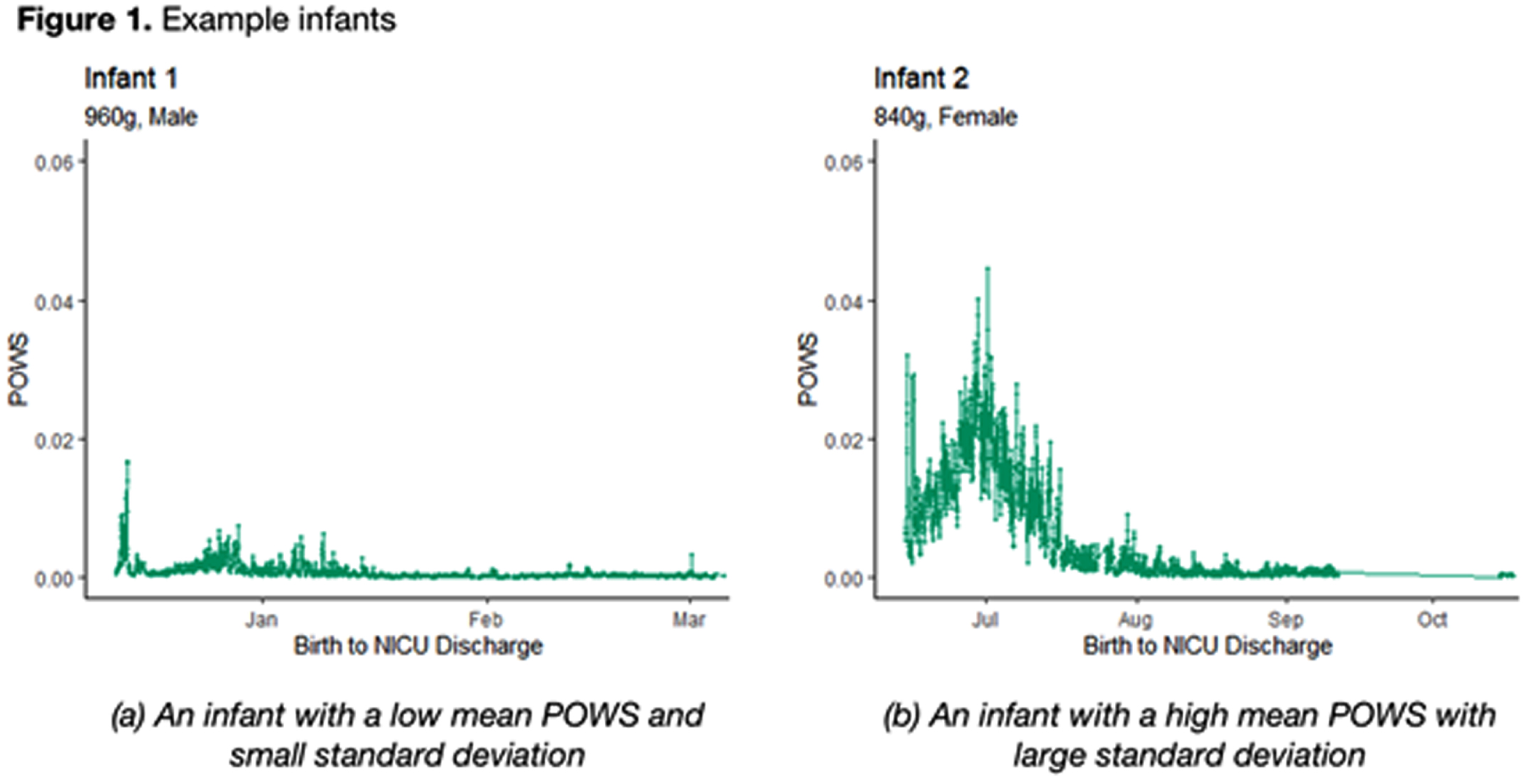
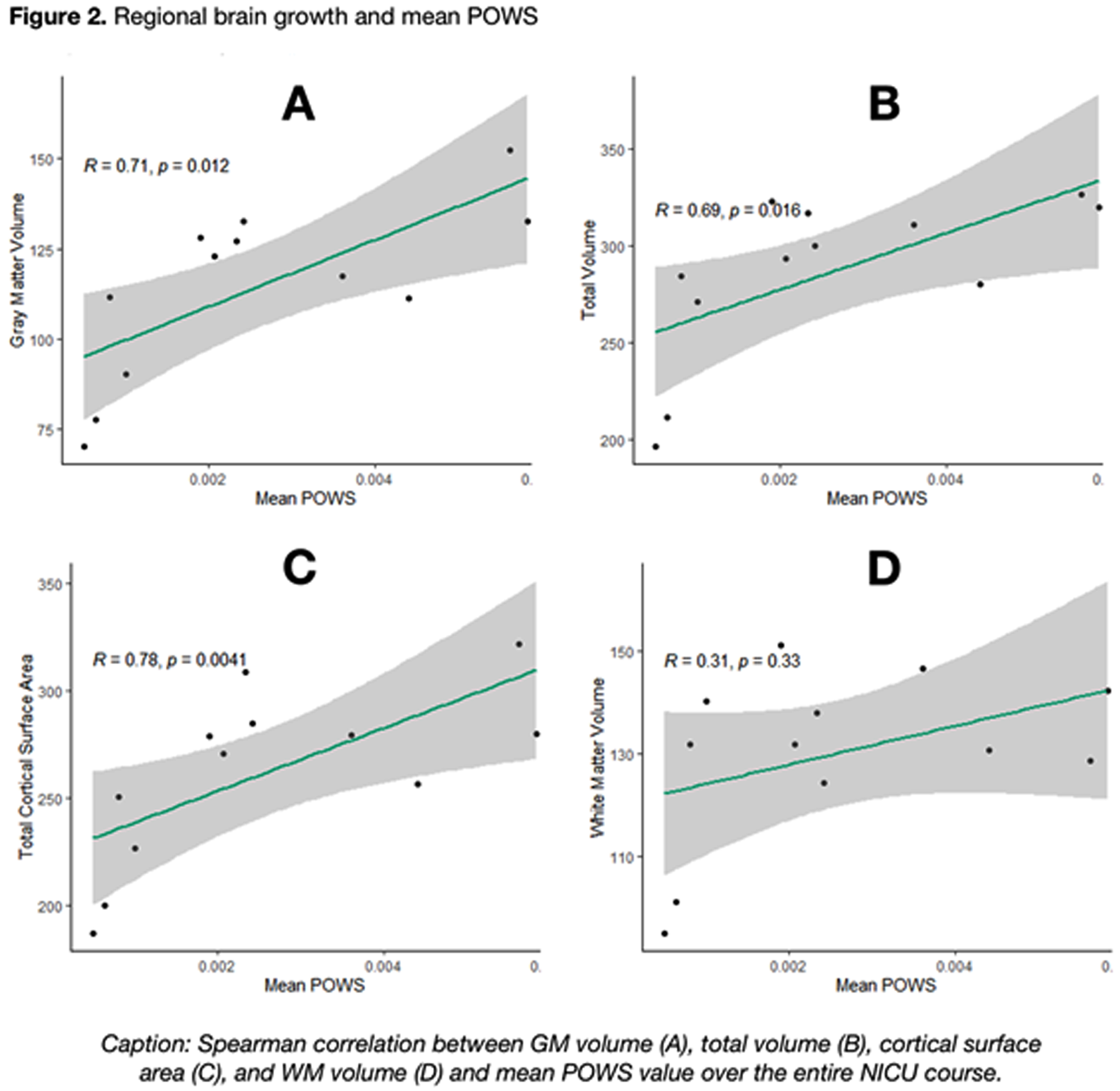
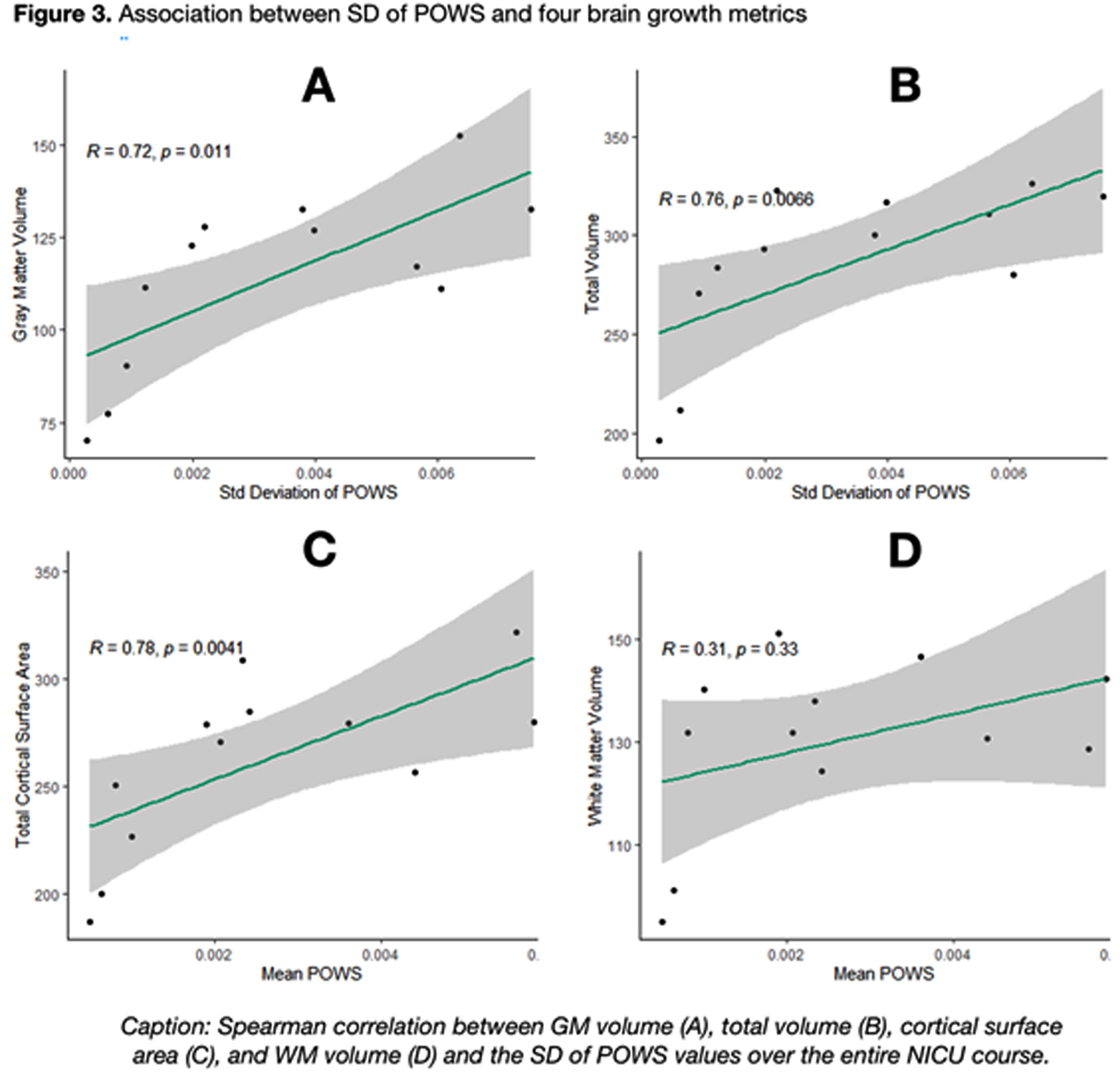
CONCLUSION: Autonomic activity and reactivity, expressed through mean and SD POWS scores, were strongly correlated with greater brain growth at TEA in preterm infants. These results generate hypotheses that warrant further study as potential tools to predict neurologic outcomes.
Post-haemorrhagic ventricular dilatation in preterm infants: How are we monitoring and which measurement is best?
Thyagarajan B2, Jefferies K1
1,2Princess Anne Hospital, Southampton, United Kingdom
BACKGROUND: Post-haemorrhagic ventricular dilatation (PHVD) secondary to severe intraventricular haemorrhage (IVH) is associated with high morbidity and mortality. Several studies have demonstrated that timely intervention can help to reduce this risk (1,2,3); However, there remains significant variation in monitoring strategies and intervention thresholds. Some centres react to serial ventricular index (VI) measurements whilst others monitor head circumference or for signs of raised intra-cranial pressure.
OBJECTIVES:
• To determine how different Tertiary Neonatal Units across the United Kingdom (UK) monitor infants with PHVD.
• To establish whether Anterior Horn Width (AHW) and Thalamo-Occipital Distance (TOD) measurements improve the early detection of moderate to severe PHVD versus Ventricular Index (VI) measurements alone when plotted on the “ventricular measurement risk zone” graphs published by El- Dib(4).
METHODS: Questionnaires were sent to Specialty Neonatal Trainees in the UK to determine how the Neonatal Intensive Care Unit (NICU) in which they currently worked measured and monitored PHVD.
Secondly, BadgerNet was used to identify infants managed for PHVD in one centre between July 2021-September 2022. Serial cranial ultrasound scans from these infants were reviewed retrospectively. VI, AHW and TOD measurements were obtained and categorized as high, moderate or low risk according to the “ventricular measurement risk zones” published by El- Dib(4). A comparison was made to see how well these values correlated.
RESULTS: 24 UK NICUs responded to the questionnaire. 18(75%) measured VIs, 2(8.3%) measured VIs and AHW, 1(4.2%) measured VIs, AHW and TOD. The remaining 3(12.5%) centres monitored head circumference alone. Between July 2021-September 2022, 8 preterm infants (23+3- 29+6) were managed with PHVD in one NICU. From these 8 individuals, 64 cranial ultrasound scans were reviewed. 5 scans were performed at <24 weeks gestation and excluded because the results could not be plotted on the chart (4). From the remaining 59 scans, 113 VI, 110 AHW and 106 TOD measurements were obtained. 110 corresponding VI and AHW measurements were obtained. These two measurements both correlated as either high, moderate or low risk in 66.3% of cases. AHW underestimated risk in 27.3% and overestimated risk in 6.4%. 103 corresponding VI and TOD measurements were obtained. These two measurements both correlated as either high, moderate or low risk in 47.5% of cases. TOD underestimated risk in 44.7% and overestimated risk in 7.8%.
CONCLUSION: Despite the high associated morbidity and mortality following severe IVH and PHVD, significant variation remains in both strategies for monitoring and consequently thresholds for intervention. Whilst AHW and TOD measurements may provide additional information related to degree of ventricular dilatation, there was a tendency to underestimate risk when compared to VI measurements so should be used with caution in isolation as they could delay intervention.
REFERENCES
[1] de Vries LS, Liem KD, van Dijk K, Smit BJ, Sie L, Rademaker KJ, et al. Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in The Netherlands. Acta Paediatr. 2002;91:212–7. [PubMed: 11952011]
[2] Leijser LM, Miller SP, van Wezel-Meijler G, Brouwer AJ, Traubici J, van Haastert IC, et al. Posthemorrhagic ventricular dilatation in preterm infants: When best to intervene? Neurology. 2018;90:e698–e706. [PubMed: 29367448]
[3] Bassan H, Eshel R, Golan I, Kohelet D, Ben Sira L, Mandel D, et al. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with posthemorrhagic hydrocephalus. Eur J Paediatr Neurol. 2012;16:662–70. [PubMed: 22591810]
[4] El-Dib,M. et al. Management of Post-haemorrhagic Ventricular Dilatation in the Preterm Infant. J Pediatr.2020;226. 16–27.
Transfontanelle photoacoustic imaging: A novel imaging modality for cerebral oxygenation measurement
Avanaki K1, Manwar R, McGuire L, Islam M, Shoo A, T Charbel F, M Pillers D
1The University Of Illinois At Chicago, 851 S Morgan St, United States
SUMMARY: The capability of photoacoustic (PA) imaging to measure oxygen saturation through a fontanelle has been demonstrated in sheep in-vivo. We call this method, transfontanelle photoacoustic imaging (TFPAI).
BACKGROUND AND PURPOSE: problem statement or hypothesis as appropriate: Tissue oxygenation is a significant biomarker of neonatal central nervous system health and functionality. However, there are no suitable techniques to directly measure tissue oxygenation. We demonstrated the capability of PA imaging using a handheld probe, i.e., TFPAI, to measure oxygenation through a fontanelle for various conditions including hypoxia and hyperoxia in-vivo.
MATERIALS OR METHODOLOGY: A 2-cm fontanelle was created in an adult sheep, and PA imaging was performed through the scalp. Photoaocutic spectroscopic analysis was performed based on four laser light wavelengths to extract the oxygen saturation at different locations within the brain tissue.
RESULTS: The performance of TFPAI was evaluated and compared to the gold standard blood gas analyzer measurement from arterial blood samples. We observed a strong correlation between the predicted measurement and the reading of the blood gas analyzer. TFPAI could measure sO2 at deep structures because (i) the light/acoustic attenuation at the cranial window was lower than skull, and (ii) illumination was maximized by simultaneous operation of two lasers, reaching to the total energy of 140mJ at 750nm wavelength while maintaining the fluence below the ANSI limit.
CONCLUSION/IMPACT: The results showed the efficacy of the TFPAI probe in determining the oxygenation level. Our next objective is to utilize the probe on neonates with an actual fontanelle. Towards that goal, at first, we plan to further refine the probe capability by developing a calibration technique based on machine learning to compensate for the deviation predicted between cerebral sO2, umbilical and capillary sO2. We expect that the TFPAI probe will be a valuable monitoring tool especially for neonates with critical conditions and admitted into intensive care unit (NICU).
Moderate-late preterm infants are not “near term”: Premature birth across the gestational age spectrum affects brain growth at term equivalent age
Verschuur A1,2,3, van Steenis A4, Boswinkel V5, Nijholt I1, Boomsma M1, Steggerda S4, Meijler G5, Leijser L3
1Department of Radiology, Isala Hospital, Zwolle, The Netherlands, 2Image Science Institute, University Medical Centre Utrecht (UMCU), Utrecht, The Netherlands, 3Department of Pediatrics, Section of Neonatology, University of Calgary, Cumming School of Medicine, Calgary, Canada, 4Department of Neonatology, Leiden University Medical Centre, Leiden, The Netherlands, 5Department of Neonatology, Isala Women and Children’s Hospital (IVKC), Zwolle, The Netherlands
BACKGROUND: Preterm infants (<37 weeks’ gestation) are at high-risk for neurodevelopmental problems, of which the incidence and severity are inversely related to gestational age (GA) at birth. Suboptimal brain growth may contribute to this risk. However, the influence of GA on early postnatal brain volumes has been insufficiently studied. Our aim was to investigate brain volumetric differences between extremely preterm (EP; <28 weeks), very preterm (VP; 28-32 weeks), moderate preterm (MP; 32-34 weeks), late preterm (LP; 34-37 weeks) and full-term (FT; >37 weeks) infants from magnetic resonance imaging (MRI) at term equivalent age (TEA).
METHODS: Data from three prospective neonatal cohorts were used: 1) EP and VP cohort from Leiden University Medical Center, The Netherlands (ED/BGT-study); 2) MP and LP cohort from Isala Women and Children’s Hospital, The Netherlands (BIMP-study); 3) open access LP and FT cohort from the developmental human connectome project (dHCP), UK. In all cohorts, 3T brain MRI was performed at TEA according to established protocols. Volumes of eight brain regions (cerebrospinal fluid [CSF], cortical and deep gray matter, white matter, hippocampus, amygdala, cerebellum, brainstem) and intracranial and total tissue volumes were automatically segmented from T2-weighed images (slice thickness ≤2mm) using an adapted version of MANTiS (Morphologically adaptive neonatal tissue segmentation toolbox). Segmentation results were checked visually and manually corrected when minor errors existed in ≤2 slices; scans with errors in >2 slices were excluded. Absolute regional volumes and CSF volumes calculated relative to intracranial volume were compared between age groups using linear regression analysis, with group as determinant, volume as outcome and FT infant volumes used as reference. Analyses were corrected for postmenstrual age at MRI. Statistical significance was set at p<0.05. A Bonferroni correction was applied to correct for multiple testing.
RESULTS: A total of 589 scans were eligible for analyses (25 EP, 48 VP, 38 MP, 92 LP, 385 FT infants). Without correction for multiple testing, most absolute volumes (except CSF and amygdala) were significantly smaller in EP, VP, MP and LP infants compared to FT infants, becoming increasingly prominent with decreasing GA (Figure 1). While absolute CSF volumes were not different between groups, relative CSF volumes were significantly larger in EP and VP than FT infants. After correction for multiple testing, white matter volumes in MP infants were no longer significantly different.
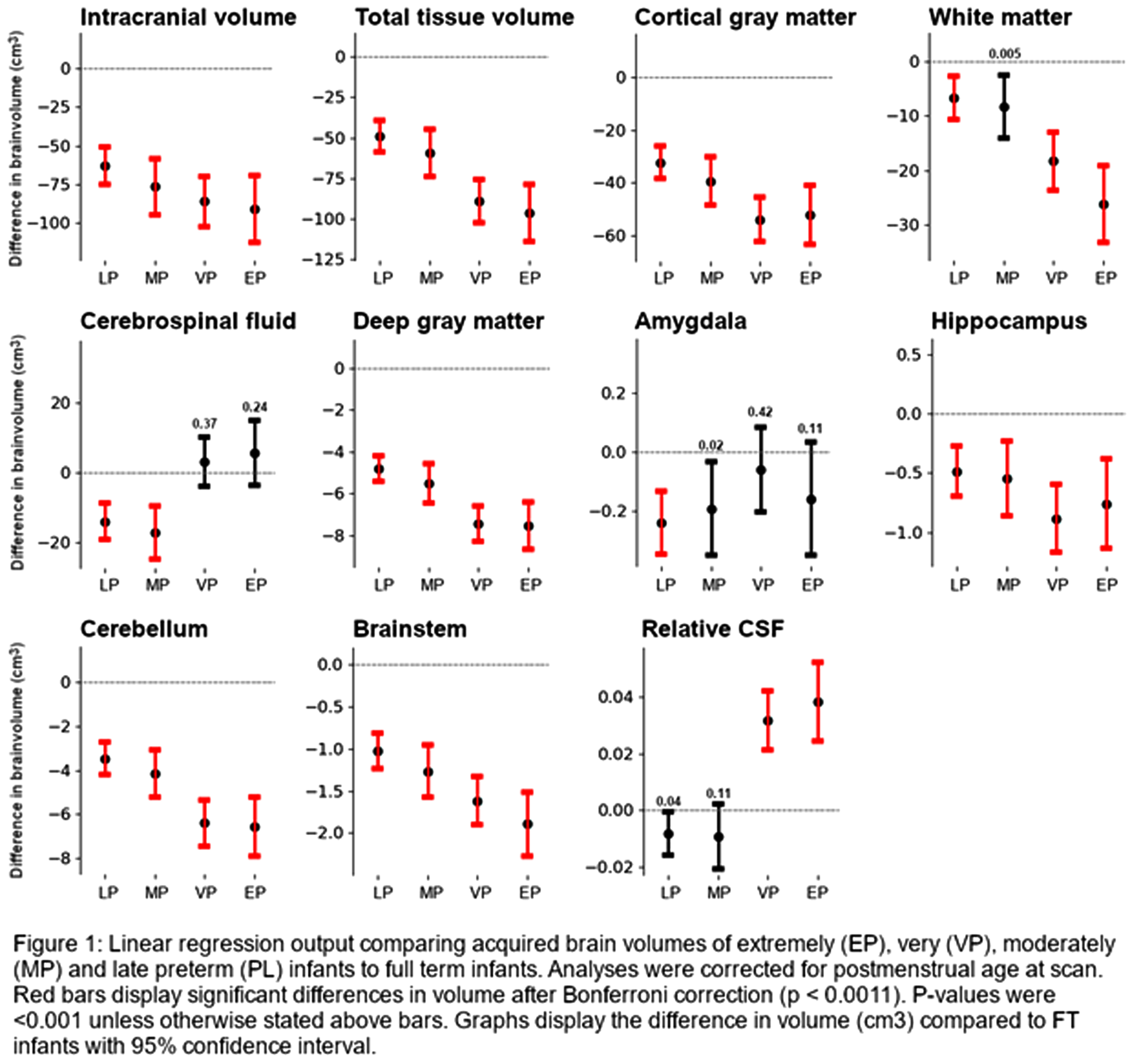
CONCLUSION: Preterm birth is associated with smaller brain volumes and larger CSF spaces at TEA. While most prominent in the youngest infants, the suboptimal brain growth affects the full spectrum of preterm infants from EP to LP. The developmentally more advanced, yet rapidly developing MP to LP brain may thus be as vulnerable to disruptions in development related to preterm birth and related exposures as the EP and VP brain.
ACKNOWLEDGEMENT: These results were obtained using data made available from the Developing Human Connectome Project funded by the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013) / ERC Grant Agreement no. [319456].
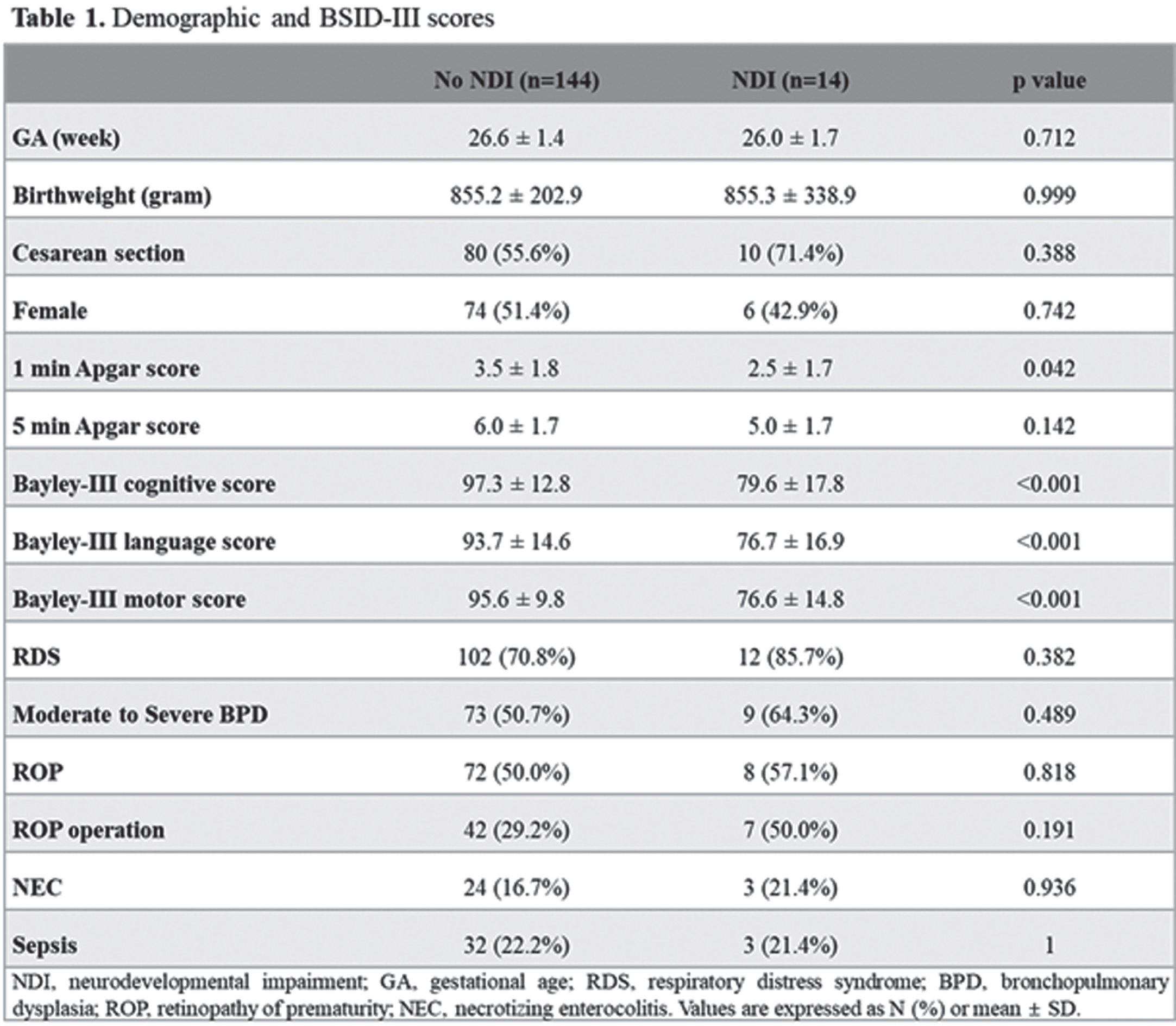
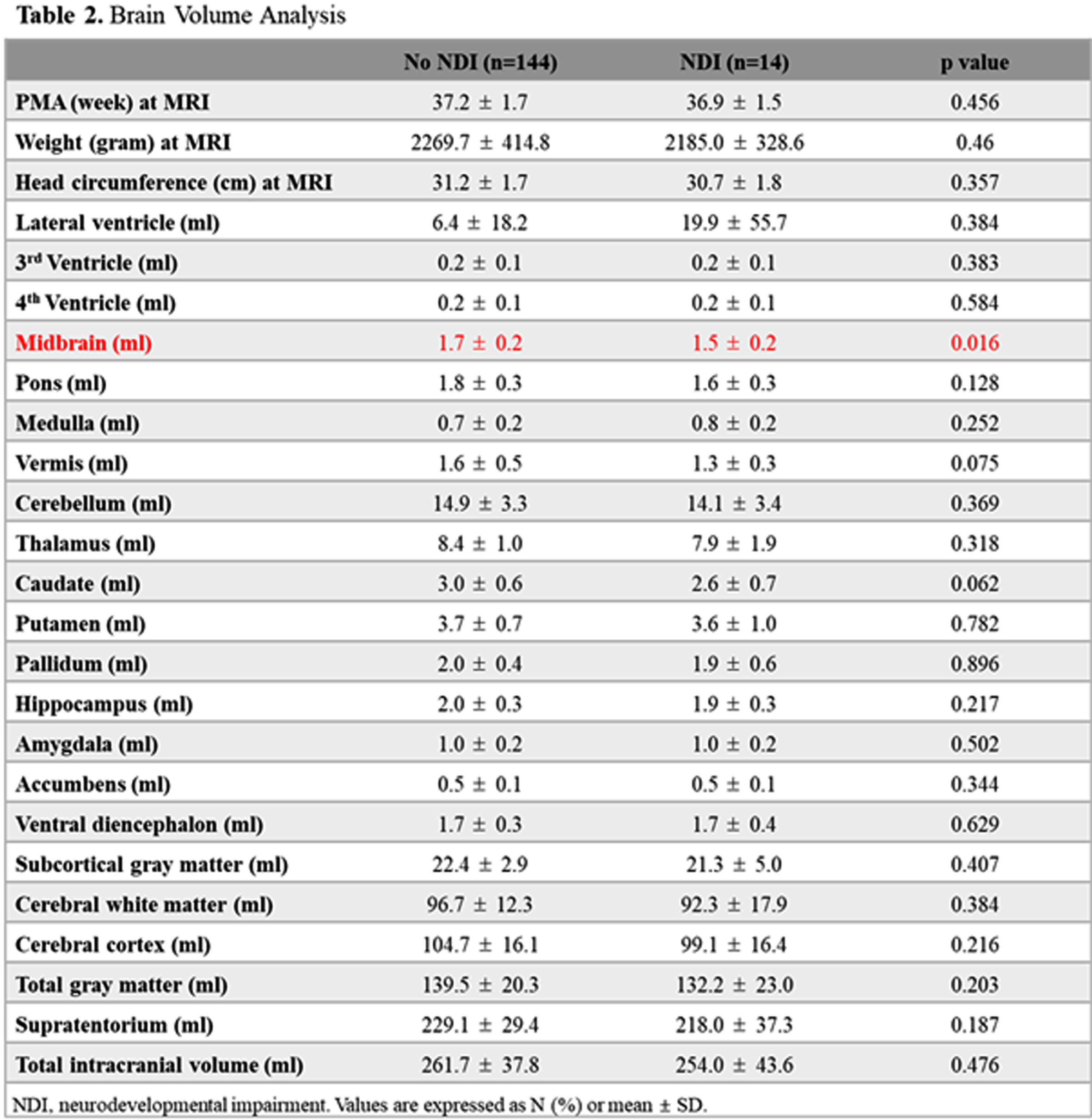
Association between the volumes of preterm brain structures, neurodevelopmental outcomes, and neonatal morbidities
Park S1, Yang H1, Lim S1, Kim S1, Shin S1, Kim E1, Kim H1
1Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, South Korea
BACKGROUND: Premature infants are prone to have impaired neurodevelopmental outcomes due to the injury or underdevelopment of the brain. However, impaired neurodevelopmental outcomes may be present without any brain lesions, and the association between neonatal morbidities and the volume of segmental brain structure has not been fully understood. The objective of this study was to analyze the association between the volumes of brain structures, neonatal morbidities, and neurodevelopmental outcomes.
METHOD: This was a retrospective cohort study of 158 preterm infants from January 2007 to December 2019 admitted to Seoul National University Children’s Hospital, among whom brain MRI at term equivalent age (TEA-MRI) and neurodevelopmental evaluation at corrected age of 18-24 months were evaluated. Patients with congenital anomalies or significant brain lesions such as high-grade intraventricular hemorrhage and periventricular leukomalacia detected by TEA-MRI were excluded. Medical records, including Bayley Scales of Infant and Toddler Development 3rd Edition (BSID-III) of preterm infants, were reviewed and analyzed. Infant Freesurfer software was used to evaluate the volumes of brain structures, and each volume of brain regions was adjusted for gestational age and post-menstrual age at MRI in multivariate regression analysis.
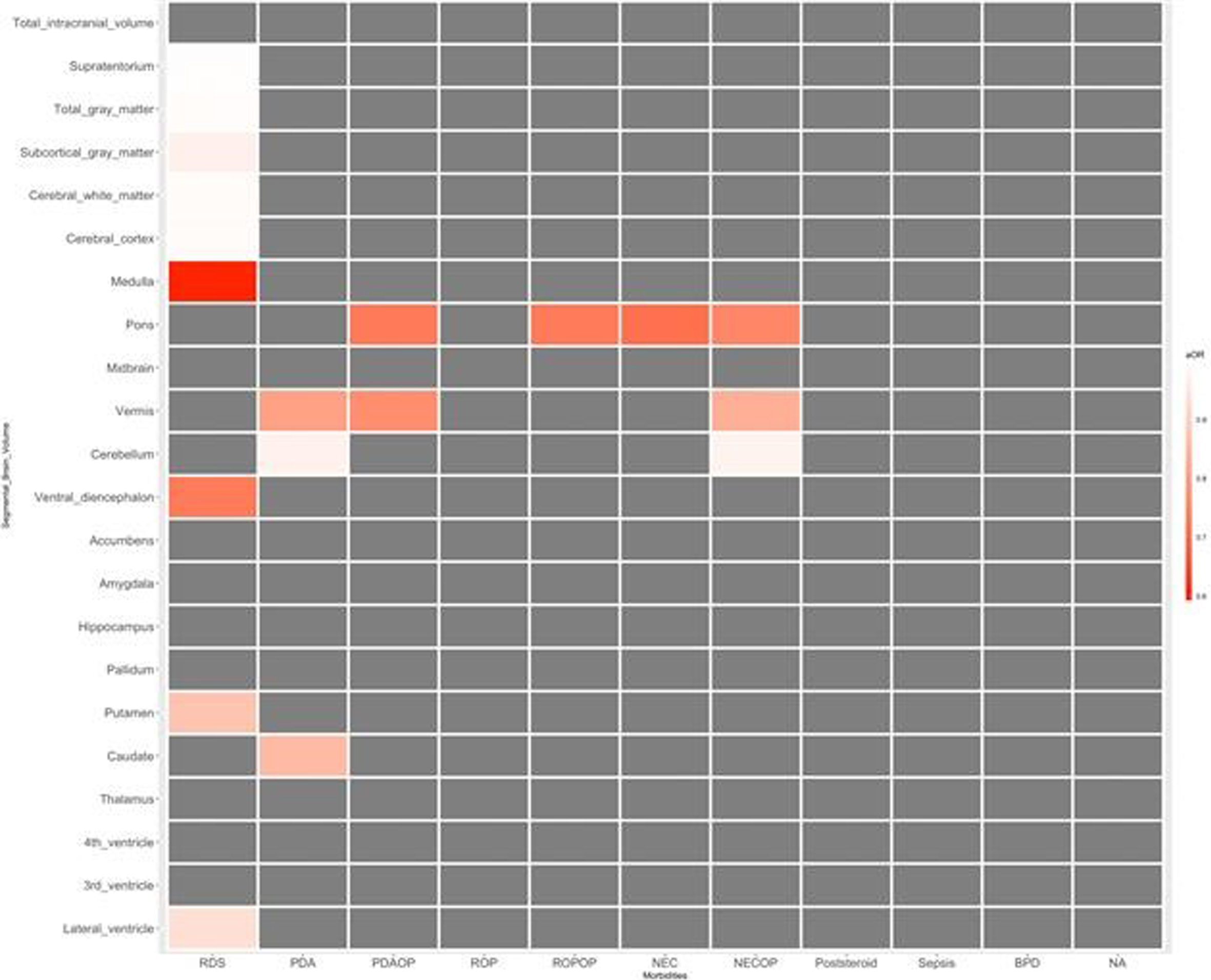
RESULT: Infant with neurodevelopmental impairment was associated with a smaller volume of the midbrain (aOR 0.804, 95% CI 0.647-0.998). Among neonatal morbidities of preterm infants, respiratory distress syndrome was associated with a smaller volume of the medulla (aOR 0.593, 95% CI 0.387-0.908), ventral diencephalon (aOR 0.730, 95% CI 0.572-0.932), and putamen (aOR 0.874, 95% CI 0.788-0.969). Patent ductus arteriosus (PDA) requiring operation was associated with a smaller volume of pons (aOR 0.727, 95% CI 0.594-0.889) and vermis (aOR 0.766, 95% CI 0.646-0.907). Retinopathy of prematurity (ROP) requiring operation was associated with a smaller volume of pons (aOR 0.729, 95% CI 0.600-0.886). Necrotizing enterocolitis (NEC) requiring operation was associated with smaller volume of pons (aOR 0.751, 95% CI 0.661-0.854), vermis (aOR 0.832, 95% CI 0.745-0.930), and cerebellum (aOR 0.974, 95% CI 0.960-0.989).
CONCLUSION: Among preterm infants without significant brain injuries, a smaller volume of the midbrain was associated with impaired neurodevelopment. RDS, PDA requiring operation, ROP requiring operation, and NEC requiring operation were associated with reduced segmental volumes of the brain in preterm infants.
REFERENCES
[1] Ecury-Goossen GM, Dudink J, Lequin M, Feijen-Roon M, Horsch S, Govaert P. The clinical presentation of preterm cerebellar haemorrhage. Eur J Pediatr 2010;169:1249-53.
[2] Hortensius LM, Dijkshoorn ABC, Ecury-Goossen GM, Steggerda SJ, Hoebeek FE, Benders M, et al. Neurodevelopmental Consequences of Preterm Isolated Cerebellar Hemorrhage: A Systematic Review. Pediatrics 2018;142.
[3] Villamor-Martinez E, Fumagalli M, Alomar YI, Passera S, Cavallaro G, Mosca F, et al. Cerebellar Hemorrhage in Preterm Infants: A Meta-Analysis on Risk Factors and Neurodevelopmental Outcome. Front Physiol 2019;10:800.
[4] Hou D, Shetty U, Phillips M, Gray PH. Cerebellar haemorrhage in the extremely preterm infant. J Paediatr Child Health 2012;48:350-5.
[5] Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 2014;134:e444-53.
[6] Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Jr., Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007;120:584-93.
[7] Zayek MM, Benjamin JT, Maertens P, Trimm RF, Lal CV, Eyal FG. Cerebellar hemorrhage: a major morbidity in extremely preterm infants. J Perinatol 2012;32:699-704.
[8] Tam EW, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr 2011;158:245-50.
[9] Steggerda SJ, De Bruine FT, van den Berg-Huysmans AA, Rijken M, Leijser LM, Walther FJ, et al. Small cerebellar hemorrhage in preterm infants: perinatal and postnatal factors and outcome. Cerebellum 2013;12:794-801.
[10] El Shimi MS, Hassanein SM, Mohamed MH, Abdou RM, Roshdy A, Atef SH, et al. Predictive value of vascular endothelial growth factor in preterm neonates with intraventricular haemorrhage. J Matern Fetal Neonatal Med 2012;25:1586-90.
Periodic breathing distorts functional connectivity analysis
Shiraki A1, Kidokoro H1, Watanabe H2, Taga G2, Narita H1, Mitsumatsu T1, Kumai S1, Suzui R1, Sawamura F1, Ito Y1, Yamamoto H1, Nakata T1, Sato Y3, Hayakawa M3, Natsume J1,4
1Department of Pediatrics, Nagoya University Graduate School of Medicine, Nagoya, Japan, 2Graduate School of Education, The University of Tokyo, Tokyo, Japan, 3Division of Neonatology, Center for Maternal-Neonatal Care, Nagoya University Hospital, Nagoya, Japan, 4 Department of Developmental Disability Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan
BACKGROUND: Periodic breathing (PB), characterized by breathing periods interrupted by short episodes of apnea, is often seen in preterm infants. Although oxy- and deoxy-hemoglobin (Hb) in local brain regions fluctuate in synchrony with the PB rhythm, it remains unclear how PB affects the functional connectivity (FC) network analysis. We evaluated the effect of PB on FC studies using simultaneous EEG and functional near-infrared spectroscopy (NIRS) recordings in preterm infants.
METHODOLOGY: Recordings containing the PB sections were selected from the 128 EEG-functional NIRS data obtained at 31–46 weeks postmenstrual age. PB was defined based on the respiration pattern according to the American Academy of Sleep Medicine Manual (Figure 1). PB and non-PB sections were defined as the longest continuous sections that lasted for > 3 min. EEGs were recorded polygraphically with at least eight electrodes and were used for active sleep (AS) and quiet sleep (QS), scoring every 30 s, and PB detection. An eight-channel NIRS device was placed around the head to detect changes in oxy- and deoxy-Hb concentrations. The grand average of the hemodynamics at apnea onset in PB was analyzed for each channel, and an average value was calculated. Additionally, we calculated average FCs and phase synchronization indices (PSIs) from 28 pairs of channels under slow (< 0.1 Hz) oxy- and deoxy-Hb fluctuations and compared them between PB and non-PB sections under the same sleep state from the same records. Spearman’s rank correlation analysis and Wilcoxon signed-rank test were used for statistical analysis. P < 0.05 was considered statistically significant.
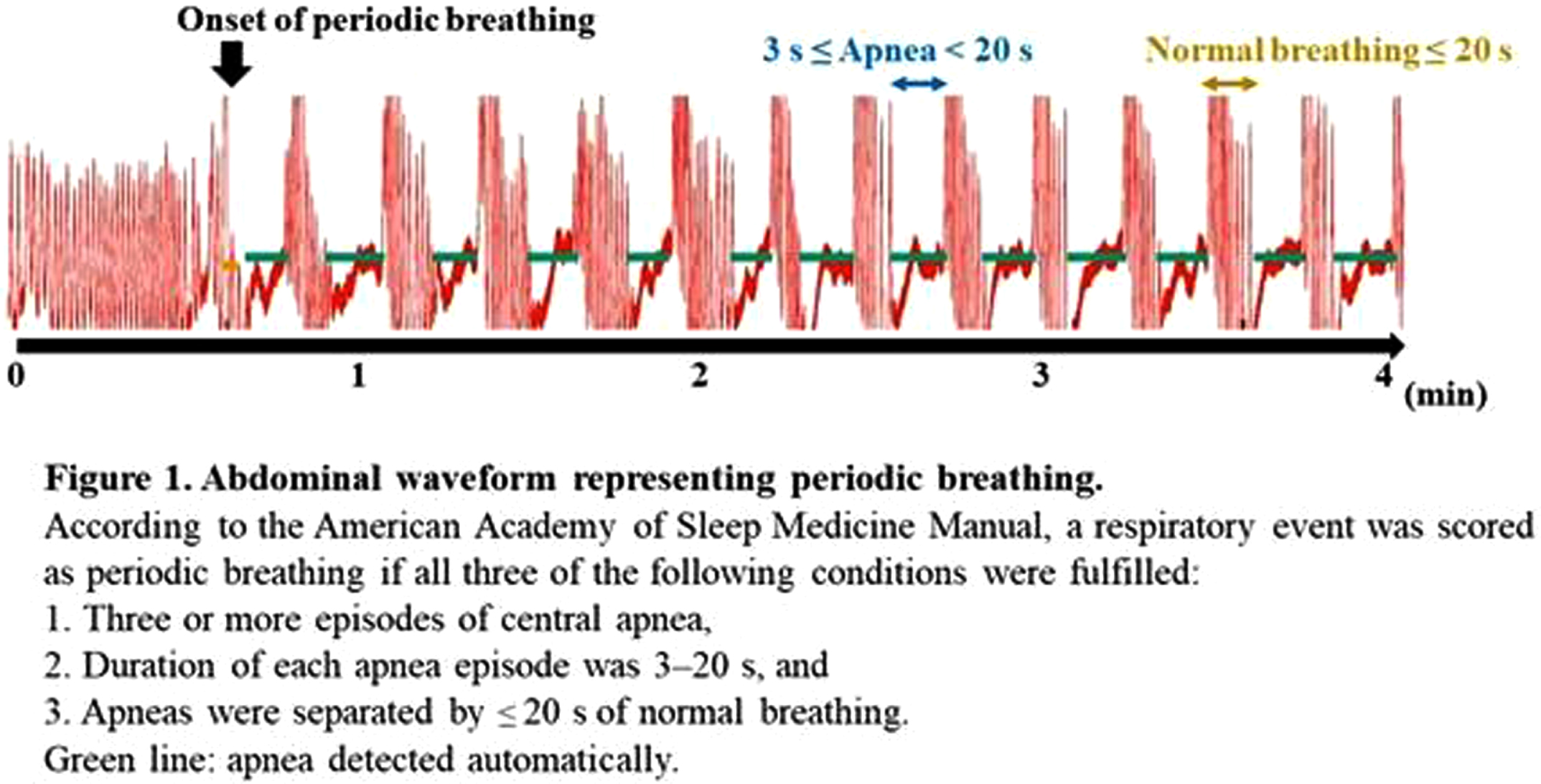
RESULTS: Of the 128 records, 24 contained PB sections that lasted > 3 min: 17 in AS and 15 in QS. The median (range) gestational and postmenstrual ages at the time of recording were 32.6 (24.9–34.4) and 36.8 (33.0–39.6) weeks, respectively. In each channel, the grand average of the NIRS data in PB showed a decrease-increase pattern in oxy-Hb and an increase-decrease pattern in deoxy-Hb (Figure 2). The period from apnea onset to the bottom of the average oxy-Hb and the period from apnea onset to the top of the average deoxy-Hb correlated significantly with mean apnea time (rs = 0.60 and 0,68, respectively). In the time series data, average FC and PSI were higher during the PB than non-PB sections in most records (Figure 3). The average FC of PB sections in QS, and PSI of PB sections in AS and QS in oxy-Hb, were significantly higher than those of non-PB sections (Figure 4). Similar trends were observed for deoxy-Hb.
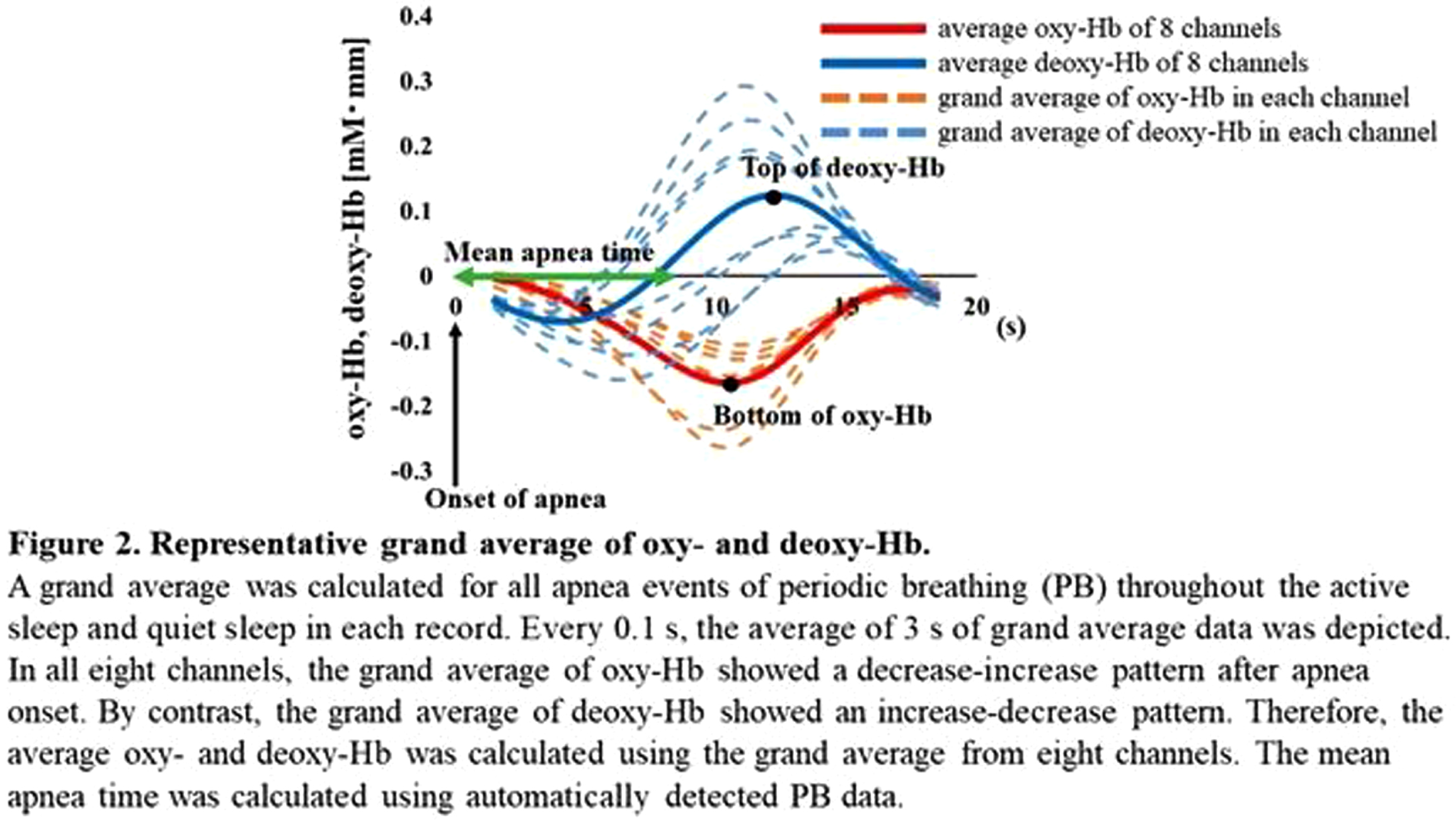
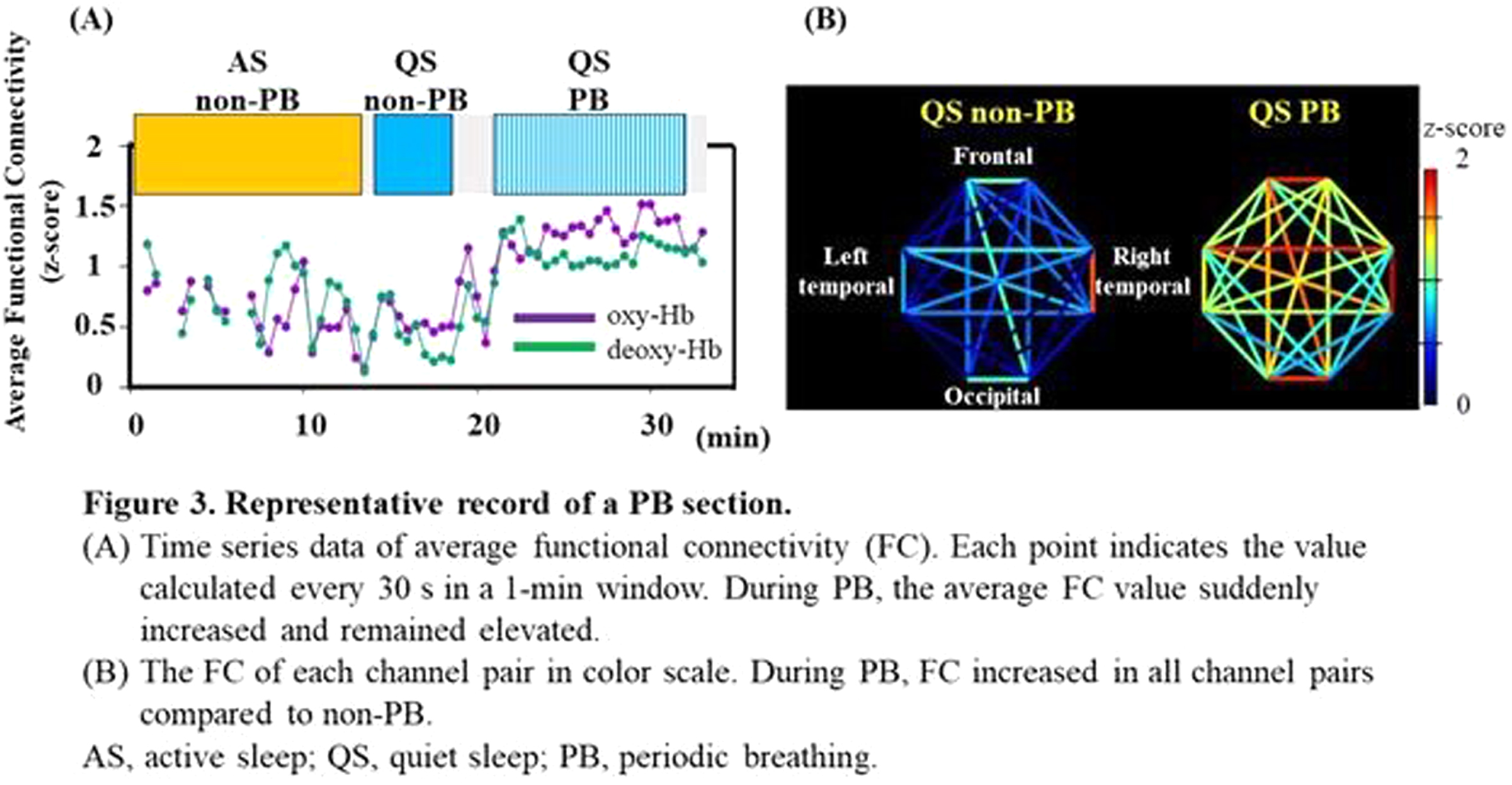
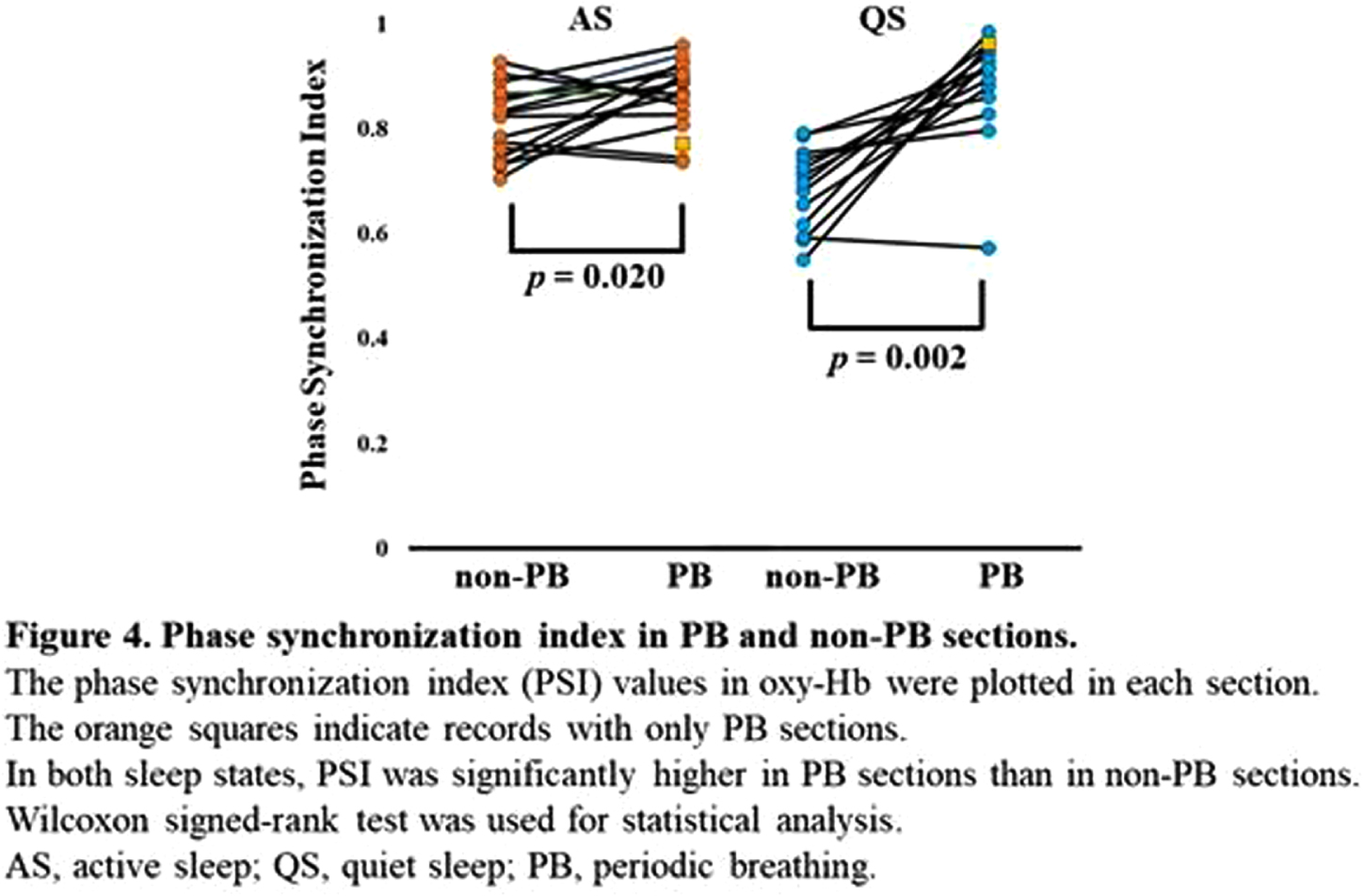
CONCLUSION: PB significantly affects the hemodynamics and FC indices, resulting in pseudo-hyperconnectivity in both sleep states. Monitoring the breathing pattern is essential for studies of resting-state FC networks, particularly for preterm infants.
Reference values for 2D quantitative brain metrics in mr images in infants born extremely preterm
Buchmayer J1, Kasprian G, Giordano V, Jernej R, Klebermass-Schrehof K, Berger A, Goeral K
1Medical University Of Vienna, Vienna, Austria
BACKGROUND AND PURPOSE: Cerebral magnetic resonance imaging (cMRI) is currently an important diagnostic tool for neonatal patients. Additional to qualitative analysis, studies showed that quantitative measurements may help identifying impaired brain growth. Through the application of volumetric techniques infants at risk for adverse development might be identified earlier leading to improved outcome prediction and later neurodevelopment. Basic two-dimensional (2D) cMRI measurements have been implemented by Garel et al1, but measurements were based on fetal cMRI, and Nguyen The Tich2, who analyzed a more mature collective.
The aim of this study was to create reference values for easy reproduceable brain metrics of various brain areas in a contemporary cohort of extremely preterm infants born before 28 weeks of gestation (wGA) born at a high-volume level IV perinatal center in Austria.
METHODOLOGY: This retrospective study analyzes imaging data, collected since the beginning of routine cMRI examinations for all preterm infants before 28 wGA at term-equivalent age in November 2017 over a four-year period. Only images of infants without severe brain pathologies were analyzed. The following 2D parameters were analyzed: cerebral and bone biparietal width (cBPW, bBPW), interhemispheric distance (IHD), transverse cerebellar diameter (TCD) and fronto-occipital diameter (FOD) (figure 1). Reference values were created.
Figure 1.
Measurements of (a) cerebral biparietal width (cBPW, light grey), bone biparietal width (bBPW, dark grey), interhemispheric distance (IHD, black); (b) transverse cerebellar diameter (tCD, black) and (c) fronto-occipital diameter (FOD, black).
RESULTS: The study cohort consisted of 112 preterm neonates, with a median gestational age of 25.9 (IQR 24.3-26.8) weeks and a median birth weight of 748 (IQR 625-896) grams. cMRI examinations were separated into five groups: 37 wGA (n=51), 38 wGA (n=27), 39 wGA (n=18), 40 wGA (n=9), 41/42 wGA (n=7). Mean values of the studied parameters are shown in table 1. Furthermore, there were significant correlations between older gestational age at birth and larger fronto-occipital diameters, as well as smaller interhemispheric distances. Reference values were created.
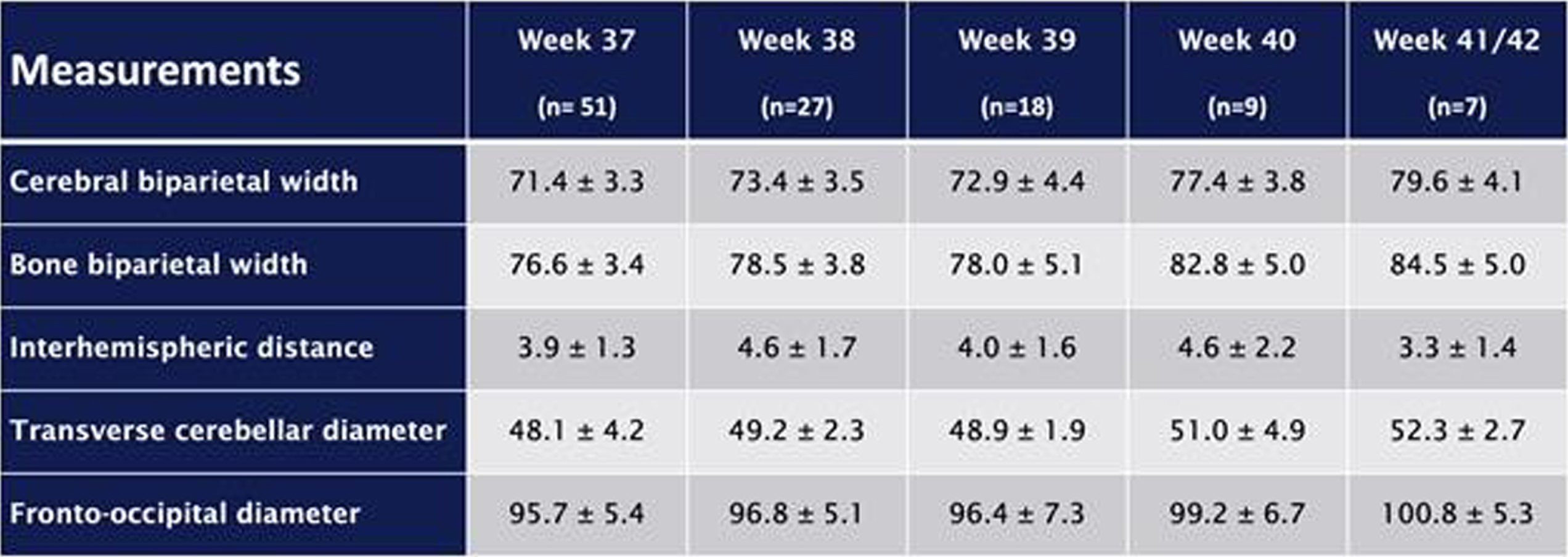
CONCLUSION/IMPACT: 2D cMRI brain measurements at term-equivalent age with reference values may result in an easy and reliable approach for the evaluation of brain size and growth in infants at high-risk for neurodevelopmental impairment. They could be easily performed by neonatologists as well as radiologists without specialized equipment or computational expertise.
REFERENCES
[1] Garel C. MRI of the fetal Brain, Normal Development an Cerebral Pathologies: Springer-Verlag Berlin Heidelberg; 2004.
[2] Nguyen The Tich S, Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol. 2009;30:125-31.
Differences between early versus late magnetic resonance imaging in infants with neonatal encephalopathy following therapeutic hypothermia
Garvey A1,2, El-Shibiny H1, Yang E3, Inder T1,4, El-Dib M1
1Department of Pediatric Medicine, Brigham And Women’s Hospital, Harvard Medical School, Boston, USA, 2INFANT Research Centre, Cork, Ireland, 3Department of Radiology, Boston Children’s Hospital, Harvard Medical School, Boston, USA, 4Childrens Hospital of Orange County, University of California Irvine, Irvine, USA
INTRODUCTION: MRI is the gold standard diagnostic test to define the nature of brain injury in infants with Neonatal Encephalopathy (NE), (1) with conventional MRI being of predictive value for subsequent neurodevelopmental outcome.(2, 3) In the first days of life, the nature of injury is informed by diffusion weighted MRI.(4) However, as both clinical features and imaging findings evolve considerably over the first week of life, early imaging may not reflect the true extent of brain injury.(5) The aim of this study is to assess the value of combining early MRI with late MRI following therapeutic hypothermia for NE.
METHODS: This retrospective cohort study included term born infants who received therapeutic hypothermia for NE at the Brigham and Women’s Hospital between 2016-2020. We identified infants who had two MRIs: one within the first week of life (early) and one beyond the first week of life (late). All MRIs were clinically reported by pediatric neuroradiologists and reviewed by study investigators to classify injury. Reports were categorized into normal or abnormal which included diffusion restriction, evidence of parenchymal or germinal matrix hemorrhage, extra-axial hemorrhage, or other signal abnormalities.
RESULTS: Ninety-four infants with NE were included (40 mild, 49 moderate, 5 severe). Median gestation age was 39.3 weeks (IQR 37.5 – 40.2) and median birth weight was 3.2kg (IQR 2.6 – 3.6). First MRI scan was performed at a median age of 4 days (IQR 4-4) with repeat scan at a median age of 15 days (IQR 11-24). A summary of MRI findings is outlined in Table 1. Twenty-four infants (26%) had a normal early scan of which 3/24 (13%) had injury noted on repeat MRI.(Figure 1) Seventy infants (74%) had abnormal findings noted on the early MRI, of which 5/70 (7%) had further evolution of injury (Figure 2) and 11/70 (16%) had complete resolution of findings.(Figure 3)
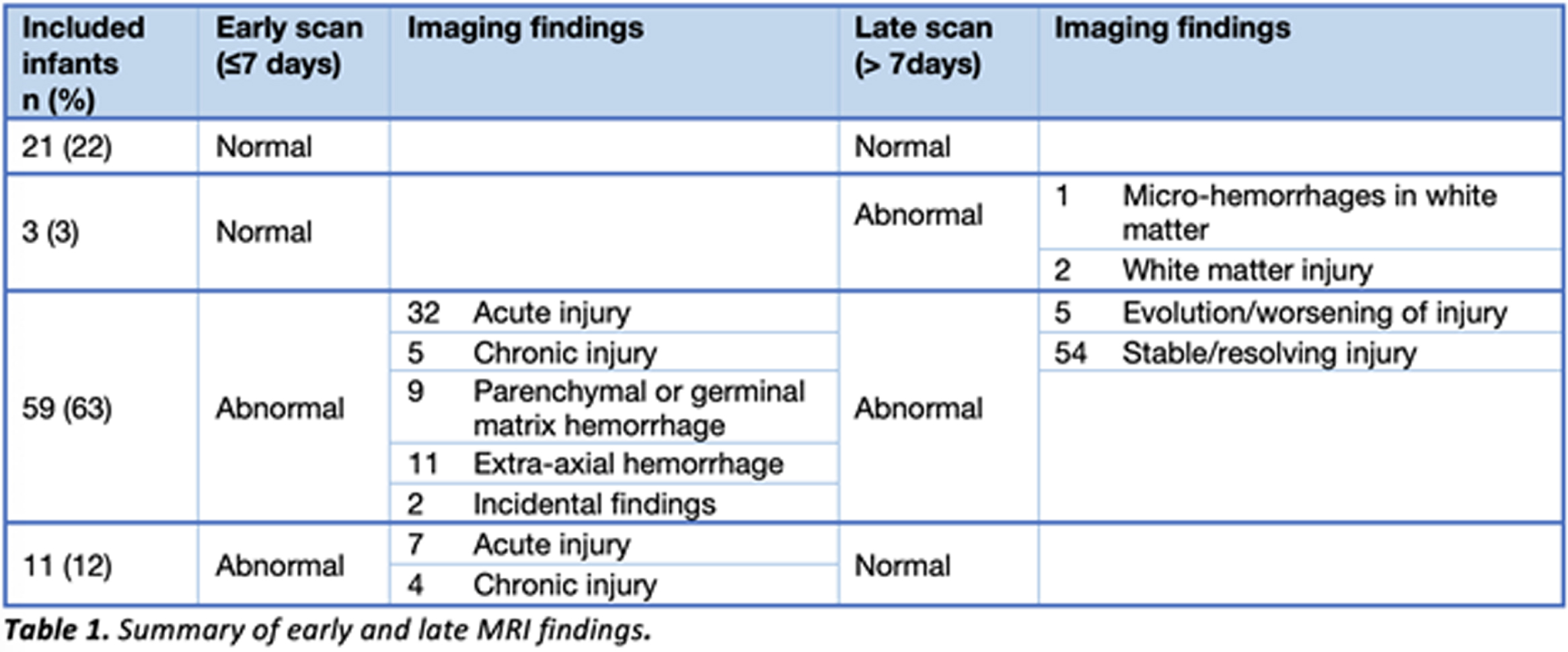
Figure 1.
Example of MRI images of normal early scan and abnormal late scan. Images A-H on Day 4. Images I-P on Day 11. In the late scan, punctate foci of diffusion restriction in the left caudate head and left putamen are seen on DWI (I and J) and on ADC (K and L). Punctate foci seen in left external capsule on axial T1 (M and N) and T2 (O and P) weighted images. These findings are not evident on the early scan (A – H).
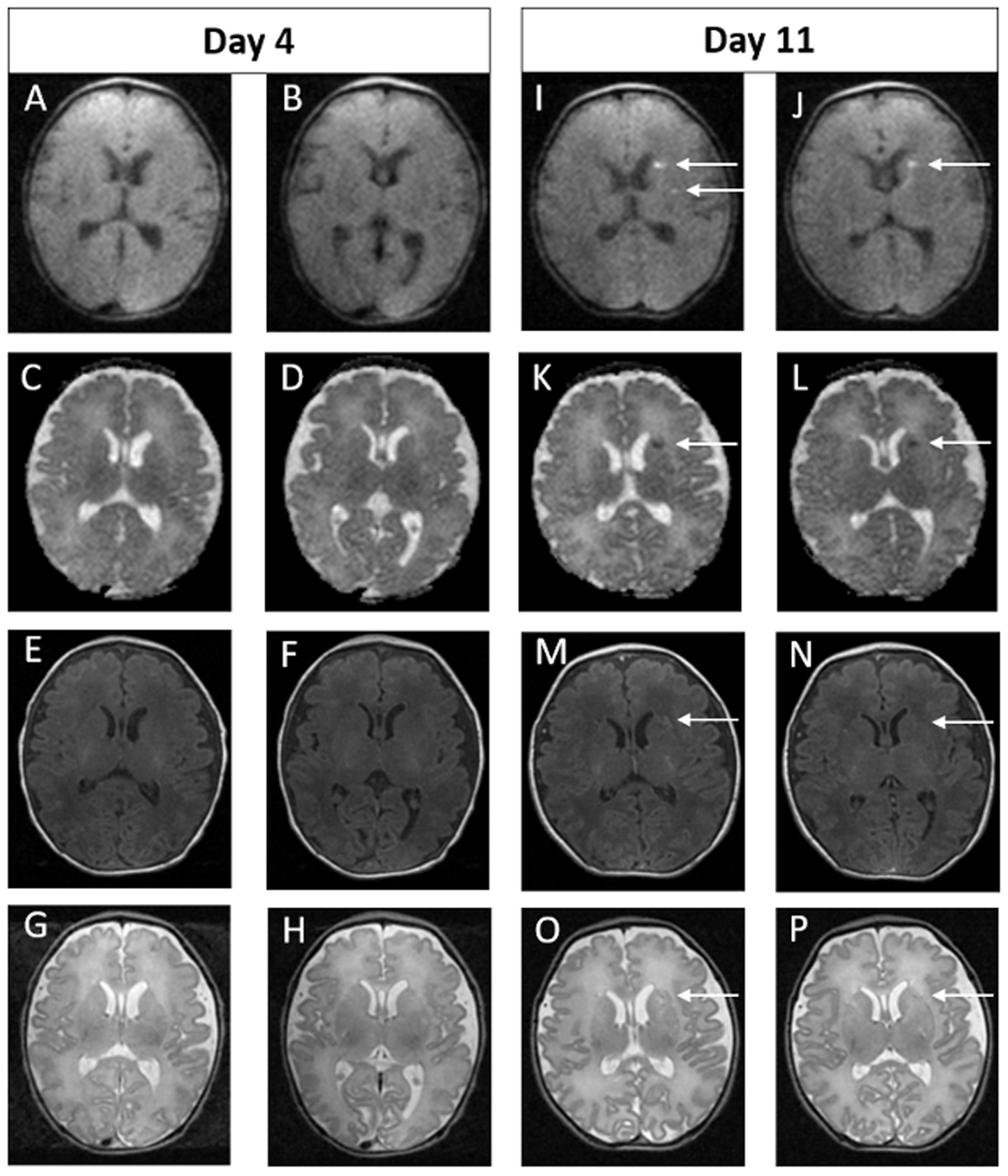
Figure 2.
Example of MRI images of abnormal early scan and evolved abnormalities on the late scan. Images A-H on Day 5. Images I-P on Day 11. In the early scan, punctate foci of signal abnormality are seen bilaterally in the frontal periventricular white matter on the axial T2 weighted image (A and B) and on DWI (C and D). These finds are again seen on the late scan (I-L). In addition, new focal punctate white matter lesions are seen on DWI in the deep bilateral frontal white matter of the centra semiovale (M and N) and in the right and left frontal periventricular white matter on sagittal T1 weighted images (O and P). These findings were not evident on the early scan (E-H).
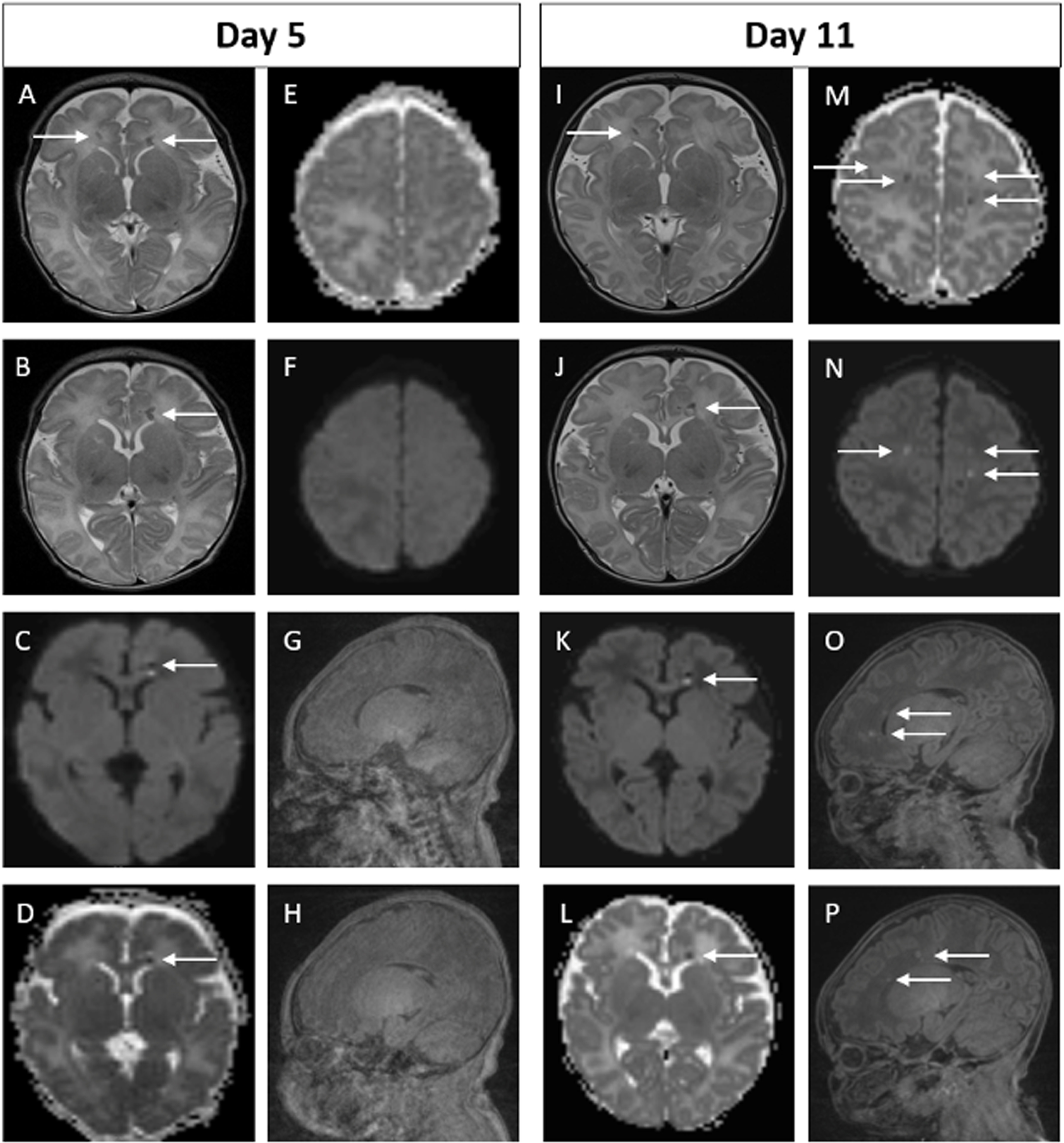
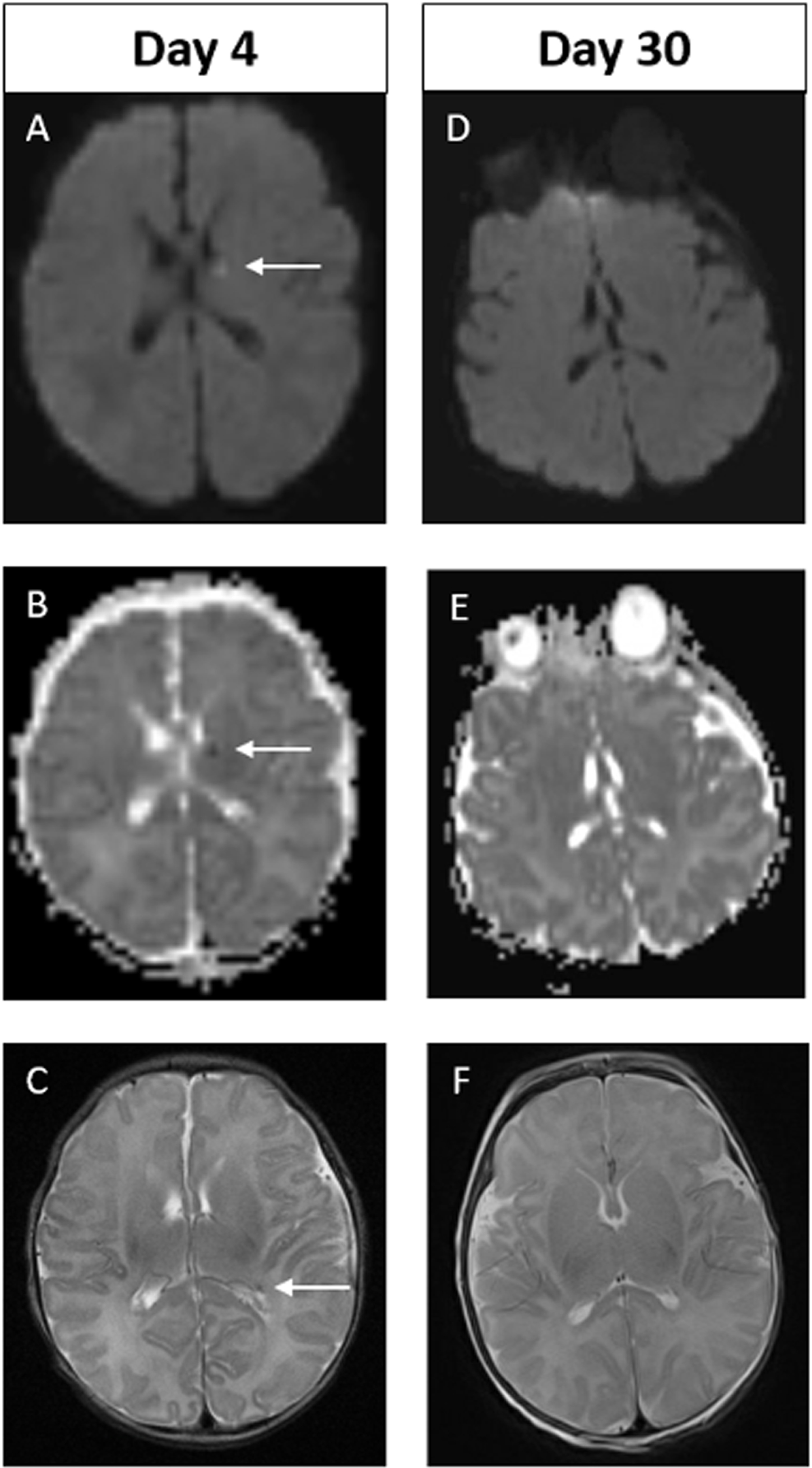
Figure 3.
Example of MRI images of abnormal early scan and normal late scan. Images A-C on Day 4. Images D-F on Day 30. In the early scan, punctate focus of diffusion restriction in the anterior, superior aspect of left thalamus is seen on DWI (A) and on ADC (B). Punctate focus of low signal intensity at left lateral ventricular atrium seen on axial T2 weighted image (C). These findings are not evident on the late scan (D-F). Of note, there are technical differences in alignment between the two scans.
CONCLUSION: In infants who received therapeutic hypothermia for NE, 20% had significant changes noted between their early and late MRIs. Relying solely on early MRI may overestimate injury in a proportion of infants and miss injury in another proportion. Caregivers should be aware of this limitation as it may impact prognostication. Combining early and late MRI following hypothermia allows for better characterization of brain injury.
REFERENCES
[1] Wisnowski JL, Wintermark P, Bonifacio SL, Smyser CD, Barkovich AJ, Edwards AD, et al. Neuroimaging in the term newborn with neonatal encephalopathy. Seminars in fetal & neonatal medicine. 2021;26(5):101304.
[2] Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. The Lancet Neurology. 2010;9(1):39-45.
[3] Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR American journal of neuroradiology. 1998;19(1):143-9.
[4] Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78(18):1420-7.
[5] Gano D, Chau V, Poskitt KJ, Hill A, Roland E, Brant R, et al. Evolution of pattern of injury and quantitative MRI on days 1 and 3 in term newborns with hypoxic-ischemic encephalopathy. Pediatric research. 2013;74(1):82-7.
Acute diffusion-weighted imaging signaling severe periventricular leukomalacia in preterm infants: Case report and review of literature
Garvey A1,2, El-Dib M1, Grant P3, Manning S1, Volpe J1,4, Inder T1,5
1Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA, 2INFANT Research Centre, Cork, Ireland, 3Departments of Radiology and Medicine, Boston Children’s Hospital, Harvard Medical School, Boston, USA, 4Department of Neurology, Boston Children’s Hospital, Harvard Medical School, Boston, USA, 5Childrens Hospital of Orange County, University of California Irvine, Irvine, USA
INTRODUCTION: Periventricular leukomalacia (PVL) occurs in up to 25% of very preterm infants resulting in adverse neurodevelopmental outcomes.(1) In its acute phase, PVL is clinically silent.(2) Although cranial ultrasound (CUS) is widely available, its sensitivity in the early detection of PVL is low.(3) Few studies have assessed the ability of diffusion weighted imaging (DWI) on magnetic resonance imaging (MRI) for the early identification of PVL. We identified a preterm infant with early DWI changes which later evolved to PVL and reviewed all available literature.
CASE REPORT: Male infant born at 30 weeks’ gestation to a G2P1 mother with a history of premature rupture of membranes for 27 hours (+antibiotic coverage) prior to spontaneous vaginal delivery. Infant required CPAP at birth. Apgar scores were 7 at 1min and 9 at 5 mins. Infant received antibiotics for 48 hours pending a negative sepsis evaluation. Physical examination revealed a depressible region on the left parietal skull concerning for a skull fracture. CUS could not determine whether depression was pathological or physiological with brain reported as normal. MRI performed on day of life 2 confirmed a normal skull but revealed multifocal areas of diffusion abnormality in the periventricular white matter and thalami bilaterally. (Figure 1) On repeat MRI 10 days later, reduced diffusivity had resolved with abnormalities more evident on T1 and T2 weighted images.(Figure 2)
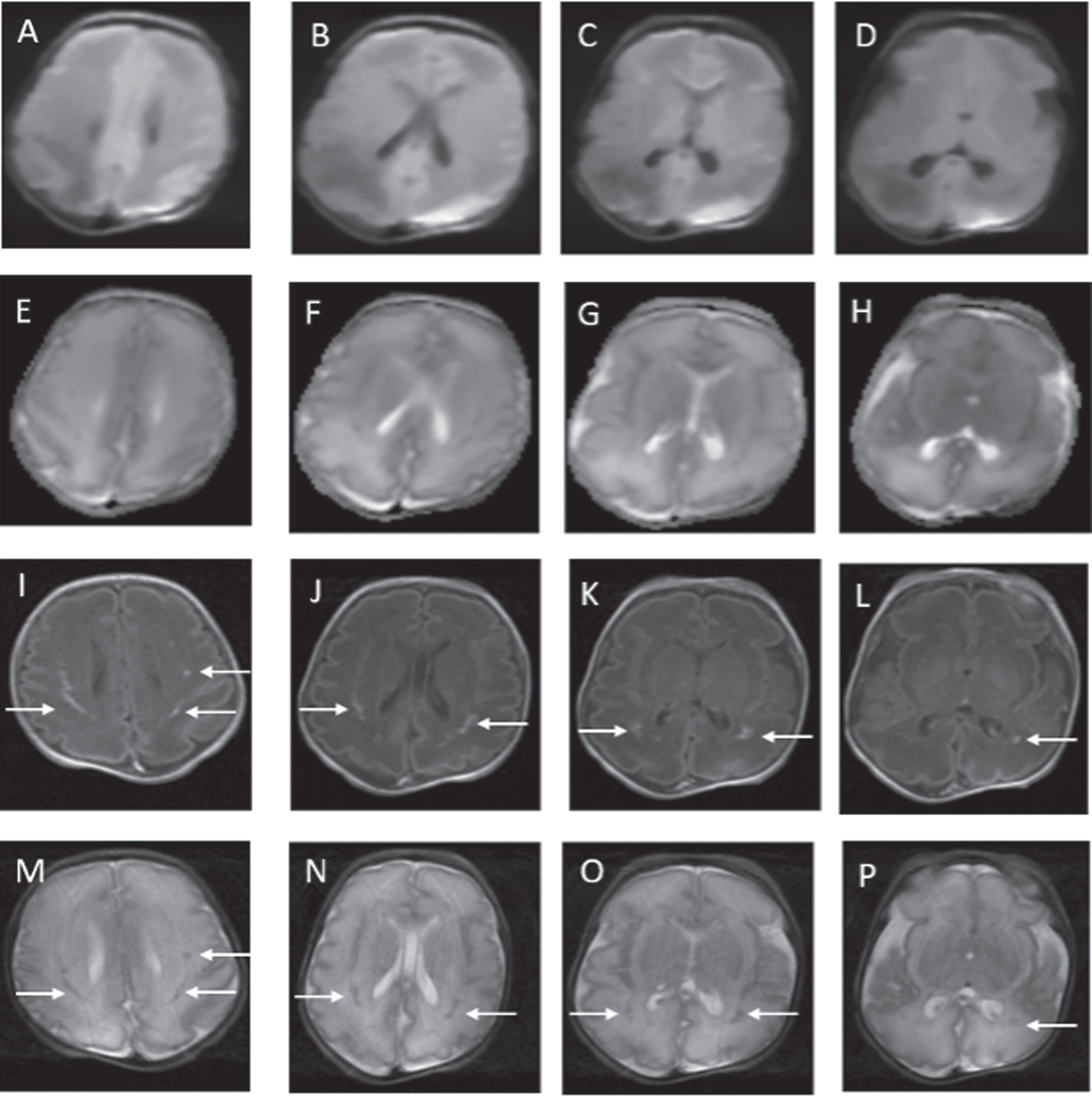
Figure 1.
MRI findings on postnatal day 2. DWI abnormalities are depicted from A to D. Apparent diffusion coefficient (ADC) abnormalities are depicted from E to H. White matter abnormalities are in the classic distribution for PVL. Minimal abnormalities seen on conventional axial T1 (I to L) and axial T2 (M to P) weighted images.
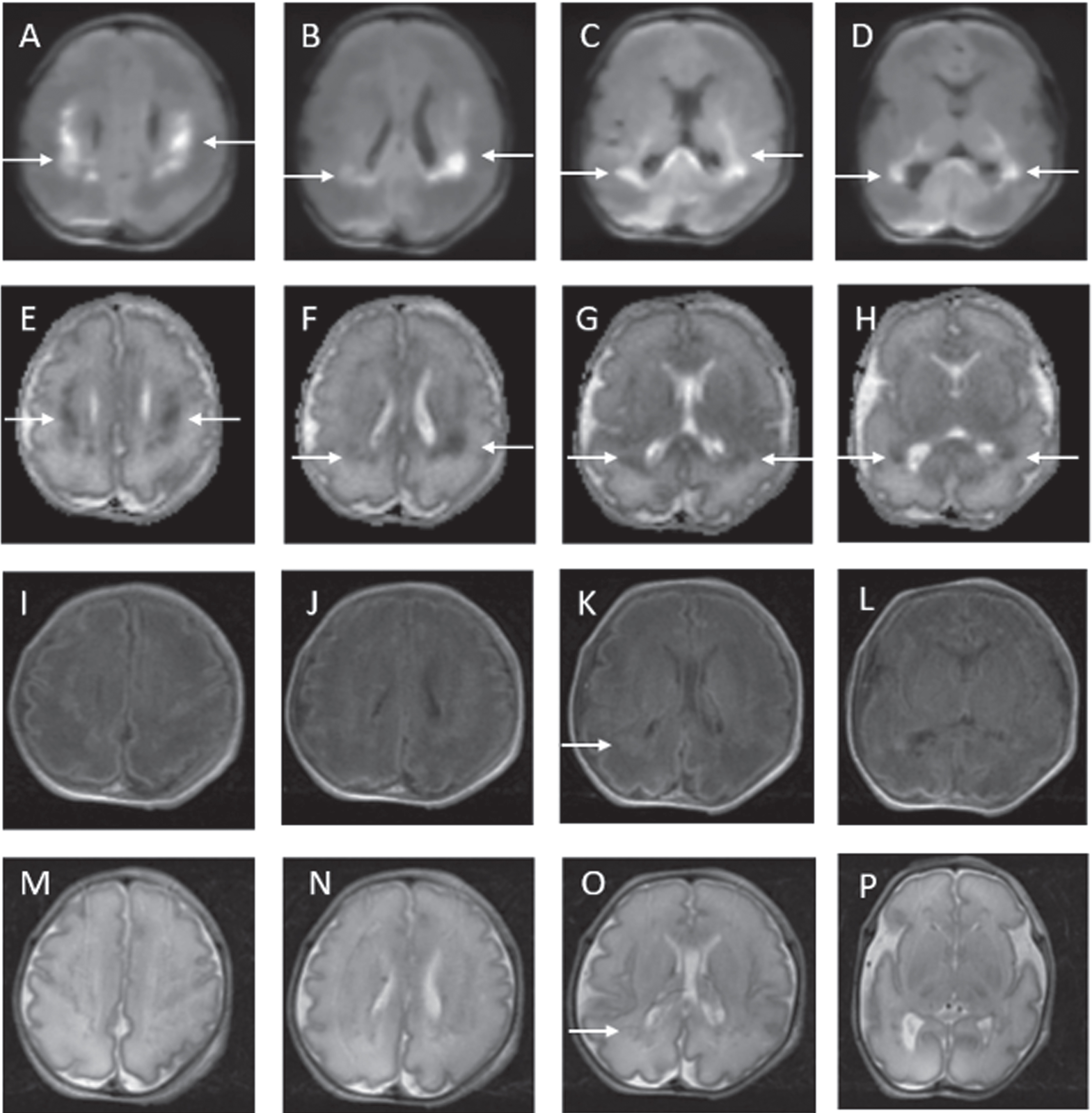
Figure 2.
MRI findings on postnatal day 12. Previously seen abnormalities on DWI and ADC have resolved (A to H). White matter injury now more evident on axial T1 (I to L) and axial T2 (M to P) images.
PUBLISHED LITERATURE: 31 cases of abnormal DWI reliably heralding severe PVL in the preterm infant have been published in the literature. (Table 1) Notable features in these reports and our case include a) infants were more mature preterm infants (29-36 weeks gestation); b) findings were often serendipitous with benign clinical courses; and c) DWI changes were the only abnormal imaging finding in the first week of life with subsequent defined PVL abnormalities seen on conventional MRI or CUS.
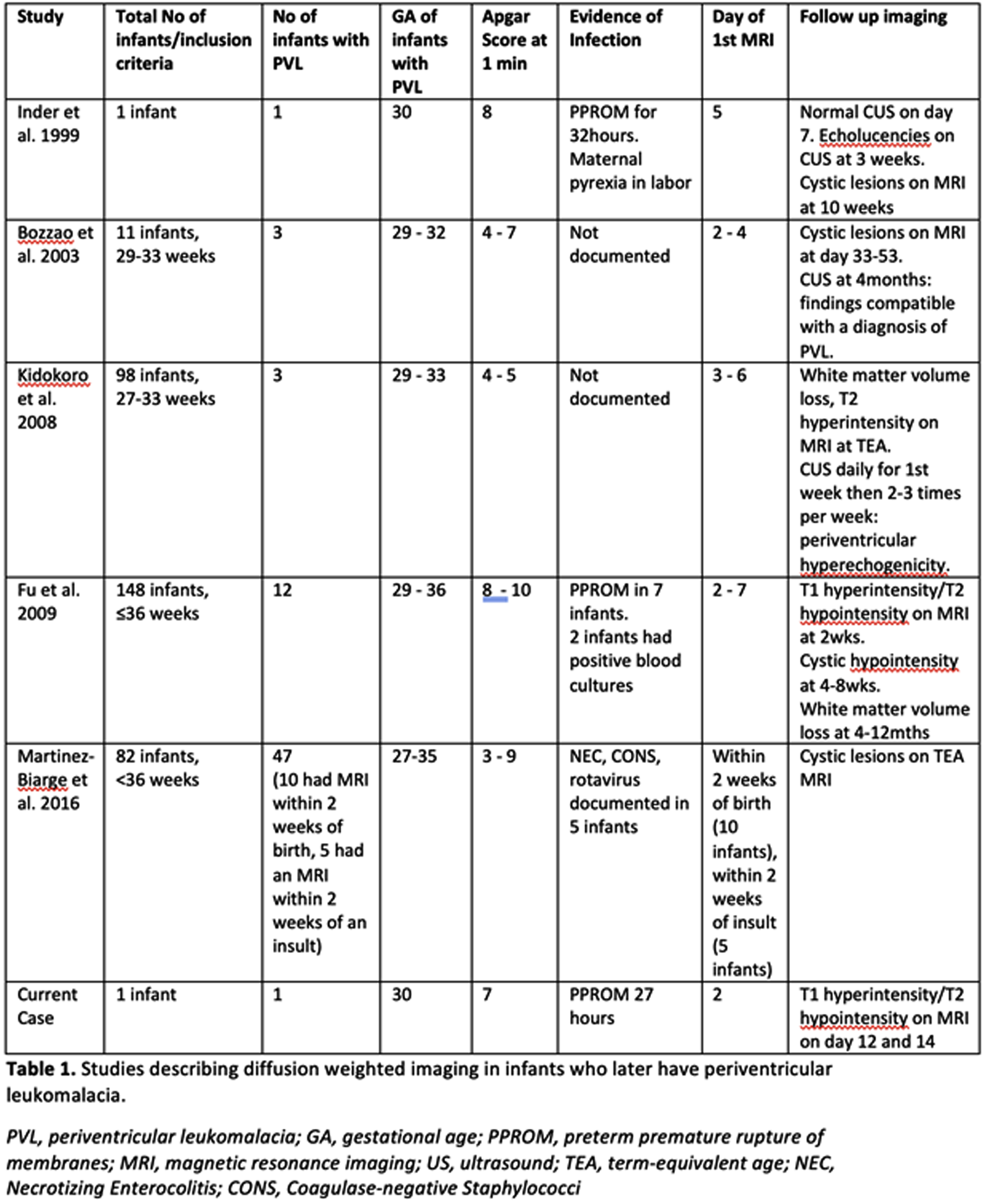
CONCLUSION: These cases reflect the detection of acute DWI injury preceding moderate-severe PVL occurring in more mature preterm infants, despite many very immature infants having been imaged in the first week of life. This may represent a maturational-dependent propensity for PVL and/or the sensitivity of diffusion to detect PVL. The affected infants had minimal clinical illness, highlighting persisting gaps in our understanding of the clinical markers for PVL. DWI in the first week of life may be a reliable early marker for PVL injury in preterm infants.
REFERENCES
[1] Volpe JJ, Inder TE, Darras BT, de Vries LS, du Plessis AJ, Neil J, et al. Volpe’s neurology of the newborn e-book: Elsevier Health Sciences; 2017.
[2] Perlman JM, Risser R, Broyles RS. Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics. 1996;97(6 Pt 1):822-7.
[3] Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR American journal of neuroradiology. 2003;24(5):805-9.
Incorporating the scores of term-equivalent age brain MRI of preterm infants in clinical practice
Garvey A1,2, Erdei C1, Inder T1,3, El-Dib M1
1Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA, 2INFANT Research Centre, Cork, Ireland, 3Childrens Hospital of Orange County, University of California Irvine, Irvine, USA
BACKGROUND: Despite advances in maternal and neonatal care, preterm infants remain at risk for adverse long-term neurodevelopmental outcomes.(1) MRI is more sensitive than cranial ultrasound at detecting white matter injury and is increasingly being used to define preterm brain injury.(2) Translating MRI findings into prognostic outcome, however, remains challenging.(3, 4)
This study aimed to improve the interpretation and clinical utility of term-equivalent age (TEA) MRIs of preterm infants by providing the clinical team with individualized MRI reports based on a previously published scoring system.(5)
METHODS: This study was conducted at the Brigham and Women’s Hospital, Boston, a 66-bed tertiary neonatal intensive care unit (NICU). In our center, all infants born <28 weeks or <32 weeks with additional risk factors undergo an MRI at TEA. While results are reported by specialized neuroradiologists, the medical providers are the ones responsible for the interpretation of these scans and subsequent communication with parents. We anonymously surveyed medical providers in the Growth and Development Unit, a neurorehabilitative 20-bed subunit where most infants undergoing TEA MRI are cared for prior to transition to home. Survey included questions about the knowledge and confidence in interpreting TEA MRIs, prediction of outcome and conveying results to parents. Over a period of 4 months, all TEA MRIs were scored using a previously published scoring system and individualized reports using evidence-based outcomes were sent to providers within 24-48 hours of the MRI. Following the intervention, providers were surveyed again to determine whether they found the reports helpful.
RESULTS: Nine medical providers were included in the initial survey, two-thirds of whom were only slightly or not confident in interpreting the MRI clinical report, how injury related to outcome and in conveying the results of the MRI to parents. During the 4-month period, 14 reports were formulated and sent to caregivers. An example of one of these reports is demonstrated in Figure 1. Following the intervention, all providers reported that the intervention was helpful and allowed for “better, more cohesive plans with families” and improved ability to interpret findings of brain growth more quantitatively and integrate this information in clinical care.
Figure 1.
Example of report sent to providers
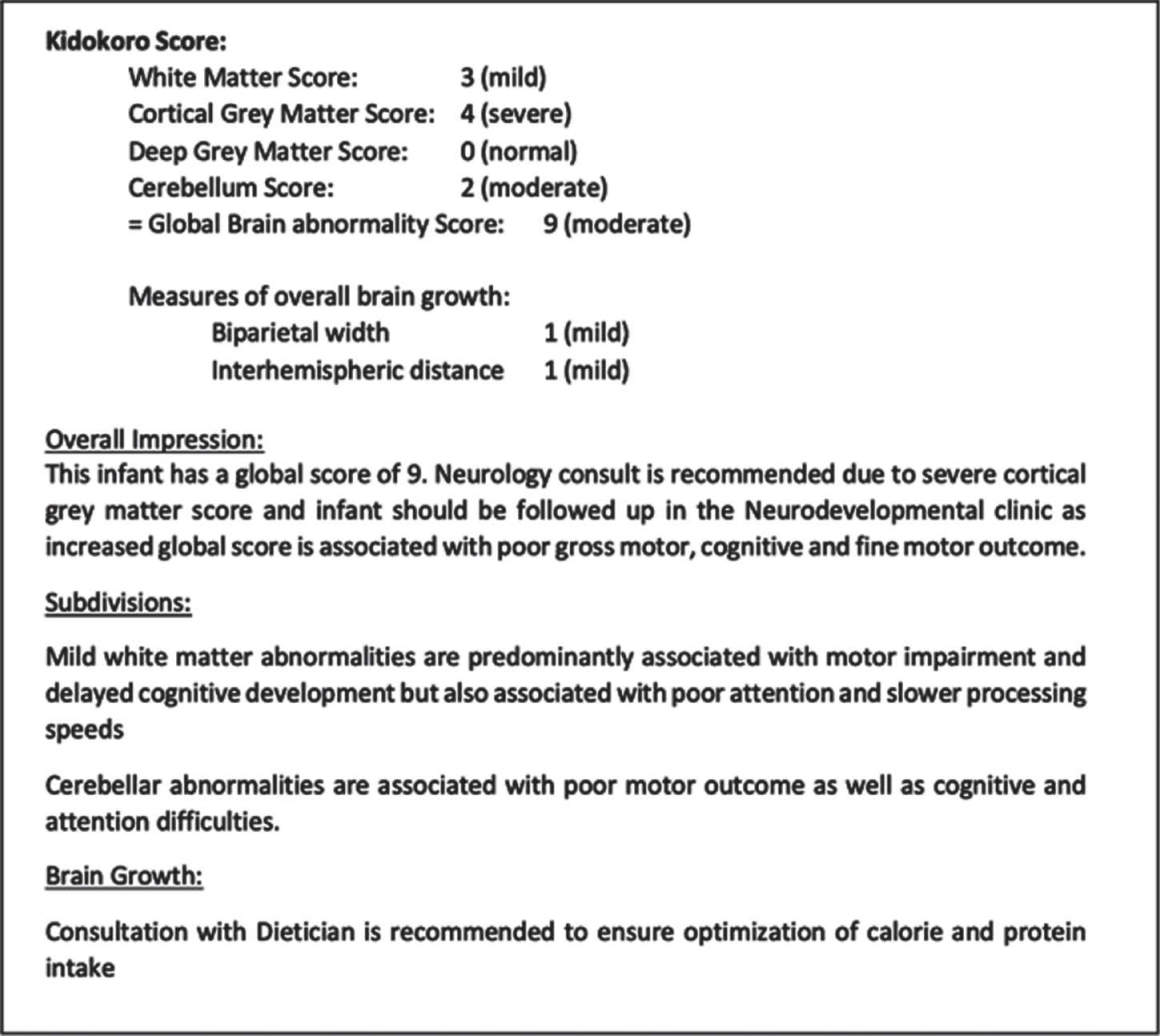
Figure 2.
Scoring sheet used to score MRIs.
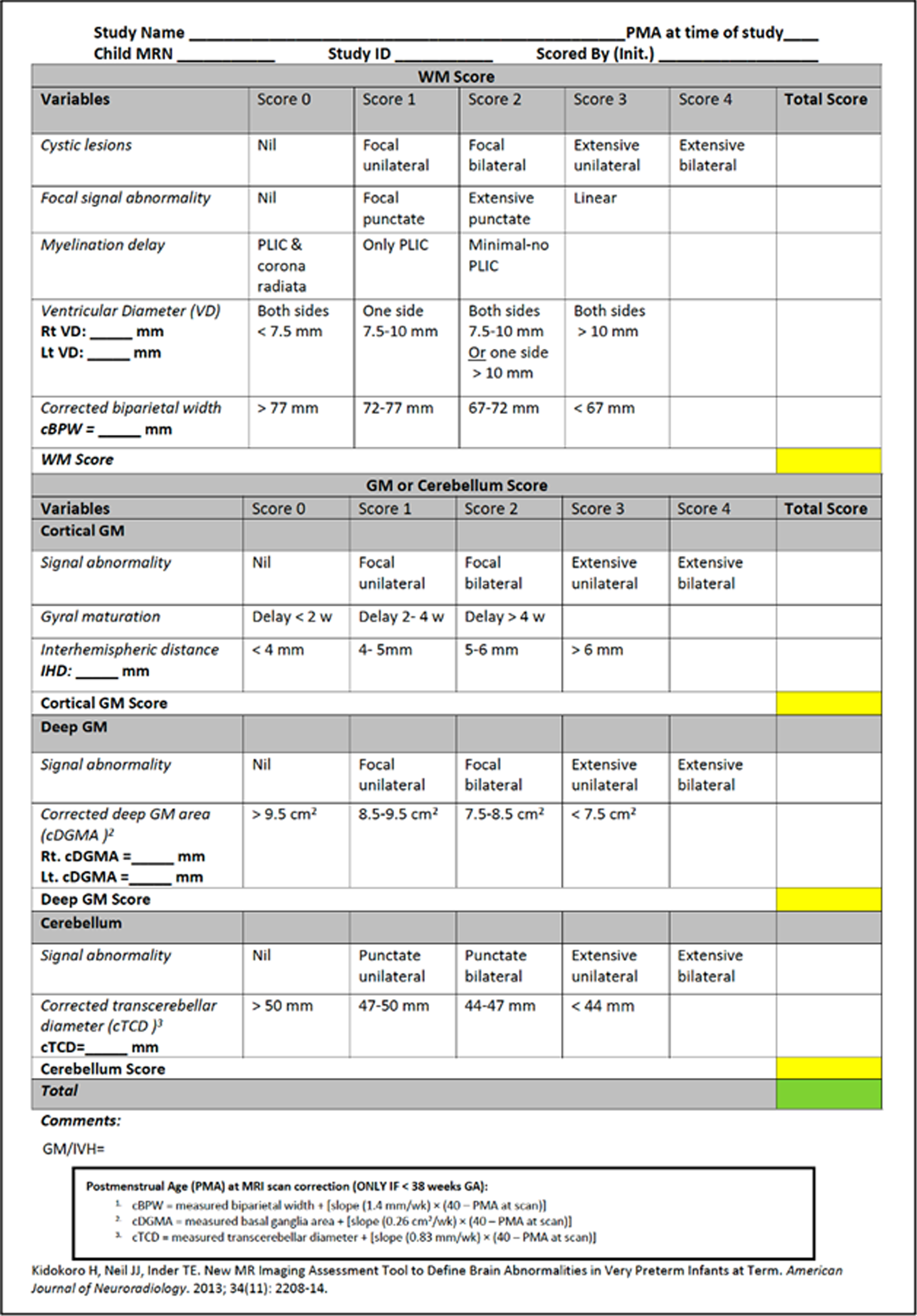
Figure 3.
Grading system used to grade the MRI scores.

CONCLUSION: To date, MRI scoring systems have predominantly been used in research settings. Its use in this clinical setting was feasible and provided an objective, quantifiable assessment of brain growth and injury in preterm infants allowing for individualized reports and subsequently targeted interventions and follow-up.
REFERENCES
[1] Cheong J. L., Spittle A. J., Burnett A. C., Anderson P. J., Doyle L. W. Have outcomes following extremely preterm birth improved over time? Seminars in Fetal and Neonatal Medicine. 2020; 25(3), 101114. https://doi.org/10.1016/j.siny.2020.101114
[2] Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR American journal of neuroradiology. 2003;24(5):805-9.
[3] Natarajan N, Pardo AC. Challenges in neurologic prognostication after neonatal brain injury. Semin Perinatol. 2017 Mar;41(2):117-123. doi: 10.1053/j.semperi.2016.11.008. Epub 2017 Jan 28. PMID: 28139254.
[4] Hinojosa-Rodríguez M, Harmony T, Carrillo-Prado C, Van Horn JD, Irimia A, Torgerson C, Jacokes Z. Clinical neuroimaging in the preterm infant: Diagnosis and prognosis. Neuroimage Clin. 2017 Aug 14;16:355-368. doi: 10.1016/j.nicl.2017.08.015. PMID: 28861337; PMCID: PMC5568883.
[5] Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013 Nov-Dec;34(11):2208-14. doi: 10.3174/ajnr.A3521. Epub 2013 Apr 25. PMID: 23620070; PMCID: PMC4163698.
Value of early and serial brain MRI in preterm infants to assess brain growth and injury and tailor neuropromotive intervention in the NICU
Roychaudhuri S1, Pineda R3, Sharon D1, Singh E1, Steele T1, Sheldon Y1, Cuddyer D1, Grant E2,4, Yang E2,4, El-Dib M1,2, Inder T1,2,5, Erdei C1,2
1Department of Pediatric Newborn Medicine, Brigham And Women’s Hospital, Boston, United States, 2Harvard Medical School, Boston, United States, 3Chan Division of Occupational Science and Occupational Therapy, University of Southern California, Los Angeles, United States, 4Division of Neuroradiology, Boston Children’s Hospital, Boston, United States, 5Division of Neonatology, Children’s Hospital of Orange County and University of California, Irvine, United States
BACKGROUND AND PURPOSE: Very preterm born infants continue to experience high rates of neurodevelopmental delays, which correlate with the severity of neurological injury in early neonatal period. (1) Cranial ultrasound (CUS) is the most common modality used to diagnose and classify degree of neurological injury of very preterm infants in the neonatal intensive care unit (NICU). Early intensive neuropromotive intervention for very preterm (VP) infants before term age is associated with improved infant neurological outcomes and greater parental satisfaction. (2)(3) The aim of this study is to use early brain magnetic resonance imaging (MRI) to better understand longitudinal brain growth, delineate neurological injury, and tailor neuropromotive intervention for VP infants prior to term age.
METHODOLOGY: All infants born less than 33 weeks gestational age (GA) in a level-III NICU at an academic institution were approached for consent excluding those with congenital infections or significant anomalies or genetic syndromes. Along with routine HUS, early MRI scans were performed, and the group was categorized into high risk and low-risk groups based on the level of neurological injury present on early imaging. Any degree of intraventricular hemorrhage (IVH) with ventricular dilatation, moderate to severe white matter injury (WMI) and significant cerebellar hemorrhage were classified as high-risk. Serial MRI scans were obtained with each baby having at least 2 and a maximum of 6 scans. They were also compared to separate controls who underwent a single term equivalent (TE) MRI.
RESULTS: Currently, 30 babies (of 75 projected) have been enrolled in this study. A total of 85 MRI scans have been performed of which 30 were early scans at 30-34 weeks GA. Interval scans were during the NICU stay with at least one TE scan at 38-42 weeks GA. A total of 10 babies met criteria for high-risk injury as per early MRI findings as opposed to 7 based on CUS. Early MRI scans were particularly useful in detecting WMI, as opposed to CUS (moderate-severe WMI 6 vs 3 cases; any WMI 12 vs 4 cases). Three significant cerebellar hemorrhages were detected on MRI as opposed to 2 on CUS. Of note, the severity of WMI became less conspicuous by TE MRI as compared to early images. TE MRI was particularly helpful in assessment of cortical maturation and quantitative volumetric assessment especially when comparing with the earlier MRI images obtained. Data collection is ongoing and will be critical to inform more definitive results.
CONCLUSION/IMPLICATIONS FOR PRACTICE: Early MRI of the brain has rarely been used to direct management in rehabilitative care. Our study shows that it could be a sensitive tool for injury detection and classification, which could help tailor intensive rehabilitative intervention for preterm infants prior to term age to optimize neurodevelopmental outcomes.
Image 1.
Normal appearing head ultrasound of a 30 week GA newborn at 7 days of life
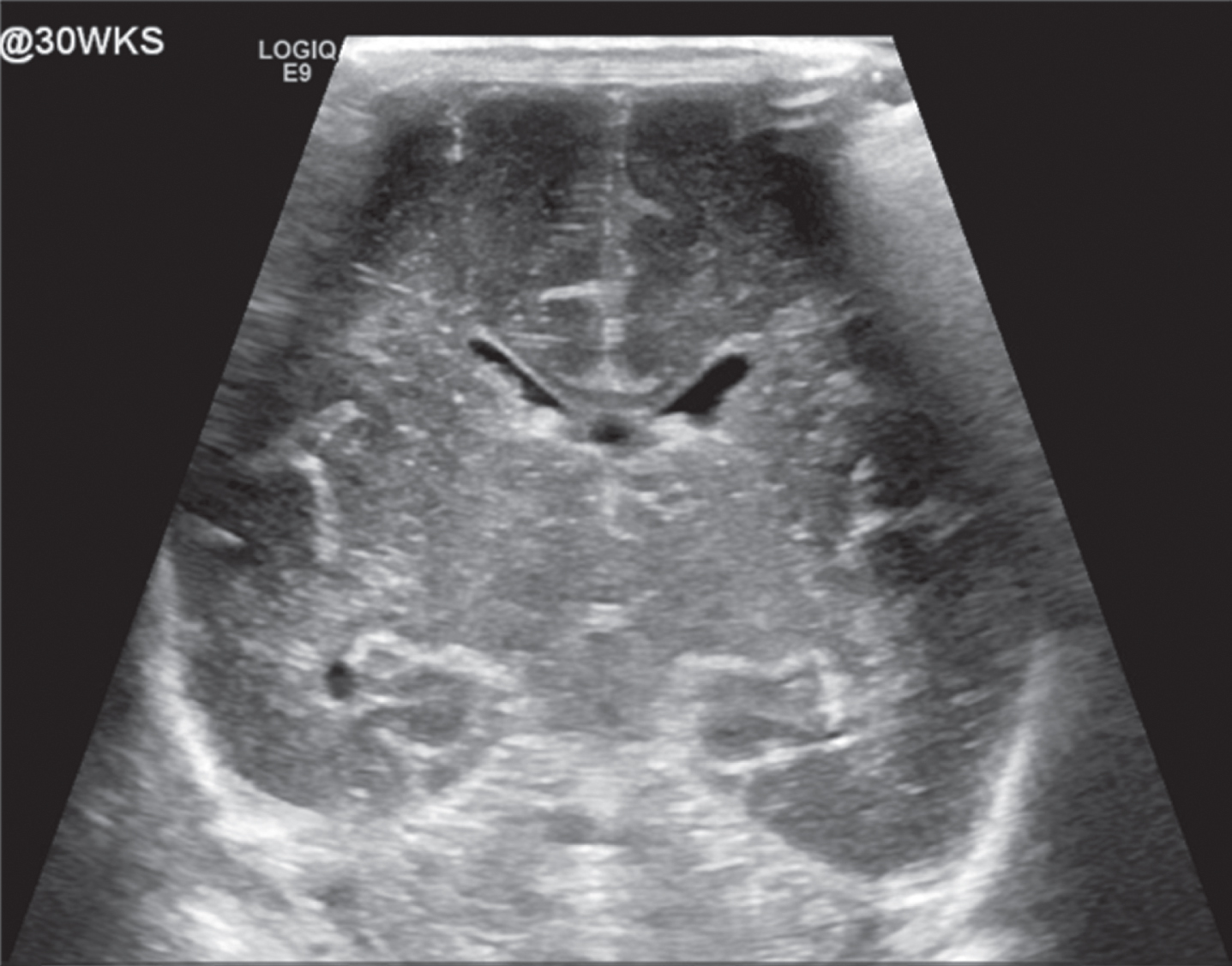
Image 2.
Diffusion weighted image of same newborn at day 3 of life showing areas of acute white matter injury with restricted diffusion
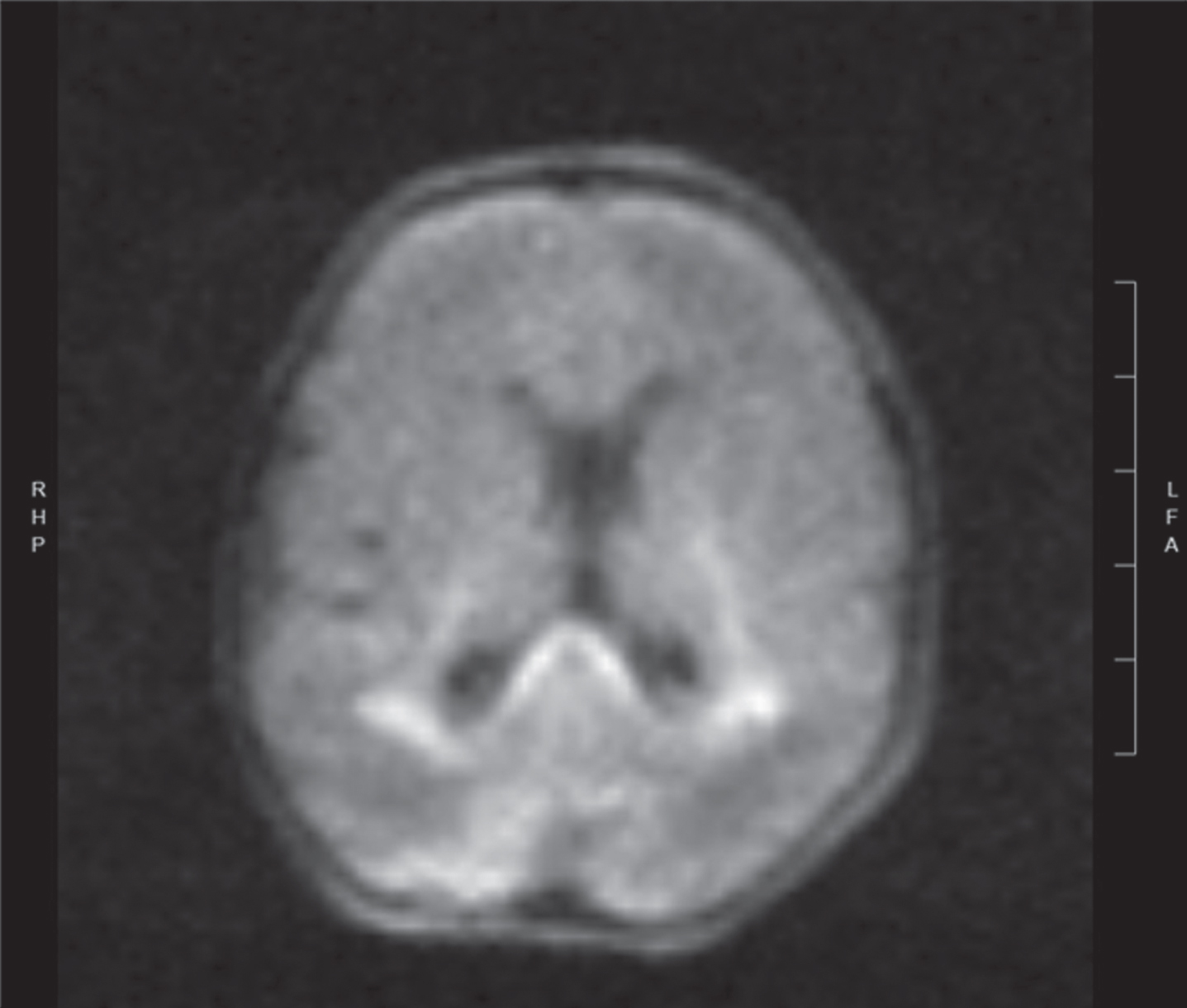
Image 3.
Normal appearing head ultrasound of a 31 week GA newborn at 4 days of life
Image 4.
Axial T1 image of same baby at day 5 showing area of gliosis in left parietal lobe
REFERENCES
[1] Pineda RG, Neil J, Dierker D., Smyser CD, Wallendorf M, Kidokoro H, Reynolds LC, Walker S, Rogers C, Mathur AM, Van Essen DC, Inder T. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. The Journal of pediatrics. 2014; 164(1), 52–60.e2.
[2] Pineda R, Wallendorf M, Smith J. A pilot study demonstrating the impact of the supporting and enhancing NICU sensory experiences (SENSE) program on the mother and infant. Early Human Development. 2020; 144: 1-8.
[3] Pineda, Smith J., Roussin J., Wallendor M., Kellner P., Colditz, G. Randomized clinical trial investigating the effect of consistent, developmentally-appropriate, and evidence-based multisensory exposures in the NICU. Journal of Perinatology. 2021; 41(10), 2449–2462. https://doi.org/10.1038/s41372-021-01078-7)
Comparative evaluation of MRI scoring systems predicting neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy
Szakmar E1, Andorka C1, Barta H1, Sesztak T1, Varga E2, Szabo M1, Jermendy A1
1Division Of Neonatology, 1st Department Of Pediatrics, Semmelweis University, Budapest, Hungary, 2Department of Neuroradiology, Medical Imaging Centre, Semmelweis University, Budapest, Hungary
BACKGROUND: Several MRI scoring systems were developed to quantify brain injury in infants with hypoxic- ischemic encephalopathy (HIE). The Barkovich scoring system uses conventional MRI sequences to assess patterns of injury involving basal ganglia, thalamus and watershed areas. (1) Weeke et al. developed a scoring system assessing brain injury of grey matter, white matter, cortex and cerebellum using diffusion weighted images (DWI) and proton magnetic resonance spectroscopy (H-MRS). (2) Our objective was to compare the predictive value of 2 MRI scoring systems for composite adverse outcome in infants receiving hypothermia for HIE.
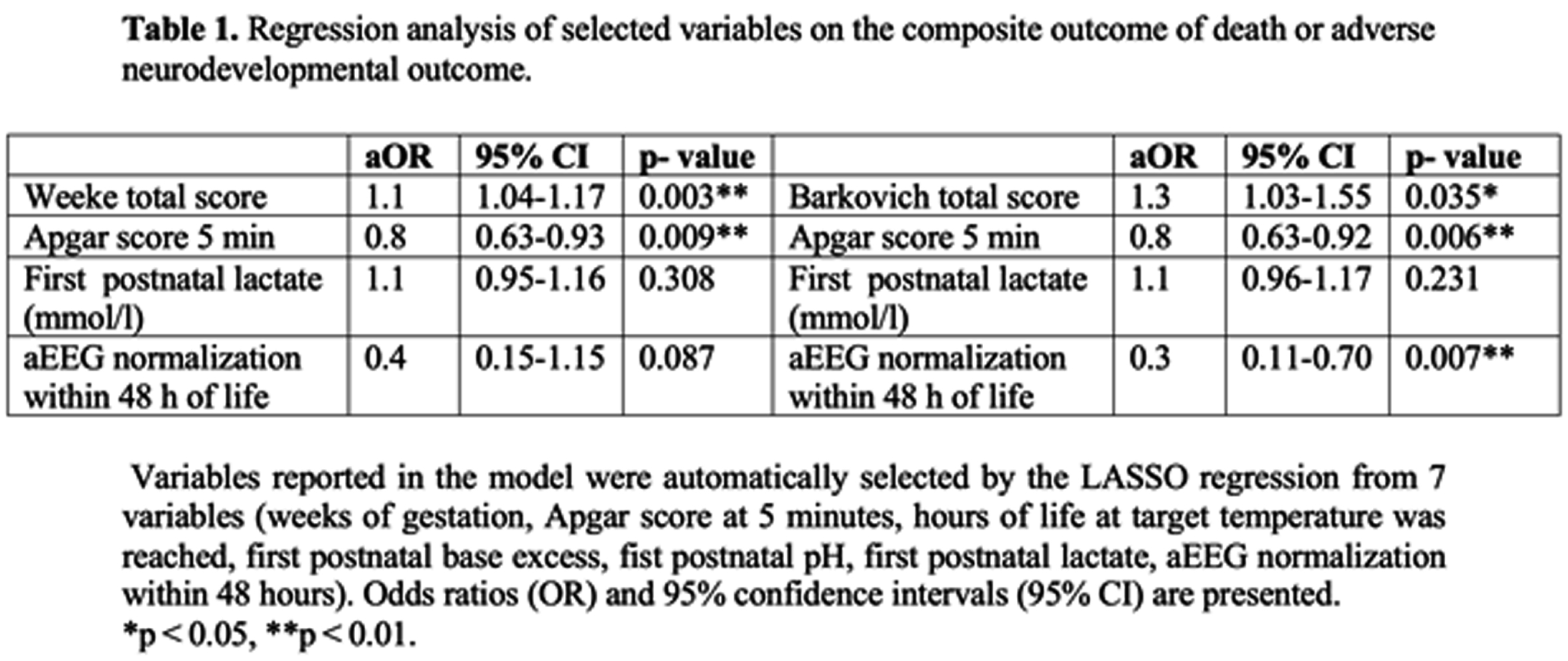
METHODS: This single center retrospective cohort study included infants who received hypothermia for HIE between 2013 and 2019. Post-rewarming brain MRI were evaluated according to Barkovich and Weeke scoring systems. Follow-up was performed using Bayley Scales of Infant Development II at 18 to 22 months of age. Composite adverse outcome was defined as death or severe disability if Bayley II score was <70 in any domain. Receiver Operating Characteristics (ROC) curve was used to calculate area under the curve (AUC), and optimal cut off point for adverse outcome. DeLong- test was carried out to compare AUCs. LASSO regression was performed to predict adverse outcome by selecting the subset of the variables that minimize prediction error. AUCs were also adjusted for selected variables.
RESULTS: A total of 163 infants with post rewarming MRI and follow up data were included in the analysis. The rate of composite adverse outcome was 33%. MRI studies were carried out at a median 5 days of life [IQR 4; 6]. Brain injury was detected with higher frequency using Weeke score compared to Barkovich (71% vs. 35%, p<0.001). The area under the ROC curve was 0.81 [95%CI 0.74; 0.88] with the optimal cutoff point of 12 (Sensitivity 57%, Specificity 93%) for adverse outcome using Weeke score. The AUC was 0.73 [95%CI 0.66; 0.81] with the optimal cutoff point of 2 (Sensitivity 57%, Specificity 88%) based on Barkovich score. DeLong- test showed a significant difference between the two AUCs (p= 0.0012). Higher Weeke and Barkovich scores were associated with increased odds ratio for adverse outcome after adjusting the model for 5 minutes Apgar score, first postnatal lactate and aEEG normalization within 48 hours of life (Table 1.) Covariate- adjusted AUC improved for Weeke (AUC 0.85, 95%CI 0.78; 0.11) and Barkovich scores (AUC 0.83, 95%CI 0.76; 0.90).
CONCLUSIONS: More detailed scoring system incorporating DWI and H-MRS, developed by Weeke et al. has a better predictive value for adverse outcome in infants with HIE compared to the Barkovich score. MRI can serve as a bridging biomarker and a surrogate end point for neurodevelopmental outcome, so it is crucial to quantify brain injuries based on standardized scoring systems.
REFERENCES
[1] Barkovich AJ, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998; 19:143-149.
[2] Weeke LC, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr. 2018; 192:33-40 e32.
Preterm infants brain injury: The predictive value of the CUS at TEA
Panzarini I1, Ventola MA1, Pietrobelli A1
1AOUI Verona, Verona, Italy
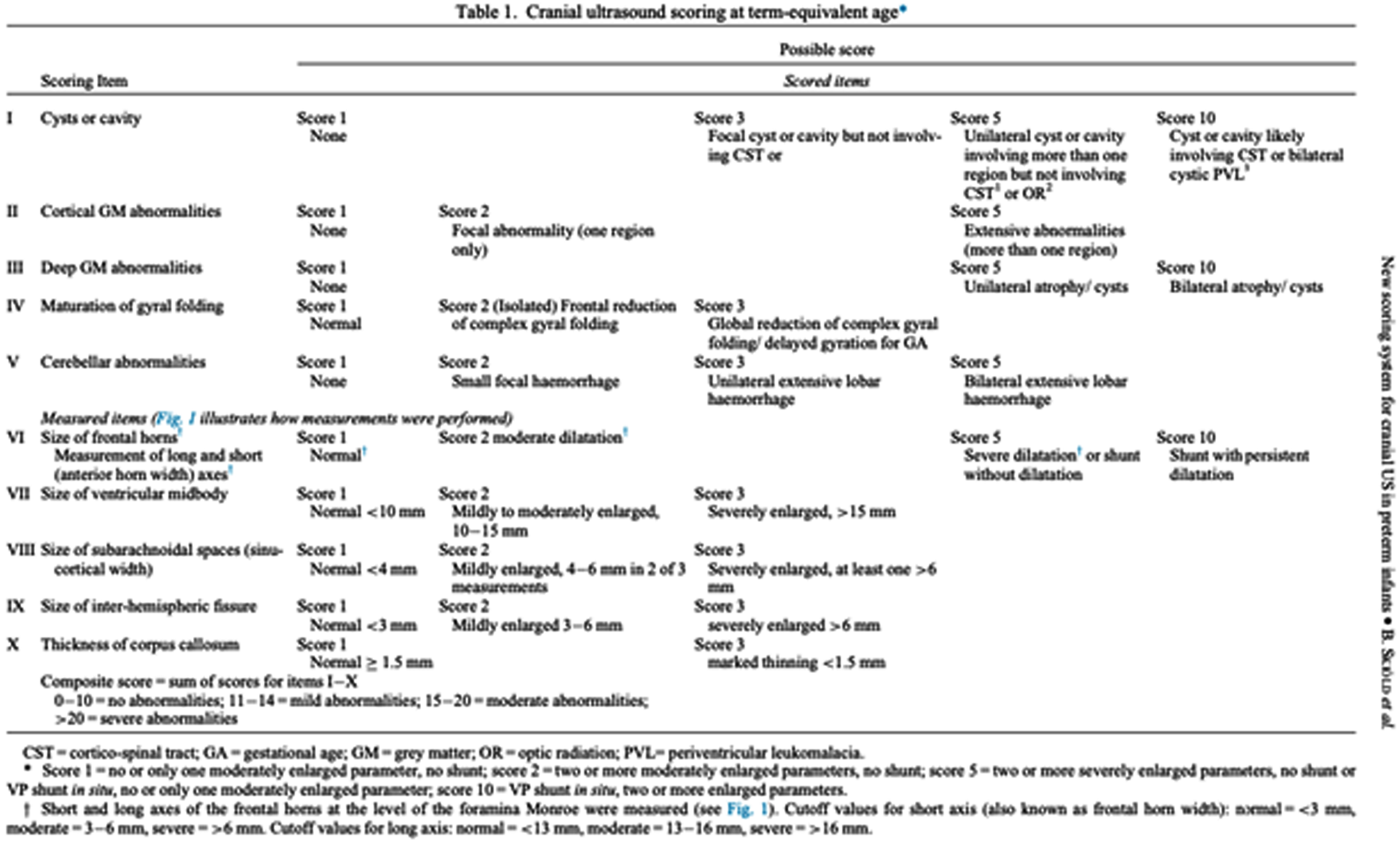
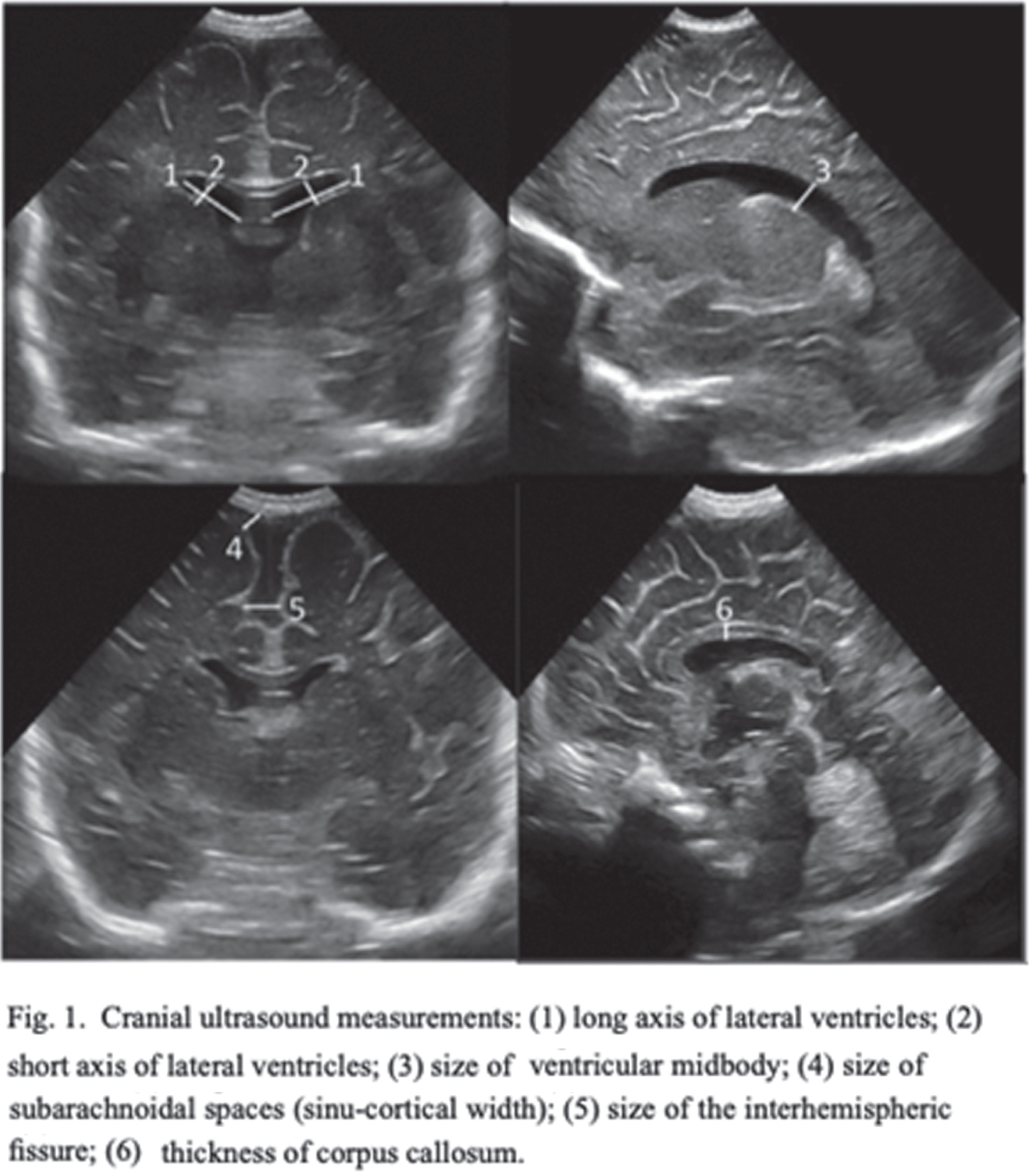
INTRODUCTION: Despite the improvement in neonatal intensive care, the neurological pathology of the preterm infant remains a frequent complication and is associated with an increased risk of a severe outcome, higher the earlier the birth is. Cranial ultrasound (CUS) is a valuable diagnostic tool for early detection and serial monitoring of brain injuries. The prognostic value of CUS performed at Term- Equivalent Age (TEA) in prediction of outcome is increasingly being demonstrated, especially regarding cystic periventricular leukomalacia. A detailed qualitative and quantitative classification could allow a reduction in subjectivity and an improvement in sensitivity and specificity of CUS in the recognition of the typical alterations of preterms, increasing the positive and negative predictive values. The aim of this study is to evaluate the prognostic value of the scoring system proposed by Skiöld et al. in 20191 in predicting motor outcome at 3 months of corrected age.
METHODS: Infants with gestational age < 32 weeks, born between March 2021 and January 2022, were included and underwent serial ultrasound scans from birth to 3 months of corrected age, with an evaluation of the scoring system at TEA. The neurological follow-up, performed at TEA and at 3 months of corrected age, was based on the assessment of the infant’s Hammersmith scale (HINE), general movements (GMA) and electroencephalogram.
RESULTS: Spearman’s correlation found a good agreement between the ultrasound score of twenty-three preterms and the HINE (ρ = -0,63, p = 0,002) and between the score and fidgety movements (FMs, ρ = -0,63, p = 0,009), with scores inversely proportional to gestational age. The CUS at TEA was predictive of HINE (linear regression coefficient -1,580, p < 0,001; odds-ratio 1,281, p = 0,299, PPV 100%, NPV 86%) and of FMs (linear regression coefficient -0,126, p = 0,001). Only 20% of CUS altered at 32 weeks of gestational age remained pathological at TEA scan.
DISCUSSION: The proposed novel CUS scoring system at TEA is a valid prognostic tool in prediction of outcome at three months of corrected age. Among the ten parameters, the presence of cyst/cavity and deep gray-matter alterations seem to be the most predictive. The earliest CUS scans revealed, in most cases, alterations that underwent spontaneous resolution.
CONCLUSIONS: Our study confirms the value of TEA CUS in predicting outcome. The use of this scoring system could prove to be a screening tool in clinical practice for preterm newborns with high neurological risk.
Skin-breaking procedures are associated with larger thalamic volume in moderate-to-late preterm infants
King R1, Verschuur A2,3, Hendson L1, Carlson H1, Scotland J1, Zein H1, Mohammed K1, Meijler G2, Leijser L1
1Department of Pediatrics, University Of Calgary, Calgary, Canada, 2Department of Neonatology, Isala Women and Children’s Hospital (IVKC, Zwolle, The Netherlands, 3Image Science Institute, University Medical Centre Utrecht, Utrecht, The Netherlands
BACKGROUND: Preterm infants (<37 weeks’ gestation) typically undergo numerous, potentially painful skin-breaking procedures (e.g., blood draws, intramuscular injections) during their stay in the neonatal intensive care unit (NICU)1. The procedures are needed for optimal, life-supporting care and treatments for sick and vulnerable preterm infants. Prior research suggests that more pain experienced in the neonatal period contributes to altered brain development, in particular of the deep gray matter, in very preterm infants (<32 weeks)2,3. The aim of our study is to assess whether a similar association exists between painful procedures and thalamic volumes in moderate to late preterm (MLPT) infants born between 32-36 weeks.
METHODS: Preliminary analysis of 15 MLPT infants who underwent a brain MRI between 40-44 (mean 41.85±0.9) weeks’ postmenstrual age (PMA). The infants were recruited as part of a larger prospective cohort study on brain imaging in MLPT infants, with inclusion criteria: gestational age (GA) between 32+0 and 35+6 weeks, admission to one of two level II NICUs in Calgary, no metabolic or chromosomal disorders. For the 15 infants, the total number of skin-breaking procedures throughout their NICU stay were measured, including central and peripheral line insertion, skin pokes for blood draw (e.g., glucose, bilirubin, complete blood count CBC, newborn metabolic screening) and vitamin K injections. T2-weighted images were segmented using the developing human connectome project (dHCP) structural pipeline4. Absolute thalamic volumes were calculated in both hemispheres. Linear regression analysis with correction for PMA was performed to assess the association between skin-breaking procedures and thalamic volume. Level of significance was p<0.05.
PRELIMINARY RESULTS: At present, the MRIs of 15 infants (10 female, 5 male), born at a median of 34.8 (range 32.0–35.9) weeks were analysed. The mean count of skin-breaking procedures was 13.3 (±9.5) per infant over a mean of 13.2 (±7.8) days NICU stay. Total thalamic volume (B = 2.51, p=0.033; 95%-CI: 0.244 – 4.772) and right thalamic volume (B = 5.52, p=0.020; 95%-CI: 1.053 – 9.995) showed a positive association with higher numbers of skin-breaking procedures. Left side thalamic volume (B=4.31, p=0.058; 95%-CI: -0.168 – 8.797) was not significantly associated. Results for a larger cohort will be available at the time of the conference.
CONCLUSIONS: Higher number of skin-breaking procedures during NICU admission may be associated with thalamic volume in MLPT infants at term equivalent age. Our findings differed from those in the very preterm group2,3, who showed reduced thalamic volume in infants who experienced higher counts of skin-breaking procedures. The contradicting results may suggest differing vulnerability or neuroplastic potential of the more mature MLPT brain. Future study in a larger sample is needed to further investigate the associations between skin-breaking procedures in early infancy and thalamic tissue volume.
REFERENCES
[1] Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008; 300:60–70. 10.1001/jama.300.1.60
[2] Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol. 2012; 71:385–396. 10.1002/ana.22267
[3] Duerden EG, Grunau RE, Guo T, et al. Early Procedural Pain Is Associated with Regionally-Specific Alterations in Thalamic Development in Preterm Neonates. J Neurosci. 2018 Jan 24;38(4):878-886.
[4] Makropoulos A, Robinson EC, Schuh A, Wright R, Fitzgibbon S, Bozek J, Counsell SJ, Steinweg J, Vecchiato K, Passerat-Palmbach J, Lenz G, Mortari F, Tenev T, Duff EP, Bastiani M, Cordero-Grande L, Hughes E, Tusor N, Tournier JD, Hutter J, Price AN, Teixeira RPAG, Murgasova M, Victor S, Kelly C, Rutherford MA, Smith SM, Edwards AD, Hajnal JV, Jenkinson M, Rueckert D. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage. 2018 Jun;173:88-112. doi: 10.1016/j.neuroimage.2018.01.054. Epub 2018 Jan 31. PMID: 29409960; PMCID: PMC6783314.
Pattern of injury on neonatal mri is associated with cognitive and language outcome at 4 years in term neonatal encephalopathy
Bach A1, Lambing H2,3, Rogers E2, Xu D4, Barkovich A4, Ferriero D2,3, Glass H2,3, Gano D2,3
1Departments of Neurology and Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, United States, 2Department of Pediatrics, University of California–San Francisco, San Francisco, United States, 3Department of Neurology, University of California–San Francisco, San Francisco, United States, 4Department of Radiology, University of California–San Francisco, San Francisco, United States
PURPOSE: Our objective is to evaluate the association of injury pattern on neonatal magnetic resonance imaging (MRI) and therapeutic hypothermia with 4-year language and cognitive outcomes in a cohort of term neonatal encephalopathy (NE). Additionally, we aim to describe the predictive value of 30-month language and cognitive outcomes for 4-year outcomes in this cohort.
METHODS: This is a cross-sectional analysis of 4-year language and cognitive outcomes (IQR:48-52 months) in a prospective cohort of term NE with MRI 4 days (IQR:3-6) after birth (2002-2017). Therapeutic hypothermia (TH) was implemented in 2007. The severity of watershed (WS) and basal ganglia/thalamus (BG/T) injury was scored by a blinded pediatric neuroradiologist using our published criteria (Figure 1). The primary outcome was verbal IQ (VIQ) and full-scale IQ (FSIQ) on the Weschler Preschool and Primary Scale of Intelligence-III at 4 years. Children were additionally evaluated at median 30.4 months (IQR:29.6-31.7) with the Bayley-II or III. Descriptive statistics and multivariate linear regression were used to evaluate the association of injury pattern and TH with VIQ and FSIQ. Covariates were baseline characteristics significantly different between the TH-treated and untreated groups that comprise eligibility for TH: cord gas pH, 10-minute APGAR score, seizure(s) within 24 hours of life, intubation at resuscitation, neonatal encephalopathy score. The predictive value of language and cognitive outcome on the last available Bayley for VIQ or FSIQ<85 at 4 years was also calculated. Bayley-II MDI<70 and Bayley-III language or cognitive score<85 were considered abnormal.
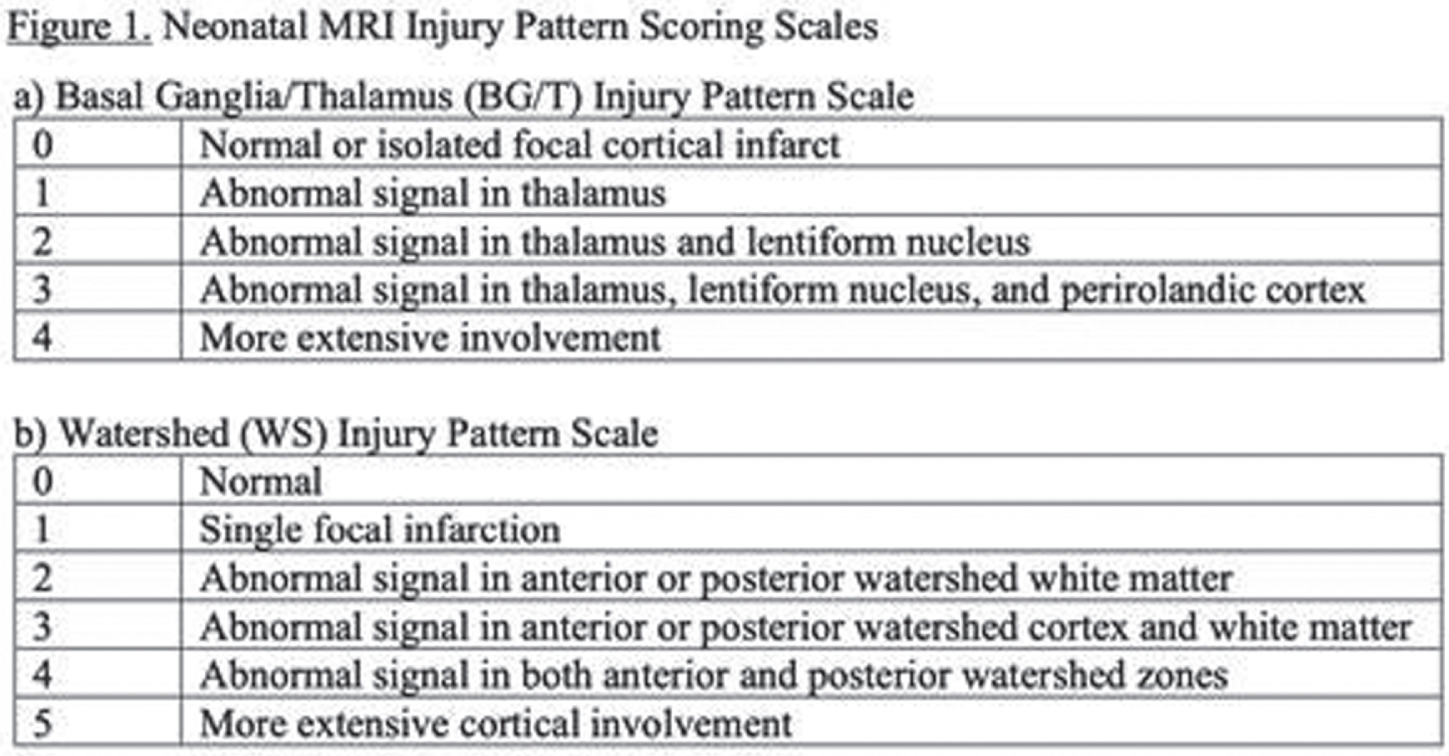
RESULTS: Among 148 children with NE seen for 4-year follow-up, 44(30%) children had TH. MRI on day 4 showed decreased frequency of injury in TH-treated newborns: no injury 25/44 vs 30/104, isolated WS injury 14/44 vs 35/104, BG/T injury 5/44 vs 39/104 (P=0.004). At 4 years, TH was associated with higher VIQ (TH: mean 102+/-3.0, 95% CI 96-108. No-TH: mean 93+/-2.3, 95% CI 89-98. T-test P=0.029) and FSIQ (TH: mean 103+/-2.6, 95% CI 98-108. No-TH: mean 95+/-2.4, 95% CI 90-100. T-test P=0.045). Distributions of VIQ and FSIQ varied by injury pattern (Figure 2). Adjusting for pattern of injury and predictors of TH, TH was not independently associated with VIQ or FSIQ. In these models, isolated severe WS injury, mild BG/T injury, and severe BG/T injury were all associated with significantly lower VIQ and FSIQ (Figure 3). The sensitivity of 30-month Bayley-III language score<85 for 4-year VIQ<85 was 0.50. The sensitivity of 30-month Bayley-II MDI<70 or Bayley-III cognitive score<85 for 4-year FSIQ<85 was 0.28 (Figure 4).
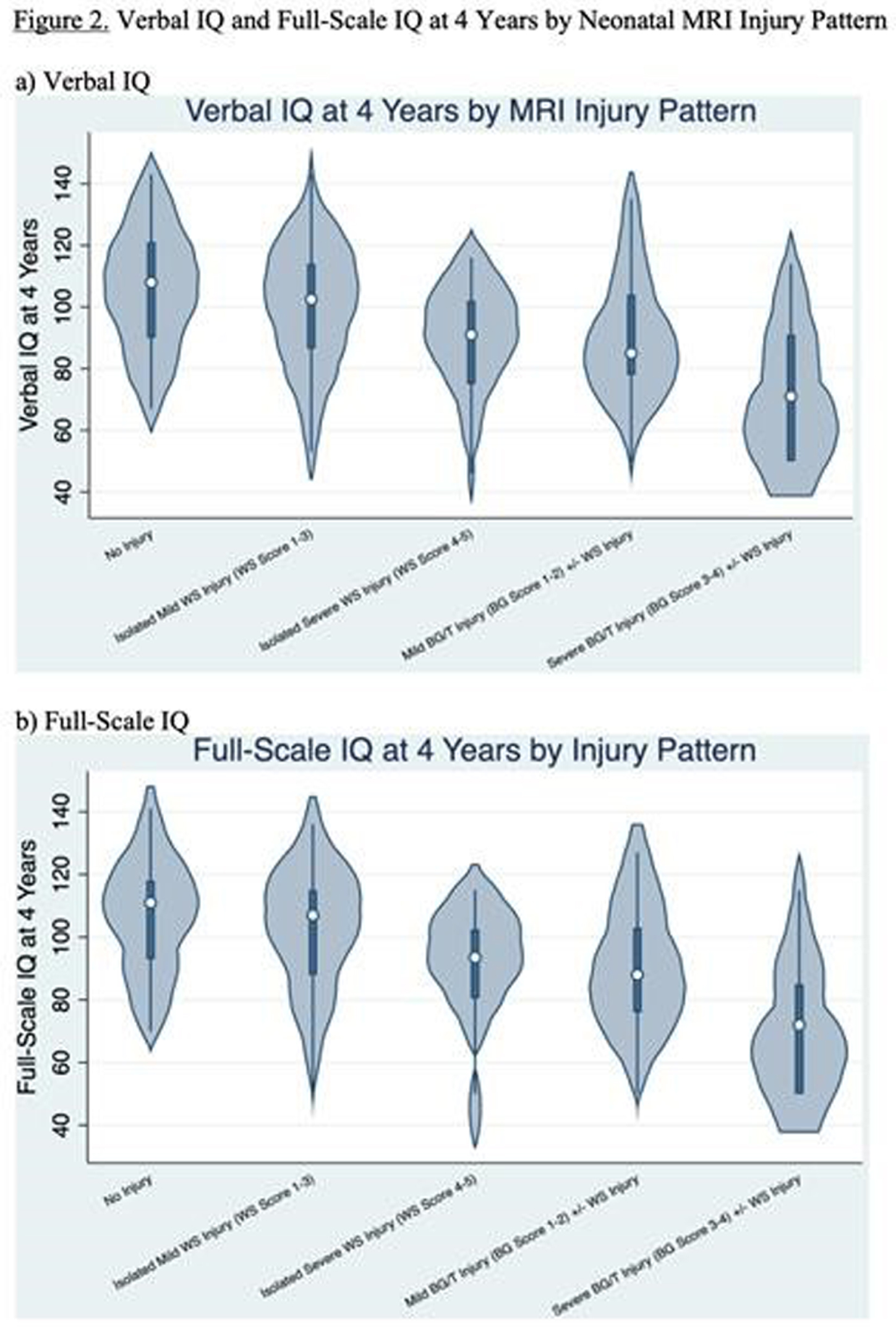
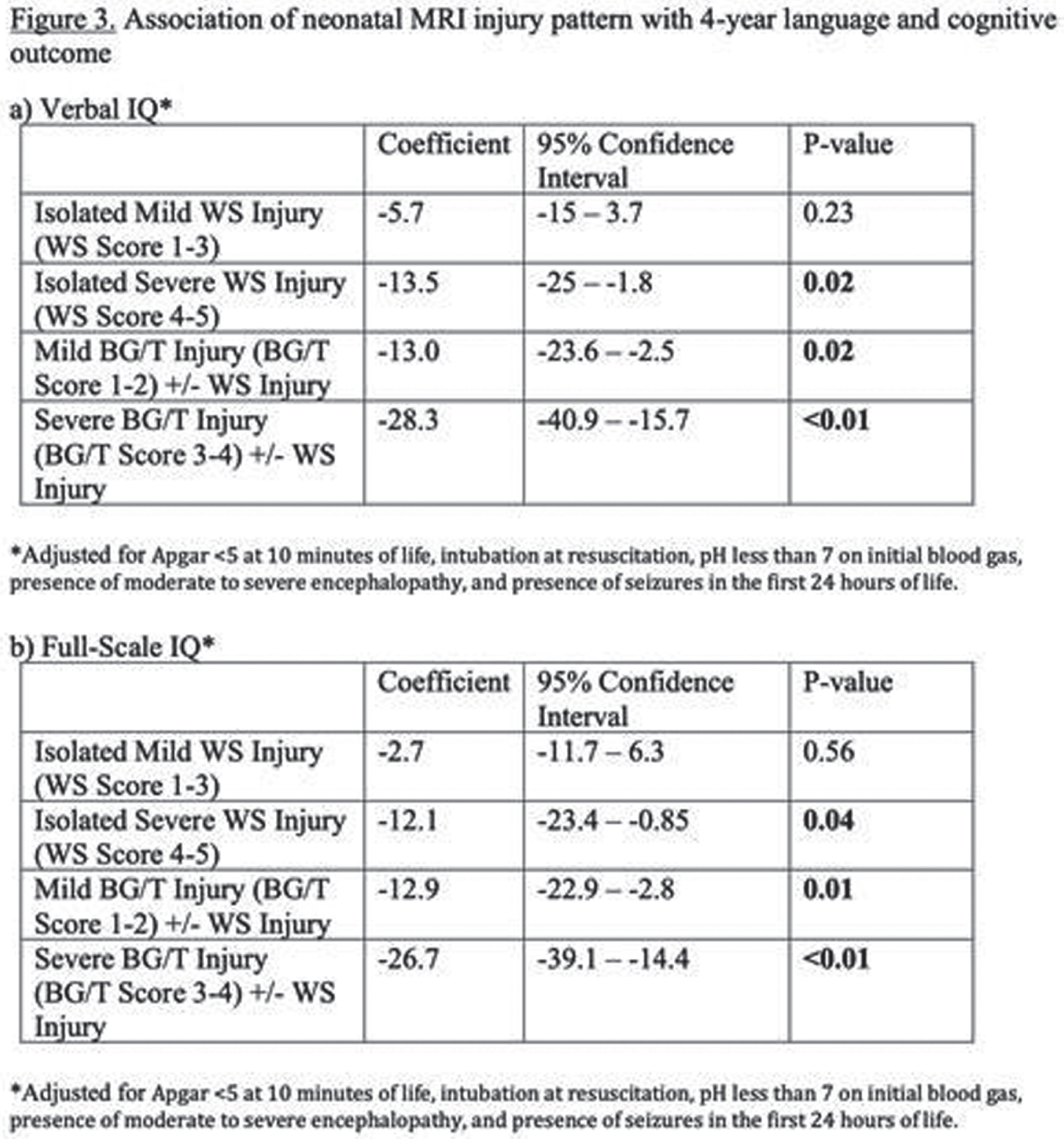
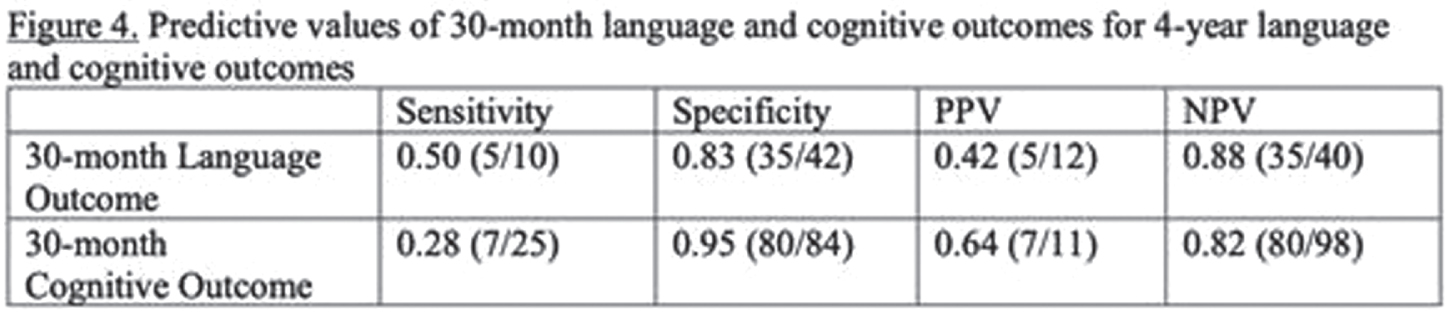
CONCLUSIONS: Severe isolated WS injury and any BG/T injury in term NE was associated with lower 4-year verbal and full-scale IQ regardless of TH treatment. In this cohort, 30-month outcomes had low sensitivity for abnormal 4-year outcomes, highlighting the importance of long-term follow-up.
The predictive value of the weeke score in the prognosis of hypoxic-ischemic encephalopathy treated with therapeutic hypothermia
Igreja L1, Ferreira A2, Rita Gomes R2,3, Sousa B2,3, Novo A2, Eduardo Alves J1, Proença E2, Carvalho C2
1Neuroradiology Department, Centro Hospitalar Universitário do Porto, Largo do Prof. Abel Salazar, 4099-001, Porto, Portugal, 2Intensive Neonatal Care Unit, Department of Neonatology and Pediatric Intensive Care, Centro Materno-Infantil do Norte, Centro Hospitalar Universitário do Porto, Largo do Prof. Abel Salazar, 4099-001, Porto, Portugal, 3Department of Pediatrics, Centro Materno-Infantil do Norte, Centro Hospitalar Universitário do Porto, Largo do Prof. Abel Salazar, 4099-001, Porto, Portugal
BACKGROUND AND PURPOSE: In hypoxic-ischemic encephalopathy (HIE) treated with therapeutic hypothermia (TH), clinical and electroencephalogram findings as well as neuroimaging may help to deliver a more accurate neurological outcome, guiding future therapeutic decisions. Different magnetic resonance imaging (MRI) scores have been validated in moderate/severe HIE, yet little applied in clinical practice.
Our aim was to retrospectively apply the Weeke score in a group of neonates clinically diagnosed with HIE treated with TH and evaluate its prognostic value.
MATERIALS OR METHODOLOGY: An analysis of patients diagnosed with HIE and who underwent TH at a tertiary neonatal intensive care unit between January/2012 and July/2020 was conducted, and the Weeke score calculated based on the MRI findings. Demographic data, pre- and perinatal information, neurological sequelae at 12 and 24 months and mortality were collected. Mann-Whitney, Kruskal-Wallis, and Pearson correlation coefficient tests were used in the statistical analysis.
RESULTS: 29 patients with a median gestational age of 39 weeks, 55% male were included and in 26 patients (90%) a sentinel event was identified: an altered cardiotocography (n=17), placental abruption (n=6) and uterine rupture (n=6). They underwent MRI on average at day 6 (3-13) of life. 21 patients were clinically assessed at 12 and 24 months. Clinical seizures on admission correlated significantly with gray matter (GM) subscore (p=0.050) and total score (p=0.046). The aEEG pattern at 48h correlated significantly with GM subscore and total score (p=0.003 and p=0.004), as did the aEEG after TH with GM, white matter (WM) and total scores (p=0.006, p=0.011, p=0.005). Thompson score after TH showed strong correlation with GM subscore (p=0.791) and WM to moderate degree (p=0.613). After therapeutic hypothermia, Thompson score, GM subscore and total score correlated with mortality (p=<0.001) and motor sequelae (p=0.048, p=0.002 and p=0.004).
CONCLUSIONS: Our results confirm the predictive value of the Weeke Score for neurological prognosis and mortality in HIE treated with TH, allowing detailed characterization of the imaging findings and grading of its severity. The implementation of this score in routine neuroimaging assessment in IHE may help to deliver a more objective and accurate neurological prognosis.
REFERENCES
[1] Weeke LC, Groenendaal F, Mudigonda K, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr. 2018;192:33-40.e2.




