Proceedings of the 14th International Newborn Brain Conference: Other forms of brain monitoring, such as NIRS, fMRI, biochemical, etc.
Urgent need for a better identification of newborn infants at risk of perinatal hypoxic-ischemic brain injury who might benefit from neuroprotective interventions and systematic follow-up
Franz A1, Segerer H2, Kittel J2, Felderhoff U3, Stein A3, Hüning B3, Pöschl J4, Waldherr S4, Keller M5, Sarközy G5, Wieg C13, Beato N13, Rüdiger M6, Winkler S6, Flemmer A7, Herber-Jonat S7, Domogalla C7, Koch L8, Kühr J9, Holz S9, Peters J10, Belker K10, Yapicioglu-Yildizdas H11, Taskin E12, Benders M14, Annink K14, Groenendaal F14, Hellström-Westas L15, Marlow N16, Saugstad O17, Meyer R18, Plum A18, Steins-Rang C18, Munk A18, Engel C1, Bartmann P19
1University Hospital Tübingen, Tübingen, Germany, 2Krankenhaus Barmherzige Brüder Regensburg - Klinik St. Hedwig, Regensburg, Germany, 3University Hospital Essen, Essen, Germany, 4University Hospital Heidelberg, Heidelberg, Germany, 5Kinderklinik Dritter Orden, Passau, Germany, 6University Hospital Carl Gustav Carus an der Technischen Universität Dresden, Dresden, Germany, 7Universitätskinderkrankenhaus und Perinalzentrum LMU, Munich, Germany, 8Katholisches Kinderkrankenhaus Wilhelmsstift gGmbH, Hamburg, Germany, 9Städtisches Krankenhaus Karlsruhe, Karlsruhe, Germany, 10Klinikum Dritter Orden München-Nymphenburg, Munich, Germany, 11Cukurova University Hospital, Adana, Turkey, 12University of Firat- Pediatrics, Elazig, Turkey, 13Klinikum Aschaffenburg-Alzenau, Aschaffenburg, Germany, 14University Medical Center Utrecht, Utrecht, The Netherlands, 15Uppsala University Dept. of Women’s and Children’s Health, Uppsala, Sweden, 16University College London - Institute for Women’s Health, London, United Kingdom, 17University of Oslo, Pediatric Research, Oslo, Norway, 18InfanDx AG, Cologne, Germany, 19University Hospital Bonn, Bonn, Germany
BACKGROUND AND PURPOSE: Hypoxic-ischemic encephalopathy (HIE) is caused by perinatal asphyxia and, until now, therapeutic hypothermia (TH) is the only evidence-based neuroprotective therapy (systematic review in Jacobs, 2013). TH needs to be started within the first 6h after birth to effectively reduce brain injury, hence requires immediate identification of patients at risk. However, current assessment of HIE severity is based on clinical parameters which cannot reliably detect all infants in need for treatment. This secondary analysis of the BANON (Biomarkers and Neurological Outcome in Neonates) study provides neurodevelopmental outcomes after 2-4 years of newborn infants with moderate to severe perinatal acidosis not being treated with TH.
METHODOLOGY: Prospective observational study at 13 academic level III NICUs in Germany (11 sites) and in Turkey (2 sites) with a follow up after 2-4 years aiming to verify metabolomic biomarkers for HIE (BANON study). 553 infants were recruited into the BANON study based on umbilical artery (or other) pH<7.10 and/or need for neonatal resuscitation. Neurodevelopmental outcomes after 2-4 years were assessed using the Bayley (Bayley III, Pearson) and the Ages and Stages Questionnaire (ASQ 3; Brookes Publishing Co.).
RESULTS: Of the 553 infants included in BANON, n=73 have been treated with TH for moderate/severe HIE. Of those 480 patients without TH, n=366 were here evaluated at follow-up. Of these n=1 had another diagnosis instead of HIE associated with impaired outcome and n=2 had incomplete assessments prohibiting outcome classification. The following numbers refer to infants that did not undergo TH and underwent follow-up. At 2-4 years, outcome was classified ‘normal’ in n=283 and ‘abnormal (likely due to HIE)’ in n=18, n=62 had ‘suspect’ 2-4-year outcome, hence requiring further evaluation. In the abnormal 2-4 years outcome group, 2 children had cerebral palsy, and 1 child cerebral palsy and ongoing seizures. Children with normal, suspect, and abnormal 2-4 years outcome, 3/283, 14/62 and 18/18 had abnormal neurological assessments, abnormal Bayley tests or (in the absence of Bayley results) ASQ-Scores below the cut-off. Infants with normal, suspect, and abnormal 2-4 years outcome had similar neonatal characteristics: in 52%, 53% and 50% of patients, there was a sentinel event indicating acute prenatal asphyxia. More detailed neonatal patient information is presented in table 1.
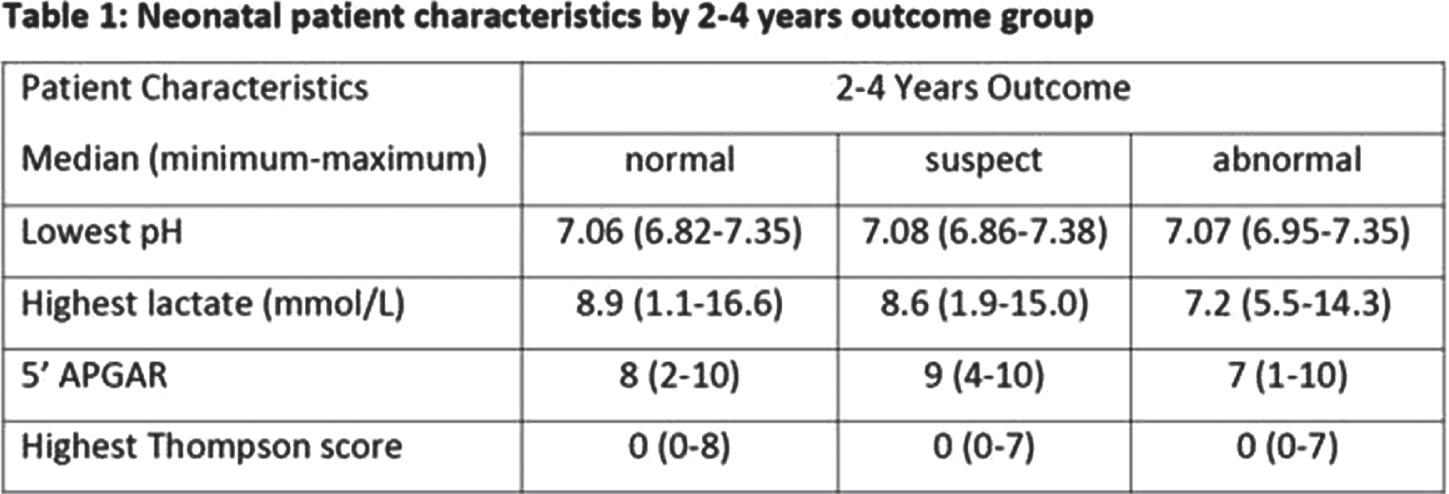
CONCLUSION: Of infants recruited based on moderate to severe perinatal acidosis and/or need for neonatal resuscitation, 5% showed impaired and 17% suspect developmental outcomes at 2-4 years. These infants might have had benefit from neonatal neuroprotective interventions and/or developmental follow-up programs and hence should have been reliably identified early postnatally.
REFERENCES
[1] Jacobs S, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews. 2013; 1. https://doi.org/10.1002/14651858.CD003311.pub3
Resting-state functional near-infrared spectroscopy reveals altered functional connectivity of neonates with hypoxic ischemic encephalopathy post cooling
Tang L1, Kebaya L2,3, Altamimi T2, Kowalczyk A2, Musabi M2, Roychaudhuri S2, Vahidi H3, Meyerink P2, Camila Mayorga P2,3, de Ribaupierre D4, Bhattacharya S2, St. Lawrence K1,5, Duerden E1,2,6
1Biomedical Engineering, Faculty of Engineering, Western University, London, Canada, 2Neonatal-Perinatal Medicine, Schulich School of Medicine and Dentistry, Western University, London, Canada, 3Neuroscience, Schulich School of Medicine and Dentistry, Western University, London, Canada, 4Clinical Neurological Sciences, Schulich School of Medicine and Dentistry, Western University, London, Canada, 5Medical Biophysics, Schulich School of Medicine and Dentistry, Western University, London, Canada, 6Applied Psychology, Faculty of Education, Western University, London, Canada
BACKGROUND AND PURPOSE: Hypoxic-Ischemic Encephalopathy (HIE) is a brain injury resulting from lack of oxygen to the brain during the antenatal period. HIE can lead to premature mortality, as well as a variety of acute and long-term morbidities.1 Therapeutic hypothermia (TH) is the standard of care for neonates with HIE and has proven to have neuroprotective effects; yet the mechanisms remain largely unknown. Functional near-infrared spectroscopy (fNIRS) offers the ability to assess resting state functional connectivity (RSFC), a key indicator of brain functional organization. Better understanding of the functional networks influenced by TH may provide insights on treatment mechanisms and predict functional outcomes in HIE. Therefore, in this study, we aim to determine whether the patterns of resting state functional connectivity in term-born neonates with HIE in the post-cooling period, differ compared to healthy newborns, using resting-state fNIRS.
METHODS: Ten neonates with HIE (median postmenstrual age [PMA]=38.8) and ten term born healthy newborns (PMA=39.1) were recruited. Newborns with HIE underwent TH during the first week of life. fNIRS system (NIRSport2, NIRx medical systems, Berlin, Germany) was set up with 20 channels covering the whole brain. Resting-state fNIRS data (6 minutes) were collected at the bedside in the post-cooling period. After denoising, RSFC was calculated as the correlation coefficients amongst the time courses of the fNIRS channels of both oxygenated and deoxygenated hemoglobin (HbO and Hbr, respectively). Local and global RSFC was calculated with graph theory based metrices, e.g., clustering coefficient, centrality, etc. A Louvain-like method2 was used for identifying clusters. T-tests were applied to determine significant group differences.
RESULTS: In the Hbr RSFC, at various network sparsity, global analysis (figure 1) showed the HIE group has significantly higher clustering coefficient and network efficiency, indicating brain regions of HIE neonates are more locally connected. Local analysis (figure 2) showed left parietal lobule has lower degree centrality, indicating the region is potentially vulnerable to injuries. Clustering analysis (figure 3) showed HIE group has greater modularity compared to controls indicating increased intrinsic functional connectivity but lower mutual information, meaning more variability in terms of clustering and disrupted functional networks.
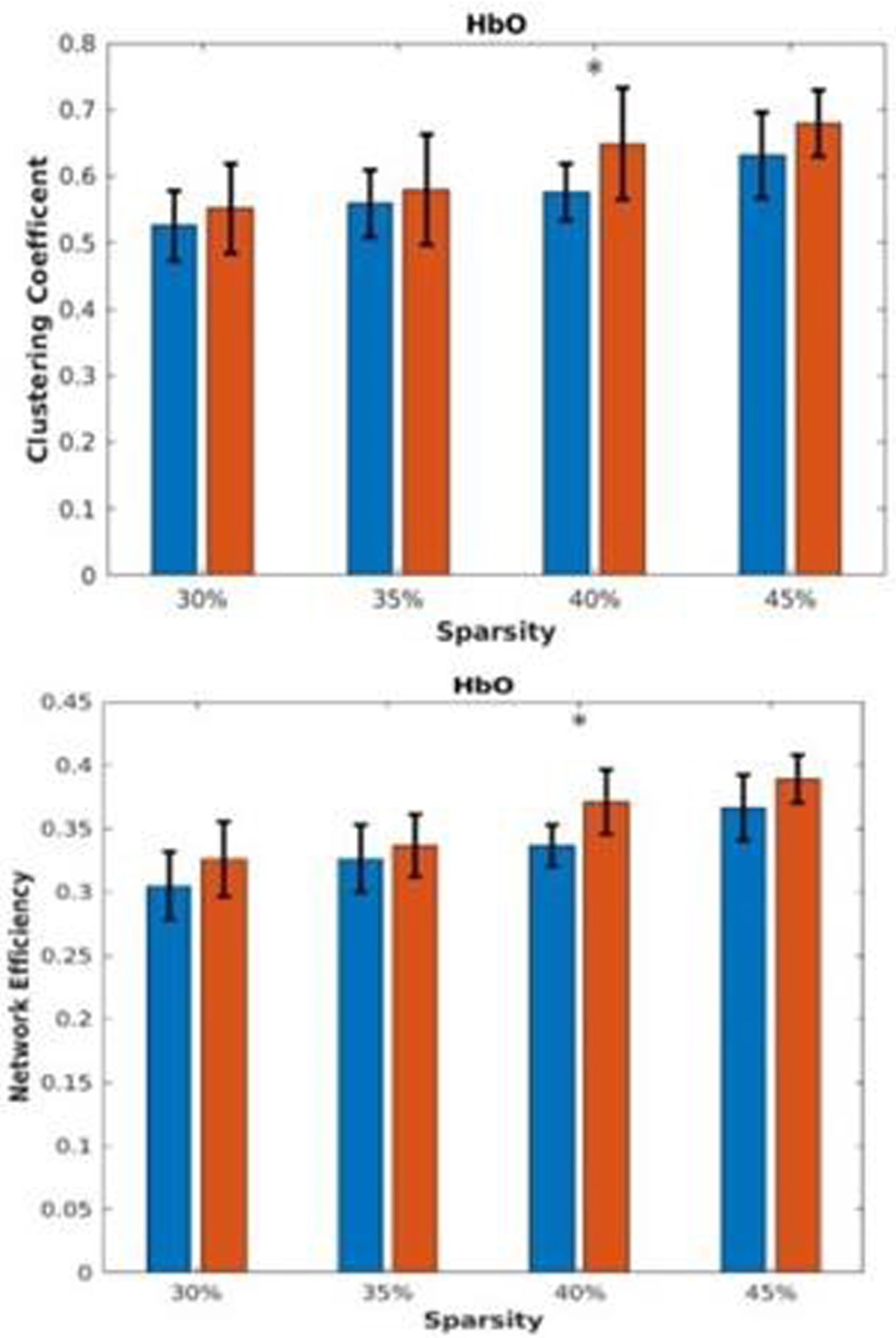
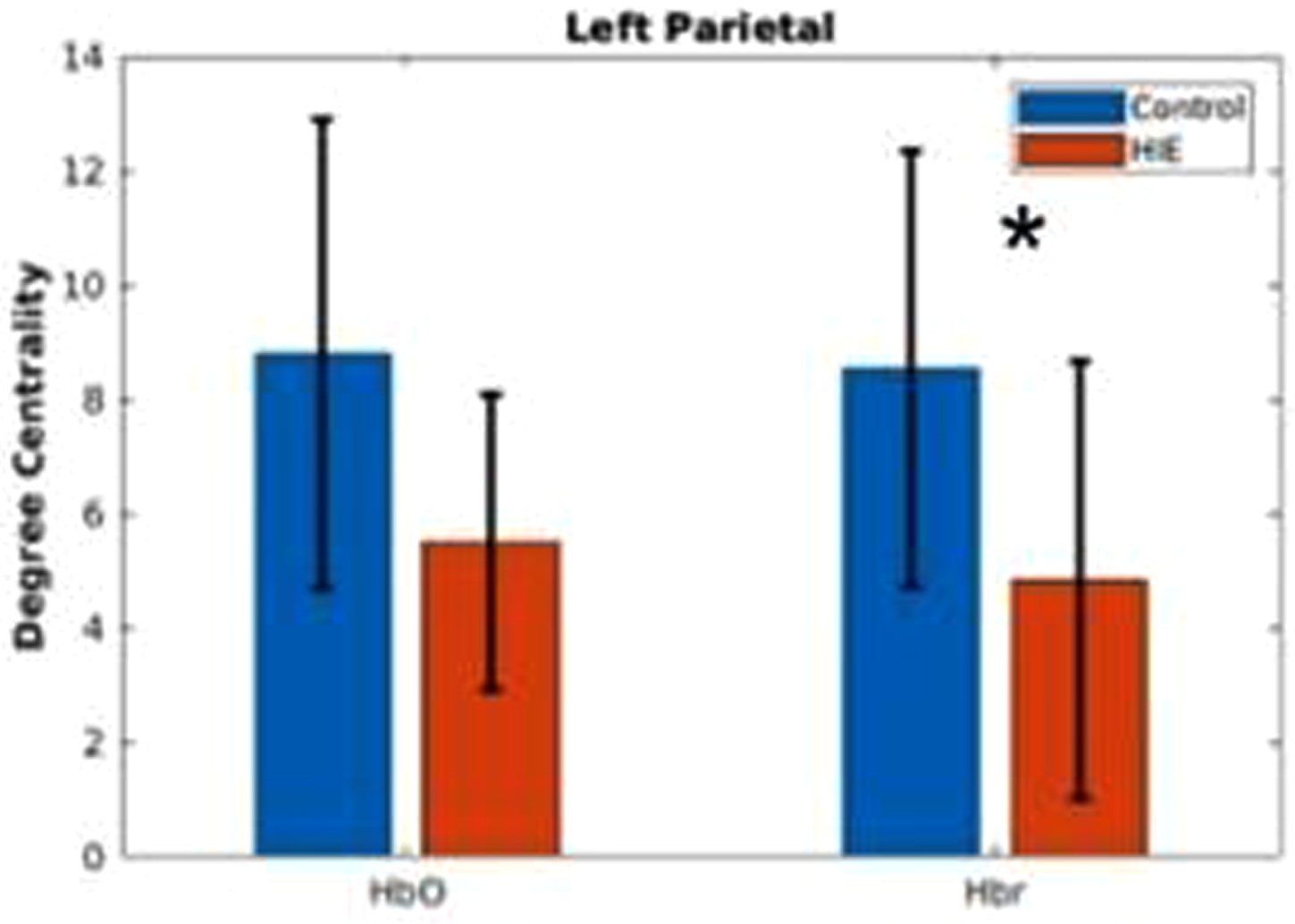
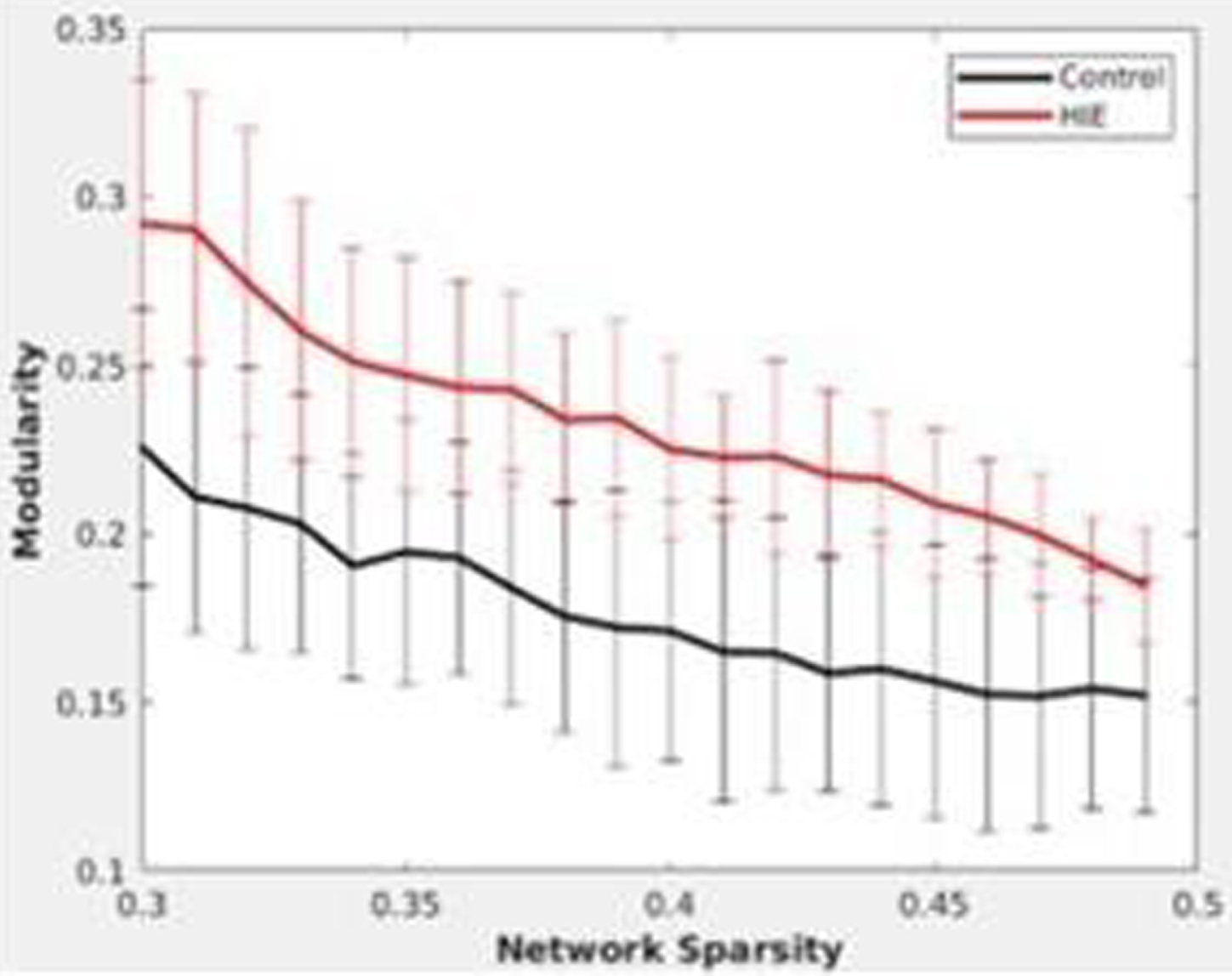
CONCLUSION: Global and regionally specific increase in clustering coefficient could reflect the neuroprotective effects of therapeutic hypothermia. Regionally decreased connectivity and inconsistency of clustering indicates the effect of injury persists. Results highlight the importance of using bedside measurements of cerebral hemodynamics to better characterize brain health in newborns with HIE.
REFERENCES
[1] Mitra S., et al., Cerebral near infrared spectroscopy monitoring in term infants with hypoxic ischemic encephalopathy—a systematic review. Front. Neurol. 2020;11, 1–17.
[2] Blondel V. D., et al., E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008.
Brain functional connectivity of term newborns with neonate abstinence syndrome: A resting-state fNIRS study
Tang L1, Roychaudhuri S2,3, Kowalczyk A2,3, Musabi M2,3, Vahidi H4, Bhattacharya S2,3, St. Lawrence K1,5, Duerden E1,4
1Biomedical Engineering, Faculty of Engineering, Western University, London, Canada, 2Division of Neonatal-Perinatal Medicine, Department of Pediatrics, London Health Sciences Centre, London, Canada, 3Neonatal - Perinatal Medicine, Schulich School of Medicine & Dentistry, Western University, London, Canada, 4Applied Psychology, Faculty of Education, Western University, London, Canada, 5Medical Biophysics, Schulich School of Medicine & Dentistry, Western University, London, Canada
BACKGROUND AND PURPOSE: Within the last decade, there are increasing number of cases of maternal opioid exposure and newborns with neonatal abstinence syndrome (NAS). Neonates with NAS can have a complicated clinical course and may be at high risk of adverse cognitive, motor and language impairments, suggesting early damage of brain function1. However, how neonatal brains are affected by NAS is yet to be examined. Functional near-infrared spectroscopy (fNIRS), due to its good accessibility and resistance to motion, is a promising tool for monitoring neonatal brain activity. In this study, we aim to use resting-state fNIRS (rs-fNIRS) to explore differences in functional connectivity between term newborns with NAS and healthy controls.
METHODS: fNIRS data were prospectively collected in 10 healthy newborns and 6 newborns with NAS (see figure 1 for detailed demographics). The fNIRS system (NIRSport2, NIRx medical systems, Berlin, Germany) was set up with 8 sources and 8 detectors covering the whole brain, yielding 20 channels in total. rs-fNIRS was applied to each subject for a 6-minute scan. fNIRS was well tolerated by both cohorts. In healthy newborns the data were collected within the first 8 hours of life, while the fNIRS data were collected on day of life 0-3 in the NAS cohort. For preprocessing of the fNIRS data, we applied spline interpolation for motion correction and band-pass filtering of 0.01-0.1 Hz to remove physiological noise, followed by modified Beer-Lambert law. Functional connectivity was calculated as the Pearson correlation coefficients amongst the time courses of the fNIRS channels. Graph theory based metrices, e.g., clustering coefficient, centrality, etc., were calculated to evaluate local and global properties of functional connectivity maps. T-test was used to evaluate the difference between cohorts.
RESULTS: With filtering out negative and low weighted connectivity, healthy control cohort showed significantly higher clustering coefficient and network efficiency compared with NAS cohort (figure 2). Potentially, this indicates disrupted integrity of functional networks within NAS cohort. Local analysis showed there were significant differences in the degree centrality and local clustering coefficient over the channels around frontal lobe on both hemispheres (figure 3), suggesting this part of brain could be particularly vulnerable to NAS.
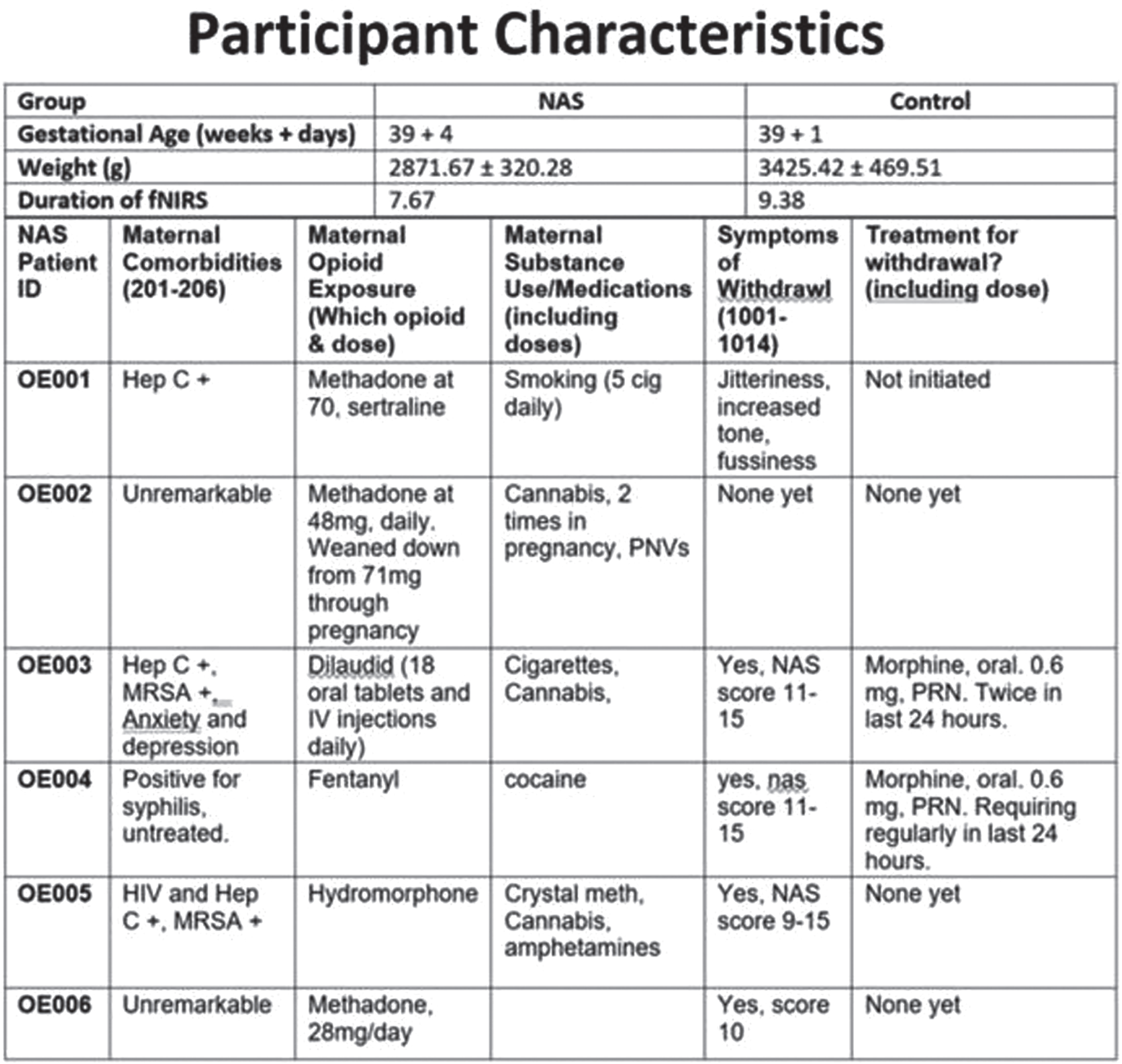
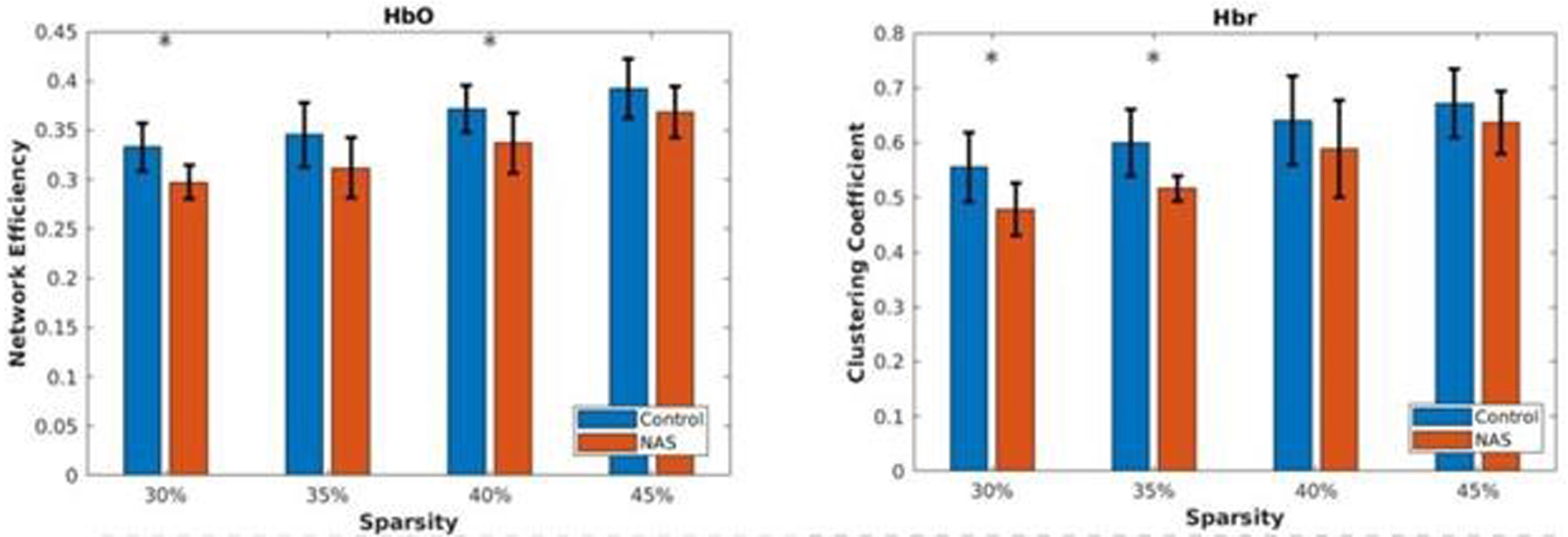
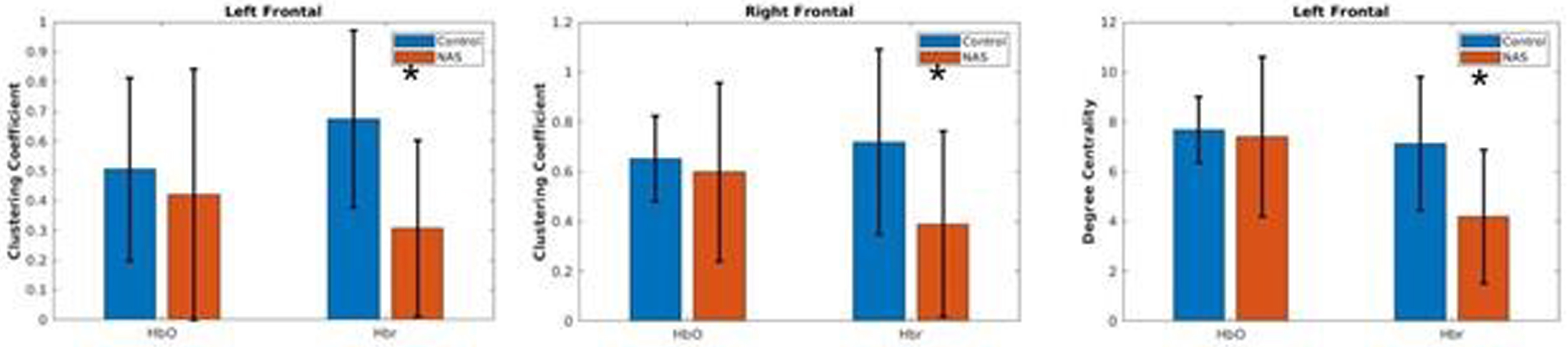
CONCLUSION: Preliminary results indicated distinct rs-fNIRS functional connectivity patterns between the two cohorts. Better characterization of early changes using portable bedside measurements in babies with NAS can aid in identifying biomarkers reflective of an increased risk for affected neurodevelopmental outcomes.
REFERENCES
[1] Logan, B. A., et al., Neonatal Abstinence Syndrome: Treatment and Pediatric Outcomes. Clin Obstet Gynecol. 2013 March ; 56(1): 186–192.
Continuous transcutaneous carbon dioxide monitoring in infants with neonatal encephalopathy
Szakmar E1, Sunwoo J3, Cherkerzian S1, Kim S1, Hannon K1, Munster C1, Garvey A4, Elshibiny H1, Lee S1, Ben-David D1, Franceschini M3, Robinson J1, Christou H1, Inder T1, El-Dib M1
1Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA, 2Division of Neonatology, 1st Department of Pediatrics, Semmelweis University, Budapest, Hungary, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, USA, 4INFANT Research Centre, Cork, Ireland; Department of Paediatrics and Child Health, University College Cork, Cork, Ireland
BACKGROUND AND PURPOSE: Increasing evidence suggests that hypocapnia is associated with brain injury on MRI and adverse neurodevelopmental outcome in infants treated with therapeutic hypothermia (TH) for neonatal encephalopathy (NE). Consequently, continuous carbon dioxide (CO2) monitoring and avoiding extreme levels of CO2 have the potential to improve neurologic outcome. Our aim was to compare changes in transcutaneous CO2 levels (tcPCO2) during hypothermia and rewarming between infants with and without brain injury on MRI in a cohort of infants with NE.
METHODS: This prospective observational trial enrolled infants, who received TH for mild to severe NE between October 2019 and June 2022 at Brigham and Women’s Hospital. The tcPCO2 data (SenTec Digital Monitor) was captured by a real-time integrated neuromonitoring system (Moberg, CNS Monitor). The pattern of brain injury was evaluated according to an MRI grading system developed by Weeke et al. We identified 2 groups of patients with and without any brain injury based on the assessment of MRI after rewarming. The median of tcPCO2 at each hour was calculated for each infant. Using these values, we calculated the mean tcPCO2 in each of seven phases of TH. The association between brain injury and tcPCO2 at each phase was analyzed using linear mixed models with fixed effects for group, phase, and their interaction and adjustment for correlation among repeated measurements. Differences between groups at each phase was determined using estimated marginal means.
RESULTS: 110 infants received TH during study period, out of those 37 infants were enrolled into this prospective study. Serial measurements from 31 patients were available for analysis. Integrated monitoring began at median 24 hours of life (HOL, min-max: 11-51) and ended at median 94 HOL (min-max: 87-120). Sixteen (51.6%) infants had evidence of any brain injury on MRI. Results from mixed models indicated a significant effect of phase on mean tcPCO2 level (F =4.4, p= 0.001), but no difference by brain injury status (F= 0.0, p=0.304) or an interaction effect (F=1.2, p=0.304) (Figure 1). Mean levels of tcPCO2 exhibited a significant decrease between the last phase of TH (> 60 HOL) and post-rewarming phase. The pairwise comparisons between each phase and the post-rewarming period are summarized in Table 1.
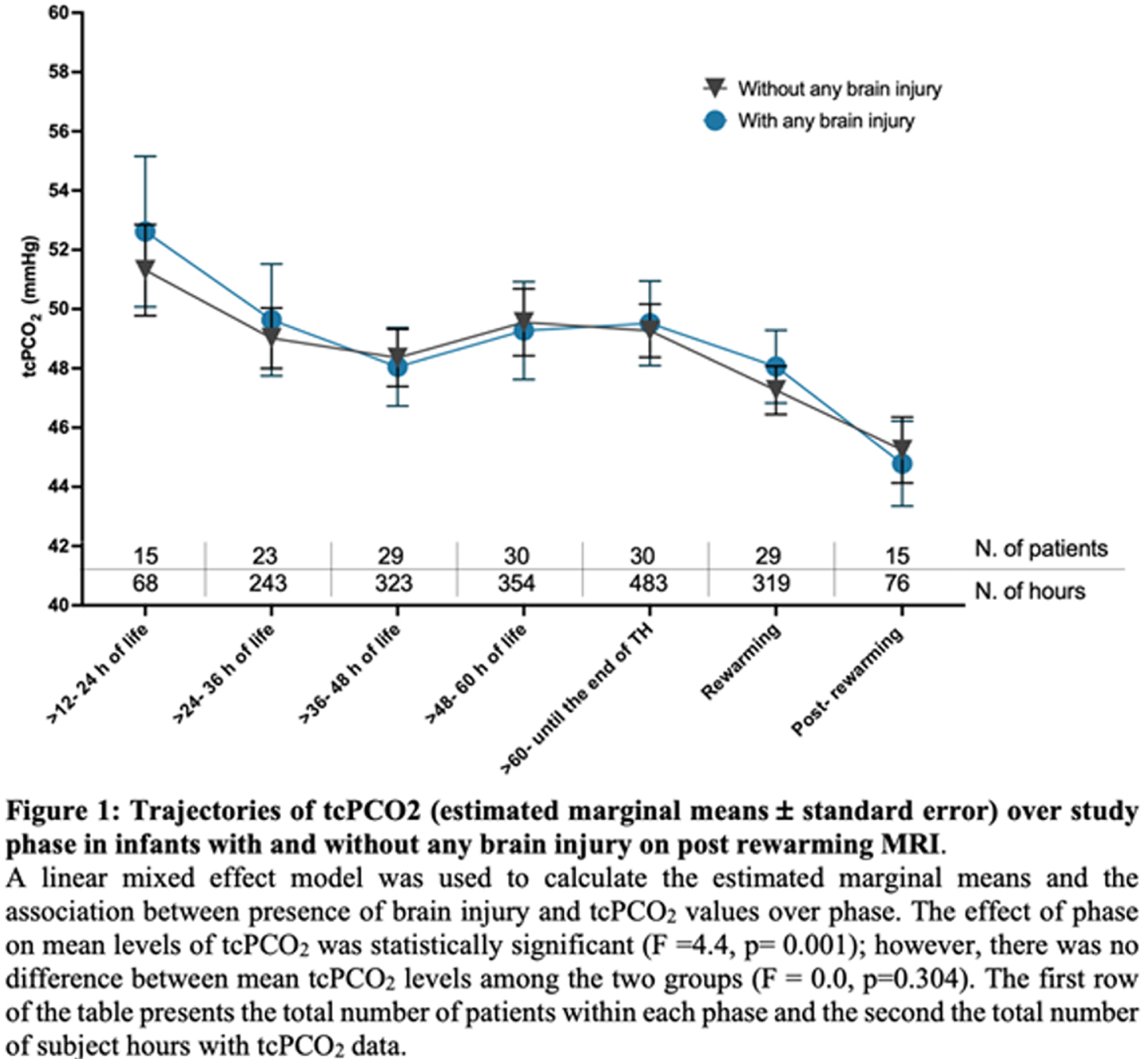
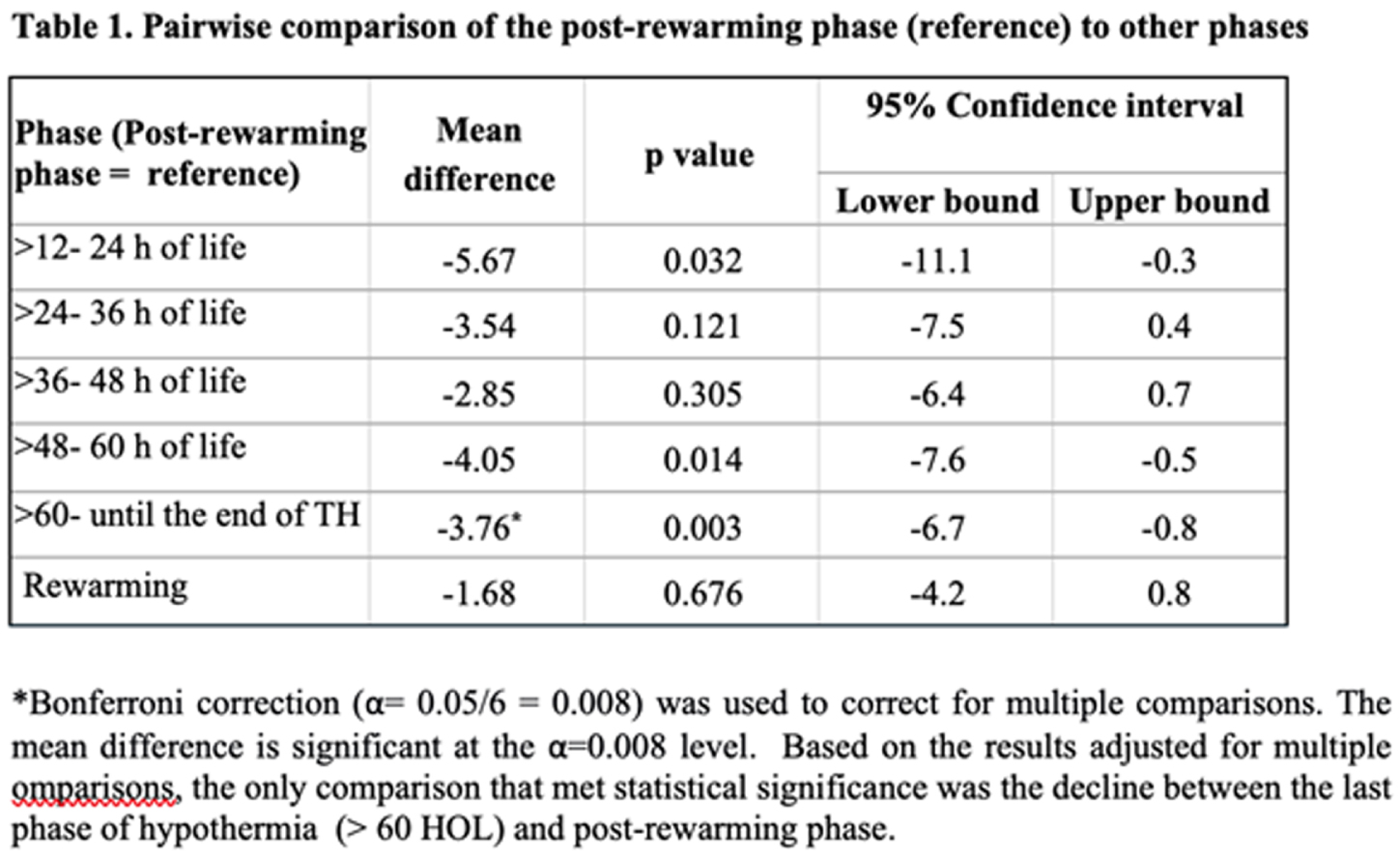
CONCLUSION: This novel longitudinal study of infants with NE across phases of TH showed relatively stable tcPCO2 levels within the normal range during hypothermia and rewarming but with a significant decline between the last phase of hypothermia and post-rewarming phase. The trajectories of tcPCO2 did not differ between infants with and without brain injury. These pilot results need to be interpreted with caution given study limitations that include no adjustment for potential confounding or lack of data during the early hours of life.
REFERENCES
[1] El-Dib M, et al. Challenges in respiratory management during therapeutic hypothermia for neonatal encephalopathy. Semin Fetal Neonatal Med. 2021:101263.
[2] Abend N, et al. Proceedings of the 13th International Newborn Brain Conference: Neonatal Neurocritical Care, Seizures, and Continuous EEG monitoring. J Neonatal Perinatal Med. 2022; 15:467-485.
[3] Weeke LC, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr. 2018; 192:33-40 e32.
Carbon dioxide reactivity in infants with neonatal encephalopathy
Szakmar E1, Sunwoo J3, Cherkerzian S1, Kim S1, Hannon K1, Munster C1, Garvey A4, Elshibiny H1, Lee S1, Ben-David D1, Franceschini M3, Robinson J1, Christou H1, Inder T1, El-Dib M1
1Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA, 2Division Of Neonatology, 1st Department Of Pediatrics, Semmelweis University, Budapest, Hu, Budapest, Hungary, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School,, Boston, USA, 4INFANT Research Centre, Cork, Ireland; Department of Paediatrics and Child Health, University College Cork, Cork, Ireland
BACKGROUND: Carbon dioxide is one of the most potent regulators of cerebral blood flow (CBF). Near infrared spectroscopy has been used in neonatal encephalopathy (NE) during therapeutic hypothermia (TH) to monitor regional cerebral oxygen saturation (rSO2) which can be a proxy for CBF. Impaired cerebral autoregulation following severe hypoxic-ischemic brain injury has been associated with poor outcome. The aim of this study was to evaluate the correlation between transcutaneous carbon dioxide (tcPCO2) and markers of cerebral oxygenation (rSO2 and fractional tissue oxygen extraction, FTOE) among infants with NE.
METHODS: This prospective observational single center trial enrolled infants, who received TH for mild to severe NE between October 2019 and June 2022. We identified 2 groups of patients with or without any brain injury based on the assessment of MRI after rewarming. The pattern of brain injury was evaluated according to an MRI grading system developed by Weeke et. al. tcPCO2, rSO2 and bedside physiological data were captured simultaneously by a real-time integrated neuromonitoring system (CNS Monitor). FTOE was calculated as (SpO2 – rSO2)/SpO2. The median of tcPCO2, rSO2 and FTOE at each hour was calculated for each infant. Using these values, we calculated the median of each variable in each of seven phases of TH for each infant. Pearson correlation coefficients (Pearson’s r) between median levels of tcPCO2 and oxygenation (rSO2 and FTOE) were computed within each phase (Figure 1). Correlation between tcPCO2 and oxygenation (rSO2 and FTOE) across the study period by brain injury status was calculated using repeated measures correlation to take into account intra-individual correlation among medians of the different phases (Figure 2).
RESULTS: Data of 31 infants captured by CNS Monitor were analyzed. Integrated monitoring began at median 24 hours of life (HOL, min-max: 11-51) and ended at median 94 HOL (min-max: 87-120). Sixteen (52 %) infants presented with any brain injury on MRI. Correlations between tcPCO2 and measures of oxygenation were small throughout the phases of TH, with positive correlations with rSO2 and negative with FTOE (Figure 1). Though small in effect size, correlations were stronger among infants with brain injury (tcPCO2/rSO2 r= 0.19; tcPCO2/FTOE r= -0.15) compared to those without brain injury on MRI (tcPCO2/rSO2 r= 0.08; tcPCO2/FTOE r=-0.04) (Figure 2).
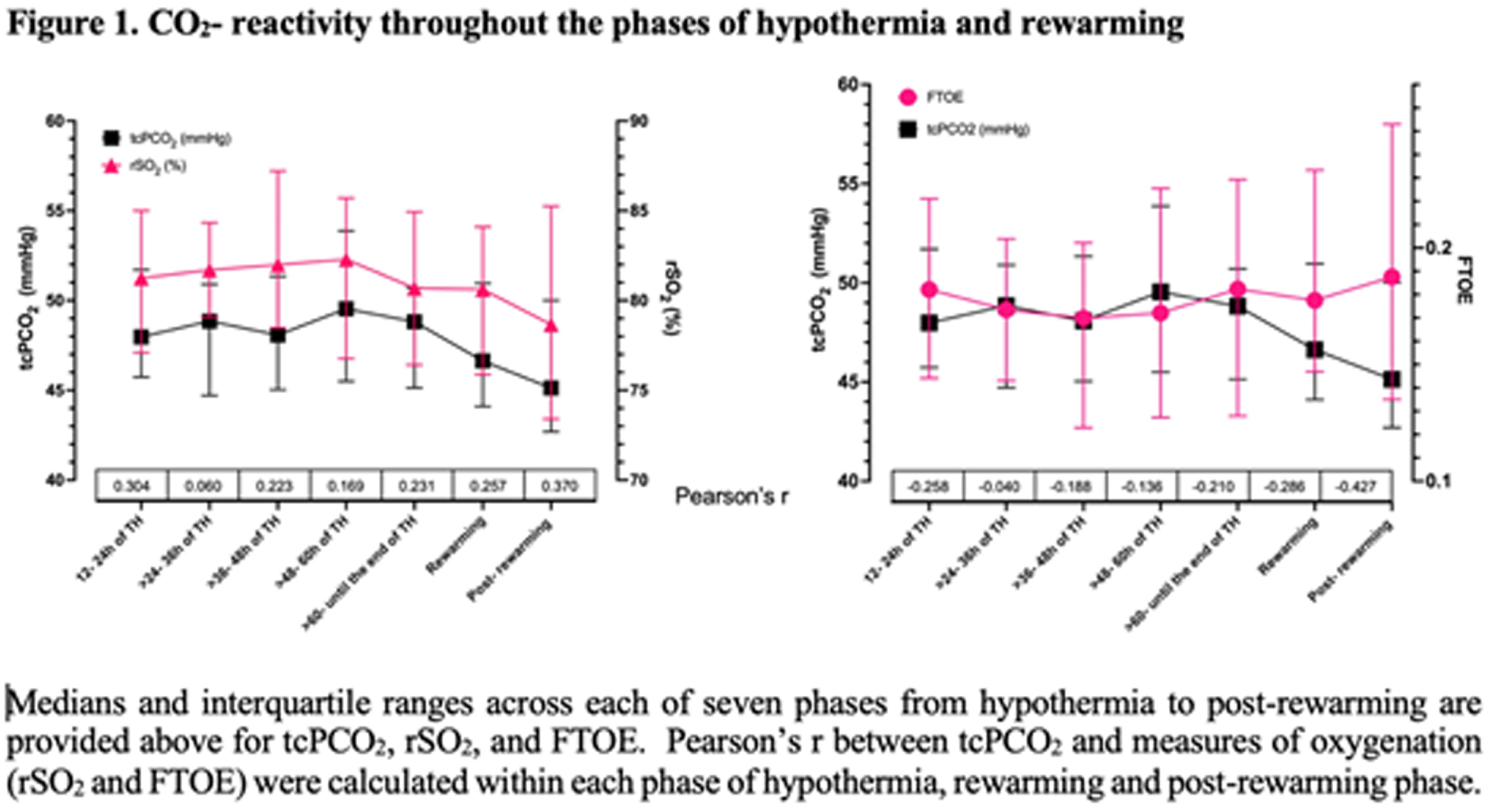
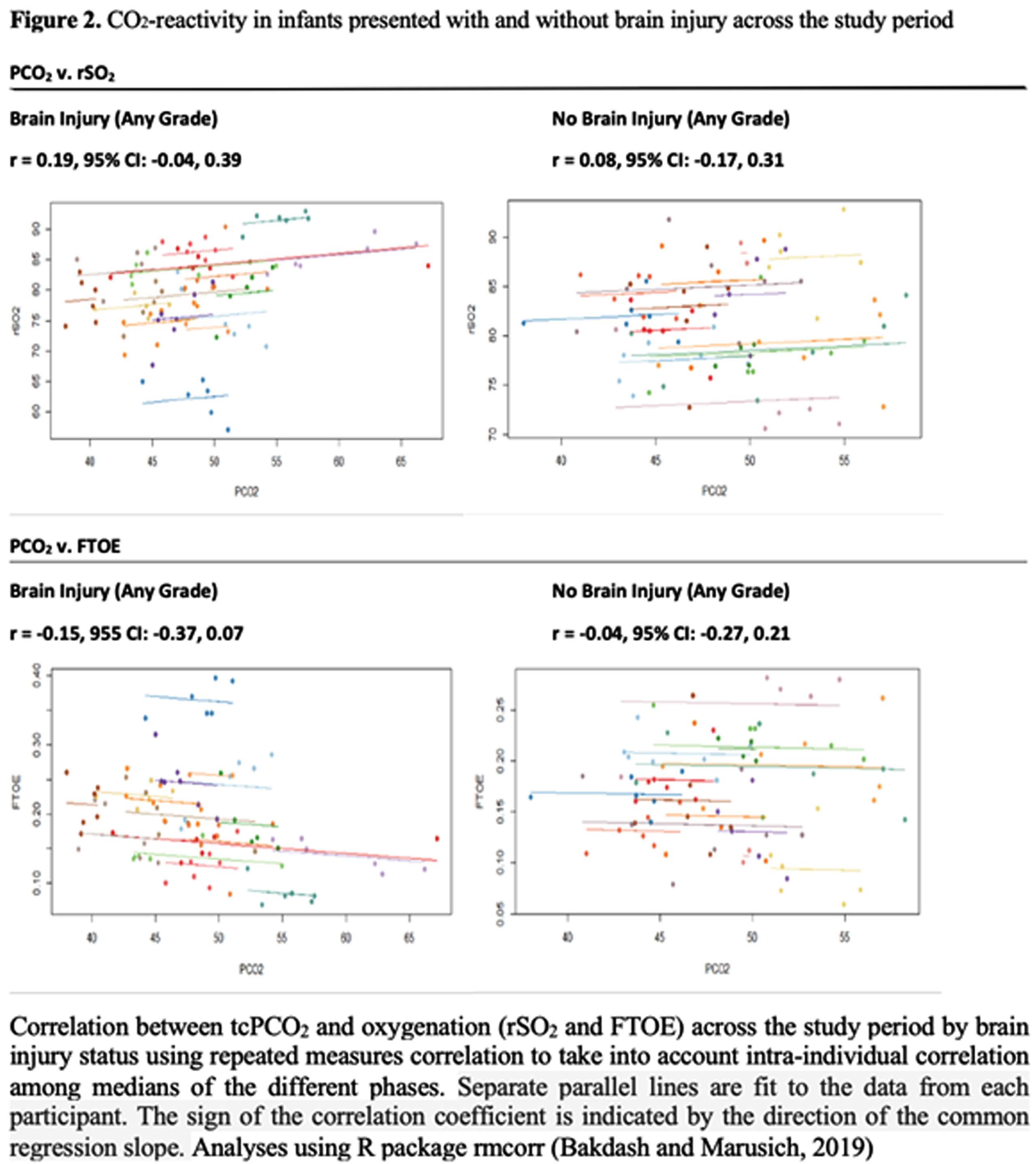
CONCLUSIONS: Correlations detected between CO2 and cerebral oxygenation were modest with a positive correlation with rSO2 and negative correlation with FTOE throughout the phases. Though small in effect size, the correlation between tcPCO2 and oxygenation (rSO2 and FTOE) over the study period was greater in magnitude among infants with brain injury compared to those without, among whom the correlation was close to zero. These pilot results need to be interpreted with caution, increasing sample size and further investigations are needed.
REFERENCES
[1] Pryds O, Greisen G, Lou H, Friis-Hansen.Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990; 117:119-125.
[2] Weeke LC, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr. 2018; 192:33-40 e32.
[3] Bakdash JZ, Marusich LR. Repeated Measures Correlation. Front Psychol. 2017 Apr 7;8:456. doi: 10.3389/fpsyg.2017.00456. Erratum in: Front Psychol. 2019 May 28;10:1201.
Does packed red blood cell transfusion (PRBCT) affect cerebral tissue oxygenation (TO) measured by near infra-red spectroscopy (NIRS) in extreme preterm neonates? A decomposing time series model analysis of minute-to-minute data
Anbu Chakkarapani A1, Anbu Chakkarapani A2, Yajamanyam P1, Soni N1, Ali S1, More K1, Elanbari M3, Jamil A3, Khalifa A3, Jilson T3, Gupta S1
1Attending Physician, Division of Neonatology, Sidra Medicine, Doha, Qatar, 2Assistant Professor, Department of Pediatrics, Weill Cornell Medicine, Doha, Qatar, 3Department of Research, Sidra Medicine, Doha, Qatar
BACKGROUND AND PURPOSE: PRBCT is a common neonatal intensive care unit (NICU) intervention. NIRS measures regional tissue oxygen saturation non-invasively (Figure 1) and continuously, reflecting the oxygen saturation in a mixed vascular bed dominated primarily by venules. Our null hypothesis is that NIRS-measured cerebral, renal, intestine, and skeletal muscle TO parameters do not trend linearly before, during, and after PRBCT in a decomposing time series model.

OBJECTIVE: To study the real-time minute-to-minute data points from NIRS to find the PRBCT effect on TO measurements from the cerebral, renal, intestine, and skeletal muscle.
METHODOLOGY: This observational, prospective cohort pilot study was conducted in a state-of-the-art Level IV NICU from May 1, 2021, to April 30, 2022. This institutional review board approved the study after parental consent. We recruited hemodynamically stable preterms born < 28 weeks gestational age (GA) who need elective PRBCT during their NICU stay. Babies with co-existing complex congenital heart disease and those given PRBC due to an emergency were excluded. The study team collected real-time minute-to-minute data from four-channel NIRS (INVOSTM 5100C) for a minimum of 4 hours before, 3 hours during, and 24 hours after PRBCT (study duration 31 hours). The primary outcome was cerebral TO. Secondary outcomes were renal, intestine, and skeletal muscle TO.
RESULTS: Among 20 preterm infants included in this study, 11 of them were <26-week GA at birth (mean + SD: 23.6 + 0.5), and 9 of them were between 26-to-28-week GA at birth (mean + SD: 26.2 + 0.9). There were 30% preterms with more than grade 2 intraventricular hemorrhage (n=6/20) and 20% preterms with post-hemorrhagic hydrocephalus (n=4/20). Pre and post-PRBCT hemoglobin levels are shown in Figure 2. We used the decompose function from the R package stats to decompose the time series of the four TO parameters (n=1860 x 4 =7440 per baby (Figure 3)) over the study period of 31 hours (overall data points for the total study population, n=148,800 (20x7440)). Finally, we used the presence/absence of linear trend data for all subjects and all TO parameters to generate a heatmap (Figure 4) that summarized similarities between subjects and parameters. Cerebral TO is the most significant in 85% (n=17/20) of the study population, followed by Renal TO (70%), Skeletal Muscle TO (55%), and Intestine TO (50%).
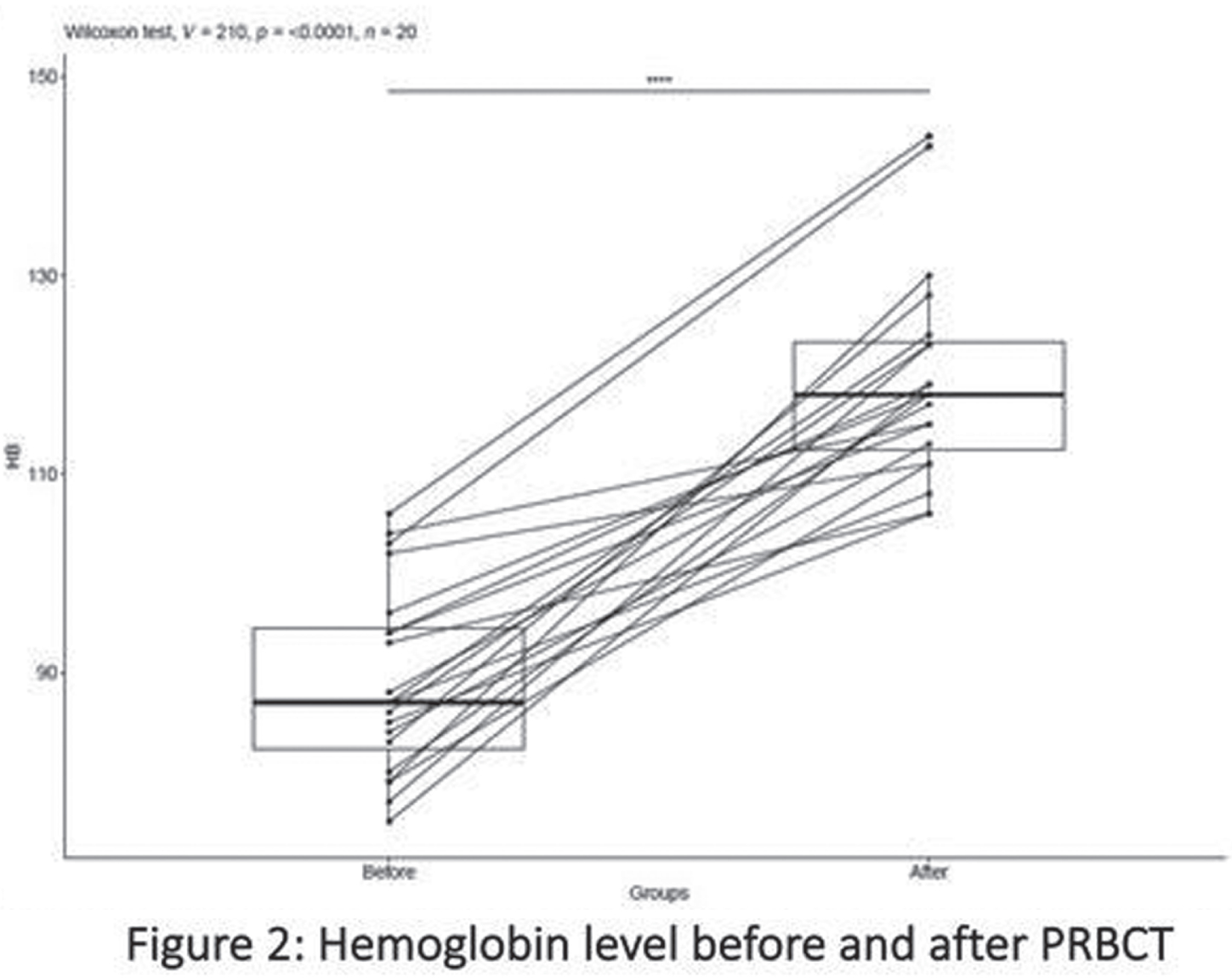
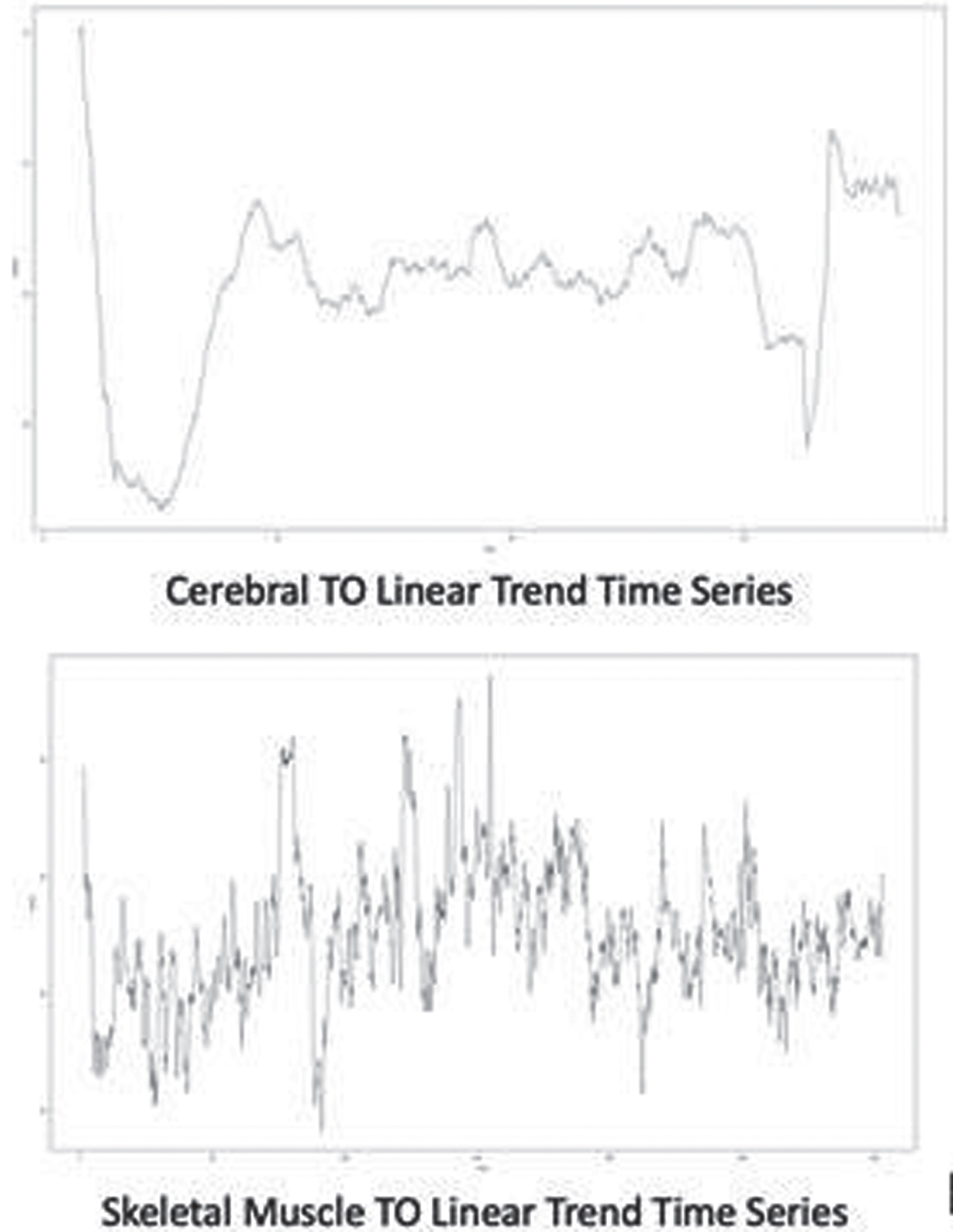
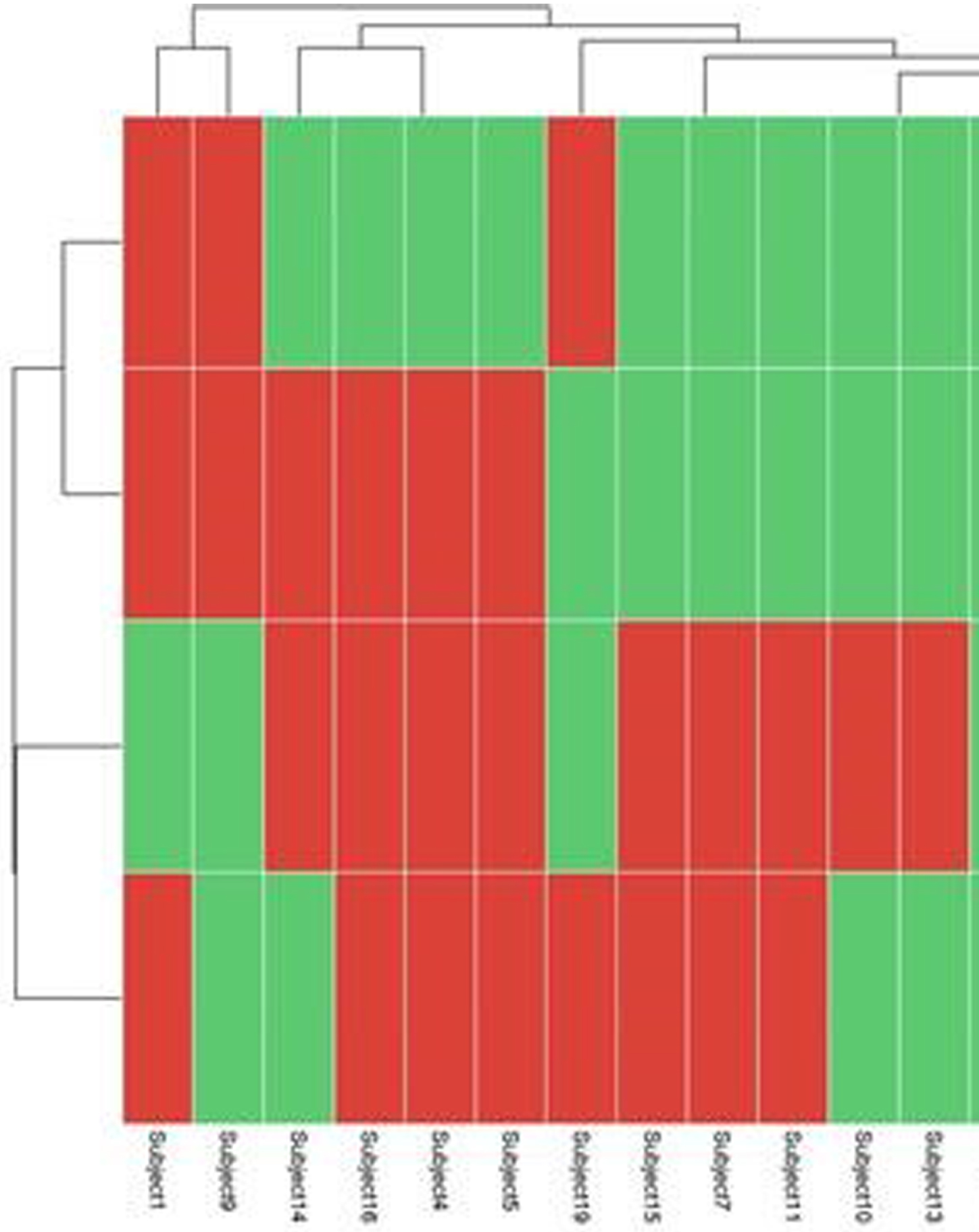
CONCLUSION: In this pilot study, we found that our primary study outcome, Cerebral TO measure by NIRS, significantly changed in most preterm infants before, during, and after PRBCT on a linear trend time series model.
REFERENCES
[1] Sadaka F. The effect of RBC transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann Intensive Care. 2011;1(1):46
[2] Kisilevsky A. Anaemia and RBC transfusion in intracranial neurosurgery: a comprehensive review. Br J Anaesth. 2018;120(5):988-98.
Fetal cardiac hemodynamics and neonatal cerebral hemodynamics and oxygen metabolism in transposition of the great arteries
Charbonneau L1,2, Arman Chowdhury R1,3, Marandyuk B1, Wu R4, Poirier N5, Miró J4,6, Nuyt A1,7, Raboisson M4,6, Dehaes M1,3,8
1Research Centre, CHU Sainte-Justine Hospital University Centre, Montreal (QC), Canada, 2Department of Biomedical Sciences, University of Montreal, Montreal (QC), Canada, 3Institute of Biomedical Engineering, University of Montreal, Montreal (QC), Canada, 4Department of Fetal Cardiology, CHU Sainte-Justine Hospital University Centre, Montreal (QC), Canada, 5Department of Cardiac Surgery, University of Montreal, Montreal (QC), Canada, 6Division of Pediatric Cardiology, University of Montreal, Montreal (QC), Canada, 7Division of Neonatology, Department of Pediatrics, University of Montreal, Montreal (QC), Canada, 8Department of Radiology, Radio-oncology and Nuclear Medicine, University of Montreal, Montreal (QC), Canada
BACKGROUND AND PURPOSE: Hemodynamics abnormalities and brain development disorders were previously reported in fetuses and children with transposition of the great arteries (TGA) and intact ventricular septum (TGA&IVS). A ventricular septal defect (VSD) is thought to be an additional risk factor for brain development, but literature describing this population is sparse. The objectives were to (1) assess fetal cardiac hemodynamics throughout pregnancy, (2) monitor cerebral hemodynamics and oxygen metabolism in neonates, and (3) compare these data between TGA&IVS, TGA&VSD and age-matched controls.
METHODOLOGY. Cardiac hemodynamics were assessed in TGA&IVS and TGA&VSD fetuses and compared with healthy controls matched for gestational age (GA) during three periods: ≤ 22.5 (GA1), 27 to 32.5 (GA2), and ≥34.5 (GA3) weeks GA. Left and right ventricular outputs (LVO and RVO, respectively), combined ventricular output (CVO), ductus arteriosus flow (DAF), diastolic DAF, transpulmonary flow (TPF), and foramen ovale (FO) diameter were measured. Aortic (AoF) and main pulmonary artery flows (MPAF) were derived in % of CVO. Bedside optical brain monitoring was used to measure cerebral hemoglobin oxygen saturation (SO2) and an index of microvascular cerebral blood flow (CBFi) along with peripheral arterial oxygen saturation (SpO2) in TGA&IVS and TGA&VSD neonates. Using hemoglobin (HGB) concentration measurements, these parameters were used to derive cerebral oxygen delivery and extraction fraction (OEF) as well as an index of cerebral oxygen metabolism (CMRO2i). These data were acquired in the early preoperative period (within 3 days of age and post Rashkind) and compared with age-matched healthy controls, as well as before discharge when vital signs were stable.
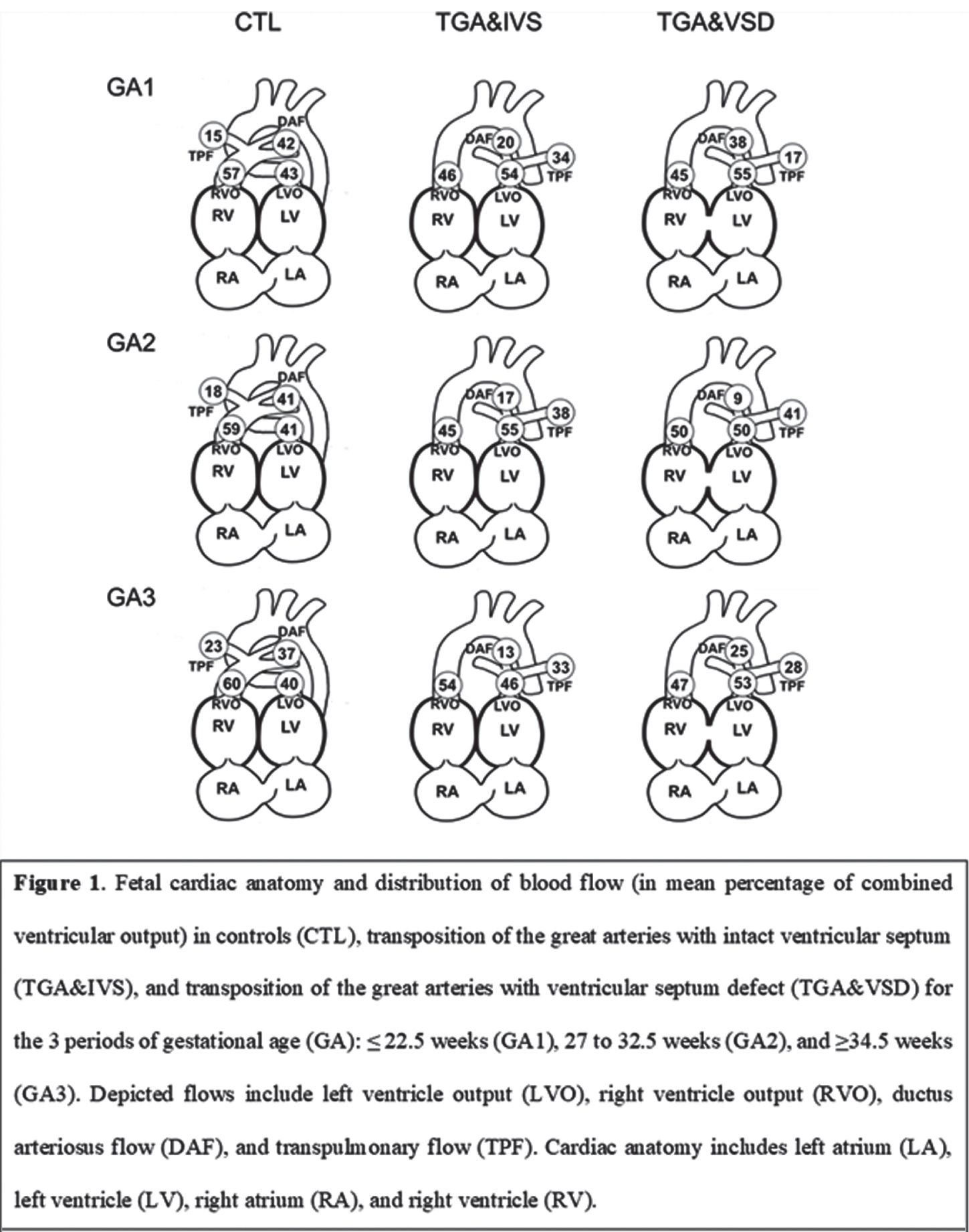
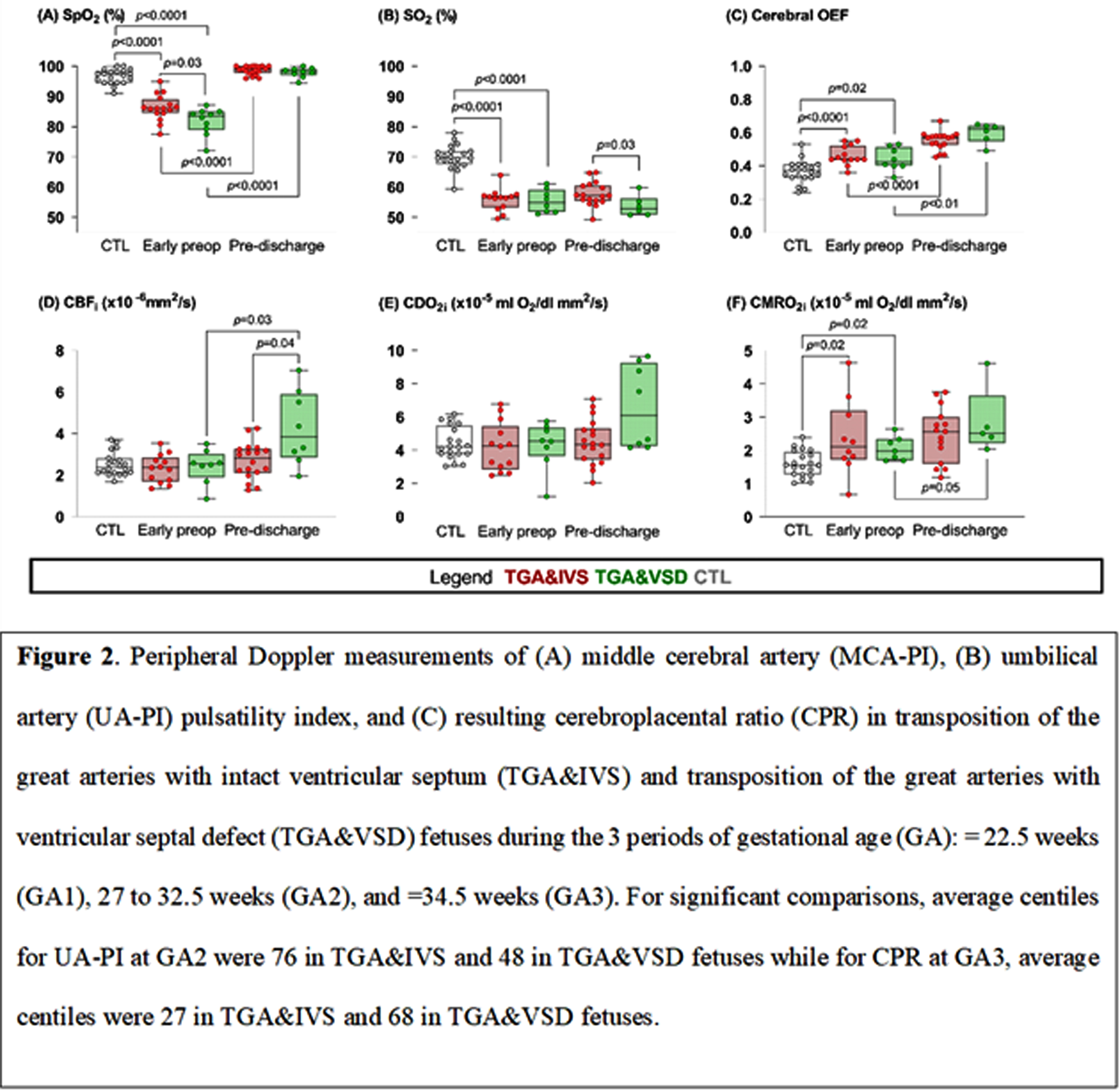
RESULTS: LVO was increased in both TGA groups compared to controls throughout pregnancy. Transpulmonary flow was increased while diastolic DAF was decreased compared to controls throughout pregnancy in TGA&IVS and during GA1 and GA2 in TGA&VSD. Compared to controls, DAF was decreased in TGA&IVS throughout pregnancy and in GA2 and GA3 for TGA&VSD. At GA2, AoF was higher in TGA&IVS and TGA&VSD compared to controls when MPAF was lower. Compared to TGA&VSD at GA3, RVO and CVO were higher while FO diameter was smaller in TGA&IVS at GA2. Within 3 days of age, SpO2 and SO2 were lower while HGB, cerebral OEF, and CMRO2i higher in both TGA groups compared to controls. Preoperative SpO2 was also lower in TGA&VSD compared to TGA&IVS. From preoperative to pre-discharge periods, SpO2 and OEF increased in both TGA groups, but CBFi and CMRO2i increased only in TGA&VSD. At pre-discharge, SO2 was higher in TGA&IVS compared to TGA&VSD while CBFi lower.
CONCLUSION: Fetal cardiac and neonatal cerebral hemodynamic alterations were observed in both TGA groups compared to controls. Compared to TGA&IVS, fetuses with TGA&VSD had lower RVO and CVO at GA3 and smaller FO diameter at GA2. A higher level of preoperative hypoxemia was observed in TGA&VSD.




