Proceedings of the 14th International Newborn Brain Conference: Neuroprotection strategies in the neonate
Breast milk stem cells as a new source for stem cells therapy in neonatal brain injury
Cornaz Buros S1, Fernandes Trigo N3, Steinlin M1, Schöberlein A2, Ganal S3, Klein A1
1Neuropediatrics, Kinderklinik, Inselspital, Bern, Switzerland, 2Departement Geburtshilfe und Gynäkologie, Labor für Pränatale Medizin & Geburtshilfe und Feto-maternale Medizin, Inselspital, Bern, Switzerland, 3Department for BioMedical Research, Inselspital, Bern, Switzerland
BACKGROUND AND PURPOSE: Human Breast milk is universally recognized as the best-adapted food for infants and toddlers. Although the chemical composition of breast milk has been extensively studied, recent evidence, describe the presence of a cellular subpopulation having stem cell properties. Neonatal cerebral lesions resulting from neonatal stroke, asphyxia and intracerebral hemorrhage in preterm infants are a major cause of disability in children worldwide. Because of the poor regenerative potential of brain tissue, few therapeutic options are currently available and stem cell therapy constitutes an avenue of hope for the future. However, such therapy is costly and requires sophisticated technology. Breast milk stem cells constitute an attractive, cost effective and universally available alternative. These cells would constitute ideal candidates for stem cell therapy in newborn but are still partly characterized.
AIM:
1. Characterize breast milk cell populations at the single cell level, determine the heterogeneity of its population and assess the proportion of cells that display stem cell features.
2. Assess through In vitro and In vivo assay the potential of breast milk derived stem cell in regenerative medicine after perinatal brain insult (perinatal stroke/birth asphyxia/intracerebral bleeding)
MATERIAL AND METHOD:
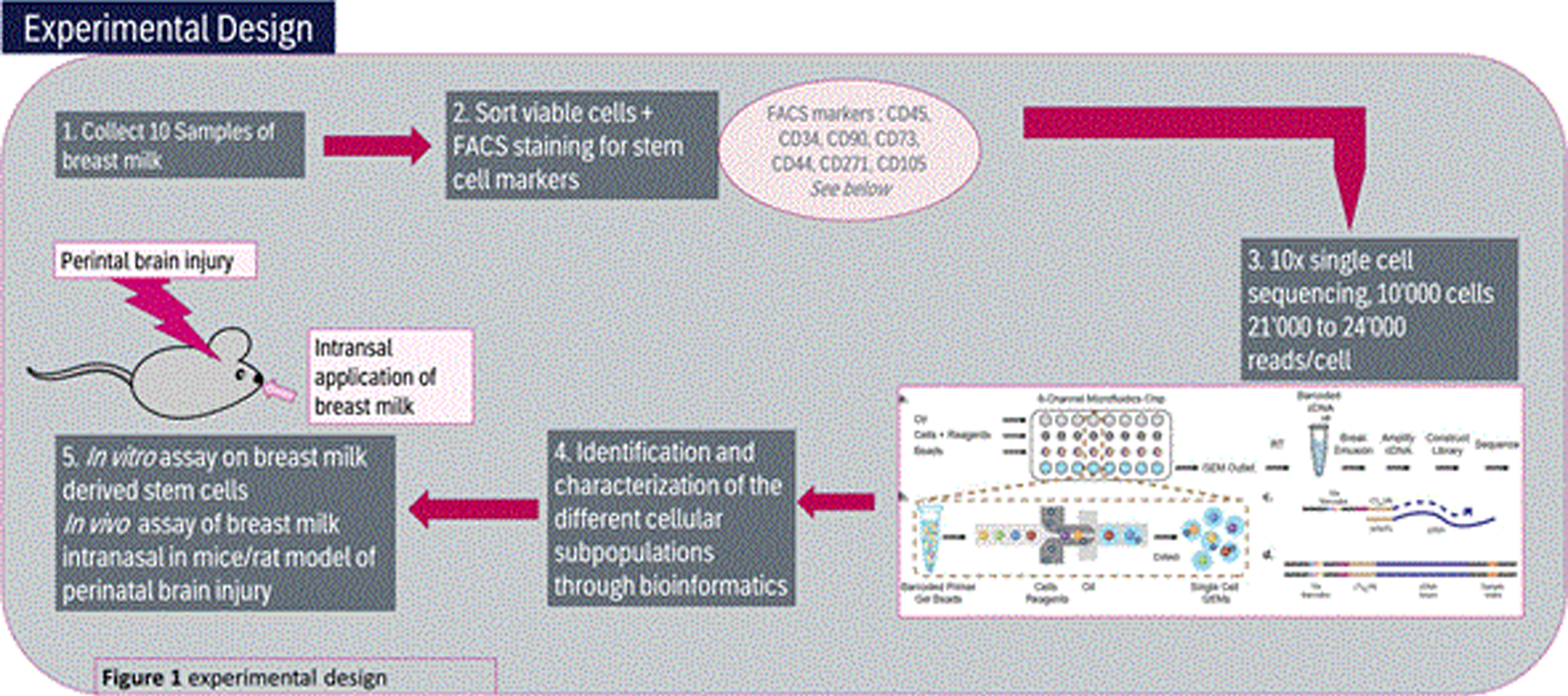
- Collect 10 Samples of fresh breast milk samples day 10 post-partum, perform FACS staining for stem cell marker and proceed to 10x single cell sequencing for 10’000 cells/20’000 reads/cell.
- Identify and characterization the different cellular subpopulations through bioinformatics analysis
- Perform in vitro assay on breast milk derived stem cells as well as in vivo assay of breast milk intranasal in mice/rat model of perinatal brain injury
(See also Figure 1)
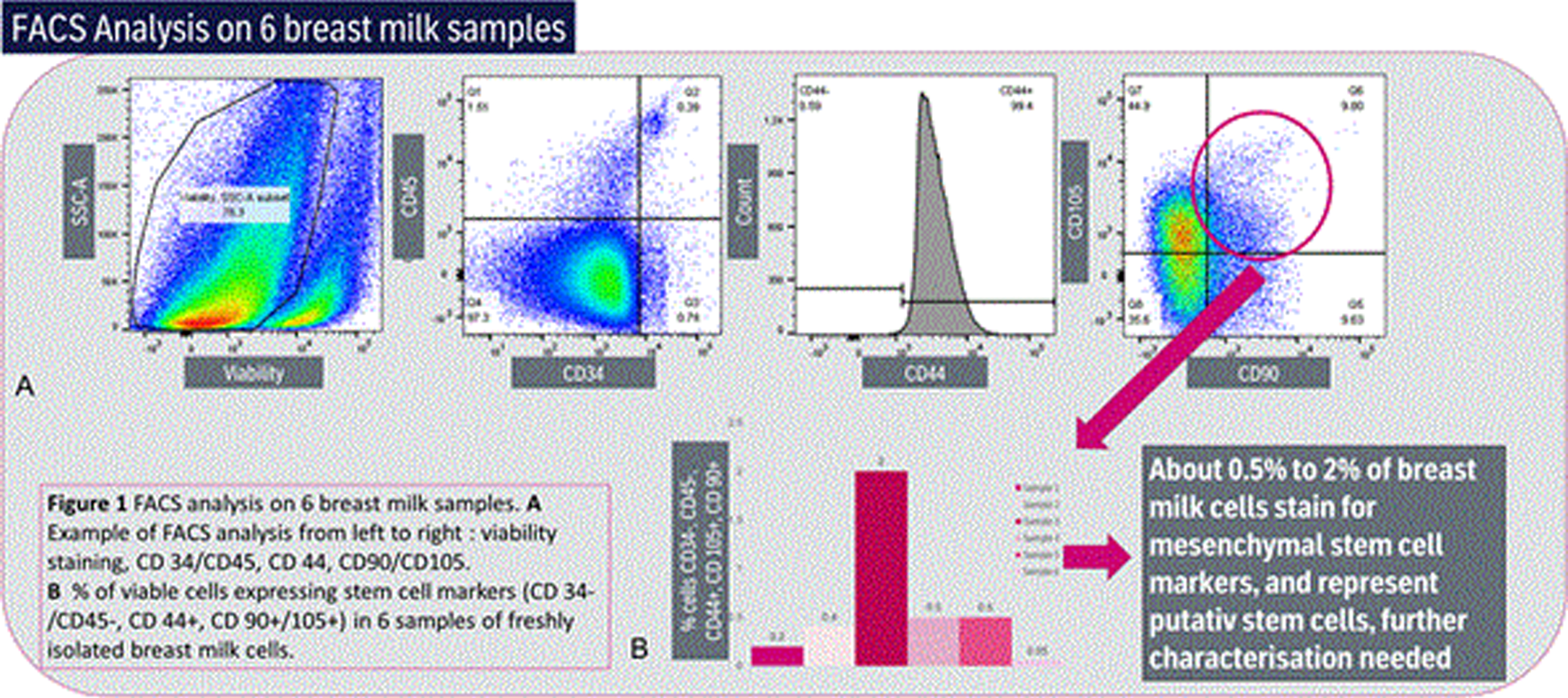
RESULTS: About 0.5% to 2% of breast milk cells stain for mesenchymal stem cell markers, and represent putative stem cells, further characterization needed (See Figure 2)
CONCLUSION AND IMPACT: If the presence of breast milk stem cells is confirmed by single cell sequencing, these cells could represent a major breakthrough in neonatal and neuro-regenerative neurology as they would represent readily available, cheap alternatives to umbilical cord stem cells and could be administered safely via the intranasal route.
REFERENCES
[1] van Velthoven et al. Mesenchymal Stem Cell Transplantation Attenuates Brain Injury After Neonatal Stroke. Stroke. 2013; 44(5): 1426–1432. doi: 10.1161/STROKEAHA.111.000326.
[2] Victora C. G. et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. The Lancet. 2016; 387(10017): 475–490. doi: 10.1016/S0140-6736(15)01024-7.
[3] Hassiotou F. et al. At the Dawn of a New Discovery: The Potential of Breast Milk Stem Cells. Advances in Nutrition. 2014; 5(6): 770–778. doi: 10.3945/an.114.006924
FGF21 therapy alters hippocampal CA2 proteins in a murine model of perinatal asphyxia
Jackson T1, Herrmann J2, Gorse K1, Vagni V2, Janesko-Feldman K2, Kochanek P2
1USF Health Heart Institute, University Of South Florida, Tampa, United States, 2Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, United States
BACKGROUND/PURPOSE: The cold-stress hormone fibroblast growth factor 21 (FGF21) is neuroprotective in the Rice-Vannucci model of perinatal asphyxia (PA) and improves long-term neurological outcome [1]. The beneficial mechanisms remain undefined but may involve a novel FGF21-CA2-hippocampal signaling axis [2]. Thus, we hypothesized that FGF21 therapy might alter CA2 subregion-specific signaling proteins in PA.
METHODOLOGY: We used our protocol of hypoxia-ischemia (HI) in newborn mice [3]. The right common carotid artery (CCA) was ligated in PND10 female pups followed by 25min of 8% O2. Age-matched shams received procedures without HI. Temperature was maintained to 36°C (age-appropriate normothermia). Mice (n=8/group) received subcutaneous injection of vehicle or 1.5mg/kg FGF21 at 5min post-HI, and the hippocampus was collected 24h later for protein analysis. A second cohort (n=8/group) received four doses of vehicle or FGF21 in 24h intervals and the hippocampus was collected at 4d post-injury. CA2-specific marker proteins (RGS14, PCP4, and NECAB2) were analyzed (Western blot). Normalized densitometric data were analyzed by Kruskal-Wallis and Dunn’s post-hoc test. Data were significant at p<0.05.
RESULTS: Hippocampal RGS14 and PCP4 levels were not different in vehicle-treated aged-matched shams vs. vehicle-treated HI-injured pups at 24h post-injury (p=0.10 and p=0.26) or 4d post-injury (p=0.29 and p>0.99). NECAB2 significantly decreased at 24h (p<0.01) and increased at 4d (p<0.01) post-injury. FGF21 therapy did not affect NECAB2 but decreased RGS14 (p<0.01) and increased PCP4 levels (p<0.01) at 24h post-injury. Conversely FGF21 therapy decreased PCP4 by 4d post-injury (p<0.01) vs. vehicle.
CONCLUSION: NECAB2 levels changed dynamically after HI suggesting its promise as a novel injury marker. FGF21 decreased RGS14 and increased PCP4 levels early post-injury. RGS14 inhibits plasticity and learning and memory in rodents [4], while PCP4 is neuroprotective [5]. Thus, FGF21 altered both proteins in a potentially beneficial manner post-PA. Our data support its further pre-clinical investigation in PA.
REFERENCES
[1] Ye L, Wang X, Cai C, et al. FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/β-klotho. Exp Neurol. 2019;317:34-50
[2] Forsström S, Jackson CB, Carroll CJ, et al. Fibroblast Growth Factor 21 Drives Dynamics of Local and Systemic Stress Responses in Mitochondrial Myopathy with mtDNA Deletions. Cell Metab. 2019;30(6):1040-1054.e7
[3] Jackson TC, Herrmann JR, Garman RH, et al. Hypoxia-ischemia-mediated effects on neurodevelopmentally regulated cold-shock proteins in neonatal mice under strict temperature control. Pediatr Res. 2022;10.1038/s41390-022-01990-4
[4] Lee SE, Simons SB, Heldt SA, et al. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci U S A. 2010;107(39):16994-16998
[5] Kanazawa Y, Makino M, Morishima Y, et al. Degradation of PEP-19, a calmodulin-binding protein, by calpain is implicated in neuronal cell death induced by intracellular Ca2+ overload. Neuroscience. 2008;154(2):473-481.
An observational study on the neurodevelopment and growth of term low birth weight neonates born from January to February 2021 in Zamboanga city medical center after oral zinc supplementation
Abdurajan S1
1Zamboanga City Medical Center, Zamboanga City, Philippines
BACKGROUND: Zinc is an important element which has a major role in the growth of neonates. It is essential for both tissue growth and neurological development. Infants of low birth weight do not have enough zinc storage, which may lead to undernourishment and poor development for these neonates. Thus, zinc supplement was provided on a number of low birth weight neonates while keeping a record of their growth and developmental status.
PURPOSE: This study aimed to describe the neuro-development and growth status of low birth weight neonates given oral zinc supplementation at Zamboanga City Medical Center.
METHODOLOGY: This is a descriptive cohort study of low-birth-weight neonates recruited on the first day of life and was given oral zinc supplementation at a dose of 2.1 mg/day daily for 6 weeks.
RESULT: The study showed that among the neonates who received zinc supplementation, there is an increase of weight from baseline, 47 percent increase on the first monitoring at 6 weeks of age and 254 percent on the second monitoring at 9 months of age. Also, these neonates had 14 percent and 40 percent increase in length at six weeks of age and nine months of age on the first and second monitoring respectively. Females had more weight gain while male neonates had higher length gain. All of the infants had no neuro-developmental concerns.
CONCLUSION: Low birthweight neonates given zinc supplementation were able to achieve expected growth and neurodevelopment for age.
KEYWORDS: low birth weight neonates, zinc supplementation, neuro-development, z score, maternal and child health, nutrition
Intranasal administration of a p75Ntr small molecule ligand as a delayed therapy for cholinergic forebrain system rescue after Hypoxic-Ischemia (HI) in mice: Feasibility and pharmacological studies
Kuter N1, Doucette L1, Turnbill V1, Flock D1, Chen M1, Alt J2, Chavez-Valdez R1, Jantzie L1, Rais R2, Martin L3, Northington F1
1Division of Neonatal-Perinatal Medicine, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, United States, 2Johns Hopkins Drug Discovery, Johns Hopkins School of Medicine, Baltimore, MD, Baltimore, United States, 3Departments of Neuroscience and Pathology, Johns Hopkins School of Medicine, Baltimore, United States
BACKGROUND: Memory and executive function are affected in child survivors of neonatal hypoxic-ischemic encephalopathy (HIE) despite treatment with therapeutic hypothermia. Forebrain cholinergic systems control these functions. p75 neurotrophin receptor (p75Ntr) modulates survival of adult cholinergic basal forebrain (cBF) neurons. We have found that cBF neurons are severely affected in a neonatal mouse model of HI. LM11A-31 is an orally deliverable, brain-penetrant, small molecule p75Ntr ligand with neuroprotective effects in mouse models of adult neurodegenerative disease. These properties make LM11A-31 an attractive drug candidate for late treatment of HI.
METHODS: Immunohistochemistry and Western Blot for p75Ntr were performed to determine p75Ntr expression in target neuron populations. Mouse pups (n=6/group) were administered LM11A-31 orally (100 mg/kg) or intranasally (50 mg/kg) at P29 and were sacrificed 15 min or 1 hour after treatment to establish whether LM11A-31 can be administered orally or intranasally in neonatal mice and whether it reaches cortical levels reported to be neuroprotective. LM11A-31 was administered intranasally (50 mg/kg) daily at P14-25 and P14-60 (n=9) to assess peak cortical and plasma concentrations with chronic dosing. Rice-Vannuci neonatal HI model was performed at P10 and cortical drug levels were compared between HI and naive mice. Plasma and cortical tissue levels of LM11A-31 were measured by liquid chromatography-tandem mass spectrometry.
RESULTS: P75Ntr was robustly expressed in cholinergic neurons in medial septal nucleus, peri-pallidum and nucleus basalis of Meynert and in crude homogenates of cortical tissue. With oral dosing, LM11A-31 levels at 15 min and 60 min were 7.5 and 1.6 nmol/ml in plasma; 1.3 and 0.83 nmol/g in cortex. Intranasal LM11A-31 levels at 15 min and 60 min were 6.1 and 1.2 nmol/ml in plasma; 0.74 and 1.4 nmol/g in cortex. Both treatment routes achieved therapeutic cortical levels at 1h with intranasal administration resulting in higher brain levels with a lower drug dose. Chronic drug administration achieved similar cortical levels to single dose administrations and there was no difference between levels in naive and HI mice. Cortical drug levels (0.96 nmol/g) were significantly higher than olfactory bulb levels (0.71 nmol/g) with chronic intranasal dosing (p<0.05). No deaths occurred during LM11A-31. Post-weaning, LM11A-31 treated HI mice weighed less than sham mice (p<0.05).
CONCLUSIONS: p75Ntr in the cBF is targetable in young mice with LM11A-31. LM11A-31 does not increase mortality and does not accumulate in tissues with chronic dosing. LM11A-31 after HI may result in slower weight gain post-weaning. Intranasal dosing of LM11A-31 after neonatal HI is feasible, generates levels in brain at or above target levels, even after HI, and if shown to be safe and efficacious could be a promising late and simple treatment for neonatal HI.
REFERENCES
[1] Northington F. J., et al. J CompNeurol. 2021.
[2] Xie Y., et al. Sci. Rep. 2019.
[3] Knowles J.K. et al. Neurobiol Aging. 2013.
Is lactate neuroprotective? Effects of lactate on acute seizure activity in neonatal hypoxic-ischemic brain injury
June A1, Matysik W1, Marlicz M1, Burnsed J1
1University Of Virginia, 500 West Main St Apt A, United States
BACKGROUND AND PURPOSE: Hypoxic-ischemic encephalopathy (HIE) is a serious brain injury that causes significant morbidity and mortality in newborns worldwide and is the most common cause of neonatal seizures. Even with therapeutic hypothermia (TH), the only approved therapy for HIE, a high percentage of infants who survive HIE have persistent neurodevelopmental deficits, including epilepsy and cerebral palsy. Lactate is a known byproduct and clinical biomarker of anaerobic glycolysis that is elevated in neonates with HIE. Emerging research suggests that lactate may have a key neuroprotective role in neonatal HIE through interactions with the lactate receptor HCAR1 (hydroxycarboxylic acid receptor). Recent animal studies have shown that elevated lactate above physiologic levels decreases neuronal excitability, thus inhibiting seizure activity. Lactate and HCAR1 may therefore be promising novel therapeutic targets for neonatal HIE. This project aims to identify if lactate receptor HCAR1 is neuroprotective against acute seizure activity caused by neonatal hypoxic-ischemic (HI) brain injury. We hypothesize that knockout (KO) neonatal mice without the lactate receptor HCAR1 will exhibit a greater seizure burden.
METHODOLOGY: Wild-type (WT) and HCAR1 KO neonatal mice were stereotactically implanted with electroencephalography (EEG) headsets on post-natal day 9 (p9). On post-natal day 10 (p10), the mice were connected to a video EEG rig and exposed to hypoxia-ischemia using a modified Rice-Vannucci model. Video EEG recordings were obtained at baseline, during ligation recovery, 8% hypoxia, and post-hypoxia for a total duration of 3 hours per mouse.
RESULTS: Video EEGs were then analyzed for electrographic seizure activity including seizure number and duration in the WT (n = 9) and HCAR1 KO (n = 9) mice. The average time of individual seizures in the WT and HCAR1 KO group were 32 and 62 seconds, respectively (p < 0.001, see Fig 1). The severity of seizure behavior, qualified by a Behavioral Seizure Score (BSS), was more severe in the HCAR1 KO group versus the WT group (p < 0.001, see Fig 2). The seizure burden, defined as the number of seizures multipled by total seizure duration (s) per animal, was higher in the HCAR1 KO group compared to the WT group (p = 0.04, see Fig 3).
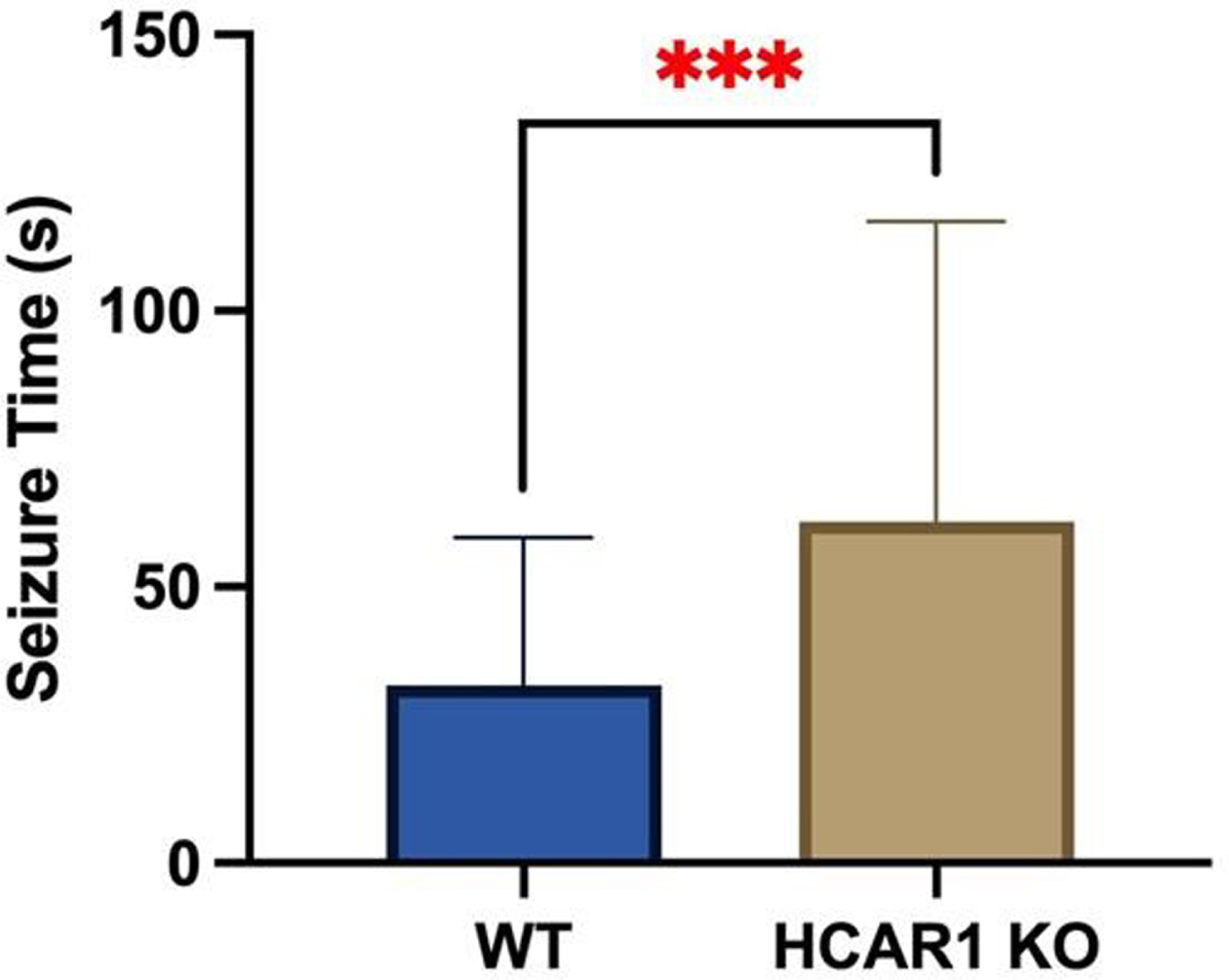
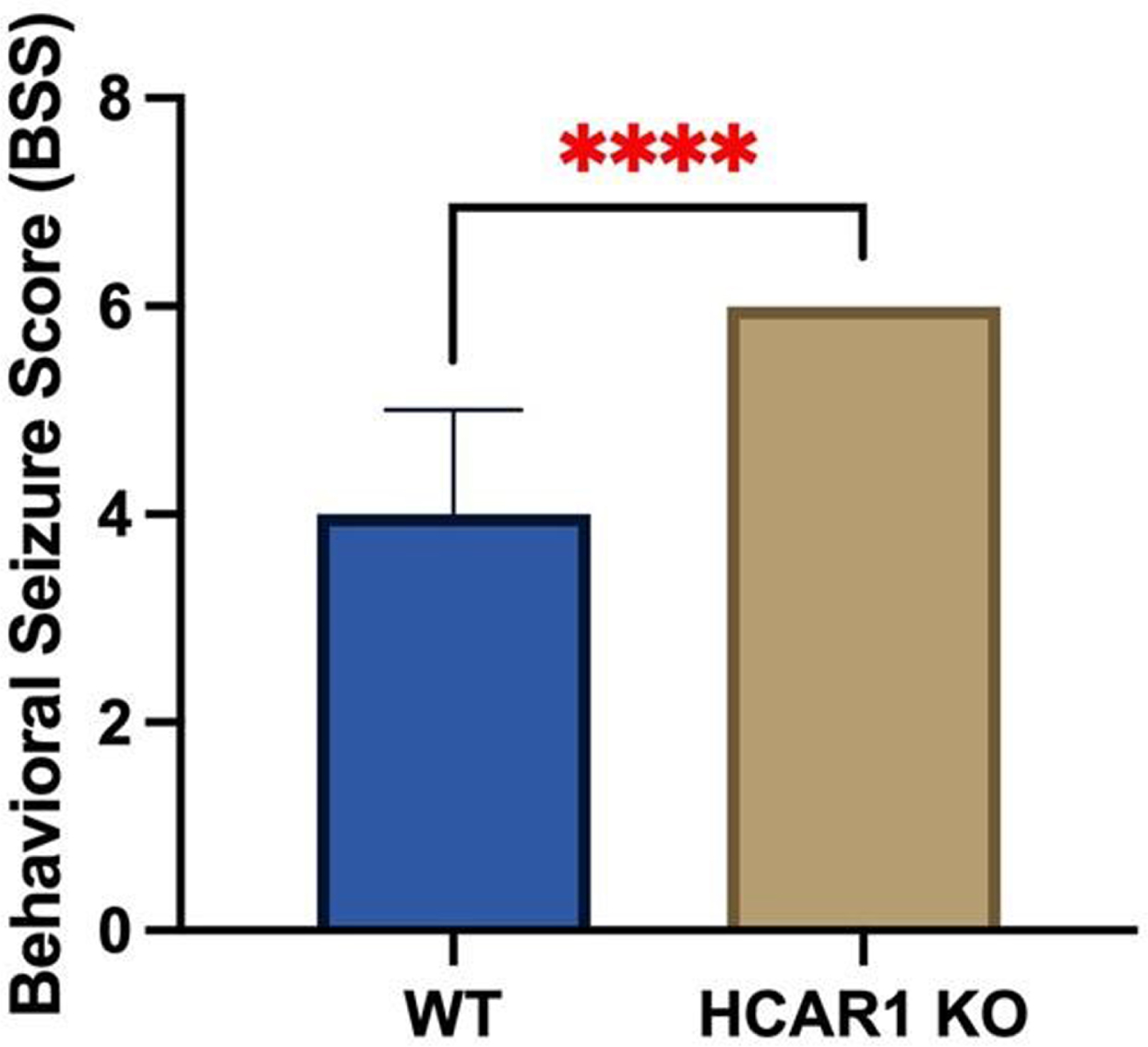
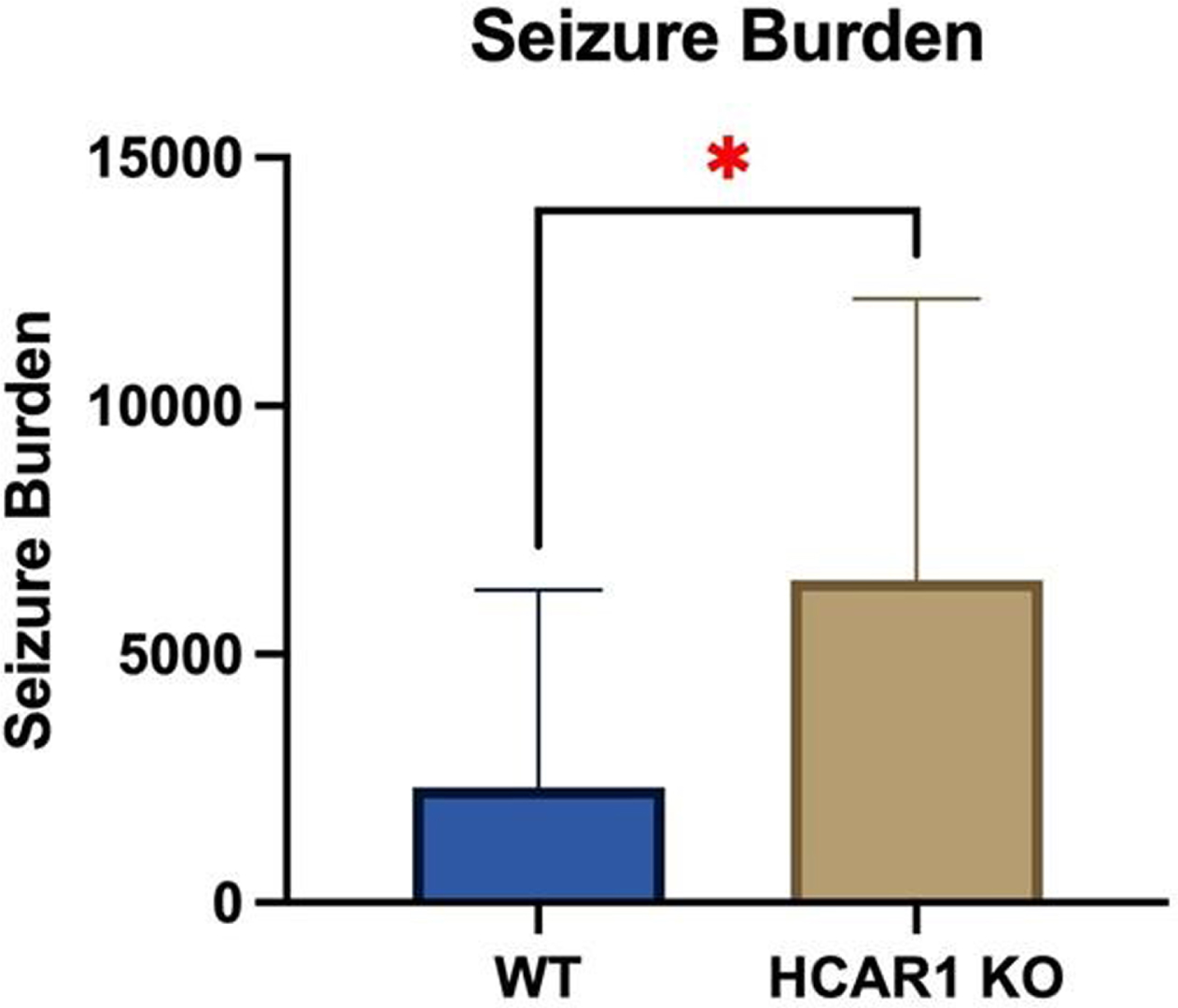
CONCLUSIONS: In a neonatal mouse model of HIE, HCAR1 KO mice have significantly longer and more severe acute seizure activity compared to WT mice. The effects of signaling pathways associated with the lactate receptor HCAR1 may be neuroprotective in neonatal HIE.
REFERENCES
[1] Abrantes H., et al. The Lactate Receptor HCAR1 Modulates Neuronal Network Activity through the Activation of G α and G βγ Subunits. J Neurosci. 2019; 39(23):4422-4433.
[2] Kang S.K., et al. Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters. Front Cell Neurosci. 2015; 9:1–16.
Staff’s attitudes towards parent-infant closeness and routines during therapeutic hypothermia
Bäcke P1, Axelin A2, Ågren J1, Thernström Blomqvist Y1
1Department Of Women’s And Children’s Health, Uppsala University, Uppsala, Sweden, 2Department of Nursing Science, University of Turku, Turku, Finland
BACKGROUND: Separation is a source of stress for both parents and infants during neonatal intensive care. Therapeutic hypothermia (TH) represents a particular challenge since skin-to-skin care is clearly not possible due to thermal management principles. However, creating a calm environment where unnecessary stimuli is minimized and needed stimuli, such as parental touch, voice and the taste of the mother’s breast milk, are still feasible and might be important to create a healing environment for the infant and support parent-infant bonding. The aim of this study was to investigate NICU staff’s attitudes towards parent-infant closeness and routines during TH.
MATERIALS AND METHODOLOGY: All Swedish NICUs providing TH (n=10) were invited and agreed to participate. Data were collected January-April 2021; a survey followed by a semi-structured interview, both with the registered nurse and the neonatologist responsible for neonatal neurology at each unit. The survey focused on different areas; Practical organization of the care space for TH, “living arrangements” for the parents, family integrated care practices, and staff estimates of parents’ participation. The interviews focused on the attitude towards parents’ involvement in their infants’ care and the parental presence at the NICU. In the material, meaning units were found, condensed, and formed into codes followed by grouping into sub-categories and categories.
RESULTS: Differences and similarities regarding practical approach during TH at the included NICUs were identified. The qualitative content analysis identified three categories: the importance of standardized routines, environmental influence on parent-infant closeness and practices around parent-infant closeness and involvement in care. All NICUs allowed parents to stay with their infant around the clock, which was a prerequisite for promoting parent-infant closeness. The staff described the importance of standardized routines and procedures regarding the preparation of the infants’ care space and how the care should be conducted for infants. Established routines worked time-efficient and staff-saving which also freed up time to focus on the families. The staff also stated that the NICU environment effected parental-infant closeness and presences. Furnishing with a parental bed instead of a crib had positive effect promoting closeness, encourage the parent’s presence and creating better conditions for the family to be together. All units were keen to encourage and support parents to be actively involved in the infants’ care.
CONCLUSION: Enabling parent-infant closeness and presences during the stay at the NICU is important, the TH-period is not an exception. This study found that standardized routines regarding the care space set up, medical and caring approach as well as the NICU environment and practices around the families can promote or curb the possibilities of parent-infant closeness and presences. A clear structure and good environmental conditions with room for flexibility regarding the family’s need is therefore needed.
Novel model of global cerebral ischemia induced by cardiac arrest reveals hippocampal dysfunction
Tiemeier E1, Dingman A1, Kathiresh S1, Quilinan N1, Dietz R1
1University Of Colorado Anschutz Medical Campus, Denver, United States
BACKGROUND: Hypoxic ischemic encephalopathy (HIE) results from global cerebral ischemia (GCI) at the perinatal timepoint and results in significant morbidity and mortality. Hippocampal injury and cognitive impairment produced by global ischemia in neonates affects significant developmental delays later in life. Current models of cerebral ischemia center around superior aortic artery occlusion followed by ischemia, producing imperfect, unsustained global injury or undesired secondary injury. To address these challenges, a novel rodent model of global cerebral ischemia was developed in neonatal rats.
METHODS: Global cerebral ischemia was induced using cardiac arrest. Cardiac arrest was initiated in post-natal day 10 rats were via intravenous 0.5M potassium chloride. Following 10-14-min of asystole, cardiopulmonary resuscitation CPR was performed with chest compressions and intravenous epinephrine with return of spontaneous resuscitation within 3 minutes. Left ventricular whole blood samples were collected and analyzed 30 minutes after reperfusion. Neuronal injury was assessed 3 days after CA/CPR by Fluorojade and NeuN staining in coronal brain sections. To assess hippocampal synaptic plasticity, the Schaffer Collateral pathway was stimulated and long-term potentiation (LTP, a cellular model of memory) was evaluated via extracellular recording from hippocampal CA1 region 14 days after GCI (in acute hippocampal slices following a theta-burst stimulation). Increase in field excitatory post-synaptic potential (fEPSP) slope 60 min after theta burst stimulation of the Schaffer-collateral pathway (40 pulses 100Hz) was analyzed as a measurement of LTP. Results reported as mean±SD.
RESULTS: Within these experiments, 10/14 rats were successfully resuscitated and survived to their endpoint. Blood gas analysis 30 min after CA/CPR revealed pH 6.7±0.14 compared to 7.3±0.05 in sham rats (n=3, p=0.02). In addition, lactate was increased 30 min following GCI (10.3±1.4 mmol/L vs 2.4±0.4 mmol/L in sham, n=3, p=0.03), as was troponin (GCI: 8.1±2.3 ng/ml vs Sham 1.2±0.4 ng/ml, n=3, p=0.04). Fluorojade revealed neuronal cell death in the hippocampus and striatum at 3 days after CA/CPR. To assess cognitive function, hippocampal LTP was assessed 14 days following GCI. In sham mice, LTP was 179±45% (n=5) of baseline (set to 100%) 14 days following surgery. In contrast, LTP was impaired 14 days after GCI (106±20%, n=5, p=0.01 compared to sham mice).
CONCLUSIONS: These results demonstrate acute cardiac injury, neuronal cell death and hippocampal dysfunction following neonatal GCI and results are consistent with clinical outcomes in HIE. Future studies will extend these results to evaluate cognitive-affective outcomes in the juvenile period of development.
Effect of implementing a clinical practice guideline for prophylactic indomethacin on reduction of severe IVH in extremely preterm infants
Harrison S1, Szakmar E1,2, El-Shibiny H1, Munster C, El-Dib M
1Brigham and Women’s, Boston, United States, 2Semmelweis University, , Hungary
BACKGROUND AND PURPOSE: The use of prophylactic indomethacin in extremely preterm infants has been associated with reduction of severe intraventricular hemorrhage (SIVH, grade III. or IV) but no improvement in neurodevelopmental outcome. Routine use of prophylactic indomethacin has been controversial with variable utilization in clinical practice. Since January 2016, Brigham and Women’s Hospital (BWH) has implemented a clinical practice guideline (CPG) for prophylactic indomethacin to reduce the rate of SIVH in extremely preterm infants. Utilizing an online calculator, indomethacin was administrated to those who had risk of ≥ 15% for SIVH. We hypothesized that the implementation of the indomethacin CPG would be associated with reduction of SIVH.
METHODS: This retrospective cohort study included infants born between 23 to 28 weeks of gestation and admitted to the neonatal intensive care unit at BWH. We compared demographics, clinical data and rate of IVH, the use of indomethacin for any reason between infants before (pre-group, January 2010-December 2015) and after the CPG implementation (post-group, January 2016-December 2021). Risk categories for SIVH were defined as the following based on the online calculator (https://www.neoqicma.org/sivh: low risk ≤15%, moderate risk ≥15% to < 25%, and high risk ≥ 25%. Multivariate logistic regression model was applied to evaluate the association between SIVH and the administration of indomethacin; the model was adjusted for gestational age, birth weight, Apgar score at 5 minutes, gender, antenatal steroids, mode of delivery and the location of delivery (inborn vs. outborn).
RESULTS: 749 infants were included in the study, 424 in the pre-group and 325 infants in the post-group. Infants in the post group presented with lower Apgar scores and higher rate of mortality. The use of indomethacin for any reason was 44% in pre-group and 62% in post-group (p<0.001) (Table 1). When the use of indomethacin was compared in different risk categories, 92% of the infants with risk of ≥ 15% for SIVH received indomethacin after CPG implementation compared to 56% in the pre-group. There was no significant difference in the risk categories predicted by the calculator and in the actual rate of SIVH in each risk category between the 2 groups (Figure 1). Moreover, there was no association between the use of indomethacin and development of SIVH in multivariate regression models (Table 2).
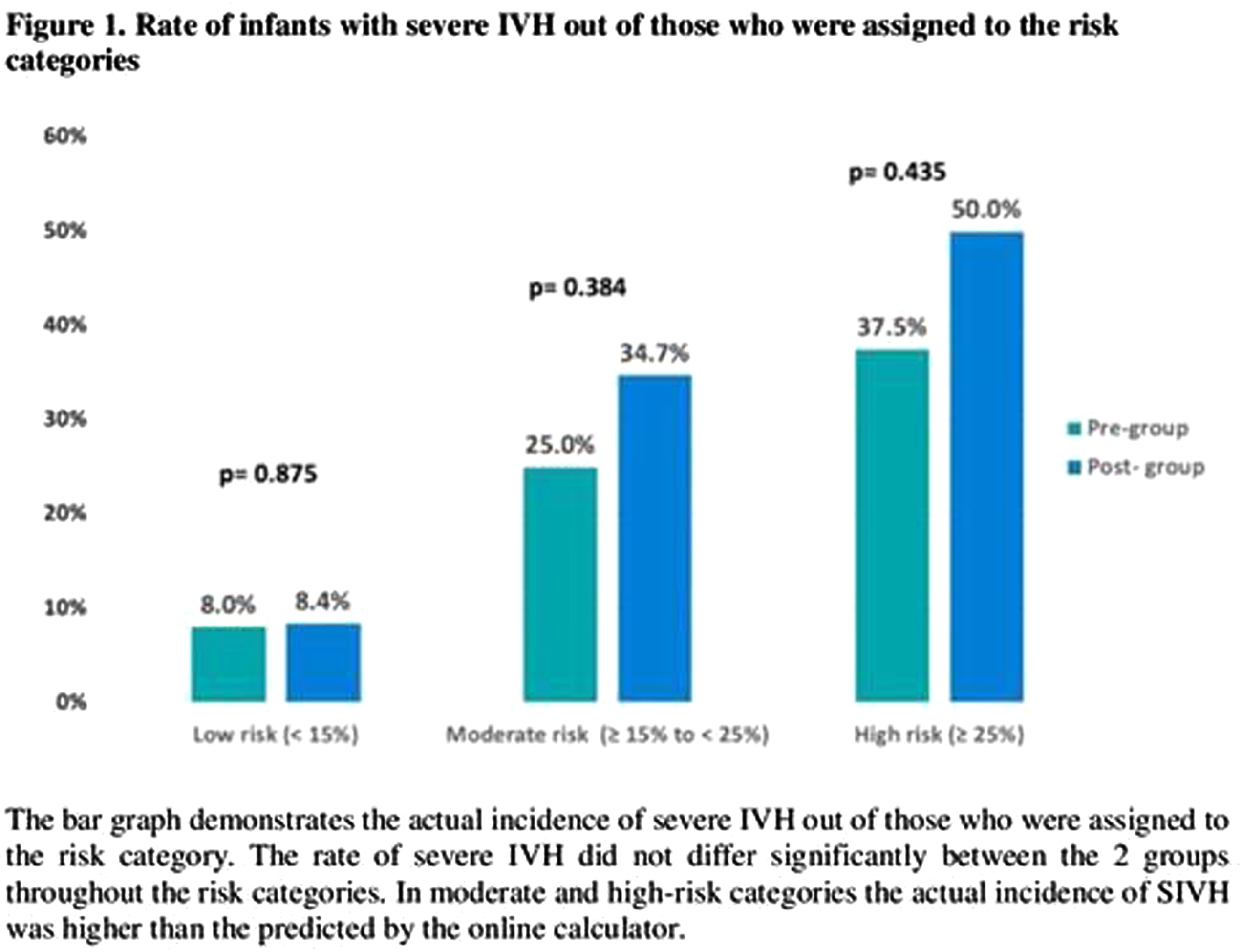
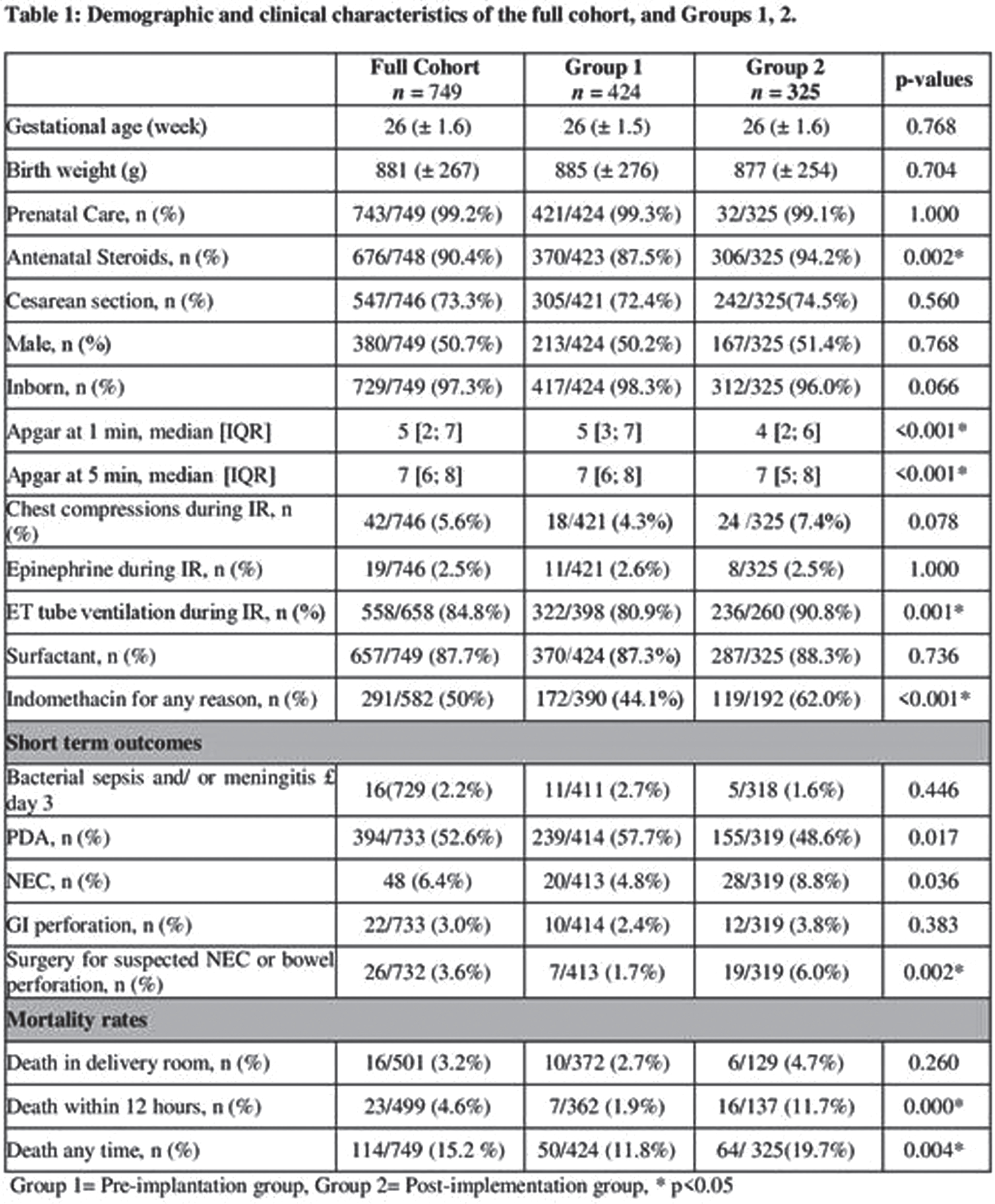
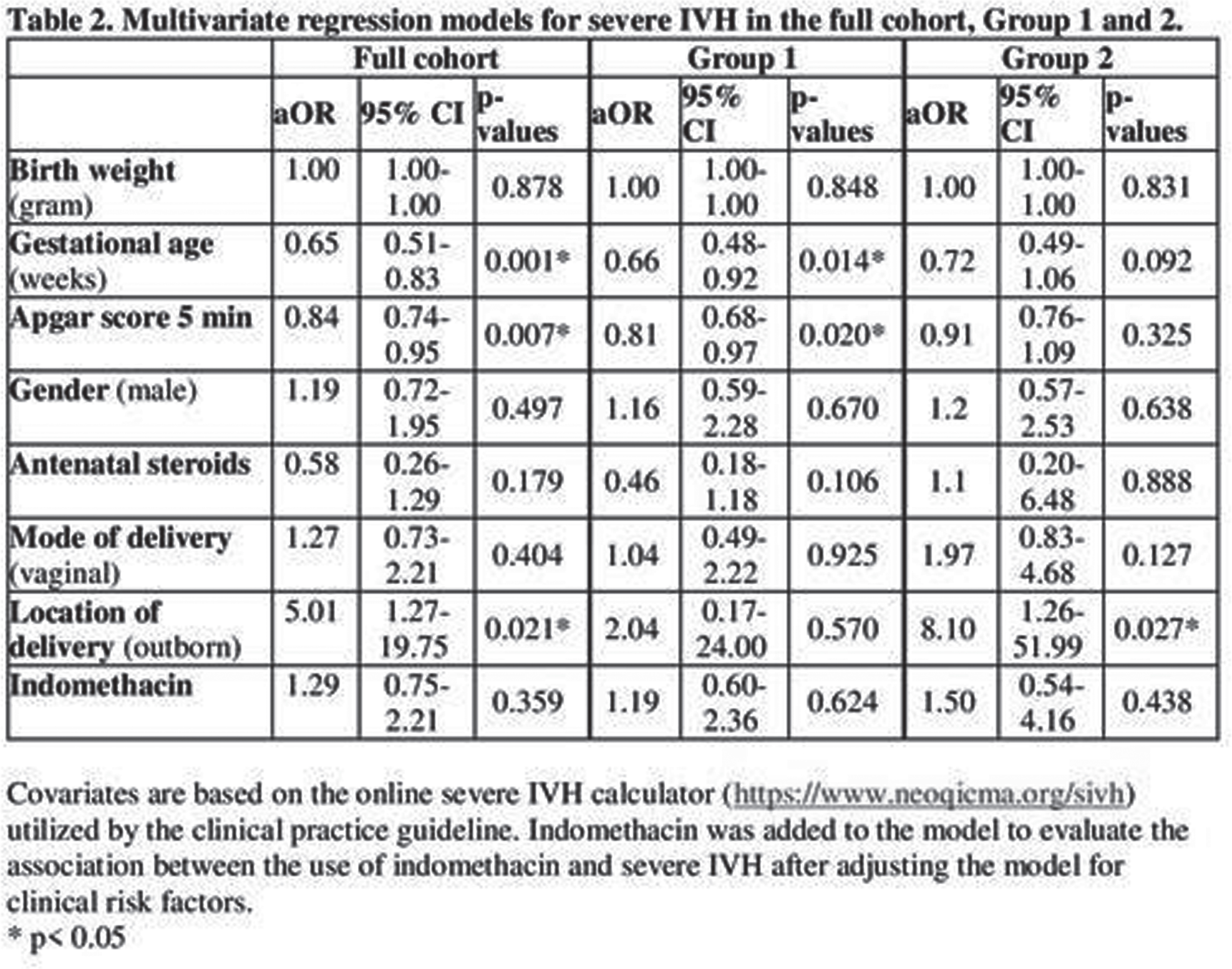
CONCLUSION: The implementation of CPG for prophylactic indomethacin in extremely preterm infants was not associated with reduction in the incidence of severe IVH and no association was found between indomethacin and development of SIVH. In presence of potential significant side effects, routine use of prophylactic indomethacin might not be encouraged.
Reducing intraventricular hemorrhaging during neonatal transport
Dickie J1, Burckhard Z1, Guo J1, Tran P1
1University Of Washington, Seattle, United States
BACKGROUND: Vibration and sensory stimulation during the transport of preterm neonates to a referral Neonatal Intensive Care Unit (NICU), may increase the risk of complications, including intraventricular hemorrhaging (IVH). Preterm infants transported in their first 72 hours of life are significantly more likely to develop IVH1, and severe IVH can lead to mortality or long-term neurodevelopmental morbidities. Current transport equipment does little to dampen or suppress vibration experienced during transport. Our objective was to develop an incubator mattress with a buckling structure that aims to attenuate vibrations experienced by neonates during intrahospital and interhospital transportation with the goal of increasing the safety and well-being of newborns during transport.
METHODS: Over the last year of problem identification, designing, and prototyping, we developed NIMBUS (Neonatal Incubator Motion Buffering Universal System), a polymer mattress that is compatible with current incubators, an example could be seen in Figure 1. NIMBUS was made with Dragon Skin 10, a high-performance silicone resin through casting. The mattress will buckle and absorb when exposed to critical loads during transport, greatly reducing vibration and sensory stimulation.

RESULTS: We examined the performance of the solution through two types of bench testing: A shaker table test to evaluate performance under constant vibration, and a drop test to simulate shocks induced during transportation. We can see that our solution performed better than the current memory foam mattress in use. In Figure 2, the shaker table test has shown a reduction in vibration peaks and noise filtration, while the drop test in Figure 3 has shown a 34.05% reduction in shock amplitude.
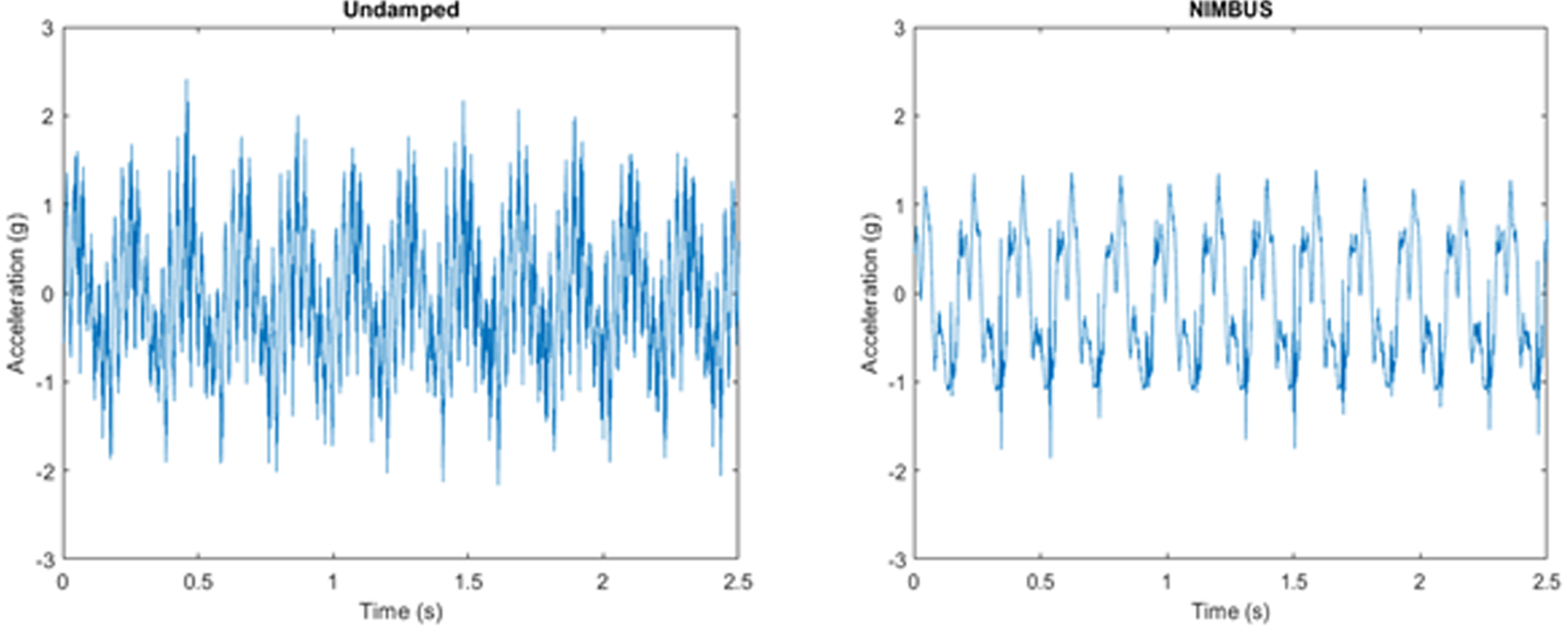
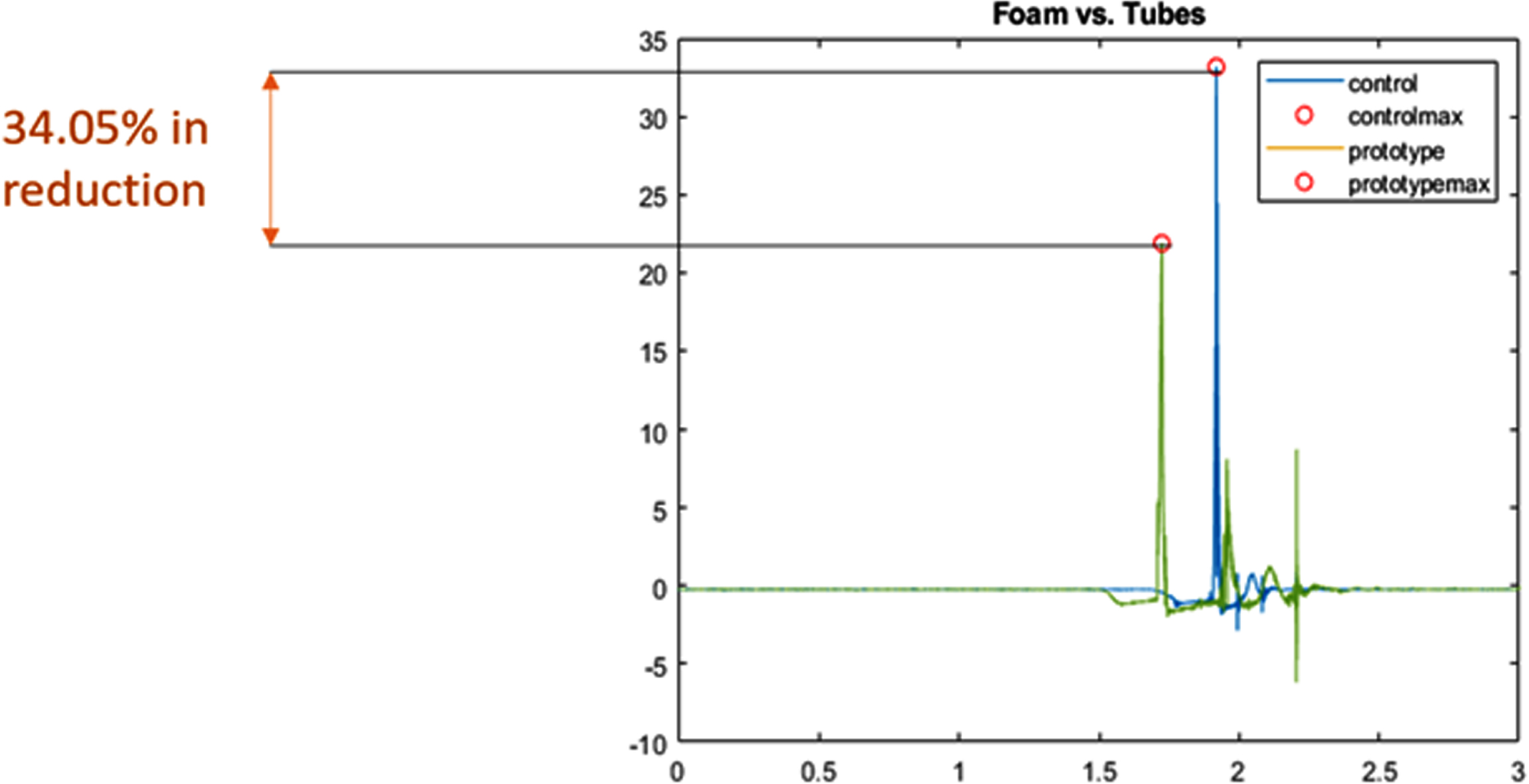
CONCLUSIONS: NIMBUS aims to reduce vibrations and mechanical shock during neonatal transportation. This will be done in such a way that does not interfere with current practices of transport teams. If NIMBUS is incorporated into neonatal transport, it will reduce mechanical stimulation that may lead to IVH, improving the lives of infants and their families.
REFERENCES
[1] L. Shipley, T. Gyorkos, J. Dorling, L. J. Tata, L. Szatkowski, D. Sharkey. Risk of severe intraventricular hemorrhage in the first week of life in preterm infants transported before 72 hours of age*. Pediatric Critical Care Medicine. 2019; 20 (no. 7): 638–644.
The role of erythropoietin for neuroprotection in extremely low birth weight infants: Randomized control study
Sharafutdinova D, Ionov OV, Balashova E, Kirtbaya A, Suvorova J, Suvorov I, Ushakova L
1National Medical Research Center for obstetrics, gynecology and perinatology named academician V.I. Kulakov, 117198, Moscow, Oparina street, 4, Russian Federation, 2Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), 119435, Moscow, Bolshaya Pirogovskaya st., 2, build. 4 , Russian Federation, 3”Research Clinical Institute of Childhood Ministry of Health of the Moscow Region”
BACKGROUND AND PURPOSE: Currently the use of promising neuroprotective technologies in premature newborns is increasingly discussed. A number of studies have shown that high doses of recombinant human erythropoietin (rhEPO) have a neuroprotective effect, but the benefits and safety of this therapy in premature infants have not been established.
Objective: to study the effectiveness of rhEPO as neuroprotective therapy in premature infants with extremely low birth weight (ELBW).
MATERIALS OR METHODOLOGY: A single-center randomized study was conducted, which included 190 ELBW infants who received various regimens of rhEPO. The gestational age of the newborns ranged from 26 to 30 weeks. The infants were divided into 3 groups depending on the treatment regimen: group 1 (n=63) – premature newborns who received 600 IU/kg per week subcutaneously; group 2 (n=76) – received 1200 IU/kg per week subcutaneously; group 3 (n=51) premature infants who did not receive rhEPO – the control group. The neurological development of newborns at the time of discharge, as well as from 22 to 30 months of life, was evaluated. 168 newborns were included in the analysis after discharge: 122 received rhEPO according to various schemes and 46 did not receive rhEPO.
RESULTS: There were no significant differences regarding short-term outcomes such as intraventricular hemorrhage, periventricular leukomalacia, retinopathy of prematurity, necrotizing enterocolitis and bronchopulmonary dysplasia, death or the frequency of serious adverse events. There was no significant difference between the rhEPO groups and the control group in the incidence of severe neurodevelopmental impairment at 22-30 months of life (group1 vs group3 relative risk (RR)=1.06; 95% confidence interval (CI) 0.66 to 1.84; p=0.735; group2 vs group3 RR=1.08; 95% confidence interval (CI) 0.42 to 1.96; p=0.613, group1 vs group2 RR=1.02; 95% confidence interval (CI) 0.22 to 2.15; p=0.875).
CONCLUSION: Treatment with different doses of rhEPO in ELBW infants (600 IU/kg per week vs 1200 IU/kg per week vs control group) did not result in a lower risk of severe neurodevelopmental impairment at the age of 22 to 30 months of life.




