Proceedings of the 14th International Newborn Brain Conference: Fetal and/or neonatal brain development, both normal and abnormal
Histological chorioamnionitis as a risk factor for early brain damage in premature babies in a referral hospital in Algiers
Arfi H1, Lebane D1, Abrouk S2
1Chu Mustapha Algiers, Ouled fayet, Algérie
2University of Algiers, 2 Rue Didouche Mourad, Alger Ctre 16000, Algeria
INTRODUCTION: The early brain damage of the premature has been correlated to several risk factors and among them the histological chorioamnionitis (HC). However, the study of the placenta is not systematically performed in the underdeveloped countries.
Our study is estimating the frequency of early brain lesions of premature infants and evaluating the attributed risk of HC among the others independent factors.
MATERIALS AND METHODS: This case control study was realized at the neonatal service of CHU Mustapha of Algiers from 01/12/2007 to 31/12/2012. 801 in born babies were included, aged between 24 to 36 GA +6 days. All the patients benefited a histological placental study and cranial ultrasonography (US) within the 72hours of birth followed by weekly controls until the patients reached the term. The MRI was realized at the acquired term for babies born before 32 weeks or if the US lesions persisted after the acquired term or if the neurological exam remained abnormal. An EEG was systematically realized during the first week of life.
24 brain risk factors were studied, among them, histological chorioamnionitis. The statistical method consisted in bi-varied analysis, logistic regression. P inferior to 0.05 was considered significant.
RESULTS: The frequency of the brain lesions was 27.3%. The lesions included peri- intraventricular hemorrhages, cystic and non-cystic leukomalacia and the sequelae (post hemorrhagic hydrocephalus, hypotrophy or atrophy of corpus callosum, and cortical atrophy). The global frequency of HC was 14% .
Bi-varied analysis revealed 24 perinatal risk factors significantly associated with the brain lesions and among them, HC with an OR=1,95 IC95=1,25-3,04 p=0,001.
After adjustment by logistic regression ,8 factors were independent and the HC was an important prognosis factor as it multiplied the risk of the lesions by about 2 with an OR= 1,92 IC95 (1,09-3,37) P=0,024.
CONCLUSION: The HC is an important pejorative risk factor in our study.
Low-income countries should recommend a systematic histological study of the placenta in the case of premature birth which is feasible in practice and will make it possible to carry out protocols for the prevention and management of parturients at high risk of chorioamnionitis.
Electroencephalographic changes in newborns with antecedents of hemodynamic alterations during intrauterine growth restriction
Cubero-Rego L1, Ricardo-Garcell J1, Cubero-Rego M1, Harmony T1, Corsi-Cabrera M1
1Institute of Neurobiology, Autonomous University of Mexico., BOULEVARD DE LAS CIENCIAS No. 3000, Querétaro Mexico
BACKGROUND: Intrauterine growth restriction (IUGR) is the fetal incapability for achieve his/her genetic potential of growing, and commonly reflects uteroplacental insufficiency. IUGR has a global prevalence of 20.5%. Its adverse outcomes include preterm birth, stillbirth, increased neonatal mortality, increased risk of metabolic syndrome in adulthood, and neurodevelopmental disability. Several reports show that 45 to 50 % of children with IUGR present inferior index of development and psychomotricity and intellectual quotient in their scholar years. It is necessary to offer early neurohabilitation therapies to these patients, but an accurate detection of damage is difficult at newborn age. The study of spontaneous electrical cerebral activity (EEG), and evoked responses to auditory (Auditory Brainstem Responses, ABR) and visual stimuli (Visual Evoked Potentials, VEPs) are very useful in newborns, because they are objective, noninvasive tests, and they do not require patient cooperation.
HYPOTHESIS: IUGR neonates will have signals of brain damage in their EEG, ABR or VEPs at the term age.
METHODS: We studied transversally a cohort of 51 newborns at postmenstrual age (PMA) between 39 and 45 weeks. They were divided in three independent groups: i) 17 Healthy neonates (HN) with normal prenatal Doppler ultrasound study and birth weight higher than 15 percentile for gestational age at birth (EG); ii) 17 newborns with Mild IUGR (fetal estimated weight inferior to the 10 percentile for EG), without other alterations in their prenatal Doppler ultrasound examination, and iii) 17 newborns with Severe IUGR, with fetal estimated weight inferior to the 10 percentile for EG, and elements suggestive of hemodynamic redistribution, as abnormal pulsatility index of middle cerebral artery and/or the umbilical artery.
Results: Severe IUGR group were born with significant decreasing in several anthropometrical measures: birth weight (HKW=34.5, p<0.0001); head circumference at birth (HKW=13.2, p=0.001); cephalization index (HKW=11.5, p=0.003). Non-significant differences were found in latencies/amplitude of ABR’s waves (p>0.16). Although 30% of severe IUGR newborns showed non-reproducible VEPs at luminous stimulation, the latencies/amplitude comparisons between groups were non-significant. In EEG studies, severe IUGR group showed subclinical spikes/sharp waves activity, (~2=12.4, p=0.001), and longer inter-burst intervals (IBIs, HKW=6.6, p=0.03), that were not found in healthy newborns. Quantitative EEG analysis showed greater differences of EEG’s absolute power in slow band between active sleep and quiet sleep in the healthy newborns, in comparison with severe IUGR neonates (F=3.19, p=0.04; post-hoc multiple planned comparisons, p<0.05).
CONCLUSION: Abnormal maturation of EEG slow band (1-6 Hz) is found in IUGR newborns with alterations of middle cerebral artery and/or umbilical artery, with increased duration of IBIs and presence of subclinical paroxysmal activity. Future longitudinal studies are necessary to assess the long-term implications of these early neurophysiological alterations.
REFERENCES
[1] Figueras F, Gratacós E. 2017. An integrated approach to fetal growth restriction. Best Practice & Research Clinical Obstetrics and Gynecology. 38: 48-58.
[2] Cohen E, Baerts W, van Bel F. 2015. Brain-Sparing in Intrauterine Growth Restriction: Considerations for the Neonatologist. Neonatology. 108: 269–276.
[3] Cubero-Rego L, Corsi-Cabrera M, Ricardo-Garcell J, Cruz-Martínez R, Harmony T., 2018. Visual evoked potentials are similar in polysomnographically defined quiet and active sleep in healthy newborns. International Journal of Developmental Neuroscience. 68: 26-34.
[4] Shellhaas RA, Burns JW, Barks JD, Chervin RD. 2014. Quantitative sleep stage analyses as a window to neonatal neurologic function. Neurology. 82: 390-5.
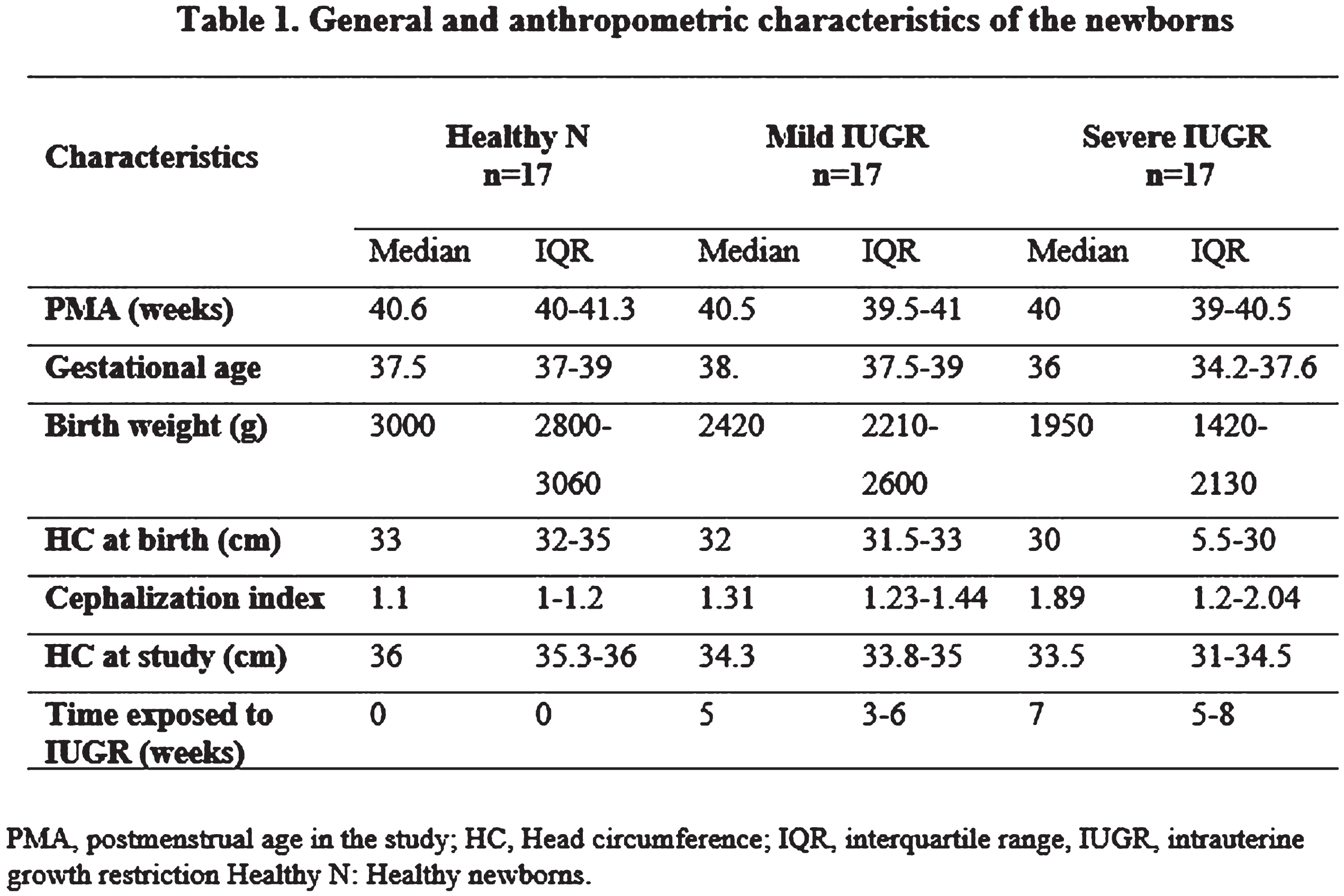
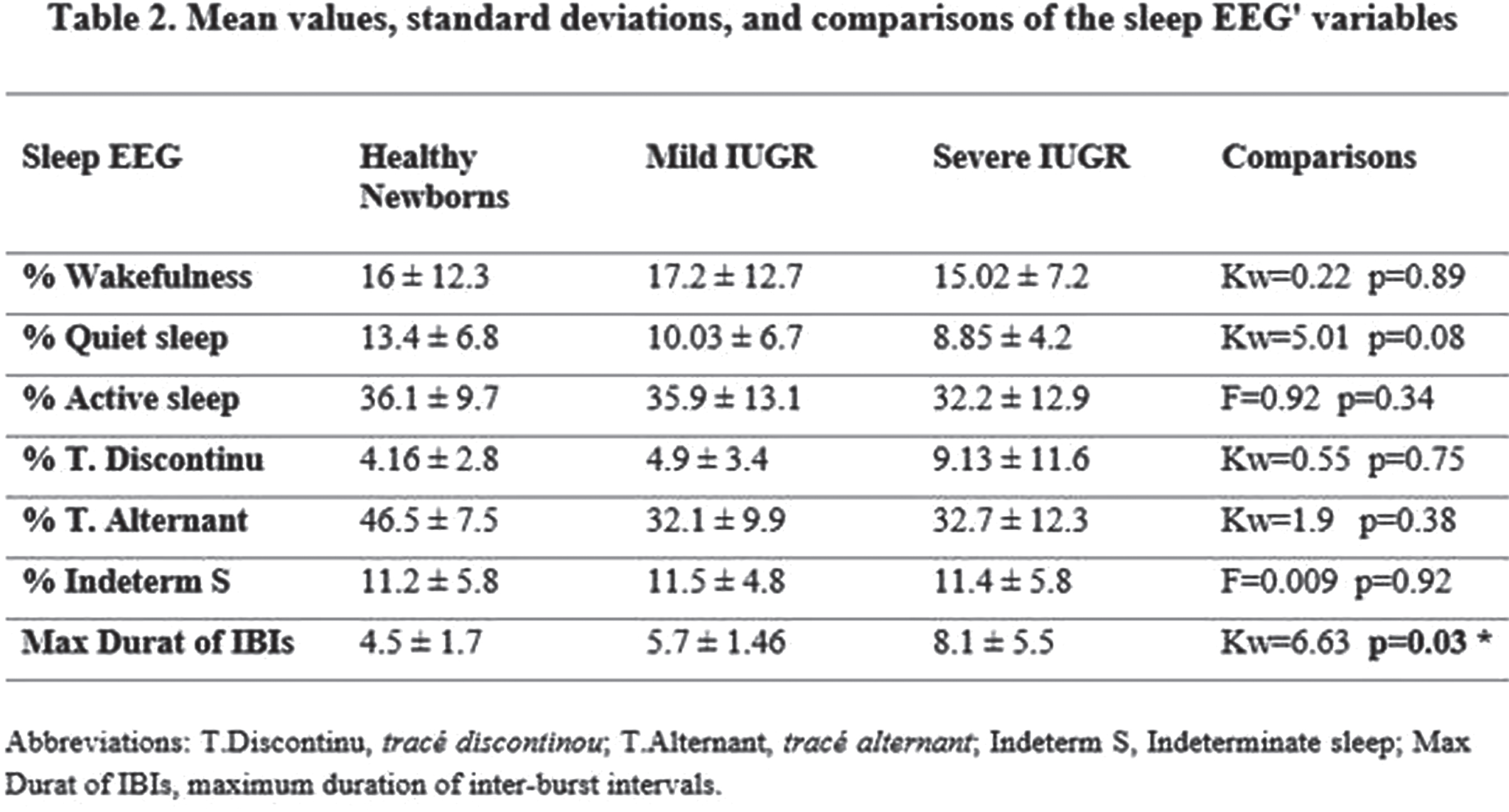
Figure 1.
The difference between quiet sleep (QS) and active sleep (AS), measured as absolute power (AP) for the EEG’ slow band (1-6 Hz) was significantly higher in healthy newborns than in the IUGR severe group (p<0.05).
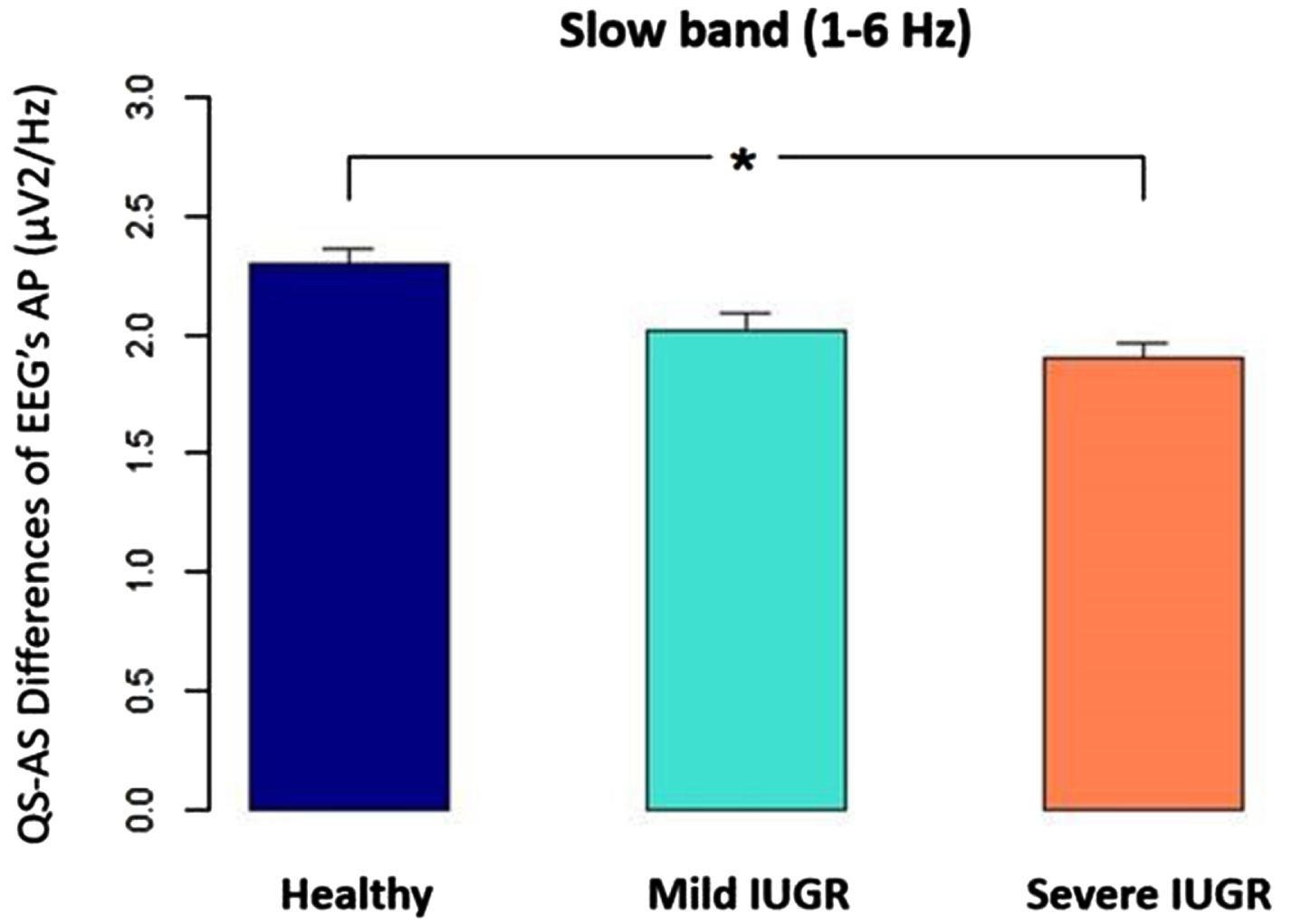
Figure 4.
The difference between quiet sleep (QS) and active sleep (AS), measured as absolute power (AP) for the EEG’ slow band (1-6 Hz) was significantly higher in healthy newborns than IUGR severe group (p<0.05).
Placental neurosteroid disruption alters somatosensory corticogenesis and function
Vacher CM1, Bakalar D, O’Reilly JJ, Lacaille H, Salzbank J, Penn AA
1Columbia University Medical Center, New York, United States
BACKGROUND AND PURPOSE: The placenta is essential to fetal growth and brain development by enabling the exchange of nutrients, gases and waste between the mother and the fetus. Growing body of evidence also suggests that it produces hormones that support not only pregnancy but also neurodevelopment. As a result, placental pathology or premature loss due to preterm delivery have been associated with short- or long-term neurological outcome. We have recently demonstrated that the placental provision of allopregnanolone (ALLO), a potent GABAergic neurosteroid synthesized from progesterone, was necessary for cerebellar white matter development and that its loss was associated with an increased risk of autistic-like behavioral traits in the male progeny only (Vacher et al., 2021). The production of ALLO by the placenta peaks in mid-to-late gestation, a period coinciding with a significant expansion of the cerebral cortex. In this study, we tested the hypothesis that placental ALLO loss alters corticogenesis and long-term cognitive functions.
Materials or Methodology. We used a mouse model characterized by placental ALLO insufficiency. In these mice (named plKO), the akr1c14 gene coding for the ALLO synthesis enzyme, is specifically ablated in trophoblasts using a tissue-specific Cre-Lox strategy (Vacher et al., 2021). Corticogenesis in control and plKO brains were examined in the S1 area of the somatosensory cortex from embryonic stages to adolescence. Somatosensory function was also evaluated in adolescent mice. In addition, post-mortem specimens comprising Brodmann Area 10 from term (>37 weeks of gestation) and preterm (<34 weeks of gestation) infants who died at an average corrected age of 6 weeks were used for comparative gene expression analysis.
RESULTS: The insufficient production of ALLO by the placenta in plKO mice is associated with a sex-linked disruption of corticogenesis in S1. Embryonic cell proliferation alterations were observed in plKO mice of both sexes, but they persisted primarily in female offspring after birth. Adolescent plKO females showed significant reduction in pyramidal neuron density in the upper cortical layers, as well as somatosensory behavioral deficits as compared with plKO males and control littermates (Bakalar et al., 2022). Assessment of layer-specific markers in human postmortem cortices indicates that preterm infants may also have female-biased abnormalities in cortical layer specification as compared with term infants.
CONCLUSION/IMPACT: This study establishes a novel and fundamental link between placental function and sex-linked long-term neurological outcomes. It shows that placental hormones such as ALLO support fetal brain development in a sex- and region-dependent manner, and that their loss may contribute to long-term neurobehavioral outcomes of prematurity, emphasizing the importance of the growing field of neuroplacentology. Further investigation of the plKO mice will provide valuable knowledge for future development of therapeutic strategies aimed at preventing long-term neurodevelopmental disorders resulting from compromised placental function.
REFERENCES
[1] Bakalar D et al., Lack of placental neurosteroid alters cortical development and female somatosensory function. Front. Endocrinol. 13:972033, 2022.
[2] Vacher CM et al., Placental endocrine function shapes cerebellar development and social behavior. Nat. Neurosci. 24(10):1392-1401, 2021.
Congenital brain malformations: Seizure incidence, EEG findings, and developmental outcomes
Armstrong L1, Cooke A1, Harshbarger J1, Armour E2, Reddy S1, Pagano L1, Carter E1
1Vanderbilt University Medical Center, Nashville, United States, 2University of Michigan Health, Ann Arbor, United States
BACKGROUND: With improvements in pre- and post-natal imaging, congenital brain malformations are increasingly found prior to onset of symptoms. Effectively counseling families requires knowledge of expected outcomes. Currently, there is limited literature to provide accurate prognostication in terms of epilepsy and developmental outcomes. Although the American Clinical Neurophysiology Society recommends EEG monitoring for these infants, evidence is lacking on whether universal screening changes outcomes.
MATERIALS/METHODOLOGY: In this retrospective study, we identified neonates with congenital brain malformations including polymicrogyria, lissencephaly, pachygyria, simplified gyral pattern, heterotopias, schizencephaly, hemimegencephaly, focal cortical dysplasias, absent septum pellucidum, holoprosencephaly, or corpus callosum dysgenesis/agenesis who received a Neurology consult in the newborn nursery or the NICU. We collected data via chart review on EEG characteristics, seizure incidence, and developmental outcomes.
RESULTS: We identified 46 patients that met inclusion/exclusion criteria and divided them into groups as follows: disorders of neuronal migration/organization (polymicrogyria, lissencephaly, pachygyria, simplified gyral pattern, heterotopias, schizencephaly, hemimegencephaly, focal cortical dysplasias), disorders of early prosencephalic development (absent septum pellucidum, holoprosencephaly), complex malformations (abnormalities in both migration and early prosencephalic development), and isolated corpus callosum dysgenesis/agenesis. Twenty patients (43%) had evaluation with EEG during the initial hospitalization. In our study, disorders of early prosencephalic development conferred the highest risk of seizures during the initial admission (43% 3/7), followed by complex malformations (25% 1/4), disorders of neuronal migration (14% 3/21), and isolated corpus callosum dysgenesis/agenesis (8% 1/13). Of the 7 patients with EEG confirmed seizures, 2 were electrographic only. After discharge, 5 patients (15% of those discharged from the hospital without seizures) later developed seizures and were started on anti-seizure medications. These 5 patients had no interictal epileptiform abnormalities on their initial EEG. Of the patients surviving to discharge 44% had developmental delay noted at follow-up, 21% had normal development, and 36% had no clinic follow-up.
CONCLUSION/IMPACT: We identified disorders of early prosencephalic development as having the highest risk of seizures during initial admission. Our results differed from previous studies because corpus callosum abnormalities were analyzed separately and had a lower risk of seizures compared to other congenital brain malformations. Our results confirmed the high rate of electrographic seizures in patients with congenital brain abnormalities, reinforcing the importance of EEG data. We identified a population of patients who developed seizures post-discharge and who could potentially benefit from early identification and intervention. However, there were no interictal epileptiform abnormalities identified on the initial EEG in these patients. This data reinforces the need for and will serve as the foundation for future studies using universal EEG screening to improve outcomes in patients with congenital brain malformations.
Apnoea and EEG brain activity in neonates: Data from a systematic review
Usman F1,2, Marchant S1, Baxter L1, Aliyu M3, Salihu H4, Adams E5, Hartley C1
1University Of Oxford, Oxford, United Kingdom, 2Bayero University, Kano and Aminu Kano Teaching Hospital, Kano, Nigeria, 3Vanderbilt University Medical Center, Nashville, USA, 4Baylor College of Medicine, Houston, USA, 5Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom
BACKGROUND AND PURPOSE: Neonates have immature respiratory control mechanisms [1] and a developing brain that is susceptible to the adverse effects of intermittent hypoxia [2]. Apnoea is a common pathology in premature infants, driven by the immaturity of the brainstem. Frequent apnoeas have been associated with reduced childhood cognitive ability [3,4]. However, it remains unclear whether episodes of apnoea, resulting in frequent hypoxic insults, lead to changes in brain development, or whether this association with reduced cognitive ability is only correlative. We conducted a systematic review to investigate how brain activity recorded using EEG in neonates studied between 28-42 weeks PMA changes during periods of normal respiration and acute respiratory events. Here, we focused on the subset of papers reporting EEG recordings and apnoea.
METHODOLOGY: We searched MEDLINE, Embase, Global Health, PsycINFO, CINAHL, Cochrane Library, Science Citation Index and Conference Proceedings Citation Index via the Web of Science database; and ProQuest. We also conducted a backward citation search for all the included articles to ensure robust literature acquisition. All studies reporting on respiratory events and EEG-recorded brain activity in human neonates studied between 28-42 weeks PMA were included. We excluded review articles, systematic reviews, commentaries, questionnaires, surveys, studies with the wrong study population and infants with cardiovascular, neurological and, in the case of control infants, respiratory confounders at the time of the study. The selection for eligible articles was performed with Rayyan software [5]. Two reviewers independently screened the articles. The Joanna Briggs Institute critical appraisal tools were used to assess study quality. The full systematic review protocol was registered in Prospero (CRD42022339873).
RESULTS: The search was conducted in January 2022 and updated in August 2022. We identified a total of 273 articles, of which 10 studies involving 210 infants reported EEG changes during apnoea. Neonatal apnoea-related EEG changes were inconsistent, with EEG suppression and amplitude reduction reported in some but not all apnoeas; these changes were more likely during prolonged episodes. One study reported a significant reduction in EEG amplitude in term infants >41weeks; this change was not significant in younger infants.
CONCLUSION: Only a small number of studies have been conducted to date investigating EEG changes during apnoea. Diverse exposure definitions and outcome measures limit inference, thus, a consensus needs to be adopted for a valid comparison of EEG-related respiratory changes.
REFERENCES
[1] Erickson G, Dobson NR, Hunt CE. Immature control of breathing and apnea of prematurity: the known and unknown. J Perinatol 2021;41:2111–23. https://doi.org/10.1038/s41372-021-01010-z.
[2] Martin RJ, Wang K, Köroglu Ö, Fiore J Di, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? . Neonatology 2011;100:303–10. https://doi.org/10.1159/000329922.
[3] Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ. Apnea Is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol 2004;24:763–8. https://doi.org/10.1038/sj.jp.7211182.
[4] Pillekamp F, Hermann C, Keller T, von Gontard A, Kribs A, Roth B. Factors influencing apnea and bradycardia of prematurity – Implications for neurodevelopment. Neonatology 2007;91:155–61. https://doi.org/10.1159/000097446.
[5] Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:1–10. https://doi.org/10.1186/s13643-016-0384-4.
Structural and functional motor development in preterm infants: Feasibility of serial MRI and motor assessment
Peyton C1, Millman R1, Yarnykh V2, Drobyshevsky A3
1Northwestern University, 645 N. Michigan Ave, United States, 2University of Washington, Seattle, USA, 3NorthShore University Health System, Evanston, USA
BACKGROUND AND PURPOSE: Children with cerebral palsy (CP) experience a loss of independent joint control (IJC), causing difficulty with dexterity and gait. Infants with CP experience altered development of IJC, but the underlying structural and functional mechanisms of this development are not well understood. Our objective was to determine the feasibility of studying the changes in movement behavior and structural motor pathways between the preterm period and first 4 months of life in a sample of very preterm infants (<32 weeks gestational age; GA) with and without CP. Our central hypothesis is that between 0 and 3 months both IJC and macromolecular proton fraction (MPF), a biomarker of myelination in neural tissue, will decline in preterm children with CP.
MATERIALS/METHODOLOGY: Infants were enrolled during the NICU stay and video-recorded for 2-3 minutes of active movement biweekly between enrollment and 58 weeks post-menstrual age (PMA). Infants received 1-2 non-sedated brain MRIs between term equivalent and 58 weeks PMA, with diffusion tensor imaging, macromolecular proton fraction (MPF), and fMRI sequences. DeepLabCut, an open source machine learning algorithm, was used to create a model for kinematic joint movement data extraction. A behavioral coding approach was also created to code instances of IJC at each joint of the upper and lower extremities.
RESULTS: 19 infants were enrolled between 12/2022 and 9/2022, with an average birthweight of 1042g (545-1400), and GA of 28wks (24-31). Five participants (26%) had ≥ grade III intraventricular hemorrhage. 143 videos were collected, with an average of 7 videos/infant. 11 MRIs were completed with a 91% success rate, at an average age of 43 wks PMA for first MRI and 54wks PMA for second MRI. 79 (55%) videos were annotated to create a machine learning model.
CONCLUSION/IMPACT: Serial collection of structural and functional markers of IJC was feasible in a preliminary sample of very preterm infants. Future analysis of data will include kinematic modeling of infant IJC, behavioral scoring of IJC, tractography of the corticospinal pathways, MPF mapping of motor regions of the brain, and resting state fMRI in infants with and without CP. Improved understanding of the structural and functional mechanisms that underlie the development of IJC may help to improve earlier and targeted interventions.
REFERENCES
[1] Cahill-Rowley K et al. Etiology of impaired selective motor control: emerging evidence and its implications for research and treatment in cerebral palsy. Developmental Medicine & Child Neurology. 2014.
[2] Yarnykh V, Knipenberg N, Tereshchenkova O. Quantitative assessment of pediatric brain myelination in a clinical setting using macromolecular proton fraction.
[3] Passmore, E., et al. Automatic identification of infants at high-risk of cerebral palsy from smart-phone videos. Gait & Posture. 2022; 97: S141-S142.
Sleep-state-dependent functional connectivity in healthy term infants
Kidokoro H1, Shiraki A1, Watanabe H2, Taga G2, Ushida T3, Narita H1, Mitsumatsu T1, Kumai S1, Suzui R1, Sawamura F1, Ito Y1, Yamamoto H1, Nakata T1, Sato Y4, Hayakawa M4, Natsume J1,5
1Department of Pediatrics, Nagoya University Graduate School of Medicine, Nagoya, Japan, 2Graduate School of Education, The University of Tokyo, Tokyo, Japan, 3Department of Obstetrics and Gynecology, Nagoya University Graduate School of Medicine, Nagoya, Japan, 4Division of Neonatology, Center for Maternal-Neonatal Care, Nagoya University Hospital, Nagoya, Japan, 5Department of Developmental Disability Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan
BACKGROUND: Recently, the relationship of the sleep state with the ontogenetic and developmental processes of the functional connectivity (FC) network in the brain has attracted significant attention. We investigated the relationship between sleep state and functional brain networks using simultaneous electroencephalography (EEG) and functional near infrared spectroscopy (fNIRS) in healthy term infants.
METHODOLOGY: Simultaneous EEG-fNIRS recordings were performed on 28 term infants born at Nagoya University Hospital. The EEGs were polygraphically recorded for at least 30–40 min and included active sleep (AS) and quiet sleep (QS). At least eight electrodes were placed at Fp1, Fp2, C3, C4, O1, O2, T3, and T4. An NIRS instrument with eight channels was used to measure the relative concentration changes of oxy- and deoxy-hemoglobin (Hb). Pairs of channels were placed at the frontal, temporal, and occipital regions to include AFz, POz, C5, and C6. AS and QS were scored every 30 s based on the EEG, rapid eye movements, breathing pattern, and behavior. The FC and phase synchronization index (PSI) for oxy- and deoxy-Hb during low-frequency fluctuations (< 0.1 Hz) were used. For FC, the correlation coefficients of 28 channel pairs were calculated and converted to z-score using Fisher’s z-transformation. After excluding the sections with periodic breathing, the longest consecutive sections of NIRS data in AS and QS were selected for subsequent analysis of FC and PSI. Global FC and PSI values of the 28 channel pairs were calculated. For group-level analysis, the FC for oxy-Hb in the longest consecutive sections of AS and QS was averaged.
RESULTS: The gestational age ranged from 37 to 41 weeks. The records ranged from 1 to 9 (median: 2) days of age. The mean global FC for oxy-Hb was 0.52 during AS and 0.43 during QS, and for deoxy-Hb it was 0.61 during AS and 0.48 during QS. The global FC for both oxy-Hb and deoxy-Hb was significantly higher during AS than during QS. The mean PSI for oxy-Hb was 0.65 during AS and 0.50 during QS, whereas the mean PSI for deoxy-Hb was 0.65 during AS and 0.51 during QS, with significantly higher values during AS than during QS. In group-level analysis, both AS and QS had higher frontal, temporal, and occipital inter-hemispheric FC than in other connections. The difference in FC between AS and QS was observed in all networks involving the occipital region after multiple comparisons (Figure 1).
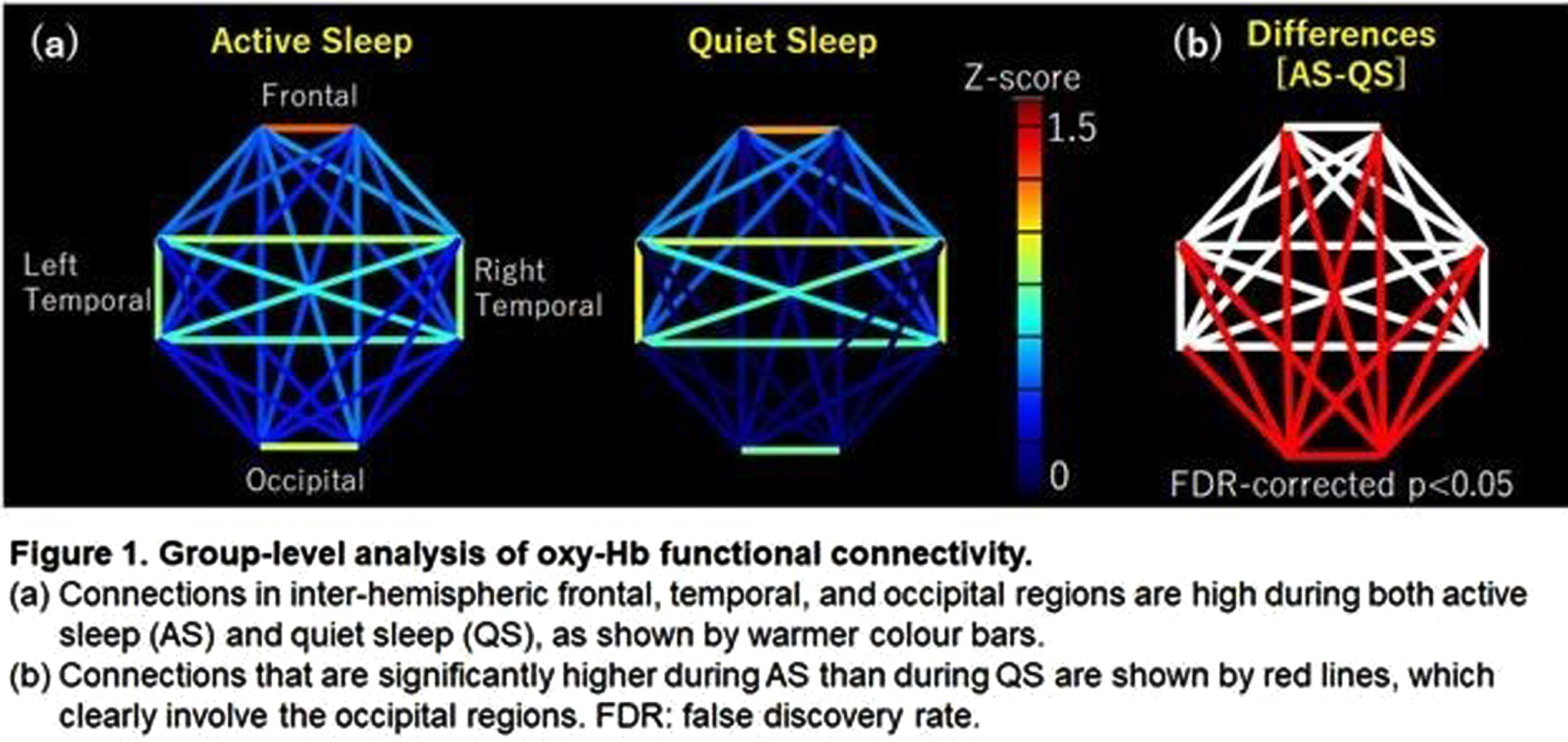
CONCLUSION: The global FC was higher during AS than during QS, which may be explained by higher FC in all networks involving the occipital region. These findings suggest that AS promotes functional connectivity across distant visual system structures during activity-dependent development, probably due to the rapid eye movements
Early catch up in growth and psychomotor development of preterm infants in low-income country
Mbayabo Gloire G1,2, Tuka D1, Bambi J1, Tady B1, Biselele T1
1University Hospital of Kinshasa, Kinshasa , Congo (the Democratic Republic of the), 2Center of Human Genetics, University of Kinshasa, Kinshasa , Congo (the Democratic Republic of the)
BACKGROUND: Prematurity remains a real public health concern in the world, especially in low-income country like the Democratic Republic of Congo (DRC), and it leads to growth retardation and psychomotor developmental deficits.
OBJECTIVES: To determine the period of catch up in growth and psychomotor development in preterm infants in low income country in order to improve their outcome.
METHODOLOGY: We followed 65 preterm infants from April 2016 to March 2017 admitted in three hospitals of Kinshasa, the capital of Democratic Republic of Congo (DRC), from the birth till 6months postnatal corrected age. These three hospitals have the same standard of the management of preterm infants. They underwent Kangaroo mother care (KMC) and classic method (incubators). We determined the catch up at 6 months postnatal corrected age in growth and psychomotor development. Psychomotor development was assessed using Bayley scale II.
RESULTS: Among 65 preterm infants included in this study, 31 underwent KMC and 34 underwent classic method; 26 (40%) were male and 39(60%) were female. At 6months postnatal corrected age, 44 (67.7%) vs 21(32.3%) caught up in weight, 42(64.6%) vs 23(35.4%) caught up in height; 32(49,2%) vs 33(58.2%) caught up in mental development age and 44(67.7%) vs 21(32.3%) caught up in motor developmental age. The majority of preterm infants who caught up in growth and psychomotor developmental had birth age >32 weeks, birth weight >1500g.
CONCLUSION: The early catch up in growth and psychomotor development is effective in preterm infants even in low-income country. This catch is mostly influenced by birth age and weight.
KEYWORDS: Preterm infants, catch up, growth, psychomotor development, DR Congo
Brain macrostructure in neonates with hypoxic-ischemic encephalopathy
Kebaya L1,2, Kapoor B3,4, Mayorga P2, Meyerink P2, Altamimi T2, Nichols E3,4, de Ribaupierre S1,5,7, Bhattacharya S2, Tristao L6, Jurkiewicz M6, Duerden E1,3,4,7
1Neuroscience, Western University, London, Canada, 2Neonatal-Perinatal Medicine, Department of Paediatrics, London Health Sciences Centre, London, Canada, 3Applied Psychology, Faculty of Education, Western University, London, Canada, 4Western Institute for Neuroscience, Western University, London, Canada, 5Clinical Neurological Sciences, London, Canada, 6Department of Medical Imaging, London Health Sciences Centre, London, Canada, 7Children’s Health Research Institute, London, Canada
BACKGROUND: Hypoxic ischemic encephalopathy (HIE) is a severe brain injury impacting term-born neonates. Despite treatment with therapeutic hypothermia (TH), HIE is associated with a myriad of adverse developmental outcomes suggesting the involvement of subcortical structures including, the thalamus and basal ganglia, which may be vulnerable to perinatal asphyxia, particularly during the acute period.
Aims: 1) To examine subcortical macrostructure in the first few days of life in neonates with HIE compared to age- and sex-matched healthy newborns. 2) To determine whether subcortical volumetric maturation is associated with HIE severity.
METHODS: A cohort of 28 newborns (19 males [67.9%], median gestational age [GA] =38.6 weeks, interquartile range [IQR]=36.8-39.6) with HIE (mild = 4, moderate =21, severe =3 based on Sarnat Staging) were scanned with MRI within the first four days of life (median postmenstrual age [PMA]=39.2, IQR=37.6-40.3), with the majority of scans occurring in the post-cooling period (n=23[82%]). The control group included 28 healthy newborns matched for GA, birth weight and PMA at scan. Subcortical volumes (thalamus, basal ganglia, hippocampus, cerebellum) were automatically extracted from T1-weighted images (Figure 1). Generalized linear models assessed between-group differences in subcortical volumes, adjusting for sex, GA, PMA, and total cerebral volumes. Within-group analyses assessed the association amongst subcortical volumes, HIE severity and cooling status.
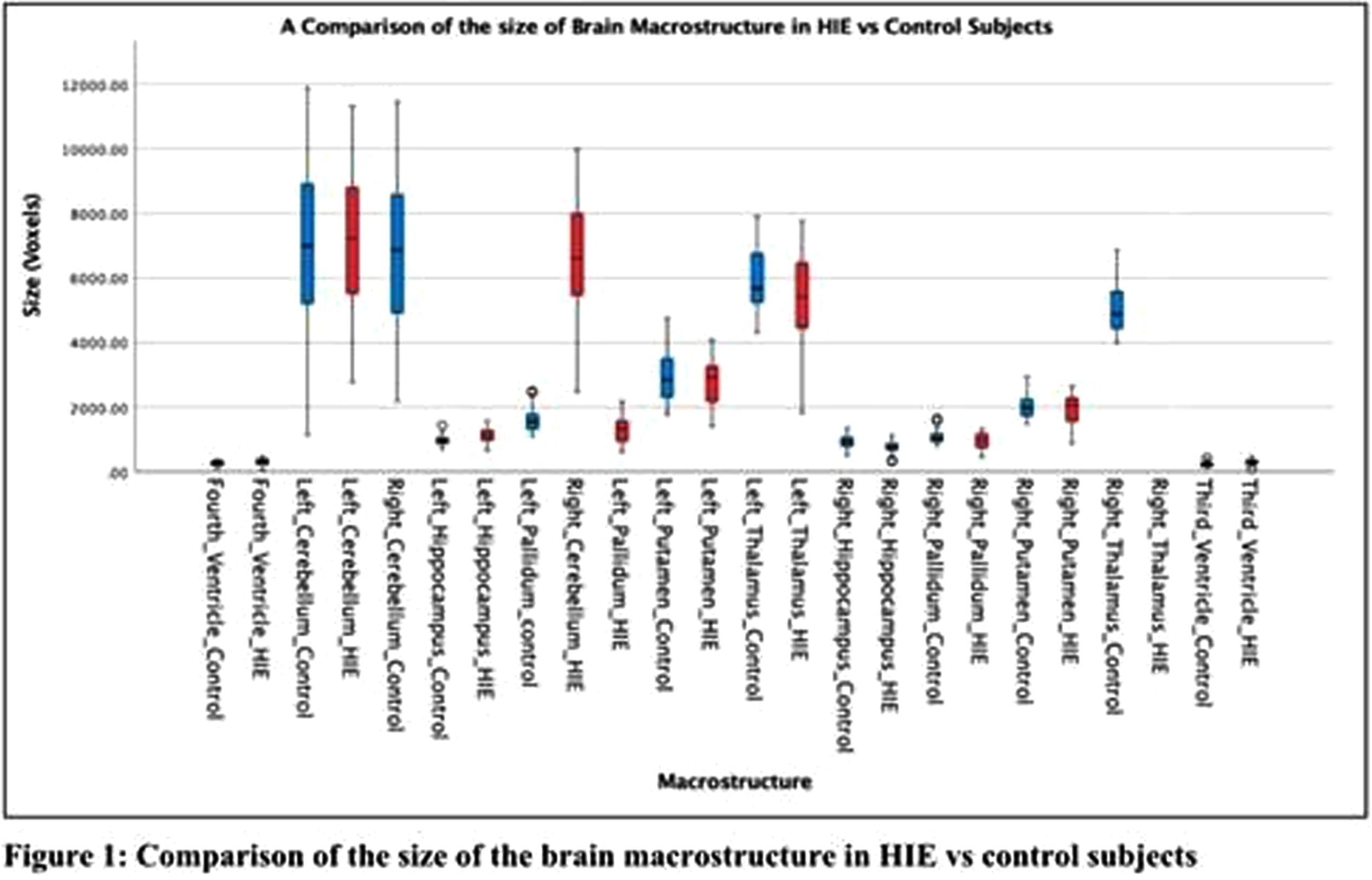
RESULTS: Newborns with HIE had significantly smaller bilateral thalamic, basal ganglia, cerebellar and right hippocampal volumes compared to healthy newborns (all, p<0.001), as shown in Table 1 and Figure 2. Newborns with HIE had significantly larger ventricular volumes (all, p<0.001) compared to controls. Greater HIE severity was associated with smaller volumes of the right putamen (p <0.001), left putamen (p<.001), right (p<0.001) and left pallidum (p=0.015) when adjusting for TH, mechanical ventilation and other clinical and demographic factors.
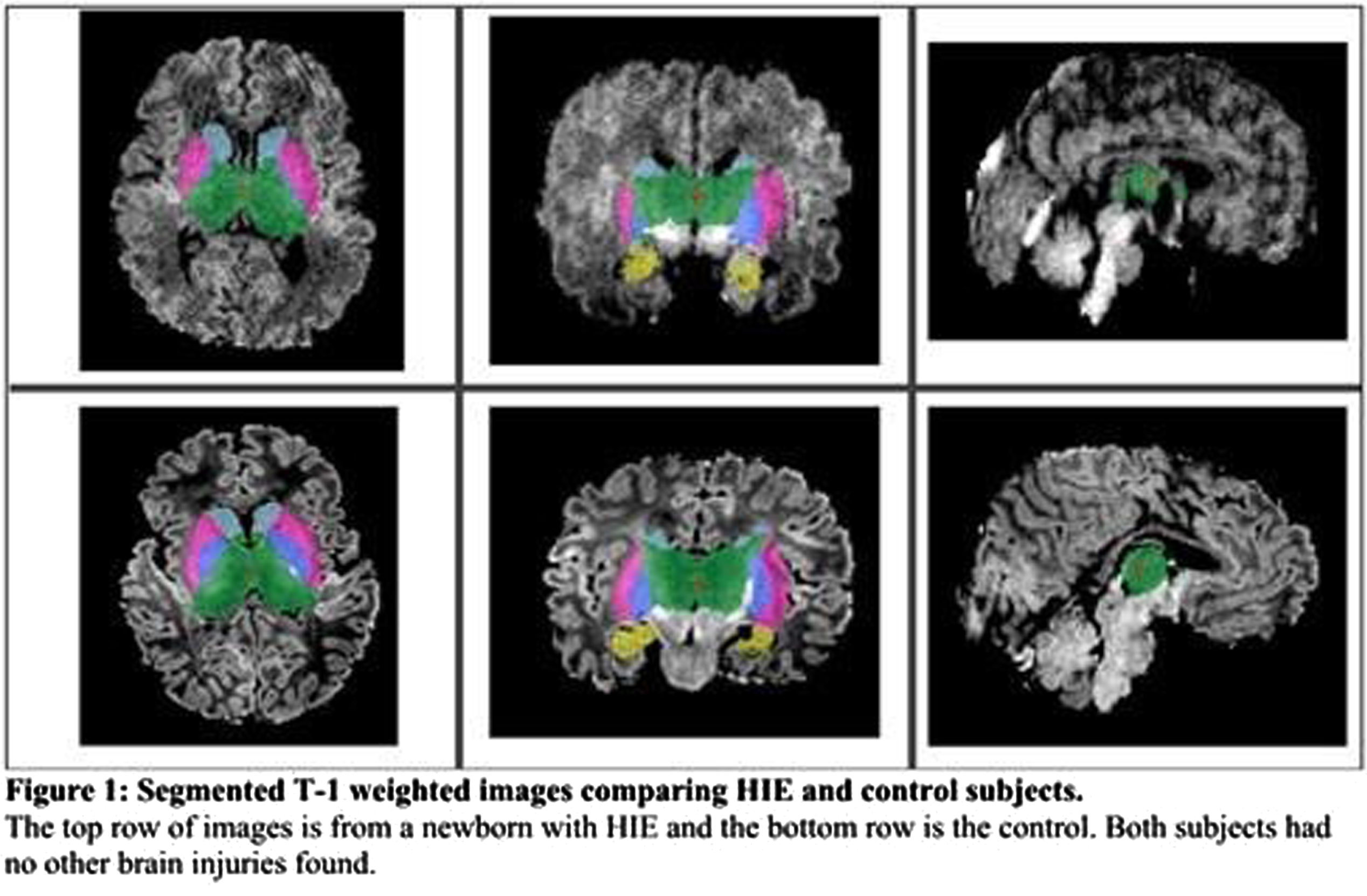
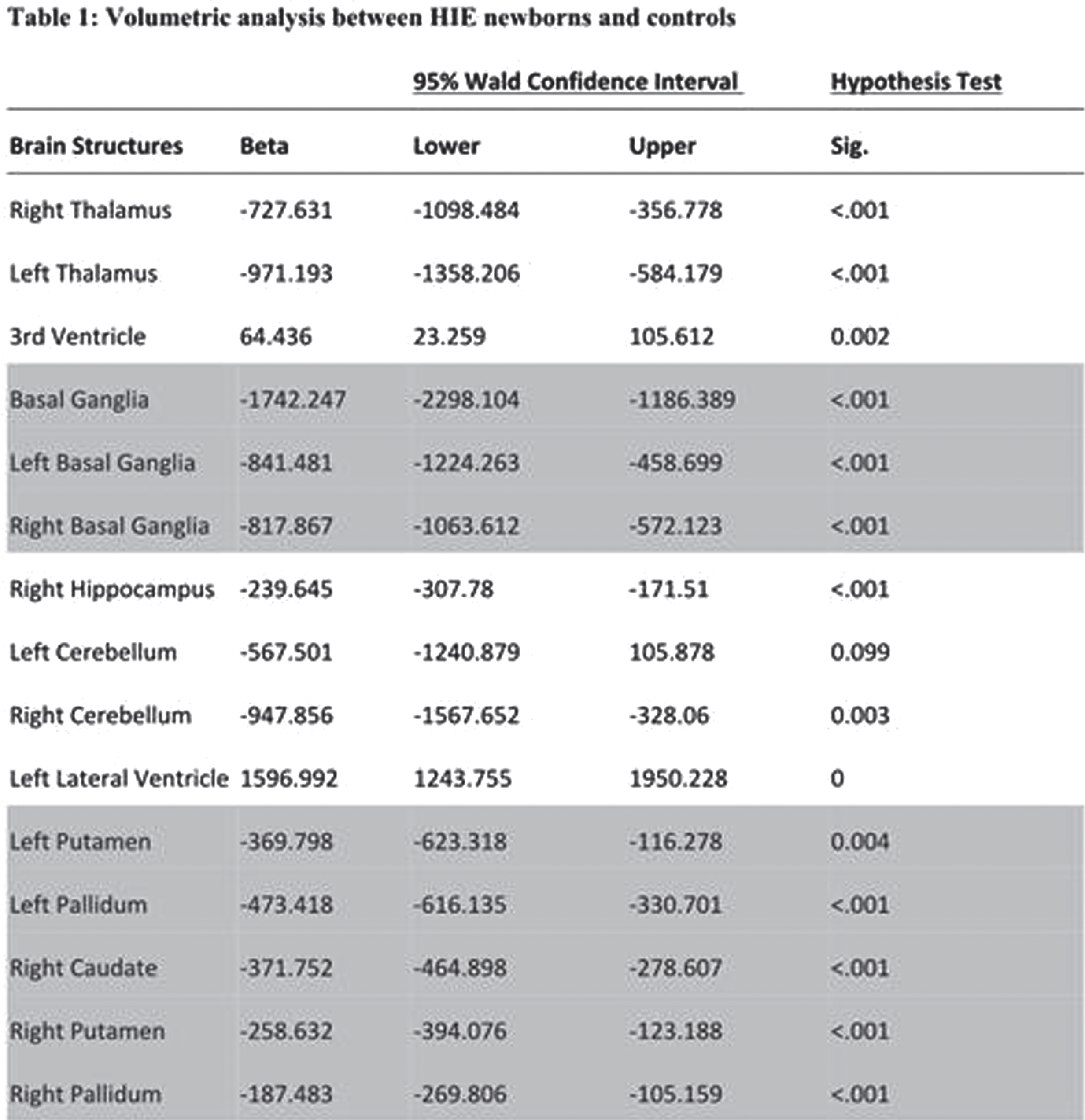
CONCLUSIONS: Newborns with HIE, scanned with MRI within the first days of life, had smaller subcortical volumes impacting sensory and motor regions, including the thalamus, basal ganglia and cerebellum, compared to healthy newborns. HIE severity was associated with smaller volumes, particularly impacting the basal ganglia, suggesting heightened vulnerability of these structures to perinatal asphyxia. The sample size was not large enough to draw conclusions on the neuroprotective advantages of TH in relation to basal ganglia volume
Evaluation of the neurodevelopmental effects and mechanisms of phthalates in glial cell culture
Keles Gulnerman E1,2, ARAL A3,7, Elmazoglu Z4, Ergun M5, Abbasoglu Topa E2, Kazan H6, Bolay H2,7,8
1Northwestern University, Feinberg School of Medicine, Hybrid And Machine Intelligence Lab, Chicago, United States, 2Gazi University Faculty of Medicine, Neuropsychiatry of Education, Research and Application Center, Ankara, Turkey, 3Izmir Demokrasi University, Faculty of Medicine, 5Department of Immunology, Izmir, Turkey, 4Medipol Ankara University, Faculty of Pharmacy, Ankara,Turkey, 5Gazi University, Faculty of Medicine,Genetics, Ankara, Turkey, 6Near East University, Faculty of Medicine, Genetics, Lefkosa, Turkish Republic North Cyprus, 7Gazi University, Faculty of Medicine, Neurology, Ankara, Turkey, 8Neuroscience and Neurotechnology Center of Excellence (NÖROM), Ankara, Turkey
BACKGROUND: Phthalates are used to convert the hard plastic polyvinyl chloride into a flexible plastic. The most common phthalate, “Di-(2-Ethylhexyl) phthalate (DEHP),” is frequently used as a plasticizer of PVC products in medical materials such as food packaging, clothing, car products, children’s products, mosquito killers, and blood transfusion bags.
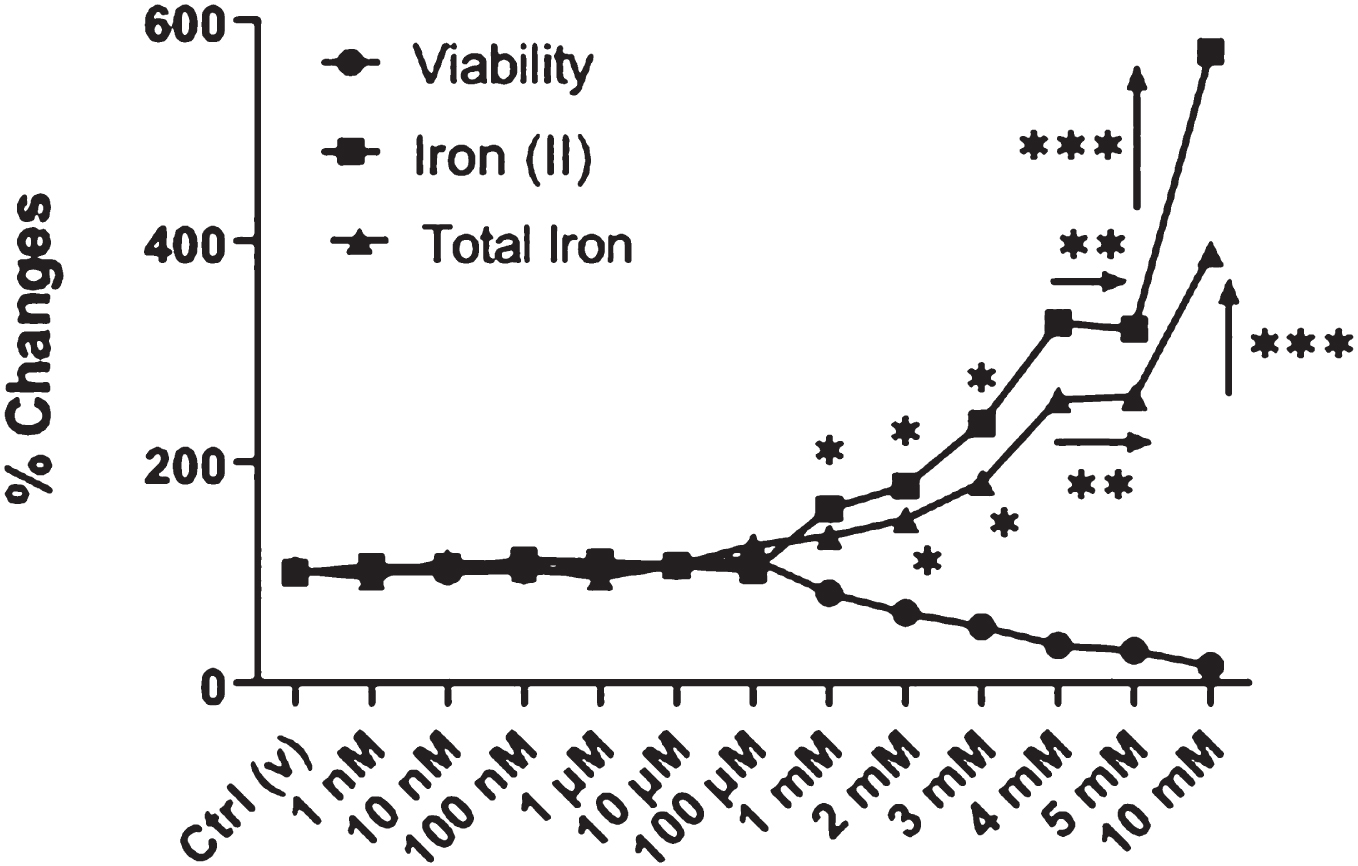
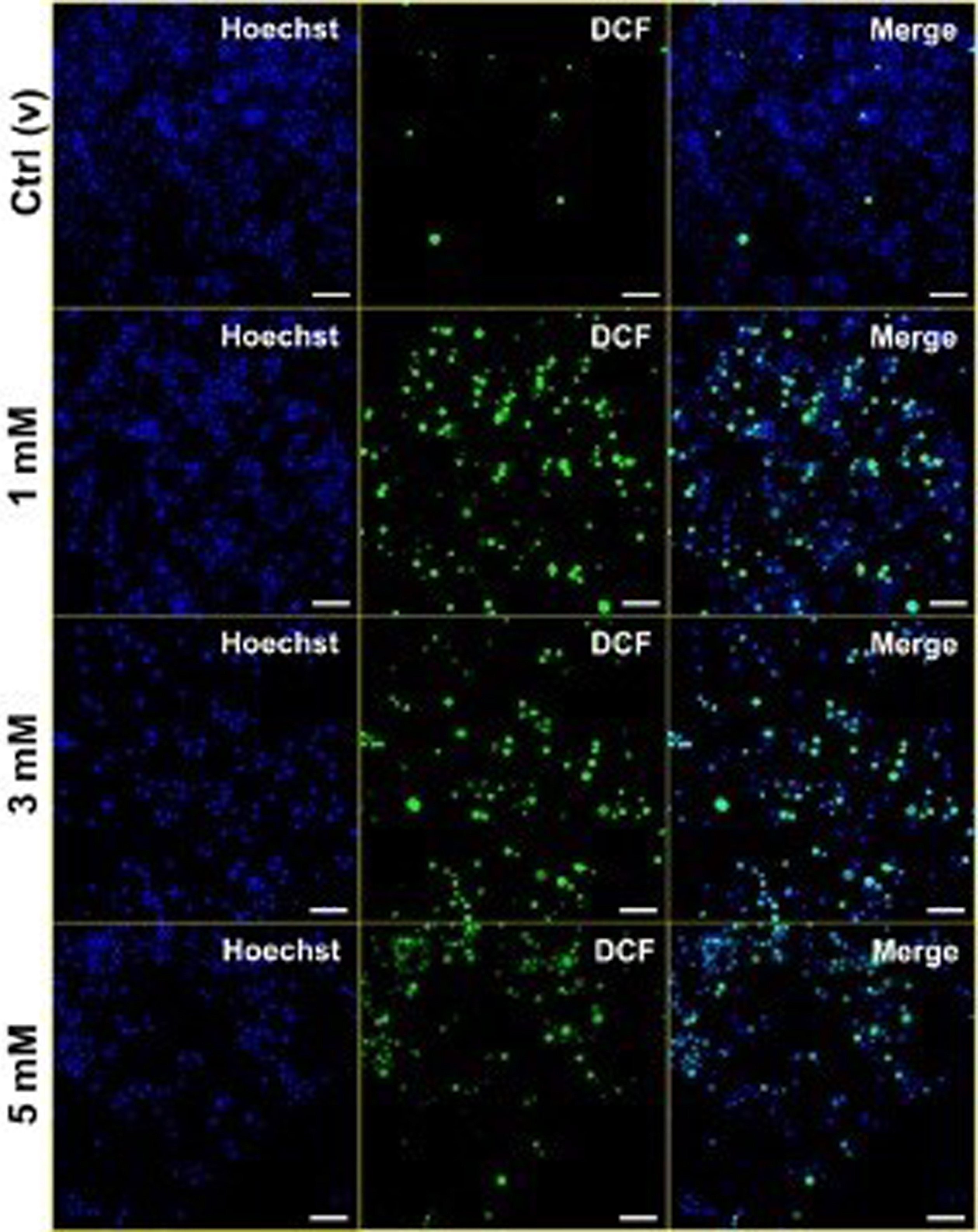
METHODS: Phthalates can cross the placenta and the blood-brain barrier. DEHP has been shown to cause neurodevelopmental impairment in vivo and in vitro, but the molecular mechanisms for this effect have not been elucidated. BV-2 neonatal microglial cells were cultured and incubated with phthalate at doses of 1 nM-10 mM overnight. Cellular toxicities were determined by MTT assay. Intracellular ferrous (Fe2+) and total iron levels, and intracellular reactive oxygen species (ROS) levels were measured by spectrophotometric approach. Cell death mechanisms were followed by Apoptosis/Necrosis Ratio by Acridine Orange/Ethidium Bromide dual staining. Lysosomal and mitochondrial integrity was figured out by Hoechst/Neutral Red/Janus Green B triple staining. Ferritin and CD11b immunoreactivity were evaluated by IFA and IL-1β, and IL-18 cytokine levels were followed by ELISA method. Expression levels of iron metabolism-related genes (ferritin analysis, DMT1 (importer), and ferroportin (exporter)) were determined by RT-qPCR To establish that it produces neuroinflammation via ferroptosis, a ferroptosis inhibitor was applied; it was observed that the increase in cell viability. The expression of GPX4 and ACSL4 was quantified using real-time polymerase chain reaction (qPCR) to determine whether the ferroptosis pathway›s essential enzymes are involved.
RESULTS: All tests were repeated three times separately, and data were analyzed by either Student’s t-test or ANOVA and were significant when p<0.05. Intracellular Fe2+ levels increased dramatically at 1 nM-10 mM concentrations of phthalate. Total iron levels, which mediate the reduction of Fe+3 ion to Fe+2 ion, also increased statistically. Free iron was found to be higher than total iron for phthalate-treated cells. At 1-5 mM of phthalate doses, apoptosis increased compared to non-treated controls. It was observed that the lysosomal and mitochondrial integrity was impaired, ferritin and CD11 staining, IL-1β and IL-18 cytokine levels, Ferritin-L, and Ferroportin expressions were increased upon phthalate treatment. GPX4 enzyme expression was also found to be statistically increased with increasing DEHP levels of exposure.
CONCLUSIONS: It has been shown that exposure to phthalate causes inflammation and microglial death via mainly ferroptosis and other inflamatory-regulated cell death pathways mediated by iron and ROS. It is predicted that exposure to DEHP is associated with neuroinflammation and microglial loss in the developing brain, especially in hospitalized children and infants.
Head ultrasound biomarkers of early language outcomes in preterm infants assessed at a multidisciplinary clinic during the first year of life
Trapp N1, Carrasco M1
1Department of Neurology, Section of Pediatric Neurology, University Of Wisconsin School Of Medicine And Public Health, Madison, United States
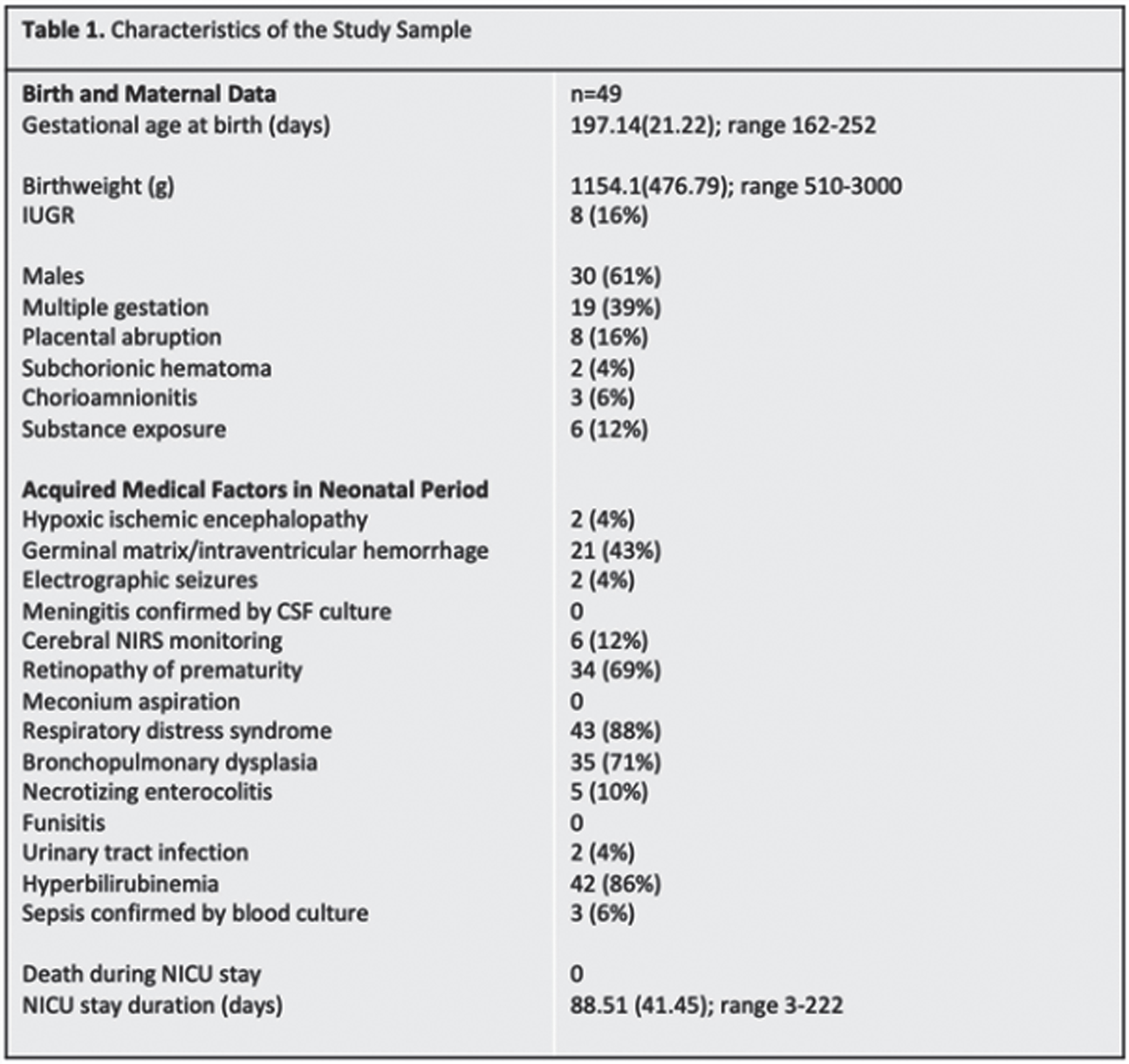
BACKGROUND/PURPOSE: Premature birth is a risk factor for a range of neurodevelopmental deficits, including receptive and expressive language delay.1 Serial head ultrasound (HUS) monitoring is readily available in most NICUs, and the ability to predict language outcomes based on easily obtainable ventricular measurements would be useful to inform clinical practice and early interventions. Our study aims to establish the utility of quantitative HUS ventricular measurements in predicting language outcomes before 1 year corrected age (CA) among infants born premature.
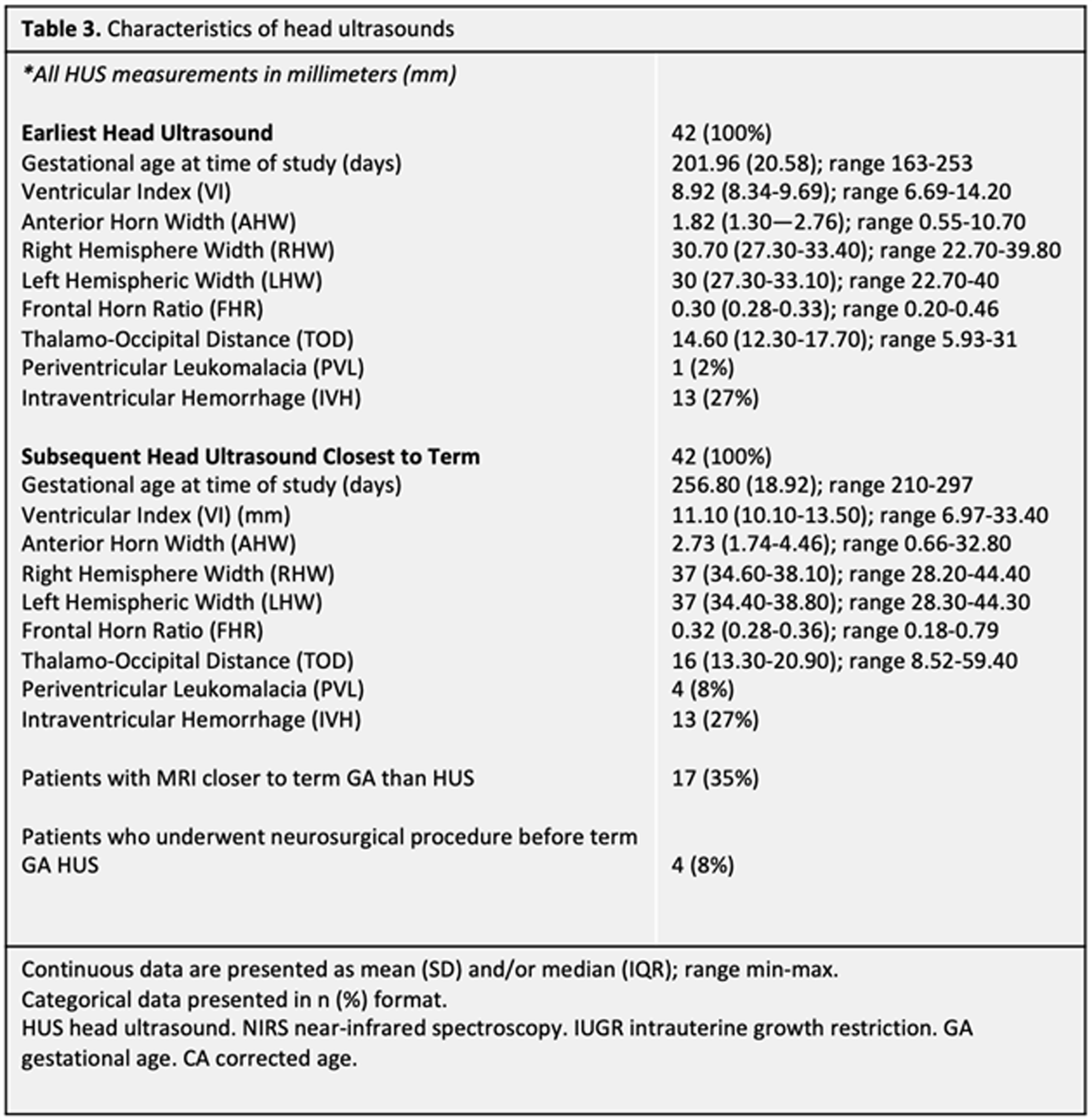
METHODS: Forty-nine neonates (30 males, 19 females) born before 37 weeks gestational age (GA) between 2014-2021 completed at least two sets of HUS. Ventricular measurements were performed on the patient’s initial HUS completed at/near birth (range 23w2d-36w1d GA), hereafter referred to as the Early HUS. Ventricular measurements were also made on the Late HUS, defined as either the last HUS prior to neurosurgical intervention for hydrocephalus management or, in patients where no surgical intervention was performed, the HUS recorded closest to term age (range 30w0d-42w3d GA). Ventricular measurements, including Ventricular Index (VI), Anterior Horn Width (AHW), Hemispheric Width (HW), and Thalamo-occipital Distance (TOD), were manually determined. Patients also completed ≥ 1 multidisciplinary newborn clinic follow-up visits before 1 year CA. Thirty patients completed a Receptive-Expressive Language Test (REEL) between 6-12 months CA, with scores assigned for Expressive, Receptive, and Total Language Ability. Logistic regressions were used to examine the predictive value of HUS ventricular measurements and evidence of language dysfunction between 6-12 months CA.
RESULTS: Twenty-two of 30 patients performed within average range for age in Receptive Ability and 23 were within this range in Expressive Ability. Logistic regressions were performed to ascertain the effects of various quantitative ventricular measurements on the likelihood that participants would have abnormal language abilities between 6-12 months CA. Increasing VI, AHW, FHR, and TOD Late HUS values were associated with an increased likelihood of patients exhibiting sub-average receptive (VI: p = 0.050; AHW: p = 0.043; FHR: p = 0.045; TOD: p =0.033, respectively) and expressive (VI: p = 0.034; AHW: p = 0.31; FHR: p = 0.034; TOD: p = 0.039, respectively) language abilities. It is important to note that findings were not significant after correcting for multiple comparisons with the Bonferroni correction.
CONCLUSIONS: Data collection is ongoing; however, this initial analysis suggests that there may be associations between quantitative HUS biomarkers and language outcomes after 6 months CA, as assessed by the REEL among children born premature. Next steps will include expansion of the sample size to strengthen the study, as well as exploration of MRI as a more sensitive prognostication tool in this setting.
REFERENCES
[1] Vohr B. R. Language and hearing outcomes of preterm infants. Seminars in Perinatology. 2016; 40(8), 510–519.
Serial brain imaging and targeted neuropromotive intervention for very preterm infants in the NICU: Study protocol and preliminary outcomes
Sharon D1, Roychaudhuri S1, Singh E1, Steele T1, Sheldon Y1, Cuddyer D1, Flynn P1, Pacheco J1, Gibson K1, Kling E1, El-Dib M1,2, Pineda R3, Inder T1,2,4, Erdei C1,2
1Brigham And Women’s Hospital, Boston, United States, 2Harvard Medical School, Boston, United States, 3Chan Division of Occupational Science and Occupational Therapy, University of Southern California, Los Angeles, United States, 4Division of Neonatology, Children’s Hospital of Orange County and University of California, Irvine, Irvine, United States
BACKGROUND AND PURPOSE: Babies born preterm undergo rapid brain growth and development prior to term age while hospitalized in the NICU (1), a critical window for intervention. Early neuropromotive intervention is essential and should be initiated as soon as possible after birth for very preterm (VP) infants in the NICU. This study aims to enhance our understanding of early brain development in VP infants in relationship to intensive neurodevelopmental care. The Supporting and Enhancing NICU Sensory Experiences (SENSE) program is a sensory-based intervention that emphasizes meaningful multisensory exposures (2,3) to improve infant and family outcomes (4).
HYPOTHESIS: Implementing targeted early neuropromotive interventions in the NICU will improve neurodevelopment of VP infants.
METHODOLOGY: We plan to enroll 75 VP infants, born before 33 weeks gestation, in a level-III NICU prospectively over two years. The infants will be divided into three groups (see Figure 1). Infants are assigned to a group (low-risk or high-risk) depending on whether significant neurological injury is present on early imaging (high risk defined as IVH with any ventricular dilatation, white matter injury, cerebellar hemorrhage). Implementation of the SENSE program is preferentially done by NICU families with coaching from developmental therapists and NICU staff (4). Serial MRIs are performed over at least three time points until term equivalent age (TEA). Infants who met inclusion criteria, but were either unable to enroll or declined enrollment, were offered enrollment in the standard care group. Enrolled babies undergo standard neurodevelopmental assessments during their NICU stay and at outpatient clinical follow-ups until two years of age.
Legend 1:
Diagram of enrollment set-up for study
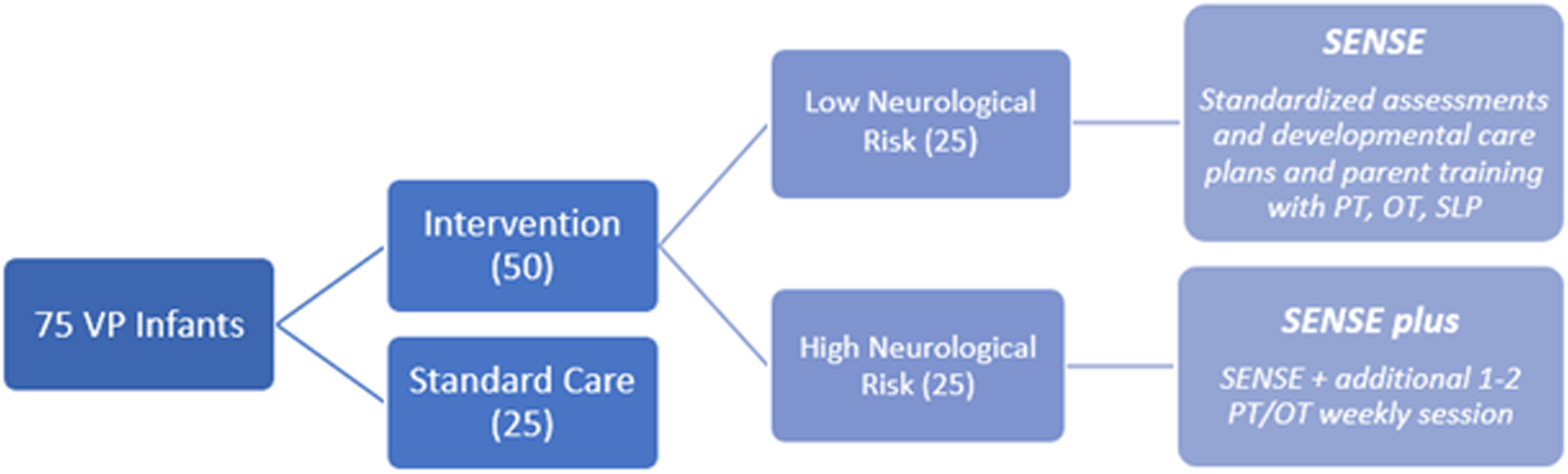
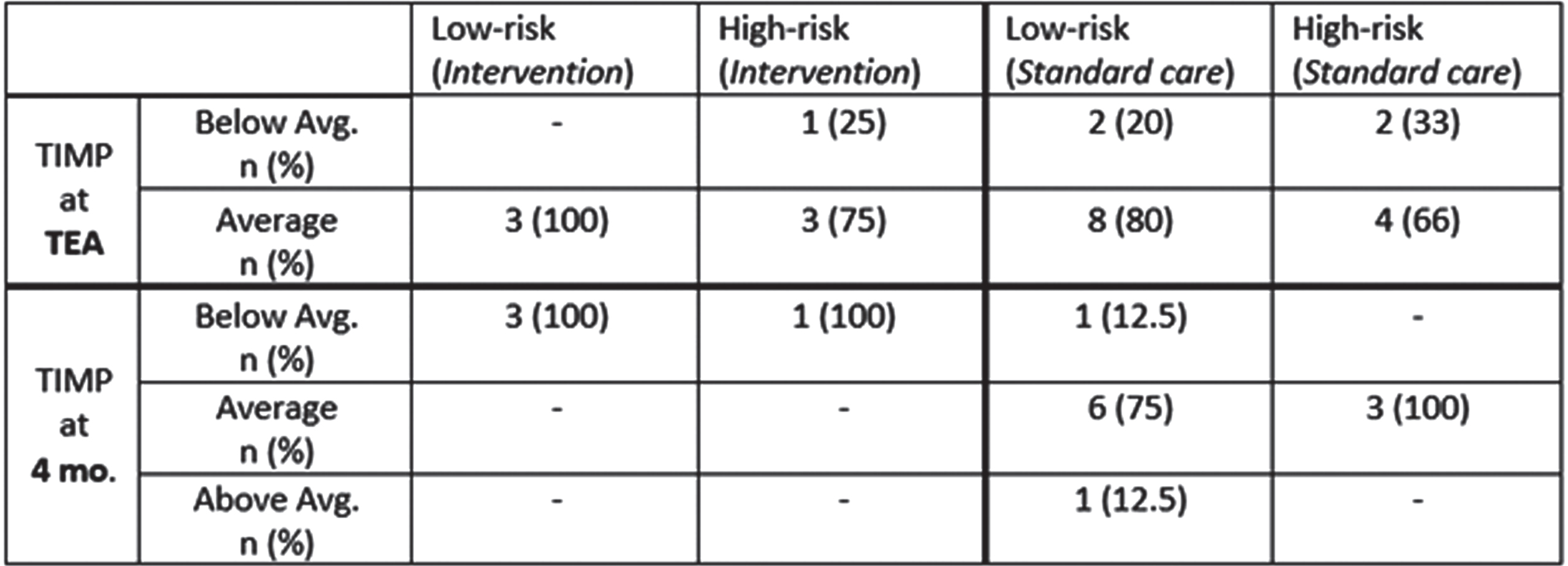
RESULTS: To date, 31 infants have been enrolled: 10 intervention (5 low-risk and 5 high-risk) and 21 standard care (15 low-risk and 6 high-risk). 87 MRI scans have been performed with 26 at TEA. Eight babies (80%) in the intervention group received all interventions with delivery of 75% or more of the targeted multisensory experiences. 23 infants (7 intervention and 16 standard care) had a TIMP (5) assessment prior to NICU discharge. To date, a total of 4 (100%) babies in the intervention group and 12 (92%) babies in the standard care group have returned to the developmental follow-up clinic at 4 months corrected age (TIMP results in Table 1). Early data show that many infants at TEA in standard care are below average while at 4 months this is less prominent. To date, too few subjects have had outcomes in the intervention group to assess impact, although many still display below average performance despite interventions.
Legend 2:
Results from the TIMP at TEA and at a 4-month follow-up visit.
CONCLUSION: Serial imaging provides valuable insight into the pattern of brain growth and neurodevelopment in VP infants. The impact of implementing targeted interventions in the NICU setting on improving the outcomes in VP infants with and without neurological injury continues to be investigated.
REFERENCES
1. Pineda S J., Roussin J., Wallendorf M., Kellner P., Colditz G. Randomized clinical trial investigating the effect of consistent, developmentally-appropriate, and evidence-based multisensory exposures in the NICU. Journal of Perinatology. 2021; 41(10), 2449–2462.
2. Pineda R, Smith J. 2017 [Parent Education PPT] Washington University in St. Louis, Missouri. Retrieved from https://chan.usc.edu/research/clinical-tools/sense/content
3. Pineda R, Smith J. 2017 [USC-SENSE Week-By-Week Guide] Washington University in St. Louis, Missouri. Retrieved from https://chan.usc.edu/research/clinical-tools/sense/content
4. Pineda R, Wallendorf M, Smith J. A pilot study demonstrating the impact of the supporting and enhancing NICU sensory experiences (SENSE) program on the mother and infant. Early Human Development. 2020; 144: 1-8.
5. Kim S. A., Lee Y. J., Lee Y. G. Predictive Value of Test of Infant Motor Performance for Infants based on Correlation between TIMP and Bayley Scales of Infant Development. Annals of rehabilitation medicine. 2011, 35(6), 860–866.
Inflammatory injury in the postnatal subventricular zone neural stem cell niche follows neonatal intestinal perforation
Epstein A1, Chao A1, Abdi K1, Jain V1, Rikard B1, Younge N1, Maxfield C1, Gregory S1, Benner E1
1Duke University School of Medicine, Durham, United States
BACKGROUND AND PURPOSE: Intestinal perforation in preterm infants has been linked to neurodevelopmental impairment. However, the mechanism(s) coupling systemic inflammatory disease to preterm brain injury are not well-defined. The subventricular zone (SVZ) is a transient neural stem cell niche residing in the germinal matrix that generates glial progenitors required for proper myelination and neuronal progenitors that migrate to the frontal lobes postnatally. Notably, our group is also presenting data on an association between SVZ echogenicity (SVE) using cranial ultrasound associated with intestinal perforations (Maxfield et al.)
Here, we investigated structural and functional changes in the SVZ using neonatal modeled intestinal perforation (MIP) in rodents.
MIP led to increased macrophage/microgliosis and increased proinflammatory cytokines in the SVZ compared to littermate controls. Disruption of multiciliated ependymal cell organization was seen in SVZ whole mounts using FoxJ1-EGFP tg-mice, as well as decreased cilia on the ventricular surface visualized using scanning electron microscopy. We employed single nuclear transcriptomic profiling (snRNAseq) to study discrete SVZ niche cell populations in both MIP and littermate control mice.
RESULTS: In silico pathway analysis showed ependymal cell and macrophage clusters significantly upregulate multiple inflammatory pathways. Analysis of the neural progenitor lineages demonstrated decreased cell frequency and downregulation of metabolic pathways while also showing increased EGF receptor signaling following MIP. Deficits in postnatal neurogenesis were confirmed using confocal microscopy of doublecortin (DCx)-positive cell chain organization across the lateral ventricular wall in young adult mice.
CONCLUSIONS: Our animal data suggests that SVZ injury following intestinal perforation leads to deficits in neurogenesis during a critical time of neurodevelopment. This previously undescribed injury may be an important molecular mechanism linking inflammatory complications following preterm birth and poor neurodevelopmental outcomes in infants.
Pain sensitivity in newborns
Witulska-Alagöz A1, Sadowska-Krawczenko I1
1Department Of Neonatology, Collegium Medicum N.c. University, Jan Biziel University Hospital No. 2, Bydgoszcz, Bydgoszcz, Poland
BACKGROUND AND PURPOSE: The Neonatal Intensive Care Unit (NICU) is a place that provides life-saving medical care for the increasing number of newborns each year. During treatment they are at high risk for repeated pain exposure[1]. The most vulnerable are neonates born prematurely that experience numerous painful procedures for a prolonged period of time.
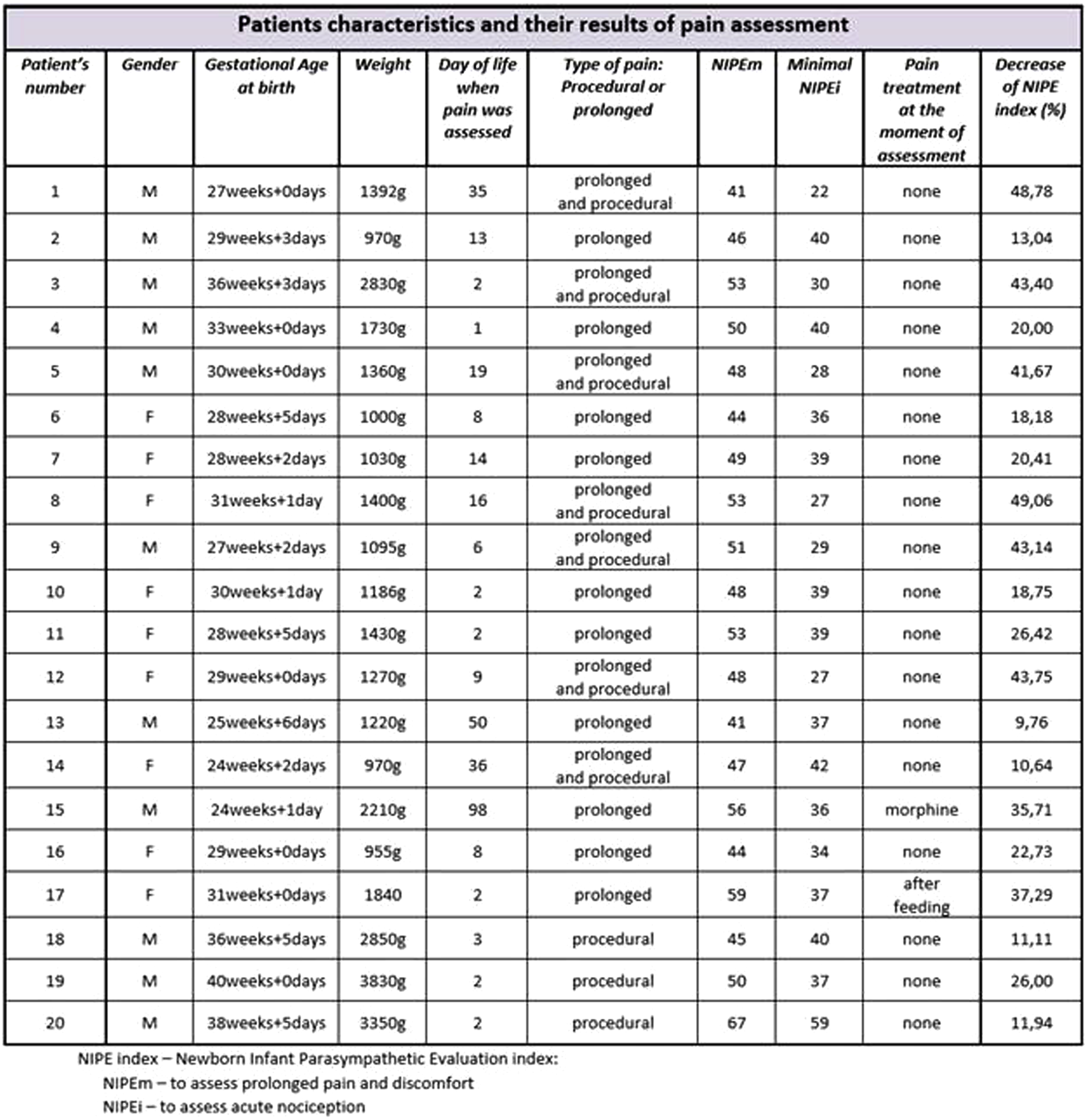
The overall objective of the research was to assess pain sensitivity in newborns during their hospitalization at the Neonatal Department using the NIPE (Newborn Infant Parasympathetic Evaluation) index that correlates with the baby’s comfort and the level of experienced pain.
METHODOLOGY: This was a prospective observational study. Patients were evaluated with the NIPE Pain Monitor. Neonates born after the beginning of the 24th week of gestational age experiencing an acute procedural pain or prolonged discomfort (ventilated invasively or non-invasively) were included in the research. Newborns with neurological or cardiac congenital anomalies; diagnosed with severe encephalopathy; suffering from cardiac arrythmia; with circulatory failure requiring infusion of vasopressive drugs or fluid resuscitation were excluded from the study.
RESULTS: 20 neonates (median gestational age 30,15weeks and 2,05days) were assessed in the study. The evaluation showed that even the youngest patients in NICU feel pain and react to painful procedures. Premature infants with relatively immature nervous systems are less reliably able to present behavioral signs of noxious stimuli in comparison with more mature infants[2]. The NIPE index was an objective result revealing their nociception. Pain sensitivity was also measured by the decrease of the NIPE index and it depended on very different factors like the intensity and type of painful stimuli, the gestational age, the duration of hospitalization and the pain treatment method.
CONCLUSIONS: All newborns are able to feel pain[3]. Early and cumulative pain exposure in neonates during a critical period of development has been associated with adverse long-term physiological, cognitive and social effects[4]. Therefore the great concern about that consequences gives the motivation to assess the pain accurately and start the optimal treatment.
The prevention of pain in neonates, minimizing the number of painful procedural interventions and providing adequate analgesia are among the primary goals of neonatal care.
REFERENCES
[1] Campbell-Yeo M, Eriksson M, Benoit B. Assessment and Management of Pain in Preterm Infants: A Practice Update. Children(Basel). 2022Feb 11;9(2):244.
[2] Green G, Hartley C, Hoskin A, Duff E, Shriver A, Wilkinson D, Adams E, Rogers R, Moultrie F, Slater R. Behavioural discrimination of noxious stimuli in infants is dependent on brain maturation. Pain. 2019Feb;160(2):493-500.
[3] Hatfield LA. Neonatal pain: What›s age got to do with it? Surg Neurol Int. 2014Nov13;5(Suppl 13):S479-89.
[4] McPherson C, Miller SP, El-Dib M, Massaro AN, Inder TE. The influence of pain, agitation, and their management on the immature brain. Pediatr Res. 2020Aug;88(2):168-175.
Fetal neurology practice survey
Tarui T1, Venkatesan C2, Gano D3, Lemmon M4, Mulkey S5, Pardo A6, Emrick L7, Scher M8, Agarwal S9
1Tufts Medical Center, Boston, United States, 2Cincinnati Children’s Hospital Medical Center, Cinncinati, United States, 3UCSF Weill Institute for Neurosciences, San Francisco, United States, 4Duke University School of Medicine, Durham, United States, 5Children’s National, DC, Washington, United States, 6Lurie Children’s Hospital of Chicago, Chicago, United States, 7Texas Children’s Hospital, Houston, United States, 8University Hospitals of Cleveland, Cleveland, United States, 9Children’s Hospital of Philadelphia, Perelman School of Medicine at University of Pennsylvania, Philadelphia, United States
BACKGROUND: Fetal neurology is a rapidly evolving subspecialty that centers on the diagnosis and treatment of neurologic conditions that impact the developing brain. Innovations in fetal diagnostic tools and therapies have resulted in an increased need for specialized training. Prenatal diagnostic input with postnatal continuity of care can better address sequelae including developmental, behavioral, epileptic and mental health disorders into adulthood. Despite the growing need for fetal neurology services, little is known about where, how, and by whom fetal neurology care is delivered in current practice.
METHODOLOGY: We distributed a survey characterizing fetal neurologic care delivery to practicing child neurologists. The survey was administered by the Fetal Neurology Consortium, which includes representatives from 9 sites across the United States. An online fetal neurology practice questionnaire using a Redcap database survey was posted to the Child Neurology Society member website in November 2021 and March 2022, inviting member child neurologists and other specialists who currently perform fetal neurology consultations. The survey results were summarized using descriptive statistics.
RESULTS: Representatives from 43 US institutions responded to the survey. Most centers had a fetal diagnostic center (n=38/43, 88%), with about half using a fetal neurology registry. Most centers performed fetal ultrasound and MRI studies on site. The annual number of fetal consultations ranged between <20 for 40% to >100 for 17% of the respondents. About 50% of respondents were subspeciality trained in fetal-neonatal neurology (18) or neurogenetics (7). Earliest gestational age of fetal MRI was between weeks 16-19 weeks in 30% of centers and 20-23 weeks in 30%. Ventriculomegaly, corpus callosal anomalies, absent septum pellucidum, and malformations of posterior fossa or cortical development were the top reasons for consultation. 25% of respondents participated in interdisciplinary consultations. Frequency and types of prenatal genetic testing varied between centers. About 40% had prenatal whole exome sequencing as an option. A majority of the respondents followed neurodevelopment clinically, but formal developmental testing was not systematically used for most respondents. Most respondents (n=39/43, 91%) were interested in participating in a collaborative national registry for prenatally diagnosed neurological disorders. The top-five priorities for the field ranked by respondents were clinical guidelines, long-term follow-up data, fetal imaging, practice improvement and prenatal counseling technique.
CONCLUSION: Fetal neurology consultations are increasingly common in the US. This survey of 43 centers highlights heterogeneity in practice, lack of clinical practice standardization, and high demand for practice guidelines and practical education. Interdisciplinary collaborations will be crucial for relevant curriculum and program development, including increasing attention to healthcare disparities. This synergy among different disciplines will improve outcomes and may reduce the burden of neurological diseases with fetal origins across the lifespan.
REFERENCES
[1] Danziger P, Laventhal N. Prenatal consultation: perspectives on training, relevance, and utilization among pediatric subspecialty program directors. J Perinatol. 2018 Aug;38(8):989-996. doi: 10.1038/s41372-018-0121-z. Epub 2018 May 8. PMID: 29740188.
[2] Kett JC, Woodrum DE, Diekema DS. A survey of fetal care centers in the United States. J Neonatal Perinatal Med. 2014 Jan 1;7(2):131-5. doi: 10.3233/NPM-14814005. PMID: 25104126.
[3] Ream MA, Mulkey SB. A Neurologist’s Practical Guide to Conducting a Fetal Consultation. Semin Pediatr Neurol. 2022 Jul;42:100957. doi: 10.1016/j.spen.2022.100957. Epub 2022 Mar 25. PMID: 35868732.
[4] Agarwal S, Scher MS. Fetal-neonatal neurology program development: Continuum of care during the first 1000 days. J Perinatol. 2022 Feb;42(2):165-168. doi: 10.1038/s41372-021-01282-5. Epub 2021 Nov 30. PMID: 34848849.




