Multiple vascular malformations in a newborn
Abstract
We describe the case of a term newborn who presented with congenital testicular torsion at 10 hours of age. During the evaluation of this problem, additional malformations were encountered. Diagnostic and therapeutic considerations are addressed.
1Case report
A term male infant was born at 40 weeks’ gestation to a 37-year-old primigravida mother. The pregnancy was achieved through in vitro fertilization. The mother was admitted twice during the pregnancy for preterm labor. She was given corticosteroids at 27 and 31 weeks of pregnancy. Labor ensued at 40 weeks, but failure to progress led to a Cesarean section. The baby was appropriate for gestational age (3130 g) and transitioned without difficulty. Apgar scores were 9 and 10 at one and five minutes, respectively.
At 10 hours of age, discoloration of the scrotum was noted. Vital signs were all normal. An arterial blood gas sample at 11 hours of age revealed pH 7.33, pCO2 42.6 torr, BE – 3.7 mEq/L, lactate 3.5 mmol/L, and glucose 48.6 mg/dL (Fig. 1).
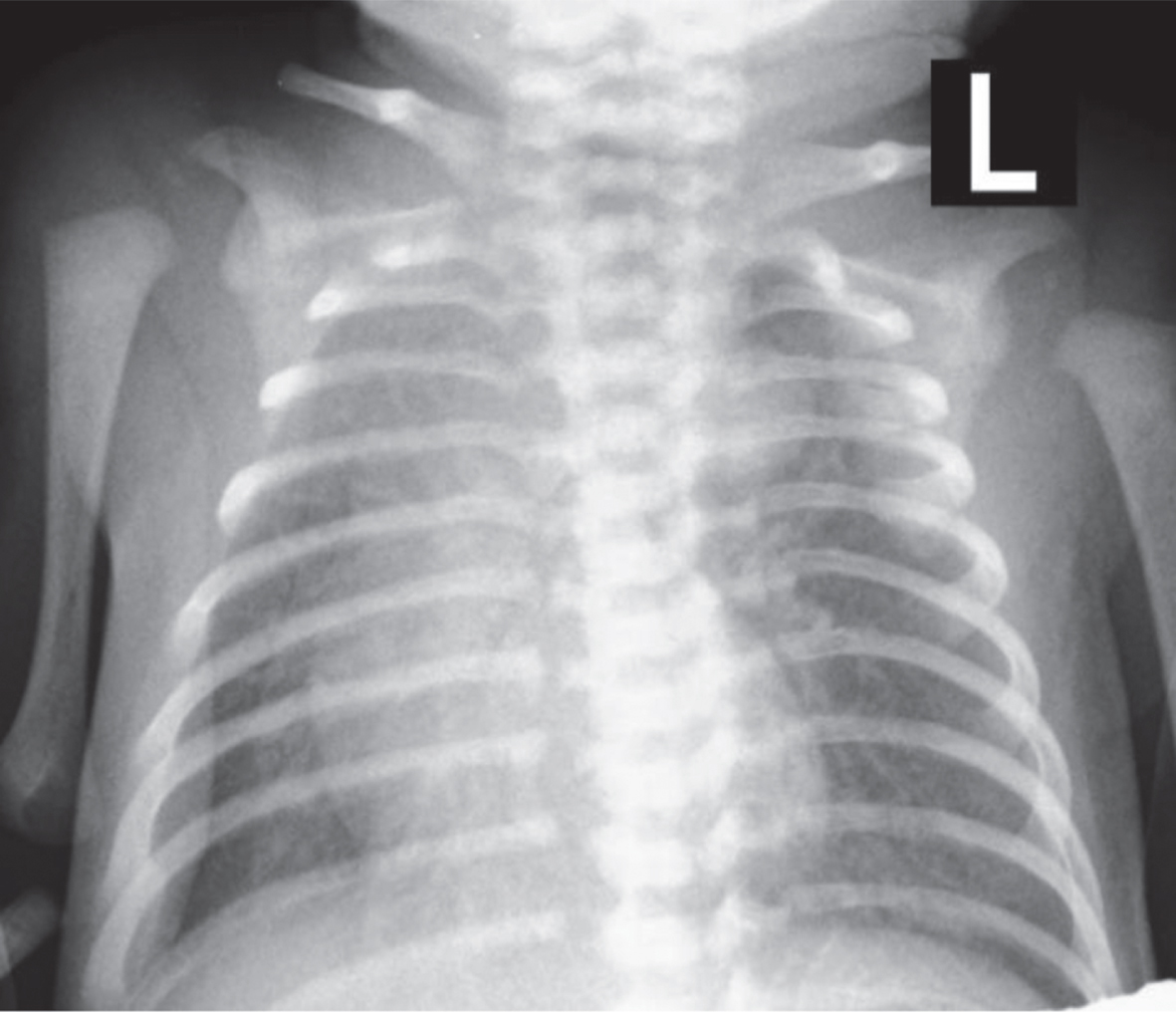
Fig. 1
Antero-posterior radiograph of the aneurysm of the ductus arteriosus, with less areation of the right upper lung lobe.
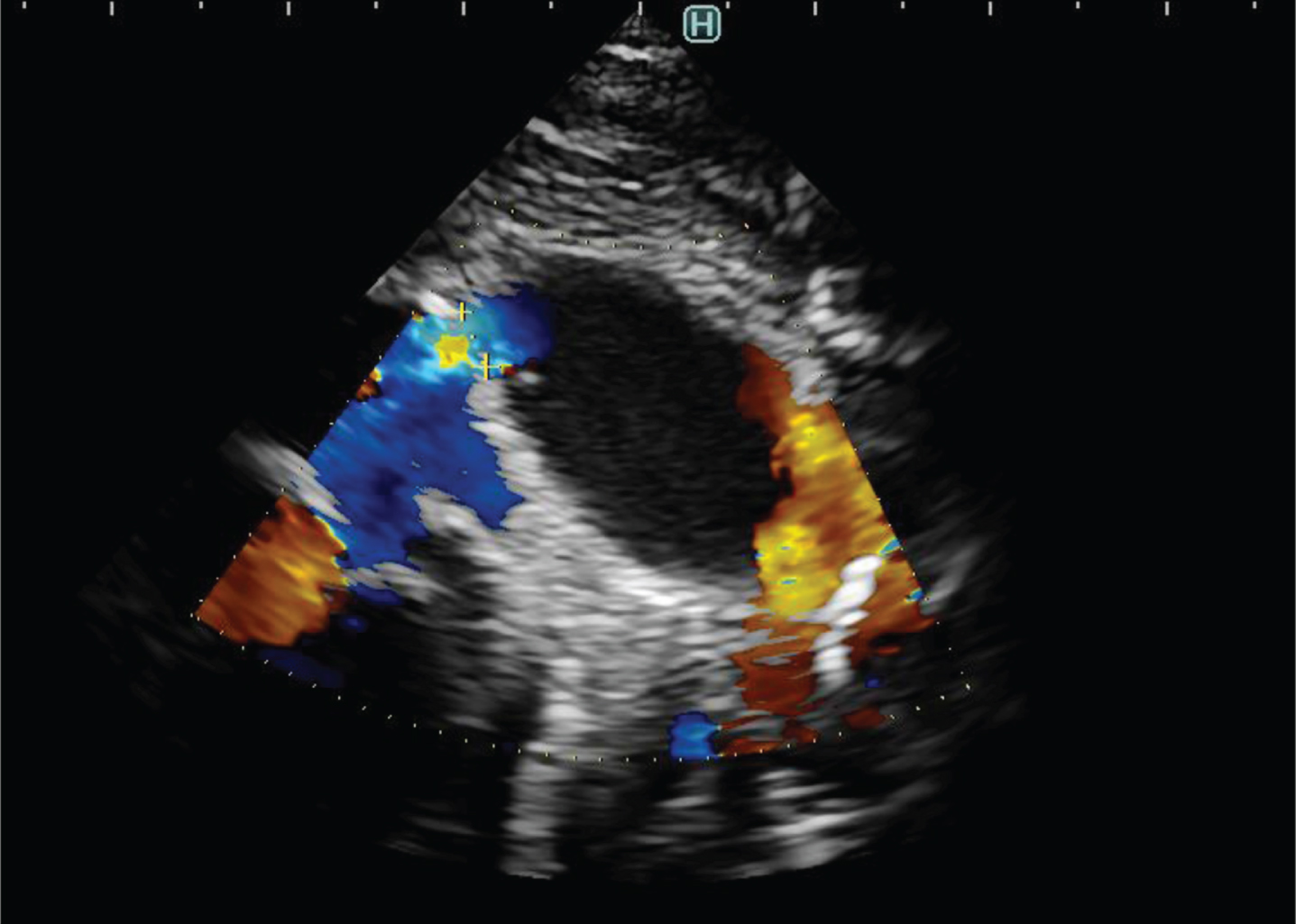
During the presurgical evaluation of the heart, an aneurysm of the ductus arteriosus was found on echocardiography. It measured 20×14 mm but was without other morphologic pathology (Fig. 2). The hemodynamic function of the heart was normal, output of the left and right ventricles was adequate. Blood gases and acid-base balance remained normal.
Fig. 2
Echocardiogram demonstrating aneurysmal extension of PDA 20×15×14 mm without signs of thrombus in the PDA cavity.
The Cardiosurgical Center was consulted and it was determined that there were no contraindications to emergency surgery for the testicular torsion. The baby successfully underwent a right orchidectomy and left orchidopexy under general anesthesia.
Post-operatively, it was also discovered that the baby had a duplex left kidney (3.8×2.4 cm and 3.2×2.2 cm) with two renal arteries and grade III hydronephrosis.
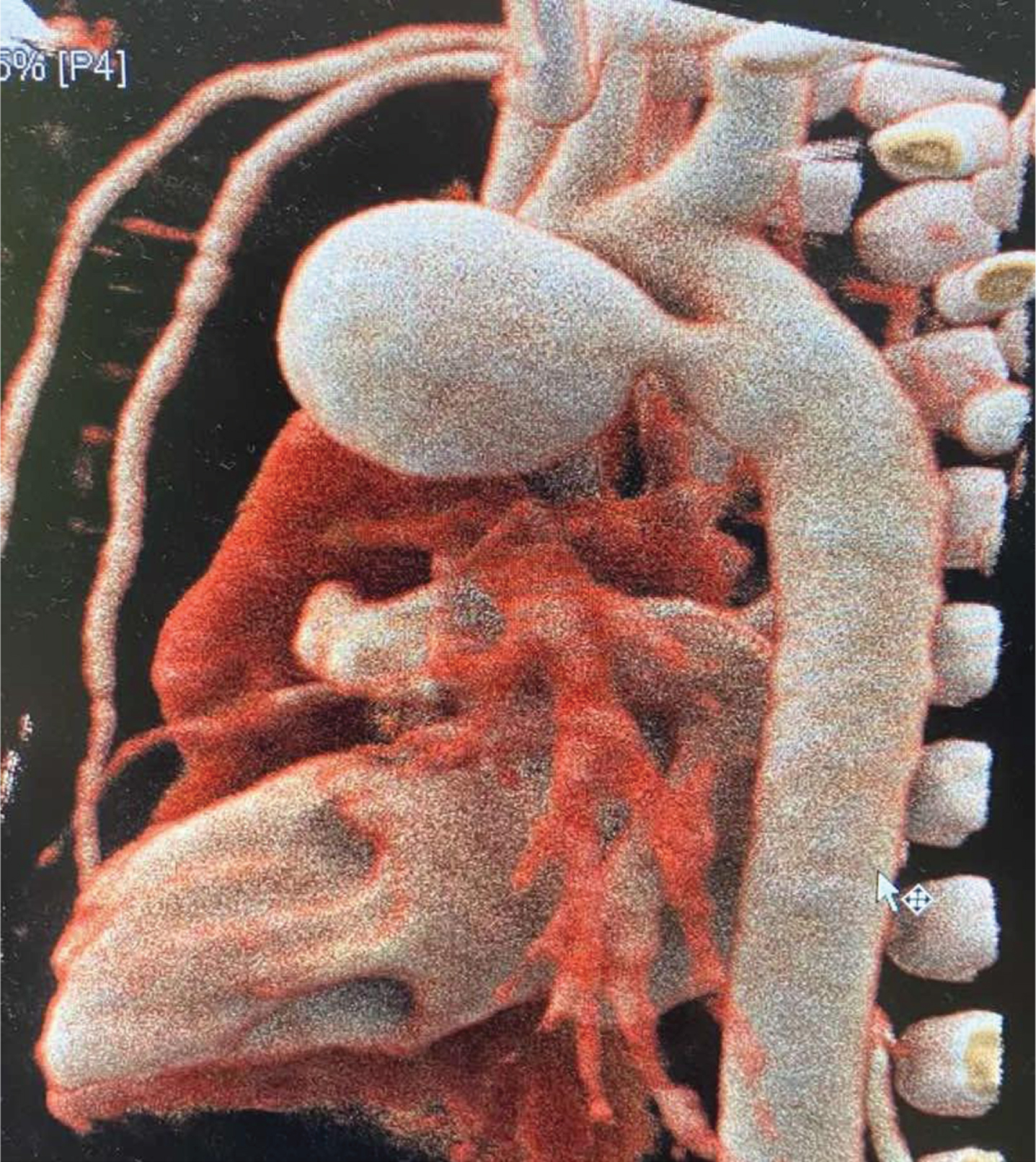
Because of the ductal aneurysm, the baby underwent computerized tomographic (CT) angiography for more detailed description. It demonstrated aneurysmal extension of the ductus arteriosus (20×14×14 mm) without signs of thrombosis in the PDA cavity, communication with the aorta and the main pulmonary artery (up to 2.5 mm in caliber), left atrium and left ventricle without dilation (indicating no hemodynamic significance), a normal ascending aorta, a mildly dilated left subclavian artery (6×5.5 mm) without signes of coarctation, a normal descending aorta without significant external compression of the central respiratory tree, and atelectatic changes in the middle/dorsal segments of the right lung. There were no signs of cardiac decompensation, no mural thrombi in the lumen of the aneurysm, no compression of surrounding structures (left bronchus, LPA), nor any signs of incipient rupture of the aneurysm with extravasation (Fig. 3).
Fig. 3
Computed tomographic angiography. Note the striking aneurysmal extension of the PDA 20×14×14 mm without signs of thrombus in the PDA cavity.
Because of absent clinical and CT signs of severity and an imminent threat to the patient, as well the reported low risk of aneurysmal rupture in the neonatal period, a conservative approach was chosen. Echocardiographic surveillance was performed every 2 to 3 days. The baby was treated with nasal CPAP, and antibiotics for postoperative atelectasis. The baby tolerated full enteral feedings beginning on day 6. The left-sided hydronephrosis was treated with bladder catheterization and prophylactic antibiotics.
By 15 days of age, the ductus arteriosus aneurysm had disappeared on sonography. The baby was discharged at 14 days of age. Six months later another CT angiography was done; the ductus arteriosus aneurysm had disappeared.
2Discussion
Most ductus arteriosus aneurysms (DAA) develop in the third trimester of fetal life or have a dilated fetal ductus arteriosus before progression to a true aneurysm postnatally [1, 2]. The process of ductal dilation and then aneurysm formation appears to originate from the aortic end with propagation toward the pulmonary end [1].
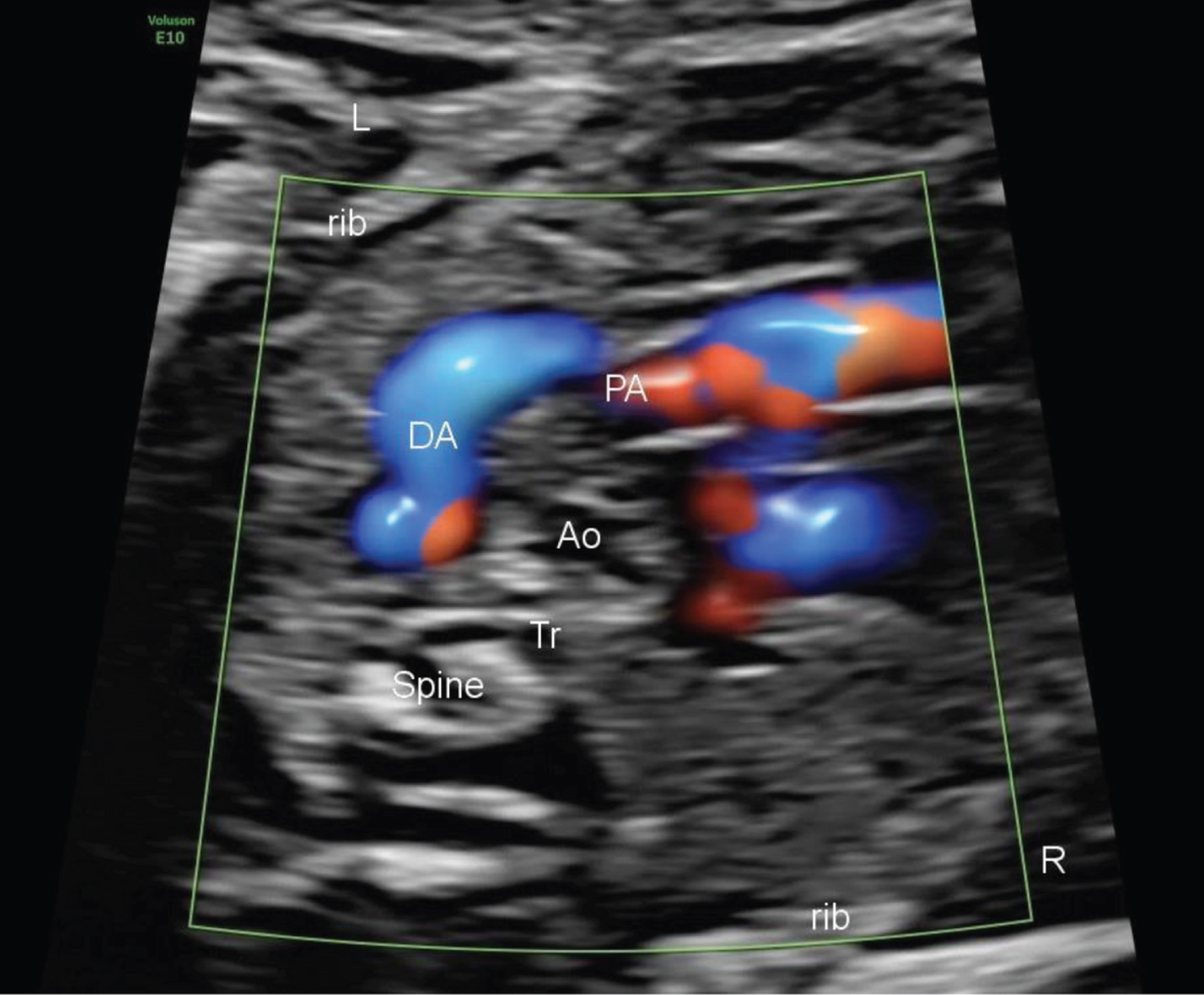
The diagnosis and surveillance is done by echocardiography [8]. It is most commonly ordered to rule out congenital heart disease before surgical intervention, in the setting of a murmur, or during the evaluation of respiratory distress. However, there are no specific consensus diagnostic criteria for the definition of a neonatal DAA. Neonatal DAA are often seen as a tortuous and dilated vascular structure protruding leftward of the aortic arch (Fig. 4).
Fig. 4
Prenatal ultrasound of the S shaped ductus arteriosus.
DAA is often discovered in the third trimester or early postnatally as a coincidental finding. Its incidence ranges from 1.5 to 8.8%. It usually regresses spontaneusly in 6 to 12 weeks. Potential complications appear to be rare and include thromboembolism, infection, airway erosion, and rupture. CT or MRI is needed to clarify ultrasonographic findings.
In prenatal cases, DAA appears as a tortuous and dilated vascular structure leftward of the aortic arch. DAA should be suspected If the dilation is >10 mm. It is important to evaluate the left and right ventricular outputs to assess the hemodynamic stability of the fetus or newborn [10, 11].
The optimal recommendation for the management of DAA remains controversial. Observation during the first two months is the most common aproach. Surgery may be indicated if it fails to regress after two months. Early detection and description of the DAA and evaluation for possible complications is important to guide the selection of the conservative or surgical approaches [3].
The complication rate of neonatal DAAs has historically been reported to be as high as 31% (4), with thromboembolism and rupture reported most commonly, accounting for 12% and 9%, respectively, in one series [2]. These rates may be overestimated because of the presentation and reporting bias. The true incidence of complications in neonatal DAAs is unknown because many affected infants have a benign course with eventual regression and resolution and are not brought to medical attention [4].
Arterial tortuosity syndrome is characterized by blood vessel abnormalities, particularly abnormal twists and turns of the blood vessels that carry blood from the heart to the rest of the body. Tortuosity arises from abnormal elongation of the arteries; since the end points of the arteries are fixed, the extra length results in twists and turns. Other blood vessel abnormalities that may occur in this disorder include constriction (stenosis) and abnormal bulging (aneurysm) of vessels, as well as small clusters of enlarged blood vessels just under the skin (telangiectasia) [3].
Complications resulting from the abnormal arteries can be life-threatening. Rupture of an aneurysm or sudden tearing (dissection) of the layers in an arterial wall can result in massive loss of blood. Blockage of blood flow to vital organs such as the heart, lungs, or brain can lead to myocardial infarction, respiratory problems, and strokes. Stenosis of the arteries forces the heart to work harder and may lead to heart failure. As a result of these complications, arterial tortuosity syndrome is often fatal in childhood, although some individuals with mild cases of the disorder live into adulthood [3, 9].
In this case, the suspicion of arterial tortuosity syndrome was raised by the compendium of findings, including the testicular torsion and DAA. Although the renal duplication and dual renal arteries are not typically seen in the disorder, it is possible that they may be a previously undescribed component. The DAA has also been reported as an isolated finding [12]. To date, no specific genetic abnormalities have been identified, but the multiplicity of anomalies suggests the possibility.
References
[1] | Tseng JJ , Jan SL . Fetal echocardiographic diagnosis of isolated ductus arteriosus aneurysm: A longitudinal study from 32 weeks of gestation to term. Ultrasound Obstet Gynecol. (2005) ;26: (1):50–6. |
[2] | Botta AM , Aquino F , Pereira C , Fin A , Nogueira A , Ricachinewsky C , Lucchese F , Molossi S . Silent patent ductus arteriosus aneurysm. Arq Bras Cardiol. (2002) ;79: (3):302–7. |
[3] | Bannan B , Aly S , Yoo SJ , Seed M , Lam CZ . The many faces of neonatal ductus arteriosus aneurysms: Multimodality imaging with an emphasis on CT and MRI appearance. Radiol Cardiothorac Imaging. (2021) ;3: (3):e210017. |
[4] | Lund JT , Jensen MB , Hjelms E . Aneurysm of the ductus arteriosus. A review of the literature and the surgical implications. Eur J Cardiothorac Surg. (1991) ;5: (11):566–70. |
[5] | Cruickshank B , Marquis RM . Spontaneous aneurysm of the ductus arteriosus. Am J Med. (1958) ;25: :140–9. |
[6] | Jan SL , Hwang B , Fu YC , Chai JW , Chi CS . Isolated neonatal ductus arteriosus aneurysm. Pediatr Cardiol. (2002) ;39: :342–7. |
[7] | Lund JT , Hansen D , Brocks V , Jensen MB , Jacobsen JR Aneurysm of the ductus arteriosus in the neonate: Three case reports with a review of the literature. Pediatr Cardiol. (1992) ;13: (4):222–6. |
[8] | Jackson CM , Sandor GG , Lim K , Duncan WJ , Potts JE . Diagnosis of fetal ductus arteriosus aneurysm: Importance of the three-vessel view. Ultrasound Obstet Gynecol. (2005) ;26: (1):57–62. |
[9] | Hornberger LK . Congenital ductus arteriosus aneurysm. J Am Coll Cardiol. (2002) ;39: (2):348–50. |
[10] | Choi SJ , Joeng HY , Lee MY , Song C . P25.11: Prenatal diagnosis of isolated ductus arteriosus aneurysm. Ultrasound Obstet Gynecol. (2009) ;34: :275–275. |
[11] | Oh SJ , Jeung IC . A case of isolated congenital ductus arteriosus aneurysm detected by fetal echocardiography at 38 weeks of gestation. J Clin Ultrasound. (2011) ;39: (9):530–3. |
[12] | Ganesan S , Hutchinson DP , Sampson AJ . Prenatal diagnosis of ductus arteriosus aneurysm. Ultrasound. (2015) ;23: (4):251–3. |




