Proceedings of the 13th International Newborn Brain Conference: Neuroprotection strategies in the neonate
Effects of combined treatment of Inter-Alpha Inhibitor Proteins (IAIPs) with hypothermia in neonatal rats exposed with hypoxic-ischemic (HI) brain injury
Chen Xa, Wu Y, Kim B, Domonoske R, Lim Y, Stonestreet B
aWomen & Infants’ Hospital Of RI, 101 Dudley Street, Providence, United States
BACKGROUND: Hypoxic-ischemic (HI) brain injury is one of the most common neurological problems observed in infants. Hypothermia is the only approved therapy for neonatal HI encephalopathy. This therapy is only partially protective, cannot be used in preterm infants, and has a narrow therapeutic window after birth. Therefore, additional adjunctive therapies are urgently required. IAIPs are naturally plasma-derived proteins. We have previously shown that IAIP alone reduces cortical neuronal cell death, improves behavior outcomes, and attenuates brain volume loss in neonatal HI rats (Chen et al., 2019; Schuffels et al., 2020). However, the therapeutic effects of the combined treatment of IAIPs with hypothermia have not been previously established in neonatal rats.
PURPOSE: To examine the effects of combined treatment of IAIPs with hypothermia on brain infarct volumes after exposure to HI insults in male and female neonatal rats.
METHODOLOGY: The Rice-Vannucci method was used to induce HI, e.g. unilateral carotid artery ligation followed by hypoxic exposure to 90 min of 8% O2 at postnatal (P) day 7. After recovery of HI, the pups were treated either with normothermia (rectal temperature 36°C) or hypothermia (rectal temperature 30°C) for 3 h. The rats were randomly assigned to 6 groups: (1) normothermia+Placebo (PL, Sham); (2) normothermia+HI-PL; (3) normothermia+HI-IAIP; (4) hypothermia (rectal temperature 30)+Sham; (5) hypothermia+HI-PL; and (6) hypothermia+HI-IAIP. HI animals received PL (phosphate-buffered saline) or human plasma-derived IAIPs (30 mg/kg) intraperitoneally (i.p.) at 0, 24, and 48 h after HI. At P14, brains were collected for infarct volume measurement (Fig. A and B).
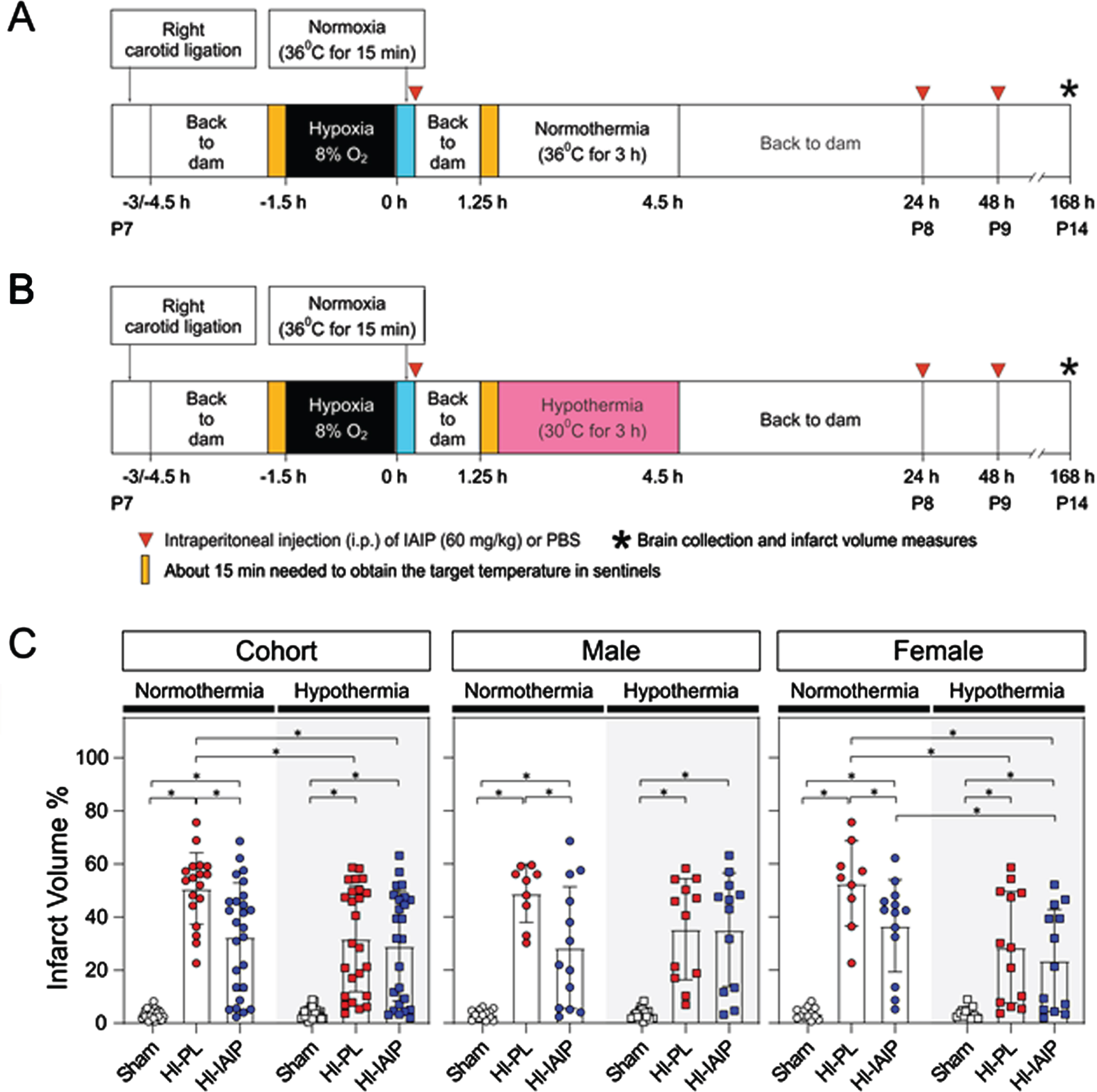
RESULTS: Under normothermia, treatment with IAIPs significantly attenuated brain volume loss in cohort (male+female), male and female neonatal rats (P<0.05, Fig. C). Compared to the normothermia+HI-PL group, the combined treatment of IAIPs with hypothermia attenuated brain volume loss in cohort and females (P<0.05), but not in males. In females, the brain volume loss in hypothermia+HI-IAIP groups was reduced compared to the normothermia+HI-IAIP, suggesting that the combined treatment of IAIPs with hypothermia enhanced IAIP neuroprotection in HI brain injury. No difference between hypothermia+HI-PL and hypothermia+HI-IAIP groups was observed.
CONCLUSIONS: The combined treatment of IAIPs with hypothermia attenuates brain volume loss in cohort and female neonatal rats after exposure to HI. Although IAIPs could be an additional adjunctive therapy to infants that currently receive therapeutic hypothermia, the optimal dose, and therapeutic windows remain to be determined.
Bibliography:
[1] Chen, X., et al., 2019. Neuroprotective effects of inter-alpha inhibitor proteins after hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 317, 244-259.
[2] Schuffels, S., et al., 2020. Effects of inter-alpha inhibitor proteins on brain injury after exposure of neonatal rats to severe hypoxia-ischemia. Exp Neurol. 334, 113442.
Therapeutic hypothermia in neonates with hypoxic ischemic encephalopathy: Update systematic review and meta-analysis
Dsouza Ja, Mathew Jb, Kaur Nb
aKasturba Medical College, Manipal, Manipal, India
bPostgraduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India
BACKGROUND: Therapeutic hypothermia (TH) is the top-ranking intervention for neonatal hypoxic encephalopathy [1]. However, the recently-published HELIX trial, reported increased short-term and long-term mortality in developing country settings [2]. This necessitates an up-to-date systematic review to evaluate therapeutic hypothermia (intervention) versus normothermia (comparison) in neonatal hypoxic encephalopathy (population), on mortality and neuro-development (outcomes); and a separate analysis in developing country settings.
METHODS: We searched multiple databases without language or date restrictions, for randomized controlled trials (RCT) comparing therapeutic hypothermia (defined as whole-body or selective head cooling, to temperature <34.5° for 48-72hr) initiated within 6hr of birth, versus no hypothermia, in neonates with hypoxic encephalopathy (defined by Apgar scoring and/or cord blood analysis, and supporting clinical findings). We included RCTs reporting mortality before discharge, mortality at 18-24mo, mortality or neurologic disability at 18-24mo, disability at 18-24mo, and cerebral palsy at 18-24mo.
RESULTS: The searches, updated to 30-September-2021, identified 36345 citations. Step-wise screening of titles, abstracts, and full-text, by two authors independently, short-listed 149 citations, of which 33 publications, reporting 29 trials, were included. Using Cochrane Risk of Bias 2 tool two authors independently categorized, 11, 8, and 10 RCT as having high, moderate, and low risk of bias. Meta-analysis yielded the relative risks (with 95% CI). Mortality before discharge: 0.83 (0.71, 0.98), 23 trials, 2221 participants, I2 37% (Figure 1). Mortality at 18-24mo: 0.88 (0.78, 1.01), 11 trials, 2042 participants, I2 51% (Figure 2). Mortality or neurologic disability at 18-24mo: 0.79 (0.72, 0.86), 10 trials, 1917 participants, I2 54% (Figure 3). Neurologic disability at 18-24mo: 0.63 (0.53, 0.75), 10 trials, 1327 participants, I2 37% (Figure 4). Cerebral palsy at 18-24mo: 0.63 (0.50, 0.78), 8 trials, 1136 participants, I2 39%. These data suggested statistically significant benefit for all outcomes except mortality at 18-24mo of age. Subgroup analysis by study setting (developed versus developing countries) showed stark differences in mortality before discharge: RR 0.68 (0.51, 0.92), 8 trials, 790 participants, I2 0% versus 0.92 (0.76, 1.11), 15 trials, 1431 participants, I2 48%; and mortality at 18-24mo: 0.79 (0.66, 0.93), 7 trials, 1212 participants, I2 7%, versus 1.05 (0.86, 1.29), 4 trials, 830 participants, I2 65%. Other outcomes showed benefit of TH in both developed and developing countries; the magnitude of effect was greater in developing countries for disability and cerebral palsy.
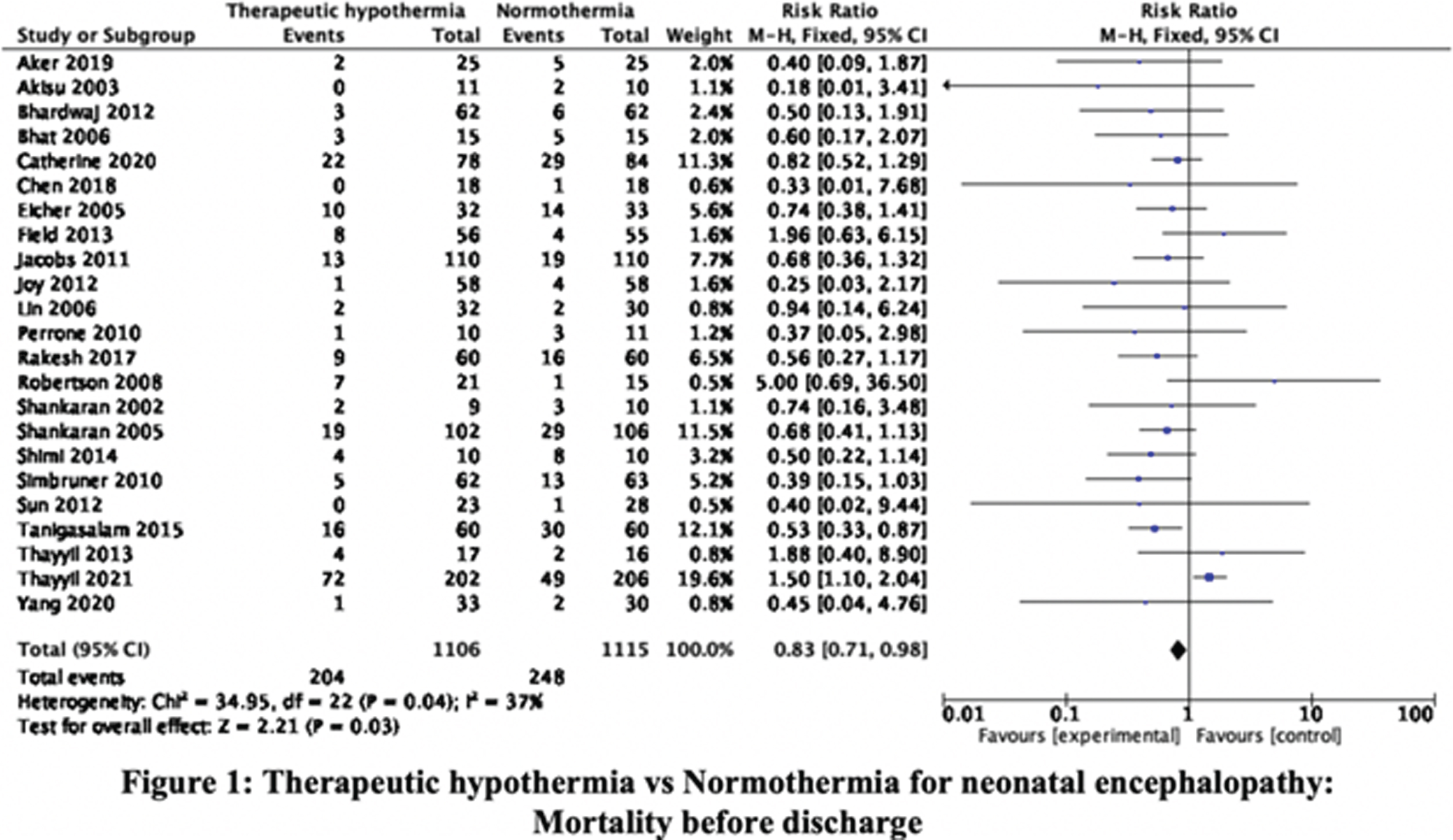
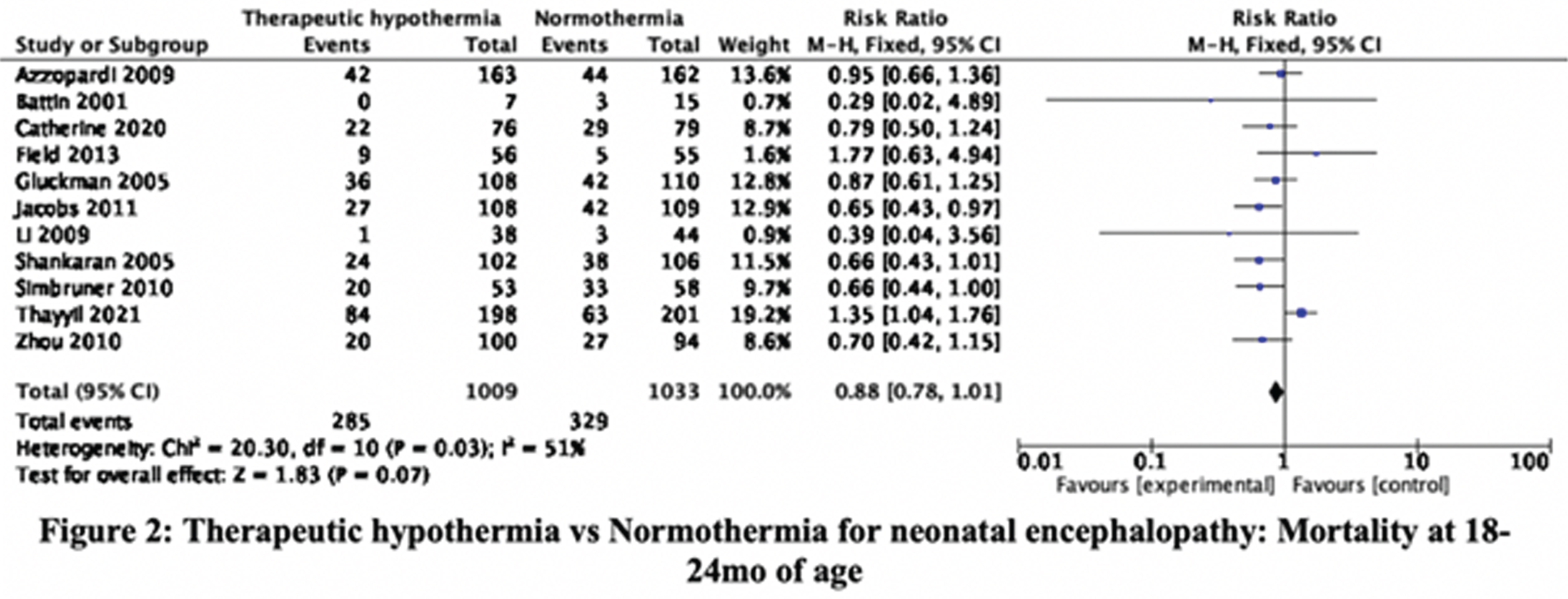
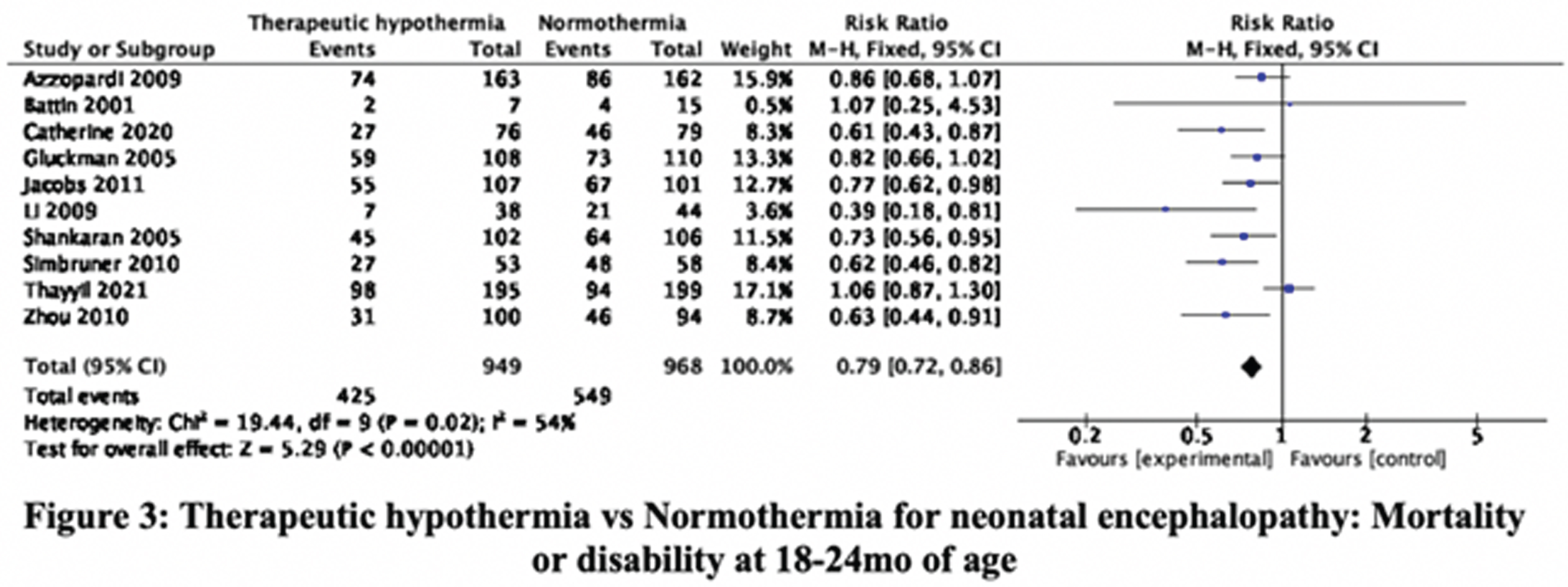
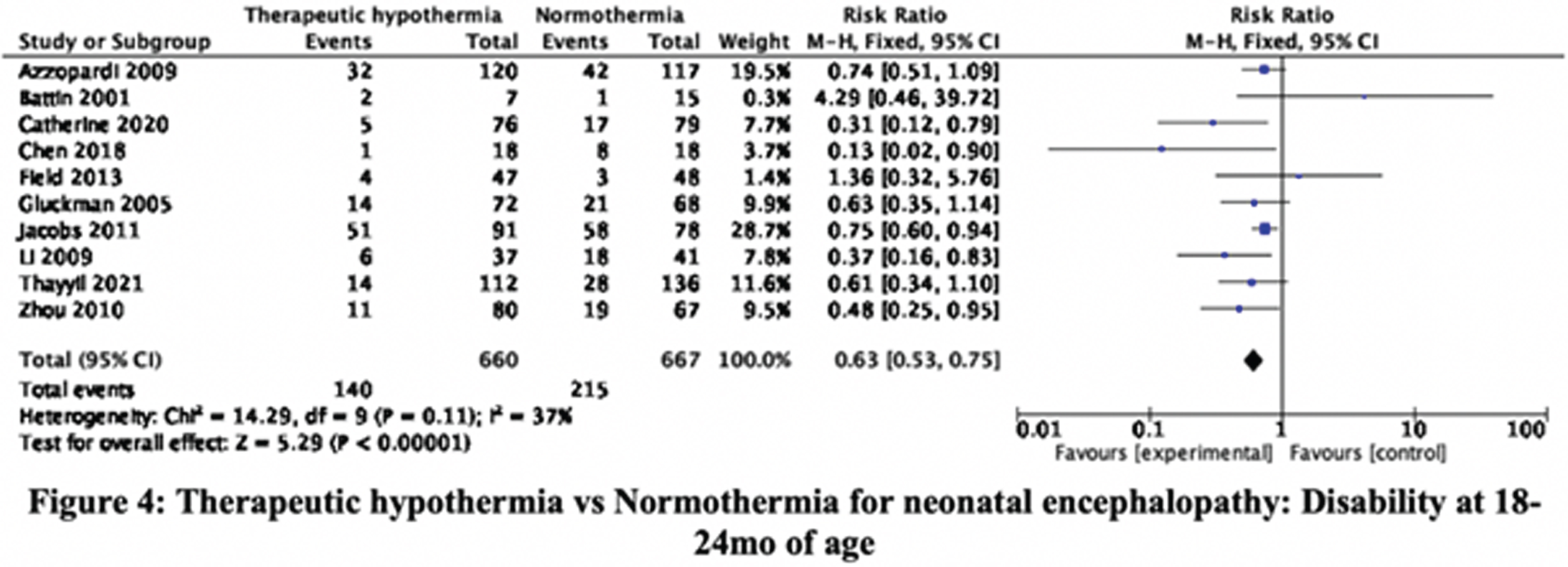
CONCLUSION/IMPACT: These data confirm that TH reduces neurologic disability and cerebral palsy in later infancy in diverse settings. However, the expected benefit on short-term and long-term mortality is uncertain in developing country settings. These findings will help physicians, families, and policy-makers, to make evidence-informed choices and decisions, about therapeutic hypothermia for neonatal encephalopathy.
Bibliography:
[1] Lee et al. Front Pharmacol. 2019 Oct 25;10:1221.
[2] Thayyil et al. Lancet Glob Health. 2021 Sep;9(9):e1273-e1285.
Perinatal stroke presenting as HIE: practical diagnostic and management challenges
El Gamal Ma, Chetcuti Ganado C
aBedfordshire Hospitals NHS Trust, Luton, United Kingdom
BACKGROUND: While therapeutic hypothermia is accepted as a standard of care for improving neurological outcome for HIE patients, evidence of its benefits for neonatal stroke is lacking. Neurological presentations of HIE and neonatal stroke can be difficult to distinguish. Our observational analysis of neonatal stroke infants highlights challenges in distinguishing between the two groups and reaching an early definitive diagnosis to inform appropriate treatment. This analysis attempts to quantify whether antenatal sentinel events, clinical presentation, CFM and cranial ultrasound help distinguish between the two groups and whether perinatal factors influence early diagnosis and management.
METHODS: A retrospective cohort study of term neonates >37 weeks gestation born between May 2011 and April 2020 at a specialized NICU network in the East of England with a principal diagnosis of neonatal stroke. A total of 16 patients were identified of which 8 had been cooled for an initial clinical diagnosis of HIE, and retrospective MRI diagnosis of neonatal stroke. MRI brain injury scores (1a to 3) were based on the National Institute of Child Health and Human Development Neonatal Research Network classification.
RESULTS: 15 infants had middle cerebral artery infarction, and one infant had cerebral sinus thrombosis. Of the 8 cooled infants only 3 (37%) of cooled infants fulfilled both Toby A and B criteria.
Infants who received therapeutic hypothermia were more likely to present with early onset seizures (5/8) than infants who were not cooled (1/8) (p=0.019). The CFM showed unilateral seizure activity in 14/16 (87.5%) of infants. Only 1 infant with underlying venous infarction presented with bilateral seizure activity. The background trace was normal in 8 (50%) of infants. There was no correlation between seizure burden and background trace. Cooled infants were more likely to present with abnormal neurology at birth but had a lower seizure burden and MRI injury score than non-cooled infants although this did not reach statistical significance. Cranial ultrasound findings were absent or non-specific.
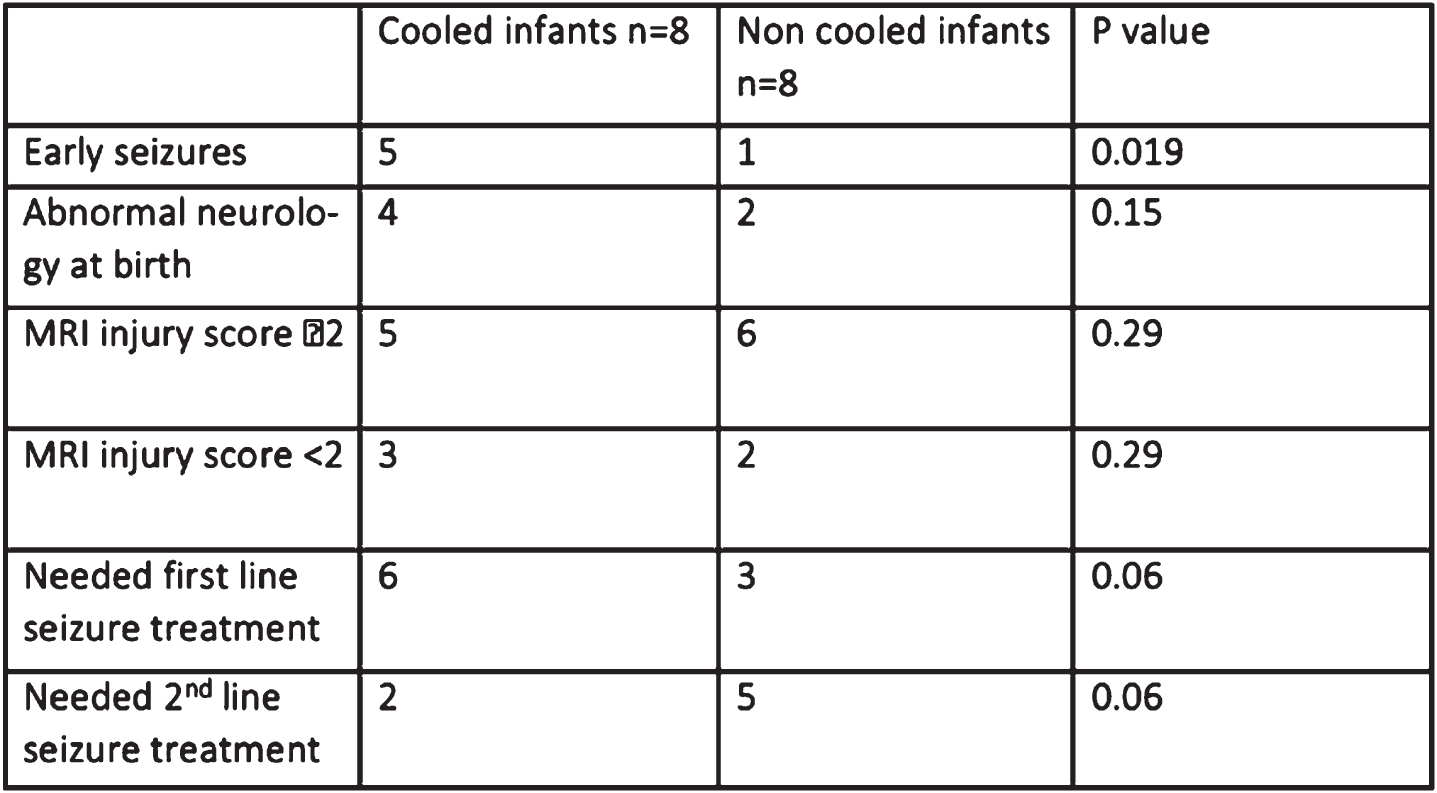
CONCLUSION: Infants with neonatal stroke who present with early seizures are more likely to receive therapeutic hypothermia despite failure to fulfil both Toby A and B criteria. Early suspicion to inform management can be obtained more effectively from CFM while cranial ultrasound findings are generally non-specific. A normal CFM background or unilateral abnormal background with unilateral seizure activity are highly suggestive of neonatal stroke. Evidence of the effectiveness and indication of therapeutic hypothermia in this setting is still lacking. The new BAPM framework for HIE with the inclusion of the CFM in the selection of infants who undergo therapeutic cooling will likely decrease the incidence of infants with neonatal stroke receiving therapeutic hypothermia. Future research in understanding the effectiveness of therapeutic hypothermia on neurological outcomes in neonatal stroke are needed.
Asphyxia Associated Metabolite Biomarker Investigation (AAMBI). Short-term clinical outcomes and at the age of 22 to 42 months
Franz Aa, Yapicioglu-Yildizdas Hb, Taskin Ec, Yaman Ad, Celik Ye, Simsek Hb, Hamitoglu Sc, Aydinol Nd, Keles Ef, Keller Mg, Benders Mh, Groenendaal Fh, Anninck Ki, Hellström-Westas Lj, Saugstad Ok, Marlow Nl, Deigner Hm, Kohl Mm, Meyer Rn, Plum An, Steins-Rang Cn, Bartmann Po
aUniversity Hospital Tübingen, Tübingen, Germany
bCukurova University Hospital Adana, Adana, Turkey
cFirat University Hospital Elazig, Elazig, Turkey
dÖzel Güngören Hastanesi Istanbul, Istanbul, Turkey
eMersin University School of Medicine, Mersin, Turkey
fGazi University Hospital Ankara, Ankara, Turkey
gKinderklinik Dritter Orden, Passau, Germany
hUniversity Medical Center Utrecht, Neonatology, Utrecht, Netherlands
iWilhelmina Children’s Hospital Utrecht, Utrecht, Netherlands
jWomen’s and Children’s Health, Uppsala University, Uppsala, Sweden
kPediatric Research, University of Oslo, Oslo, Norway
lInstitute for Women’s Health, UCL, London, United Kingdom
mHochschule Furtwangen, Furtwangen, Germany
nInfanDx AG, Cologne, Germany, 15University Hospital Bonn, Neonatology, Bonn, Germany
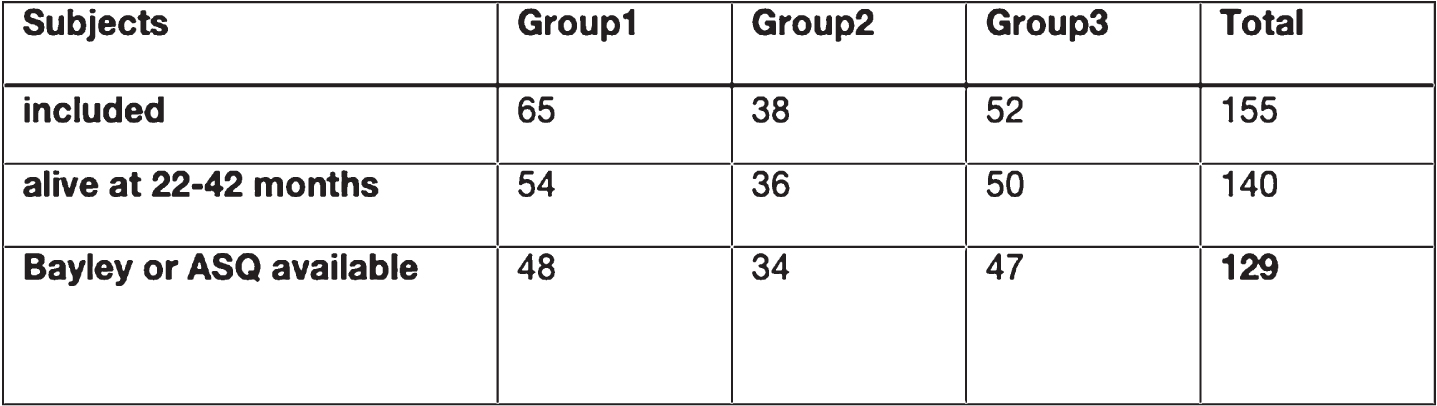
BACKGROUND AND PURPOSE: Perinatal hypoxic-ischemic encephalopathy (HIE) can be ameliorated by Therapeutic Hypothermia (TH) and potentially additionally by pharmacological neuroprotective interventions. Early identification of neonates at risk is not sufficiently reliable based on pH, base deficit and APGAR scores. The AAMBI study aims to define a set of metabolomic biomarkers best suited to identify infants that might benefit from neuroprotective interventions. Here we present a comparison of neonatal short-term clinical outcomes of the AAMBI cohort with neurodevelopmental status at the age of 22 to 42 months (see Table 1).
Table 1
– Selection of Subjects for this presentation
 |
METHODOLOGY: Prospective observational study. Following written informed consent, three groups of infants were recruited:
Group 1: 65 infants meeting local criteria for TH;
Group 2: 38 infants with suspected perinatal brain injury based on moderate-to-severe perinatal acidosis (pH≤7.10 or a base excess ≤-12mmol/l) or resuscitation within 30min after birth but not undergoing TH;
Group 3: 52 Infants with pH ≥ 7.25 and adaptation disorder of the newborn and need of postnatal clinical surveillance.
The neonatal clinical outcome was determined based on Thompson Scores at 2±0.5h and 6±1h as well as results of central assessments of cMRI and aEEG and graded as normal, suspect, potentially abnormal, abnormal HIE, or abnormal non-HIE. In addition, results of Bayley-Test and/or Ages and Stages Questionnaire (ASQ) and neurological assessment, as well as questions about visual and hearing capabilities, were used for outcome classification at 22-42 months graded normal, suspect, abnormal-HIE and abnormal non-HIE.
RESULTS: Figure 2 provides the distribution of inclusion groups to neonatal and 22-42 months neuro-developmental clinical outcomes.
Figure 2 –
Changes in Outcome
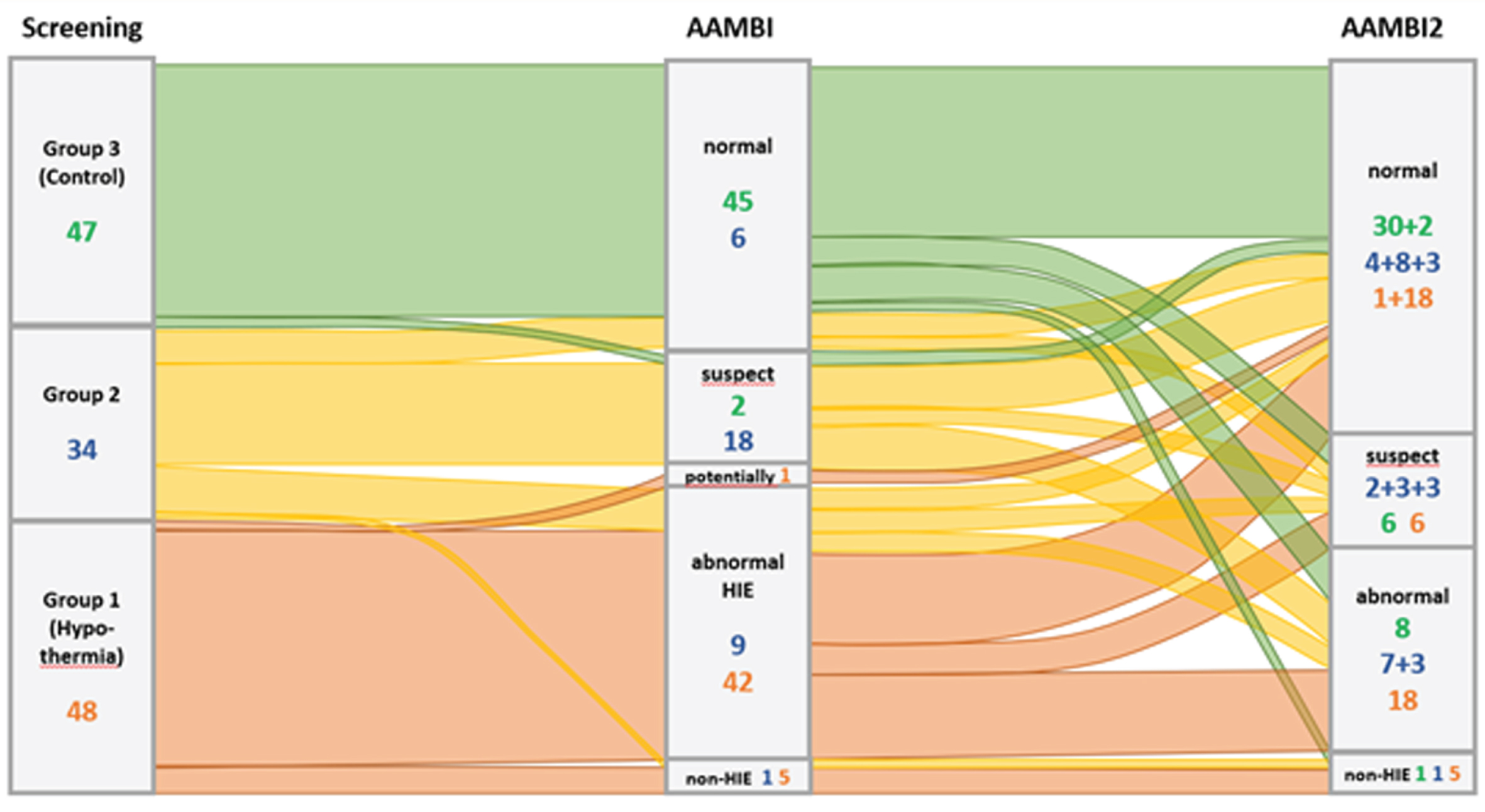
DISCUSSION AND CONCLUSION: For all three patient groups, the clinical diagnosis in the neonatal period does not allow a sufficiently reliable prognosis of the developmental status at 22-42 months. Therefore, classic diagnostic instruments commonly applied in the newborn period (e.g. pH) cannot reliably indicate need for neuroprotective therapy. The AAMBI project aims to achieve improved diagnostic confidence by identifying metabolomic biomarkers. Therefore, blood samples assayed at birth and at 2 and 6h of age will now be subjected to a comprehensive metabolomic analysis.
Short term outcomes of newborn infants with Hypoxic Ischemic Encephalopathy treated with therapeutic hypothermia in a low resource setting: A retrospective cohort study
Kricitober Ja, Mukiza Nc, Sebunya Rb, Nakibukka Vb
aUganda Martrys University, Kampala, Uganda
bSt. Francis Hospital, Nsambya, Kampala, Uganda
cKing Caesar University, Kampala, Uganda
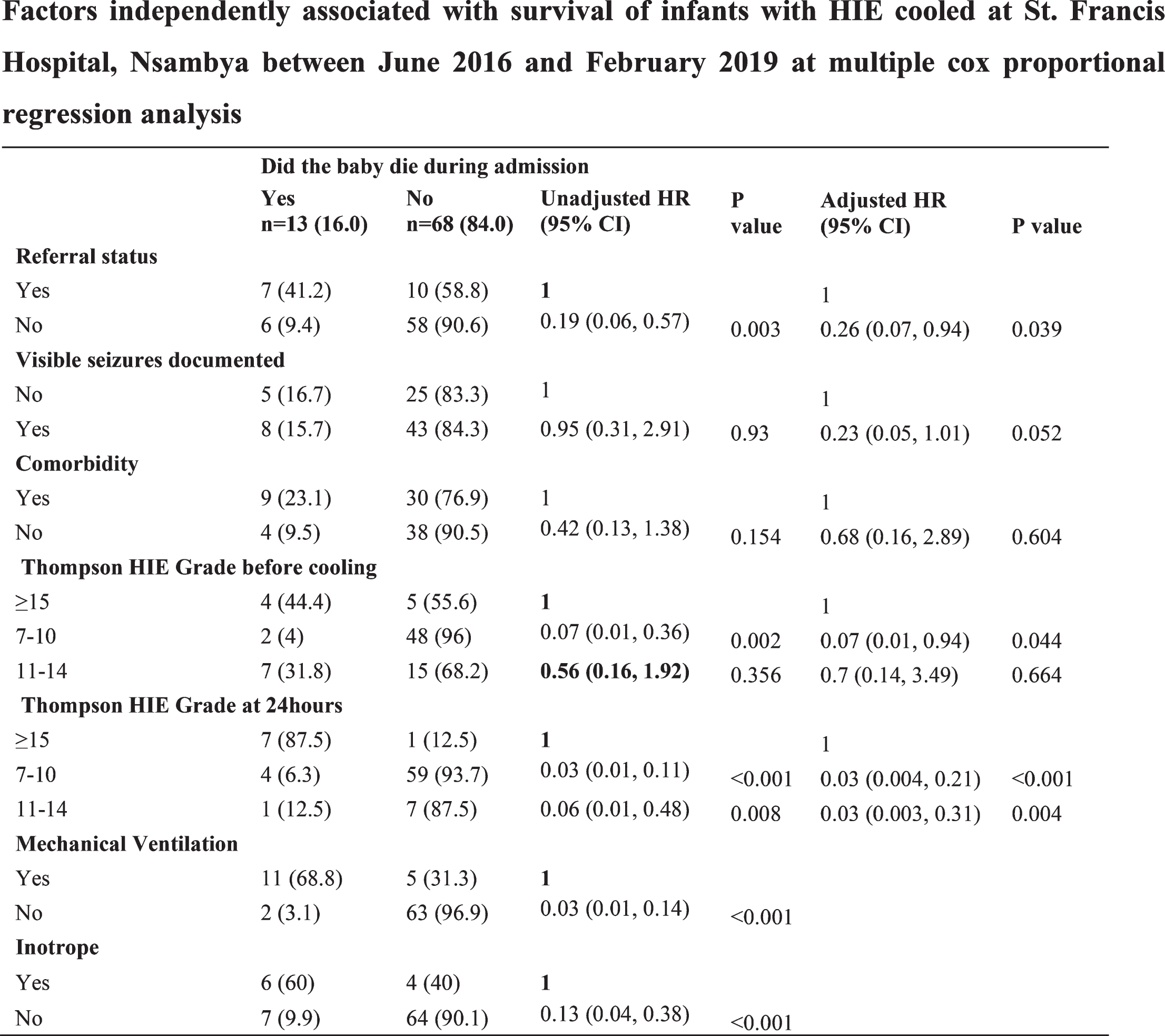
BACKGROUND: Hypoxic Ischemic Encephalopathy (HIE) remains a significant cause of death and neuro-developmental deficits among children, especially in the low and middle income countries. Therapeutic hypothermia which is the mainstay of treatment for hypoxic ischemic encephalopathy in developed countries is not widely practiced in resource limited settings. We aimed to determine the short term outcomes and factors associated with survival among newborn infants with moderate to severe hypoxic ischemic encephalopathy treated with therapeutic hypothermia at a tertiary teaching hospital.
METHODS: This was a retrospective cohort study of 81 newborn infants with moderate to severe HIE who were 36 weeks and above that were treated with therapeutic hypothermia between June 2016 and February 2019. Data on maternal and infant characteristics, clinical outcomes were extracted from the HIE registry and HIE follow up forms.
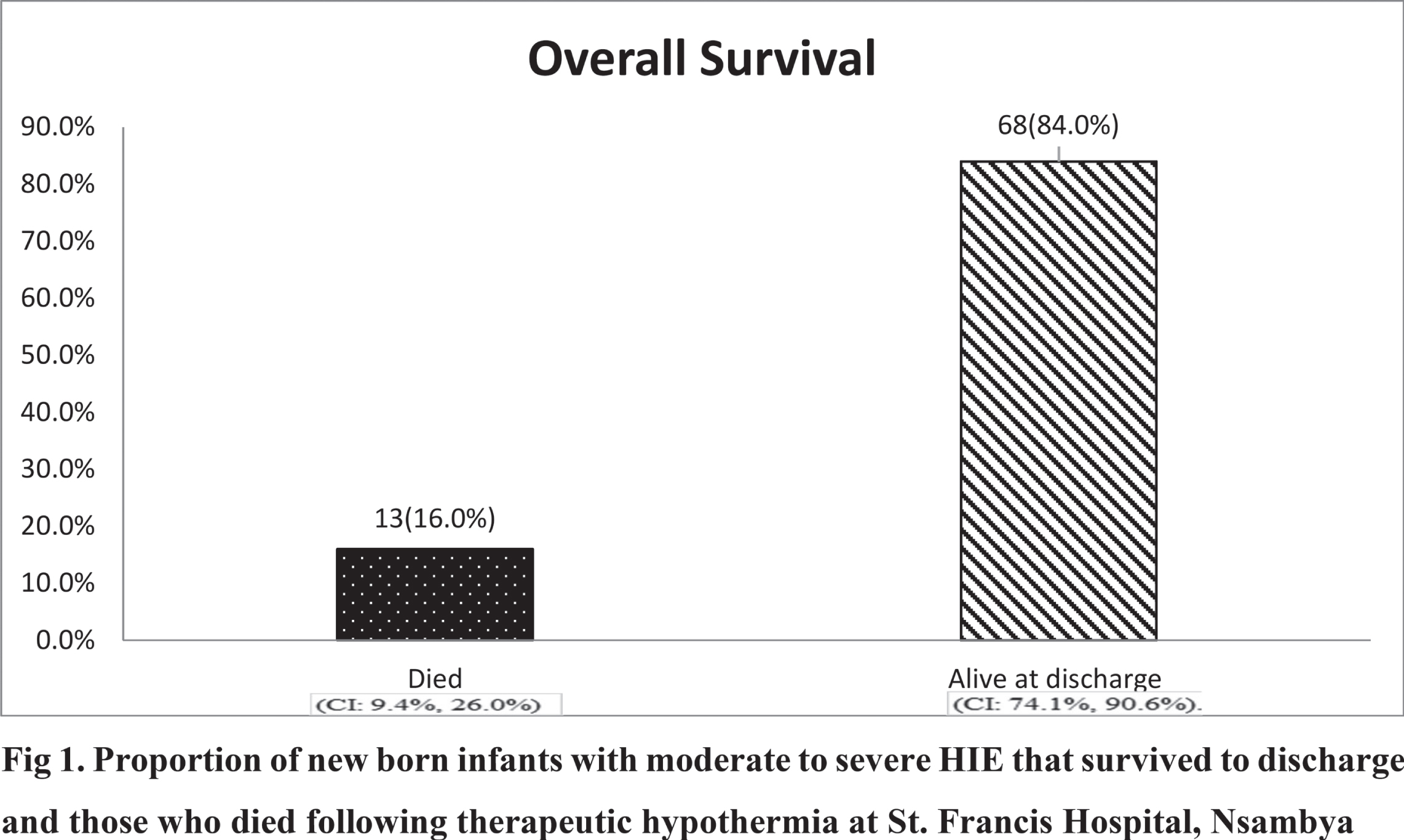
RESULTS: The proportion of newborn infants who survived was 68/81(84%), (95%: CI: 0.74, 0.91). The factors associated with survival were a Thompson score of 7 to10 at initiation of the therapeutic Hypothermia (HR: 0.07, 95% CI: 0.01, 0.94) and at 24 hours of cooling (HR: 0.03, 95%CI: 0.004, 0.21), being born within the hospital providing therapeutic hypothermia (HR: 0.26, 95% CI: 0.07, 0.94) and not needing mechanical ventilator (HR: 0.03, 95% CI: 0.01, 0.14) or inotropic support. (HR: 0.13, 95%CI: 0.04, 0.38). The median time to attainment of full cup feeds was 6 days, with the majority 43/68(63%) attaining full feeds from 5 to 8 days. The median time to discharge was 7 days, and the median time to death was 3 days. The median Thompson score at discharge was 1 and at death was 16
CONCLUSION: The survival rate of the infants treated with therapeutic Hypothermia in our setting at 84% is comparably high. Rolling out therapeutic hypothermia in resource limited settings is recommended under strict protocols.
Key words: Short term outcomes, Newborn infants, Hypoxic ischaemic encephalopathy, Therapeutic hypothermia
Use of recombinant human erythropoietin as neuroprotective agent in Hypoxic Ischemic Encephalopathy in term newborns- An open label, randomized controlled trial
Londhe Aa, Deshmukh La
aGovernment Medical College, Aurangabad (MS) India, Aurangabad, India
BACKGROUND: Perinatal asphyxia is the fifth largest cause of child death. In developing countries, like India, the incidence of perinatal asphyxia was 5% (1). Patients with moderate HIE have a 10% risk of death and 30% risk of disability among survivors. Among patients with severe HIE, 60% patients die and almost all survivors develop disability (2). Babies who received Therapeutic Hypothermia, one third developed disability and another one third didn’t survive (3,4). So, there is a need of other therapeutic modalities which have neuroprotective and neurorestorative properties.
MATERIAL AND METHODS: Sixty-two term neonates with moderate or severe HIE were randomized by random allocation to receive either EPO 500 U/ kg/ dose in 2 ml saline intravenously (31 neonates) on alternate days for a total of five doses with the first dose given within 24 h of age (treatment group) or 2 ml of normal saline (31 neonates) similarly for a total of five doses (placebo group) along with standard treatment. No hypothermia was given. The primary outcome was end point of death. Secondary outcomes were seizure frequency and need of ventilation.
RESULTS: Death occurred in 39% of neonates in the treatment group vs 71% in the placebo group (risk ratio, 0.55; 95% confidence interval (CI) 0.33 to 0.9; P = 0.01). 29% babies in EPO required 2 or more anticonvulsants as compared to 42% in control group (Risk ratio 0.74; 95% confidence interval (CI) 0.38 to 1.46; p = 0.38). Also, we didn’t find any significant difference in need of mechanical ventilation in both groups (Risk ratio 0.77 95% confidence interval (CI) 0.52 to 1.14; p= 0.19).
CONCLUSION: EPO reduces risk of death in term newborns with moderate to severe encephalopathy.
Bibliography:
[1] Lawn JE CS, Zupan J, for the Lancet Neonatal Survival Steering Team (2005) 4 million neonatal deaths: When? Where? Why? Lancet 365: 891–900.
[2] Shankaran S, Woldt E, Koepke T, Bedard MP, Nandayal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev 1991; 25: 135–148.
[3] Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ2010; 340: c363.
[4] Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009; 361: 1349–1358.
Neuroprotection care bundle improved long term neurodevelopmental outcomes in infants born extremely premature preterm
Benlamri Aa, Murthy P, Zein H, Thomas S, Scott J, Abou Mehrem A, Esser M, Lodha A, Noort J, Tang S, Metcalfe C, Kowal D, Irvine L, Scotland J, Leijser L, Mohammad Ka
aUniversity Of Calgary, Calgary, Canada
OBJECTIVE: To study the impact of an evidence-based neuroprotection care (NPC) bundle on long-term neurodevelopmental impairment (NDI) in infants born extremely premature.
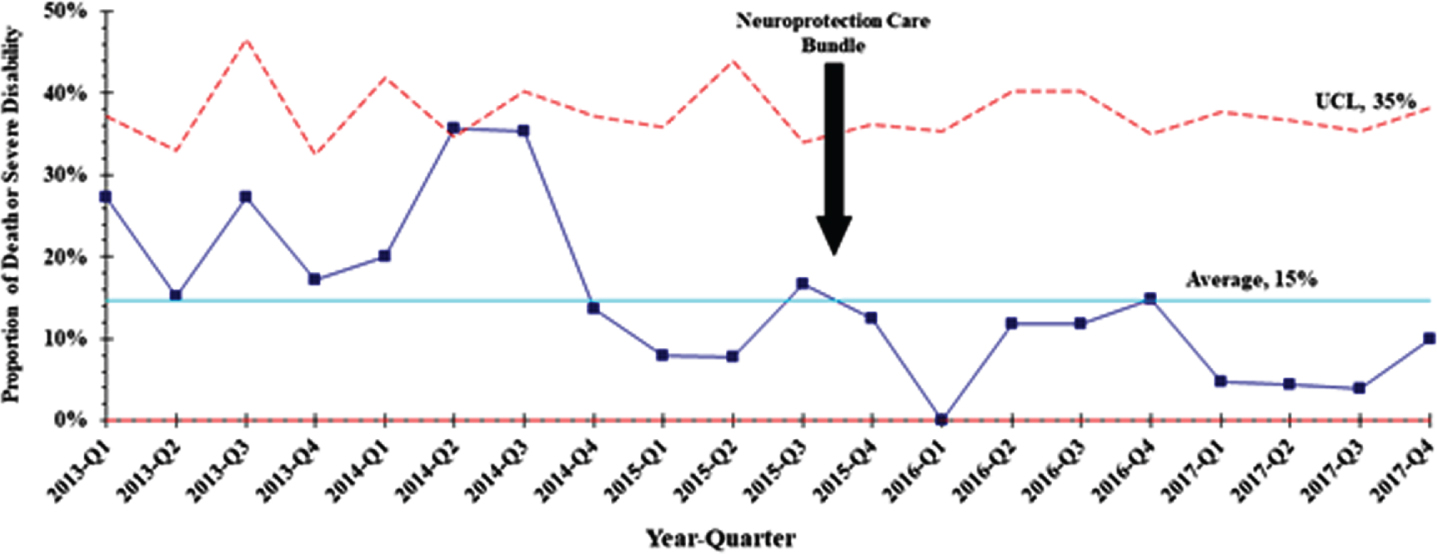
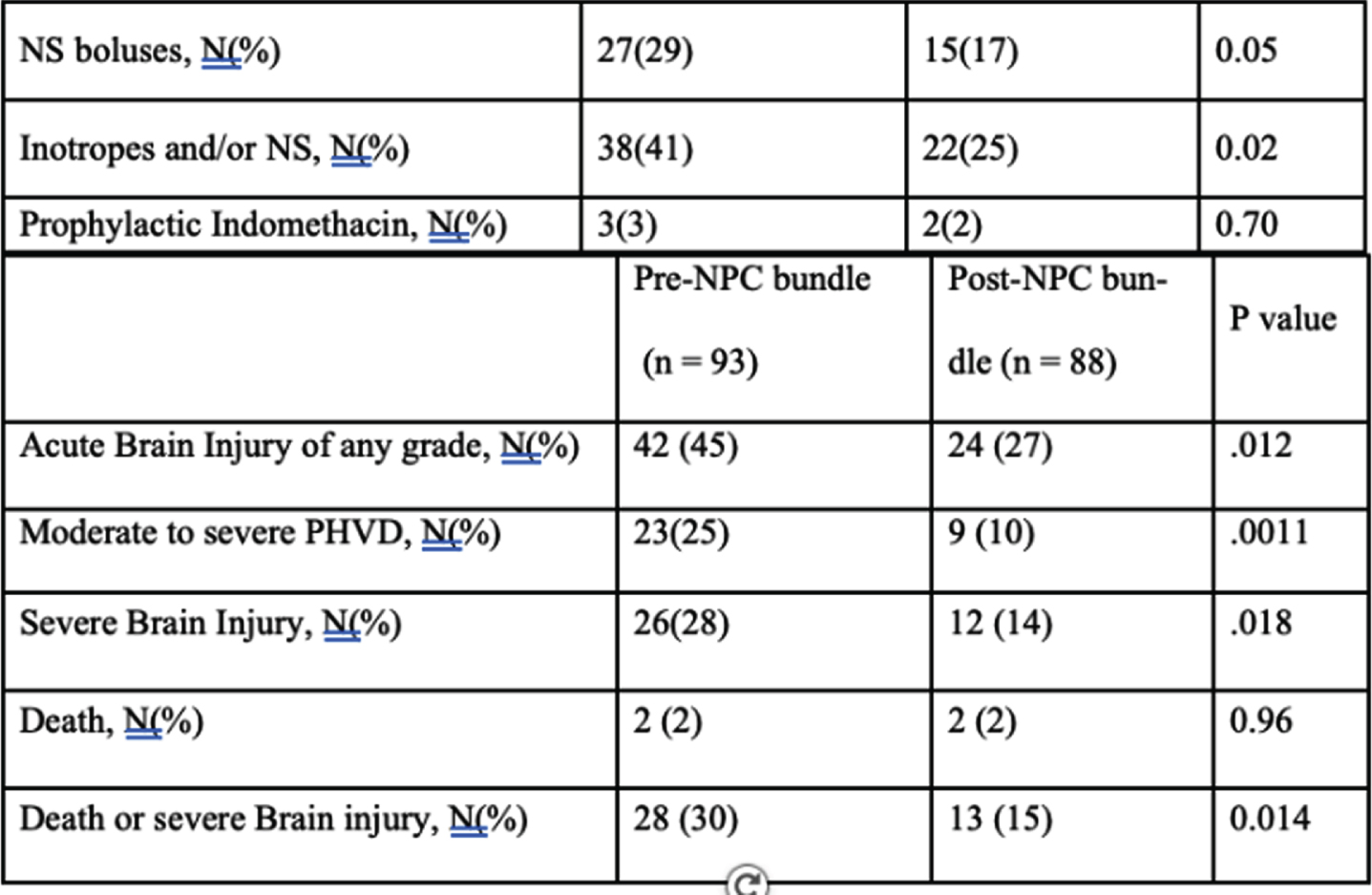
METHODS: A quality improvement study where an NPC bundle consisted of interventions targeting predefined risk factors for acute brain injury in extremely preterm infants (born at gestational age less than 29 weeks) prenatally and during the first three days of birth was developed. This included a combination of neuroprotection interventions such as minimal handling, midline head position, deferred cord clamping, and protocolization of hemodynamic and respiratory managements. Implementation occurred in a stepwise manner, including care bundle development by a multidisciplinary care team based on previous evidence and experience, end-user engagement and education. We compared the incidence of the composite outcome of death or severe neurodevelopmental impairment (sNDI) at 21 months adjusted age pre and post bundle implementation.
RESULTS: Adjusting for confounding factors, NPC bundle implementation was associated with a significant reduction in death or sNDI at 21 months adjusted age (aOR, 0.35; 95% CI 0.19 – 0.65; P < 0.001), mortality (aOR, 0.35; 95% CI 0.17 – 0.72; P = 0.005), sNDI (aOR, 0.27; 95% CI 0.09 – 0.76; P = 0.013), any Bayley III motor, language, or cognitive composite score < 70 (aOR, 0.46; 95% CI 0.25 – 0.84; P = 0.012).
CONCLUSIONS: Implementation of NPC bundle targeting predefined risk factors is feasible and effective in reducing death or sNDI in extremely preterm infants.
Impact of outreach education on the incidence of acute brain injury in transported premature neonates
Mohammad Ka, Momin Sa, Murthy Pa, Zein Ha, N Scott Ja, Abou Mehrem Aa, Ghosh Aa, Javadyan Aa, Al Awad Ea, Gurram Venkata Sa, Paul Ra, Rombough Ba, Bolderheij La, Dossani Sa, Montpetit Ja, Eshemokhai Pa, Fiedrich Ea, Thomas Sa
aUniversity Of Calgary, Calgary, Canada
AIM: To evaluate the impact of outreach education on the incidence of acute brain injury in transported premature neonates.
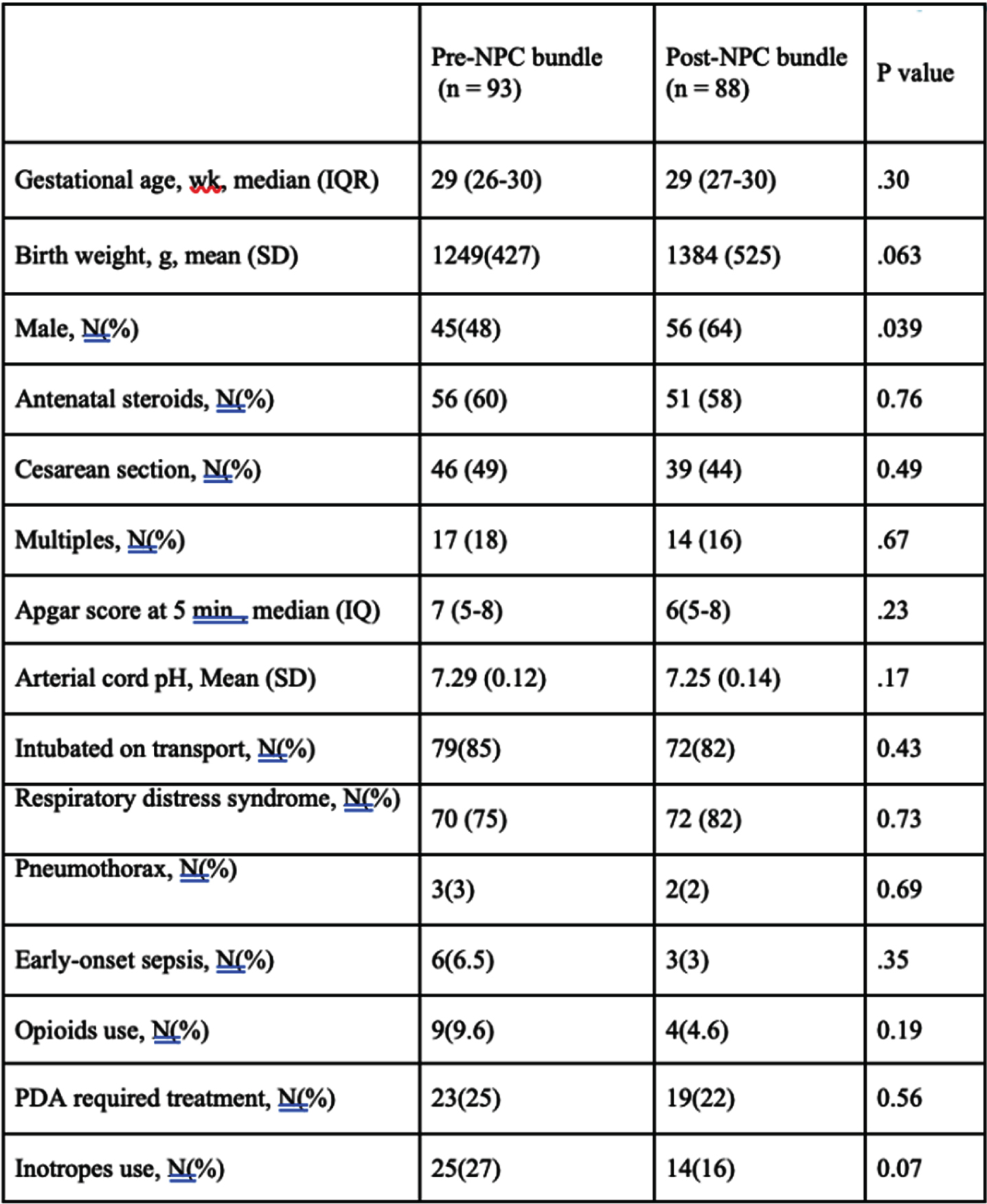
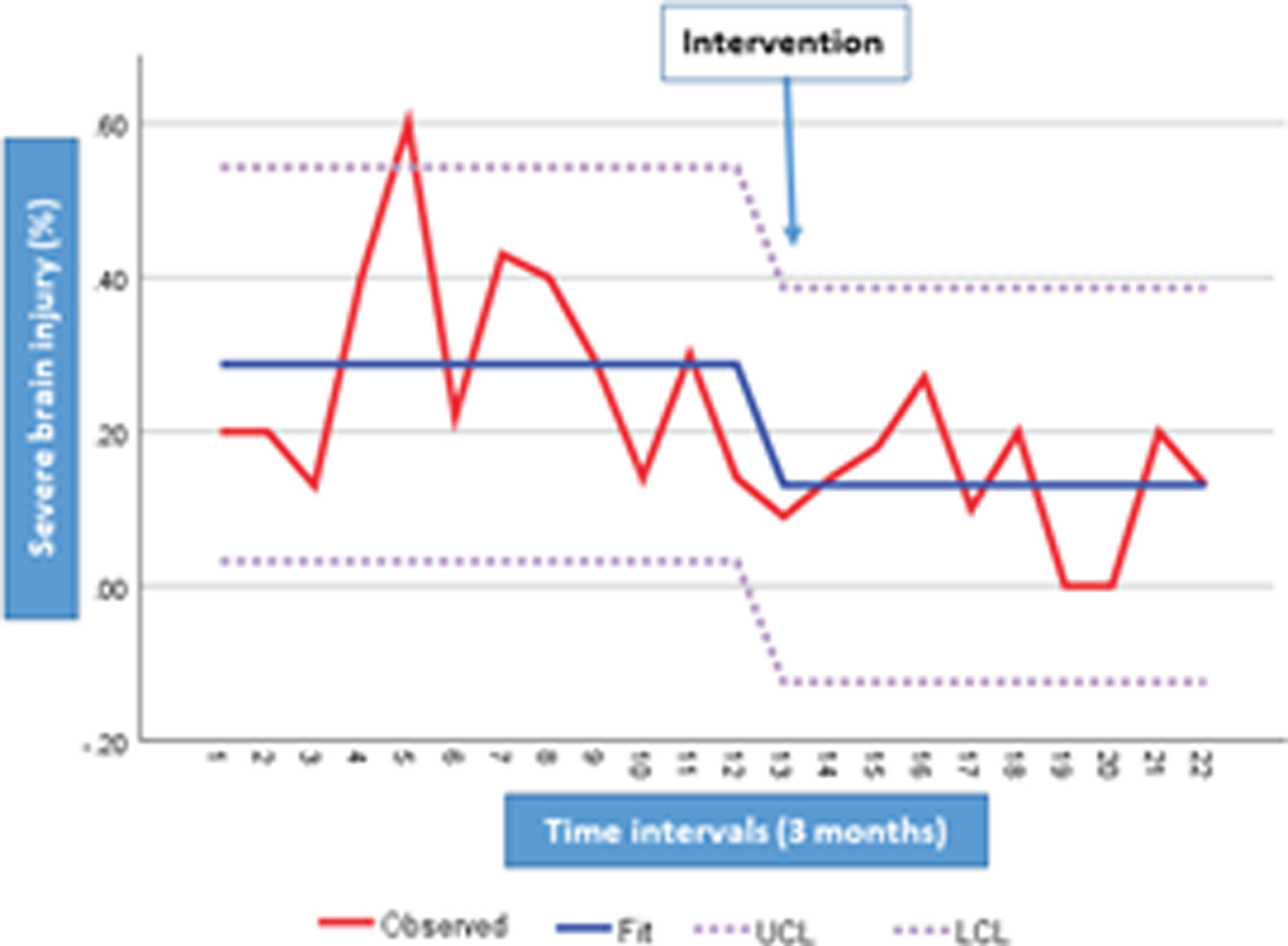
METHOD: A prospective cohort study of premature neonates born at < 33 weeks gestational age (GA) and transported to a tertiary center conducted between January 2013 and December 2018 before and following the implementation of neonatal outreach education. The program comprised of recommendations and interventions aimed at minimizing the risk of acute brain injury through in-person visits, telemedicine, simulation, and the development of a neuroprotection care bundle for very preterm neonates requiring neonatal transport.
RESULTS: A total of 181 neonates were included (93 prior and 88 post-intervention). Basic characteristics were comparable between the two groups. Adjusted for confounding factors, the implementation of outreach education was associated with a significant reduction in the rate of composite death and/or severe brain injury (AOR 0.36, 95% CI, 0.15-0.85, P=0.02), severe brain injury (AOR 0.34, 95% CI, 0.14-0.83, P=0.02), moderate to severe post hemorrhagic ventricular dilatation (AOR 0.21, 95% CI, 0.08-0.61, P=0.004), and brain injury of any grade (AOR 0.41, 95% CI, 0.21-0.83, P=0.01).
Conclusions: Implementation of neonatal outreach education targeting neuroprotection can reduce acute brain injury in infants born at < 33 weeks GA who require neonatal transport.
Characteristics and mortality in neonates with moderate to severe encephalopathy managed with induced hypothermia in a tertiary public hospital in south africa
Nakwa Fa, Sepeng La, Thomas Ra, van Kwawegen Aa, Kamanga Na, Seake Ka, Ntuli Na, Kgwadi Da, Ondongo-Ezhet Ca, Kesting Sa, Mogajane Ta, Coetser Ab, van Rensburg Jb, Pepper Mb, Velaphi Sa
aChris Hani Baragwanath Academic Hospital And University Of The Witwatersrand, Johannesburg, South Africa
bInstitute for Cellular and Molecular Medicine, Department of Immunology, SAMRC Extramural Unit for Stem Research and Therapy, Faculty of Health Sciences, University of Pretoria, Tshwane, Pretoria, South Africa
BACKGROUND: Induced hypothermia (IH) has been shown to reduce death and/or moderate to severe disability in neonates with hypoxic ischemic encephalopathy (HIE). A recent meta-analysis has shown that use of induced hypothermia in neonates with HIE from low- and middle-income countries (LMICs) is associated with reduction in mortality. To the contrary, a recent randomized control trial evaluating IH in South Asia has shown that IH is detrimental and associated with a higher mortality. This study reports on our 5-year experience on neonates with HIE managed with IH in a tertiary public hospital from a LMIC.
OBJECTIVES: To determine the characteristics and mortality at hospital discharge in neonates with moderate to severe encephalopathy managed with IH
METHODS: Records of neonates diagnosed with intrapartum asphyxia were reviewed for clinical data, use of IH (cooled or not cooled) and outcomes at hospital discharge. Neonates included in the study were those with a birthweight ≥ 1800g, gestational age ≥ 36 weeks and had moderate to severe encephalopathy. Comparisons were made between the cooled and not cooled neonates; and between survivors and non survivors within each group (cooled and not cooled).
RESULTS: Sixty-nine percent (595/856) of the 856 neonates that had intrapartum asphyxia, had moderate to severe HIE, 67% (399/592) were managed with IH. Common reasons among the 193 not cooled were; neonate being moribund, unavailability of equipment, presence of pulmonary artery hypertension, improvement in encephalopathy and postnatal age being >6 hours at assessment. Mortality was 17% and 53.4% in cooled and not cooled infants respectively. Amongst both groups non-survivors were more likely to be severely encephalopathic, taken a longer time to spontaneous respiration, had higher Thompson scores and lower pH. On multivariate logistic regression analysis,
odds of having severe HIE were 51 fold amongst the non-survivors in the non-cooled group [OR 51.5 (CI: 5.86-453), p<0.001].
CONCLUSION: Mortality rate in neonates who managed with IH was 17%. Factors associated with mortality were delays in onset of spontaneous respiration, severe acidosis and severity of encephalopathy. Predictor of mortality amongst those who were not cooled was severity of encephalopathy.
Bibliography:
[1] Jacobs SB, M. Hunt, R., Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews. 2013(1).
[2] Abate BB, Bimerew M, Gebremichael B, et al Effects of therapeutic hypothermia on death among asphyxiated neonates with hypoxic-ischemic encephalopathy: A systematic review and meta-analysis of randomized control trials. PLoS One. 2021 Feb 25;16(2):e0247229.
[3] Thayyil S, Pant S, Montaldo P, Shukla D, Oliveira V, Ivain P, et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health. 2021;9(9):e1273-e85.
Cerebral oxygenation and body position in the preterm infant: systematic review and meta-analysis
Maes Ea, Cools Fb, Dereymaeker Aa, Jansen Kc, Naulaers Ga, Thewissen La
aDepartment of Neonatology, University Hospitals Leuven, Leuven, Belgium
bDepartment of Neonatology, University Hospitals Brussels, Brussels, Belgium
cDepartment of Pediatric neurology, University Hospitals Leuven, Leuven, Belgium
BACKGROUND AND PURPOSE: The ideal body position after preterm birth to promote neurological outcome is still unclear in literature. The prone compared to supine position could lead to a change in near-infrared spectroscopy measured cerebral oxygenation (rSc02), which is a surrogate for cerebral blood flow (CBF). Preterms with an intraventricular hemorrhage (IVH) have lower rScO2 and spent more time in cerebral hypoxia. Furthermore, low values are associated with impaired neurological outcome. A possible hypothesis is that rotating the head, that occurs in the prone position, gives an obstruction of the ipsilateral internal jugular vein with venous congestion. A higher intracranial pressure leads to a reduction in CBF and consequently in a decrease in rSc02 with a higher risk of IVH. Some studies promote this prone position in order to support respiratory function and provide kangaroo mother care. In contrast, the supine position, with the head in a neutral midline position, facilitates the cerebral venous drainage and could thereby reduce the risk of an IVH. We conducted a systematic review and meta-analysis to evaluate the effect of body position on cerebral oxygenation. Therefore we compared cerebral oxygenation in prone with supine position in the preterm infant in the first two weeks after birth.
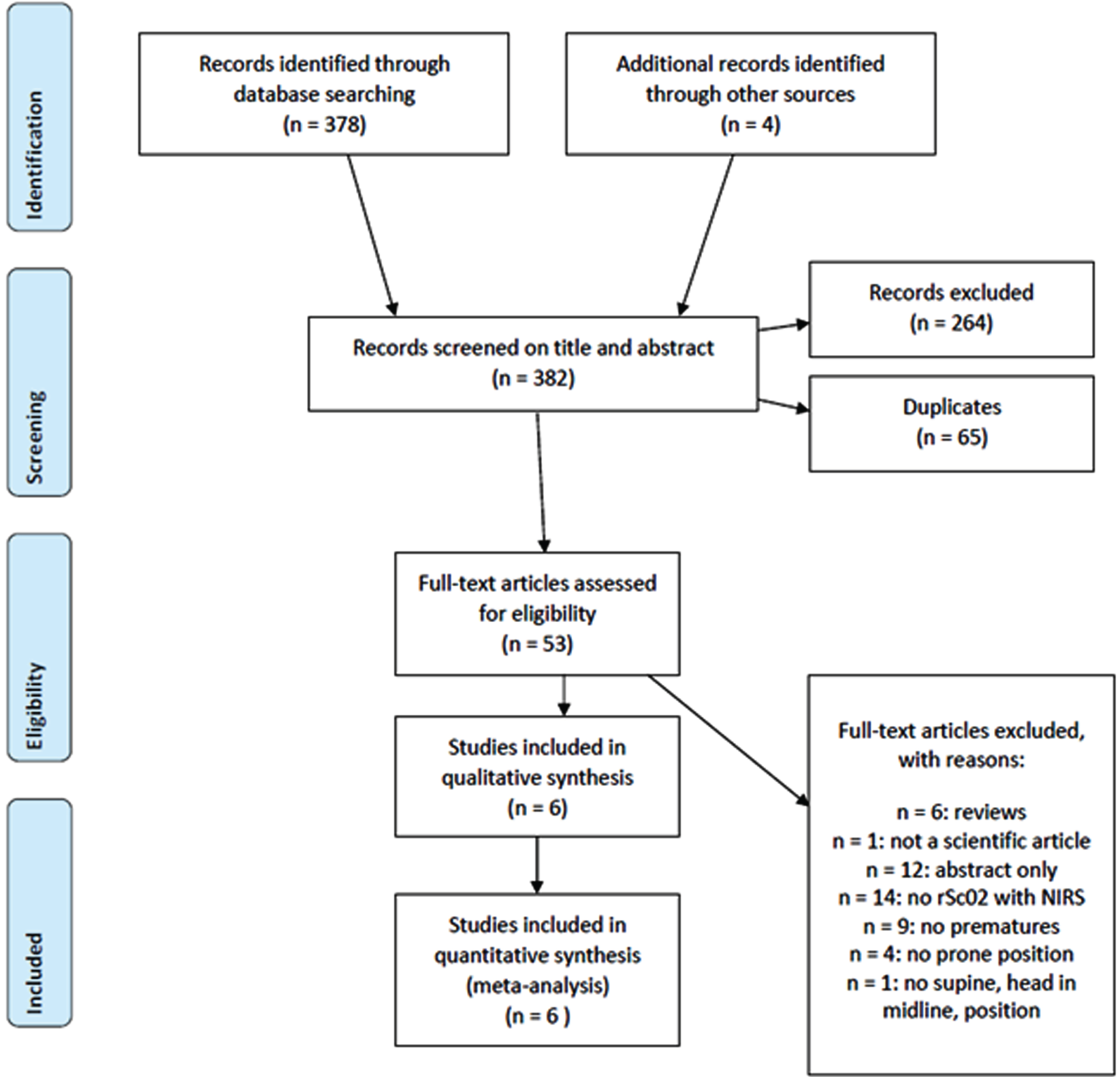
METHODS: A comprehensive literature search was performed to identify all trials that included preterm infants in the first two weeks after birth, and compared rSc02 in the prone versus supine head in midline position of the infant. A meta-analysis, including two subgroup-analyses based on postnatal age (PNA) and gestational age (GA), was performed.
RESULTS: Six observational cohort studies with moderate to strong methodological quality were included (Fig 1). Only one study showed a significantly higher rSc02 in the prone compared to the supine position. Because of the high degree of heterogeneity, we only report the predefined subanalyses. In the first week PNA, there was no difference in rSc02 between the two positions. In the second week, there was a significantly higher rSc02 in the prone position with a mean difference of 1.97% (95% CI 0.87-3.07)(Fig 2). In the subgroup analysis by GA, no difference was observed in rScO2 between both positions in the extremely preterm nor the preterm group (Fig 3).
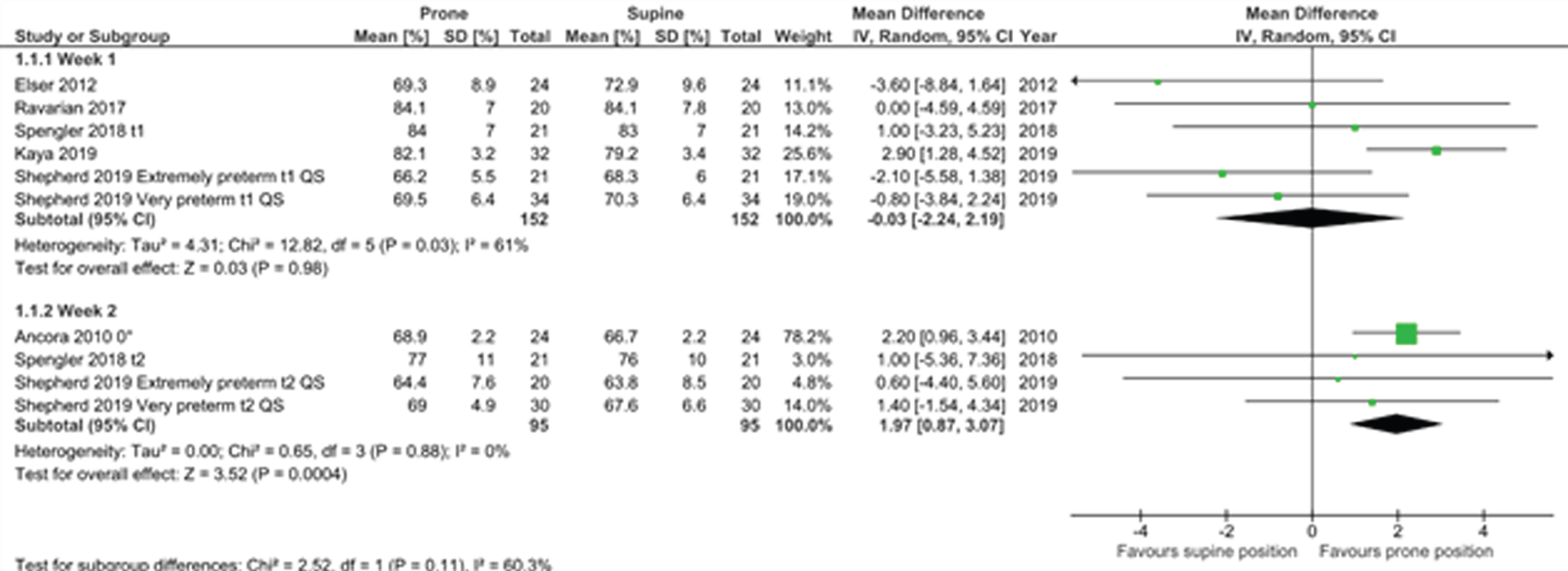
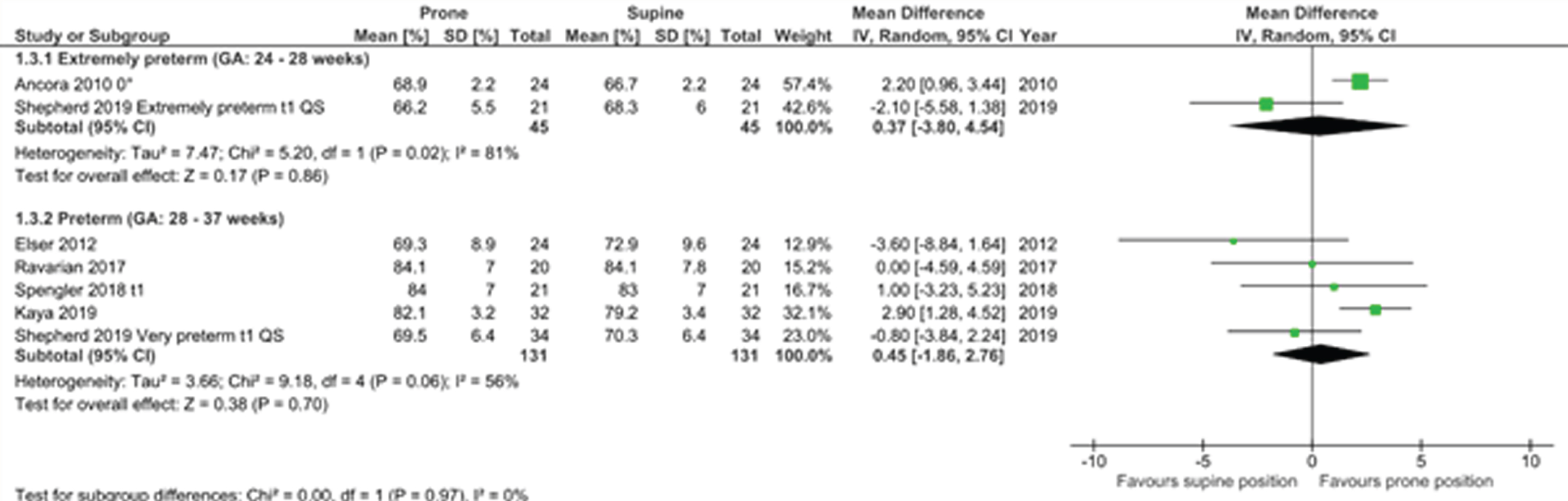
CONCLUSION: There is no consistent evidence that for preterm infants in the first 2 weeks after birth their body position influences rSc02. There was important heterogeneity between studies, however a subgroup-analysis suggests that in the second week after birth, the prone position might result in higher cerebral rSc02 than the supine position with head in midline. Multiple factors such as SaO2 and sleep state influence the rSc02 and determine the best body position in preterms.
Cross-site comparisons of early exposure to analgesia in preterm neonates
Zaki Pa, Selvanathan Ta, Au-Young Sa, Da Rocha Ga, Chau Cb, LePine Ma, Chau Va, McLean Mb, Chan Nb, Ly La, Kelly Ea,c, Grunau Rb, Miller Sa
aDepartment of Pediatrics, The Hospital for Sick Children and University of Toronto, Toronto, Canada
bDepartment of Pediatrics, University of British Columbia, BC Children’s Hospital Research Institute, and BC Women’s Hospital, Vancouver, Canada
cDepartment of Pediatrics, Mount Sinai Hospital and University of Toronto, Toronto, Canada
BACKGROUND AND PURPOSE: Early exposure to frequent invasive procedures is related to long-term alterations in brain development in very preterm neonates. Thus, adequate analgesia is important for neuroprotection during this vulnerable time of brain development. However, there is a lack of consensus around the safe and effective management of neonatal pain. Our study aims to characterize cross-site differences in analgesic practices, accounting for exposure to invasive procedures and major surgery, across three tertiary NICUs in Canada.
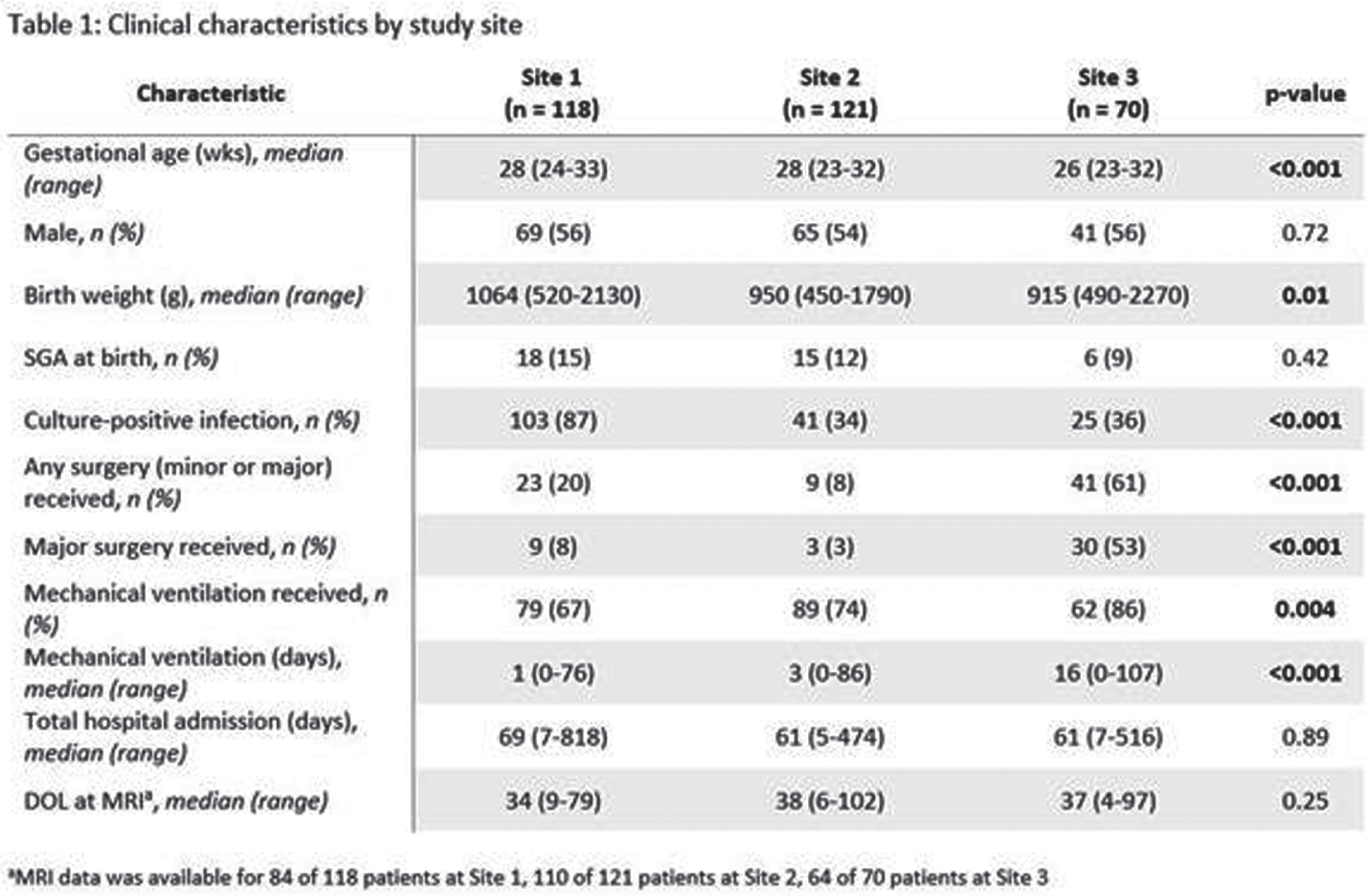
METHODS: Detailed clinical data were collected for 309 very preterm neonates across three tertiary NICUs (Site 1 n=118; Site 2 n=121; Site 3 n=70) between birth and early-life MRI, including daily quantification of invasive procedures as described previously (Vinall et al., 2014), major surgeries, and analgesic medications. Kruskal-Wallis and Fisher’s exact tests were used to compare differences in clinical characteristics, number of invasive procedures and cumulative analgesic dose per invasive procedure between sites. To determine whether analgesic use differed by study site for a given number of invasive procedures, we used multivariate linear regression models with the outcome of cumulative exposure to analgesic medications and the primary predictor as the interaction term between study site and number of invasive procedures; all models accounted for gestational age, days from birth to early MRI and major surgeries.
RESULTS: There were significant differences in gestational age, clinical comorbidities, number of invasive procedures, number of surgeries, and analgesia use across sites (Table 1 and Fig 1-2). Infants at site 1 were not exposed to sucrose (Fig 2). Infants at site 3 received higher cumulative doses of morphine and sucrose relative to the number of invasive procedures compared to the other two NICUs, although there was more variability in morphine exposure per number of invasive procedures at Site 1 (Fig 2-3).
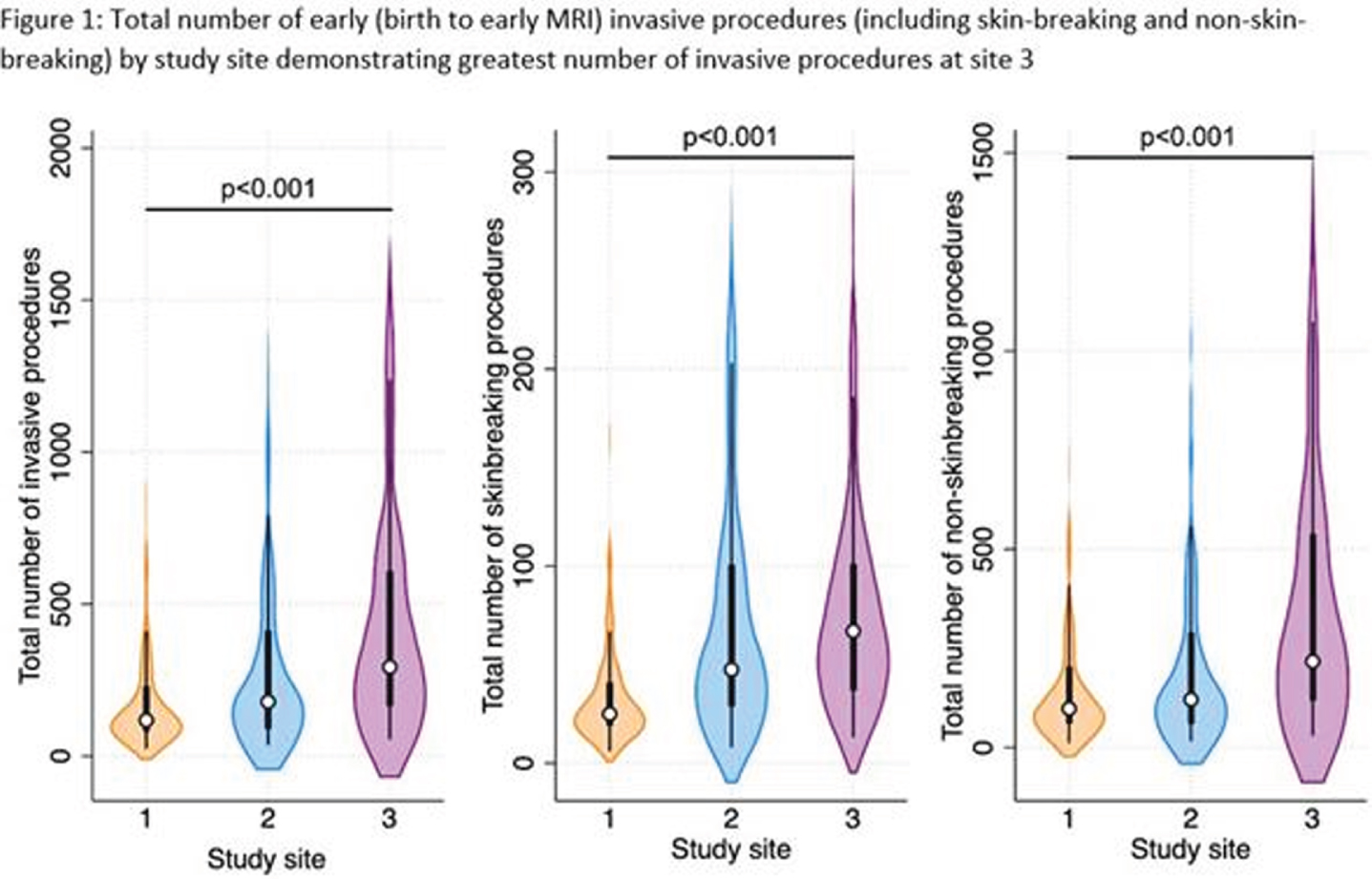
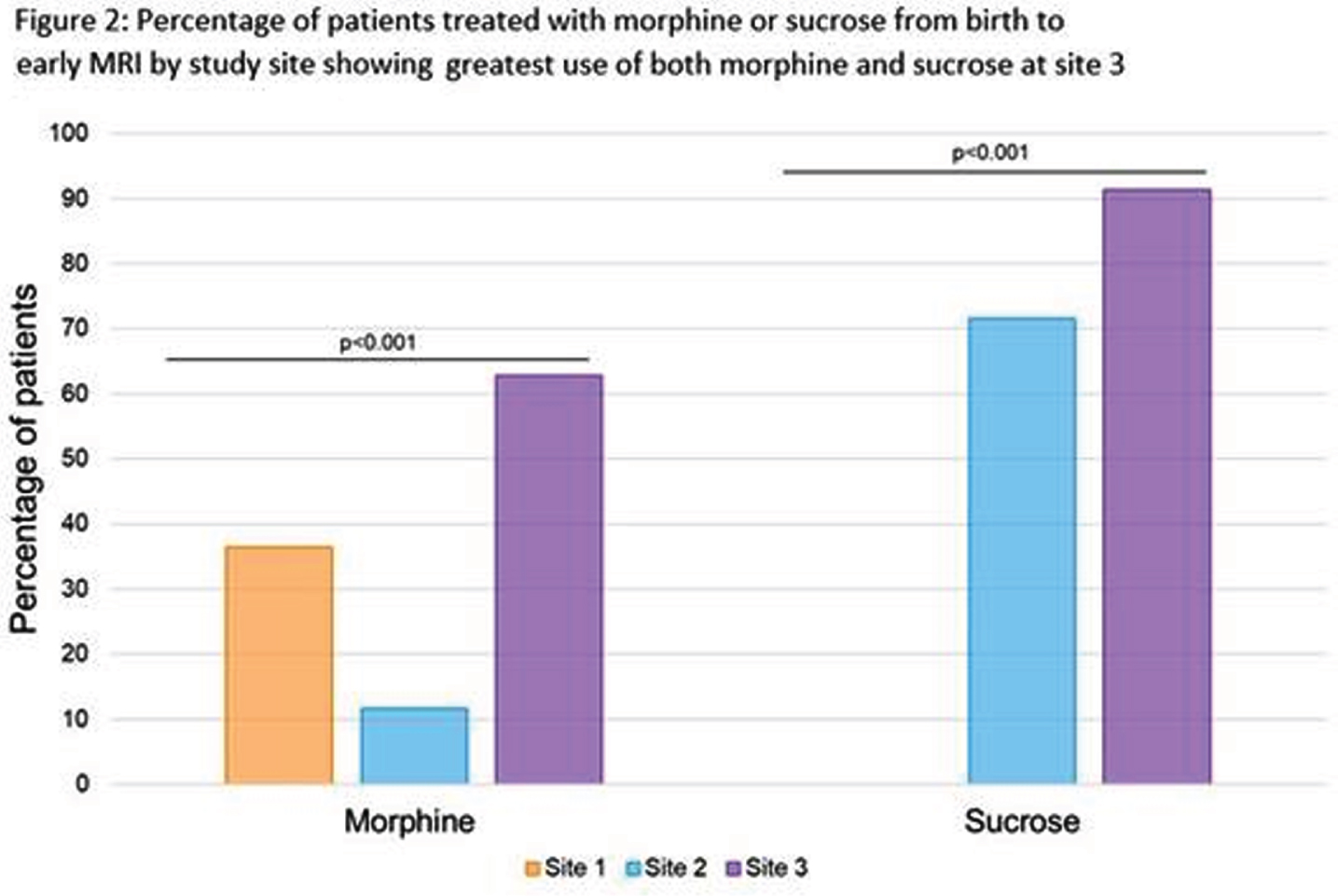
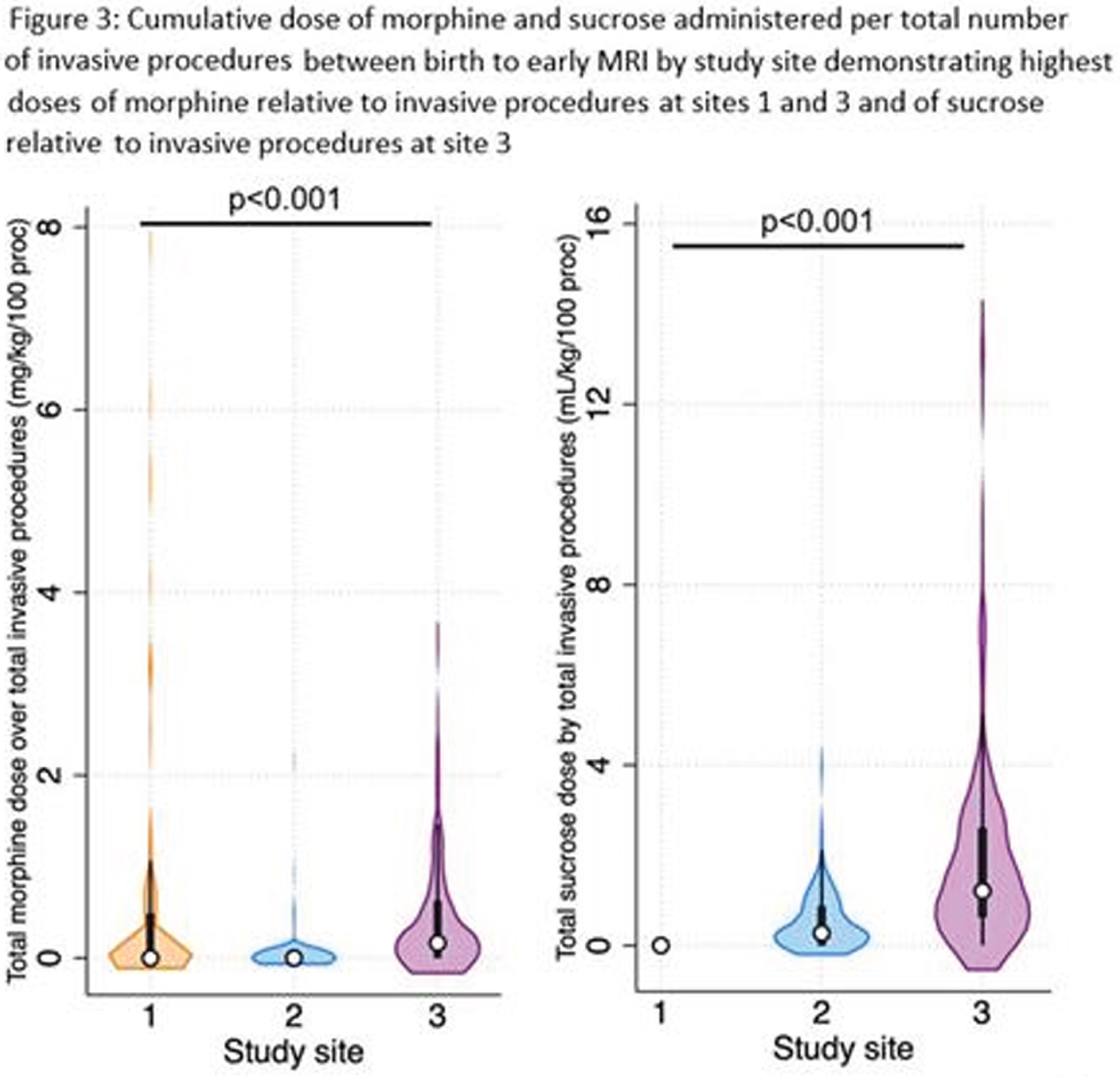
MORPHINE: Multivariate linear regression models revealed a significant interaction between study site and number of invasive procedures (p<0.001) in predicting early cumulative morphine exposure. Morphine use was also significantly higher in neonates undergoing major surgeries (b=0.1, 95%CI 0.04-0.2, p=0.005). There was also an association between cumulative morphine and cumulative sucrose exposure (b=-0.01, 95%CI 0.001-0.01, p=0.03).
SUCROSE: Multivariate linear regression models revealed a significant interaction between study site and number of invasive procedures (p=0.07) in predicting early cumulative sucrose exposure. In contrast with morphine, sucrose use was not associated with surgery exposure.
CONCLUSION: Our findings demonstrate that the use of morphine and sucrose significantly varies across three tertiary NICUs in Canada for a given exposure to early-life pain, even when accounting gestational age at birth and exposure to surgery. These findings highlight important differences in clinical practice and the need to develop clinical guidelines to standardize pain management during this sensitive period of brain development in very preterm newborns.
Feasibility of umbilical cord blood-derived stem cell collection for autologous use in preterm brain injury
Zhou La,b,c, McDonald Cb, Yawno Tb, Jenkin Gb,d, Miller Sb,d, Malhotra Aa,b,c
aDepartment Of Paediatrics, Monash University, Melbourne, Australia
bHudson Institute of Medical Research, The Ritchie Centre, Melbourne, Australia
cMonash Children’s Hospital, Melbourne, Australia
dDepartment of Obstetrics, Monash University, Melbourne, Australia
BACKGROUND: Over 1500 infants are born extremely prematurely (28 weeks or less) every year across the Australia New Zealand Neonatal Network, and suffer high rates of brain injury, notably intraventricular haemorrhage and periventricular leukomalacia1. Periventricular leukomalacia is the leading contributor to development of cerebral palsy in this population, resulting in life-long disability2-3. Currently, there are no available treatments for prevention or cure of preterm brain injury and its sequelae. Umbilical cord blood (UCB)-derived cells have shown promise as a treatment for preterm brain injury in pre-clinical models, including neuro-inflammation4, hypoxia-ischaemia5 and perinatal stroke6. These cells have now been given intravenously as an autologous therapy in early-phase trials for hypoxic ischaemic encephalopathy7, congenital hydrocephalus8, and overall preterm morbidity9.While UCB collection and storage in term infants is well established, there is limited data relating to collection of UCB from preterm infants, particularly those born at less than 28 weeks10. We aimed to establish feasibility of collection, processing and storage of UCB-derived stem cells for autologous use for preterm brain injury.
METHODS: We conducted a prospective study of UCB collection for infants born at <28 weeks at a tertiary maternity hospital in Melbourne, Australia. UCB was collected using 18 gauge needles and 20ml syringes, and placed into heparinised Falcon tubes (Corning, New York, USA), or cord blood collection bags (Macopharma, Tourcoing, France) if the parents elected to have it stored. UCB volume and demographic data was documented, and samples processed to obtain total nuclear cell count and viability by fluorescence-activated cell sorting (BD FACSymphony, BD Biosciences, Melbourne, Australia).
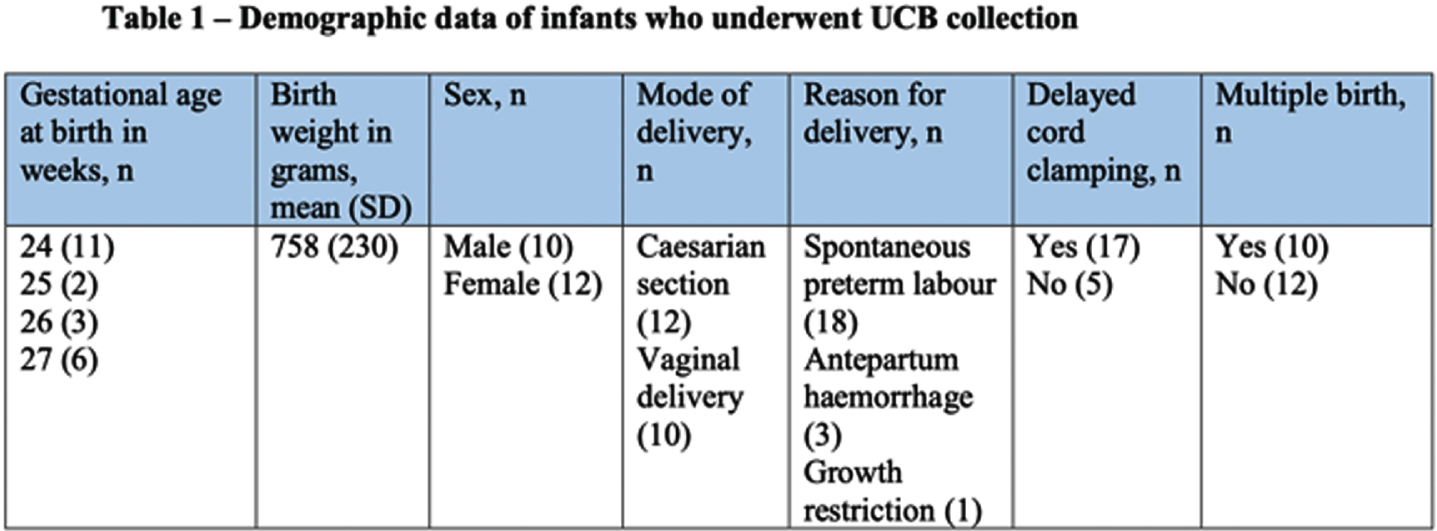
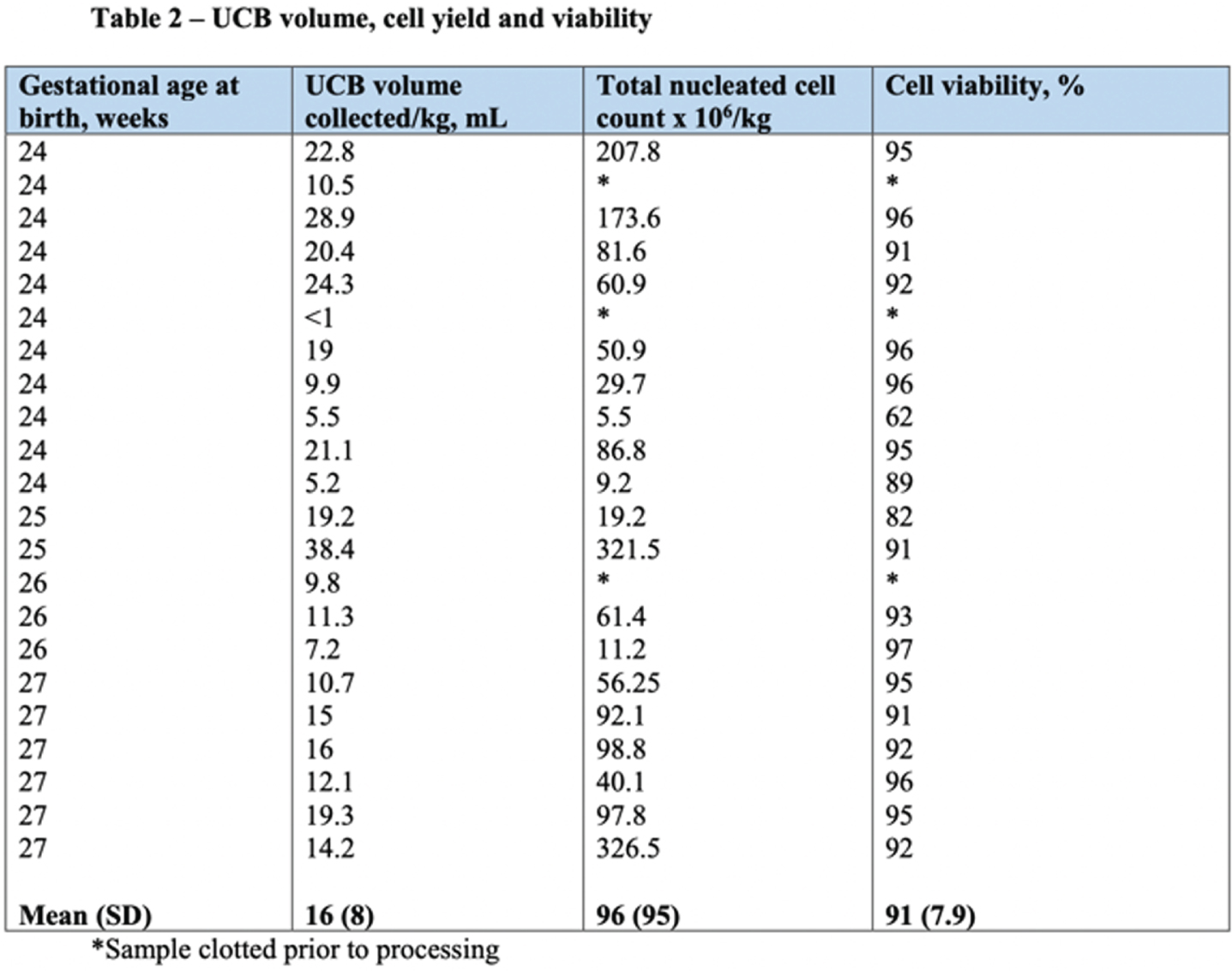
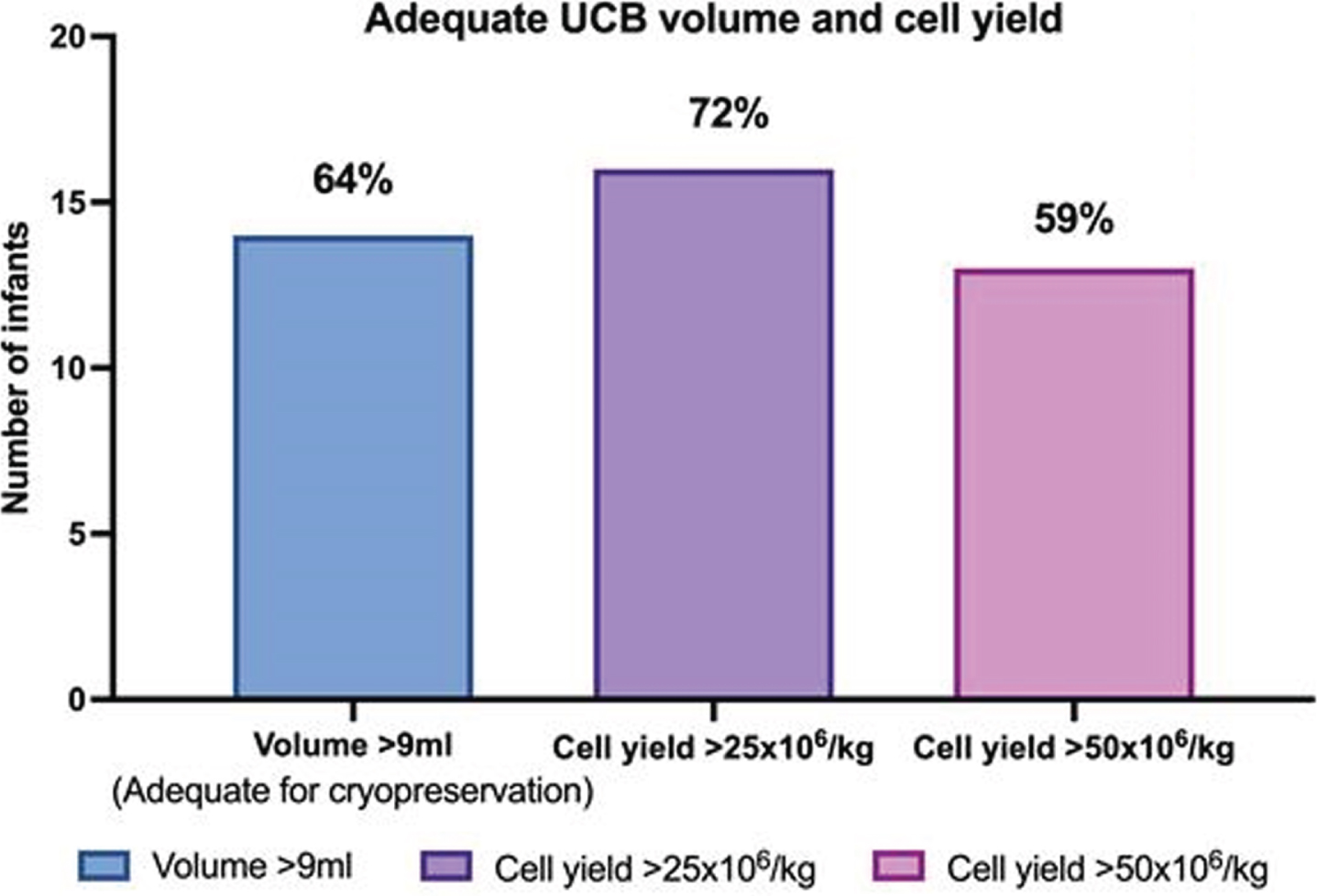
RESULTS: Thirty-six women gave antenatal consent for UCB collection, and 22 (61%) had collection attempted and were included in the study. Of those who did not have UCB collected, 8 progressed beyond 28 weeks, and 6 were missed due to sudden delivery and research staff availability. Of infants included in the study, 10 were male and 12 female; 12 (54%) were delivered via caesarean section, 17 (77%) had delayed cord clamping, and 10 (45%) were part of multiple births. Eighteen (81%) were delivered due to spontaneous preterm labour, 3 (13%) due to antepartum haemorrhage, and one (4%) due to severe growth restriction. Average gestational age and birth weight was 25 weeks, and 758 grams respectively (Table 1). Average UCB volume collected was 12ml/kg, and 14 (64%) were adequate volume for cryopreservation (>9ml). Average total nucleated cell yield was 96x106/kg, with 16 (72%) yielding > 25x106/kg, and 13(59%) >50x106/kg. Average cell viability was 91% (Table 2 and Figure 1).
CONCLUSION: UCB collection yielding adequate cells for autologous use was achievable in approximately two thirds of extremely preterm infants. This has enabled progression to our phase 1 clinical trial for preterm brain injury, the CordSaFe study11.
Bibliography
1. Chow S, Creighton P, Chambers G, Lui K et al. Report of the Australia New Zealand Neonatal Network 2017. ANZNN 2019.
2. Yoon B, Park C, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG: An International Journal of Obstetrics and Gynaecology Dec 2003 110 (20) 124-127.
3. Maclenan A, Thompson S, Gecz J. Cerebral palsy: causes, pathways, and role of genetic variants. American Journal of Obstetrics and Gynaecology 213(6) 779-788.
4. Paton MCB, Allison BJ, Li J, Fahey MC, Sutherland AE, Nitsos I, et al. Human Umbilical Cord Blood Therapy Protects Cerebral White Matter from Systemic LPS Exposure in Preterm Fetal Sheep. Developmental Neuroscience. 2018;40(3):258-70.
5. Aridas JD, McDonald CA, Paton MC, Yawno T, Sutherland AE, Nitsos I, et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb. J Physiol. 2016;594(5):1421-35.
6. Kim e, Anh S, Sung D, Park Y et al. Human umbilical cord derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatric Research 2012; 72: 277-284.
7. Cotten C, Murtha A, Goldberg R, Grotegut C et al. Feasibility of autologous cord blood cells for infants with hypoxic ischaemic encephalopathy. The Journal of Pediatrics 2014; 164(5): 973-979.
8. Sun J, Grant G, McLaughlin C, Allison J et al. Repeated autologous umbilical cord blood infusions are feasible and had no acute safety issues in young babies with congenital hydrocephalus. Pediatric Research 2015; 78: 712-716.
9. Ren Z, Xu F, Zhang C, Miao J et al. Autologous cord blood cell infusion in preterm neonates safely reduces respiratory support duration and potentially preterm complications. Stem Cells Translational Medicine 2019; 9(2): 169-176.
10. Segler A, Braun T, Fischer HS, Dukatz r, Weiss CR et al. Feasibility of umbilical cord blood collection in neonates at risk of brain damage – a step toward autologous cell therapy for a high-risk population. Cell Transplantation Feb 2021 epub ahead of print doi.org/10.1177/0963689721992065.
11. Malhotra A, Novak I, Miller S, Jenkin G. Autologous transplantation of umbilical cord blood-derived cells in extreme preterm infants: protocol for a safety and feasibility study. BMJ Open 2020; 10; e036065.




