Proceedings of the 13th International Newborn Brain Conference: Fetal and/or neonatal brain development, both normal and abnormal
Breast milk-associated oxysterol reverses neonatal white matter injury through Gli2-dependent oligodendrogenesis
Chao Aa, Matak Pa, Pegram Ka, Dubois Lb, Thompson Jb, Jain Vc, Younge Na, Gregory Sc, Goldberg Ra, Benner Ea
aDivision of Neonatology, Department of Pediatrics, Duke University Medical Center, Durham, United States
bDuke Proteomics and Metabolomics Shared Resource, Durham, United States
cDuke Molecular Physiology Institute, Durham, United States
BACKGROUND AND PURPOSE: White matter injury (WMI) is the most common brain injury leading to poor neurologic outcomes in premature infants. Sepsis and inflammation are significant risk factors and there are no treatment options available. Developing novel drug therapies for neonates is challenged by appropriate concerns for safety. Here, we identified oxysterols in human maternal breast milk and explored their therapeutic potential in directing neural stem cells (NSCs) into the oligodendrocyte (OL) lineage via the Sonic Hedgehog (Shh) pathway in vitro. Further, we investigated whether breast milk-associated oxysterols can rescue neonatal WMI in vivo.
METHODOLOGY: Oxysterols were identified in breast milk using Liquid Chromatography/Electrospray Ionization/Tandem Mass Spectrometry. NSCs were cultured from the subventricular zone (SVZ) of mice and treated with oxysterols. Cells were analyzed by immunohistochemistry, western blot, flow cytometry, and scRNAseq, looking for markers of the OL lineage. Shh pathway activation was established by quantifying upregulation of target gene, Gli1, by western blot and RT-PCR. Gli-dependence was explored using pharmacological and genetic approaches. Neonatal sepsis leading to WMI was induced in mice on postnatal day 5, using an intraperitoneal stool injection. Mice then received either breast milk-associated 20-αhydroxycholesterol (20HC) or vehicle. Stereology determined OL numbers in the periventricular white matter regions. Myelination was evaluated by performing g-ratios to determine myelin thickness. Motor function in mice was analyzed using the CatWalk gait analysis system. To lineage trace postnatal nestin+ SVZ NSCs in vivo, neonatal sepsis was induced in Nestin-CreERT2;R26r-TdTomato pups as above, and then treated with either vehicle or 20HC.
RESULTS: Multiple oxysterols were identified in human maternal breast milk that induced oligodendrocyte production from NSCs in vitro. We found that Gli2 is functionally required for oxysterol-induced oligodendrogenesis. Following neonatal WMI in vivo, 20HC treatment increased numbers of mature OLs, improved myelination and rescued motor deficits in mice. Lineage tracing experiments showed that 20HC-mediated recovery of OL deficit is mediated in part through 20HC-induced SVZ-derived oligodendrogenesis in vivo. Additional recovery may be due to the impact of 20HC on oligodendrocyte progenitor cell maturation.
Supplemental IGF-1 promotes neuronal maturation and myelination in preterm pigs
Christiansen La, Holmqvist Bb, Lindholm Sa, Pan Xa, Holgersen Ka, Henriksen Na, Carey Gc, Ley Dd, Thymann Ta, Sangild Pa, Pankratova Sa
aComparative Pediatrics and Nutrition, Department of Veterinary and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
bImaGene-iT, Medicon Village, Lund, Sweden
cTakeda, Cambridge, United States
dPediatrics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
BACKGROUND: Preterm infants are born with low circulating insulin-like growth factor 1 (IGF-1) levels and in cohort studies, low IGF-1 levels have been associated with morbidities, including poor neurodevelopment (1). It remains unclear if supplemental IGF-1 promotes neurodevelopment in preterm infants (2). Using preterm pigs as a model for preterm infants (3,4), we investigated how supplemental IGF-1, in complex with the binding protein IGFBP3, affects brain maturation in the early postnatal period.
METHODS: Preterm-born pigs were treated with 2.25 mg/(kg*day) IGF-1/IGFBP3 for 5 or 9 days starting at birth, then pigs were euthanized for collection of brain sections for immunohistochemistry, RNAseq and qPCR analyses. The locomotor activity was evaluated by open field test at day 7.
RESULTS: IGF1R was expressed in immature neurons and exogenous IGF-1/IGFBP3 stimulated neuronal maturation, subcortical myelination and attenuated synaptogenesis in region- and time-dependent manners. Expression of genes involved in neuronal and oligodendrocyte maturation (EEF1A2, EEF1A1, Opalin), angiogenic (CNMD) and transport functions (AQP4, TTR) were altered, reflecting enhanced maturation in response to treatment. No effects on regional brain weights, early motor development or hippocampal expression of genes related to IGF-1 signaling (IGF1, IGF2, IGF1R, IGF2R, IRS1, IGFBP3) were observed. Likewise, the populations of immature neurons and microglia were not affected by treatment.
CONCLUSION: Supplemental IGF-1/IGFBP3 promotes neurodevelopment in the early postnatal period in preterm pigs. Possibly, supplemental IGF-1/IGFBP3 may improve brain development in preterm infants.
Bibliography:
[1] Hellström, A. et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatrica, International Journal of Paediatrics vol. 105 576–586 (2016).
[2] Ley, D. et al. rhIGF-1/rhIGFBP-3 in Preterm Infants: A Phase 2 Randomized Controlled Trial. J. Pediatr. 206, 56-65.e8 (2019).
[3] Holme Nielsen, C. et al. Rapid Postnatal Adaptation of Neurodevelopment in Pigs Born Late Preterm. in Developmental Neuroscience vol. 40 586–600 (S. Karger AG, 2019).
[4] Bæk, O. et al. Sex-Specific Survival, Growth, Immunity and Organ Development in Preterm Pigs as Models for Immature Newborns. Front. Pediatr. 9, 626101 (2021).
Fully automated quiet sleep quantification in preterm infants
Smets La,b, Comani Sb,c, De Vos Ma,d, Dereymaeker Ad
aDepartment of Electrical Engineering (ESAT), STADIUS Center for Dynamical Systems, Signal Processing and Data Analytics, KU Leuven, Leuven, Belgium
bDepartment of Neuroscience, Imaging and Clinical Sciences, University “G. d’Annunzio” of Chieti-Pescara, Chieti, Italy
cBehavioral Imaging and Neural Dynamics Center, University “G. d’Annunzio” of Chieti-Pescara, Chieti, Italy
dDepartment of Development and Regeneration, UZ Leuven, Leuven, Belgium
BACKGROUND: Preterm infants are born during a crucial period of rapid brain development [1]. Neonates spend most of their time in the sleeping state, crucial for endogenous driven brain activity. Their sleep structure is developmentally uniquely regulated with a predominance of Active Sleep (AS) compared to Quiet Sleep (QS) in younger preterm infants [2]. Generally, as the newborn brain matures, the duration of sleep segments and the percentage of time in QS increases, whereas the percentage in AS decreases by term-equivalent age [2]. However, preterm infants experience sleep disturbances in the Neonatal Intensive Care Unit (NICU), affecting further normal functional brain maturation [2]. Therefore, quantifying sleep in preterm infants is needed as a surrogate for brain health, which is done in this study by using a fully automated sleep classification algorithm and additional representation with survival curve analysis.
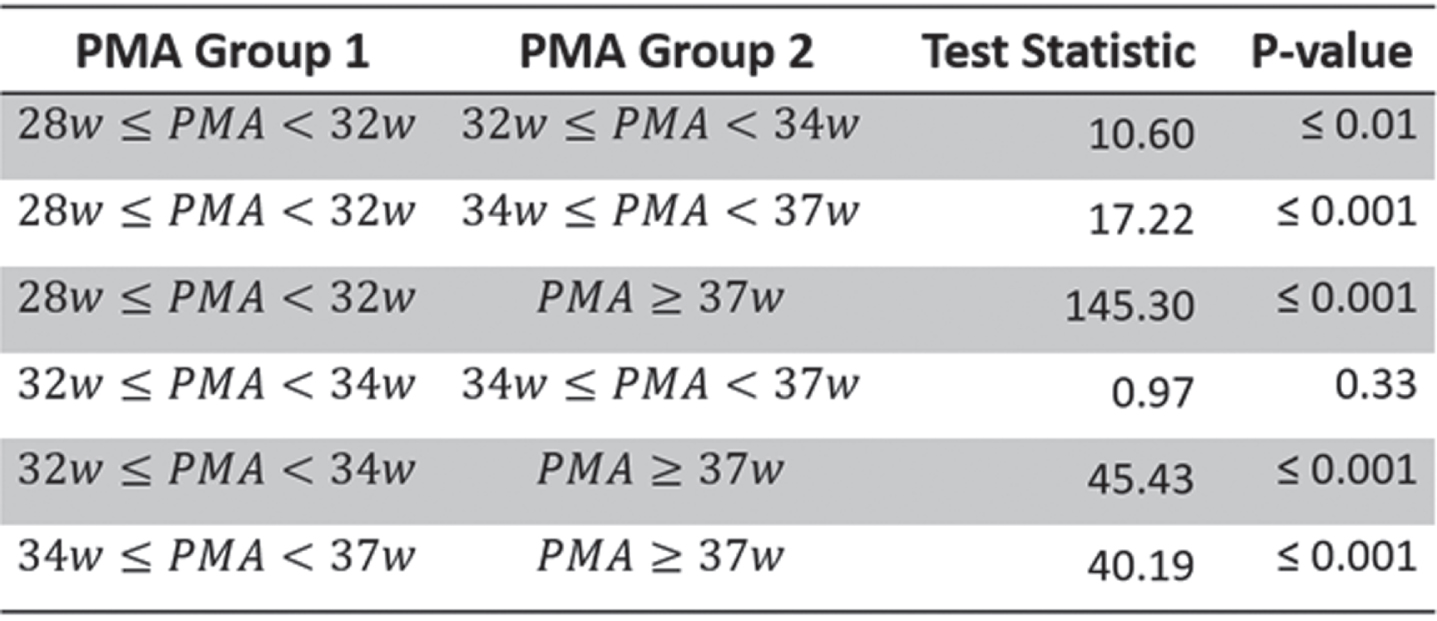
MATERIALS AND METHODOLOGY: Long-duration EEG measurements of 133 preterm infants (gestational age = [23.86-33.86] weeks), recorded at the NICU in University Hospitals Leuven, were used for automated sleep state classification with the CNN developed by Ansari et al. [3] in AS and QS epochs. Preterm neonates were divided in four groups, based on the postmenstrual age (PMA) at time of recording: 28w≤PMA<32w; 32w≤PMA<34w; 34w≤PMA<37w; and PMA≥37w. The duration of each QS segment was considered and plotted using the Kaplan Meier function. Statistical differences were tested with a pairwise logrank test with a significance level of 5%.
RESULTS: Figure 1 shows the Kaplan Meier function of the persistence of QS for each PMA group. This figure clearly shows that the persistence of QS increases with increasing PMA. This is in line with the findings of Georgoulas et al. [4] and normal brain maturation patterns. All but one pairwise comparisons between the four curves are significant (Table 1).
CONCLUSION: For the first time, sleep state analyses from preterm newborns are processed in a fully automated way [4]. This automated sleep quantification approach in preterm infants using survival curve analysis, opens up the way to further study objectively the effects of physiological stress, family intervention programs and drugs on sleep development to optimize sleep-wake regulation in NICU.
Bibliography:
[1] R. Grunau, “Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity.,” Rambam Maimonides Med. J., vol. 4, no. 4, pp. 1–13, 2013.
[2] D. Barbeau and M. Weiss, “Sleep Disturbances in Newborns,” Children, vol. 4, no. 10, p. 90, Oct. 2017.
[3] A. Ansari et al., “A convolutional neural network outperforming state-of-the-art sleep staging algorithms for both preterm and term infants,” J. Neural Eng., vol. 17, p. 016028, 2020.
[4] A. Georgoula et al., “Sleep-wake regulation in preterm and term infants,” Sleep, vol. 44, no. 1, pp. 1–10, 2021.
Functional brain maturation and sleep organisation in neonates with Congenital Heart Disease
Dereymaeker Aa, Hermans T, Thewissen L, Gewillig M, De Vos M, Naulaers G, Breckpot J, Jansen K
aDepartment of Development and Regeneration, Neonatal Intensive Care Unit, University Hospitals Leuven, KU Leuven (University of Leuven), Leuven, Belgium
bDepartment of Development and Regeneration, Neonatal Intensive Care Unit, University Hospitals Leuven, KU Leuven (University of Leuven), Leuven, Belgium
cDepartment of Cardiovascular Science, Paediatric Cardiology, University Hospitals Leuven, KU Leuven (University of Leuven), Leuven, Belgium
dDepartment of Development and Regeneration, Child Neurology, University Hospitals Leuven, KU Leuven (University of Leuven), Leuven, Belgium
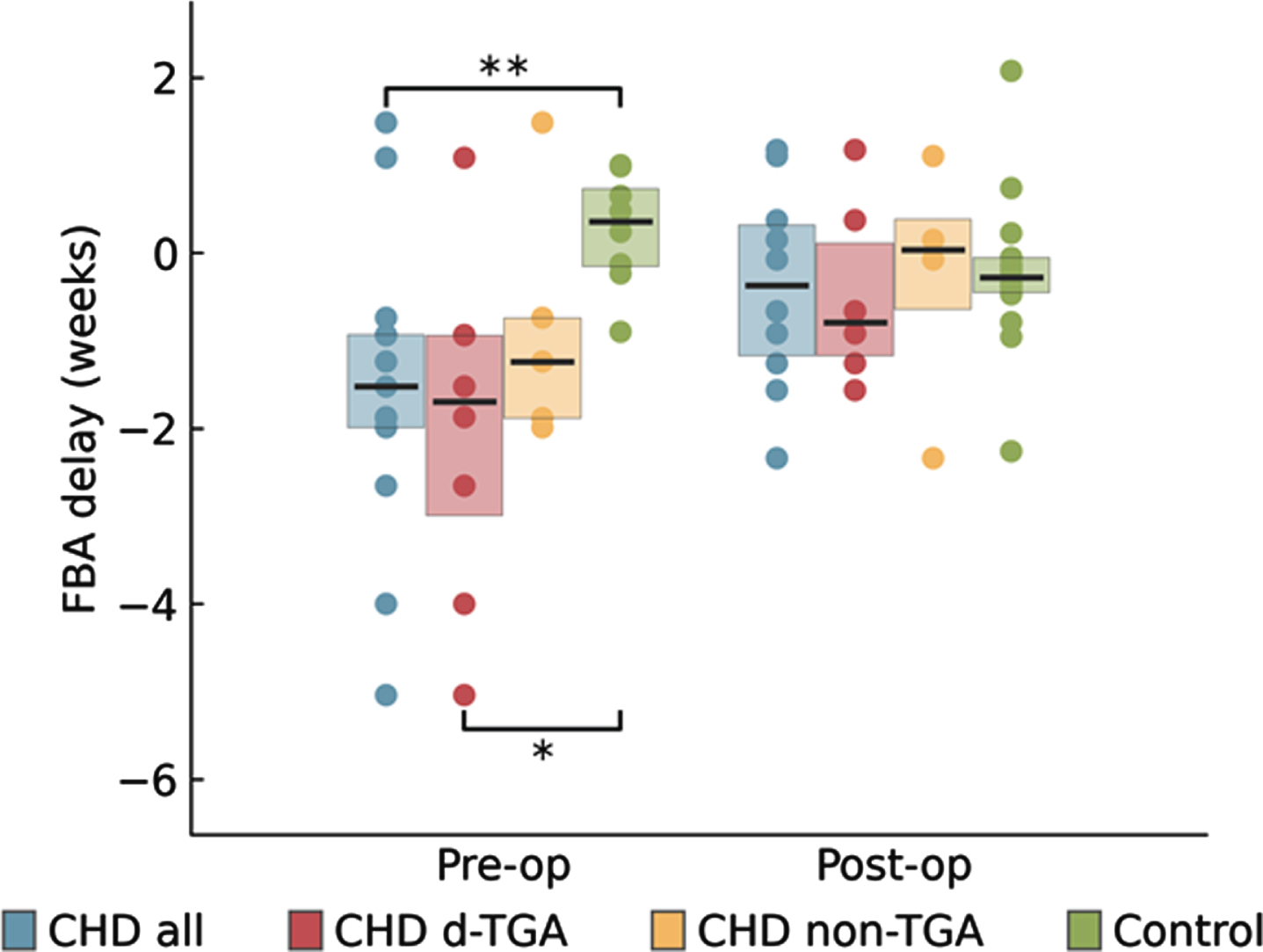
OBJECTIVE: Neuroimaging studies have demonstrated structural delays in brain development in neonates with Congenital Heart Disease (CHD) (1). To evaluate whether this is also reflected in early alterations of functional brain maturation on EEG, analysis of Functional Brain Age (FBA) and (2) sleep organisation (3) during the neonatal period is investigated.
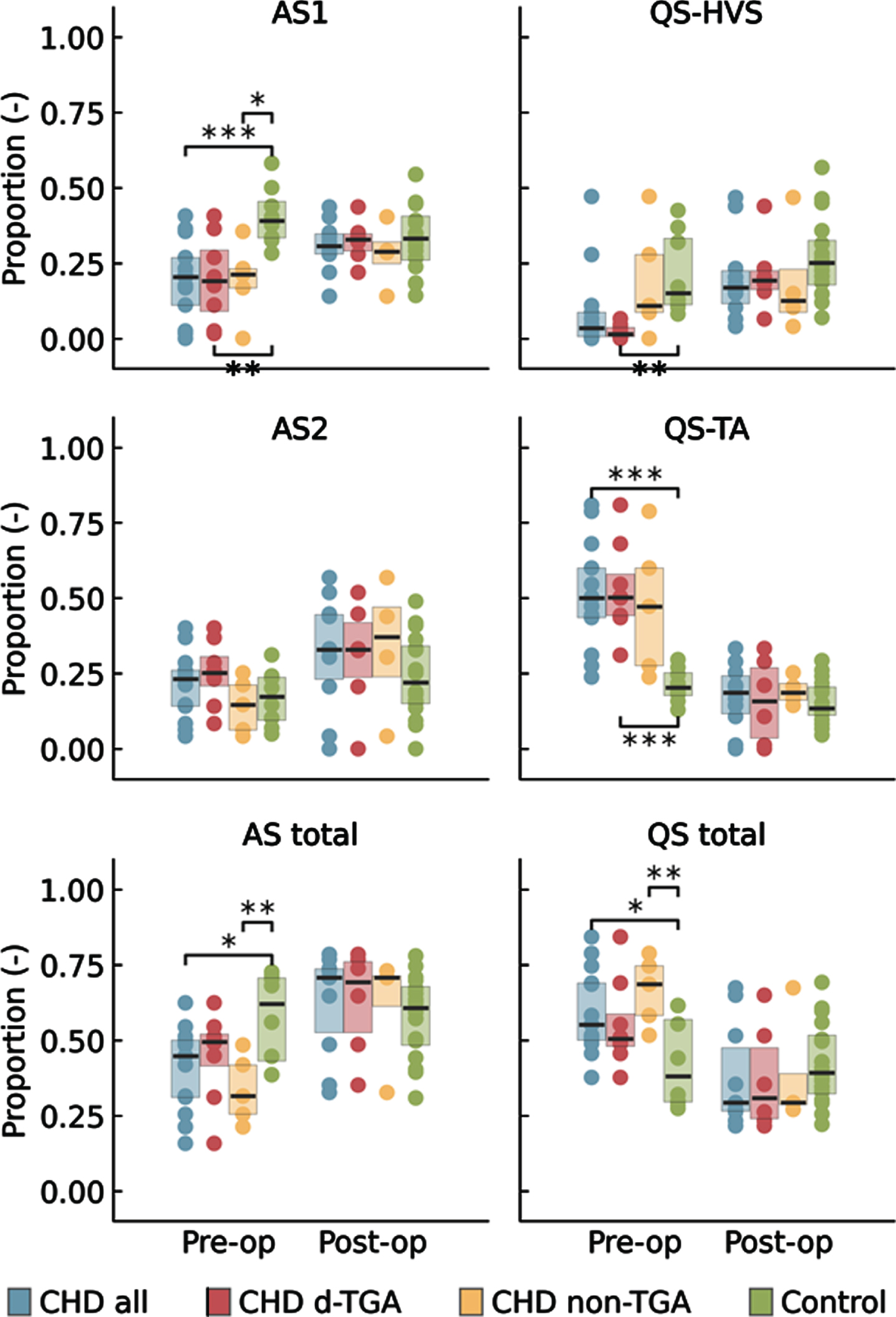
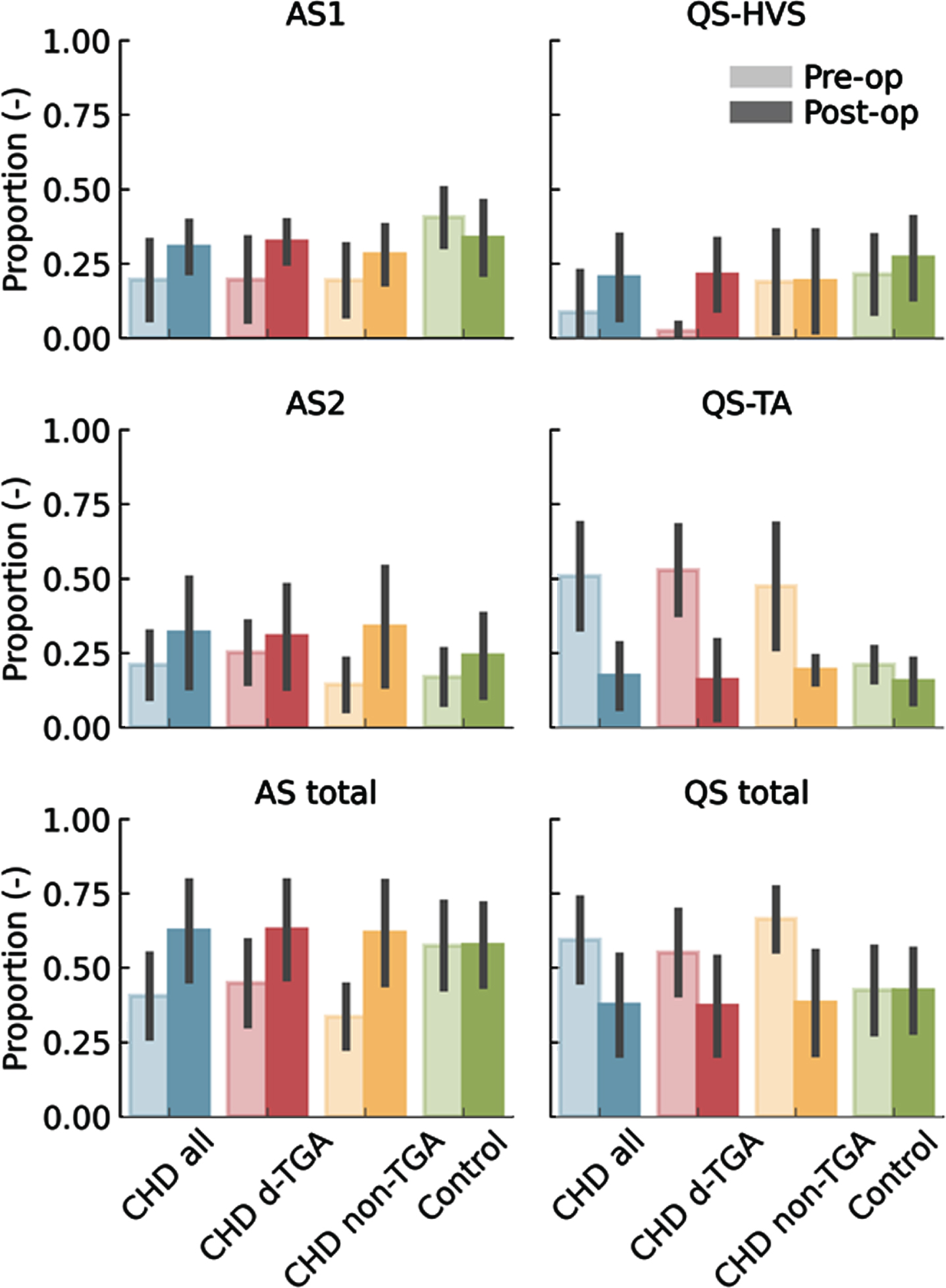
METHODS: We compared prospectively 15 neonates with CHD who underwent multichannel EEG, pre -and postoperatively, with healthy term newborns of the same postmenstrual age (PMA). Subgroup analysis for d-Transposition of the Great Arteries (d-TGA) was performed (n=8). To estimate FBA, a prediction tool using quantitative EEG features as input, was applied. Second, the EEG was automatically classified as Active Sleep Stage 1 (AS1), Quiet Sleep Tracé Alternant (QS-TA), Quiet Sleep High Voltage Slow Wave Sleep (QS-HVS) and Active Sleep Stage 2 (AS2). Neonates with CHD underwent neurodevelopmental testing at 24 months with Bayley Scale of Infant Development (BSID-III).
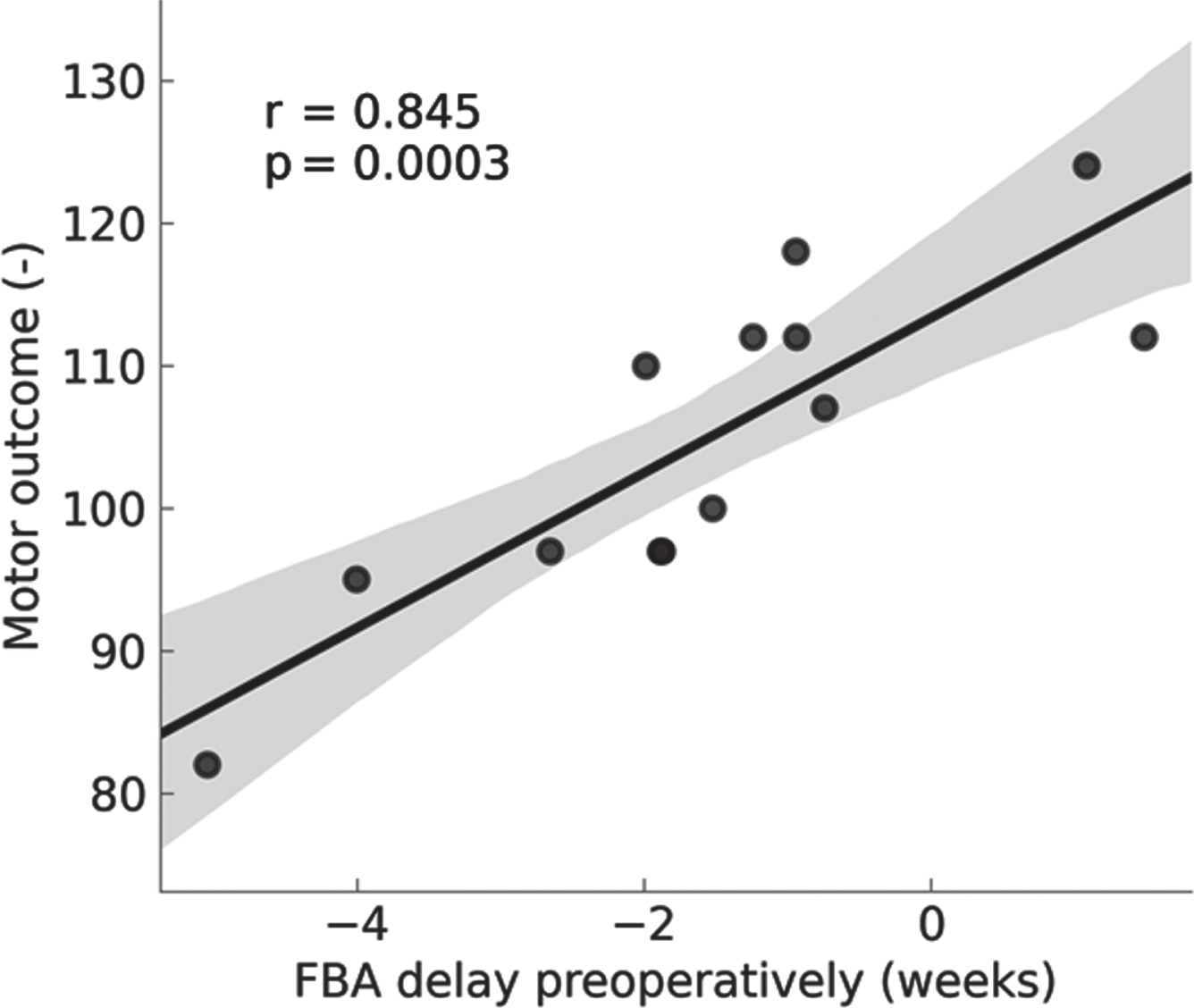
RESULTS: The pre-operative FBA was delayed in the CHD infants (n=15) and more so in the d-TGA infants. The FBA was positively correlated with BSID-III motor scores.
Sleep organisation was also significantly influenced during the first days in neonates with CHD. The duration of the sleep cycle and the proportion of AS1 was decreased, again more marked in the d-TGA infants. Neonates with d-TGA spent less time in QS-HVS and more in QS-TA compared to healthy terms. Both FBA and sleep organisation normalised post-operatively to be similar to control infants. Duration of QS-HVS was positively correlated with higher motor scores in d-TGA infants.
The FBA could also be predicted on reduced 2-channel EEG montage.
INTERPRETATION: Altered early brain function and sleep is present in neonates with CHD, with significant changes after early interventions which improved cerebral oxygenation delivery. These results are intruiging, as inefficient neonatal sleep, expressed as increased QS-TA, has been linked with adverse long-term outcome in newborns at risk for cerebral dysfunction (4). Identifying how these rapid alterations in brain function are mitigated through early surgery, drugs and nutrition may have relevance for clinical practice and long-term outcome.
Bibliography:
[1] Peyvandi S, Latal B, Miller SP, McQuillen PS. The neonatal brain in critical congenital heart disease: Insights and future directions. Neuroimage. 2019 Jan 15;18
[2] Pillay K, Dereymaeker A, Jansen K, Naulaers G, De Vos M. Applying a data-driven approach to quantify EEG maturational deviations in preterms with normal and abnormal neurodevelopmental outcomes. Sci Rep. 2020 Apr 29;10(1):7288.
[3] Ansari AH, De Wel O, Pillay K, et al. A convolutional neural network outperforming state-of-the-art sleep staging algorithms for both preterm and term infants. J Neural Eng. 2020 Jan 14;17(1):016028.
[4] Shellhaas R, Burns JW, Hassan F, et al. Neonatal Sleep-Wake Analyses Predict 18-month Neurodevelopmental Outcomes
Bowel perforation model leads to postnatal subventricular zone inflammation and neural stem cell niche injury
Epstein Aa,c, Rikard Bc, Jain Vb, Abdi Kc, Younge Na,c, Chao Aa,c, Pegram Kc, Gregory Sb, Benner Ea,c
aDivision of Neonatology, Department of Pediatrics, Duke University Medical Center, Duke University, Durham, United States
bDuke Molecular Physiology Institute, Duke University, Durham, United States
cDuke University, Durham, United States
OBJECTIVE: High rates of neurodevelopmental impairments are associated with sequalae of extreme premature birth, especially necrotizing enterocolitis and bowel perforations. The mechanisms of brain injury in this population are poorly understood. In this study we investigated the impact of a novel injury to the postnatal subventricular zone following modeled bowel perforation. Altered gliogenic and neurogenic functionality of the subventricular zone may contribute to neurologic deficits observed in preterm infants.
METHODS: Using a clinically relevant rodent model of neonatal bowel perforation leading to polymicrobial sepsis we replicated many features of brain injuries seen among preterm-born infants. These injuries include diffuse white matter injury as well as motor deficits. Our experiments focused on neuroimmune responses and histological changes at the subventricular zone (SVZ) neural stem cell niche that resides in the germinal matrix of human preterm infants. qPCR was utilized to measure localized inflammatory cytokine production. We employed single nuclear transcriptomic profiling (snRNAseq) to identify shifts in SVZ ependymal, glial, and neural progenitors in septic mice. Observations from transcriptomic data were validated using immunohistology of SVZ whole mounts.
RESULTS: Focal inflammation was seen in the SVZ following modeled bowel perforation. Immunohistology confirms localized periventricular microgliosis and qPCR on isolated SVZ tissue shows increased proinflammatory IL-1 and TNFalpha cytokine expression following sepsis compared to littermate controls. SVZ whole mounts using FoxJ1-EGFP tg-mice expressing green fluorescence protein in ependymal cells prepared following neonatal sepsis show diffuse loss of classical ependymal cell organization, as well as broad loss of cilia visualized using electron microscopy. Transcriptomic profiling on SVZ tissues identified 42 clusters of distinct cell populations in both sepsis and control animals. Preliminary identification of clusters revealed a loss of postnatal neurogenesis. To confirm the deficits in neurogenesis we observed in the transcriptomic profiling data, we next employed confocal microscopy to image doublecortin (DCx)-positive cells in SVZ whole mounts to identify neuroblast chain development in control animals and septic animals at 18 days after sepsis induction. SVZ whole mounts in control mice at postnatal day 23 (P23) show DCx-positive cells arranged in chains moving up the wall in an organized pattern towards the striatal-cortical junction. However, these neuroblast chains are grossly deficient and disorganized in SVZ whole mounts from age-matched animals with neonatal sepsis.
CONCLUSION: Our animal data suggests that neonatal bowel perforations injure the neural stem cell niche leading to deficits in neurogenesis during a critical time of neurodevelopment. This previously undescribed injury may be an important molecular mechanism linking inflammatory complications following preterm birth and poor neurodevelopmental outcomes in infants.
Association of Fetal tracing patterns with cerebral MRI findings of infants with neonatal encephalopathy
Geva Na, Geva Yb, Yaniv Salem Sb, Rotem Rc, Abramsky Rd, Hershkovitz Rb, Weintraub Ab, Shelef Ie, Shany Ed
aDepartment of Pediatrics, Soroka University Medical Center Beer-Sheva, Israel and Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel
bDepartment of Obstetrics and Gynecology, Soroka University Medical Center, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel
cDivision of Obstetrics and Gynecology, Shaare Zedek Medical Center, Hebrew University Medical School, Jerusalem, Israel
dDepartment of Neonatology, Soroka University Medical Center Beer-Sheva, Israel and Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel
eDepartment of Medical Imaging, Soroka University Medical Center and the Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, Beer-Sheva, Israel
BACKGROUND AND PURPOSE: Neonatal encephalopathy (NE) of hypoxic-ischemic origin, is the single greatest contributor to overall disability worldwide and accounts for one-tenth of all disability-adjusted life years 1. During the last decades advances in the treatment of NE were made, mainly by implementing therapeutic hypothermia (TH) as a routine practice, in cases of moderate to severe HIE. Still, the ability to predict which neonates are expected to suffer from MRI abnormalities according to intrapartum continuous fetal cardiotocography (CTG) analysis is lacking.
The use of CTG became a mainstay in obstetrics in high income countries. Though its use has resulted in a rise of obstetrical interventions, using the traditional category system of interpretation has very little ability to predict neonatal outcome.
We hypothesized that intrapartum deceleration and acceleration areas are associated with the degree of cerebral insult of the neonatal brain.
The aim of this study was to assess the association of intrapartum deceleration and accelerations area under the curve with the degree of cerebral injury in cerebral MRI of infants with NE.
MATERIALS AND METHODOLOGY: A single center retrospective cohort study including all NE cases in a tertiary hospital between 2013 and 2021. Included in the analysis were all term and near-term antepartum low-risk pregnancies that resulted in NE treated with TH and that had an intrapartum CTG record of 60 minutes and above.
We calculated acceleration and deceleration areas, minimum and maximum depth and duration of accelerations and decelerations and the acceleration-to-deceleration ratio during the 120 minutes prior to delivery.
MRI scans performed before 10d of age were categorized as normal-mild or moderate-severe according to a modified Rutherford score2.
RESULTS: During the study period, 137 infants were treated for NE at our center. In 44 (32.1%) infants CTG records were insufficient, 3 were excluded due to congenital malformations or breach deliveries and 13 (14.4%) did not have an MRI scan available. Overall, 77 infants were included in the final analysis, 26 with severe findings on brain MRI.
CTG tracing analysis revealed a significant association between increased acceleration area and better MRI results and an even stronger association between a higher acceleration/deceleration ratio and better MRI results.
CONCLUSION: In the presence of a pathologic CTG record, lower total acceleration area and especially lower acceleration-deceleration are associated with a higher risk of brain MRI abnormalities.
In order to increase the role of these measurements as a relevant clinical tool, larger, more powered prospective trials are needed and computerized real-time analysis of the fetal heart rate needs to be made available.
Bibliography:
[1] Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb Clin Neurol. 2019;
[2] Association of cerebral activity with MRI scans in infants with neonatal encephalopathy undergoing therapeutic hypothermia. Eur J Pediatr. 2019;
Male mice have larger macrophage response than female mice 3 days after P10 hypoxia-ischemia
Guez-Barber Da, Gillis Sb, Kiffer Fa, Yun Sa,b, Eisch Aa,b
aChildren’s Hospital Of Philadelphia, Philadelphia, United States
bUniversity of Pennsylvania, Philadelphia, United States
BACKGROUND: In rodent models of hypoxia-ischemia (HI), males show larger anatomic injuries, distinct motor deficits, and worse cognition after HI relative to females. Microglia, the brain’s resident macrophages, have sex-dependent developmental trajectories and gene expression patterns. Lesion (1) and transplantation (2) studies show microglia play different roles in male and female brains after HI or stroke. While one study (3) demonstrated that male mice have a higher activated microglia response than female mice over the entire lesioned hemisphere 3 days after HI, no studies have examined this in a brain region-specific manner. Our initial study focuses on the hippocampus, a hypoxia-sensitive brain region critical for learning that may not be protected by therapeutic hypothermia.
METHODOLOGY: In the modified Vannucci model at postnatal day 10 (P10, approximates human term gestation), the right carotid artery is ligated under isoflurane anesthesia. Mice recover on a heating pad for 60-80 minutes with temperatures maintained between 36.0 - 37.9C. Mice then undergo hypoxia at FiO2 8% for 45 minutes on the same heating pads. The procedure for Sham mice is identical except that the carotid artery is isolated but not ligated, and the chamber remains in normoxia (FiO2 21%). HI and Sham littermate mice are returned to the dam in their home cage 10 minutes after hypoxia is complete. At P13, mice are euthanized and lesion size, if present, is measured. Brains are either sectioned fresh for TTC (Figure 1) or fixed in PFA then sectioned for immunohistochemistry (Figure 2).
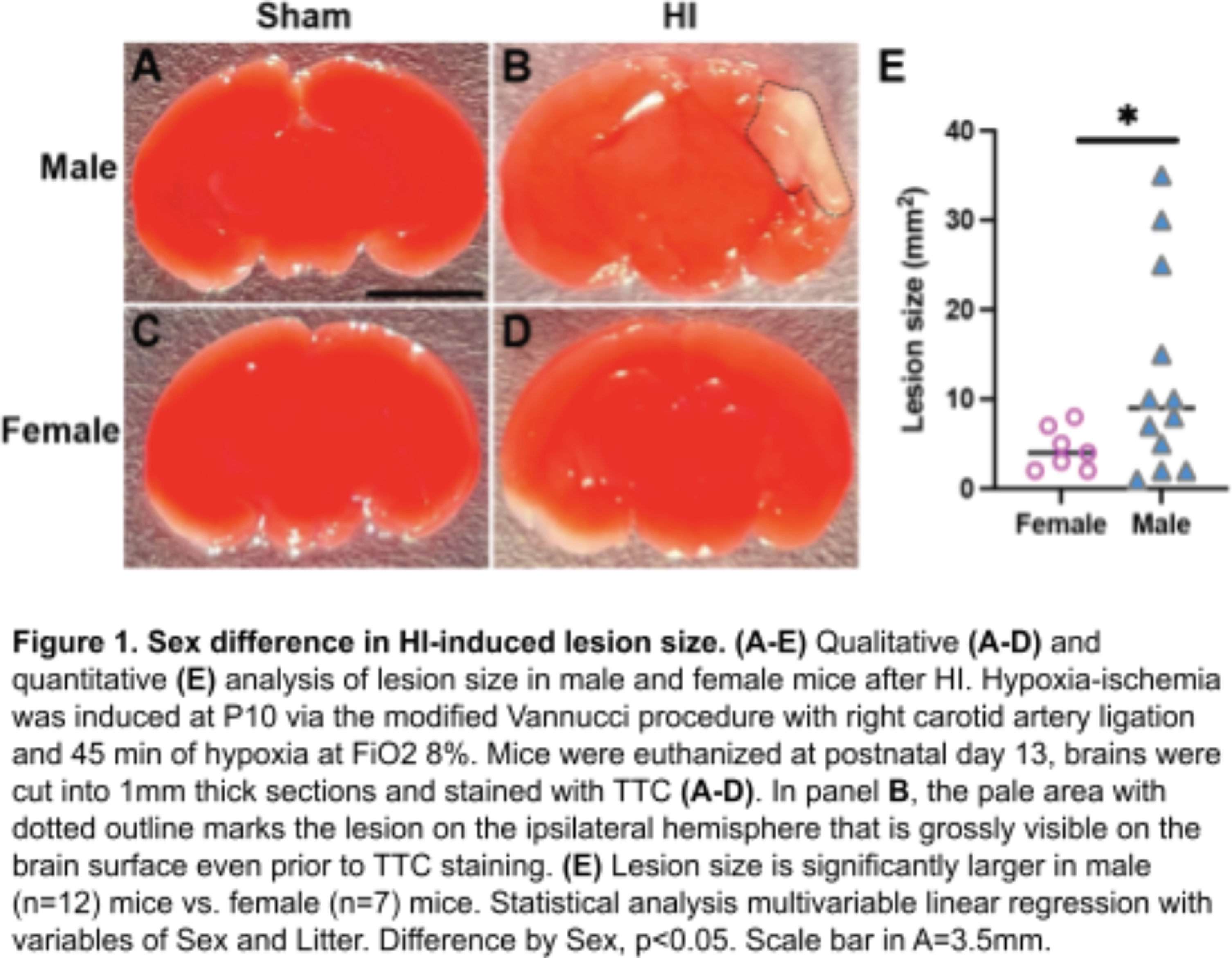
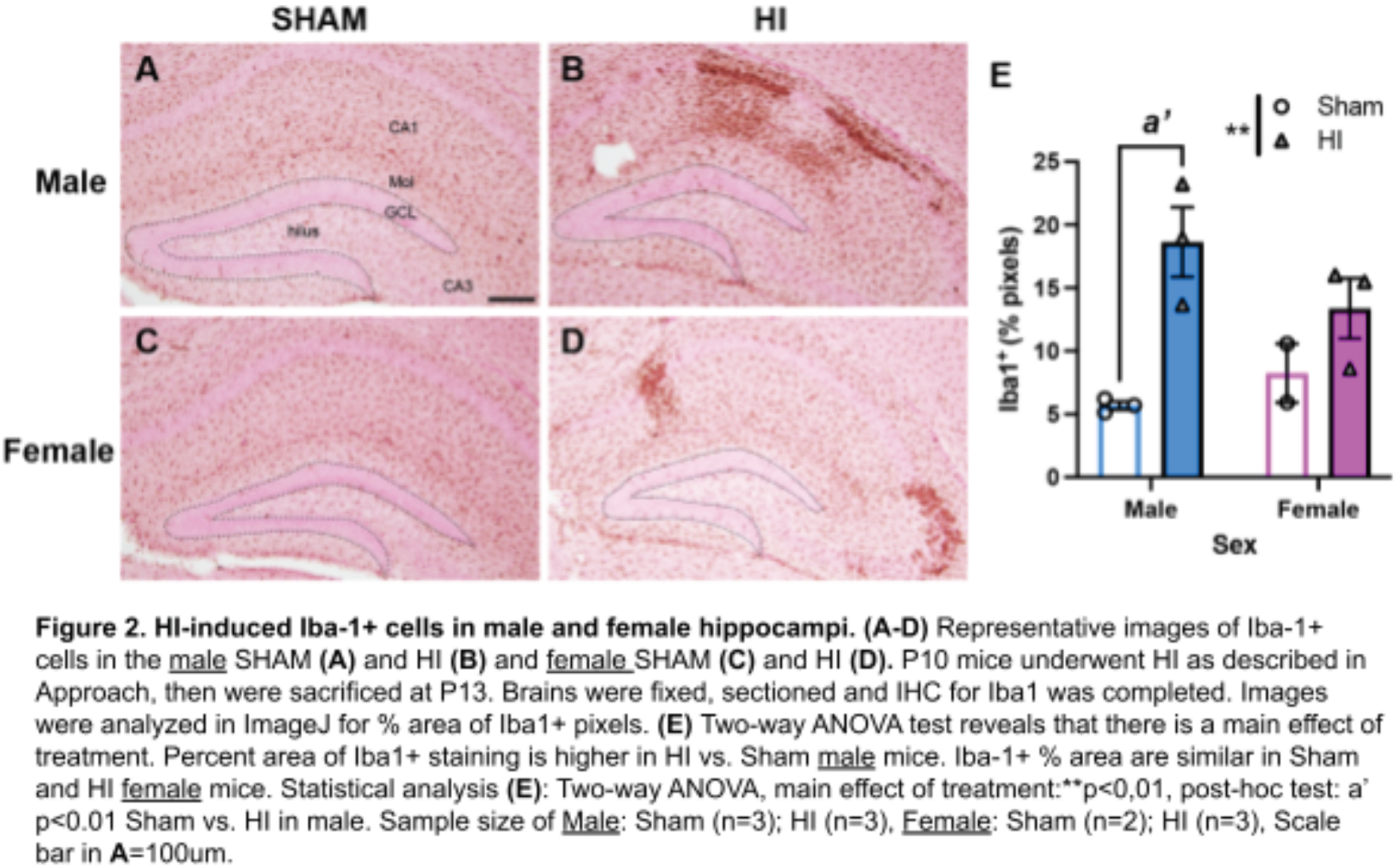
RESULTS: Of 95 mice treated with Hypoxia-Ischemia (HI), 12 (27%) male HI mice and 7 (18%) female HI mice have had a pale, grossly visible lesion on the surface of the brain. Of the mice who did have a grossly visible lesion, the lesion size is ~2 fold-larger in males than females (Figure 1; mean males 12.3mm2, mean females 5.5mm2); this is consistent with the literature. My pilot data show that, in mice that do not have a grossly visible lesion on the surface of the brain, the Iba1+ response (which labels both brain resident microglia and peripheral macrophages) 3 days after HI is larger in the male hippocampus than female hippocampus (Figure 2).
CONCLUSION / IMPACT: Male mice have a larger macrophage response than female mice in the hippocampus 3 days after P10 HI. Next steps include differentiating what part of this response is due to brain resident microglia vs. macrophages infiltrating from the periphery. Understanding the mechanisms underlying these sex differences could reveal new therapeutic targets for sex-based or individualized treatments to improve outcomes for all children with HIE.
Bibliography:
[1] Tsuji, et al. J. Neuroinflammation (2020).
[2] Villa, et al. Cell Rep. (2018).
[3] Mirza, et al. J. Neuroinflammation (2015).
Assessing maturational effects on brain dynamics in preterm neonates using microstate analysis
Hermans Ta, Khazaei Mb, Raeisi Kb, Croce Pb,c, Tamburro Gb,c, Dereymaeker Ad,e, De Vos Ma,d, Zappasodi Fb,c, Comani Sb,c
aDepartment of Electrical Engineering (ESAT), STADIUS Center for Dynamical Systems, Signal Processing and Data Analytics, KU Leuven, Leuven, Belgium
bDepartment of Neuroscience Imaging and Clinical Sciences, G. d’Annunzio University of Chieti–Pescara, Chieti, Italy
cBehavioral Imaging and Neural Dynamics Center, G. d’Annunzio University of Chieti–Pescara, Italy
dDepartment of Development and Regeneration, UZ Leuven, Leuven, Belgium
eNeonatal Intensive Care Unit, UZ Leuven, Leuven, Belgium
BACKGROUND AND PURPOSE: One fundamental objective in preterm neonates admitted to the Neonatal Intensive Care Unit (NICU) is to ensure a normal brain development, because abnormal brain development can cause neurodevelopmental disorders in later life. Multi-channel electroencephalography (EEG) is commonly used to monitor brain development as it captures changes in the neonatal brain function. Common analytical methods for automatic detection of trends in EEG dynamics that can be related to maturation include timeseries analysis, frequency analysis and pairwise connectivity analysis, but their main limitation is that they do not capture global multi-channel brain dynamics. Microstate (MS) analysis is a framework that overcomes this limitation as it characterizes global brain dynamics by exploiting the information in all EEG channels. This is achieved by modeling the EEG as a sequence of transient, non-overlapping patterns of quasi-stable electrical potentials (microstates) [1, 2]. Whereas quite common in adult studies, no microstate studies have investigated the developing preterm brain. Therefore, the goal of this study is to characterize changes in microstate-derived parameters due to maturation in a dataset of preterm neonates with normal neurodevelopmental outcome.
METHODOLOGY: Longitudinal 9-channel EEG recordings (UZ Leuven NICU) of 48 neonates with normal neurodevelopmental outcome were categorized into four age groups based on post menstrual age (PMA): ≤31 w (n=34), 32-33 w (n=36), 34-36 w (n=38), ≥37 w (n=27). An automatic sleep detection algorithm was used to identify quiet (QS) and active (AS) sleep segments. For each recording, a 5-minute artefact-free epoch was selected for QS and AS. Finally, microstates were identified using a modified k-means algorithm.
RESULTS: We identified 4 dominant MS templates in the neonatal EEG (Fig. 1). Whereas the templates for the youngest three age groups were similar, the templates for the fourth age group differed significantly from all other groups (Fig. 2). PMA had a significant effect on mean MS duration and occurrence, which decreased and increased with PMA, respectively (Figs 3 and 4).
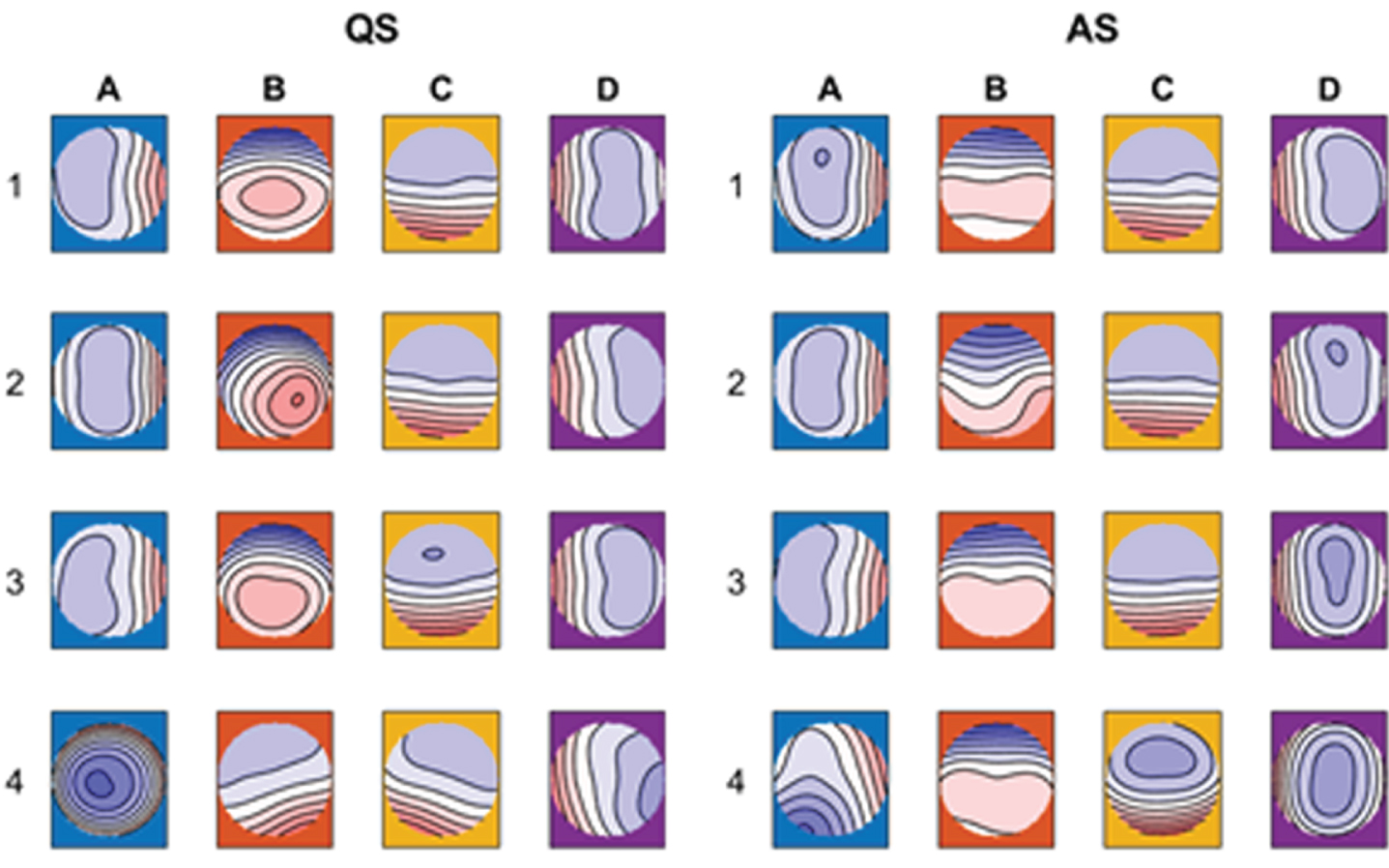
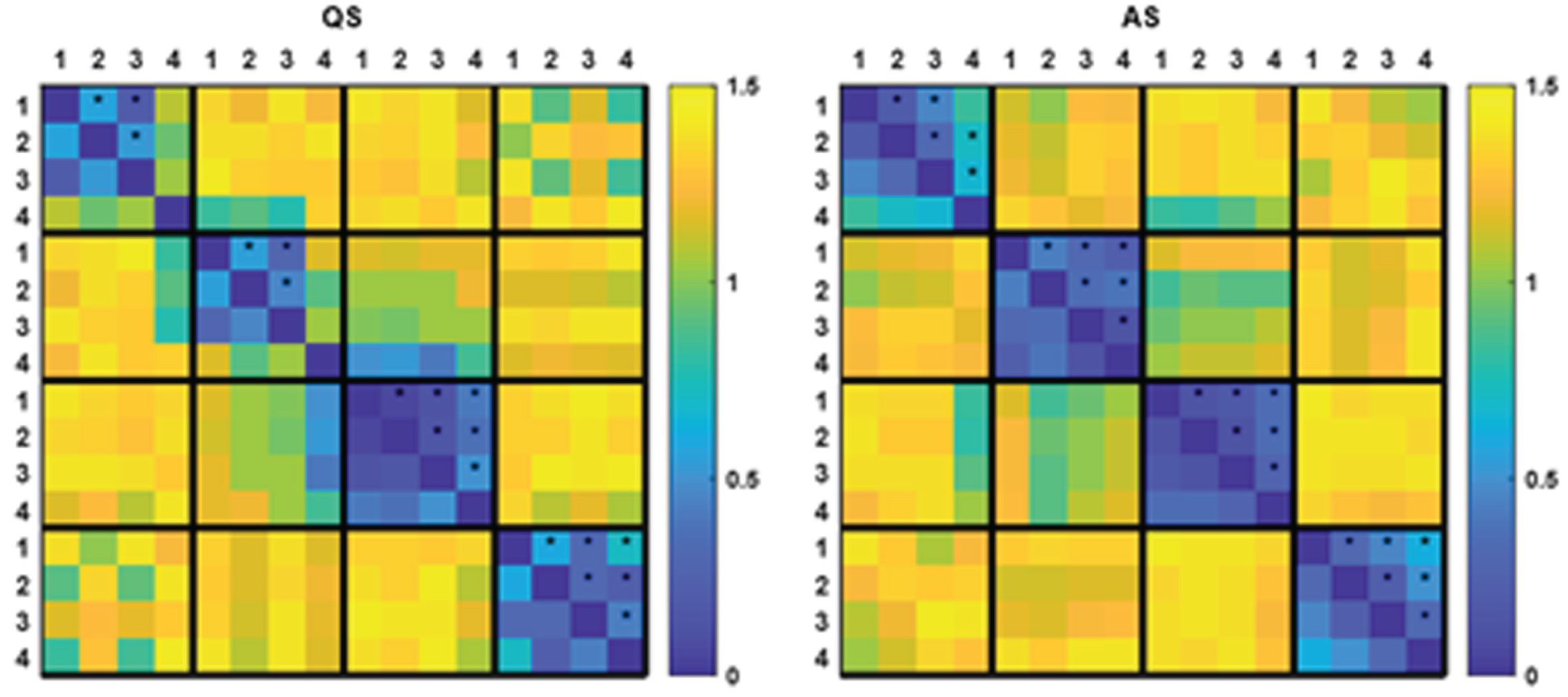
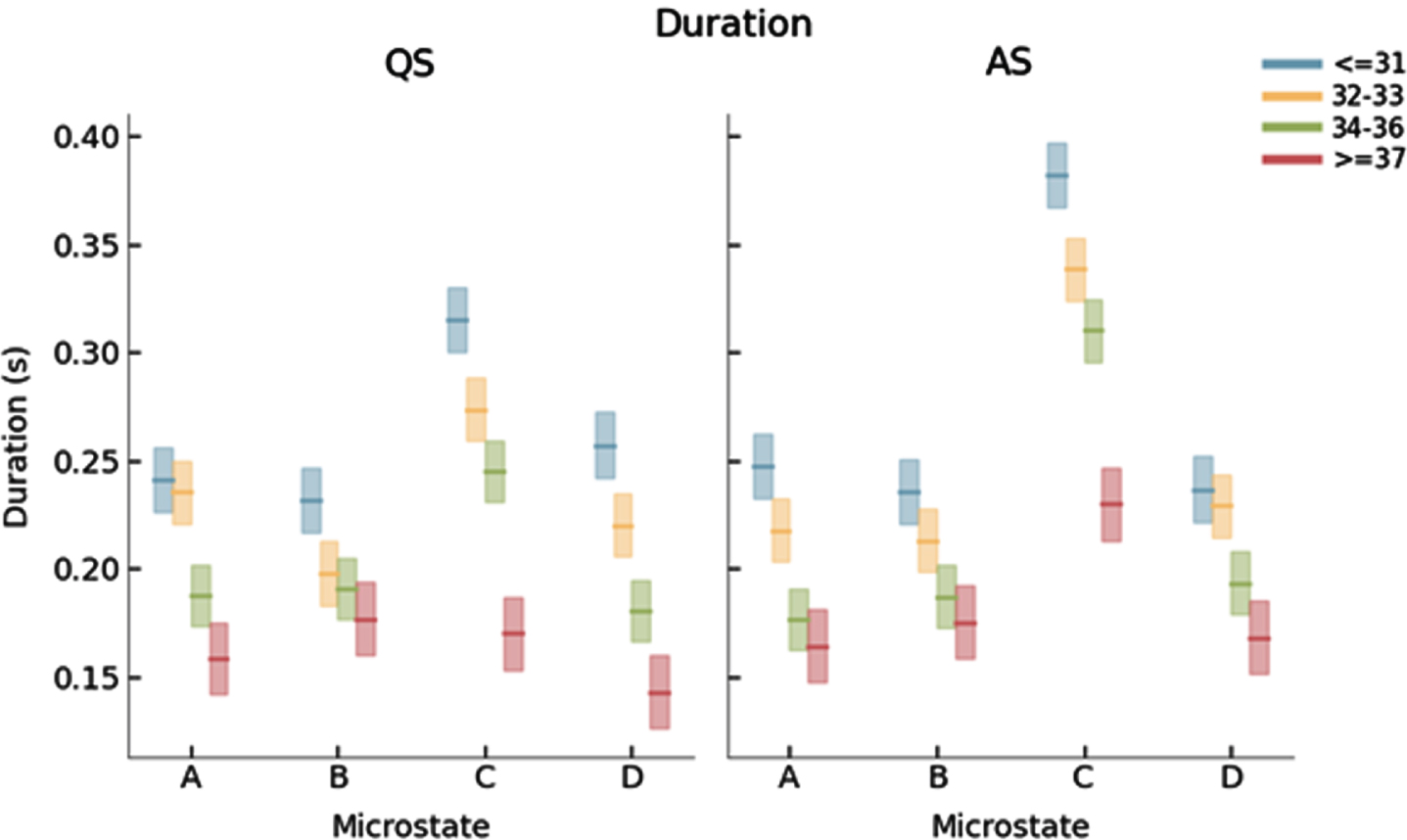
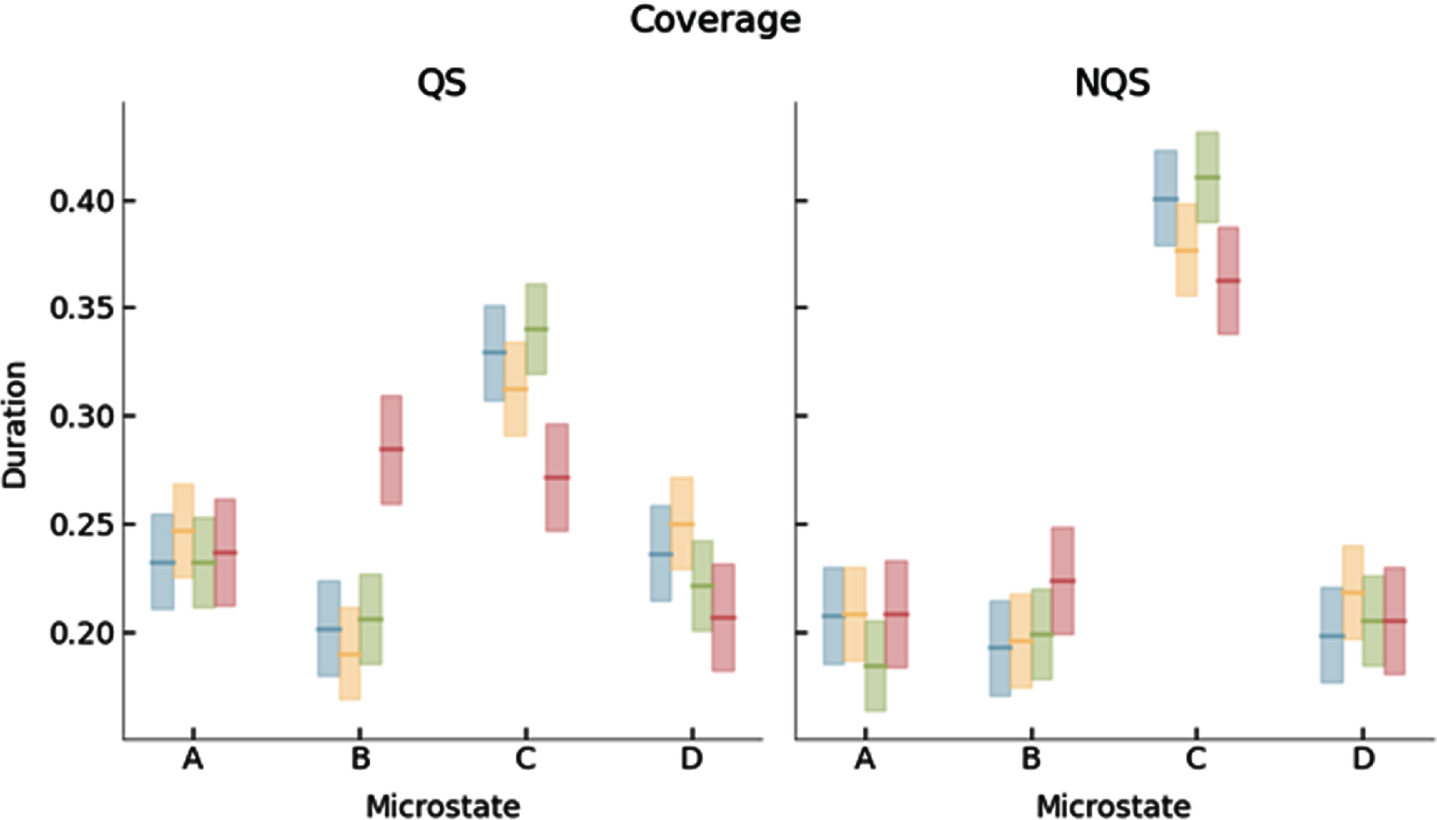
CONCLUSION: In this study, reference results for MS analysis in preterm neonates were presented for different age groups. MS duration and occurrence were found to be correlated with PMA, suggesting that MS analysis effectively captures maturational changes in the neonatal brain and could serve as an additional tool in automated brain maturation monitoring.
Bibliography:
[1] Michel CM, Koenig T (2018) EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage 180:577–593. https://doi.org/10.1016/j.neuroimage.2017.11.062
[2] Khazaei M, Raeisi K, Croce P, et al (2021) Characterization of the Functional Dynamics in the Neonatal Brain during REM and NREM Sleep States by means of Microstate Analysis. Brain Topogr. https://doi.org/10.1007/s10548-021-00861-1
Expanding the spectrum of fetal neuroimaging findings and clinical outcomes in neonates with Congenital Aqueductal Stenosis, a 10 year single tertiary center experience
Khair Aa, Nikam R, Kandula V
aNemours Children’s Health / Thomas Jefferson University, Wilmington, United States
BACKGROUND: Congenital Aqueductal Stenosis (CAS) is a known cause of congenital ventriculomegaly. It affects about 1 in 1000 births and accounts for up to 25 % of cases of congenital hydrocephalus. List of underlying etiologies are not completely known but genetic disorders are thought to represent the majority. Early diagnosis is crucial for confirming the diagnosis, identifying possible associated anomalies, outlining family counselling plan and directing potential intervention strategies. Fetal MR based neuroimaging is a rising diagnostic technique with promising precision ability in diagnosis AS.
METHODOLOGY: We have reviewed infants who were diagnosed with CAD via fetal MRI in our tertiary center over 10 years (2011-2021). Demographic data, postnatal clinical findings, genetic testing results, procedural intervention, and short-term outcomes have also been reviewed.
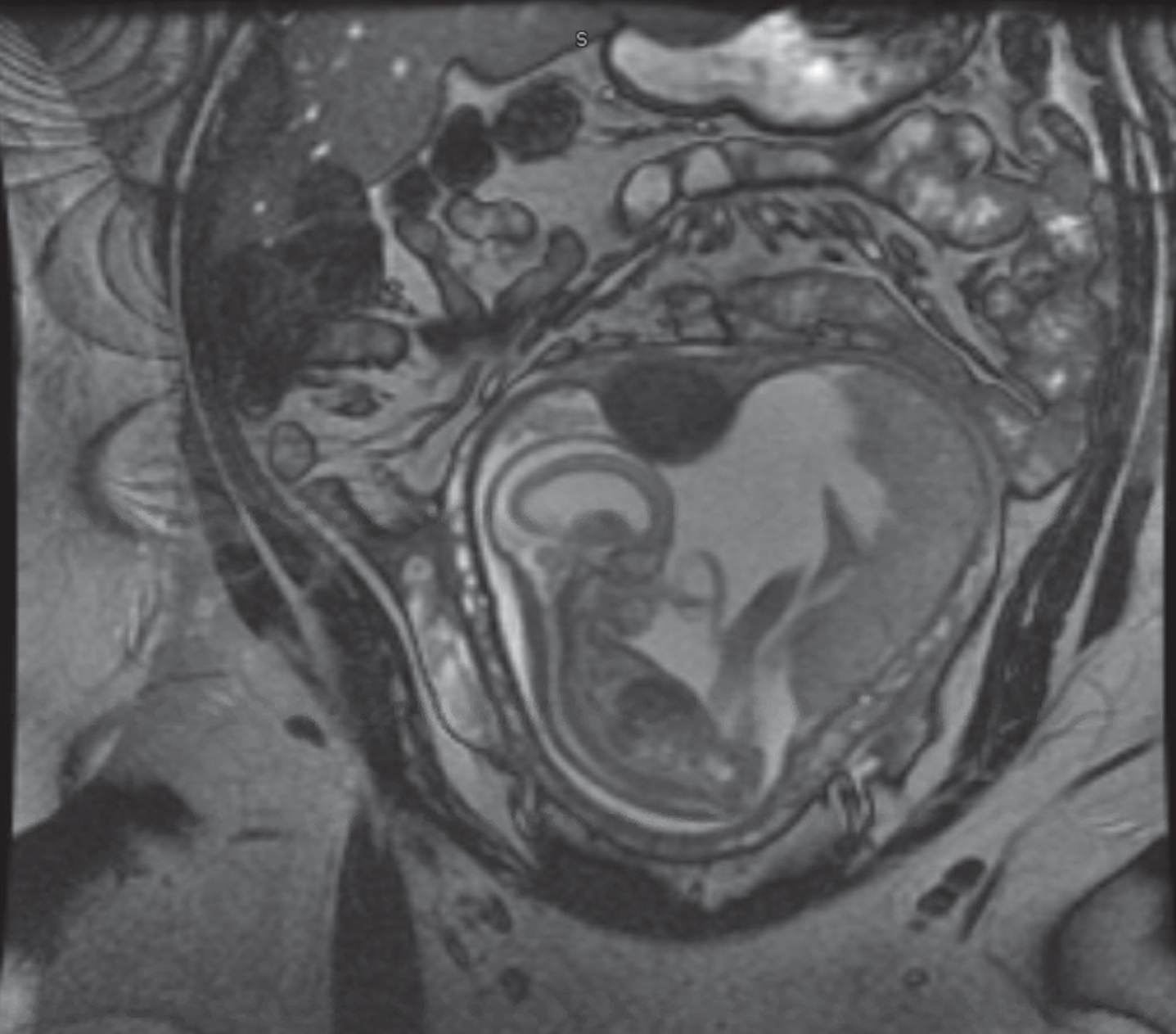
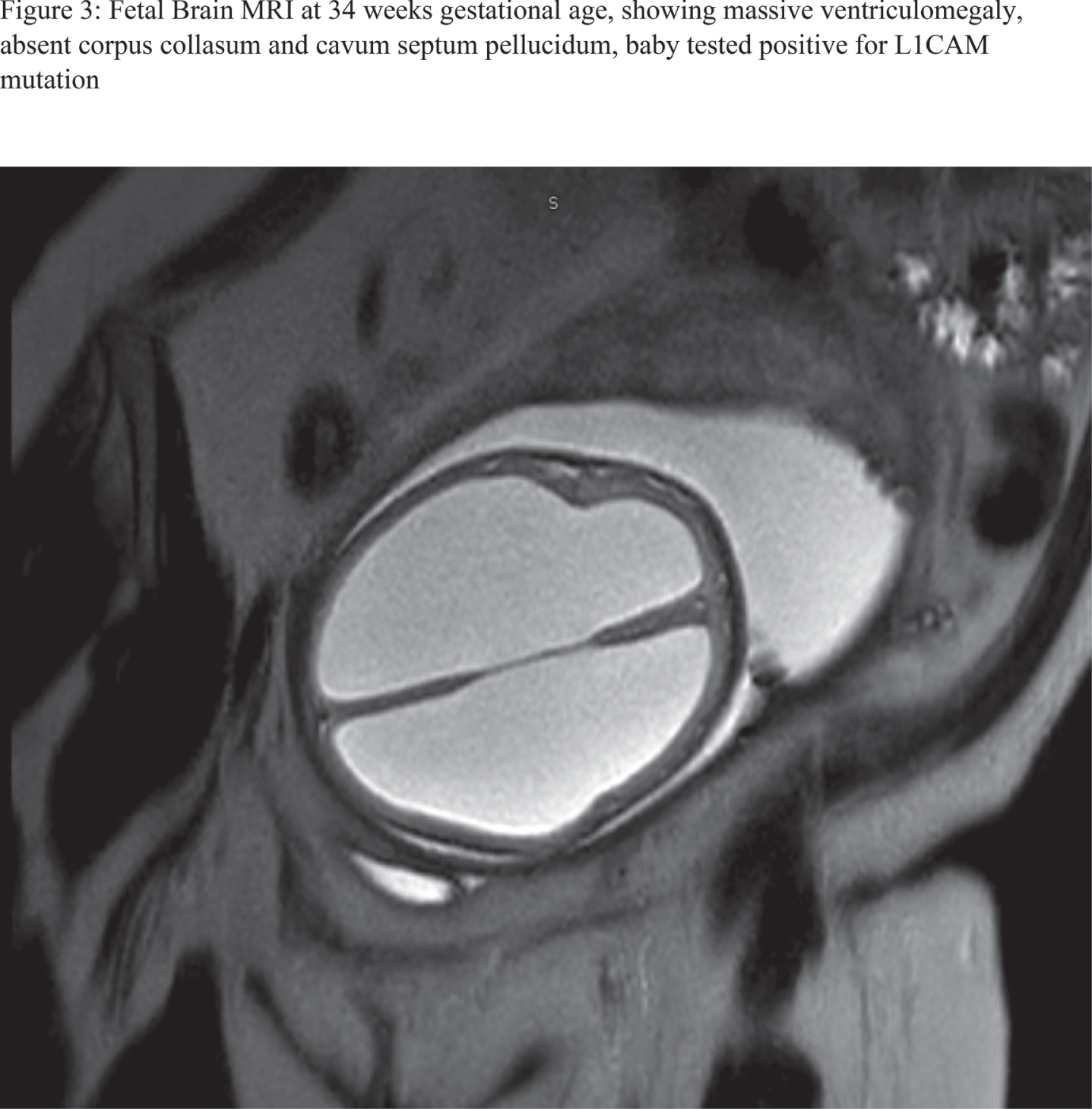
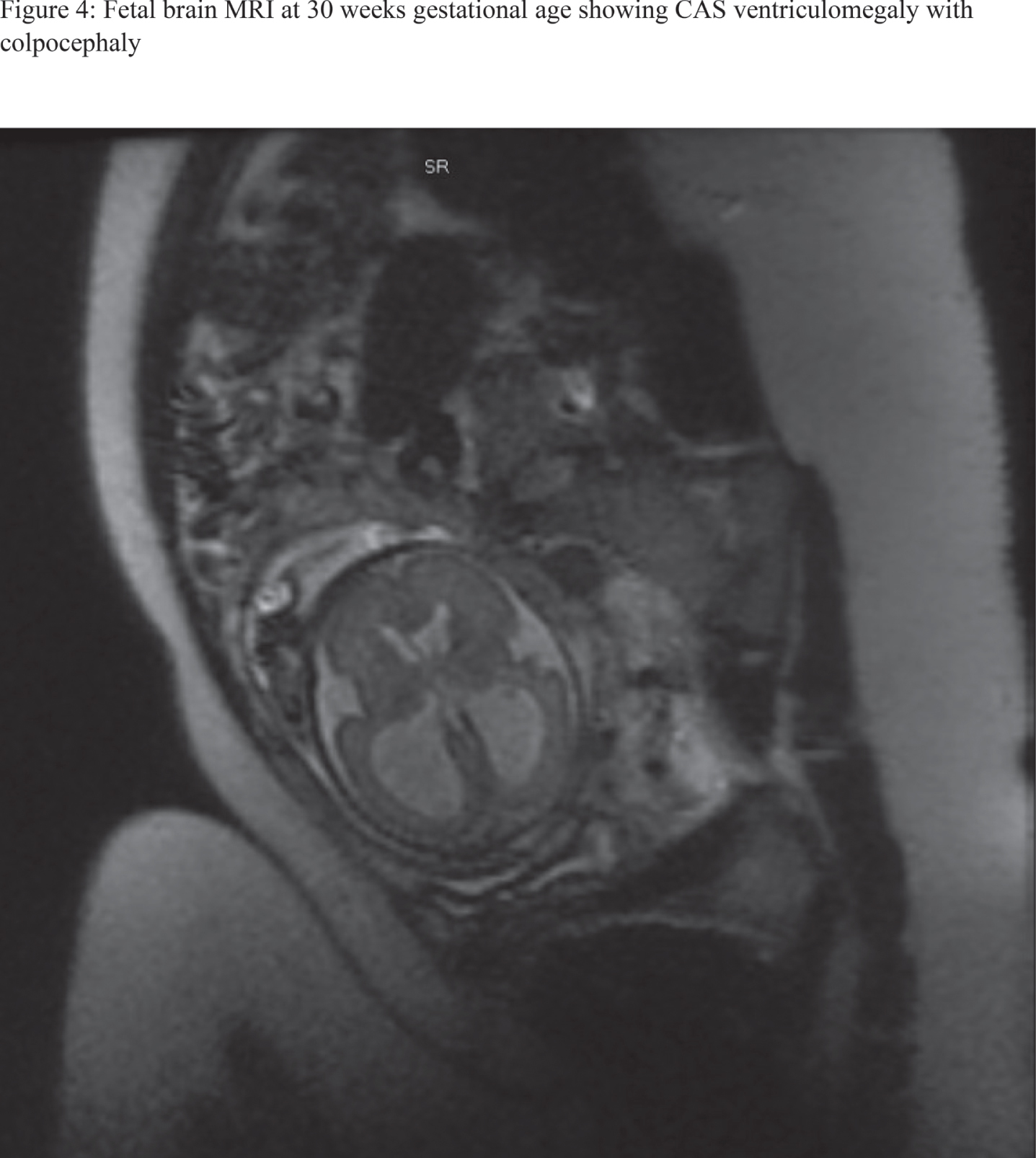
RESULTS: 28 fetal MRI studies with findings consistent with significant CAS were identified. Of them, 18 patients (64%) were ultimately assigned as males, whereas females constituted 10 patients (34%). 21 patients (75%) were diagnosed before the 3rd trimester with mean gestational age of 21.5 +/- 0.87 for 95% confidence interval. 12 patients (42%) had isolated CAS. Severe cortical thinning and hypoplastic corpus collasum were the most frequently associated anomaly as noted in 6 patients (21%), likely to be attributed to severity of ventriculomegaly. Rare and interesting associated anomalies included Dandy walker malformation, middle cerebral artery stroke, grey matter heterotopias, under-developed lower extremities and microcephaly with micrognathia. Out of 17 patients who had postnatal MRI at our center to further characterize their ventriculomegaly, diagnosis of CAS was confirmed in 15 patients (88%). From the latter, 9 patients (60%) underwent neurosurgical ventricular shunting procedures. Figures 1-4 are examples of 4 fetal MRI studies showing congenital CAS and other associated anomalies in those fetus patients.
CONCLUSION: Fetal brain MRI appears to be a useful tool in predicting postnatal clinical and radiological outcome in cohort of patients with CAS as early as before the start of 3rd trimester. In our cohort, Fetal MRI showed high reliability and validity in diagnosing CAS. Prenatally diagnosed CAS should alert clinicians to possibility of other associated anomalies which may have a major impact on family counseling and treatment options.
Figure 1:
27 weeks fetal MRI showing massive ventriculomegaly, with associated cystic dilatation of posterior fossa consistent with Dandy Walker malformation
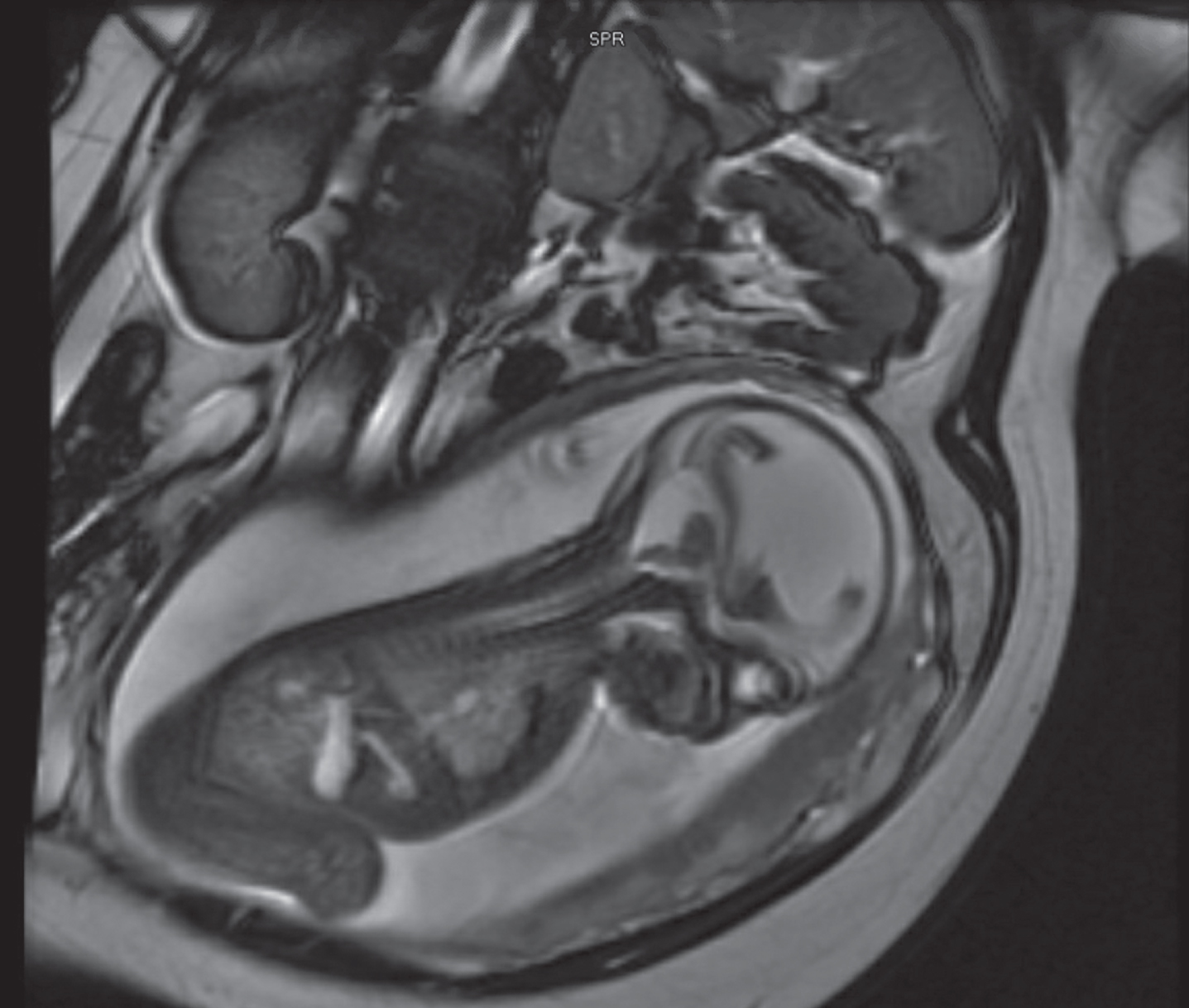
Figure 2:
19 weeks old fetal brain MRI showing CAS associated severe ventriculomegaly and cerebellar atrophy
Preterm birth alters the maturation of the GABAergic system in the human prefrontal cortex
Lacaille Ha, Vacher Ca, Penn Aa
aColumbia University Medical Center, New York, United States
BACKGROUND AND PURPOSE: Alterations in neurotransmitter systems, particularly changes in GABAergic and glutamatergic systems, during frontal lobe development have been hypothesized to play a key role in neurodevelopmental disorders seen in children born very preterm (<32 weeks gestation) and at very low birth weight [1]. Prematurity frequently results in neurodevelopmental disorders such as autism, attention deficit disorders or epilepsy [2, 3] in the absence of radiologically visible lesions, suggesting that long-term physiological alterations might underlie these pathologies. Mid-to-late gestational brain development includes neurogenesis, migration, dendrite arborization and synaptogenesis, which are heavily dependent on GABA and glutamatergic neural systems and are at risk of disruption in the extra-uterine environment after preterm birth [4]. Recent magnetic resonance spectroscopy imaging studies have suggested that GABA concentrations may be lower in preterm than term infants [1, 5, 6], but cannot assess the components of the GABAergic system that may have altered development. Here we studied the molecular development of the GABAergic system specifically in the dorsolateral prefrontal cortex (Brodmann area 10), a region that plays a role in attention and working memory.
MATERIALS OR METHODOLOGY: First, we delineated the molecular development of the GABAergic system in the dorsolateral PFC (Brodmann area 10; BA10), which plays a role in attention and working memory and is altered in preterm infants and cases of psychiatric disorders. The maturation state of the GABAergic system in this region was assessed in human post-mortem brain samples ranging in age from 0 to 8 months obtained from the NIH NeuroBiobank of 17 male and 9 female term infants. Gene expression levels were measured for 47 genes related to GABAergic development and this gene expression data was used to calculate a maturation index. To evaluate the impact of premature birth on the GABAergic system development, samples from one-month-old term (n=9 male, 4 female) and one-month corrected-age (n=8 male, 6 female) very preterm infants, were compared using the same gene list and methodology.
RESULTS: In term infants, the maturation index correlated with the age of the donor and was more dynamic in male than in female infants, suggesting a link to the increased male infant susceptibility to impaired neurodevelopment. When comparing term and preterm infants, the maturation index for the GABAergic system was significantly lower (–50% p<0.05) in male preterm infants, with major alterations in genes linked to GABAergic function in astrocytes.
Conclusion/Impact: Taken together, this study suggests that the development of GABAergic system is more dynamic in male infants, potentially contributing to their increased susceptibility to perinatal neurological insults. We have identified a potential key mechanism in the alteration of astrocytic GABAergic development that we are now investigating.
Early parenteral nutrients support cerebellar metabolic maturation using 1H-MRS in preterm newborns
Lundberg Ra, Basu Sa, Andescavage Na, Pradhan Sa, Ottolini K, Kapse Ka, Murnick Ja, Limperopoulos Ca
aChildren’s National Medical Center, Washington, United States
BACKGROUND/PURPOSE: Nutrition in premature infants influences brain development and neurodevelopmental outcomes. Early initiation of total parenteral nutrition (TPN) improves early weight gain and decreases length of stay in the hospital [1]. Further investigation is needed for optimization of TPN for brain development and growth [2]. Magnetic proton spectroscopy (1H-MRS) allows for non-invasive in-vivo assessment of the metabolic milieu in the developing brain [3]. Our objective was to examine the association between early TPN nutrient intake and brain biochemistry in preterm infant at term equivalent age (TEA). We hypothesized that early TPN nutrient intake in the first 2 weeks of life would positively correlate with neuro-metabolite concentrations measured at TEA MR.
METHOD: Premature infants (<35 wga) admitted to our quaternary level NICU between 2016 and 2021 were enrolled in a prospective cohort study and underwent term equivalent age MRI. Exclusion criteria included Grade 2 or higher intraventricular hemorrhage (IVH), known congenital anomalies and genetic syndromes. Point Resolved Spectroscopy 1H-MRS was acquired at echo time = 68ms, relaxation time = 2000ms on a 3 Tesla GE MRI and analyzed by LCModel for quantitative measurements of neuro-metabolites. We report an interim analysis of data from 28 enrolled preterm infants investigating for relationship (Pearson correlation) of the 2-week consolidated TPN nutrient intake (in mg/kg/d for dextrose, lipid and protein) with TEA MRI cerebellar metabolite concentrations. Bivariate significant associations were adjusted for plausible co-variates using multivariate linear regression models.
RESULTS: 28 preterm newborns born at a mean GA of 27.9 weeks underwent term 1H-MRS. Baseline characteristics, mean cerebellar metabolite concentrations and first 2-week TPN nutrient intake are reported in Table 1. On Pearson correlation, 2-week TPN Dextrose mg/kg/d intake positively correlated with TEA cerebellar NAA/Cr [rho=0.56, p value=0.004], Glx/Cr [rho =0.45, p=0.034] and trended with Cho/Cr [rho=0.37, p=0.07] (Table 2). Adjusted for GA at birth, PMA at term MRI and sex, the TPN Dextrose/kg/d remained the strongest predictor of TEA cerebellar metabolites [NAA (β=0.034, p=0.002), Glx/Cr (β=0.1, p=0.035) and Cho/Cr (β=0.03, p=0.031)].
Table 1:
Baseline characteristics of cohort
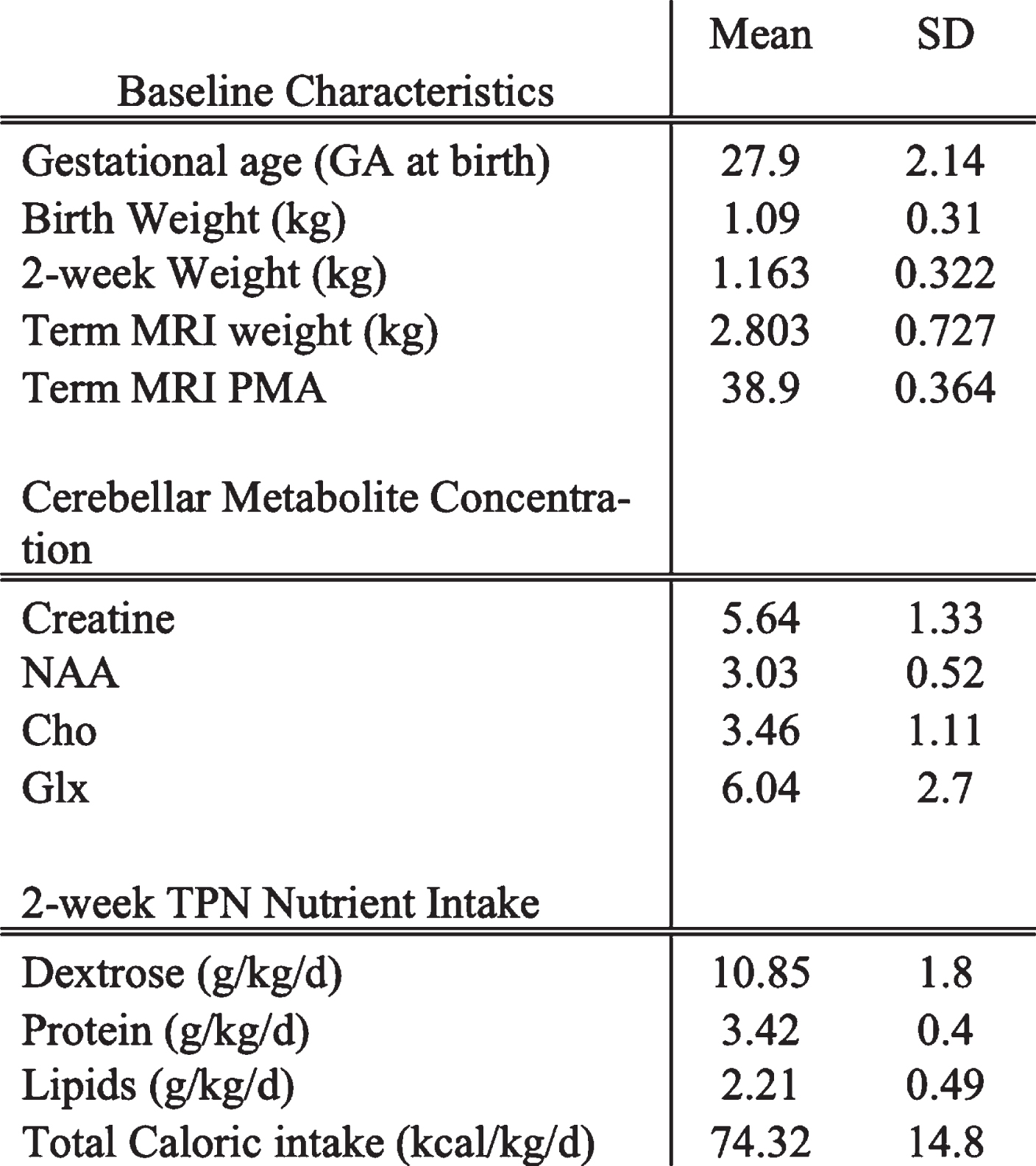 |
Table 2:
Summary of TPN nutrient intake and MRS outcomes
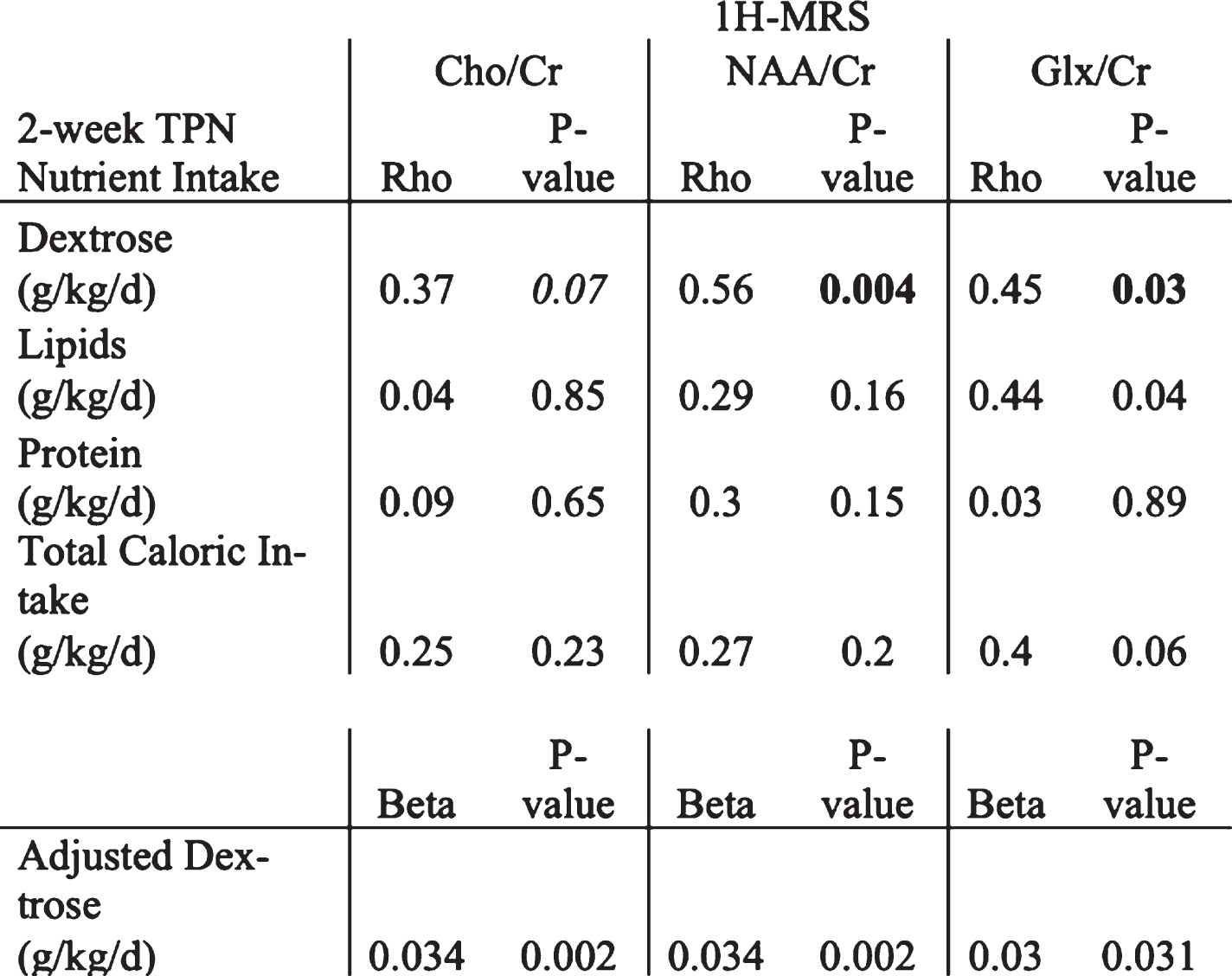 |
Figure 1.
Representative 1-H-MRS PRESS spectra (echo time = 68 ms). Footnote: The spectra were acquired on a 3T scanner with TE=68 ms, TR=2000ms, NSA = 256.
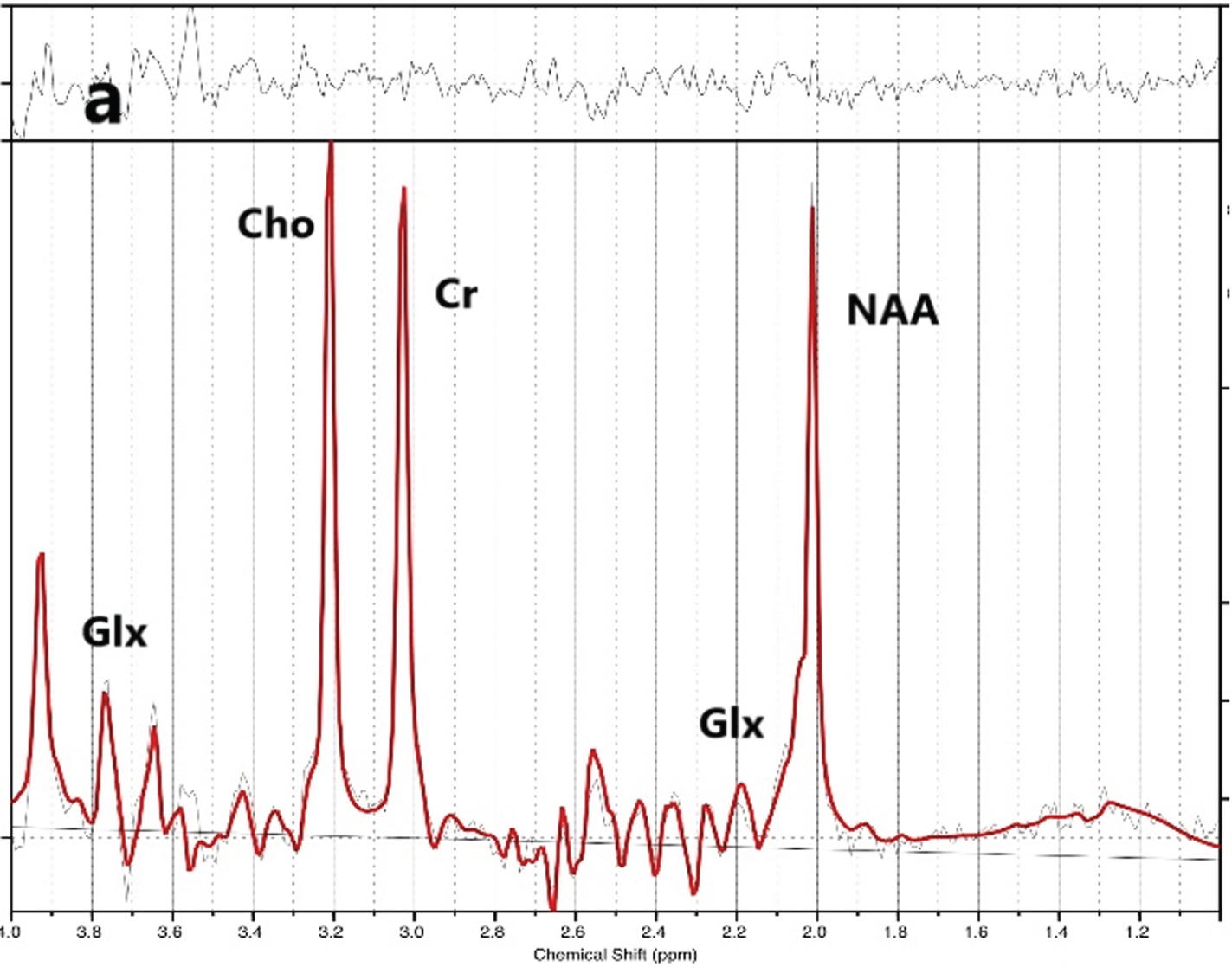
Figure 2.
Cerebellar 1-H-MRS voxel placement.
CONCLUSION: Early TPN nutrient intake, particularly dextrose is associated with higher cerebellar neuro-metabolite concentrations at TEA age. Whether the relationship between nutritional intake and metabolic development of the cerebellum influences neurodevelopmental outcomes will be the focus of our subsequent investigation.
Bibliography:
[1] Hay WW. Aggressive nutrition of the preterm infant. Curr Pediatr Rep. 2013;1(4):229-239.
[2] Stephens BE, Walden RV, Gargus RA, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123(5):1337-1343.
[3] Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Hüppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1 H magnetic resonance spectroscopy: Brain Composition in Early Human Development. Magn Reson Med. 2002;48(6):949-958.
Beneficial effects of Kangaroo care on preterm infants in growth and psychomotor outcomes in a low-income country
Mbayabo Gloire Ga, Mubungu Gb, Bambi Ja, Mavinga La, Lukusa Pb, Tady Ba, Biselele Ta
aNeonatology Service, Department of Pediatrics, University Hospitals of Kinshasa, Kinshasa, Congo (the Democratic Republic of the)
bCenter of Human Genetic, University of Kinshasa, Kinshasa, Congo (the Democratic Republic of the)
INTRODUCTION: Prematurity remains a real public health concern in the world and in Africa, especially in the Democratic Republic of Congo (DRC) due to its high morbidity and mortality rate. Growth and psychomotor outcomes of preterm infant remain worrying. Although several researches have shown the beneficial effects of Kangaroo care (KC), few data exist in low-income countries such as the DRC on short-term effects of KC in growth and psychomotor outcomes on premature infants.
OBJECTIVES: To assess the beneficial effects of KC on growth and psychomotor outcomes in preterm infants in a low-income country.
METHODOLOGY: Sixty-five preterm infants who were born in three public hospitals in Kinshasa were included in this study. We included those preterm infants weighted below 2000g, who had ability to have oral alimentation and have cardiovascular stability. We excluded those with congenital abnormalities that may influence the neurodevelopmental outcomes and the growth. Neurodevelopmental outcomes were assessed by the Bayley scale II. Thirty-one preterm infants underwent KC and thirty-four the traditional method (incubators). KC was performed in two of the three public hospitals and traditional care was done in one hospital. The age and weight of the infant, the education and socioeconomic status of the family were matched into the two groups.
RESULTS: Among sixty-five preterm infants included in this study, 31 underwent KC and 34 traditional method. The growth and psychomotor outcomes were better in the KC group than in the traditional method group, especially in mental development index (MDI: 91.6±12.1 vs 81.2±12.6, p 0.001). Birth weight was found as a determinant of the growth. Sex, birth age, APGAR at 5th minute, socioeconomic status and nutritional status were found as determinants of a good psychomotor outcome.
CONCLUSION: In our setting KC offers more beneficial effects to the preterm infant according to growth and psychomotor outcomes than the traditional method.
KEYWORDS: Preterm infant, growth, psychomotor outcome, Kinshasa
Bibliography:
[1] Organisation mondiale de la Santé. Naissances prématurés, Aide-mémoire N°363, Novembre 2016
[2] Lawn JE, Mwansa-Kambafwile J, Horta BL, Barros FC, Cousens S. ‘Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol 2010;39 (Suppl 1):1144-54
[3] Mattia F.R., De Regnier R.A. Chronic physiologic instability is associated with neurodevelopmental morbidity at one and two years in extremely premature infants. Pediatrics, 1998. 102: 35
[4] Feldman R, Eidelman Al. Skin-to-skin contact (Kangaroo care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol 2003; 45(4):274-81
[5] Ruth Feldman R, Arthur I. Eidelman. Comparison of Skin-to-Skin (Kangaroo) and traditional care: Parenting outcomes and preterm infant development. Pediatrics 2002; 110,16.
Insulin-like-growth factor 1 stimulated release of extracellular vesicles from the choroid plexus – novel mode of blood-brain-barrier signalling
Ortenlöf Na, Vallius Sa, Pankratova Sb, Gram Ma, Ley Da
aPediatrics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
bDepartment of Veterinary and Animal Sciences and Department of Neuroscience, University of Copenhagen, Denmark
BACKGROUND AND PURPOSE: It is well accepted that insulin-like growth factor 1 (IGF-1) plays a crucial role in brain development. However, previous studies have inferred that the transfer of systemically administered IGF-1 over the blood-brain-barrier is restricted, which would present a limitation for beneficial treatment effects within the brain (1). Extracellular vesicles (EVs) are small, cell-derived phospholipid membrane enclosed vesicles that constitute important cell-to-cell messengers, thereby regulating diverse cellular functions of recipient cells (2). EVs released from the choroid plexus (CP) to intraventricular cerebrospinal fluid (CSF) have been shown to cross the ependyme and exert a regulatory function in periventricular brain tissue (3). We hypothesized that exposure of the CP to blood-born IGF-1 induces release of EVs into intraventricular CSF destined to affect the surrounding brain parenchyma. We evaluated this in a primary culture of CP epithelial (CPE) cells using a Transwell in vitro model.
METHODOLOGY: CPE cells were collected from P3-P9 mice pups and plated on Transwell membranes. Cells were stimulated with 40 ng/ml IGF-1 at the basal (blood) side for 24 hours, and subsequently the apical supernatant was collected, and cells were fixed. EVs were prepared from cell culture supernatant. The size and quantity of EVs were measured with nanoparticle tracking analysis. EV morphology and protein immunolabeling were evaluated with transmission electron microscopy (TEM). CP protein markers were analyzed using immunocytochemistry.
RESULTS: Functional characteristics of CPE cells were ascertained by positive staining for transthyretin (TTR) and zonula occludens-1 (ZO-1) (Fig.1a, b, TTR green and ZO-1 red), absent staining for several fibroblast markers, and an increasing transepithelial electric resistance during culture. The CPE cells stained positively for IGF-1, the IGF-1 receptor and the exosomal marker CD63, (Fig.1c,d, IGF-1 magenta, IGF-1 receptor magenta and CD63 red). TEM of purified EVs from apical supernatant displayed typical exosome and microvesicle morphology, positive immunolabeling for IGF-1 and the exosomal marker Flotilin-2 (Fig.1e,f). Exposure of CPE cells to IGF-1 caused an increased release of EVs into the apical supernatant (p = 0.004) (Fig.1g,h).
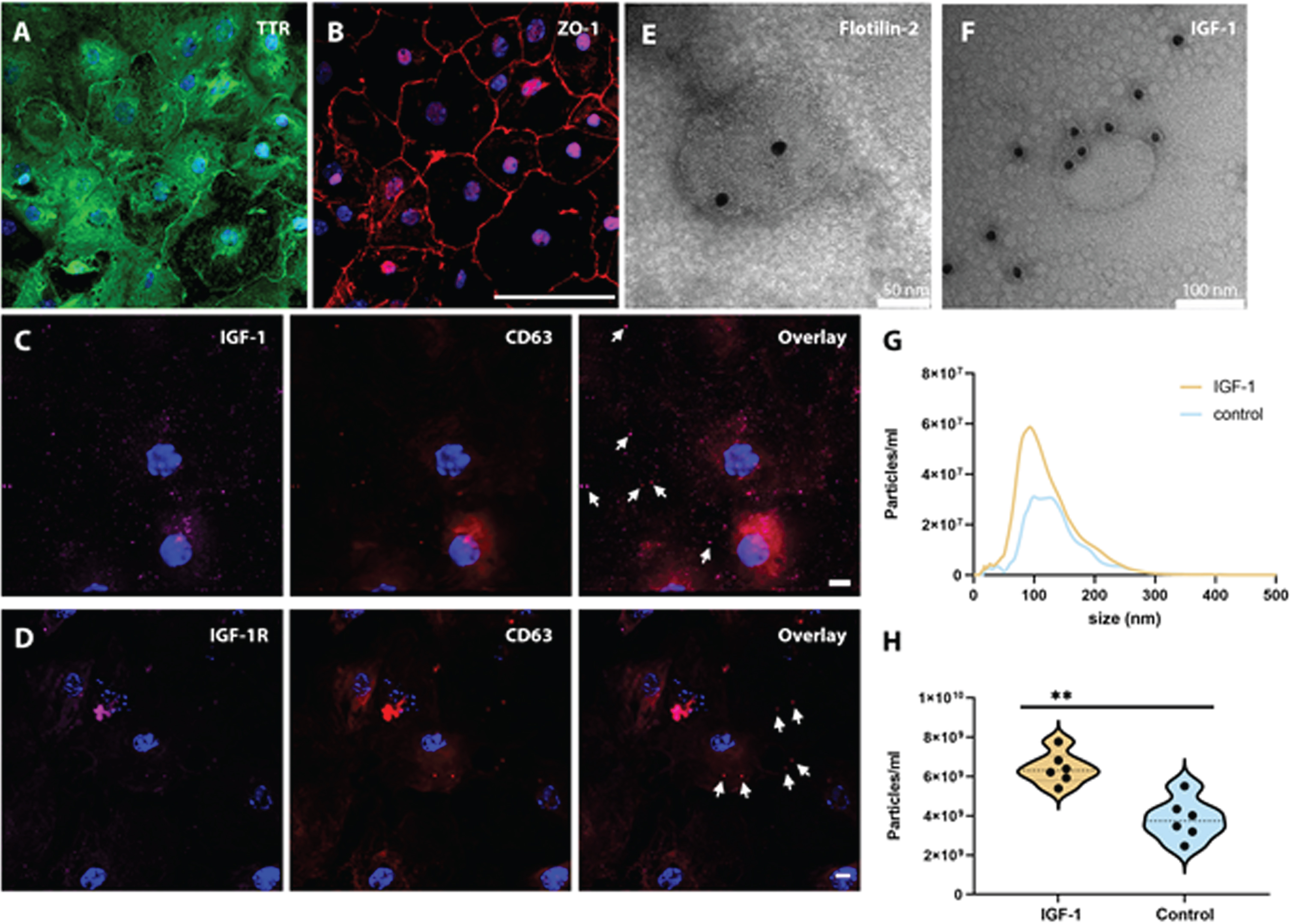
CONCLUSION/IMPACT: Simulated blood-borne exposure of IGF-1 stimulates CP epithelial cells to secrete IGF-1 positive EVs in a Transwell in vitro system. This postulated transport of IGF-1 from the CPE to the CSF via EVs could provide a mechanism for IGF-1 to penetrate the brain ependyma and affect parenchymal cells. We will further expand this study to investigate the miRNA secretome of CP derived EVs upon IGF-1 stimulation, both in vitro and in vivo.
Bibliography:
[1] Pulford, B. E. & Ishii, D. N. (2001).
[2] Kalra, H., et al (2016).
[3] Balusu, S., et al (2016).
Ethanol exposure in a rat model of preterm neonates leads to dampened hypoxic ventilatory drive
Rickman Na, Mayer Ca, Vatolin Sa, Tokat C, MacFarlane Pa, Bearer Ca
aDepartment of Pediatrics, Case Western Reserve University School of Medicine, Cleveland, United States
BACKGROUND AND PURPOSE: Healthy development of the central nervous system (CNS) that controls respiration is crucial for the transition from a fetus to a neonate, especially for preterm neonates.1 Fetal alcohol spectrum disorder (FASD) has been linked to numerous poor outcomes of the CNS, such as microcephaly, severe learning disorders and sudden infant death syndrome (SIDS).2 SIDS is thought to be caused by abnormal respiratory control.3 In this study, we investigated whether ethanol exposure disrupts various aspects of respiratory neural control. Specifically, using a rodent model of preterm neonates, we hypothesized that ethanol exposure would alter the hypoxic and/or the hypercapnic drive in 5-day-old (P5) rats, compared to saline treated animals.
METHODOLOGY: P5 Sprague-Dawley rat pups were treated with 0.8 or 4.4 mg/g of ethanol delivered via IP injection, a validated model of FASD4,5,6. Pups’ hypoxic and hypercapnic ventilatory responses were measured using plethysmography. Individual pups were allowed to acclimatize in the plethysmography chamber for 1 h, while normoxic air flowed through the chamber. A baseline minute ventilation was recorded at 1 h, pups were then subjected to hypoxia (10% O2) for 5 min; their minute ventilation at each minute mark was recorded. Following 5 min of hypoxia, pups were allowed to recover in normoxia for 5 min, and were then subjected to 5 min of hypercapnia (5% CO2); their minute ventilation was recorded at each minute.
RESULTS: We found that ethanol exposure significantly dampened the initial (1-2 min) phase of the hypoxic ventilatory response in females, but not in males (Figures 1, 2). The late (5 min) phase of the hypoxic ventilatory response was not affected by ethanol in either sex. Exposure had no effect on the hypercapnic response in males, but dampened the late phase (5th minute) of the hypercapnic response in females.
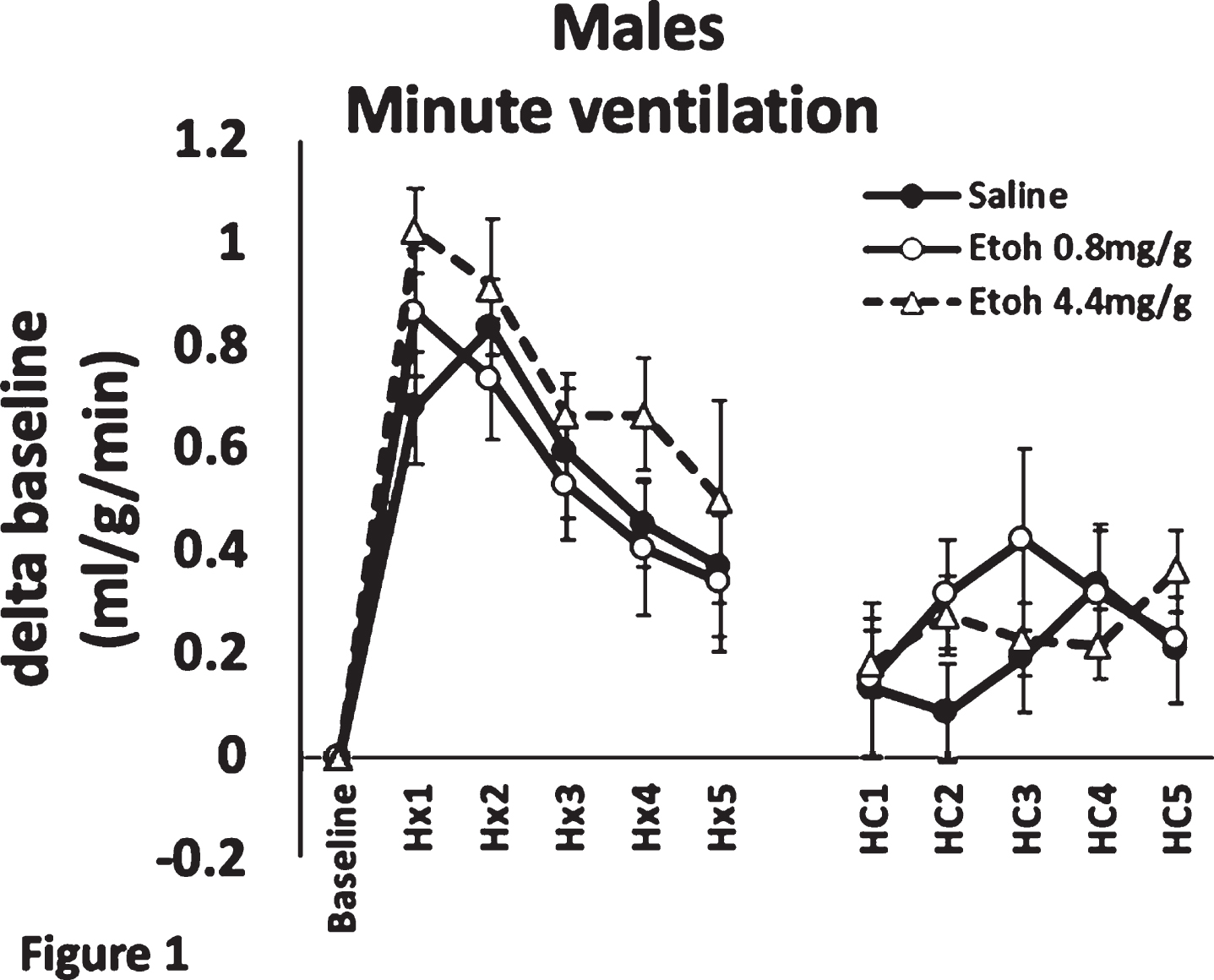
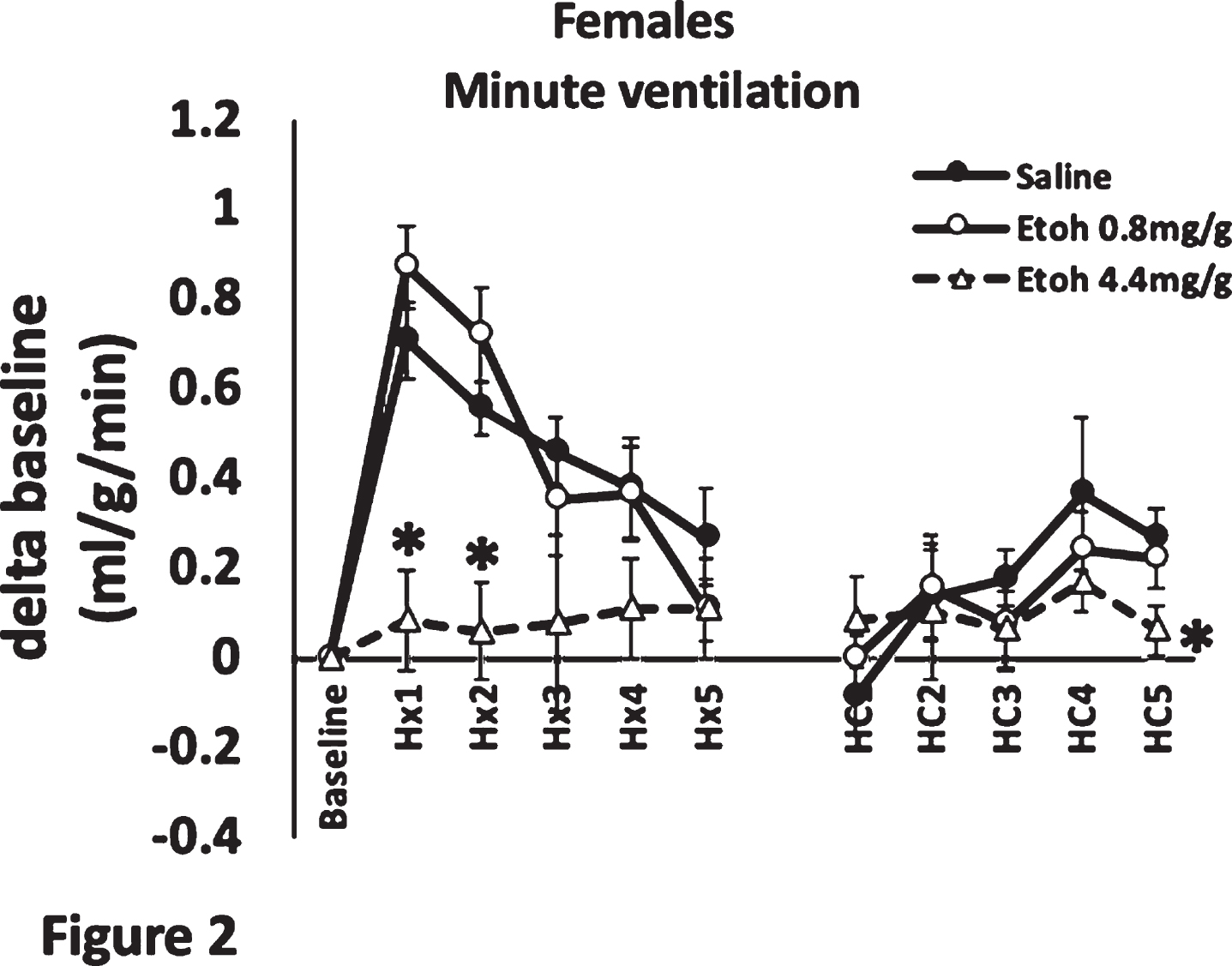
CONCLUSION/IMPACT: The effect on the early phase of the hypoxic response could imply that the carotid body chemoreceptors are affected by ethanol exposure1, whereas the attenuated hypercapnic response suggests a central mechanism of respiratory control dysfunction. The affected respiratory drive may imply that ethanol exposure may lead to SIDS. Future studies will attempt to elucidate the mechanism by which ethanol causes underdevelopment of respiratory control neurons in female pups.
Development of sleep-state-dependent functional connectivity in preterm infants
Shiraki Aa, Kidokoro Ha, Watanabe Hb, Taga Gb, Narita Ha, Mitsumatsu Ta, Kumai Sa, Suzui Ra, Sawamura Fa, Kawaguchi Ma, Suzuki Ta, Yamamoto Ha, Nakata Ta, Sato Yc, Hayakawa Mc, Natsume Ja,d
aDepartment of Pediatrics, Nagoya University Graduate School of Medicine, Nagoya, Japan
bGraduate School of Education, The University of Tokyo, Tokyo, Japan
cDivision of Neonatology, Center for Maternal-Neonatal Care, Nagoya University Hospital, Nagoya, Japan
dDepartment of Developmental Disability Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan
BACKGROUND: It is a pressing challenge to understand whether functional connectivity networks (FCN) changes depending on sleep states and how FCN develops in preterm infants. The aim of this study is to elucidate developmental changes of sleep-state-dependent FCN in preterm infants using simultaneous EEG and functional NIRS recordings.
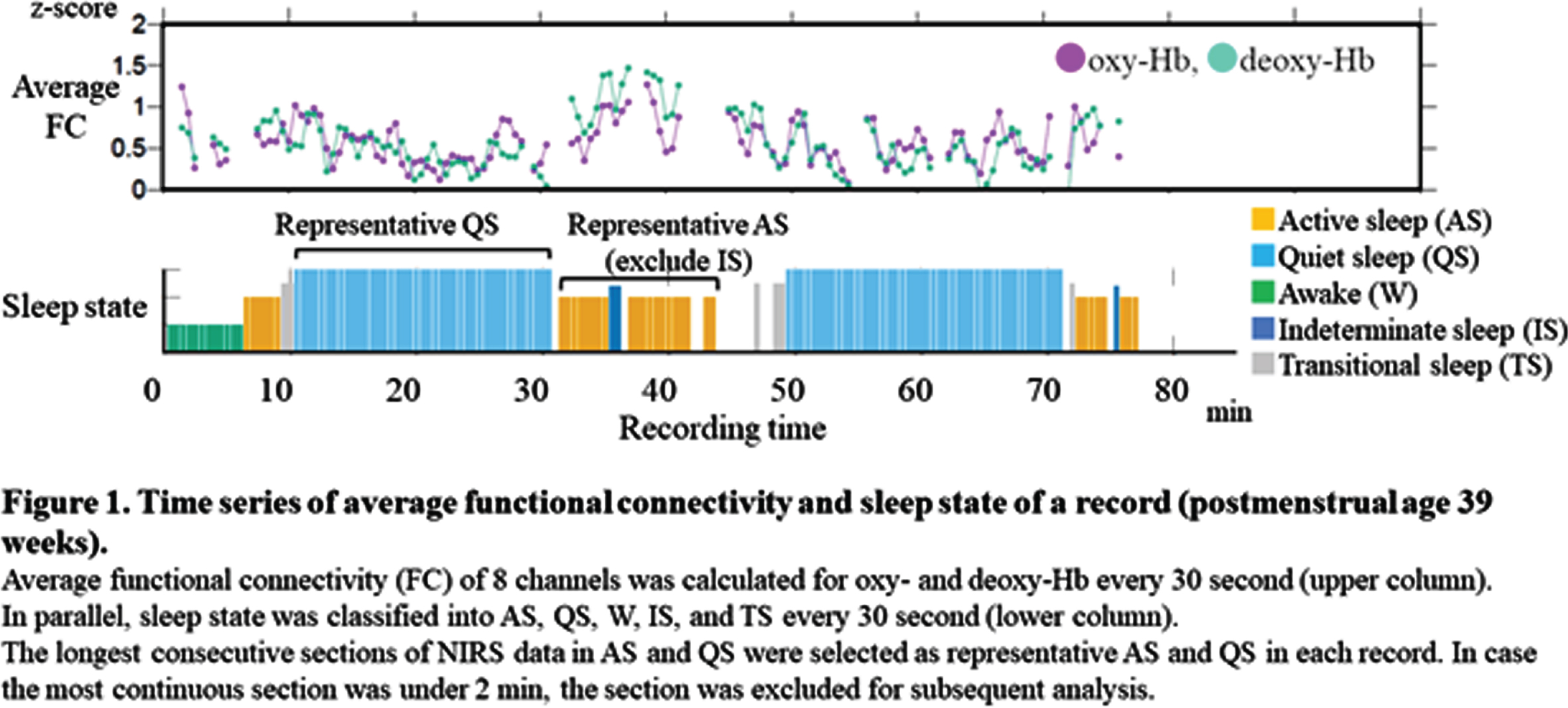
METHODOLOGY: We recruited preterm infants (gestational age of 34 weeks or younger) who were born at Nagoya University Hospital between April 2020 and August 2021. All EEGs were recorded polygraphically for at least 30 to 40 min containing active sleep (AS) and quiet sleep (QS). At least 8 electrodes were placed at Fp1, Fp2, C3, C4, O1, O2, T3 and T4. An NIRS instrument with 8 channels was used to detect relative concentration changes in oxy- and deoxy-Hb. A pair of two channels were placed over the frontal, temporal and occipital regions to sandwich AFz, POz, C5, and C6, respectively. AS and QS were scored every 30 s based on EEG, electrooculography, respiration pattern and behaviors (Figure 1). As parameters of FCN, we used functional connectivity (FC) and phase synchronization index (PSI) of oxy- and deoxy-Hb in low frequency (<0.1Hz) fluctuations. Average FC and PSI of each pair of channels were calculated every 30 s with 1 min duration. The longest consecutive sections of NIRS data in AS and QS were selected for subsequent analysis in FC and PSI. In group level analysis, we averaged FC of oxy-Hb of representative AS and QS from each record, divided into three postmenstrual age (PMA) groups of 31-34, 35-37, and 38-40 weeks.
RESULTS: Seventy-three recordings from 32 preterm infants without severe brain injury were included. Their median gestational age was 32.6 (range, 24.9-34.9) weeks (Table 1). Average FC of oxy-Hb gradually increased with PMA in AS, whereas it decreased in QS (Figure 2A). PSI of oxy-Hb showed the same trend as with average FC (Figure 2B). In group level analysis, the patterns of FC were similar between AS and QS in 31-34 weeks of PMA and both showed stronger short-range FC and long-range inter-hemispheric FC. In 35-37 weeks of PMA, long-range intra-hemispheric FC and inter-hemispheric FC increased in AS, while the pattern of FC in QS was similar to that in 31-34 weeks. The patterns of FC in 38-40 weeks were similar to those in 35-37 weeks of PMA (Figure 3). The trends of FC and PSI on deoxy-Hb were similar to those of oxy-Hb.

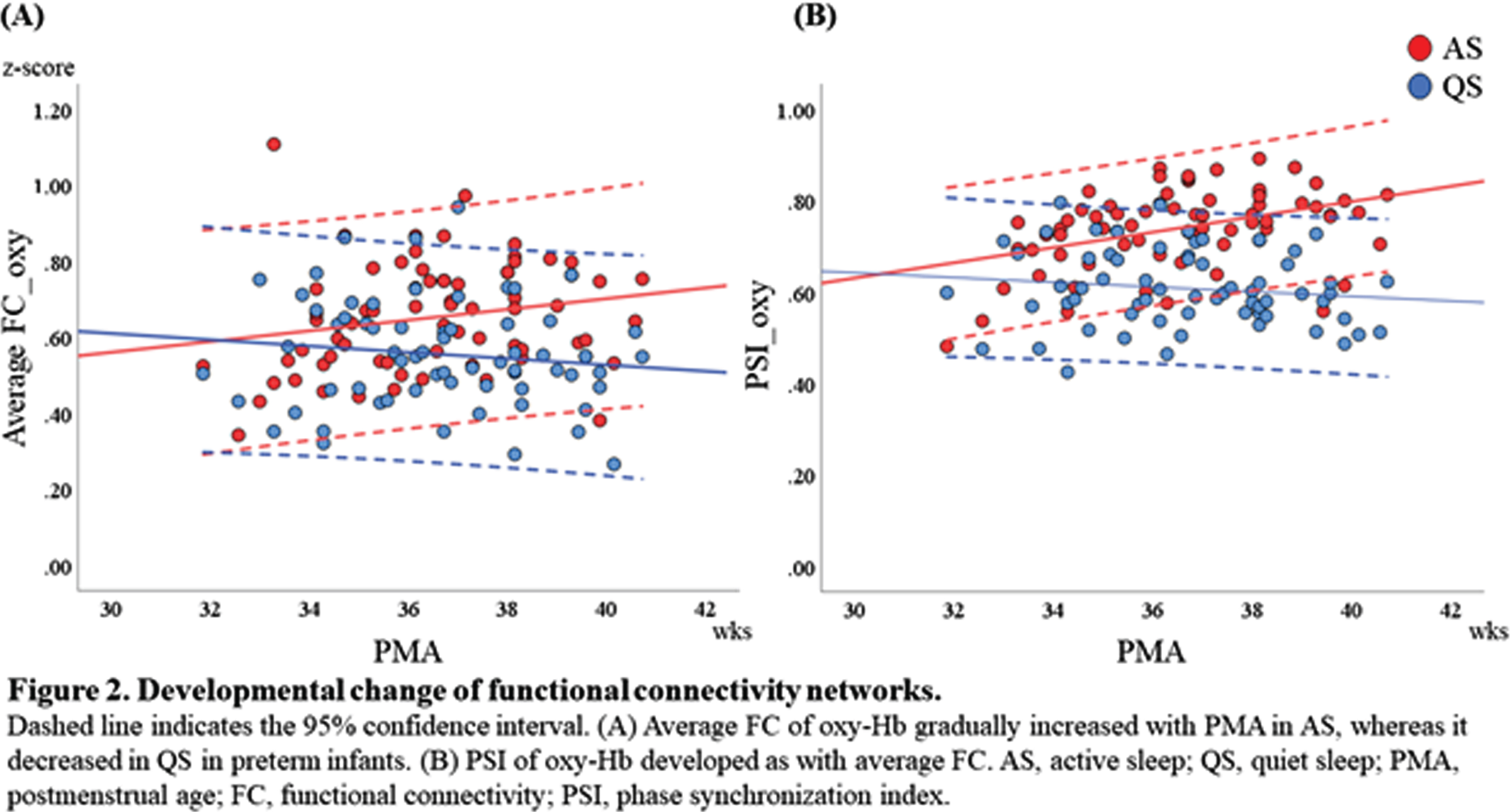
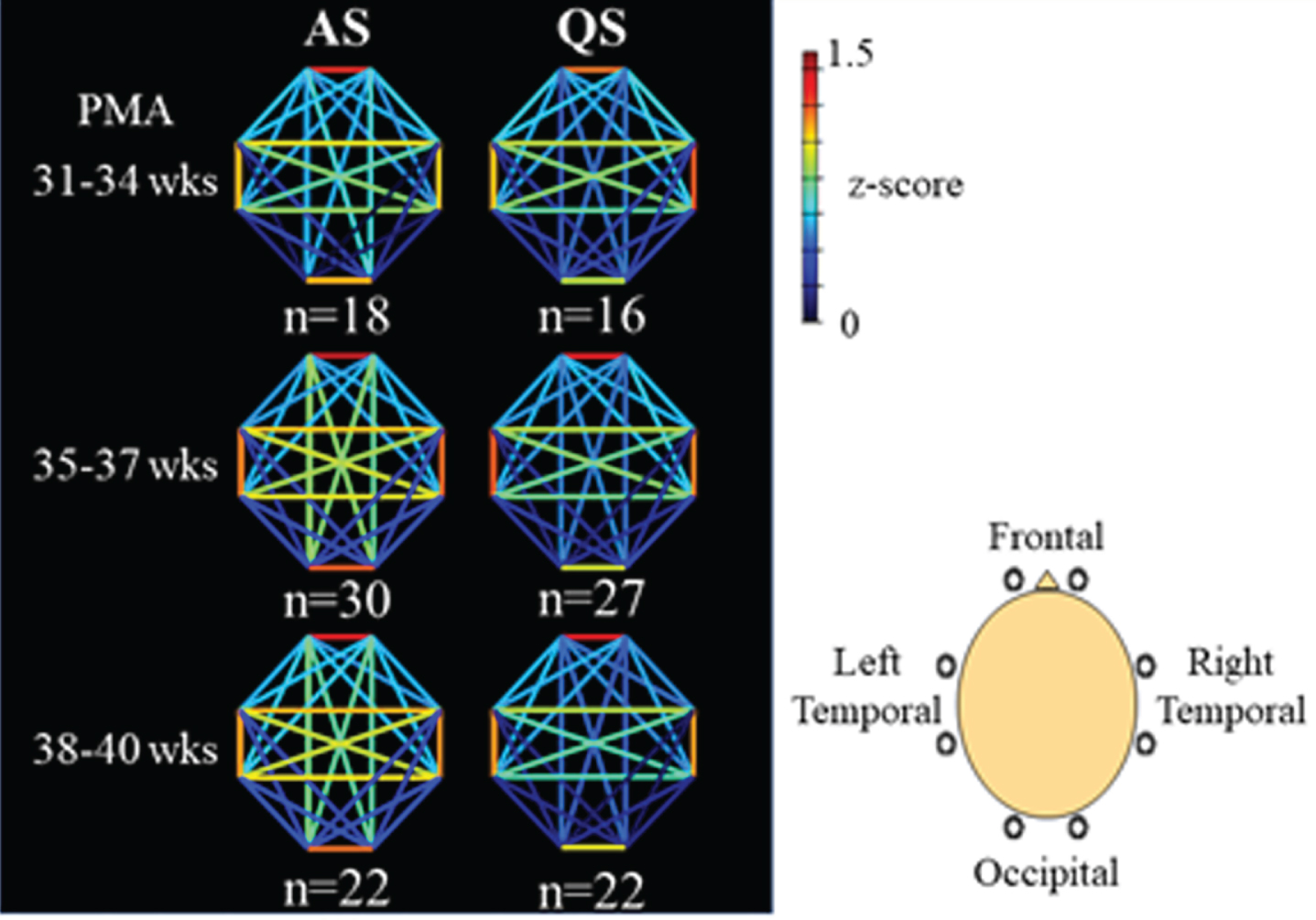
CONCLUSION: FCN can be changeable according to sleep states in preterm infants; higher FC in AS and lower FC in QS, and this difference would be more obvious over 34 weeks of PMA. This study highlights the importance of distinguishing sleep states when studying FCN in preterm and term infants.
Placental allopregnanolone insufficiency leads to long-term neurobehavioral changes
Vacher Ca, Lacaille Ha, Penn Aa
aColumbia University Medical Center, New York, United States
BACKGROUND AND PURPOSE: Early environmental insults may predispose individuals to later neurodevelopmental diseases. In particular, placental dysfunction or early loss resulting from preterm birth may represent significant perinatal risk factors for cognitive and psychiatric disorders. The placenta produces hormones that pervade the fetal brain and potentially support its development. One key potential placental hormone supporting fetal brain development may be allopregnanolone (ALLO), a progesterone derivative neurosteroid. Fetal brain ALLO levels in utero are higher than at any other time in life, peaking in late gestation. ALLO is a potent positive modulator of the GABA-A receptor (GABAAR). GABAAR signaling being a major regulator of numerous neurodevelopmental processes, we hypothesized that placental ALLO loss alters fetal neurodevelopment and increases the risk for long-term behavioral disorders.
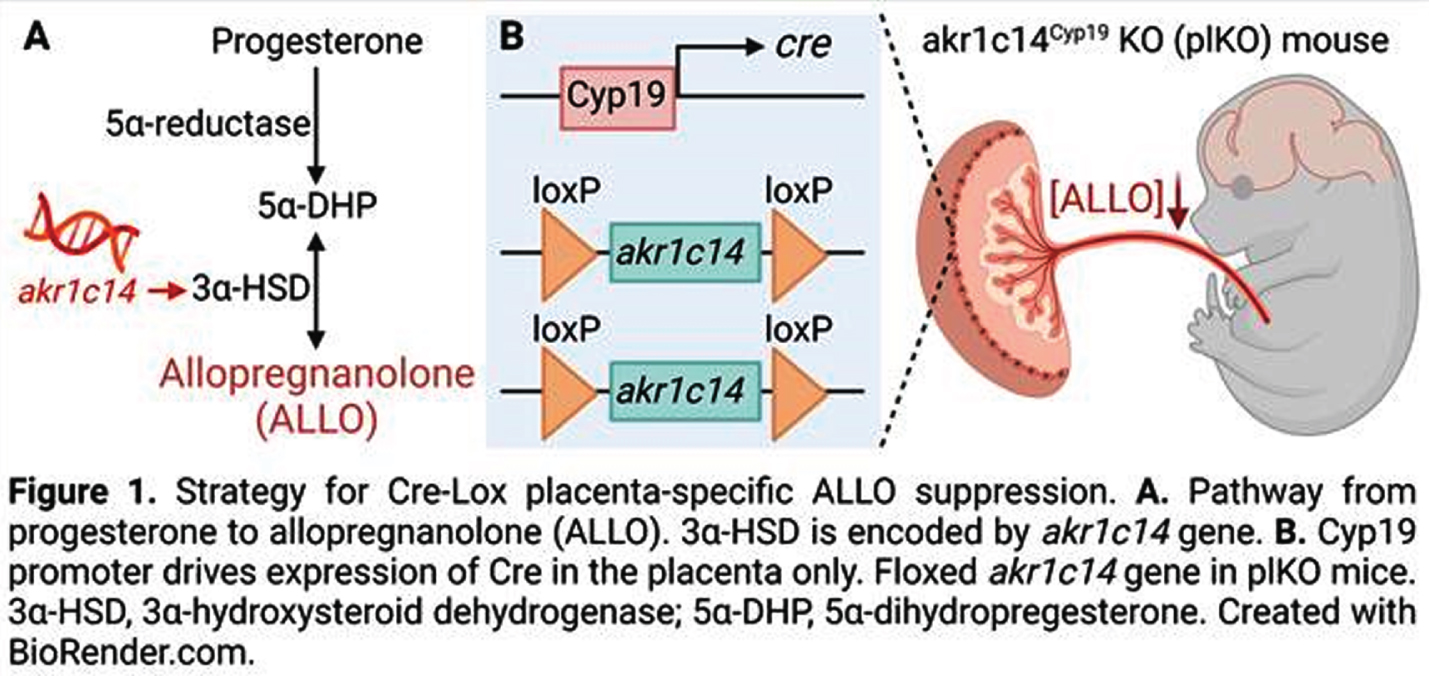
MATERIALS AND METHODOLOGY: We have generated a mouse model in which the gene encoding the synthesis enzyme for ALLO (Akr1c14) is specifically deleted in trophoblasts using a tissue-specific Cre-Lox strategy (Fig 1). In this model, the mice lacking placental Akr1c14 (plKO mice) show a ~50% reduction in placenta and brain ALLO levels(1). The plKO brains were analyzed from molecular to behavioral aspects, in a sex-specific manner. In addition, we challenged the translational potential of our mouse model by comparing some of the molecular signatures found in the plKO brain with the preterm infant brain.
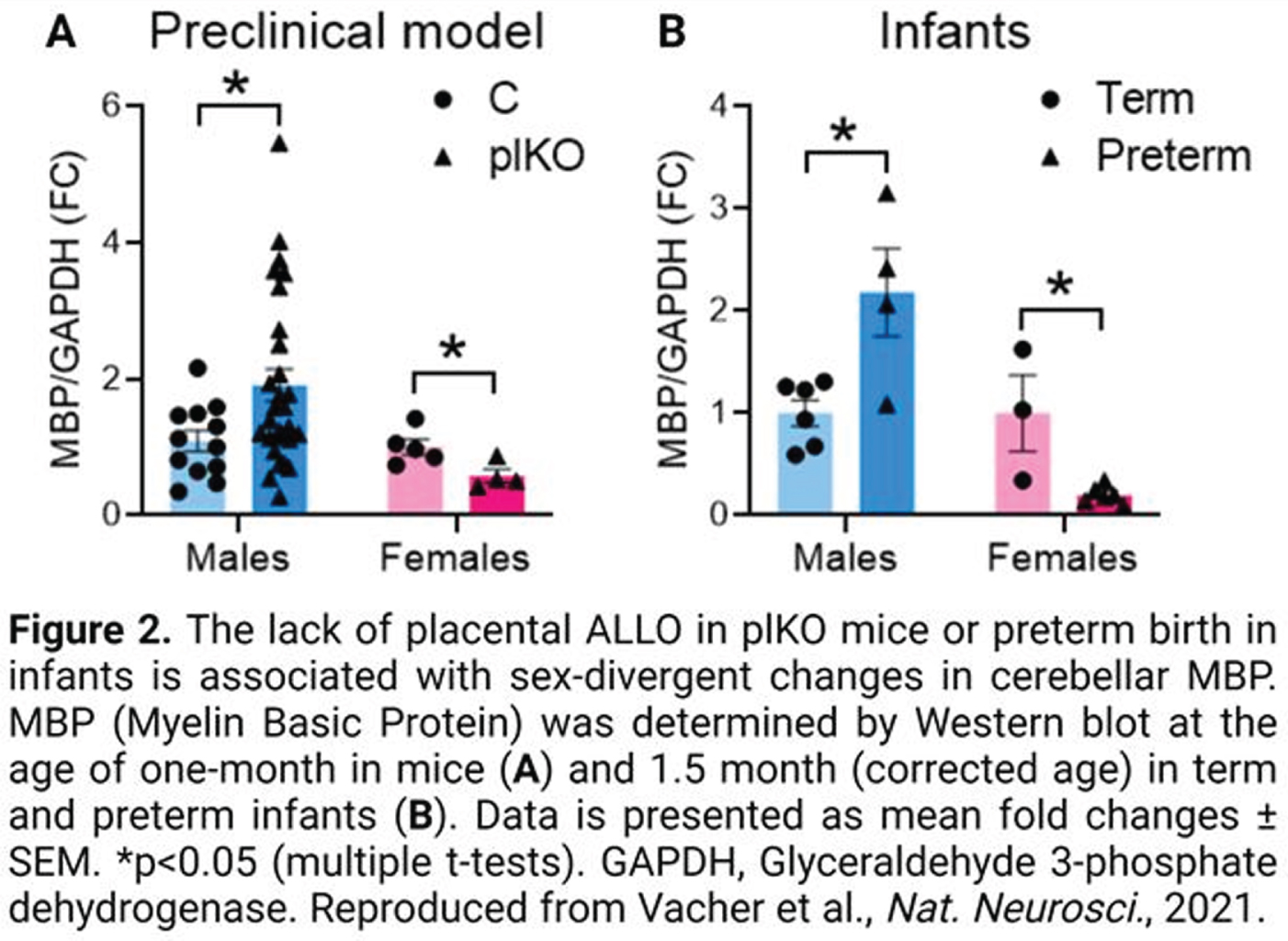
RESULTS: A non-biased transcriptomic analysis of various brain regions in one-month-old plKO mice suggested that cerebellar white matter (WM) might represent one of the most impacted structures after placental ALLO insufficiency. Molecular, ultrastructural and cellular analysis of the cerebellar WM revealed sexually divergent alterations, with the male cerebellum being hypermyelinated, in contrast to hypomyelination in the female cerebellum (Fig 2A). Interestingly, similar sex-linked dysregulation of the Myelin Basic Protein (MBP), were found in the preterm infant cerebellum (Fig 2B). Behavioral testing of plKO mice demonstrated male-specific ASD-like features including sociability deficits and motor stereotypies. A positive correlation between cerebellar MBP contents and autistic symptom severity was seen. Supporting the translational potential of our model, a significant overlap between the dysregulated genes in plKO cerebellum and ASD-linked genes was found (Fig 3). Finally, a single injection of ALLO or muscimol (a GABAAR agonist) during gestation rescued both cerebellar MBP levels and behavior in plKO littermates.

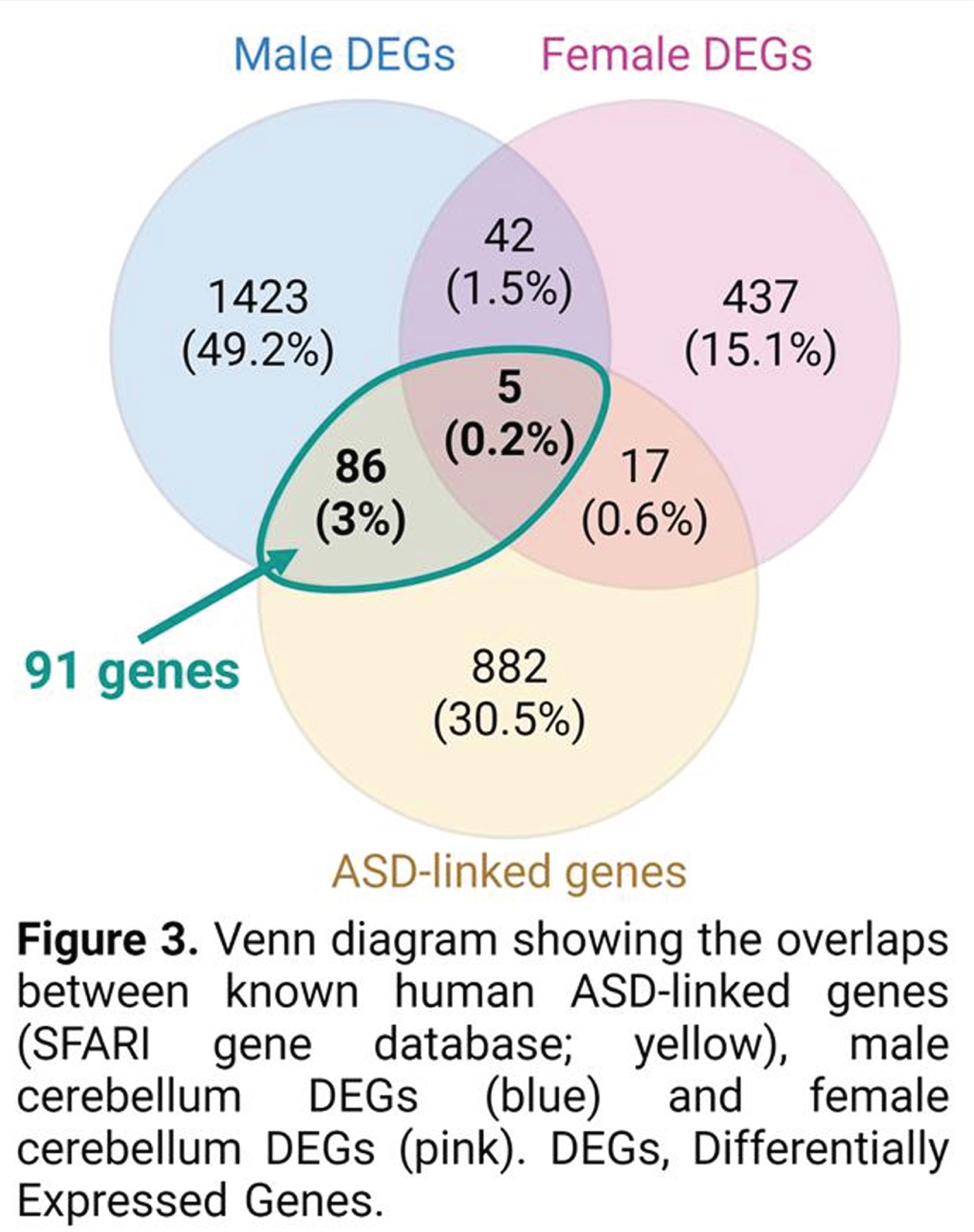
CONCLUSION/IMPACT: Our findings demonstrate that the lack of placental ALLO alters fetal neurodevelopment and increases the risk for long-term behavioral disorders. The potential translational value of this new model paves the way for further investigation in the field of neuroplacentology and allows us to design novel therapeutic approaches to prevent the neurological outcomes of preterm birth or placental dysfunction.
Bibliography:
[1] Vacher CM, Lacaille H..., Penn AA. 2021. Placental endocrine function shapes cerebellar development and social behavior. Nature Neuroscience, 24(10):1392-1401.
Impact of nutritional status on brain volumes of preterm infants
Valdes Ca, Nataraj Pa, Kisilewicz Ka, Bliznyuk Nb, Sura La, Weiss Ma
aDepartment of Pediatrics, Division of Neonatology, University of Florida, Gainesville, United States
bDepartment of Agricultural & Biological Engineering, Biostatistics and Statistics, University of Florida, Gainesville, United States
BACKGROUND: Preterm infants are at risk of malnutrition due to their innate organ immaturity, underdeveloped nutrient absorption and cautious enteral feeding advancement. The brain is a highly metabolic organ that consumes a great number of nutritional resources for growth and function. The purpose of this study is to evaluate if nutritional status is associated with brain growth utilizing recently published guidelines for malnutrition.
METHODS: The study was a retrospective chart review enrolling infants born <30 weeks’ gestation admitted to the NICU at UF Health between the years 2018-2019. Thirty three variables were obtained from the chart, including gestational age, information on enteral nutrition and malnutrition scores. MRIs obtained at term equivalent age (TEA) were analyzed using T2 weighted axial sequences with ITK-SNAP software to perform manual segmentation by 2 different investigators to ensure optimal brain volume reconstruction, excluding ventricles, brainstem and cerebellum. We compared the obtained volumes with the maximum change in weight and length over time, type of enteral feed, intracranial hemorrhages and gestational age at birth.
RESULTS: A total of 64 infants < 30 weeks’ gestation met the criteria for enrollment. Exclusion criteria included death, incomplete nutritional data or intracranial anomalies. Linear regression models were used and when feasible, these were reaffirmed using generalized additive models (GAMs) capable of capturing nonlinear associations between the response variable and the covariate(s). Linear associations with brain volume at TEA and gestational age at birth and maximum change in weight over time were found (p<0.05). Neonates that were small for gestational age (head sparing) had lower brain volumes at TEA compared to those that were appropriate for gestational age (p<0.05).
CONCLUSIONS: With modeling using gestational age at birth and maximum change in weight over time (Z- scores), infants tend to have a positive association with brain volumes at TEA. Further detailed analysis is ongoing to include other potential confounding variables to delineate the role of malnutrition on brain growth.




