Proceedings of the 13th International Newborn Brain Conference: Neuro-imaging studies
Association between anterior cerebral artery resistive index on ultrasound, brain injury on MRI, and outcome in newborns who received therapeutic hypothermia
Acosta La, Maltais-Bilodeau Ca, Reddy Da, Mitsakakis Na, Davila Ja, Lemyre Ba, Ben Fadel Na, Redpath Sa, Miller Ea
aCHEO, University of Ottawa, Ottawa, Canada
BACKGROUND/PURPOSE: Therapeutic hypothermia (TH) initiated within the first 6 hours after birth, is proven to decrease brain tissue injury in moderate to severe neonatal encephalopathy (NE) and improve 18-24 month neurological outcomes. Recently, a correlation between the resistive index (RI) of the anterior cerebral artery on head ultrasound (HUS) performed after rewarming and severity of injury on MRI was reported. It remains unknown whether early RI anomalies (before rewarming) may be associated with severity of injury on MRI and as such, might have prognostic value for infants too unstable to undergo an MRI within the optimal time window. The aim of this study was to evaluate: (i) the association between a low anterior cerebral artery RI on HUS obtained during active TH and the severity of brain injury on MRI, and to examine the association between the RI value and outcomes at 48 months of age.
METHODOLOGY: A retrospective study was performed. Electronic patient data records and patient charts were reviewed to identify infants born ≥35 weeks of gestation between October 2009 and December 2016, who received TH, at the Children’s Hospital of Eastern Ontario. RI on HUS in the first three days of life were compared to brain MRI findings based on the scoring system by Weeke et al. Outcomes at 48 months of life were based on the Ages and Stages questionnaire administered during a clinic visit.
RESULTS: Of the 101 newborns (median 39 weeks (IQR 38, 40); median birth weight 3.4 kg (IQR 3.0-3.8)) who underwent TH, 74 had doppler ultrasound of the anterior cerebral artery. The median age at which TH was achieved was 5.8 hours of life (IQR = 4.0 – 8.1). The median (IQR) for time to first ultrasound was 16.5 (10.7-24.3) hours. The mean RI in the initial ultrasound was 0.65 (IQR 0.59 – 0.73). 62 newborns had brain MRI, with a median of the total Weeke score of 2.5 (IQR = 0 – 11); the median grey matter score was 0 (IQR 0.0 – 4.0). Overall, there is little to no correlation between the mean RI and total Weeke score. There is a weak negative correlation between the mean RI and the Weeke grey matter score (Pearson(95% CI): -0.37 (-0.57–0.13; p-value=0.05). The logistic regression model, did not show any significant association between mean RI and neurologic outcome at 48 months (p=0.39).
CONCLUSION: Despite the small sample size of the study cohort, the mean RI of the anterior cerebral artery shows some degree of correlation with the Weeke grey matter score on MRI. This suggests it may be helpful as a biomarker in neonates with encephalopathy under TH, especially those that are severely compromise where an MRI may be unsafe.
Association between morphine exposure and brain injury on term-equivalent age brain magnetic resonance imaging in very preterm infants
Almouqdad Ma, Jamjoom Db, Khalil Ta, Asfour Yc, Albeshri Ba, Asfour Sa
aKing Saud medical city, Riyadh, Saudi Arabia bKing Saud University, Riyadh, Saudi Arabia cFamily Care Hospital, Riyadh, Saudi Arabia
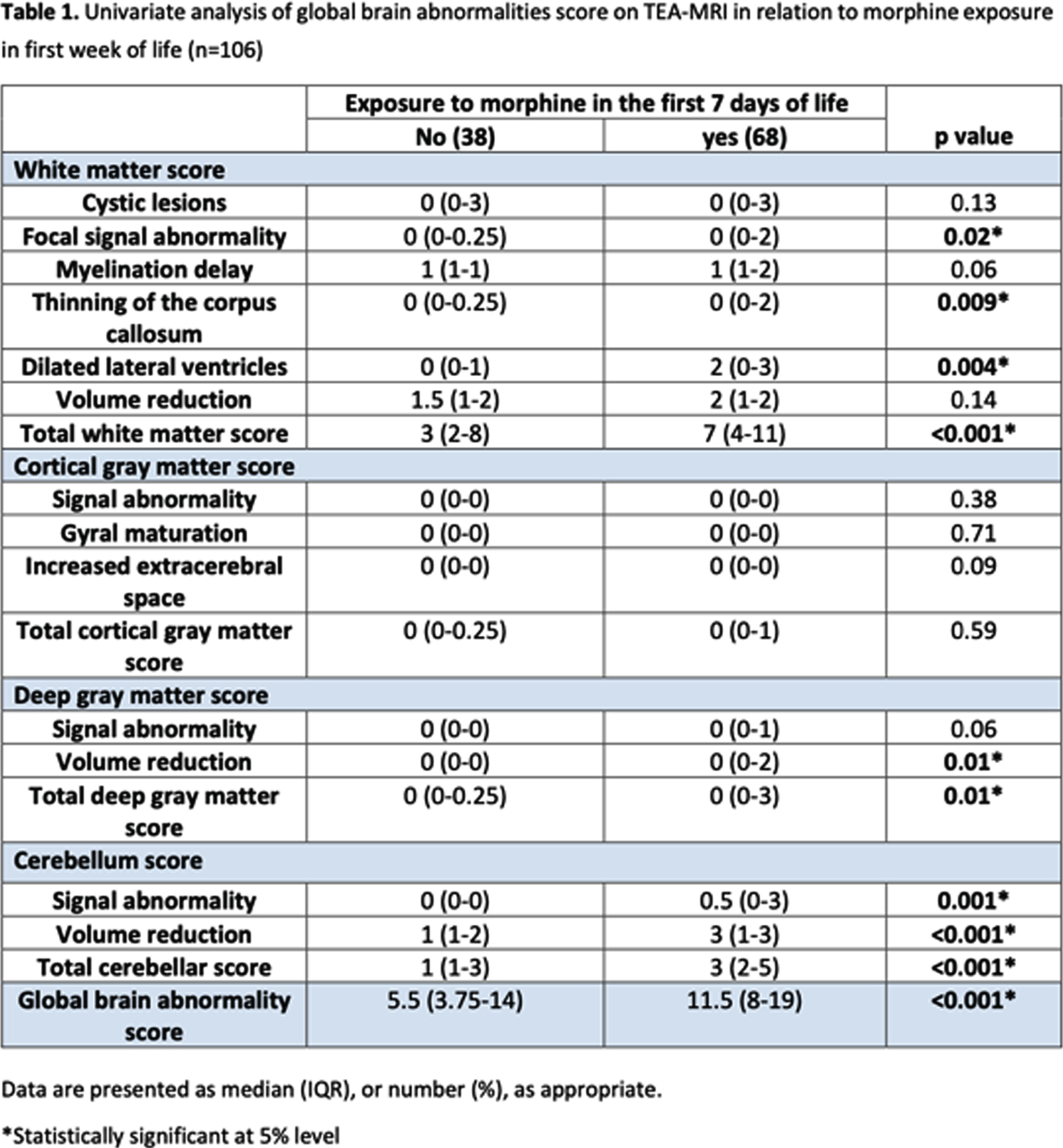
OBJECTIVE: To investigate the relationship between morphine exposure in the first week of life and brain injury on term-equivalent age magnetic resonance imaging (MRI) in very preterm infants.
METHODS: We included 106 infants with a birth weight of < 1500 g who were born at King Saud Medical City at ≤ 32 gestational weeks, were admitted to the neonatal intensive care unit, and underwent term-equivalent age and pre-discharge brain MRI. Modified log-Poisson regression with a robust variance estimator was applied, and the effect of early morphine exposure on brain morphology and growth at term-equivalent age was determined using the Kidokoro score.
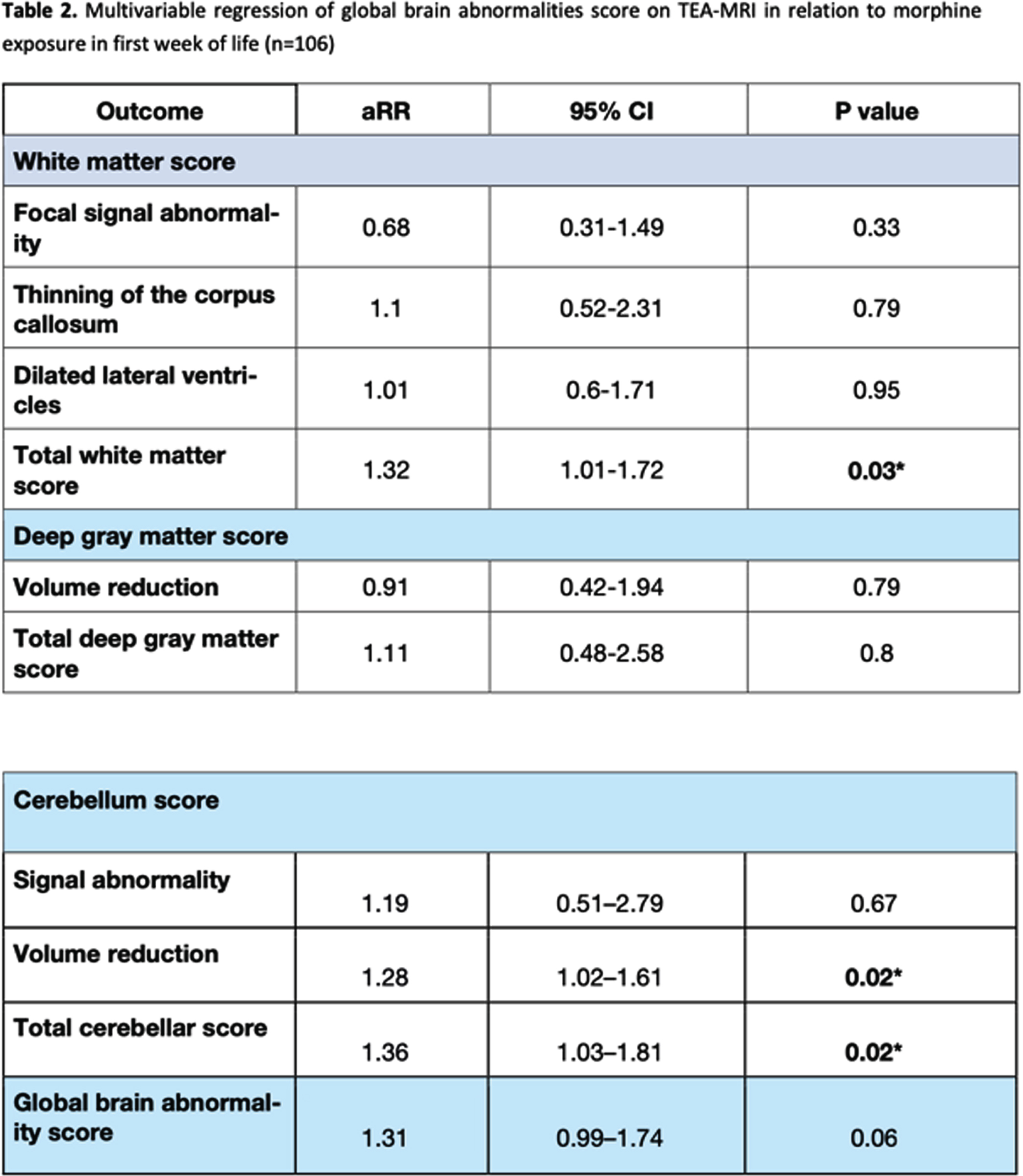
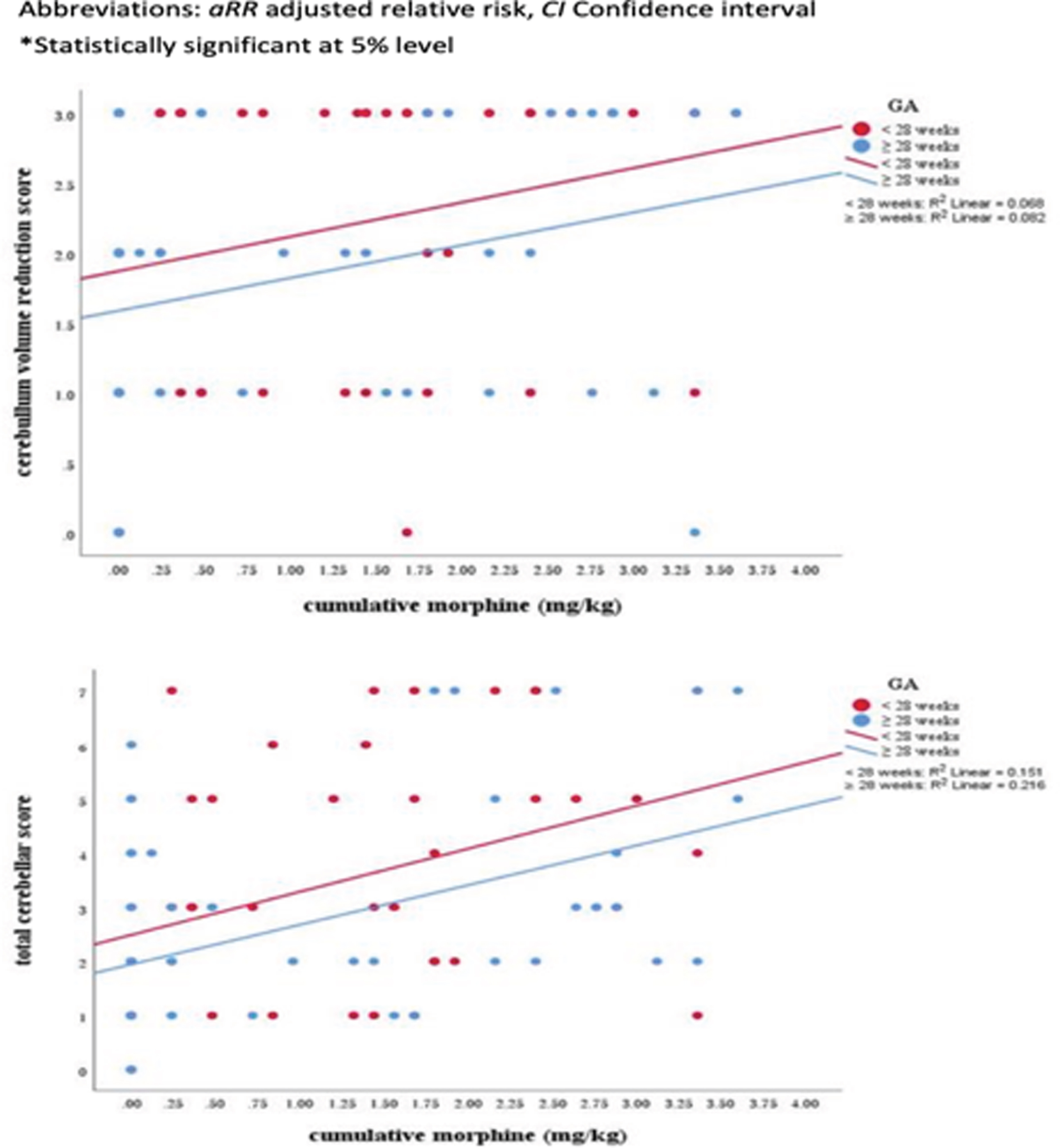
RESULTS: Sixty-eight (64.2%) infants had received morphine in the first week of life (median cumulative dose: 1.68 mg/kg, interquartile range: 0.48–2.52 mg/kg). Early initiation of morphine administration was significantly associated with high total white matter (adjusted relative risk [aRR]: 1.32, 95% confidence interval [CI]: 1.01–1.72) and cerebellum (aRR: 1.36, 95% CI: 1.03–1.81) scores and a low cerebellar volume (aRR: 1.28, 95% CI: 1.02–1.61).
CONCLUSION: Morphine exposure in the first week of life was independently associated with white matter and cerebellar injury on term-equivalent age brain MRI in very preterm infants.
Bibliography:
[1] Smith, G.C. et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 70, 541-549 (2011).
[2] Kumar, P., Walker, J.K., Hurt, K.M., Bennett, K.M., Grosshans, N. & Fotis, M.A. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J. Pediatr. 152, 412-415 (2008).
[3] Anand, K.J. et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 363,1673-1682 (2004).
[4] Steinhorn, R., McPherson, C., Anderson, P.J., Neil, J., Doyle, L.W. & Inder, T. Neonatal morphine exposure in very preterm infants-cerebral development and outcomes. J. Pediatr. 166, 1200-1207 (2015).
[5] Zwicker, J.G. et al. Smaller Cerebellar Growth and Poorer Neurodevelopmental Outcomes in Very Preterm Infants Exposed to Neonatal Morphine. J. Pediatr. 172, 81-87 (2016).
[6] Ranger, M. et al. Neonatal Pain and Infection Relate to Smaller Cerebellum in Very Preterm Children at School Age. J. Pediatr. 167, 292-298 (2015).
[7] Simons, S.H. et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 290, 2419-2427 (2003).
[8] de Graaf, J. et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 154, 449-458 (2013).
[9] van den Bosch, G.E. et al. Prematurity, Opioid Exposure and Neonatal Pain: Do They Affect the Developing Brain? Neonatology 108, 8-15 (2015).
[10] Miller, S.P. et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J. Pediatr. 147, 609-616 (2005).
Prenatal cerebral abnormalities in congenital heart disease detected on structural magnetic resonance imaging
Dagur Ga,b, Ortinau Cc, Chin Ta, Kleinmahon Jb,d, Gurvitz Me,f, Bora Sf
aMothers, Babies and Women’s Health, Mater Research Institute, Faculty of Medicine, The University of Queensland, Brisbane, Australia
bOchsner Clinical School, Faculty of Medicine, The University of Queensland, New Orleans, United States
cPediatrics, Washington University School of Medicine, St. Louis, United States
dPediatric Cardiology, Ochsner Hospital for Children, New Orleans, United States
ePediatrics, Harvard Medical School, Boston, United States
fCardiology, Boston Children’s Hospital, Boston, United States
BACKGROUND: Fetuses with congenital heart disease (CHD) are vulnerable to alterations of the developing brain. 1Despite growing recognition of prenatal cerebral abnormalities, there is limited understanding primarily due to small sample sizes and varying neuroimaging modalities. 2,3 The aim of this study was to conduct a systematic review and meta-analysis, appraising evidence to estimate the prevalence of prenatal cerebral abnormalities in CHD detected on magnetic resonance imaging (MRI).
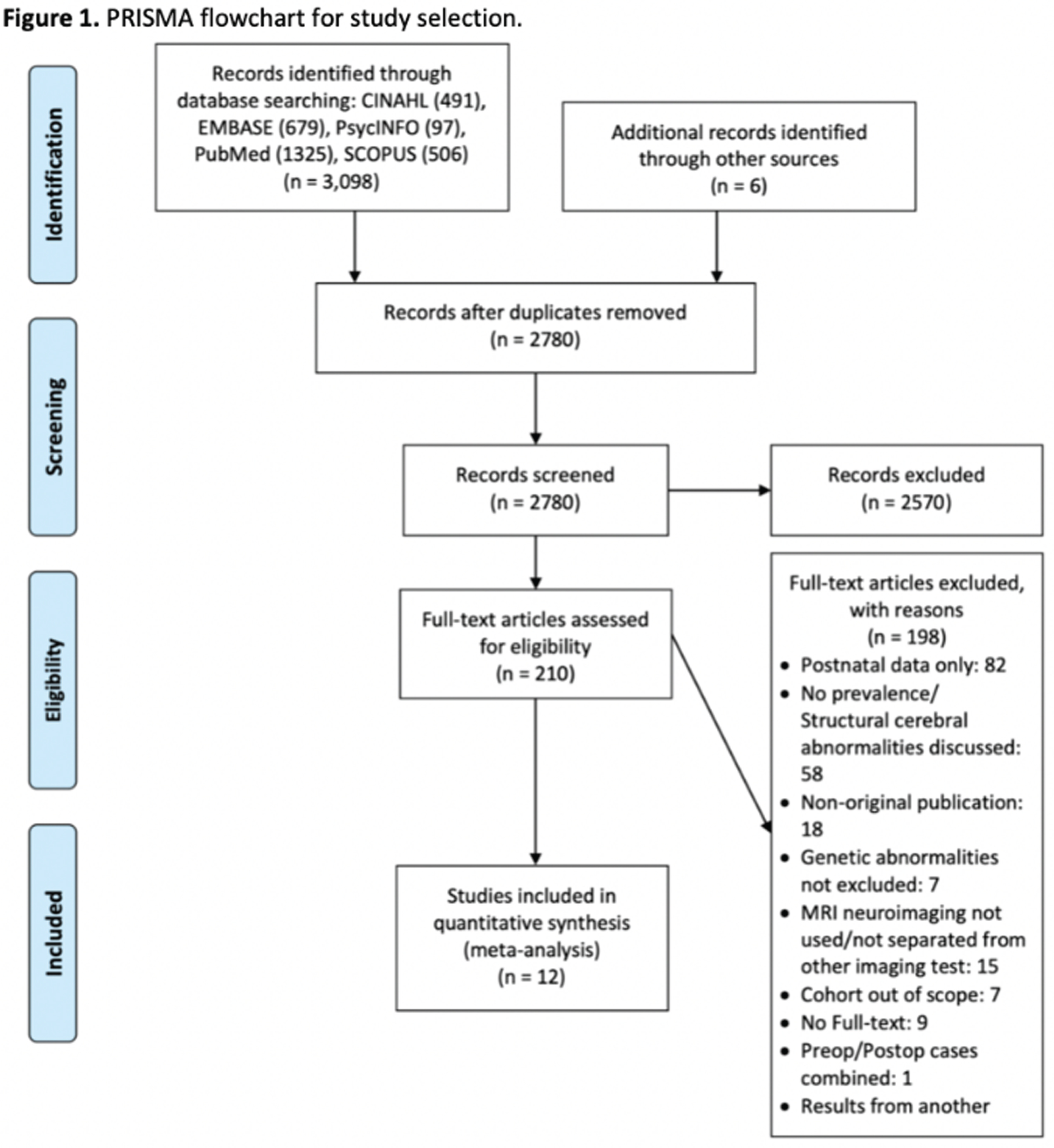
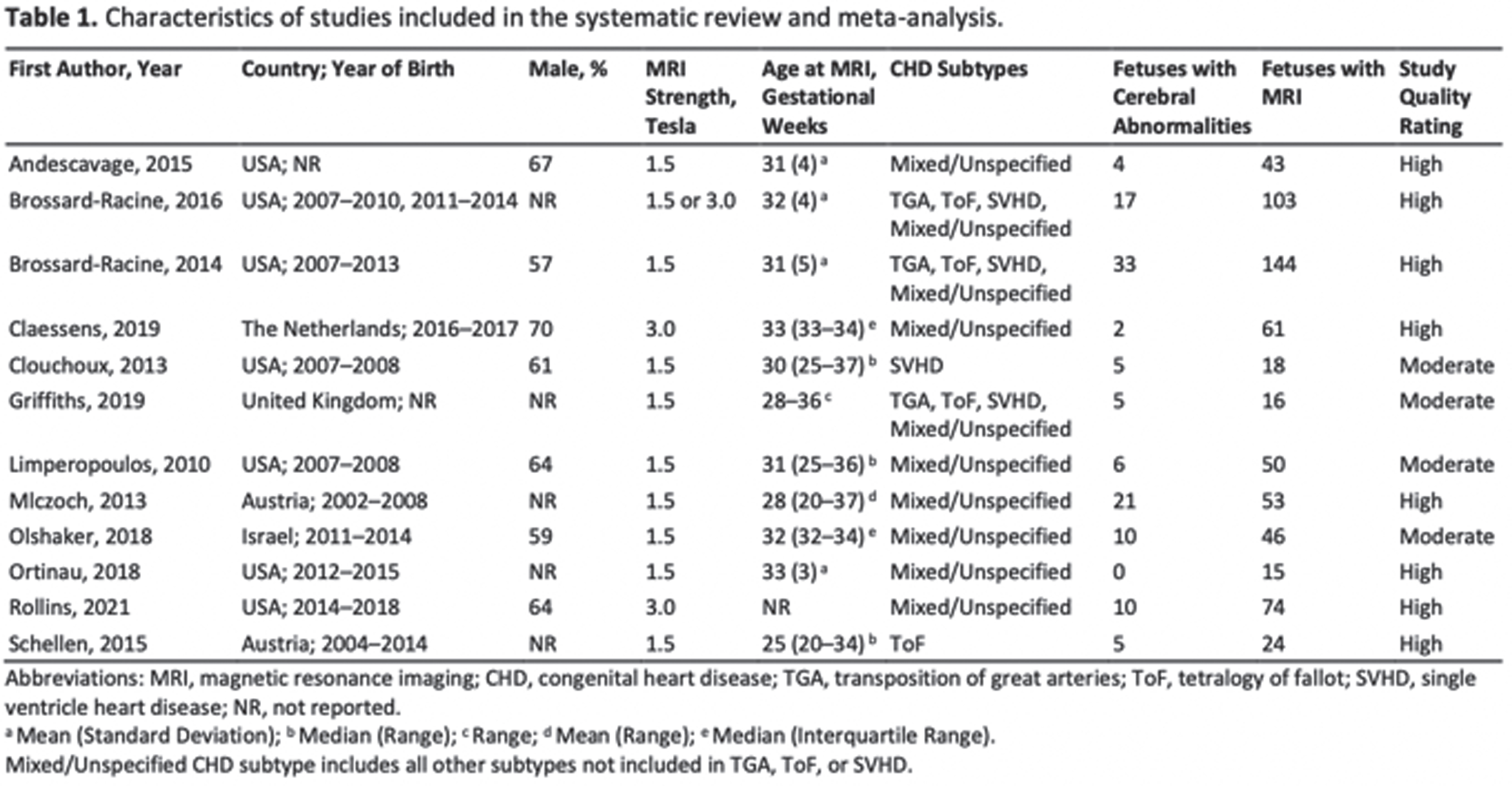
METHODOLOGY: This study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guideline. Two independent reviewers screened five electronic databases (CINAHL, EMBASE, PsycINFO, PubMed/MEDLINE, and SCOPUS) using combinations of MeSH terms and keywords in the format of population, exposure, outcome, and neuroimaging modality to identify peer-reviewed English language publications from inception to February 2021. Inclusion criteria were: 1) sample aged 24 weeks gestation to birth, with 2) isolated (non-syndromic) CHD, reporting 3) prenatal cerebral abnormalities on structural MRI, and using 4) cohort, case-control, or cross-sectional study designs. CHD subtypes and cerebral abnormalities were classified as reported in the original studies. Studies were excluded if they reported 1) outcomes on neuroimaging modalities other than MRI, or 2) structural MRI measures (e.g., volumetrics and surface-based analyses) without rates of abnormalities. For studies reporting outcomes of the same cases, the study with the largest sample size was included in the meta-analysis, to ensure sample independence. Random-effects modeling was used for pooled prevalence estimates and heterogeneity based on I2.
RESULTS: As shown in Figure 1, the systematic search yielded 12 studies fulfilling the inclusion criteria, comprising 435 fetuses with isolated CHD. Study characteristics are summarized in Table 1. As shown in Figure 2, the overall prevalence of prenatal cerebral abnormalities in CHD detected on structural MRI was 22% (95% CI=9%–35%), with 25% in single ventricle heart disease, 24% tetralogy of fallot, 19% in transposition of great arteries, and 16% in mixed/unspecified lesions.
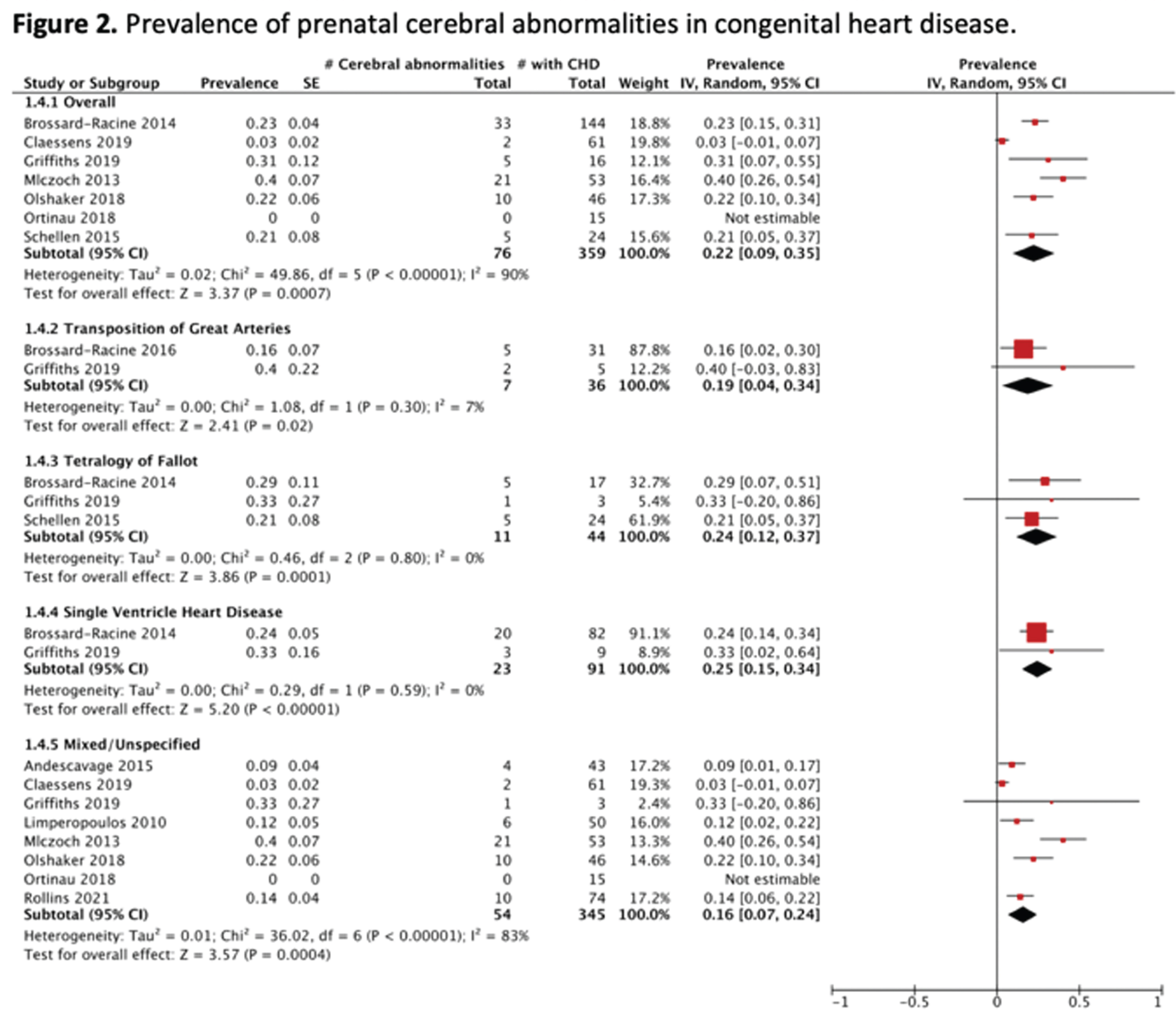
CONCLUSION: Findings provide evidence that CHD is associated with prenatal cerebral abnormalities, with more than a fifth of this high-risk population at risk. Further studies are warranted to better understand the longitudinal progression of these abnormalities and their association with neurodevelopmental outcomes. This study was limited by reliance on cerebral abnormalities as defined in the original studies, thereby resulting in a heterogeneous profile of prenatal cerebral injuries and/or alterations.
Bibliography:
[1] Bonthrone AF, et al. MRI studies of brain size and growth in individuals with congenital heart disease. Translational Pediatrics. 2021;10(8):2171-2181.
[2] Jansen FA, et al. Fetal brain imaging in isolated congenital heart defects – a systematic review and meta-analysis. Prenatal Diagnosis. 2016;36(7):601-613.
[3] Khalil A, et al. Prevalence of prenatal brain abnormalities in fetuses with congenital heart disease: a systematic review. Ultrasound in Obstetrics & Gynecology. 2016;48(3):296-307.
Facilitators and barriers of neuroimaging in children with congenital heart disease
Dagur Ga,b, Kleinmahon Jb,c, Gurvitz Md,e, Acosta Rf, Bora Sa
aMothers, Babies and Women’s Health, Mater Research Institute, Faculty of Medicine, The University of Queensland, Brisbane, Australia
bOchsner Clinical School, Faculty of Medicine, The University of Queensland, New Orleans, United States
cPediatric Cardiology, Ochsner Hospital for Children, New Orleans, United States
dPediatrics, Harvard Medical School, Boston, United States
eCardiology, Boston Children’s Hospital, Boston, United States
fOn Behalf of Conquering CHD, United States
BACKGROUND: There has been increasing recognition of the prognostic utility of neuroimaging in high-risk pediatric neurodevelopment.1,2 It is now well-documented that brain injuries or abnormalities are associated with neurodevelopmental delays/deficits in children with congenital heart disease (CHD).3 Thus, the aim of this study was to identify the facilitators and barriers to attending pediatric neuroimaging, as reported by primary caregivers of children with CHD.
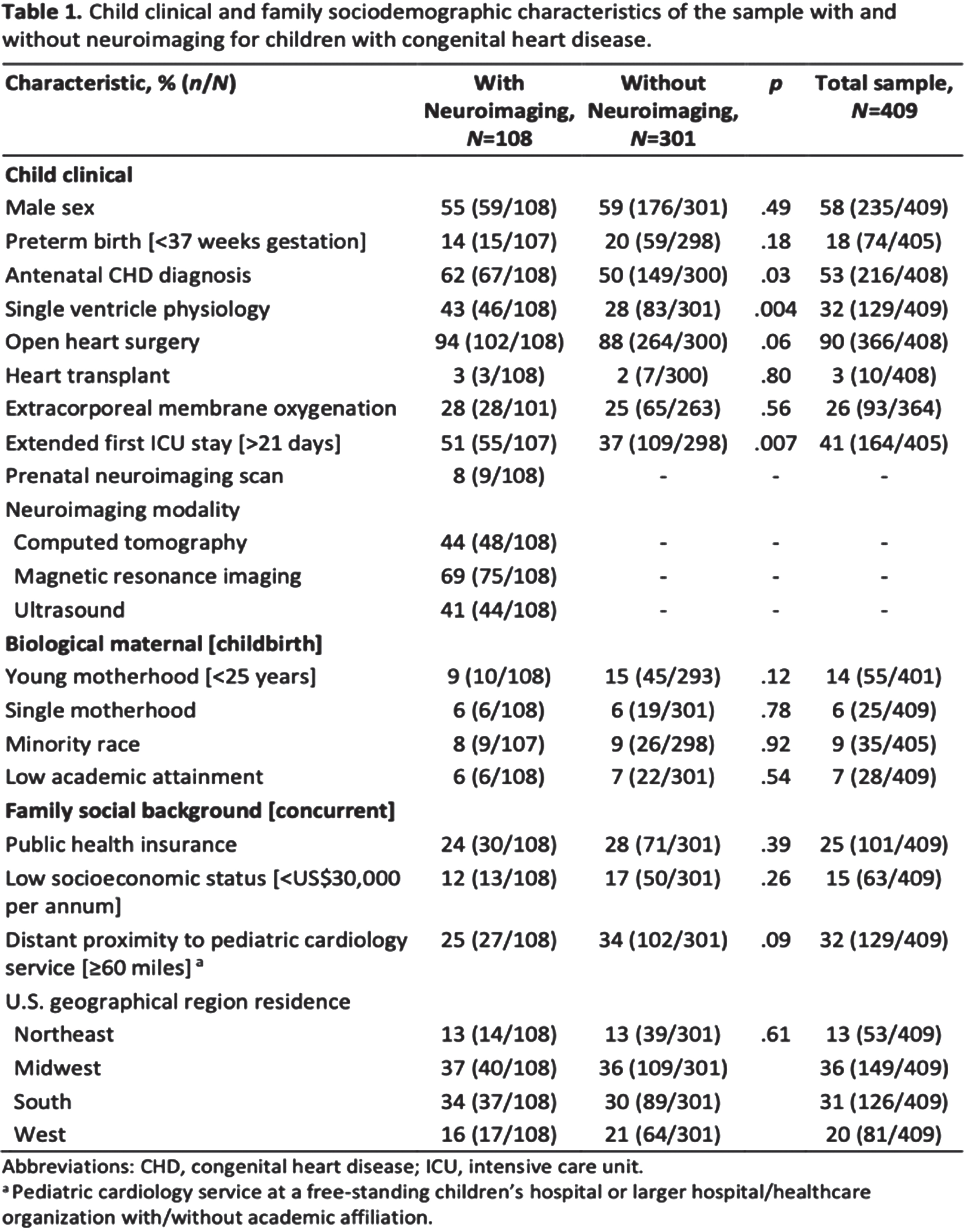
METHODOLOGY: As part of a cross-sectional study, 409 primary caregivers of children with CHD residing in the U.S. completed an online survey, with 108 and 301 children with and without neuroimaging for CHD, respectively. The survey was disseminated between November 2020 and January 2021. Sample recruitment was facilitated by Conquering CHD, the largest CHD patient and family advocacy organization in the U.S. Responses of participants regarding facilitators and barriers to attending neuroimaging were classified into 15 categories. Further, these were stratified into three themes corresponding to healthcare systems, health care professionals, and primary caregivers.
RESULTS: A descriptive profile of child clinical and family sociodemographic characteristics is provided in Table 1. Relative to children without neuroimaging, those with neuroimaging had significantly higher rates of antenatal CHD diagnosis (50% vs. 62%), single ventricle physiology (28% vs. 43%), and extended first ICU stay (37% vs. 51%). Further, 34% of children without neuroimaging, compared with 25% of children with neuroimaging, were living ≥60 miles from the nearest pediatric cardiology center. As shown in Figure 1a–c, factors related to primary caregivers and health care professionals were the most common determinants of neuroimaging for children with CHD. Among those who underwent neuroimaging, the biggest facilitator was the perceived benefits of neuroimaging by primary caregivers, reported by 67% of respondents. Further, 63% indicated the importance of health care professionals’ communication regarding neuroimaging as a major facilitator. Correspondingly, for children without neuroimaging, the biggest reported barriers were health care professionals’ communication (79%) and limited understanding of the primary caregiver (54%) concerning neuroimaging.
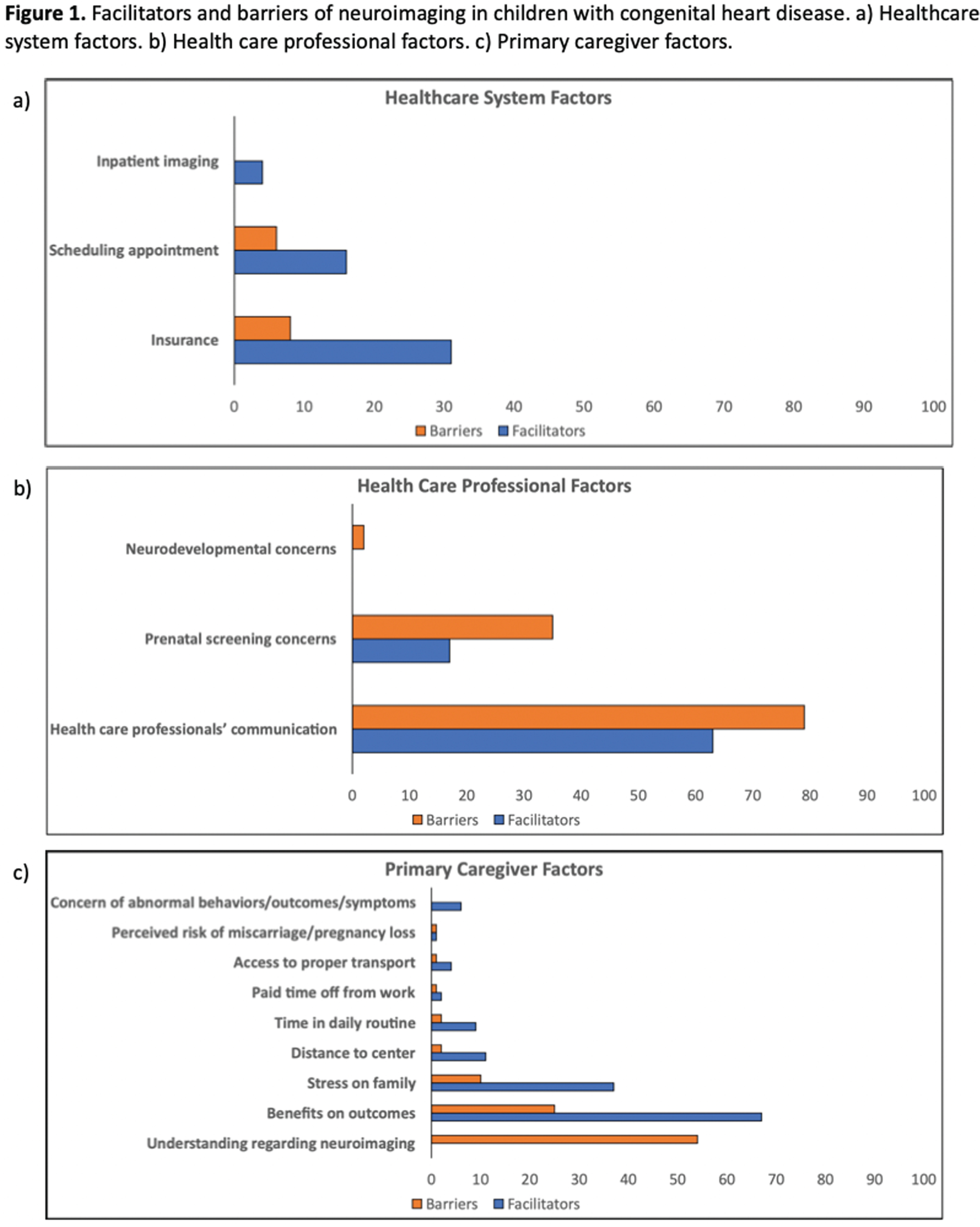
CONCLUSION: To facilitate increased uptake of neuroimaging, study findings highlight the need to enhance awareness and knowledge of primary caregivers regarding the utility of neuroimaging for children with CHD. Further, the critical role of health care providers’ communication is emphasized, particularly highlighting the importance of neuroimaging in relation to neurodevelopmental outcomes. Taken together, a multifactorial approach is required to support follow-up care of children with CHD and their families.
Bibliography:
[1] Guillot M, et al. Comparative performance of head ultrasound and MRI in detecting preterm brain injury and predicting outcomes: A systematic review. Acta Paediatrica. 2021;110(5):1425-1432.
[2] Ouwehand S, et al. Predictors of outcomes in hypoxic-ischemic encephalopathy following hypothermia: A meta-analysis. Neonatology. 2020;117(4):411-427.
[3] Mebius MJ, et al. Brain injury and neurodevelopmental outcome in congenital heart disease: A systematic review. Pediatrics. 2017;140(1):e20164055.
Conventional neonatal MRI predicts gross motor disability at four years in children with neonatal encephalopathy
Lambing Ha, Gano Da, Li Ya, Bach Ab, Girvan Oc, Rogers Ea, Ferriero Da, Barkovich Ja, McCulloch Ca, Glass Ha
aUniversity Of California, San Francisco, San Francisco, United States
bChildren’s Hospital of Philadelphia, Philadelphia, United States
cJohns Hopkins University, Baltimore, United States
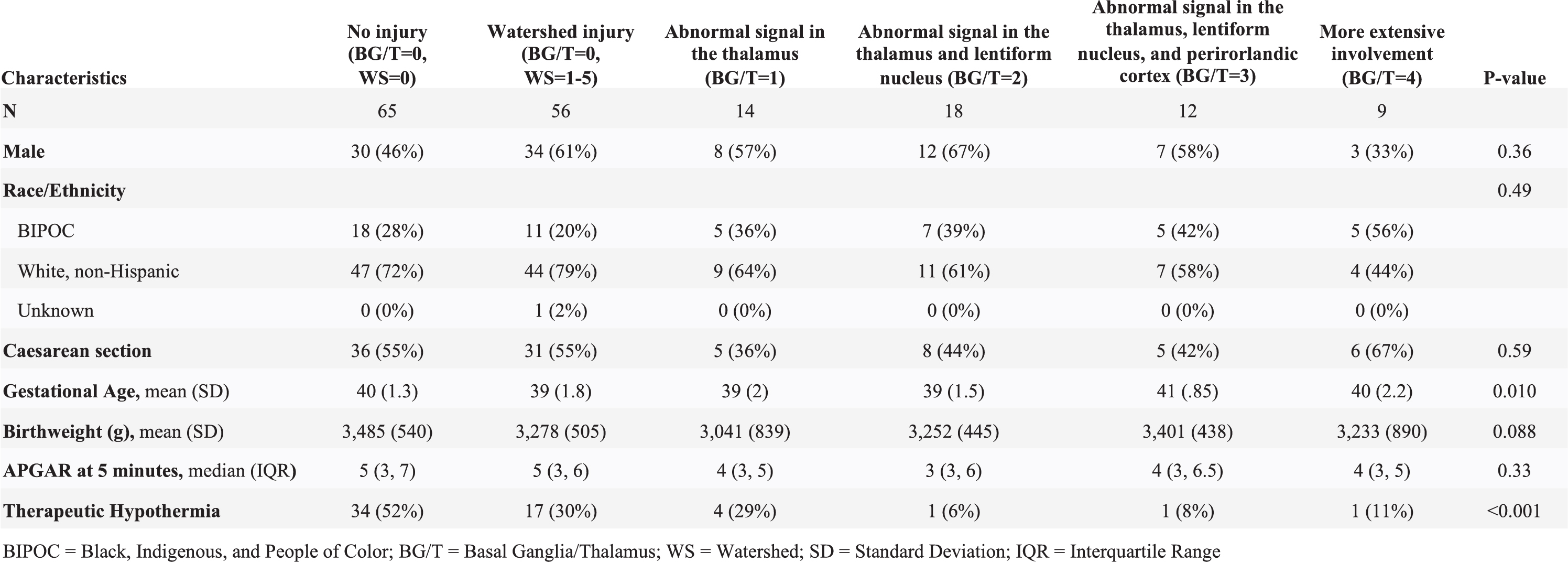
BACKGROUND: Children with neonatal encephalopathy due to hypoxic-ischemic encephalopathy are at risk for basal ganglia/thalamus (BG/T) and watershed (WS) patterns of brain injury. We previously showed that neuromotor impairment at 30 months of age is more common in infants with BG/T than with WS injury [Miller S.P., 2005]. The relationship between neonatal imaging and early childhood outcome is not well characterized.
METHODS: Term neonates at risk for brain injury due to neonatal encephalopathy were prospectively enrolled at UCSF Benioff Children’s Hospital from 1993-2014 and received MRI within two weeks after birth. A neuroradiologist blinded to clinical condition scored brain injury in the BG/T pattern (from 0, no injury to the deep gray nuclei, to 4, basal ganglia, thalamic, perirolandic and additional cortical injury) [Barkovich A.J., 1998]. Brain injury was quantified as a full-scale (no injury, WS injury only, and BG/T scores 1-4) and dichotomized BG/T (0-1 vs. 2-4). Two researchers independently assigned a Gross Motor Function Classification System (GMFCS) score for cerebral palsy (CP) at age four years based on a structured neurological examination (linearly weighted kappa=0.881). To describe the predictive value of the MRI, the relationship between BG/T injury and dichotomized GMFCS (0-2=none/mild vs. 3-5=moderate/severe CP) was evaluated with logistic regression. Covariates associated with injury were added stepwise to the model and cross-validated AUROC was used to determine the model with the best predictive performance.
RESULTS: Of the 408 newborns enrolled and imaged, 356 (93%) survived and 174 (49%) of survivors attended the four-year follow-up (Figure 1). MRI at a median age of 4 days (IQR 3-6) showed no injury in 65 (38%), WS injury only in 56 (32%), and BG/T injury of varying severity in 53 (31%, Table 1). At four years (50 +/- 5 months), 145 children (83%) had no CP, 18 children (10%) had mild CP (GMFCS=1-2), and 11 children (6%) had moderate-severe CP (GMFCS=3-5). Higher BG/T scores were associated with higher GMFCS (Figure 2). Both the binary and full-scale predictor had high AUROC (0.8865 and 0.8945, respectively); clinical covariates did not meaningfully add to the model. Risk of moderate-severe CP was low (<20%) in all patterns of brain injury except BG/T=4, which carried 67% probability (95% CI 36-98%) of moderate-severe CP (Figure 3).
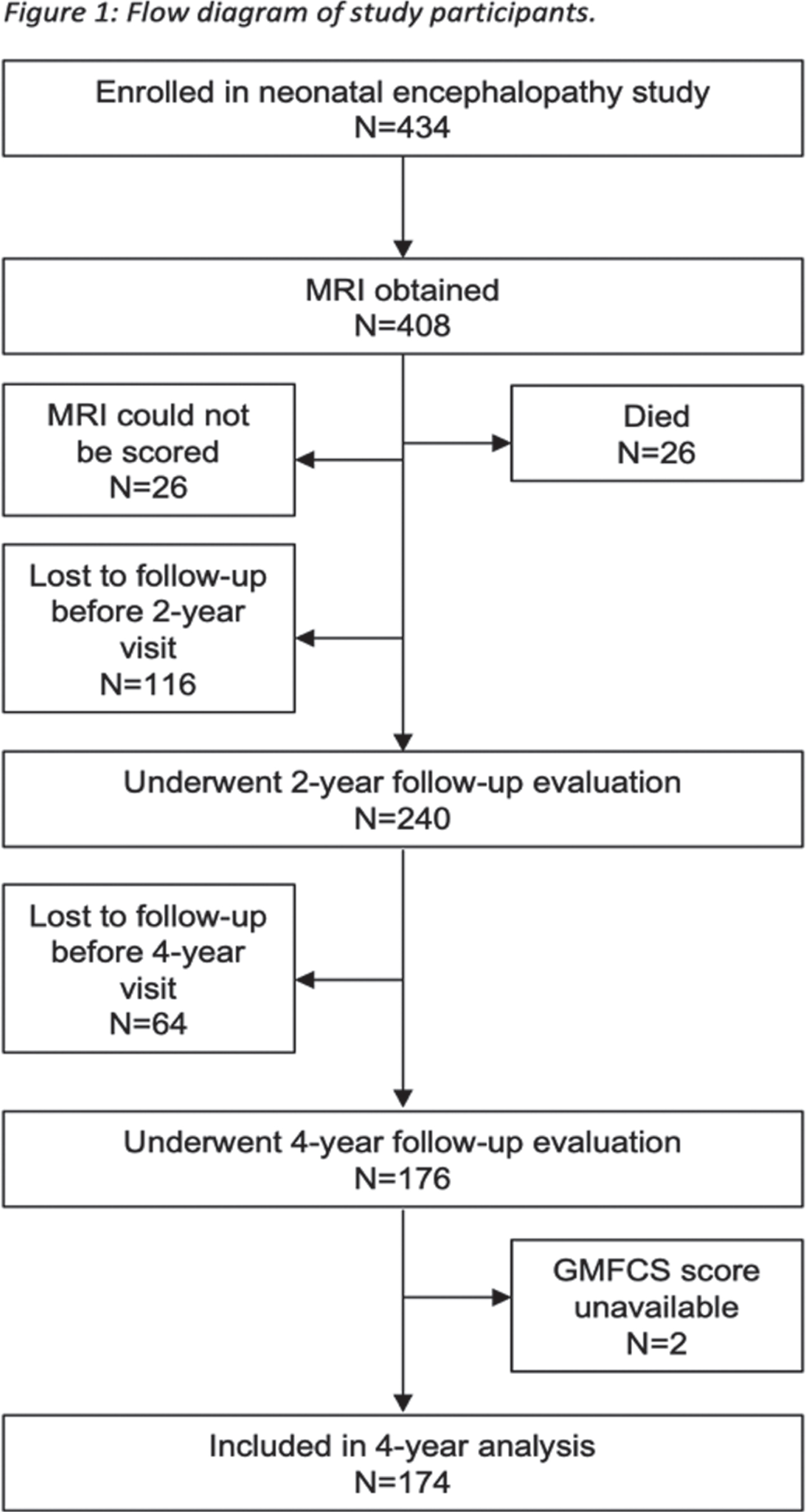
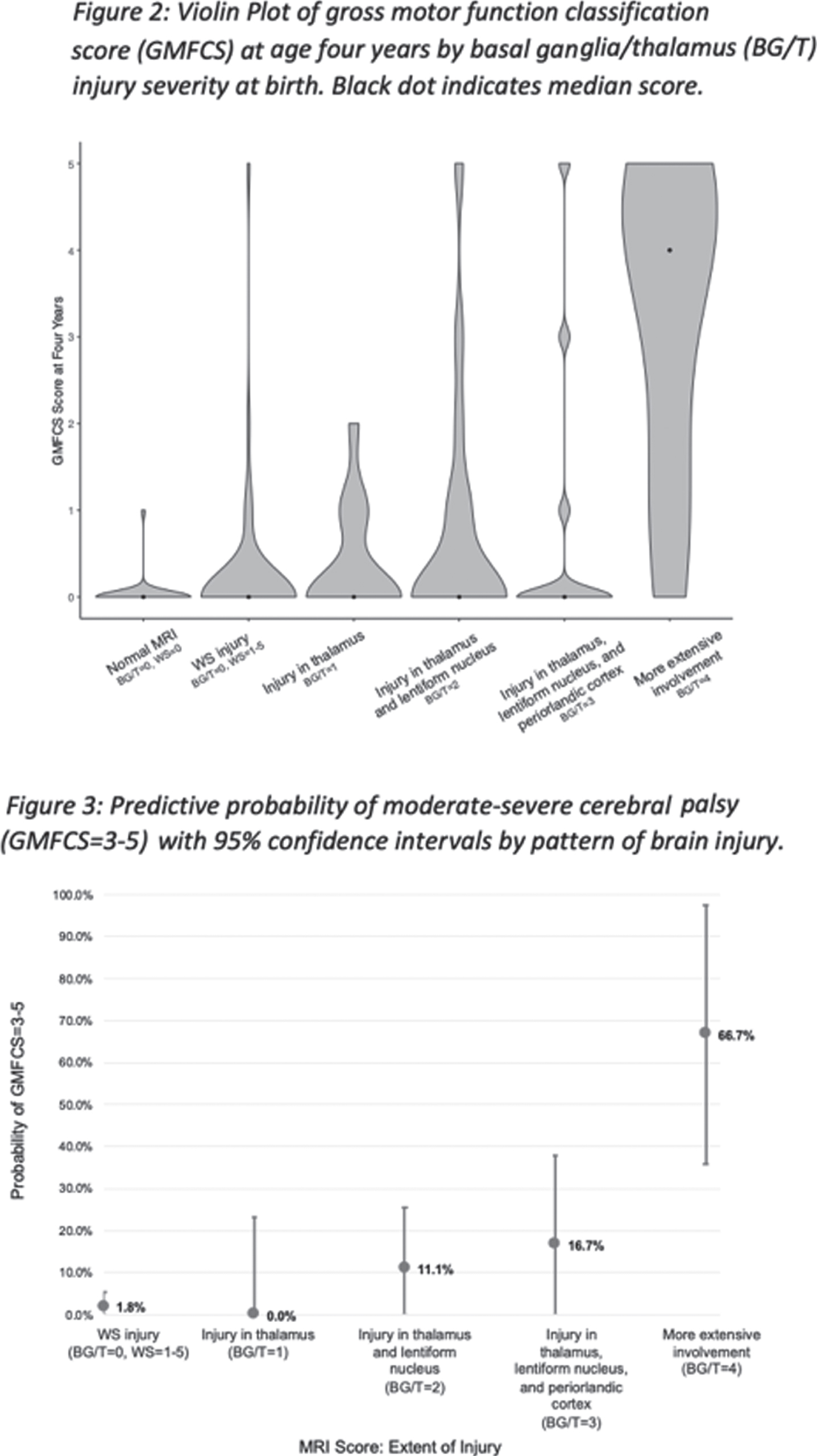
CONCLUSIONS: In this large, prospective cohort of children with neonatal encephalopathy, the risk of moderate-severe CP was very low, particularly for children with BG/T=0-3. However, among children with BG/T=4, two-thirds had moderate-severe CP indicating inability to ambulate independently at age four years. Understanding the relationship between neonatal MRI and motor outcome is important for clinicians to provide recommendations for treatment and resources to families at the time of birth and diagnosis, rather than when the motor abnormalities become apparent in early childhood.
Effectiveness of the Canadian consensus approach for diagnosis and grading of preterm brain injury from cranial Ultrasound
Leijser La, Scott Jb, Zein Ha, Mohammad Ka
aUniversity of Calgary, Cumming School of Medicine, Department of Pediatrics, Section of Neonatology, Calgary, Canada
bUniversity of Calgary, Cumming School of Medicine, Department of Radiology, Calgary, Canada
BACKGROUND AND PURPOSE: Cranial ultrasound (cUS) is the first-line tool to screen the vulnerable preterm infant’s brain for injury. Concerns, however, remain regarding variability in diagnosis and grading of common preterm brain injury types from cUS between Neonatologists and Radiologists within and across neonatal centers. We recently developed a Canadian consensus approach to decrease this variability.1 The current study assessed the interobserver agreement in diagnosis and grading of preterm brain injury from cUS before and after the implementation of the consensus approach in our centers.
METHODS: Retrospective observational study in extremely preterm infants (<29 weeks) who were admitted to Calgary neonatal centers between 2010-2019 and had ≥3 cUS scans throughout the neonatal period. All available serial cUS scans were assessed by a Neonatologist and Radiologist before and after implementation of the consensus approach. Assessments included: Presence and grade (1 to 3) of germinal matrix-intraventricular hemorrhage (GMH-IVH; with grade 3 defined as IVH with anterior horn width of lateral ventricle >6mm), and associated presence of periventricular hemorrhagic infarction (PVHI), post-hemorrhagic ventricular dilatation (PHVD; defined as enlarged ventricular measurements on repeated scans), cystic white matter injury (cWMI) and cerebellar hemorrhage (CBH). Interobserver agreement (intra-class correlation coefficient [ICC]) after consensus approach implementation was tested for all injury types. In addition, ICCs before and after implementation were compared for GMH-IVH presence and grading. In case of inconsistency in observers’ assessment, consensus on injury diagnosis and grading was reached through case discussion.
RESULTS: As per consensus, 292 out of 1452 (20%) eligible infants showed unilateral or bilateral GMH-IVH, with a total of 460 GMH-IVHs diagnosed among these infants (Table). Interobserver agreement for GMH-IVH presence and grading was good (ICC 0.85) before, while excellent (ICC 0.93) after consensus approach implementation. Of the 33 (7%) inconsistencies between observers in GMH-IVH grading, only four (0.9%) involved a grade 3 IVH, of potential clinical significance. Among the 292 infants, associated PVHI, PHVD, cWMI and/or CBH was diagnosed in 88 (30%), 65 (22%), 7 (2%) and 8 (3%) infants, respectively. Interobserver agreement after implementation was excellent (ICC ≥0.92) for all these injury types (Table).
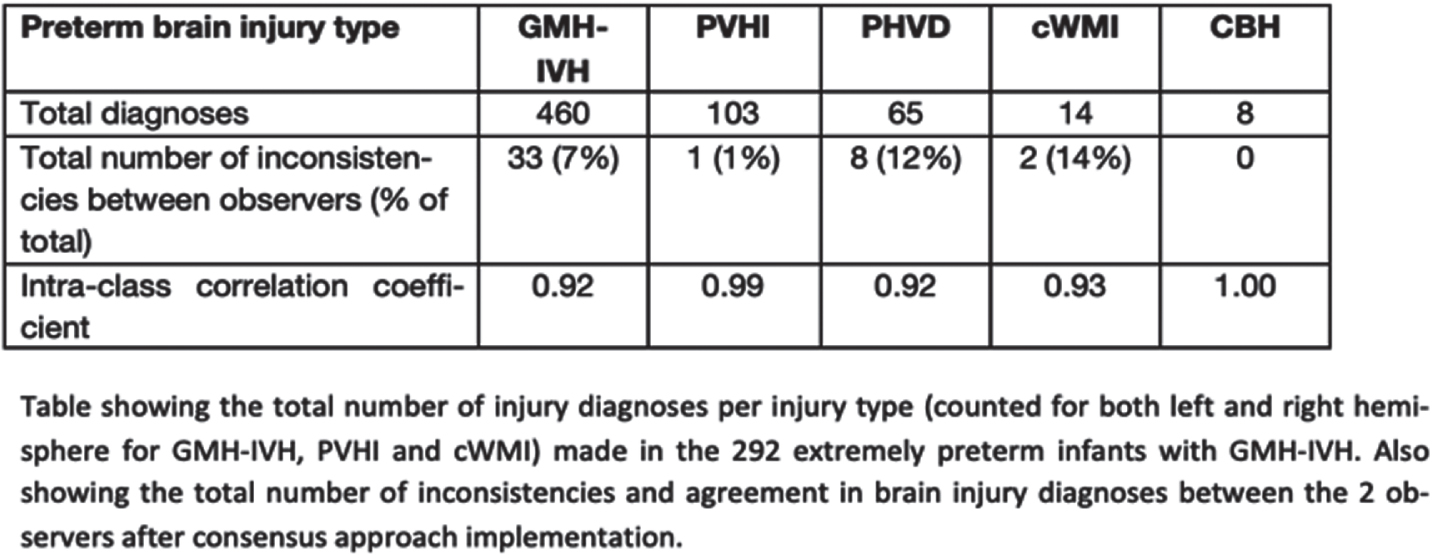
CONCLUSIONS: Our study shows that the development of a consensus approach facilitates a high consistency in diagnosis and grading of common and clinically significant brain injury types from cUS in extremely preterm infants. The consensus approach may contribute to better identification of risk factors for preterm brain injury and therewith improved clinical care. Also, it has the potential of improving prediction of long-term neurodevelopmental outcomes of preterm infants from serial cUS. Further study to this end is required.
Bibliography:
[1] Mohammad K, et al. Consensus Approach for Standardizing the Screening and Classification of Preterm Brain Injury Diagnosed with Cranial Ultrasound: A Canadian Perspective. Front Pediatr. 2021;9:618236
Incidence of brain lesions in moderate-late preterm infants assessed by cranial ultrasound and MRI: The BIMP-study
Boswinkel Va, Nijboer J, Krüse-Ruijter Ma, Wu-Smit Ma, Mulder-de Tollenaer Sa, Boomsma Ma, de Vries Lb,c, Meijler Ga
aIsala, Zwolle, Netherlands
bWilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, Netherlands
cLeiden University Medical Center, Leiden, Netherlands
BACKGROUND/PURPOSE: Moderate-late preterm (MLPT) infants, born at 32 – 36 weeks’ gestation, do not routinely undergo neuro-imaging. The aim of this study was to investigate the incidence and characteristics of brain lesions in MLPT infants using cranial ultrasound (cUS) and magnetic resonance imaging (MRI).
METHODS: Between August 2017 and November 2019, MLPT infants born between 32+0 and 35+6 weeks’ gestation were included in a prospective cohort study that was carried out at Isala Women and Children’s Hospital, Zwolle, the Netherlands. Neuroimaging was performed at three time points: cUS at postnatal day 3–4 (early-cUS), before discharge and repeated at term equivalent age (TEA). In addition, infants underwent an MRI at TEA. CUS and MRI were scored using a scoring system for abnormalities e.g. hemorrhages, white matter and deep gray matter injury, including mild lesions. Furthermore, brain maturation and gyration were visually evaluated. Brain lesions were classified as mild or moderate-severe. Incidences and confidence intervals were calculated.
RESULTS: In total, 166 MLPT infants participated and underwent cUS. Of these, 127 infants underwent MRI. The overall incidence of mild brain lesions was 71.7% (119/166) and of moderate-severe lesions 3.6% (6/166) on cUS and/or MRI. The most frequent lesions were signs suggestive of white matter injury: inhomogeneous echogenicity in 30.5% (50/164) infants at early-cUS, in 8.1% (12/148) infants at TEA-cUS and diffuse white matter signal changes (MRI) in 25.5% (27/127) infants. On MRI, cerebellar hemorrhage was observed in 12.6% (16/127) infants and delayed maturation in 13.4% (17/117) infants. Small hemorrhages and punctate white matter lesions were better detected on MRI than on cUS.
CONCLUSIONS: Mild brain lesions were frequently encountered in MLPT infants, especially signs suggestive of white matter injury and small hemorrhages. Moderate-severe lesions were less frequently seen.
Resistive index in early onset neonatal sepsis
Mishra Aa, Deshmukh La
aGovernment Medical College Aurangabad Maharashtra India, Government Medical College Aurangabad, India
BACKGROUND: Cerebral hemodynamics in early onset neonatal sepsis is complex and poorly understood. There is dearth of adequate knowledge in the literature regarding cerebral blood flow(CBF) changes in early onset neonatal sepsis(EONS). The aim of our study was to study CBF by measuring the resistive index (RI) of the anterior cerebral artery in EONS.
METHODOLOGY: All newborn admitted in our intensive care unit having suspicion of EONS during the period of January 2021 to June 2021 underwent point of care transcranial doppler ultrasonography to measure the RI of the anterior cerebral artery within 24hrs of onset of signs and symptoms before starting inotrope if at all recommended. Neonates with congenital malformations, genetic syndromes, perinatal asphyxia and metabolic disorders were excluded. Babies with positive blood culture were included in the final analysis.
RESULTS: out of 88 suspected EONS, 60 babies were analyzed, of which 25 were culture positive and included in the final study. The mean gestational age was 33.3 ± 2.4 week and the mean birth weight being 1.94 ± 0.77 kg. Most common organism isolated was Escherichia coli (72%) followed by klebsiella pneumoniae ( 14%). Lower RI was documented in 52% (13 of 25), normal in 40% (10 of 25) and variable in 8% (2 of 25) cases.
CONCLUSION: In the present study, we have documented a decrease in RI suggestive of increased CBF in EONS. However, a larger scale of data would help in better understanding of the hemodynamics in EONS.
Value of routine magnetic resonance venography for the diagnosis of cerebral venous sinus thrombosis in neonates with encephalopathy receiving therapeutic hypothermia
Munster Ca, Elshibiny Ha, Szakmar Ea,b, Yang Ec, Inder Ta, El-Dib Ma
aDepartment of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, United States
b1st Department of Pediatrics, Semmelweis University, Budapest, Hungary
cDepartment of Radiology, Boston Children’s Hospital, Harvard Medical School, Boston, United States
BACKGROUND AND PURPOSE: Neonatal encephalopathy (NE) may be associated with increased incidence of cerebral venous sinus thrombosis (CVST), a diagnosis with significant therapeutic implications. A recent study by Radicioni et al. demonstrated that 10 out of 37 asphyxiated neonates (27%) undergoing therapeutic hypothermia (TH) were diagnosed with CVST using magnetic resonance venography (MRV) [1]. For this reason, MRV was routinely added to the imaging protocol for NE infants in a tertiary care center providing TH for neonates with mild, moderate, and severe encephalopathy. We investigated the frequency of CVST diagnoses before and after this change in practice to determine the frequency of CVST and whether routine MRV increased the diagnosis of this entity.
METHODOLOGY: We performed a retrospective chart review for all neonates who received TH from January 2014-March 2020 at Brigham and Women’s Hospital. Demographic and clinical data of the study cohort were compared between those who had an MRV and those who did not have one. MRV was routinely performed for cooled neonates with NE between April 2018 and March 2020. Incidence of CSVT detection was compared before and after implementation of routine MRV.
RESULTS: There were 291 babies cooled during the study period. Demographic and clinical characteristics of the study cohort are provided in Table 1. Before the routine use of MRV, there were 209 babies cooled, and 25 (12%) of them had MRV. Only one baby was diagnosed with CVST in this period, and it was initially detected with MRI and then confirmed with MRV. Following the routine inclusion of MRV in the imaging protocol in April 2018, 82 neonates were cooled. Of these 82, 74 (90%) had MRV performed. None of these neonates were diagnosed with CVST. Out of a total of 99 neonates with MRV, 15 showed severe cerebral veins asymmetry. Representative MRI and MRV scans of the infant with CVST and another infant with asymmetry but no CVST are shown in Figure 1. Case 1 demonstrates the importance of intraluminal signal abnormality (T1 and SWI) as well as vessel expansion in diagnosing CVST. While asymmetry of the transverse/sigmoid sinuses on MRV is a common anatomic variant (Case 2), it might be difficult to differentiate it from CVST in cases with complete absence of flow.
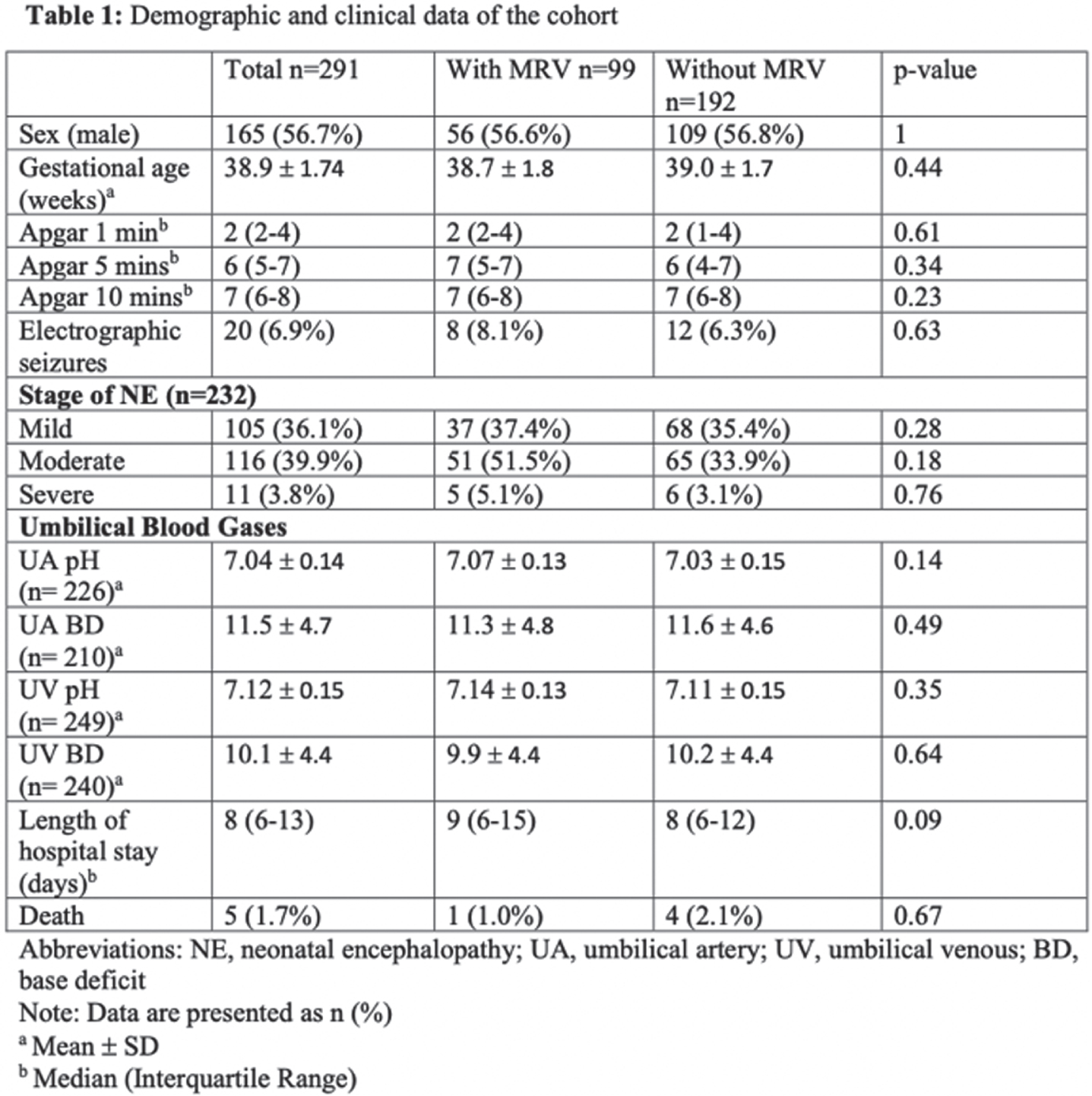
CONCLUSION: Incidence of venous thrombosis in neonates receiving TH for NE may not be as common as previously reported. Routine MRV has not been associated with increased detection of venous thrombosis.
Bibliography:
[1] Radicioni, M., et al., Cerebral Sinovenous Thrombosis in the Asphyxiated Cooled Infants: A Prospective Observational Study. Pediatr Neurol, 2017. 66: p. 63-68.
White matter maturity of female and male extremely preterm neonates - A quantitative MRI study
Schmidbauer Va, Dovjak Ga, Goeral Ka, Klebermass-Schrehof Ka, Berger Aa, Prayer Da, Kasprian Ga
aMedical University Of Vienna, Vienna, Austria
BACKGROUND AND PURPOSE: Former preterm born males are at higher risk for neurodevelopmental disabilities compared to female infants born at the same gestational age (GA) [1]. This study investigated sex-related differences in the maturity of the brainstem and internal capsule regions in infants born before 28 weeks GA, using diffusion-tensor- and relaxometry-based MRI techniques.
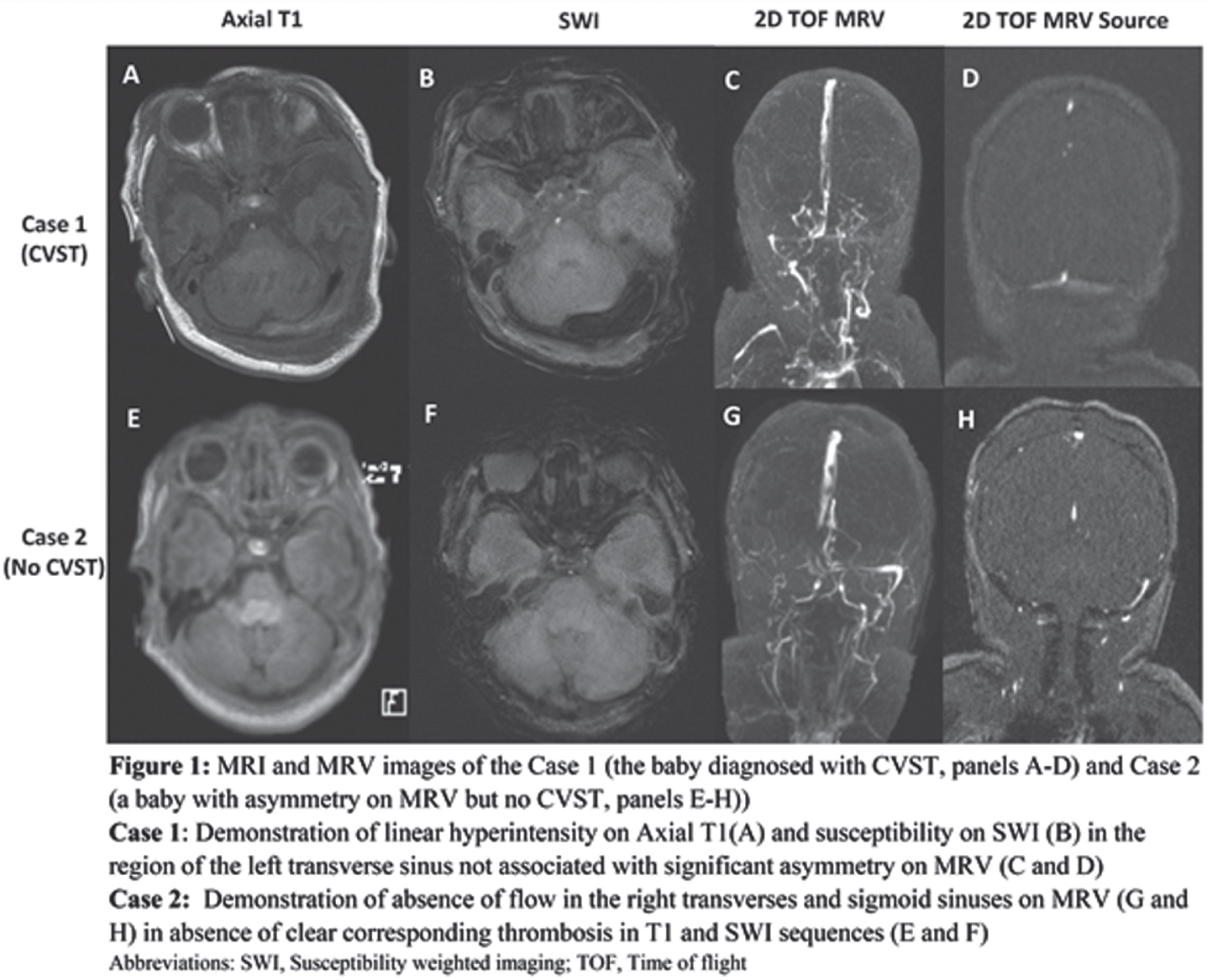
MATERIALS AND METHODOLOGY: In this study, quantitative MRI sequence acquisitions were analyzed in a sample of 35 extremely preterm neonates imaged at term-equivalent ages. Quantitative MRI metrics [fractional anisotropy (FA); ADC (10-3mm2/s); and T1-/T2-relaxation time (T1R/T2R) (ms)] of the medulla oblongata, pontine tegmentum, midbrain and the right/left posterior limb of the internal capsule (PLIC) were determined on diffusion-tensor- and multi-dynamic multi-echo (MDME) sequence-based imaging data [2]. For each brain section of interest, two separate ROIs were drawn at different levels (Figure 1). ANCOVA and a paired t-test were used to compare female and male infants and to detect hemispheric developmental asymmetries.
RESULTS: Seventeen female [mean GA at birth: 26+0 (±1+4)] and 18 male [mean GA at birth: 26+1 (±1+3)] infants were enrolled in this study.
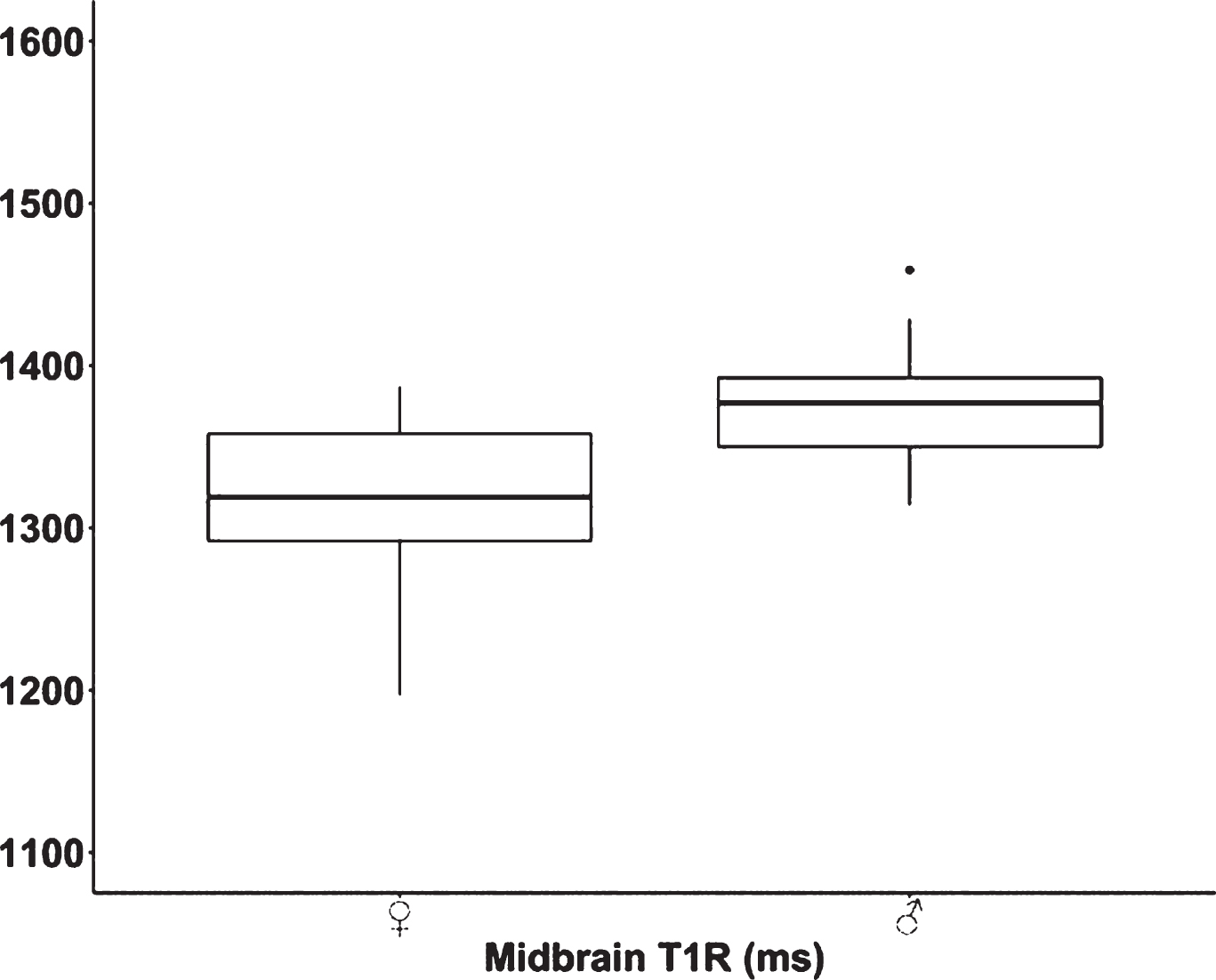
Significant differences were observed in T1R of the midbrain between female and male neonates (p<.001) (Figure 2). In both sexes, FA [p(♀)<.001/p(♂)<.001], ADC [p(♀)<.001/p(♂)<.001], and T1R [p(♀)<.001/p(♂)<.001] differed significantly between the right and left PLIC.
CONCLUSION AND IMPACT: T1R and diffusion-tensor metrics are sensitive markers for the detection of sex-related and interhemispheric differences of white matter maturity. The midbrain of male preterm neonates is more immature compared to female infants at term-equivalent ages. Sex differences in white matter maturation need further attention for the personalization of neonatal brain imaging.
Bibliography:
[1] Skiöld B, Alexandrou G, Padilla N, et al. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr 2014;164:1012–1018
[2] Schmidbauer V, Geisl G, Diogo M, et al. SyMRI detects delayed myelination in preterm neonates. Eur Radiol 2019;29:7063–7072
Collaborative curriculum for neonatal neuroradiology conferences
Sewell Ea, Milla Sb, Kadom Na
aEmory University; Children’s Healthcare of Atlanta, Atlanta, United States
bChildren’s Hospital Colorado, Aurora, United States
PURPOSE: Traditionally, some neonatal intensive care units briefly review neuroimaging cases with a radiologist on a regular schedule as part of routine clinical care. Additionally, trainees may rotate through radiology as a visitor in the reading room. The trainee education during these experiences can be highly variable and depends on the ability and willingness of the radiologist to engage the trainee, as well as the variety and relevance of cases occurring on any given day. We aimed to create a formal collaborative neuroimaging curriculum between pediatric neuroradiology and neonatal medicine geared towards neonatology fellows that was clinically relevant and reviewed key board concepts.
METHODS AND MATERIALS: This program engages a single pediatric neuroradiologist and neonatologist throughout the fellowship year and focuses on neuroimaging studies in neonates. This curriculum for neonatology fellows was implemented in 2018. Between 7-8 neonatology fellows participate each year. The curriculum consists of four 1-hour sessions throughout the academic year. The American Board of Pediatrics (ABP) Neonatal-Perinatal Medicine board content outline specifications for neuroimaging and neurological disorders were used to create the curriculum.
RESULTS: Fifteen neonatology fellows have participated since the beginning of the program. A total of 12 sessions have been taught to date. The attending time effort per year was 10 hours for the neonatology attending (session case selection, scheduling, session attendance, session feedback review) and 6 hours for the pediatric neuroradiologist (30 min/session case review prior to presentation, session attendance). The curriculum consisted of preparatory reading relevant to the session topic, case studies focusing on key neuroimaging findings, and relevance of imaging to clinical management. The sessions evolved to the following format: (1) head US with focus on preterm brain injury; (2) brain MRI with focus on hypoxic-ischemic encephalopathy; (3) brain MRI with focus on other brain injury in the term infant including stroke and congenital malformations); and (4) fellow case presentations including neuroimaging with attending feedback. Standardized evaluations consisting of Likert scale and open-ended questions were collected intermittently starting in 2019; a sample is provided in Figure 1. A median of 4 evaluations were completed on average for 6 sessions (22 total evaluations). 100% of evaluators agreed or strongly agreed that the sessions delivered important teaching topics and that the presentation was practical and useful. The most common suggested improvement was alterations to the session format.

CONCLUSIONS: ABP certification in Neonatal-Perinatal Medicine requires that neonatology fellows understand the indications and limitations of specific neuroimaging studies and gain competence in interpreting imaging studies and major patterns of injury. Real-life case studies with targeted incorporation of neuroimaging and high-yield neurological disorders are an effective teaching method for adult learners. This knowledge will aid future neonatologists who may practice in hospitals without trained pediatric radiologists.
Diffusion tensor tractography of the corticospinal tracts in neonates after hypoxic ischemic encephalopathy
Eshel Da, Shelef Ia,b, Abramsky Ra,c, Eshel Ra,d, Novoa Ra,b, Golan Aa,c, Marks Ka,c, Shany Ea,c
aFaculty of Health sciences, Be’er Sheva, Israel
bRadiology Department, Soroka Medical Center, Be’er Sheva, Israel
cNeonatal Department, Soroka Medical Center, Be’er Sheva, Israel
dClinical Research Center, Soroka Medical Center, Be’er Sheva, Israel
BACKGROUND AND PURPOSE: Neonatal Hypoxic Ischemic Encephalopathy (HIE) is a major cause of neurologic disabilities in term neonates. MRI scans of infants who suffered from HIE is commonly used to predict long term neurodevelopmental outcome [1-5]. Diffusion tensor imaging (DTI) is a quantitative MRI technique that measures the magnitude and direction of water molecules movement in a medium which are characterized respectively by the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) [6-8]. Diffusion tensor tractography (DTT) is a validated fiber-tracking method that utilizes DTI generated vectors to visualize and model neuronal fiber tracts within the brain [9,10]
HYPOTHESIS: Cerebral tracts’ diffusion parameters are negatively affected by the HIE-related neurological insult and can be used to predict long term neurodevelopmental outcome.
MAIN OBJECTIVE: Evaluate the associations of corticospinal tracts’ diffusion values with the degree of MRI brain injury in infants with neonatal HIE. Secondary objectives were to evaluate the associations of the tract’s diffusion values with cerebral activity in the first week of life.
METHODOLOGY: Retrospective study of a prospective cohort of infants with neonatal HIE admitted to the Soroka Medical Center NICU between 2014-2020. Included were infants underwent an MRI scan prior to discharge and within the first 10 days of life. Scans were prospectively scored for evidence of brain injury according to the Rutherford et al. severity scoring system that was modified to include a diffusion weighted sequence [11,12]. Explore DTI software [13] streamline deterministic method was used for CST tractography (figure 1). Volume, fractional anisotropy (FA) and apparent diffusion coefficient (ADC) data were extracted. Neonatal data for seizures, abnormal aEEG, abnormal neurologic status at discharge, or death was retrieved.
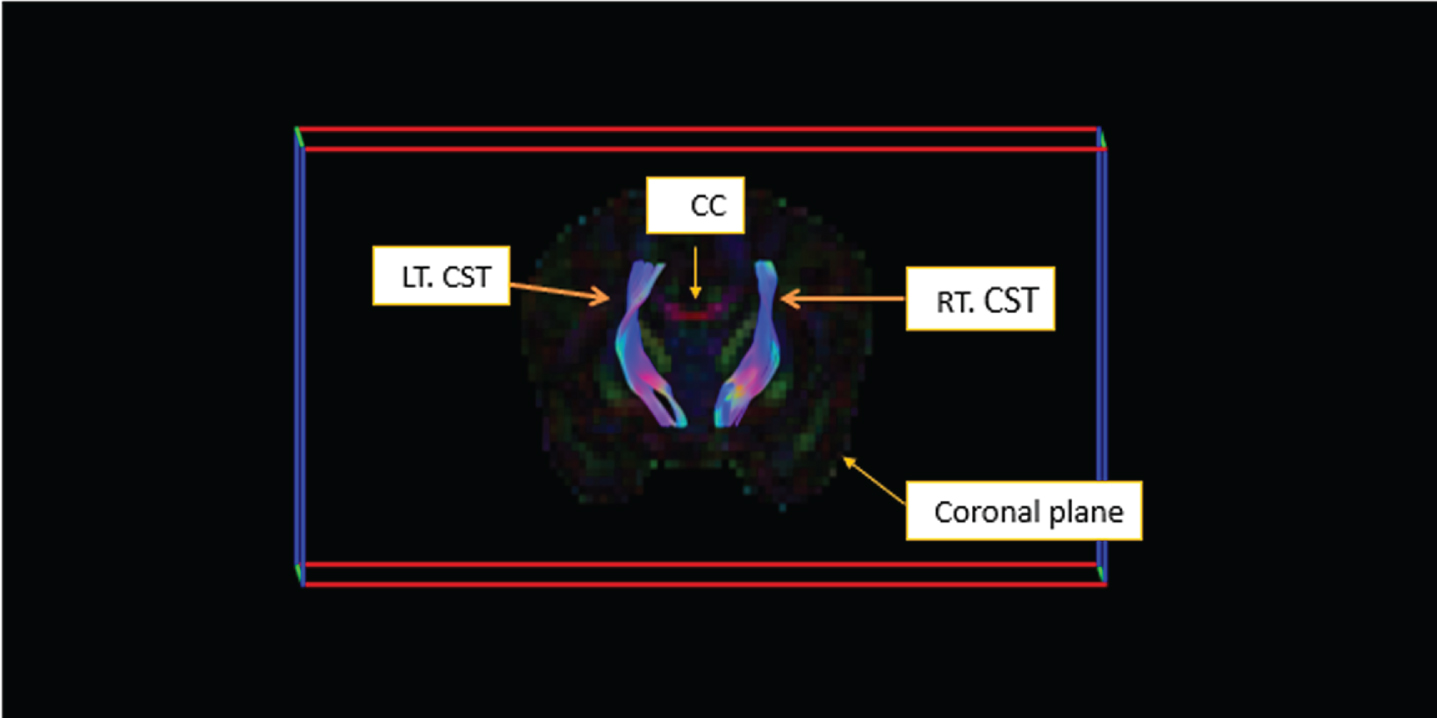
RESULTS: Sixty-six infants were included in the study with a median gestational age of 40W+1D. Significant association was found between a worse MRI score and a longer stay in the ICU (8 vs. 9 vs. 20, p=0.002), seizures (p=0.012), abnormal aEEG background (p<0.001), abnormal neurologic status at discharge (2.6% vs. 36.4% vs. 55.6%, p<0.001) and death (p=0.001). A significant negative association was found between a higher MRI category score, tract volume and diffusion values (Table 1). These associations remained significant after adjustment for infants’ gestational age, age at MRI scan (in days) and aEEG seizures.
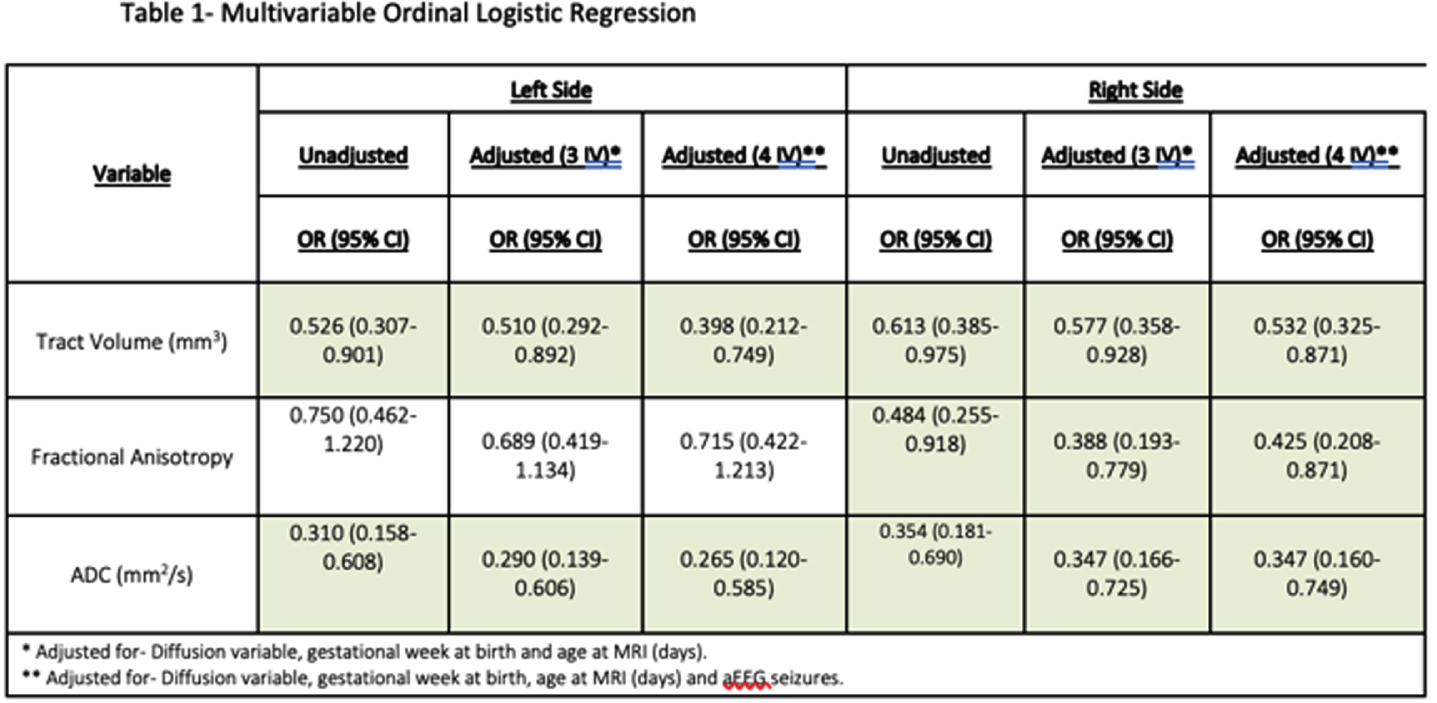
CONCLUSIONS: We demonstrated that a decrease in diffusion values: ADC bilaterally and FA on the left side, was significantly associated with a higher likelihood of worse MRI category scores. These findings support a possible prognostic role of the CST tracts diffusion values early after birth. More work is needed t in order to assess the association of tracts’ diffusion values with long term outcome in neonatal HIE.
Bibliography:
[1] Twomey E, Twomey A, Ryan S, Murphy J, Donoghue VB. MR imaging of term infants with hypoxic-ischaemic encephalopathy as a predictor of neurodevelopmental outcome and late MRI appearances. Pediatr Radiol. 2010;40(9):1526–35.
[2] Hayes BC, Ryan S, Mcgarvey C, Mulvany S, Doherty E, Grehan A, et al. Brain magnetic resonance imaging and outcome after hypoxic ischaemic encephalopathy. J Matern Neonatal Med. 2016;29(5):777–82.
[3] Laptook AR, Shankaran S, Barnes P, Rollins N, Do BT, Parikh NA, et al. Limitations of Conventional Magnetic Resonance Imaging as a Predictor of Death or Disability Following Neonatal Hypoxic-Ischemic Encephalopathy in the Late Hypothermia Trial. J Pediatr. 2020 Nov;
[4] Goergen SK, Ang H, Wong F, Carse EA, Charlton M, Evans R, et al. Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: interobserver agreement and MRI predictors of outcome at 2 years. Clin Radiol. 2014 Jan;69(1):72–81.
[5] Rutherford MA, Pennock JM, Counsell SJ, Mercuri E, Cowan FM, Dubowitz LM, et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics. 1998 Aug;102(2 Pt 1):323–8.
[6] Mori S, Zhang J. Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research. Neuron. 2006 Sep;51(5):527–39.
[7] Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–67.
[8] Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. J Magn Reson. 1996;213(2):560–70.
[9] Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in Vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94.
[10] Stieltjes B, Kaufmann WE, Van Zijl PCM, Fredericksen K, Pearlson GD, Solaiyappan M, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14(3):723–35.
[11] Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39–45.
[12] Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, et al. Patterns of Brain Injury in Neonates Exposed to Perinatal Sentinel Events. Pediatrics. 2008;121(5):906–14.
13) Leemans A, Jeurissen B, Sijbers J, Jones D. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc 17th Sci Meet Int Soc Magn Reson Med. 2009;17(2):3537.
Characterization of MRI-defined short-term outcomes for infants evaluated for but not treated with therapeutic hypothermia
Thiim Ka, Lee Sa, Singh Ea, Steele Ta, Elshibiny Ha, Inder Ta, El-Dib Ma
aBrigham and Women’s Hospital, Boston, United States
BACKGROUND AND PURPOSE: Therapeutic hypothermia (TH) is the standard of care for neonatal encephalopathy (NE). 1In recent years, many centers, including ours at Brigham and Women’s Hospital (BWH), have broadened their TH treatment criteria to include infants with mild NE. Our protocol uses both evidence of perinatal insult and a quantitative Neonatal Encephalopathy Score (NES) of four or more to decide on TH treatment eligibility. Amplitude integrated EEG (aEEG) is used as an adjunct where only infants with a continuous normal voltage (CNV) are excluded from cooling.2 Whether these criteria, inclusive of mild encephalopathy, are capable of screening those with no brain injury who might benefit from TH is unknown. Our primary aim was to evaluate short term MRI outcomes in infants evaluated for TH.
METHODOLOGY: We enrolled infants who were screened for but not treated with TH between April 2019 and August 2021. All brain MRIs were obtained within the first week of life at a BWH in-hospital scanner. Clinical MRI reports were used in this analysis.
RESULTS: Thirty-nine infants enrolled in the study had a mean gestational age of 39.0 ± 1.4 weeks. Their detailed demographic and clinical characteristics are reported in Table 1. Of the 39 infants, 34 (87.2%) had no evidence of acute perinatally acquired hypoxic ischemic brain injury. Of note, three of these infants had evidence of chronic punctate white matter injury, and one infant showed an incidental finding of a medullary tumor. Five infants (12.8%) had MRI findings suggestive of acute brain injury displayed by acute diffusion weighted imaging. Three of these cases were small foci of restricted diffusion in the fronto-parietal white matter. The fourth case displayed restriction focally in the left posterior cerebral artery territory. The fifth case displayed acute watershed ischemic injury most notably in the left parieto-occipital lobe. Clinical characteristics of these five cases, along with their MRI findings, are described in Table 2 and displayed in Figure 1. Notably, cases 4 and 5 had normal centro-parietal aEEG on initial evaluation with a posterior injury pattern on MRI.
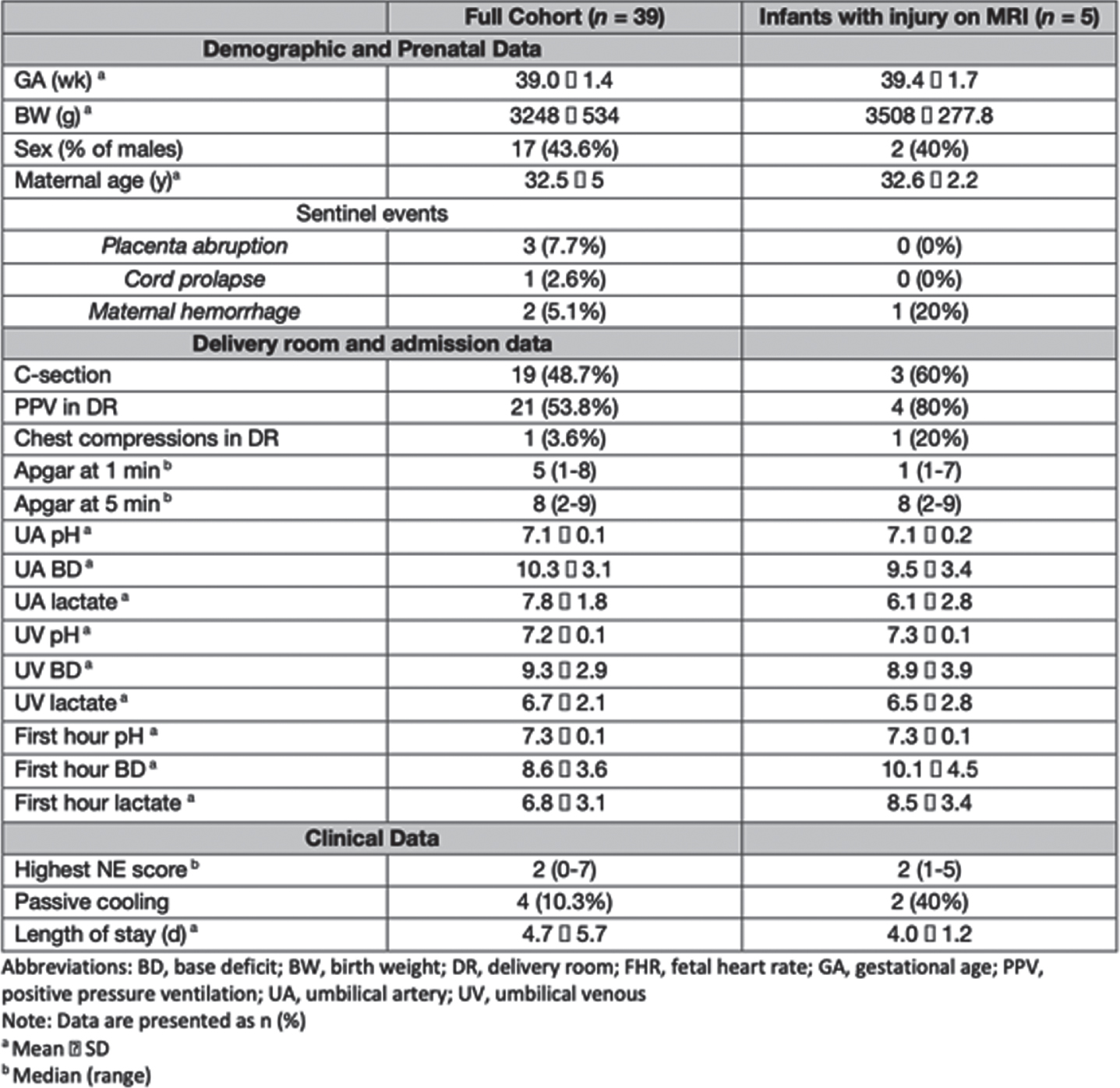
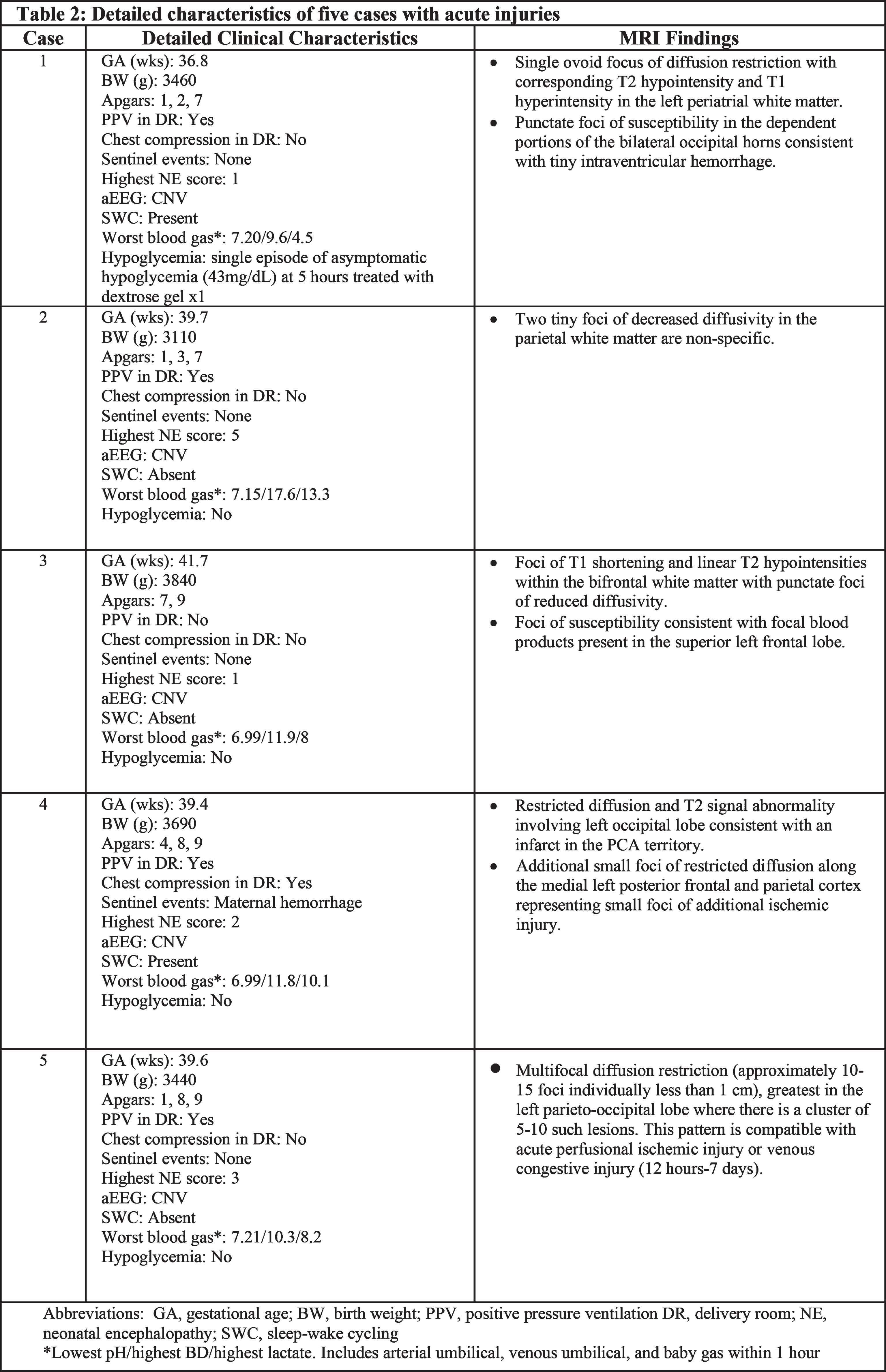
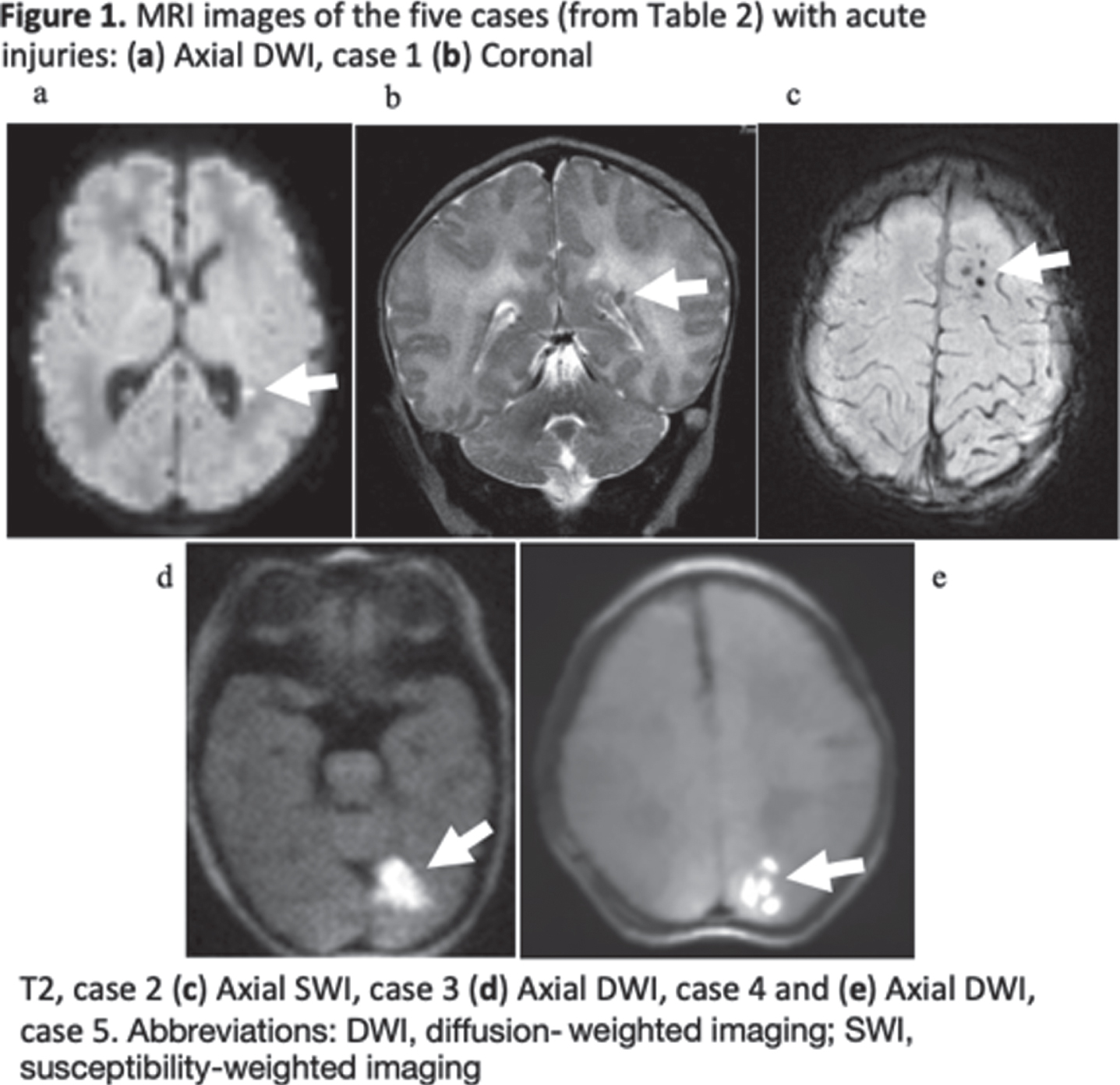
CONCLUSION(S): These results suggest that the current expanded TH criteria, inclusive of mild NE at a threshold on a systematic neurological examination, is effective at detecting most infants with acute hypoxic ischemic injuries. Notably, two infants with more significant injury displayed a posterior predominant lesion suggesting that clinical exam and aEEG may be less reliable for such focal lesions. Application of neuroimaging in infants considered for cooling but not receiving treatment remains worthy of consideration.
Bibliography:
01) Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. The Cochrane database of systematic reviews. 2013;1:CD003311.
02) Brigham and Women’s Hospital TH Clinical Practice Guidelines. https://www.brighamandwomens.org/assets/BWH/pediatric-newborn-medicine/pdfs/th-cpg-amended.pdf
Investigating functional connectivity in the newborn brain cot-side using wearable high-density diffuse optical tomography
Uchitel Ja,c, Blanco Bb, Collins-Jones Lc, Porter Ed, Edwards Ad, Cooper Rc, Austin Td
aDepartment of Paediatrics, University of Cambridge, Cambridge, United Kingdom
bDepartment of Psychology, University of Cambridge, Cambridge, United Kingdom
cDOT-HUB, Department of Medical Physics and Biomedical Optics, University College London, London, United Kingdom
dNeonatal Intensive Care Unit, Rosie Hospital, Cambridge NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, United Kingdom
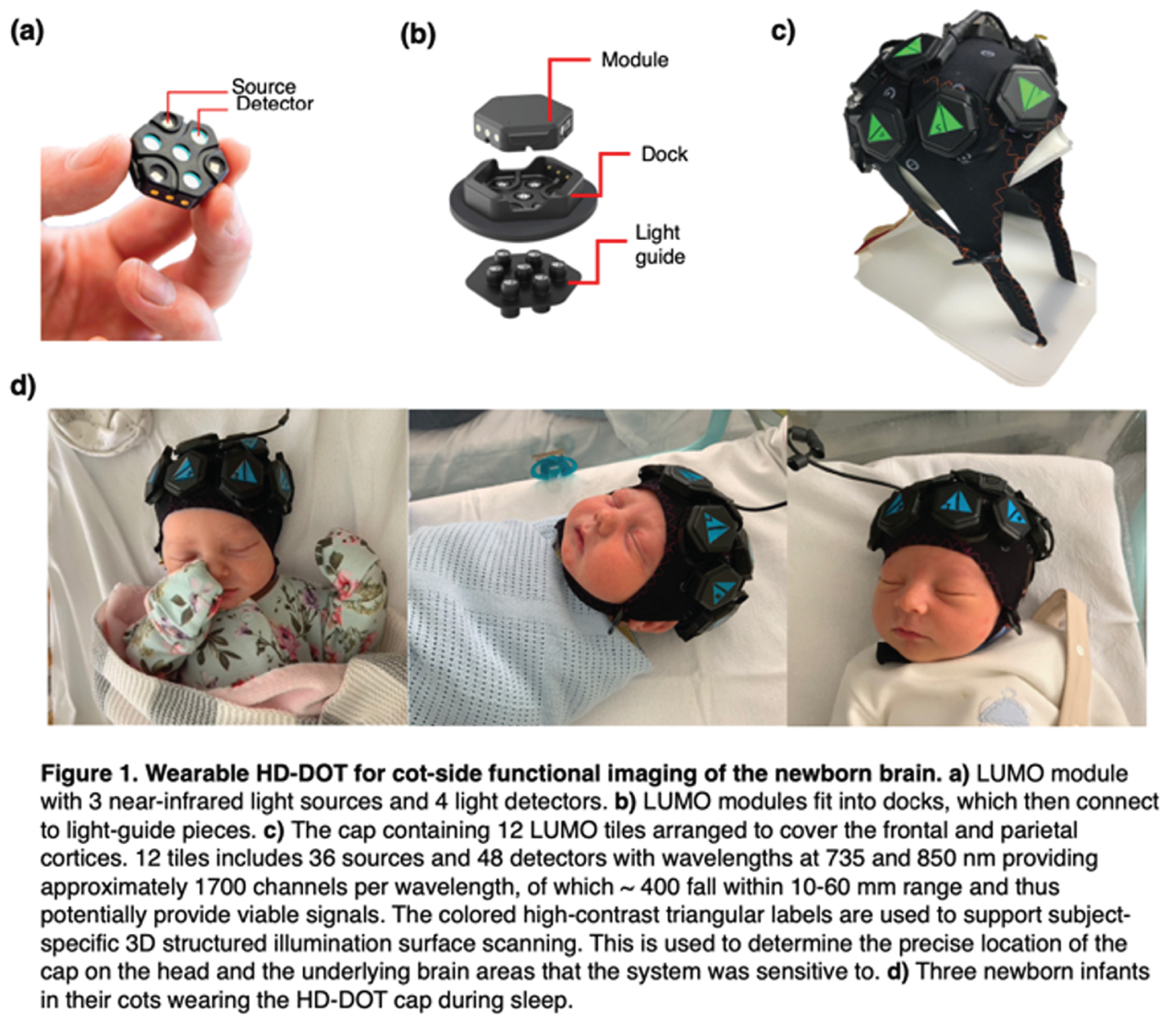
BACKGROUND/OBJECTIVE: Sleep is an important biomarker of brain development for newborn infants. Newborn infants possess two sleep states: active sleep (AS) and quiet sleep (QS). Like sleep, functional brain connectivity (FC) is tightly linked to brain development, yet the relationship between sleep and FC is poorly understood [1]. This relationship is difficult to investigate using traditional neuroimaging approaches, which often require sedation and cannot be performed cot-side. This study aimed to 1) investigate FC in newborn infants across infant sleep states using high-density diffuse optical tomography (HD-DOT) and 2) demonstrate the utility of a new generation of modular wearable HD-DOT for non-invasive, cot-side brain imaging in clinical settings.
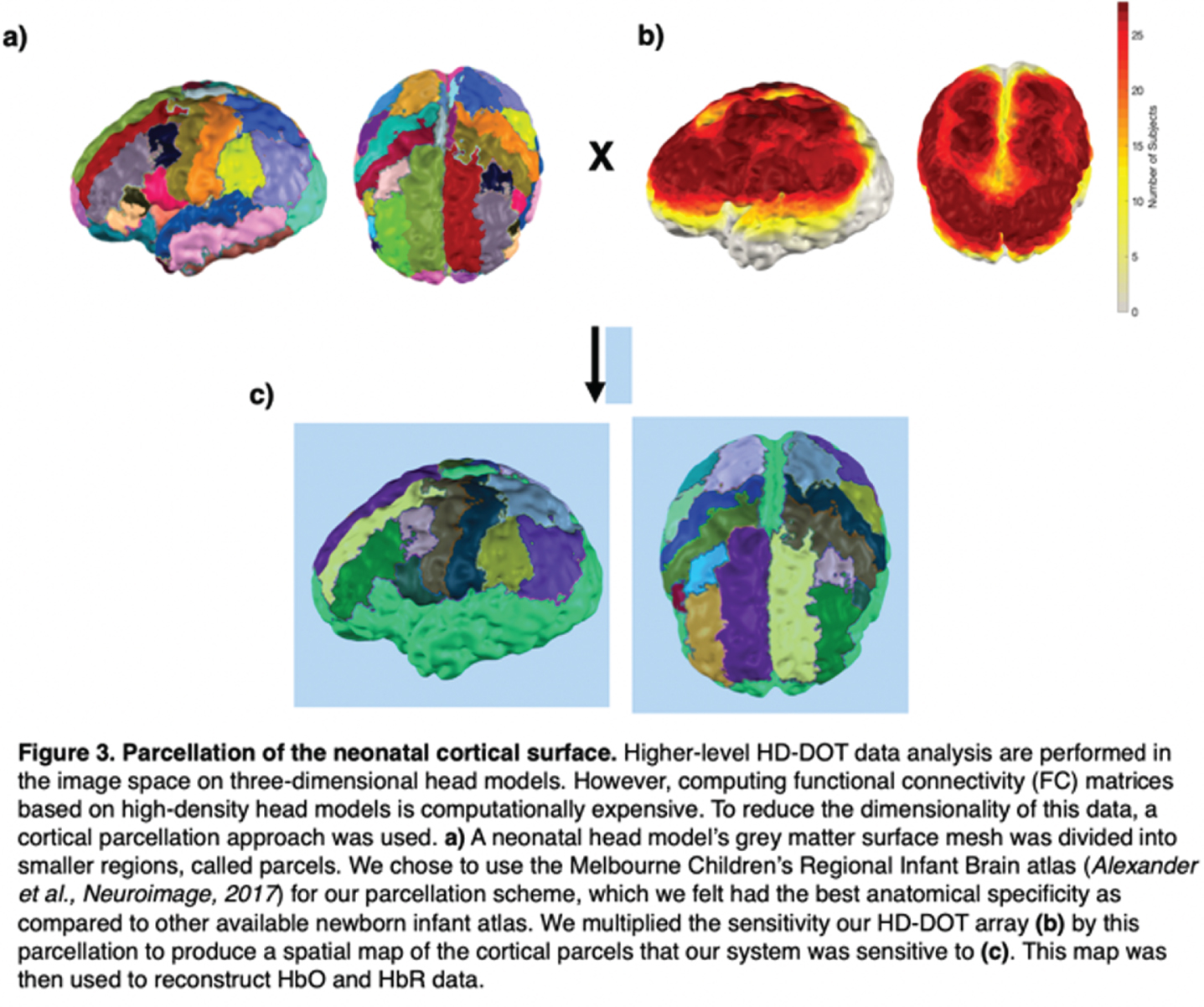
METHODS: We applied a 12-module wearable HD-DOT system (LUMO, Gowerlabs Ltd.) to study 45 healthy term-born newborn infants during natural sleep (Fig. 1). Video recording of behavior was taken for scoring of sleep state. Three-dimensional structured-illumination images of the head surface were also taken for identification of cranial landmarks, source, and detector positions. A common neonatal head model was registered to these positions, resulting in a 3D mesh model for each subject. From recordings, clean data segments that were at least 3 mins in duration were extracted and preprocessed (Fig 2). Preprocessed data segments were then reconstructed to produce cortical surface images of changes in oxygenated and deoxygenated hemoglobin (HbO and HbR) concentrations. In this image space, HbO and HbR data were parcellated using the neonatal M-CRIB atlas [2] to reduce data dimensionality and determine the underlying brain regions that the system was sensitive to (Fig. 3). Group-level functional connectivity matrices and connectome-based independent component analysis (connICA) [3] were computed based on parcellated data to further explore the presence of FC.
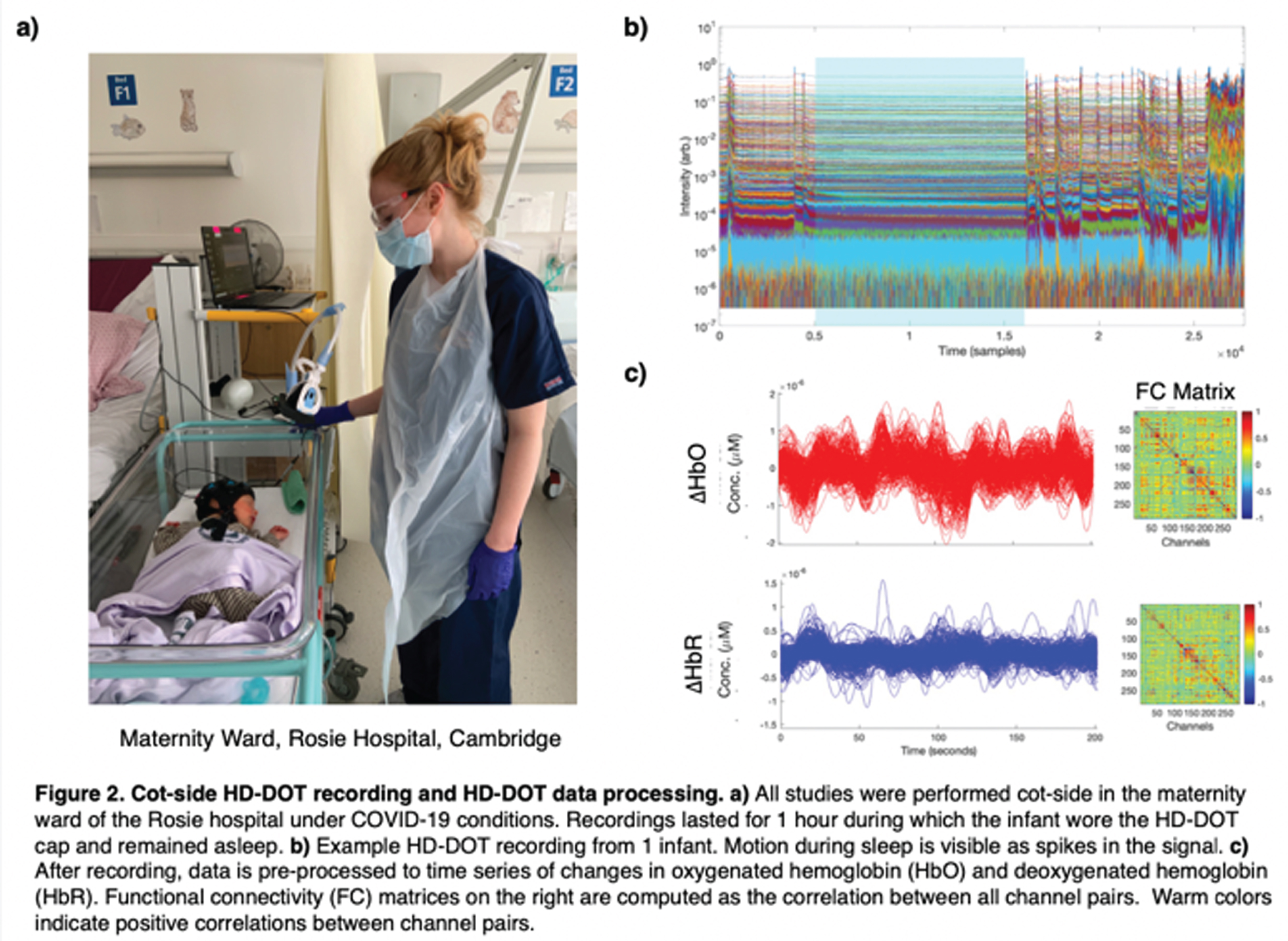
RESULTS: Of the 45 infants, 28 had at least one clean data segment (62% subject retention rate) of at least 3 mins (mean: 5.2±3.0). Of the 63 segments, 18 were during AS (mean: 4.2±1.1 min), 40 were QS (mean: 5.6±3.7 min), and 4 were in transitional sleep. Group-level functional correlation matrices using these segments also support the presence of FC in this dataset given strong correlation between HbO-HbO and HbR-HbR (Fig. 4). Preliminary connICA analysis of data during quiet sleep demonstrates the presence of significant interhemispheric connectivity (Fig. 4).
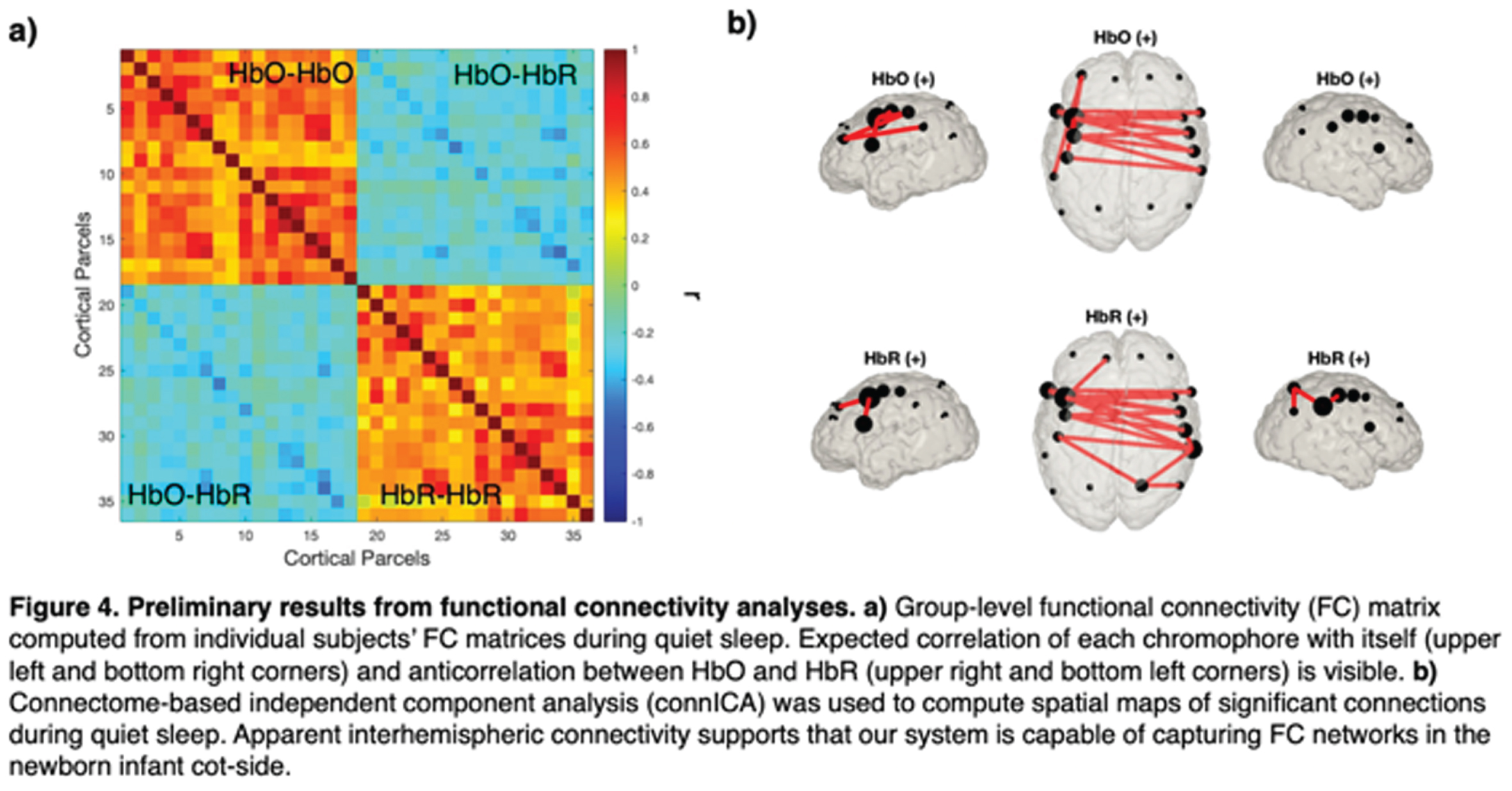
CONCLUSION: Preliminary results support that HD-DOT is a suitable and well-tolerated method to obtain high-quality FC data in newborn infants in clinical settings. Additional higher-level analyses currently underway are spatial independent component analysis (spatial ICA), network-based statistics, and statistically comparing FC features across AS and QS. Pilot studies of this system on preterm infants are also currently underway using an 8-module LUMO system.
[1] Lee et al., Front. Neurosci., 2020
[2] Alexander et al., Neuroimage, 2017
[3] Amico et al., Neuroimage, 2017.
Improved segmentation of neonatal brain MRI scans by addressing motion artifacts with data interpolation
Verschuur Aa,b, Boswinkel Vc,d, Tax Cb,e, van Osch Ja, Nijholt Ia, Slump Cf, de Vries Lg,h, van Wezel-Meijler Gc, Leemans Ab, Boomsma Ma
aDepartment of Radiology, Isala Hospital, Zwolle, Netherlands
bImage Sciences Institute, University Medical Centre Utrecht (UMCU), Utrecht, Netherlands
cDepartment of Neonatology, Isala Women and Children’s Hospital (IVKC), Zwolle, Netherlands
dUMC Utrecht Brain Center, University of Utrecht, Utrecht, Netherlands
eCardiff University Brain Research Imaging Centre, Cardiff, United Kingdom
fDepartment of Robotics and Mechatronics, University of Twente, Enschede, Netherlands
gDepartment of Neonatology, Wilhelmina Children’s Hospital, Utrecht, Netherlands
hDepartment of Neonatology, Leiden University Medical Centre (LUMC), Leiden, Netherlands
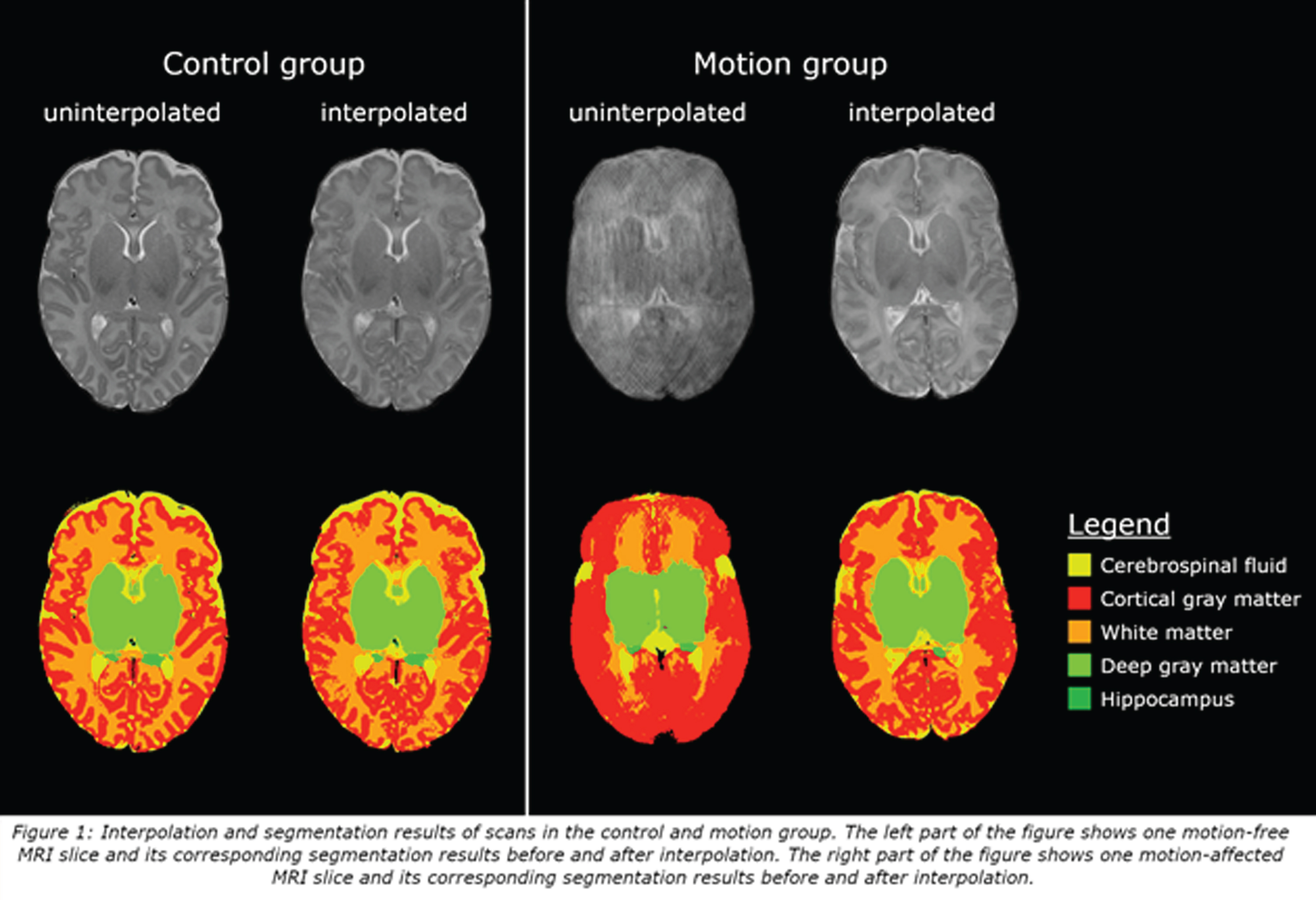
BACKGROUND: Motion artifacts are a common problem in neonatal brain MRI and may negatively affect segmentation. The purpose of this study was to investigate whether motion-affected slices can be replaced by interpolated slices to enhance segmentation of neonatal brain MRI scans.
METHODS: From August 2017 to November 2019, moderate-late preterm infants were enrolled in a prospective cohort study entitled Brain Imaging in Moderate-late Preterm infants (BIMP-study). Around term equivalent age, MRI of the brain was performed using a 3 Tesla MRI. T2-weighed (voxel size 0.35x0.35x2mm) transverse images were automatically segmented into eight brain structures with a neonatal segmentation toolbox [1]. Upon visual inspection, scans with motion artifacts that affected segmentation (25/112; motion group) and scans without motion artifacts (27/112; control group) were selected and used for analysis. Slices with motion artifacts were re-estimated using shape-preserving cubic spline interpolation [2, 3], followed by automatic segmentation of the interpolated scan. Analysis was performed in three stages. Firstly, scans from the control group were used to test interpolation reliability: 18/54 axial slices of these scans were interpolated. Segmentation results of uninterpolated and interpolated scans were compared using the Sørensen-Dice coefficient. Secondly, uninterpolated and interpolated volumes of the motion group were compared using the Wilcoxon Signed-Ranks test. Thirdly, interpolated volumes of the motion group were compared to uninterpolated volumes of the control group using the Mann-Whitney U test.
RESULTS: In the control group, Sørensen-Dice coefficients ranged between 0.87 and 0.97. In the motion group, interpolation resulted in a significant decrease of cortical (Z=-2.9, p=0.004) and deep gray matter (Z=-3.30, p<0.001), and a significant increase of white matter (Z=2.84, p=0.005) volumes. No significant differences were found between interpolated volumes of the motion group and uninterpolated volumes of the control group.
CONCLUSION: Shape preserving cubic spline interpolation enables reliable segmentation of motion-affected MRI scans in moderate-late preterm infants.
References:
[1] Beare RJ, Chen J, Kelly CE, et al. Neonatal Brain Tissue Classification with Morphological Adaptation and Unified Segmentation. Front Neuroinform 2016;10:12. DOI:10.3389/fninf.2016.00012.
[2] Wolberg G, Alfy I. Monotonic Cubic Spline Interpolation. Proceedings Computer Graphics International 1999:188-195. DOI: 10.1016/S0377-0427(01)00506-4 .
[3] Fritsch FN, Carlson RE. Monotone Piecewise Cubic Interpolation. SIAM J Numer Anal 1980;17(2):238-246. DOI:10.1137/0717021.
Mild brain lesions do not affect brain volumes in moderate-late preterm infants
Boswinkel Va,b, Verschuur Ac,d, Nijholt Ic, van Osch Jc, Nijboer-Oosterveld Jc, Beare Re,f, Slump Cg, de Vries Lh,i, Boomsma Mc, van Wezel-Meijler Ga
aDepartment of Neonatology, Isala Women and Children’s Hospital (IVKC), Zwolle, Netherlands
bUMC Utrecht Brain Center, University of Utrecht, Utrecht, Netherlands
cDepartment of Radiology, Isala Hospital, Zwolle, Netherlands
dImage Sciences Institute, University Medical Centre Utrecht (UMCU), Utrecht, Netherlands
eMurdoch Children’s Research Institute, The Royal Children’s Hospital, Melbourne, Australia
fDepartment of Medicine, Monash Medical Centre, Monash University, Melbourne, Australia
gDepartment of Robotics and Mechatronics, University of Twente, Enschede, Netherlands
hDepartment of Neonatology, Wilhelmina Children’s Hospital, Utrecht, Netherlands
iDepartment of Neonatology, Leiden University Medical Centre (LUMC), Leiden, Netherlands
BACKGROUND: The influence of frequently occurring mild brain lesions on brain volumes in moderate (MP; 32+0-33+6 weeks’ gestation) and late (LP; 34+0-35+6 weeks’ gestation) preterm infants is unknown. The purpose of this study was to investigate the effect of mild brain lesions on brain volumes in moderate-late preterm (MLPT) infants and to compare brain volumes between MP and LP infants.
METHODS: Eligible MLPT infants born in Isala Women and Children’s Hospital were enrolled in a prospective cohort study (Brain Imaging in Moderate-late Preterm infants ‘BIMP-study’) between August 2017 and November 2019. Term equivalent age MRI was performed using a 3T MRI system. MRI scans were assessed for brain lesions by three investigators in consensus. Automatic segmentation of eight brain structures was performed using T2-weighted images and an adapted version of MANTiS (Morphologically adaptive neonatal tissue segmentation toolbox). Absolute and relative (proportion of intracranial volume) brain volumes were compared between infants with and without mild brain lesions and between MP and LP infants using linear regression analysis. Analyses were adjusted for postmenstrual age and weight at MRI. Statistical significance levels were set at p<0.05.
RESULTS: In total, 104 infants were included in this part of the BIMP-study (68 with and 36 without mild brain lesions; 36 MP and 68 LP). Univariate analyses showed significantly larger intracranial (B=27.4cm3, p=0.02), cerebrospinal fluid (B=8.78cm3, p=0.01) and cerebellar volumes (B=1.70cm3, p=0.03) in infants with mild brain lesions compared to infants without mild brain lesions. Multivariate analyses were not significant. Mean brain volumes were larger in LP infants compared to MP infants, but differences were not significant. Relative brain volumes were not significantly different in both analyses.
CONCLUSION: Neither having mild brain lesions, nor being born moderately prematurely has a significant effect on brain volumes at term equivalent age in MLPT infants.




