Incidence of congenital complications related to COVID-19 infection during pregnancy
Abstract
PURPOSE:
Infection with COVID-19 during pregnancy has been associated with a hypercoagulable state. It is unknown if maternal COVID-19 infection results in congenital anomalies secondary to intrauterine vascular accidents. This study sought to determine if the rate of in-utero vascular complications (intestinal atresia and limb abnormalities) that may be attributable to the hypercoagulable states associated with COVID-19 and pregnancy increased after the onset of the pandemic.
METHODS:
Pregnancy, neonatal, and congenital defect data from a single academic medical center and the partner’s children’s hospital were collected and compared to the period prior to onset of the pandemic. A subanalysis including pregnant woman 18 years or greater with documented COVID-19 infection during gestation between March 2020-2021 was performed.
RESULTS:
Rates of intestinal atresia did not differ prior to or after the onset of the pandemic (3.78% vs 7.23%, p = 0.21) nor did rates of limb deficiency disorders (4.41% vs 9.65%, p = 0.09). On subanalysis, there were 194 women with COVID-19 infection included in analysis: 135 (69.6%) were positive during delivery admission and 59 (30.4%) were positive earlier in their pregnancy. There was one infant born with intestinal atresia.
CONCLUSION:
We report a low incidence of congenital anomalies in infants born to mothers with COVID-19 infection. It remains unclear if the impact of COVID-19 on the coagulative state augments the normal pro-thrombotic state of pregnancy; ongoing surveillance is warranted.
1Introduction
Since the onset of the coronavirus 2019 (COVID-19) global pandemic, millions of pregnant women have been exposed to and infected with the virus. Given that pregnant women and fetuses are considered vulnerable populations, substantial research has focused on the impact of COVID-19 on pregnancy and complications for the fetus and newborn. Many recent studies demonstrate a low incidence of vertical transmission of COVID-19, with less than 5% of neonates with in-utero exposure to COVID-19 demonstrating clinical evidence of infection immediately after birth; however, there are a few case reports documenting perinatal infection [1–4]. Even in the absence of vertical transmission of the virus to the fetus, maternal infection has a variety of negative implications on the health of the developing fetus.
Prior studies have shown neonates born to mothers infected with COVID-19 have increased rates of respiratory disorders, invasive ventilation following birth, hyperbilirubinemia, as well as higher rates of preterm delivery [4–6]. There have not been reports of a teratogenic effect of COVID-19. Studies assessing the placental pathology from COVID-19 mothers have demonstrated evidence of fetal vascular malperfusion, which is associated with fetal vascular thrombosis and maternal hypercoagulable states [7–9]. Intestinal atresias, specifically ileal, jejunal and colonic atresias, are thought to be secondary to intrauterine vascular accidents and evidence has suggested placental vascular compromise may be implicated [10, 11]. Additionally, congenital limb deficiency defects, which are known to have multiple etiologies, are also thought to be due to in-utero vascular accidents in some instances [12, 13]. Any association between hypercoagulable state from maternal infection of COVID-19 and incidence of congenital anomalies secondary to intrauterine vascular accidents has yet to be investigated.
Both pregnancy and COVID-19 are associated with pro-thrombotic states [14, 15]. We hypothesize that there is a synergistic relationship of pregnancy prothrombotic state as well as COVID-19 related hypercoagulability leading to increased congenital gastrointestinal and limb anomalies. This study sought to therefore evaluate if the incidence of in-utero vascular complications in the developing fetus increased after the onset of the COVID-19 pandemic, which could indicate there is a synergistic relationship between to the hypercoagulable states associated with COVID-19 infection and pregnancy.
2Material and methods
This was a prospective, multi-institutional observational study with a retrospective data collection component performed at Children’s Hospital Colorado (CHCO) and University of Colorado Hospital (UCH). Infants born at UCH with surgical congenital anomalies are transferred to CHCO (approximately 0.25 miles away).
Overall rates of congenital anomalies of interest were abstracted from the CHCO electronic health record (EHR) for the study period and compared to the pre-COVID period, which was defined as February 1, 2019 –February 29, 2020, and served as the historical control. Anomalies of interest were identified using ICD-10 codes and then manually reviewed to confirm diagnoses were correct. Rates of these congenital anomalies were calculated based on total neonatal intensive care unit admissions during the associated time periods and compared. Notably, only anomalies requiring NICU admission were included in this query.
Pregnant woman 18 years or greater with documented COVID-19 infection during gestation between March 1, 2020, and March 31, 2021, were included in this study. Newborns included in this study were the child of patients provided by a prospectively maintained patient registry of women who contracted COVID-19 during gestation, which was generated by an Epic EHR data extraction analyst [16]. Positive COVID-19 infection was confirmed on antigen, polymerase chain reaction, or by presence of antibodies in a biological sample. This study was approved by the Colorado Multiple Institution Review Board.
Data was abstracted retrospectively from the EHR and included demographic data, pregnancy history information, exposure to smoking, cocaine, methamphetamine, and alcohol during pregnancy, pregnancy outcome data, COVID-19 status of infant, and data on congenital outcomes in the infant. The primary outcome of this study was rates of intestinal atresia and limb abnormalities in infants born to mothers with known COVID-19 infection during pregnancy. Secondary outcomes include rates of other surgical gastrointestinal conditions such as necrotizing enterocolitis, gastroschisis, meconium peritonitis, intestinal perforation, and intestinal stricture, as well as rates of preterm delivery and small for gestation age. Preterm delivery was reported as delivery prior to 37 weeks gestation. Very low birth weight (VLBW) was defined as < 1500 grams and extremely low birth weight (ELBW) was defined as < 1000 grams.
Descriptive summaries of measures were provided as mean (standard deviation [SD]) for continuous measures and as frequency (%) for categorical measures. Chi-Square tests were used to compare groups for categorical outcomes. Missing data were not imputed and a lack of diagnosis codes in the electronic health record were assumed to indicate the absence of the diagnosis. Pregnancy data and infant outcomes were stratified by maternal COVID-19 status at birth. All analyses are completed used R v4.1.0 (Vienna, Austria).
3Results
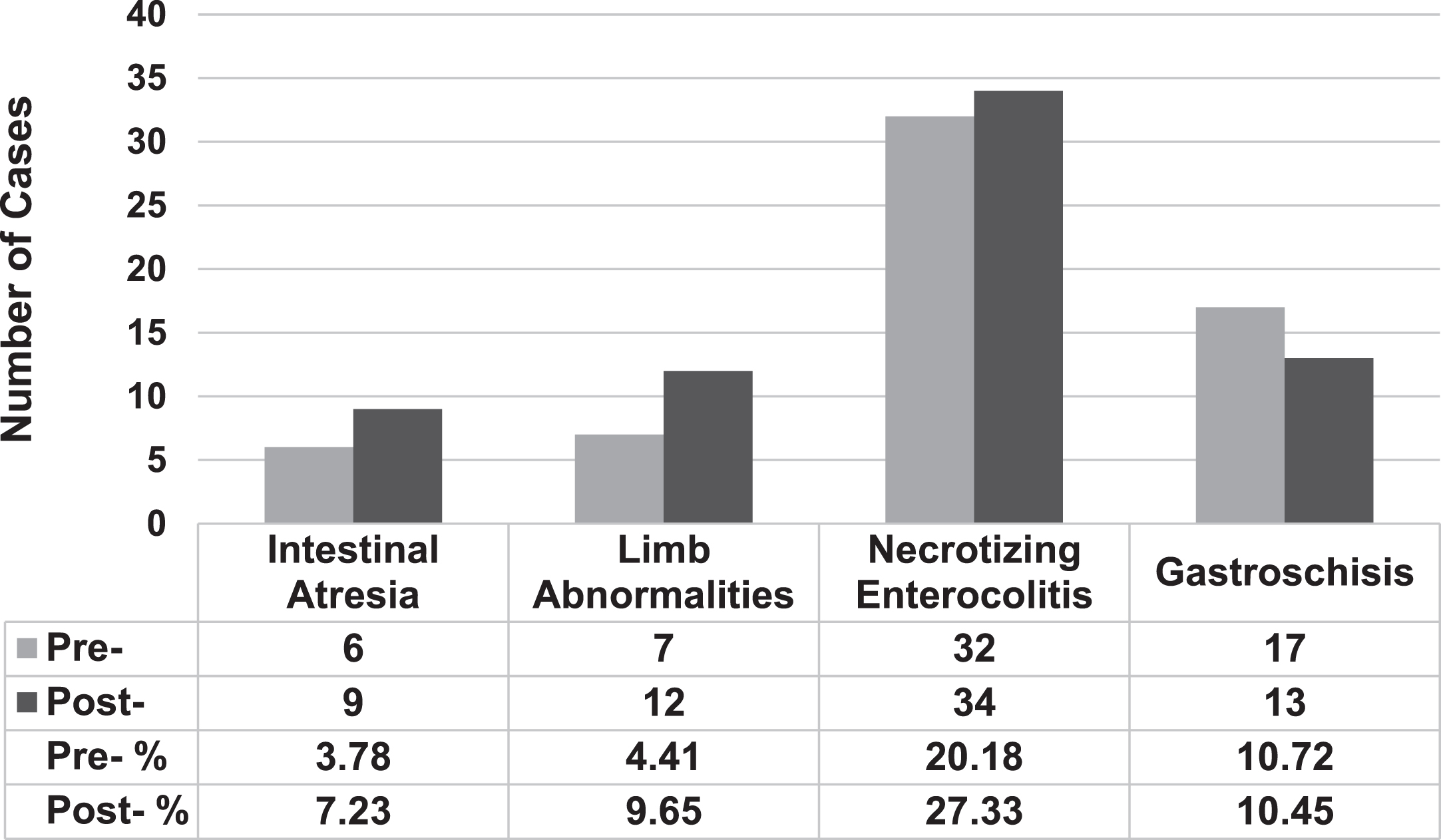
When determining overall incidence of the congenital anomalies of interest, there were six cases of intestinal atresia among infants in the historical pre-COVID period. There were nine cases during the study period; one of whom was born to a mother with COVID-19 infection during delivery. Seven infants in the NICU were born with limb abnormalities prior to the COVID-19 period and 12 were born with them after the start of the pandemic. Prior to and after the pandemic, rates of NEC (n = 32 and n = 39, respectively) and gastroschisis (n = 17 and n = 13, respectively) were similar (Fig. 1). These incidences were compared to overall NICU admissions at both CHCO and UCH during the defined study periods, which were 1586 for the pre-COVID and 1244 after the start of the pandemic. Using the overall NICU admissions rate as the denominator, the rate of intestinal atresia pre-COVID was 3.78% compared to 7.23% (p = 0.21) after and the rate of limb abnormalities was 4.41% versus 9.65% (p = 0.09). There were no statistically significant differences in rates of necrotizing enterocolitis or gastroschisis. The rates of intestinal perforation, intestinal stricture and meconium peritonitis were not collected due to unreliable ICD-10 codes for querying.
Fig. 1
Overall Rates of Congenital Outcomes Prior To and After Start of Pandemic. “Pre-” defined as February 2019-2020, and “Post-” defined as after start of pandemic, March 2020-2021.
There were 215 women who were identified as having active coronavirus infection during pregnancy, of which 20 women had documented coronavirus infection that was not COVID-19 and were excluded. There were 195 women confirmed to have COVID-19 infection at some point during their pregnancy. One woman had a spontaneous abortion during pregnancy and was excluded from analysis, resulting in 194 patients in final analysis. This woman reported a spontaneous abortion at 19 weeks gestation. She had a history of type 1 diabetes mellitus and developed diabetic ketoacidosis following her COVID-19 infection. She had a spontaneous abortion five days later that required a dilation and curettage.
Within the study population, the majority of women were White (43.3%, n = 84) or other (38.1%, n = 74) race, and the majority were Hispanic ethnicity (53.1%, n = 103). The mean (SD) age at delivery was 28.0 (6.0) years. The mean (SD) BMI was 33.0 (6.5) kg/m2. This birth was the first gestation for 55 (28.4%) of women. Five women reported prenatal exposure to drugs or alcohol; smoking was most frequently reported (1.5%, n = 3). Most women delivered vaginally (74.7%, n = 145) and the mean (SD) gestational age at birth was 38.7 (2.2) weeks. All were singleton pregnancies. The proportion of preterm infants was 11.3% (n = 22). All demographic data are summarized in Table 1.
Table 1
Demographics and pregnancy data of overall population
| Overall Population (n = 194) | |
| Maternal Race | |
| White | 84 (43.3%) |
| Black or African American | 15 (7.7%) |
| American Indian or Alaska Native | 2 (1.0%) |
| Asian | 13 (6.7%) |
| Native Hawaiian or Other Pacific Islander | 2 (1.0%) |
| Other | 74 (38.1%) |
| Unknown | 4 (2.1%) |
| Maternal Ethnicity | |
| Hispanic | 103 (53.1%) |
| Non-Hispanic | 88 (45.4%) |
| Unknown | 3 (1.5%) |
| Mom’s Age at Delivery (years) | 28.0 (6.0) |
| Mom’s BMI at Delivery (kg/m2) | 33.0 (6.5) |
| Parity | |
| Nulliparous | 55 (28.4%) |
| Multiparous | 139 (71.6%) |
| Maternal Prenatal Exposure | |
| Smoking | 3 (1.5%) |
| Cocaine | 1 (0.5%) |
| Methamphetamine | 0 (0.0%) |
| Alcohol Abuse | 1 (0.5%) |
| Vaginal Delivery | 145 (74.7%) |
| Gestational Age at Birth (weeks) | 38.7 (2.2) |
| Preterm Infants (<37 Weeks) | 22 (11.3%) |
| Gestation Age of Preterm Infants (weeks) | 33.9 (3.1) |
| Delivery Site: | |
| UCH | 185 (95.4%) |
| CHCO | 1 (0.5%) |
| Outside Hospital | 7 (3.6%) |
| Missing | 1 (0.5%) |
Data is reported as mean (standard deviation) or frequency (%). BMI = body mass index; UCH = University of Colorado Hospital; CHCO = Children’s Hospital Colorado.
There were 135 (69.6%) women who were positive for COVID-19 at time of birth. Fifty-nine women were positive during pregnancy, of which 48 (24.7%) were negative during delivery and 11 (5.7%) had an unknown COVID-19 status at time of delivery. The majority of neonates were not tested for COVID (70.6%, n = 137). One (0.5%) was positive at birth (Table 2).
Table 2
COVID-19 status of mother and infants, overall population
| Overall Population (n = 194) | |
| COVID-19 Status of Mother: | |
| Positive During Pregnancy, Negative at Birth | 48 (24.7%) |
| Positive During Pregnancy, Unknown at Birth | 11 (5.7%) |
| Positive at Birth | 135 (69.6%) |
| Baby COVID-19 Status: | |
| Positive | 1 (0.5%) |
| Negative | 54 (27.8%) |
| Not Tested | 137 (70.6%) |
| Missing | 2 (1.0%) |
Data is reported frequency (%).
When stratifying by maternal COVID-19 status at time of delivery, the one infant who was positive at time of delivery was born to a COVID-19 positive mother. The proportion of preterm infants was 12.6% and 10.4% among women who were COVID-19 positive and negative at time of birth, respectively (p = 0.80). The mean (SD) birth weight was 3160 (587.6) grams for women who were positive at time of delivery and 3160 (527.8) grams for women who were negative at time of delivery. Infants who were considered VLBW or ELBW were only born to mothers who were positive at time of delivery, but these differences were not significant (p = 0.57 and p = 1.0, respectively). These deliveries were all pre-term; one was indicated due to maternal COVID-19 status. Full data is summarized in Table 3.
Table 3
Pregnancy outcomes stratified by COVID-19 status of mother at birth
| Positive | Negative | Unknown | |
| (n = 135) | (n = 48) | (n = 11) | |
| Baby COVID-19 Status: | |||
| Positive | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Negative | 51 (37.8%) | 1 (2.1%) | 2 (18.2%) |
| Not Tested | 82 (60.7%) | 46 (95.8%) | 9 (81.8%) |
| Missing | 1 (0.7%) | 1 (2.1%) | 0 (0.0%) |
| Vaginal Delivery | 100 (74.1%) | 36 (75.0%) | 9 (81.8%) |
| Baby Sex (% female) | 81 (60.0%) | 26 (54.2%) | 5 (45.5%) |
| Gestational Age at Birth (weeks) | 38.5 (2.4) | 38.8 (1.7) | 39.4 (0.86) |
| Preterm Infants (<37 Weeks) | 17 (12.6%) | 5 (10.4%) | 0 (0.0%) |
| Gestational Age of Preterm Infants (weeks) | 33.6 (3.4) | 34.6 (1.3) | – |
| Baby’s Birth Weight (g) | 3160 (587.6) | 3160 (537.8) | 3301 (316.1) |
| VLBW Infants (<1500 g) | 3 (2.2%) | 0 (0.0%) | 0 (0.0%) |
| ELBW Infants (<1000 g) | 2 (1.5%) | 0 (0.0%) | 0 (0.0%) |
| Congenital Outcomes | |||
| Intestinal Atresia | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Limb Abnormalities | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Necrotizing Enterocolitis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Gastroschisis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Intestinal Stricture | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Intestinal Perforation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Meconium Peritonitis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Data is reported as mean (standard deviation) or frequency (%). VLBW = very low birth weight; ELBW = extremely low birth weight.
Within this cohort, there was only one patient born with a study-specific congenital anomaly. This infant was born with intestinal atresia to a mother who had active COVID-19 infection at the time of delivery. The mother was symptomatic with cough and fever, and she did not receive anticoagulation. No infants had limb abnormalities, necrotizing enterocolitis, gastroschisis, intestinal stricture, intestinal perforation, or meconium peritonitis (Table 3).
4Discussion
Among women with documented COVID-19 infection during pregnancy, there was only one instance of an infant being born with intestinal atresia. No other anomalies of interest were observed within our study cohort during study period. Despite knowledge of pro-thrombotic states during pregnancy and COVID-19, based on our small series, we did not find evidence that maternal COVID-19 infection during pregnancy resulted in higher rates of congenital anomalies compared to in-utero vascular accidents.
Among our cohort of COVID-19 positive moms, there was only one instance of intestinal atresia in infants within this cohort and no infants born with limb anomalies. The infant born with intestinal atresia was born to a mother who was COVID-19 positive at birth. Though the exact mechanism for intestinal atresia is not fully elucidated, the leading hypothesis remains that it is caused by an intra-uterine ischemic event [6, 10, 11]. The exact timing of such a mesenteric vascular occlusion is not known, but such an occurrence is believed to be a late intrauterine event [16]. The hematologic complications of COVID-19 have been reported since the onset of the pandemic, including hypercoagulability and prolongation of coagulation times [18]. Though management of COVID-19 has changed, especially as the virus has evolved and mutated, pharmacologic anticoagulation during in-patient hospitalizations, and occasionally following discharge from the hospital, has been part of the management of individuals infected with COVID-19. Anticoagulation, however, was not indicated when a woman is admitted for labor and tests positive for COVID-19 due to routine testing, but was asymptomatic, which was likely the case of many within our cohort. However, despite evidence of the thrombotic effects of COVID-19 and fetal vascular malperfusion in COVID-19 positive pregnancies, the low rate of intestinal atresia and limb anomalies in infants born to women who contracted COVID-19 is encouraging. This as well as the lack of overall difference during the pandemic compared to prior, suggests there is likely no synergistic effect of these two pro-thrombotic states.
There are limited reported data on the incidence of congenital anomalies or neonatal gastrointestinal disorders in infants born to women with COVID-19 infection. One prior study found no differences in necrotizing enterocolitis rates between preterm infants born to COVID-19 positive mothers compared to controls [19]. Another study reported increased risk for severe neonatal morbidity, which was defined to include necrotizing enterocolitis, in infants born to COVID-19 positive women after adjusting for preterm birth, but absolute rates were not reported [20]. However, there has been substantial data on maternal and fetal outcomes in women with active COVID-19 infection overall [6, 20, 21]. These data have largely reported pregnancy related complications, such as gestational hypertension, preeclampsia, and maternal admission to the intensive care unit (ICU). Neonatal outcomes reported include preterm birth, neonatal intensive care unit stay, low birth weight, and symptoms secondary to COVID-19 [17]. Multiple studies have reported that women with COVID-19 diagnosis are at increased risk of preterm birth, both medically indicated and not, and low birth weight [1, 5, 6, 19, 20]. Within our cohort, the rate of preterm birth was 11.4%, which is consistent with prior reports and slightly greater than the preterm rate of 9.1% in Colorado [21]. There were three infants in this cohort with very low birth weights. Rates of low birth weight have been reported previously in those with COVID-19 infection [6, 19]. Exact rates are difficult to interpret given the heterogeneity in parameters to define low-birth weight and differences in prior COVID-19 study populations. It is further complicated by the fact that low-birth weights may be due to lower gestational ages influenced by treatment for maternal infection, for example, induction in the context of severe COVID-19, but data suggests COVID-19 infection is associated with lower birth weight and earlier delivery [1, 5, 6, 17, 19, 20].
Of substantial interest has been the transmissibility of COVID-19 infection from mother to fetus through transplacental transmission. Though this is likely the most plausible mechanism for transmission of the virus, it is a rarely reported event [1, 6, 20]. Within our cohort, we only report one (0.5%) infant with a positive COVID-19 test at birth; the mother of this infant also tested positive at time of delivery. It is unknown exactly how the infant within this study contracted COVID-19, but this low rate of viral detect in our cohort is reassuring and supportive of prior studies, that infection of the fetus is unlikely when the mother has active infection. Our institution additionally has policies in place aimed to prevent horizonal transmission from close contact of mom and baby after birth if the mom tested positive for COVID-19. It is important to note that most positive maternal COVID-19 tests may have been related to hospital policy for universal testing at time of admission, which would capture positive asymptomatic patients; this is of relevance given that neonatal outcomes have been shown to vary with severity of maternal infection [23].
There are several limitations to this study. Our study is limited by its small sample and lack of a contemporary comparative study group. We relied on historical data before COVID-19 to compare rates of intestinal atresia and limb atresia before and during the pandemic. Ideally, data on all women who delivered within our health care system would be available to determine the relationship between COVID-19 infection during pregnancy and outcomes. An initial component of this study was to obtain blood samples of women who delivered infants with the outcomes of interest to test for prior COVID-19 infection. A monetary incentive was offered for those that participated in this aspect of the study, but this, however, ultimately resulted in only one woman enrolling so was not included. Thus, much of our data is limited to women who tested positive during delivery. Given that vascular accidents of incident occur earlier in pregnancy, there may be decreased utility in having such a large proportion of women with active COVID-19 infection during delivery. Furthermore, our reported rates of congenital anomalies are limited by those infants who were seen within our health care institution. As most limb anomalies are non-life threatening, infants would likely not be transferred to CHCO for evaluation and could follow-up outside the healthcare system, which would impact our reported numbers. Additionally, our primary outcomes of interest have low incidence rates, which limits the likelihood to detect such anomalies in our study cohort [24–26]. Pooling data from multiple institutions may allow for greater power to detect such outcomes. We also used March 1, 2020 as the beginning of our pandemic but recognize COVID-19 was prevalent in the United States prior to this date. It is important to also consider that throughout the pandemic there have been multiple variants of COVID-19 and these different strains could impact clinical outcomes; stillbirth rates have reported higher during period of Delta predominance compared to pre-Delta period [27]. It is possible that more severe variants of the virus have a higher likelihood of impacting fetal development compared to more mild strains.
5Conclusion
In conclusion, we report a low incidence of congenital anomalies in infants born to mothers with COVID-19 infection. Though it is unclear if the pathophysiological impact of COVID-19 on the coagulative state impacts the normal pro-thrombotic state of pregnancy, COVID-19 during pregnancy is not a benign state. This supports ongoing surveillance and efforts to control the virus, specifically among these vulnerable patients.
Acknowledgments
Internal institutional funding for this work was provided by the Center for Children’s Surgery, University of Colorado and Children’s Hospital Colorado and the Children’s Hospital Center for Research in Outcomes in Children’s Surgery.
Conflicts of interest
The authors have no conflicts of interest, financial or otherwise, to disclose.
References
[1] | Kyle MH , Hussain M , Saltz V , Mollicone I , Bence M , Dumitriu D . Vertical transmission and neonatal outcomes following maternal SARS-CoV-2 infection during pregnancy. Clin Obstet Gynecol. (2022) ;65: (1):195–202. |
[2] | Pashaei Z , Seyed Alinaghi S , Qaderi K , Barzegary A , Karimi A , Mirghaderi SP , Mirzapour P , et al. Prenatal and neonatal complications of COVID- A systematic review. Health Sci Rep. (2022) ;5: (2):e510. |
[3] | Kotlyar AM , Grechukhina O , Chen A , Popkhadze S , Grimshaw A , Tal O , et al. Vertical transmission of coronavirus disease A systematic review and meta-analysis. Am J Obstet Gynecol. (2021) ;224: (1):35–53.e3. |
[4] | Mullins E , Hudak ML , Banerjee J , Getzlaff T , Townson J , Barnette K , et al. Pregnancy and neonatal outcomes of COVID- Coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. (2021) ;57: (4):573–81. |
[5] | Norman M , Navér L , Söderling J , Ahlberg M , Hervius Askling H , Aronsson B , et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA. (2021) ;325: (20):2076–86. |
[6] | Angelidou A , Sullivan K , Melvin PR , Shui JE , Goldfarb IT , Bartolome R , et al. Association of maternal perinatal SARS-CoV-2 infection with neonatal outcomes during the COVID-19 pandemic in Massachusetts. JAMA Netw Open. (2021) ;4: (4):e217523. |
[7] | Wong YP , Khong TY , Tan GC . The effects of COVID-19 on placenta and pregnancy: What do we know so far? Diagnostics (Basel). (2021) ;11: (1):94. |
[8] | Menter T , Mertz KD , Jiang S , Chen H , Monod C , Tzankov A , et al. Placental pathology findings during and after SARS-CoV-2 infection: Features of villitis and malperfusion. Pathobiology. (2021) ;88: (1):69–77. |
[9] | Heider A . Fetal vascular malperfusion. Arch Pathol Lab Med. (2017) ;141: (11):1484–9. |
[10] | Komuro H , Amagai T , Hori T , Hirai M , Matoba K , Watanabe M , et al. Placental vascular compromise in jejunoileal atresia. J Pediatr Surg. (2004) ;39: (11):1701–5. |
[11] | Nixon HH , Tawes R . Etiology and treatment of small intestinal atresia: Analysis of a series of 127 jejunoileal atresias and comparison with 62 duodenal atresias. Surgery. (1971) ;69: (1):41–51. |
[12] | Van Allen MI , Hoyme HE , Jones KL . Vascular pathogenesis of limb defects. I. Radial artery anatomy in radial aplasia. J Pediatr. (1982) ;101: (5):832–8. |
[13] | Soltan HC , Holmes LB . Familial occurrence of malformations possibly attributable to vascular abnormalities. J Pediatr. (1986) ;108: (1):112–4. |
[14] | Terpos E , Ntanasis-Stathopoulos I , Elalamy I , Kastritis E , Sergentanis TN , Politou M , et al. Hematological findings and complications of COVID-19. Am J Hematol. (2020) ;95: (7):834–47. |
[15] | Middleton P , Shepherd E , Gomersall JC . Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period. Cochrane Database Syst Rev. (2021) ;3: (3):CD001689. |
[16] | Epic Systems Corporation. EPIC. Verona: Epic Systems Corporation (2022) . |
[17] | Louw JH , Barnard CN . Congenital intestinal atresia; observations on its origin. Lancet. (1955) ;269: (6899):1065–7. |
[18] | Indrio F , Salatto A , Amato O , Bartoli F , Capasso L , Corvaglia L , et al. Neonatal outcomes of premature infants born to women with the novel coronavirus (SARS-CoV-2) infection: A case control study. Am J Perinatol. 2021. |
[19] | Villar J , Ariff S , Gunier RB , Thiruvengadam R , Rauch S , Kholin A , et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. (2021) ;175: (8):817–26. |
[20] | Di Toro F , Gjoka M , Di Lorenzo G , De Santo D , De Seta F , Maso G , et al. Impact of COVID-19 on maternal and neonatal outcomes: A systematic review and meta-analysis. Clin Microbiol Infect. (2021) ;27: (1):36–46. Epub 2022 Nov 2. |
[21] | Reproductive health: Preterm birth. Centers for Disease Control and Prevention https://www.cdc.gov/nchs/pressroom/states/colorado/co.htm; (2021) [8 March 2022]. |
[22] | Best KE , Tennant PW , Addor MC , Bianchi F , Boyd P , Calzolari E , et al. Epidemiology of small intestinal atresia in Europe: A register-based study. Arch Dis Child Fetal Neonatal Ed. (2012) ;97: (5):F353–8. |
[23] | Metz TD , Clifton RG , Hughes BL , Sandoval G , Saade GR , Grobman WA , et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease (COVID-19). Obstet Gynecol. (2021) ;137: (4):571–80. |
[24] | Adams SD , Stanton MP . Malrotation and intestinal atresias. Early Hum Dev. (2014) ;90: (12):921–5. Epub 2014 Oct 13. |
[25] | Dalla Vecchia LK , Grosfeld JL , West KW , Rescorla FJ , Scherer LR , Engum SA . Intestinal atresia and stenosis: A 25-year experience with 277 cases. Arch Surg. (1998) ;133: (5):490–6; discussion 490–6. |
[26] | Tayel SM , Fawzia MM , Al-Naqeeb NA , Gouda S , Al Awadi SA , Naguib KK . A morpho-etiological description of congenital limb anomalies. Ann Saudi Med. (2005) ;25: (3):219–27. |
[27] | DeSisto CL , Wallace B , Simeone RM , Polen K , Ko JY , Meaney-Delman D , et al. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization —United States, March –September 2021. MMWR Morb Mortal Wkly Rep. ((2021) ) 70: , 1640–5. |




