A case report: Hypoxic ischemic encephalopathy & pneumonia in a neonate after SARS-CoV-2 intrauterine transmission
Abstract
Severe acute respiratory coronavirus 2 (SARS-CoV-2) is primarily transmitted via respiratory droplet or aerosol route. However, there is mounting evidence for intrauterine transmission. We report on a late preterm infant with suspected intrauterine acquisition of SARS-CoV-2 who experienced birth depression, hypoxic ischemic encephalopathy, multisystem organ involvement, and late onset COVID-19 pneumonia [22].
Abbreviations
SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
COVID-19 | Coronavirus disease |
FIRS | Fetal inflammatory response syndrome |
MIS-N | Neonatal multi-inflammatory syndrome in children |
PCR | Polymerase chain reaction |
NICU | Neonatal intensive care unit |
HIE | Hypoxic ischemic encephalopathy |
APGAR | Appearance, pulse, grimace, activity, respiration |
TH | Therapeutic hypothermia |
PPHN | Persistent pulmonary hypertension of the Newborn |
iNO | Inhaled nitric oxide |
EEG | Electroencephalogram |
DOL | Day of life |
MRSA | Methicillin resistant staph aureus |
NIPPV | Non-invasive positive pressure ventilation |
CRP | C-reactive protein |
MRI | Magnetic resonance imaging |
FiO2 | Fraction of inspired oxygen |
CXR | Chest x-ray |
ARDS | Acute respiratory distress syndrome |
HIV | Human immunodeficiency virus |
HCV | Hepatitis C virus |
GBS | Group B streptococcus |
CD68 | Cluster of differentiation 68 |
IVIG | Intravenous immunoglobulin |
RNA | Ribonucleic acid |
1Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has been well described as a cause of severe respiratory illness with potential to affect other organ systems. However, data on its effects during pregnancy and in neonates remains insufficient and inconclusive [1]. Intrauterine transmission of SARS-CoV-2 in the third trimester has been reported to be 3.2% [2]. While most of these infants are asymptomatic, recent case reports demonstrate that some infants born to mothers with COVID-19 can become severely ill [3, 4]. Systematic reviews have shown that the incidence of preterm birth and perinatal fetal distress has increased in pregnant women affected by COVID-19 [5]. It has also been reported that infants born to mothers with COVID-19 can develop multi-organ involvement resulting from a fetal inflammatory response syndrome (FIRS). FIRS is similar to neonatal multi-inflammatory syndrome (MIS-N), but historically these infants tested negative for SARS-CoV-2 [6, 7].
In this case report, we present an infant delivered at 35 weeks gestation with placental changes associated with COVID-19 infection, positive SARS-CoV-2 polymerase chain reaction (PCR) results, complications of hypoxic ischemic encephalopathy (HIE), and late onset symptoms of both severe acute COVID-19 infection and pneumonia.
2Patient presentation
A 35-year-old gravida 3 para 2 mother with a history of positive SARS-CoV-2 PCR via nasal swab testing 12 days prior to admission presented at 35 weeks gestation with worsening shortness of breath and decreased fetal movement for several hours. Additional symptoms included dyspnea on exertion, chest tightness on deep inspiration, nonproductive cough, fevers, chills, night sweats, and nausea. She was unvaccinated against COVID-19. Her pregnancy had been notable for hypothyroidism but was otherwise uncomplicated until 12 days prior to delivery when she developed symptoms of COVID-19 with confirmed infection during pregnancy [8]. No history of obesity, diabetes, hypertension, or pre-eclampsia were noted. Her first trimester labs included: Hepatitis B surface antigen, Human Immunodeficiency Virus (HIV), hepatitis C, chlamydia, and gonorrhea, and Group B streptococcus culture all resulted negative. On admission her syphilis rapid plasma reagin test was negative and SARS-CoV-2 rapid antigen positive. Prenatal medications included ibuprofen, acetaminophen, desiccated thyroid, and prenatal vitamins.
On evaluation, she was not hypoxemic. Maternal chest X-ray was notable for faint bilateral patchy interstitial infiltrates consistent with COVID-19 pneumonia. She denied leakage of amniotic fluid, abdominal pain, uterine contractions, or vaginal bleeding. Fetal monitoring showed a non-reassuring fetal heart tracing, resulting in urgent cesarean-section delivery. The obstetrician noted that the placenta was very friable and suspected a partial placental abruption.
A depressed female infant was born weighing 2680 grams. The infant was pale, hypotonic, and initially without a heart rate. Resuscitation was performed per Neonatal Resuscitation Program Guidelines [9]. At 11 minutes of life, the infant’s heart rate recovered to >80, and she was transferred to the neonatal intensive care unit (NICU). APGAR scores were assigned as 0, 1, 3, 4 and 6 at 1, 5, 10, 15, and 20 minutes, respectively. The umbilical cord and arterial blood gases at 30 minutes of life both showed a pH of 6.8 with a base deficit of –18 and –27 respectively and a lactate of 17.
Based on the blood gases, APGAR scores, and modified Sarnat scale neurologic exam demonstrating severe encephalopathy, the infant qualified for therapeutic hypothermia (TH) for suspected severe HIE [10]. Although at our institution TH is routinely offered for moderate to severe HIE in infants born at ≥36wks gestation age, it can be considered for babies born at 34-35 weeks who meet all other criteria [11–13]. Therefore, following discussion of risk and benefits, consent was obtained, and the infant underwent TH treatment. A baseline head ultrasound revealed no structural abnormalities or hemorrhage.
During the 72-hour TH protocol, the infant remained intubated and mechanically ventilated, and received two doses of surfactant due to chest x-rays (CXR) consistent with surfactant deficiency (Fig. 1A). Echocardiogram revealed persistent pulmonary hypertension of the newborn (PPHN) and decreased right ventricular function requiring inhaled nitric oxide (iNO), milrinone, and dopamine. The infant received 72 hours of antibiotics for suspected sepsis with a negative blood culture. Routine methicillin resistant staph aureus (MRSA) screenings were negative on day of life (DOL) 2 and 4. SARS-CoV-2 PCR testing of the nasopharynx at 24 and 48 hours of life were both positive. The video electroencephalogram (EEG) demonstrated a discontinuous pattern, mostly severe voltage attenuation and some irritability in the right frontal lobe. In addition, the infant demonstrated liver dysfunction, acute kidney injury, hypoglycemia and was coagulopathic and thrombocytopenic requiring multiple blood products (Table 1). Though some of these conditions are known complications associated with TH in infants, SARS-CoV-2 infection may have contributed (Table 2) [11–14].
Fig. 1
Case report X-ray findings. Chest X-ray revealing coarse interstitial opacities and air bronchograms bilaterally (A). Nine days after birth, chest X-ray revealed bilateral diffuse interstitial infiltrates worse on the right (B). Bilateral opacities and multifocal infiltrates along the right upper lobe persisted on day of life 14 (C).
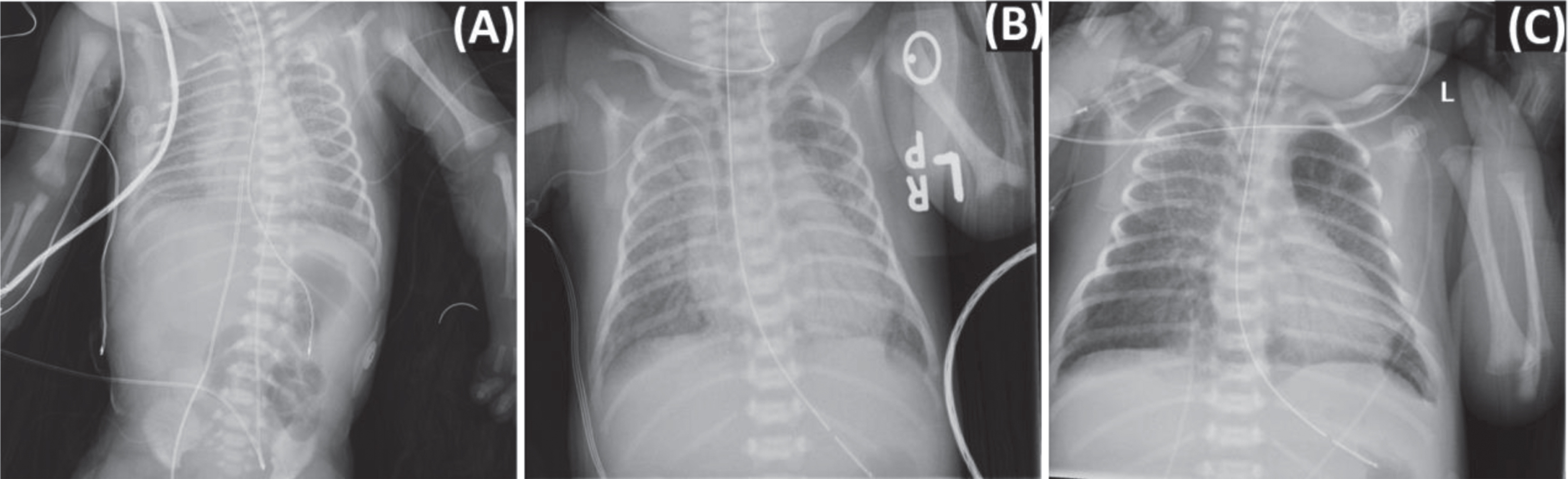
Table 1
Case study laboratory results
| FIRST 72 HOURS | LATE ONSET INFECTION | ||||||||
| LAB | BIRTH | DOL 1 | DOL 2 | DOL 3 | DOL 6 | DOL 9 | DOL 10 | DOL 11 | DOL13 |
| White Cell Count (9.1–16×103/uL) | 35.1 | 22.3 | 11.3 | 7.6 | 4.7 | 17.4 | 16.5 | 15.9 | 14.8 |
| Hemoglobin (15–22 G/DL) | 17 | 16.8 | 13.1 | 13.5 | 10.8 | 16.6 | 18.7 | 19 | 14.9 |
| Platelets (150–450 103 3/uL) | 203 | 159 | 163 | 126 | 119 | 105 | 118 | 64 | 146 |
| Neutrophils (15–30%) | 33 | 49 | 68 | 52 | 28 | 54 | 58 | ||
| Band Neutrophils (0–11%) | 8 | 7 | 5 | 5 | 1 | 10 | 1 | ||
| Lymphocytes (41–71%) | 46 | 32 | 21 | 42 | 59 | 23 | 29 | ||
| Creatinine (0.55–1.02 mg/dL) | 0.76 | 0.96 | 0.77 | 0.57 | 0.47 | 0.39 | <0.15 | <0.15 | <0.15 |
| Lactic Acid (0.36–0.75 mmol/L) | 18.4 | 9.1 | 4.5 | 1.7 | 1.5 | 3.3 | 1.8 | 2.4 | 1.4 |
| Aspartate Amino Transferase (AST) | 192 | 580 | 689 | 485 | 67 | ||||
| (15–37 Units/L) | |||||||||
| Alanine Aminotransferase (ALT) | 34 | 199 | 383 | 416 | 70 | ||||
| (13–56 Units/L) | |||||||||
| Troponin (0–0.045 ng/mL) | 0.277 | 0.573 | |||||||
| Prothrombin Time (PT) (10–12.8 seconds) | 22 | 21.7 | 22.9 | 16.3 | 11.4 | ||||
| Activated Partial Thromboplastin Time (PTT) | 68 | 35 | 30 | 32 | 27 | ||||
| (25–38 seconds) | |||||||||
| Fibrinogen (207–493 mg/dL) | 232 | 217 | 178 | 203 | 351 | ||||
| C-Reactive Protein (0–0.3 mg/dl) | 3.11 | 3.08 | 1.83 | ||||||
| SARS-CoV-2 PCR (negative) | Positive | Positive | Positive | ||||||
DOL, Day of Life; PCR, polymerase chain reaction.
Table 2
Overlapping of multi-system organ involvement in FIRS, HIE, MIS-N compared to index case
| ORGAN SYSTEM | FIRST 72 HOURS | LATE ONSET SEPSIS | |||
| FIRS | HIE | CASE | MIS-N | CASE | |
| Respiratory | X | X | X | X | X |
| Hematologic | X | X | X | X | |
| Cardiac | X | X | X | X | X |
| Neurologic | X | X | X | X | X |
| Renal | X | X | X | ||
| Dermatologic | X | X | |||
| Visual/Ophthalmologic | X | ||||
| Gastrointestinal | X | ||||
| Endocrine | X | X | X | ||
| Immune | X | ||||
FIRS, fetal inflammatory response syndrome HIE, Hypoxic Ischemic Encephalopathy MIS-N, neonatal multi-inflammatory syndrome in children.
Upon rewarming, the infant was noted to have an electrographic seizure emanating from right frontal lobe. Phenobarbital was initiated for seizure suppression and no further seizures followed. Over the next four days, the infant was weaned off vasopressors and iNO, acid/base status stabilized, coagulopathy resolved, liver function improved, and she was extubated to non-invasive positive pressure ventilation (NIPPV). Brain magnetic resonance imaging (MRI) on DOL 8 demonstrated marked supratentorial hydrocephalus with extensive grade 3 intraventricular hemorrhage bilaterally and areas concerning for diffuse ischemic changes.
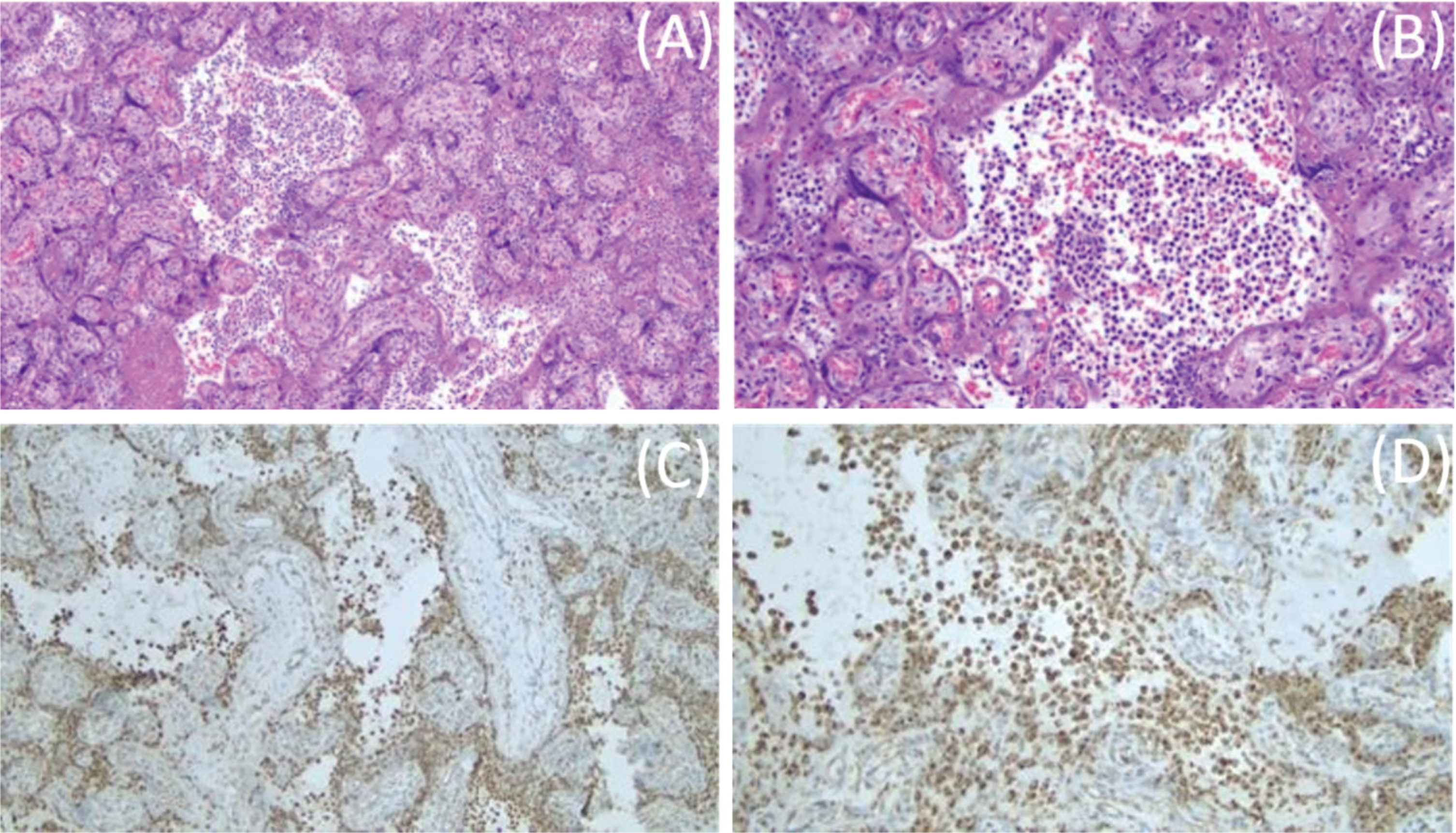
Maternal placental pathology showed chronic histiocytic intervillositis (stage 1, grade 1) highlighted by cluster of differentiation 68 (CD68) immunostaining, no fetal inflammatory response, three vessel umbilical cord with no evidence of funisitis, and mature chorionic villi with fibrin. Per the pathology report, these findings may be related to SARS-CoV-2 infection (Fig. 2) and are consistent with findings of inflammatory changes previously reported in SARS-CoV-2 positive mothers [15–19]. The clinically suspected abruption was not confirmed.
Fig. 2
Placental histopathology. Placental hematoxylin and eosin staining showing mononuclear cell inflammation of the intervillous space, chronic histiocytic intervillositis, at 10X (A). Chronic histiocytic intervillositis in the intervillous space at 20X (B). CD68+ histiocytes in the intervillous space at 10X (C). CD68+ histiocytes in the intervillous space at 20X (D).
On DOL 9, the infant presented with acute respiratory deterioration and respiratory failure requiring reintubation and ventilatory support with an increase in oxygen requirement to 100%, and resumption of iNO for suspected pulmonary hypertension. Echocardiogram did not demonstrate pulmonary hypertension, but the oxygen requirement did improve on iNO. The CXR demonstrated bilateral diffuse infiltrates concerning for pneumonia (Fig. 1B). A blood culture and tracheal aspirate were sent for gram stain and culture. A C-reactive protein (CRP) at this time was elevated at 3.11 mg/dl, and quantitative real-time PCR analysis of respiratory secretions identified no other common respiratory viruses (see Table 1 for additional laboratory evaluation). As a superimposed bacterial pneumonia could not be ruled out, the infant was started on vancomycin and cefepime and completed 14 days of antibiotics with negative tracheal culture and gram stain as well as a negative blood culture. Routine MRSA screening was negative on DOL 11. In addition, a 10-day course of dexamethasone was initiated based on clinical consensus and published pediatric COVID-19 cases [20, 21]. Remdesivir was considered for use as compassionate care but was not given due to a lack of recommended dosing for infant with a birth weight of less than 3.5 kg, and concern that some of her symptoms were consistent with MIS-N (Table 2) [22].
A repeat SARS-CoV-2 PCR was positive on DOL 13, and on DOL 14 the CXR demonstrated bilateral lung opacities and multifocal infiltrates along the right upper lobe (Fig. 1C). The infant had continued worsening of respiratory status despite high frequency oscillatory ventilation support and iNO therapy as well as treatment with broad spectrum antibiotics and dexamethasone. Therefore, the infant received one dose of Intravenous immunoglobulin (IVIG) as an effort to dampen the inflammatory response given worsening respiratory status while on high frequency ventilatory support and a CXR demonstrating bilateral lung opacities and multifocal infiltrates along the right upper lobe (Fig. 1C). The infant remained on broad spectrum antibiotics and dexamethasone. Over the course of the next week, the infant’s respiratory status slowly improved. The infant was weaned off iNO and extubated to NIPPV on DOL 23, at which time a SARS-CoV-2 PCR of the nasopharynx was negative. During this time, serial head ultrasounds revealed worsening intraventricular hemorrhage with parenchymal extension and worsening ventriculomegaly. Therefore, on DOL 23, the infant was transferred to a referral center for a pediatric neurosurgery evaluation and required subgaleal shunt placement on DOL 32. On DOL 54, the infant was weaned to unassisted room air and was discharged home on DOL 67.
3Discussion
We report a case of an infant with confirmed intrauterine acquired SARS-CoV-2 infection born to an unvaccinated, symptomatic, confirmed SARS-CoV-2 positive mother with COVID-19 [23, 24]. Because the infant was isolated immediately from the mother at birth and following strict infection control and prevention procedures in our unit, postnatal transmission was deemed unlikely. There are increasing published case reports of suspected intrauterine transmission of COVID-19 [2, 25, 26]. This infant was screened for SARS-CoV-2 per the American Academy of Pediatrics guidelines at 24 and 48 hours of age using nasal swab PCR, and both were positive, confirming a neonatal intrauterine infection based on Blumberg’s classification [23]. Additionally, findings on placental pathology were pathognomonic for SARS-CoV-2 infection without confirming the previously suspected abruption.
The placental findings of chronic histiocytic intervillositis, trophoblast necrosis and CD68 histiocytes have been previously described in cases of maternal COVID-19 infection [15–19]. Placental PCR testing is not standard of care for diagnosis of maternal COVID-19 infection and the COVID-19 PCR yield could be affected by handling of placental tissue and ribonucleic acid (RNA) degradation [27]. In addition, the clinical presentation, the maternal COVID-19 status, and the placental pathology findings support a strong likelihood of intrauterine transmission of SARS-CoV-2 even in the absence of placental PCR. This case highlights that the placental dysfunction seen in SARS-CoV-2 infection may have sequelae such as fetal distress and resulting HIE. Signs of maternal vascular malperfusion have been seen in placentas of SARS-CoV-2 positive mothers including infarction and thrombosis, which we hypothesize could be a contributing factor. Schoenmakers et al. [19] report a case of metabolic acidosis and low APGAR scores in a 31-week infant born to an asymptomatic SARS-CoV-2 positive mother. The placenta revealed evidence of inflammation histiocytic intervillositis, and the infant presented with severe multi-organ failure, even though the infant tested negative for SARS-CoV-2. The authors hypothesize that the infant’s adverse neonatal outcomes were not associated with asphyxia alone but were related to SARS-CoV-2 related placental inflammation and dysfunction, suggesting that SARS-CoV-2 associated damage to the placenta early in pregnancy potentially can lead to fetal distress, and possible fetal demise if not recognized in time. These placental findings were also seen in our case, with both a positive test for SARS-CoV-2 and the presence of perinatal asphyxia supporting the likelihood of the infection affecting placental function leading to fetal distress.
This infant’s multisystem organ failure seen at birth overlaps with common problems seen in HIE, and FIRS and MIS-N related to SARS-CoV-2 infections (Table 2), creating a challenge for diagnosis. While HIE is likely the etiology of the infant’s initial symptoms, there was no identifiable perinatal sentinel event. While sentinel events are not always present in infants with HIE, given findings on placental pathology as well as the infant’s initial positive testing, we suspect that the placental dysfunction from SARS-CoV-2 infection attributed to birth asphyxia and multi-organ involvement of the infant at birth. This has been seen in other cases of infants with FIRS related to maternal SARS-CoV-2 infection [6, 28]. FIRS is characterized with fetal deployment of a local or systemic inflammatory response and elevation of fetal plasma interleukin-6 when exposed to infections [29]. In our case, the absence of a fetal inflammatory response and funisitis in the placental pathology make a diagnosis of FIRS less likely to be reason for development of the late onset symptoms in the infant.
The organ involvement in reported cases of MIS-N, associated with maternal COVID-19 and our infant are similar (Table 2) [30, 31]. MIS-N is believed to develop because of immune-mediated multisystem injury due to transplacental transfer of maternal SAR-CoV-2 antibodies or due to antibodies mounted by the newborn to SARS-CoV-2 infection. The Center for Disease Control and Prevention and the World Health Organization have developed a definition for MIS-C with some authors modifying this definition to include MIS-N. They define MIS-N as those infants with confirmed infection or exposure to SARS-CoV-2 infection before 28 days of age, who presented without fever and had three-organ involvement, with other etiologies such as sepsis and birth depression excluded [32]. MIS-N may be further classified as early MIS-N, with features presenting within 72 hours after birth, or late MIS-N, which occurs beyond the first 72 hours of age [33]. Even though our infant may meet some criteria for MIS-N, significant elevation of pro-inflammatory markers are seen in more than 70% of babies with MIS-N with CRP ranges of 20–78 mg/dl, but in our case, the CRP was only slightly elevated at 3.11 mg/dl [32–35]. Though this infant’s clinical presentation in the first few days of life is likely multifactorial, we suspect the infant’s respiratory decompensation at DOL 9 and 14 were due to SARS-CoV-2 related infection [36–38]. Other possible etiologies such as blood and tracheal aspirate cultures and respiratory viral panel were evaluated and reported negative making the diagnosis of bacterial or other viral sepsis less likely. The infants SARS-CoV-2 PCR on DOL 13 remained positive. Also, the infant’s initial CXR at birth revealed bilateral ground glass opacities, which are classic findings in RDS but are also common findings in children diagnosed with SARS-CoV-2 pneumonia [39–41]. The CXRs when the infant decompensated on DOL 9 revealed bilateral airspace disease consistent with acute respiratory distress syndrome (ARDS). Both the CXR findings and timeline of illness are typical of pediatric patients with COVID-19 pneumonia [39–41]. While we did not obtain viral load or PCR cycle threshold, these have not shown to be predictive of clinical outcomes in pediatric cases [42].
Our case, supports findings of maternal SARS-COV-2 infection affecting the placenta and fetus, resulting in neonatal complications including preterm delivery, neonatal birth depression and HIE. This has implications for the prenatal care of women including routine counseling, individual risk evaluation, vaccine recommendations, and close monitoring irrespective of the severity of COVID-19 symptoms during pregnancy. The positive SARS-CoV-2 testing of the infant at 24 and 48 hours of life in conjunction with the clinical decline, respiratory failure on DOL 9 and persistent positive SARS-CoV-2 PCR in this case are consistent with COVID-19 infection/pneumonia and less likely to MIS-N even in the presence of some overlapping clinical features, which has implications for the postnatal management of neonates born to SARS-CoV-2 positive mothers both in the NICU and potentially in outpatient pediatric settings.
Acknowledgments
Thank you to Dr. Allison Messina, Pediatric Infectious Disease, for her contributions to the clinical case and review of manuscript.
Financial disclosure statement
There are no potential or actual interests to disclose for all authors involved in this publication.
References
[1] | The-nCo V , Outbreak Joint Field Epidemiology Investigation Team Li Q . An outbreak of NCIP -nCoV) infection inChina –Wuhan, Hubei Province, -China CDC Wkly (2020) ;2: (5):79–80. |
[2] | Kotlyar AM , Grechukhina O , Chen A , Popkhadze S , Grimshaw A , Tal O ,et al. Vertical transmission of coronavirus disease A systematic review and meta-analysis, Am J Obstet Gynecol (2021) ;224: (1):35–53.e3. |
[3] | Saikia B , Tang J , Robinson S , Nichani S , Lawman KB , Katre M , et al. Neonates with SARS-CoV-2 infection and pulmonary disease safely treated with remdesivir, Pediatr Infect Dis J (2021) ;40: (5):e194–6. |
[4] | Sagheb S , Lamsehchi A , Jafary M , Atef-Yekta R , Sadeghi K . Two seriously ill neonates born to mothers with COVID-19 pneumonia- a case report, Ital J Pediatr (2020) ;46: (1):137. |
[5] | Diriba K , Awulachew E , Getu E . The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: A systematic review and meta-analysis, Eur J Med Res (2020) ;25: (1):39. |
[6] | McCarty KL , Tucker M , Lee G , Pandey V . Fetal inflammatory response syndrome associated with maternal SARS-CoV-2 infection, Pediatrics (2021) ;147: (4). |
[7] | Pawar R , Gavade V , Patil N , Mali V , Girwalkar A , Tarkasband V , et al. Neonatal Multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV- A case series, Children (Basel) (2021) ;8: (7):572. |
[8] | Shah PS , Diambomba Y , Acharya G , Morris SK , Bitnun A . Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates, Acta Obstet Gynecol Scand (2020) ;99: (5):565–8. |
[9] | Weiner G , editor. Textbook of neonatal resuscitation. 8th ed. Elk GroveVillage, IL: American Academy of Pediatrics and American Heart Association; 2021. |
[10] | Power BD , McGinley J , Sweetman D , Murphy JFA . The modified Sarnat score in the assessment of neonatal encephalopathy: A quality improvement initiative, Ir Med J (2019) ;112: (7):976. |
[11] | Rao R , Trivedi S , Vesoulis Z , Liao SM , Smyser CD , Mathur AM . Safety and short-term outcomes of therapeutic hypothermia in preterm neonates 34-35 weeks gestational age with hypoxic-ischemic encephalopathy, J Pediatr (2017) ;183: :37–42. |
[12] | Jacobs SE , Morley CJ , Inder TE , Stewart MJ , Smith KR , McNamara PJ , et al. Infant Cooling Evaluation Collaboration, Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: A randomized controlled trial. Arch Pediatr Adolesc Med (2011) ;165: (8):692–700. |
[13] | Eicher DJ , Wagner CL , Katikaneni LP , Hulsey TC , Bass WT , Kaufman DA , et al. Moderate hypothermia in neonatal encephalopathy: Safety outcomes, Pediatr Neurol (2005) ;32: (1):18–24. |
[14] | Yan ES , Chock VY , Bonifacio SL , Dahlen A , Guimaraes CV , Altit G , et al. Association between multi-organ dysfunction and adverse outcome in infants with hypoxic ischemic encephalopathy. J Perinatol. 2022. 10.1038/s41372-022-01413-6. |
[15] | Husen MF , van der Meeren LE , Verdijk RM , Fraaij PLA , van der Eijk AA , Koopmans MPG , et al. Unique severe COVID-19 placental signature independent of severity of clinical maternal symptoms, Viruses (2021) ;13: (8):1670. |
[16] | Bouachba A , Allias F , Nadaud B , Massardier J , Mekki Y , Bouscambert Duchamp M , et al. Placental lesions and SARS-Cov-2 infection: Diffuse placenta damage associated to poor fetal outcome, Placenta (2021) ;112: :97–104. |
[17] | Marton T , Hargitai B , Hunter K , Pugh M , Murray P . Massive perivillous fibrin deposition and chronic histiocytic intervillositis a complication of SARS-CoV-2 infection, Pediatr Dev Pathol (2021) ;24: (5):450–4. |
[18] | Shanes ED , Mithal LB , Otero S , Azad HA , Miller ES , Goldstein JA . Placental pathology in COVID-19, Am J Clin Pathol (2020) ;154: (1):23–32. |
[19] | Schoenmakers S , Snijder P , Verdijk RM , Kuiken T , Kamphuis SSM , Koopman LP , et al. Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman, J Pediatric Infect Dis Soc (2021) ;10: (5):556–61. |
[20] | Deepali A , Rijwana S . SARS-CoV-2 Infection in neonates presenting as sepsis with deranged inflammatory markers. Pediatr Oncall J. 2021. |
[21] | Covid-19 Treatment guideline special considerations in children. https://www.covid19treatmentguidelines.nih.gov/special-populations/children/. Published 2021. Accessed October 12, 2021. |
[22] | Jenco M , editor. CDC details COVID-19-related inflammatory syndrome in children. AAP News. https://www.aappublications.org/news/2020/05/14/covid19inflammatory051420. Published May 14, 2020. Accessed October 18, 2020. |
[23] | Blumberg DA , Underwood MA , Hedriana HL , Lakshminrusimha S . Vertical transmission of SARS-CoV- What is the optimal definition? Am J Perinatol (2020) ;37: (8):769–72. |
[24] | Vigil-Vázquez S , Carrasco-García I , Hernanz-Lobo A , Manzanares Á , Pérez-Pérez A , Toledano-Revenga J , et al. GESNEO-COVID cohort working grouImpact of gestational COVID-19 on neonatal outcomes: Is vertical infection possible? Pediatr Infect Dis J (2022) ;41: (6):466–72. |
[25] | Hopwood AJ , Jordan-Villegas A , Gutierrez LD , Cowart MC , Vega-Montalvo W , Cheung WL , et al. Severe acute respiratory syndrome coronavirus-2 pneumonia in a newborn treated with remdesivir and coronavirus disease convalescent plasma, J Pediatric Infect Dis Soc (2021) ;10: (5):691–4. |
[26] | Wyckoff AS , editor. AAP issues guidance on infant born to mothers with suspected or confirmed COVID- 19. AAP News. https://www.aappublications.org/news/2020/04/02/infantcovidguidance040220. Published April 2, 2020. Accessed Oct 15, 2021. |
[27] | Fajardy I , Moitrot E , Vambergue A , Vandersippe-Millot M , Deruelle P , Rousseaux J . Time course analysis of RNA stability in human placenta, BMC Mol Biol (2009) ;10: :21. |
[28] | Jung E , Romero R , Yeo L , Diaz-Primera R , Marin-Concha J , Para R , Lopez AM , et al. The fetal inflammatory response syndrome: The origins of a concept, pathophysiology, diagnosis, and obstetrical implications, Semin Fetal Neonatal Med (2020) ;25: (4):101146. |
[29] | Gotsch F , Romero R , Kusanovic JP , Mazaki-Tovi S , Pineles BL , Erez O , et al. The fetal inflammatory response syndrome, Clin Obstet Gynecol (2007) ;50: (3):652–83. |
[30] | Godfred-Cato S , Tsang CA , Giovanni J , Abrams J , Oster ME , Lee EH , et al. Multisystem inflammatory syndrome in infants <12 months of Age, United States, May -January Pediatr Infect Dis J, 601-5. Erratum in: Pediatr Infect Dis J (2022) ;41: (3):274. |
[31] | Divekar AA , Patamasucon P , Benjamin JS . Presumptive neonatal multisystem inflammatory syndrome in children associated with coronavirus disease 2019 Am J Perinatol. (2021) ;38: (6):632–6. |
[32] | Shaiba LA , More K , Hadid A , Almaghrabi R , Al Marri M , Alnamnakani M , et al. Multisystemic inflammatory syndrome in neonates: A systematic review. Neonatology. 2022;1-13. |
[33] | More K , Aiyer S , Goti A , Parikh M , Sheikh S , Patel G , et al. Multisystem inflammatory syndrome in neonates (MISN) associated with SARS-CoV2 infection: A case series [published online ahead of print, 2022 Jan 14]. Eur J Pediatr. 2022;1-16. |
[34] | Saha S , Pal P , Mukherjee D . Neonatal MIS-C: Managing the cytokine storm, Pediatrics (2021) ;148: (5):e2020042093. |
[35] | Zhao Y , Yin L , Patel J , Tang L , Huang Y . The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID- A meta-analysis, J Med Virol (2021) ;93: (7):4358–69. |
[36] | Abasse S , Essabar L , Costin T , Mahisatra V , Kaci M , Braconnier A , et al. , A Neonatal COVID-19 pneumonia: Report of the first case in a preterm neonate in mayotte, an overseas department of france, Children (Basel, Switzerland) (2020) ;7: (8):87. |
[37] | De Bernardo G , Giordano M , Zollo G , Chiatto F , Sordino D , De Santis R , et al. The clinical course of SARS-CoV-2 positive neonates, JPerinatol (2020) ;40: (10):1462–9. |
[38] | Norman M , Navér L , Söderling J , Ahlberg M , Hervius Askling H , et al. Association of maternal SARS-CoV-2 infection in pregnancywith neonatal outcomes, JAMA (2021) ;325: (20):2076–86. |
[39] | Nino G , Molto J , Aguilar H , Zember J , Sanchez-Jacob R , Diez CT , et al. Chest X-ray lung imaging features in pediatric COVID-19 and comparison with viral lower respiratory infections in young children, Pediatr Pulmonol (2021) ;56: (12):3891–8. |
[40] | Parisi GF , Indolfi C , Decimo F , Leonardi S , Miraglia Del Giudice M . COVID-19 pneumonia in children: From etiology to management, Front Pediatr (2020) ;8: :616622. |
[41] | Palabiyik F , Kokurcan SO , Hatipoglu N , Cebeci SO , Inci E . Imaging of COVID-19 pneumonia in children, Br J Radiol (2020) ;93: (1113):20200647. |
[42] | Trunfio M , Longo BM , Alladio F , Venuti F , Cerutti F , Ghisetti V , et al. On the SARS-CoV-2 “Variolation Hypothesis": No association between viral load of index cases and COVID-19 severity of secondary cases, Front Microbiol (2021) ;12: :646679. |




