Multisystem inflammatory syndrome in neonates associated with SARS-CoV-2 infection, a different entity?
Abstract
BACKGROUND:
Multisystemic inflammatory syndrome in children (MIS-C) is a novel disease that is associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). MIS-C usually affects children older than 5 years of age and adolescents, with a median of 8-years and an interquartile range of 3 to 11 years. A multisystemic inflammatory disease has been described in neonates and named MIS-N (multisystemic inflammatory syndrome in Neonates). We report three cases of Mexican newborns with MIS-N presenting with multiorgan compromise and a positive anti-SARS-CoV-2 IgG who developed Kawasaki disease (KD)-like cardiac features and discuss the current dilemma regarding diagnosis and treatment in these patients.
1Introduction
Multisystemic inflammatory syndrome in children (MIS-C) is a novel disease that is associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). MIS-C usually affects children older than 5 years of age and adolescents, with a median of 8-years and an interquartile range of 3 to 11 years [1]. MIS-C is extremely rare in infants, particularly in neonates [1]. Neonates born to mothers with SARS-CoV2 infection during pregnancy demonstrate multisystemic inflammatory disease with cardiac dysfunction and has been named Multisystemic Inflammatory Syndrome in Neonate (MIS-N) [2–21]. We report three cases of Mexican newborns with MIS-N showing multiorgan compromise and a positive anti-SARS-CoV-2 IgG who developed Kawasaki disease (KD)-like cardiac features. We performed a literature search of MIS-C, MIS-C-like or MIS-N cases reported in the literature and compared them with our cases. We discuss the peculiarities of MIS-N compared to MIS-C.
2Case 1
A 32-year-old female with a history of diabetes mellitus was admitted for cesarean delivery due to macrosomic product. She had no history of COVID-19 symptoms and SARS CoV2 antibodies showed IgG positive and IgM negative. The female newborn weighed 3.7 kg, the Apgar scores were 8 at 1 minute and 9 at 5 minutes and required routine care. The infant developed tachypnea and hypoxemia at birth intra-uterine pneumonia was suspected and was admitted to the neonatal intensive care unit. The patient developed respiratory failure requiring mechanical ventilation on her first day of life. Antibiotic therapy was started, and cardiac evaluation was performed due to a heart murmur. Transthoracic echocardiogram showed left ventricle ejection fraction of 61% and minimal pericardial effusion with coronary artery dilation, left coronary artery 1.5 mm (Z score+1.83); left anterior descending (LAD) 1.6 mm (Z score+2.26); right coronary artery proximal region 1.5 mm (z score+1.52); medium region (z score+2.51) and distal region (z score+2.56). Laboratory tests revealed hemoglobin 12.9 g/dL, leukocytes 10200 cells /μL, neutrophils 4000 cells/mcl, 5100 cells/μL, albumin 3.2 g/dL, platelet count 457×109/L, AST 22 UI/L, ALT 18 UI/L, normal CRP (5.9 mg/L), elevated D-dimer (23974 ng/mL), elevated CK-MB (26.3 U/L), and elevated ferritin (174 mg/dL). The patient had a negative nasopharyngeal swab RT-PCR SARS-CoV2 and a positive SARS-CoV-2 IgG with negative IgM. Due to multiorgan involvement and elevation in inflammatory markers, the patient was diagnosed with MIS-C-like manifestations. She was treated with intravenous immunoglobulin (IVIG) 2 g/kg, methylprednisolone 1 mg/kg/day, low-dose aspirin and enoxaparin, with improvement and the patient was discharged at 15th day of life.
3Case 2
A premature male infant weighing 1.3 kg was delivered by cesarean section at 32-weeks of gestation due to severe preeclampsia. Apgar scores were 6 at 1 minute and 7 at 5 minutes. Due to respiratory distress, cyanosis and increase in oxygen requirements the patient was admitted to the NICU. On arrival the infant was intubated and received mechanical ventilation with 100% oxygen. The patient developed hemodynamic instability and required vasopressors (dobutamine). The patient’s mother had respiratory symptoms at the time of cesarean delivery (cough, dyspnea, hypoxemia) and had a positive RT-PCR SARS-CoV2 test. On day 24 after cesarean delivery, the patient’s mother had a positive anti-SARS-CoV-2 IgG and negative anti-SARS-CoV-2 IgM test.
Laboratory tests show hemoglobin 15.9 g/dL, leukocytes 12700 cells/μL, neutrophils 4600 cells/mcl, 4400 cells/μL, albumin 3.0 g/dL, platelet count 246×109/L, 1225×109/L at day 12, AST 20 IU/L, ALT 6.4 IU/L, normal CRP (0.4 mg/L), elevated D-dimer (1983 ng/mL), elevated CK-MB (22 U/L) and elevated ferritin (365 mg/dL). Nasopharyngeal swab, for SARS-CoV-2 RT-PCR was negative. Transthoracic echocardiogram showed normal biventricular systolic function but identified coronary artery dilation, left coronary artery 1.5 mm (Z score+2.6); left anterior descending (LAD) 1.3 mm (Z score 2.78); right coronary artery proximal region 1.4 mm (z score+3.0) and hypertrophic myocardiopathy (left ventricular hypertrophy) with ejection fraction 82%. He was treated with IVIG 2 g/kg, methylprednisolone 1 mg/kg/day, low-dose aspirin and enoxaparin. The patient required prolonged mechanical ventilation and had neurologic complications (Grade II intraventricular hemorrhage, Papile classification) and severe prematurity retinopathy.
4Case 3
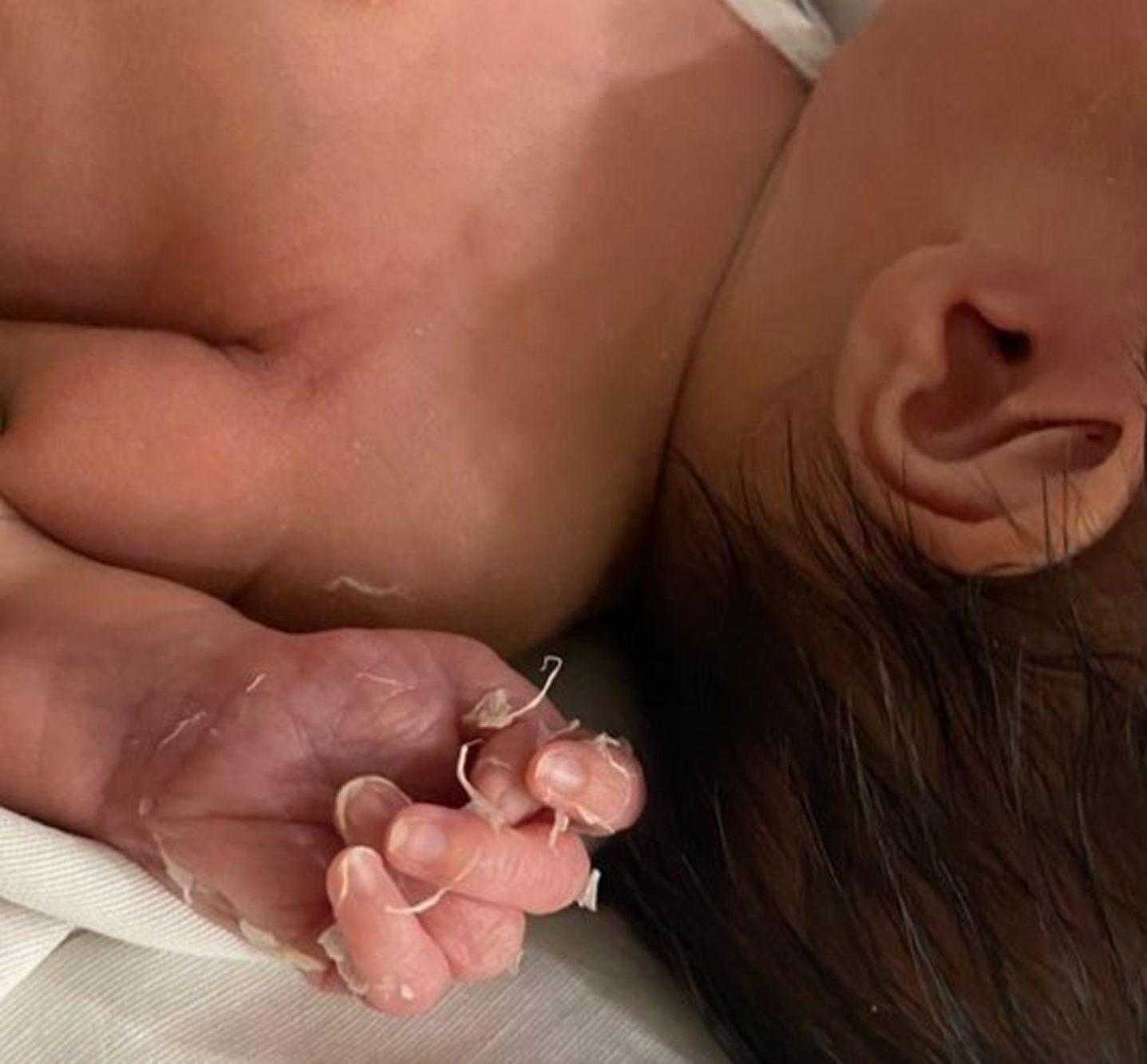
A preterm (gestational age 35 weeks, 1875 g) infant female was delivered by urgent cesarean section due to anhydramnios. Her Apgar scores were 7 at 1 minute and 8 at 5 minutes requiring one cycle of positive ventilation. Her mother was a 25-year-old, gravida 1 with a history of COVID-19 infection (positive RT-PCR test) at 18 weeks of gestational age. The neonate developed respiratory distress at birth requiring admission to the neonatal intensive care unit with early initiation of continuous positive airway pressure therapy. Intra-uterine pneumonia was suspected, antibiotic therapy was started, and cardiac evaluation was performed due to cardiomegaly seen on a chest X-ray. The patient developed hemodynamic instability and was started on dobutamine. Laboratory tests at birth revealed hemoglobin 11 g/dL, leukocytes 128000 cells /μL, albumin 3.3 g/dL, platelet count 30×109/L, CRP 2.56 mg/L, troponin level 84.3 ng/ pro-BNP 12,700 pg/mL; AST 29 IU/L, ALT 20 IU/L. She received platelet transfusions. Oncologic evaluation was permormed because severe leukocytosis, and oncologic pathology was rule out. The neonatal nasopharyngeal swab for SARS-CoV-2 RT-PCR was negative, anti-SARS-CoV-2 IgG was positive and anti-SARS-CoV-2 IgM was negative, 24 hours after birth. Echocardiogram showed tricuspid and pulmonary regurgitation with normal ventricular function. She was treated with IVIG2 g/kg, methylprednisolone 3 mg/kg/day, low-dose aspirin and dobutamine 5mcg/kg/min with improvement and normalization of cardiac enzymes. The patient developed desquamation of fingers of the hands at 14th day of life (Fig. 1). Currently she is alive, with normal cardiac function.
Fig. 1
Desquamation of the fingers.
5Search strategy
Using the PubMed/MEDLINE, Scopus, and Web of Science databases, we searched existing literature using the following strategy: (COVID-19 OR SARS-CoV-2 OR coronavirus OR Multisystemic Inflammatory Syndrome in Children (MIS-C) OR Paediatric Inflammatory Multisystemic Syndrome (PIMS)) AND (neonate) OR (newborn) OR (infant). Only publications involving humans were reviewed. 336 publications were retrieved from PubMed/MEDLINe, 2048 from Scopus, 616 from Web of Science. After excluding non-relevant papers, all individual case reports and case series published before 08/26/2022 were reviewed.
6Discussion
Newborns of mothers with positive SARS-CoV2 infection rarely acquire the disease or show adverse clinical outcomes. Several reviews analyzing neonatal SARS-CoV2 infections have been published [10, 11].
Godfred-Cato et al. described the clinical course of 85 infants < 12 months with MIS-C, including one 14 days-old patient and concluded that as a group they present a milder course compared to older patients [12]. Out of 3000 newborns born in our maternity hospital during the pandemic, only three presented the clinical features described above. Few cases of MIS-C or MIS-C-like neonates have been described so far (Table 1). Borkotoky et al. report a term infant with persistent pulmonary hypertension with features of MIS-C [5]. Divekar et al. report a 1,300 g female whose mother presented asymptomatic SARS-CoV2 infection [6]. The patient presented multisystemic dysfunction and the echocardiogram showed pericardial effusion, mitral regurgitation and dilated coronary arteries. Kappanayil et al. report a 24-day-old female with cardiogenic shock with elevated transaminases and ferritin who responded to IVIG, corticosteroids and anticoagulants [8]. Savic et al. report a SARS-CoV2 infected newborn who presented a “cytokine storm syndrome” with multiorgan failure and died despite treatment with steroids and tocilizumab [9]. Baidoun et al. report a 4-week-old patient believed to have dilated cardiomyopathy associated with SARS-CoV2 infection, without fulfilling MIS-C criteria [13]. Important to note is that few patients have presented with fever, absence of abdominal manifestations in the majority of patients and two of our patients presented a normal CRP, all important data to diagnose MIS-C in older patients. Bakhle et al described an eight-day-old neonate with fever [14]. The mother was positive for COVID-19 in the 29th week. COVID-19 reverse-transcription polymerase chain reaction was negative and antibodies were positive. He had increased leukocyte count, and elevated levels of C-reactive protein (CRP), procalcitonin, ferritin, lactate dehydrogenase, and D-dimers along with bilateral reticulonodular opacities on chest radiograph and multiple nodules with evidence of cavitation in both lungs on chest tomography, the authors conclude that thromboembolic complications secondary to inflammatory response after SARS-CoV2 exposure should be considered in neonates [14]. Agrawal et al, present the first case report of a neonate presenting within 48 hours of life with predominant abdominal signs mimicking surgical abdomen. Clinical picture comprised fever, multiorgan dysfunction (gastrointestinal, cardiorespiratory, hepatic and dermatological), and positive inflammatory markers [15]. Finally, Saha et al, reported a very severe case of a term infant girl with fever from day 8 and Reverse transcriptase–polymerase chain reaction results for coronavirus disease positive who developed cardiogenic shock with pulmonary edema and needed invasive ventilation. She developed seizures, pulmonary hemorrhage, cardiac arrest and acute kidney injury [16]. Interestingly, the majority of reported patients in the literature are from India, raising the possibility of a genetic predisposition in this population (Table 1).
Table 1
Cases of Multisystem Inflammatory Syndrome in neonates associated with SARS-CoV-2 infection
| Author | Borkotoky | Divekar | Orlasnki-Meyer | Kappanayil | Savic | Bakhle | Agrawal | Saha | Arun | Gupta | Gupta | Costa | Voddapelli | Case 1 | Case 2 | Case 3 |
| Country | India | USA | Israel | India | Serbia | India | India | India | India | India | India | Italy | India | Mexico | Mexico | Mexico |
| Gender | Male | Female | Female | Female | NR | male | Male | Female | Male | Male | Female | Male | Female | Female | Male | Female |
| Age | 4 hour male 38 weeks of gestation | 30 weeks of gestation | 8 weeks-old | 24 day-old | NR | 8 day_old 37 weeks of gestation | 39 weeks of gestation | 8 day-old | 2 days-old | At birth | 6th day of life | At birth | 3 day | 38 weeks of gestation | 32 weeks of gestation | 35 weeks of gestation |
| Clinical picture | Respiratory distress, feed intolerance, fever, vomiting | Respiratory failure, hepatic and renal dysfunction | Diarrhea, bloody stool, vomiting, fever, lethargic | Cardiogenic shock, hepatomegaly, gluteal skin lesions | Tachypnea, tachycardia, fever, grunting | multiple cavitary lesions in lung | Fever and predominant abdominal signs mimicking surgical abdomen | Fever, Cardiogenic Shock, seizures, pulmonary hemorrhage, cardiac arrest and acute kidney injury | Swelling left thigh, poor feeding, lethargy, seizures, IC bleeding (Hemo philia) | Persistent pulmonary hypertension, cardiac dysfunction, coagulopathy | Cardiac dysfuncyion, intracardiac thrombosis | Respiratory distress syndrome, seizures, desquamation | Fever, abdominal distention, vomiting, hepatomegaly, shock | Respiratory distress | Respiratory distress | Respiratory distress |
| Kawasaki disease clinical features | No | No | Cracked lips | No | No | No | No | No | No | NR | NR | No | No | No | No | Finger desquamation |
| Symptomatic Mother | Yes (fever and cough) | No | NR | Yes (mild COVID-19) | No | Yes (mild COVID -19) | No history of contact of mother with COVID-19 4weeks before delivery | No | NR | Asymptomatic | NR | Fever, anosmia, ageusia | COVID-19 three weeks before delivery | No | No | No |
| RT-PCR SARS-CoV2 mother | Negative | Positive | Positive | Negative | Positive | Negative | Negative | NR | NR | NR | NR | NR | NR | Negative | Positive | Positive |
| PCR SARS-CoV2 patient | Negative | Negative | Negative | Negative | Positive | NR | Negative | Positive | NR | NR | NR | NR | NR | Negative | Negative | Negative |
| Serology in mother | Positive IgG SARS-CoV2 | Positive | NR | Positive IgG SARS-CoV2 | NR | NR | SARS-CoV-2 IgG positive | NR | NR | NR | NR | Positive | NR | Positive | Positive | Positive |
| Serology in patient | Positive | Positive | Positive | Positive IgG SARS-CoV2 | NR | Positive IgG antibodies against SARS-CoV-2 spike protein | SARS-CoV-2 IgG positive | NR | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Laboratory | Leukocytosis, thrombocytosis, elevated CRP, IL-6, ferritin, D-dimers, CK-MB, BNP, troponin | Leukopenia, lymphopenia, thrombocytopenia, elevated D-dimers | Elevated CRP, BNP, leukocytosis, thrombocytosis | Leukocytosis, elevated BNP, troponin, D-dimers, transaminases, ferritin, CRP | Elevated D-dimers, CRP, IL-6 | Increased leucocyte count, and elevated levels of C-reactive protein (CRP), procalcitonin, ferritin, lactate dehydrogenase, and D-dimer | Elevated CRP, procalcitonin, D Dimer and N-terminal-pro-B-type natriuretic peptide | Elevated CRP, D Dimer, Pro B type natriuretic peptide. Ferritin. Thrombocytopenia | Leukocytosis, neutrophilia, elevated CRP, conjugated hyperbilirubinemia, elevated LDH, elevated D-dimers. | Elevated troponin, CPK, ferritin, LDH, proBNP | Elevated D-dimers, ferritin, troponin, LDH | Thrombocytopenia, neutropenia, elevated proBNP | Elevated CRP, IL-6, proBNP, leukocytosis, neutrophilia | Elevated CK-MB, D-dimers, normal CRP | Leukocytosis, thrombocytosis, normal CRP, | Anemia, leukocytosis, elevated troponin, CRP and BNP |
| ECHO | Pulmonary hyprtension | Small pericardial effusion | Mitral regurgitation | Biventricular dysfunction, hyperechoic coronary arteries | NR | NR | Normal at 2 weeks | Systolic dysfunction, with ejection fraction of 40% and mild pericardial effusion. | Left coronary artery dilatation | Dilated hyperechhogenic coronaries | INTRACARDIAC THROMBUS | Dilated right coronary artery, dilated lefct coronary artery | Biventricular dysfunction, normal coronary arteries | Coronary artery dilation, pericardial effusion | Coronary artery dilation | Pulmonary and tricuspid regurgitation, |
| Treatment | Sildenafil, furosemide, tazobactam/piperacilin, dexamethasone | IVIG, hydrocrtisone, dopamine | IVIG, MPD, anakinra | IVIG, heparin, MPD | Tocilizumab, dexamethasone, enoxaparin | IVIG 1 g/kg/ day for 3 days | IVIG (2/gr/kg) 2 doses methylprednisolone (1 mg/kg/dose) Enoxaparin, Inotropic treatment | IVIG 2 g/kg, along with methylprednisolone at 2 mg/kg/day. Enoxaparin Inotropic support | Methylprednisolone, IVIG | IVIG, steroids, inotropic, diuretics, sildenafil, bosentan | IVIG enoxaparin, aspirin | IVIG, methylprednislone, enoxaparin | IVIV, dexamethasone | IVIG, MPD, enoxaparin | IVIG, MPD, enoxaparin, aspirin, dobutamine | IVIG, MPD, aspirin, dobutamine |
| Outcome | Good | Good | Good | Good | Died | Good | Good | Good | Good | Death | NR | Good | Good | Good | Good | Stable |
Molloy et al. recommend using the term MIS-N to describe neonatal inflammation illness involving >2 organ systems along with laboratory evidence of inflammation and a maternal history of SARS-CoV2 infection during pregnancy with the exception of fever which is uncommon in neonates [4]. Alternate diagnosis has to be excluded. Important to note is that Kawasaki disease clinical features are not found in MIS-N. A consensus definition of MIS-N must be implemented [4].
Raschetti et al. report multisystemic involvement with MIS-C-like manifestations including rash, conjunctivitis, gastrointestinal, neurological and hemodynamic manifestationsin neonates with SARS-CoV2 transmitted postnatally [11].
It is unclear whether MIS-C develops secondary to a direct effect of SARS-CoV2 infection with ongoing viral replication, postinfectious immune-dysregulation or a combination of this factors. Most children with MIS-C respond to immunomodulatory therapy, consistent with a pathogenesis primarily mediated by inappropriate immune system activation. Antibodies from mothers infected with SARS-CoV2 may passively cross the placental barrier and it is believed that confer protection to the newborn, however in MIS-N patients it may trigger the disease. Maternal adaptive immune response t SARS-CoV2 infection may generate protective antibodies and, in some cases, pathogenic antibodies directed toward neonatal antigens responsible for cytokine release and multisystemic inflammation. Multiple autoantibodies have been proposed to be implicated in the pathogenesis of MIS-C [21]. Anti-receptor binding domain (RBD) antibodies have been shown to be higher in children with severe MIS-C which correlate with erythrocyte sedimentation rate [22]. Patients with MIS-C humoral response present enhanced monocyte activating capacity with dysregulated proinflammatory IgG response to SARS-CoV2 [23]. Also, antibody dependent enhancement is thought to play a role in the pathogenesis of COVID-19. Alternatively, SARS-CoV2 have been proved to be transmitted trans-placentally [24]. It has been suggested that SARS-CoV2 has superantigen properties leading to a hyperinflammatory response [25].
Neonatal KD is extremely rare too. Li et al. reviewed 20 cases of neonatal KD, most of them present incomplete presentation, 55% of them with coronary changes [26]. Interestingly 31% of them presented normal CRP [22]. MIS-N present unique characteristics compared to MIS-C older patients. Recent MIS-N reviews have come to the same conclusions. Shaiba et al. report cardiovascular compromise in 77% of patients that included cardiac dysfunction, arrhythmias, coronary abnormalities, pericardial effusion, pulmonary hypertension and intracardiac thrombus [2]. Two of our patients presented coronary artery abnormalities. Patients with MIS-N usually don't present fever, CRP may be normal, and KD-features are subtle or absent in the majority of patients. Clinical features are varied including gastrointestinal, pulmonary and neurological involvement but most importantly cardiological manifestations. All neonates present with positive IgG antiSARS-CoV2 antibodies. Echocardiographic evaluation is crucial, with development of coronary abnormalities despite not having Kawasaki disease clinical features MIS-N is extremely rare and may have a different physiopathology compared to MIS-C in older patients. MIS-N may constitute a distinct entity, with diverse and different clinical and laboratory manifestations, probably triggered by trans-placental pathogenic antibodies. Neonatologist should perform specific investigation in patients born to mother with COVID-19 and presenting with at least two systems involved. A high index of suspicion is key in neonates from SARS-CoV2 infected mothers during the present pandemic.
Data availability
All data relevant to the study are included in the article.
Declarations
Ethics approval: All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgement
We thank Dr. Alberto Unzueta for useful discussions.
References
[1] | Belot A , Antona D , Renolleau S , Javouhey E , Hentgen V , Angoulvant F , et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May Eur Surveill (2020) ;25: (22):2001010. |
[2] | Shaiba LA , More K , Hadid A , Almaghrabi R , Al Marri M , Alnamnakani M , et al., Multisystemic inflammatory syndrome in neonates: A systematic review, Neonatology (2022) ;119: (4):405–17. |
[3] | De Rose DU , Pugnaloni F , Calì M , Ronci S , Caoci S , Maddaloni C , et al., Multisystem inflammatory syndrome in neonates born to mothers with SARS-CoV-2 infection (MIS-N) and in neonates and infants younger than 6 months with acquired COVID-19 (MIS-C): A Systematic Review, Viruses (2022) ;14: (4):750. |
[4] | Molloy EJ , Nakra N , Gale C , Dimitriades VR , Lakshminrusimha S , Multisystem inflammatory syndrome in children (MIS-C) and neonates (MIS-N) associated with COVID- optimizing definition and management, Pediatr Res (2022) ;1: :1–10. |
[5] | Borkotoky RK , Barua PB , Paul SP , Heaton P , COVID-19-related potential multisystem inflammatory syndrome in childhood in a neonate presenting as persistent pulmonary hypertension of the newborn, Pediatr Infect Dis J (2021) ;40: (4):e162–e164. |
[6] | Divekar AA , Patamasunon P , Benjamin JS , Presumptive Neonatal Multisystem Inflammatory Syndrome in Children Associated with Coronavirus Disease Am J Perinatol (2021) ;38: (6):632–6. |
[7] | Orlanski-Meyer E , Yogev D , Auerbach A , Megged O , Glikman D , Hashkes PJ , et al., Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus-2 in an 8-week-old infant, J Pediatric Infect Dis Soc (2020) ;9: (6):781–4. |
[8] | Kappanayil M , Balan S , Alawani S , Mohanty S , Leeladharan SP , Gangadharan S , et al., Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-CoV- a case report, Lancet Child Adolesc Health (2021) ;5: (4):304–8. |
[9] | Savić D , Simović A , Ristić D , Stojković T , Zivojinović S , Prodanović T , et al., Fatal outcome of COVID-19 in a Newborn, Indian J Pediatr (2021) ;6: :1. doi: 10.1007/s12098-021-03860-zEpub ahead of print. |
[10] | Kyle MH , Glassman ME , Khan A , Fernández CR , Hanft E , Emeruwa UN , et al., A review of newborn outcomes during the COVID-19 pandemic, Semin Perinatol (2020) ;44: (7):151286. doi: 10.1016/j.semperi.2020.151286Epub 2020 Jul 23.. |
[11] | Raschetti R , Vivanti A , Vaulopu-Fellous C , et al., Synthesis and systematic review of reported neonatal SARS-CoV-2 infections, Nat Commun (2020) ;15;11: (1):5164. |
[12] | Godfred-Cato S , Tsang CA , Giovanni J , Abrams J , Oster ME , Lee EH , et al., Multisystem Inflammatory Syndrome in Infants <12 months of Age, United States, May –January 2021. Pediatr Infect Dis J (2021) ;40: (7):601–5. |
[13] | Baidoun M , Elgendy M , Al-Maajali D , Fountain R , SARS-CoV-2 infection associated severe dilated cardiomyopathy in a 4-week-old infant, IDCases (2021) ;25: :e01178. doi: 10.1016/j.idcr.2021.e01178Epub 2021 Jun 10. |
[14] | Bakhle A , Sreekumar K , Baracho B , Sardessai S , Silveira MP , Cavitary lung lesions in a neonate: Potential manifestation of COVID-19 related multisystem inflammatory syndrome, Pediatr Pulmonol (2022) ;57: (1):311–4. |
[15] | Agrawal G , Wazir S , Arora A , Sethi SK , Síndrome inflamatoriomultisistémico en un neonato enmascarado como abdomenquirúrgico, BMJ Case Ree (2021) ;14: (10):246579. |
[16] | Saha S , Pal P , Mukherjee D , Neonatal MIS-C: Managing the Cytokine Storm, Pediatrics (2021) ;148: (5):e2020042093. |
[17] | Voddapelli SK , Murki S , Rao VP , Neonatal multisystem inflammatory syndrome (MIS-N) presenting as necrotizing enterocolitis and cardiac dysfunction, Indian Pediatr (2022) ;59: (6):502–3. |
[18] | Arun S , Cherian TG , Philip C , Multisystem inflammatory syndrome in a neonate with severe hemophilia –a diagnostic challenge in COVID times: A case report, BMC Pediatr (2022) ;22: (1):397. |
[19] | Gupta P , B SA , Tamatam PR , Dhulipudi B , Vardhelli V , Deshabhotla S , Oleti TP , Neonatal Multisystem Inflammatory Syndrome (MIS-N) Associated with Maternal SARS-CoV-2 Exposure, Indian J Pediatr (2022) ;89: (8):827–8. |
[20] | Costa S , Delogu AB , Bottoni A , Purcaro V , D’Andrea V , Paladini A , et al., COVID-19-associated multisystem inflammatory syndrome in a neonate with atypical coronary artery involvement, Am J Perinatol (2022) ;29: (14):1514–8. doi: 10.1055/a-1733-4163Epub ahead of print. |
[21] | Consiglio CR , Cotugno N , Sardh F , Pou C , Amodio D , Rodriguez L , et al., The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19, Cell (2020) ;183: (4):968–981.e7. |
[22] | Bartsch YC , Wang C , Zohar T , Fischinger S , Atyeo C , Burke JS ,et al. Humoral signatures of protective and pathological SARS-CoV-2infection in children, Nat Med (2021) ;27: (3):454–62. |
[23] | Rostad CA , Chahroudi A , Mantus G , Lapp SA , Teherani M , Macoy L , et al., Quantitative SARS-CoV-2 Serology in Children With MultisystemInflammatory Syndrome (MIS-C), Pediatrics (2020) ;146: (6):e2020018242. |
[24] | Vivanti AJ , Vauloup-Fellous C , Prevot S , Zupan V , Suffee C , Do Cao J , et al., Transplacental transmission of SARS-CoV-2 infection, NatCommun (2020) ;11: (1):3572. |
[25] | Noval Rivas M , Porritt RA , Cheng MH , Bahar I , Arditi M , COVID-19-associated multisystem inflammatory syndrome in children (MIS-C): A novel disease that mimics toxic shock syndrome-the superantigen hypothesis, J Allergy Clin Immunol (2021) ;147: (1):57–9. |
[26] | Li C , Du Y , Wang H , Wu G , Zhu X , Neonatal Kawasaki disease: Case report and literature review, Medicine (2021) ;100: (7):e24624. |




